Abstract
Background
Arcobacter butzleri (A. butzleri) is an emerging enteric pathogen increasingly identified in Europe and is likely under-reported in other global regions. We describe to our knowledge the first case report of A. butzleri in an AIDS patient, along with the first documented local (Singapore) case of A. butzleri infection. Case Presentation. A 38-year-old AIDS patient presented with diarrhoea of 2 weeks' duration. Stool cultures yielded A. butzleri. The patient was treated with 3 days of ciprofloxacin with clinical resolution of diarrhoea.
Conclusion
A. butzleri is likely to be present, although under-reported in AIDS patients, and it should be noted as a pathogen of increasing significance.
1. Introduction
Patients with human immunodeficiency virus (HIV) are more prone to acquiring enteric infections from food-borne and zoonotic pathogens [1]. Arcobacter butzleri (A. butzleri) is a Campylobacter-like organism of the genus Arcobacter [2] that has been increasingly identified as an emerging enteric pathogen [2, 3].
We report a case of A. butzleri infection in an HIV-positive patient with acquired immunodeficiency syndrome (AIDS). This is likely to be the first case reported of its kind in an AIDS patient and in Singapore based on literature review. Clinicians need to be aware of this pathogen and its treatment and complications in immunocompromised hosts.
2. Case Presentation
A 38-year-old man with a history of HIV infection diagnosed in 2016 but only on intermittent antiviral treatment presented with symptoms of acute diarrhoea lasting 2 weeks. The diarrhoeal episodes occurred about ten to twenty times per day and stools were neither bloody nor mucoid. The diarrhoeal episode was associated with clinically significant loss of weight and cramping abdominal pain. The patient had been on boosted protease inhibitor (darunavir/ritonavir) monotherapy for 6 months prior to admission. There was no recent travel history or intake of raw or uncooked food, and he had worked as a freelance architect prior to losing his job at the start of the pandemic. Clinical examination revealed a hypovolemic fluid state with significant cachexia, while bowel sounds were hyperactive. There was no abdominal tenderness, and digital rectal examination was unremarkable.
Laboratory investigations showed evidence of mild leukocytosis (10.03 × 109/L) with neutrophilia (9.36 × 109/L); his absolute CD4 count was <20. His viral load was 87 × 106 U/mL. There was no elevated creatinine to suggest acute kidney injury, although his urea level was slightly elevated, indicating dehydration. Blood cultures were negative for bacterial organisms. Stool cultures were positive for A. butzleri on 2 separate occasions (Figure 1). Sensitivity testing yielded intermediate sensitivities to ciprofloxacin (34 mm) and high sensitivities to erythromycin (20 mm) (Figure 2).
Figure 1.
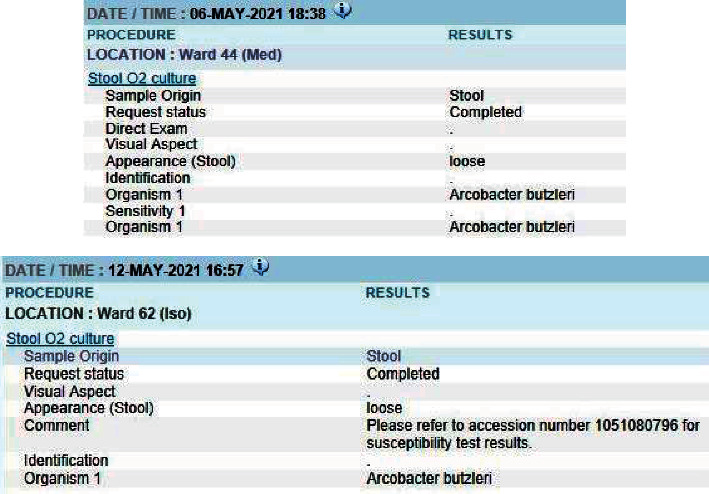
Stool culture reports indicating presence of A. butzleri.
Figure 2.

Sensitivities of identified A. butzleri in our patient.
The patient was subsequently treated presumptively with 3 days of oral ciprofloxacin 500 mg twice daily with complete clinical resolution of diarrhoea and abdominal discomfort. Repeat stool cultures after the completion of antibiotics were also negative (Figure 3). Unfortunately, he developed other infective complications such as nosocomial pneumonia, cytomegalovirus (CMV) colitis, and disseminated Mycobacterium avium complex (MAC) infection. He passed away from respiratory failure from severe pneumonia 4 weeks after admission.
Figure 3.
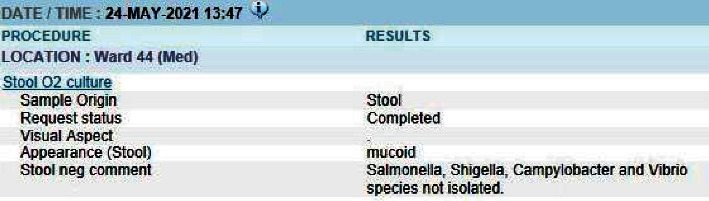
Stool culture report after 3-day course of ciprofloxacin.
3. Discussion and Conclusions
A. butzleri is a Gram-negative, motile, aerotolerant microbe of the Epislonproteo bacteria class and Campylobacteraceae family [2] and is one of 27 recognised species of Arcobacter characterised to date [3]. It was first isolated in aborted bovine foetuses [4] and was later linked to causing reproductive disorders and late-term abortions in cattle, pigs, and sheep [5]. It has been present in a range of commonly consumed meat including poultry and red meat [6, 7] and contains virulence genes such as ciaB (Campylobacter invasive antigen B) and hecB (encoding a haemolysin activation protein) [8], in addition to possessing adhesive and invasive properties toward multiple human cell lines [9]. These virulence genes are nonetheless not routinely tested in our laboratory and thus were not tested in this patient's specimen.
As a result, A. butzleri has been identified as an emerging enteropathogen and a zoonotic agent of increasing significance [10]. Taylor et al. identified A. butzleri in 2.4% of diarrhoeal samples in Thai children in 1991 [11], while several sporadic outbreaks of A. butzleri gastroenteritis have been observed in Europe and South Africa across the 1990s and 2000s [12–14]. Gastrointestinal infection and colonisation with A. butzleri have since demonstrated increasing prevalence; A. butzleri was identified as a causative organism for 24 out of 4636 cases of gastroenteritis in a prospective study in Germany [15], while several studies have identified A. butzleri to be amongst the most frequently isolated Campylobacteraceae strain in human clinical samples [16, 17].
While gastrointestinal infections with A. butzleri are becoming more common, severe infections such as bacteraemia remain rare. A case of A. butzleri bacteraemia was observed in a neonate in the United Kingdom [18], while in Hong Kong, two cases of A. butzleri bacteraemia have been reported, both patients with underlying diseases of liver cirrhosis and gangrenous appendicitis, respectively [19, 20]. A single case of Arcobacter causing peritonitis has been reported in a patient on peritoneal dialysis, occurring after repositioning of a Tenckhoff catheter [21]. Case studies documenting A. butzleri infection, ranging from gastrointestinal infections to bacteraemia, are apprised in Table 1. Despite the well-documented phenomenon of enteral infections with A. butzleri, there have yet to be documented cases of infection in HIV patients, although it is highly likely that the true global prevalence is underestimated. We believe that this is the first documented case of its kind, in addition to being the first in Singapore to document a case of A. butzleri infection locally.
Table 1.
Case series of documented A. butzleri infections.
| Case studies | Treatment | Reference |
|---|---|---|
| Diarrhoea in rural children, Thailand (93) | Not stated | [11] |
| Gastrointestinal symptoms campylobacter-like infections, France (29) | 2 treated with oral (PO) fluoroquinolones (ciprofloxacin), 1 treated with PO co-amoxiclav | [17] |
| Enteritis (46), of which 19 had acute gastroenteritis, 30 had co-existing conditions and 8 had chronic colitis, Belgium | Not stated | [22] |
| Gastroenteritis (24) out of 3884 outpatients and 752 inpatients | Not stated; recommendations were for fluoroquinolones | [15] |
| Bactaraemia in an immunocompromised host (1) (85 y/o CLL on idelalisib) | Intravenous (IV) piperacillin-tazobactam and vancomycin | [23] |
| Bactaraemia in a neonate (1) causing neonatal sepsis | Not stated | [18] |
| Bactaraemia in a patient with acute gangrenous appendicitis (1) | IV cefuroxime and metronidazole | [20] |
| Bactaraemia in a liver cirrhosis patient (1) | IV cefuroxime | [19] |
| Peritoneal dialysis (PD) peritonitis (1) | IV ticarcillin-clavulanate x 2 weeks | [21] |
Differential diagnoses to be considered for this patient would include cytomegalovirus (CMV) colitis, Mycobacterium avium enteric infection, as well as HIV-associated enteropathy [24], along with other Campylobacter species that are found more commonly in HIV patients [25, 26].
The majority of A. butzleri infections are self-limiting and do not require treatment with antibiotics unless clinically indicated, such as in cases of severe and persistent symptoms [17], as in our patient. Despite its status as an emerging pathogen, data on antibiotic susceptibility of A. butzleri remain sparse. The most optimal antibiotic choice for treatment of A. butzleri is still unclear. The species has been classified as a potential multidrug resistant (MDR) organism as it has demonstrated varying levels of susceptibility toward different classes of agents [27]. In a Belgian antibiotic susceptibility study of 63 strains of A. butzleri, most strains (87%) were susceptible to ciprofloxacin (MIC90 32 mg/L), while moderate levels (36%) of resistance to doxycycline (MIC90 4 mg/L) were reported [22]. Combined resistance to erythromycin and doxycycline was observed in 10 out of 13 strains of A. butzleri [22]. Bruckner et al. also showed that ciprofloxacin was the most effective antibiotic agent for treatment of A. butzleri as compared to other antibiotics such as azithromycin [15], which are commonly used for Campylobacter. However, another meta-regression analysis revealed emerging resistance of Arcobacter species against fluoroquinolones [28]; this was corroborated in the case of our patient, whose specimens instead demonstrated the greater efficacy of erythromycin as opposed to ciprofloxacin (Figure 2).
In summary, A. butzleri is an emerging pathogen which is recognised increasingly in Europe and probably underrecognised in the rest of the world. Ours is the first documented case of A. butzleri in Singapore and possibly the first in an HIV patient. Clinicians should recognise the pathogenicity of A. butzleri in immunocompromised hosts and should always send stool cultures to test for bacterial strains especially in cases of persistent diarrhoea. Given that A. butzleri's antibiotic susceptibility pattern is different from Campylobacter's, laboratories in centres treating individuals with HIV should also be alert to this treatable pathogen to reduce morbidity.
Acknowledgments
The authors express their gratitude toward the staff of National University Hospital for their expert care of the patient.
Abbreviations
- A. butzleri:
Arcobacter butzleri.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Ethical Approval
The Institutional Review Board of National University Hospital waived the need for ethics approval for the purpose of this case report.
Consent
Verbal informed consent for publication of this case report was obtained from the patient. Written informed consent from the patient's next-of-kin was also obtained for publication of this case report as the patient had unfortunately passed away at the time of writing.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
THY Tan conducted the conception of the work, design of the work, acquisition of data, analysis of data, interpretation of data, drafting, and submission of the work. The author was also responsible for final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SM Tham conducted analysis and interpretation of data, revising of the work critically for important intellectual content. The author agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. PA Tambyah conducted conception of the work, design of the work, revising of the work critically for important intellectual content. The author agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Trevor Hwee Yong Tan, Sai Meng Tham, and Tambyah Paul Anatharajah have contributed equally to the work.
References
- 1. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=400125 .
- 2.Vandamme P. Microbiology Of Campylobacter Infections: Taxonomy Of The Family Campylobacteraceae . Washington, DC, USA: ASM Press; 2000. [Google Scholar]
- 3.Perez-Cataluna A., Salas-Masso N., Dieguez A. L., et al. Revisiting the taxonomy of the genus Arcobacter: getting order from the chaos. Frontiers in Microbiology . 2018;9:p. 2077. doi: 10.3389/fmicb.2018.02077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis W. A., Neill S. D., O’Brien J. J., Ferguson H. W., Hanna J. Isolation of Spirillum/Vibrio-like organisms from bovine fetuses. The Veterinary Record . 1977;100(21):451–452. doi: 10.1136/vr.100.21.451. [DOI] [PubMed] [Google Scholar]
- 5.Ho H. T., Lipman L. J., Gaastra W. Arcobacter, what is known and unknown about a potential foodborne zoonotic agent. Veterinary Microbiology . 2006;115(1-3):1–13. doi: 10.1016/j.vetmic.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Houf K., De Zutter L., Verbeke B., Van Hoof J., Vandamme P. Molecular characterization of Arcobacter isolates collected in a poultry slaughterhouse. Journal of Food Protection . 2003;66(3):364–369. doi: 10.4315/0362-028x-66.3.364. [DOI] [PubMed] [Google Scholar]
- 7.Wesley I. V. Encyclopedia of Food Microbiology . Academic Press, Cambridge, MA, USA: 2014. [Google Scholar]
- 8.Karadas G., Sharbati S., Hanel I., et al. Presence of virulence genes, adhesion and invasion of Arcobacter butzleri. Journal of Applied Microbiology . 2013;115(2):583–590. doi: 10.1111/jam.12245. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira S., Queiroz J. A., Oleastro M., Domingues F. C. Insights in the pathogenesis and resistance of Arcobacter: a review. Critical Reviews in Microbiology . 2016;42(3):364–383. doi: 10.3109/1040841X.2014.954523. [DOI] [PubMed] [Google Scholar]
- 10.Snelling W. J., Matsuda M., Moore J. E., Dooley J. S. Under the microscope: Arcobacter. Letters in Applied Microbiology . 2006;42(1):7–14. doi: 10.1111/j.1472-765x.2005.01841.x. [DOI] [PubMed] [Google Scholar]
- 11.Taylor D. N., Kiehlbauch J. A., Tee W., Pitarangsi C., Echeverria P. Isolation of group 2 aerotolerant Campylobacter species from Thai children with diarrhea. Journal of Infectious Diseases . 1991;163(5):1062–1067. doi: 10.1093/infdis/163.5.1062. [DOI] [PubMed] [Google Scholar]
- 12.Vandamme P., Pugina P., Benzi G., et al. Outbreak of recurrent abdominal cramps associated with Arcobacter butzleri in an Italian school. Journal of Clinical Microbiology . 1992;30(9):2335–2337. doi: 10.1128/jcm.30.9.2335-2337.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lerner J., Brumberger V., Preac-Mursic V. Severe diarrhea associated with Arcobacter butzleri. European Journal of Clinical Microbiology & Infectious Diseases . 1994;13(8):660–662. doi: 10.1007/BF01973994. [DOI] [PubMed] [Google Scholar]
- 14.Samie A., Obi C. L., Barrett L. J., Powell S. M., Guerrant R. L. Prevalence of Campylobacter species, Helicobacter pylori and Arcobacter species in stool samples from the Venda region, Limpopo, South Africa: studies using molecular diagnostic methods. Journal of Infection . 2007;54(6):558–566. doi: 10.1016/j.jinf.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 15.Brückner V., Fiebiger U., Ignatius R., et al. Prevalence and antimicrobial susceptibility of Arcobacter species in human stool samples derived from out- and inpatients: the prospective German Arcobacter prevalence study Arcopath. Gut Pathogens . 2020;12(1):p. 21. doi: 10.1186/s13099-020-00360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandenberg O., Dediste A., Houf K., et al. ArcobacterSpecies in Humans1. Emerging Infectious Diseases . 2004;10:1863–1867. doi: 10.3201/eid1010.040241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prouzet-Mauléon V., Labadi L., Bouges N., Ménard A., Mégraud F. Arcobacter butzleri: underestimated enteropathogen. Emerging Infectious Diseases . 2006;12(2):307–309. doi: 10.3201/eid1202.050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.On S. L., Stacey A., Smyth J. Isolation of Arcobacter butzleri from a neonate with bacteraemia. Journal of Infection . 1995;31(3):225–227. doi: 10.1016/S0163-4453(95)80031-X. [DOI] [PubMed] [Google Scholar]
- 19.Yan J. J., Ko W. C., Huang A. H., Chen H. M., Jin Y. T., Wu J. J. Arcobacter butzleri bacteremia in a patient with liver cirrhosis. Journal of the Formosan Medical Association . 2000;99(2):166–169. [PubMed] [Google Scholar]
- 20.Lau S. K. P., Woo P. C. Y., Teng J. L. L., Leung K. W., Yuen K. Y. Identification by 16S ribosomal RNA gene sequencing of Arcobacter butzleri bacteraemia in a patient with acute gangrenous appendicitis. Molecular Pathology . 2002;55(3):182–185. doi: 10.1136/mp.55.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yap D. Y. H., Kwan L. P. Y., To K. K. W., Chan T. M. Arcobacter peritonitis after fluoroscopic repositioning of a Tenckhoff catheter. Peritoneal Dialysis International: Journal of the International Society for Peritoneal Dialysis . 2013;33(2):222–223. doi: 10.3747/pdi.2012.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van den Abeele A. M., Vogelaers D., Vanlaere E., Houf K. Antimicrobial susceptibility testing of Arcobacter butzleri and Arcobacter cryaerophilus strains isolated from Belgian patients. Journal of Antimicrobial Chemotherapy . May;71(5):1241–1244. doi: 10.1093/jac/dkv483. [DOI] [PubMed] [Google Scholar]
- 23.Arguello E., Otto C. C., Mead P., Babady N. E., Riecken E. O., Schulzke J. D. Bacteremia caused by Arcobacter butzleri in an immunocompromised host. Journal of Clinical Microbiology . 2015;53(4):1448–1451. doi: 10.1097/00002030-199801000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stockmann M., Fromm M., Schmitz H., Schmidt W., Riecken E. O., Schulzke J. D. Duodenal biopsies of HIV‐infected patients with diarrhoea exhibit epithelial barrier defects but no active secretion. AIDS . 1998;12(1):43–51. doi: 10.1097/00002030-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Larsen I. K., Gradel K. O., Helms M., et al. Non-typhoidal Salmonella and Campylobacter infections among HIV-positive patients in Denmark. Scandinavian Journal of Infectious Diseases . 2011;43(1):3–7. doi: 10.3109/00365548.2010.517780. [DOI] [PubMed] [Google Scholar]
- 26.Kebede A., Aragie S., Shimelis T. The common enteric bacterial pathogens and their antimicrobial susceptibility pattern among HIV-infected individuals attending the antiretroviral therapy clinic of Hawassa university hospital, southern Ethiopia. Antimicrobial Resistance and Infection Control . 2017;6(1):p. 128. doi: 10.1186/s13756-017-0288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Šilha D., Pejchalová M., Šilhová L. Susceptibility to 18 drugs and multidrug resistance of Arcobacter isolates from different sources within the Czech Republic. Journal of global antimicrobial resistance . 2017;9:74–77. doi: 10.1016/j.jgar.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira S., Luis A., Oleastro M., Pereira L., Domingues F. C. A meta-analytic perspective on Arcobacter spp. antibiotic resistance. Journal of Global Antimicrobial Resistance . 2019;16:130–139. doi: 10.1016/j.jgar.2018.12.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.


