Summary
Background
Appropriate feeding of infants and young children is essential for healthy growth and the prevention of stunting, wasting, and overweight. We aimed to assess the beneficial versus harmful effects of providing fortified complementary foods to children in the complementary feeding period.
Methods
In this systematic review and meta-analysis, we searched the databases Cochrane Central Register of Controlled Trials, MEDLINE, Embase, Cumulative Index to Nursing and Allied Health Literature, Global Index Medicus, Web of Science, ClinicalTrials.gov, and WHO International Clinical Trials Registry Platform from inception to March 9, 2021. We included randomised controlled trials and controlled clinical trials done in infants and children aged 6–23 months with no identified health problems. Consumption of foods fortified centrally (ie, during industrial processing) with one micronutrient or a combination of vitamins, minerals, or both was compared with the same complementary foods, but without micronutrient fortification. Two review authors independently screened studies for eligibility, extracted data, assessed risk of bias, and rated the certainty of the evidence. The main outcomes were growth (measured by Z scores for weight for age, weight for height or length, and height or length for age, or other growth measures), stunting, wasting, nutrient adequacy or excess, anaemia, haemoglobin concentration, iron status, serum zinc concentration, and serum retinol concentration. We used a random-effects meta-analysis for combining data. This study is registered with PROSPERO, CRD42021245876.
Findings
We included 16 studies with 6423 participants, 13 of which were done in malaria-endemic areas. Overall, 12 studies were included in the quantitative syntheses. We identified five further ongoing studies. There was no difference between participants who received fortified complementary foods and those who received non-fortified complementary foods in weight-for-age Z scores (mean difference −0·01, 95% CI −0·07 to 0·06; five trials; 1206 participants; moderate-certainty evidence), weight-for-height or length Z scores (−0·05, −0·19 to 0·10; four trials; 1109 participants; moderate-certainty evidence), and height or length-for-age Z scores (−0·01, −0·21 to 0·20; four trials; 811 participants; low-certainty evidence); stunting and wasting were not assessed in any study as outcomes. Moderate-certainty evidence from six trials with 1209 patients showed that providing fortified complementary foods to children aged 6–23 months reduced the risk of anaemia (risk ratio 0·57, 95% CI 0·39 to 0·82). Those who received fortified complementary foods compared with those who did not had higher haemoglobin concentrations (mean difference 3·44 g/L, 95% CI 1·33 to 5·55; 11 trials; 2175 participants; moderate-certainty evidence) and ferritin concentration (0·43 μg/L on log scale, 0·14 to 0·72; six trials; 903 participants; low-certainty evidence). The intervention led to no effects on serum zinc concentration (−0·13 g/dL, −0·82 to 0·56; two trials; 333 participants; low-certainty evidence) and serum retinol concentration (0·03 μmol/L, −0·02 to 0·08; five trials; 475 participants; moderate-certainty evidence).
Interpretation
Fortified complementary foods are effective strategies to prevent anaemia in infants and young children aged 6–23 months in malaria-endemic regions. Effects of complementary food fortification should be further investigated in low-income and middle-income countries, but should also be assessed in high-income countries, and in regions where malaria is not endemic.
Funding
WHO.
Introduction
Complementary feeding is the transition from exclusive breastfeeding to family foods, typically from 6 months to 23 months of age.1 This period is crucial for physical, cognitive, and motor development, and during this time infants and young children need a great dietary diversity to ensure their nutrient needs are met.2
The low mineral content of breast milk, the relatively small amount of complementary foods consumed, their potentially inadequate nutrient density, low consumption of haem iron-containing meat, and the relatively high requirement of micronutrients can lead to nutrient deficiencies during this period of growth.3 Micronutrient deficiencies are common in low-income and middle-income countries, affecting more than 2 billion people worldwide.4, 5 The highest nutrient gaps in the complementary feeding period have been described for iron, vitamin A, vitamin B12, zinc, and calcium.4, 5
Research in context.
Evidence before this study
Fortified complementary foods are processed foods enriched with essential micronutrients, with the aim of preventing or correcting deficiency of one or more micronutrients in the critical period of complementary feeding. They provide an alternative to home or point-of-use fortification with micronutrient powders or crushable or soluble micronutrient tablets, which were shown in recent systematic reviews to be effective tools to reduce anaemia in children in the complementary feeding period in low-income and middle-income countries. We did a preliminary search of PubMed, Embase, and the Cochrane Library for existing systematic reviews and meta-analyses evaluating the health impact of fortified complementary foods in infants and young children aged 6–23 months using the search terms “fortified” OR “fortification” AND “complementary” published up to Jan 15, 2021. No language restriction was applied. The preliminary investigation revealed systematic reviews assessing the effects of other existing nutritional strategies, including interventions with small-quantity lipid-based nutrient supplements, fortified milk, and home fortification with micronutrient powders. Some systematic reviews included a wide range of interventions, including industrially fortified foods. However, these reviews were narrow in terms of investigated outcomes and described studies from 2006 to 2014. The present systematic review and meta-analysis was done to address the existing gap and to inform the process of updating WHO guidance on feeding of infants and young children aged 6–23 months.
Added value of this study
To our knowledge, this is the first systematic review summarising evidence on the consumption of fortified complementary foods compared with the unfortified version of the same complementary foods in infants and young children aged 6–23 months. We showed that fortified complementary foods probably reduce anaemia in infants and young children in malaria-endemic regions. We showed that fortification with the applied micronutrient composition and doses probably makes little or no difference to growth outcomes. However, the diversity of foods fortified and the micronutrients used for fortification, and the differences in micronutrient doses used for fortification and in baseline characteristics of children, including their anaemia and malaria status, limit our ability to understand how, when, and where this intervention is the most effective and safe.
Implications of all the available evidence
Our study suggests that interventions with fortified complementary foods are effective strategies to prevent anaemia in infants and young children aged 6 months to 2 years in malaria-endemic regions. Our findings support the need for further studies investigating health-related outcomes associated with fortified complementary foods in regions where malaria is not endemic and in high-income countries. More evidence is needed to better understand what level of fortification can lead to adequate or excess nutrient intake and related safety issues. It should be further investigated whether these products lead to the displacement of other foods and how they affect vitamin and mineral status, stunting, and wasting.
Several strategies have been proposed to provide target nutrients to infants and young children. These strategies include, besides diversified diets, fortified complementary foods, fortified animal milk,6 micronutrient powders,7 and small quantities of lipid-based nutrient supplements.8
Food fortification is defined as the addition of micronutrients to processed foods with the aim to increase the intake of these micronutrients and thereby correct or prevent micronutrient deficiencies.9 Complementary foods can be fortified either centrally (ie, during industrial processing) or at the point of use (home fortification).9
In malaria-endemic regions, the safety of iron preparations administered through home fortification has raised many questions,10 as the malaria parasite requires iron for growth11 and randomised controlled trials have indicated that a bolus of iron taken in a single dose might lead to adverse effects, such as increased risk of hospital admission, primarily due to malaria and infectious disease, and mortality, in these regions.12, 13 As fortified processed complementary foods enable iron to be consumed in smaller amounts throughout the day, and therefore absorbed more slowly, this form of iron administration might be a preferred alternative in these regions.10
Health effects of point-of-use fortification of complementary foods in children aged 6–23 months were assessed in a recent systematic review.7 Several trials exist on the potential beneficial and harmful effects of complementary feeding with centrally fortified foods,14, 15, 16, 17, 18, 19, 20, 21 although there is currently no up-to-date systematic review summarising potential health effects of adding micronutrients to industrially processed and widely consumed complementary food products.22
We aimed to assess the effects of micronutrient-fortified complementary food compared with the unfortified version of the same complementary food on beneficial or harmful dietary and health outcomes in infants and young children aged 6–23 months.
Methods
Search strategy and selection criteria
For this systematic review and meta-analysis, an information specialist experienced in systematic reviews (M-IM) searched the following electronic databases and trial registers from the inception of each database up to March 9, 2021, without restrictions on the language of publication: Ovid MEDLINE, Cochrane Central Register of Controlled Trials, Cumulative Index to Nursing and Allied Health Literature, Global Index Medicus (comprising African Index Medicus, Index Medicus for the Eastern Mediterranean Region, Index Medicus for the South-East Asia Region, Latin America and the Caribbean Literature on Health Science, and Western Pacific Region Index Medicus), Embase, Web of Science (comprising Science Citation Index and Emerging Citation Index), ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform. Details for all search strategies, including search terms, are available in the appendix (pp 2–4). We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of included trials and related systematic reviews, meta-analyses, and health technology assessment reports. Study authors were not contacted, and articles were translated into English if necessary.
We included randomised controlled trials with both individual and cluster randomisation and controlled clinical trials, if concurrently controlled. Included children were aged 6–23 months at the start of the intervention. We intended to include apparently healthy children from the general population, although some might have been at risk of having highly prevalent diseases (eg, malaria, HIV, diarrhoea, and undernutrition). The eligible intervention was consumption of fortified complementary products (excluding milk and milk-based formula), fortified centrally with one micronutrient or a combination of vitamins, minerals, or both. We excluded food supplements, micronutrient powders, or any other ways of home (point-of-use) fortification. The comparator was consumption of an unfortified version of the same complementary product.
Pairs of review authors (IC, RF, ÉS, and SL) independently screened titles and abstracts of every retrieved record using Covidence. Full texts of all potentially relevant records were screened for eligibility. Any disagreements were resolved through consensus or by recourse to a third author (IC or SL). All records excluded after full-text assessment are listed in the appendix (pp 5–36).
From full-text publications, we extracted data on study methods, participants, interventions, controls, outcomes, sources of funding, and potential conflict of interest statements. Data were extracted by one reviewer (IC or RF) and checked for completeness, accuracy, and consistency by a second reviewer (IC or RF).
We included abstracts and conference proceedings, but did not use them for data extraction, because they do not fulfil the CONSORT requirements.23 Data available as study results in trials registries were also extracted.
Two review authors (ÉS and SL) independently assessed the risk of bias of each included trial. Any disagreements were resolved by consensus. Risk of bias was evaluated with version 2 of the Cochrane risk of bias tool for randomised trials (RoB 2).24 Overall risk of bias was defined for each trial as the least favourable assessment across the domains of bias.
Outcomes
The outcomes of interest reflect the actual public health need on information and were defined by the WHO guideline development group (GDG) on complementary feeding of infants and young children aged 6–23 months. The main outcomes were growth (measured by Z scores for weight for age, weight for height or length, and height or length for age, or other growth measures), stunting, wasting, nutrient adequacy or excess, anaemia, haemoglobin concentration, iron status, serum zinc concentration, and serum retinol concentration. Additional outcomes were all-cause mortality, adverse effects, mental and motor skill development, morbidity, ferritin concentration, serum or urine concentration of other vitamins or minerals, gut microbiota composition, taste preference, and displacement of other foods. We included outcomes as measured at any given timepoint.
Data analysis
We a priori planned to undertake a meta-analysis for all outcomes for which we judged the participants, interventions, comparisons, and outcomes to be sufficiently similar to ensure a result that was clinically meaningful.
For dichotomous data, we present results as risk ratios or odds ratios (ORs) with 95% CIs. For continuous data, we use mean differences with 95% CIs for studies measuring outcomes in the same way, and standardised mean differences with 95% CIs for studies measuring outcomes in various ways.
We combined results from cluster-randomised and individually randomised studies. Where trial authors had adjusted their results for the effect of clustering, we aimed to extract the cluster-adjusted risk ratios and SE and enter the natural log of these into Review Manager (RevMan) using the generic inverse variance method, as recommended by Higgins and colleagues.25 Otherwise, we extracted the simple summary data for all relevant outcomes and calculated crude risk ratios and 95% CIs using RevMan. We adjusted for the effects of clustering using the approximate analysis method.25 This involves inflating the SE of the risk ratio using an estimate of the design effect and entering the natural logs of the adjusted risk ratios and corresponding SEs into RevMan using the generic inverse variance method. The intracluster correlation coefficient was not reported in any of the trials, so the value of 0·03 was used, as suggested by Leyrat and colleagues.26 A sensitivity analysis with respect to the intracluster correlation coefficient was not undertaken. We examined the potential effects of clustering using sensitivity analyses.
For outcomes with skewed data (presented as geometric means or medians), we calculated log-transformed data for all studies and did a meta-analysis on the scale of the log-transformed data. For multi-arm studies, we included the directly relevant arm only.27 If a study compared two possible fortified products with one non-fortified comparator, we combined groups to create a single pairwise comparison.28
We assessed methodological heterogeneity by examining risk of bias, and clinical heterogeneity by examining similarities and differences between studies regarding types of participants, interventions, and outcomes. We considered the size and direction of effect and used a standard χ2 test with a significance level of α=0·119 and the I2 statistic, which quantifies inconsistency across trials, to assess the effect of heterogeneity on the meta-analysis.29, 30 We explored heterogeneity through prespecified subgroup analyses.
We used funnel plots to assess reporting bias and to investigate the relationship between effect size and SE when at least ten studies were included in a meta-analysis. The degree of funnel plot asymmetry was quantified using Egger's test.31 The Robvis tool was used to visualise risk of bias.
As we expected differences between studies in both the population and the intervention, we decided to combine the data using a random-effects model. We used Mantel-Haenszel weighting for dichotomous outcomes and inverse variance for continuous outcomes or in case both individually and cluster-randomised trials were included in a meta-analysis.
We planned to do subgroup analyses for the following characteristics: age groups, different types of nutrients added through fortification, different types of products fortified, duration of intervention, the country income classification (defined according to the World Bank country income classification32), anaemia status at the start of the intervention, and sponsor. The potential effects of clustering were examined by sensitivity analyses.
We did statistical analyses using RevMan 5 (version 5.4.1). We followed the GRADE approach to rate the certainty of evidence.33 The methodology and the results are reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) reporting guideline.34 This study is registered with PROSPERO, CRD42021245876.
Role of the funding source
The present work serves as a background evidence review for the WHO guidelines on feeding of infants and young children aged 6–23 months. The questions guiding the review were discussed and developed, and the study protocol was approved, by the WHO GDG on complementary feeding of infants and young children aged 6–23 months. WHO and its GDG had no role in data collection, analysis, or interpretation. The WHO GDG members peer reviewed the systematic review report. The present manuscript is based on the report. WHO and its GDG did not participate in the writing of the present manuscript.
Results
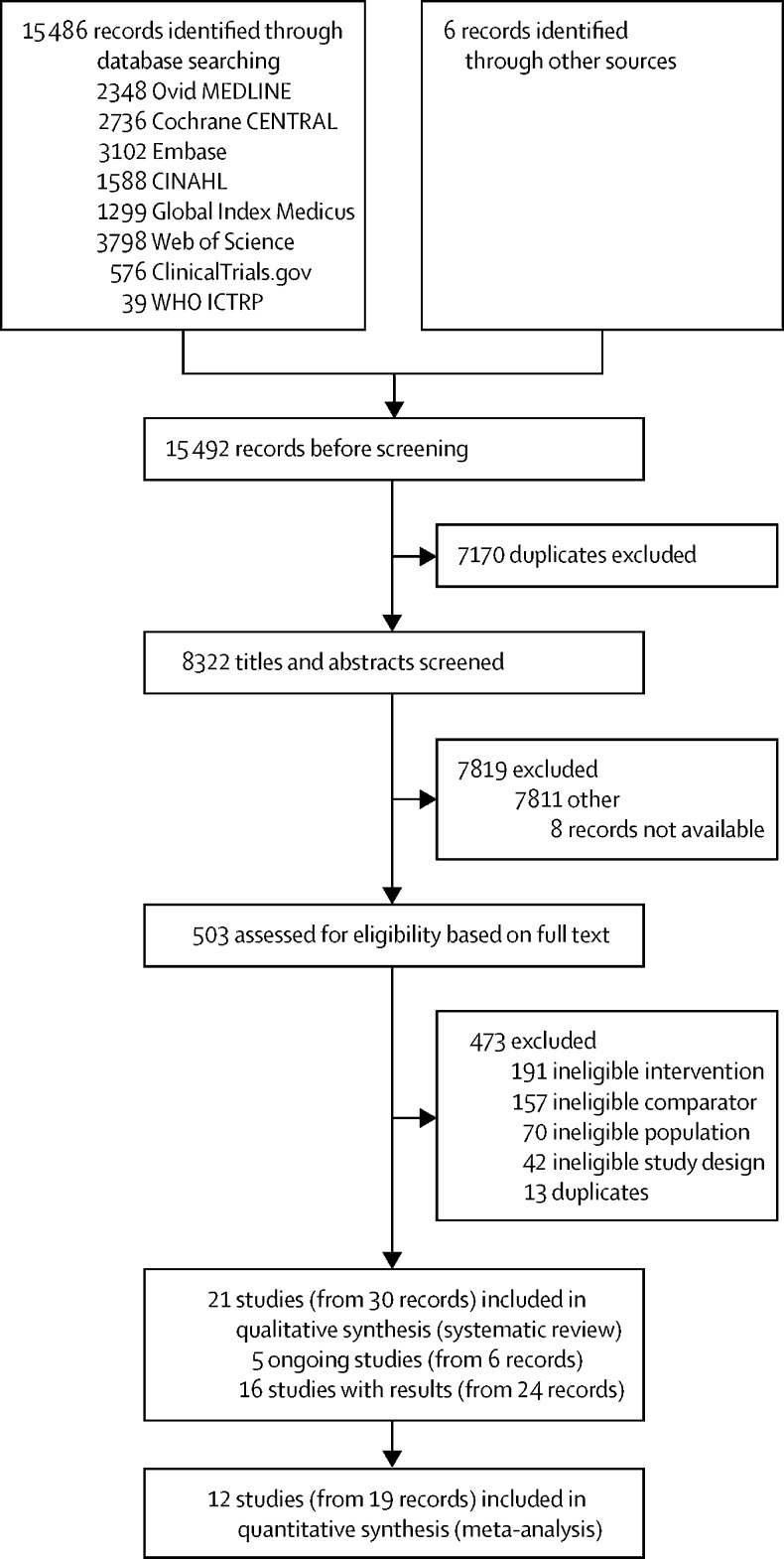
We retrieved 15 486 unique records through database searching and six through citation searching (figure 1). After removing duplicates, 8322 records were screened on the basis of their titles and abstracts (figure 1). We evaluated 503 full texts or records to determine their eligibility for inclusion in the review. 21 studies met our inclusion criteria (16 studies with full-text publications and five further studies that are not yet published, including key data from abstracts; appendix pp 37–41).
Figure 1.
Study selection
CENTRAL=Central Register of Controlled Trials. CINAHL=Cumulative Index to Nursing and Allied Health Literature. ICTRP=International Clinical Trials Registry Platform.
We included 16 studies with 6423 participants (table; appendix pp 42–56). Eight studies were randomised controlled trials randomised at the individual level,14, 35, 37, 44, 46, 47, 48, 50 one was a controlled clinical trial,38 and seven were cluster-randomised controlled trials.2, 19, 36, 39, 42, 43, 45 Most of the studies were done in upper or lower-middle-income countries. One study was done in a high-income country,50 and no study was done in a low-income country. Three studies were done in non-malaria-endemic areas (appendix pp 57–58).52
Table.
Key characteristics of included studies
| Country | Study design | Sample size | Age at start of intervention (months) | Fortified product | Micronutrients added to the fortified products* | Duration of intervention | Outcomes | |
|---|---|---|---|---|---|---|---|---|
| Palmer et al (2021)35 | Zambia | RCT, parallel | 255 | 9–12 | Maize meal | Retinyl palmitateor β-carotene (biofortified) | 90 days | Plasma retinol, vitamin A total body stores, liver retinol |
| Ekoe et al (2020)36 | East Cameroon | RCT, cluster | 205 | 18–59 | Infant cereal | Iron (ferrous fumarate) | 6 months | Haemoglobin, serum ferritin, serum iron, C-reactive protein, transferrin, anaemia, nutrition status, iron deficiency, iron deficiency anaemia, weight, height, weight-for-age Z scores, height or length-for-age Z scores, weight-for-height or length Z scores |
| Gannon et al (2019)37 | India | RCT, cross-over | 52 | 6–24 | Crops | Biofortified crops (not further specified) | 3 consecutive days | Acceptability |
| Huey et al (2017)38 | India | CCT, cross-over | 125 | 12–24 | Pearl millet | Iron and zinc (biofortified) | 3 consecutive days | Acceptability |
| Ma et al (2016),39 Krebs et al (2013),40 Sheng et al (2019)41 | China | RCT, cluster | 1465 | 6 | Rice cereal | Iron (ferrous fumarate), zinc (zinc sulphate), vitamin B12 | 12 months | Weight-for-age Z scores, height or length-for-age Z scores, weight-for-height or length Z scores, serum vitamin B12, haemoglobin, body iron, anaemia, ferritin, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, cognitive score, fine motor score, gross motor score |
| Nogueira Arcanjo et al (2012)42 | Brazil | RCT, cluster | 216 | 10–23 | Rice | Iron (ferric pyrophosphate) | 18 weeks | Haemoglobin, anaemia |
| Nogueira Arcanjo et al (2013)43 | Brazil | RCT, cluster | 171 | 10–23 | Rice | Iron (ferric pyrophosphate) | 18 weeks | Haemoglobin, anaemia |
| del Refugio Carrasco Quintero et al (2011)44 | Mexico | RCT, parallel | 395 | 7–24 | Corn flour | Iron, zinc, vitamin A, vitamin B3, folic acid | 10 months | Weight, height, nutritional status, weight-for-age Z scores, weight-for-height or length Z scores, mental and psychomotor development, haemoglobin |
| Bagni et al (2009)45 | Brazil | RCT, cluster | 354 | 12–60 | Rice | Iron (bisglycine chelate) | 16 weeks | Haemoglobin, anaemia |
| Nesamvuni et al (2005)46 | South Africa | RCT, parallel | 44 | 12–36 | Maize meal | Vitamins A, B1, B2, B6 | 12 months | Weight, height, haemoglobin, haematocrit, serum retinol, serum retinol-binding protein |
| Faber et al (2005)47 | South Africa | RCT, parallel | 361 | 6–12 | Porridge | β-carotene, iron (ferrous fumarate), zinc (zinc sulphate), vitamin C (sodium ascorbate), copper, selenium, vitamin B2, vitamin B6, vitamin B12, vitamin E | 6 months | Motor development, weight, length, height or length-for-age Z scores, weight-for-age Z scores, weight-for-height or length Z scores, haemoglobin, serum ferritin, serum retinol, serum zinc |
| Schümann et al (2005)48 | Guatemala | RCT, parallel | 110 | 12–36 | Beans | Iron (ferrous sulphate; inorganic salt) or haem iron (from bovin blood) | 10 weeks | Haemoglobin, ferritin |
| Lartey et al (1999),14 Lartey et al (2000)49 | Ghana | RCT, parallel | 216 | 6 | Cereal–legume blend | Calcium, iron, zinc, copper, magnesium, potassium, sodium, phosphorus, vitamin C, vitamin B3, vitamin B6, vitamin B2, vitamin B1, vitamin B12, folic acid, vitamin A | 6 months | Plasma zinc, plasma retinol, erythrocyte vitamin B2, haemoglobin, haematocrit, plasma ferritin, plasma transferrin, plasma transferrin saturation, diarrhoea, fever, respiratory illness, dietary iron intake, zinc intake, anaemia, vitamin A intake, vitamin B2 intake, weight-for-age Z scores, height or length-for-age Z scores, weight gain, length gain, mid-upper arm circumference, head circumference, tricep skinfold thickness, subscapular skinfold thickness, mid-upper arm fat area, mid-upper arm muscle area |
| Bovell-Benjamin et al (1999)50 | USA | RCT, cross-over | 40 | 6–24 | Whole-maize meal | Iron (ferrous bisglycinate) | 3 subsequent sessions | Degree of liking |
| Liu et al (1993)19 | China | RCT, cluster | 164 | 6–13 | Rusk | Calcium, iron, zinc, vitamin A (retinyl acetate), vitamin D3, vitamin B1, vitamin B2, vitamin B3, vitamin B12, folic acid | 3 months | Weight, length, free erythrocyte porphyrin, plasma ferritin, erythrocyte glutathione reductase activation coefficient, plasma vitamin E, plasma vitamin A, haemoglobin |
| Gershoff et al (1977),2 Gershoff et al (1975)51 | Thailand | RCT, cluster | 2250 | 6–60 | Rice | Vitamin B1 (thiamin naphthalene disulphonate), vitamin B2, vitamin A (retinol acetate), iron (ferric phosphate) | 1–4 years | Length, weight, bone age, head circumference, chest circumference, arm circumference, tricep skinfold, subscapular skinfold, haemoglobin, haematocrit, morbidity, hand–wrist x-ray |
Vitamin A1 is also known as retinol. Vitamin B1 is also known as thiamine. Vitamin B2 is also known as riboflavin. Vitamin B3 is also known as niacin. Vitamin B6 is also known as pyridoxine. Vitamin B12 is also known as cyanocobalamin. Vitamin C is also known as ascorbic acid. Vitamin D3 is also known as cholecalciferol. CCT=controlled clinical trial. RCT=randomised controlled trial.
Further details on the amount of micronutrients used for fortification can be found in the appendix (pp 59–60).
Participant age ranged from six to 60 months (three studies included children aged up to 60 months). When possible, we only included data for children younger than 24 months. Sample sizes ranged from 4050 to 2250.2 Three studies had an intervention duration of three subsequent feeding sessions50 to 3 consecutive days.37, 38 Among studies investigating longer-term effects of fortified complementary food consumption, the intervention duration lasted between 10 weeks48 and 4 years.2, 51 Fortified products were cereals in most of the cases (appendix pp 59–62).
Overall, eight (50%) studies were rated with high risk of bias due to the randomisation process and carry-over effects,38 deviations from the intended interventions,2, 45, 46, 47, 48 or missing outcome data2, 19, 46, 47, 48, 50 (appendix p 63). The evidence profiles are shown in the appendix (pp 64–72).
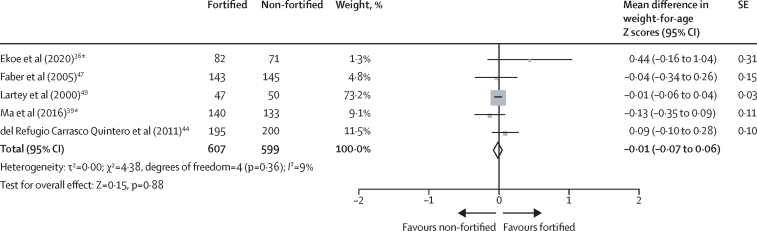
Five trials with an intervention duration of 6 months or longer measured growth.36, 39, 44, 47, 49 Available evidence showed no difference between groups in weight-for-age Z scores (mean difference −0·01, 95% CI −0·07 to 0·06; five trials; 1206 participants; moderate-certainty evidence; figure 2), weight-for-height or length Z scores (−0·05, −0·19 to 0·10; four trials; 1109 participants; moderate-certainty evidence; appendix p 82), and height or length-for-age Z scores (−0·01, −0·21 to 0·20; four trials; 811 participants; low-certainty evidence; appendix p 85).
Figure 2.
Effect of fortified versus non-fortified complementary food on weight for age (in Z scores)
*Cluster-randomised controlled trial.
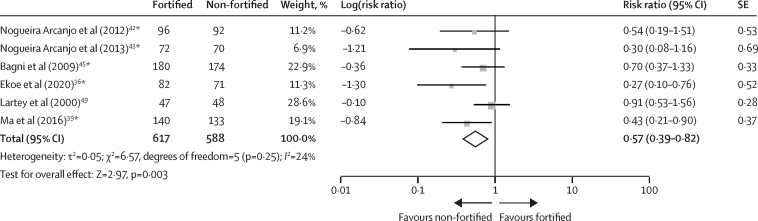
Anaemia was assessed in six trials (including 1205 participants) with 4–12 months duration.36, 39, 42, 43, 45, 49 Children receiving fortified complementary food products were significantly less likely to have anaemia at follow-up than children receiving non-fortified complementary foods (risk ratio 0·57, 95% CI 0·39–0·82; moderate-certainty evidence; figure 3).
Figure 3.
Effect of fortified versus non-fortified complementary food on anaemia prevalence
*Cluster-randomised controlled trial.
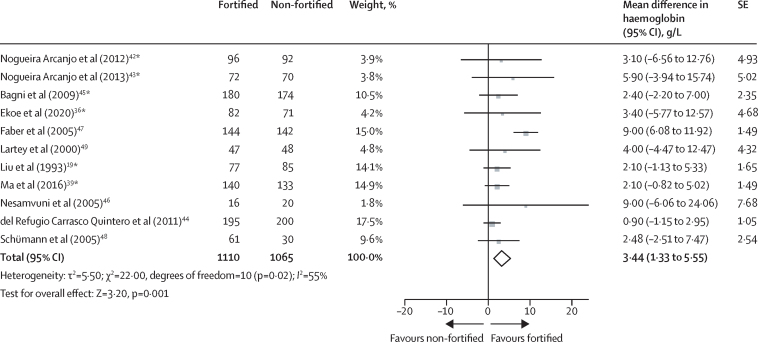
Compared with children receiving a non-fortified complementary food product, children consuming fortified complementary foods for 10 weeks to 12 months had higher haemoglobin concentrations at follow-up (mean difference 3·44 g/L, 95% CI 1·33–5·55; 11 trials; 2175 participants; moderate-certainty evidence; figure 4). We found no evidence of reporting bias (Egger's test p=0·37; appendix p 92).
Figure 4.
Effect of fortified versus non-fortified complementary food on haemoglobin (g/L)
*Cluster-randomised controlled trial.
Iron deficiency, defined as ferritin concentrations of less than 12 μg/L, was investigated in three studies with 571 participants.36, 39, 49 Children consuming iron-fortified complementary foods for 6 or 12 months were significantly less likely to have iron deficiency at follow-up than children consuming non-fortified complementary foods (risk ratio 0·39, 95% CI 0·21–0·75; moderate-certainty evidence; appendix p 91).
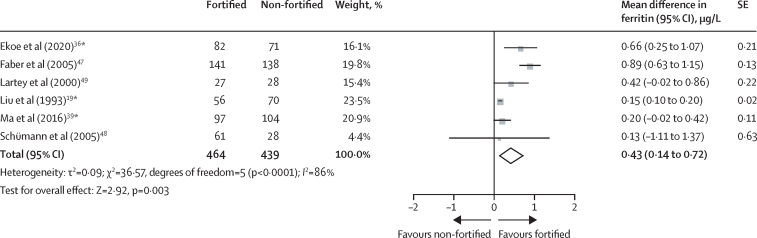
Iron status was measured as ferritin concentration, body iron, or free erythrocyte porphyrin. A daily dose of iron added to the complementary food products via fortification varied among studies, with a daily amount of 0·36 mg,39 2·5 mg,36 3·66 mg,47 4·6 mg,48 or 4·93 mg19 of elemental iron consumed, or with more than 10·9 mg per day or less than 21·9 mg per day.49 Children receiving iron-fortified complementary foods for 12 weeks to 12 months had, on average, higher ferritin concentrations at follow-up than children consuming non-fortified complementary food (mean difference 0·43 μg/L on log scale, 95% CI 0·14–0·72; six trials; 903 participants; low-certainty evidence; figure 5). One study39 described rice cereal fortified with iron, zinc, and vitamin B12 to be effective in increasing body iron, whereas another study19 found no effects of rusks fortified with iron, zinc, and calcium on free erythrocyte porphyrin (appendix pp 89–90).
Figure 5.
Effect of fortified versus non-fortified complementary food on iron status (ferritin concentrations in μg/L)
*Cluster-randomised controlled trial.
Serum zinc concentration was measured in two studies, in which less than 10·26 mg per day49 and 6 mg per day47 daily doses of zinc were provided, in combination with other micronutrients. The percentage of children with low serum zinc concentrations (cutoff values defined as serum zinc <10·7 μmol/L49 or <9·9 μmol/L47) at baseline was 3·6% (among 120 participants)49 and 43–48% (among 289 participants).47 Children consumed fortified food for 6 months in both studies. These studies found no effect of the provision of a zinc-fortified complementary food on children's serum zinc concentrations (mean difference −0·13 g/dL, 95% CI −0·82 to 0·56; two trials; 333 participants; low-certainty evidence; appendix p 90).
Prevalence of children with low zinc values (defined as <10·7 μmol/L) decreased from 6·5% to 3·2% in the non-fortified, and increased from 3·3% to 10·0% in the fortified groups (appendix p 91) in one study with 61 children assessing zinc deficiency.49
In trials reporting on serum retinol concentrations, the percentage of children with serum retinol concentrations of less than 0·7 μmol/L at baseline were 17% for those who received fortified complementary food versus 19% for those who received non-fortified complementary food,47 21·6 versus 34·5%,14 0 versus 7%,46 19·6 versus 34·5%,35 and not reported for one study.19 Overall, the provision of fortified complementary foods compared with non-fortified complementary food products had no effect on serum retinol concentrations (mean difference 0·03 μmol/L, 95% CI −0·02 to 0·08; five trials; 475 participants; moderate-certainty evidence; appendix p 90).
In three studies assessing vitamin A deficiency after an intervention period from 3 months to 12 months,35, 46, 49 there were no differences in the likelihood of having vitamin A deficiency (defined as serum retinol concentrations of <0·70 μmol/L) at follow-up between children consuming fortified and unfortified complementary foods (risk ratio 0·97, 95% CI 0·24–3·90; three trials; 257 participants; very-low-certainty evidence; appendix p 91). One study reported on nutrient adequacy and described that children in the fortified cereal–legume blend group consumed two to three times the recommended intake from zinc and vitamin A as a result of fortification.14
Mental skill development was assessed in two studies (508 participants), one44 measuring it on the first version of the Bayley Scales of Infant Development (BSID I), and the other39 on BSID III. Overall, children consuming fortified complementary foods had higher scores than children consuming the unfortified version of the same complementary food (mean difference 0·80, 95% CI 0·12–1·48; moderate-certainty evidence; appendix p 90). Motor skill development was reported as fine motor and gross motor score (measured on BSID III) in one study,39 as psychomotor score (measure on BSID I) in one study,44 and 25-item motor development score (BSID II) in one study.47 Psychomotor development was improved in children consuming fortified complementary food (mean difference 1·13, 95% CI 0·35–1·91; two trials; 661 participants; low-certainty evidence; appendix p 91). This effect was not seen in the study investigating fine and gross motor scales separately (appendix p 91).39
One trial with 97 children reported on morbidity,49 defined as number of new episodes per 100 days at risk. The number of cases of diarrhoea, acute respiratory tract infection, and fever disease episodes did not differ between children consuming fortified and non-fortified complementary foods (very-low-certainty evidence; appendix p 90).
Acceptability of fortified compared with non-fortified complementary foods was measured in two randomised controlled trials37, 50 and one controlled clinical trial38 with a total of 215 children. All studies evaluated acceptance on a 9-point hedonic scale (answers of toddlers interpreted by mothers), with a higher score representing better acceptance. Degree of liking did not differ significantly in any of these studies between fortified and non-fortified groups.
No studies reported data on the following prespecified outcomes: stunting and wasting, nutrient intakes above the upper limit, all-cause mortality, gut microbiota composition, or displacement of other foods.
Adverse effects were reported in one study, in which fortified complementary foods “were well tolerated and no side effects were reported in either group”.36
For the growth outcome, no relevant subgroup effects were detected, and cluster-randomised controlled trials were shown to have no effect on the direction of the pooled estimate in sensitivity analyses. For the outcomes of anaemia prevalence, haemoglobin concentration, and iron status, inconsistent differences were seen among age subgroups and the types of products that were fortified. Baseline anaemia status did not affect favourable effects of fortified complementary foods on post-intervention anaemia prevalence and iron status, but had effects on haemoglobin concentrations (all subgroup analyses are shown in the appendix pp 73–91). The beneficial effect of fortified complementary foods on haemoglobin concentration was no longer significant when all of the cluster-randomised controlled trials were excluded in the sensitivity analysis (mean difference 4·48 g/L, 95% CI −0·10 to 9·05; five trials;44, 46, 47, 48, 49 903 participants), but were still significant in the case of iron status (0·64 μg/L on log scale, 95% CI 0·23 to 1·05; three trials;47, 48, 49 423 participants).
Discussion
To our knowledge, this is the first systematic review summarising evidence on the consumption of fortified complementary foods compared with the unfortified version of the same complementary foods in infants and children aged 6–23 months. Results showed that providing fortified complementary foods to children probably reduces anaemia by 43%. Participants who receive fortified complementary foods probably have higher haemoglobin and might have higher ferritin concentrations than those who receive non-fortified complementary foods. Fortification with the applied micronutrient composition and doses probably makes little or no difference to growth outcomes. The intervention might result in little to no effect on zinc and vitamin A concentrations; there is no available evidence of effects on other vitamin and mineral concentrations. Acceptability of fortified and non-fortified complementary products in terms of sensory characteristics might not differ. Results of this systematic review are limited to preventive purposes; effectiveness of food fortification in treating any form of malnutrition was not evaluated.
As of March 30, 2022, two of five ongoing studies had been published as full-text papers.53, 54 One of them proved to be ineligible to be included in this systematic review because it compared two different fortified food products.53 The other study provides additional data for the outcomes haemoglobin, ferritin, zinc, and retinol concentrations, and on growth measures,54 which do not change the conclusions.
WHO guidelines recommend that: “In populations where anaemia is a public health problem, point-of-use fortification of complementary foods with iron-containing micronutrient powders in infants and young children aged 6–23 months is recommended, to improve iron status and reduce anaemia”.55 Our systematic review and meta-analysis shows that fortified foods formulated specifically for infants and young children might be an effective alternative.
This study has several strengths. First, we used a broad search strategy in both electronic databases and trial registries without applying date or language restrictions. It is unlikely that published trials have been missed; however, unpublished or ongoing trials not registered in clinical trial registries could be missing. Second, we aimed to reduce bias wherever possible by having at least two review authors work independently on trial selection, data extraction, and risk of bias and GRADE assessments.
A limitation of this systematic review is that several prespecified outcomes were investigated in a small number of trials or no data were available at all. Second, authors of ongoing studies were not contacted. We were able to explore the potential for publication bias using funnel plots only for the outcome haemoglobin concentration. Included studies were published between 1977 and 2020. Both the foods fortified and the micronutrients used for fortification were diverse; most of the studies provided iron either alone or in combination with other micronutrients.
We checked other systematic reviews and meta-analyses and investigated their agreements and disagreements with our findings. We found two studies: one review assessed the effects of a wide range of iron-containing interventions on anaemia and iron status in infants and children younger than 3 years,56 and the other assessed the health effect of centrally processed micronutrient-fortified dairy products and fortified cereal in children aged 6 months to 5 years.57 These two reviews found that iron fortification increased haemoglobin concentrations and reduced anaemia. Developmental effects of a range of interventions, including micronutrient-fortified complementary foods, were assessed in a systematic review in infants and children aged between 6 months and 2 years,58 with only two included studies assessing the effects of fortified processed foods, both finding no effect on growth.
The findings of our review are generalisable to apparently healthy children in middle-income settings in Asia and Africa, although some children might have been at risk of having highly prevalent diseases such as malaria, diarrhoea, or malnutrition. In malaria-endemic regions, malaria might be an additional cause of anaemia besides iron deficiency, infections, or other nutrient deficiencies, as the malaria parasite destroys erythrocytes.9 In such regions, nutritional status results have to be interpreted cautiously because malaria substantially reduces haemoglobin concentrations59 and affects many other nutritional status indicators, including serum ferritin, serum transferrin, and plasma retinol.60 Therefore, biomarkers of iron and vitamin A status should be statistically adjusted for malaria and the severity of infection.61 Most of the included studies in our systematic review were done in malaria-endemic areas; however, none reported adjustments for malaria. Thus, the effect of malaria on our results cannot be accurately judged.
Some studies suggest that fetal iron deficiency might be detrimental to early neurodevelopment, but early iron supplementation in anaemic infants can have a positive effect on developmental scores (both cognitive and motor), regardless of a country's income status.62 Micronutrients as a sole intervention (ie, without an additional increase in macronutrient intake), however, seem to have no effect on growth.
Micronutrient deficiencies and their health consequences remain a global health problem, and several factors, including age of the target population, baseline anaemia status, malaria prevalence, and presence of other causes of infection or inflammation, need to be considered.22, 63 Beside products from multinational companies, locally manufactured, lower-cost fortified complementary food products are available in low-income countries; however, access to these products is still far from optimal in several settings.64, 65 WHO states: “To ensure their success and sustainability, especially in resource-poor countries, food fortification programmes should be implemented in concert with poverty reduction programmes and other agricultural, health, education and social intervention programmes that promote the consumption and utilization of adequate quantities of good quality nutritious foods among the nutritionally vulnerable.”9
Several research gaps have to be further explored. As provision of iron in any product that is given to infants and young children raises questions of safety, potential adverse effects have to be further explored in both malaria-endemic and non-malaria-endemic settings. Studies should investigate whether consumption of fortified complementary foods has an effect on all-cause mortality. It would be important to know what level of fortification can lead to adequate or excess nutrient intakes, affect stunting or wasting, or influence vitamin and mineral status other than iron. Further studies are required to answer the question under what circumstances the provision of fortified complementary foods to infants and young children is able to affect mental and motor skill development. Microbiota composition has not yet been investigated at all. How the consumption of such foods affects the intake of other foods, diet diversity, or breastfeeding time—ie, whether and to what extent they displace other foods—should also be determined.
Studies with both higher and lower nutrient content have to be done to determine dose–response effects. Effects of complementary food fortification should be further investigated in low-income and middle-income countries, but should also be assessed in high-income countries, and in regions where malaria is not endemic. Planned randomised controlled trials should be done with rigorous methodology and large sample sizes. Furthermore, there is a need to investigate the sustainability of different evidence-based interventions and factors that enhance or impede their implementation.63
In conclusion, micronutrient deficiencies and their health consequences are a global problem, and fortified complementary food products might be one effective strategy to improve the micronutrient status of young children. There is a need to enhance these targeted, evidence-based interventions to prevent micronutrient deficiencies in infants and young children aged 6–23 months.
For the Robvis tool see https://mcguinlu.shinyapps.io/robvis/
For Covidence see https://www.covidence.org/
Data sharing
Full datasets can be obtained from the corresponding author.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
The University of Pécs (Pécs, Hungary) received funding from WHO to conduct this research.
Contributors
M-IM and SL were responsible for the development of the search strategy. M-IM did the systematic searches. IC, RF, ÉS, and SL screened titles, abstracts, and full text. IC and RF extracted data. ÉS and SL assessed risk of bias. TF and SL did the statistical analyses. LS and SL did the GRADE assessment. IC, RF, ÉS, and SL prepared the first draft of the manuscript. All authors approved the final manuscript. SL was responsible for the coordination of tasks. IC and RF accessed and verified all data. The corresponding author had the final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Fewtrell M, Bronsky J, Campoy C, et al. Complementary feeding: a position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2017;64:119–132. doi: 10.1097/MPG.0000000000001454. [DOI] [PubMed] [Google Scholar]
- 2.Gershoff SN, McGandy RB, Suttapreyasri D, et al. Nutrition studied in Thailand. II. Effects of fortification of rice with lysine, threonine, thiamin, riboflavin, vitamin A, and iron on preschool children. Am J Clin Nutr. 1977;30:1185–1195. doi: 10.1093/ajcn/30.7.1185. [DOI] [PubMed] [Google Scholar]
- 3.WHO Guiding principles for complementary feeding of the breastfed child. Jan 1, 2003. https://www.who.int/publications/i/item/9275124604
- 4.Beal T, White JM, Arsenault JE, et al. Micronutrient gaps during the complementary feeding period in South Asia: a Comprehensive Nutrient Gap Assessment. Nutr Rev. 2021;79(suppl 1):26–34. doi: 10.1093/nutrit/nuaa144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White JM, Beal T, Arsenault JE, et al. Micronutrient gaps during the complementary feeding period in 6 countries in Eastern and Southern Africa: a Comprehensive Nutrient Gap Assessment. Nutr Rev. 2021;79(suppl 1):16–25. doi: 10.1093/nutrit/nuaa142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuyama M, Harb T, David M, Davies PS, Hill RJ. Effect of fortified milk on growth and nutritional status in young children: a systematic review and meta-analysis. Public Health Nutr. 2017;20:1214–1225. doi: 10.1017/S1368980016003189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suchdev PS, Jefferds MED, Ota E, da Silva Lopes K, De-Regil LM. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database Syst Rev. 2020;2 doi: 10.1002/14651858.CD008959.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das JK, Salam RA, Hadi YB, et al. Preventive lipid-based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes. Cochrane Database Syst Rev. 2019;5 doi: 10.1002/14651858.CD012611.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen L, de Benoist B, Dary O, Hurrell R. Guidelines on food fortification with micronutrients. Nov 25, 2006. https://www.who.int/publications/i/item/9241594012
- 10.WHO Conclusions and recommendations of the WHO Consultation on prevention and control of iron deficiency in infants and young children in malaria-endemic areas. Food Nutr Bull. 2007;28(suppl):S621–S627. doi: 10.1177/15648265070284s414. [DOI] [PubMed] [Google Scholar]
- 11.Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr. 2001;131:616S–6133. doi: 10.1093/jn/131.2.616S. [DOI] [PubMed] [Google Scholar]
- 12.Sazawal S, Black RE, Ramsan M, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 13.Tielsch JM, Khatry SK, Stoltzfus RJ, et al. Effect of routine prophylactic supplementation with iron and folic acid on preschool child mortality in southern Nepal: community-based, cluster-randomised, placebo-controlled trial. Lancet. 2006;367:144–152. doi: 10.1016/S0140-6736(06)67963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lartey A, Manu A, Brown KH, Peerson JM, Dewey KG. A randomized, community-based trial of the effects of improved, centrally processed complementary foods on growth and micronutrient status of Ghanaian infants from 6 to 12 mo of age. Am J Clin Nutr. 1999;70:391–404. doi: 10.1093/ajcn/70.3.391. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler EE, Fomon SJ, Nelson SE, Jeter JM, Theuer RC. Dry cereals fortified with electrolytic iron or ferrous fumarate are equally effective in breast-fed infants. J Nutr. 2011;141:243–248. doi: 10.3945/jn.110.127266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidsson L, Sarker SA, Jamil KA, Sultana S, Hurrell R. Regular consumption of a complementary food fortified with ascorbic acid and ferrous fumarate or ferric pyrophosphate is as useful as ferrous sulfate in maintaining hemoglobin concentrations >105 g/L in young Bangladeshi children. Am J Clin Nutr. 2009;89:1815–1820. doi: 10.3945/ajcn.2008.27353. [DOI] [PubMed] [Google Scholar]
- 17.Glinz D, Hurrell RF, Ouattara M, et al. The effect of iron-fortified complementary food and intermittent preventive treatment of malaria on anaemia in 12- to 36-month-old children: a cluster-randomised controlled trial. Malar J. 2015;14:347. doi: 10.1186/s12936-015-0872-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glinz D, Wegmüller R, Ouattara M, et al. Iron fortified complementary foods containing a mixture of sodium iron EDTA with either ferrous fumarate or ferric pyrophosphate reduce iron deficiency anemia in 12- to 36-month-old children in a malaria endemic setting: a secondary analysis of a cluster-randomized controlled trial. Nutrients. 2017;9:e759. doi: 10.3390/nu9070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu DS, Bates CJ, Yin TA, Wang XB, Lu CQ. Nutritional efficacy of a fortified weaning rusk in a rural area near Beijing. Am J Clin Nutr. 1993;57:506–511. doi: 10.1093/ajcn/57.4.506. [DOI] [PubMed] [Google Scholar]
- 20.Manno D, Kowa PK, Bwalya HK, et al. Rich micronutrient fortification of locally produced infant food does not improve mental and motor development of Zambian infants: a randomised controlled trial. Br J Nutr. 2012;107:556–566. doi: 10.1017/S0007114511003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oelofse A, Van Raaij JM, Benade AJ, Dhansay MA, Tolboom JJ, Hautvast JG. The effect of a micronutrient-fortified complementary food on micronutrient status, growth and development of 6- to 12-month-old disadvantaged urban South African infants. Int J Food Sci Nutr. 2003;54:399–407. doi: 10.1080/0963748031000092161. [DOI] [PubMed] [Google Scholar]
- 22.da Silva Lopes K, Yamaji N, Rahman MO, et al. Nutrition-specific interventions for preventing and controlling anaemia throughout the life cycle: an overview of systematic reviews. Cochrane Database Syst Rev. 2021;9 doi: 10.1002/14651858.CD013092.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. In: Cochrane Handbook for Systematic Reviews of Interventions, version 6.2 (updated February, 2021) Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane; London, UK: 2021. Chapter 8: assessing risk of bias in a randomized trial. [Google Scholar]
- 25.Higgins JPT, Eldridge S, Li T, Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions, version 6.2 (updated February, 2021) Cochrane; London, UK: 2021. Chapter 23: including variants on randomized trials. [Google Scholar]
- 26.Leyrat C, Caille A, Eldridge S, Kerry S, Dechartres A, Giraudeau B. Intervention effect estimates in cluster randomized versus individually randomized trials: a meta-epidemiological study. Int J Epidemiol. 2019;48:609–619. doi: 10.1093/ije/dyy229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JPT, Eldridge S, Li T, Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions, version 6.0 (updated July, 2019) Cochrane; London, UK: 2019. Chapter 23: including variants on randomized trials. [Google Scholar]
- 28.Higgins JPT, Li T, Deeks JJ, Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions, version 6.2 (updated February, 2021) Cochrane; London, UK: 2021. Chapter 6: choosing effect measures and computing estimates of effect. [Google Scholar]
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The World Bank Group Country and Lending Groups. http://data.worldbank.org/about/country-classifications/country-and-lending-groups#Lower_middle_income
- 33.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer AC, Jobarteh ML, Chipili M, et al. Biofortified and fortified maize consumption reduces prevalence of low milk retinol, but does not increase vitamin A stores of breastfeeding Zambian infants with adequate reserves: a randomized controlled trial. Am J Clin Nutr. 2021;113:1209–1220. doi: 10.1093/ajcn/nqaa429. [DOI] [PubMed] [Google Scholar]
- 36.Ekoe T, Bianpambe OI, Nguefack F, et al. Efficacy of an iron-fortified infant cereal to reduce the risk of iron deficiency anemia in young children in East Cameroon. Food Sci Nutr. 2020;8:3566–3577. doi: 10.1002/fsn3.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gannon BM, Thakker V, Bonam VS, et al. A randomized crossover study to evaluate recipe acceptability in breastfeeding mothers and young children in India targeted for a multiple biofortified food crop intervention. Food Nutr Bull. 2019;40:460–470. doi: 10.1177/0379572119855588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huey SL, Venkatramanan S, Udipi SA, et al. Acceptability of iron- and zinc-biofortified pearl millet (ICTP-8203)-based complementary foods among children in an urban slum of Mumbai, India. Front Nutr. 2017;4:39. doi: 10.3389/fnut.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma J, Sun Q, Liu J, et al. The effect of iron fortification on iron (Fe) status and inflammation: a randomized controlled trial. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krebs NF, Sheng XY, Westcott JE, Hu YQ, Liu SS, Hambidge KM. Higher cognitive and gross motor scores in Chinese toddlers randomized to meat compared to either micro-nutrient fortified or unfortified infant cereal as first complementary food. FASEB J. 2013;27:1. [Google Scholar]
- 41.Sheng X, Wang J, Li F, Ouyang F, Ma J. Effects of dietary intervention on vitamin B12 status and cognitive level of 18-month-old toddlers in high-poverty areas: a cluster-randomized controlled trial. BMC Pediatr. 2019;19:334. doi: 10.1186/s12887-019-1716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nogueira Arcanjo FP, Santos PR, Arcanjo CP, Amancio OM, Braga JA. Use of iron-fortified rice reduces anemia in infants. J Trop Pediatr. 2012;58:475–480. doi: 10.1093/tropej/fms021. [DOI] [PubMed] [Google Scholar]
- 43.Nogueira Arcanjo FP, Roberto Santos P, Madeiro Leite AJ, Bastos Mota FS, Duarte Segall S. Rice fortified with iron given weekly increases hemoglobin levels and reduces anemia in infants: a community intervention trial. Int J Vitam Nutr Res. 2013;83:59–66. doi: 10.1024/0300-9831/a000145. [DOI] [PubMed] [Google Scholar]
- 44.del Refugio Carrasco Quintero M, Ortiz Hernández L, Chávez Villasana A, et al. [Impact of consumption of corn flour with low level enrichment in children of rural zones] Nutr Hosp. 2011;26:1097–1104. doi: 10.1590/S0212-16112011000500026. (in Spanish). [DOI] [PubMed] [Google Scholar]
- 45.Bagni UV, Baião MR, Santos MM, Luiz RR, Veiga GV. [Effect of weekly rice fortification with iron on anemia prevalence and hemoglobin concentration among children attending public daycare centers in Rio de Janeiro, Brazil] Cad Saude Publica. 2009;25:291–302. doi: 10.1590/s0102-311x2009000200007. (in Portuguese). [DOI] [PubMed] [Google Scholar]
- 46.Nesamvuni AE, Vorster HH, Margetts BM, Kruger A. Fortification of maize meal improved the nutritional status of 1–3-year-old African children. Public Health Nutr. 2005;8:461–467. doi: 10.1079/phn2005782. [DOI] [PubMed] [Google Scholar]
- 47.Faber M, Kvalsvig JD, Lombard CJ, Benadé AJ. Effect of a fortified maize-meal porridge on anemia, micronutrient status, and motor development of infants. Am J Clin Nutr. 2005;82:1032–1039. doi: 10.1093/ajcn/82.5.1032. [DOI] [PubMed] [Google Scholar]
- 48.Schümann K, Romero-Abal ME, Mäurer A, et al. Haematological response to haem iron or ferrous sulphate mixed with refried black beans in moderately anaemic Guatemalan pre-school children. Public Health Nutr. 2005;8:572–581. doi: 10.1079/phn2004708. [DOI] [PubMed] [Google Scholar]
- 49.Lartey A, Manu A, Brown KH, Dewey KG. Predictors of micronutrient status among six- to twelve-month-old breast-fed Ghanaian infants. J Nutr. 2000;130:199–207. doi: 10.1093/jn/130.2.199. [DOI] [PubMed] [Google Scholar]
- 50.Bovell-Benjamin AC, Allen LH, Guinard JX. Toddlers' acceptance of whole maize meal porridge fortified with ferrous bisglycinate. Food Qual Prefer. 1999;10:123–128. [Google Scholar]
- 51.Gershoff SN, McGandy RB, Suttapreyasri D, Nondasuta A, Pisolyabutra U, Tantiwongse P. Amino acid fortification of rice studies in Thailand. I. Background and baseline data. Am J Clin Nutr. 1975;28:170–182. doi: 10.1093/ajcn/28.2.170. [DOI] [PubMed] [Google Scholar]
- 52.WHO World Malaria Report 2020. 2020 years of global progress & challenges. Nov 30, 2020. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2020/
- 53.Harrison OA, Hays NP, Ansong RS, Datoghe D, Vuvor F, Steiner-Asiedu M. Effect of iron-fortified infant cereal on nutritional status of infants in Ghana. Food Sci Nutr. 2021;10:286–294. doi: 10.1002/fsn3.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehta S, Huey SL, Ghugre PS, et al. A randomized trial of iron- and zinc-biofortified pearl millet-based complementary feeding in children aged 12 to 18 months living in urban slums. Clin Nutr. 2022;41:937–947. doi: 10.1016/j.clnu.2022.02.014. [DOI] [PubMed] [Google Scholar]
- 55.WHO . World Health Organization; Geneva: 2016. WHO guideline: use of multiple micronutrient powders for point-of-use fortification of foods consumed by infants and young children aged 6–23 months and children aged 2–12 years. [PubMed] [Google Scholar]
- 56.Pratt O. A review of the strategies used to reduce the prevalence of iron deficiency and iron deficiency anaemia in infants aged 6–36 months. Nutr Bull. 2015;40:257–267. [Google Scholar]
- 57.Eichler K, Wieser S, Rüthemann I, Brügger U. Effects of micronutrient fortified milk and cereal food for infants and children: a systematic review. BMC Public Health. 2012;12:506. doi: 10.1186/1471-2458-12-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dewey KG, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutri. 2008;4(suppl 1):24–85. doi: 10.1111/j.1740-8709.2007.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asobayire FS, Adou P, Davidsson L, Cook JD, Hurrell RF. Prevalence of iron deficiency with and without concurrent anemia in population groups with high prevalences of malaria and other infections: a study in Côte d'Ivoire. Am J Clin Nutr. 2001;74:776–782. doi: 10.1093/ajcn/74.6.776. [DOI] [PubMed] [Google Scholar]
- 60.Siekmann JH, Allen LH, Bwibo NO, Demment MW, Murphy SP, Neumann CG. Kenyan school children have multiple micronutrient deficiencies, but increased plasma vitamin B-12 is the only detectable micronutrient response to meat or milk supplementation. J Nutr. 2003;133(suppl 2):3972S–39780. doi: 10.1093/jn/133.11.3972S. [DOI] [PubMed] [Google Scholar]
- 61.Sandalinas F, Filteau S, Joy EJM, Segovia de la Revilla L, MacDougall A, Hopkins H. Measuring the impact of malaria infection on indicators of iron and vitamin A status: a systematic literature review and meta-analysis. Br J Nutr. 2022 doi: 10.1017/S0007114522000757. published online March 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCann S, Perapoch Amadó M, Moore SE. The role of iron in brain development: a systematic review. Nutrients. 2020;12 doi: 10.3390/nu12072001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zlotkin S, Dewey KG. Perspective: putting the youngest among us into the nutrition “call for action” for food fortification strategies. Am J Clin Nutr. 2021;114:1257–1260. doi: 10.1093/ajcn/nqab207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dimaria SA, Schwartz H, Icard-Vernière C, Picq C, Zagre NM, Mouquet-Rivier C. Adequacy of some locally produced complementary foods marketed in Benin, Burkina Faso, Ghana, and Senegal. Nutrients. 2018;10:785. doi: 10.3390/nu10060785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dewey KG. Formulations for fortified complementary foods and supplements: review of successful products for improving the nutritional status of infants and young children. Food Nutr Bull. 2009;30(suppl):S239–S255. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Full datasets can be obtained from the corresponding author.