Abstract
The Agrocybe aegerita mitochondrial genome contains a truncated family B DNA polymerase gene (Aa-polB P1) whose nucleotide sequence is 86% identical to the previously described and potentially functional Aa-polB gene. A tRNAMet gene occurs at the 3′ end of the Aa-polB P1 gene. The Aa-polB P1 gene could result from reverse transcription of an Aa-polB mRNA primed by a tRNAMet followed by the integration of the cDNA after recombination at the mitochondrial tRNA locus. Two naturally occurring alleles of Aa-polB P1 carry one or two copies of the disrupted sequence. In strains with two copies of Aa-polB P1, these copies are inverted relative to one another and separated by a short sequence carrying the tRNAMet gene. Both A. aegerita mitochondrial family B DNA polymerases were found to be related to other family B DNA polymerases (36 to 53% amino acid similarity), including the three enzymes of the archaebacterium Sulfolobus solfataricus. If mitochondria originated from a fusion between a Clostridium-like eubacterium and a Sulfolobus-like archaebacterium, then the A. aegerita family B DNA polymerase genes could be remnants of the archaebacterial genes.
The genes in the fungal mitochondrial genome generally belong to a small set of highly conserved genes that probably originated from a prokaryotic ancestral endosymbiont (for a review, see reference 12). They encode components of complexes I to V of the electron transport chain, as well as the RNA portion of the translation system, mitochondrial rRNAs, and mitochondrial tRNAs. Most other genes encoding mitochondrial proteins are located on the nuclear chromosomes. In particular, the highly conserved γ DNA polymerases (17) responsible for the replication of the mitochondrial DNA (mtDNA) are nucleus encoded.
Agrocybe aegerita is a cultivated basidiomycete whose mitochondrial genome has been cloned and mapped (21) and from which three mitochondrial genes have been sequenced: cox1 (10) and the small-subunit (SSU) and large-subunit rRNA genes (rDNAs) (9, 11). A potentially functional family B DNA polymerase gene named Aa-polB is near the SSU rDNA, on the opposite strand, in the 20 A. aegerita wild strains that have been studied (4). This gene putatively encodes a 571-amino-acid (aa) protein possessing all the conserved domains and residues involved in 3′-5′ exonucleolytic and polymerization activities (4). Other sequences similar to Aa-polB are present in the mitochondrial genome about 20 kb from the Aa-polB gene. This region, named H4, has two alleles, H4-1 and H4-2, that differ in length and that are present in 28 and 8 strains, respectively, of 36 A. aegerita field strains (3).
Our objectives in this study were to determine the relationship between Aa-polB and a putative copy of this gene some 20 kb distant and to determine if the distal copy of this sequence affects allelic variability. The duplicated copies of this family B DNA polymerase gene may have arisen by reverse transcription of mRNAs primed by a mitochondrial tRNA.
MATERIALS AND METHODS
Strains, media, and culture conditions.
We sequenced the HindIII restriction fragments H4 and H4a, corresponding respectively to the allelic forms H4-2 and H4-1. The 4.2-kb H4 fragment from the H4-2 allele was previously isolated from an mtDNA library from A. aegerita strain WT-3 (= SM47) (21); the 3.5-kb H4a fragment from the H4-1 allele was isolated from a library of mtDNA from strain WT-11 (= SM751002). Both strains are preserved on CYM medium (23) in the International Culture Collection of the Laboratory of Molecular Genetics and Breeding of Cultivated Mushrooms (collection number GMACC WDCM 786) and were previously described (3). Escherichia coli JM83 (29) was used for cloning and propagation of plasmids in Luria-Bertani medium (20).
DNA manipulations.
HindIII or HaeIII mitochondrial fragments were cloned into the HindIII or SmaI sites of pUC18, respectively, by using conventional cloning procedures (8, 20). The H4a restriction fragment was isolated by colony hybridization, using H4 as a probe. Probes were digested with the appropriate restriction endonucleases, separated by electrophoresis in a 0.8% (wt/vol) Nusieve GTG agarose gel (FMC Bioproducts, Rockland, Maine), recovered by using a Geneclean kit (Bio 101 Inc., Vista, Calif.), and labeled with [α-32P]dCTP (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, United Kingdom) by using a Promega (Madison, Wis.) Random Primer DNA labeling kit.
A. aegerita DNA was extracted from vegetative dikaryotic mycelium by N-cetyl-N,N,N-trimethylammonium bromide extraction (22). Digested total DNA (10 μg) was transferred after agarose (0.8% wt/vol) gel electrophoresis (20) to Hybond N+ (Amersham) membranes by the Southern method (28), with the help of a vacuum transfer system (Appligène, Illkirch, France), in the presence of 0.4 N NaOH. Prehybridizations, hybridizations, and high-stringency washings were carried out as previously described (3). Colony hybridizations were performed as described elsewhere (25).
DNA sequencing and sequence analyses.
Mitochondrial sequences were subcloned in both orientations in pUC18 (29), then processed to generate nested deletions by using the Erase-a-Base system according to the manufacturer's (Promega) recommendations. Recombinant plasmids were purified from the E. coli JM83 clones by a conventional miniprep method (20). Both strands were sequenced in reactions using the M13-40 primer, the M13 reverse primer, or specific 18-mer oligonucleotides (Eurogentec, Seraing, Belgium). The sequencing reactions were performed by the dideoxy-chain termination method (26) with a Sequenase II kit (United States Biochemical Corp., Cleveland, Ohio). Labeled fragments were denatured, separated by electrophoresis on 6% (wt/vol) polyacrylamide gels, and identified by autoradiography. Sequence analyses were performed with the DNA Strider 1.2 software (Commissariat, à l'Energie Atomique, Gif-sur-Yvette, France). Comparisons with sequences in the GenBank and EMBL databases were performed by using the BLAST search algorithm (1), and nucleotide and protein sequences were aligned with the Clustal W package (13, 14).
RESULTS
Hybridization of the Aa-polB gene with a sequence located in a polymorphic region of the A. aegerita mtDNA.
The Aa-polB gene is carried on a 4.2-kb HaeIII and a 7.2-kb HindIII restriction fragment (4). Four fragments were identified in Southern hybridizations of the HindIII- or HaeIII-digested mtDNA from WT-3, using the 4.2-kb HaeIII fragment or the H4 fragment (21) as a probe: 7.2- and 4.2-kb (H4) HindIII fragments and 4.2- and 11-kb HaeIII fragments. If mtDNA from WT-11 was probed in a similar manner, then the DNA fragments typical of the H4-2 allele (4.2-kb HindIII and 11-kb HaeIII) were not seen and those typical of the H4-1 allele (3.5-kb HindIII and 5.3-kb HaeIII) were evident instead. From these results, we concluded that a sequence hybridizing with the Aa-polB gene was present in two locations in both the WT-3 and WT-11 mtDNAs.
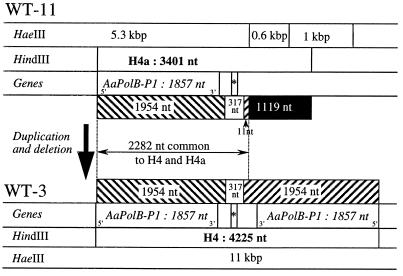
We sequenced the 4.2-kb HindIII fragment from WT-3, termed H4 (GenBank accession no. AF269234), and the 3.5-kb HindIII fragment from WT-11, termed H4a (GenBank accession no. AF269233). H4a was 3,401 nucleotides (nt) in length and was 77% A+T. H4 was 4,225 nt in length and 78% A+T, and it carried two long inverted repeats of 1,954 nt (R), each beginning at a HindIII restriction site, separated by a nonrepeated central sequence of 317 nt (Fig. 1). H4 and H4a have 2,282 nt of identical sequence which contains (i) a complete sequence of the inverted repeat R (1,954 nt), (ii) the central nonrepeated sequence (317 nt), and (iii) the last 11 nucleotides of the second copy of the inverted repeat. H4 has a complete second copy of the inverted repeat, while H4a has a 1,119-nt sequence not found on H4. We used the 0.6-kb HaeIII fragment from H4a to probe total DNA of strains WT-3 and WT-11. This fragment hybridized as expected with the 3.5-kb HindIII and 0.6-kb HaeIII fragments from H4a but did not hybridize with any fragments from WT-11.
FIG. 1.
Comparison of the restriction maps and molecular organizations of the A. aegerita mtDNA H4 regions carrying the Aa-polB P1 genes of strain WT-11 (allelic form H4-1) and WT-3 (allelic form H4-2). The sizes of the restriction fragments are indicated in kilobase pairs when established by agarose gel electrophoresis or in nucleotides when deduced from the sequence. The location of the mitochondrial tRNAMet gene is indicated by an asterisk. Duplicated sequences are indicated by hatched bars; the sequence specific to the H4a fragment is indicated by a black bar.
Sequence analysis.
We identified open reading frames (ORFs) in the H4 and H4a sequences by following coding rules for Neurospora crassa mtDNA (9). In H4a, we found a single large ORF of 1,857 nt, beginning at the HindIII site. In H4, this ORF was found in both inverted copies; no additional ORF was present in the 317-nt central nonrepeated sequence. The ORF was interrupted at its 5′ end by the HindIII site. We sequenced the region surrounding the H4a fragment on the overlapping 5.3-kb HaeIII fragment (Fig. 1) and found a TAA stop codon in the same reading frame immediately before the HindIII site. Thus, the ORF is entirely contained in the H4a sequence. The putatively encoded protein had a size of 500 aa from the first ATG (nt 355 to 357) codon or of 617 aa from the termination codon just before the HindIII site.
The ORF is 86% (nt) and 96% (aa) identical to the previously described Aa-polB gene and gene product (4), respectively, from the A. aegerita mitochondrial genome (GenBank accession no. AF061244). Lower percentages of amino acid similarity were obtained with other family B DNA polymerases, encoded by linear protein-primed replicating genomes (24, 27), such as the bacteriophage Φ 29 (GenBank accession no. X53370; 42% aa identity), or fungal linear mitochondrial plasmids such as the plasmid pEM of Agaricus bitorquis (GenBank accession no. P30322; 40% aa similarity), the plasmid pHC2 of Hebeloma circinans (GenBank accession no. Y11504; 39% aa similarity), or the kalilo plasmid of Neurospora intermedia (SwissProt accession no. P33538; 37% aa similarity).
The 1,119 nt specific to the H4a fragment has no significant sequence identity at the nucleotide level to sequences in the GenBank and EMBL databases. The largest ORF it contained that began with an ATG codon was 210 nt.
The central nonrepeated sequence from H4 (Fig. 1) has 72% identity with two Saccharomyces cerevisiae mitochondrial tRNA genes, tRNAfMet and tRNAPro. Based on primary sequence and on secondary structure, both H4 and H4a carry the same mitochondrial tRNAMet, located between nt 2071 (5′ end) and nt 2141 (3′ end), i.e., 213 nt following the first stop codon of the ORF with sequence similarity to the family B DNA polymerase genes.
Comparison of the large ORF of the polymorphic region with Aa-polB and other related family B DNA polymerase genes.
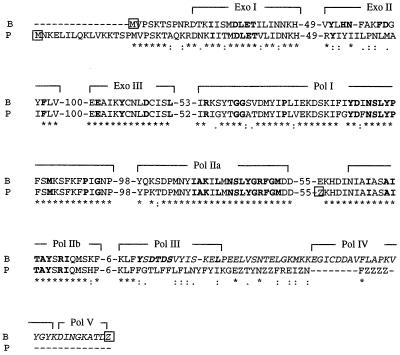
In the 2,282-nt region from the HindIII site (nt 1) to the end of the tRNAMet (nt 2071) in both H4 and H4a, the ORF region (nt 1 to 1955) can be aligned with a part (nt 644 to 2602) of the A. aegerita mitochondrial sequence (GenBank accession no. AF061244) containing the Aa-polB gene preceded by the intergenic region between the 5′ end of the SSU rDNA and Aa-polB (86% sequence identity). The remaining 116 nt of H4 or H4a, from nt 1956 to the 5′ end of the tRNAMet (nt 2071), had no significant sequence identity with the Aa-polB gene sequence, suggesting that the 3′ end of this gene was missing from the H4 or H4a sequence. If the two protein sequences are aligned (Fig. 2), then there is 96% aa similarity and 78% aa identity from the methionine (aa 1) constituting the Aa-POLB protein NH2 terminus to the KLF motif (aa 515 to 517) located in the DNA polymerase III (Pol III) domain. Thus, the Aa-polB P1 paralog is a truncated copy that should produce a nonfunctional protein that lacks the Pol III, Pol IV, and Pol V polymerization domains. The Aa-POLB P1 protein also should contain 16 aa before the methionine residue aligning with the NH2 terminus of Aa-POLB, since there is another Met amino acid codon in frame there.
FIG. 2.
Partial aa alignment of the family B DNA polymerases encoded by the Aa-polB (B) and Aa-polB P1 (P) genes (GenBank accession no. AF061244 and AF269233, respectively), carried out with the Clustal W package (13, 14). The conserved aa are indicated by stars, and the similar and equivalent aa residues are indicated by periods and colons, respectively. The conserved domains involved in the exonuclease (Exo) and polymerase activities previously defined on the Aa-POLB protein (4) and the numbers of amino acids between conserved domains are indicated. For each protein, the first Met aa (M) and the Z, corresponding to the first stop codon TAA, are framed. The COOH-terminal region of the Aa-POLB protein truncated in Aa-POLB P1 is in italics.
We compared the amino acid sequence similarities of Aa-POLB, Aa-POLB P1, the bacteriophage Φ 29 replicase, the DNA polymerase of the mitochondrial linear plasmid pEM from Agaricus bitorquis, and three family B DNA polymerases of Sulfolobus solfataricus in the portion of each protein between the highly conserved Pol I and Pol III domains (Table 1). Aa-POLB and Aa-POLB P1 were found to be distantly related (36 to 53% aa similarity) to the other family B DNA polymerases in the GenBank database. The A. aegerita proteins are about as closely related to the three S. solfataricus DNA polymerases (36 to 44%) as these proteins are to each other (44 to 50% similarity).
TABLE 1.
Comparison of the A. aegerita family B DNA polymerases Aa-POLB and Aa-POLB P1 with five related family B DNA polymerases
| Microorganism | Family B DNA polymerase (GenBank accession no.) | Size (aa) | Size (aa) and location of the Pol I to Pol III sequences | % aa similarityb
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aa-POLB | Aa-POLB P1 | Ss-B1 | Ss-B2 | Ss-B3 | Φ 29 | pEM | ||||
| A. aegerita strain WT-3a | Aa-POLB (AF061244) | 571 | 271 (IRKS–SKEL) | 100 | 96 | 44 | 38 | 40 | 44 | 53 |
| Aa-POLB P1 (AF268233) | 500 | 272 (IRIG–YFYI) | 100 | 41 | 36 | 39 | 45 | 51 | ||
| S. solfataricus strain P2 | Ss-B1 (U92875) | 882 | 327 (RTSA–VNVL) | 100 | 50 | 47 | 40 | 32 | ||
| Ss-B2 (X71597) | 626 | 298 (IMMV–RVYD) | 100 | 44 | 37 | 34 | ||||
| Ss-B3 (Y08257) | 764 | 242 (IRFI–SKKK) | 100 | 35 | 40 | |||||
| Bacteriophage Φ 29 | Replicase (X53370) | 575 | 245 (VRYA–TGTE) | 100 | 47 | |||||
| Agaricus bitorquis plasmid pEM | Replicase (P30322) | 773 | 286 (IRSS–RKPL) | 100 | ||||||
The complete sequences of Aa-polB as well as those of Aa-polB P1 in strains WT-3 and WT-11 were identical (see text and reference 4).
Based on the amino acid sequences located between the highly conserved Pol I and Pol III domains.
DISCUSSION
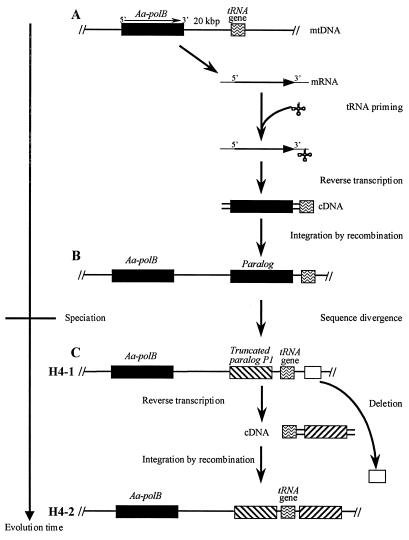
The A. aegerita mitochondrial genome contains the previously described Aa-polB gene, which encodes a putatively functional family B DNA polymerase (4), and a truncated, probably nonfunctional paralog, Aa-polB P1. Moreover, two widely distributed mitochondrial alleles carry one or two copies of the truncated Aa-polB P1 sequence. These DNA polymerase genes may have arisen by duplications occurring in two steps (Fig. 3). A first duplication of Aa-polB, occurring before A. aegerita speciation, led to the divergent (14%) paralog Aa-polB P1 present in all strains (Fig. 3A and B); then a duplication of Aa-polB P1, occurring after A. aegerita speciation, led to the copies accounting for the allelic variability of the H4 region (Fig. 3C).
FIG. 3.
Hypothetical model for the duplications of the family B DNA polymerase genes in the A. aegerita mitochondrial genome. (A) Distal (20-kb) duplication involving reverse transcription (RT) of the Aa-polB mRNA primed by the tRNAMet followed by integration of the cDNA (Aa-polB-tRNAMet) by recombination at the tRNAMet locus. (B) Sequence divergence (up to 14%) between Aa-polB and its paralog, leading to the disruption of the Aa-polB P1 copy. (C) Proximal (317-nt) duplication generating two inverted copies of Aa-polB P1 by recombinational integration at the tRNAMet locus of a cDNA (Aa-polB P1-tRNA) obtained after RT of the Aa-polB P1 mRNA. This duplication is accompanied by a large (>0.6-kb) deletion of neighboring mitochondrial sequences.
The putative origin of both duplications could be due to an illegitimate recombination between two mitochondrial genomes or, more probably, to the integration of a cDNA at a tRNA mitochondrial locus (Fig. 3). The first hypothesis is supported only by the fact that recombinant mtDNA molecules in A. aegerita heteroplasmons have been described (2). Because the Aa-polB P1 gene is followed by a tRNAMet, it seems more probable that an mRNA of the Aa-polB gene captured a tRNAMet whose 3′-OH end was used as a primer for a mitochondrial reverse transcriptase activity (Fig. 3A) (see, e.g., references 5 and 18). The resulting cDNA (Aa-polB-tRNAMet) was integrated into the mtDNA by recombination at the tRNAMet locus (Fig. 3A and B). We have no information about the other recombination site on the mtDNA; this region of recombination could be nonhomologous (illegitimate recombination) or, more probably, consist of a short homologous sequence present both on the cDNA and on the mtDNA near the tRNAMet locus. The high percentage of A+T in the recombining molecules favors the presence of such a homologous small sequence.
The second duplication event leading to the H4-2 allele appears to require a complex mechanism involving the duplication of Aa-polB P1 and the deletion of a large sequence of size greater than 0.6 kb (Fig. 3C). Since this duplication could have affected the regions preceding the sequenced fragment, our results do not allow the determination of the size of the deleted sequence or the size of the duplicated one.
The mechanism(s) leading to the formation of the Aa-polB P1 truncated gene is unknown; in particular, we failed to find in the short sequence separating the truncation site and the tRNAMet any sequence or secondary structure reminiscent of a group I or group II mitochondrial intron.
The γ DNA polymerases represent a highly conserved family of nucleus-encoded DNA polymerases responsible for the replication of circular mitochondrial genomes of all eukaryotic organisms. However, recent reports on plant or fungal mitochondria have described the presence of additional DNA polymerase activities. For example, a nucleus-encoded β DNA polymerase activity in yeast mitochondria has been recently reported (19), and family B DNA polymerase genes in the chrysophyte alga Ochromonas danica (6) and in the plant Beta vulgaris (GenBank accession no. Z34298) have been described. The products of these family B DNA polymerase genes could be involved in the replication of linear genomes, and it has been recently reported that most of the plant and fungal genomes are present as linear multimeric molecules (16).
Comparison of Aa-POLB and Aa-POLB P1 sequences with other family B DNA polymerases shows that they are distantly related to the polymerases of Sulfolobus solfataricus (7). If mitochondria originated from a fusion between a Clostridium-like eubacterium and a Sulfolobus-like archaebacterium (15), the A. aegerita family B DNA polymerase genes could be remnants of the archaebacterial genes.
The presence of Aa-polB and its paralog Aa-polB P1 in the mtDNA of the basidiomycete A. aegerita suggests that a family B DNA polymerase activity could exist in a fungal mitochondrion; the lack of such a gene in all the ascomycete mitochondrial genomes sequenced to date leads us to hypothesize that in these fungi this gene was eliminated after transfer of a duplicated copy to the nucleus. The search for sequences homologous to Aa-polB in other basidiomycetes and the determination of their genomic location(s) (nucleus and/or mitochondria) will allow us to assess this hypothesis and to reconstruct the evolutionary histories of such mitochondrial family B DNA polymerase genes.
ACKNOWLEDGMENTS
This work was supported by grants from the European Community (Fond Européen de Développement Régional), the Conseil Scientifique de l'Université Victor Ségalen Bordeaux 2, the Conseil Régional d'Aquitaine, Monsieur le Préfet de la région Aquitaine, Préfet de la Gironde (Fond National d'Aménagement et de Développement du Territoire), the Conseil Régional d'Aquitaine, and the Institut National de la Recherche Agronomique.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Barroso G, Labarère J. Genetic evidence for nonrandom sorting of mitochondria in the basidiomycete Agrocybe aegerita. Appl Environ Microbiol. 1997;63:4686–4691. doi: 10.1128/aem.63.12.4686-4691.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barroso G, Blesa S, Labarère J. Wide distribution of mitochondrial genome rearrangements in wild strains of the cultivated basidiomycete Agrocybe aegerita. Appl Environ Microbiol. 1995;61:1187–1193. doi: 10.1128/aem.61.4.1187-1193.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bois F, Barroso G, Gonzalez P, Labarère J. Molecular cloning, sequence and expression of Aa-polB, a mitochondrial gene encoding a family B DNA polymerase from the edible basidiomycete Agrocybe aegerita. Mol Gen Genet. 1999;261:508–513. doi: 10.1007/s004380050994. [DOI] [PubMed] [Google Scholar]
- 5.Chiang C-C, Lambowitz A M. The Mauriceville retroplasmid reverse transcriptase initiates cDNA synthesis de novo at the 3′ end of tRNAs. Mol Cell Biol. 1997;17:4526–4535. doi: 10.1128/mcb.17.8.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman A W, Thompson W F, Goff L J. Identification of the mitochondrial genome in the chrysophyte alga Ochromonas danica. J Protozool. 1991;38:129–135. [Google Scholar]
- 7.Edgell D R, Klenk H-P, Doolittle W F. Gene duplications in evolution of archaeal family B DNA polymerases. J Bacteriol. 1997;179:2632–2640. doi: 10.1128/jb.179.8.2632-2640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukumasa-Nakai Y, Matsumoto T, Fukuda M. Efficient isolation of mitochondrial DNA from higher basidiomycetes for restriction endonuclease analysis. Rep Tottori Mycol Inst. 1992;30:60–68. [Google Scholar]
- 9.Gonzalez P, Barroso G, Labarère J. DNA sequence and secondary structure of the mitochondrial small subunit ribosomal RNA coding region including a group-IC2 intron from the cultivated basidiomycete Agrocybe aegerita. Gene. 1997;184:55–63. doi: 10.1016/s0378-1119(96)00573-2. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez P, Barroso G, Labarère J. Molecular analysis of the split cox1 gene from the Basidiomycota Agrocybe aegerita: relationship of its introns with homologous Ascomycota introns and divergence levels from common ancestral copies. Gene. 1998;220:45–53. doi: 10.1016/s0378-1119(98)00421-1. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez P, Barroso G, Labarère J. Molecular gene organization and secondary structure of the mitochondrial large subunit ribosomal RNA from the cultivated Basidiomycota Agrocybe aegerita: a 13 kb gene possessing six unusual nucleotide extensions and eight introns. Nucleic Acids Res. 1999;27:1754–1761. doi: 10.1093/nar/27.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray M W, Burger G, Lang B F. Mitochondrial evolution. Science. 1999;283:1476–1481. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- 13.Higgins D G, Sharp P M. Clustal V: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988;73:237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 14.Higgins D G, Sharp P M. Fast and sensitive multiple alignment sequence on a microcomputer. Comput Appl Biosci. 1989;5:151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- 15.Karlin S, Brocchieri L, Mrazek J, Campbell A M, Spormann A M. A chimeric prokaryotic ancestry of mitochondria and primitive eukaryotes. Proc Natl Acad Sci USA. 1999;96:9190–9195. doi: 10.1073/pnas.96.16.9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lecrenier N, Foury F. New features of mitochondrial DNA replication in yeast and man. Gene. 2000;246:37–48. doi: 10.1016/s0378-1119(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 17.Lecrenier N, Van der Bruggen P, Foury F. Mitochondrial DNA polymerases from yeast to man: a new family of polymerases. Gene. 1997;185:147–152. doi: 10.1016/s0378-1119(96)00663-4. [DOI] [PubMed] [Google Scholar]
- 18.Leon P, Walbot V, Bedinger P. Molecular analysis of the linear 2.3 kb plasmid of maize mitochondria: apparent capture of tRNA genes. Nucleic Acids Res. 1989;17:4089–4099. doi: 10.1093/nar/17.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucas P, Laquel-Robert P, Plissonneau J, Schaeffer J, Tarrago-Litvak L M, Castroviejo M. A second DNA polymerase activity in yeast mitochondria. C R Acad Sci Ser III Life Sci. 1997;320:299–305. doi: 10.1016/s0764-4469(97)82771-0. [DOI] [PubMed] [Google Scholar]
- 20.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 21.Moulinier T, Barroso G, Labarère J. The mitochondrial genome of the basidiomycete Agrocybe aegerita: molecular cloning, physical mapping and gene location. Curr Genet. 1992;21:499–505. doi: 10.1007/BF00351660. [DOI] [PubMed] [Google Scholar]
- 22.Noël T, Labarère J. Isolation of DNA from Agrocybe aegerita for the construction of a genomic library in Escherichia coli. Mushroom Sci. 1987;12:187–201. [Google Scholar]
- 23.Raper J R, Hoffman R M. Schizophyllum commune. In: King R C, editor. Handbook of genetics. Vol. 1. New York, N.Y: Plenum Press; 1974. pp. 597–626. [Google Scholar]
- 24.Rohe M, Schründer J, Tudzynski P, Meinhardt F. Phylogenetic relationships of linear protein-primed replicating genomes. Curr Genet. 1992;21:173–176. doi: 10.1007/BF00318478. [DOI] [PubMed] [Google Scholar]
- 25.Salvado J C, Labarère J. Isolation of transcripts preferentially expressed during fruit body primordia differentiation in the basidiomycete Agrocybe aegerita. Curr Genet. 1991;2:1135–1137. doi: 10.1007/BF00326234. [DOI] [PubMed] [Google Scholar]
- 26.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiu-Shing B, Court D, Vierula J, Bertrand H. The kalilo linear senescence-inducing plasmid of Neurospora is an invertron and encodes DNA and RNA polymerase. Curr Genet. 1991;20:225–237. doi: 10.1007/BF00326237. [DOI] [PubMed] [Google Scholar]
- 28.Southern E. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Biol Chem. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 29.Yanish-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]