Abstract
Purpose
Diet may play an essential role in the aetiology of bladder cancer (BC). The B group complex vitamins involve diverse biological functions that could be influential in cancer prevention. The aim of the present study was to investigate the association between various components of the B group vitamin complex and BC risk.
Methods
Dietary data were pooled from four cohort studies. Food item intake was converted to daily intakes of B group vitamins and pooled multivariate hazard ratios (HRs), with corresponding 95% confidence intervals (CIs), were obtained using Cox-regression models. Dose–response relationships were examined using a nonparametric test for trend.
Results
In total, 2915 BC cases and 530,012 non-cases were included in the analyses. The present study showed an increased BC risk for moderate intake of vitamin B1 (HRB1: 1.13, 95% CI: 1.00–1.20). In men, moderate intake of the vitamins B1, B2, energy-related vitamins and high intake of vitamin B1 were associated with an increased BC risk (HR (95% CI): 1.13 (1.02–1.26), 1.14 (1.02–1.26), 1.13 (1.02–1.26; 1.13 (1.02–1.26), respectively). In women, high intake of all vitamins and vitamin combinations, except for the entire complex, showed an inverse association (HR (95% CI): 0.80 (0.67–0.97), 0.83 (0.70–1.00); 0.77 (0.63–0.93), 0.73 (0.61–0.88), 0.82 (0.68–0.99), 0.79 (0.66–0.95), 0.80 (0.66–0.96), 0.74 (0.62–0.89), 0.76 (0.63–0.92), respectively). Dose–response analyses showed an increased BC risk for higher intake of vitamin B1 and B12.
Conclusion
Our findings highlight the importance of future research on the food sources of B group vitamins in the context of the overall and sex-stratified diet.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00394-022-02805-2.
Keywords: Nutritional oncology, Bladder cancer, Pooled cohort analysis, B group vitamins
Introduction
With an estimated 549,393 new cases and 199,922 deaths in 2018, bladder cancer (BC) is the tenth most common cancer worldwide [1]. More than half of all BC cases occur in higher income countries, with the highest incidence rates in North America and Europe and the lowest in Africa. Due to high recurrence rates, BC is an expensive malignancy to treat from diagnosis to death, with estimated costs ranging from USD 89,287 to USD 202,203 per patient [2]. In fact, bladder cancer is the most expensive malignancy to treat of all cancers [3]. Therefore, BC is an important public health problem.
BC is a complex disease not only influenced by genetic predisposition, but also lifestyle, environmental, and occupational exposures could potentially play an important role in the development of this disease [4]. The more established risk factors associated with the development of BC include smoking and deleterious occupational exposure [5, 6] and male gender [5, 7]. Since the bladder is an important excretion organ through which dietary metabolites are filtered, diet may play an essential role in the development of BC. According to the United States National Cancer institute, one third of all BC cases could have been prevented by adherence to dietary recommendations, hence the salient need to investigate potential associations between foods, nutrients and BC [8].
Previous epidemiological research on diet and BC reported that high amount of fluid, fruit, vegetable, yogurt, whole grain and dietary fiber intake were associated with a reduced risk of BC [9–11], while higher intake of barbecued meat, pork, and total fat may increase BC risk [8]. At last, organ meat consumption has also been associated with BC development [12].
Although these findings for individual food items lead to useful dietary recommendations, it remains unclear what nutrients or bioactive compounds are responsible for the observed effects on BC risk [13].
The B group complex vitamins are found in a variety of foods, including meat, whole grains, and are especially in rich supply in fruits and vegetables [14]. The B group complex vitamins include (1) thiamine (B1), (2) riboflavin (B2) and (3) niacin (B3), which mainly play a role in energy metabolism [15–17], (4) pyridoxine (B6), which reduces oxidative stress (as does vitamin B2), thereby preventing DNA damage [18, 19], (5) folate (B9), and (6) cyanocobalamin (B12), which play a significant role in protecting and maintaining the stability of the human genome by the maintenance of one-carbon metabolism (i.e. a set of interconnected biochemical pathways driven by both vitamin B9 and B12 to generate methyl groups for DNA methylation [20]), thereby possessing the potential to lower the chance of a neoplastic events [14, 21]. However, despite the biological plausibility that these B group vitamins play a role in cancer prevention, epidemiological evidence on the effect of these B group vitamin compounds on BC development is lacking or inconclusive [4, 14, 22]. Inverse associations with BC risk have only been reported for high intake of the vitamins B9, B12 and B6 [4, 23, 24]. A meta-analysis, however, concluded that the evidence for this protective effect is limited [14].
This lack of evidence could be due to the small sample sizes of previous studies and their consequent lack of statistical power to detect associations. The present study, therefore, aims to provide a more precise quantitative estimate for the associations between the various components of the B group complex vitamins and BC risk by pooling data from four cohort studies.
Materials and methods
Study population
Data were derived from the BLadder cancer Epidemiology and Nutritional Determinants study (BLEND): a large international consortium on dietary factors and BC risk, compromising a total of 11,000 cases and over 680,000 non-cases aged between 18 and 100 years from different countries in Europe, America, Asia and Australia [25]. Currently, BLEND consists of 19 case–control and seven cohort studies.
The present study pooled data from the BLEND cohort studies only. Four cohort studies, including a total of 2915 cases and 530,012 non-cases, had sufficient information on dietary B group vitamin intake to be eligible for inclusion in our analyses. Informed consent was obtained from all individual participants included in each study. Included studies were the Netherlands Cohort Study on diet and cancer (NLCS) [26], the Radiation Effects Research Foundation (RERF) atomic bomb survivors study [27], the VITamins And Lifestyle study (VITAL) [28] and the European Prospective Investigation into Cancer and nutrition (EPIC) [29, 30] (Table 1).
Table 1.
Baseline characteristics of the four included cohort studies
| NLCS | RERF | VITAL | EPIC | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | No | % | No | % | |
| Country | The Netherlands | Japan | USA | Europe | ||||||
| Recruitment period | 1986–2003 | 1950–2000 | 2000–2008 | 1993–2006 | ||||||
| Food items (n) | 150 | 102 | 126 | 260 | ||||||
| Mean follow-up, years (± SD) | 14.06 (± 4.81) | 27.78 (± 12.74) | 6.74 (± 1.50) | 11.04 (± 2.81) | 11.20 (± 4.50) | |||||
| Subject status | ||||||||||
| Total | 5247 | 100 | 6582 | 100 | 69,489 | 100 | 451,609 | 100 | 532,927 | 100 |
| Cases | 877 | 16.71 | 42 | 0.64 | 346 | 0.50 | 1,650 | 0.37 | 2915 | 0.55 |
| Men | 734 | 83.69 | 32 | 76.19 | 269 | 77.75 | 1,154 | 69.94 | 2,189 | 75.09 |
| Women | 143 | 16.31 | 10 | 23.81 | 77 | 22.25 | 496 | 30.06 | 726 | 24.91 |
| Non-cases | 4370 | 83.29 | 6540 | 99.36 | 69,143 | 99.50 | 449,959 | 99.63 | 530,012 | 99.45 |
| Men | 2134 | 48.83 | 2475 | 37.84 | 33,470 | 48.41 | 131,503 | 29.23 | 169,582 | 32.00 |
| Women | 2236 | 51.17 | 4065 | 62.16 | 35,673 | 51.59 | 318,456 | 70.77 | 360,430 | 68.00 |
| Sex | ||||||||||
| Men | 2868 | 54.66 | 2507 | 38.09 | 33,739 | 48.55 | 132,657 | 29.37 | 171,771 | 32.23 |
| Women | 2379 | 45.34 | 4075 | 61.91 | 35,750 | 51.45 | 318,952 | 70.63 | 361,156 | 67.77 |
| Age (years) | ||||||||||
| < 50 | ||||||||||
| Cases | 18 | 42.86 | 2 | 0.58 | 200 | 12.12 | 220 | 7.55 | ||
| Non-cases | 3703 | 56.62 | 1812 | 2.62 | 191,060 | 42.46 | 196,575 | 37.09 | ||
| 50–59 | ||||||||||
| Cases | 195 | 22.23 | 10 | 23.81 | 71 | 20.52 | 617 | 37.39 | 893 | 30.63 |
| Non-cases | 1355 | 31.01 | 1179 | 18.03 | 30,845 | 44.61 | 160,674 | 35.71 | 194,053 | 36.61 |
| 60–69 | ||||||||||
| Cases | 631 | 71.95 | 10 | 23.81 | 132 | 38.15 | 725 | 43.94 | 1498 | 51.39 |
| Non-cases | 2781 | 63.64 | 1198 | 18.32 | 23,862 | 34.51 | 88,032 | 19.56 | 115,873 | 21.86 |
| > = 70 | ||||||||||
| Cases | 51 | 5.82 | 4 | 9.52 | 141 | 40.75 | 108 | 6.55 | 304 | 10.43 |
| Non-cases | 234 | 5.35 | 460 | 7.03 | 12,624 | 18.26 | 10,193 | 2.27 | 23,511 | 4.44 |
| TNM stage | ||||||||||
| MIBC | 413 | 51.69 | 110 | 32.74 | 133 | 21.63 | 656 | 37.49 | ||
| Male | 333 | 80.63 | 91 | 82.73 | 98 | 73.68 | 522 | 79.57 | ||
| Female | 80 | 19.37 | 19 | 17.27 | 35 | 26.32 | 134 | 20.43 | ||
| NMIBC | 386 | 48.31 | 226 | 67.26 | 482 | 78.37 | 1094 | 62.51 | ||
| Male | 335 | 86.79 | 169 | 74.78 | 313 | 64.94 | 817 | 74.68 | ||
| Female | 51 | 13.21 | 57 | 25.22 | 169 | 35.06 | 277 | 25.32 | ||
| Smoking status | ||||||||||
| Cases | ||||||||||
| Never | 117 | 13.34 | 7 | 16.67 | 85 | 24.57 | 349 | 21.15 | 558 | 19.14 |
| Current light | 63 | 7.18 | 164 | 9.94 | 227 | 7.79 | ||||
| Current heavy | 287 | 32.73 | 433 | 26.24 | 720 | 24.70 | ||||
| Current unknown | 36 | 4.10 | 28 | 66.67 | 53 | 15.32 | 92 | 5.58 | 209 | 7.17 |
| Former light | 127 | 14.48 | 127 | 4.36 | ||||||
| Former heavy | 224 | 25.54 | 224 | 7.68 | ||||||
| Former unknown | 23 | 2.62 | 7 | 16.67 | 208 | 60.12 | 612 | 37.09 | 850 | 29.16 |
| Non-cases | ||||||||||
| Never | 1581 | 36.18 | 3579 | 54.72 | 32,982 | 47.70 | 223,234 | 49.61 | 261,376 | 49.32 |
| Current light | 353 | 8.08 | 48,134 | 10.70 | 48,487 | 9.15 | ||||
| Current heavy | 765 | 17.51 | 43,435 | 9.65 | 44,200 | 8.34 | ||||
| Current unknown | 115 | 2.63 | 2580 | 39.45 | 5621 | 8.13 | 10,546 | 2.3 | 18,862 | 3.56 |
| Former light | 820 | 18.76 | 820 | 0.15 | ||||||
| Former heavy | 647 | 14.81 | 647 | 0.12 | ||||||
| Former unknown | 89 | 2.04 | 381 | 5.83 | 30,540 | 44.17 | 124,610 | 27.69 | 155,620 | 29.36 |
| Thiamine (B1)a | ||||||||||
| Mean (± SD) | ||||||||||
| Cases | 2.80 (± 0.63) | 0.12 (± 0.08) | 0.42 (±0.11) | 1.33 (± 0.45) | 1.65 (± 0.35) | |||||
| Non-cases | 2.65 (± 0.26) | 0.12 (± 0.01) | 0.40 (±0.01) | 1.10 (± 0.02) | 1.01 (± 0.01) | |||||
| t (p) | 2.40 (0.02) | 0.70 (0.48) | 1.87 (0.06) | 9.12 (< 0.001) | 34.11 (< 0.001) | |||||
| Riboflavin (B2) a | ||||||||||
| Mean (± SD) | 1.42 (± 0.09) | 3.63 (± 0.13) | ||||||||
| Cases | 9.17 (± 0.32) | 0.13 (± 0.01) | 0.56 (± 0.02) | 0.98 (± 0.003) | 0.97 (± 0.003) | |||||
| Non-cases | 8.60 (± 0.13) | 0.12 (± 0.001) | 0.53 (± 0.002) | 9.60 (< 0.001) | 68.32 (< 0.001) | |||||
| t (p) | 1.71 (0.09) | 0.45 (0.65) | 1.21 (0.23) | |||||||
| Niacin (B3) a | ||||||||||
| Mean (± SD) | ||||||||||
| Cases | 22.23 (± 0.29) | 0.73 (± 0.06) | 6.87 (±0.18) | 16.45 (±0.64) | 16.83 (±0.38) | |||||
| Non-cases | 20.89 (± 0.12) | 0.71 (± 0.01) | 6.54 (±0.01) | 13.05 (±0.02) | 12.11 (±0.02) | |||||
| t (p) | 4.41 (< 0.001) | 0.33 (0.74) | 1.86 (0.06) | 9.98 (<0.001) | 19.38 (<0.001) | |||||
| Pyridoxine (B6) a | ||||||||||
| Mean (± SD) | ||||||||||
| Cases | 2.73 (± 0.07) | 0.04 (± 0.003) | 0.61 (± 0.02) | 2.04 (± 0.09) | 2.05 (± 0.06) | |||||
| Non-cases | 2.60 (± 0.03) | 0.03 (± 0.00002) | 0.65 (± 0.001) | 1.66 (± 0.003) | 1.51 (± 0.003) | |||||
| t (p) | 1.96 (0.05) | 1.19 (0.23) | − 2.02 (0.04) | 7.58 (< 0.001) | 14.96 (< 0.001) | |||||
| Folate (B9) b | ||||||||||
| Mean (± SD) | ||||||||||
| Cases | 512.85 (± 7.73) | 97.55 (± 2.70) | 199.39 (± 2.87) | 282.81 (± 4.10) | ||||||
| Non-cases | 488.49 (± 3.29) | 97.54 (± 0.20) | 196.18 (± 0.15) | 185.59 (± 0.15) | ||||||
| t (p) | 3.00 (0.003) | 0.005 (1.00) | 1.29 (0.20) | 48.27 (< 0.001) | ||||||
| Cyanocobalamin (B12)b | ||||||||||
| Mean (± SD) | ||||||||||
| Cases | 9.64 (± 0.44) | 0.03 (± 0.003) | 3.92 (± 0.20) | 3.10 (± 0.09) | 5.12 (± 0.15) | |||||
| Non-cases | 9.67 (± 0.18) | 0.03 (± 0.0003) | 4.26 (± 0.01) | 5.32 (± 0.01) | 5.15 (± 0.01) | |||||
| t (p) | − 0.06 (0.95) | 0.14 (0.89) | − 1.68 (0.09) | − 14.10 (< 0.001) | − 0.28 (0.78) | |||||
NLCS Netherlands Cohort Study on diet and cancer, RERF the Radiation Effects Research Foundation, VITAL the VITamins And Lifestyle study, EPIC European Prospective Investigation into Cancer and nutrition, TNM Classification of Malignant tumors, SD standard deviation
aNutrient values measured per day in milligrams
bNutrient values measured per day in micrograms
Data collection and coding
Details on the methodology of the BLEND consortium have been described elsewhere [25]. All included studies used a self-administered or trained interviewer administered food frequency questionnaire (FFQ) that was validated on either food groups [28, 30–33], and/or energy intake [33, 34]. The period of recalling the dietary intake and the method used to validate the intake differed per study. In brief, (a) in the NLCS participants were asked to report on their dietary intake during the preceding year before study enrolment. This method was validated by a 5-year reproducibility test [35]; (b) the Vital study used a time reference for all dietary questions of “in the last 3 months” and used 24-h dietary recalls and a 4-day food record to validate the dietary intake of the participants [28]; (c) studies included in the EPIC study used either a 7-day food consumption diary or 24-h dietary recalls to validate the reported dietary intake of the participants during the preceding year [29]; (d) the RERF also used 24-h recalls to assess the bias and precision of their FFQ in which a 1 year time reference was used [32]. The collected dietary data were harmonized and categorized using the hierarchical Eurocode 2 food coding system (ECC) developed by the European Union, besides, weekly, monthly or yearly intake were converted to weekly food intake.
As a second step, all recoded food items were converted into nutrients using the United States Department of Agriculture (USDA) food composition database [36]. This database has been validated for B group vitamins [37]. For this, we chose the nutrient content per 100 g of generic food items where possible. Raw products were preferred over cooked/boiled for fruits, whereas for meat, fish, vegetables (except for salad vegetables—see below) and pulses roasted/cooked/boiled/grilled was preferred over raw. For example, for “Whitefish” we chose "Fish, whitefish, mixed species, cooked, dry heat" (code 15223) and for “Chicken breast” we chose “Chicken, broilers or fryers, breast, skinless, boneless, meat only, cooked, grilled” (code 05747). The nutrient content for endive, lettuce, fennel, chicory, garlic, chives, radish, cucumber, avocado, morel, and cress was selected as raw, for tomatoes the raw and cooked values were averaged, and for olives “canned” was selected.
Two approaches were used for food items not available in the USDA food composition database: (1) American terms were matched (i.e. the ECC is written in British English and the USDA database in American English). For example, “Courgette” was matched to zucchini; (2) if food items remained not found, we searched the internet for a description of the food item. For example, “Runner beans” were matched to kidney beans based on the information that kidney beans are the mature seed of the runner bean plant. For ECC pooled food items (e.g. salmon and trout), individual items were searched in the USDA database and the nutrient content was averaged.
The final nutrient intake was converted from weekly intake to daily intake (i.e. for each nutrient a nutrient in micrograms/day was created). If possible, portion sizes were adapted from individual studies, and otherwise based on USDA database information.
Person-years of follow-up for each participant was calculated from date of study enrolment until date of BC diagnosis, or date of ending follow-up (e.g. date of death, loss to follow-up, or study exit), whichever came first. For the NLCS study, a nested case–cohort approach was applied, in which the number of person-years at risk was estimated based on a sub-cohort that was randomly sampled [26].
Each study ascertained incident BC with International Classification of Diseases for Oncology (ICD-O-3 code C67) using population-based cancer registries, health insurance records, or medical records. The term BC was used for all urinary bladder neoplasms. BCs were classified into non-muscle invasive bladder cancer (NMIBC) and muscle invasive bladder cancer (MIBC). NMIBC included non-invasive papillary carcinomas confined to the urothelium (stage Ta), and carcinomas that invaded the lamina propria of the bladder wall (stage T1). High-grade flat non-invasive carcinomas confined to the urothelium (carcinoma in situ; CIS) without other concomitant tumour stages [i.e. T1/Ta (classified to non-muscle invasive prior) or MIBC] were also classified as NMIBC. MIBC included carcinomas that invaded into the detrusor muscle (stage T2), carcinomas that invaded into the peri-vesical tissue (stage T3), and carcinomas that invaded adjacent tissues and organs (most often the prostate or uterus, stage T4).
In addition to information on dietary intake, the BLEND data also included study characteristics (design, method of dietary assessment, recall time of dietary intake and geographical region), participant demographics (age, sex and ethnicity), BC pathology (MNIBC and MIBC), and smoking status (current/former/never and pack years), which were all measured at baseline [25].
Statistical analysis
The differences between the different exposure categories were examined by t test for continuous variables. To assess the association between dietary B group vitamins and BC risk, cox proportional hazard regression analysis was used to obtain hazard ratios (HRs) and corresponding 95% confidence intervals, stratified by study centre. Scaled Schoenfeld residuals were estimated for each covariate to test the proportional hazards assumption. In addition, the appropriateness of the use of the log-normal distribution was tested using a Wald test, and again no evidence of violation was found [38].
Separate analyses were undertaken for each B group vitamin compound individually, for all B group vitamins combined, and for combinations of B group vitamins based on their biological function. Combining of B group vitamin intakes was done by multiplying the individual compound intakes. B group vitamin intake was classified in tertiles (low/moderate/high intake) based on the distribution of all included study participants per study separately, since not every study measured an equal amount of food items of focussed on the same food groups. The Cox-regression model used low consumers as the reference group and was adjusted for previously shown BC risk factors: model 1: adjusted for the confounders age, sex (male/female), smoking status (was defined as a dummy variable: 0 (never smokers); 1 [current light smokers (i.e. smoking less than 20 pack-years)]; 2 [current heavy smokers (i.e. smoking more than 20 pack-years)]; 3 [current smokers (no information on pack-years)]; 4 [former light smokers (i.e. smokers who ceased smoking over 1 year prior and smoked less than 20 pack-years)]; 5 [former heavy smokers (i.e. smokers who ceased smoking over 1 year prior and smoked more than 20 pack-years)]; 6 [former smokers (smokers who ceased smoking over 1 year prior and no information on pack-years)])) [5, 6] and model 2: additionally, adjusted for water (low/high) (because of the water-soluble origin of B group vitamins [23]).
The Wald-test was used to derive the p value for linear trend. To understand the relevance of the effect modification, the main interaction terms between B group vitamin consumption (low/moderate/high) and sex, smoking and water were added to the model. P interaction < 0.05 was considered statistically significant where upon all analyses were stratified for the covariate of interest. In addition, sensitivity analyses were performed in which BC cases diagnosed within the first 5 years after recruitment were excluded.
Based on a priori hypothesis, additional supplemental analyses were performed by BC subtype (i.e. NMIBC and MIBC). In addition, dose–response analyses of vitamin intake (plotted on the x-axis) and HR (model 2, plotted on the y-axis) were performed using a nonparametric test for trend.
All statistical analyses were performed using Stata14 (StataCorp LLC, College Station, TX).
Results
Baseline characteristics
Baseline characteristics of the study population are described in Table 1. In total, 3265 cases and 580,634 non-cases were included in our analyses. Cases were on average older than non-cases (60.3 vs 52.6 years), more likely to be current or former smoker (37.58% and 42.02% vs 20.82% and 29.15%, respectively) and there were approximately three times more male than female cases (2189 vs 726). Compared to non-cases, cases consumed on average more vitamin B1 (1.65 mg vs 1.01 mg), B2 (3.63 mg vs 0.97 mg), B3 (16.83 mg vs 12.11 mg), B6 (2.05 mg vs 1.51 mg) and B9 (282.81 µg vs 185.59 µg).
B group vitamins and their associations with BC
Overall results
Overall, no significant associations of higher intakes of any of the B group complex vitamins and BC risk were observed (Table 2), except for a moderate intake of vitamin B1, which showed to slightly increase the risk of BC (HRB1: 1.10, 95% CI: 1.00–1.20) (Table 2). No other significant associations were observed.
Table 2.
Hazard ratios and 95% confidence intervals for BC according to dietary B group vitamin intake in the BLEND study
| Overall (n = 532,927) | Men (n = 171,771) | Women (n = 361,156) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Moderate | High | P | Low | Moderate | High | P | Low | Moderate | High | p | |
| Thiamin (B1) | ||||||||||||
| Model 1a | 1 |
1.10 (1.00–1.20) |
1.04 (0.95–1.14) |
< 0.001 | 1 |
1.13 (1.02–1.26) |
1.13 (1.02–1.26) |
< 0.001 | 1 |
1.03 (0.87–1.22) |
0.80 (0.67–0.97) |
< 0.001 |
| Model 2b | 1 |
1.14 (1.03–1.26) |
1.14 (1.01–1.29) |
< 0.001 | 1 |
1.16 (1.03–1.30) |
1.20 (1.05–1.38) |
< 0.001 | 1 |
1.13 (0.94–1.36) |
0.96 (0.75–1.23) |
< 0.001 |
| Riboflavin (B2) | ||||||||||||
| Model 1a | 1 |
1.07 (0.98–1.17) |
0.99 (0.91–1.08) |
< 0.001 | 1 |
1.14 (1.02–1.26) |
1.06 (0.95–1.17) |
< 0.001 | 1 |
0.91 (0.76–1.08) |
0.83 (0.70–1.00) |
< 0.001 |
| Model 2b | 1 |
1.10 (1.00–1.21) |
1.06 (0.93–1.21) |
< 0.001 | 1 |
1.14 (1.02–1.28) |
1.06 (0.91–1.23) |
< 0.001 | 1 |
1.01 (0.83–1.22) |
1.04 (0.80–1.35) |
< 0.001 |
| Niacin (B3) | ||||||||||||
| Model 1a | 1 |
1.02 (0.93–1.12) |
0.98 (0.90–1.07) |
< 0.001 | 1 |
1.05 (0.94–1.17) |
1.06 (0.95–1.18) |
< 0.001 | 1 |
0.97 (0.82–1.15) |
0.77 (0.63–0.93) |
< 0.001 |
| Model 2b | 1 |
1.03 (0.94–1.14) |
1.02 (0.91–1.14) |
< 0.001 | 1 |
1.05 (0.94–1.18) |
1.07 (0.94–1.21) |
< 0.001 | 1 |
1.03 (0.86–1.24) |
0.87 (0.68–1.10) |
< 0.001 |
| Pyridoxine (B6) | ||||||||||||
| Model 1a | 1 |
1.00 (0.92–1.09) |
0.95 (0.87–1.04) |
< 0.001 | 1 |
1.05 (0.95–1.17) |
1.05 (0.94–1.16) |
< 0.001 | 1 |
0.89 (0.75–1.06) |
0.73 (0.61–0.88) |
< 0.001 |
| Model 2b | 1 |
1.01 (0.92–1.12) |
0.99 (0.86–1.13) |
< 0.001 | 1 |
1.06 (0.94–1.19) |
1.06 (0.91–1.25) |
< 0.001 | 1 |
0.92 (0.75–1.12) |
0.79 (0.59–1.04) |
< 0.001 |
| Folate (B9) | ||||||||||||
| Model 1a | 1 |
1.04 (0.95–1.14) |
0.99 (0.90–1.08) |
< 0.001 | 1 |
1.09 (0.98–1.21) |
1.05 (0.95–1.17) |
< 0.001 | 1 |
0.93 (0.79–1.11) |
0.82 (0.68–0.99) |
< 0.001 |
| Model 2b | 1 |
1.06 (0.96–1.16) |
1.03 (0.93–1.14) |
< 0.001 | 1 |
1.09 (0.98–1.22) |
1.06 (0.94–1.20) |
< 0.001 | 1 |
0.99 (0.83–1.19) |
0.93 (0.75–1.16) |
< 0.001 |
| Cyanocobalamin (B12) | ||||||||||||
| Model 1a | 1 |
0.97 (0.89–1.05) |
0.94 (0.85–1.03) |
< 0.001 | 1 |
0.96 (0.87–1.06) |
1.01 (0.91–1.13) |
< 0.001 | 1 |
0.99 (0.83–1.18) |
0.79 (0.66–0.95) |
< 0.001 |
| Model 2b | 1 |
0.97 (0.89–1.07) |
0.95 (0.85–1.06) |
< 0.001 | 1 |
0.95 (0.86–1.06) |
1.00 (0.88–1.13) |
< 0.001 | 1 |
1.04 (0.87–1.25) |
0.88 (0.71–1.08) |
< 0.001 |
| Energy metabolism (B1*B2*B3) | ||||||||||||
| Model 1a | 1 |
1.07 (0.98–1.17) |
1.01 (0.92–1.10) |
< 0.001 | 1 |
1.13 (1.02–1.26) |
1.09 (0.98–1.21) |
< 0.001 | 1 |
0.94 (0.79–1.12) |
0.80 (0.66–0.96) |
< 0.001 |
| Model 2b | 1 |
1.10 (1.00–1.22) |
1.08 (0.96–1.23) |
< 0.001 | 1 |
1.15 (1.02–1.28) |
1.13 (0.98–1.30) |
< 0.001 | 1 |
1.02 (0.84–1.24) |
0.94 (0.73–1.21) |
< 0.001 |
| Oxidative stress reduction (B2*B6) | ||||||||||||
| Model 1a | 1 |
1.02 (0.93–1.11) |
0.96 (0.88–1.05) |
< 0.001 | 1 |
1.08 (0.97–1.20) |
1.05 (0.95–1.17) |
< 0.001 | 1 |
0.89 (0.74–1.05) |
0.74 (0.62–0.89) |
< 0.001 |
| Model 2b | 1 |
1.04 (0.94–1.15) |
1.01 (0.87–1.16) |
< 0.001 | 1 |
1.08 (0.97–1.22) |
1.08 (0.92–1.26) |
< 0.001 | 1 |
0.92 (0.75–1.13) |
0.80 (0.60–1.07) |
< 0.001 |
| DNA stability (B9*B12) | ||||||||||||
| Model 1a | 1 |
1.00 (0.92–1.10) |
0.95 (0.87–1.05) |
< 0.001 | 1 |
1.01 (0.91–1.12) |
1.04 (0.94–1.16) |
< 0.001 | 1 |
0.99 (0.84–1.18) |
0.76 (0.63–0.92) |
< 0.001 |
| Model 2b | 1 |
1.02 (0.93–1.12) |
0.99 (0.88–1.11) |
< 0.001 | 1 |
1.02 (0.91–1.13) |
1.06 (0.92–1.21) |
< 0.001 | 1 |
1.04 (0.86–1.24) |
0.84 (0.67–1.06) |
< 0.001 |
| Vitamin B complex (B1*B2*B3*B6*B9*B12) | ||||||||||||
| Model 1a | 1 |
1.06 (0.97–1.15) |
0.93 (0.84–1.03) |
< 0.001 | 1 |
1.07 (0.97–1.18) |
0.94 (0.84–1.06) |
< 0.001 | 1 |
1.03 (0.86–1.24) |
0.93 (0.77–1.11) |
< 0.001 |
| Model 2b | 1 |
1.05 (0.96–1.14) |
0.91 (0.82–1.01) |
< 0.001 | 1 |
0.97 (0.97–1.18) |
0.94 (0.83–1.06) |
< 0.001 | 1 |
1.01 (0.84–1.20) |
0.91 (0.76–1.09) |
< 0.001 |
BC Bladder Cancer, BLEND BLadder cancer Epidemiology and Nutritional Determinants
aAdjusted for sex, age, and smoking status
bAdjusted for sex, age, smoking status and water
Stratified analyses
Sex
A significant interaction was shown for sex and B vitamins (Table 3).
Table 3.
P interaction values of possible confounders
| Thiamin (B1) | Riboflavin (B2) | Niacin (B3) | Pyridoxine (B6) | Folate (B9) | Cyanocobal amin (B12) |
Energy metabolism (B1*B2*B3) | Oxidative stress reduction (B2*B6) | DNA stability (B9*B12) | Vitamin B complex (B1*B2*B3* B6*B9*B12) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||||
| Moderate | 0.33 | 0.03 | 0.44 | 0.11 | 0.13 | 0.64 | 0.07 | 0.06 | 0.96 | 0.29 |
| High | 0.001 | 0.02 | 0.003 | 0.001 | 0.02 | 0.02 | 0.003 | 0.001 | 0.004 | 0.002 |
| Water | 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.001 |
| Moderate | 0.96 | 0.87 | 0.09 | 0.40 | 0.76 | 0.66 | 0.61 | 0.39 | 0.58 | 0.83 |
| High | 0.59 | 0.41 | 0.53 | 0.70 | 0.80 | 0.42 | 0.41 | 0.68 | 0.35 | 0.181 |
| Smoking | ||||||||||
| Current light*moderate | 0.48 | 0.65 | 0.16 | 0.73 | 0.44 | 0.71 | 0.33 | 0.14 | 0.94 | 0.40 |
| Current light*high | 0.16 | 0.15 | 0.13 | 0.39 | 0.31 | 0.91 | 0.20 | 0.43 | 0.88 | 0.21 |
| Current heavy*moderate | 0.23 | 0.91 | 0.91 | 0.09 | 0.88 | 0.56 | 0.82 | 0.68 | 0.41 | 0.69 |
| Current heavy*high | 0.88 | 0.88 | 0.60 | 0.96 | 0.14 | 0.02 | 0.92 | 0.82 | 0.02 | 0.93 |
| Current unknown*moderate | 0.05 | 0.21 | 0.29 | 0.83 | 0.50 | 0.29 | 0.16 | 0.16 | 0.90 | 0.05 |
| Current unknown*high | 0.04 | 0.17 | 0.03 | 0.25 | 0.53 | 0.95 | 0.09 | 0.10 | 0.78 | 0.20 |
| Former light*moderate | 0.79 | 0.75 | 0.71 | 0.85 | 0.57 | 0.19 | 0.59 | 0.55 | 0.08 | 0.73 |
| Former light*high | 0.60 | 0.77 | 0.67 | 0.98 | 0.52 | 0.74 | 0.71 | 0.82 | 0.86 | 0.47 |
| Former heavy*moderate | 0.69 | 0.95 | 0.82 | 0.54 | 0.63 | 0.40 | 0.87 | 0.54 | 0.94 | 0.02 |
| Former heavy*high | 0.31 | 0.23 | 0.70 | 0.17 | 0.26 | 0.31 | 0.32 | 0.20 | 0.46 | 0.21 |
| Former unknown*moderate | 0.57 | 0.64 | 0.23 | 0.84 | 0.28 | 0.29 | 0.28 | 0.93 | 0.41 | 0.82 |
| Former unknown*high | 0.15 | 0.23 | 0.12 | 0.30 | 0.54 | 0.26 | 0.18 | 0.14 | 0.37 | 0.11 |
| BC type | ||||||||||
| Moderate | 0.56 | 0.81 | 0.46 | 0.24 | 0.04 | 0.73 | 0.87 | 0.76 | 0.63 | 0.72 |
| High | 0.30 | 0.78 | 0.32 | 0.95 | 0.65 | 0.95 | 0.52 | 0.41 | 0.25 | 0.33 |
Moderate intake of the vitamins B1, B2 and the vitamins related to energy metabolism showed to be associated with a small increased BC risk among men (HRB1: 1.13, 95% CI: 1.02–1.26, HRB2: 1.14, 95%CI: 1.02–1.26, HRenergy metabolism: 1.13, 95% CI: 1.02–1.26). In addition, for vitamin B1 also high intake was related to an increased BC risk (HRB1: 1.13, 95% CI: 1.02–1.26) (Table 2).
No such positive associations were observed for women. In contrast, we observed an inverse associations between high intake of all B vitamins and B vitamin combinations and BC risk (HRB1: 0.80, 95% CI: 0.67–0.97, HRB2: 0.83, 95% CI: 0.70–1.00, HRB3: 0.77, 95% CI: 0.63–0.93, HRB6: 0.73, 95% CI: 0.61–0.88, HRB9: 0.82, 95% CI: 0.68–0.99, HRB12: 0.79, 95% CI: 0.66–0.95, HRenergy metabolism: 0.80, 95% CI: 0.66–0.96, HRoxidative stress: 0.74, 95% CI: 0.62–0.89, HRDNA stability: 0.76, 95% CI: 0.63–0.92), except for the entire B group vitamin complex (Table 2).
BC subtypes
MIBC
No significant interaction was shown for BC type and B vitamins (Table 3).
Overall, a decreased MIBC risk was observed for high intake of the vitamins B1, B2, B3, B6, B9 the vitamins related to energy metabolism, oxidative stress reduction and DNA stability (HRB1: 0.86, 95% CI: 0.77–0.97, HRB2: 0.88, 95% CI: 0.78–0.98, HRB3: 0.80, 95% CI: 0.72–0.90, HRB6: 0.88, 95% CI: 0.79–0.99, HRB9: 0.86, 95% CI: 0.77–0.96, HRenergy metabolism: 0.84, 95% CI: 0.75–0.95, HRoxidative stress: 0.88, 95% CI: 0.79–0.98, HRDNA stability: 0.85, 95% CI: 0.75–0.95) (Table 4).
Table 4.
Hazard ratios and 95% confidence intervals for MIBC according to dietary B group vitamin intake in the BLEND study
| Overall (n = 531,883) | Men (n = 170,954) | Women (n = 360,879) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Moderate | High | P | Low | Moderate | High | P | Low | Moderate | High | p | |
| Thiamin (B1) | ||||||||||||
| Model 1a | 1 |
0.98 (0.87–1.09) |
0.86 (0.77–0.97) |
< 0.001 | 1 |
1.03 (0.90–1.17) |
0.93 (0.82–1.06) |
< 0.001 | 1 |
0.87 (0.70–1.08) |
0.70 (0.55–0.88) |
< 0.001 |
| Model 22 | 1 |
0.95 (0.84–1.07) |
0.81 (0.70–0.95) |
< 0.001 | 1 |
1.00 (0.87–1.15) |
0.87 (0.73–1.04) |
< 0.001 | 1 |
0.83 (0.65–1.06) |
0.63 (0.46–0.87) |
< 0.001 |
| Riboflavin (B2) | ||||||||||||
| Model 1a | 1 |
0.96 (0.86–1.08) |
0.88 (0.78–0.98) |
< 0.001 | 1 |
0.99 (0.87–1.13) |
0.91 (0.80–1.03) |
< 0.001 | 1 |
0.87 (0.70–1.08) |
0.80 (0.63–1.00) |
< 0.001 |
| Model 22 | 1 |
0.93 (0.82–1.05) |
0.81 (0.69–0.96) |
< 0.001 | 1 |
0.95 (0.82–1.10) |
0.81 (0.67–0.98) |
< 0.001 | 1 |
0.86 (0.67–1.11) |
0.78 (0.56–1.09) |
< 0.001 |
| Niacin (B3) | ||||||||||||
| Model 11 | 1 |
0.90 (0.81–1.01) |
0.80 (0.72–0.90) |
< 0.001 | 1 |
0.97 (0.85–1.10) |
0.87 (0.77–1.00) |
< 0.001 | 1 |
0.78 (0.63–0.97) |
0.63 (0.50–0.82) |
< 0.001 |
| Model 22 | 1 |
0.88 (0.78–0.99) |
0.74 (0.64–0.86) |
< 0.001 | 1 |
0.94 (0.82–1.08) |
0.81 (0.69–0.95) |
< 0.001 | 1 |
0.74 (0.58–0.93) |
0.57 (0.42–0.77) |
< 0.001 |
| Pyridoxine (B6) | ||||||||||||
| Model 11 | 1 |
0.89 (0.80–1.00) |
0.88 (0.79–0.99) |
< 0.001 | 1 |
0.94 (0.83–1.08) |
0.96 (0.84–1.09) |
< 0.001 | 1 |
0.79 (0.63–0.98) |
0.71 (0.57–0.90) |
< 0.001 |
| Model 22 | 1 |
0.86 (0.76–0.98) |
0.80 (0.67–0.96) |
< 0.001 | 1 |
0.92 (0.79–1.07) |
0.90 (0.74–1.11) |
< 0.001 | 1 |
0.70 (0.54–0.92) |
0.57 (0.40–0.82) |
< 0.001 |
| Folate (B9) | ||||||||||||
| Model 11 | 1 |
0.96 (0.86–1.08) |
0.86 (0.77–0.96) |
< 0.001 | 1 |
1.00 (0.88–1.14) |
0.90 (0.78–1.02) |
< 0.001 | 1 |
0.88 (0.71–1.10) |
0.77 (0.60–0.98) |
< 0.001 |
| Model 22 | 1 |
0.96 (0.85–1.08) |
0.85 (0.74–0.97) |
< 0.001 | 1 |
0.99 (0.86–1.14) |
0.87 (0.75–1.02) |
< 0.001 | 1 |
0.89 (0.70–1.11) |
0.77 (0.58–1.02) |
< 0.001 |
| Cyanocobalamin (B12) | ||||||||||||
| Model 11 | 1 |
0.93 (0.83–1.03) |
0.90 (0.80–1.02) |
< 0.001 | 1 |
0.93 (0.82–1.05) |
0.96 (0.83–1.10) |
< 0.001 | 1 |
0.93 (0.74–1.17) |
0.82 (0.65–1.03) |
< 0.001 |
| Model 22 | 1 |
0.93 (0.83–1.04) |
0.90 (0.78–1.03) |
< 0.001 | 1 |
0.92 (0.81–1.05) |
0.94 (0.80–1.11) |
< 0.001 | 1 |
0.94 (0.75–1.20) |
0.85 (0.65–1.11) |
< 0.001 |
| Energy metabolism (B1*B2*B3) | ||||||||||||
| Model 11 | 1 |
0.94 (0.84–1.06) |
0.84 (0.75–0.95) |
< 0.001 | 1 |
1.00 (0.87–1.14) |
0.90 (0.79–1.03) |
< 0.001 | 1 |
0.82 (0.66–1.02) |
0.69 (0.55–0.88) |
< 0.001 |
| Model 22 | 1 |
0.91 (0.80–1.03) |
0.77 (0.66–0.90) |
< 0.001 | 1 |
0.96 (0.83–1.11) |
0.82 (0.68–0.98) |
< 0.001 | 1 |
0.77 (0.60–0.98) |
0.61 (0.44–0.85) |
< 0.001 |
| Oxidative stress reduction (B2*B6) | ||||||||||||
| Model 11 | 1 |
0.89 (0.80–1.00) |
0.88 (0.79–0.98) |
< 0.001 | 1 |
0.92 (0.81–1.06) |
0.94 (0.83–1.07) |
< 0.001 | 1 |
0.82 (0.65–1.02) |
0.73 (0.58–0.92) |
< 0.001 |
| Model 22 | 1 |
0.86 (0.75–0.98) |
0.80 (0.75–0.98) |
< 0.001 | 1 |
0.90 (0.77–1.04) |
0.87 (0.71–1.07) |
< 0.001 | 1 |
0.74 (0.56–0.97) |
0.60 (0.42–0.86) |
< 0.001 |
| DNA stability (B9*B12) | ||||||||||||
| Model 11 | 1 |
0.89 (0.80–1.00) |
0.85 (0.75–0.95) |
< 0.001 | 1 |
0.90 (0.79–1.02) |
0.90 (0.78–1.03) |
< 0.001 | 1 |
0.90 (0.72–1.13) |
0.76 (0.60–0.96) |
< 0.001 |
| Model 22 | 1 |
0.88 (0.78–0.99) |
0.82 (0.71–0.95) |
< 0.001 | 1 |
0.88 (0.76–1.01) |
0.86 (0.73–1.03) |
< 0.001 | 1 |
0.89 (0.70–1.13) |
0.73 (0.55–0.98) |
< 0.001 |
| Vitamin B complex (B1*B2*B3*B6*B9*B12) | ||||||||||||
| Model 11 | 1 |
0.93 (0.83–1.04) |
0.89 (0.79–1.01) |
< 0.001 | 1 |
0.94 (0.83–1.07) |
0.91 (0.78–1.05) |
< 0.001 | 1 |
0.90 (0.71–1.13) |
0.88 (0.70–1.10) |
< 0.001 |
| Model 22 | 1 |
0.92 (0.82–1.02) |
0.87 (0.76–0.99) |
< 0.001 | 1 |
0.93 (0.82–1.06) |
0.89 (0.76–1.04) |
< 0.001 | 1 |
0.89 (0.70–1.12) |
0.87 (0.69–1.09) |
< 0.001 |
MIBC Muscle-Invasive Bladder Cancer, BLEND BLadder cancer Epidemiology and Nutritional Determinants
1Adjusted for sex, age, and smoking status
2Adjusted for sex, age, smoking status and water
Among men, a decreased MIBC risk was observed for high intake of vitamin B3 (HRB3: 0.87, 95% CI: 0.77–1.00) (Table 4).
Among women, a decreased MIBC risk was observed for moderate intake of vitamin B3 and B6 (HRB3: 0.78, 95% CI: 0.63–0.97, HRB6: 0.79, 95% CI: 0.63–0.98) and for high intakes of vitamin B1, B3, B6, B9, vitamins related to energy metabolism, oxidative stress reduction and DNA stability (HRB1: 0.70, 95% CI: 0.55–0.88, HRB3: 0.63, 95% CI: 0.50–0.82, HRB6: 0.71, 95% CI: 0.57–0.90, HRB9: 0.77, 95% CI: 0.60–0.98, HRenergy metabolism: 0.69, 95% CI: 0.55–0.88, HRoxidative stress: 0.73, 95% CI: 0.589–0.92, HRDNA stability: 0.76, 95% CI: 0.60–0.96) (Table 4).
NMIBC
Overall, no significant association was observed between dietary vitamin B intake and NNMIBC risk (Table 5).
Table 5.
Hazard ratios and 95% confidence intervals for NMIBC according to dietary B group vitamin intake in the BLEND study
| Overall (n = 532,271) | Men (n = 171,249) | Women (n = 361,022) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Moderate | High | P | Low | Moderate | High | P | Low | Moderate | High | p | |
| Thiamin (B1) | ||||||||||||
| Model 11 | 1 |
1.06 (0.95–1.17) |
1.02 (0.92–1.13) |
< 0.001 | 1 |
1.09 (0.96–1.23) |
1.10 (0.98–1.24) |
< 0.001 | 1 |
1.00 (0.83–1.21) |
0.79 (0.64–0.97) |
< 0.001 |
| Model 22 | 1 |
1.10 (0.99–1.23) |
1.12 (0.98–1.28) |
< 0.001 | 1 |
1.11 (0.97–1.26) |
1.17 (1.00–1.37) |
< 0.001 | 1 |
1.11 (0.91–1.37) |
0.98 (0.74–1.28) |
< 0.001 |
| Riboflavin (B2) | ||||||||||||
| Model 11 | 1 |
1.03 (0.93–1.14) |
0.96 (0.86–1.06) |
< 0.001 | 1 |
1.09 (0.96–1.23) |
1.02 (0.91–1.15) |
< 0.001 | 1 |
0.89 (0.74–1.08) |
0.80 (0.65–0.98) |
< 0.001 |
| Model 22 | 1 |
1.05 (0.94–1.18) |
1.01 (0.88–1.17) |
< 0.001 | 1 |
1.08 (0.95–1.23) |
1.01 (0.85–1.20) |
< 0.001 | 1 |
1.00 (0.81–1.24) |
1.02 (0.76–1.36) |
< 0.001 |
| Niacin (B3) | ||||||||||||
| Model 11 | 1 |
0.97 (0.87–1.07) |
0.95 (0.86–1.05) |
< 0.001 | 1 |
0.99 (0.87–1.12) |
1.03 (0.92–1.16) |
< 0.001 | 1 |
0.94 (0.78–1.13) |
0.73 (0.59–0.90) |
< 0.001 |
| Model 22 | 1 |
0.98 (0.88–1.10) |
0.99 (0.87–1.13) |
< 0.001 | 1 |
0.99 (0.87–1.13) |
1.04 (0.90–1.21) |
< 0.001 | 1 |
1.00 (0.82–1.22) |
0.83 (0.64–1.08) |
< 0.001 |
| Pyridoxine (B6) | ||||||||||||
| Model 11 | 1 |
0.95 (0.85–1.05) |
0.91 (0.82–1.00) |
< 0.001 | 1 |
0.97 (0.86–1.09) |
1.00 (0.89–1.12) |
< 0.001 | 1 |
0.89 (0.74–1.08) |
0.69 (0.56–0.84) |
< 0.001 |
| Model 22 | 1 |
0.95 (0.85–1.06) |
0.91 (0.78–1.07) |
< 0.001 | 1 |
0.96 (0.84–1.10) |
0.98 (0.81–1.18) |
< 0.001 | 1 |
0.92 (0.74–1.15) |
0.74 (0.54–1.01) |
< 0.001 |
| Folate (B9) | ||||||||||||
| Model 11 | 1 |
1.02 (0.92–1.13) |
0.97 (0.88–1.08) |
< 0.001 | 1 |
1.07 (0.95–1.21) |
1.04 (0.92–1.17) |
< 0.001 | 1 |
0.90 (0.75–1.09) |
0.79 (0.64–0.98) |
< 0.001 |
| Model 22 | 1 |
1.04 (0.93–1.16) |
1.02 (0.91–1.15) |
< 0.001 | 1 |
1.08 (0.95–1.22) |
1.06 (0.92–1.21) |
< 0.001 | 1 |
0.97 (0.79–1.18) |
0.91 (0.71–1.15) |
< 0.001 |
| Cyanocobalamin (B12) | ||||||||||||
| Model 11 | 1 |
0.91 (0.83–1.00) |
0.94 (0.84–1.04) |
< 0.001 | 1 |
0.89 (0.80–1.00) |
1.01 (0.89–1.15) |
< 0.001 | 1 |
0.98 (0.80–1.19) |
0.80 (0.65–0.97) |
< 0.001 |
| Model 22 | 1 |
0.92 (0.83–1.02) |
0.96 (0.85–1.09) |
< 0.001 | 1 |
0.88 (0.79–1.00) |
1.00 (0.86–1.15) |
< 0.001 | 1 |
1.03 (0.84–1.26) |
0.91 (0.72–1.15) |
< 0.001 |
| Energy metabolism (B1*B2*B3) | ||||||||||||
| Model 11 | 1 |
1.04 (0.93–1.15) |
0.98 (0.88–1.08) |
< 0.001 | 1 |
1.09 (0.97–1.23) |
1.06 (0.94–1.19) |
< 0.001 | 1 |
0.92 (0.76–1.11) |
0.77 (0.62–0.94) |
< 0.001 |
| Model 22 | 1 |
1.07 (0.96–1.19) |
1.05 (0.91–1.20) |
< 0.001 | 1 |
1.10 (0.97–1.25) |
1.09 (0.93–1.28) |
< 0.001 | 1 |
1.01 (0.82–1.25) |
0.93 (0.70–1.23) |
< 0.001 |
| Oxidative stress reduction (B2*B6) | ||||||||||||
| Model 11 | 1 |
0.98 (0.89–1.09) |
0.91 (0.83–1.01) |
< 0.001 | 1 |
1.04 (0.92–1.17) |
1.00 (0.89–1.13) |
< 0.001 | 1 |
0.87 (0.72–1.05) |
0.69 (0.57–0.85) |
< 0.001 |
| Model 22 | 1 |
0.99 (0.88–1.11) |
0.92 (0.79–1.08) |
< 0.001 | 1 |
1.03 (0.90–1.17) |
0.98 (0.82–1.18) |
< 0.001 | 1 |
0.91 (0.72–1.13) |
0.75 (0.55–1.03) |
< 0.001 |
| DNA stability (B9*B12) | ||||||||||||
| Model 11 | 1 |
0.98 (0.89–1.08) |
0.95 (0.85–1.05) |
< 0.001 | 1 |
0.98 (0.87–1.10) |
1.02 (0.90–1.16) |
< 0.001 | 1 |
0.99 (0.82–1.20) |
0.77 (0.63–0.95) |
< 0.001 |
| Model 22 | 1 |
1.00 (0.90–1.11) |
0.99 (0.87–1.13) |
< 0.001 | 1 |
0.98 (0.87–1.11) |
1.04 (0.89–1.21) |
< 0.001 | 1 |
1.05 (0.86–1.28) |
0.88 (0.69–1.14) |
< 0.001 |
| Vitamin B complex (B1*B2*B3*B6*B9*B12) | ||||||||||||
| Model 11 | 1 |
1.01 (0.92–1.11) |
0.92 (0.83–1.03) |
< 0.001 | 1 |
1.03 (0.92–1.15) |
0.93 (0.81–1.07) |
< 0.001 | 1 |
0.95 (0.78–1.16) |
0.90 (0.74–1.10) |
< 0.001 |
| Model 22 | 1 |
0.99 (0.90–1.10) |
0.90 (0.81–1.01) |
< 0.001 | 1 |
1.03 (0.92–1.15) |
0.92 (0.80–1.06) |
< 0.001 | 1 |
0.93 (0.76–1.14) |
0.91 (0.74–1.11) |
< 0.001 |
NMIBC Non-Muscle Invasive Bladder Cancer, BLEND BLadder cancer Epidemiology and Nutritional Determinants
1Adjusted for sex, age, and smoking status
2Adjusted for sex, age, smoking status and water
Among men, moderate intake of vitamin B12 reduced the risk of NMIBC (HRB12: 0.89, 95% CI: 0.80–1.00) (Table 5).
Among women, high intake of the vitamins B1, B2, B3, B6, B9, B12 and vitamins related to energy metabolism, oxidative stress reduction and DNA stability decreased NMIBC risk (HRB1: 0.79, 95% CI: 0.64–0.97, HRB2: 0.80, 95% CI: 0.65–0.98, HRB3: 0.73, 95% CI: 0.59–0.90, HRB6: 0.69, 95% CI: 0.56–0.85, HRB9: 0.79, 95% CI: 0.64–0.98, HRB12: 0.80, 95% CI: 0.65–0.97, HRBenergy metabolism: 0.77, 95% CI: 0.62–0.94, HRBoxidative stress: 0.69, 95% CI: 0.57–0.85, HRBDNA stability: 0.77, 95% CI: 0.63–0.95) (Table 5).
Removal of early BC cases
After removing BC cases diagnosed within the first 5 years after recruitment, similar increased BC risks were observed (Supplementary Table 1).
Dose–response analyses
Linear associations
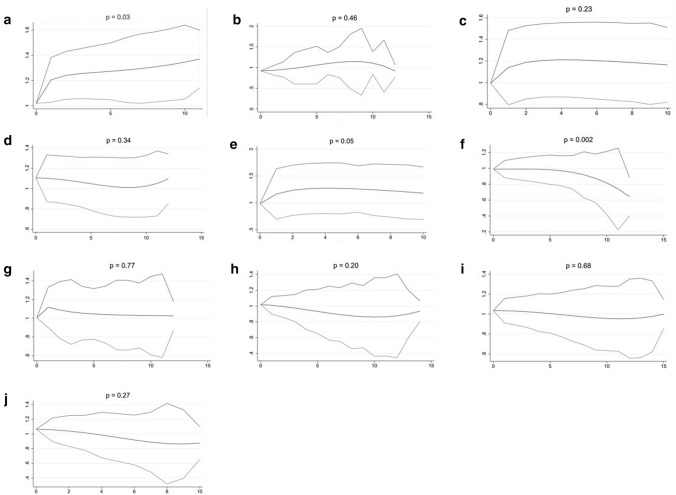
Overall dose–response curves are shown in Fig. 1. A slightly increased BC risk was observed for higher intake of vitamin B1 (p = 0.03) and a decreased risk for higher intake of vitamin B12 (p = 0.002). No other compound or combination showed a significant association with BC risk.
Fig. 1.
Dose–response analyses (x-axis: dose (defined per vitamin or vitamin group, see below), y-axis: HR for BC, with 95% confidence interval); a thiamin (B1) (dose = 300 µg), b riboflavin (B2) (dose = 800 µg), c niacin (B3) (dose = 2500 µg), d pyridoxine (B6) (dose = 300 µg), e folate (B9 (dose = 30 µg)), f cyanocobalamin (B12) (dose = 1.5 µg), g vitamins related to energy metabolism (dose = 1.25 × 1011 µg), h vitamins related to the reduction of oxidative stress (dose = 5.0 × 106 µg), i vitamins related to DNA stability (dose = 200 µg) and j the entire B group vitamin complex (dose = 1.0 × 1016 µg)
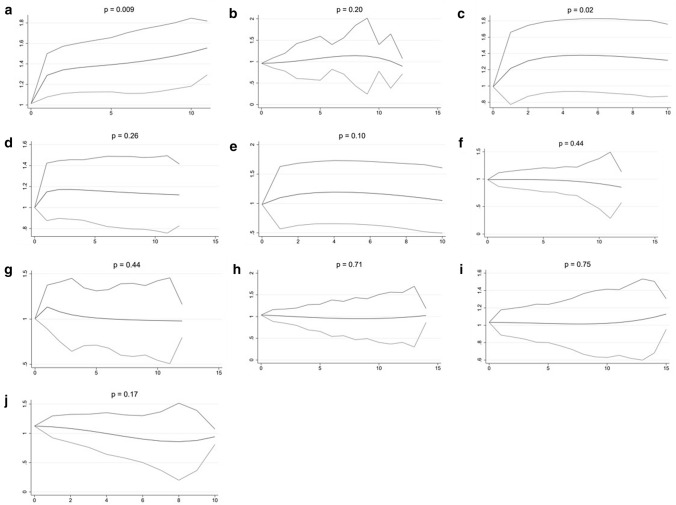
For men, a significant increased BC risk was observed for high intake of the vitamins B1 (p = 0.009) and B3 (p = 0.02) (Fig. 2).
Fig. 2.
Dose–response analyses for men (x-axis: dose (defined per vitamin or vitamin group, see below), y-axis: HR for BC, with 95% confidence interval); a thiamin (B1) (dose = 300 µg), b riboflavin (B2) (dose = 800 µg), c niacin (B3) (dose = 2500 µg), d pyridoxine (B6) (dose = 300 µg), e Folate (B9 (dose = 30 µg)), f cyanocobalamin (B12) (dose = 1,5 µg), g vitamins related to energy metabolism (dose = 1.25 × 1011 µg), h vitamins related to the reduction of oxidative stress (dose = 5.0 × 106 µg), i vitamins related to DNA stability (dose = 200 µg) and j the entire B group vitamin complex (dose = 1.0 × 1016 µg)
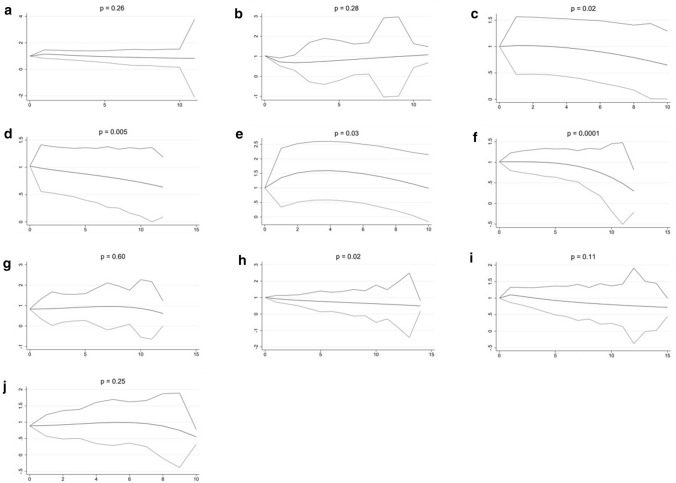
Among women, significant decreased risks were observed for high intake of the vitamins B3 (p = 0.02), B6 (p = 0.005), B9 (p = 0.03), B12 (p = 0.0001) and for the vitamins related to the reduction of oxidative stress (p = 0.02) (Fig. 3).
Fig. 3.
Dose–response analyses for women (x-axis: dose (defined per vitamin or vitamin group, see below), y-axis: HR for BC, with 95% confidence interval); a thiamin (B1) (dose = 300 µg), b riboflavin (B2) (dose = 800 µg), c niacin (B3) (dose = 2500 µg), d pyridoxine (B6) (dose = 300 µg), e folate (B9 (dose = 30 µg)), f cyanocobalamin (B12) (dose = 1.5 µg), g vitamins related to energy metabolism (dose = 1.25 × 1011 µg), h vitamins related to the reduction of oxidative stress (dose = 5.0 × 106 µg), i vitamins related to DNA stability (dose = 200 µg) and j the entire B group vitamin complex (dose = 1.0 × 1016 µg)
Discussion
The present study showed a slightly increased BC risk for moderate vitamin B1 consumption. In men, moderate intake of the vitamins B1, B2, and the vitamins related to energy metabolism and higher intake of vitamin B1 were associated with an increased BC risk, while in women, higher intake of all vitamins and vitamin combinations, except for the entire B group vitamin complex, showed an inverse association.
B group vitamins have essential roles in preventing transmission from a normal cell to a malignant cancer cell, by involving into the pathways of energy metabolism, oxidative stress reduction, and methylation regulation [39, 40].
B group vitamins and energy metabolism
Experimental studies show that vitamin B1 is needed for the metabolism of glucose [41], thereby delivering the fuel of our cells [42]. Contrary, the present study showed a slight increased BC risk for moderate and high vitamin B1 intake among male, while among female high intake was associated with a decreased BC risk. No previous observational studies were conducted on the influence of vitamin B1 on BC risk, nonetheless, several attempts have been made to correlate vitamin B1 intake to other cancer types. However, results remain inconclusive [43, 44]. A possible explanation for the discrepant findings between male and female might be the different source from which participants retrieved their vitamin B1. While male mainly retrieved their vitamin B1 intake from meat consumption, the main vitamin B1 source for female are vegetables (Supplementary Table 2). Meat consumption has previously been associated with an increased BC risk [45–50], while high vegetable consumption showed a decreased BC risk [9, 10]. Moreover, after adjustment of the main food resources (i.e. meat and vegetables) results for men and women were similar and did not reach statistical significance, thereby strengthening the hypothesis that indeed the different food source might explain the observed gender difference. However, future research is needed to further clarify this gender difference.
Vitamin B2 is a coenzyme for many metabolic processes in the body [18, 41]. Although this suggests an inverse effect of vitamin B2 on BC risk, in current observational studies this effect is controversial [51]. The present study also shows conflicting results; among men moderate vitamin B2 intake showed an increased BC risk, while among women a decreased risk was observed. This gender difference might be explained by the source. It is expected that men mainly derive vitamin B2 from meat, while women might derive it mainly from other sources. This is confirmed by the higher vitamin B2 meat-derived intake in men compared to women (Mmeat-B2 men: 0.70 mg, SD = 0.63, Mmeat-B2 women: 0.55 mg, SD = 0.50, t = 90.96, p < 0.001) (Supplementary Table 2). Meat has previously been associated with an increased BC risk due to its pro-carcinogenic components [45–50], which could abolish the positive effect of vitamin B2. However, adjustment for the main food sources did not significantly change the findings. Therefore, future research is needed to clarify this gender difference.
Vitamin B3 plays a role in energy metabolism, which is essential to maintain cellular metabolism and respiration [52] and it is important for genetic and epigenetic regulators [52]. Studies of the consequences of DNA damage in cultured mouse and human cells as a function of vitamin B3 status have supported the hypothesis that vitamin B3 may be a protective factor in limiting carcinogenic events [53]. In line with this, we observed a decreased BC risk for high dietary vitamin B3 intake among women. Since meat is a rich source of vitamin B3 [54], this again could explain the observed gender differences. In our study, men retrieved on average more vitamin B3 from meat than women (Mmeat-B3, men: 17.83 mg, SD = 15.71, Mmeat-B3 women: 14.54 mg, SD = 13.44, t = 78.49, p < 0.001) (Supplementary Table 2). However, adjustment for the main food sources did not significantly change the findings. Therefore, future research is needed to clarify this gender difference. When analyzing the effect of the vitamins B1, B2 and B3 (i.e. the vitamins related to energy metabolism) together, moderate intake among men showed an increased risk on BC and high intake a decreased risk among women.
B group vitamins and oxidative stress
Oxidative stress is an imbalance in the cell which leads to DNA damage [55], thereby increasing cancer risk [39]. Experimental studies showed that the vitamins B6 and B2 are involved in oxidative stress reduction by catalyzing regulatory enzymes [55, 56]. Results of the present study confirm these findings by showing an inverse association of high intake of vitamin B6 on BC risk among women, in line with a previously conducted cohort study [23].
B group vitamins and DNA stability
Vitamin B9 plays a pivotal role in cell metabolism which is necessary for DNA synthesis and repair, as well as for methylation [57]. Previous epidemiological studies and a meta-analysis confirm this protective role of vitamin B9 in the development of BC [14]. The present study also shows a reduced BC risk among women (for both vitamin B9 separate as well as combined with vitamin B12). In men, this protective effect could not be observed. This is possibly the result of the fact that men are less responsive to vitamin B9 than women, due to a lower body mass in which the folate dose distributes over a larger volume [58]. The difference might also be explained by androgens, which are involved in one-carbon metabolism [59]. In our study, men did consume on average more vitamin B9 than women (MB9, men: 194.27 µg, SD = 128.66, MB9 women: 182.12 µg, SD = 96.28, t = 37.76, p < 0.001).
Vitamin B12 is essential for the reduction of DNA damage [60]. Experimental studies show that vitamin B12 deficiency mimics radiation damage to DNA, possibly leading to cancer [61]. However, observational studies showed no effect of high intake of vitamin B12 on BC risk [62]. The present study only observed a significant protective effect of high vitamin B12 intake among women. The main sources of vitamin B12 is meat [54]. Since meat is mainly consumed by men (MB12, meat men: 3.86 µg, SD = 4.30, MB12 meat women: 3.35 µg, SD = 3.85, t = 43.03, p < 0.001) (Supplementary Table 2), it might be argued that the observed effect in men are caused by the earlier mentioned negative effects of other (micro)nutrients in meat [63]. However, adjustment for the main food sources did not significantly change the findings. Therefore, future research is needed to clarify this gender difference.
Limitations
Although BLEND is one of the largest known pooled cohort studies investigating the association between dietary B group vitamin intake and the risk of developing BC, allowing for detailed analyses with enough statistical power, it has several limitations. First, limited information was available for possible BC risk factors, such as body mass index, physical activity, socioeconomic status, and occupational exposures. Nevertheless, current literature shows only a small proportion of BC cases can be attributed to these factors [23, 64–66]. In addition, no information was available on comorbidities that may make people alter their diet [67], or of which the drugs may influence the bioavailability of B group vitamins in the body [68]. At last, information on alcohol was lacking which might influence B vitamins’ absorption [56].
A second limitation arises from the use of FFQs, which could lead to recall bias, systematic and random error when estimating vitamin intake. However, since the dietary intake of all included studies were validated, recall bias has likely only played a minor role. In addition, measurement error could be negligible, considering our large sample size.
Thirdly, although people are less likely to change their dietary habits at an older age, most of the included studies only measured their participants at baseline and we were, therefore, unable to take possible changes of dietary habits over time into account. This could have led to misclassification of long-term exposure [69]. However, the included NLCS study repeated the questionnaire 5 year after baseline, and showed only a minor decline in average intake for all food items [35].
Fourthly, most of the included studies did not provide information on supplement use. Therefore, we were unable to take supplemental vitamin intake into account, which may have led to an underestimation of the true effect of B group vitamins.
Fifthly, a single database was used for the conversion of food into nutrient intake. Since the food composition of similar food items and the food fortification may differ between different countries, the use of country specific food composition tables might be more accurate. Previous studies, however, showed that the use of a common food composition database advantages over the use of country-specific food composition databases in that errors are consistent between the countries [70]. In addition, all our main regression analyses were study centre stratified.
Besides, results obtained from cohort studies on diet and cancer risk cannot always rule out the possibility of reversed causality. Since there is no evidence that people are likely to alter their diet in the period before BC diagnosis, we decided to not exclude study participants who received a BC diagnosis within a short period of follow-up.
Finally, in view of multiple testing, it could indeed be debated whether, for instance, Bonferroni p value adjustments should have been applied. However, it previously has been argued that the use of Bonferroni p value adjustments is impractical and likely too conservative when testing a priori hypotheses [71]. Since we were able to formulate plausible a priori hypotheses regarding all the included analyses, based on data from previous studies, we did not apply Bonferroni correction in our analyses. Moreover, if we had adjusted for the number of main analyses being performed (n = 10) the significance level would have been 0.005. In that case, most of the observed associations between the vitamin B intake and BC risk would still be statistically significant.
Conclusion
The present study showed a slight increased BC risk for moderate intake of vitamin B1. In men, moderate intake of the vitamins B1, B2 and the vitamins related to energy metabolism and high intake of vitamin B1 were found to be associated with an increased BC risk. In women, high intake of all vitamins and vitamin combinations, except for the entire complex, showed to have a protective effect. These findings may be helpful for informing BC prevention strategies. It should be noted, however, that dietary recommendations on vitamin B intake carefully consider the food sources from which this nutrient is retrieved. In addition, future studies should focus on nutritional patterns and look deeper into B group vitamins’ interactions with other nutrients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We gratefully acknowledge all principal investigators for their willingness to participate in this joined project [4].
Author contributions
Study conception and design: AW, IB and MB; analyses and interpretation of data: IB, AW and EYY; drafting of the manuscript: IB and AW; revised the manuscript: AW, MB, MPZ and EYY; provided the data: PvdB, EG, EW, EW, PF, HF, MS, BBM, MJS, and BG; approved the manuscript: all authors.
Funding
This work was partly funded by the World Cancer Research Fund International (WCRF 2012/590) and European Commission (FP7-PEOPLE-618308). The Netherlands Cohort Study on diet and cancer was supported by the Dutch Cancer Society. The RERF atomic bomb survivors Study was supported by The Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan, a public interest foundation funded by the Japanese Ministry of Health, Labour and Welfare (MHLW) and the US Department of Energy (DOE). The research was also funded in part through DOE award DE-HS0000031 to the National Academy of Sciences. This publication was supported by RERF Research Protocol RP-A5-12. The VITamins and Lifestyle Study (VITAL) was supported by a grant (R01CA74846) from the National Cancer Institute. The European Prospective Investigation into Cancer and Nutrition (EPIC) was carried out with financial support of the ‘Europe Against Cancer’ Programme of the European Commission (SANCO); Ligue contre le Cancer (France); Société 3 M (France); Mutuelle Générale de l’Éducation Nationale; Institut National de la Santé et de la Recherche Médicale (INSERM); Institute Gustave Roussy; German Cancer Aid; German Cancer Research Centre; German Federal Ministry of Education and Research; Danish Cancer Society; Health Research Fund (FIS) of the Spanish Ministry of Health; the Spanish Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra; Cancer Research UK; Medical Research Council, UK; Stroke Association, UK; British Heart Foundation; Department of Health, UK; Food Standards Agency, UK; Wellcome Trust, UK; Greek Ministry of Health; Greek Ministry of Education; Italian Association for Research on Cancer; Italian National Research Council; Dutch Ministry of Public Health, Welfare and Sports; Dutch Prevention Funds; LK Research Funds; Dutch ZON (Zorg Onderzoek Nederland); World Cancer Research Fund; Swedish Cancer Society; Swedish Scientific Council; Regional Government of Skane, Sweden; Norwegian Cancer Society; Norwegian Research Council. Partial support for the publication of this supplement was provided by the Centre de Recherche et d’Information Nutritionnelles (CERIN).
Availability of data and material
The paper uses data of the BLEND study [25]. The data and code that support the findings of this study are available on reasonable request pending approval from the corresponding author, AW.
Declarations
Conflict of interest
All the authors declare no conflict of interest.
Ethical approval
Each participating study has been approved by the local ethic committee.
Consent to participate
Informed consent was obtained from all individual participants included in each study.
Consent for publication
All authors consent to publish this paper.
References
- 1.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018 doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Sievert KD, et al. Economic aspects of bladder cancer: what are the benefits and costs? World J Urol. 2009 doi: 10.1007/s00345-009-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cumberbatch MG, Noon AP. Epidemiology, aetiology and screening of bladder cancer. Transl Androl Urol. 2019;8(1):5–11. doi: 10.21037/tau.2018.09.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schabath MB, et al. Case-control analysis of dietary folate and risk of bladder cancer. Nutr Cancer. 2005 doi: 10.1207/s15327914nc5302_3. [DOI] [PubMed] [Google Scholar]
- 5.Al-Zalabani AH, et al. Modifiable risk factors for the prevention of bladder cancer: a systematic review of meta-analyses. Eur J Epidemiol. 2016 doi: 10.1007/s10654-016-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Osch FH, et al. Quantified relations between exposure to tobacco smoking and bladder cancer risk: a meta-analysis of 89 observational studies. Int J Epidemiol. 2016 doi: 10.1093/ije/dyw044. [DOI] [PubMed] [Google Scholar]
- 7.Lenis AT, et al. Bladder cancer: a review. JAMA. 2020 doi: 10.1001/jama.2020.17598. [DOI] [PubMed] [Google Scholar]
- 8.Research, W.C.R.F.A.I.f.C., Continuous update project expert report 2018. Diet, nutrition, physical activity and bladder cancer. https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018.pdf
- 9.Acham M, et al. Intake of milk and other dairy products and the risk of bladder cancer: a pooled analysis of 13 cohort studies. Eur J Clin Nutr. 2019 doi: 10.1038/s41430-019-0453-6. [DOI] [PubMed] [Google Scholar]
- 10.Park S-Y, et al. Fruit and vegetable intakes are associated with lower risk of bladder cancer among women in the Multiethnic Cohort Study. J Nutr. 2013 doi: 10.3945/jn.113.174920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu EYW, et al. Grain and dietary fiber intake and bladder cancer risk: a pooled analysis of prospective cohort studies. Am J Clin Nutr. 2020 doi: 10.1093/ajcn/nqaa215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dianatinasab M, et al. The association between meat and fish consumption and bladder cancer risk: a pooled analysis of 11 cohort studies. Eur J Epidemiol. 2021 doi: 10.1007/s10654-021-00762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao A, et al. Optimal nutrition and the ever-changing dietary landscape: a conference report. Eur J Nutr. 2017 doi: 10.1007/s00394-017-1460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He H, Shui B. Folate intake and risk of bladder cancer: a meta-analysis of epidemiological studies. Int J Food Sci Nutr. 2014 doi: 10.3109/09637486.2013.866641. [DOI] [PubMed] [Google Scholar]
- 15.Depeint F, et al. Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chem Biol Interact. 2006 doi: 10.1016/j.cbi.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Winter SL, Boyer JL. Hepatic toxicity from large doses of vitamin B3 (nicotinamide) N Engl J Med. 1973 doi: 10.1056/NEJM197311292892208. [DOI] [PubMed] [Google Scholar]
- 17.Zastre JA, et al. Linking vitamin B1 with cancer cell metabolism. Cancer Metab. 2013 doi: 10.1186/2049-3002-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rank O (2007) Vitamin B2 (Riboflavin). The Health Professional's Guide to Dietary Supplements. p 103. https://ods.od.nih.gov/factsheets/Riboflavin-HealthProfessional/
- 19.Zhang X-H, et al. Vitamin B6 and colorectal cancer: current evidence and future directions. World J Gastroenterol. 2013 doi: 10.3748/wjg.v19.i7.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyon P, et al. B Vitamins and one-carbon metabolism: implications in human health and disease. Nutrients. 2020 doi: 10.3390/nu12092867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenech M. Folate (vitamin B9) and vitamin B12 and their function in the maintenance of nuclear and mitochondrial genome integrity. Mutat Res. 2012 doi: 10.1016/j.mrfmmm.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 22.García-Closas R, et al. Food, nutrient and heterocyclic amine intake and the risk of bladder cancer. Eur J Cancer. 2007 doi: 10.1016/j.ejca.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Dugué PA, et al. Dietary intake of nutrients involved in one-carbon metabolism and risk of urothelial cell carcinoma: a prospective cohort study. Int J Cancer. 2018 doi: 10.1002/ijc.31319. [DOI] [PubMed] [Google Scholar]
- 24.Wu J, et al. Dietary intake of meat, fruits, vegetables, and selective micronutrients and risk of bladder cancer in the New England region of the United States. Br J Cancer. 2012 doi: 10.1038/bjc.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goossens ME, et al. International pooled study on diet and bladder cancer: the bladder cancer, epidemiology and nutritional determinants (BLEND) study: design and baseline characteristics. Arch Public Health. 2016 doi: 10.1186/s13690-016-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Brandt PA, et al. A large-scale prospective cohort study on diet and cancer in the Netherlands. J Clin Epidemiol. 1990 doi: 10.1016/0895-4356(90)90009-e. [DOI] [PubMed] [Google Scholar]
- 27.Ozasa K, et al. Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res. 2011 doi: 10.1667/rr2629.1. [DOI] [PubMed] [Google Scholar]
- 28.White E, et al. VITamins and lifestyle cohort study: study design and characteristics of supplement users. Am J Epidemiol. 2004 doi: 10.1093/aje/kwh010. [DOI] [PubMed] [Google Scholar]
- 29.Riboli E, et al. European prospective investigation into cancer and nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002 doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 30.Riboli E, Kaaks R. The EPIC Project: rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997 doi: 10.1093/ije/26.suppl_1.s6. [DOI] [PubMed] [Google Scholar]
- 31.Satia-Abouta J, et al. Reliability and validity of self-report of vitamin and mineral supplement use in the vitamins and lifestyle study. Am J Epidemiol. 2003 doi: 10.1093/aje/kwg039. [DOI] [PubMed] [Google Scholar]
- 32.Sauvaget C, et al. Validation of a food frequency questionnaire in the Hiroshima/Nagasaki Life Span Study. J Epidemiol. 2002 doi: 10.2188/jea.12.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeegers M, Goldbohm R, Van Den Brandt P. Are retinol, vitamin C, vitamin E, folate and carotenoids intake associated with bladder cancer risk? Results from the Netherlands Cohort Study. Br J Cancer. 2001 doi: 10.1054/bjoc.2001.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrari P, et al. Evaluation of under- and overreporting of energy intake in the 24-hour diet recalls in the European Prospective Investigation into Cancer and Nutrition (EPIC) Public Health Nutr. 2002 doi: 10.1079/PHN2002409. [DOI] [PubMed] [Google Scholar]
- 35.Goldbohm RA, et al. Reproducibility of a food frequency questionnaire and stability of dietary habits determined from five annually repeated measurements. Eur J Clin Nutr. 1995;49(6):420–429. [PubMed] [Google Scholar]
- 36.US Department of Agriculture, A.R.S., Nutrient Data Laboratory, USDA National Nutrient Database for Standard Reference, Release 27 (Slightly Revised). 2015, US Department of Agriculture Washington, DC
- 37.Ahuja JK, et al. USDA food and nutrient databases provide the infrastructure for food and nutrition research, policy, and practice. J Nutr. 2013 doi: 10.3945/jn.112.170043. [DOI] [PubMed] [Google Scholar]
- 38.Kassambara A. Cox Model Assumptions. http://www.sthda.com/english/wiki/cox-model-assumptions. Accessed 30 June 2020
- 39.Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. NatRev Genet. 2006 doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 40.Unknown, Cancer Research Product Guide. 3 ed. unknown: Tocris
- 41.Bryd-Bredbenner C, Beshgetoor D, Moe G. Wardlaw's perspectives in Nutrition (Eight Edition) Abebooks; 2007. [Google Scholar]
- 42.Beck K (2019) What happens when glucose enters a cell? https://sciencing.com/happens-glucose-enters-cell-5158995.html. Accessed 30 June 2020
- 43.Kabat G, et al. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008 doi: 10.1038/sj.bjc.6604540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaul L, et al. The role of diet in prostate cancer. Nutr Cancer. 1987 doi: 10.1080/01635588709513919. [DOI] [PubMed] [Google Scholar]
- 45.Genkinger JM, Koushik A. Meat consumption and cancer risk. PLoS Med. 2007 doi: 10.1371/journal.pmed.0040345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito N, et al. A new colon and mammary carcinogen in cooked food, 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP) Carcinogenesis. 1991 doi: 10.1093/carcin/12.8.1503. [DOI] [PubMed] [Google Scholar]
- 47.Joosen AM, et al. Effect of processed and red meat on endogenous nitrosation and DNA damage. Carcinogenesis. 2009 doi: 10.1093/carcin/bgp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santarelli RL, et al. Meat processing and colon carcinogenesis: cooked, nitrite-treated, and oxidized high-heme cured meat promotes mucin-depleted foci in rats. Cancer Prev Res (Phila) 2010 doi: 10.1158/1940-6207.CAPR-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tricker A. N-nitroso compounds and man: sources of exposure, endogenous formation and occurrence in body fluids. Eur J Cancer Prev. 1997;6(3):226–268. doi: 10.1097/00008469-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Crippa A, et al. Red and processed meat consumption and risk of bladder cancer: a dose–response meta-analysis of epidemiological studies. Eur J Nutr. 2018 doi: 10.1007/s00394-016-1356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr. 2003 doi: 10.1093/ajcn/77.6.1352. [DOI] [PubMed] [Google Scholar]
- 52.Kirkland JB, Meyer-Ficca ML. Niacin. Adv Food Nutr Res. 2018 doi: 10.1016/bs.afnr.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Jacobson EL. Niacin deficiency and cancer in women. J Am Coll Nutr. 1993 doi: 10.1080/07315724.1993.10718330. [DOI] [PubMed] [Google Scholar]
- 54.Subar AF, et al. Dietary sources of nutrients among US adults, 1989 to 1991. J Am Diet Assoc. 1998 doi: 10.1016/S0002-8223(98)00122-9. [DOI] [PubMed] [Google Scholar]
- 55.Ashoori M, Saedisomeolia A. Riboflavin (vitamin B 2) and oxidative stress: a review. Br J Nutr. 2014 doi: 10.1017/S0007114514000178. [DOI] [PubMed] [Google Scholar]
- 56.Shen J, et al. Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: the Boston Puerto Rican Health Study. Am J Clin Nutr. 2010 doi: 10.3945/ajcn.2009.28571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stanisławska-Sachadyn A, et al. Folate/homocysteine metabolism and lung cancer risk among smokers. PLoS ONE. 2019 doi: 10.1371/journal.pone.0214462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winkels RM, et al. Gender and body size affect the response of erythrocyte folate to folic acid treatment. J Nutr. 2008 doi: 10.1093/jn/138.8.1456. [DOI] [PubMed] [Google Scholar]
- 59.Corbin JM, Ruiz-Echevarría MJ. One-carbon metabolism in prostate cancer: the role of androgen signaling. Int J Mol Sci. 2016 doi: 10.3390/ijms17081208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zingg J-M, Jones PA. Genetic and epigenetic aspects of DNA methylation on genome expression, evolution, mutation and carcinogenesis. Carcinogenesis. 1997 doi: 10.1093/carcin/18.5.869. [DOI] [PubMed] [Google Scholar]
- 61.Ames BN. DNA damage from micronutrient deficiencies is likely to be a major cause of cancer. Mutat Res. 2001 doi: 10.1016/s0027-5107(01)00070-7. [DOI] [PubMed] [Google Scholar]
- 62.Brinkman MT, et al. Minerals and vitamins and the risk of bladder cancer: results from the New Hampshire Study. Cancer Causes Control. 2010 doi: 10.1007/s10552-009-9490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Love HJ, Sulikowski D. Of meat and men: sex differences in implicit and explicit attitudes toward meat. Front Psychol. 2018 doi: 10.3389/fpsyg.2018.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burger M, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013 doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 65.Koebnick C, et al. Body mass index, physical activity, and bladder cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev. 2008 doi: 10.1158/1055-9965.EPI-08-0026. [DOI] [PubMed] [Google Scholar]
- 66.Madeb R, Messing EM. Gender, racial and age differences in bladder cancer incidence and mortality. Urol Oncol. 2004 doi: 10.1016/S1078-1439(03)00139-X. [DOI] [PubMed] [Google Scholar]
- 67.Bassett JK, et al. Dietary intake of B vitamins and methionine and prostate cancer incidence and mortality. Cancer Causes Control. 2012 doi: 10.1007/s10552-012-9954-5. [DOI] [PubMed] [Google Scholar]
- 68.Suter P, et al. Diuretic use: a risk for subclinical thiamine deficiency in elderly patients. J Nutr Health Aging. 2000;4(2):69–71. [PubMed] [Google Scholar]
- 69.Hennekens C, Buring JE. Analysis of epidemiologic studies: evaluating the role of confounding. Epidemiol Med. 1987;287:323. [Google Scholar]
- 70.Slimani N, et al. The EPIC nutrient database project (ENDB): a first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur J Clin Nutr. 2007 doi: 10.1038/sj.ejcn.1602679. [DOI] [PubMed] [Google Scholar]
- 71.Moran MD. Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos. 2003 doi: 10.1034/j.1600-0706.2003.12010.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The paper uses data of the BLEND study [25]. The data and code that support the findings of this study are available on reasonable request pending approval from the corresponding author, AW.