Abstract
This case series describes 9 patients diagnosed with myelin oligodendrocyte glycoprotein (MOG)-IgG associated disorder (MOGAD) following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Patients developed neurological symptoms between 4 days and 5 weeks following SARS-CoV-2 infection. Myelitis was observed in 4 patients; 4 presented with optic neuritis; and encephalopathy was observed in 3. Serum MOG-IgG cell-based assay was medium or high positive in each case. The majority of patients had near-complete recovery following acute immunosuppression. This series adds to the growing number of cases of central nervous system demyelination following SARS-CoV-2 infection and highlights a potential role of infection in the immunopathogenesis of MOGAD.
Keywords: MOGAD, SARS-CoV-2, COVID-19, Transverse myelitis, Optic neuritis
Abbreviations: CBA, cell-based assay; FLAIR, fluid attenuated inversion recovery; FACS, fluorescence-activated cell sorting; IVIg, intravenous immunoglobulin; IVMP, intravenous methylprednisolone; MOG, myelin oligodendrocyte glycoprotein; MOGAD, myelin oligodendrocyte glycoprotein-IgG associated disorder; MRI, magnetic resonance imaging; N/A, not available; OCBs, oligoclonal bands; ON, optic neuritis; PLEX, plasma exchange; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VA, visual acuity; WBC, white blood cells
1. Introduction
Neurological complications of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are increasingly recognized. Myelin oligodendrocyte glycoprotein (MOG)-IgG associated disorder (MOGAD) is a neuroinflammatory disorder predominantly affecting the optic nerves, brain, and spinal cord, a subset of which have a post-infectious history. (Marignier et al., 2021) Here we present 9 de novo cases of MOGAD post-SARS-CoV-2 infection.
2. Methods
Patients were individually identified from our respective neuroimmunology centers with a clinical phenotype of MOGAD and positive MOG-IgG within 6 weeks of SARS-CoV-2 infection. MOG-IgG antibodies were measured by fluorescence-activated cell sorting assay (FACS) for 6 patients, and MOG-IgG antibodies were measured by live cell-based immunofluorescence assay (CBA) for 3 patients, as described elsewhere. (Mariotto et al., 2017; Waters et al., 2019) All patients provided informed consent.
3. Results
Clinical characteristics of patients are outlined in Table 1 . Nine patients were identified (7 females, 2 males), with a median age of 44 (range 20–85) years.
Table 1.
Characteristics of patients diagnosed with MOGAD following SARS-CoV-2 infection.
| Case | Age (years)/ Gender | Comorbidities | SARS-CoV-2 Severity⁎ | Latency⁎⁎ | Neurological presentation | MRI findings | CSF findings | MOG-IgG | Treatment | Outcome | Relapsing course | Follow-up time |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21/ Female | None | Non-severe | 11 days | Acute myelitis; Brainstem syndrome | T2 hyperintensity throughout cervical and thoracic spine, periaqueductal gray matter, pons, posterior corpus callosum (Fig. 1A-C) | WBC 108 cells/mm3, protein 38 mg/dL, glucose 77 mg/dL, OCBs absent | 1:1000 † | IVMP for 5 days, PLEX for 5 sessions | Complete recovery | No | 3 months |
| 2 | 20/ Female | None | Non-severe | 2 weeks | Acute myelitis; Encephalopathy | T2 hyperintensity throughout cervical spinal cord and conus; Diffuse, poorly demarcated cortical, periventricular and juxtacortical T2 hyperintensities also involving thalamus and brainstem (Fig. 1D-F) | WBC 144 cells/mm3, protein 45 mg/dL, glucose 45 mg/dL, no unique OCBs |
1:2560 ‡ | IVMP for 5 days, oral prednisone taper | Near-complete recovery (mild residual paraparesis) |
No | 1 month |
| 3 | 30/ Female |
Major depressive disorder | Non-severe | 2 weeks | Acute myelitis | T2 hyperintensity throughout cervical spine (C2-C7), with heterogenous signal throughout the thoracic spine | WBC 68 cells/mm3, protein 46 mg/dL, glucose 55 mg/dL, OCBs absent | 1:1000 † | IVMP for 5 days | Near-complete recovery (mild ataxia, mild sensory deficit) | No | 18 months |
| 4 | 36/ Female | Hypertension | Non-severe | 3 weeks | Acute myelitis | No abnormalities | WBC 139 cells/mm3, protein 37 mg/dL, glucose 55 mg/dL, OCBs absent | 1:1000 † | IVMP for 3 days, IVIg 2 g over 5 days, maintenance IVIg | Near-complete recovery (residual bladder dysfunction) | No | 3 months |
| 5 | 57/ Male |
Parkinson's Disease | Non-severe | 2 weeks | Left ON | Left optic nerve enhancement (anterior, with perineural sheath enhancement; Fig. 1H) | WBC 1 cells/mm3, protein 30 mg/dL, glucose 58 mg/dL, OCBs absent | 1:320 † | Oral prednisone taper, maintenance IVIg | Complete recovery | No | 5 months |
| 6 | 73/ Female | Hypertension; type 2 diabetes mellitus; thyroiditis | Non-severe | 5 weeks | Right ON | Right optic nerve T2 hyperintensity without contrast enhancement | N/A | 1:5120 ‡ | IVMP for 3 days, oral prednisone taper | Partial recovery (20/25 VA) | No | 5 months |
| 7 | 74/ Male |
Type 2 diabetes mellitus; hypertension | Non-severe | 4 days | Bilateral ON | Bilateral (left > right) optic nerve enhancement (anterior; Fig. 1I) | WBC 2 cells/mm3, protein 53 mg/dL, glucose 105 mg/dL, OCBs absent | 1:100 † | IVMP for 3 days | Complete recovery | No | 12 months |
| 8 | 44/ Female | None | Non-severe | 3 weeks | Encephalopathy; Bilateral ON | Diffuse, poorly demarcated cortical/subcortical T2 hyperintensities in the bilateral parietal lobes | WBC 63 cells/mm3, protein 61 mg/dL, glucose 66 mg/dL | 1:100 † | IVMP for 5 days, PLEX for 5 sessions | Complete recovery | No | 8 months |
| 9 | 85/ Female | Hypertension | Severe | 2 weeks | Encephalopathy; Seizures |
Diffuse, poorly demarcated bilateral cortical/subcortical hyperintensities (Fig. 1G) |
WBC 2 cells/mm3, protein 45 mg/dL, glucose 50 mg/dL OCBs absent |
1:2560 ‡ | IVMP, ceftriaxone, acyclovir | Death (severe SARS-CoV-2) |
No | 3 weeks |
CSF = cerebrospinal fluid; IVIg = intravenous immunoglobulin; IVMP = intravenous methylprednisolone; MOG = myelin oligodendrocyte glycoprotein; MOGAD = myelin oligodendrocyte glycoprotein-IgG1 associated disorder; MRI = magnetic resonance imaging; N/A = not available; OCBs = oligoclonal bands; ON = optic neuritis; PLEX = plasma exchange; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; VA = visual acuity; WBC = white blood cells.
All patients tested positive by nasopharyngeal polymerase chain reaction.
Latency = time from SARS-CoV-2 infection to onset of neurological symptoms.
MOG-IgG1 quantified by fluorescence-activated cell sorting assay.
MOG-IgG quantified by live cell-based immunofluorescence assay.
3.1. SARS-CoV-2 infection
The median latency from SARS-CoV-2 infection to neurological symptom onset was 2 weeks (range 4 days-5 weeks). SARS-CoV-2 infection was non-severe in 8/9 patients; one had severe SARS-CoV-2-related pneumonia.
3.2. Clinical and MRI features
Optic neuritis (ON) was observed in 4 patients (unilateral [2], bilateral [2]). Myelitis was observed in 4. Encephalopathy/encephalitis was observed in 3, of whom 1 developed status epilepticus. One patient experienced a brainstem attack with nausea, vertigo, and multidirectional nystagmus.
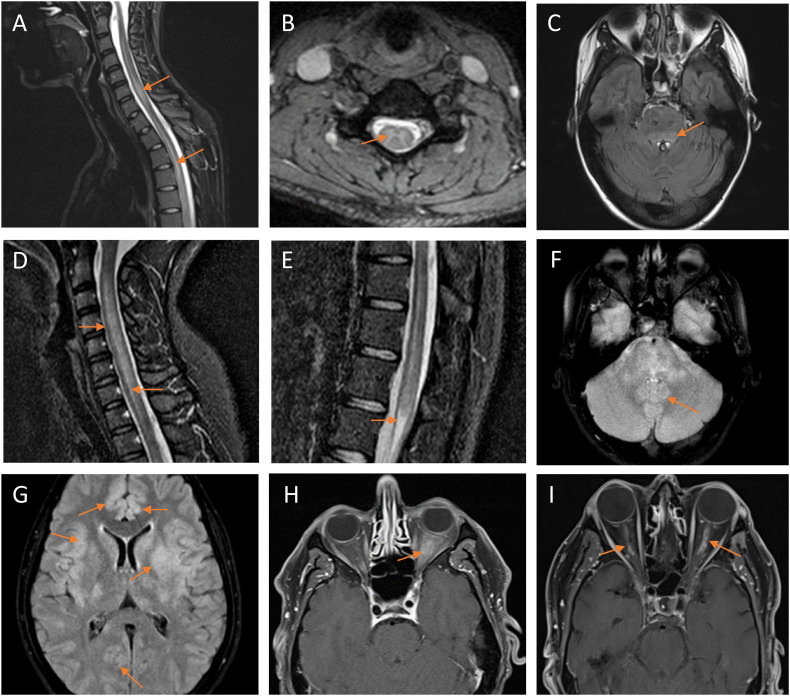
Magnetic resonance imaging (MRI) spine features of myelitis included non-enhancing, longitudinally extensive T2 hyperintensities (Fig. 1A–B and 1D–E). MRI brain lesions were observed in 4 patients, including T2 hyperintensity in the periaqueductal gray matter and brainstem (Fig. 1C and F), cortex and juxtacortical regions (Fig. 1G), and other subcortical regions including the parietal lobes and caudate nucleus. MRI features of ON included anterior-predominant contrast-enhancement with perineural sheath enhancement of >50% of the length of the optic nerves (Fig. 1H–I).
Fig. 1.
MRI findings.
Patient #1 (A–C) exhibited longitudinally extensive T2-weighted hyperintensities in the cervical and thoracic spine on sagittal short-tau inversion recovery (STIR; A) and axial (B) sequences, and T2-weighted fluid-attenuated inversion recovery (FLAIR) hyperintensities on axial sequences in the periaqueductal gray matter/pons (C). Patient #2 (D–F) similarly demonstrated longitudinally extensive T2-weighted hyperintensities in the cervical spine (D) and conus medullaris (E) on sagittal STIR sequences, and T2-weighted FLAIR hyperintensities in the brainstem on axial sequences (F). Patient #9 exhibited diffuse, poorly demarcated bilateral cortical and juxtacortical T2-weighted FLAIR hyperintensities (G). Patient #5 demonstrated T1-weighted thickening and contrast-enhancement of the anterior segment of the left optic nerve and nerve sheath on axial sequences (H). Patient #7 exhibited T1-weighted contrast-enhancement of the anterior segment of the optic nerves bilaterally (left greater than right) on axial sequences (I).
3.3. Cerebrospinal fluid and serology
Cerebrospinal fluid analyses were performed in 8/9 patients. White blood cell count was elevated among 5/8 with a median of 65.5 (elevated range 63–144) cells/mm3. Oligoclonal bands were absent (7/8) or matched (1). Serum MOG-IgG titers ranged from 1:100-1:1000; FACS [6]), and 1:2560-1:5120; live CBA [3]). All patients were negative for serum aquaporin-4-IgG.
3.4. Treatment and outcomes
All patients were initially treated with corticosteroids (intravenous methylprednisolone [8], high-dose oral prednisone [1]). Two patients received plasma exhange, and 1 received intravenous immunoglobulin (IVIg) acutely. Two patients received maintenance IVIg. The majority (7/9) of patients exhibited complete or near-complete recovery at a median follow-up of 5 months (range 23 days-18 months). No patients had a relapsing course. One patient with seizures died of severe SARS-CoV-2 pneumonia.
4. Discussion
Here we describe 9 de novo MOGAD attacks that developed post-SARS-CoV-2 infection with typical clinical and radiological features and good recovery. To date, few cases of MOGAD following SARS-CoV-2 infection have been reported. Similar to the patients in this series, cases described have generally been young or middle-aged, with mild SARS-CoV-2 severity, presenting with features typical of MOGAD (ON, myelitis and brain involvement). Latency period from SARS-CoV-2 infection to development of neurological symptoms ranged from several days to weeks, with generally good recovery and no clinical/radiologic relapses over limited follow-up periods ranging from several days to 2 months ( Table 2 ).(Durovic et al., 2021; Kogure et al., 2021; Peters et al., 2021; Sawalha et al., 2020; Zhou et al., 2020).
Table 2.
Characteristics of previously described adult patients diagnosed with MOGAD following SARS-CoV-2 infection.
| Case report | Age (years)/ Gender | Comorbidities | SARS-CoV-2 Severity⁎ | Latency⁎⁎ | Neurological presentation | MRI findings | CSF findings | MOG-IgG | Treatment | Outcome | Relapsing course | Follow-up time |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhou et al., 2020 | 26/ Male |
None | Non-severe | 2 days | Bilateral ON; acute myelitis | Bilateral optic nerve enhancement (globe to pre-chiasm); patchy contrast-enhancing hyperintensities in lower cervical and upper thoracic spinal cord | WBC 55 cells/mm3, protein 31 mg/dL, glucose 57 mg/dL, no unique OCBs | 1:1000† | IVMP for 5 days, oral prednisone taper | Partial recovery (residual 20/30 VA bilaterally) | No | 3 weeks |
| Sawalha et al., 2020 | 44/ Male |
None | Non-severe | 14 days | Bilateral ON | Bilateral optic nerve enhancement (pre-chiasmal) | WBC 3 cells/mm3, protein 50 mg/dL, glucose 88 mg/dL, OCBs absent | 1:160§ | IVMP for 5 days, oral prednisone taper | Near-complete recovery (right eye impaired VA) | No | 5 days |
| Kogure et al., 2021 | 47/ Male |
Right adrenal resection; recurrent sinusitis | Non-severe | 2 days | Left ON | Bilateral (left>right) optic nerve contrast enhancement | Reportedly normal WBC, normal protein, SARS-CoV-2 PCR negative | 1:128§ | IVMP for 3 days | Partial recovery (left eye residual VA 20/160) |
No | 2 weeks |
| Durovic et al., 2021 | 22/ Male |
None | Non-severe | 3 days | Meningism | Cortical T2 hyperintensities without contrast enhancement | WBC 31 cells/mm3, protein 40 mg/dL, glucose 64 mg/dL, SARS-CoV-2 PCR negative | 1:640‡ | IVMP for 5 days | Complete recovery | No | 2 months |
| Peters et al., 2021 | 23/ Male |
Childhood non-febrile seizures | Non-severe | 0–14 days | Seizures; encephalopathy | Diffuse left-hemispheric cortical FLAIR hyperintensity with left hemispheric leptomeningeal enhancement | WBC 57 cells/mm3, protein 40 mg/dL, glucose 60 mg/dL, OCBs absent, SARS-CoV-2 PCR negative | 1:100† | IVMP for 5 days, oral prednisone taper | Near-complete recovery (residual cognitive impairment) | No | 8 weeks |
CSF = cerebrospinal fluid; FLAIR = fluid attenuated inversion recovery; IVMP = intravenous methylprednisolone; MOG = myelin oligodendrocyte glycoprotein; MOGAD = myelin oligodendrocyte glycoprotein-IgG1 associated disorder; MRI = magnetic resonance imaging; OCBs = oligoclonal bands; ON = optic neuritis; PLEX = plasma exchange; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; VA = visual acuity; WBC = white blood cells.
All patients tested positive by nasopharyngeal polymerase chain reaction.
Latency = time from SARS-CoV-2 infection to onset of neurological symptoms.
MOG-IgG1 quantified by fluorescence-activated cell sorting assay.
MOG-IgG quantified by live cell-based immunofluorescence assay.
Method of MOG-IgG quantification not stated.
Several pathophysiological mechanisms of SARS-CoV-2-related CNS inflammatory disorders have been proposed, including direct viral neuroinvasion, cytokine-induced CNS inflammation, hypercoagulability, and/or hypoxia.(Durovic et al., 2021; Kogure et al., 2021) MOG-IgG antibodies are postulated to cause demyelination through various mechanisms, including antibody-dependent cytotoxicity and encephalitogenic T-cells.(Marignier et al., 2021).
In our cases, SARS-CoV-2 infection was generally non-severe, suggesting that viral-mediated cytokine storming and/or systemic inflammation are unlikely to underpin the development of MOGAD. The temporal relationship observed between SARS-CoV-2 infection and MOGAD may suggest an autoimmune or inflammatory para-infectious or post-infectious phenomenon, with viral triggering of autoantibodies to CNS antigens. While the global incidence of MOGAD since the beginning of the SARS-CoV-2 pandemic remains unknown, there are reports of increased detection of MOGAD by some neuroimmunological laboratories.(Mariotto et al., 2022) However, to date there has been a relative paucity of reported cases of MOGAD following SARS-CoV-2 infection, suggesting that clinicians have not observed a significant rise in the incidence of MOGAD during this time. Differences in the host response to SARS-CoV-2 may explain why only a small subgroup of individuals develop MOGAD following infection. Viral infection and a subsequent immune-mediated phenomenon may trigger an initial clinical event in individuals that have a predilection towards developing MOGAD, potentially due to underlying immune dysregulation or genetic susceptibility. This hypothesis is supported by observations of MOGAD occurring in a subset of patients following viral infections, including herpes simplex virus, Epstein-Barr virus, and Borrelia.(Marignier et al., 2021) The diversity of these potential triggers further supports a host-mediated response, rather than factors specific to a particular inciting pathogen. Potential mechanisms may include molecular mimicry (as outlined by Vojdani and Kharrazian, 2020), bystander activation, epitope spreading, and B-cell receptor-mediated co-capture of antigens, among others.(Marignier et al., 2021; Vojdani and Kharrazian, 2020).
5. Conclusions
We present a series of 9 patients diagnosed with MOGAD in the period following SARS-CoV-2 infection. These cases highlight a potential role of infection in the immunopathogenesis of MOGAD. Further studies are needed to evaluate whether the incidence of MOGAD has increased during the SARS-CoV-2 pandemic, and to elucidate whether host or disease factors may contribute to this association.
References
- Durovic E., Bien C., Bien C.G., Isenmann S. MOG antibody-associated encephalitis secondary to Covid-19: case report. BMC Neurol. 2021;21:414. doi: 10.1186/s12883-021-02449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure C., Kikushima W., Fukuda Y., Hasebe Y., Takahashi T., Shibuya T., Sakurada Y., Kashiwagi K. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis in a COVID-19 patient: a case report. Medicine (Baltimore) 2021;100 doi: 10.1097/MD.0000000000025865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marignier R., Hacohen Y., Cobo-Calvo A., Pröbstel A.-K., Aktas O., Alexopoulos H., Amato M.-P., Asgari N., Banwell B., Bennett J., Brilot F., Capobianco M., Chitnis T., Ciccarelli O., Deiva K., De Sèze J., Fujihara K., Jacob A., Kim H.J., Kleiter I., Lassmann H., Leite M.-I., Linington C., Meinl E., Palace J., Paul F., Petzold A., Pittock S., Reindl M., Sato D.K., Selmaj K., Siva A., Stankoff B., Tintore M., Traboulsee A., Waters P., Waubant E., Weinshenker B., Derfuss T., Vukusic S., Hemmer B. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021;20:762–772. doi: 10.1016/S1474-4422(21)00218-0. [DOI] [PubMed] [Google Scholar]
- Mariotto S., Ferrari S., Monaco S., Benedetti M.D., Schanda K., Alberti D., Farinazzo A., Capra R., Mancinelli C., De Rossi N., Bombardi R., Zuliani L., Zoccarato M., Tanel R., Bonora A., Turatti M., Calabrese M., Polo A., Pavone A., Grazian L., Sechi G., Sechi E., Urso D., Delogu R., Janes F., Deotto L., Cadaldini M., Bianchi M.R., Cantalupo G., Reindl M., Gajofatto A. Clinical spectrum and IgG subclass analysis of anti-myelin oligodendrocyte glycoprotein antibody-associated syndromes: a multicenter study. J. Neurol. 2017;264:2420–2430. doi: 10.1007/s00415-017-8635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotto S., Carta S., Dinoto A., Lippi G., Salvagno G.L., Masin L., Alberti D., Marignier R., Ferrari S. Is there a correlation between MOG-associated disorder and SARS-CoV-2 infection? Eur. J. Neurol. 2022;ene.15304 doi: 10.1111/ene.15304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Alhasan S., Vogels C.B.F., Grubaugh N.D., Farhadian S., Longbrake E.E. MOG-associated encephalitis following SARS-COV-2 infection. Mult. Scler. Relat. Disord. 2021;50 doi: 10.1016/j.msard.2021.102857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawalha K., Adeodokun S., Kamoga G.-R. COVID-19-induced acute bilateral optic neuritis. J. Investig. Med. High Impact Case Rep. 2020;8 doi: 10.1177/2324709620976018. 232470962097601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020;217 doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters P.J., Komorowski L., Woodhall M., Lederer S., Majed M., Fryer J., Mills J., Flanagan E.P., Irani S.R., Kunchok A.C., McKeon A., Pittock S.J. A multicenter comparison of MOG-IgG cell-based assays. Neurology. 2019 doi: 10.1212/WNL.0000000000007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Jones-Lopez E.C., Soneji D.J., Azevedo C.J., Patel V.R. Myelin oligodendrocyte glycoprotein antibody–associated optic neuritis and myelitis in COVID-19. J. Neuroophthalmol. 2020;40:398–402. doi: 10.1097/WNO.0000000000001049. [DOI] [PMC free article] [PubMed] [Google Scholar]