Abstract
Lanthanide-doped upconversion nanoparticles (UCNPs) have aroused extraordinary interest due to the unique physical and chemical properties. Combining UCNPs with other functional materials to construct nanocomposites and achieve synergistic effect abound recently, and the resulting nanocomposites have shown great potentials in various fields based on the specific design and components. This review presents a summary of diverse designs and synthesis strategies of UCNPs-based nanocomposites, including self-assembly, in-situ growth and epitaxial growth, as well as the emerging applications in bioimaging, cancer treatments, anti-counterfeiting, and photocatalytic fields. We then discuss the challenges, opportunities, and development tendency for developing UCNPs-based nanocomposites.
Subject terms: Nanoparticles, Nonlinear optics
This review presents a summary of diverse designs and applications of nanocomposites based on lanthanide-doped upconversion nanoparticles.
Introduction
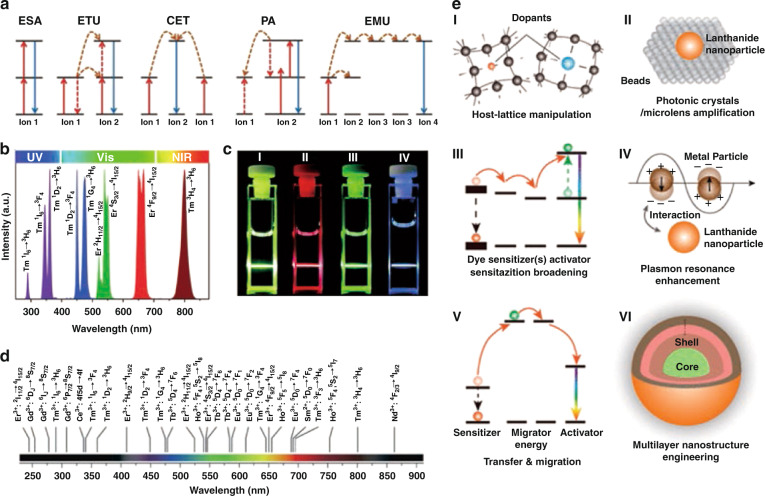
Lanthanide-doped upconversion nanoparticles (UCNPs) which can absorb two or more low-energy photons and radiate a high-energy photon are favored by researchers in various fields1,2. The 4f electrons of rare-earth ions constitute the rich metastable energy levels, which makes the upconversion process of rare-earth-doped nanocrystals diversified. In general, the upconversion luminescence (UCL) processes associated with rare-earth ions mainly involve five types of energy transfer (ET) pathways: excited state absorption (ESA), energy transfer upconversion (ETU), cooperative energy transfer (CET), photon avalanche (PA), and energy migration upconversion (EMU), as shown in Fig. 1a3,4. In UCNPs, lanthanide ions (Ln3+) with ladder-like electronic energy levels are often co-doped as activators for UCL process. Before in-depth understanding of EMU process, efficient activators were limited to Er3+, Tm3+, and Ho3+ (especially Er3+ and Tm3+). For UCNPs co-doped with Yb3+–Er3+, the energy transitions of 2H11/2 → 4I15/2, 4S3/2 → 4I15/2, and 4F9/2 → 4I15/2 occurred under 980 nm excitation, green (525 nm and 542 nm) and red (655 nm) UCL were observed. For Tm3+ ions, under 980 nm excitation, ultraviolet (UV) emissions (290, 345, and 362 nm), visible (Vis) emissions (450, 475, 644, and 694 nm) and near infrared (NIR) emission (800 nm) could be observed. The UCL spectral of Er3+ and Tm3+ in UCNPs are shown in Fig. 1b, and the photograph of UCL is shown in Fig. 1c3. As the EMU process is proposed, the UCL of rare-earth ions (Ce3+, Gd3+, Tb3+, Dy3+, Eu3+, Sm3+, and Sm2+ ions) without suitable intermediate energy levels has been realized through the construction of core–shell structures (Fig. 1d)5,6. The modulation of emission wavelengths in UV-to-NIR spectral region could be easily achieved by selecting appropriate Ln3+ within UCNPs. As known, Ln3+ have inherent limitations such as narrow absorption cross-sections and inefficient nonlinear multiphoton processes. Many strategies, including host lattice modulation, photonic crystal and microlens magnification, molecular sensitization, plasmon resonance enhancement, energy transfer/transfer manipulation, core–shell engineering, etc., are currently used to overcome these difficulties (Fig. 1e)7. Reassuringly, compared with conventional materials with downshifting luminescence (DSL), such as luminescent complexes, organic dyes, and quantum dots (QDs), upconversion materials have excellent physical, chemical and biological characteristics, such as narrow-band emission, long fluorescence lifetime, large anti-Stokes shifts, superior light and chemical stability, low biological toxicity, and so on8–12. Due to the above advantages, UCNPs have a wide range of applications in three-dimensional display13,14, solar spectrum conversion15, anti-counterfeiting technology16, optical sensing17, and biomedicine18–20.
Fig. 1. Upconversion processes and optical modulation.
a Schematic diagrams of five upconversion processes4. b Representative UCL emissions of UCNPs doped with Yb–Er and Yb–Tm ranging from UV to NIR region under irradiation at 980 nm. c Photographs of UCL of UCNPs in colloidal solution. (I) Total UCL of NaYF4:Yb,Er sample. (II, III) The UCL of NaYF4:Yb,Er sample through red and green filters, respectively. (IV) Total UCL of NaYF4:Yb,Tm sample3. d By selecting the proper type of doping Ln3+ within UCNPs, a broad range of emission wavelengths from UV to NIR spectral region that could be modulated5. e Several photoluminescence enhancement strategies in Ln3+-doped UCNPs. (I) host lattice modulation, (II) photonic crystal and microlens magnification, (III) molecular sensitization, (IV) plasmon resonance enhancement, (V) energy transfer/transfer manipulation, (VI) core–shell engineering7. a Reprinted with permission from ref. 4 Copyright 2020, Elsevier. b, c Reprinted with permission from ref. 3 Copyright 2015, The Royal Society of Chemistry. d Reprinted with permission from ref. 5 Copyright 2019, Elsevier. e Reprinted with permission from ref. 7 Copyright 2020, American Chemical Society.
On account of the restricted features, single nanomaterial is often unable to meet the needs of practical applications. During the last decade, the design and fabrication of multifunctional nanocomposites have aroused immense research interests. Particularly, scientists have made much effort to develop UCNPs-based nanocomposites, which are composed of UCNPs and other functional materials. In terms of design and synthesis, self-assembly and in-situ growth are usually used to obtain UCNPs-based nanocomposites with core/satellite structures. In addition, UCNPs-based nanocomposites with core–shell structure could be prepared by epitaxial growth. In terms of multifunctional properties, UCNPs-based nanocomposites often retain the unique photoluminescence properties of UCNPs, and could be endowed with variable properties of other various functional materials. In terms of applications, combine UCNPs with bioimaging contrast agents, chemotherapy drugs, photothermal agents, photodynamic agents, and chemodynamic agents could be used for diagnosis and treatment of malignant tumor; UCNPs integrated with other fluorescent materials are potential candidates for multi-modal anti-counterfeiting; The integration of UCNPs and semiconductor photocatalysts have great potentials in NIR light-induced photocatalysis.
In this review, we firstly summarize the synthesis methods of UCNPs-based nanocomposites for various design purposes, i.e., self-assembly (electrostatic adsorption, specific recognition reaction, and covalent bonding), in-situ growth, and epitaxial growth. Then, we systematically introduce the applications of such nanocomposites, i.e., bioimaging, cancer treatments (chemotherapy, photothermal therapy, photodynamic therapy, synergistic cancer therapeutics), anti-counterfeiting, and photocatalysis. Finally, we discuss the challenges, future directions, and suggestions for UCNPs-based nanocomposites.
Strategies toward design and synthesis of UCNPs-based nanocomposites
To integrate UCNPs and other functional materials into one nanosystem, many strategies have been developed. Herein, we review the recent studies on the construction strategies and synthesis approaches of UCNPs-based nanocomposites, mainly classified into three categories: self-assembly, in-situ growth, and epitaxial growth.
Self-assembly
Self-assembly methods play an important role in the construction of multifunctional nanocomposites, mainly preparing various monomer components in advance and then combining such different components to form one nanosystem21. Recently, the common self-assembly methods for preparing UCNPs-based nanocomposites primarily contain electrostatic adsorption, specific recognition reaction, and covalent bonding.
Electrostatic adsorption
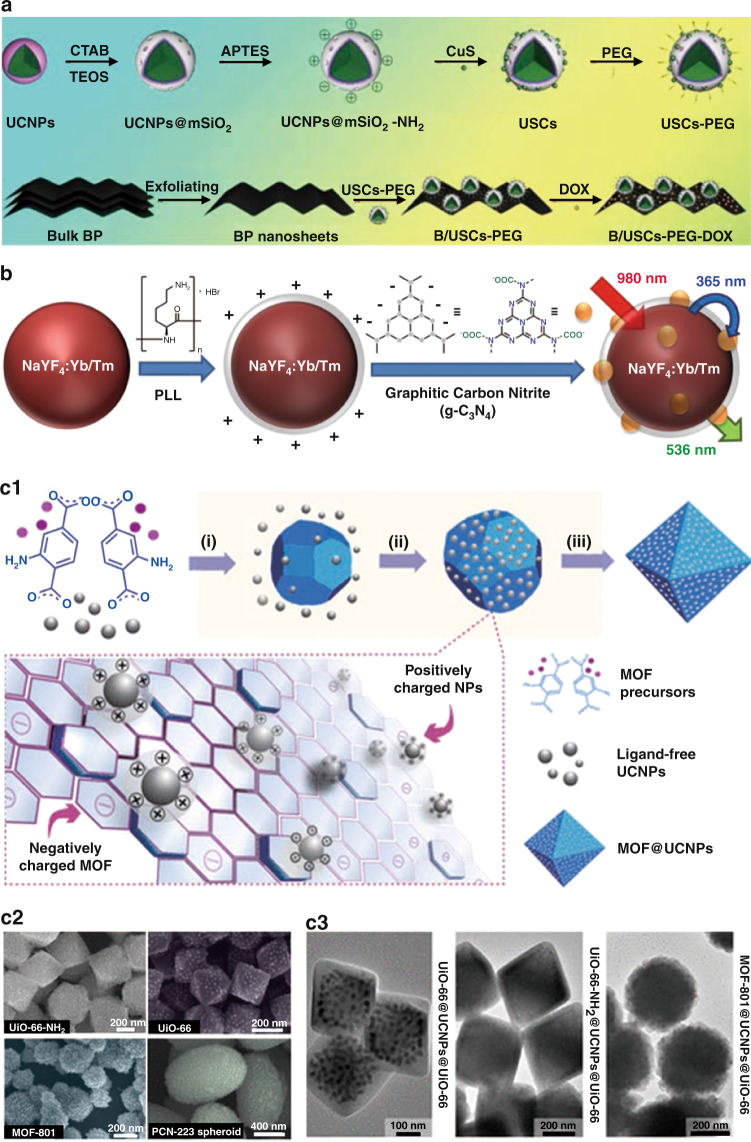
Electrostatic adsorption is a typical strategy for the synthesis of nanocomposites. Specifically, nano-monomers with different charges were synthesized separately, and then combined through electrostatic interaction to form nanocomposites22–27. Shi et al. 22 reported a core/satellite nanocomposite by decorating negatively charged CuS nanoparticles onto positively charged UCNPs@SiO2–NH2 nanoparticles. Yang et al.24 developed an intelligent nanoplatform by conjugating mesoporous silica (mSiO2)-coated UCNPs (UCNPs@mSiO2) with CuS nanoparticles and black phosphorus (BP) nanosheets. In Fig. 2a, positively charged UCNPs@mSiO2–NH2 were obtained by (3-aminopropyl)triethoxysilane (APTES) modification. Then, CuS was conjugated further through electrostatic adsorption and PEG was introduced to improve the water solubility. Finally, BP nanosheets with negative charges were coupled with UCNPs@mSiO2–CuS–PEG (USCs–PEG). This study takes full advantage of electrostatic adsorption to integrate several components with different charge modifications into one nanoplatform. Liu et al. 25 reported a novel NaYF4:Yb/Tm-PLL@g-C3N4 nanoplatform. In Fig. 2b, NaYF4:Yb/Tm core was decorated with positive ligand poly(l-lysine) (PLL). Graphitic carbon nitride (g-C3N4) with negatively charged COO− groups could combine with NaYF4:Yb/Tm-PLL through electrostatic interaction. Recently, new nanomaterials have been developed by combining metal-organic frameworks (MOFs) with UCNPs26,27. Huang et al. 26 used electrostatic interactions to spread UCNPs onto MOFs surfaces (Fig. 2c1). Various MOFs such as UiO-66-NH2, UiO-66, MOF-801, and PCN-223 are paved with UCNPs through this method (Fig. 2c2). Furthermore, MOF@UCNPs@MOF with sandwich structure could be obtained through the epitaxial growth of MOF (Fig. 2c3). This work provides a simple route to construct the nanocomposites combined MOFs and UCNPs with unique structures.
Fig. 2. Electrostatic adsorption technique.
a Schematic diagram of the synthetic process of B/USCs-PEG-DOX24. b Schematic illustration of the preparation of NaYF4:Yb/Tm-PLL@g-C3N425. c1 Schematic of the synthetic process of UCNPs and MOF nanocomposites. c2 SEM images of UiO-66-NH2@NaYF4:Yb/Er, UiO-66@NaYF4:Yb/Er, MOF-801@NaYF4:Yb/Er, PCN-223@NaYF4:Yb/Er nanocomposites. c3 TEM images of MOF@UCNPs@MOF sandwiched nanocomposites26. a Reprinted with permission from ref. 24 Copyright 2020, Elsevier. b Reprinted with permission from ref. 25 Copyright 2016, American Chemical Society. c1–c3 Reprinted with permission from ref. 26 Copyright 2018, American Chemical Society
Specific recognition reaction
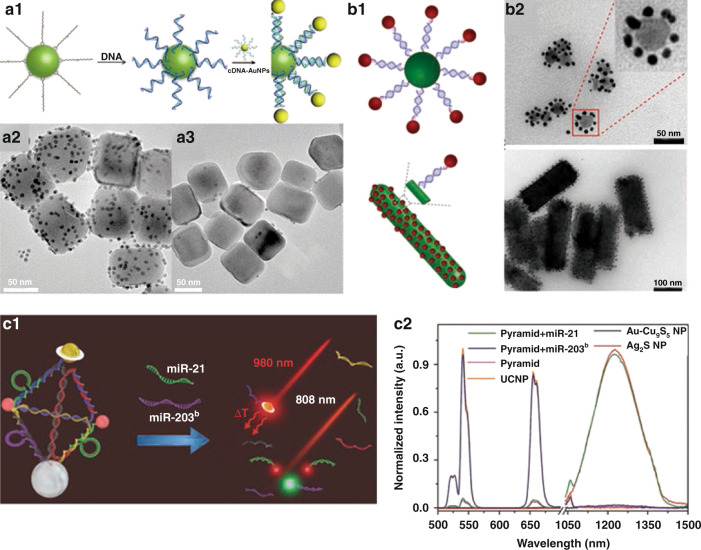
Specific recognition reaction mainly uses certain specific biological molecules (DNA and RNA aptamers28,29, avidin and biotin30, antigens and antibodies31, etc.) to combine multiple components together. DNA is an ideal programmable self-assembling agent due to its precise length and well-known Watson–Crick base pairing. Lu et al. 32 fabricated DNA-modified UCNPs directly by a simple method, and then integrated with AuNPs modified with complementary DNA strand. In Fig. 3a1, T30 oligonucleotides modified UCNPs (T30-UCNPs) could assemble with complementary DNA strand modified AuNPs (A27-AuNPs). The satellite structure could be constructed by surrounding T30-UCNPs with several A27-AuNPs (Fig. 3a2). In contrast, when non-complementary DNA-modified AuNPs were incubated with T30-UCNPs, the self-assembly could not be achieved owing to lack of specific recognition between the two parts (Fig. 3a3). This work proposes a novel combinatorial approach and further extend the applications in biomimetic nano-assembly and biomedicine. Huang et al. 33 proved the molecular recognition and programmable assembly capabilities of the as-prepared monodispersed DNA-UCNPs by mixing them with DNA-AuNPs together. DNA strands on UCNPs and AuNPs will hybridize to form the duplex, forming the satellite-like structure with UCNPs in the center and AuNPs outside (Fig. 3b1). AuNPs can be uniformly coated on NaYF4:Yb/Er nanospheres and NaYF4:Gd/Yb/Er nanorods through DNA-guided assembly (Fig. 3b2). The obtained nanocomposites could realize the adjustment of the distance between nanoparticles, which provides new thought for the subsequent synthesis of other nanocomposites. Kuang et al. 34 assembled nanopyramids with Au–Cu9S5, UCNPs, and Ag2S by using the complementary DNA hybridization between the recognition sequences and DNA skeleton (Fig. 3c1). When miR-21 and miR-203b appeared, the recognition sequences connected to Ag2S and UCNPs in the pyramid were completely complementary and separated from DNA framework based on competitive hybridization. Two luminescent signals could be restored simultaneously under 808 nm excitation, which arise from UCNPs at 541 nm and Ag2S at 1227 nm in the Vis and second window of NIR (NIR-II) region, respectively (Fig. 3c2). This proposed strategy opens extensive opportunities for self-assembled nanostructures in the biological field.
Fig. 3. Specific recognition reaction.
a1 Schematic illustration of DNA-directed assembly of UCNPs and AuNPs. TEM images of the assembly of T30-UCNPs with AuNPs containing complementary DNA (a2) and non-complementary DNA (a3)32. b1 Schematic illustrations of the DNA-mediated assemblies of UCNPs and AuNPs (5 nm). b2 TEM images of DNA-mediated assemblies UCNPs (NaYF4:Yb/Er nanospheres and NaYF4:Gd/Yb/Er nanorods) with AuNPs33. c1 Nanopyramids for dual miRs detection. c2 Luminescence spectra of Ag2S NPs, Au-Cu9S5 NPs, UCNPs, pyramids, pyramids with miR-21, and pyramids with miR-203b excited by 808 nm laser34. a1–a3 Reprinted with permission from ref. 32 Copyright 2013, American Chemical Society. b1, b2 Reprinted with permission from ref. 33 Copyright 2020, John Wiley and Sons. c1, c2 Reprinted with permission from ref. 34 Copyright 2017, John Wiley and Sons
Covalent bonding
In some UCNPs-based nanocomposites, UCNPs are coupled with other components through amidation reaction, in which the two parts containing functional groups could share electron pairs to form stable covalent bonds. In the amidation reaction, 1-ethyl-3-(3’-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS) are usually served as cross-linking agents to activate the carboxyl group (EDC/NHS method)35–38. Our group35 constructed ZnO-gated UCNPs@mSiO2 nanoplatform. EDC was acted as the cross-linking agent to facilitate the formation of amide bonds between carboxylic acid-functionalized UCNPs@mSiO2 and amine-capped ZnO nanodots, enabling ZnO nanodots to be firmly anchored to UCNPs surface. Shan et al. 36 covalently combined amino-functionalized UCNPs with carboxylated nanodiamond by using EDC and NHS as cross-linking agents to prepare multifunctional nanoplatform. Compared with the electrostatic adsorption, the nanocomposites obtained by covalent bonding have high structural stability and biocompatibility.
In-situ growth
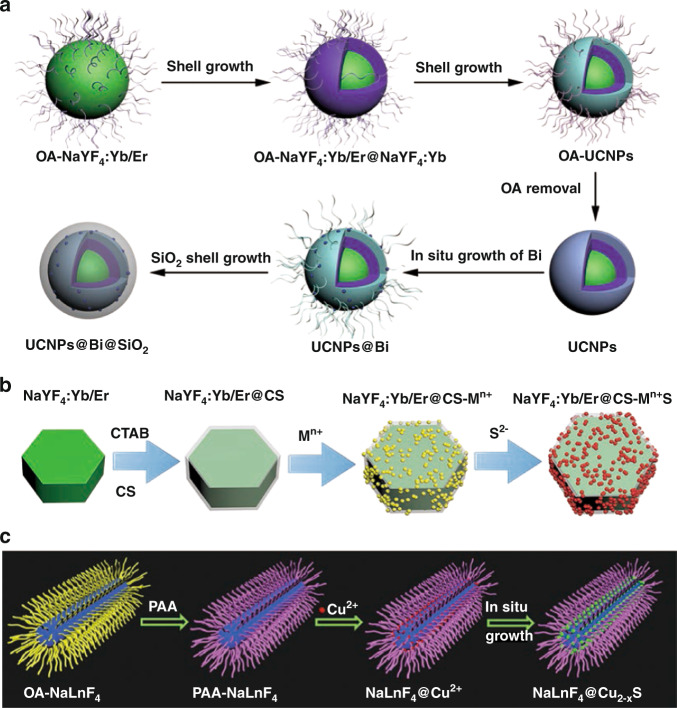
In-situ growth strategy is mainly summarized as a method of first synthesizing one of the components, and then uniform growing the other component to its surface39–49. Up to now, most nanocomposites based on UCNPs and single “element” nanoparticles (such as Au and Bi) have been synthesized through in-situ growth strategy39–41. You et al. 41 used in-situ growth method to synthesize UCNPs@Bi and finally obtain UCNPs@Bi@SiO2 nanocomposites through surface modification of SiO2. Oleic acid (OA) coated UCNPs were first obtained and ligand-free UCNPs were prepared through the acid-induced ligand removal process. Then, Bi nanoparticles could be well decorated on UCNPs through in-situ growth. Finally, the outermost layer of UCNPs@Bi nanoparticles is coated with dense SiO2 shell to improve the stability (Fig. 4a). Nowadays, conjugating transition-metal chalcogenide (Mn+S/Se) and UCNPs has aroused immense research interests42–47. Chitosan (CS) with glucosamine and hydroxyl groups could chelate metal ions (Ag+, Cu2+, Cd2+, etc.) and improve the biocompatibility of UCNPs. Our group43 reported a general in-situ growth method that can make (Mn+S, M = Ag, Cu, Cd) nanodots to uniformly conjugate on CS-coated UCNPs (Fig. 4b). First, OA-modified NaYF4:Yb/Er UCNPs were prepared and transferred into hydrophilic phase with the assistance of cetyltrimethylammonium bromide (CTAB). Then, CS was introduced to further immobilize Mn+. Finally, Mn+S QDs could be well decorated on UCNPs@CS through in-situ growth after adding the sulfur source. Such facile in-situ growth strategy could also be used to combine Ag2Se nanodots with CS-coated UCNPs, only using selenium source instead of sulfur source44. Besides, Hao et al. 47 synthesized NaLnF4@Cu2-xS nanocomposites using in-situ growth strategy (Fig. 4c). OA-coated NaLnF4 was firstly synthesized by hydrothermal method. The oil-phase core could be transferred to the water phase and negatively charged by PAA modification. Then, Cu2+ could be absorbed by PAA-NaLnF4. Finally, Cu2−xS grow uniformly in-situ on NaLnF4 by adding sulfur source. For in-situ growth strategy, polymers or complexes are firstly modified on UCNPs, which will then act as nucleation and growth centers to induce further growth of other nanodots. This method simplifies the multi-step additional loading steps, and could control the growth behavior of nanodots on UCNPs by adjusting the feeding ratio.
Fig. 4. In-situ growth strategy.
a Schematic diagram of synthetic process of UCNPs@Bi@SiO2 nanocomposites41. b Schematic illustration of preparation of NaYF4:Yb/Er@CS@Mn+S nanocomposites43. c Schematic illustration of designing NaLnF4@Cu2-xS theranostic nanoplatform47. a Reprinted with permission from ref. 41 Copyright 2019, American Chemical Society. b Reprinted with permission from ref. 43 Copyright 2020, The Royal Society of Chemistry. c Reprinted with permission from ref. 47 Copyright 2019, John Wiley and Sons
Epitaxial growth
Growing homogeneous shell on UCNPs through epitaxial growth is generally considered to be an effective approach to reduce the original nanocrystal surface defect density, thereby improving the UCL efficiency. Various core–shell UCNPs have been constructed, such as inert core–shell structures (NaYF4:Yb/Ln@NaYF4 (Ln = Er, Tm)50–52, NaGdF4:Yb/Tm@NaGdF453,54, LaF3:Yb/Tm@LaF355) or active core–shell structures (NaYF4:Yb/Tm@NaYF4:Yb/Er56, NaGdF4:Yb/Er@NaGdF4:Yb57, NaGdF4:Yb/Tm@NaGdF4:Eu58, and BaGdF5:Yb/Er@BaGdF5:Yb59). The heterogeneous core–shell structure refers to the different host lattices between the core and shell, which has been proven to be an effective strategy to form hybrid nanostructures60–69. Yan et al. 61 found that the UCL efficiency of NaYF4:Ln3+@CaF2 nanoparticles with heterogeneous core–shell structure could be increased by more than 300 times compared with shell-free NaYF4:Ln3+ nanoparticles. This indicates that heterogeneous core–shell structure can effectively prevent the influence of nanoparticle surface defects and environmental quenching effects. Han et al. 62 found that UCL intensity of NaYF4:(20–100%)Yb/Tm@CaF2 was enhanced by two orders of magnitude compared with the nanoparticles without CaF2 shell. Yang et al. 63 developed α-NaYbF4:Tm@CaF2:Nd@ZnO nanoplatform via hetero-epitaxial growth manner. CaF2 shell grow epitaxially on α-NaYbF4:Tm core, which can not only effectively control the particle size, but also greatly promote UCL intensity. Cubic ZnO layer firmly assembled on α-NaYbF4:Tm@CaF2:Nd via the epitaxy manner to form core–shell–shell nanostructure. Gao et al. 64 epitaxially grown ZnS on KMnF3:Yb/Er based on the high affinity of Mn2+ for chalcogen ions. The formation of KMnF3:Yb,Er@ZnS enhances UCL by suppressing the surface-quenching effects. The core–shell particles also exhibit intense DSL of Mn2+ under UV excitation, attributing to Mn2+ doping into the ZnS lattice through the core–shell interface.
Emerging applications of UCNPs-based nanocomposites
Scientific interest in UCNPs-based nanocomposites has rapidly increased and some newly emerging sectors have seen the applications of them including bioimaging, cancer treatments, anti-counterfeiting, photocatalysis, etc.
Bioimaging
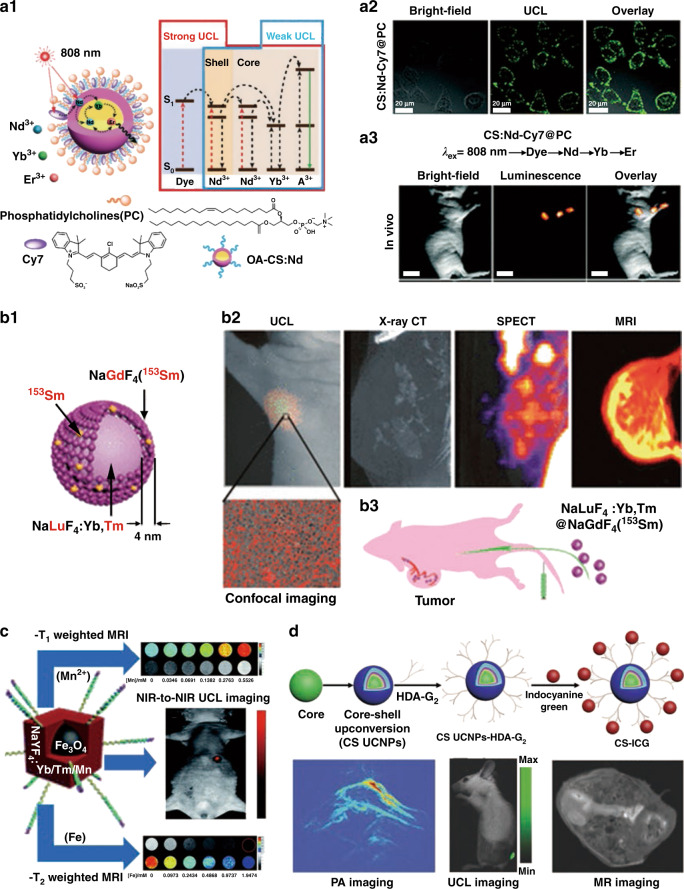
UCNPs with good chemical and optical stability, low toxicity and good biocompatibility could normally be radiated by NIR light, which have higher tissue penetration depth than short-wavelength UV or Vis light and avoid interference from organism background fluorescence70–74. Li’s group70 designed phosphatidylcholine (PC) modified dye-sensitized UCNPs-based nanocomposite for biological UCL imaging. A sulfonic functionalized cyanine dye derivative (Cy7) attached on NaYF4:30%Yb,1%Nd,0.5%Er@NaYF4:20%Nd (CS:Nd) was used as the antenna dye, which could broadly harvest NIR energy and enhance the UCL (Fig. 5a1). The intense UCL signal could be detected in HeLa cells incubated with CS:Nd-Cy7@PC nanocomposite (Fig. 5a2). Moreover, CS:Nd-Cy7@PC was successfully applied in lymphatic imaging (Fig. 5a3), suggesting the feasibility of dye-sensitized upconversion nanocomposite for bioimaging. However, optical imaging still has the limits of resolution and three-dimensional reconstruction. It is necessary to combine other imaging technologies widely used in the medical field, such as magnetic resonance (MR) imaging (MRI), X-ray computed tomography (CT), single-photon emission computed tomography (SPECT), and photoacoustic imaging (PAI), to improve the accuracy and sensitivity of imaging. UCNPs-based nanocomposites offer great opportunities for multi-modal imaging. Especially, UCNPs themselves could achieve various imaging such as UCL imaging, CT imaging, MRI, etc. by modulating lanthanide elements and structure of UCNPs. Li et al. 75 developed NaLuF4:Yb,Tm@NaGdF4:153Sm as an optimized multi-modal imaging probe, in which Lu was used for CT imaging, Tm was used for UCL imaging, Gd was applied for MRI and radioisotope 153Sm for improving SPECT imaging (Fig. 5b1–b3). It is worth noting that the desired sensitivity and resolution in vivo could be greatly improved by combining MRI and UCL imaging. UCNP-based nanocomposites for UCL/MR dual-modal imaging have been well studied76–80. Yang et al. 76 synthesized Fe3O4@Mn2+-doped NaYF4:Yb/Tm nanoplatform, which can not only offer contrast signal in T1/T2-weighted MRI due to the co-existence of Fe3O4 and Mn2+, but also exhibit pronounced NIR-to-NIR UCL (Yb3+–Tm3+) in vivo fluorescence imaging, (Fig. 5c). As known, MRI is more proper for soft tissue examination, while CT shows great advantages in bone, lung, and chest imaging, as well as cancer detection. Therefore, the combination of MRI, CT, and UCL imaging can undoubtedly provide more comprehensive information of tissues81,82. Li’s group81 developed Fe3O4@NaLuF4:Yb,Er/Tm nanocomposites as multi-modal (MR, CT and UCL) imaging probe, which could provide the detailed imaging information of tumor-bearing mice. NaGdF4:Yb,Er–Ag hybrid nanocomposites were designed for UCL/CT/MR multi-modal bioimaging82. PAI with characteristics of non-invasiveness, rapidness, and accurate quantification has attracted immense attention specifically in diagnosis of tumor pathophysiological status. Indocyanine Green (ICG) exhibits strong PAI signal at low concentration owing to the strong absorbance in the range of 740–800 nm83,84. Nie et al. 83 synthesized high-efficiency UCNPs with 800 nm excitation, and then ICG was loaded onto UCNPs to enhance the total absorption and UCL, achieving PAI and UCL imaging, as well as MRI (Fig. 5d).
Fig. 5. Bioimaging.
a1 Schematic diagram and energy level diagram of PC modified dye-sensitized UCNPs-based nanocomposite for photon upconversion upon 808 nm irradiation. a2 UCL images of HeLa cells incubated with CS:Nd-Cy7@PC. a3 UCL lymphatic imaging 30 min after injection of CS:Nd-Cy7@PC under 808 nm excitation70. b1 Composition of NaLuF4:Yb,Tm@NaGdF4(153Sm). b2 Four-modal imaging of the tumor-bearing nude mouse at 1 h post intravenous injection of NaLuF4:Yb,Tm@NaGdF4(153Sm). b3 Schematic diagram of tumor angiogenesis imaging using NaLuF4:Yb,Tm@NaGdF4 (153Sm) as the probe75. c T1/T2-weighted MR and NIR-to-NIR UCL imaging of Fe3O4@NaYF4:Yb/Tm/Mn nanoplatform76. d Schematic of the synthesis of ICG modified UCNPs (CS-ICG) and the PAI, UCL imaging, and MRI of CS-ICG nanocomposites in vivo83. a1–a3 Reprinted with permission from ref. 70 Copyright 2016, The Royal Society of Chemistry. b1–b3 Reprinted with permission from ref. 75 Copyright 2013, American Chemical Society. c Reprinted with permission from ref. 76 Copyright 2017, The Royal Society of Chemistry. d Reprinted with permission from ref. 83 Copyright 2016, John Wiley and Sons
Cancer treatments
How to achieve accurate treatment of cancer has always been a difficult problem and research hotspot in the medical field. After careful modification, UCNPs could be used as promising carriers of multiple functional probes (such as chemotherapeutic agents, NIR photothermal agents, photosensitizer, Fenton nanocatalysts, etc.) for cancer treatments.
Chemotherapy
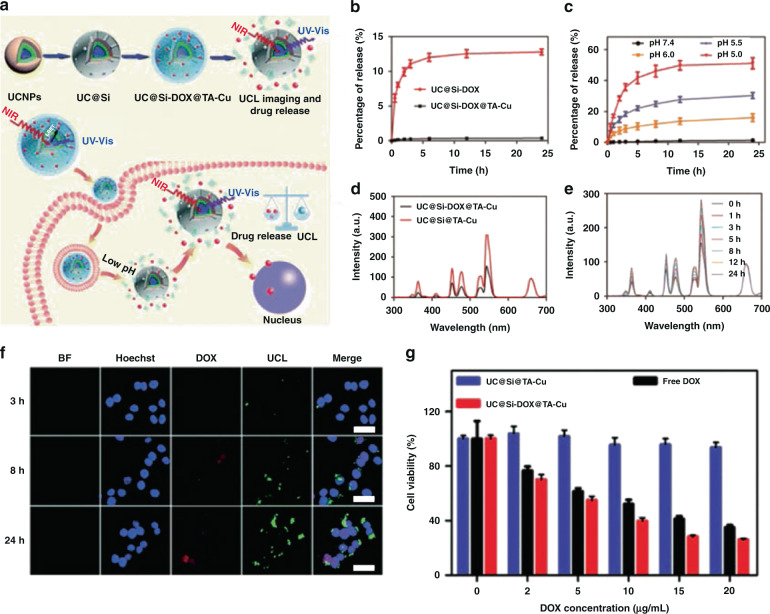
Chemotherapy is the way of destroying cancer cells with one or more anti-cancer drugs, which have achieved great success in improving the prognosis of patients. However, its severe toxic side effects are life-threatening, which present new challenges to people to alleviate the risk85,86. Various drug vectors such as polymer micelles87, vesicles88, and inorganic nanoparticles89 have been extensively studied in drug loading to reduce adverse reactions. Chemotherapeutic applications of UCNP-based nanocomposites have been studied extensively90–98. Our group35 designed ZnO-gated UCNPs@mSiO2@DOX (DOX = doxorubicin) nanoplatform for specific pH-triggered on-demand drug release. The “gatekeeper” ZnO exists stably in normal tissue environment to prevent premature drug leakage and decomposes in the acidic tumor microenvironment to realize pH-triggered on-demand release of DOX. Li et al. 94 designed UC@Si-DOX@TA–Cu (TA = tannic acid) nanoplatform to realize real-time UCL monitoring of pH-responsive drug release in live cells (Fig. 6a). UC@Si-DOX exhibits rapid release behavior at pH 7.4 while UC@Si-DOX@TA–Cu shows negligible DOX leakage, which indicates that TA–Cu protective shell can effectively encapsulate DOX within the mesopores (Fig. 6b). For UC@Si-DOX@TA–Cu, DOX release increases with decreasing pH, attributing to the efficient decomposition of TA–Cu complexes under acidic condition (Fig. 6c). Moreover, the luminescence resonance energy transfer from UCNPs to DOX occurs, resulting in the emission quenching of UCNPs (Fig. 6d). Upon pH triggered DOX release, the luminescence resonance energy transfer is gradually eliminated, resulting in an increase in UCL to monitor DOX release in real-time (Fig. 6e). The real-time monitoring of intracellular DOX release from UC@Si-DOX@TA–Cu exhibits that time-dependent enhancement of DOX fluorescence could be detected with the extension of incubation time. The significant recovery of UCL can be clearly detected under 980 nm excitation (Fig. 6f). The cytotoxicity assay proves that UC@Si-DOX@TA–Cu shows good cancer therapeutic effect (Fig. 6g).
Fig. 6. Chemotherapy.
a Schematic illustration of the fabrication of UC@Si-DOX@TA–Cu and pH-responsive drug release monitored by UCL imaging in real time. b Drug release profiles for UC@Si-DOX@TA–Cu and UC@Si-DOX in phosphate buffer saline (PBS) at pH 7.4. c Drug release profiles for UC@Si-DOX@TA–Cu in PBS at pH 7.4, 6.0, 5.5 and 5.0. d The UCL spectra of UC@Si@TA–Cu and UC@Si-DOX@TA–Cu. e Time-dependent UCL spectra of UC@Si-DOX@TA–Cu after immersing in PBS solution (pH = 5.5). f Confocal fluorescence images of HeLa cells after incubation with UC@Si-DOX@TA–Cu for 3, 8 and 24 h. Scale bar: 40 μm. g Viability of HeLa cells incubated with UC@Si@TA–Cu, DOX and UC@Si-DOX@TA–Cu at varying concentrations94. Reprinted with permission from ref. 94 Copyright 2019, The Royal Society of Chemistry
Photothermal therapy
Photothermal therapy (PTT) is an efficient noninvasive therapy approach, based on the photothermal conversion effect of photothermal agents to increase the temperature of tumor site for killing the tumor cells under the irradiation of external light. Compare with UV and Vis light, NIR light possesses much higher tissue penetration ability99. To date, various organic or inorganic NIR-absorbed photothermal agents have been developed for PTT100–109.
NIR light-activated nanocomposites combining UCNPs with photothermal agents show great prospects in PTT of tumor45,110–115. Our group44 reported multifunctional UCNPs@CS@Ag2Se nanocomposites for multi-modal imaging-guided PTT of tumor. Benefiting from the excellent absorption coefficient of Ag2Se, the nanocomposites exhibit high photothermal conversion efficiency, and excellent photothermal killing effect. You et al. 45 designed UCNP–Bi2Se3 nanohybrid for UCL/CT imaging-guided PTT. Bi2Se3 nanodots connected on UCNPs possess strong NIR absorption and efficient photothermal performance as well as distinct cancer cell ablation under single-wavelength NIR laser irradiation. Additionally, Shi et al. 110 reported novel UCNP@Al(OH)3/Au nanohybrids for synergistic-targeted PTT and UCL imaging under NIR light irradiation.
Photodynamic therapy
Photodynamic therapy (PDT) is a noninvasive and site-specific cancer treatment based on photochemistry. Typical PDT system involves three major elements: photosensitizer, suitable excitation of light, and oxygen molecules at the site of the disease tissue. Upon light excitation at specified wavelength, photosensitizer could be selectively activated to generate reactive oxygen species (ROS), which can induce cancer cell death, nevertheless, its application is limited by the penetration depth of the excitation light116.
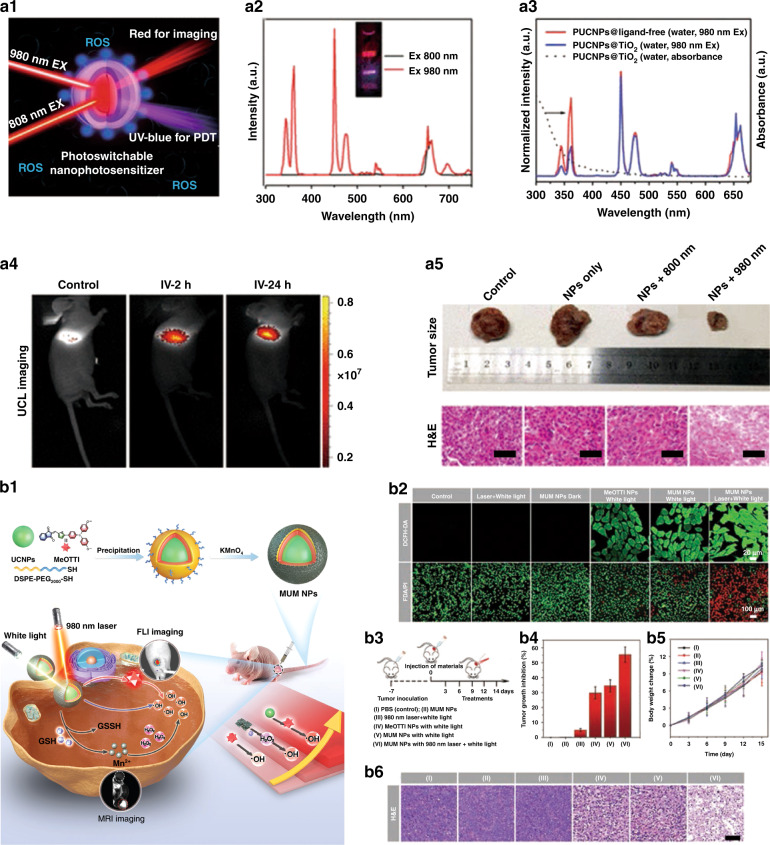
As UCNPs could convert NIR light to UV/Vis light, the tissue penetration depth in their PDT application could be improved. Based on this, UCNPs-based nanocomposites become the ideal candidates for PDT of deep tissue cancer117–124. Kong et al. 118 designed NIR light switchable PUCNPs@TiO2 nanocomposites to realize imaging guided accurate PDT of tumor (Fig. 7a1). PUCNPs (NaErF4@NaYF4@NaYbF4:0.5%Tm@NaYF4) possess the superior photoswitching feature. The intense UV-blue emission of Tm3+ could be observed under 980 nm excitation while it could be completely turned off when irradiated by 800 nm light. Additionally, red emission could be detected under 800 nm laser irradiation, attributing to the multi-wavelength excitation (800, 980, and 1530 nm) property of NaErF4 (Fig. 7a2). Using this switching characteristic, TiO2 photosensitizer could absorb UV light selectively resulting from PUCNPs. The ET efficiency between PUCNPs and TiO2 is calculated as 63% based on the UV/red emission ratio (Fig. 7a3). In vivo results show that the upconversion photoswitching nanocomposites are appropriate for UCL imaging in real-time and PDT of cancer under spatio-temporal control (Fig. 7a4, a5). This work promotes the application of UCNPs optical switching nanomaterials in biological field. Tang et al. 119 developed switchable DNA/UCNPs nanocomposite with chlorin e6 (Ce6) functionalization, which could produce singlet oxygen (1O2) and perform effective PDT for cancer under 980 nm excitation, providing new insights for precise targeting and highly efficient cancer therapy. At present, photosensitizers with aggregation-induced emission (AIE) features do not need to rely on O2 and have better therapeutic effects on the hypoxic regions of tumors125. However, AIE-active photosensitizers could often be irradiated by UV light with limited tissue penetration and the preparation of AIE-active photosensitizers with long-wavelength optical windows tends to be very complicated126. To optimize the diagnosis and treatment performance of AIE active photosensitizers, Tang et al. 127 constructed tumor microenvironment-responsive multifunctional nanoplatform (MUM NPs), which is composed of AIE-active photosensitizer (MeOTTI), UCNPs, and MnO2. The fluorescence resonance energy transfer (FRET) between UCNPs and MeOTTI could extend the wavelength of excitation light from UV–Vis to NIR region, which greatly enhances the tissue penetration depth and hydroxyl radicals (•OH) production efficiency. In the tumor microenvironment, MnO2 shells could decompose and effectively reduce the expression of intracellular glutathione (GSH), thereby increasing the level of intracellular •OH. Mn2+ generated by the reaction can catalyze the generation of •OH from intracellular H2O2, and finally realize the “triple jump” of ROS level and effective PDT guided by UCL/MR imaging (Fig. 7b1). Both the intracellular ROS generation assay and the corresponding live–dead staining assay demonstrated the extraordinary PDT performance of MUM NPs (Fig. 7b2). MUM NPs can strongly inhibit tumor proliferation and destroy tumor tissue, achieving highly effective antitumor therapy (Fig. 7b3–b6). This work inspires researchers to further explore therapeutic nanoplatforms with diversified AIE-active photosensitizers for prospective clinical translation.
Fig. 7. Photodynamic therapy.
a1 Schematic of PUCNPs@TiO2 nanocomposites for imaging guided PDT. a2 Upconversion emission spectra of PUCNPs excited by 800 or 980 nm laser (5 W cm−2). Inset: luminescence photograph of PUCNPs in cyclohexane under irradiation by two laser beams (0.8 W cm−2). a3 UCL spectra of PUCNPs@ligand-free (red line) and PUCNPs@TiO2 (blue line), the absorbance spectra of PUCNPs@TiO2 (dotted line). a4 UCL imaging in LLC tumor-bearing mouse after intravenous injection of PUCNPs@TiO2 for 2 and 24 h (800 nm irradiation). a5 The digital photographs of excised tumors after various treatments and H&E-stained slices of tumor tissues collected from different groups. The scale bars stand for 50 μm118. b1 Schematic illustration of fabrication of MUM NPs and dual-modal imaging guided triple-jump PDT. b2 DCFH-DA assay for intracellular ROS level (upper row) and FDA/PI assay for live/dead cell staining (lower row) of 4T1 cells after different treatments. b3 Schematic diagram of the operation process of antitumor therapy. b4 Tumor inhibition ratios of different groups after various treatments on day 14. b5 Body weight profiles of mice under different treatments. b6 H&E staining analysis of tumor tissues collected from different groups127. a1–a5 Reprinted with permission from ref. 118 Copyright 2018, American Chemical Society. b1–b6 Reprinted with permission from ref. 127 Copyright 2021, John Wiley and Sons
Synergistic cancer therapeutics
Except chemotherapy, PTT and PDT mentioned above, UCNPs-based nanocomposites could also be applied for radiotherapy (RT), chemodynamic therapy (CDT), gas therapy as well as immunotherapy. In order to better control tumor progression and prevent tumor metastasis and recurrence, the combination of multiple treatments has become an inevitable trend in cancer treatment.
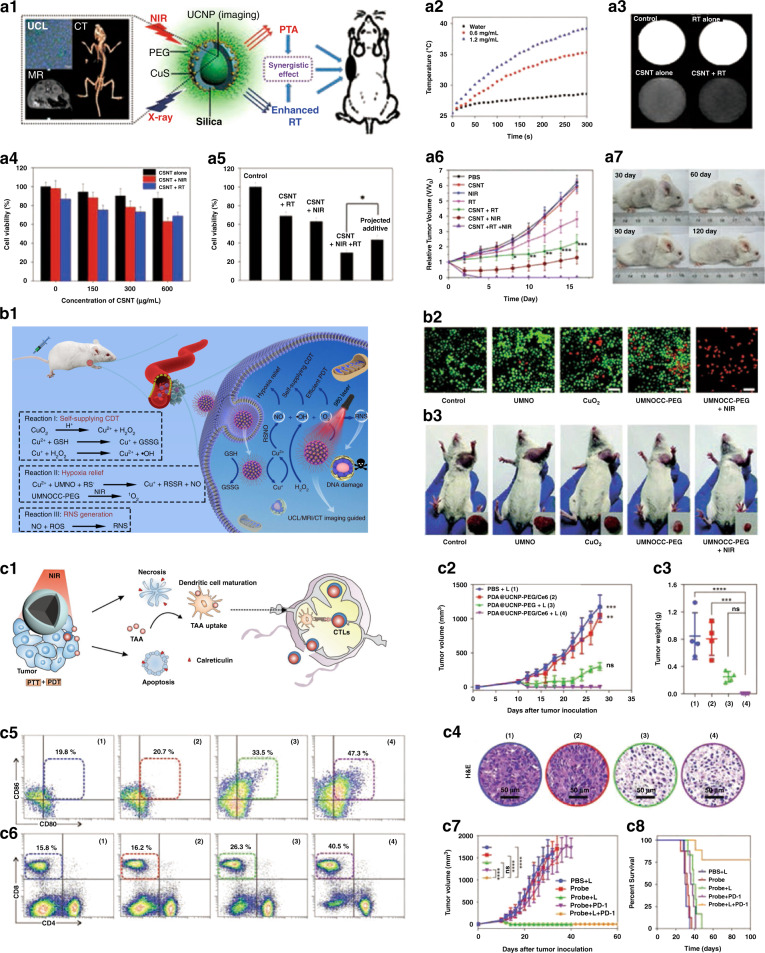
RT is one of the most commonly used therapies using high energy electromagnetic radiation to inhibit tumor growth, which has better results in patients who cannot undergo surgical treatment or have difficult resection128,129. As known, the combination of RT and PTT will counteract the disadvantages of PTT alone for deep tumors. To improve therapeutic effect of cancer by integrating photothermal ablation (PTA) with RT, Shi et al. 22 designed UCNPs@SiO2@CuS (CSNT) nanocomposites for UCL/CT/MR imaging guided RT/PTA synergistic therapy (Fig. 8a1). Such nanocomposites possess distinct photothermal conversion performance under 980 nm excitation through the adherence of CuS satellites (Fig. 8a2). CSNT can be used as a radiation sensitizer to produce dose enhancement effect due to the existence of high Z elements (Yb, Gd, and Er) (Fig. 8a3). The applicability of CSNT as efficient photothermal conversion agent and radiosensitizer was well demonstrated (Fig. 8a4, a5). Through the synergistic therapeutic effect of RT/PTA, tumor tissue could be completely eradicated without late recurrence (Fig. 8a6, a7), laying a foundation for future early diagnosis and multi-modal imaging-guided synergistic tumor therapy.
Fig. 8. Synergistic cancer therapeutics.
a1 Schematic illustration of CSNT for imaging-guided RT/PTA synergistic therapy. a2 Temperature variation of CSNT solutions irradiated by a 980 nm NIR laser (1.5 W cm−2, 5 min). a3 The impact of CSNT on the X-ray radiation dose. a4 Viability of HeLa cells incubated with CSNTs at different concentrations with or without 980 nm laser irradiation and RT. a5 Viability of HeLa cells that have taken up CSNTs treated with RT, PTA, and RT/PTA. a6 Relative tumor growth curves of different groups. a7 Digital photographs of mice from group 7 after 30, 60, 90, and 120 days of treatment22. b1 Schematic illustration of UMNOCC-PEG for imaging-guided tumor therapy. b2 CLSM images of HeLa cells co-stained with calcein AM (live cells, green) and PI (dead cells, red) after different treatments (0.5 W cm−2, 500 μg mL−1). b3 Photographs of the representative mice and excised tumors137. c1 Schematic illustration of synergistic phototherapy to enhance antitumor immunity. Tumor growth curve (c2), tumor weight (c3), and representative H&E staining (c4) of 4T1 tumor-bearing mice after different treatments. Detection of DC maturity (CD80+CD86+ gated on CD11c+) in tumor-draining lymph nodes (c5) and CTLs (CD4−CD8+ gated on CD3+) in the spleen (c6) by flow cytometry. Mean tumor growth kinetics (c7) and corresponding survival rates (c8) of mice after different treatments139. a1–a7 Reprinted with permission from ref. 22 Copyright 2013, American Chemical Society. b1–b7 Reprinted with permission from ref. 137 Copyright 2020, The Royal Society of Chemistry. c1–c8 Reprinted with permission from ref. 139 Copyright 2019, John Wiley and Sons
CDT, as a burgeoning type of technology for tumor treatment, has aroused great research interest. CDT takes the acidic microenvironment in the tumor as the reaction condition, over expressed H2O2 as the reaction raw material and transition metal nanomaterials as the catalyst to trigger Fenton or Fenton-like reaction in cancer cells, catalyze H2O2 to produce highly cytotoxic •OH and induce irreversible mitochondrial damage, DNA strand breakage, and protein and membrane oxidation130–132. Lin et al. 133 designed UCNPs–Pt(IV)–ZnFe2O4 nanoplatform for collaborative PDT/CDT/chemotherapy of cancer. NaGdF4:Yb/Tm@NaGdF4:Yb UCNPs triggered by NIR light could serve as UV–Vis light source to induce PDT effect and Fenton reaction of ZnFe2O4. Pt(IV) prodrugs could be reduced to highly toxic Pt(II) through GSH in tumor cells. This nanoplatform provide a comprehensive way for synergetic anticancer therapy. Besides, our group134 designed Cu2−xS decorated NaYF4:Yb/Er@NaYF4:Yb UCNPs to achieve synergistic enhanced CDT/PTT of cancer.
Gas therapy is promising for the treatment of many diseases due to its inherent biosafety and insignificant side effects. So far, gaseous molecules including NO, H2, H2S, SO2, and CO have shown significant anticancer effect135–137. Yang et al. 137 reported a versatile Cu2+-initiated NO nanotheranostic system (UMNOCC–PEG) for UCL/CT/MR imaging guided CDT/PDT/gas combination therapy. When UMNOCC–PEG nanocomposite is endocytosed by tumor cells, pH-sensitive CuO2 nanodots are decomposed, allowing the release of Cu2+ ions and H2O2. This not only triggers the Fenton-like reaction of Cu2+ and H2O2, but also realizes efficient CDT by solving the problem of limited endogenous H2O2 content. It can alleviate the antioxidant capacity and hypoxia of tumor through NO production and GSH consumption, to further boost the therapeutic effect of CDT and PDT (Fig. 8b1). In vitro and in vivo experimental results demonstrate that UMNOCC–PEG has excellent synergistic anticancer ability (Fig. 8b2, b3).
Immunotherapy is a treatment mode to improve the intrinsic ability against tumor by activating the body’s own immune system, which can not only effectively inhibit tumor recurrence and metastasis, but also specifically kill tumor cells that have relapsed and metastasized138–140. Liu and co-authors synthesized polydopamine (PDA) coated with NaGdF4:Yb/Er shell, and then loaded the photosensitizer Ce6 on its surface (PDA@UCNP–PEG/Ce6)139. The nanocomposites could elicit robust systemic and humoral antitumor immune responses by synergistic phototherapy (Fig. 8c1). The synergistic treatment group (group 4) could effectively eradicate tumors (Fig. 8c2–c4), confirming that synergistic phototherapy performed better than PDT or PTT alone in tumor ablation. The cell maturation efficacy and cytotoxic T lymphocytes (CTLs) in the spleen in the synergistic treatment group were much higher than those in the control group (Fig. 8c5, c6), proving that synergistic phototherapy could induce systemic antitumor immune response. Importantly, the combination of PDA@UCNP–PEG/Ce6 and PD-1 blocking antibody can effectively inhibit tumor recurrence and metastasis (Fig. 8c7) and prolong the survival period of tumor-bearing mice (Fig. 8c8). This study establishes an innovative paradigm for increasing immunogenic cell death by synergistic phototherapeutic nanoplatforms.
Anti-counterfeiting applications
Counterfeit and shoddy currencies, drugs and valuables are increasingly damaging to the market economy, bringing immeasurable economic loss to consumers and copyright holders. Therefore, a variety of anti-counterfeiting materials such as digital watermark, diffraction grating, photonic structure, stimulus response materials and luminescent materials have been developed and widely used in the current anti-counterfeiting technology. However, the security level of traditional anti-counterfeiting materials is relatively low and easy to be copied141–143.
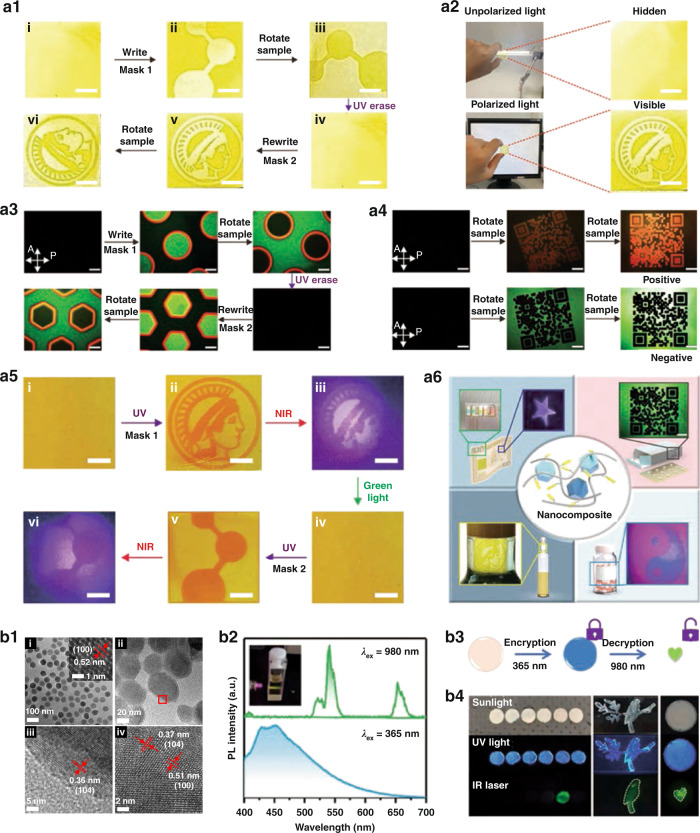
Lanthanide-doped UCNPs are especially suitable for anti-counterfeiting because of their rich intermediate state energy levels and distinguishable spectral characteristics144–149. Multicolor dual-modal excitation can be realized simultaneously by controlling the species and distribution of Ln3+ ions in the core and shells or developing new nanocomposites in combination with other luminescent materials150–152. Recently, Wu et al. 150 introduced UCNPs in photoresponsive azobenzene-containing polymer (azopolymer) to form PAzo/UCNPs nanocomposites, which have the characteristics of various anti-counterfeiting manners and read-out methods, as well as easy processing. First, different color patterns were prepared by using the photoisomerization properties of PAzo/UCNPs. Based on the differences of mechanical features between trans- and cis-azopolymers, the periodic arranged photonic crystal structures could be obtained by embossing. Due to Bragg diffraction, a pattern with structural color is finally presented. According to photoinduced orientation properties of azopolymers, macroscopic and microscopic polarization related patterns were further prepared (Fig. 9a1–a4). Because cis-azopolymers can absorb upconversion blue light, the photochromic pattern can be recognized under irradiation of NIR light according to the synergistic effect of azopolymers and UCNPs (Fig. 9a5). According to practical needs of anti-counterfeiting, the nanocomposites could be coated with various patterns on flexible substrates and applied in banknotes, medicine boxes, wine bottles, and medicine bottles (Fig. 9a6). The research work is important for designing the high-end anti-counterfeiting materials.
Fig. 9. Anti-counterfeiting applications.
a1 Photographs of erasable and rewritable macropolarization-related patterns of nanocomposite film. Scale bars stand for 0.5 mm. a2 Photographs of macroscopic polarization-dependent patterns under unpolarized and polarized light. Scale bars stand for 0.5 mm. a3 Polarization microscope photographs of microscopic polarization correlation patterns. Scale bars: 200 μm. a4 QR code pattern on different backgrounds under polarized light microscope. Scale bars: 200 μm. a5 Photographs of photochromic UCL patterns of PAzo/UCNPs under NIR light. Scale bars stand for 5 mm. a6 PAzo/UCNPs nanocomposite for different anti-counterfeiting applications150. b1 (i) TEM image of NaYF4:Yb3+,Er3+ UCNPs. (ii) TEM image of UCNP@CsMnCl3 nanocomposites. (iii) The magnified TEM images enclosed by the red frame in (ii). (iv) High-resolution image of UCNP@CsMnCl3 recorded by spherical aberration electron microscopy. b2 Photoluminescence spectra of UCNP@CsMnCl3 excited by 980 nm laser and 365 nm UV light. Inset: luminescence photograph of UCNP@CsMnCl3 under irradiation by 980 nm laser. b3 Dual-modal light-emitting anticounterfeiting principle diagram. b4 Images of security patterns made of pure CsMnCl3 and UCNP@CsMnCl3 under diverse excitation modes (sunlight, UV light, NIR laser)158. a1–a6 Reprinted with permission from ref. 150 Copyright 2021, John Wiley and Sons. b1–b4 Reprinted with permission from ref. 158 Copyright 2021, John Wiley and Sons
Recently, a series of nanocomposites have been developed by coupling UCNPs with perovskite quantum dots, which play the important roles in multi-modal anti-counterfeiting153–158. Our group153 designed UCNPs–CsPbX3 (UCX3) nanocomposites, emitting multicolor UCL/DSL under 980 nm laser or 365 nm lamp excitation. In addition, UCX3/polystyrene with multicolor fluorescence and dual-modal luminescence features could be used as a quick drying fluorescent ink for writing and printing, which greatly increase the difficulty of fraud and provide insight for the practical application in anti-counterfeiting. Lin et al. 158 synthesized UCNP@CsMnCl3 nanocomposites, which play an important role in high-quality optical anti-counterfeiting. NaYF4:Yb3+,Er3+ was used as core, followed by heteroepitaxial growth of CsMnCl3 to obtain the core–shell structure (Fig. 9b1). UCNP@CsMnCl3 exhibits the characteristic UCL of Er3+ (980 nm excitation, Fig. 9b2, top). Broad blue DSL of CsMnCl3 is realized upon excitation with 365 nm light (Fig. 9b2, bottom). To verify the anti-counterfeiting capability, CsMnCl3 and UCNP@CsMnCl3 are used initially to prepare dot-based patterns. Fig. 9b3 shows the relevant encryption and decryption process, and Fig. 9b4 shows the three anti-counterfeiting modes based on this design. The decryption cannot be completed under sunlight and UV light irradiation. Under excitation at 980 nm, only UCNP@CsMnCl3 exhibits green emission, enabling decryption. Subsequently, they utilize UCNP@CsMnCl3 to make more complex graphs, and use CsMnCl3 to fill the blank space, then encryption mode with high encryption level could be obtained. It is concluded that UCNP@CsMnCl3 are undoubtedly suitable for high-level anti-counterfeiting and high-capacity information encryption.
UCNPs/CDs (CDs = carbon dots) dual-modal luminescent materials have attracted extensive attention159,160. Xu et al. 160 prepared UCNPs@CDs@mSiO2 nanocomposites that can produce red, green, and blue UCL (980 nm laser irradiation) and blue DSL (365 nm light excitation). The nanocomposites could be further manufactured into different luminescent inks to produce highly safe anti-counterfeiting barcodes. Besides, luminescent composites with DSL and UCL such as Gd2O3:Yb3+/Er3+/Eu(DBM)3Phen161, YVO4:Er3+,Yb3+@YPO4:Eu3+ 162, and Y2O3:Er3+,Yb3+@SiO2@HPU-19b@Eu3+(Tb3+)163, have been used as dual-modal fluorescent inks and barcode, which are potential nanomaterials for anti-counterfeiting.
Photocatalysis
Recently, the preparation of photocatalysts with broad-spectrum (UV to NIR range) absorption properties to realize the effective utilization of solar energy in various fields (photocatalytic hydrogen production, elimination of environmental pollutants, antibacterial, etc.) has been a hot topic of research164–168. Upconversion luminescent materials could absorb NIR light and convert it into UV/Vis light. Therefore, the photocatalysts can be constructed by combining the upconversion material and semiconductor. The resulting nanocomposites could be excited by NIR light and generate photogenerated holes (h+) and electrons (e−), which could take advantage of sunlight and enhance the photocatalytic efficiency. A variety of UCNPs/semiconductor nanocomposites have been fabricated, such as UCNPs/TiO2, UCNPs/CdS, UCNPs/ZnO, etc. complex systems.
UCNPs/TiO2
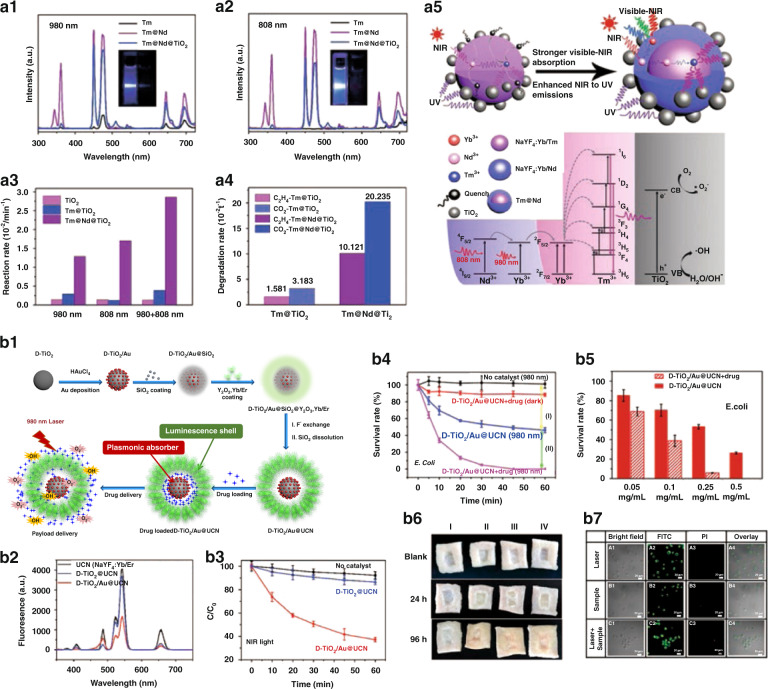
TiO2 has been widely studied in photocatalytic degradation of organic and inorganic pollutants due to its high catalytic activity, non-toxic and low cost. However, TiO2 could only be excited by UV light resulting from its band gap (3.2 eV), which greatly limits its application of photocatalysis. UCNPs/TiO2 nanocomposites could avoid the above problem and be served as the photocatalytic materials irradiated by NIR light169–174. Huang et al. 171 designed NaYF4:Yb3+,Tm3+@NaYF4:Yb3+,Nd3+@TiO2 (Tm@Nd@TiO2) nanocomposites for NIR photocatalysis. When Tm@Nd was modified with TiO2, UV emission of nanocomposites was greatly reduced irradiated at 980 or 808 nm (Fig. 10a1, a2). The Rhodamine B degradation rate constants of nanocomposites under 980, 808, and 980 + 808 nm laser excitation are 4.40, 5.84 and 9.83 times as high as that of Tm@TiO2 under 980 nm excitation, respectively (Fig. 10a3). Under 980 + 808 nm laser excitation, the ethylene degradation rate constant of Tm@Nd@TiO2 is 6.4 times higher than that of Tm@TiO2 (Fig. 10a4). The enhanced photocatalytic activity of nanocomposites could be attributed to strong NIR absorption of Nd3+ and intense upconversion emission of UCNPs (Fig. 10a5). This study provides the route for further improving NIR light-mediated photocatalytic activity of TiO2-based upconversion photocatalysts. Song and co-workers172 prepared D-TiO2/Au@UCN nanocomposites and antibiotic drug ampicillin sodium (AMP) was loaded into D-TiO2/Au@UCN, which can be used as NIR-activated photocatalytic platform for bacterial inactivation (Fig. 10b1). Under 980 nm excitation, the UCL of D-TiO2/Au@UCN is significantly lower than that of pristine UCN, resulting from the efficient Vis light harvesting of D-TiO2@Au (Fig. 10b2). D-TiO2/Au@UCN can decompose more than 60% of rhodamine 6G (Fig. 10b3), which is much higher than that of the control and D-TiO2/UCN groups (degradation rate: 13.3%). Under NIR-light irradiation, almost no viable E. coli could be observed after incubation for 60 min, showing stronger bactericidal activity of AMP-loaded D-TiO2/Au@UCN (Fig. 10b4, b5). Such NIR light-triggered system exhibits excellent photocatalytic bactericidal performance under deep tissue penetration conditions (Fig. 10b6). The negligible cytotoxicity of AMP-loaded D-TiO2/Au@UCN was verified by cytotoxicity assay (Fig. 10b7). It could greatly expand TiO2-based photocatalysis in destroying antibiotic-resistant and heat-resistant microorganisms.
Fig. 10. Photocatalysis.
UCL spectra of NaYF4:Yb3+,Tm3+ (Tm), Tm@Nd, and Tm@Nd@TiO2 under 980 nm excitation (a1) and 808 nm excitation (a2). The insets of a1 and a2 are luminescence photographs of Tm@Nd (left) and NaYF4:Yb3+,Tm3+ (right) with the same concentration dispersed in cyclohexane under 980 and 808 nm excitation, respectively. Insets of a1 and a2: UCL photographs of Tm@Nd (left) and NaYF4:Yb3+,Tm3+ (right) under 980 and 808 nm laser excitation, respectively. a3 The degradation rates of Rhodamine B by different samples under excitation at 980, 808, and 980 + 808 nm. a4 Degradation rates of C2H4 by Tm@TiO2 and Tm@Nd@TiO2 photocatalysts. a5 Schematic diagram of enhanced photocatalytic activity and upconversion photocatalytic mechanism171. b1 Schematic diagram of preparation of drug-loaded D-TiO2/Au@UCN nanocomposites and NIR light-controlled drug release. b2 UCL spectra of UCN, D-TiO2@UCN, and D-TiO2/Au@UCN. b3 The time-dependent ratios of C/C0 in the presence of D-TiO2@UCN and D-TiO2/Au@UCN or without photocatalyst for rhodamine 6G degradation under NIR light irradiation. b4 E. coli viability treated with D-TiO2/Au@UCN and AMP-loaded D-TiO2/Au@UCN in the dark or under 980 nm irradiation. b5 Survival rate of E. coli under different sample concentrations under NIR light irradiation for 30 min. b6 Photographs of the bacterial contamination for pig skin in 96 h. Grouping of infected skin (I) drug-loaded D-TiO2/Au@UCN + NIR light, (II) drug-loaded D-TiO2/Au@UCN in the dark; (III) NIR light irradiation, and (IV) in the dark. b7 Confocal fluorescence images of HaCaT cells co-stained with V-fluorescein isothiocyanate and propidium iodide after different treatments172. a1–a5 Reprinted with permission from ref. 171 Copyright 2019, Elsevier. b1–b7 Reprinted with permission from ref. 172. Copyright 2020, American Chemical Society
UCNPs/ZnO
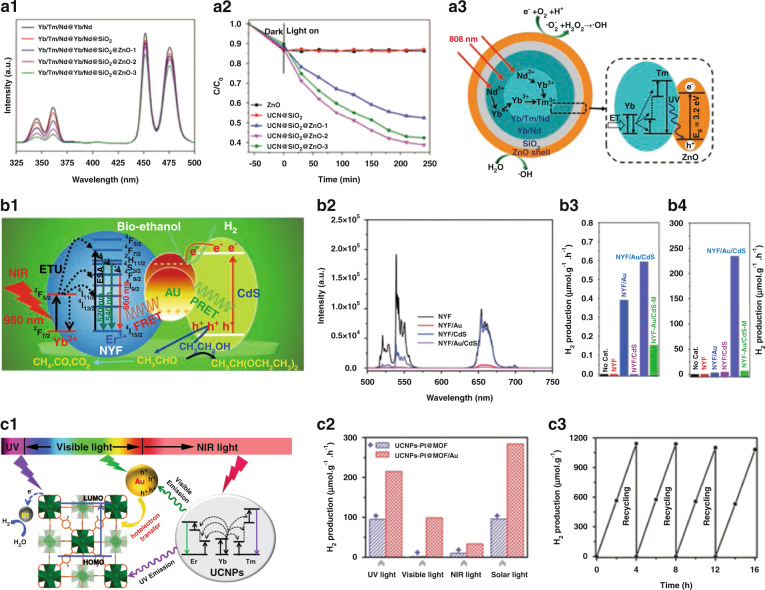
ZnO with a wide bandgap of ~3.37 eV is also widely used in photocatalytic and antibacterial fields. ZnO produces photocatalytic effect under UV light excitation, which restricts its applications in Vis and NIR range. To date, UCNPs@ZnO heterojunction structures have been constructed, which have widened the light response range of ZnO-based photocatalytic materials and improved the photocatalytic activity under NIR light irradiation175–178. Li’s group175 developed NaYF4:Yb/Tm@SiO2@ZnO nanocomposites with ideal NIR photocatalytic activity in degradation of organic pollutants and antibacterial activity. Qiao et al. 176 prepared NaYF4:Yb,Tm,Nd@NaYF4:Yb,Nd@SiO2@ZnO (UCN@SiO2@ZnO), which presented good NIR-induced photocatalytic activity. The UV UCL of UCN@SiO2@ZnO could be effectively harvested by ZnO to activate photocatalytic process (Fig. 11a1). UCN@SiO2@ZnO shows high degradation rate of Rhodamine B (61.2%) after 808 nm irradiation for 250 min (Fig. 11a2). The upconversion UV emission absorbed by ZnO could effectively generate photogenerated e− and h+. With the oxidation–reduction reaction occurs between the substances adsorbed on the surface of ZnO and photogenerated carrier, •OH radicals could be generated to participate in the photocatalytic reaction process as an active substance (Fig. 11a3). This study has important value for developing composite photocatalysts with excellent NIR photoresponsive properties.
Fig. 11. Photocatalysis.
a1 UCL spectra of different samples. a2 Time-dependent ratios of C/C0 for Rhodamine B with different samples as photocatalysts. a3 ET process in UCNPs and the proposed photocatalytic mechanism for UCN@SiO2@ZnO176. b1 ET processes between NYF, Au, and CdS, and evolution process of bio-ethanol photoreformed H2 under NIR irradiation. b2 UCL spectra of NYF, NYF/Au, NYF/CdS and NYF/Au/CdS. Photocatalytic H2 evolution rates of different samples under NIR (b3) and simulated sunlight (b4)179. c1 Schematic diagram of photocatalytic H2 production mechanism of UCNPs-Pt@MOF/Au. c2 H2 production rates of UCNPs-Pt@MOF and UCNPs-Pt@MOF/Au under excitation by UV, Vis, NIR, and solar light. c3 Recycling test for H2 production of UCNPs-Pt@MOF/Au under simulated solar light164. a1–a3 Reprinted with permission from ref. 176 Copyright 2020, American Chemical Society. b1–b4 Reprinted with permission from ref. 179 Copyright 2017, The Royal Society of Chemistry. c1–c8 Reprinted with permission from ref. 164 Copyright 2018, John Wiley and Sons
UCNPs/CdS
CdS with narrow band gap is used to replace TiO2 or ZnO and coated on UCNPs, which can make full use of the converted UV and Vis light, improve the utilization of light and enhance the photocatalytic effect46,179,180. Li et al. 46 developed NaYF4:Yb,Tm@C@CdS nanocomposites for NIR-light enhanced photocatalysis. The photocatalytic activity of such nanocomposite was higher than that of CdS and the mixture of NaYF4:Yb,Tm and CdS. Liu et al. 179 prepared NaYF4:Yb/Er@Au@CdS (NYF/Au/CdS) for H2 production by photoreforming of bio-ethanol, where Au component promoted the electron–hole separation through FRET and plasmonic resonance energy transfer (PRET) (Fig. 11b1). The UCL of NaYF4:Yb/Er significantly reduced after modification with Au or CdS. For NYF/Au/CdS, the upconversion emissions further diminish (Fig. 11b2). NYF/Au/CdS exhibits promoted NIR light-induced photocatalytic bio-ethanol-reforming activity and has the highest H2 yield (0.59 μmol g−1 h−1) compared with NYF, NYF/Au, and NYF/CdS (Fig. 11b3). Furthermore, NYF/Au/CdS exhibits the largest H2 evolution rate under simulated sunlight illumination (Fig. 11b4). This work provides new strategy for developing efficient NIR-driven UC photocatalytic systems.
Other UCNPs/semiconductor nanocomposite photocatalysts
Recently, Jiang’s group164 prepared UCNPs-Pt@MOF/Au with broad-spectrum absorption characteristic. MOF mainly responds to UV light and Au nanoparticles with plasma resonance effect absorb Vis light, while UCNPs convert NIR light into UV and Vis light, which could be captured by adjacent MOF and Au again, to realize the absorption and utilization of composite materials from UV to NIR range (Fig. 11c1). UCNPs–Pt@MOF/Au nanocomposites show considerable H2 production rate irradiated by UV, Vis, and even NIR laser (Fig. 11c2). Importantly, UCNPs–Pt@MOF/Au exhibits good recovery performance under simulated sunlight irradiation, and H2 production rate do not change significantly during four catalytic cycles of 16 h (Fig. 11c3). This work opens a way to harness NIR light for photocatalysis. g-C3N4 has become one of the most promising photocatalysts driven by Vis light. Park et al. 181 designed UCNPs/g-C3N4 nanocomposites with photocatalytic activity much better than pure g-C3N4.
Conclusions and outlook
In summary, UCNPs-based nanocomposites are versatile candidates to utilize the UCL characteristics and have great potentials in various applications. This review summarizes the main methods for constructing UCNPs-based nanocomposites, and the applications of such nanocomposites in bioimaging, cancer treatments, anti-counterfeiting, and photocatalysis. Notably, although promising advance has been made in the preparation strategies and applications of UCNPs-based nanocomposites, there are still great challenges in the following aspects.
The existing synthesis methods for UCNPs-based nanocomposites still have shortcomings and improvements. Self-assembly method often has disadvantages of time-consuming, easy aggregation, weak adsorption, and the structure is easily to be destroyed under the action of some solvents. In-situ growth method often needs to modify or coat polymers or complexes on UCNPs as precursors, and then the precursors act as nucleation and growth centers to induce other nanodots to be grown further. This also motivates us to develop more novel modified materials, which can not only ensure that the luminescence of UCNPs will not be quenched too much, but also pave the way for the further growth of other materials. The epitaxial growth method usually uses toxic, expensive precursors or organic solvents, and the products are hydrophobic, and the synthesis temperature is relatively high. Heteroepitaxial growth method requires more severe conditions, and is impossible to track the reaction process in situ. Thus, it is difficult to expound the reaction mechanism exactly. Other facile methods to synthesize UCNPs-based nanocomposites remain to be optimized and explored.
The biological applications of UCNPs-based nanocomposites are still in the preliminary stage of research, and there are many problems to be solved before realizing clinical transformation. It is necessary to further rationally optimize the chemical composition and structure, reasonable particle size, and surface properties of UCNPs-based nanocomposites to construct nanoplatforms with high uniformity and excellent biocompatibility. Most importantly, to quantitatively load functional molecules (such as photosensitive molecules, anticancer drugs, etc.) and achieve the controllable release are of great significance. It cannot be ignored that reducing the biotoxicity, improving metabolic efficiency in vivo, and ensuring the reproducibility of diagnosis and treatment effects are the prerequisites for the future biological applications of UCNPs-based nanocomposites.
The development of superior luminescent nanomaterials and high-tech fluorescent anti-counterfeiting technologies is of great significance for the global economy, security, and human health, which has been proven to be a huge challenge. Tunable multicolor, multi-modal luminescent nanocomposites have been achieved by combining UCNPs with other luminescent components, which could greatly improve the anti-counterfeiting level. Although multiple anti-counterfeiting materials could be used simultaneously to impart multiple security features, the implementation process is complex and leads to low efficiency of production. This motivates researchers to develop novel UCNPs-based nanocomposites with multiple anti-counterfeiting features, different identification methods and easy processing to promote their practical applications. To improve the overall photostability of UCNPs-based nanocomposites is an important direction.
UCNPs@semiconductor photocatalysts have unique core-shell structure and good photocatalytic activity under sunlight. Although researchers have made great progress in UCNPs-based nanocomposites with certain photocatalytic activity in the UV, Vis and NIR regions by enhancing UCL of UCNPs, introducing semiconductors, and constructing heterostructures, etc., the utilization rate of Vis and NIR light is still unsatisfactory. The following problems need to be considered and solved: (a) The photocatalytic mechanism of nanocomposites needs to be further studied. (b) The photocatalytic efficiency is not satisfactory. The fluorescence intensity of UCNPs needs to be improved. The surface-enhanced fluorescence phenomenon can be generated by introducing nano-precious metals to improve its fluorescence intensity; new fluorescent materials should be developed to absorb much NIR energy and improve the conversion rate.
The design and applications of UCNPs-based nanocomposites are still in the infancy, the research of fundamental theoretical and practical applications will face complex challenges. This requires close collaboration between researchers from different disciplines to overcome the problems. It is believed that UCNPs-based nanocomposites will lead to major changes in life sciences, anti-counterfeiting technology, optoelectronics, energy catalysis, and other fields.
Acknowledgements
We acknowledge financial support from the National Key Research and Development Program of China (2021YFF0701800), National Natural Science Foundation of China (21871248, 21834007, and 22020102003), the International Partnership Program of Chinese Academy of Sciences (121522KYSB20190022), and K. C. Wong Education Foundation (GJTD-2018-09).
Conflict of interest
The authors declare no competing interests.
Contributor Information
Jing Feng, Email: fengj@ciac.ac.cn.
Hongjie Zhang, Email: hongjie@ciac.ac.cn.
References
- 1.Wang F, Liu XG. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem. Soc. Rev. 2009;38:976–989. doi: 10.1039/b809132n. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, et al. Light upconverting core–shell nanostructures: nanophotonic control for emerging applications. Chem. Soc. Rev. 2015;44:1680–1713. doi: 10.1039/C4CS00170B. [DOI] [PubMed] [Google Scholar]
- 3.Dong H, Sun LD, Yan CH. Energy transfer in lanthanide upconversion studies for extended optical applications. Chem. Soc. Rev. 2015;44:1608–1634. doi: 10.1039/C4CS00188E. [DOI] [PubMed] [Google Scholar]
- 4.Lei PP, Feng J, Zhang HJ. Emerging biomaterials: taking full advantage of the intrinsic properties of rare earth elements. Nano Today. 2020;35:100952. doi: 10.1016/j.nantod.2020.100952. [DOI] [Google Scholar]
- 5.Zheng KZ, et al. Recent advances in upconversion nanocrystals: expanding the kaleidoscopic toolbox for emerging applications. Nano Today. 2019;29:100797. doi: 10.1016/j.nantod.2019.100797. [DOI] [Google Scholar]
- 6.Zhang Z, Zhang Y. Orthogonal emissive upconversion nanoparticles: material design and applications. Small. 2021;17:2004552. doi: 10.1002/smll.202004552. [DOI] [PubMed] [Google Scholar]
- 7.Yi ZG, et al. Lanthanide-activated nanoparticles: a toolbox for bioimaging, therapeutics, and neuromodulation. Acc. Chem. Res. 2020;53:2692–2704. doi: 10.1021/acs.accounts.0c00513. [DOI] [PubMed] [Google Scholar]
- 8.Chen GY, et al. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem. Rev. 2014;114:5161–5214. doi: 10.1021/cr400425h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou B, et al. Controlling upconversion nanocrystals for emerging applications. Nat. Nanotechnol. 2015;10:924–936. doi: 10.1038/nnano.2015.251. [DOI] [PubMed] [Google Scholar]
- 10.Tsang MK, Bai GX, Hao JH. Stimuli responsive upconversion luminescence nanomaterials and films for various applications. Chem. Soc. Rev. 2015;44:1585–1607. doi: 10.1039/C4CS00171K. [DOI] [PubMed] [Google Scholar]
- 11.Wang F, et al. Microscopic inspection and tracking of single upconversion nanoparticles in living cells. Light: Sci. Appl. 2018;7:18007. doi: 10.1038/lsa.2018.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudry D, et al. Structure–property relationships in lanthanide-doped upconverting nanocrystals: recent advances in understanding core–shell structures. Adv. Mater. 2019;31:1900623. doi: 10.1002/adma.201900623. [DOI] [PubMed] [Google Scholar]
- 13.Deng RR, et al. Temporal full-colour tuning through non-steady-state upconversion. Nat. Nanotechnol. 2015;10:237–242. doi: 10.1038/nnano.2014.317. [DOI] [PubMed] [Google Scholar]
- 14.Downing E, et al. A three-color, solid-state, three-dimensional display. Science. 1996;273:1185–1189. doi: 10.1126/science.273.5279.1185. [DOI] [Google Scholar]
- 15.Huang XY, et al. Enhancing solar cell efficiency: the search for luminescent materials as spectral converters. Chem. Soc. Rev. 2013;42:173–201. doi: 10.1039/C2CS35288E. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, et al. Universal process-inert encoding architecture for polymer microparticles. Nat. Mater. 2014;13:524–529. doi: 10.1038/nmat3938. [DOI] [PubMed] [Google Scholar]
- 17.Rabie H, et al. NIR biosensing of neurotransmitters in stem cell-derived neural interface using advanced core-shell upconversion nanoparticles. Adv. Mater. 2019;31:1806991. doi: 10.1002/adma.201806991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, et al. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science. 2018;359:679–684. doi: 10.1126/science.aaq1144. [DOI] [PubMed] [Google Scholar]
- 19.Sedlmeier A, Gorris HH. Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications. Chem. Soc. Rev. 2015;44:1526–1560. doi: 10.1039/C4CS00186A. [DOI] [PubMed] [Google Scholar]
- 20.Mahata MK, De R, Lee KT. Near-infrared-triggered upconverting nanoparticles for biomedicine applications. Biomedicines. 2021;9:756. doi: 10.3390/biomedicines9070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boles MA, Engel M, Talapin DV. Self-assembly of colloidal nanocrystals: from intricate structures to functional materials. Chem. Rev. 2016;116:11220–11289. doi: 10.1021/acs.chemrev.6b00196. [DOI] [PubMed] [Google Scholar]
- 22.Xiao QF, et al. A core/satellite multifunctional nanotheranostic for in vivo imaging and tumor eradication by radiation/photothermal synergistic therapy. J. Am. Chem. Soc. 2013;135:13041–13048. doi: 10.1021/ja404985w. [DOI] [PubMed] [Google Scholar]
- 23.Lv RC, et al. Integration of upconversion nanoparticles and ultrathin black phosphorus for efficient photodynamic theranostics under 808 nm near-infrared light irradiation. Chem. Mater. 2016;28:4724–4734. doi: 10.1021/acs.chemmater.6b01720. [DOI] [Google Scholar]
- 24.Xu MS, et al. An intelligent nanoplatform for imaging-guided photodynamic/photothermal/chemo-therapy based on upconversion nanoparticles and CuS integrated black phosphorus. Chem. Eng. J. 2020;382:122822. doi: 10.1016/j.cej.2019.122822. [DOI] [Google Scholar]
- 25.Chan MH, et al. Near-infrared light-mediated photodynamic therapy nanoplatform by the electrostatic assembly of upconversion nanoparticles with graphitic carbon nitride quantum dots. Inorg. Chem. 2016;55:10267–10277. doi: 10.1021/acs.inorgchem.6b01522. [DOI] [PubMed] [Google Scholar]
- 26.Yuan Z, et al. Paving metal-organic frameworks with upconversion nanoparticles via self-assembly. J. Am. Chem. Soc. 2018;140:15507–15515. doi: 10.1021/jacs.8b10122. [DOI] [PubMed] [Google Scholar]
- 27.Li ZK, et al. Core-satellite metal-organic framework@upconversion nanoparticle superstructures via electrostatic self-assembly for efficient photodynamic theranostics. Nano Res. 2020;13:3377–3386. doi: 10.1007/s12274-020-3025-0. [DOI] [Google Scholar]
- 28.Li QF, et al. Efficient detection of environmental estrogens bisphenol A and estradiol by sensing system based on AuNP–AuNP–UCNP triple structure. Chin. J. Anal. Chem. 2018;46:486–492. doi: 10.1016/S1872-2040(17)61079-X. [DOI] [Google Scholar]
- 29.Kong JL, et al. Specific biosensing using DNA aptamers and nanopores. Adv. Funct. Mater. 2019;29:1807555. doi: 10.1002/adfm.201807555. [DOI] [Google Scholar]
- 30.Wang M, et al. Immunoassay of goat antihuman immunoglobulin G antibody based on luminescence resonance energy transfer between near-infrared responsive NaYF4: Yb, Er upconversion fluorescent nanoparticles and gold nanoparticles. Anal. Chem. 2009;81:8783–8789. doi: 10.1021/ac901808q. [DOI] [PubMed] [Google Scholar]
- 31.Wang LY, et al. Fluorescence resonant energy transfer biosensor based on upconversion-luminescent nanoparticles. Angew. Chem. Int. Ed. 2005;44:6054–6057. doi: 10.1002/anie.200501907. [DOI] [PubMed] [Google Scholar]
- 32.Li LL, et al. An exceptionally simple strategy for DNA-functionalized up-conversion nanoparticles as biocompatible agents for nanoassembly, DNA delivery, and imaging. J. Am. Chem. Soc. 2013;135:2411–2414. doi: 10.1021/ja310432u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ge H, et al. Sequence-dependent DNA functionalization of upconversion nanoparticles and their programmable assemblies. Angew. Chem. Int. Ed. 2020;59:8133–8137. doi: 10.1002/anie.202000831. [DOI] [PubMed] [Google Scholar]
- 34.Li S, et al. Hybrid nanoparticle pyramids for intracellular dual MicroRNAs biosensing and bioimaging. Adv. Mater. 2017;29:1606086. doi: 10.1002/adma.201606086. [DOI] [PubMed] [Google Scholar]
- 35.Wang YH, et al. ZnO-functionalized upconverting nanotheranostic agent: multi-modality imaging-guided chemotherapy with on-demand drug release triggered by pH. Angew. Chem. Int. Ed. 2015;54:536–540. doi: 10.1002/anie.201409519. [DOI] [PubMed] [Google Scholar]
- 36.Zhang KK, et al. Nanodiamonds conjugated upconversion nanoparticles for bio-imaging and drug delivery. J. Colloid Interface Sci. 2019;537:316–324. doi: 10.1016/j.jcis.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 37.Yang GX, et al. A core/shell/satellite anticancer platform for 808 NIR light-driven multimodal imaging and combined chemo-/photothermal therapy. Nanoscale. 2015;7:13747–13758. doi: 10.1039/C5NR03085D. [DOI] [PubMed] [Google Scholar]
- 38.Yang D, et al. Y2O3: Yb, Er@mSiO2-CuxS double-shelled hollow spheres for enhanced chemo-/photothermal anti-cancer therapy and dual-modal imaging. Nanoscale. 2015;7:12180–12191. doi: 10.1039/C5NR02269J. [DOI] [PubMed] [Google Scholar]
- 39.Wei RY, et al. In situ crystal growth of gold nanocrystals on upconversion nanoparticles for synergistic chemo-photothermal therapy. Nanoscale. 2017;9:12885–12896. doi: 10.1039/C7NR02280H. [DOI] [PubMed] [Google Scholar]
- 40.Wu QQ, et al. Universal multifunctional nanoplatform based on target-induced in situ promoting Au seeds growth to quench fluorescence of upconversion nanoparticles. ACS Sens. 2017;2:1805–1813. doi: 10.1021/acssensors.7b00616. [DOI] [PubMed] [Google Scholar]
- 41.Zhao S, et al. Designing of UCNPs@Bi@SiO2 hybrid theranostic nanoplatforms for simultaneous multimodal imaging and photothermal therapy. ACS Appl. Mater. Interfaces. 2019;11:394–402. doi: 10.1021/acsami.8b19304. [DOI] [PubMed] [Google Scholar]
- 42.Lv RC, et al. In situ growth strategy to integrate up-conversion nanoparticles with ultrasmall CuS for photothermal theranostics. ACS Nano. 2017;11:1064–1072. doi: 10.1021/acsnano.6b07990. [DOI] [PubMed] [Google Scholar]
- 43.Du KM, et al. Decoration of upconversion nanocrystals with metal sulfide quantum dots by a universal in situ controlled growth strategy. Nanoscale. 2020;12:3977–3987. doi: 10.1039/C9NR08708G. [DOI] [PubMed] [Google Scholar]
- 44.Du KM, et al. In situ decorating of ultrasmall Ag2Se on upconversion nanoparticles as novel nanotheranostic agent for multimodal imaging-guided cancer photothermal therapy. Appl. Mater. Today. 2020;18:100497. doi: 10.1016/j.apmt.2019.100497. [DOI] [Google Scholar]
- 45.Zhao S, et al. UCNP-Bi2Se3 upconverting nanohybrid for upconversion luminescence and CT imaging and photothermal therapy. Chemistry. 2020;26:1127–1135. doi: 10.1002/chem.201904586. [DOI] [PubMed] [Google Scholar]
- 46.Tou MJ, et al. Depositing CdS nanoclusters on carbon-modified NaYF4: Yb, Tm upconversion nanocrystals for NIR-light enhanced photocatalysis. Nanoscale. 2016;8:553–562. doi: 10.1039/C5NR06806A. [DOI] [PubMed] [Google Scholar]
- 47.Jiang MY, et al. A general in situ growth strategy of designing theranostic NaLnF4@Cu2-xS nanoplatform for in vivo NIR-II optical imaging beyond 1500 nm and photothermal therapy. Adv. Therap. 2019;2:1800153. doi: 10.1002/adtp.201800153. [DOI] [Google Scholar]
- 48.Liu SK, et al. Degradable calcium phosphate-coated upconversion nanoparticles for highly efficient chemo-photodynamic therapy. ACS Appl. Mater. Interfaces. 2019;11:47659–47670. doi: 10.1021/acsami.9b11973. [DOI] [PubMed] [Google Scholar]
- 49.Hu LJ, et al. The in situ “grafting from” approach for the synthesis of polymer brushes on upconversion nanoparticles via NIR-mediated RAFT polymerization. Polym. Chem. 2021;12:545–553. doi: 10.1039/D0PY01550D. [DOI] [Google Scholar]
- 50.Yi GS, Chow GM. Water-soluble NaYF4: Yb, Er(Tm)/NaYF4/polymer core/shell/shell nanoparticles with significant enhancement of upconversion fluorescence. Chem. Mater. 2007;19:341–343. doi: 10.1021/cm062447y. [DOI] [Google Scholar]
- 51.Homann C, et al. NaYF4: Yb, Er/NaYF4 core/shell nanocrystals with high upconversion luminescence quantum yield. Angew. Chem. Int. Ed. 2018;57:8765–8769. doi: 10.1002/anie.201803083. [DOI] [PubMed] [Google Scholar]
- 52.Johnson NJJ, et al. Self-focusing by Ostwald ripening: a strategy for layer-by-layer epitaxial growth on upconverting nanocrystals. J. Am. Chem. Soc. 2012;134:11068–11071. doi: 10.1021/ja302717u. [DOI] [PubMed] [Google Scholar]
- 53.Wang F, Wang J, Liu XG. Direct evidence of a surface quenching effect on size-dependent luminescence of upconversion nanoparticles. Angew. Chem. Int. Ed. 2010;49:7456–7460. doi: 10.1002/anie.201003959. [DOI] [PubMed] [Google Scholar]
- 54.Wu SL, et al. Morphology control of the NaGdF4: Yb, Tm@NaGdF4 core–shell nanostructure by tailoring the ratio of core to shell. CrystEngComm. 2017;19:5022–5027. doi: 10.1039/C7CE01232B. [DOI] [Google Scholar]
- 55.Tek S, et al. Atomic-scale structural analysis of homoepitaxial LaF3: Yb, Tm core–shell upconversion nanoparticles synthesized through a microwave route. Cryst. Growth Des. 2020;20:2153–2163. doi: 10.1021/acs.cgd.9b00902. [DOI] [Google Scholar]
- 56.Qian HS, Zhang Y. Synthesis of hexagonal-phase core-shell NaYF4 nanocrystals with tunable upconversion fluorescence. Langmuir. 2008;24:12123–12125. doi: 10.1021/la802343f. [DOI] [PubMed] [Google Scholar]
- 57.Vetrone F, et al. The active-core/active-shell approach: a strategy to enhance the upconversion luminescence in lanthanide-doped nanoparticles. Adv. Funct. Mater. 2009;19:2924–2929. doi: 10.1002/adfm.200900234. [DOI] [Google Scholar]
- 58.Liu YS, et al. A strategy to achieve efficient dual-mode luminescence of Eu3+ in lanthanides doped multifunctional NaGdF4 nanocrystals. Adv. Mater. 2010;22:3266–3271. doi: 10.1002/adma.201000128. [DOI] [PubMed] [Google Scholar]
- 59.Yang DM, et al. Colloidal synthesis and remarkable enhancement of the upconversion luminescence of BaGdF5: Yb3+/Er3+ nanoparticles by active-shell modification. J. Mater. Chem. 2011;21:5923–5927. doi: 10.1039/c0jm04179c. [DOI] [Google Scholar]
- 60.Su Y, et al. Core–shell–shell heterostructures of α-NaLuF4: Yb/Er@NaLuF4: Yb@MF2 (M = Ca, Sr, Ba) with remarkably enhanced upconversion luminescence. Dalton Trans. 2016;45:11129–11136. doi: 10.1039/C6DT01005A. [DOI] [PubMed] [Google Scholar]
- 61.Wang YF, et al. Rare-earth nanoparticles with enhanced upconversion emission and suppressed rare-earth-ion leakage. Chemistry. 2012;18:5558–5564. doi: 10.1002/chem.201103485. [DOI] [PubMed] [Google Scholar]
- 62.Shen J, et al. Tunable near infrared to ultraviolet upconversion luminescence enhancement in (α-NaYF4: Yb, Tm)/CaF2 core/shell nanoparticles for in situ real-time recorded biocompatible photoactivation. Small. 2013;9:3213–3217. doi: 10.1002/smll.201370117. [DOI] [PubMed] [Google Scholar]
- 63.Cai Q, et al. A pH-activable chemo-photodynamic therapy based on cube-wrapped-cube α-NaYbF4: Tm@CaF2/Nd@ZnO nanoparticles mediated by 808 nm light. Chem. Mater. 2020;32:7492–7506. doi: 10.1021/acs.chemmater.0c02624. [DOI] [Google Scholar]
- 64.Ning HR, et al. Manganese-mediated growth of ZnS shell on KMnF3: Yb, Er cores toward enhanced up/downconversion luminescence. ACS Appl. Mater. Interfaces. 2020;12:11934–11944. doi: 10.1021/acsami.9b21832. [DOI] [PubMed] [Google Scholar]
- 65.Swabeck JK, et al. Broadband sensitization of lanthanide emission with indium phosphide quantum dots for visible to near-infrared downshifting. J. Am. Chem. Soc. 2018;140:9120–9126. doi: 10.1021/jacs.8b02612. [DOI] [PubMed] [Google Scholar]
- 66.Gu YY, et al. High-sensitivity imaging of time-domain near-infrared light transducer. Nat. Photonics. 2019;13:525–531. doi: 10.1038/s41566-019-0437-z. [DOI] [Google Scholar]
- 67.Ruan LF, Zhang Y. NIR-excitable heterostructured upconversion perovskite nanodots with improved stability. Nat. Commun. 2021;12:219. doi: 10.1038/s41467-020-20551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Idris NM, et al. Photoactivation of core–shell titania coated upconversion nanoparticles and their effect on cell death. J. Mater. Chem. B. 2014;2:7017–7026. doi: 10.1039/C4TB01169D. [DOI] [PubMed] [Google Scholar]
- 69.Lucky SS, et al. Titania coated upconversion nanoparticles for near-infrared light triggered photodynamic therapy. ACS Nano. 2015;9:191–205. doi: 10.1021/nn503450t. [DOI] [PubMed] [Google Scholar]
- 70.Zou XM, et al. A water-dispersible dye-sensitized upconversion nanocomposite modified with phosphatidylcholine for lymphatic imaging. Chem. Commun. 2016;52:13389–13392. doi: 10.1039/C6CC07180E. [DOI] [PubMed] [Google Scholar]
- 71.Park YI, et al. Upconverting nanoparticles: a versatile platform for wide-field two-photon microscopy and multi-modal in vivo imaging. Chem. Soc. Rev. 2015;44:1302–1317. doi: 10.1039/C4CS00173G. [DOI] [PubMed] [Google Scholar]
- 72.Li P, et al. Thermo-activatable PNIPAM-functionalized lanthanide-doped upconversion luminescence nanocomposites used for in vitro imaging. RSC Adv. 2017;7:50643–50647. doi: 10.1039/C7RA10859A. [DOI] [Google Scholar]
- 73.Choi JE, et al. 800 nm near-infrared light-excitable intense green-emitting Li(Gd, Y)F4: Yb, Er-based core/shell/shell upconversion nanophosphors for efficient liver cancer cell imaging. Mater. Des. 2020;195:108941. doi: 10.1016/j.matdes.2020.108941. [DOI] [Google Scholar]
- 74.Liang T, et al. Removing the obstacle of dye-sensitized upconversion luminescence in aqueous phase to achieve high-contrast deep imaging in vivo. Adv. Funct. Mater. 2020;30:1910765. doi: 10.1002/adfm.201910765. [DOI] [Google Scholar]
- 75.Sun Y, et al. Core-shell lanthanide upconversion nanophosphors as four-modal probes for tumor angiogenesis imaging. ACS Nano. 2013;7:11290–11300. doi: 10.1021/nn405082y. [DOI] [PubMed] [Google Scholar]
- 76.Luo Y, et al. Core@shell Fe3O4@Mn2+-doped NaYF4: Yb/Tm nanoparticles for triple-modality T1/T2-weighted MRI and NIR-to-NIR upconversion luminescence imaging agents. RSC Adv. 2017;7:37929–37937. doi: 10.1039/C7RA07460C. [DOI] [Google Scholar]
- 77.Mukherjee P, et al. Facile strategy to synthesize magnetic upconversion nanoscale metal–organic framework composites for theranostics application. ACS Appl. Bio Mater. 2020;3:869–880. doi: 10.1021/acsabm.9b00949. [DOI] [PubMed] [Google Scholar]
- 78.Ding BB, et al. MnO2-disguised upconversion hybrid nanocomposite: an ideal architecture for tumor microenvironment-triggered UCL/MR bioimaging and enhanced chemodynamic therapy. Chem. Mater. 2019;31:2651–2660. doi: 10.1021/acs.chemmater.9b00893. [DOI] [Google Scholar]
- 79.Li XX, et al. Peptide-enhanced tumor accumulation of upconversion nanoparticles for sensitive upconversion luminescence/magnetic resonance dual-mode bioimaging of colorectal tumors. Acta Biomater. 2020;104:167–175. doi: 10.1016/j.actbio.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 80.Qiao H, et al. Targeting osteocytes to attenuate early breast cancer bone metastasis by theranostic upconversion nanoparticles with responsive plumbagin release. ACS Nano. 2017;11:7259–7273. doi: 10.1021/acsnano.7b03197. [DOI] [PubMed] [Google Scholar]
- 81.Zhu XJ, et al. Core–shell Fe3O4@NaLuF4: Yb, Er/Tm nanostructure for MRI, CT and upconversion luminescence tri-modality imaging. Biomaterials. 2012;33:4618–4627. doi: 10.1016/j.biomaterials.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 82.Yamini S, et al. NaGdF4: Yb, Er-Ag nanowire hybrid nanocomposite for multifunctional upconversion emission, optical imaging, MRI and CT imaging applications. Microchim. Acta. 2020;187:317. doi: 10.1007/s00604-020-04285-9. [DOI] [PubMed] [Google Scholar]
- 83.Liu Y, et al. Deep photoacoustic/luminescence/magnetic resonance multimodal imaging in living subjects using high-efficiency upconversion nanocomposites. Adv. Mater. 2016;28:6411–6419. doi: 10.1002/adma.201506460. [DOI] [PubMed] [Google Scholar]
- 84.Lv RC, et al. Stable ICG-loaded upconversion nanoparticles: silica core/shell theranostic nanoplatform for dual-modal upconversion and photoacoustic imaging together with photothermal therapy. Sci. Rep. 2017;7:15753. doi: 10.1038/s41598-017-16016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carvalho C, et al. Doxorubicin: the good, the bad and the ugly effect. Curr. Med. Chem. 2009;16:3267–3285. doi: 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- 86.Upadhyay KK, et al. The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly(γ-benzyl l-glutamate)-b-hyaluronan polymersomes. Biomaterials. 2010;31:2882–2892. doi: 10.1016/j.biomaterials.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 87.Campillos M, et al. Drug target identification using side-effect similarity. Science. 2008;321:263–266. doi: 10.1126/science.1158140. [DOI] [PubMed] [Google Scholar]
- 88.Wang KY, et al. Novel vesicles self-assembled from amphiphilic star-armed PEG/polypeptide hybrid copolymers for drug delivery. Macromol. Biosci. 2011;11:65–71. doi: 10.1002/mabi.201000247. [DOI] [PubMed] [Google Scholar]
- 89.Xu CY, et al. Biodegradable nanoparticles of polyacrylic acid-stabilized amorphous CaCO3 for tunable pH-responsive drug delivery and enhanced tumor inhibition. Adv. Funct. Mater. 2019;29:1808146. doi: 10.1002/adfm.201808146. [DOI] [Google Scholar]
- 90.Hou ZY, et al. Up-conversion luminescent and porous NaYF4: Yb3+, Er3+@SiO2 nanocomposite fibers for anti-cancer drug delivery and cell imaging. Adv. Funct. Mater. 2012;22:2713–2722. doi: 10.1002/adfm.201200082. [DOI] [Google Scholar]
- 91.Liu B, et al. Poly(acrylic acid) modification of Nd3+-sensitized upconversion nanophosphors for highly efficient UCL imaging and pH-responsive drug delivery. Adv. Funct. Mater. 2015;25:4717–4729. doi: 10.1002/adfm.201501582. [DOI] [Google Scholar]
- 92.Zhang L, Jin DY, Stenzel MH. Polymer-functionalized upconversion nanoparticles for light/imaging-guided drug delivery. Biomacromolecules. 2021;22:3168–3201. doi: 10.1021/acs.biomac.1c00669. [DOI] [PubMed] [Google Scholar]
- 93.Zhao S, et al. One-pot synthesis of Ln3+-doped porous BiF3@PAA nanospheres for temperature sensing and pH-responsive drug delivery guided by CT imaging. Nanoscale. 2020;12:695–702. doi: 10.1039/C9NR09401F. [DOI] [PubMed] [Google Scholar]
- 94.Hu F, et al. Real-time monitoring of pH-responsive drug release using a metal-phenolic network-functionalized upconversion nanoconstruct. Nanoscale. 2019;11:9201–9206. doi: 10.1039/C9NR01892A. [DOI] [PubMed] [Google Scholar]
- 95.Chen Y, et al. NIR-light-activated ratiometric fluorescent hybrid micelles for high spatiotemporally controlled biological imaging and chemotherapy. Small. 2020;16:2005667. doi: 10.1002/smll.202005667. [DOI] [PubMed] [Google Scholar]
- 96.Ling DP, et al. Heterodimers made of metal-organic frameworks and upconversion nanoparticles for bioimaging and pH-responsive dual-drug delivery. J. Mater. Chem. B. 2020;8:1316–1325. doi: 10.1039/C9TB02753J. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Z, et al. Modularly assembled upconversion nanoparticles for orthogonally controlled cell imaging and drug delivery. ACS Appl. Mater. Interfaces. 2020;12:12549–12556. doi: 10.1021/acsami.0c00672. [DOI] [PubMed] [Google Scholar]
- 98.Han RL, et al. Facilitating drug release in mesoporous silica coated upconversion nanoparticles by photoacid assistance upon near-infrared irradiation. Adv. Powder Technol. 2020;31:3860–3866. doi: 10.1016/j.apt.2020.07.025. [DOI] [Google Scholar]
- 99.Zhou J, et al. NIR photothermal therapy using polyaniline nanoparticles. Biomaterials. 2013;34:9584–9592. doi: 10.1016/j.biomaterials.2013.08.075. [DOI] [PubMed] [Google Scholar]
- 100.Choi WI, et al. Tumor regression in vivo by photothermal therapy based on gold-nanorod-loaded, functional nanocarriers. ACS Nano. 2011;5:1995–2003. doi: 10.1021/nn103047r. [DOI] [PubMed] [Google Scholar]
- 101.Xiao ZY, et al. DNA self-assembly of targeted near-infrared-responsive gold nanoparticles for cancer thermo-chemotherapy. Angew. Chem. Int. Ed. 2012;51:11853–11857. doi: 10.1002/anie.201204018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang W, et al. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials. 2011;32:8555–8561. doi: 10.1016/j.biomaterials.2011.07.071. [DOI] [PubMed] [Google Scholar]
- 103.Kam NWS, et al. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc. Natl Acad. Sci. USA. 2005;102:11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang WT, et al. Albumin-bioinspired Gd:CuS nanotheranostic agent for in vivo photoacoustic/magnetic resonance imaging-guided tumor-targeted photothermal therapy. ACS Nano. 2016;10:10245–10257. doi: 10.1021/acsnano.6b05760. [DOI] [PubMed] [Google Scholar]
- 105.Liu T, et al. Drug delivery with PEGylated MoS2 nano-sheets for combined photothermal and chemotherapy of cancer. Adv. Mater. 2014;26:3433–3440. doi: 10.1002/adma.201305256. [DOI] [PubMed] [Google Scholar]
- 106.Cheng L, et al. FeSe2-decorated Bi2Se3 nanosheets fabricated via cation exchange for chelator-free 64Cu-labeling and multimodal image-guided photothermal-radiation therapy. Adv. Funct. Mater. 2016;26:2185–2197. doi: 10.1002/adfm.201504810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sheng ZH, et al. Smart human serum albumin-indocyanine green nanoparticles generated by programmed assembly for dual-modal imaging-guided cancer synergistic phototherapy. ACS Nano. 2014;8:12310–12322. doi: 10.1021/nn5062386. [DOI] [PubMed] [Google Scholar]
- 108.Zha ZB, et al. Uniform polypyrrole nanoparticles with high photothermal conversion efficiency for photothermal ablation of cancer cells. Adv. Mater. 2013;25:777–782. doi: 10.1002/adma.201202211. [DOI] [PubMed] [Google Scholar]
- 109.Liu YL, et al. Dopamine-melanin colloidal nanospheres: an efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013;25:1353–1359. doi: 10.1002/adma.201204683. [DOI] [PubMed] [Google Scholar]
- 110.Chen J, et al. Developing a pH-sensitive Al(OH)3 layer-mediated UCNP@Al(OH)3/Au nanohybrid for photothermal therapy and fluorescence imaging in vivo. J. Mater. Chem. B. 2018;6:7862–7870. doi: 10.1039/C8TB02213E. [DOI] [PubMed] [Google Scholar]
- 111.Wang C, et al. A novel core–shell structured upconversion nanorod as a multimodal bioimaging and photothermal ablation agent for cancer theranostics. J. Mater. Chem. B. 2018;6:2597–2607. doi: 10.1039/C7TB02842C. [DOI] [PubMed] [Google Scholar]
- 112.Meng XF, et al. Accurate and real-time temperature monitoring during MR imaging guided PTT. Nano Lett. 2020;20:2522–2529. doi: 10.1021/acs.nanolett.9b05267. [DOI] [PubMed] [Google Scholar]
- 113.Wang SH, et al. Nanostructures based on vanadium disulfide growing on UCNPs: simple synthesis, dual-mode imaging, and photothermal therapy. J. Mater. Chem. B. 2020;8:5883–5891. doi: 10.1039/D0TB00993H. [DOI] [PubMed] [Google Scholar]
- 114.Ding X, et al. Multifunctional core/satellite polydopamine@Nd3+-sensitized upconversion nanocomposite: a single 808 nm near-infrared light-triggered theranostic platform for in vivo imaging-guided photothermal therapy. Nano Res. 2017;10:3434–3446. doi: 10.1007/s12274-017-1555-x. [DOI] [Google Scholar]
- 115.Ramírez-García G, et al. Theranostic nanocomplex of gold-decorated upconversion nanoparticles for optical imaging and temperature-controlled photothermal therapy. J. Photochem. Photobiol. A: Chem. 2019;384:112053. doi: 10.1016/j.jphotochem.2019.112053. [DOI] [Google Scholar]
- 116.Lucky SS, Soo KC, Zhang Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015;115:1990–2042. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- 117.Cai HJ, et al. A core–shell metal-organic-framework (MOF)-based smart nanocomposite for efficient NIR/H2O2-responsive photodynamic therapy against hypoxic tumor cells. J. Mater. Chem. B. 2017;5:2390–2394. doi: 10.1039/C7TB00314E. [DOI] [PubMed] [Google Scholar]
- 118.Zuo J, et al. Near infrared light sensitive ultraviolet-blue nanophotoswitch for imaging-guided “off–on” therapy. ACS Nano. 2018;12:3217–3225. doi: 10.1021/acsnano.7b07393. [DOI] [PubMed] [Google Scholar]
- 119.Yu ZZ, et al. A pre-protective strategy for precise tumor targeting and efficient photodynamic therapy with a switchable DNA/upconversion nanocomposite. Chem. Sci. 2018;9:3563–3569. doi: 10.1039/C8SC00098K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Z, et al. Controllable assembly of upconversion nanoparticles enhanced tumor cell penetration and killing efficiency. Adv. Sci. 2020;7:2001831. doi: 10.1002/advs.202001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang Y, et al. Upconverting nanocomposites with combined photothermal and photodynamic effects. Nanoscale. 2018;10:791–799. doi: 10.1039/C7NR05499H. [DOI] [PubMed] [Google Scholar]
- 122.Wang M, et al. One-pot synthesis of theranostic nanocapsules with lanthanide doped nanoparticles. Chem. Sci. 2020;11:6653–6661. doi: 10.1039/D0SC01033B. [DOI] [Google Scholar]
- 123.He LC, et al. DNA-assembled core-satellite upconverting-metal-organic framework nanoparticle superstructures for efficient photodynamic therapy. Small. 2017;13:1700504. doi: 10.1002/smll.201700504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kowalik P, et al. The ROS-generating photosensitizer-free NaYF4: Yb, Tm@SiO2 upconverting nanoparticles for photodynamic therapy application. Nanotechnology. 2021;32:475101. doi: 10.1088/1361-6528/abe892. [DOI] [PubMed] [Google Scholar]
- 125.Xu WH, et al. Three-pronged attack by homologous far-red/NIR AIEgens to achieve 1 + 1 +1 > 3 synergistic enhanced photodynamic therapy. Angew. Chem. Int. Ed. 2020;59:9610–9616. doi: 10.1002/anie.202000740. [DOI] [PubMed] [Google Scholar]
- 126.Wu WB, et al. A highly efficient and photostable photosensitizer with near-infrared aggregation-induced emission for image-guided photodynamic anticancer therapy. Adv. Mater. 2017;29:1700548. doi: 10.1002/adma.201700548. [DOI] [PubMed] [Google Scholar]
- 127.Wang YW, et al. Triple-jump photodynamic theranostics: MnO2 combined upconversion nanoplatforms involving a type-I photosensitizer with aggregation-induced emission characteristics for potent cancer treatment. Adv. Mater. 2021;33:2103748. doi: 10.1002/adma.202103748. [DOI] [PubMed] [Google Scholar]
- 128.Noordijk EM, et al. Radiotherapy as an alternative to surgery in elderly patients with resectable lung cancer. Radiother. Oncol. 1988;13:83–89. doi: 10.1016/0167-8140(88)90029-1. [DOI] [PubMed] [Google Scholar]
- 129.Fan WP, et al. Intelligent MnO2 nanosheets anchored with upconversion nanoprobes for concurrent pH-/H2O2-responsive UCL imaging and oxygen-elevated synergetic therapy. Adv. Mater. 2015;27:4155–4161. doi: 10.1002/adma.201405141. [DOI] [PubMed] [Google Scholar]
- 130.Ma BJ, et al. Self-assembled copper amino acid nanoparticles for in situ glutathione “AND” H2O2 sequentially triggered chemodynamic therapy. J. Am. Chem. Soc. 2019;141:849–857. doi: 10.1021/jacs.8b08714. [DOI] [PubMed] [Google Scholar]
- 131.Lin LS, et al. Simultaneous fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew. Chem. Int. Ed. 2018;57:4902–4906. doi: 10.1002/anie.201712027. [DOI] [PubMed] [Google Scholar]
- 132.Tang ZM, et al. Chemodynamic therapy: tumour microenvironment-mediated Fenton and Fenton-like reactions. Angew. Chem. Int. Ed. 2019;58:946–956. doi: 10.1002/anie.201805664. [DOI] [PubMed] [Google Scholar]
- 133.Bi HT, et al. Glutathione mediated size-tunable UCNPs-Pt(IV)-ZnFe2O4 nanocomposite for multiple bioimaging guided synergetic therapy. Small. 2018;14:1703809. doi: 10.1002/smll.201703809. [DOI] [PubMed] [Google Scholar]
- 134.Du KM, et al. Engineering Cu2−xS-conjugated upconverting nanocomposites for NIR-II light-induced enhanced chemodynamic/photothermal therapy of cancer. J. Mater. Chem. B. 2021;9:7216–7228. doi: 10.1039/D1TB00337B. [DOI] [PubMed] [Google Scholar]
- 135.Li SH, et al. Near-infrared light-triggered sulfur dioxide gas therapy of cancer. ACS Nano. 2019;13:2103–2113. doi: 10.1021/acsnano.8b08700. [DOI] [PubMed] [Google Scholar]
- 136.Fan WP, Yung BC, Chen XY. Stimuli-responsive NO release for on-demand gas-sensitized synergistic cancer therapy. Angew. Chem. Int. Ed. 2018;57:8383–8394. doi: 10.1002/anie.201800594. [DOI] [PubMed] [Google Scholar]
- 137.Liu SK, et al. An all-in-one theranostic nanoplatform based on upconversion dendritic mesoporous silica nanocomposites for synergistic chemodynamic/photodynamic/gas therapy. Nanoscale. 2020;12:24146–24161. doi: 10.1039/D0NR06790C. [DOI] [PubMed] [Google Scholar]
- 138.Ding BB, et al. Large-pore mesoporous-silica-coated upconversion nanoparticles as multifunctional immunoadjuvants with ultrahigh photosensitizer and antigen loading efficiency for improved cancer photodynamic immunotherapy. Adv. Mater. 2018;30:1802479. doi: 10.1002/adma.201802479. [DOI] [PubMed] [Google Scholar]
- 139.Yan SQ, et al. Activating antitumor immunity and antimetastatic effect through polydopamine-encapsulated core-shell upconversion nanoparticles. Adv. Mater. 2019;31:1905825. doi: 10.1002/adma.201905825. [DOI] [PubMed] [Google Scholar]
- 140.Xu MZ, et al. Temperature-feedback nanoplatform for NIR-II penta-modal imaging-guided synergistic photothermal therapy and CAR-NK immunotherapy of lung cancer. Small. 2021;17:2101397. doi: 10.1002/smll.202101397. [DOI] [PubMed] [Google Scholar]
- 141.Qi QK, et al. Solid-state photoinduced luminescence switch for advanced anticounterfeiting and super-resolution imaging applications. J. Am. Chem. Soc. 2017;139:16036–16039. doi: 10.1021/jacs.7b07738. [DOI] [PubMed] [Google Scholar]
- 142.Liu Y, et al. Inkjet-printed unclonable quantum dot fluorescent anti-counterfeiting labels with artificial intelligence authentication. Nat. Commun. 2019;10:2409. doi: 10.1038/s41467-019-10406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhuang YX, et al. X-ray-charged bright persistent luminescence in NaYF4: Ln3+@NaYF4 nanoparticles for multidimensional optical information storage. Light.: Sci. Appl. 2021;10:132. doi: 10.1038/s41377-021-00575-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu XW, et al. Binary temporal upconversion codes of Mn2+-activated nanoparticles for multilevel anti-counterfeiting. Nat. Commun. 2017;8:899. doi: 10.1038/s41467-017-00916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lei ZD, et al. An excitation navigating energy migration of lanthanide ions in upconversion nanoparticles. Adv. Mater. 2020;32:1906225. doi: 10.1002/adma.201906225. [DOI] [PubMed] [Google Scholar]
- 146.Jia H, et al. High color-purity red, green, and blue-emissive core-shell upconversion nanoparticles using ternary near-infrared quadrature excitations. ACS Appl. Mater. Interfaces. 2021;13:4402–4409. doi: 10.1021/acsami.0c19902. [DOI] [PubMed] [Google Scholar]
- 147.Erol E, et al. The synergistic effect of Er3+ and Ho3+ on temporal color tuning of upconversion emission in a glass host via a facile excitation modulation technique for anti-counterfeiting applications. Phys. Chem. Chem. Phys. 2020;22:25963–25972. doi: 10.1039/D0CP04809G. [DOI] [PubMed] [Google Scholar]
- 148.Gao GJ, et al. Highly efficient La2O3: Yb3+, Tm3+ single-band NIR-to-NIR upconverting microcrystals for anti-counterfeiting applications. ACS Appl. Mater. Interfaces. 2018;10:39851–39859. doi: 10.1021/acsami.8b11196. [DOI] [PubMed] [Google Scholar]
- 149.Huang R, et al. Tunable upconversion of holmium sublattice through interfacial energy transfer for anti-counterfeiting. Nanoscale. 2021;13:4812–4820. doi: 10.1039/D0NR09068A. [DOI] [PubMed] [Google Scholar]
- 150.Liu YZ, et al. Fabrication of anticounterfeiting nanocomposites with multiple security features via integration of a photoresponsive polymer and upconverting nanoparticles. Adv. Funct. Mater. 2021;31:2103908. doi: 10.1002/adfm.202103908. [DOI] [Google Scholar]
- 151.Ni ML, et al. Orthogonal reconstruction of upconversion and holographic images for anticounterfeiting based on energy transfer. ACS Appl. Mater. Interfaces. 2021;13:19159–19167. doi: 10.1021/acsami.1c02561. [DOI] [PubMed] [Google Scholar]
- 152.Huang H, et al. Lanthanide-doped core@multishell nanoarchitectures: multimodal excitable upconverting/downshifting luminescence and high-level anti-counterfeiting. Small. 2020;16:2000708. doi: 10.1002/smll.202000708. [DOI] [PubMed] [Google Scholar]
- 153.Du KM, et al. Embellishment of upconversion nanoparticles with ultrasmall perovskite quantum dots for full-color tunable, dual-modal luminescence anticounterfeiting. Adv. Opt. Mater. 2021;9:2100814. doi: 10.1002/adom.202100814. [DOI] [Google Scholar]
- 154.Wan W, et al. Constructing perovskite-like oxide CsCa2Ta3O10: Yb, Er@Cs(PbxMn1-x)(ClyBr1-y)3 perovskite halide composites for five-dimensional anti-counterfeiting barcodes applications. Chem. Eng. J. 2021;409:128165. doi: 10.1016/j.cej.2020.128165. [DOI] [Google Scholar]
- 155.Zeng M, et al. Strong upconversion emission in CsPbBr3 perovskite quantum dots through efficient BaYF5: Yb, Ln sensitization. J. Mater. Chem. C. 2019;7:2014–2021. doi: 10.1039/C8TC06063K. [DOI] [Google Scholar]
- 156.Naresh V, et al. NIR triggered NaYF4: Yb3+, Tm3+@NaYF4/CsPb(Br1-x/Ix)3 composite for up-converted white-light emission and dual-model anti-counterfeiting applications. Mater. Today Chem. 2022;23:100752. doi: 10.1016/j.mtchem.2021.100752. [DOI] [Google Scholar]
- 157.Zhao J, et al. Strong upconverting and downshifting emission of Mn2+ ions in a Yb, Tm: NaYF4@NaLuF4/Mn: CsPbCl3 core/shell heterostructure towards dual-model anti-counterfeiting. Chem. Commun. 2020;56:14609–14612. doi: 10.1039/D0CC05663D. [DOI] [PubMed] [Google Scholar]
- 158.Xiao H, et al. Core–shell structured upconversion/lead-free perovskite nanoparticles for anticounterfeiting applications. Angew. Chem. Int. Ed. 2022;134:e202115136. doi: 10.1002/anie.202115136. [DOI] [PubMed] [Google Scholar]
- 159.Li MX, et al. Facile synthesis and screen printing of dual-mode luminescent NaYF4: Er, Yb (Tm)/carbon dots for anti-counterfeiting applications. J. Mater. Chem. C. 2017;5:6512–6520. doi: 10.1039/C7TC01585B. [DOI] [Google Scholar]
- 160.Tan HH, et al. Upconversion nanoparticles@carbon dots@Meso-SiO2 sandwiched core–shell nanohybrids with tunable dual-mode luminescence for 3D anti-counterfeiting barcodes. Langmuir. 2019;35:11503–11511. doi: 10.1021/acs.langmuir.9b01919. [DOI] [PubMed] [Google Scholar]
- 161.Singh SK, Singh AK, Rai SB. Efficient dual mode multicolor luminescence in a lanthanide doped hybrid nanostructure: a multifunctional material. Nanotechnology. 2011;22:275703. doi: 10.1088/0957-4484/22/27/275703. [DOI] [PubMed] [Google Scholar]
- 162.Lin F, et al. Core–shell mutual enhanced luminescence based on space isolation strategy for anti-counterfeiting applications. J. Lumin. 2020;218:116862. doi: 10.1016/j.jlumin.2019.116862. [DOI] [Google Scholar]
- 163.He XL, et al. Tunable dual-mode MOF-based composite fluorescent materials: stimuli-responsive and anti-counterfeiting application. Cryst. Growth Des. 2021;21:1625–1635. doi: 10.1021/acs.cgd.0c01465. [DOI] [Google Scholar]
- 164.Li DD, Yu SH, Jiang HL. From UV to near-infrared light-responsive metal-organic framework composites: plasmon and upconversion enhanced photocatalysis. Adv. Mater. 2018;30:1707377. doi: 10.1002/adma.201707377. [DOI] [PubMed] [Google Scholar]
- 165.Sang YH, Liu H, Umar A. Photocatalysis from UV/Vis to near-infrared light: towards full solar-light spectrum activity. ChemCatChem. 2015;7:559–573. doi: 10.1002/cctc.201402812. [DOI] [Google Scholar]
- 166.Wang G, et al. Cu2(OH)PO4, a near-infrared-activated photocatalyst. Angew. Chem. Int. Ed. 2013;52:4810–4813. doi: 10.1002/anie.201301306. [DOI] [PubMed] [Google Scholar]
- 167.He WW, et al. Photogenerated charge carriers and reactive oxygen species in ZnO/Au hybrid nanostructures with enhanced photocatalytic and antibacterial activity. J. Am. Chem. Soc. 2014;136:750–757. doi: 10.1021/ja410800y. [DOI] [PubMed] [Google Scholar]
- 168.Wu JM, Kao WT. Heterojunction nanowires of AgxZn1–xO–ZnO photocatalytic and antibacterial activities under visible-light and dark conditions. J. Phys. Chem. C. 2015;119:1433–1441. doi: 10.1021/jp510259j. [DOI] [Google Scholar]
- 169.Tang YN, et al. NIR-responsive photocatalytic activity and mechanism of NaYF4: Yb, Tm@TiO2 core–shell nanoparticles. ACS Catal. 2013;3:405–412. doi: 10.1021/cs300808r. [DOI] [Google Scholar]
- 170.Xu ZH, et al. Harvesting lost photons: plasmon and upconversion enhanced broadband photocatalytic activity in core@shell microspheres based on lanthanide-doped NaYF4, TiO2, and Au. Adv. Funct. Mater. 2015;25:2950–2960. doi: 10.1002/adfm.201500810. [DOI] [Google Scholar]
- 171.Huang HN, et al. Efficient near-infrared photocatalysts based on NaYF4: Yb3+, Tm3+@NaYF4: Yb3+, Nd3+@TiO2 core@shell nanoparticles. Chem. Eng. J. 2019;361:1089–1097. doi: 10.1016/j.cej.2018.12.174. [DOI] [Google Scholar]
- 172.Xu JW, et al. Upconversion nanoparticle-assisted payload delivery from TiO2 under near-infrared light irradiation for bacterial inactivation. ACS Nano. 2020;14:337–346. doi: 10.1021/acsnano.9b05386. [DOI] [PubMed] [Google Scholar]
- 173.Nie ZY, et al. NaYF4: Yb, Er, Nd@NaYF4: Nd upconversion nanocrystals capped with Mn: TiO2 for 808 nm NIR-triggered photocatalytic applications. J. Phys. Chem. C. 2019;123:22959–22970. doi: 10.1021/acs.jpcc.9b05234. [DOI] [Google Scholar]
- 174.Ghorashi MS, et al. Enhanced TiO2 broadband photocatalytic activity based on very small upconversion nanosystems. J. Phys. Chem. C. 2021;125:13788–13801. doi: 10.1021/acs.jpcc.1c01403. [DOI] [Google Scholar]
- 175.Tou MJ, et al. Sequential coating upconversion NaYF4: Yb, Tm nanocrystals with SiO2 and ZnO layers for NIR-driven photocatalytic and antibacterial applications. Mater. Sci. Eng. C. 2017;70:1141–1148. doi: 10.1016/j.msec.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 176.Tan LX, et al. Preparation of multishell-structured NaYF4: Yb, Tm, Nd@NaYF4: Yb, Nd@SiO2@ZnO nanospheres with effective NIR-induced photocatalytic activity. J. Phys. Chem. C. 2020;124:18081–18090. doi: 10.1021/acs.jpcc.0c04528. [DOI] [Google Scholar]
- 177.Zhao YL, et al. ZnO flowerlike microspheres loaded with upconversion nanoparticles for high-performance NIR-driven photocatalytic activity. Mater. Lett. 2021;293:129719. doi: 10.1016/j.matlet.2021.129719. [DOI] [Google Scholar]
- 178.Jain A, et al. Strategic combination of ultra violet–visible–near infrared light active materials towards maximum utilization of full solar spectrum for photocatalytic chromium reduction. Chemosphere. 2021;267:128884. doi: 10.1016/j.chemosphere.2020.128884. [DOI] [PubMed] [Google Scholar]
- 179.Feng WH, et al. Near-infrared-activated NaYF4: Yb3+, Er3+/Au/CdS for H2 production via photoreforming of bio-ethanol: plasmonic Au as light nanoantenna, energy relay, electron sink and co-catalyst. J. Mater. Chem. A. 2017;5:10311–10320. doi: 10.1039/C7TA02402A. [DOI] [Google Scholar]
- 180.Zhu HZ, et al. Strong interface contact between NaYF4: Yb, Er and CdS promoting photocatalytic hydrogen evolution of NaYF4: Yb, Er/CdS composites. J. Mater. Sci. Technol. 2022;102:1–7. doi: 10.1016/j.jmst.2021.05.069. [DOI] [Google Scholar]
- 181.Lee SY, et al. Visible/near-infrared driven highly efficient photocatalyst based on upconversion nanoparticles/g-C3N4 nanocomposite. Appl. Surf. Sci. 2020;508:144839. doi: 10.1016/j.apsusc.2019.144839. [DOI] [Google Scholar]