Abstract
Protocadherin 9 (Pcdh9) is a member of the cadherin superfamily and is uniquely expressed in the vestibular and limbic systems; however, its physiological role remains unclear. Here, we studied the expression of Pcdh9 in the limbic system and phenotypes of Pcdh9-knock-out mice (Pcdh9 KO mice). Pcdh9 mRNA was expressed in the fear extinction neurons that express protein phosphatase 1 regulatory subunit 1 B (Ppp1r1b) in the posterior part of the basolateral amygdala (pBLA), as well as in the Cornu Ammonis (CA) and Dentate Gyrus (DG) neurons of the hippocampus. We show that the Pcdh9 protein was often localised at synapses. Phenotypic analysis of Pcdh9 KO mice revealed no apparent morphological abnormalities in the pBLA but a decrease in the spine number of CA neurons. Further, the Pcdh9 KO mice were related to features such as the abnormal optokinetic response, less approach to novel objects, and reduced fear extinction during recovery from the fear. These results suggest that Pcdh9 is involved in eliciting positive emotional behaviours, possibly via fear extinction neurons in the pBLA and/or synaptic activity in the hippocampal neurons, and normal optokinetic eye movement in brainstem optokinetic system-related neurons.
Subject terms: Cadherins, Cellular neuroscience, Development of the nervous system, Neural circuits, Amygdala
Introduction
The protocadherin (Pcdh) family forms the largest subgroup in the cadherin superfamily; Pcdhs are divided into clustered- (α, β, γ) and non-clustered (δ)-types according to their genomic organisation1–3, and are known to be involved in the neural circuit formation, synaptogenesis, and neural activity. Pcdh9, a member of δ group, is uniquely expressed in various neural circuits, including the limbic, vestibular, and oculomotor systems4. The Pcdh9 gene was reported to be a susceptibility gene for autism spectrum disorder and other psychiatric diseases5. Furthermore, it has been reported that Pcdh9 is involved in the cortical development and long-term social and object recognition6; however, the exact physiological role of Pcdh9 in the nervous system remains largely unknown.
Emotional behaviours are controlled by the limbic system that includes the amygdala and hippocampus. Amygdala, in particular, is critical for eliciting the emotional behaviours; the positive and negative emotional valences are combined in the basolateral amygdala (BLA), and the BLA sends this information to the output neurons in the central amygdala, leading to appetitive or non-appetitive behaviours7. It was reported that the neurons with expressed protein phosphatase 1 regulatory subunit 1 B (Ppp1r1b, also known as Dopamine- and cAMP-regulated phosphoprotein, Mr 32 kDa; DARPP-32)+neurons in the posterior part of the BLA (pBLA) and R-spondin 2 (Rspo2) in the anterior part of the BLA (aBLA) are involved in eliciting the appetitive and non-appetitive behaviours, respectively7.
Here, we examined the expression and distribution of Pcdh9 in the amygdala and hippocampus. We also determined Pcdh9 localisation in neurons and studied the phenotypes associated with neural circuit formation, neural functions, and behaviours using the Pcdh9-knock-out mice (Pcdh9 KO mice). We revealed that Pcdh9 was mainly expressed in Ppp1r1b+ neurons in the pBLA, and Cornu Ammonis (CA) and Dentate Gyrus (DG) neurons of the hippocampal formation; we found that the protein is often localised at the contact sites of neurites and synapses, along with other puncta over the cell surface. Histological analyses of the nervous system showed that the brains of the Pcdh9 KO mice were largely normal, including projections of major neural pathways. However, neuronal defects such as larger ventricles and reduced synaptic density were detected in the hippocampus. Physiological analyses revealed that the Pcdh9 KO mice had an abnormal optokinetic response (no changes were observed in the vestibular response). In the behavioural analyses, we found that the deficient mice tended not to approach novel objects, and the fear extinction was reduced during the recovery of fear. Our results suggest that Pcdh9 is involved in eliciting emotional behaviours, possibly via cell–cell interactions among the pBLA Ppp1r1b+ and hippocampal neurons, and in normal optokinetic eye movement in brainstem optokinetic system-related neurons.
Results
Expression of Pcdh9 in the amygdala and hippocampus
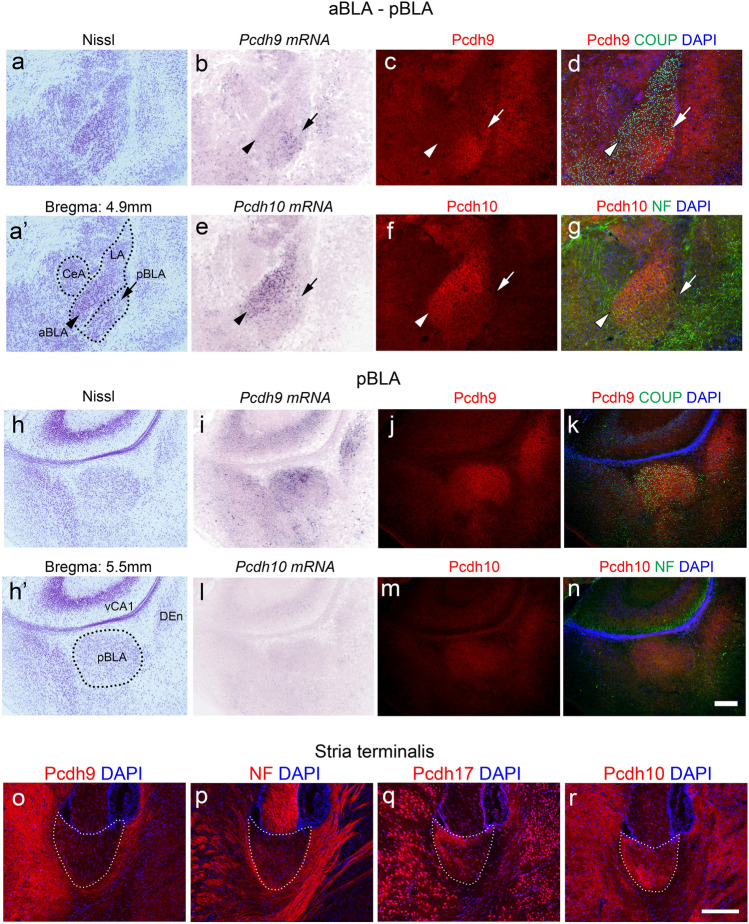
Previously, we reported that the Pcdh9 protein was distributed in various brain regions, including the vestibular and oculomotor systems4. Because Pcdh9 KO mice showed abnormalities in emotional behaviours (see below), we focused on the expression of Pcdh9 in the amygdala and related regions using in situ hybridization and immunohistochemistry (Fig. 1). Expression of Pcdh9 mRNA was specifically detected in pBLA, but no or faint in aBLA (Fig. 1b,e,i,l). The distribution of the Pcdh9 protein was consistent with the mRNA expression patterns whereas no or faint signal could be detected in the anterior part of the BLA (aBLA) where the staining signal of Pcdh10 was strong (Fig. 1b,c,i,j). Although the Pcdh9 protein was broadly distributed, its co-localisation with synapsin I was limited (see Supplementary Fig. S1a–c online). Other amygdala regions, including the central amygdala, showed no Pcdh9-specific mRNA or protein (Fig. 1b). In the stria terminalis that contains fibres from the various amygdala regions, we could not observe prominent Pcdh9+ fibres (Fig. 1o).
Figure 1.
Expression and distribution of Pcdh9 gene products in the amygdala and stria terminalis. (a–n) Expression and distribution of the Pcdh9 gene products in the amygdala region in comparison to that of the Pcdh10. (b–d) and (i–k) Show the Pcdh9 gene products in the aBLA and pBLA regions, respectively. Similarly, (e–g) and (l–n) Show the Pcdh10 gene product in the aBLA and pBLA regions, respectively. (o–r) Staining of the stria terminalis with various anti-protocadherin antibodies. Most of the fibres in the stria terminalis were Pcdh9 negative, whereas Pcdh10 and Pcdh17 were positive in the subsets of fibres. Abbreviation: NF, neurofilament. Scale bars, 200 μm in (a–n) and 100 μm in (o–r).
In the brain regions that are known to be connected with the pBLA8, we observed a moderate signal in the ventral part of the CA1 (vCA1) of the hippocampus, while faint or no signals were observed in other regions such as the infralimbic cortical area (ILA) and medial part mediodorsal nucleus of the thalamus (MD) (Supplementary Fig. S2a; see Supplementary Fig. S1f, k online). In the hippocampal formation, Pcdh9 was expressed in CA and DG neurons, but the staining of the Pcdh9 protein was weak (Supplementary Fig. S2b). Among the Pcdh9+ signals, a stronger signal was observed in the calbindin+ mossy fibres in the CA3 region (Supplementary Fig. S2b, e, h); most of the Pcdh9 staining did not overlap with that of synapsin I (Supplementary Fig. S2g–i) in this region.
Identification of Pcdh9+ neurons in the pBLA
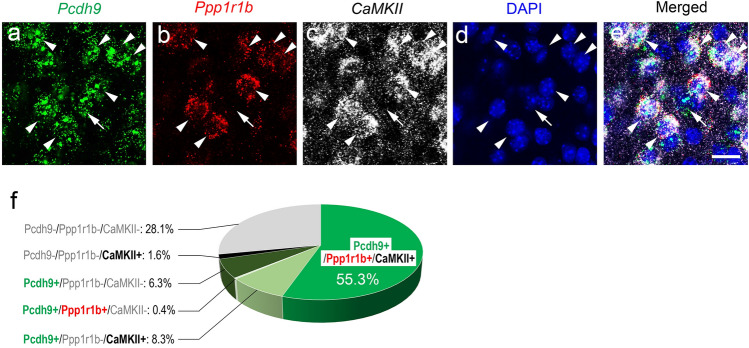
To identify the cell type of Pcdh9+ neurons in the pBLA, we employed a multiple in situ hybridization method (Fig. 2a–e). Previously, it was reported that the “fear-extinction” neurons, which are involved in valence coding for appetitive behaviour, exist in the pBLA7. Since the fear recovery was reduced in the behavioural analysis of Pcdh9 KO mice (see below), we compared the expression of Pcdh9 with that of the Ppp1r1b (also known as DARPP-32; a marker of "fear-extinction" neurons) and calcium/calmodulin-dependent protein kinase II (CamKII; a marker of excitatory neurons). In the pBLA, Pcdh9+ neurons accounted for approximately 70% of the total neurons, and approximately 91% of the Pcdh9+ neurons were CamKII+ (CamKII+/total Pcdh9+), whereas approximately 8.5% were CamKII- (CamKII-/total Pcdh9+) (Fig. 2f). Ppp1r1b+ neurons almost overlapped with that of the Pcdh9+ neurons (Ppp1r1b+/total Pcdh9+). These results suggest that a large proportion of Pcdh9+ neurons are excitatory fear extinction neurons, while only a small proportion are inhibitory neurons.
Figure 2.
Identification of Pcdh9-expressing neurons. (a–e) Expression of Pcdh9 mRNA; Pcdh9 (a) was compared with Ppp1r1b (b) and CamKII (c) using multi-color in situ hybridization. Arrowheads indicate Pcdh9+ neurons that also expressed Ppp1r1bmRNA, whereas arrows indicate Pcdh9+ neurons that did not express Ppp1r1b mRNA. (f) Composition of pBLA neurons that expressed Pcdh9, Ppp1r1b, and/or CamKII in different combinations. Scale bar, 20 μm in (a–e).
Subcellular localization of the Pcdh9 protein in vitro
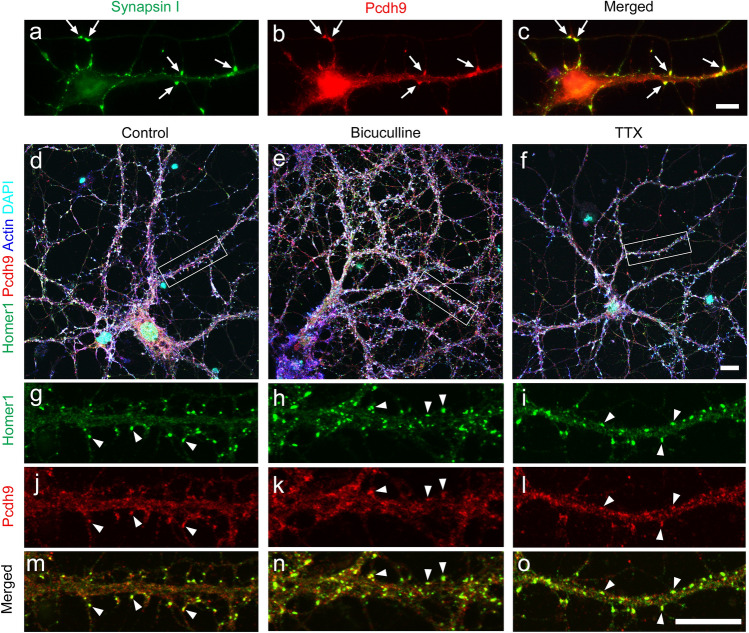
To understand the relationship of Pcdh9 with synapses, we examined the subcellular localisation of Pcdh9 protein in cultured hippocampal neurons. As reported previously with dorsal thalamus neurons4, we observed many puncta and/or clusters of Pcdh9 signals along neurites, growth cones, and contact sites in short cultures around 5 days in vitro (5DIV; Fig. 3a–c). In 14–21 DIV neurons, Pcdh9 protein was often co-localised with synapse markers at the contact sites among neurites (Fig. 3d,g,j,m). In addition to synaptic localisation, many Pcdh9+ puncta and clusters were distributed broadly along the axons. The subcellular localisation of Pcdh9 did not change in an activity-dependent manner following treatment of tetrodotoxin (TTX) and bicuculline (Fig. 3d–o).
Figure 3.
Localization of Pcdh9 proteins in cultured hippocampal neurons. (a–c) In 5-days in vitro (5-DIV) cultures, Pcdh9 protein was often localized at contact sites between neurites (arrows). (d–o) In 20-DIV cultures, Pcdh9 protein was often localized at synapses (arrowheads) in addition to other regions of neurites. Synaptic localization was not changed in normal medium (d, g, j, m), medium containing bicuculline (e, h, k, n), and TTX (f, i, l, o). Scale bars, 10 μm in (a–c), 10 μm in (d–f) and 10 μm in (g–o).
Defects of the nervous system in the Pcdh9-deficient mouse
To understand Pcdh9 roles in vivo, we generated Pcdh9 KO mice and examined the phenotypes. Generally, the bodyweight of the KO mice was lower than that of the wild-type (wt) mice (see Supplementary Fig. S3c online). We could not find any major defects in various tissues, including the lungs, kidneys, and heart, using general histological examinations. The overall structure of the Pcdh9 KO brain did not show gross defects; however, larger ventricles in the posterior region were observed than that of the wt mice (see Supplementary Fig. S4e, f, g online). The thickness of the cerebral cortex in the Pcdh9 KO mice was identical to that of the wt mice (see Supplementary Fig. S5 online). Furthermore, we also could not find abnormalities in the major neural tracts (see Supplementary Fig. S6 online).
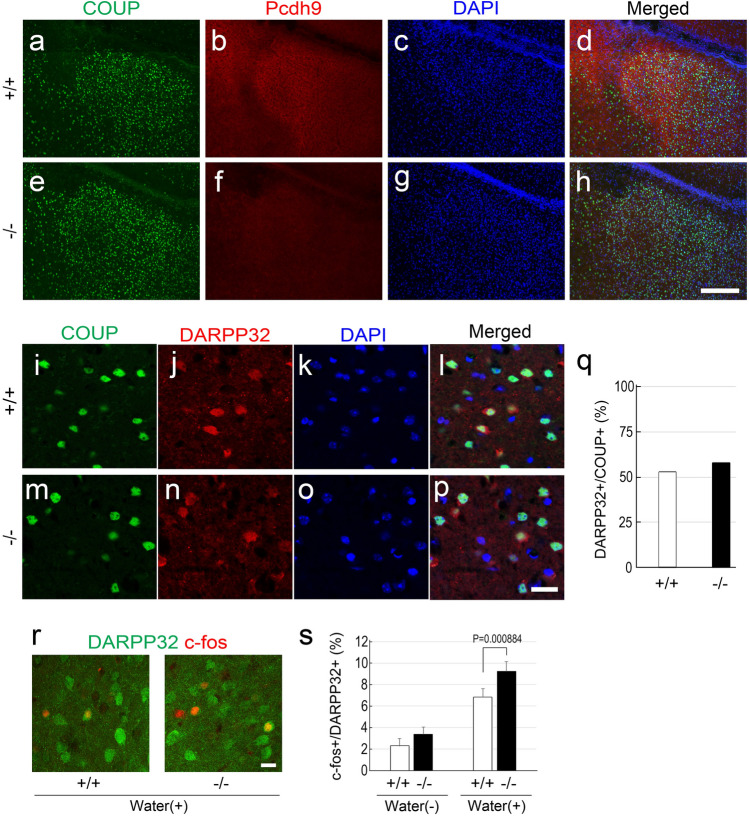
In comparison to the wt mice, the size of pBLA was identical in Pcdh9 KO mice (see Fig. 4a–h); further, no changes were observed in the number of Ppp1r1b (DARPP-32)+ neurons in the pBLA (Fig. 4i–q). Previously, it was reported that Ppp1r1b+ and "reward neurons" overlap significantly in the BLA, and are activated by rewards9. Hence, we examined c-fos expression in the Ppp1r1b+ neurons after a water reward. The results showed that a water reward induced higher c-fos expression in Pcdh9 KO mice than that in the wt mice (Fig. 4r,s). Spine density and branching of the primary dendrites of the pyramidal neurons of the pBLA with conventional Golgi staining also did not show any detectable changes in the Pcdh9 KO mice as compared to the wt mice (Fig. 5a–d).
Figure 4.
pBLA in the Pcdh9 KO mice. (a–h) The size of pBLA, visualized with a marker of BLA neurons (COUP-TFII), was not affected in the KO mice. (i–p) Double staining of COUP-TFII and DARPP-32 (Ppp1r1b), a marker of fear extinction neurons. (q) The number of DARPP-32+ neurons was not changed in the pBLA of the KO mice. (r) Double staining of c-fos (red) and DARPP32 (green) after a water reward. (s) c-fos expression in the DARPP-32+ neurons was not different between the wt mice and the Pcdh9 KO mice without a water reward, whereas it was significantly higher in the Pcdh9 KO mice than that of the wt mice after a water reward. In the case without water reward, the numbers of c-fos+ and DARPP-32+ were counted with 10 sections from two wt mice and 12 sections from two Pcdh9 KO mice, whereas in the case with water reward, the numbers of c-fos+ and DARPP-32+ were counted with 28 sections from five wt mice and 30 sections from six Pcdh9 KO mice. Scale bars, 200 μm in (a–h) and 20 μm in (i–p, r).
Figure 5.
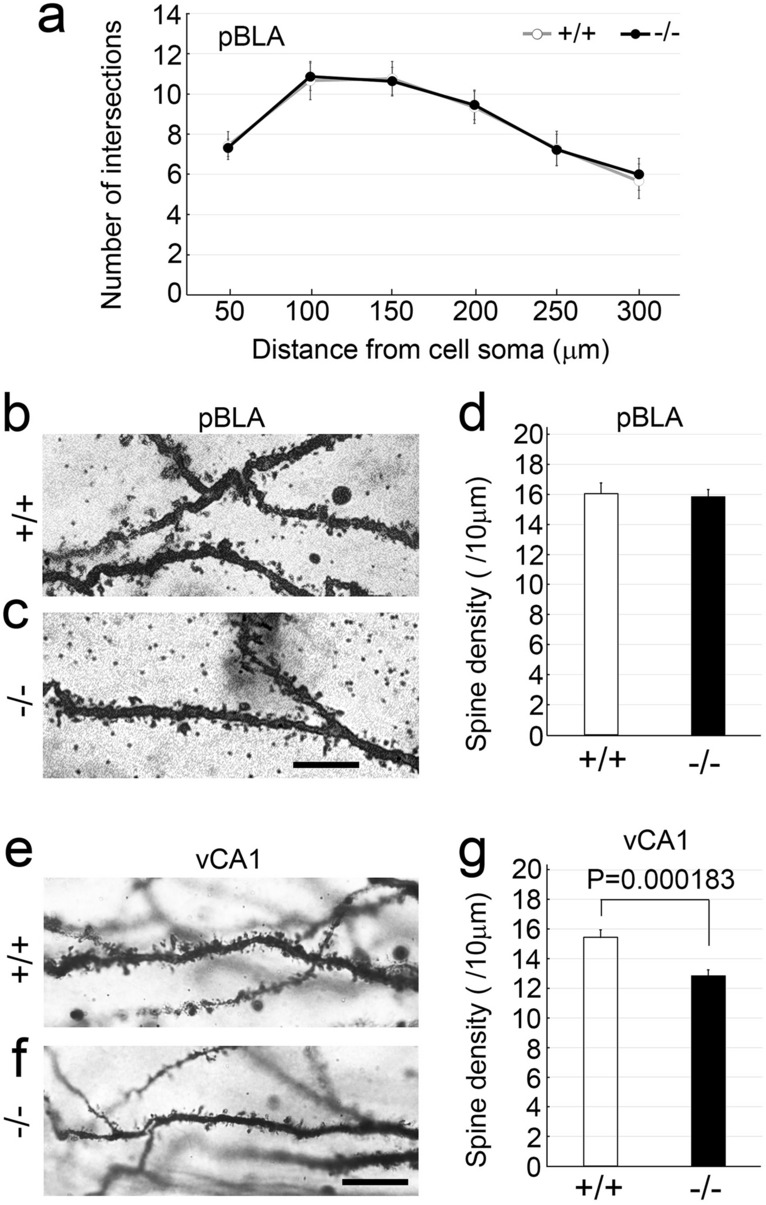
Morphological analyses of neurons in the Pcdh9 KO mice with Golgi staining. (a) Statistics of dendritic arborisation of neurons in the pBLA. The number of branches from the primary dendrite was counted with 21 neurons from two wt mice and 22 neurons from four Pcdh9 KO mice. Two-way ANOVA p = 0.76832. (b–d) The spine density of neurons in the pBLA was not changed in the Pcdh9 KO mice. The number of spines was counted with 31 neurons from two wt mice and 65 dendrites from four Pcdh9 KO mice. (e–g) Spine density in the vCA1 was significantly reduced in the Pcdh9 KO mice. The number of spines was counted with 38 neurons from two wt mice and 94 neurons from four Pcdh9 KO mice. Scale bars, 10 μm in (b, c) and 100 μm in (e, f).
In comparison to the wt mice, spine density was decreased in pyramidal neurons in the CA1 region, including the ventral part of the hippocampus in the Pcdh9 KO mice (Fig. 5e–g). When the boutons of mossy fibres were visualised using the Thy-1 GFP transgene in the CA3 region, Pcdh9 KO mice showed reduced bouton size in comparison to the wt mice (see Supplementary Fig. S7c, d, e online).
Deficient of Pcdh9 gene affected the optokinetic response in eye movement
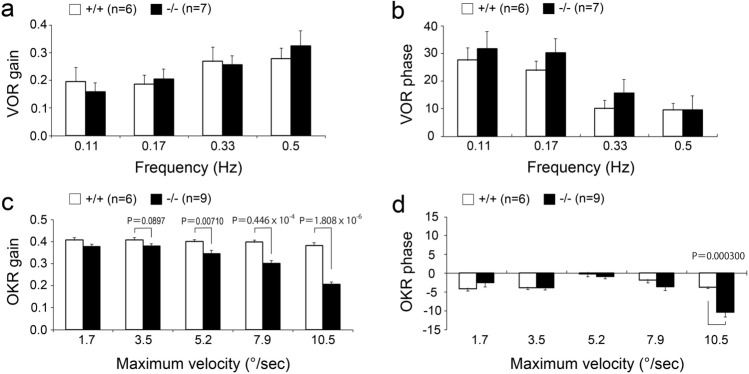
Because Pcdh9 is prominently expressed in the vestibular and oculomotor systems4, we first examined the sense of balance in Pcdh9 KO mice. No changes were observed in gait, righting reflex, and contact righting reflex of the Pcdh9 KO mice in comparison to the wt mice (Supplementary Table S1 online). Further, the auditory brainstem response was the same as the wt mice. We also examined the vestibular and optokinetic eye movement responses of Pcdh9 KO mice according to a previously described method10. The results showed that the vestibulo-ocular reflex (VOR) was not affected in the gains and phases in Pcdh9 KO mice (Fig. 6a,b). In contrast, the optokinetic response (OKR) was significantly affected in the gains and phases in the Pcdh9 KO mice (Fig. 6c,d). A significant difference was seen (P < 0.05, Welch's T-test) in the OKR gains at relatively higher screen velocities (5.2–10.5 deg/s). Further, the OKR phase was delayed at 10.5 deg/screen oscillation in the Pcdh9 KO mice.
Figure 6.
Abnormal optokinetic response (OKR) in the Pcdh9 KO mice. (a, b) Vestibulo-ocular reflex (VOR) gain and VOR phase were not changed in the Pcdh9 KO mice. (c, d) OKR gain and OKR phase were reduced at the higher maximum velocities in the Pcdh9 KO mice. Scale bar, 2 mm.
Pcdh9 KO mice showed abnormal emotional behaviours
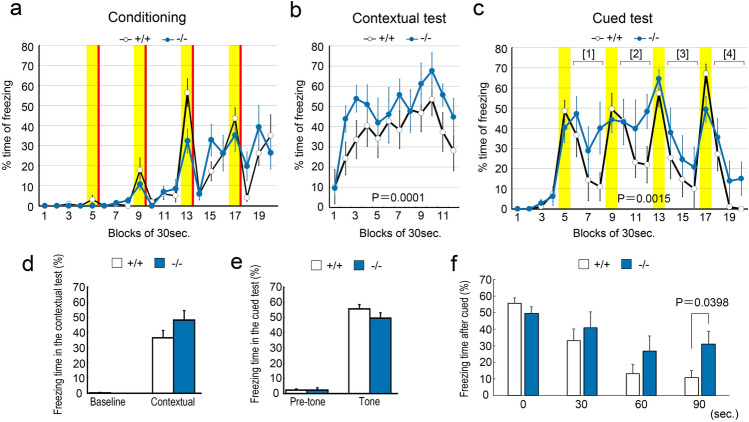
The behaviour of Pcdh9 KO mice was evaluated according to the behavioural test battery of the Mouse Clinic (RIKEN BRC Web site)11, and the comparison was made with reference to the wt mice. In the light/dark transition test, we observed that the Pcdh9 KO mice tended to delay entering the lightroom, but moved more frequently between bright and dark rooms (see Supplementary Fig. S8a, d online). In the open-field test, the Pcdh9 KO mice showed normal behaviour in most parameters, except for the centre distance travelled (see Supplementary Fig. S8e, f online). We also performed Crawley’s social interaction test using three-chamber cages (Fig. 7). The Pcdh9 KO mice approached the stranger mice in one of the chambers, similar to the wt mice (Fig. 7c,f,i). In contrast, we observed a significant reduction in the staying time of Pcdh9 KO mice near the novel objects (Fig. 7b,e,h). In the home cage activity test, Pcdh9 KO mice showed higher activity during the light period, although the total activity was identical to that of the wt mice (see Supplementary Fig. S9a–d online). In addition, Pcdh9 KO mice showed lower activity transiently at the beginning of the dark period (see Supplementary Fig.S9f, g online). In the Y-maze test, we could not detect any differences in the alternation ratio and distance travelled between the wt and Pcdh9 KO mice (see Supplementary Fig. S8g, h online). In the fear conditioning test (Fig. 8a–f), a fear memory was established in the Pcdh9 KO mice, similar to the wt mice (Fig. 8d,e). However, fear was higher in the Pcdh9 KO mice than that of the wt mice in the contextual cue (Fig. 8b), while the recovery from fear by cue was significantly delayed in the Pcdh9 KO mice (Fig. 8c,f). In the pre-pulse inhibition test, Pcdh9 KO mice generally showed normal responses, except for a stronger response at PP2 and a weaker response at PP2S (see Supplementary Fig. S10a online). Finally, we did not observe differences in the ratio of prepulse inhibition (see Supplementary Fig. S10b online).
Figure 7.
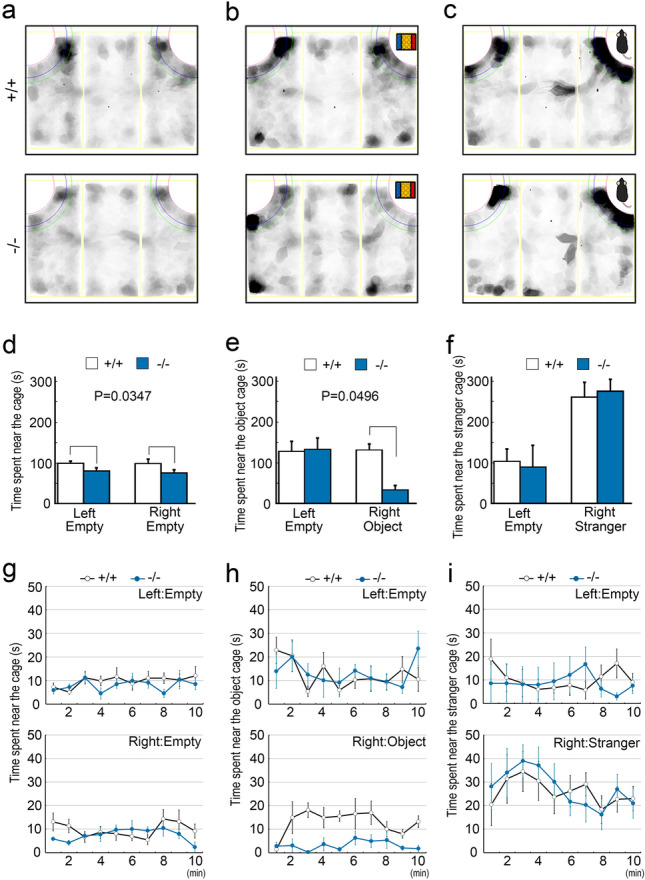
The reduced approach of the Pcdh9 KO mice to a novel object. (a–c) Schematic diagrams of the three-chamber cages. The mouse behaviours were traced with shadows in each experiment. (a) Mice were habituated in the case with two empty chambers at the corners for 10 min before experiments. (d–f) Statistics (Two-way ANOVA) of the results in each experiment. (d) During habituation, the Pcdh9 KO mice showed a tendency not to approach the empty chambers. (e) With a novel object in one side of the chambers, the approach of the Pcdh9 KO mice to a novel object was significantly reduced. (f) In contrast, with a novel mouse in one side of the chambers, the Pcdh9 KO mice behaved normally. (g–i) Time courses of staying time at each chamber. (h) Pcdh9 KO mice tended to stay away from a novel object for all time. Scale bars, 100 μm in (a, b) and 10 μm in (c, d).
Figure 8.
Reduced fear extinction of the Pcdh9 KO mice. (a–c) Time course of freezing during conditioning, contextual, and cued tests in the conventional fear-conditioned memory test. The yellow shadows indicate the periods with tone stimulations whereas the red bars indicate the points of electric shocks. In both contextual and cued tests, a fear memory was established. In the recovery phases (Period [1]–[4]), freezing time of the Pcdh9 KO mice was significantly longer than that of the wt mice (2-way ANOVA). (d, e) In both contextual and cued tests, the total freezing time of the Pcdh9 KO mice was not different from that of the wt mice. However, during the time course, the freezing time of the Pcdh9 KO mice was longer than that of the wt mice in the contextual test with 2-way ANOVA analysis (b). (f) Comparison of the freezing time during the recovery phase of the cued test. The sum of the freezing times of each bin during four recovery phases (Period [1]–[4]) in the cued test is shown. The degree of freezing of the Pcdh9 KO mice was significantly larger than that of the wt mice during the recovery from fear.
Based on the observation that the Pcdh9 KO mice stayed away from a novel object but not a novel mouse, we next performed a short-term object recognition test between the familiar and novel objects to examine the non-social specificity. We found a tendency of reduction in sniffing in Pcdh9 KO mice (discrimination index ((time novel-time familiar)/(time novel + time familiar); p = 0.0908) and novel/familiar ratio (time novel/time familiar; p = 0.0391)) (see Supplementary Fig. S11 online). In addition, we performed a marble-burying test to examine whether fear was elevated in Pcdh9 KO mice. During the test, the Pcdh9 KO mice hid marbles in slightly smaller numbers (p = 0.119) (see Supplementary Fig. S12a online), but the timing of hiding the first marble was significantly delayed in the Pcdh9 KO mice (see Supplementary Fig. S12b online).
Discussion
We have previously reported that Pcdh9 is expressed in various brain regions, including the vestibular and oculomotor systems4. In this study, we analysed the physiological role of Pcdh9 using the Pcdh9 KO mice. We did not observe any major morphological abnormalities in the nervous system of the Pcdh9 KO mice except for the wider ventricles. Previously, Bruining et al. produced a similar null Pcdh9 KO mouse line and reported a reduction in the thickness of the cortex, including the somatosensory cortex6. However, we did not observe a reduction in the thickness of the cortex in our Pcdh9 KO mice. One possible reason could be related to the difference between the genomes; our Pcdh9 KO mice lacked the neomycin resistance gene that might have affected neural cells in Pcdh9 KO mice developed by Bruining et al.
We did not observe clear abnormalities in the balance of walking in Pcdh9 KO mice. Since most neurons express several cadherins, it is possible that they compensate for the lack of Pcdh9 in the vestibular system. In contrast, we observed abnormal OKR (but not VOR) in Pcdh9 KO mice, suggesting that the OKR-specific neural circuits, including the brainstem accessory optic tract (AOT) and nucleus reticularis tegmenti pontis (NRTP), which specifically relay OKR to the extraocular muscle motor neurons via the vestibular nuclei in rodents12–14, might have some defects in Pcdh9 KO mice. Hence, Pcdh9 may be involved in neural connections and/or synapse formation in these circuits.
Few abnormalities were detected in the Pcdh9 KO mice during the behavioural analyses; although general fear did not increase in the light–dark transition, open field, and Y-maze tests, the fear extinction was significantly delayed in the Pcdh9 KO mice during the recovery from the fear induced in the fear-conditioned memory test. It is reported that Ppp1r1b+ "fear-extinction" neurons exist in the pBLA, and the decision of appetitive or nonappetitive behaviour depends on the balance of positive and negative emotions via valence coding in the amygdala where the Ppp1r1b+ neurons in the pBLA led to a positive emotional value, while the Rspo2+ neurons in the aBLA led to a negative emotional value7,15. Expression of Pcdh9 in Ppp1r1b+ neurons in the pBLA indicates that the deficiency of Pcdh9 may cause abnormalities in fear-extinction neurons via a lack of Pcdh9-mediated cell–cell interactions. However, we also observed that the c-fos expression was significantly higher in Ppp1r1b+ neurons of Pcdh9 KO mice than that of the wt mice after a reward. Since it was reported that Ppp1r1b+ neurons and reward neurons functionally overlap significantly in the pBLA9, the result did not seem consistent with the hypothesis that the activation of Ppp1r1b+ neurons is involved in fear extinction. We were not able to explain this contradiction; however, it is possible that they are not identical although the fear extinction process and the reward system significantly overlap.
Another interesting phenotype of Pcdh9 KO mice is a significantly reduced approach to a novel object, but not to a novel mouse (Fig. 7). This observation is consistent with the results of the object recognition test and the delay in the starting time to hide marbles in the marble-burying test (Supplementary Figs. S11, S12 online). It is difficult to discriminate whether the reduction in approach is a result of avoidance with fear or ignorance without interest, but it is possible that fear was elevated in the Pcdh9 KO mice via a defect in fear extinction. Because the fear of novel mice seemed not elevated, fear extinction may be specific to novel objects via proper recognition of objects.
Bruining et al. reported various phenotypes of their Pcdh9 KO mice, including abnormal cortical development, abnormal long-term social and object recognition, hyperactivity, and abnormal sensorimotor functions6. We found differences in phenotypes such as cortical development and emotional behaviour between Bruining et al. and our study. These differences may be due to the different conditions and sensitivity of analyses in these studies. Further research is needed to reveal the exact function of Pcdh9 in the nervous system. Since Pcdh9 is expressed in various brain regions, it is likely involved in various neural functions including fear extinction, OKR, cognitive function, and sensory processing.
In this study, we could not elucidate the mechanism of Pcdh9 involvement in the fear extinction neural circuits. Although the synaptic localization of Pcdh9 is limited in tissue section, Pcdh9 is likely to be involved in the neural connections because of its clear localization at cell–cell contact sites and synapses in neural culture. Further, if the Pcdh9 protein function as homophilic adhesion molecules, the targets of Ppp1r1b+ neurons should express Pcdh9. Among the fear extinction circuits, Pcdh9 is expressed in vCA1, whereas IL, aBLA, and CeL are faint or negative. Thus, Pcdh9 may be involved in the long projection between the pBLA and vCA1. In addition, because of the localisation of Pcdh9 protein at the synapse in wt neuron cultures, and the reduction in synapse number in the KO CA1, Pcdh9 is likely to be involved in the earlier phase of synapse formation, and it might be decreased during maturation of the synapse. On the other hand, Pcdh9 puncta were distributed over the cell surface outside the synaptic region in cultured neurons and were also abundant outside the synaptic region in mature neural tissue. Therefore, Pcdh9 might have some unknown functions in addition to synapse formation. To explore some of the underlying mechanisms of Pcdh9-mediated neural functions and associated phenotypes identified in this study, further studies are needed.
Material and methods
Animals
ICR mice (Japan SLC) were used for in situ hybridization, immunohistochemical analyses, and primary cell cultures. To analyse the phenotypes of the Pcdh9 KO mice, we used the wt siblings of the c57BL/6N background as controls.
To generate Pcdh9 null mice (accession no. CDB0920K: http://www2.clst.riken.jp/arg/mutant%20mice%20list.html), the 2nd exon of the Pcdh9 gene, that encodes the large part of the molecule (1–1016 amino acids), was replaced with a neomycin resistant gene flanked with loxP sequence in the TT2 ES cell lines with conventional homologous recombination16 (see Supplementary Fig. S2a online). Homologous recombinant ES cells were injected into the ICR 8-cell stage embryos to generate chimeric mice. The resulting heterozygote mice derived from the targeted ES cells were backcrossed with a c57BL/6N strain for more than 10 generations. The neomycin gene was then removed from the genome by crossing it with Cre-expressing mice (B6.Cg-Tg(CAG-Cre) CZ-MO2Osb mouse line, RIKEN RBRC01828). We confirmed the targeted deletion of the 2nd exon of the Pcdh9 gene by sequencing the targeted region and the boundaries between the vector arms and the genome. We also confirmed the loss of Pcdh9 protein in the KO mice by Western blot analysis (see Supplementary Fig. S1b online). After back-crossing with C57BL/6N for several generations, we used this heterozygote line to obtain homozygotes. For genotyping with PCR, we used two pairs of primers; TCTGGGCAAATGCAACAAGC and GAAGCCCCAGGAGCAGAATTG to detect the KO allele, and CGAGCTGGAGGAACAGGGTATAGG and GAAGCCCCAGGAGCAGAATTG to detect wt allele. These mice together with the wt mice were used for all the phenotypic analyses, except for the physiological tests for vestibular and optokinetic responses, where we used Pcdh9 KO mice with a neomycin-resistant gene. To visualise mossy fibre boutons in the CA3 region, Thy1-GFP (line M) transgenic mice (Jackson Laboratory No: 007788, Tg (Thy1-EGFP) MJrs/J) were crossed with Pcdh9 KO mice to obtain Thy1-GFP/PCDH9 double mutant mice.
All animals were kept under specific-pathogen-free (SPF) conditions until experimental use. All procedures described here were reviewed and approved by the Institutional Animal Care and Use Committee of Kansai Medical University and the RIKEN Tsukuba, Wako, and Kobe branches, and experiments were performed under the institutional guiding principles for the care and use of laboratory animals. All methods are reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Preparation of brain sections and slices
Mice of various ages were deeply anaesthetised and transcardially fixed with 4% paraformaldehyde solution in HEPES-buffered balanced salt solution (HBSS). The brains were post-fixed in 4% paraformaldehyde (PFA) solution at 4 °C for 4–24 h depending on the mouse age. After fixation, the solution was replaced with a 20% sucrose solution for cryoprotection. The brains were frozen in embedding medium (Sakura, 4583). Serial brain sections were made at 14 μm thickness using a cryostat (Leica CM3050S) at − 20 °C, and they were attached onto the Poly-l-Lysine (PLL)-coated glass slides. To prepare brain slices, the fixed brains were sliced to 150 μm thickness using a vibratome (Dosaka EM, Japan, DTK-3000 W).
Immunohistochemistry
Brain sections and slices were permeabilised using 0.25% Triton-X-100 in 50 mM Tris-buffered salt solution containing 1 mM Ca2+ (TBS-Ca) and washed with the TBS-Ca. After blocking with 5% skim milk in TBS-Ca, the following primary antibodies were applied in the blocking solution overnight at 4 °C. To detect Pcdh9, we used a 2D3 rat monoclonal antibody (mAb)4. Rat anti-OL-protocadherin (also known as Pcdh10; mAb 5G10) mAb and anti-Pcdh17 polyclonal antibodies (pAbs) have been previously described17,18. Mouse mAb 2H3 against neurofilament was obtained from the Developmental Studies Hybridoma Bank (University of Iowa). Other primary antibodies (and dilutions) used were: mouse anti-FoxP2 (Millipore, MAB415, 1:500), mouse anti-COUP-TFII (Perseus Proteomics, PP-H7147-00, 1:400), mouse anti-calbindin (Swant, CB300, 1:200), rabbit anti-DARPP-32 (Santa Cruz Biotechnology, sc11365, 1:500), rabbit anti-c-fos (Abcam, ab208942, 1:1000), rabbit anti-Synapsin I (Millipore, AP1543P, 1:1,000), rabbit anti-Homer (Synaptic Systems, 160003, 1:400). Primary antibodies were detected with appropriate Alexa488- or Alexa555-conjugated (Thermo Fisher A11029, A11034, A21424, and A21429) or Cy3-conjugated antibodies (Chemicon, AP183C, or AP189C, 1:500) as secondary antibodies. Actin filaments in the cultured neurons were visualized using Alexa647 conjugated phalloidin (Thermo Fisher, A22287).
In situ hybridization
Conventional in situ hybridization was performed according to Suzuki et al.19 We synthesised digoxigenin-labelled sense- and antisense RNA probes for Pcdh9 and Pcdh10 using a DIG RNA labelling kit (Merck 11175025910). The probes corresponded to the 800 bp cDNA fragment (2680–3480 bp, MGC189939) of Pcdh9 and 900 bp cDNA fragment of Pcdh1020. Multicolour fluorescent in situ hybridization (FISH) was performed using the RNAscope Fluorescent Multiplex R Kit (Advanced Cell Diagnostics, ACDBio). Pcdh9 (Cat#524921), Ppp1r1b (Cat #405901), Esr1 (Cat #432861), and CaMkIIa (Cat#445231) FISH probes were obtained from ACDBio. Single-plane and z-series FISH images were acquired using a confocal microscope (LSM700, Carl Zeiss).
Micro Computed Tomography (CT) imaging
Micro-CT imaging was performed using Inveon (Siemens, Germany). CT scanningwas performed according to Zikmund et al.21. After transcardial perfusion with 4% PFA, the mouse brains were dissected from the skull and post-fixed with 4% PFA overnight at 4 °C. The brains were impregnated with a 1% iodine and 95% ethanol solution for 24 h after dehydration using a methanol series. Through the hydration step with a methanol series, excess iodine was washed, and brain samples were embedded in a 1% agarose block. Micro-CT scans were performed at 50 kV acceleration voltage and 500 μA tube current conditions. CT images were acquired at15.83 μm pixel size under 2000 ms exposure time without binning. Tomographic reconstruction was performed using OsiriX software (Newton Graphics, Inc., Japan). Masking of target neural tissue for 3D reconstruction and volume analysis of brain structures was carried out using Mimics software (Materialise, Belgium).
Primary hippocampal neuron culture
Primary cultures of hippocampal neurons were performed according to a conventional method22. Briefly, hippocampal tissue dissected from the E17.5 mouse cortex was treated with 0.25% trypsin solution and then triturated with calcium- and magnesium-free HEPES buffered salt solution (HCMF). Hippocampal neurons were plated on PLL or laminin-coated glass coverslips at a density of 3.0–6.0 × 105 cells/mL in neurobasal medium (GIBCO) supplemented with B27 (GIBCO), 2 mM L-glutamine, and 20 µg/mL gentamicin (GIBCO). For immunochemical analysis, neurons were fixed with 2% PFA and 4% sucrose in HBSS solution for 5 min at 37 °C. Neurons were permeabilised with 0.25% Triton-X-100 in TBS-Ca solution for 10 min and then blocked with 5% skim milk for 1 h. The neurons were incubated with primary and secondary antibodies. Fluorescent images were taken using a fluorescence microscope (Zeiss Axioplan and Axioscope) and a confocal microscope (Olympus FV3000).
Analysis of spine density and dendritic arborization in the Golgi-stained brain
We performed the Golgi staining of adult brains using a Rapid Golgi Stain kit (FD Neuro Technologies), according to the manufacturer's protocol. The brains were impregnated with kit solutions and then sliced at 200 μm thickness using a cryostat at − 25 °C. The slices were mounted on gratin-coated slide grasses (FD Neuro Technologies). Tissue staining was performed using the kit solutions and mounted on glass slides after washing. Z-stack images of dendritic spines of neurons stained with Golgi solutions were obtained using a BZ-9000 microscope (Keyence, Japan) with a 100 × objective lens. Spine density and dendritic arborisation of neurons were analysed using ImageJ software.
Physiological and behavioural tests
c-fos expression after a reward was examined according to the method described previously9. Acute water deprivation was performed against wt and mutant mice overnight, and then the mice were supplied with a sufficient amount of water using a conventional bottle the next early morning. The mice were perfused with 4% PFA solution at 90 min after the first access to water. c-fos and DARPP-32 expressing neurons were visualised by immunostaining in the pBLA region, and the percentage of positive c-fos was calculated in the DARPP-32 positive neurons.
Optokinetic response (OKR) and vestibulo-ocular reflex (VOR) were analysed as described before10.
All behavioural tests with male mice were performed at the Japan Mouse Clinic of RIKEN BRC. A battery of the following seven tests was carried out for comprehensive behavioural phenotyping: light/dark transition test (6-weeks old), open-field test (7-weeks old), Crawley’s social interaction test (3-chamber test, 9-weeks old), home-cage activity test (10 to 11-weeks old), Y-maze test (12-weeks old), fear conditioning test (13-weeks old), and pre-pulse inhibition test (14-weeks old). The results of the behavioural tests are sensitive to prior experience23. Therefore, to suppress the effects of prior experience, these tests were started from the least invasive test in the battery. For all the tests in the battery, mice were transferred from their husbandry room to the testing room for at least 30 min before the experiment. Seven males of each genotype were used as subjects in the test battery. We performed these behavioural analyses as described previously, with slight modifications24, and the detailed procedures are disclosed on the website11. In addition, we performed additional tests using another batch of male mice (n = 16), including the open field, object recognition, and marble-burying tests. The marble-burying and the object recognition tests were performed as previously reported with modifications24,25.
Supplementary Information
Acknowledgements
We started this project when S.H. was at Kochi Medical University, and we thank Ms. Miori Nakato for her technical assistance and Dr. Yasufumi Sato for his involvement in the initial phase of the KO mouse analysis. We also thank Drs. Tayo Katano and Kazuhiko Nishida for their technical advice. This work was supported by grants from the Takeda Science Foundation, Grants-in-Aid for Scientific Research of the Ministry of Education, Science, Sports, and Culture of Japan (18K06230), RIKEN Cooperative Research, the Sumitomo Foundation, the MEXT-Supported Program for the Strategic Research Foundation at Private Universities (Grant No. S1201038), and Kansai Medical University MolecularImaging Center of Diseases. A part of the results of this study was presented in Neuroscience 2017 (annual meeting of the Society for Neuroscience)26.
Author contributions
M.U. performed most histological analyses and wrote the manuscript. T.F., I.Y., and T.K. performed behavioural analyses under the supervision of S.W. and M.T. Pcdh9 KO mice were generated by T.A. and H.K. S.N. and M.K. performed the physiological examinations of OKR and VOR. K.I. performed the Western blot analysis and part of the molecular biology analyses, including in situ hybridization. K.Y. examined the general pathology of Pcdh9 KO mice. S.H. was involved at all stages of this study, from the planning of this project to writing the paper.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-16106-5.
References
- 1.Hirano S, Takeichi M. Cadherins in brain morphogenesis and wiring. Physiol. Rev. 2012;92:597–634. doi: 10.1152/physrev.00014.2011. [DOI] [PubMed] [Google Scholar]
- 2.Weiner KMMJA. In: The cadherin superfamily. Suzuki ST, Hirano S, editors. Springer; 2016. pp. 195–221. [Google Scholar]
- 3.Jontes JD. In: The cadherin superfamily. Suzuki ST, Hirano S, editors. Springer; 2016. pp. 223–249. [Google Scholar]
- 4.Asahina H, Masuba A, Hirano S, Yuri K. Distribution of protocadherin 9 protein in the developing mouse nervous system. Neuroscience. 2012;225:88–104. doi: 10.1016/j.neuroscience.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Hirano S, Imai-Okano K. In: The cadherin superfamily. Suzuki ST, Hirano S, editors. Springer; 2016. pp. 399–421. [Google Scholar]
- 6.Bruining H, et al. Genetic mapping in mice reveals the involvement of Pcdh9 in long-term social and object recognition and sensorimotor development. Biol. Psychiatry. 2015;78:485–495. doi: 10.1016/j.biopsych.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Pignatelli M, Xu S, Itohara S, Tonegawa S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci. 2016;19:1636–1646. doi: 10.1038/nn.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hintiryan H, et al. Connectivity characterization of the mouse basolateral amygdalar complex. Nat. Commun. 2021;12:2859. doi: 10.1038/s41467-021-22915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Kim J, Tonegawa S. Amygdala reward neurons form and store fear extinction memory. Neuron. 2020;105:1077–1093. doi: 10.1016/j.neuron.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 10.Shutoh F, Ohki M, Kitazawa H, Itohara S, Nagao S. Memory trace of motor learning shifts transsynaptically from cerebellar cortex to nuclei for consolidation. Neuroscience. 2006;139:767–777. doi: 10.1016/j.neuroscience.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 11.https://ja.brc.riken.jp/lab/jmc/mouse_clinic/en/pipeline_en/pipeline_02.htm#p2-00
- 12.Miyashita Y, Ito M, Jastreboff PJ, Maekawa K, Nagao S. Effect upon eye movements of rabbits induced by severance of mossy fiber visual pathway to the cerebellar flocculus. Brain Res. 1980;198:210–215. doi: 10.1016/0006-8993(80)90357-1. [DOI] [PubMed] [Google Scholar]
- 13.Kano M, Iino K, Maekawa K, Kano MS. Optokinetic response of cells in the nucleus reticularis tegmenti pontis of the pigmented rabbit. Exp. Brain Res. 1991;87:239–244. doi: 10.1007/BF00231840. [DOI] [PubMed] [Google Scholar]
- 14.Nagao S. Ocular reflex adaptation as an experimental model of cerebellar learning: In memory of Masao Ito. Neuroscience. 2021;462:191–204. doi: 10.1016/j.neuroscience.2020.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S. Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron. 2017;93:1464–1479. doi: 10.1016/j.neuron.2017.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yagi T, et al. A novel ES cell line, TT2, with high germline-differentiating potency. Anal. Biochem. 1993;214:70–76. doi: 10.1006/abio.1993.1458. [DOI] [PubMed] [Google Scholar]
- 17.Aoki E, Kimura R, Suzuki ST, Hirano S. Distribution of OL-protocadherin protein in correlation with specific neural compartments and local circuits in the postnatal mouse brain. Neuroscience. 2003;117:593–614. doi: 10.1016/s0306-4522(02)00944-2. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi S, et al. Protocadherin-17 mediates collective axon extension by recruiting actin regulator complexes to interaxonal contacts. Dev. Cell. 2014;30:673–687. doi: 10.1016/j.devcel.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M. Neuronal circuits are subdivided by differential expression of type-II classic cadherins in postnatal mouse brains. Mol. Cell Neurosci. 1997;9:433–447. doi: 10.1006/mcne.1997.0626. [DOI] [PubMed] [Google Scholar]
- 20.Hirano S, Yan Q, Suzuki ST. Expression of a novel protocadherin, OL-protocadherin, in a subset of functional systems of the developing mouse brain. J. Neurosci. 1999;19:995–1005. doi: 10.1523/JNEUROSCI.19-03-00995.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zikmund T, Novotná M, Kavková M, Tesařová M, Kaucká M, Szarowská B, Adameyko I, Hrubá E, Buchtová M, Dražanová E, Starčuke Z, Kaiser J. High-contrast differentiation resolution 3D imaging of rodent brain by X-ray computed microtomography. JINST. 2017;13:C02039. doi: 10.1088/1748-0221/13/02/C02039. [DOI] [Google Scholar]
- 22.Togashi H, et al. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- 23.Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Kim K, et al. Autophosphorylation of F-actin binding domain of CaMKIIbeta is required for fear learning. Neurobiol. Learn. Mem. 2019;157:86–95. doi: 10.1016/j.nlm.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Deacon RM. Digging and marble burying in mice: Simple methods for in vivo identification of biological impacts. Nat. Protoc. 2006;1:122–124. doi: 10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- 26.Hirano, S. et al. Deficient of autism susceptibility gene Protocadherin 9 leads to abnormalities in vestibular response and emotional behavior in mice. Annual meeting of the Society for Neuroscience (Neuroscience 2017), 651.614 (2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.