Abstract
Objectives
This study aims to explore the benefits and harms of Chinese Herbal Medicine (CHM) for mild cognitive impairment (MCI).
Methods
Electronic searching was conducted in two English and four Chinese databases till 2021 December. Randomized clinical trials on CHM compared to no intervention, placebo or other therapies for MCI were included.
Results
Forty-nine RCTs (48 finished trials and 1 protocol) were identified. The overall methodological quality of included trials was relatively low. This review found that compared to no intervention or placebo, CHM can significantly decrease the number of patients who progressed to dementia (RR 0.36, 95% CI 0.22–0.58) and increase the cognitive function assessed by MMSE (MD 1.96, 95% CI 1.41–2.50) and MoCA (MD 2.44, 95% CI 1.57–3.31). The subgroup analysis of different CHM showed that Ginko leaf tablets can significantly improve the cognitive function compared to no intervention or placebo when assessed by MMSE (MD 2.03, 95% CI 1.18–2.88) and MoCA (MD 3.11, 95% CI 1.90–4.33). Compared to western medicine, CHM can significantly increase the score of MMSE (MD 0.88 95% CI 0.46–1.30) and MoCA (MD 0.87, 95% CI 0.33–1.41), but there was no significant difference on the score of ADL (SMD −0.61, 95% CI −1.49 to 0.27). None of the RCTs reported on the quality of life. Of 22 RCTs that reported adverse events, there was no statistical difference between the CHM and the control group.
Conclusions
CHM, Ginko leaf extracts in particular, could help to prevent progression into dementia and to improve cognitive function and ability of daily living activities. More qualified RCTs were needed to confirm the conclusion due to the low quality of current trials.
Systematic Review Registration
Unique Identifier: CRD42020157148.
Keywords: mild cognitive impairment (MCI), Chinese herbal medicine, systematic review, meta-analysis, traditional Chinese medicine
Introduction
Mild cognitive impairment (MCI) represents a transitional state between normal aging and dementia (1). It is diagnosed by the presence of one or more domains of cognitive impairment without fulfilling the diagnostic criteria for dementia (2). If impairment involves memory, it is known as amnestic MCI, and if not, non-amnestic MCI (2). The population-based studies showed that the frequency of MCI is estimated to be 15–20% in persons 60 years and older (3). The annual rate of development of dementia in people with MCI varied between 5 and 15% (3). People with MCI are at higher risk of progression to dementia than age-matched general people, therefore, it is critical to find treatments that may improve symptoms or prevent or delay progression to dementia (2).
Currently, no treatments, pharmacologic or non-pharmacologic, are approved specifically for MCI by the Food and Drug Administration (2). Doctors sometimes prescribe approved drugs that were for Alzheimer's disease to patients with MCI, such as cholinesterase inhibitors, but according to the practice guideline by the American Academy of Neurology, doctors may not choose cholinesterase inhibitors and must first discuss lack of evidence when offering it to people with MCI (2). The guideline found evidence on 12 pharmacologic treatments (e.g., Donepezil, Galantamine, Rivastigmine), but none was recommended to be used for MCI as no high-quality evidence exists (2). For non-pharmacologic treatments, exercise training and cognitive training may help improve cognitive measures (2).
In recent years, Chinese herbal extracts and preparations have received considerable research attention for the management of cognition impairment. Previously published systematic reviews of Chinese herbal medicine (CHM) for MCI have been limited by enrolling people with mixed cognitive impairment (including MCI and other memory impairment), or focusing only on Montreal Cognitive Assessment (MoCA) (4), Mini-mental state examination (MMSE) (5), or Alzheimer's Disease Assessment Scale-Cognitive subscale (ADAS-Cog) (5), but not on suggested clinically meaningful patient outcomes such as development into dementia or quality of life (5). These reviews were likely not to provide dependable results because of possible flaws in methodology, such as lack of protocol (4–6), the inadequate technique for risk of bias assessment (6), or synthesis of data regardless of significant clinical heterogeneity (5). An update of evidence is also warranted as these systematic reviews were based on evidence up till December 2017 (4–6). Therefore, this study was conducted to summarize the up-to-date evidence and to explore the benefits and harms of CHM for MCI in a systematic review of randomized clinical trials (RCTs) with rigorous and reasonable methodology.
Methods
The protocol of this review was registered on PROSPERO (registration number: CRD42020157148). We followed the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (7).
Inclusion Criteria
Types of Studies
We searched for RCTs regardless of language, publication year, publication format, and publication status.
Types of Participants
We included participants of any gender and at any age diagnosed as MCI by trialists or according to guidelines.
Types of Interventions
We included CHM at any dose and form, compared with placebo, no intervention or other commonly used therapies (e.g., western medication, cognitive training, or lifestyle change) for MCI. CHM with fixed production process and controllable quality, approved by national medical products administration and state administration for market regulation (SAMR) were included. Comparison between two different CHM was excluded. We allowed co-intervention when it was administered equally to the intervention and the control group.
Types of Outcome Measures
Primary outcomes were the number of participants progressing into dementia, cognitive function assessed by authority scales such as MoCA and MMSE, and the number of participants with adverse events. Secondary outcomes were quality of life and assessment of behavior or psychiatric symptoms.
Search Strategy
We searched MEDLINE, Cochrane CENTRAL, China National Knowledge Infrastructure (CNKI), Chongqing VIP (CQVIP), Wanfang Data, and Sinomed from their inception to 31 December 2021. We also searched the reference lists of the meta-analyses on this topic and the references of the included trials. The search strategy (see Supplementary Table 1 for details) included the following key medical keywords: “mild cognitive impairment”, “Chinese herbal medicine”, “Chinese patent medicine”, “random”, “randomization”, and “randomized clinical trials”.
Data Selection and Extraction
Reviewers in pairs (SH Yang, CH Liang, LD Gao, S Wang) independently screened titles and abstracts to retrieve the potentially eligible trials, and read full-text papers to identify the trials that should be included. Reviewers (YX Chen, SH Yang) extracted the information from the included trials through a pre-piloted table created in Microsoft Excel. Another author (N Liang) rechecked the extracted information. The extracted information included publication data (e.g., publication year, authors); study characteristics and design; characteristics of the participants (e.g., age, gender, diagnostic and inclusion criteria); interventions and controls (e.g., dose, form, duration); and outcomes. We extracted data at maximum follow-up.
Assessment of Risk of Bias in Included Trials
Reviewers in pair (SH Yang, CH Liang, LD Gao, S Wang) independently assessed the risk of bias according to the Cochrane “risk of bias” tool (7). We evaluated the following items: allocation sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias.
Statistical Analysis
RevMan 5.3 was used to perform statistical analyses. We used the risk ratio (RR) for measuring dichotomous outcomes and mean difference (MD) for continuous data, with 95% confidence intervals (CIs). When studies used different instruments to measure the same continuous outcome, we calculated the standard mean difference (SMD), with 95% CI. We assessed clinical and methodological heterogeneity by carefully examining trial participant and intervention characteristics and the design of the included trials. We assessed our intervention effects with both fixed-effect model and random-effects model, and we reported both results when results differed (e.g., one giving a significant intervention effect, while the other no significant intervention effect); otherwise, we reported the estimate closest to the zero effect (the highest P-value). We started by looking at the forest plots for signs of statistical heterogeneity. Next, we used the Chi2 test with a significance threshold set as P < 0.10 and measured the amount of heterogeneity with the I2 statistic to assess to what extent variation is from heterogeneity rather than chance. We interpreted the I2 statistic as suggested in Cochrane Handbook: 0–40%: might not be important; 30–60%: might represent moderate heterogeneity; 50–90%: might represent substantial heterogeneity; 75–100%: considerable heterogeneity (7). In case of available data, we performed the subgroup analyses in terms of different interventions and controls, and different causes of MCI. We used sensitivity analyses whenever we wanted to test the robustness of our findings. We planned to assess reporting bias using funnel plots if we obtained data from at least 10 trials per comparison. To assess the risk of publication bias, we intended to look for symmetry or asymmetry of each funnel plot.
Results
Description of the Search
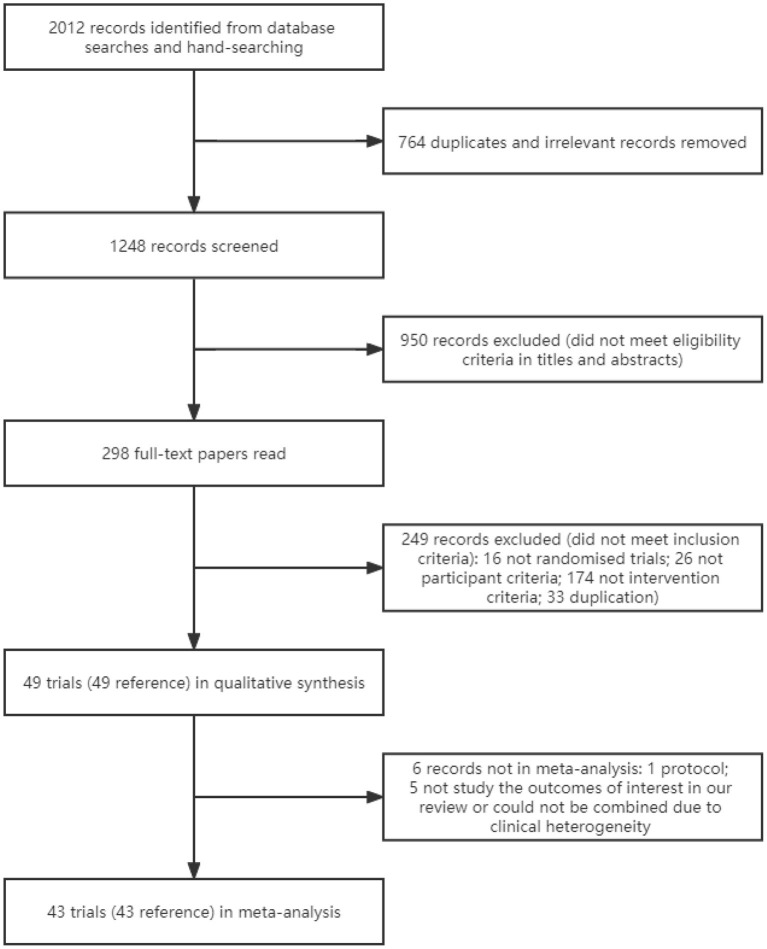
We identified 2,012 references through searching the databases and the reference lists of meta-analyses on this topic and included trials (Figure 1). After excluding duplicates and irrelevant studies, titles and abstracts of 1,248 references were screened; of these, 298 full-text papers were read. Totally, 49 references of 49 trials were included (8–56).
Figure 1.
Flow chart of included studies in this systematic review.
Basic Characteristics of Included Studies
We included 48 finished trials and one protocol (30). Of the 48 finished trials, 43 trials provided data for meta-analyses. The remaining five trials either did not study the outcomes of interest in our review or could not be combined due to clinical heterogeneity (19, 34, 38, 53, 55); hence we used the provided information only in a narrative way. Fifteen trials received funding from government or academic institution; four trials were funded by pharmaceutical companies, and high risk of financial supports was suspected even though no conflict of interest was declared (33, 42, 48, 53); the remaining 30 trials did not provide information on funding. Undisclosed funding may influence trial results and may lead to poor trial design.
Sample size ranged from 18 to 245 participants, and a total of 4,656 participants were randomized. For 42 trials that reported the gender of participants, the total ratio of female to male was 1,897:1,892. Age of participants was reported in 44 trials, and mean age of participants in these trials was 67.82 years old (Table 1).
Table 1.
Characteristics of trials included in the review.
| Study ID | Participants | CHM group | Control group | Sample size (I/C), dropout (I/C) | Age (I/C) | No. of female (I/C) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Su et al. (15) | MCI | Ginkgo leaf tablets, 19.2 mg, t.i.d; + Donepezil hydrochloride; 2 m | Donepezil hydrochloride, 5 mg (1st month); then 10 mg; 2 m | 100 (50/50), 0 | 53.5 ± 8.5/51.8 ± 9.6 | 44/100 | MoCA; EEG; ERP P300 |
| Dong (19) | MCI | Ginkgo leaf tablets, 19.2 mg, t.i.d; + Donepezil hydrochloride; 2 m | Donepezil hydrochloride, 5 mg, q.d, 2 m | 114 (57/57), 0 | 66.2 ± 2.6/65.4 ± 2.5 | 48/114 | MMSE; BI; SAS; SDS; symptom score (fail to recognize; visual space disorder; misuse; aphasia; memory) |
| Xiao (23) | MCI | Ginkgo leaf tablets, 19.2 mg, t.i.d; + Basic treatment; 12 m | Basic treatment (health education, moderate exercise, nutrition support), 12 m | 113 (58/55), 0 | 72.78 ± 5.03/73.53 ± 5.97 | 73/113 | MMSE; ADL; CDT; WMS; number of patients progressed to dementia |
| Han (14) | MCI | Ginkgo leaf tablets, 1 tablet, t.i.d; +Basic treatment; 12 m | Basic treatment (health education, moderate exercise, nutrition support), 12 m | 120 (60/60), 0 | 72.3 ± 5.3/71.7 ± 4.9 | 79/120 | MQ; number of patients progressed to dementia; MMSE; serum index (MDA, AchE); AE |
| Zhang (27) | Vascular MCI | Compound Congrong Yizhi, 4 capsules, t.i.d, 6 m | No intervention | 18 (9/9), 0 | 60–69: 6/4 70–80: 3/5 | 7/18 | MoCA; TCM symptom score; AE |
| Wang (20) | Post-stroke MCI | Compound Congrong Yizhi, 4 capsules, t.i.d; +Health education; 6 m | Health education | 20 (10/10), 0 | 64.1 ± 5.9 | 5/20 | MMSE; MoCA; ADL |
| Ye (21) | Amnestic MCI | Tongxinluo, 6 capsules, add to 12 capsules if no AE; + Memantine; 6 m | Memantine, 5 mg, add to 20 mg if no AE, 6 m | 70 (35/35), 7 (5/2) | 69.8 ± 6.3/68.5 ± 5.7 | 37/70 | MMSE; DSR; AE |
| Jia et al. (25) | MCI | Tongxinluo, 2-4 capsules, b.i.d; + Oxiracetam; 1 m | Oxiracetam, 800 mg, b.i.d, 1 m | 48 (25/23), 0 | 72 ± 4/70 ± 5 | 21/48 | Cognitive function; CGI-EI; number of patients progressed to dementia; AE |
| Huang et al. (17) | MCI | Liuwei Dihuang, 8 pills, t.i.d; +Cognitive training; 12 m | Cognitive training, 12 m (2 m treatment then 1 m rest) | 86 (43/43), 48 (24/24) | 79.5 ± 7.80 | 62/86 | MoCA; AE |
| Liu et al. (30) | Vascular MCI | Yangxue Qingnao granules, 4 g, t.i.d; +Basic treatment; 4 m | Basic treatment for vascular diseases, 4 m | 45 (24/21), 0 | 76.5/73.5 | 24/45 | MMSE; MQ; AE |
| Dong (11) | MCI | Gui Ling Ji capsule, 0.6 g, q.d; +Cognitive training; 3 m; follow up of 12 m | Cognitive training, 3 m; follow up of 12 m | 100 (50/50), 0 | NR | NR | MMSE; ADL |
| Lin (29) | Vascular MCI | Astragalus injection, 10 mL, q.d; Nimodipine; 1 m | Nimodipine, 30 mg, t.i.d, 1 m | 59 (30/29), 0 | 65.03 ± 6.51/64.27 ± 6.63 | 28/59 | MMSE; CDT; ERP P300; AE; TCM symptom score; serum index (TC, TG, HDL-C, LDL-C, CRP); insuline resistance index |
| Lan et al. (16) | Vascular MCI | Qizhi Tongluo capsule, 800 mg, b.i.d; Nimodipine; 3 m | Nimodipine, 30 mg, t.i.d, 3 m | 60 (30/30), 0 | 66.22 ± 2.85/67.43 ± 1.86 | NR | MMSE; TCM symptom score |
| Wu et al. (10) | MCI | Tianmeng oral liquid, 20 mL, b.i.d; Citicoline sodium tablets; 2 m | Citicoline sodium tablets, 0.2 g, t.i.d, 2 m | 102 (53/49), 0 | 69.6 ± 7.8 | 41/102 | MMSE; AE; transcranial doppler test; TCM symptoms score |
| Yu et al. (9) | Amnestic MCI | Compound sea snake capsule, 0.3 g (1st week), 0.6 g (2nd week), then 0.9 g, t.i.d, 24 m | No intervention, 24 m | 120 (60/60), 27 (12/15) | 76.1 ± 5.9/75.7 ± 5.9 | 52/93 | MMSE; MoCA; ADAS-Cog |
| Meng (22) | Post-stroke MCI | Compound Congrong Yizhi, 4 capsules, t.i.d, 6 m | Nimodipine, 30 mg, t.i.d, 6 m | 60 (30/30), 0 | 67.19 ± 7.56 | 29/60 | MoCA; ADL- Barthel; AE; core symptom scores |
| Gao et al. (18) | Post-stroke MCI | Compound Congrong Yizhi, 4 capsules, t.i.d; +Basic treatment; 6 m | Nimodipine, 30 mg, t.i.d; +Basic treatment for stroke; 6 m | 140 (70/70), 7 (3/4) | 67.1 ± 5.3/66.3 ± 4.9 | 65/133 | MMSE; MoCA; AE |
| Chen et al. (12) | MCI | Compound Congrong Yizhi, 4 capsules, t.i.d, 3 m | Donepezil hydrochloride, 1 tablet, q.d, 3 m | 60 (30/30), 0 | 67.9 ± 7.6/67.5 ± 8.1 | 35/60 | MMSE; ADAS-Cog; ADL; AE |
| Ouyang and Ouyang (24) | Ischemic post-stroke MCI | Jiannao Bushen pill, 15 capsules, b.i.d, 2 m | Nimodipine, 60 mg, t.i.d, 2 m | 75 (39/36), 0 | 69.3 ± 7.7/67.6 ± 6.7 | 27/75 | MMSE |
| Chen et al. (13) | MCI | Shenzhiling oral liquid, one piece, b.i.d, 6 m | Huperzine A tablets, 100 μg, b.i.d, 6 m | 69 (35/34), 0 | 66.46 ± 3.85/65.94 ± 4.10 | 35/69 | MMSE; MoCA; CDT; ADL; ADAS-Cog; RVR; DS; visual reaction time test; hand coordination test |
| Tian et al. (31) | MCI | Jinsiwei pill, 4 pills, t.i.d; +Piracetam tablets placebo; 3 m | Piracetam tablets, 2 tablets, t.i.d; +Jinsiwei pill placebo; 3 m | 60 (30/30), 0 | 57.23 ± 8.14/60.43 ± 7.05 | NR | MMSE; memory function scores |
| Xie et al. (28) | Amnestic MCI | Tianzhi granules, 5 g, t.i.d, 1 m | Donepezil hydrochloride, 5mg, q.d, 1m | 96 (68/28), 5 (5/0) | 69 ± 7/65 ± 6 | 46/96 | MMSE; ADL; TCM symptom score |
| Zhu (26) | MCI | Xuanyunning tablet, 2 tablets, t.i.d, 3 m | Piracetam tablet, 0.8 g, t.i.d, 3 m | 100 (50/50), 0 | 75.38 ± 6.79/76.21 ± 5.98 | 47/100 | MSEE; TCM symptom score; CGI-EI; AE; hippocampal volume |
| Zhang et al. (8) | Parkinson related MCI | Fufang Huonao Shu, 2 tablets, t.i.d; +Madopar; 6 m | Oxiracetam, 800 mg, t.i.d; + Madopar; 6 m | 60 (30/30), 0 | 65.13 ± 7.85/64.89 ± 8.29 | 31/60 | MMSE; MoCA; ADL; TCM symptom score |
| Zhang et al. (46) | Parkinson related MCI | Tebonin (Ginkgo biloba extract tablet), 80 mg, t.i.d; +Basic treatment; 6 m | Basic treatment (Madopar +Health education and lifestyle guidance), 6 m | 83 (43/40), 4 (1/3) | 62.25 ± 8.51 | 37/83 | MMSE; MoCA; number of patients progressed to dementia |
| Xia (45) | MCI | Tanakan (Ginkgo biloba extract tablet), 40 mg, t.i.d; + Basic treatment;12 m | Basic treatment (Medopar + Nimodipine + health education, exercises and nutrition support), 12 m | 245 (123/122), 0 | 64.1 ± 7.1/63.7 ± 6.5 | 122/245 | MMSE; CDT; RBC-Ach; RBC-AchE; number of patients progressed to dementia; AE |
| Zhang et al. (36) | MCI | Ginkgo biloba extract injection, 20 mL; + GIK; 14 d | GIK, 250 mL, 14 d | 200 (100/100), 0 | 75.7 ± 7.3/73.1 ± 5.7 | NR | MMSE; MoCA; serum index (hs-CRP, IL-6) |
| Wang et al. (50) | MCI | Styron (Ginkgo biloba extract tablet), 19.2 mg, t.i.d; +Basic treatment; 12 m | Basic treatment (health education and lifestyle guidance), 12 m | 87 (45/42), 0 | 80.86 ± 5.99 | NR | MMSE; ERP P300 |
| Zheng and Zheng (34) | MCI | Ginkgo biloba extract tablet, 19.2 mg, t.i.d; +Basic treatment; 12 m | Basic treatment (health education, exercise and nutrition support), 12 m | 100 (50/50), 0 | 66.57 ± 2.56/66.38 ± 2.47 | 47/100 | CDT |
| Li et al. (44) | MCI with CHD and hyperuricemia | Ginkgo biloba extract tablet, 9.6 mg, t.i.d; +Basic treatment; 3 m | Basic treatment (Allopurinol, 0.1 g, t.i.d; exercise), 3 m | 69 (35/34), 0 | 71.36 ± 4.97/72.58 ± 5.09 | 39/69 | MMSE; CDT; number of patients progressed to dementia |
| Zhang et al. (40) | SVD related MCI | Ginkgo bilobate dispersible tablets, 0.15 g, t.i.d; +Donepezil +Basic treatment; 6 m | Donepezil, 5 mg, q.d; Basic treatment for primary disease; 6 m | 86 (43/43), 0 | 45–69/46–70 | 44/86 | MMSE; ADL |
| Zheng et al. (41) | MCI | Yindanxinnaotong soft capsule, 4 capsules, t.i.d; + Aniracetam; 3 m, follow up of 6 m | Aniracetam, 0.2 g, t.i.d, 3 m; follow up of 6 m | 149 (75/74), 0 | 72.7 ± 7.2/71.9 ± 8.5 | 65/149 | MMSE; ADL; TCM symptom score; AE |
| Cai et al. (37) | MCI with depression | Shuganjieyu capsule, 3 capsules, b.i.d, 6 m | Fluoxetine hydrochloride, 20 mg, 6 m | 120 (60/60), 0 | 66.7 ± 9.6/65.8 ± 10.3 | 54/120 | TCM symptom score; HAMD; MMSE; AE |
| Zhu and Zhang (53) | MCI | Nao Li Bao pills, 4 pills, t.i.d; + Piracetam tablets placebo | Piracetam tablets, 2 tablets, t.i.d; + Nao Li Bao pills placebo | 76 (41/35), 0 | 59.63 ± 8.23/61.25 ± 8.05 | 43/76 | Effective rate |
| Yuan et al. (47) | MCI | Huan Shao capsules, 2.1 g, t.i.d, 6 m | Nimodipine, 40 mg, t.i.d, 6 m | 76 (38/38), 0 | 79.85 ± 9.58/78.69 ± 9.32 | 40/76 | MoCA; TCM symptom score; AE |
| Zhang et al. (38) | Post-stroke MCI | Compound Congrong Yizhi, 4 capsules, t.i.d, 3 m | Nimodipine, 30 mg, t.i.d, 3 m | 140 (70/70), 7 (3/4) | 67.1 ± 5.3/66.3 ± 4.9 | 65/133 | Vascular endothelial function index; cerebral blood flow index |
| Zhao and Xiang (51) | Diabetic MCI | Yang Xue Qing Nao granules, 4 g, t.i.d; + Basic treatment; 4 m | Basic treatment, 4 m | 70, 0 | NR | NR | MMSE; serum index (CRP, LDL-C, SOD) |
| Gavrilova et al. (42) | Amnestic MCI | Ebb 761 (Ginkgo biloba extracts tablets), 240 mg, q.d, 6 m | Placebo, 240 mg, q.d, 6 m | 160 (80/80), 5 (2/3) | 65 ± 7/63 ± 7 | 124/159 | STAI-X1; GDS; TMT; CGI; AE; Neuropsychiatric inventory |
| Steiner et al. (32) | MCI | Sailuotong, 2 capsules, b.i.d, 3 m | Placebo, 2 capsules, b.i.d, 3 m | – | – | – | Logical memory story A; Digit symbol; D-KEFS, RCFS; Neuropsychological test battery; QOL; DASS-21; physiological tests; inflammatory tests |
| Yakoot et al. (48) | MCI | Memo, 750 mg, q.d, 1 m | Placebo, 750 mg, q.d, 1 m | 66 (33/33), 6 (3/3) | 65.97 ± 6.52/66.43 ± 5.79 | 27/60 | MMSE; AE |
| Zhang et al. (35) | Amnestic MCI | Bushen capsule (Congrong Yizhi capsule), 4 capsules, t.i.d, 24 m | Placebo, 4 capsules, t.i.d, 24 m | 60 (30/30), 2 (0/2) | 64.67 ± 6.83 | 32/60 | MMSE; AVLT; ROCF; Digit Span test; TMT; SDMT; SCWT; CVFT; BNT; AE; MRI |
| Gschwind et al. (33) | MCI with dual-task-related gait impairment | GBE 1370 (Ginkgo biloba extract), 120 mg, b.i.d, 6 m | Placebo, 120 mg, b.i.d, 6 m | 50 (25/25), 8 (24/18) | 68.5 ± 8.4 | 25/50 | Spatio-temporal gait parameters; AE |
| Guo et al. (39) | Vascular MCI | Xinnaoning capsule, 1.35 g, t.i.d; +Basic treatment; 6 m | Nimodipine, 30 mg, t.i.d; +Basic treatment (health education and exercise); 6 m | 80 (40/40), 2 (0/2) | 70.13 ± 6.02/70.00 ± 5.47 | 37/78 | MMSE; MoCA |
| Li et al. (49) | MCI | Ginkgo biloba tablets, 80 mg, t.i.d, 3 m | Nicergoline tablets, 10 mg, t.i.d, 3 m | 150 (75/75), 0 | 68.2 ± 4.4 | 65/150 | MMSE |
| He (43) | Post stroke MCI | Ginkgo Leaf Extract and dipyridamole injection, 20 mL, with sodium chloride; 14 d | Sodium chloride injection, 250 mL, q.d, 14 d | 80 (40/40), 0 | 60.7 ± 5.84/61.35 ± 5.75 | 30/80 | MMSE; MoCA; AE |
| Li (52) | MCI | Quan Tian Ma capsules, 2.5 g, t.i.d; +Basic treatment; 3 m | Basic treatment, 3 m | 60 (30/30), 0 | 71.6 ± 6.6/71.2 ± 6.7 | 26/60 | MMSE; AE |
| Guo et al. (54) | MCI with Type 2 Diabetic | Jinlida granules, 9 g, tid; +Sitagliptin, 100 mg, qd; 3 m | Sitagliptin, 100 mg, qd; 3 m | 102 (51/51), 6 (3, 3) |
67.8 ± 5.4/68.2 ± 5.8 | 51/102 | MMSE; MoCA; symptom score; effective rate; inflammatory tests; AE |
| Liu (55) | Post stroke MCI | Zhongfeng Huichun tablet, 5 tablets, tid; + Butylphthalide and Sodium, 25 mg, bid; 14 d | Butylphthalide and Sodium Chloride injection, 25 mg, bid; 14 d; | 110 (55/55), 0 | 54.2 ± 2.6/53.8 ± 2.4 | 49/110 | Effective rate (NIHSS); LOTCA |
| Yang et al. (56) | Vascular MCI | Yinxing Tongzhi tablets, 0.15 g, tid; + Butylphthalide Soft capsules, 0.2 g, tid; 3 m | Butylphthalide Soft capsules, 0.2 g, tid; 3 m | 102 (51/51)0.0 | 57.04 ± 6.81/57.18 ± 6.52 | 39/102 | MMSE; inflammatory tests; ADL; AE |
CHM, Chinese herbal medicine; I, Intervention; C, Control; MCI, Mild cognitive impairment; MMSE, Mini-mental state examination; MoCA, Montreal cognitive assessment; ADL, Activities of daily living scale; IADL, Instrumental activities of daily living scale; ERP P300, Event-related potentials P300; EEG, Electroencephalogram; SAS, Self-rating anxiety scale; SDS, Self-rating depression scale; BI, Barthel index; CDT, Clock drawing task; WMS, Wechsler memory scale; MQ, Memory quotient; MDA, Maloudialdehyde; AchE, Acetylcholinesterase; DSR, Delayed story recall; AE, Adverse events; CGI- EI, Clinical global impression-efficacy index; ADAS-Cog, The Alzheimer's disease assessment scale-cognitive subscale; RVR, Rapid verbal retrieve; DS, Digit span; TCM, Traditional Chinese medicine; TG, Triglyceride; TC, Total cholesterol; HDL-C, High density liptein-cholesterol; LDL-C, Low density lipoprotein-cholesterol; CRP, C-reactive protein; NR, Not reported; NHFPC, National Health and Family Planning Commission; CHD, Coronary heart disease; SVD, Cerebral small vessel disease; HAMD, Hamilton Depression Scale; CACMS, China Academy of Chinese Medical Sciences; IBRCM, Institute of Basic Research in Clinical Medicine; CVFT, Category verbal fluency test; AVLT, Auditory verbal learning test; MRI, Magnetic resonance imaging; STAI-X1, State-Trait anxiety inventory; GDS, Geriatric depression scale; TMT, Trail-Making test; ROCF, Rey-Osterrieth complex figure test; BNT, Boston naming test; SDMT, Symbol digit modalities test; SCWT, Stroop color and word test; RCFS, Rey complex figure test; D-KEFS, Delis-Keplan executive function system; DASS-21, 21-item Depression, Anxiety, Stress scale.
Twenty-seven CHM were explored in the included trials (see Supplementary Table 2 for details). The dosage form included capsules (n = 13), pills (n = 4), tablets (n = 4), granules (n = 3), oral liquids (n = 2), and injection (n = 2). The duration of CHM treatment ranged from 14 to 24 months.
Risk of Bias in Included Studies
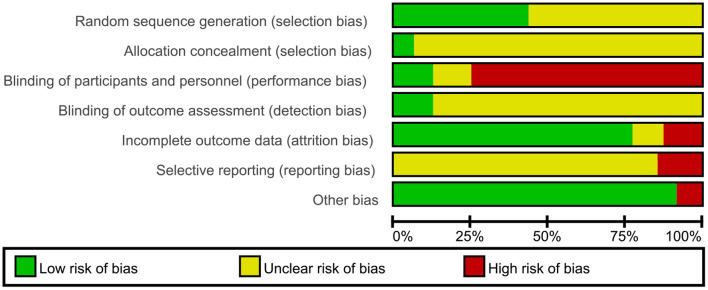
We carried out the risk of bias assessment for 48 finished trials. We judged many trials to have unclear risk of bias in certain domains because we could not obtain additional information from the trial authors after we contacted them (Figures 2, 3).
Figure 2.
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Figure 3.
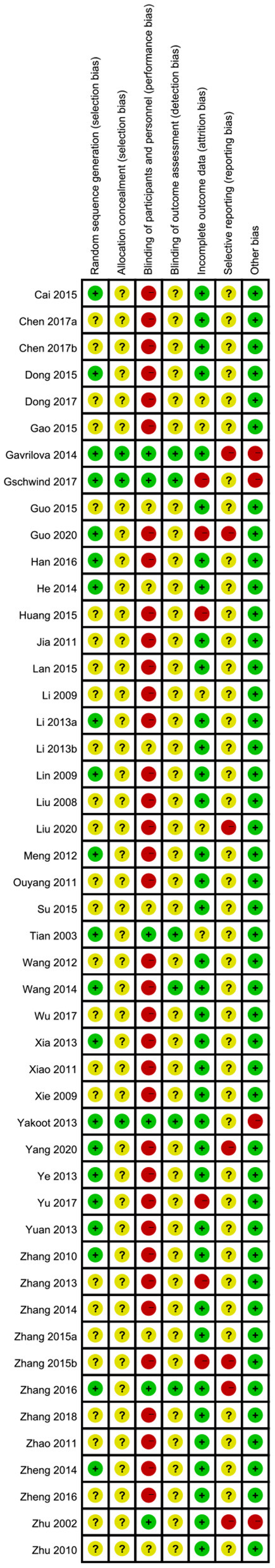
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
For selection bias, 21 trials (9, 14, 19, 21, 27, 29, 33, 35, 37, 41–45, 47, 48, 54, 56) which either used SAS software or the randomization table to generate a randomization sequence were assessed at low risk of bias; the remaining 27 trials did not specify the randomization method, and therefore we assessed at unclear risk of bias. The method used to conceal allocation was not reported in most of the included trials, except for three trials (33, 42, 48) assessed at low risk of bias regarding allocation concealment. Six trials (31, 33, 35, 42, 48, 53) adequately performed blinding of participants and personnel; the remaining 42 trials did not adequately describe any blinding methods of participants and personnel; moreover, 36 trials of them were assessed at high risk of bias as CHM in addition to a co-intervention was compared to a co-intervention or the form of CHM was quite different from that of control intervention. Blinding of outcome assessors was adequately performed in six trials (20, 31, 33, 35, 42, 48); the remaining 42 trials did not describe the method used for blinding of outcome assessors. 38 trials reported having no missing data and included all participants in data analyses, showing low risk of attribution bias; six trials (9, 17, 33, 38, 46, 54) had high dropout rates and excluded dropouts from the analyses, so we assessed them at high risk of bias as they did not properly deal with the missing data; four trials (11, 18, 31, 52) did not report the information of dropouts, and therefore were assessed at unclear risk of bias. The risk of selective reporting of one trial was low as the published paper was consistent with the protocol; 40 trials was unclear because of lack of pre-published trial protocols though one primary outcome was reported; seven trials (35, 38, 42, 53–56) was assessed to be of high risk as no pre-published protocols and no primary outcome was reported. Except four trials (33, 42, 48, 53) which was funded by pharmaceutical companies, and resulted our assessments of high risk of bias, the remaining 44 trials appeared to be free of other factors that could put them at risk of bias.
Effect Estimates
All 48 finished trials employed parallel design, 33 of which compared CHM with no intervention/placebo allowing for co-intervention; the remaining 15 trials (9, 12, 13, 22, 24, 26–28, 33, 35, 37, 38, 42, 47, 48) compared CHM with western drugs allowing for co-intervention.
CHM vs. No Intervention/Placebo (Co-intervention Was Allowed)
Number of Participants Progressed to Dementia
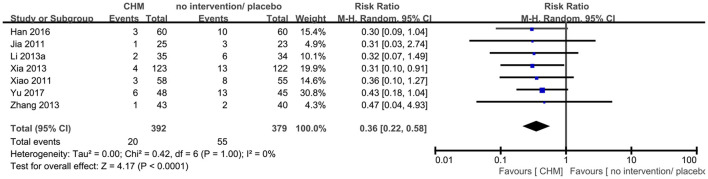
Seven RCTs (9, 14, 23, 25, 44–46) reported this outcome, and the meta-analysis showed that CHM significantly decreased the number of patients who progressed to dementia compared to no intervention or placebo (RR 0.36, 95% CI 0.22–0.58, 7 trials, 771 participants, I2 = 0%) (Figure 4).
Figure 4.
Forest plot of comparison: CHM vs. no intervention/placebo, outcome: number of participants progressed to dementia.
Cognitive Function
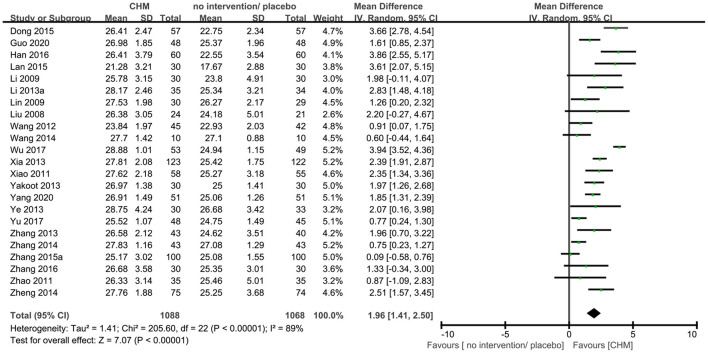
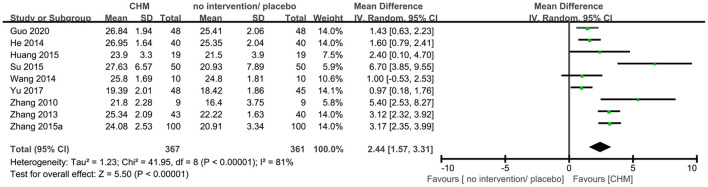
Twenty-three trials (9, 10, 14, 16, 19, 20, 23, 25, 29, 30, 35, 36, 40, 41, 44–46, 48, 50–52, 54, 56) reported MMSE outcome, and the meta-analysis showed that that CHM can significantly improve the cognitive function compared to no intervention or placebo when assessed by MMSE (MD 1.96, 95% CI 1.41–2.50, 23 trials, 2156 participants, I2 = 89%, Figure 5). Nine trials (9, 15, 17, 20, 27, 36, 43, 46, 54) reported MoCA outcome, and the meta-analysis showed that CHM can significantly improve the cognitive function compared to no intervention or placebo when assessed by MoCA (MD 2.44, 95% CI 1.57–3.31, 728 participants, I2 = 81%, Figure 6). The heterogeneity was high. Therefore, we tried to conduct subgroup analyses in attempts to identify the difference in the effect. The results showed statistically significant differences when comparing trials with different CHM (test for subgroup difference: MMSE: P < 0.00001, I2 = 89.6%; MoCA: P = 0.05, I2 = 57.8%, Supplementary Figures 1, 2), trials with different causes of MCI (test for subgroup difference: MMSE: P < 0.00001, I2 = 89.1%; MoCA: P = 0.0002, I2 = 84.4%, Supplementary Figures 3, 4), and trials with different treatment duration (test for subgroup difference: MMSE: P = 0.06, I2 = 72.4%; MoCA: P < 0.00004, I2 = 92.1%, Supplementary Figures 5, 6). The subgroup analysis of different CHM showed that Ginko leaf tablets can significantly improve the cognitive function compared to no intervention or placebo when assessed by MMSE (MD 2.03, 95% CI 1.18–2.88, 1117 participants, I2 = 90%, Supplementary Figure 1) and MoCA (MD 3.11, 95% CI 1.90–4.33, 463 participants, I2 = 82%, Supplementary Figure 2). The sensitivity analysis by including only trials with relatively lower risk of bias found similar results (MD 1.87, 95% CI 1.22–2.52, 2 trials, 120 participants) with that including all trials (MD 1.96, 95% CI 1.41–2.50, 23 trials, 2,156 participants) showing robustness of the results on MMSE (Supplementary Figure 13).
Figure 5.
Forest plot of comparison: CHM vs. no intervention/placebo, outcome: MMSE.
Figure 6.
Forest plot of comparison: CHM vs. no intervention/placebo, outcome: MoCA.
ADL
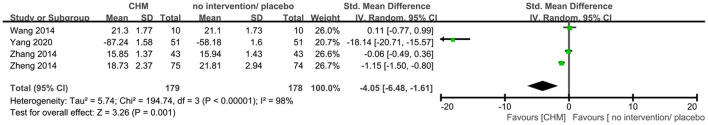
Four trials (20, 40, 41, 56) reported ADL outcome, of which one trial (56) used 0–100 ADL scale with higher score showing better results while the other three trials used the ADL scale with lower score showing better results. As ADL scales with differences in the direction have been used, we first multiplied the mean value of the trial set (56) by −1 to ensure the scales in the same direction, and then used SMD to synthesize data as recommended by Cochrane handbook. The meta-analysis showed that CHM significantly decreased the ADL score compared to no intervention and placebo (SMD −4.05, 95% CI −6.48 to −1.61, 357 participants, I2 = 100%, Figure 7).
Figure 7.
Forest plot of comparison: CHM vs. no intervention/placebo, outcome: ADL.
CHM vs. Western Medicine (Co-intervention Was Allowed)
Cognitive Function
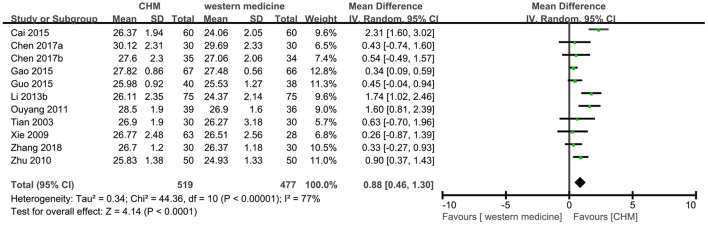
Eleven trials (8, 12, 13, 18, 24, 26, 28, 31, 37, 39, 49) reported MMSE outcome, and the meta-analysis showed that TCM had a significant increase on the score of MMSE compared to western medicine (MD 0.88, 95% CI 0.46–1.30, 996 participants, I2 = 77%, Figure 8). Because of the high heterogeneity, the subgroup analyses were conducted according to different interventions and causes of MCI. The statistically significant subgroup difference was found when comparing different interventions (test for subgroup difference: P < 0.0001, I2 = 77.5%, Supplementary Figure 7), and when comparing different causes of MCI (test for subgroup difference: P < 0.0001, I2 = 82.9%, Supplementary Figure 8).
Figure 8.
Forest plot of comparison: CHM vs. western medicine, outcome: MMSE.
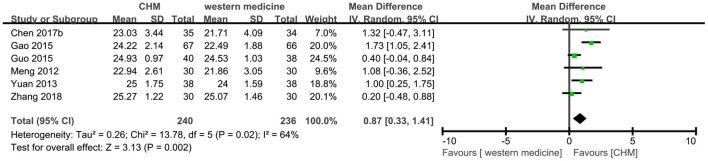
Six trials (8, 13, 18, 22, 39, 47) reported the MoCA score, and the meta-analysis showed that CHM had a significant increase on the score of MoCA compared to western medicine (MD 0.87, 95% CI 0.33–1.41, I2 = 64%, Figure 9). Because of the high heterogeneity, the subgroup analyses were conducted according to different interventions and causes of MCI. The statistically significant subgroup difference was found when comparing different interventions (test for subgroup difference: P = 0.01, I2 = 69.6%, Supplementary Figure 9), and when comparing different causes of MCI (test for subgroup difference: P = 0.17, I2 = 42.8%, Supplementary Figure 10).
Figure 9.
Forest plot of comparison: CHM vs. western medicine, outcome: MoCA.
ADL
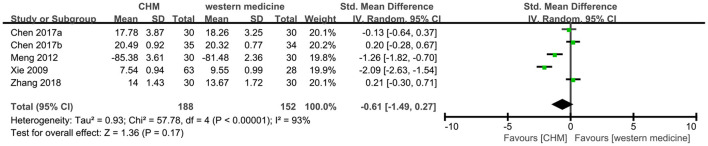
Five trials (8, 12, 13, 22, 28) reported this outcome, of which one trial (22) used ADL scale (higher score indicates better results) in the different direction with other scales (lower score indicates better results). We multiplied the mean value of 22 and used SMD model to synthesize the data. And the meta-analysis showed that CHM had no significant difference in the score of ADL compared to western medicine (SMD −0.61, 95% CI −1.49 to 0.27, I2 = 93%, Figure 10).
Figure 10.
Forest plot of comparison: CHM vs. western medicine, outcome: ADL.
Adverse Events
Twenty-four trials reported adverse events, of which 13 trials reported that no adverse events ever occurred in both the CHM group and the control group. The remaining 11 trials found no significant difference between two groups on adverse events.
Discussion
This review summarized current evidence on the comparative effectiveness of CHM, either individually tested or in combined remedies, for treatment of MCI, and finally identified 49 trials (48 finished trials and 1 protocol). This study found that CHM was better than no intervention or placebo (when co-intervention was allowed) in improving the number of participants who progressed to dementia and the score of MMSE, MoCA, ADL. And CHM was better than western medicine (when co-intervention was allowed) on improving the score of MMSE and MoCA but the significant difference was not found on the score of ADL. Considering the high heterogeneity, the subgroup analysis was conducted according to intervention, cause of MCI, and treatment duration.
In the subgroup analysis of intervention, we found that CHM Gingko leaf extracts were explored most. The combined effect from meta-analyses showed that Gingko leaf extracts as adjuvant therapy could slow down the progression to dementia, improve the MMSE score and MoCA score. Previous study showed that Gingko leaf extracts have been widely used in treating neuropsychiatric disorders (57). A previous systematic review published in 2016 (58) evaluated Ginkgo biloba in MCI and Alzheimer's diseases, and provided a similar result with our review stating that Ginkgo biloba in combination with conventional medicine is superior to conventional medicine alone in improving MMSE scores for people with MCI. The results of our study may provide support that Ginkgo leaf extracts can be considered for use in patients with MCI. However, still more qualified RCTs were needed to confirm the effect and optimize the use of Gingko leaf extracts. In the subgroup analysis of the cause of MCI, we found that CHM can improve the number of participants who progressed to dementia and the score of MMSE and MoCA in Parkinson related MCI patients compared to no intervention or placebo; CHM can improve the score of MMSE and MoCA of post-stroke and vascular MCI patients compared to no intervention or placebo; CHM can improve the score of MMSE, MoCA and ADL of post-stroke MCI patients compared to western medicine. However, more high-quality RCTs with large samples were needed to further confirm the effectiveness of CHM for the specific type of MCI. In the subgroup analysis of treatment duration, we found that CHM can improve the score of MMSE and MoCA in both subgroups, more than 6 months group and ≤ 6 months group.
Our review identified that the recommended outcomes by the guideline, including progression to dementia, reduction of ability to undertake daily activities, and quality of life (59), were infrequently assessed in the included trials than scales/ tools for cognition. Seven out of the included 45 trials have used a long-term endpoint progression to dementia as an outcome; eight trials have used scales of daily life activities; none of the trials has used quality of life. Oppositely, many different scales/ tools for cognitive function and global function have been used. The various scales/tools being used within the included trials limited the synthesis of data from different clinical trials. Moreover, the superiority of one scale/tool over another one has not been proved and should be explored in future (59). Besides, preservation of the patient's personality or the accessibility of disease information and health services should also be focused on future MCI studies from the voices of stakeholders (60). Outcome recommendations for dementia or cognitive function have been reported though (59–61), core outcome set specifically for MCI is still needed and should be developed.
As for methodological quality, future trials are recommended to be reported according to the CONSORT statement and its extension for herbal medicinal interventions (62, 63). Placebo-controlled design should be introduced. For trials comparing TCM with other western medicine, the double-dummy technique should be adopted to compare drugs with very different appearances to reduce observer and patients bias (64). The high risk of other bias was mainly shown in the funding and personnel team of the included trials. Six trials had only one author and we could not obtain any information of acknowledgment. Although the quality of RCTs cannot be decided by the number of the authors, for RCTs if conducted by only one author, the blinding was impossible. Four trials were suspected of sponsorship bias either because the authors were from the pharmaceutical companies or the companies fully or partially funded the trials. The previous review has suggested that industry-sponsored studies were biased in favor of the sponsor's products (65). The small sample size effect on publication bias should be cautioned as the included studies seemed to be not large, ranging from 48 to 245 participants. Sample size estimation is not available in most of the included studies, thereby whether the statistical power is enough seems to be unclear. Large sample size trials with strong power is warranted in future CHM studies for MCI.
There were similar systematic reviews previously published. One systematic review published in 2009 (6) explored CHM for MCI and age-associated memory impairment, and found the effects of the CHM was at least equivalent to piracetam on MMSE scores. Two systematic reviews by Lin et al. (4, 5) explored the effects of CHM for MCI by focusing either on MMSE and Alzheimer's disease assessment scale-cognitive subscale (4) or on MoCA (5). Our review was registered on PROSPERO and conducted a comprehensive up-to-date search of Chinese and English language databases to 2021 December. In this review, we focused on CHM, which is characterized by refined dosage forms and relative standardization and is approved by the State Administration for Market Regulation (66). To explore the benefits and harms of CHM, in this review, we focused on primary outcomes including the number of participants who progressed into dementia, cognitive function measured by accepted scales/tools, and adverse events.
There were some limitations in this review. Firstly, many included trials were judged to have the unclear risk of bias because we could not obtain enough information from the trial authors. Furthermore, there was certain clinical heterogeneity in the included RCTs because of causes of MCI, treatment duration.
Conclusion
CHM, Ginko leaf extracts in particular, could help to prevent progression into dementia and to improve cognitive function and ability of daily living activities. However, due to the low quality of current trials, more qualified large-sample randomized controlled trials were needed to confirm the conclusion.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
NS was the project leader and initiated the study. YW and ZZ contributed to the conception of the study. NL provided the methodological guidance, trained the reviewers, and drafted the manuscript. SY, CL, LG, and SW searched the literature, collected the data, and evaluated the quality of the included studies. YC performed the meta-analysis and drafted the manuscript. All authors read and approved the final manuscript.
Funding
The study was supported by National Key R&D Plan (2019YFC1712000) and National Science and Technology Major Project (2018ZX10101001-005).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
Abbreviations
- AchE
Acetylcholinesterase
- ADAS-Cog
Alzheimer's Disease Assessment Scale-Cognitive subscale
- ADL
Activities of daily living scale
- AE
Adverse events
- AVLT
Auditory verbal learning test
- BI
Barthel index
- BNT
Boston naming test
- C
Control
- CACMS
China Academy of Chinese Medical Sciences
- CDT
Clock drawing task
- CGI- EI
Clinical global impression-efficacy index
- CHD
Coronary heart disease
- CHM
Chinese herbal medicine
- CIs
confidence intervals
- CNKI
China National Knowledge Infrastructure
- CQVIP
Chongqing VIP
- CRP
C-reactive protein
- CVFT
Category verbal fluency test
- DASS-21
21-item Depression
- Anxiety
Stress scale
- D-KEFS
Delis-Keplan executive function system
- DS
Digit span
- DSR
Delayed story recall
- EEG
Electroencephalogram
- ERP P300
Event-related potentials P300
- GDS
Geriatric depression scale
- HAMD
Hamilton Depression Scale
- HDL-C
High density liptein-cholesterol
- I
Intervention
- IADL
Instrumental activities of daily living scale
- IBRCM
Institute of Basic Research in Clinical Medicine
- LDL-C
Low density lipoprotein-cholesterol
- MCI
mild cognitive impairment
- MD
mean difference
- MDA
Maloudialdehyde
- MMSE
Mini-mental state examination
- MoCA
Montreal Cognitive Assessment
- MQ
Memory quotient
- MRI
Magnetic resonance imaging
- NHFPC
National Health and Family Planning Commission
- NR
Not reported
- RCFS
Rey complex figure test
- RCTs
randomized clinical trials
- ROCF
Rey-Osterrieth complex figure test
- RR
risk ratio
- RVR
Rapid verbal retrieve
- SAMR
State Administration for Market Regulation
- SAS
Self-rating anxiety scale
- SCWT
Stroop color and word test
- SDMT
Symbol digit modalities test
- SDS
Self-rating depression scale
- SMD
standard mean difference
- STAI-X1
State-Trait anxiety inventory
- SVD
Cerebral small vessel disease
- TC
Total cholesterol
- TCM
Traditional Chinese medicine
- TG
Triglyceride
- TMT
Trail-Making test
- WMS
Wechsler memory scale.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.903224/full#supplementary-material
References
- 1.Petersen RC. Clinical practice. Mild cognitive impairment. N Eng J Med. (2011) 364:2227–34. 10.1056/NEJMcp0910237 [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. (2018) 90:126–35. 10.1212/WNL.0000000000004826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen RC. Mild cognitive impairment. Continuum. (2016) 22:404–18. 10.1212/CON.0000000000000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin D, Hyde AJ, Zhang AL, Xue CC, May BH. Chinese herbal medicine for mild cognitive impairment using montreal cognitive assessment: a systematic review. J Altern Complement Med. (2019) 25:578–92. 10.1089/acm.2018.0346 [DOI] [PubMed] [Google Scholar]
- 5.Lin D, May BH, Feng M, Hyde AJ, Tan HY, Guo X, et al. Chinese herbal medicine for mild cognitive impairment: a systematic review and meta-analysis of cognitive outcomes. Phytother Res. (2016) 30:1592–604. 10.1002/ptr.5679 [DOI] [PubMed] [Google Scholar]
- 6.May BH, Yang AW, Zhang AL, Owens MD, Bennett L, Head R, et al. Chinese herbal medicine for mild cognitive impairment and age associated memory impairment: a review of randomised controlled trials. Biogerontology. (2009) 10:109–23. 10.1007/s10522-008-9163-5 [DOI] [PubMed] [Google Scholar]
- 7.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester: John Wiley & Sons; (2019). [Google Scholar]
- 8.Zhang J, Wang C, Li J. Therapeutic effect of compound huo nao shu on mild cognitive impairment of insufficiency Parkinson's disease. Chin J Clinical Rational Drug Use. (2018) 1:68–9. 10.15887/j.cnki.13-1389/r.2018.01.035 [DOI] [Google Scholar]
- 9.Yu E, Liao Z, Tan Y, Qiu Y, Zhu J, Shi M, et al. Preventive effects of Haishe capsule on the conversion of amnestic mild cognitive impairment to Alzheimer's disease. Chin J Geriatrics. (2017) 36:278–81. 10.3760/cma.j.issn.0254-9026.2017.03.013 [DOI] [Google Scholar]
- 10.Wu D, Guo R, Wang Y. Clinical observation of sweet dream liquid curing deficiency of spleen and kidney style MCI. Chin Traditional Herb Drugs. (2017) 23:4958–62. 10.7501/j.issn.0253-2670.2017.23.022 [DOI] [Google Scholar]
- 11.Dong C. The clinical effect of cognitive function training combined with Bushenyang Chinese patent medicine on prevention and treatment of cognitive impairment in old people. Chin. Control Endemic Dis. (2017) 32:568–71. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2017&filename=DYBF201705051&uniplatform=NZKPT&v=8NNOONuj0KBgFI-V19YY2SIS-rLEsvlN2xoBeupj1NX0vMaIn0xuJiLcAS6JvBCj [Google Scholar]
- 12.Chen K, Chen L, Hu W. Clinical efficacy observation on Bushen Huatan Quyu method in the treatment of mild cognitive impairment of kidney deficiency and phlegm type. China Mod Dr. (2017) 55:97–100. Available online at: https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjIwNDE1EhZ6d2tqemxtbC15eXdzMjAxNzI1MDI4GggydmE5NHJubA%3D%3D [Google Scholar]
- 13.Chen J, Sun S, Yu Z, Sheng F, Zhou L. Clinical observation on Shenzhiling oral liquid in the treatment of mild cognitive impairment. Chin J Integr Med Cardiocerebrovasc Dis. (2017) 15:365–8. 10.3969/j.issn.1672-1349.2017.03.032 [DOI] [Google Scholar]
- 14.Han Z. Clinical effect of Ginkgo Biloba tablet on mild cognitive impairment. Practical J Card Cereb Pneumal Vasc Dis. (2016) 24:91–4. 10.3969/j.issn.1008-5971.2016.01.027 [DOI] [Google Scholar]
- 15.Su W, Liu B, Su Y. Ginkgo biloba preparation combined with donepezil hydrochloride in the treatment of old people cognitive impairment and its effect on EEG and ERPP300. Shaanxi J TCM. (2015) 36:542–4. 10.3969/j.issn.1000-7369.2015.05.012 [DOI] [Google Scholar]
- 16.Lan P, Pan F, Su J. Clinical study on Qizhi Tongluo capsule intervention for mild cognitive impairment. Med Inf. (2015) 28:59–60. 10.13192/j.issn.1000-1719.2016.04.029 [DOI] [Google Scholar]
- 17.Huang Y, Chen C, Qin X, Lan H. Modified Liuwei Dihuang Pills to treat mild cognitive dysfunction with kidney essence deficiency syndrome. J Guangxi University of Chin Med. (2015) 18:8–10. Available online at: https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjIwNDE1EhFneHp5eHl4YjIwMTUwMzAwMxoIcWl2cnM2dGs%3D [Google Scholar]
- 18.Gao L, Zhang X, Liu X, Xu Y, Wang M, Chen L, et al. Clinical research of mild cognitive impairment after stroke treated with Fufang Congrong Yizhi capsules. World J Integr Traditional and Western Med. (2015) 10:65–8. 10.13935/j.cnki.sjzx.150120 [DOI] [Google Scholar]
- 19.Dong J. Effects of Ginkgo biloba combined with donepezil hydrochloride on cognitive function, mental state and negative emotions in elderly patients with mild cognitive dysfunction. Shaanxi J TCM. (2015) 36:820–2. 10.3969/j.issn.1000-7369.2015.07.026 [DOI] [Google Scholar]
- 20.Wang Y. Kidney Phlegm and Activating Blood Method in the Treatment of Mild Cognitive Impairment After Stroke. Shijiazhuang: Hebei Medical University; (2014). [Google Scholar]
- 21.Ye H. Study on memantine combined with tongxinluo in treatment for amnestic mild cognitive impairment. Int Med Health Guidance News. (2013) 19:1158–61. 10.3760/cma.J.issn.1007-1245.2013.08.040 [DOI] [Google Scholar]
- 22.Meng C. The Tonifying Kidney and Promoting Blood Circulation and Resolving Phlegm Method in Treatment of Stroke After Mild Cognitive Impairment Clinical Study. Changchun: Changchun University of Chinese Medicine; (2012). [Google Scholar]
- 23.Xiao S. The Clinical Research of Ginkgo Biloba Extract on Mild Cognitive Impairment and the Conversion Rate to Dementia. Shanghai: Fudan University; (2011). [Google Scholar]
- 24.Ouyang S, Ouyang Z. Therapeutic effects of Jiannao Bushen pills for mild cognitive dysfunction after ischemic stroke in 39 cases. Guiding J TCM. (2011) 17:41–2. 10.3969/j.issn.1672-951X.2011.05.019 [DOI] [Google Scholar]
- 25.Jia X, Kang X, Yan Z. Research on treatment of mild cognitive impairment by Tongxinluo combined with oxiracetam. Chin J Difficult and Complicated Cases. (2011) 10:495–7. 10.3969/j.issn.1671-6450.2011.07.006 [DOI] [Google Scholar]
- 26.Zhu L. Clinical effect of Xuanyunning tablet in treating senile patients with mild cognitive impairment of turbid-phlegm blocking orifice syndrome. Chin J Med Guide. (2010) 12:2107–9. [PubMed] [Google Scholar]
- 27.Zhang Y. Clinical Study on TCM Intervention for Mild Vascular-Derived Cognitive Impairment With Kidney Deficiency, Phlegm Turbidity and Blood Stasis Syndrome Based on the Theory of Brain Marrow. Changchun: Changchun University of Chinese Medicine; (2010). [Google Scholar]
- 28.Xie Z, Xie N, Feng Y. Clinical study on Tianzhi granules in treating amnesia mild cognitive impairment with liver yang hyperactivity syndrome. Chin J Exp Trad Med Formulae. (2009) 15:95–6. 10.3969/j.issn.1005-9903.2009.09.033 [DOI] [Google Scholar]
- 29.Lin L. The Clinical Study of Mongolian Milkvetch Root Injection for Improving the Congnitive Disorder of Mild Vascular Cognitive Impairment Patients. Nanning: Guangxi University of Chinese Medicine; (2009). [Google Scholar]
- 30.Liu N, Deng Y, Zhou B, Tang Z. Clinical study on the effect of Yangxueqingnao Granules on patients with mild vascular cognitive dysfunction. Chin J Integr Med Cardiocerebrovasc Dis. (2008) 6:407–9. 10.3969/j.issn.1672-1349.2008.04.018 [DOI] [Google Scholar]
- 31.Tian J, Zhu A, Zhong J. A follow-up study on a randomized, single-blind control of King's Brain Pills in treatment of memory disorder in elderly people with MCI in a Beijing community. Zhongguo Zhong Yao Za Zhi. (2003) 28:987–91. 10.3321/j.issn:1001-5302.2003.10.031 [DOI] [PubMed] [Google Scholar]
- 32.Steiner G, Bensoussan A, Liu J, Hohenberg M, Chang D. Study protocol for a randomised, double-blind, placebo-controlled 12-week pilot phase II trial of Sailuotong (SLT) for cognitive function in older adults with mild cognitive impairment. Trials. (2018) 19:510–22. 10.1186/s13063-018-2912-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gschwind Y, Bridenbaugh S, Reinhard S, Granacher U, Monsch A, Kressig R. Ginkgo biloba special extract LI 1370 improves dual-task walking in patients with MCI: a randomised, double-blind, placebo-controlled exploratory study. Aging Clin Exp Res. (2017) 29:609–19. 10.1007/s40520-016-0699-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng W, Zheng W. Effects of Ginkgo biloba leaves on cognitive function in patients with mild cognitive impairment. World Latest Med Inf. (2016) 16:127. 10.3969/j.issn.1671-3141.2016.12.08722704410 [DOI] [Google Scholar]
- 35.Zhang J., Liu Z., Zhang H., Yang C., Heli, Li X., Chen K., et al. (2016). A two-year treatment of amnestic mild cognitive impairment using a compound Chinese medicine: a placebo controlled randomized trial. Sci. Rep. 6, 28982. 10.1038/srep28982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Wu Q, Chang L, Huang G. The effect of Ginkgo biloba leaves injection on elderly patients with mild cognitive impairment and influence onplasmahs-CRPandIL-6. Chin J Clin Healthc. (2015) 18:167–9. 10.3969/J.issn.1672-6790.2015.02.018 [DOI] [Google Scholar]
- 37.Cai Z, Mai J, Wang F. Therapeutic effect of Shuganjieyu capsules on mild cognitive impairment associated with depression in elderly patients. Chin J Difficult Comp Cases. (2015) 14:458–61. 10.3969/j.issn.1671-6450.2015.05.08 [DOI] [Google Scholar]
- 38.Zhang X, Gao L, Jiao J, Sui X, Song W, Mou S, et al. Impacts of Fufang Congrong Yizhi capsules on cerebral blood flow and vascular endothelial function in the patients of post-stroke mild cognitive impairment. World J Integr Trad Western Med. (2015) 10:533–6. 10.13935/j.cnki.sjzx.150426 [DOI] [Google Scholar]
- 39.Guo M, Xue Q, An Y. Curative effect of Xinnaoning capsule-on the treatment of mild vascular cognitive impairment no dementia. Beijing Med J. (2015) 37:97–9. [Google Scholar]
- 40.Zhang Q, Chen A, Chen Y, Liu W. The effect of Ginkgo ketone dispersible tablets on mild cognitive impairment caused by cerebrovascular disease. Chin J Integr Med Cardiocerebrovasc Dis. (2014) 12:1231–2. 10.3969/j.issn.16721349.2014.10.034 [DOI] [Google Scholar]
- 41.Zheng E, Wang X, Zhou S. Clinical study of Yindanxinnaotong soft capsule combined with Aniracetam on elder mild cognitive impairment with phlegm and blood stasis into resistance syndrome. Guiding J TCM and Pharm. (2014) 20:46–9. 10.13862/j.cnki.cn43-1446/r.2014.06.016 [DOI] [Google Scholar]
- 42.Gavrilova SI, Preuss UW, Wong JWM, Hoerr R, Kaschel R, Bachinskaya N, et al. Efficacy and safety of Ginkgo biloba extract EGb 761® in mild cognitive impairment with neuropsychiatric symptoms: a randomized, placebo-controlled, double-blind, multi-center trial. Int J Geriatr Psychiatry. (2014) 29:1087–95. 10.1002/gps.4103 [DOI] [PubMed] [Google Scholar]
- 43.He H. Clinical Observation Yinxingdamo Injection Treatment of Ischemic Stroke With Mild Cognitive Impairment. Nanning: Guangxi University of Chinese Medicine; (2014). [Google Scholar]
- 44.Li W, Tian D, Yan W, Liu X, Zhu Y. Therapeutic observation on Ginkgo Biloba tablet for cognitive impairment in hyperuricemia patients. World Chin Med. (2013) 8:48–50. 10.3969/j.issn.1673-7202.2013.01.018 [DOI] [Google Scholar]
- 45.Xia X. Effect of Ginkgo Biloba extract on mild cognitive impairment. Zhejiang Zhejiang J Integ Trad Chin West Med. (2013) 23:876–8. 10.3969/j.issn.1005-4561.2013.11.004 [DOI] [Google Scholar]
- 46.Zhang C, Feng J, Li H, Wang J, Ye J, Wang X. Efficacy observation on Ginkgo biloba extract in the treatment of Parkinson's disease with mild cognitive impairment. China Mod Dr. (2013) 51:109–11. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDHIS2&filename=ZDYS201331047&uniplatform=NZKPT&v=23OcE9a5MOgRhwmRPFzcXnL62ZZoB1X8ALyCZ2ajz7KMtAobIbEtxbUQW8NcWpuK [Google Scholar]
- 47.Yuan J, Shao Y, Xie W, Ou S. Huan Shao capsule in adjuvants for treating mild cognitive impairment. J Guangxi Univ Chin Med. (2013) 16:14–5. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDHIS2&filename=GSZB201304007&uniplatform=NZKPT&v=yc3Xoba7a0GGWE-yZFKGj4dU–w2x9KEhm-oLvRYV1e7xjHyJvsVjo3YkaEYBZO3 [Google Scholar]
- 48.Yakoot M, Salem A, Helmy S. Effect of Memo®, a natural formula combination, on mini-mental state examination scores in patients with mild cognitive impairment. Clin Interv Aging. (2013) 8:975–81. 10.2147/CIA.S44777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li B, Lai H, Li G. The clinical efficacy of Niergoline tablets in the treatment of cognitive impairment of elder people. Natl Med Front China. (2013) 8:66–7. [Google Scholar]
- 50.Wang B, Zhong Y, Yan H. Ginkgo biloba in treating elderly people with cognitive dysfunction and its clinical efficacy and effects on change of event-related potential P300. Chin J Gerontol. (2012) 32:2495–6. [Google Scholar]
- 51.Zhao L, Xiang K. The effects of Yangxueqingnao granules on mild cognitive dysfunction in elderly diabetic people from the perspective of oxidative stress. J China TCM Inf. (2011) 3:20–1. Available online at: https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjIwNDE1EhB6Z3p5eXp4MjAxMTIyMDE0Ggh3YWIzNmRhYg%3D%3D [Google Scholar]
- 52.Li P. The therapeutic effect of Quantianma capsule on mild cognitive dysfunction. Chin J Prim Med and Pharm. (2009) 16:728–9. 10.3760/cma.j.issn.1008-6706.2009.04.114 [DOI] [Google Scholar]
- 53.Zhu X, Zhang B. 41 cases of mild senile cognitive impairment of liver and kidney Yin deficiency syndrome treated by Naolibao pills. J TCM. (2002) 43:204–5. 10.3321/j.issn:1001-1668.2002.03.032 [DOI] [Google Scholar]
- 54.Guo W, Peng C, Li W, Xie H. Clinical Research on sitagliptin combined with jinlida granules in the treatment of elderly type 2 diabetic patients with mild cognitive impairment. Chin J Pharmacoepidemiol. (2020) 29:657–61. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2020&filename=YWLX202010001&uniplatform=NZKPT&v=YREcSwk7ikKFijbenO9WanUAqykoNd9hIioBiSuw9cc0peWJBuq3IZMmz796VP8O [Google Scholar]
- 55.Liu H. Effect of Zhongfeng Huichun Tablet combined with Butylphthalide and Sodium Chloride Injection on mild cognitive dysfunction after acute cerebral infarction. Pract Clin J Integ Trad Chin West Med. (2020) 20:95–6. 10.13638/j.issn.1671-4040.2020.17.048 [DOI] [Google Scholar]
- 56.Yang X, Fan X, Yang X. Clinical study on Yinxing Tongzhi tablets combined with Butylphthalide Soft capsules in treatment of vascular mild cognitive impairment. Drugs Clinic. (2020) 35:1089–92. 10.7501/j.issn.1674-5515.2020.06.007 [DOI] [Google Scholar]
- 57.Kandiah N, Ong PA, Yuda T, Ng L-L, Mamun K, Merchant RA, et al. Treatment of dementia and mild cognitive impairment without cerebrovascular disease: expert consensus of Ginkgo biloba extract, EGb 761. CNS Neurosci Ther. (2019) 25:288–98. 10.1111/cns.13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang G, Wang Y, Sun J, Zhang K, Liu J. Ginkgo biloba for mild cognitive impairment and Alzheimer's disease: a systematic review and meta-analysis of randomized controlled trials. Curr Top Med Chem. (2016) 16:520–8. 10.2174/1568026615666150813143520 [DOI] [PubMed] [Google Scholar]
- 59.Tochel C, Smith M, Baldwin H, Gustavsson A, Ly A, Bexelius C, et al. What outcomes are important to patients with mild cognitive impairment or Alzheimer's disease, their caregivers, and health-care professionals? A systematic review. Alzheimers Dementia. (2019) 11:231–47. 10.1016/j.dadm.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bossers W, Woude LVD, Boersma F, Scherder E, Heuvelen MV. Recommended measures for the assessment of cognitive and physical performance in older patients with dementia: a systematic review. Dement Geriatr Cogn Dis Extra. (2012) 2:589–609. 10.1159/000345038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lees R, Fearon P, Harrison J, Broomfield N, Quinn T. Cognitive and mood assessment in stroke research. Stroke. (2012) 43:1678–80. 10.1161/STROKEAHA.112.653303 [DOI] [PubMed] [Google Scholar]
- 62.Schulz K, Altman D, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. (2010) 152:726–32. 10.7326/0003-4819-152-11-201006010-00232 [DOI] [PubMed] [Google Scholar]
- 63.Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J, Bombardier C, et al. Recommendations for reporting randomized controlled trials of herbal interventions: explanation and elaboration. J Clin Epidemiol. (2006) 59:1134–49. 10.1016/j.jclinepi.2005.12.020 [DOI] [PubMed] [Google Scholar]
- 64.Marušić A, Ferenčić S. Adoption of the double dummy trial design to reduce observer bias in testing treatments. J R Soc Med. (2013) 106:196–8. 10.1177/0141076813485350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lundh A, Lexchin J, Mintzes B, Schroll J, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. (2017) 2:MR000033. 10.1002/14651858.MR000033.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhan T, Wei X, Chen Z-Q, Wang D-S, Dai X-P. A systematic review of RCTs and quasi-RCTs on traditional chinese patent medicines for treatment of chronic hepatitis B. J Tradit Chin Med. (2011) 31:288–96. 10.1016/S0254-6272(12)60006-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.