Abstract
We generated CD4+ T cell lines (TCLs) reactive to either SARS-CoV-2 spike (S) or membrane (M) proteins from unexposed naïve T cells from six healthy donor volunteers to understand in fine detail whether the S and M structural proteins have intrinsic differences in driving antigen-specific CD4+ T cell responses. Having shown that each of the TCLs were antigen-specific and antigen-reactive, single cell mRNA analyses demonstrated that SARS-CoV-2 S and M proteins drive strikingly distinct molecular signatures. Whereas the S-specific CD4+ T cell transcriptional signature showed a marked upregulation of CCL1, CD44, IL17RB, TNFRSF18 (GITR) and IGLC3 genes, in general their overall transcriptome signature was more similar to CD4+ T cell responses induced by other viral antigens (e.g. CMV). However, the M protein-specific CD4+ TCLs have a transcriptomic signature that indicate a marked suppression of interferon signaling, characterized by a downregulation of the genes encoding ISG15, IFITM1, IFI6, MX1, STAT1, OAS1, IFI35, IFIT3 and IRF7 (a molecular signature which is not dissimilar to that found in severe COVID-19). Our study suggests a potential link between the antigen specificity of the SARS-CoV-2-reactive CD4+ T cells and the development of specific sets of adaptive immune responses. Moreover, the balance between T cells of significantly different specificities may be the key to understand how CD4+ T cell dysregulation can determine the clinical outcomes of COVID-19.
Keywords: Sars-CoV-2, spike (S) glycoprotein, membrane protein, interferon signaling, immune regulation
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) the novel viral agent of the coronavirus disease 2019 (COVID-19) has resulted in widespread global morbidity and mortality (1). Infection with SARS-CoV-2 is characterized by a broad spectrum of clinical syndromes, which may range from asymptomatic infection to mild symptoms to severe pneumonia and acute respiratory distress syndrome (2, 3). According to the World Health Organization weekly epidemiological updates on COVID-19 (Edition 87), the current cumulative number of cases and deaths reported globally is almost 496 million and over 6 million respectively.
Immunological and clinical studies of acute and convalescent COVID-19 patients have observed that SARS-CoV-2-specific antibodies and T cell responses are strongly associated with milder disease and accelerated viral clearance (4–6). Indeed, SARS-CoV-2–specific CD4+ and CD8+ T cell responses have been reported as crucial for the control and resolution of primary SARS-CoV-2 infection (7). Varying approaches have been taken to quantify and characterize virus-specific T cell responses in acute, convalescent, and severe patients, in a quest to understand the nature of antigen specificity and function of the adaptive response to SARS-CoV-2 (3, 6, 8, 9). The response to the structural proteins, including spike (S), nucleocapsid (N), membrane (M), and non-structural proteins (nsp3, nsp4, ORF3a, and ORF8), has been the main targets for study. Using HLA predicted peptide megapools (MP) of the SARS-CoV-2 proteome, Grifoni et al. (10) demonstrated that SARS-CoV-2-specific CD4+ T cell responses were found in 100% of patients convalescing from COVID-19, with the majority of the CD4+ T cell reactivity directed to SARS-CoV-2 spike, M, and N proteins. On average, these antigens accounted for 27%, 21%, and 11% of the total CD4+ T cell response, respectively.
The CD4+ T cell responses to the SARS-CoV-2 S or N proteins have been shown to correlate with the magnitude of the anti-SARS-CoV-2 neutralizing antibodies in recovered patients (8, 10), a finding suggesting a potential role for the S protein in triggering a protective response to COVID-19. The SARS-CoV-2 M protein, in contrast, has been implicated in driving evasion of protective immune responses, a process felt to occur by the manipulation of innate antiviral immune responses,most specifically by interfering with interferon (IFN) signaling pathways and by antagonizing the production of type I and III IFN production (11–14). This M-driven antiviral immune suppression appears to favor SARS CoV-2 viral replication.
To understand in fine detail the molecular nature of the virus-specific CD4+ T cell response to the SARS CoV-2 structural proteins, here, we generated (in a non-biased manner) 18 human CD4+ T cell lines (TCLs) reactive to SARS-CoV-2 S protein (6), M protein (6), and 6 human TCLs reactive or to Cytomegalovirus (CMV). Each CD4+ TCL cell line was generated from naïve T cells obtained well prior to the COVID19 pandemic from 6 healthy donors with the aim of comparing their molecular properties and function. Our data suggest that SARS-CoV-2 S and M proteins each drive a strikingly distinct molecular signature in TCLs generated under neutral conditions. The S-specific CD4+ TCLs had a molecular profile with marked upregulation of CCL1, CD44, IL17RB, TNFRSF18 (GITR) and IGLC3 genes. In contrast, the M protein-specific CD4+ TCLs had a transcriptomic signature of marked suppression of STAT1-IFRs-interferon pathway signaling, a signature that is virtually indistinguishable from the molecular signature seen associated with severe COVID-19.
Methods
CMV-Specific or SARS-CoV-2 Spike or Membrane Protein-Specific CD4+ T Cell Lines Generation From Unexposed Individuals
The protocol for generating an antigen (CMV, SARS-CoV-2-Spike, SARS-CoV-2 Membrane)-specific CD4+ T cell line were adapted from previous studies (15, 16). Briefly, naïve CD4+ T cells, as well as monocytes were purified from cryopreserved PBMCs of 6 healthy donor individuals unexposed to SARS-CoV-2 (obtained prior to 2019), using magnetic cell sorting (MACS Miltenyi Biotec, USA). Purified monocytes were differentiated into dendritic cells (DCs) by a 6 day-culture in complete R10 media [RPMI 1640 medium (Gibco) supplemented with 10% heat-inactivated AB serum (Pan Biotech), 1% nonessential amino acids, 1% Hepes 1M, 1 mM sodium pyruvate, 2 mM fresh l-glutamine, 100 μg/ml streptomycin, 100 units/ml penicillin (all from Life Technologies, USA)] at 37°C, 5% CO2. 10µL of GM-CSF (1mg/mL) and 10µL of IL-4 (1mg/mL) were added at days 1, 3 and 5. At day 6, immature DCs were harvest, washed and incubated overnight with 10µL of IFN-γ (1,000 units/mL) and LPS (100ng/mL) for maturation. At day 7, 1x105 matured DCs were separately seeded in 24 wells plate, and then loaded for 2-4 hours with 1µg/mL of SARS-CoV-2 peptide megapools (MPs) covering either the entire sequence of SARS-CoV-2 spike protein (MP-S), consisted by a 15-mer peptides overlapping by 10-residues (253 peptides) (10) or covering the complete sequence of SARS-CoV-2 membrane protein (MP-M), consisting of 15-mer sequences with 11 amino acids overlap (PepTivator® SARS-CoV-2 Prot_M, Miltenyi Biotec, USA) (53 peptides) or 1µg/mL of CMV peptide megapools (~187 peptides) ( Supplementary Table 1 ). On the top of the either MP-S, MP-M or CMV pulsed-DCs, 1x106 naïve CD4+ T cells (1:10 ratio) were added and incubated for 12 days in complete R10 media. On days 2, 5 and 8, 60units of human rIL-2 (Cetus, USA) were added at each well. At day 12, cells in culture were washed, counted, and fed (1:1 ratio) with irradiated (40GY) autologous PBMCs (feeder cells) in the presence of either 1µg/mL of MP-S or MP-M for another 12-day round culture in the same conditions, including the IL-2 stimulation. After three 12-day rounds of peptide pool stimulation/expansion in vitro, we generated a total of 18, 6 MP-S-specific, 6 MP-M-specific and 6 MP-CMV-specific CD4+ TCLs, which were profiled for antigen-specificity and reactivity using multiparameter flow cytometry.
Responsiveness and Specificity of SARS-CoV-2-Specific CD4+ T Cell Line by Flow Cytometry
The generation of the different SARS-Cov-2-specific CD4+ T cell lines and their specificity and reactivity were confirmed by an immunophenotypic and functional assay where the cell lines were stimulated overnight in 5% CO2 at 37°C with their irradiated autologous feeder cells in the absence (media) or in the presence of their respective antigens (1µg/mL of MP-S or 1µg/mL of MP-M). Both CD4+ TCLs were also stimulated with 1µg/mL MP-CMV and PMA/ionomycin (Sigma-Aldrich) (0.5/0.05 pg/ml). The cells were then stained for viability (Live/Dead fixable blue (UV450), Molecular Probes), and then incubated with anti-CD3 (BV421), anti-CD4 (PerCP-Cy5.5) for 30 min in the dark at room temperature. The cells were next washed twice with FACS buffer, then fixed and permeabilized using a Fix/Perm buffer kit (BioLegend) for 30 min at 4°C. The cells were washed twice with Perm buffer (BioLegend) and resuspended with the intracellular antibody pool containing anti-CD69 (FITC), anti-CD154 (APC), anti-TNF-α (Alexa Fluor 700) and anti-IFN-γ (BUV737) for 30 min at 4°C. Finally, the cells were washed twice with Perm buffer and then acquired using the BD LSRFortessa flow cytometer (BD Biosciences) and FACSDiva software (BD Biosciences) for acquisition. All analyses were performed using FlowJo v10.5.3.
DNA Purification for Whole Exome Sequencing
Genomic DNA purified (Promega) from 30x106 PBMCs of the 6 healthy donor individuals unexposed to SARS-CoV-2 (the donors we used to generate the SARS-CoV-2-specific CD4+ TCLs) was sent for whole exome sequencing at Psomagen, Inc. The whole exome sequences were screened by the computational algorithm Demuxlet (17) to identify the genetic variations [(nucleotide polymorphisms (SNPs)] of the 6 healthy donor individuals, which were used to demultiplex the pooled samples by the genetic identity of each cell to allow statistical and visual comparisons of individual-specific scRNA-seq profiles.
Sample Preparation and Single-Cell RNA-Seq Libraries for Next Generation Sequencing
In summary, 1x105 live CD3+CD4+ T cells were sorted from the 6 MP-S, MP-M and CMV-specific CD4+ T cell lines cultures using a BD FACSAria Cell Sorter (BD Biosciences). 1 x 104 cells of each CD4+ TCLs were pooled together for both conditions separately. Approximately 6 x 103 multiplexed cells (a thousand cells per donor for each cell line) were loaded in three lanes of the Chromium Next GEM Chip G (10x Genomics) respectively, one for MP-S, another for MP-M and another for CMV-specific-CD4+ TCLs, resulting in three 10x Genomics Single Cell Chromium 3’ mRNA libraries, made in accordance with Chromium Single Cell 3’ Reagent Kits User Guide (v3.1).
Single Cell RNA-Seq Analysis
Three 10x Genomics Single Cell Chromium 3’ mRNA libraries were made and sequenced as part of one Illumina NextSeq run. Each sample had a sequencing yield of greater than 68 million reads. The sequencing run was setup with 150 cycles + 150 cycles symmetric run. Initial processing of the two samples included removal of the cells with extremely low number of UMI counts using Cellranger v4.0.0 using default parameters except for the forced cell counts which were 1,979 for S and 1,461 for M and 1,495 for CMV. Demuxlet (17) was used with matching exome SNP data to call cell genotypes and multiplet annotations. The remainder of the single cell RNA-Seq (scRNA) analysis was performed with Seurat v3.2.2 (18). SingleR (19) utilizing data available from the Novershtern Hematopoietic database (20) was used for cell type identification. All cells in all samples were filtered for quality in Seurat using the following quality metrics: Sample S cells were excluded that had less than 500, greater than 6000 detected genes, and higher than 15% mitochondrial gene expression. Sample M cells were excluded that had less than 200, greater than 5000 detected genes, and higher than 15% mitochondrial gene expression. Sample CMV cells were excluded that had less than 200 and greater than 4000 detected genes, higher than 15% mitochondrial gene expression. Cells were further filtered by the Novershtern database’s fine SingleR annotation and everything except for a CD4+ T cell was excluded. Finally, cells that contained multiplets called by Demuxlet were also excluded. This left Sample S with 713 cells, Sample M with 1,979 cells, and Sample CMV with 1,190 cells.
Samples S, M and CMV were merged using Seurat and standard scRNA analysis was done using 20 principal components (PCs) to visualize the UMAP diagrams. Initial clustering with UMAP identified 2 cell clusters separating the S and M samples. Differential expression analysis was performed to identify cluster-specific markers and to compare the S and M cell populations using MAST (21), or “Model-based Analysis of Single-cell Transcriptomics”, whereas the cluster-specific canonical pathway enrichment profiles were generated using Ingenuity Pathway Analysis (IPA, Qiagen, Redwood City, CA, USA).
Results
Specificity and Reactivity of CD4+ TCLs to SARS-CoV-2 Spike and Membrane Proteins
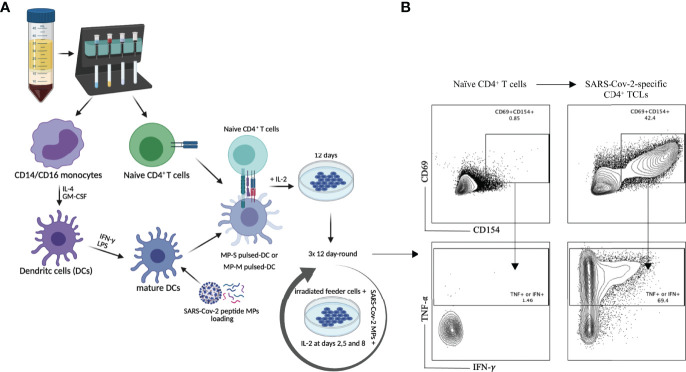
Purified naïve (CD45RA+CD45RO-) CD4+ T cells from the PBMCs of 6 healthy (unexposed to SARS-CoV-2) donor volunteers, obtained prior to 2019, were differentiated in vitro into SARS-CoV-2-specific CD4+ TCLs by rounds of stimulation and expansion using autologous dendritic cells loaded with peptide megapools (MP) covering either the entire sequence of SARS-CoV-2 spike protein (MP-S), or covering the complete sequence of SARS-CoV-2 membrane protein (MP-M) ( Figure 1A ). After three rounds of in vitro antigenic stimulation with either S- or M- specific MP in the presence of IL-2 and feeder cells, the CD4+ TCLs were assessed. Following in vitro stimulation, the generation of the SARS-CoV-2-reactive CD4+ T cells to MP-S or MP-M were identified based on the co-expression of CD154 and CD69 and their capacity to produce either IFN-γ or TNF-α in an antigen-specific manner upon stimulation. Figure 1B illustrates a representative analysis from one healthy donor’s PBMCs to demonstrate the conversion from the unresponsive naïve CD4+ T cell population into a SARS-CoV-2-reactive CD4+ TCLs which showed 44.6% of CD69+CD154+ co-expression in comparison to only 0.87% with their respective naïve CD4+ T cells, with the vast majority of CD4+ TCLs were either IFN-γ+ or TNF-α+ producers upon antigenic stimulation.
Figure 1.
SARS-Cov-2 antigen-specific CD4+ T-cell line generation from healthy donor PBMC. Panel (A) demonstrates the methodology used for generation of the various TCLs beginning with healthy donor naive T-cells and driven by SARS-Cov-2 structural spike (S) and membrane (M) protein-based peptide megapools. Panel (B) shows a representative flow cytometric dot plot highlighting the immunophenotypic conversion from naïve T cells to SARS-CoV-2-reactive CD4+ TCLs expressing CD69+CD154+ and producing either IFN-γ or TNF-α upon stimulation with SARS-CoV-2 peptide pools. Figure 1A was created with BioRender.com
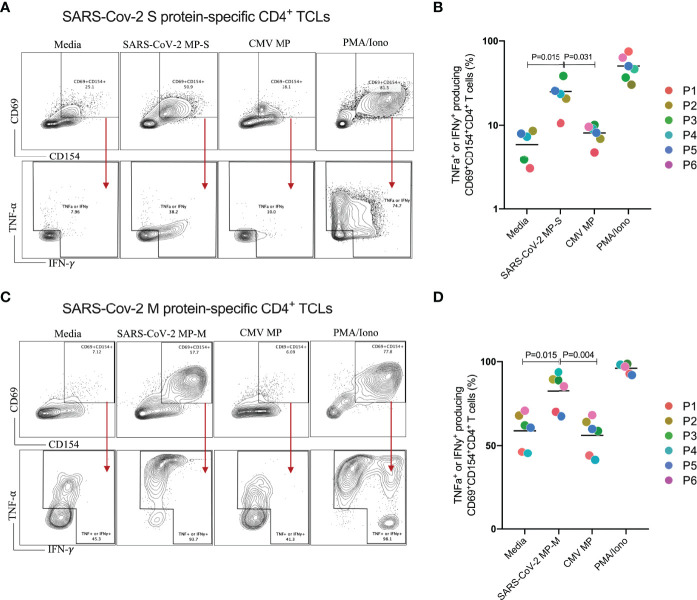
We next characterized both SARS-CoV-2 S and M protein-specific CD4+ TCLs for antigen specificity and reactivity ( Figure 2 ). All 6 MP-S specific- and MP-M specific-CD4+ TCLs were cultured in vitro in the absence or in the presence of the respective MP to test their antigen reactivity and CMV MP to test their specificity. PMA/ionomycin was also used as the positive control for the assay. When MP-S-specific CD4+ TCLs were stimulated with MP-S, it was observed a significant increase in the frequency in each of the 6 individual CD4+ TCLs expressing CD69+CD154+ and producing either IFN-γ or TNF-α when compared with non-stimulated cells (24.9 ± 9.5% vs 5.9 ± 2.3%, p=0.002). This activation failed to occur when the TCLs were stimulated with MP-CMV (8.0 ± 1.9% vs 5.9 ± 2.3%, p=0.360).
Figure 2.
Profiling of SARS-CoV-2 spike (S) and membrane (M) proteins-specific CD4+ T cell line for antigen specificity. SARS-Cov-2 spike protein-specific CD4+ TCLs from 6 different donors were cultured in vitro in the absence (media) or in the presence of different stimulation conditions including: either SARS-Cov-2 MP-S (1 µg/mL) or MP-M (1 µg/mL); CMV MP-M (1µg/mL), and PMA/ionomycin (0.5/0.05pg/mL). Panels (A–C) show a representative flow cytometric analysis of the of both MP-S or MP-M-specific CD4+ TCLs expressing CD69+CD154+ and the subsequent antigen-specific cells expressing either IFN-γ+ or TNF-α+ after the different stimulation conditions. Panels B and D reveal the specificity and the reactivity of the six MP-S specific CD4+ TCLs (B) or the six MP-M specific CD4+ TCLs (D) demonstrating the frequency of CD69+CD154+ TCLs producing either IFN-γ or TNF-α upon stimulation, in comparison with the unstimulated condition. Each plot represents the CD4+ TCLs from each donor (colored from P1-P6). All differences by Wilcoxon matched-pairs test with P < 0.05 are indicated in the graph.
The stimulation of MP-M-specific CD4+ TCLs with MP-M, also induced a marked increase in the frequency in each of the 6 CD4+ TCLs expressing CD69+CD154+ and producing either IFN-γ or TNF-α when compared with non-stimulated cells (82.4 ± 11.8% vs 58.8 ± 10.8%, p=0.015). Similar to S-reactive responses, there was no reactivity to MP-CMV stimulation (55.8 ± 10.8% vs 58.8 ± 10.8%, p=0.484) when compared with the media control ( Figures 2C, D ).
Finally, to further demonstrate their antigen specificity and that S-specific CD4+T cells do not respond to M peptide pool and conversely, S-reactive TCLs and M-reactive TCLs were cross-stimulated with MP-M and MP-S respectively and their responses assessed by their capacity to produce either IFN-γ and TNF-α upon antigenic stimulation. As shown in Supplementary Figure 1 , we were able to confirm that each CD4+ TCLs were able to respond only to its respective peptide MP. S-reactive TCLs or MP-M reactive TCLs did not respond to MP-M or MP-S respectively, as well as to the unrelated CMV MP.
CD4+ TCLs Reactive to S and M Proteins Reveals Distinct Transcriptomic Signature
Having confirmed the specificity and reactivity of each MP-S- and MP-M-specific CD4+ TCL, we sorted live CD3+CD4+ T cells from each TCL (to avoid any contamination with remaining feeder cells or CD8+ T cells) and used single cell RNAseq to understand more fully the nature (and heterogeneity) of these TCLs.
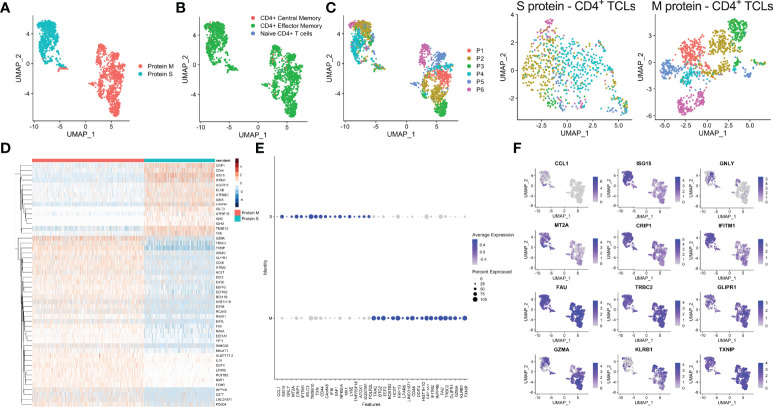
Analyses of the single-cell mRNA data among all the SARS-CoV-2 spike protein-reactive CD4+ TCLs with the SARS-Cov-2 membrane protein-reactive CD4+ TCLs revealed that M- and S-reactive TCLs had unique transcriptional signatures ( Figure 3A ). SingleR analysis utilizing data available from the Novershtern Hematopoietic database for cell identification annotated virtually all cells present in all TCLs as effector memory CD4+ T cells ( Figure 3B ). Exome SNP data was used to demultiplex the aggregate analysis in a patient-specific manner which allowed statistical and visual comparisons of donor-specific scRNA-seq profiles ( Figure 3C ). Differential gene expression analysis of the 50 topmost ( Figure 3D ) and 40 topmost significant genes showed that in contrast to CD4+ TCLs reactive to SARS-CoV-2 M protein, the S protein specific-CD4+ T cell lines expressed higher levels of CCL1, GNLY (Granulysin), TNFRSF18 (GITR), NFKBIA, IGLC3, among other genes ( Figures 3E, F ), suggesting an effector cytotoxic role for MP-S-reactive CD4+ T cells. In marked contrast, CD4+ TCLs reactive to SARS-CoV-2 M protein showed increased expression of GZMA (Granzyme A) and the EEF (eukaryotic elongation factors) gene family, including EEF1A1, EEF1B2, EEF1G, EEF2, EIF3E. Most striking, however, the MP-M reactive CD4+ T cell lines revealed a marked and significant overall suppressed expression of interferon-inducible genes, including ISG15 (interferon-stimulated gene 15) and IFITM1 (Interferon Induced Transmembrane Protein 1) ( Figure 3F ).
Figure 3.
Single-cell transcriptional profiling of SARS-CoV-2 spike (S) and membrane (M) protein-specific CD4+ T cell lines (A). SingleR analysis utilizing Novershtern Hematopoietic database for cell identification (B). Exome SNP data by Demuxlet algorithm was used to call cell genotypes in order to demultiplex the aggregate analysis of both S-reactive or M-reactive TCLs by patient-specific manner (colored from P1-P6) is displayed by manifold approximation and projection (UMAP) (C). (D) Heatmap showing expression of the most significantly 50 enriched transcripts in each cluster. Dot and feature graph highlighting the average expression and percent expression of selected marker transcripts in each cluster (E, F).
The comparative analyses between the single-cell transcriptional profiling of SARS-CoV-2 S and M proteins-specific CD4+ TCLs and those from CMV-specific-CD4+ TCLs revealed that S-responsive T-cells were more closely related (by UMAP) to CMV-reactive T-cells than to M-responsive TCLs ( Supplementary Figure 2A ). However, there were still many S-specific differentially expressed genes, including CCL11, IGLC3, IGHM, IL17RB and CD44 genes, when compared to CMV-reactive CD4+ TCLs ( Supplementary Figure 2B ). Not surprisingly then, the SARS-CoV-2 M-specific responses continue to demonstrate a profoundly marked suppression of the interferon signaling pathway in comparison to CMV-specific CD4+ TCLs ( Supplementary Figure 2C ), a suppression characterized by a significant downregulation of BAX, IFI6, IFI35, IFIT3, IRF9, ISG15, MX1, OAS1 and STAT1 ( Supplementary Figure 2D ).
SARS-CoV-2 Protein M-Specific CD4+ T Cells Reveals Marked Downregulation of Interferon Pathway Signaling
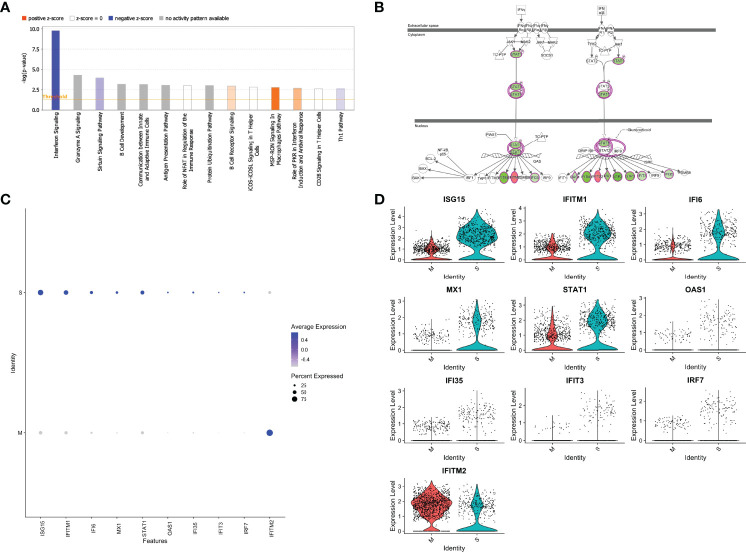
We next performed Ingenuity Pathway Analysis (IPA®) on the significantly differentially expressed genes in MP-M- compared with MP-S-reactive CD4+ TCLs. Interestingly, the IPA analysis showed that the canonical pathway signaling with immunological relevance most affected in the MP-M-specific CD4+ TCLs was the interferon signaling pathway (p=1.59-10; z-score =-2.333) ( Figure 4A ) indicating an extensive and comprehensive suppression of the genes related to types I, II, and III interferon signaling induced by SARS-CoV-2 M protein ( Figure 4B ) and ( Figure 5A ), including ISG15, IFITM1, IFI6, MX1, STAT1, OAS1, IFI35, IFIT3 and IRF7 ( Figures 4C, D ). To minimize any potential confounder factor from donor variability or influence of sample representation into the analysis, we have also analyzed the capacity of the M protein to dysregulate interferon pathway signaling in a patient-specific manner. As shown either by the heatmap or dot graph in Supplementary Figure 3 , the expression level of the interferon associated genes (ISG15, IFITM1, IFI6, MX1, STAT1, OAS1, IFI35, IFIT3 and IRF7) were markedly downregulated consistently in all 6 M protein-reactive TCLs when compared with the respective S-reactive TCLs from the same donor. In addition, upstream analysis also revealed that the MP-M-reactive CD4+ TCLs show a marked inhibition of the IFNA2- (interferon alpha 2, z-score= -3.561, p=5.7-18), IFNB1- (interferon beta, z-score= -3.312, p=9.6-14), IFNG- (interferon gamma, z-score= -2.899, p=7.2-17) and IFNL1- (interferon lamba, z-score= -3.646, p=8.3-17) associated pathways.
Figure 4.
SARS-CoV-2 membrane (M) protein downregulates types I, II, III interferon pathway signaling in CD4+ T cell lines generated from unexposed individuals. Canonical signaling pathways of immunological relevance affected in M protein-specific TCLs (A) indicating a marked suppression interferon signaling pathway and others, including Th1 pathway. (B) Map of the interferon signaling pathway indicating the 9 downstream molecules that were associated with the suppression of interferon, where in green are the genes affected negatively or in red the genes upregulated by SARS-CoV-2 M protein. Feature graph (C) and the violin plot graph (D) showing the average expression and the percent expression of the interferon signaling pathway genes in the respective S-protein-specific CD4+ T cell lines and M-protein-specific CD4+ T cell lines. Figures 4A and 4B were generated by QIAGEN Ingenuity Pathway Analysis.
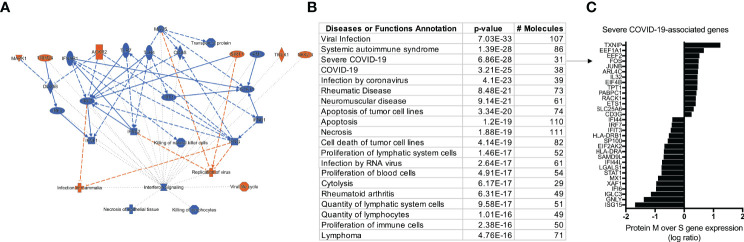
Figure 5.
IPA analysis demonstrating an overview summary of the major biological events in the transcriptome of SARS-CoV-2 M protein-reactive CD4+ TCLs (A) and The Disease or Function View highlighting how the SARS-Cov-2 specific-CD4+ TCLs relate to other diseases and functions through the ingenuity ontology (B). Severe COVID-19 associated genes that overlap with the marked differentially expressed genes in the SARS-Cov-2 M protein-reactive CD4+ TCLs (C). Figure 5A was generated by QIAGEN Ingenuity Pathway Analysis.
The Molecular Signature of SARS-CoV-2 M Protein-Reactive CD4+ TCLs Associates With the Transcriptional Profile Seen in Severe Covid-19
After characterizing the transcriptional profile of SARS-Cov-2 specific CD4+ TCLs, the next step was to use the molecular signature of M protein-reactive T cells to understand how they relate to other diseases and functions through the IPA™ ontology. The transcriptome of SARS-Cov-2 M protein-reactive CD4+ TCLs revealed pathways associated with a diverse list of inflammatory diseases and functions, including the top 3: viral infection (107 overlapping molecules, p=7.0-33), systemic autoimmune syndrome (86 overlapping molecules, p=1.3-28), and most strikingly, Severe COVID-19 (31 overlapping molecules, p=6.8-28) ( Figure 5B ). The 31 severe COVID-19-associated genes that overlapped with the differentially expressed genes in the SARS-Cov-2 M protein-reactive CD4+ TCLs were identified and plotted based on their expression level ( Figure 5C ). Interestingly, the upregulation of the inflammatory genes FOS, JUNB, the downregulation of the interferon-induced genes, including ISG15, IFI6, IFI44, IFIT3, IFI44L, and suppression of the interferon regulatory factors, including (IRF7) driven by M protein were implicated as the key players for this association, suggesting that the molecular signature of SARS-CoV-2 M protein-reactive CD4+ TCLs is characterized by suppression of the same interferon pathways seen in the transcriptional profile of severe COVID-19.
Discussion
There is a critical need for elucidating the nature of antigen specificity and function of the memory T cell responses to SARS-CoV-2. Understanding the contribution of adaptive immunity to a protective or pathogenic role in SARS-CoV-2 infection may lead the way to a fundamental knowledge that can possibly be used therapeutically in COVID-19 patients or as vaccine targets.
SARS-CoV-2 infected-patients develop specific antibodies, CD4+ T cells, and CD8+ T cells in response to the infection (3, 6, 8, 9), although, CD4+ T cells had the strongest association with diminished COVID-19 disease severity compared with the other two arms (B cells, CD8+ T cells) of the adaptive immunity (6). Strikingly, the absence of SARS-CoV-2-specific CD4+ T cells was associated with severe or fatal COVID-19 (9). Data from another coronavirus (SARS-CoV-1) reported that SARS-CoV-1 spike protein was responsible for nearly two-thirds of the CD4+ T cell reactivity with limited reactivity for M and N proteins (22). It seems, however, that the pattern of antigen predominance of SARS-CoV-2-driven immune responses is different from SARS-CoV-1 in that there is strong reactivity of CD4+ T cells to viral S, M and N structural proteins, as well as, to other non-structural proteins and open reading frames, including ORF3 and NSP3 (3, 10, 23–25).
The relationship between antigen-specific CD4+ T cell responses and COVID-19 severity remains unclear. First it has been demonstrated that mild COVID-19 patients, who typically recover without special treatment, showed broad SARS-CoV-2-specific CD4+ T cell responses to S and N proteins, responses that were highly correlated with specific antibody titers (25). However, T cell responses were imbalanced in critical ICU patients with a functionally impaired CD4+ T cell response showing reduced production of IFN-γ and TNF-α (26).
Indeed, an inflammatory cytokine and chemokine signature (elevated CXCL10, IL-6, and IL-8) accompanied by ineffective interferon responses has been strongly associated with failure to control a primary SARS-CoV-2 infection and with a higher risk of fatal COVID-19 (27–29). Moreover, impaired, and delayed type I and type III IFN responses have been associated with a higher risk of severe COVID-19 (30). Interferons (IFNs), including type I (IFN-α and IFN-β) and type III (IFN-λ) are central to both combating virus infection and modulating the antiviral immune response (31, 32). While type I IFNs are widely expressed and can result in immune-mediated pathology during viral infections, type III IFN (IFN-λ) responses are primarily restricted to mucosal surfaces and are associated with protection to viruses without driving damaging proinflammatory responses (33).
Interestingly, coronaviruses develop efficient immune evasion mechanisms by manipulating immune responses and by interfering with the IFN-related pathways (34). Indeed, several structural (M and N) and non-structural (NSP1 and NSP3) proteins from SARS-CoV and MERS-CoV can act as interferon antagonists (35). Notwithstanding, SARS-CoV-2 M protein has also been implicated to antagonize type I and III IFN production by affecting the formation of the RIG-I/MDA-5–MAVS–TRAF3–TBK1 signalosome that has been shown to attenuate antiviral immunity and enhance viral replication (11). Here, using single-cell transcriptomes of human CD4+ TCLs reactive to either S protein or M protein, we have shown that SARS-CoV-2 M protein-reactive CD4+ TCLs in comparison with S protein, expressed higher levels of the inflammatory genes FOS, JUNB and lower levels of ISGs, including ISG15, IFI6, IFI35, IFI44, IFIT3, IFITM1, STAT1, OAS1, and interferon regulatory factors, including (IRF7). Viral recognition elicits IFN production, which in turn triggers the transcription of IFN-stimulated genes (ISGs), which engage in various antiviral functions. ISGs have a central role to regulate the type I interferon (36). Among these ISGs, ubiquitin-like protein ISG15 is one of the most strongly and rapidly induced, and recent work has shown that it can directly inhibit viral replication and modulate host immunity (37–39). Similarly, we also observed that the molecular signature of SARS-CoV-2 M protein-reactive CD4+ TCLs, is characterized by suppression in the interferon pathways, genetically associates with the transcriptional profile of severe Covid-19.
Notably, through single cell RNAseq analysis of T-cell dysregulation in severe COVID-19 it has been demonstrated that CD4+ T cells from severe COVID-19 patients expressed higher levels of a set of inflammatory genes that include FOS, FOSB, JUN and others, gene expression (40) not dissimilar to those found in our M-specific TCLs derived from SARS-CoV-2 unexposed individuals. In parallel, this same study showed that CD4+ T cells from patients with severe COVID-19 showed decreased expression of interferon-induced genes including IFIT1, IFIT2, IFIT3, and IFITM1 and those downstream from interferon signaling (40), again striking similar to the molecular signature seen in the M protein-reactive CD4+ TCLs in this present study. Finally, the mechanisms by how the peptide megapools of M and S proteins underlies different responses of naïve CD4+ T cells remains unclear. Future studies are needed to elucidate if the M-driven dysregulation of interferon signaling pathway in the adaptive immunity resemble to the mechanisms already described including the interaction with pattern recognition receptors (PRRs)-downstream molecules of innate cells, or if it is induced by the interaction of the class II MHC-peptide complex with the restricted TCR repertoire of naïve T cells.
In conclusion, although it has been poorly understood how CD4+ T cell dysregulation can contribute to the immunopathogenesis of severe COVID-19, our study suggests a potential link between the antigen specificity of the reactive CD4+ T cells to SARS-CoV-2 with the development of a functional and efficient adaptive immune response. The discordant response to S compared to M proteins suggest that the balance between the T cells of different specificities may alter immune evasion mechanisms that may, in turn, drive disease severity. Therefore, one could envision therapeutic approaches that also targets the SARS-CoV-2 M protein may also be important for amelioration of severity of COVID-19.
Of note, continuing our studies in the immunobiology of the SARS-CoV-2, the next step is to utilize the SARS-CoV-2-reactive TCLs and reagents generated in this paper for a better understating of the heterogeneity of the effector’s CD4+ T cells, including TCR/HLA repertoire diversity, epitope mapping, and antigen reactivity/specificity against current and future SARS-CoV-2 variants. The unsupervised approach for the development of SARS-CoV-2-reactive TCLs from PBMCs of unexposed individuals allowed us to understand not only the molecular nature of the virus specific CD4 memory/effector human T cell response to the SARS CoV2 virus, but also to the antigens/epitopes driving such responses, ultimately serving as T cell-centric reagent that can potentially be used therapeutically and/or as biomarkers for future studies.
Data Availability Statement
The datasets presented in this study can be found in an online repository at the following address: https://www.ncbi.nlm.nih.gov/, GSE201463.
Ethics Statement
The studies involving human participants were reviewed and approved by The National Institute of Allergy and Infectious Diseases (NIAID) Institutional Review Board (study NCT00001230) under the Clinical Protocol Number 88-I-83 of the National Institutes of Health. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization: PG-G, GS, AS, DW, and TN. Methodology: PG-G, GS, AS, DW, PS, and JL. Investigation: PG-G, GS, PS, and JL. Visualization: PG-G, GS, and PS. Funding acquisition: PG-G and TN. Project administration: PG-G and TN. Supervision: PG-G and TN. Writing – original draft: PG-G. Writing – review and editing: PG-G, GS, AS, DW, PS, JL, and TN. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Division of Intramural Research, NIAID, NIH and partly by NIH contract Nr. 75N9301900065 (DW, AS).
Conflict of Interest
AS is a consultant for Gritstone Bio, Flow Pharma, Arcturus Therapeutics, ImmunoScape, CellCarta, Oxford Immunotec, and Avalia Immunotherapies. JL has filed for patent protection for various aspects of T cell epitope and vaccine design work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.883159/full#supplementary-material
References
- 1. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol (2021) 19(3):141–54. doi: 10.1038/s41579-020-00459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raoult D, Zumla A, Locatelli F, Ippolito G, Kroemer G. Coronavirus Infections: Epidemiological, Clinical and Immunological Features and Hypotheses. Cell Stress (2020) 4(4):66–75. doi: 10.15698/cst2020.04.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, et al. SARS-CoV-2-Specific T Cell Immunity in Cases of COVID-19 and SARS, and Uninfected Controls. Nature (2020) 584(7821):457–62. doi: 10.1038/s41586-020-2550-z [DOI] [PubMed] [Google Scholar]
- 4. Tan AT, Linster M, Tan CW, Le Bert N, Chia WN, Kunasegaran K, et al. Early Induction of Functional SARS-CoV-2-Specific T Cells Associates With Rapid Viral Clearance and Mild Disease in COVID-19 Patients. Cell Rep (2021) 34(6):108728. doi: 10.1016/j.celrep.2021.108728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single-Cell Landscape of Bronchoalveolar Immune Cells in Patients With COVID-19. Nat Med (2020) 26(6):842–4. doi: 10.1038/s41591-020-0901-9 [DOI] [PubMed] [Google Scholar]
- 6. Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations With Age and Disease Severity. Cell (2020) 183(4):996–1012.e19. doi: 10.1016/j.cell.2020.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological Memory to SARS-CoV-2 Assessed for Up to 8 Months After Infection. Science (2021) 371(6529). doi: 10.1101/2020.11.15.383323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ni L, Ye F, Cheng ML, Feng Y, Deng YQ, Zhao H, et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity (2020) 52(6):971–7.e3. doi: 10.1016/j.immuni.2020.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sette A, Crotty S. Adaptive Immunity to SARS-CoV-2 and COVID-19. Cell (2021) 184(4):861–80. doi: 10.1016/j.cell.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans With COVID-19 Disease and Unexposed Individuals. Cell (2020) 181(7):1489–501.e15. doi: 10.1016/j.cell.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng Y, Zhuang MW, Han L, Zhang J, Nan ML, Zhan P, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Membrane (M) Protein Inhibits Type I and III Interferon Production by Targeting RIG-I/MDA-5 Signaling. Signal Transduct Target Ther (2020) 5(1):299. doi: 10.1038/s41392-020-00438-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siu KL, Kok KH, Ng MJ, Poon VKM, Yuen KY, Zheng BJ, et al. Severe Acute Respiratory Syndrome Coronavirus M Protein Inhibits Type I Interferon Production by Impeding the Formation of TRAF3.TANK.TBK1/IKKepsilon Complex. J Biol Chem (2009) 284(24):16202–9. doi: 10.1074/jbc.M109.008227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xia H, Cao Z, Xie X, Zhang X, Chen JY, Wang H, et al. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep (2020) 33(1):108234. doi: 10.1016/j.celrep.2020.108234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fu YZ, Wang SY, Zheng ZQ, Yi HuangLi WW, Xu ZS, et al. SARS-CoV-2 Membrane Glycoprotein M Antagonizes the MAVS-Mediated Innate Antiviral Response. Cell Mol Immunol (2021) 18(3):613–20. doi: 10.1038/s41423-020-00571-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nutman TB, Ottesen EA, Fauci AS, Volkman DJ. Parasite Antigen-Specific Human T Cell Lines and Clones. Major Histocompatibility Complex Restriction and B Cell Helper Function. J Clin Invest (1984) 73(6):1754–62. doi: 10.1172/JCI111384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kahlert H. Production of T-Cell Lines. Methods Mol Med (2008) 138:31–41. doi: 10.1007/978-1-59745-366-0_3 [DOI] [PubMed] [Google Scholar]
- 17. Kang HM, Subramaniam M, Targ S, Nguyen M, Maliskova L, McCarthy E, et al. Multiplexed Droplet Single-Cell RNA-Sequencing Using Natural Genetic Variation. Nat Biotechnol (2018) 36(1):89–94. doi: 10.1038/nbt.4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM, et al. Comprehensive Integration of Single-Cell Data. Cell (2019) 177(7):1888–902.e21. doi: 10.1016/j.cell.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aran D, Looney AP, Liu L, Wu E, Fong V, Hsu A, et al. Reference-Based Analysis of Lung Single-Cell Sequencing Reveals a Transitional Profibrotic Macrophage. Nat Immunol (2019) 20(2):163–72. doi: 10.1038/s41590-018-0276-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Novershtern N, Subramanian A, Lawton LN, Mak RH, Haining WN, McConkey ME, et al. Densely Interconnected Transcriptional Circuits Control Cell States in Human Hematopoiesis. Cell (2011) 144(2):296–309. doi: 10.1016/j.cell.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Finak G, McDavid A, Yajima M, Deng J, Gersuk V, Shalek AK, et al. MAST: A Flexible Statistical Framework for Assessing Transcriptional Changes and Characterizing Heterogeneity in Single-Cell RNA Sequencing Data. Genome Biol (2015) 16:278. doi: 10.1186/s13059-015-0844-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li CK, Wu H, Yan H, Ma S, Wang L, Zhang M, et al. T Cell Responses to Whole SARS Coronavirus in Humans. J Immunol (2008) 181(8):5490–500. doi: 10.4049/jimmunol.181.8.5490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nelde A, Bilich T, Heitmann JS, Maringer Y, Salih HR, Roerden M, et al. SARS-CoV-2-Derived Peptides Define Heterologous and COVID-19-Induced T Cell Recognition. Nat Immunol (2021) 22(1):74–85. doi: 10.1038/s41590-020-00808-x [DOI] [PubMed] [Google Scholar]
- 24. Peng Y, Mentzer AJ, Liu G, Yao X, Yin Z, Dong D, et al. Broad and Strong Memory CD4(+) and CD8(+) T Cells Induced by SARS-CoV-2 in UK Convalescent Individuals Following COVID-19. Nat Immunol (2020) 21(11):1336–45. doi: 10.1038/s41590-020-0782-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oja AE, Saris A, Ghandour CA, Kragten NAM, Hogema BM, Nossent EJ, et al. Divergent SARS-CoV-2-Specific T- and B-Cell Responses in Severe But Not Mild COVID-19 Patients. Eur J Immunol (2020) 50(12):1998–2012. doi: 10.1002/eji.202048908 [DOI] [PubMed] [Google Scholar]
- 26. Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, et al. Elevated Exhaustion Levels and Reduced Functional Diversity of T Cells in Peripheral Blood May Predict Severe Progression in COVID-19 Patients. Cell Mol Immunol (2020) 17(5):541–3. doi: 10.1038/s41423-020-0401-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aid M, Busman-Sahay K, Vidal SJ, Maliga Z, Bondoc S, Starke C, et al. Vascular Disease and Thrombosis in SARS-CoV-2-Infected Rhesus Macaques. Cell (2020) 183(5):1354–66.e13. doi: 10.1016/j.cell.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuri-Cervantes L, Pampena MB, Meng W, Rosenfeld AM, Ittner CAG, Weisman AR, et al. Comprehensive Mapping of Immune Perturbations Associated With Severe COVID-19. Sci Immunol (2020) 5(49). doi: 10.1126/sciimmunol.abd7114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, et al. An Inflammatory Cytokine Signature Predicts COVID-19 Severity and Survival. Nat Med (2020) 26(10):1636–43. doi: 10.1038/s41591-020-1051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim YM, Shin EC. Type I and III Interferon Responses in SARS-CoV-2 Infection. Exp Mol Med (2021) 53(5):750–60. doi: 10.1038/s12276-021-00592-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, et al. Functional Role of Type I and Type II Interferons in Antiviral Defense. Science (1994) 264(5167):1918–21. doi: 10.1126/science.8009221 [DOI] [PubMed] [Google Scholar]
- 32. Lee AJ, Ashkar AA. The Dual Nature of Type I and Type II Interferons. Front Immunol (2018) 9:2061. doi: 10.3389/fimmu.2018.02061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired Type I Interferon Activity and Inflammatory Responses in Severe COVID-19 Patients. Science (2020) 369(6504):718–24. doi: 10.1126/science.abc6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taefehshokr N, Taefehshokr S, Hemmat N, Heit B. Covid-19: Perspectives on Innate Immune Evasion. Front Immunol (2020) 11:580641. doi: 10.3389/fimmu.2020.580641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fung TS, Liu DX. Human Coronavirus: Host-Pathogen Interaction. Annu Rev Microbiol (2019) 73:529–57. doi: 10.1146/annurev-micro-020518-115759 [DOI] [PubMed] [Google Scholar]
- 36. Schneider WM, Chevillotte MD, Rice CM. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu Rev Immunol (2014) 32:513–45. doi: 10.1146/annurev-immunol-032713-120231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Der SD, Zhou A, Williams BR, Silverman RH. Identification of Genes Differentially Regulated by Interferon Alpha, Beta, or Gamma Using Oligonucleotide Arrays. Proc Natl Acad Sci USA (1998) 95(26):15623–8. doi: 10.1073/pnas.95.26.15623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Loeb KR, Haas AL. The Interferon-Inducible 15-kDa Ubiquitin Homolog Conjugates to Intracellular Proteins. J Biol Chem (1992) 267(11):7806–13. doi: 10.1016/S0021-9258(18)42585-9 [DOI] [PubMed] [Google Scholar]
- 39. Perng YC, Lenschow DJ. ISG15 in Antiviral Immunity and Beyond. Nat Rev Microbiol (2018) 16(7):423–39. doi: 10.1038/s41579-018-0020-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kalfaoglu B, Almeida-Santos J, Tye CA, Satou Y, Ono M. T-Cell Hyperactivation and Paralysis in Severe COVID-19 Infection Revealed by Single-Cell Analysis. Front Immunol (2020) 11:589380. doi: 10.3389/fimmu.2020.589380 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in an online repository at the following address: https://www.ncbi.nlm.nih.gov/, GSE201463.