Abstract
The presence of fucose on IgG1 Asn-297 N-linked glycan is the modification of the human IgG1 Fc structure with the most significant impact on FcɣRIII affinity. It also significantly enhances the efficacy of antibody dependent cellular cytotoxicity (ADCC) by natural killer (NK) cells in vitro, induced by IgG1 therapeutic monoclonal antibodies (mAbs). The effect of afucosylation on ADCC or antibody dependent phagocytosis (ADCP) mediated by macrophages or polymorphonuclear neutrophils (PMN) is less clear. Evidence for enhanced efficacy of afucosylated therapeutic mAbs in vivo has also been reported. This has led to the development of several therapeutic antibodies with low Fc core fucose to treat cancer and inflammatory diseases, seven of which have already been approved for clinical use. More recently, the regulation of IgG Fc core fucosylation has been shown to take place naturally during the B-cell immune response: A decrease in α-1,6 fucose has been observed in polyclonal, antigen-specific IgG1 antibodies which are generated during alloimmunization of pregnant women by fetal erythrocyte or platelet antigens and following infection by some enveloped viruses and parasites. Low IgG1 Fc core fucose on antigen-specific polyclonal IgG1 has been linked to disease severity in several cases, such as SARS-CoV 2 and Dengue virus infection and during alloimmunization, highlighting the in vivo significance of this phenomenon. This review aims to summarize the current knowledge about human IgG1 Fc core fucosylation and its regulation and function in vivo, in the context of both therapeutic antibodies and the natural immune response. The parallels in these two areas are informative about the mechanisms and in vivo effects of Fc core fucosylation, and may allow to further exploit the desired properties of this modification in different clinical contexts.
Keywords: therapeutic antibodies, IgG, N-glycan, fucosylation, ADCC, NK cells, virus, humoral response
Introduction
IgGs are among the most abundant proteins in the circulation (700-1600 mg/dl in healthy adults), and specific IgGs are induced in response to infection, endogenous or allogeneic challenges, or by vaccination. Different IgG subclasses are found in man, which are very similar structurally but have distinct functions due to their differential binding to FcɣRs, complement components as well as other proteins. About 60% of plasma IgG is IgG1, 32% IgG2 and 4% each IgG3 and IgG4 in humans (1). IgGs are glycoproteins and their glycosylation pattern can change during time, due to age, diseases or environmental factors (2, 3).
Therapeutic monoclonal antibodies (mAbs) have emerged as an important therapeutic option in cancer since the approval in 1997 of the anti-CD20 antibody rituximab for the treatment of B-non Hodgkin's lymphoma (B-NHL). Since then, antibodies directed against different antigens expressed by cancer, immune cells or infectious agents have been developed to treat a variety of diseases. Indeed, so far, over 130 antibodies have been approved by the US and EU Drug Agencies, with 45% for oncological disorders, 27% for immune- or inflammation-related conditions and the rest for infectious or other diseases (4).
Most unconjugated therapeutic mAbs are IgG1 or in some cases IgG4 or IgG2. This is because the human IgG1 Fc moiety interacts efficiently with activating FcɣRs (FcɣRI, IIA, IIC, IIIA and IIIB), expressed on the surface of immune cells (1, 5). This interaction leads to antibody-dependent cellular cytotoxicity (ADCC) by NK cells (mostly via FcɣRIIIA, CD16A) (6, 7), antibody dependent phagocytosis (ADCP) by macrophages (mostly through FcɣRI, CD64 and to some extent FcɣRIIA, CD32A) (8–12) and ADCC/ADCP by polymorphonuclear neutrophils (PMN)(mostly via FcɣRIIA, CD32A) (13–15). IgG1 also interacts with FcɣRIIIB (CD16B), a GPI-linked molecules lacking activating domain, highly expressed by PMN and involved in PMN mediated ADCC and ADCP, but whose role may be either activating or inhibiting, perhaps depending on stimulus (13–16). Immune cell activation via FcɣRs also induces the release of cytokines and chemokines that may cooperate in eliminating the target cells but also induce unwanted side-effects (17). Finally the Fc region of human IgG1 can bind to the first component of the complement cascade C1q and activate the classical pathway of complement which may lead to cell lysis and death through complement dependent cytotoxicity (CDC), as well as phagocytosis by macrophages and PMN through complement receptors on these cells (18). Therefore, many therapeutic antibodies against cancer cells or other targets are of the IgG1 isotype to allow activation of a panoply of immune-mediated mechanisms, many of which rely on FcɣRs.
When the activation of the immune system is not desired, for example when a therapeutic antibody is required only to neutralize the antigen, such as a growth factor or checkpoint inhibitor, then the human IgG4 or IgG2 subclasses are often chosen, because they do not interact efficiently with FcɣRs or with C1q. The more recent human IgG4 formats include a mutation in Fc (S228A) to avoid Fab arm exchange, a natural phenomenon that leads to IgG4 instability (19).
Over the last 10-15 years, various modifications of antibody structures have been introduced to increase the efficacy of therapeutic mAbs in vitro and in vivo: these include extensively modified Abs with additional effector functions, such as bispecific antibodies (bsAbs), antibody-drug conjugates (ADCs) and fusion proteins carrying for example cytokines (17, 20). Less dramatic modifications of therapeutic mAbs include the introduction of point mutations in the Fc domain, as well as modification of Fc N-linked glycans that modulate IgG binding to FcɣRs and therefore enhance or abolish Fc mediated immune activation (ADCC, ADCP and/or CDC) (17, 20).
In this paper, we will summarize the knowledge gained about the role of IgG1 N-glycan core fucosylation in the in vitro and in vivo functions of IgG1 antibodies. Ig isotypes or subclasses other than IgG1 bear N-glycans, but less is known about the role of core Fc fucosylation in their case and these will not be further discussed here. Interestingly, the studies on the role of Fc core fucose in therapeutic IgG1 mAbs has facilitated the detection and understanding of the significance of this modification, observed during the polyclonal IgG1 response to some infectious agents, alloimmunization and in some autoimmune conditions. The knowledge on these aspects will therefore also be summarized and discussed.
The IgG N-Linked Glycans
Human IgGs are glycosylated proteins with a complex and variable glycosylation pattern. An important and extensively studied N-glycosylation site is present at conserved Asparagine 297 (Asn 297) in the CH2 domain, that interacts with FcɣRs. 20-30% of IgGs also bear N-glycans on Fab arms (21). Although there are reports of functional effects of different Fab N-glycosylation patterns in some antibodies (22), these are likely to be mostly antibody specific (23). Detailed structural studies of several commercial therapeutic mAbs has revealed that although Fab interacts with FcɣRIIIA and stabilizes the Fc-FcɣRIIIA binding, Fab fucosylation has a limited effect on the affinity of IgG for FcɣRIIIA (23, 24). The modulation of Fab fucosylation will therefore not be further discussed here.
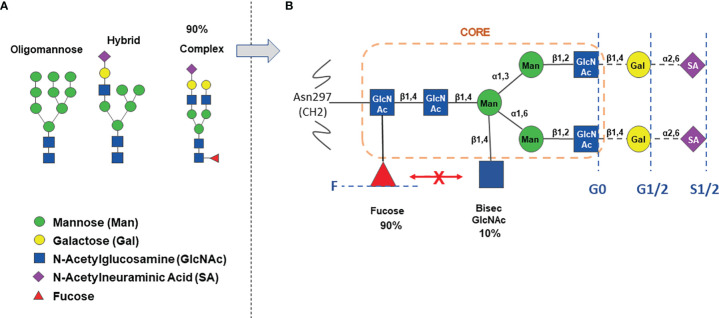
The IgG Asn 297 N-glycans show a high degree of microheterogeneity, and they can be grouped in oligomannose, hybrid or complex type, the latter being the most abundant (about 90%) in IgG, either circulating or produced by cell lines in vitro (25) ( Figure 1A ). The presence of Fc N-glycan induces in general a more open structure compared to aglycosylated IgG, favors binding to activating FcɣRs and promotes antibody stability in vitro and in vivo (1, 26). The complex type N-glycosylation itself shows microheterogeneity: whereas it always contains a heptaglycan biantennary core structure (four GlcNAc and three mannose residues), the core can bear an additional bisecting GlcNAc (in about 10% of IgGs), and 1 or 2 galactose residues (in 35% and 15% of IgG, respectively) and 1-2 terminal N-acetylneuraminic acid (sialic acid, SA), on 10-15% of IgGs ( Figure 1B ). Finally, an α-1,6 fucose residue (core fucose) is present in 90% of complex type IgG N-glycans ( Figure 1B ). Interestingly the presence of bisecting GlcNAc inhibits α-1,6 core fucosylation due to steric hindrance and therefore IgGs generally contain either a bisecting GlcNAc or a core fucose residue, although some IgGs may have both bisecting GlcNAc and fucose (2, 27–29). IgGs are composed of least 30 glycovariants, to which specific abbreviations have been assigned: G0 (no Gal residue), G1 (1 Gal), G2 (2 Gals), F (fucose) etc ( Figure 1B ) ( 2, 27–29).
Figure 1.
The IgG Asn-297 N-glycan heterogeneity. Panel (A) major types of N-glycosylation observed in IgG. Panel (B) detail of complex type glycosylation with percentage of circulating IgG containing either fucose or bisecting N-Acetylglucosamine. The orange circle indicates the core structure. The blue broken lines and text indicate the heterogeneity of complex N-glycans, carrying either no Gal (G0), 1-2 Gal (G1/2), 1-2 Sialic acids (S1/2), with (F) or without core α-1,6-fucose.
The Biosynthesis of Human IgG N-Linked Glycans
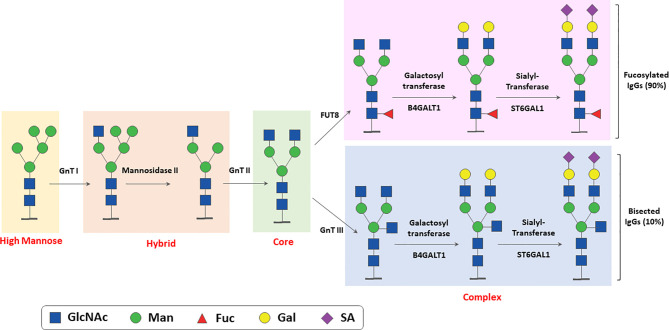
N-glycosylation is a multi-step enzyme-mediated biochemical process. IgG N-glycan biosynthesis starts in the endoplasmic reticulum with the addition of a pyrophosphate-dolichol precursor (Dol-P, Glc3Man9GlcNAc2) to the Asn 297 N-glycosylation site of IgG. This structure is then trimmed by glucosidases and mannosidases, as the process moves to the Golgi, leading to the formation of high mannose, hybrid and the complex types N-glycans (26, 30–33). The main enzymatic reactions taking place in the Golgi are summarized in Figure 2 . The stable overexpression or reduction/inhibition of some of these enzymes in antibody producing cell lines have been used to modify IgG glycan composition and perform structure-function studies of N-glycan microheterogeneity ( (31); and see below). The structure of N-linked and other IgG glycans and glycosylation pathways have already been extensively reviewed by others (30, 32, 34, 35) and we refer the readers to these more complete descriptions of the process.
Figure 2.
Simplified scheme of enzymatic reactions involved in N-linked glycosylation of IgG. The major glycosylation steps and enzymes involved in N- linked glycosylation of IgGs taking place in ER and Golgi are shown. GnT, N-Acetyl glucosamine transferase; FUT, Fucosyl transferase; Man, Mannose; Gal, Galactose; GlcNAcm N-acetylglucosamine; SA, sialic acid (N-Acetylneuraminic Acid).
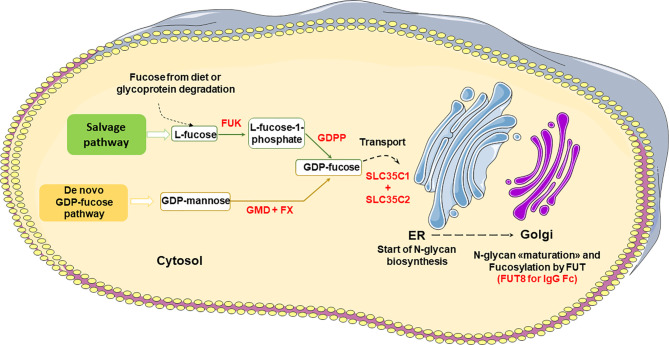
The enzymatic pathways for GDP-fucose biosynthesis and Fc core fucosylation are shown in Figure 3 (26, 30, 36). L-Fucose (6-deoxy-L-galactose) is a monosaccharide obtained by glycoprotein degradation or diet. In the salvage pathway, L-fucose is phosphorylated in the cytosol to fucose-1-phosphate by fucokinase (FUK), and then converted to GDP-fucose by GDP-fucose-pyrophosphorylase (GDPP), an essential substrate for the fucosylation of proteins ( Figure 3 ). Alternatively, and most commonly, de novo GDP-fucose is synthesized from GDP-mannose by GDP-mannose 4,6 dehydratase (GMD) and then GDP-4-keto 6-deoxymannose 3,5-epimerase-4-reductase (FX) ( Figure 3 ). GDP-fucose is transported to the ER via the SLC35C1 and SLC35C2 transporters and used by several fucosyltransferases (FUT) in the Golgi to fucosylate glycoproteins. There are 11 different FUTs, but only FUT 8 catalyzes IgG Fc core fucosylation via an α-1,6 linkage ( Figure 3 ). As mentioned above, the presence of bisected GlcNAc inhibits the addition of core α-1,6 fucose (26, 30, 36).
Figure 3.
Major pathways of GDP-fucose biosynthesis and IgG Fc core fucosylation. FUK, Fucose Kinase; GDPP, GDP-fucose-pyrophosphorylase; GMD, GDP-mannose 4,6 dehydratase; FX, GDP-4-keto 6-deoxymannose 3,5-epimerase-4-reductase; FUT, Fucosyltransferase.
These pathways show that there are multiple steps where a modulation of fucosylation can take place including the supply of diet fucose (36). The mechanisms of IgG Fc glycosylation regulation in B cells in healthy individuals and in disease are however still little understood.
Strategies to Generate Therapeutic IgG1 With Low Levels of Fucose Levels
In the last 10-15 years, removal of IgGs Fc core fucose has been shown to be an important method to enhance ADCC by therapeutic IgG1 mAbs (37–39). Based on the knowledge of the pathways of N-glycan fucosylation described above, several strategies have been used to generate therapeutic IgGs with low or no fucose [reviewed by Pereira et al. (40)]. These are briefly summarized below:
i. The selection of cell lines that naturally have low FUT8 enzyme, such as the Y2B/0 rat cell line (38).
ii. The creation of FUT8 knock out cell lines such as CHO FUT8-/- (41, 42). These produce antibodies completely lacking fucose, but may have a low growth rate unless specifically adapted.
iii. The selection of natural cell lines with defects in other enzymes involved in fucosylation, such as Lec 13, which has defective GDP mannose 4,6 dehydratase (GMD) which converts GDP mannose to GDP-fucose, the substrate for N-glycan core fucosylation ( Figure 3 ) (37, 43).
iv. The creation of engineered cell lines lacking GMD, GDP-L-fucose synthase (FX) and/or the GDP-fucose transporter SCL35C1 ( Figure 3 ) (44, 45). For example, the FX-/- and GMD-/- CHOZN® cell clones produced IgG1 antibody with core fucosylation reduced to 6-8% or 1-3%, respectively. The FX-/- clones also showed some aberrant glycan forms suggesting the GMD-/- CHOZN® cell line may be the best choice (44).
v. Another strategy is to engineer CHO clones with higher β-1,4-N-acetylglucoseaminyl-transferase III (GnTIII), best with a Golgi localization domain (2, 31, 46, 47). GnTIII catalyzes the transfer of GlcNAc to a core mannose residue in N-linked oligosaccharides via a β-1,4 linkage, which results in the formation of a bisected sugar chain. The bisection GlcNAc inhibits the transfer of fucose by FUT8, so that GnTIII overexpressing cells produce antibodies with lower levels of core fucose.
vi. Another modality is to use non-mammalian cells such as plant or insect cells that are engineered to synthesize human-type N-glycans, to reduce potential immunogenicity, but lack core fucosylation capacity (48).
vii. Cells can be treated with inhibitors of FUT8 such as 2F-Peracetyl-Fucose (49) or anti-FUT8 antibody (50).
viii. Finally, antibodies can also be enzymatically modified in vitro by treatment with glycosidase and glycosynthase enzymes. This is somewhat more complex since it requires antibody deglycosylation followed by a controlled glycosylation (51, 52).
The major strategies based on engineered mammalian cell lines are listed in Table 1 .
Table 1.
Examples of cell lines and strategies developed for low fucose antibody production.
| Cell line or system | Enzyme defect | Approximate Fc core fucosylation level (normal is >90%) | References |
|---|---|---|---|
| Lec 13 (CHO mutant) | Defective GMD | 10% | (37, 43) |
| YB2/0 (rat) | Low FUT 8 | 9-30% | (38) |
| CHO FUT8-/-
(e.g. Potelligent®) |
FUT8 KO | 0% | (41, 42) |
| CHO GMD-/-GFT-/- | GMD+SLC35C1 KO | 0% | (45) |
| CHO FX-/- | FX KO | 6-8% | (44) |
| CHO GMD-/- | GMD KO | 1-3% | (44) |
| CHO GnTIII+++
(e.g. GlycomAbs®) |
GnTIII overexpression | 10-15% | (46, 47) |
FUT, Fucosyltransferase; GMD, GDP-mannose 4,6 dehydratase; FX, GDP-4-keto 6-deoxymannose 3,5-epimerase-4-reductase; GnTIII, N-acetylglucosamine transferases III; SLC35C1, GDP-fucose transporter; KO, knock out.
It is worth noting that, depending on the system used, the amount of fucose may vary from 0% for cell lines lacking FUT8 enzyme to 10-30% for the those with reduced FUT8 or other enzymatic modifications ( Table 1 ). Also, different production cell lines and systems may lead to antibodies with different N-glycan profiles, not only regarding fucose residues. These may in turn affect function of antibodies since carbohydrates other than fucose, for example galactose or sialic acid may affect CDC or inflammation, respectively, among others (33, 53). A more detailed description of the role of galactosylation and sialic acid is beyond the scope of this review.
Functional Consequences of Low Core Fucose on IgG1 Asn 297 N-Glycan
Binding to FcγRIIIA and FcγRIIIB
Human, humanized or chimeric IgG1 antibodies lacking fucose on the Asn N-glycan bind with 10-100 fold higher affinity to human FcɣRIIIA and FcɣRIIIB (CD16B) (37, 54, 55). Structural studies have shown an increased carbohydrate-carbohydrate interaction between the N-glycans of FcγRIIIA and Fc, explaining the higher affinity of afucosylated IgG1 (56, 57).
NK Cells and ADCC
Since FcɣRIIIA is the major activating receptor on NK cells and mediates ADCC, the net result of increased binding to FcγRIIIA is a significant enhancement of ADCC by afucosylated IgG1 antibodies with respect to their fully fucosylated counterpart (2-40 fold, also depending on galactosylation) (37–39) ( Table 2 ). In addition, FcɣRIIIA has relatively low-medium affinity for IgG so that ADCC is inhibited by excess IgG in plasma. In contrast, ADCC induced by afucosylated IgG1, which has a significantly higher affinity for FcɣRIIIA, is not significantly inhibited by plasma IgG. Thus afucosylated anti-CD20 antibody may be more effective in inducing ADCC in whole blood by 2 mechanisms: 1) higher affinity for FcɣRIIIA and 2) significantly reduced inhibition by serum IgG (99). Afucosylated IgG1 has also been reported to induce greater FcɣRIIIA downmodulation from the NK cell surface, a phenomenon which takes place via shedding of the extracellular domain by the ADAM 17 metalloproteinase and may participate in the serial target cell killing by NK cells (100).
Table 2.
Selected therapeutic antibodies with low or no fucose, approved by FDA/EU or in clinical development.
| Antibody name (code) | Antigen | Antibody isotype | Method of defucosylation | % fucose | Diseases | Major findings | references |
|---|---|---|---|---|---|---|---|
| 1.1. Approved antibodies (FDA/EU) | |||||||
| Obinutuzumab (GA101) | CD20 | Humanized IgG1 | CHO overexpressing GnTIII (GlycoMAb) | About 15% | B-NHL | Higher ADCC by NK and ɣδ Tells, more effective than RTX in vivo in some mice models or in primates | (47, 55, 58–62) (63) |
| Phase III in CLL compared to RTX in combination with CLb | (64) | ||||||
| Phase III studies with different chemotherapy regimen in CLL and compared to RTX | (65) | ||||||
| Phase III studies in diff chemo combinations compared to RTX in untreated FL. | (66) | ||||||
| Mogamulizumab (KW-7061) |
CCR4 | Humanized IgG1 | CHO FUT8 -/- (Potelligent) | 0% | Cutaneous T cell lymphoma | Equivalent ADCC by afucosylated mAb, but with 10-fold lower antigen expression on target compared to fucosylated mAb | (67, 68) |
| Inebilizumab (MEDI 551) |
CD19 | Humanized IgG1 | CHO FUT8 -/-
(Potelligent) |
0% | Neuromyelitis optica | Increased ADCC in vitro. Depletes B cells more effectively than fucosylated antibody in hCD19 transgenic mice (PB, spleen and BM) | (69–72) |
| Benralizumab (MEDI-563) | IL-5Rα | Humanized IgG1 | CHO FUT8 -/-
(Potelligent) |
0% | Severe asthma with eosinophilia | Increased ADCC in vitro. Efficacy in depleting eosinophil in non-human primates and in clinical Phase III studies | (73–75) |
| Margetuximab (MGAH22) |
HER2 | Chimeric IgG1 | CHO FUT8 -/-
Also mutation in Fc to decreased CD32B binding |
0% | Advanced metastatic HER2+++ breast cancer | Increased ADCC. In vivo increased activity in hFcɣRIII+ mice. Phase III trial in breast cancer compared to trastuzumab |
(76, 77) |
| Belantamab vedotin (GSK2857916) |
BCMA | IgG1-MMAF ADC | CHO FUT8 -/- | 0% | Multiple myeloma | Increased ADCC of naked mAbs. Phase II ORR 31%. 72% survival at 6 months | (78) |
| Amivantamab (JNJ-61186372) |
EGFRxMET | Humanized bispecificIgG1 | Low fucose producing cell line | <10% | Non-small cell lung cancer (NSCLC) | Increased ADCC, not ADCP compared to high fucose variant. Phase III NSCLC | (79) |
| 1.2. Selected antibodies in clinical studies | |||||||
| Ublituximab (Emab-6) | CD20 | Chimeric IgG1 | YB2/0 | 24% | CLL, B-NHL, multiple sclerosis, neuromyelitis optica | High ADCC and ADCP (not compared with fully fucosylated antibodies). Phase I and II trials in B-NHL, CLL and autoimmune diseases + neuromyelitis optica. Phase III in CLL with or w/o ibrutinib (ORR 85% vs 65%) | (80–82) |
| Tomuzotuximab (cetuGEX) | EGFR | Humanized IgG1 (cetuximab seq) | Glyco Express System® | 0% | Advanced carcinoma | Increased ADCC in vitro, Phase I study | |
| Phase II study comparing CetuGEX with cetuximab combined with chemo: no difference observed | (83) | ||||||
| Imgatuzumab GA201 (RG7160) | EGFR | Humanized rat IgG1 (ICR62) | CHO stably expressing GnTIII (GlycomAb) | 15% | Carcinoma | Increased ADCC in vitro. Higher efficacy in mouse models (SCID beige or SCID hFcɣRIIIA tg) also in combination with chemotherapy | (84) |
| Phase I study in EGF+++ solid tumors | (85) | ||||||
| Open label study in advanced CRC. Decrease NK post treatment in PB | (86) | ||||||
| Enhanced ADCC in vitro | (87) | ||||||
| Favorable combination of GA201 and chemo in vitro and in carcinoma models in SCID mice | (88) | ||||||
| Open label study of GA201 vs cetuximab in head & neck squamous carcinoma (N=44). Greater decrease in NK in PB and greater cytokine release with GA201 vs CTX. No difference in clinical response. | (89) | ||||||
| KHK4083 | OX40 | Human IgG1 | FUT8 -/-Potelligent | 0% | Ulcerative colitis | Phase I | (90) |
| Tragex | HER2 | Humanized IgG1 | Glyco Express system®
(FUT8-/-) |
HER2+++ tumors | Increased ADCC in vitro. Enhanced activity in vivo in hFcɣRIIIA tg mice. Phase I in HER2+++ solid tumors |
(37, 91–94) | |
| Cusatuzumab (JNJ-74494550, ARGX-110) | CD70 | Humanized IgG1 | CHO FUT8 ko (Potelligent) | 0% | Hematological and solid cancers | Phase I study | (95, 96) |
| Bemarituzumab (AMG 522) | FGFR2b | Humanized IgG1 | CHO FUT8 | 0% | Gastric cancer FGFR2b+++ | Increased ADCC in vitro. Efficacy in vivo in mouse sc SCID model. Recruitment of NK and T cells into tumor. | (97, 98) |
ADCC, Antibody dependent cellular cytotoxicity; ADCP, Antibody dependent cellular phagocytosis; BM, Bone marrow; B-NHL, B-Non Hodgkin’s lymphoma; CLb, chlorambucil; CLL, Chronic lymphocytic leukemia; CR, Complete response; EGFR, Epidermal growth factor receptor; FL, follicular lymphoma; PB, Peripheral blood; NSCLC, Non-small cell lung cancer; ORR, Overall response rate; RTX, Rituximab; SCID, Severe combined immunodeficient.
Phagocytosis by Macrophages
FcɣRIIIA is expressed by monocytes/macrophages, particularly M2 and red pulp macrophages as well as microglial cells. However, macrophages also express the activating FcɣRI and FcɣRIIA, and FcγRI is thought to be the major mediator of phagocytosis of IgG1 opsonized targets (10, 11, 101). Therefore, afucosylated therapeutic mAbs, despite binding with higher affinity to FcɣRIIIA, are not generally reported to significantly enhance phagocytosis, at least in vitro (10, 11, 102). There is some evidence that phagocytosis of targets opsonized with anti-CD20 by liver Kupfer cells in vivo is enhanced by the afucosylated mAb (103), but this point still needs to be further investigated and confirmed. In the context of polyclonal alloimmune anti-HPA1a IgG1 antibodies (see below), increased phagocytosis of target platelets in vitro by both monocytes (via FcɣRIIIA) and PMN (via FcɣRIIIB) has been reported (104).
ADCC by ɣδ T Cells
FcɣRIIIA is also expressed by ɣδ T cells and these can mediate ADCC in presence of IgG1 antibodies (105, 106). There are reports of increased ADCC by ɣδ T cells in presence of low fucose anti-CD20 mAb obinutuzumab compared to rituximab (58, 107). ɣδ T cells represent <5% of T cells in the circulation of healthy individuals; they are also localized in non-lymphoid tissues and constitute the majority of immune cells in some epithelia (108). The role of ɣδ T cells in the response to IgG1 antibody in vivo is not well understood.
ADCC and Trogocytosis by PMN
IgG1 with low Fc core fucose also binds more strongly than fucosylated antibody to FcɣRIIIB, which has >97% sequence identity with FcɣRIIIA in its extracellular IgG binding domain (14, 15, 55). FcɣRIIIB is a GPI-linked receptor lacking activating module (ITAM), is expressed only by PMN and at high levels on these cells (5, 55, 109). Low fucose anti-CD20 therapeutic antibody obinutuzumab was shown to activate PMN more effectively than rituximab, which is ≥90% fucosylated, initially suggesting that enhanced FcɣRIIIB binding was responsible for this effect (55). However, subsequent experiments performed with PMN isolated from a rare FcɣRIIIB null donor, as well as the observation that PMN may express very low levels of FcɣRIIIA, indicated that PMN activation by afucosylated anti-CD20 antibodies may be mediated by FcɣRIIIA, FcɣRIIIB, or both, depending on conditions (110). The presence of low levels of FcγRIIIA on resting or activated PMN however still needs to be confirmed. The activation of PMN by anti-CD20 antibodies does not induce ADCC or phagocytosis, but only trogocytosis and cytokine production (55, 110). Interestingly other antibodies, such as anti-EGFR antibodies do mediate ADCC of tumor targets by PMN, but this function strictly requires FcɣRIIA (15). Furthermore, PMN and FcɣRIIA mediated ADCC is inhibited by FcɣRIIIB (111, 112). Indeed, ADCC by PMN is diminished in the presence of afucosylated anti-EGFR, because the latter binds more strongly to FcɣRIIIB, which is highly abundant on PMN and is thought to compete with FcɣRIIA. Therefore, at least in this context, FcɣRIIIB may act as a decoy receptor (111, 112). It remains to be established whether and how the level of fucosylation of other antibodies, for example those directed against microbes, affect PMN functions.
Cytokine Release by Immune Cells
Several studies indicate that afucosylated IgG1 antibodies induce a more rapid and intense cytokine release by NK cells, monocytes/macrophages, PMN and/or ɣδ T cells compared to their fully fucosylated counterparts. Induced cytokines include IFN-ɣ, MCP1, IL-6, TNF, MIP1αβ, Rantes, IL-8 (55, 100, 107, 113–115). The level of cytokines induced in vitro is however generally quite low and the significance of such release in vivo is not fully clear. Nonetheless, the more frequent or severe immediate reaction syndrome observed in patients treated with obinutuzumab compared to rituximab indicates that increased cytokine release, particularly IL-6 and IFN-ɣ by afucosylated antibodies may be relevant in vivo (64, 116, 117). Cytokine release should therefore be carefully studied during the pre-clinical and clinical development of afucosylated antibodies.
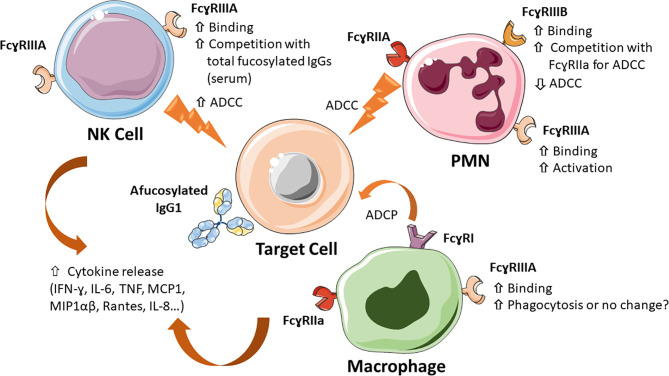
The functional effects of IgG1 Fc core afucosylation are summarized in Figure 4 .
Figure 4.
Major mechanisms of action of Fc core afucosylated IgG1 antibodies. IgG1 antibodies lacking Fc core α-1,6-fucose show a 10-100 fold increased binding to FcɣRIIIA and FcɣRIIIB on the indicated immune cells (NK, monocytes/macrophages and PMN), which results in increased ADCC by NK cells, enhanced competition with plasma IgGs, increased PMN activation, increased inhibition of FcγRIIA-mediated ADCC by PMN, induced by some antibodies (e.g. anti-EGFR mAbs). The effect of monocyte/macrophage induced ADCP is less clear. Low Fc core fucose can also increase release of cytokines, such as IL-6, TNF-α and IL-8, both in vitro and in vivo.
Evidence That Afucosylation Enhances the Efficacy of Therapeutic mAbs In Vivo in Animal Models
Studying the role of Fc core fucosylation using small animal models is complicated by the fact that mice express different set of FcɣRs compared to humans (5, 118–120). The more recently discovered mouse FcɣRIV molecule has been shown several years ago to be the murine ortholog of human FcɣRIIIA and to be an important mediator of human IgG1 efficacy in mice. Afucosylated human IgG1 binds with higher affinity also murine FcɣRIV (121). This suggests that, despite species differences, human IgG1 therapeutic antibodies can be tested in mice, at least for some aspects of their functions. However, because of differences in FcɣR expression pattern in mice, humanized mice or human FcɣRIIIA transgenic mice may be used more appropriately. Nonetheless one should bear in mind that mice do not have the equivalent of FcɣRIIIB, which therefore makes this species not completely adequate to test the role of PMN in antibody efficacy in vivo, unless fully humanized models are used (119). Despite these caveats, it is worth noting that afucosylated or low fucose anti-cancer mAbs have consistently been shown to control tumor growth more efficiently than their fully fucosylated parent molecules in mice (69, 84, 92, 122), as well as macaque, the latter having a more similar pattern of FcɣRs to humans (123). These results have led to an increased attention to the N-glycan profile of therapeutic mAbs (124), as well as the development of new afucosylated therapeutic antibodies in a variety of clinical contexts. These are discussed below.
Anti-Cancer mAbs With Low Core Fucose in the Clinic
Many antibodies with no or low Fc core fucose have entered pre-clinical or clinical development to treat different diseases. Seven such antibodies have been approved so far by FDA/EMA ( Table 2 ). They include one bispecific and one ADC, the others being unconjugated IgG1 antibodies. Five are approved for cancer therapy and two for immune/inflammatory diseases. Many other antibodies are in clinical trial ( Table 2 , part 2) or in pre-clinical development (47, 55, 58–79). In all cases, a significantly enhanced ADCC activity by NK cells has been shown in vitro and, in some, more effective target depleting activity in vivo in mice or non-human primates, as discussed above ( Table 2 ).
It is obviously difficult to evaluate whether removal of Fc core fucose significantly increases the efficacy of therapeutic anti-cancer mAbs in patients without performing phase III comparative studies of parent and afucosylated counterpart, studies that are rarely performed. Glycoengineered anti-CD20 antibody obinutuzumab was the first therapeutic antibody with low fucose to be approved in 2013 for the treatment of B-cell neoplasia. It has been extensively compared with rituximab in vitro and in clinical trials in Phase III studies in chronic lymphocytic leukemia and B-NHL, usually in combination with chemotherapy. Obinutuzumab has shown either enhanced activity or, in some cases, non-inferior activity to rituximab depending on disease contexts and treatment protocols (64, 116, 125). However, these two anti-CD20 antibodies differ from each other in aspects other than level of core fucosylation. They differ in epitope recognition, binding mode to CD20, and to some extent mechanisms of action (125). Rituximab induces higher CDC whereas obinutuzumab induces homotypic adhesion and direct cell death, in addition to the higher ADCC due to low fucose (62, 102, 126). It is therefore difficult to clearly identify the mechanism of higher efficacy of obinutuzumab in some disease contexts, compared to rituximab.
Another way to understand whether FcɣRIIIA binding affinity (and therefore the degree of IgG1 core fucosylation) is important for antibody efficacy is to study the response of patients carrying different FcɣRIIIA variants, in particular the 158V (high affinity) and 158F polymorphism (lower affinity). The studies of rituximab activity in follicular lymphoma patients treated with rituximab as single agent and the correlation between clinical response and the FcɣRIIIA-158V carriers suggested that FcɣRIIIA and ADCC by NK cells are important for the in vivo efficacy of this antibody (127). Similar data have been obtained in HER2 overexpressing breast cancer patients treated with trastuzumab (128). Nonetheless, for both rituximab and trastuzumab, these data have not been confirmed in all clinical contexts, perhaps also due to the fact that these therapeutic mAbs are generally used in combination with chemotherapy, which itself may modulate cell-mediated mechanisms (9, 129). Thus, both FcγRIIIA-dependent and independent mechanisms are likely to be important for anti-cancer IgG1 efficacy and these may vary in different clinical and therapeutic conditions.
Importantly, defucosylation has been shown not to alter significantly FcRn binding or pharmacokinetics in vivo, encouraging the development of afucosylated antibodies for clinical use (30).
To summarize, the fact that afucosylated IgG1 have consistently shown higher FcɣRIIIA binding and ADCC in vitro as well as often a greater efficacy in vivo in animal models without significant changes in antibody pharmacokinetics in vivo (69, 92) has led many groups to design and generate afucosylated therapeutic IgG1 mAbs or BsAb, as well as ADCs.
Modulation of IgG Fucosylation in Physiological States and Disease
Physiological Modulation of Fc N-Glycan Fucosylation
The glycosylation pattern of IgGs has been shown to be modulated during life. Children starting to produce their own IgGs show higher N-glycan core fucose levels, which decrease during childhood (130, 131). In adults, the levels of IgG fucosylation is quite stable in absence of pathology or interventions, but quite variable between individuals (ranging from 1.3 to 19.3% afucosylation), presumably due to hereditary as well as environmental factors (29).
How changes during pregnancy or aging are brought about is not clear. During pregnancy, chorionic gonadotropin (hCG) has been shown to promote the development of IL-10 producing B cells which are associated with production of highly core fucosylated IgG (132). The B cell response to infections that induce afucosylated IgGs probably explains the increased afucosylation during childhood (see below), but the mechanisms are unclear. Recently the spleen has been suggested to be an important site of fucosylation regulation: IgG1-Fc core fucosylation was observed to be increased in trauma splenectomized healthy individuals as well as in spherocytosis or immune thrombocytopenia (ITP) patients that were splenectomized, compared to non-splenectomized controls (133). There are several possible explanations for this observation: 1) The spleen may be a site of afucosylated IgG generation. Indeed in ITP, the spleen is also a source of anti-platelets IgGs which are highly afucosylated compared to total IgGs. 2) The spleen removes preferentially immune complexes containing afucosylated IgGs via FcɣRIIIA positive myeloid cells, so that splenectomy induces a change in the ratio of fucosylated/afucosylated IgGs (133). Further investigation of these possible mechanisms is warranted.
Several gene array analyses correlating IgG N-glycan traits with genome-wide polymorphic variants have allowed to identify gene pathways involved in N-glycome regulation, several of which were confirmed in more than one study (134–140). Some of the genes identified are glycosyltransferases, most significantly FUT8, B4GALT1 and MGAT3 (the latter encoding the GnTIII enzyme)( Figure 2 ), as well as transcription factors known or suspected to regulate these genes, such as RUNX1, RUNX3, IKZ1, IKZ3, IRF1, SMARCB1, TBX21O and HFN1 (138). Others are novel genes with no known role in glycosylation (135). With specific regard to fucosylation, FUT8 itself, the fucosidase FUCA2 and transcription factors regulating FUT8, most notably IKZF1, have been implicated in several studies (135, 137, 141). Altogether these results suggest that the expression level, localization and perhaps substrate availability of glycosylation enzymes define IgG glycosylation patterns. Clearly, further work will be required to fully identify the pathways involved and their role in the physiological regulation of IgG Fc N-glycan composition and core fucosylation in B cells.
Since N-glycosylation and core fucosylation take place in the ER and Golgi ( Figures 2 and 3 ), the physiological state, pH, ionic and redox of ER and Golgi can also affect glycosylation (142, 143). Indeed some studies have evidenced the role of ER stress in modifying protein glycosylation patterns (144). In particular in B cells, the mutation or deletion of the ER protein Jagn1 leads to ER stress, reduced IgG core fucosylation and increased sialylation, indicating that afucosylated IgG1 may be induced during ER stress (145).
Interestingly many loci identified to play a role in regulating the glycosylation pattern of IgGs and other plasma proteins are also associated with autoimmune and inflammatory diseases (135, 137). The specific role of IgG Fc core fucosylation in these pathological conditions is discussed below.
IgG1 Fc Core Fucosylation in Alloimmunity
The fact that IgG Fc core fucosylation is physiologically modulated was first observed in the context of alloimmunization, frequently occurring during pregnancy, and mostly involving red blood cells (RBC) and platelets. Rhesus D antigen alloimmunization during pregnancy and delivery of a RhD+ fetus by a RhD negative mother has been in the past the most common cause of hemolytic disease of the fetus and newborn (HDFN), occurring in sensitized mothers who had given birth to a previous RhD+ baby. The disease is due to anti-RhD IgG antibodies crossing the placenta and destroying the RhD+ RBC in the reticuloendothelial system of the fetus and newborn, causing severe fetal anemia and hydrops fetalis in severe cases. Before the advent of prophylaxis of RhD negative women with anti-RhD immunoglobulin, which prevents allo-immunization, HDFN affected 150 per 100 000 births and 10% of perinatal deaths in the Caucasian population. Analysis of IgG glycosylation in alloimmunized pregnant mothers has revealed a variable decrease in IgG1 Fc core fucosylation (even down to 12%), whereas the total IgG remained highly fucosylated, as in healthy individuals (>90%). Furthermore, the level of anti-RhD Fc core fucosylation correlated significantly with FcγRIIIA-mediated ADCC and with low fetal-neonatal hemoglobin levels (146).
A later in vitro study investigated the functional activity of monoclonal anti-RhD antibodies of different subclasses (IgG1, 2, 3 or 4), bearing either low (usually <30%) or high (>90%) Fc core fucose levels (147). Whereas reduced fucosylation increased FcɣRIIIA binding of all IgG subclasses, only defucosylated IgG1 and IgG3 showed a 12- and 7-fold increase in ADCC by NK cells, respectively, presumably due to the fact that IgG2 and IgG4 are poor binders of FcɣRIIIA (147). Afucosylation of the anti-RhD mAbs did not enhance in vitro phagocytosis of RBC by GM-CSF (M1) or M-CSF (M2) induced macrophages, reminiscent of what has been most often observed with anti-tumor antibodies (see above). Another study of an anti-RhD mAb with low core fucose showed a higher RBC clearance capacity in vivo in mice, compared to highly fucosylated antibody (148).
These data altogether show that IgG Fc core fucosylation is likely to be an important pathological feature in HDFN with diagnostic potential, and that a significant mechanism of the disease may be RBC destruction by NK cells (149), in addition to ADCP by the reticuloendothelial system.
RBC alloantigens other than Rhesus D (c, E, Kell) can induce an antibody response and HDFN. The level of defucosylation of anti-Kell, but not anti-c or anti-E, was shown to correlate with severe fetal anemia. These data suggest that the antigens shape the type of response (150).
Alloimmunization of mothers by fetal platelets can also occur during birth or pregnancy, leading to the generation of antibodies directed against polymorphic human platelet antigens (HPAs), the phagocytosis of opsonized platelets in fetal spleen and liver and the development of fetal and neonatal alloimmune thrombocytopenia (FNAIT), a rare but potentially fatal diseases of the fetus and newborn (151). FNAIT can also occur during first pregnancy of incompatible mothers and can have severe consequences, including intracranial hemorrhage. In 80% of FNAIT cases, the targeted antigen is human platelet antigen-1a (HPA-1a), i.e. a polymorphic epitope of the β3 integrin (152). The HPA-1a antigen is presented to maternal T and B cells in combination with the HLA DRB3*01:01 molecule and leads to production of anti-platelets, mostly IgG1 antibodies (151, 153). Several groups have shown that pregnant women have decreased, although variable, IgG1 Fc core fucosylation, which is specific for anti-HPA-1a as opposed to total IgG. Low core fucose on anti-platelet antibodies appeared to increase phagocytosis by monocytes and PMN (104). Reduced anti-HPA-1a Fc core fucosylation as well as antibody levels correlated with decreased neonatal platelet counts and increased disease severity in FNAIT patients (104, 152, 154). Interestingly, low fucosylation of anti-HPA antibodies was observed in patients with FNAIT but not in those with refractory thrombocytopenia following platelet transfusion, indicating that the level of fucosylation is antigen dependent and/or related to the immune milieu defined by pregnancy (104).
IgG1 Fc Core Fucosylation in Autoimmune and Inflammatory Diseases
The IgG Fc N-glycosylation pattern of total or antigen specific IgGs has also been investigated in several autoimmune and inflammatory diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple sclerosis (MS), autoimmune thyroid diseases, inflammatory bowel diseases (ICB), chronic obstructive pulmonary disease (COPD), ulcerative colitis (UC), etc. In these clinical contexts the greatest changes have been observed in galactosylation and sialylation, which in some cases such as in RA correlate with disease activity (155, 156). Fc core fucosylation has been observed to be modulated in only few autoimmune diseases, with an increase observed in RA, juvenile idiopathic arthritis and Crohn's diseases and a decrease in SLE, MS, and Lambert-Eaton myasthenic syndrome, immune thrombocytopenia (ITP) and either increase or decrease in different thyroid autoimmune diseases (157–159). It is worth noting that in this context, total IgGs rather than antigen-specific IgG have generally been analyzed. The pathological significance of these results is still unclear, except that low galactosylation and sialylation as well as high fucosylation of IgGs have been associated with a pro-inflammatory state (160). Several reviews of the state of the art regarding Fc N-glycosylation in autoimmune and inflammatory diseases has been published recently and we suggest the reader to consult these articles for further details on the subject (157, 161–163).
Interestingly, intravenous immunoglobulins (IVIGs) are extensively used as an immunomodulatory agent to treat autoimmune and inflammatory diseases. One mechanism of action of IVIGs is thought to be through competition with pathogenic IgG for Fcɣ receptors on immune cells. A very recent report analyzed the functional effects of different IVIGs glycoforms (i.e. IVIGs carrying 2 galactose, 2 sialic acids or no galactose, with or without α-1,6-fucose). Galactosylated and afucosylated IVIGs [(G2)2] had the highest affinity for FcɣRIIIA. The greatest effect was given by fucose removal as expected, but presence of 2 Gal residues increased affinity still further. Interestingly, (G2)2 IVIGs were also most potent in blocking ADCC in vitro and in reducing inflammation and serum IL-6 levels in a collagen antibody-induced arthritis model in mice (164). These data confirm that the anti-inflammatory activity of IVIGs is in part mediated via blockade of FcɣRIIIA by their galactosylated, non-fucosylated IgG component, at least in some clinical settings (165). They also corroborate the immunomodulatory capacity of Fc N-glycan composition in health and disease, particularly regarding core α-1,6-fucose and galactose. Finally, they suggest that modified IVIGs [(G2)2] may be a more effective immunomodulatory agent than unmodified IVIGs. This hypothesis will need to be verified.
IgG1 Core Fucosylation During the Humoral Response to Infectious Agents
Evidence for an important role of antibodies in the control viral or parasitic infections has gained strength over the last decade. IgG1 antibodies in infection, just like for anti-cancer mAbs, can act through a variety of mechanisms: via Fab they can neutralize viruses and parasites by blocking cellular entry and the replication cycle; through Fc they induce complement activation and phagocytosis by macrophages or PMN of opsonized immune complexes, clearing the infectious agent, as well as activate ADCC by NK cells which will destroy infected cells (166–170). Fab- and Fc-mediated activities probably synergize with each other, at least in some cases, although some antibodies under some circumstances can also enhance infection probably by facilitating the entry of the virus into FcɣR positive target cells, a phenomenon called antibody dependent enhancement (ADE). This has been particularly well described following secondary infection by a different Dengue virus serotype (166, 167, 171–173).
The role of the different FcɣRs and immune cells in protection from viral or parasitic diseases is not fully established, also because establishing suitable models is particularly challenging. Nonetheless FcɣRs as well monocytes and macrophages have been implicated as important effectors in controlling viral diseases caused by Influenza A virus, SARS-CoV and SARS-CoV-2 viruses, Chikunguya virus, West Nile virus and Yellow Fever virus (166, 170, 172, 174). Less frequently a role of NK cells or NKT cells has been demonstrated (166).
Interestingly, recent technical improvements in dissecting glycan structures have allowed to demonstrate that during natural antibody responses to viral or parasitic infections, there may be in some cases a decreased Fc core fucosylation of antigen-specific IgGs. Table 3 lists the major examples of this phenomenon and indicates the levels of afucosylation and duration that has been reported (whenever known). IgG1 antibodies against cytomegalovirus (CMV) (175) or against the plasmodium falciparum erythrocyte membrane antigen 1 (pfEMP1), a parasite antigen expressed on infected erythrocytes (183), show the highest levels of afucosylation following infection (median 30-75%), although this was highly variable between individuals. The effect appeared to be quite stable during time, perhaps due to continuous stimulation and/or formation of long-lived plasma cells secreting these afucosylated antibodies (175, 183). Other infectious agents, such as SARS-CoV-2 virus (175–179, 184), Dengue virus (180–182), Hepatitis B virus and Mump virus (175), induce lower (median 12-18%), although significant, levels of afucosylated antigen-specific IgG1s ( Table 3 ). Worth noting is that afucosylation has been demonstrated to be specific for the IgG1s that recognize the viral or parasite membrane antigen and has not been observed in total circulating IgG1 or IgGs. The mechanism involved is not yet clear. Nonetheless, present evidence suggests that antigens presented in the context of host cell membranes may induce specific Fc core afucosylation. Indeed, infection by enveloped viruses or by plasmodium falciparum decreases Fc core fucosylation of IgGs induced by membrane associated antigens, whereas vaccination with the soluble antigens from the same microorganisms does not (175, 183).
Table 3.
Examples of modulation of Fc core fucosylation of antigen-specific IgGs in infectious diseases.
| Antigen | Afucosylation level | % afucosylation(median) | IgG involved | Time frame | Clinical significance of afucosylation | ref |
|---|---|---|---|---|---|---|
| CMV | Low | 35% | IgG1 | Stable over time | Not known | (175) |
| SARS-CoV-2 Spike | Fast upon sero-conversion | 12-18% | IgG1 | Reversible | Correlates with ARDS, IL-6 and CRP levels, correlates with IL6 induction by macrophages in vitro |
(175–179) |
| Dengue E and NS1 proteins | Induced by infection (1° and 2°) |
12-18% | IgG1 | Stable over time | Correlates with disease severity; correlates with low platelets and RBC | (180–182) |
| HIV-1 | low | 12% | IgG1 | Stable over time | Unknown | (175) |
| HBV | Low | 16% | IgG1 | – | Unknown | (175) |
| Mumps virus | Low | 12% | IgG1 | – | Unknown | (175) |
| Plasmodium falciparum EMP1 | High | 30-75% | IgG1 | Stable w/o antigen boost, further decreases with multiple exposure | Induces higher degranulation of FcɣRIIIA+ cell line ex vivo | (183) |
ARDS, Acute Respiratory Distress Syndrome; CRP, C Reactive Protein; CMV, cytomegalovirus.
A recent paper investigating total plasma IgG N-glycosylation patterns in patients with asymptomatic filariasis (MF+) or with chronic disease (CP), compared to non-infected local control donors showed that CP patients had higher N-glycan heterogeneity. Afucosylation was highest in the CP group and lowest in MF+ group compared to controls. Other changes in glycosylation were observed, which may also have affected the immune and inflammatory state of the patients (185). Further studies will be required to investigate N-glycosylation pattern also on antigen-specific IgGs in the context of filariasis.
An important question is the functional effect of an increased level of antibody afucosylation during viral or parasitic infections and whether modulation of Fc core fucosylation is important for the control of the infecting microorganisms. This is obviously a difficult question to answer, also because afucosylation may be accompanied by other modifications in glycan structure and because glycosylation of other proteins could also be modified during infection (175, 186). Fc core fucosylation also affects the B cell receptor assembly (BCR) and BCR signaling (32). There is however some evidence that Fc core afucosylation may help in the control of infection, since the humoral response, ADCC and NK cells can play a role on the control of viral spread (187–190). Nonetheless afucosylation may also lead to major side effects, through pro-inflammatory mediators released by FcɣRIII expressing immune cells (see paragraph 5.5). Indeed, a correlation has been observed in some studies between degree of Fc core afucosylation and acute respiratory syndrome (ARDS) and inflammatory markers following SARS-CoV-2 virus infection (175, 177). Pongracz et al. confirmed decreased core fucosylation of anti-Spike protein in Covid-19 hospitalized patients, but could not observe a difference between those recovered or not in intensive care unit (178). Nor did they observe a negative correlation with inflammatory markers. In another study, degree of core afucosylation appeared to correlate with younger age of hospitalized patients (179). Recent work suggests that antibody titers, modifications of the IgG1 N-glycome profile other than afucosylation and FcɣRs other than FcɣIIIA, such as FcɣIIA, may contribute to Covid-19 disease severity (177–179, 191, 192), which would be in line with the complexity of the immune response. Clearly much work still needs to be done to fully define the factors contributing to SARS-CoV-2 infection control and ARDS.
During secondary infection with Dengue virus, the degree of Fc core afucosylation correlated with disease severity and with low platelets and RBCs (180–182).
Discussion
Afucosylation of Asn-297 N-glycan of human IgG1 is the glycan modification with the highest impact on the effector functions of therapeutic IgG1 antibodies, notably a 10-100 fold increase in affinity for human FcɣRIIIA and 2-40 fold increased ADCC by NK cells. The effect of Fc core afucosylation on phagocytosis by macrophages is less clear and may depend on context, because these cells express several other activating FcɣRs that participate in the process of phagocytosis, in addition to FcɣRIIIA. Afucosylation of IgG1 also enhances its binding affinity to human FcɣRIIIB, a major receptor on PMN. The functional effect of this on the PMN response to therapeutic antibodies is not well understood, since many data suggest that FcγRIIIB is an activating receptor for PMN, whereas others suggest that it may act as a decoy receptor in some circumstances.
The strong enhancement of ADCC by Fc core afucosylated IgG1 antibodies has led to the development of many novel therapeutic antibodies, with the idea that enhancing their activity in vivo. How much more effective these antibodies are in vivo in the clinic, compared to their fully fucosylated counterparts is still not completely clear. Despite these uncertainties, given that animal models suggest greater efficacy of therapeutic mAbs with lower Fc core fucosylation and that afucosylation does not significantly affect the pharmacokinetics of antibodies, this modification is frequently used as a method to try and enhance the efficacy of therapeutic IgG1 mAbs. Removal of fucose can also be combined with point mutations of IgG1 antibodies to further enhance their immune-mediated mechanisms of action (193).
Novel data have accumulated showing an increase in IgG1 Fc core afucosylation during the polyclonal antibody response to infections by some enveloped viruses and parasites and during alloimmunization by RBC or platelets in pregnancy. The correlation between disease severity in alloimmunization points to a more effective removal of target cells by the core afucosylated antibodies, mediated by NK cells and/or the mononuclear phagocyte system. In the case of infectious diseases, a decreased in core fucose in IgG1 antibodies directed against membrane-associated antigens has been demonstrated in several cases. A correlation with disease severity is suggested in some cases, such as SARS-CoV-2 infection, in which the modified N-glycome may indeed induce higher immune cells activation and inflammatory cytokine release. This observation is interesting in view of the more severe or frequent cytokine release syndrome reported with some therapeutic anti-tumor afucosylated antibodies compared to fucosylated antibodies, indicating that similar mechanisms may play a role in these phenomena.
These results emphasize the strong parallelism between the activities of therapeutic IgG1 mAbs and polyclonal IgGs naturally induced in health and disease. Still much remains to be investigated and clarified about the in vivo role of Fc core fucosylation, not only of IgG1 but also other Ig isotypes. Further studies on the physiological mechanisms of regulation of IgG core fucosylation in B cells in vivo is another exciting area of research, that may allow in the future to control pharmacologically this phenomenon in different pathological conditions.
Author Contributions
JG took the lead in writing the manuscript. AA and IC have critically revised the manuscript, designed and completed the figures and tables. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Fondazione Cariplo (Covid-Bank project). IC was supported by AIRC 5x1000 (Project n. 21147).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Vidarsson G, Dekkers G, Rispens T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front Immunol (2014) 5:520. doi: 10.3389/fimmu.2014.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pezer M, Pezer M. Immunoglobulin G Glycosylation in Diseases. In: Antibody Glycosylation, vol. 112. . Cham: Springer International Publishing; (2021). doi: 10.1007/978-3-030-76912-3_13 [DOI] [Google Scholar]
- 3. Gudelj I, Lauc G, Pezer M. Immunoglobulin G Glycosylation in Aging and Diseases. Cell Immunol (2018) 333:65−79. doi: 10.1016/j.cellimm.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 4. Kaplon H, Chenoweth A, Crescioli S, Reichert JM. Antibodies to Watch in 2022. mAbs (2022) 14(1):2014296. doi: 10.1080/19420862.2021.2014296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and Affinity of Human Fcgamma Receptors and Their Polymorphic Variants for Human IgG Subclasses. Blood (2009) 113(16):3716–25. doi: 10.1182/blood-2008-09-179754 [DOI] [PubMed] [Google Scholar]
- 6. Weiner GJ. Rituximab: Mechanism of Action. Semin Hematol (2010) 47(2):115−23. doi: 10.1053/j.seminhematol.2010.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cartron G, Watier H, Golay J, Solal-Celigny P. From the Bench to the Bedside: Ways to Improve Rituximab Efficacy. Blood (2004) 104(9):2635−42. doi: 10.1182/blood-2004-03-1110 [DOI] [PubMed] [Google Scholar]
- 8. Hussain K, Cragg MS, Beers SA. Remodeling the Tumor Myeloid Landscape to Enhance Antitumor Antibody Immunotherapies. Cancers (2021) 13(19):4904. doi: 10.3390/cancers13194904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Musolino A, Gradishar WJ, Rugo HS, Nordstrom JL, Rock EP, Arnaldez F, et al. Role of Fcγ Receptors in HER2-Targeted Breast Cancer Therapy. J Immunother Cancer (2022) 10(1):e003171. doi: 10.1136/jitc-2021-003171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gordan S, Albert H, Danzer H, Lux A, Biburger M, Nimmerjahn F. The Immunological Organ Environment Dictates the Molecular and Cellular Pathways of Cytotoxic Antibody Activity. Cell Rep (2019) 29(10):3033–46.e4. doi: 10.1016/j.celrep.2019.10.111 [DOI] [PubMed] [Google Scholar]
- 11. Nagelkerke SQ, Bruggeman CW, den Haan JMM, Mul EPJ, van den Berg TK, van Bruggen R, et al. Red Pulp Macrophages in the Human Spleen are a Distinct Cell Population With a Unique Expression of Fc-γ Receptors. Blood Adv (2018) 2(8):941−53. doi: 10.1182/bloodadvances.2017015008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiener E, Abeyakoon O, Benchetrit G, Lyall M, Keler T, Rodeck CH. Anti-HPA-1a-Mediated Platelet Phagocytosis by Monocytes In Vitro and its Inhibition by Fc Gamma Receptor (FcgammaR) Reactive Reagents. Eur J Haematol (2003) 70(2):67−74. doi: 10.1034/j.1600-0609.2003.00025.x [DOI] [PubMed] [Google Scholar]
- 13. Golay J, Valgardsdottir R, Musaraj G, Giupponi D, Spinelli O, Introna M. Human Neutrophils Express Low Levels of Fcγriiia, Which Plays a Role in PMN Activation. Blood (2019) 133(13):1395−405. doi: 10.1182/blood-2018-07-864538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Jönsson F. Expression, Role, and Regulation of Neutrophil Fcγ Receptors. Front Immunol (2019) 10:1958. doi: 10.3389/fimmu.2019.01958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Derer S, Glorius P, Schlaeth M, Lohse S, Klausz K, Muchhal U, et al. Increasing Fcγriia Affinity of an Fcγriii-Optimized Anti-EGFR Antibody Restores Neutrophil-Mediated Cytotoxicity. mAbs (2014) 6(2):409−21. doi: 10.4161/mabs.27457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Unkeless JC, Shen Z, Lin CW, DeBeus E. Function of Human Fc Gamma RIIA and Fc Gamma RIIIB. Semin Immunol (1995) 7(1):37−44. doi: 10.1016/1044-5323(95)90006-3 [DOI] [PubMed] [Google Scholar]
- 17. Bournazos S, Ravetch JV. Diversification of IgG Effector Functions. Int Immunol (2017) 29(7):303−10. doi: 10.1093/intimm/dxx025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Golay J, Taylor RP. The Role of Complement in the Mechanism of Action of Therapeutic Anti-Cancer Mabs. Antibodies (2020) 9(4):58. doi: 10.3390/antib9040058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: An Odd Antibody. Clin Exp Allergy (2009) 39(4):469−77. doi: 10.1111/j.1365-2222.2009.03207.x [DOI] [PubMed] [Google Scholar]
- 20. Carter PJ, Lazar GA. Next Generation Antibody Drugs: Pursuit of the « High-Hanging Fruit ». Nat Rev Drug Discovery (2018) 17(3):197−223. doi: 10.1038/nrd.2017.227 [DOI] [PubMed] [Google Scholar]
- 21. Bondt A, Rombouts Y, Selman MHJ, Hensbergen PJ, Reiding KR, Hazes JMW, et al. Immunoglobulin G (IgG) Fab Glycosylation Analysis Using a New Mass Spectrometric High-Throughput Profiling Method Reveals Pregnancy-Associated Changes. Mol Cell Proteomics (2014) 13(11):3029−39. doi: 10.1074/mcp.M114.039537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giddens JP, Lomino JV, DiLillo DJ, Ravetch JV, Wang L-X. Site-Selective Chemoenzymatic Glycoengineering of Fab and Fc Glycans of a Therapeutic Antibody. Proc Natl Acad Sci U S A (2018) 115(47):12023−7. doi: 10.1073/pnas.1812833115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamaguchi Y, Wakaizumi N, Irisa M, Maruno T, Shimada M, Shintani K, et al. The Fab Portion of Immunoglobulin G has Sites in the CL Domain That Interact With Fc Gamma Receptor IIIa. mAbs (2022) 14(1):2038531. doi: 10.1080/19420862.2022.2038531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yogo R, Yamaguchi Y, Watanabe H, Yagi H, Satoh T, Nakanishi M, et al. The Fab Portion of Immunoglobulin G Contributes to its Binding to Fcγ Receptor III. Sci Rep (2019) 9(1):11957. doi: 10.1038/s41598-019-48323-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Z, Shah B, Richardson J. Impact of Fc N-Glycan Sialylation on IgG Structure. mAbs (2019) 11(8):1381−90. doi: 10.1080/19420862.2019.1655377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li W, Zhu Z, Chen W, Feng Y, Dimitrov DS. Crystallizable Fragment Glycoengineering for Therapeutic Antibodies Development. Front Immunol (2017) 8:1554. doi: 10.3389/fimmu.2017.01554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jefferis R. Glycosylation as a Strategy to Improve Antibody-Based Therapeutics. Nat Rev Drug Discovery (2009) 8(3):226−34. doi: 10.1038/nrd2804 [DOI] [PubMed] [Google Scholar]
- 28. Jefferis R. Glycosylation of Natural and Recombinant Antibody Molecules. Adv Exp Med Biol (2005) 564:143−8. doi: 10.1007/0-387-25515-X_26 [DOI] [PubMed] [Google Scholar]
- 29. Pucić M, Knezević A, Vidic J, Adamczyk B, Novokmet M, Polasek O, et al. High Throughput Isolation and Glycosylation Analysis of IgG-Variability and Heritability of the IgG Glycome in Three Isolated Human Populations. Mol Cell Proteomics (2011) 10(10):M111.010090. doi: 10.1074/mcp.M111.010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boune S, Hu P, Epstein AL, Khawli LA. Principles of N-Linked Glycosylation Variations of IgG-Based Therapeutics: Pharmacokinetic and Functional Considerations. Antibodies (2020) 9(2):22. doi: 10.3390/antib9020022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Popp O, Moser S, Zielonka J, Rüger P, Hansen S, Plöttner O. Development of a Pre-Glycoengineered CHO-K1 Host Cell Line for the Expression of Antibodies With Enhanced Fc Mediated Effector Function. mAbs (2018) 10(2):290−303. doi: 10.1080/19420862.2017.1405203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun Y, Li X, Wang T, Li W. Core Fucosylation Regulates the Function of Pre-BCR, BCR and IgG in Humoral Immunity. Front Immunol (2022) 13:844427. doi: 10.3389/fimmu.2022.844427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Q, Wang T, Zhang R, Yang S, McFarland KS, Chung C-Y, et al. The Interplay of Protein Engineering and Glycoengineering to Fine-Tune Antibody Glycosylation and its Impact on Effector Functions. Biotechnol Bioeng (2022) 119(1):102−17. doi: 10.1002/bit.27953 [DOI] [PubMed] [Google Scholar]
- 34. Jefferis R. Glycosylation of Recombinant Antibody Therapeutics. Biotechnol Prog (2008) 21(1):11−6. doi: 10.1021/bp040016j [DOI] [PubMed] [Google Scholar]
- 35. Zlatina K, Galuska SP. Immunoglobulin Glycosylation – An Unexploited Potential for Immunomodulatory Strategies in Farm Animals. Front Immunol (2021) 12:753294. doi: 10.3389/fimmu.2021.753294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Becker DJ, Lowe JB. Fucose: Biosynthesis and Biological Function in Mammals. Glycobiology (2003) 13(7):41R–53R. doi: 10.1093/glycob/cwg054 [DOI] [PubMed] [Google Scholar]
- 37. Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, et al. Lack of Fucose on Human IgG1 N-Linked Oligosaccharide Improves Binding to Human Fcγriii and Antibody-Dependent Cellular Toxicity. J Biol Chem (2002) 277(30):26733−40. doi: 10.1074/jbc.M202069200 [DOI] [PubMed] [Google Scholar]
- 38. Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, et al. The Absence of Fucose But Not the Presence of Galactose or Bisecting N-Acetylglucosamine of Human IgG1 Complex-Type Oligosaccharides Shows the Critical Role of Enhancing Antibody-Dependent Cellular Cytotoxicity*. J Biol Chem (2003) 278(5):3466−73. doi: 10.1074/jbc.M210665200 [DOI] [PubMed] [Google Scholar]
- 39. Umaña P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered Glycoforms of an Antineuroblastoma IgG1 With Optimized Antibody-Dependent Cellular Cytotoxic Activity. Nat Biotechnol (1999) 17(2):176−80. doi: 10.1038/6179 [DOI] [PubMed] [Google Scholar]
- 40. Pereira NA, Chan KF, Lin PC, Song Z. The « Less-is-More » in Therapeutic Antibodies: Afucosylated Anti-Cancer Antibodies With Enhanced Antibody-Dependent Cellular Cytotoxicity. mAbs (2018) 10(5):693−711. doi: 10.1080/19420862.2018.1466767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang G, Wang Q, Chen L, Betenbaugh MJ, Zhang H. Glycoproteomic Characterization of FUT8 Knock-Out CHO Cells Reveals Roles of FUT8 in the Glycosylation. Front Chem (2021) 9:755238. doi: 10.3389/fchem.2021.755238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yuan Y, Zong H, Bai J, Han L, Wang L, Zhang X, et al. Bioprocess Development of a Stable FUT8-/–CHO Cell Line to Produce Defucosylated Anti-HER2 Antibody. Bioprocess Biosyst Eng (2019) 42(8):1263−71. doi: 10.1007/s00449-019-02124-7 [DOI] [PubMed] [Google Scholar]
- 43. Ripka J, Adamany A, Stanley P. Two Chinese Hamster Ovary Glycosylation Mutants Affected in the Conversion of GDP-Mannose to GDP-Fucose. Arch Biochem Biophys (1986) 249(2):533−45. doi: 10.1016/0003-9861(86)90031-7 [DOI] [PubMed] [Google Scholar]
- 44. Liu W, Padmashali R, Monzon OQ, Lundberg D, Jin S, Dwyer B, et al. Generation of FX-/- and Gmds-/- CHOZN Host Cell Lines for the Production of Afucosylated Therapeutic Antibodies. Biotechnol Prog (2021) 37(1):e3061. doi: 10.1002/btpr.3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Misaki R, Iwasaki M, Takechi H, Yamano-Adachi N, Ohashi T, Kajiura H, et al. Establishment of Serum-Free Adapted Chinese Hamster Ovary Cells With Double Knockout of GDP-Mannose-4,6-Dehydratase and GDP-Fucose Transporter. Cytotechnology (2022) 74(1):163−79. doi: 10.1007/s10616-021-00501-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Davies J, Jiang L, Pan LZ, LaBarre MJ, Anderson D, Reff M. Expression of GnTIII in a Recombinant Anti-CD20 CHO Production Cell Line: Expression of Antibodies With Altered Glycoforms Leads to an Increase in ADCC Through Higher Affinity for FC Gamma RIII. Biotechnol Bioeng (2001) 74(4):288−94. doi: 10.1002/bit.1119 [DOI] [PubMed] [Google Scholar]
- 47. Ferrara C, Brünker P, Suter T, Moser S, Püntener U, Umaña P. Modulation of Therapeutic Antibody Effector Functions by Glycosylation Engineering: Influence of Golgi Enzyme Localization Domain and Co-Expression of Heterologous Beta1, 4-N-Acetylglucosaminyltransferase III and Golgi Alpha-Mannosidase II. Biotechnol Bioeng (2006) 93(5):851−61. doi: 10.1002/bit.20777 [DOI] [PubMed] [Google Scholar]
- 48. Beck A, Reichert JM. Marketing Approval of Mogamulizumab. mAbs (2012) 4(4):419−25. doi: 10.4161/mabs.20996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Okeley NM, Alley SC, Anderson ME, Boursalian TE, Burke PJ, Emmerton KM, et al. Development of Orally Active Inhibitors of Protein and Cellular Fucosylation. Proc Natl Acad Sci U S A (2013) 110(14):5404−9. doi: 10.1073/pnas.1222263110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Joubert S, Guimond J, Perret S, Malenfant F, Elahi SM, Marcil A, et al. Production of Afucosylated Antibodies in CHO Cells by Coexpression of an Anti-FUT8 Intrabody. Biotechnol Bioeng (2022). doi: 10.1002/bit.28127 [DOI] [PubMed] [Google Scholar]
- 51. Li C, Li T, Wang L-X. Chemoenzymatic Defucosylation of Therapeutic Antibodies for Enhanced Effector Functions Using Bacterial α-Fucosidases. Methods Mol Biol (2018) 1827:367−80. doi: 10.1007/978-1-4939-8648-4_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Giddens JP, Wang L-X. Chemoenzymatic Glyco-Engineering of Monoclonal Antibodies. Methods Mol Biol (2015) 1321:375−87. doi: 10.1007/978-1-4939-2760-9_25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang TT, Ravetch JV. Functional Diversification of IgGs Through Fc Glycosylation. J Clin Invest (2019) 129(9):3492−8. doi: 10.1172/JCI130029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Subedi GP, Barb AW. The Immunoglobulin G1 N-Glycan Composition Affects Binding to Each Low Affinity Fc γ Receptor. mAbs (2016) 8(8):1512−24. doi: 10.1080/19420862.2016.1218586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Golay J, Da Roit F, Bologna L, Ferrara C, Leusen JH, Rambaldi A, et al. Glycoengineered CD20 Antibody Obinutuzumab Activates Neutrophils and Mediates Phagocytosis Through CD16B More Efficiently Than Rituximab. Blood (2013) 122(20):3482−91. doi: 10.1182/blood-2013-05-504043 [DOI] [PubMed] [Google Scholar]
- 56. Ferrara C, Stuart F, Sondermann P, Brünker P, Umaña P. The Carbohydrate at FcgammaRIIIa Asn-162. An Element Required for High Affinity Binding to non-Fucosylated IgG Glycoforms. J Biol Chem (2006) 281(8):5032−6. doi: 10.1074/jbc.M510171200 [DOI] [PubMed] [Google Scholar]
- 57. Ferrara C, Grau S, Jäger C, Sondermann P, Brünker P, Waldhauer I, et al. Unique Carbohydrate-Carbohydrate Interactions are Required for High Affinity Binding Between FcgammaRIII and Antibodies Lacking Core Fucose. Proc Natl Acad Sci U S A (2011) 108(31):12669−74. doi: 10.1073/pnas.1108455108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hoeres T, Pretscher D, Holzmann E, Smetak M, Birkmann J, Triebel J, et al. Improving Immunotherapy Against B-Cell Malignancies Using γδ T-Cell-Specific Stimulation and Therapeutic Monoclonal Antibodies. J Immunother (2019) 42(9):331−44. doi: 10.1097/CJI.0000000000000289 [DOI] [PubMed] [Google Scholar]
- 59. Chu Y, Awasthi A, Lee S, Edani D, Yin C, Hochberg J, et al. Obinutuzumab (GA101) vs. Rituximab Significantly Enhances Cell Death, Antibody-Dependent Cytotoxicity and Improves Overall Survival Against CD20+ Primary Mediastinal B-Cell Lymphoma (PMBL) in a Xenograft NOD-Scid IL2Rgnull (NSG) Mouse Model: A Potential Targeted Agent in the Treatment of PMBL. Oncotarget (2020) 11(32):3035−47. doi: 10.18632/oncotarget.27691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Decaup E, Rossi C, Gravelle P, Laurent C, Bordenave J, Tosolini M, et al. A Tridimensional Model for NK Cell-Mediated ADCC of Follicular Lymphoma. Front Immunol (2019) 10:1943. doi: 10.3389/fimmu.2019.01943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Herter S, Birk MC, Klein C, Gerdes C, Umana P, Bacac M. Glycoengineering of Therapeutic Antibodies Enhances Monocyte/Macrophage-Mediated Phagocytosis and Cytotoxicity. J Immunol (2014) 192(5):2252−60. doi: 10.4049/jimmunol.1301249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mössner E, Brünker P, Moser S, Püntener U, Schmidt C, Herter S, et al. Increasing the Efficacy of CD20 Antibody Therapy Through the Engineering of a New Type II Anti-CD20 Antibody With Enhanced Direct and Immune Effector Cell-Mediated B-Cell Cytotoxicity. Blood (2010) 115(22):4393−402. doi: 10.1182/blood-2009-06-225979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Herter S, Herting F, Muth G, van Puijenbroek E, Schlothauer T, Ferrara C, et al. GA101 P329GLALA, a Variant of Obinutuzumab With Abolished ADCC, ADCP and CDC Function But Retained Cell Death Induction, is as Efficient as Rituximab in B-Cell Depletion and Antitumor Activity. Haematologica (2018) 103(2):e78−81. doi: 10.3324/haematol.2017.178996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab Plus Chlorambucil in Patients With CLL and Coexisting Conditions. N Engl J Med (2014) 370(12):1101−10. doi: 10.1056/NEJMoa1313984 [DOI] [PubMed] [Google Scholar]
- 65. Stilgenbauer S, Bosch F, Ilhan O, Kisro J, Mahé B, Mikuskova E, et al. Safety and Efficacy of Obinutuzumab Alone or With Chemotherapy in Previously Untreated or Relapsed/Refractory Chronic Lymphocytic Leukaemia Patients: Final Analysis of the Phase IIIb GREEN Study. Br J Haematol (2021) 193(2):325−38. doi: 10.1111/bjh.17326 [DOI] [PubMed] [Google Scholar]
- 66. Hiddemann W, Barbui AM, Canales MA, Cannell PK, Collins GP, Dürig J, et al. Immunochemotherapy With Obinutuzumab or Rituximab for Previously Untreated Follicular Lymphoma in the GALLIUM Study: Influence of Chemotherapy on Efficacy and Safety. J Clin Oncol (2018) 36(23):2395−404. doi: 10.1200/JCO.2017.76.8960 [DOI] [PubMed] [Google Scholar]
- 67. Ishii T, Ishida T, Utsunomiya A, Inagaki A, Yano H, Komatsu H, et al. Defucosylated Humanized Anti-CCR4 Monoclonal Antibody KW-0761 as a Novel Immunotherapeutic Agent for Adult T-Cell Leukemia/Lymphoma. Clin Cancer Res (2010) 16(5):1520−31. doi: 10.1158/1078-0432.CCR-09-2697 [DOI] [PubMed] [Google Scholar]
- 68. Niwa R, Sakurada M, Kobayashi Y, Uehara A, Matsushima K, Ueda R, et al. Enhanced Natural Killer Cell Binding and Activation by Low-Fucose IgG1 Antibody Results in Potent Antibody-Dependent Cellular Cytotoxicity Induction at Lower Antigen Density. Clin Cancer Res (2005) 11(6):2327−36. doi: 10.1158/1078-0432.CCR-04-2263 [DOI] [PubMed] [Google Scholar]
- 69. Gallagher S, Turman S, Yusuf I, Akhgar A, Wu Y, Roskos LK, et al. Pharmacological Profile of MEDI-551, a Novel Anti-CD19 Antibody, in Human CD19 Transgenic Mice. Int Immunopharmacol (2016) 36:205−12. doi: 10.1016/j.intimp.2016.04.035 [DOI] [PubMed] [Google Scholar]
- 70. Frampton JE. Inebilizumab: First Approval. Drugs (2020) 80(12):1259−64. doi: 10.1007/s40265-020-01370-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Herbst R, Wang Y, Gallagher S, Mittereder N, Kuta E, Damschroder M, et al. B-Cell Depletion In Vitro and In Vivo With an Afucosylated Anti-CD19 Antibody. J Pharmacol Exp Ther (2010) 335(1):213−22. doi: 10.1124/jpet.110.168062 [DOI] [PubMed] [Google Scholar]
- 72. Rensel M, Zabeti A, Mealy MA, Cimbora D, She D, Drappa J, et al. Long-Term Efficacy and Safety of Inebilizumab in Neuromyelitis Optica Spectrum Disorder: Analysis of Aquaporin-4-Immunoglobulin G-Seropositive Participants Taking Inebilizumab for ≧̸4 Years in the N-MOmentum Trial. Mult Scler (2022) 28(6):925−32. doi: 10.1177/13524585211047223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an Anti-Interleukin-5 Receptor α Monoclonal Antibody, as Add-on Treatment for Patients With Severe, Uncontrolled, Eosinophilic Asthma (CALIMA): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet (2016) 388(10056):2128−41. doi: 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- 74. Ghazi A, Trikha A, Calhoun WJ. Benralizumab–a Humanized mAb to IL-5rα With Enhanced Antibody-Dependent Cell-Mediated Cytotoxicity–a Novel Approach for the Treatment of Asthma. Expert Opin Biol Ther (2012) 12(1):113−8. doi: 10.1517/14712598.2012.642359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM, et al. MEDI-563, a Humanized Anti-IL-5 Receptor Alpha mAb With Enhanced Antibody-Dependent Cell-Mediated Cytotoxicity Function. J Allergy Clin Immunol (2010) 125(6):1344–53.e2. doi: 10.1016/j.jaci.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 76. Nordstrom JL, Gorlatov S, Zhang W, Yang Y, Huang L, Burke S, et al. Anti-Tumor Activity and Toxicokinetics Analysis of MGAH22, an Anti-HER2 Monoclonal Antibody With Enhanced Fcγ Receptor Binding Properties. Breast Cancer Res (2011) 13(6):R123. doi: 10.1186/bcr3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rugo HS, Im S-A, Cardoso F, Cortés J, Curigliano G, Musolino A, et al. Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol (2021) 7(4):573−84. doi: 10.1001/jamaoncol.2020.7932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tai Y-T, Mayes PA, Acharya C, Zhong MY, Cea M, Cagnetta A, et al. Novel Anti–B-Cell Maturation Antigen Antibody-Drug Conjugate (GSK2857916) Selectively Induces Killing of Multiple Myeloma. Blood (2014) 123(20):3128−38. doi: 10.1182/blood-2013-10-535088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Grugan KD, Dorn K, Jarantow SW, Bushey BS, Pardinas JR, Laquerre S, et al. Fc-Mediated Activity of EGFR X C-Met Bispecific Antibody JNJ-61186372 Enhanced Killing of Lung Cancer Cells. mAbs (2017) 9(1):114−26. doi: 10.1080/19420862.2016.1249079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Church AK, VanDerMeid KR, Baig NA, Baran AM, Witzig TE, Nowakowski GS, et al. Anti-CD20 Monoclonal Antibody-Dependent Phagocytosis of Chronic Lymphocytic Leukaemia Cells by Autologous Macrophages. Clin Exp Immunol (2015) 183(1):90−101. doi: 10.1111/cei.12697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. de Romeuf C, Dutertre C-A, Le Garff-Tavernier M, Fournier N, Gaucher C, Glacet A, et al. Chronic Lymphocytic Leukaemia Cells are Efficiently Killed by an Anti-CD20 Monoclonal Antibody Selected for Improved Engagement of FcgammaRIIIA/Cd16. Br J Haematol (2008) 140(6):635−43. doi: 10.1111/j.1365-2141.2007.06974.x [DOI] [PubMed] [Google Scholar]
- 82. Klein C, Jamois C, Nielsen T. Anti-CD20 Treatment for B-Cell Malignancies: Current Status and Future Directions. Expert Opin Biol Ther (2021) 21(2):161−81. doi: 10.1080/14712598.2020.1822318 [DOI] [PubMed] [Google Scholar]
- 83. Klinghammer K, Fayette J, Kawecki A, Dietz A, Schafhausen P, Folprecht G, et al. A Randomized Phase II Study Comparing the Efficacy and Safety of the Glyco-Optimized Anti-EGFR Antibody Tomuzotuximab Against Cetuximab in Patients With Recurrent and/or Metastatic Squamous Cell Cancer of the Head and Neck - the RESGEX Study. ESMO Open (2021) 6(5):100242. doi: 10.1016/j.esmoop.2021.100242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gerdes CA, Nicolini VG, Herter S, van Puijenbroek E, Lang S, Roemmele M, et al. GA201 (RG7160): A Novel, Humanized, Glycoengineered Anti-EGFR Antibody With Enhanced ADCC and Superior In Vivo Efficacy Compared With Cetuximab. Clin Cancer Res (2013) 19(5):1126−38. doi: 10.1158/1078-0432.CCR-12-0989 [DOI] [PubMed] [Google Scholar]
- 85. Paz-Ares LG, Gomez-Roca C, Delord J-P, Cervantes A, Markman B, Corral J, et al. Phase I Pharmacokinetic and Pharmacodynamic Dose-Escalation Study of RG7160 (GA201), the First Glycoengineered Monoclonal Antibody Against the Epidermal Growth Factor Receptor, in Patients With Advanced Solid Tumors. J Clin Oncol (2011) 29(28):3783−90. doi: 10.1200/JCO.2011.34.8888 [DOI] [PubMed] [Google Scholar]
- 86. Delord J-P, Tabernero J, García-Carbonero R, Cervantes A, Gomez-Roca C, Bergé Y, et al. Open-Label, Multicentre Expansion Cohort to Evaluate Imgatuzumab in Pre-Treated Patients With KRAS-Mutant Advanced Colorectal Carcinoma. Eur J Cancer (2014) 50(3):496−505. doi: 10.1016/j.ejca.2013.10.015 [DOI] [PubMed] [Google Scholar]
- 87. Oppenheim DE, Spreafico R, Etuk A, Malone D, Amofah E, Peña-Murillo C, et al. Glyco-Engineered Anti-EGFR mAb Elicits ADCC by NK Cells From Colorectal Cancer Patients Irrespective of Chemotherapy. Br J Cancer (2014) 110(5):1221−7. doi: 10.1038/bjc.2014.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gonzalez-Nicolini V, Herter S, Lang S, Waldhauer I, Bacac M, Roemmele M, et al. Premedication and Chemotherapy Agents do Not Impair Imgatuzumab (GA201)-Mediated Antibody-Dependent Cellular Cytotoxicity and Combination Therapies Enhance Efficacy. Clin Cancer Res (2016) 22(10):2453−61. doi: 10.1158/1078-0432.CCR-14-2579 [DOI] [PubMed] [Google Scholar]
- 89. Temam S, Spicer J, Farzaneh F, Soria JC, Oppenheim D, McGurk M, et al. An Exploratory, Open-Label, Randomized, Multicenter Study to Investigate the Pharmacodynamics of a Glycoengineered Antibody (Imgatuzumab) and Cetuximab in Patients With Operable Head and Neck Squamous Cell Carcinoma. Ann Oncol (2017) 28(11):2827−35. doi: 10.1093/annonc/mdx489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Furihata K, Ishiguro Y, Yoshimura N, Ito H, Katsushima S, Kaneko E, et al. A Phase 1 Study of KHK4083: A Single-Blind, Randomized, Placebo-Controlled Single-Ascending-Dose Study in Healthy Adults and an Open-Label Multiple-Dose Study in Patients With Ulcerative Colitis. Clin Pharmacol Drug Dev (2021) 10(8):870−83. doi: 10.1002/cpdd.918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fiedler W, Stoeger H, Perotti A, Gastl G, Weidmann J, Dietrich B, et al. Phase I Study of TrasGEX, a Glyco-Optimised Anti-HER2 Monoclonal Antibody, in Patients With HER2-Positive Solid Tumours. ESMO Open (2018) 3(4):e000381. doi: 10.1136/esmoopen-2018-000381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Junttila TT, Parsons K, Olsson C, Lu Y, Xin Y, Theriault J, et al. Superior In Vivo Efficacy of Afucosylated Trastuzumab in the Treatment of HER2-Amplified Breast Cancer. Cancer Res (2010) 70(11):4481−9. doi: 10.1158/0008-5472.CAN-09-3704 [DOI] [PubMed] [Google Scholar]
- 93. Suzuki E, Niwa R, Saji S, Muta M, Hirose M, Iida S, et al. A Nonfucosylated Anti-HER2 Antibody Augments Antibody-Dependent Cellular Cytotoxicity in Breast Cancer Patients. Clin Cancer Res (2007) 13(6):1875−82. doi: 10.1158/1078-0432.CCR-06-1335 [DOI] [PubMed] [Google Scholar]
- 94. Nakajima T, Okayama H, Ashizawa M, Noda M, Aoto K, Saito M, et al. Augmentation of Antibody-Dependent Cellular Cytotoxicity With Defucosylated Monoclonal Antibodies in Patients With GI-Tract Cancer. Oncol Lett (2018) 15(2):2604−10. doi: 10.3892/ol.2017.7556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. De Meulenaere A, Vermassen T, Creytens D, De Keukeleire S, Delahaye T, Deron P, et al. An Open-Label, Nonrandomized, Phase Ib Feasibility Study of Cusatuzumab in Patients With Nasopharyngeal Carcinoma. Clin Transl Sci (2021) 14(6):2300−13. doi: 10.1111/cts.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Leupin N, Zinzani PL, Morschhauser F, Dalle S, Maerevoet M, Michot J-M, et al. Cusatuzumab for Treatment of CD70-Positive Relapsed or Refractory Cutaneous T-Cell Lymphoma. Cancer (2022) 128(5):1004−14. doi: 10.1002/cncr.34005 [DOI] [PubMed] [Google Scholar]
- 97. Xiang H, Chan AG, Ahene A, Bellovin DI, Deng R, Hsu AW, et al. Preclinical Characterization of Bemarituzumab, an Anti-FGFR2b Antibody for the Treatment of Cancer. mAbs (2021) 13(1):1981202. doi: 10.1080/19420862.2021.1981202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Catenacci DVT, Rasco D, Lee J, Rha SY, Lee K-W, Bang YJ, et al. Phase I Escalation and Expansion Study of Bemarituzumab (FPA144) in Patients With Advanced Solid Tumors and FGFR2b-Selected Gastroesophageal Adenocarcinoma. J Clin Oncol (2020) 38(21):2418−26. doi: 10.1200/JCO.19.01834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Iida S, Kuni-Kamochi R, Mori K, Misaka H, Inoue M, Okazaki A, et al. Two Mechanisms of the Enhanced Antibody-Dependent Cellular Cytotoxicity (ADCC) Efficacy of non-Fucosylated Therapeutic Antibodies in Human Blood. BMC Cancer (2009) 9:58. doi: 10.1186/1471-2407-9-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Karampatzakis A, Brož P, Rey C, Önfelt B, Cruz De Matos GDS, Rycroft D, et al. Antibody Afucosylation Augments CD16-Mediated Serial Killing and Ifnγ Secretion by Human Natural Killer Cells. Front Immunol (2021) 12:641521. doi: 10.3389/fimmu.2021.641521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, Sica A, et al. M2 Macrophages Phagocytose Rituximab-Opsonized Leukemic Targets More Efficiently Than M1 Cells In Vitro . J Immunol (2009) 182(7):4415−22. doi: 10.4049/jimmunol.0713732 [DOI] [PubMed] [Google Scholar]
- 102. Bologna L, Gotti E, Manganini M, Rambaldi A, Intermesoli T, Introna M, et al. Mechanism of Action of Type II, Glycoengineered, Anti-CD20 Monoclonal Antibody GA101 in B-Chronic Lymphocytic Leukemia Whole Blood Assays in Comparison With Rituximab and Alemtuzumab. J Immunol (2011) 186(6):3762−9. doi: 10.4049/jimmunol.1000303 [DOI] [PubMed] [Google Scholar]
- 103. Grandjean CL, Montalvao F, Celli S, Michonneau D, Breart B, Garcia Z, et al. Intravital Imaging Reveals Improved Kupffer Cell-Mediated Phagocytosis as a Mode of Action of Glycoengineered Anti-CD20 Antibodies. Sci Rep (2016) 6(1):34382. doi: 10.1038/srep34382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kapur R, Kustiawan I, Vestrheim A, Koeleman CAM, Visser R, Einarsdottir HK, et al. A Prominent Lack of IgG1-Fc Fucosylation of Platelet Alloantibodies in Pregnancy. Blood (2014) 123(4):471−80. doi: 10.1182/blood-2013-09-527978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Oberg HH, Kellner C, Gonnermann D, Sebens S, Bauerschlag D, Gramatzki M, et al. Tribody [(HER2)2xCD16] Is More Effective Than Trastuzumab in Enhancing γδ T Cell and Natural Killer Cell Cytotoxicity Against HER2-Expressing Cancer Cells. Front Immunol (2018) 9:814. doi: 10.3389/fimmu.2018.00814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gertner-Dardenne J, Bonnafous C, Bezombes C, Capietto A-H, Scaglione V, Ingoure S, et al. Bromohydrin Pyrophosphate Enhances Antibody-Dependent Cell-Mediated Cytotoxicity Induced by Therapeutic Antibodies. Blood (2009) 113(20):4875−84. doi: 10.1182/blood-2008-08-172296 [DOI] [PubMed] [Google Scholar]
- 107. Braza MS, Klein B, Fiol G, Rossi J-F. γδ T-Cell Killing of Primary Follicular Lymphoma Cells is Dramatically Potentiated by GA101, a Type II Glycoengineered Anti-CD20 Monoclonal Antibody. Haematologica (2011) 96(3):400−7. doi: 10.3324/haematol.2010.029520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Shiromizu CM, Jancic CC. γδ T Lymphocytes: An Effector Cell in Autoimmunity and Infection. Front Immunol (2018) 9:2389. doi: 10.3389/fimmu.2018.02389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shibata-Koyama M, Iida S, Misaka H, Mori K, Yano K, Shitara K, et al. Nonfucosylated Rituximab Potentiates Human Neutrophil Phagocytosis Through its High Binding for FcgammaRIIIb and MHC Class II Expression on the Phagocytotic Neutrophils. Exp Hematol (2009) 37(3):309−21. doi: 10.1016/j.exphem.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 110. Valgardsdottir R, Cattaneo I, Klein C, Introna M, Figliuzzi M, Golay J. Human Neutrophils Mediate Trogocytosis Rather Than Phagocytosis of CLL B Cells Opsonized With Anti-CD20 Antibodies. Blood (2017) 129(19):2636−44. doi: 10.1182/blood-2016-08-735605 [DOI] [PubMed] [Google Scholar]
- 111. Peipp M, Lammerts van Bueren JJ, Schneider-Merck T, Bleeker WWK, Dechant M, Beyer T, et al. Antibody Fucosylation Differentially Impacts Cytotoxicity Mediated by NK and PMN Effector Cells. Blood (2008) 112(6):2390−9. doi: 10.1182/blood-2008-03-144600 [DOI] [PubMed] [Google Scholar]
- 112. Treffers LW, van Houdt M, Bruggeman CW, Heineke MH, Zhao XW, van der Heijden J, et al. Fcγriiib Restricts Antibody-Dependent Destruction of Cancer Cells by Human Neutrophils. Front Immunol (2018) 9:3124. doi: 10.3389/fimmu.2018.03124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kircheis R, Halanek N, Koller I, Jost W, Schuster M, Gorr G, et al. Correlation of ADCC Activity With Cytokine Release Induced by the Stably Expressed, Glyco-Engineered Humanized Lewis Y-Specific Monoclonal Antibody MB314. mAbs (2012) 4(4):532−41. doi: 10.4161/mabs.20577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Capuano C, Pighi C, Molfetta R, Paolini R, Battella S, Palmieri G, et al. Obinutuzumab-Mediated High-Affinity Ligation of Fcγriiia/CD16 Primes NK Cells for Ifnγ Production. Oncoimmunology (2017) 6(3):e1290037. doi: 10.1080/2162402X.2017.1290037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Romeo V, Gierke S, Edgar KA, Liu SD. Effects of PI3K Inhibition on Afucosylated Antibody-Driven Fcγriiia Events and Phospho-S6 Activity in NK Cells. J Immunol (2019) 203(1):137−47. doi: 10.4049/jimmunol.1801418 [DOI] [PubMed] [Google Scholar]
- 116. Sehn LH, Martelli M, Trněný M, Liu W, Bolen CR, Knapp A, et al. A Randomized, Open-Label, Phase III Study of Obinutuzumab or Rituximab Plus CHOP in Patients With Previously Untreated Diffuse Large B-Cell Lymphoma: Final Analysis of GOYA. J Hematol Oncol (2020) 13(1):71. doi: 10.1186/s13045-020-00900-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med (2017) 377(14):1331−44. doi: 10.1056/NEJMoa1614598 [DOI] [PubMed] [Google Scholar]
- 118. Crowley AR, Ackerman ME. Mind the Gap: How Interspecies Variability in IgG and Its Receptors May Complicate Comparisons of Human and Non-Human Primate Effector Function. Front Immunol (2019) 10:697. doi: 10.3389/fimmu.2019.00697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Nimmerjahn F, Ravetch JV. Fcgamma Receptors: Old Friends and New Family Members. Immunity (2006) 24(1):19−28. doi: 10.1016/j.immuni.2005.11.010 [DOI] [PubMed] [Google Scholar]
- 120. Wang Y, Krémer V, Iannascoli B, Goff OR-L, Mancardi DA, Ramke L, et al. Specificity of Mouse and Human Fcgamma Receptors and Their Polymorphic Variants for IgG Subclasses of Different Species. Eur J Immunol (2022) 52(5):753–9. doi: 10.1002/eji.202149766 [DOI] [PubMed] [Google Scholar]
- 121. Dekkers G, Bentlage AEH, Plomp R, Visser R, Koeleman CAM, Beentjes A, et al. Conserved Fcγr- Glycan Discriminates Between Fucosylated and Afucosylated IgG in Humans and Mice. Mol Immunol (2018) 94:54−60. doi: 10.1016/j.molimm.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 122. Braster R, Bögels M, Benonisson H, Wuhrer M, Plomp R, Bentlage AEH, et al. Afucosylated IgG Targets Fcγriv for Enhanced Tumor Therapy in Mice. Cancers (2021) 13(10):2372. doi: 10.3390/cancers13102372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Crowley AR, Osei-Owusu NY, Dekkers G, Gao W, Wuhrer M, Magnani DM, et al. Biophysical Evaluation of Rhesus Macaque Fc Gamma Receptors Reveals Similar IgG Fc Glycoform Preferences to Human Receptors. Front Immunol (2021) 12:754710. doi: 10.3389/fimmu.2021.754710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kaur H. Characterization of Glycosylation in Monoclonal Antibodies and its Importance in Therapeutic Antibody Development. Crit Rev Biotechnol (2021) 41(2):300−15. doi: 10.1080/07388551.2020.1869684 [DOI] [PubMed] [Google Scholar]
- 125. Tobinai K, Klein C, Oya N, Fingerle-Rowson G. A Review of Obinutuzumab (GA101), a Novel Type II Anti-CD20 Monoclonal Antibody, for the Treatment of Patients With B-Cell Malignancies. Adv Ther (2017) 34(2):324−56. doi: 10.1007/s12325-016-0451-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Alduaij W, Ivanov A, Honeychurch J, Cheadle EJ, Potluri S, Lim SH, et al. Novel Type II Anti-CD20 Monoclonal Antibody (GA101) Evokes Homotypic Adhesion and Actin-Dependent, Lysosome-Mediated Cell Death in B-Cell Malignancies. Blood (2011) 117(17):4519−29. doi: 10.1182/blood-2010-07-296913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic Activity of Humanized Anti-CD20 Monoclonal Antibody and Polymorphism in IgG Fc Receptor FcgammaRIIIa Gene. Blood (2002) 99(3):754−8. doi: 10.1182/blood.v99.3.754 [DOI] [PubMed] [Google Scholar]
- 128. Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G Fragment C Receptor Polymorphisms and Clinical Efficacy of Trastuzumab-Based Therapy in Patients With HER-2/Neu-Positive Metastatic Breast Cancer. J Clin Oncol (2008) 26(11):1789−96. doi: 10.1200/JCO.2007.14.8957 [DOI] [PubMed] [Google Scholar]
- 129. Roca L, Diéras V, Roché H, Lappartient E, Kerbrat P, Cany L, et al. Correlation of HER2, FCGR2A, and FCGR3A Gene Polymorphisms With Trastuzumab Related Cardiac Toxicity and Efficacy in a Subgroup of Patients From UNICANCER-PACS 04 Trial. Breast Cancer Res Treat (2013) 139(3):789−800. doi: 10.1007/s10549-013-2587-x [DOI] [PubMed] [Google Scholar]
- 130. de Haan N, Reiding KR, Driessen G, van der Burg M, Wuhrer M. Changes in Healthy Human IgG Fc-Glycosylation After Birth and During Early Childhood. J Proteome Res (2016) 15(6):1853−61. doi: 10.1021/acs.jproteome.6b00038 [DOI] [PubMed] [Google Scholar]
- 131. Pucic M, Muzinic A, Novokmet M, Skledar M, Pivac N, Lauc G, et al. Changes in Plasma and IgG N-Glycome During Childhood and Adolescence. Glycobiology (2012) 22(7):975−82. doi: 10.1093/glycob/cws062 [DOI] [PubMed] [Google Scholar]
- 132. Fettke F, Schumacher A, Canellada A, Toledo N, Bekeredjian-Ding I, Bondt A, et al. Maternal and Fetal Mechanisms of B Cell Regulation During Pregnancy: Human Chorionic Gonadotropin Stimulates B Cells to Produce IL-10 While Alpha-Fetoprotein Drives Them Into Apoptosis. Front Immunol (2016) 7:495. doi: 10.3389/fimmu.2016.00495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wojcik I, Schmidt DE, de Neef LA, Rab MAE, Meek B, de Weerdt O, et al. A Functional Spleen Contributes to Afucosylated IgG in Humans. Sci Rep (2021) 11(1):24045. doi: 10.1038/s41598-021-03196-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Huffman JE, Knezevic A, Vitart V, Kattla J, Adamczyk B, Novokmet M, et al. Polymorphisms in B3GAT1, SLC9A9 and MGAT5 are Associated With Variation Within the Human Plasma N-Glycome of 3533 European Adults. Hum Mol Genet (2011) 20(24):5000−11. doi: 10.1093/hmg/ddr414 [DOI] [PubMed] [Google Scholar]
- 135. Klarić L, Tsepilov YA, Stanton CM, Mangino M, Sikka TT, Esko T, et al. Glycosylation of Immunoglobulin G is Regulated by a Large Network of Genes Pleiotropic With Inflammatory Diseases. Sci Adv (2020) 6(8):eaax0301. doi: 10.1126/sciadv.aax0301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Landini A, Trbojević-Akmačić I, Navarro P, Tsepilov YA, Sharapov SZ, Vučković F, et al. Genetic Regulation of Post-Translational Modification of Two Distinct Proteins. Nat Commun (2022) 13(1):1586. doi: 10.1038/s41467-022-29189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lauc G, Huffman JE, Pučić M, Zgaga L, Adamczyk B, Mužinić A, et al. Loci Associated With N-Glycosylation of Human Immunoglobulin G Show Pleiotropy With Autoimmune Diseases and Haematological Cancers. PLoS Genet (2013) 9(1):e1003225. doi: 10.1371/journal.pgen.1003225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sharapov SZ, Tsepilov YA, Klaric L, Mangino M, Thareja G, Shadrina AS, et al. Defining the Genetic Control of Human Blood Plasma N-Glycome Using Genome-Wide Association Study. Hum Mol Genet (2019) 28(12):2062−77. doi: 10.1093/hmg/ddz054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Shen X, Klarić L, Sharapov S, Mangino M, Ning Z, Wu D, et al. Multivariate Discovery and Replication of Five Novel Loci Associated With Immunoglobulin G N-Glycosylation. Nat Commun (2017) 8(1):447. doi: 10.1038/s41467-017-00453-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wahl A, van den Akker E, Klaric L, Štambuk J, Benedetti E, Plomp R, et al. Genome-Wide Association Study on Immunoglobulin G Glycosylation Patterns. Front Immunol (2018) 9:277. doi: 10.3389/fimmu.2018.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Matsumoto K, Shimizu C, Arao T, Andoh M, Katsumata N, Kohno T, et al. Identification of Predictive Biomarkers for Response to Trastuzumab Using Plasma FUCA Activity and N-Glycan Identified by MALDI-TOF-Ms. J Proteome Res (2009) 8(2):457−62. doi: 10.1021/pr800655p [DOI] [PubMed] [Google Scholar]
- 142. Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, et al. Natural Variation in Fc Glycosylation of HIV-Specific Antibodies Impacts Antiviral Activity. J Clin Invest (2013) 123(5):2183−92. doi: 10.1172/JCI65708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Viinikangas T, Khosrowabadi E, Kellokumpu S. N-Glycan Biosynthesis: Basic Principles and Factors Affecting Its Outcome. Exp Suppl 2012 (2021) 112:237−57. doi: 10.1007/978-3-030-76912-3_7 [DOI] [PubMed] [Google Scholar]
- 144. Wong MY, Chen K, Antonopoulos A, Kasper BT, Dewal MB, Taylor RJ, et al. XBP1s Activation can Globally Remodel N-Glycan Structure Distribution Patterns. Proc Natl Acad Sci U S A (2018) 115(43):E10089−98. doi: 10.1073/pnas.1805425115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Hagelkruys A, Wirnsberger G, Stadlmann J, Wöhner M, Horrer M, Vilagos B, et al. A Crucial Role for Jagunal Homolog 1 in Humoral Immunity and Antibody Glycosylation in Mice and Humans. J Exp Med (2021) 218(1):e20200559. doi: 10.1084/jem.20200559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kapur R, Della Valle L, Sonneveld M, Hipgrave Ederveen A, Visser R, Ligthart P, et al. Low Anti-RhD IgG-Fc-Fucosylation in Pregnancy: A New Variable Predicting Severity in Haemolytic Disease of the Fetus and Newborn. Br J Haematol (2014) 166(6):936−45. doi: 10.1111/bjh.12965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bruggeman CW, Dekkers G, Bentlage AEH, Treffers LW, Nagelkerke SQ, Lissenberg-Thunnissen S, et al. Enhanced Effector Functions Due to Antibody Defucosylation Depend on the Effector Cell Fcγ Receptor Profile. J Immunol (2017) 199(1):204−11. doi: 10.4049/jimmunol.1700116 [DOI] [PubMed] [Google Scholar]
- 148. Sibéril S, de Romeuf C, Bihoreau N, Fernandez N, Meterreau J-L, Regenman A, et al. Selection of a Human Anti-RhD Monoclonal Antibody for Therapeutic Use: Impact of IgG Glycosylation on Activating and Inhibitory Fc Gamma R Functions. Clin Immunol (2006) 118(2−3):170−9. doi: 10.1016/j.clim.2005.10.008 [DOI] [PubMed] [Google Scholar]
- 149. Oepkes D, van Kamp IL, Simon MJ, Mesman J, Overbeeke MA, Kanhai HH. Clinical Value of an Antibody-Dependent Cell-Mediated Cytotoxicity Assay in the Management of Rh D Alloimmunization. Am J Obstet Gynecol (2001) 184(5):1015−20. doi: 10.1067/mob.2001.112970 [DOI] [PubMed] [Google Scholar]
- 150. Sonneveld ME, Koelewijn J, de Haas M, Admiraal J, Plomp R, Koeleman CAM, et al. Antigen Specificity Determines Anti-Red Blood Cell IgG-Fc Alloantibody Glycosylation and Thereby Severity of Haemolytic Disease of the Fetus and Newborn. Br J Haematol (2017) 176(4):651−60. doi: 10.1111/bjh.14438 [DOI] [PubMed] [Google Scholar]
- 151. Brojer E, Husebekk A, Dębska M, Uhrynowska M, Guz K, Orzińska A, et al. Fetal/Neonatal Alloimmune Thrombocytopenia: Pathogenesis, Diagnostics and Prevention. Arch Immunol Ther Exp (Warsz) (2016) 64(4):279−90. doi: 10.1007/s00005-015-0371-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Wuhrer M, Porcelijn L, Kapur R, Koeleman CAM, Deelder AM, de Haas M, et al. Regulated Glycosylation Patterns of IgG During Alloimmune Responses Against Human Platelet Antigens. J Proteome Res (2009) 8(2):450−6. doi: 10.1021/pr800651j [DOI] [PubMed] [Google Scholar]
- 153. Kjeldsen-Kragh J, Bengtsson J. Fetal and Neonatal Alloimmune Thrombocytopenia-New Prospects for Fetal Risk Assessment of HPA-1a-Negative Pregnant Women. Transfus Med Rev (2020) 34(4):270−6. doi: 10.1016/j.tmrv.2020.09.004 [DOI] [PubMed] [Google Scholar]
- 154. Sonneveld ME, Natunen S, Sainio S, Koeleman CAM, Holst S, Dekkers G, et al. Glycosylation Pattern of Anti-Platelet IgG is Stable During Pregnancy and Predicts Clinical Outcome in Alloimmune Thrombocytopenia. Br J Haematol (2016) 174(2):310−20. doi: 10.1111/bjh.14053 [DOI] [PubMed] [Google Scholar]
- 155. Ercan A, Cui J, Chatterton DEW, Deane KD, Hazen MM, Brintnell W, et al. Aberrant IgG Galactosylation Precedes Disease Onset, Correlates With Disease Activity, and is Prevalent in Autoantibodies in Rheumatoid Arthritis. Arthritis Rheum (2010) 62(8):2239−48. doi: 10.1002/art.27533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Huang C, Liu Y, Wu H, Sun D, Li Y. Characterization of IgG Glycosylation in Rheumatoid Arthritis Patients by MALDI-TOF-MSn and Capillary Electrophoresis. Anal Bioanal Chem (2017) 409(15):3731−9. doi: 10.1007/s00216-017-0302-1 [DOI] [PubMed] [Google Scholar]
- 157. Flevaris K, Kontoravdi C. Immunoglobulin G N-Glycan Biomarkers for Autoimmune Diseases: Current State and a Glycoinformatics Perspective. Int J Mol Sci (2022) 23(9):5180. doi: 10.3390/ijms23095180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Martin TC, Šimurina M, Ząbczyńska M, Martinic Kavur M, Rydlewska M, Pezer M, et al. Decreased Immunoglobulin G Core Fucosylation, A Player in Antibody-Dependent Cell-Mediated Cytotoxicity, is Associated With Autoimmune Thyroid Diseases. Mol Cell Proteomics (2020) 19(5):774−92. doi: 10.1074/mcp.RA119.001860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Wang W, Xu X, Huang C, Gao C. N-Glycan Profiling Alterations of Serum and Immunoglobulin G in Immune Thrombocytopenia. J Clin Lab Anal (2022) 36(2):e24201. doi: 10.1002/jcla.24201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Plomp R, Ruhaak LR, Uh H-W, Reiding KR, Selman M, Houwing-Duistermaat JJ, et al. Subclass-Specific IgG Glycosylation is Associated With Markers of Inflammation and Metabolic Health. Sci Rep (2017) 7(1):12325. doi: 10.1038/s41598-017-12495-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Alter G, Ottenhoff THM, Joosten SA. Antibody Glycosylation in Inflammation, Disease and Vaccination. Semin Immunol (2018) 39:102−10. doi: 10.1016/j.smim.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Dekkers G, Rispens T, Vidarsson G. Novel Concepts of Altered Immunoglobulin G Galactosylation in Autoimmune Diseases. Front Immunol (2018) 9:553. doi: 10.3389/fimmu.2018.00553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Seeling M, Brückner C, Nimmerjahn F. Differential Antibody Glycosylation in Autoimmunity: Sweet Biomarker or Modulator of Disease Activity? Nat Rev Rheumatol (2017) 13(10):621−30. doi: 10.1038/nrrheum.2017.146 [DOI] [PubMed] [Google Scholar]
- 164. Mimura Y, Mimura-Kimura Y, Saldova R, Rudd PM, Jefferis R. Enhanced Immunomodulatory Effect of Intravenous Immunoglobulin by Fc Galactosylation and Nonfucosylation. Front Immunol (2022) 13:818382. doi: 10.3389/fimmu.2022.818382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Schwab I, Nimmerjahn F. Intravenous Immunoglobulin Therapy: How Does IgG Modulate the Immune System? Nat Rev Immunol (2013) 13(3):176−89. doi: 10.1038/nri3401 [DOI] [PubMed] [Google Scholar]
- 166. Keeler SP, Fox JM. Requirement of Fc-Fc Gamma Receptor Interaction for Antibody-Based Protection Against Emerging Virus Infections. Viruses (2021) 13(6):1037. doi: 10.3390/v13061037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Khandia R, Munjal A, Dhama K, Karthik K, Tiwari R, Malik YS, et al. Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection. Front Immunol (2018) 9:597. doi: 10.3389/fimmu.2018.00597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Sicca F, Neppelenbroek S, Huckriede A. Effector Mechanisms of Influenza-Specific Antibodies: Neutralization and Beyond. Expert Rev Vaccines (2018) 17(9):785−95. doi: 10.1080/14760584.2018.1516553 [DOI] [PubMed] [Google Scholar]
- 169. Bournazos S, Ravetch JV. Anti-Retroviral Antibody Fcγr-Mediated Effector Functions. Immunol Rev (2017) 275(1):285−95. doi: 10.1111/imr.12482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bernard NF, Kiani Z, Tremblay-McLean A, Kant SA, Leeks CE, Dupuy FP. Natural Killer (NK) Cell Education Differentially Influences HIV Antibody-Dependent NK Cell Activation and Antibody-Dependent Cellular Cytotoxicity. Front Immunol (2017) 8:1033. doi: 10.3389/fimmu.2017.01033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, Mehlhop E, et al. Lethal Antibody Enhancement of Dengue Disease in Mice is Prevented by Fc Modification. PLoS Pathog (2010) 6(2):e1000790. doi: 10.1371/journal.ppat.1000790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Bournazos S, Gupta A, Ravetch JV. The Role of IgG Fc Receptors in Antibody-Dependent Enhancement. Nat Rev Immunol (2020) 20(10):633−43. doi: 10.1038/s41577-020-00410-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Halstead SB, Mahalingam S, Marovich MA, Ubol S, Mosser DM. Intrinsic Antibody-Dependent Enhancement of Microbial Infection in Macrophages: Disease Regulation by Immune Complexes. Lancet Infect Dis (2010) 10(10):712−22. doi: 10.1016/S1473-3099(10)70166-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Nanaware N, Banerjee A, Mullick Bagchi S, Bagchi P, Mukherjee A. Dengue Virus Infection: A Tale of Viral Exploitations and Host Responses. Viruses (2021) 13(10):1967. doi: 10.3390/v13101967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Larsen MD, de Graaf EL, Sonneveld ME, Plomp HR, Nouta J, Hoepel W, et al. Afucosylated IgG Characterizes Enveloped Viral Responses and Correlates With COVID-19 Severity. Science (2021) 371(6532):eabc8378. doi: 10.1126/science.abc8378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Chakraborty S, Gonzalez J, Edwards K, Mallajosyula V, Buzzanco AS, Sherwood R, et al. Proinflammatory IgG Fc Structures in Patients With Severe COVID-19. Nat Immunol (2021) 22(1):67−73. doi: 10.1038/s41590-020-00828-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Hoepel W, Chen H-J, Geyer CE, Allahverdiyeva S, Manz XD, de Taeye SW, et al. High Titers and Low Fucosylation of Early Human Anti–SARS-CoV-2 IgG Promote Inflammation by Alveolar Macrophages. Sci Transl Med (2021) 13(596):eabf8654. doi: 10.1126/scitranslmed.abf8654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Pongracz T, Nouta J, Wang W, van Meijgaarden KE, Linty F, Vidarsson G, et al. Immunoglobulin G1 Fc Glycosylation as an Early Hallmark of Severe COVID-19. EBioMedicine (2022) 78:103957. doi: 10.1016/j.ebiom.2022.103957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Schwedler C, Grzeski M, Kappert K, Rust J, Heymann G, Hoppe B, et al. Coronavirus Disease 2019-Related Alterations of Total and Anti-Spike IgG Glycosylation in Relation to Age and Anti-Spike IgG Titer. Front Microbiol (2022) 13:775186. doi: 10.3389/fmicb.2022.775186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Bournazos S, Vo HTM, Duong V, Auerswald H, Ly S, Sakuntabhai A, et al. Antibody Fucosylation Predicts Disease Severity in Secondary Dengue Infection. Science (2021) 372(6546):1102−5. doi: 10.1126/science.abc7303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Thulin NK, Brewer RC, Sherwood R, Bournazos S, Edwards KG, Ramadoss NS, et al. Maternal Anti-Dengue IgG Fucosylation Predicts Susceptibility to Dengue Disease in Infants. Cell Rep (2020) 31(6):107642. doi: 10.1016/j.celrep.2020.107642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Wang TT, Sewatanon J, Memoli MJ, Wrammert J, Bournazos S, Bhaumik SK, et al. IgG Antibodies to Dengue Enhanced for Fcγriiia Binding Determine Disease Severity. Science (2017) 355(6323):395−8. doi: 10.1126/science.aai8128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Larsen MD, Lopez-Perez M, Dickson EK, Ampomah P, Tuikue Ndam N, Nouta J, et al. Afucosylated Plasmodium Falciparum-Specific IgG is Induced by Infection But Not by Subunit Vaccination. Nat Commun (2021) 12(1):5838. doi: 10.1038/s41467-021-26118-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Chakraborty S, Gonzalez JC, Sievers BL, Mallajosyula V, Chakraborty S, Dubey M, et al. Early non-Neutralizing, Afucosylated Antibody Responses are Associated With COVID-19 Severity. Sci Transl Med (2022) 14(635):eabm7853. doi: 10.1126/scitranslmed.abm7853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Adjobimey T, Hoerauf A. Distinct N-Linked Immunoglobulin G Glycosylation Patterns Are Associated With Chronic Pathology and Asymptomatic Infections in Human Lymphatic Filariasis. Front Immunol (2022) 13:790895. doi: 10.3389/fimmu.2022.790895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Gutiérrez-Huante K, Salinas-Marín R, Mora-Montes HM, Gonzalez RA, Martínez-Duncker I. Human Adenovirus Type 5 Increases Host Cell Fucosylation and Modifies Ley Antigen Expression. Glycobiology (2019) 29(6):469−78. doi: 10.1093/glycob/cwz017 [DOI] [PubMed] [Google Scholar]
- 187. Sun Y, Zhou J, Jiang Y. Negative Regulation and Protective Function of Natural Killer Cells in HIV Infection: Two Sides of a Coin. Front Immunol (2022) 13:842831. doi: 10.3389/fimmu.2022.842831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Cao J, Wang L, Yu C, Wang K, Wang W, Yan J, et al. Development of an Antibody-Dependent Cellular Cytotoxicity Reporter Assay for Measuring Anti-Middle East Respiratory Syndrome Antibody Bioactivity. Sci Rep (2020) 10(1):16615. doi: 10.1038/s41598-020-73960-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Oswald WB, Geisbert TW, Davis KJ, Geisbert JB, Sullivan NJ, Jahrling PB, et al. Neutralizing Antibody Fails to Impact the Course of Ebola Virus Infection in Monkeys. PLoS Pathog (2007) 3(1):e9. doi: 10.1371/journal.ppat.0030009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Liu Q, Fan C, Li Q, Zhou S, Huang W, Wang L, et al. Antibody-Dependent-Cellular-Cytotoxicity-Inducing Antibodies Significantly Affect the Post-Exposure Treatment of Ebola Virus Infection. Sci Rep (2017) 7:45552. doi: 10.1038/srep45552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Hou H, Yang H, Liu P, Huang C, Wang M, Li Y, et al. Profile of Immunoglobulin G N-Glycome in COVID-19 Patients: A Case-Control Study. Front Immunol (2021) 12:748566. doi: 10.3389/fimmu.2021.748566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Reis CA, Tauber R, Blanchard V. Glycosylation is a Key in SARS-CoV-2 Infection. J Mol Med Berl Ger (2021) 99(8):1023−31. doi: 10.1007/s00109-021-02092-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Roßkopf S, Eichholz KM, Winterberg D, Diemer KJ, Lutz S, Münnich IA, et al. Enhancing CDC and ADCC of CD19 Antibodies by Combining Fc Protein-Engineering With Fc Glyco-Engineering. Antibodies (2020) 9(4):63. doi: 10.3390/antib9040063 [DOI] [PMC free article] [PubMed] [Google Scholar]