Abstract
HIV reverse transcriptase (RT) inhibitors are the important components of highly active antiretroviral therapies (HAARTs) for anti-HIV treatment and pre-exposure prophylaxis in clinical practice. Many RT inhibitors and their combination regimens have been approved in the past ten years, but a review on their drug discovery, pharmacology, and clinical efficacy is lacking. Here, we provide a comprehensive review of RT inhibitors (tenofovir alafenamide, rilpivirine, doravirine, dapivirine, azvudine and elsulfavirine) approved in the past decade, regarding their drug discovery, pharmacology, and clinical efficacy in randomized controlled trials. Novel RT inhibitors such as islatravir, MK-8504, MK-8507, MK8583, IQP-0528, and MIV-150 will be also highlighted. Future development may focus on the new generation of novel antiretroviral inhibitors with higher bioavailability, longer elimination half-life, more favorable side-effect profiles, fewer drug–drug interactions, and higher activities against circulating drug-resistant strains.
Keywords: HIV treatment, HAART, NRTI, NNRTI, Clinical efficacy
Abbreviations: 3TC, (−)-2′,3′-dideoxy-3′-thiacytidine (common name, lamivudine); ABC, abacavir; ATV, atazanavir; AZT, 3′-azido-3′-deoxy-thymidine (common name, zidovudine); BIC, bictegravir; CAB, cabotegravir; CC50, the 50% cytotoxic concentration; COBI, cobicistat; DOR, doravirine; DPV, dapivirine; DRV, darunavir; DTG, dolutegravir; EACS, European AIDS Clinical Society; EC50, half maximal effective concentration; EFV, efavirenz; ESV, elsulfavirine; EVG, elvitegravir; F, bioavailability; FDA, US Food and Drug Administration; FTC, (−)-2′,3′-dideoxy-5-fluoro-3′-thiacytidine (common name, emtricitabine); HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; IAS-USA, International Antiviral Society-USA; IC50, half maximal inhibitory concentration; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; MSM, men who have sex with men; RPV, rilpivirine; t1/2, elimination half-life; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate
Graphical abstract
Approved anti-HIV regimens in the past decade. Most regimens are composed of two nucleoside/nucleotide reverse transcriptase inhibitors plus one integrase inhibitor, one non-nucleoside reverse transcriptase inhibitor, or one protease inhibitor.
1. Introduction
In 2020, 37.7 million people were living with HIV infections according to the recent update from The Joint United Nations Programme on HIV/AIDS (www.unaids.org). Although HIV cure is still unavailable, antiretroviral therapies were accessible to 27.5 million patients living with HIV in 2020, and nearly 66% of patients living with HIV had virological suppression (www.unaids.org). Due to the large population of new cases (1.5 million) and untreated patients (10.2 million), it remains important to develop effective therapies to control HIV infections and transmission.
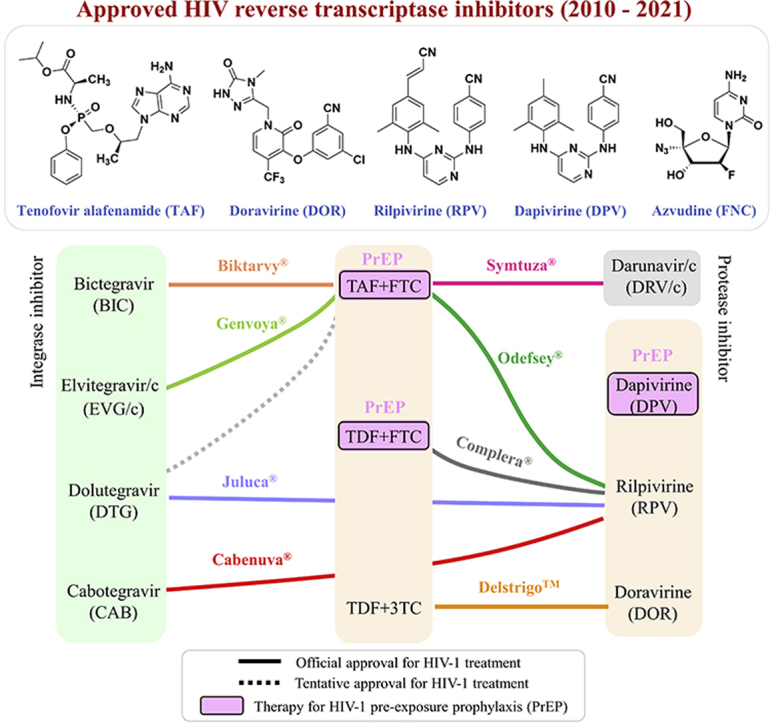
Highly active antiretroviral therapy (HAART) is the current standard of care for the management of HIV infections in clinical practice. To interrupt multiple steps of the HIV life cycle, HAARTs are mostly comprised of three compounds (Fig. 1), including two nucleos(t)ide reverse transcriptase inhibitors (NRTIs) plus one non-nucleoside reverse transcriptase inhibitor (NNRTI), one integrase inhibitor, or one protease inhibitor (boosted by ritonavir or cobicistat)1, 2, 3, 4, 5, 6, 7. As shown in Fig. 2, NRTIs and NNRTIs target different binding pockets near the catalytic site of HIV reverse transcriptase (RT) to block the viral transcription of a double-stranded viral DNA genome from a single-stranded viral RNA genome1,4,5,8. NNRTIs can inhibit the reverse transcriptase initiation complex even during the early viral transcription9. Integrase inhibitors such as bictegravir (BIC), dolutegravir (DTG), and elvitegravir (EVG) target the catalytic site of HIV integrase to inhibit the viral integration of proviral DNA into host genomes1. Protease inhibitors such as darunavir (DRV) compete with natural substrates of HIV protease to inhibit the protease-mediated cleavage of gag and gagpol precursors1. Among these four drug classes, NRTIs are the key backbones of combination regimens according to the recent update of the International Antiviral Society-USA (IAS-USA) guidelines10, the European AIDS Clinical Society (EACS) guidelines11, the NIH HIV/AIDS guidelines (https://hivinfo.nih.gov), and the WHO guidelines on HIV/AIDS (www.who.int).
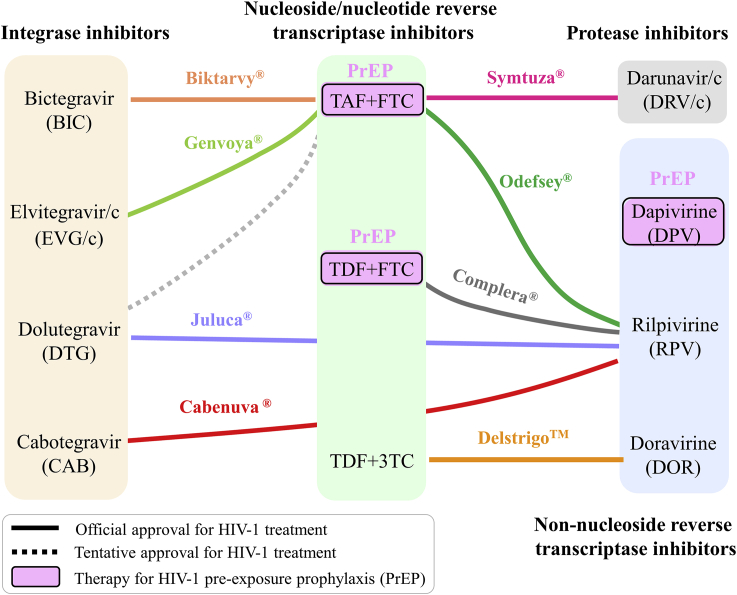
Figure 1.
Approved antiretroviral regimens in the past decade (2010 to present). Most combination regimens are composed of two HIV nucleoside/nucleotide reverse transcriptase inhibitors plus one integrase inhibitor, one non-nucleoside reverse transcriptase inhibitor, or one protease inhibitor. Dapivirine vaginal ring 25 mg was approved by the European Medicines Agency on 24 July 2020, while the other regimens have been (tentatively) approved by the FDA.
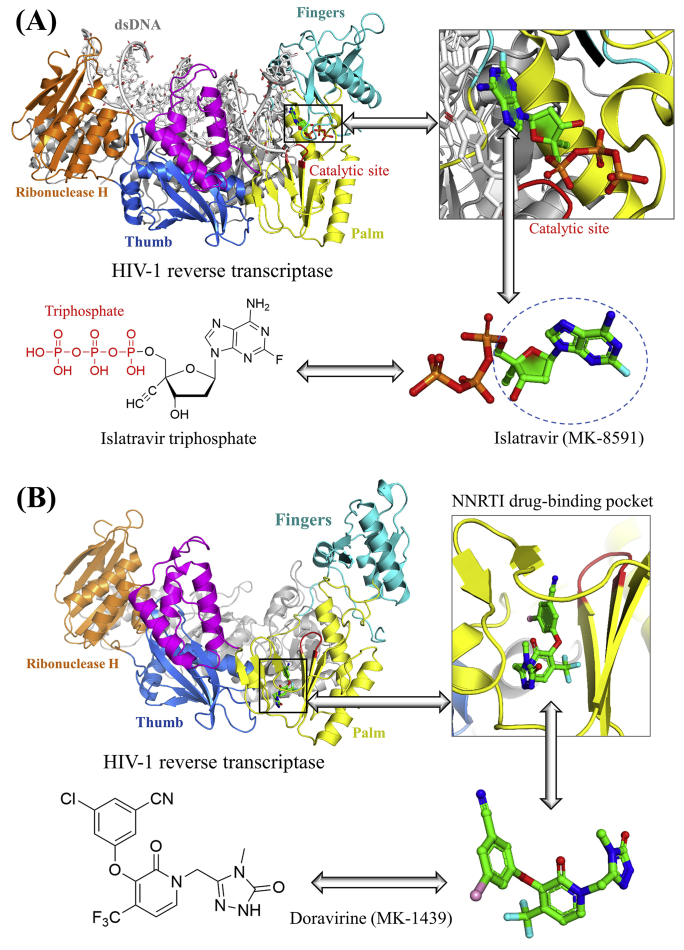
Figure 2.
Structural basis of NRTI (islatravir) and NNRTI (doravirine). (A) Chemical and 3D structures of islatravir. The drug binding pocket of islatravir is highlighted in HIV-1 reverse transcriptase (PDB code: 5J2M). Islatravir triphosphate interferes with the translocation of HIV reverse transcription on the nucleic acid substrate to slow down the viral DNA synthesis. (B) Chemical and 3D structures of doravirine. Drug binding pocket of doravirine is highlighted at the palm domain of the P66 subunit in HIV-1 reverse transcriptase (PDB code: 4NCG). PDB codes were obtained from the RCSB protein data bank (www.rcsb.org/). Protein 3D structures were visualized by PyMOL V1.7 (www.pymol.org/).
From 1987 to 2010, eight NRTIs and four NNRTIs were officially approved to combat HIV infections. In the drug class of NRTIs, the US Food and Drug Administration (FDA) approved zidovudine (AZT) in March 1987, followed by didanosine (ddI) in October 1991, zalcitabine (ddC) in June 1992, stavudine (d4T) in June 1994, lamivudine (3TC) in November 1995, abacavir (ABC) in December 1998, tenofovir disoproxil fumarate (TDF) in October 2001, and emtricitabine (FTC) in July 2003. In the drug class of NNRTIs, nevirapine (NVP), delavirdine (DLV), efavirenz (EFV), and etravirine (ETR) were approved by the FDA4,12, 13, 14. The “old” generation of the preceding RT inhibitors is often challenged by the emergence of drug resistance and adverse events. For example, (i) zalcitabine, stavudine, and delavirdine were discontinued because of severe adverse effects, inconvenient administration (three times daily), and low genetic barrier to resistance1. (ii) Zidovudine, didanosine, stavudine, and nevirapine are no longer recommended due to their toxicity, low efficacy, and severe drug resistance10,11. (iii) Abacavir-based therapies may increase the risk of cardiovascular diseases compared with abacavir-free regimens15. (iv) Efavirenz is associated with a high incidence of neuropsychiatric complications16. (v) TDF may cause renal impairment and reduce bone mineral density17. Drug discovery, drug resistance, and pharmacological features of the above NRTIs and NNRTIs have been reviewed by previous studies1, 2, 3, 4, 5, 6,8,18, 19, 20, 21, 22, 23, 24.
From 2010 to October 2021, the FDA approved three RT inhibitors: rilpivirine (RPV, Edurant®, approval date: 2011-05-20), tenofovir alafenamide (TAF, Vemlidy®, approval date: 2016-11-10), and doravirine (DOR, Pifeltro™, approval date: 2018-08-30). These RT inhibitors are further integrated into 10 fixed-dose combination regimens that have been (tentatively) approved by the FDA (Table 1). In addition to these FDA-approved drugs, elsulfavirine (Elpida®) was approved by the Russian Ministry of Health in June 2017. A vaginal ring containing dapivirine 25 mg was approved by the European Medicines Agency in July 2020. On 21 July 2021, azvudine was conditionally approved by the National Medical Products Administration in China.
Table 1.
List of approved HIV RT inhibitors and their regimens in the past decade.
| Antiretroviral drug or regimen | Usage | Trade name | Region | First approval |
|---|---|---|---|---|
| Tenofovir alafenamide (TAF) 25 mg | With other drugs | Vemlidy® | US, EU | 2016-11-10 |
| TAF 25 mg + Emtricitabine (FTC) 200 mg | Pre-exposure prophylaxis | Descovy® | US, EU | 2019-10-03b |
| TAF 25 mg + FTC 200 mg + RPV 25 mg | Complete regimen | Odefsey® | US, EU | 2016-03-01 |
| TAF 25 mg + FTC 200 mg + BIC 50 mg | Complete regimen | Biktarvy® | US, EU | 2018-02-07 |
| TAF 25 mg + FTC 200 mg + DTG 50 mg | Complete regimen | Acriptega®a | USa, India | 2020-12-04a |
| TAF 10 mg + FTC 200 mg + EVG 150 mg + COBI 150 mg | Complete regimen | Genvoya® | US, EU | 2015-11-05 |
| TAF 10 mg + FTC 200 mg + DRV 800 mg + COBI 150 mg | Complete regimen | Symtuza® | US, EU | 2018-07-17 |
| Rilpivirine (RPV) 25 mg | With other drugs | Edurant® | US, EU | 2011-05-20 |
| RPV 25 mg + TDF 300 mg + FTC 200 mg | Complete regimen | Complera® | US, EU | 2011-08-10 |
| RPV 25 mg + DTG 50 mg | Complete regimen | Juluca® | US, EU | 2017-11-21 |
| RPV 300 mg/mL + CAB 200 mg/mL | Complete regimen | Cabenuva® | US, EU | 2021-01-21 |
| Doravirine (DOR) 100 mg | With other drugs | Pifeltro™ | US, EU | 2018-08-30 |
| DOR 100 mg + TDF 300 mg + 3TC 300 mg | Complete regimen | Delstrigo™ | US, EU | 2018-08-30 |
| Elsulfavirine 20 mg | With other drugs | Elpida® | Russia | 2017-06-30 |
| Dapivirine 25 mg vaginal ring | Pre-exposure prophylaxis | – | EU | 2020-07-24 |
| Azvudine 3 mg | With other drugs | Azvudine Tablet | Chinac | 2021-07-21 |
The TAF + FTC + DTG regimen is marked as the trade name of Acriptega® in India. The FDA granted the tentative approval of dolutegravir, emtricitabine, and tenofovir alafenamide tablets on 2020-12-04.
Descovy® was approved by the FDA for HIV-1 treatment and pre-exposure prophylaxis in 2016 and 2019, respectively.
Azvudine was conditionally approved in China.
To the best of our knowledge, no review has comprehensively characterized the drug discovery, clinical efficacy, and pharmacological profiles of newly-approved HIV RT inhibitors in the past decade. Based on a large body of randomized clinical trials, this review will summarize the clinical efficacy and pharmacological profiles of approved HIV RT inhibitors (tenofovir alafenamide, dapivirine, rilpivirine, doravirine, azvudine, elsulfavirine) and their combination regimens (Descovy®, Biktarvy®, Genvoya® Acriptega®, Symtuza®, Complera®, Juluca®, Cabenuva®, Delstrigo™).
This review is organized as follows. First, our strategies for literature search and selection are described. Second, for each approved RT inhibitor, we will highlight the (i) drug discovery; (ii) antiviral activity, drug resistance, and pharmacokinetics; and (iii) clinical efficacy and safety of their approved combination regimens in randomized clinical trials. Third, novel regimens will be highlighted. Our drug movies and teaching slides that highlight HIV RT inhibitors are shared online (www.virusface.com).
2. Search strategy and selection criteria
To collect relevant literature, we searched PubMed, Google Scholar, and Journal websites using the keywords of individual drug names. We extracted clinical trial data from ClinicalTrials.gov (https://clinicaltrials.gov), drug labeling resources from the FDA database (www.accessdata.fda.gov), and drug resistance mutations from the IAS-USA guidelines25. To summarize the clinical efficacy of approved combination regimens, we collected clinical studies that fulfilled four conditions: (i) randomized, controlled clinical trials must be at the stage of phases 2, 3, and/or 4; (ii) studies recruited adults in the absence of pregnant women; (iii) recruited adults were confirmed with HIV infections without other infectious diseases (e.g., tuberculosis, HBV26, HCV27,28) or non-communicable diseases such as liver/kidney impairment; (iv) patients were treated with the exact dosage of approved regimens according to the drug labeling; and (v) viral suppression of plasma HIV-1 RNA <50 copies/mL was measured at Week 48.
3. Tenofovir alafenamide (Vemlidy®)
In 2015, the FDA approved Genvoya®―the first combination regimen that contains tenofovir alafenamide (GS-7340) for the treatment of HIV-1 infections. Since then, five TAF-based combination regimens (Genvoya®, Odefsey®, Biktarvy®, Symtuza®, Descovy®) have been approved by the FDA and the European Medicines Agency (Table 1). The tentative approval of dolutegravir, emtricitabine, and tenofovir alafenamide tablets was granted by the FDA in December 2020, and this regimen is currently marked with the trade name of Acriptega® in India.
3.1. Drug discovery
As shown in Fig. 3A, the discovery of tenofovir alafenamide began with the synthesis of an adenosine nucleoside analogue called tenofovir [(R)-9-(2-phosphonylmethoxypropyl)adenine] at the Rega Institute in Belgium29. Tenofovir showed potent activities against the replications of HIV-1 (EC50: 5.9 ± 0.45 μmol/L)29, HIV-2 (EC50: 4.9 ± 0.45 μmol/L)29, and HBV (EC50: 6.5 ± 1.1 μmol/L) in cell cultures30. However, tenofovir carries negative charges on the phosphonate moiety, resulting in poor cellular permeability and limited oral bioavailability31,32. Many prodrugs of tenofovir such as TDF were subsequently developed to enhance the permeability across the intestinal wall33. For instance, phosphoramidate prodrugs of tenofovir could be designed by the “ProTide” strategy that offers the efficient intracellular delivery of monophosphates and monophosphonates of nucleoside analogues across the cell membrane by passive diffusion34,35.
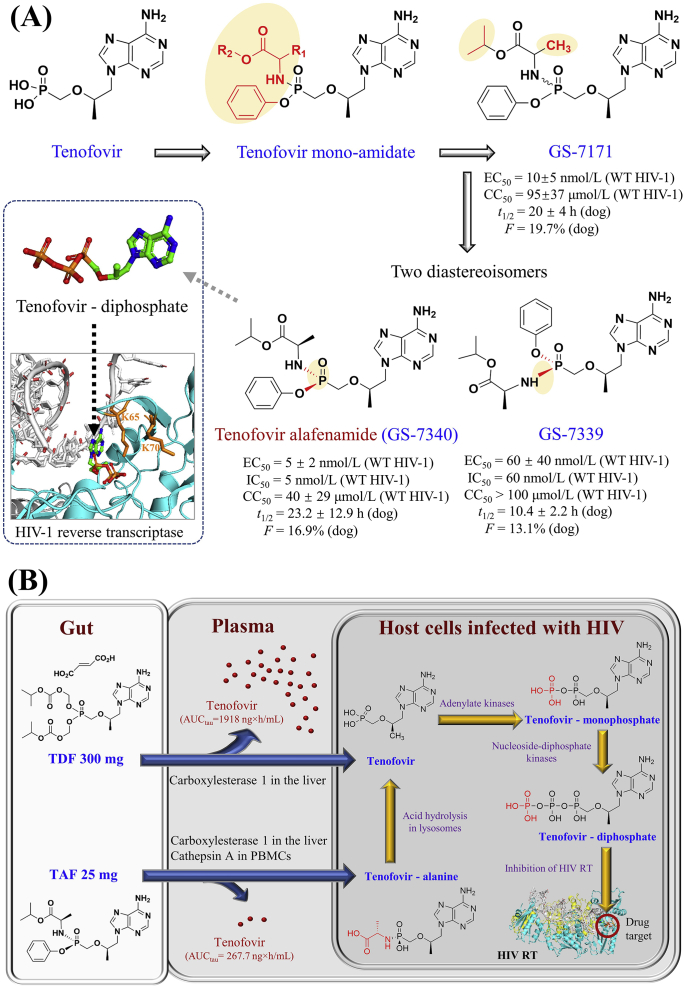
Figure 3.
Discovery and metabolic pathway of tenofovir alafenamide. (A) Discovery of tenofovir alafenamide. Anti-HIV-1 parameters and pharmacokinetic values are extracted from38. (B) Metabolic pathways of TDF 300 mg and TAF 25 mg from the gut to the blood plasma and lymphoid cells infected with HIV. At the steady-state, the plasma exposure of tenofovir at Day 10 was lower in the treatment of TAF 25 mg (AUCtau: 267.7 ng·h/mL) compared with TDF 300 mg (1918.0 ng·h/mL)43. TAF in blood enters primary hepatocytes and undertakes hydrolysis primarily by carboxylesterase one and cathepsin A that produce tenofovir–alanine conjugates within lymphocytes. TDF and TAF are converted to tenofovir and then phosphorylated to the intracellular active metabolite tenofovir diphosphate that blocks the catalytic site of HIV reverse transcriptase.
A series of aryl phenoxy-amidate derivatives of tenofovir was synthesized and one potent candidate, designated as GS-7171, showed promising anti-HIV activity in vitro and oral bioavailability in dogs36. Further purification of GS-7171 unexpectedly led to a 1:1 diastereomeric mixture of GS-7339 and GS-7340 due to the asymmetric center at the phosphorus atom of GS-7171 and the non-stereoselective synthetic process37. Each stereoisomer was isolated using batch elution chromatography37, and in vitro evaluations suggested that the anti-HIV-1 activity and pharmacokinetic profiles of GS-7340 were better than that of GS-733938. In fasted dogs, a single oral dose of TAF 10 mg-eq/kg preferentially produced a high concentration of tenofovir in lymphatic tissues and the peripheral blood mononuclear cells (PBMCs)–the primary location for HIV replication and latency38. The intracellular concentrations of tenofovir, tenofovir-monophosphate, tenofovir-diphosphate, and TAF were respectively 138 ± 55, 89 ± 8, 73 ± 15, and 0 μmol/L in monocytes that were isolated from human whole blood after the incubation with TAF 17.4 μmol/L for 1 h38. Based on the above findings, GS-7340 was selected as a potent anti-HIV inhibitor for further evaluations in clinical trials.
As the prodrugs of tenofovir, both TAF and TDF efficiently delivers tenofovir into lymphoid cells and tissues, and their structural differences can be traced to the phosphonate masking groups of TAF that harbor the phenol and alanine isopropyl ester (Fig. 3B). Compared with TDF, TAF is not only more stable in blood and plasma, but also maintains antiviral potency for a longer time39 and leads to higher levels of triglycerides, low-density and high-density lipoprotein cholesterol40. Because the prolonged exposure to TDF 300 mg may increase the risk of renal impairment and a loss of bone mineral density17, TAF 25 mg offers lower systemic tenofovir exposure at reduced doses, thereby reducing renal and bone impairments41,42. TAF 10 or 25 mg/day is currently considered to replace TDF 300 mg/day in fixed-dose combinations.
3.2. Antiviral activity, drug resistance, and pharmacokinetics
TAF exhibited a strong potency against broad-spectrum isolates from HIV-1 groups/subtypes (EC50 range: 0.1–12 nmol/L) and HIV-2 (EC50 range: 0.91–2.63 nmol/L)39. In MT-2 cells infected with HIV-1 IIIb strain, the EC50 value of TAF (0.005 ± 0.002 μmol/L) was lower than that of TDF (0.05 ± 0.03 μmol/L) and tenofovir (5.0 ± 2.6 μmol/L)38. Despite its potency against HIV and HBV strains, TAF does not inhibit HCV, HSV-1, HCMV, dengue, or influenza A virus39. According to the recent update of IAS-USA guideline, TAF and TDF share similar drug-resistance profiles that include four primary amino acid substitutions: K65 R/E/N and K70E25.
In a phase 1b study of treatment-naïve adults who received the monotherapy for 10 days, TAF 25 mg was quickly absorbed with the median Tmax of 0.5 h43. At the steady-state, the plasma exposure of tenofovir on Day 10 was much lower in the treatment of TAF 25 mg (AUCtau: 267.7 ng·h/mL) compared with TDF 300 mg (1918.0 ng·h/mL)43. At approximately one-tenth of TDF 300 mg, TAF 25 mg offered a higher concentration of tenofovir-diphosphate in intracellular peripheral blood mononuclear cells (AUCtau: 21.4 vs 3.0 μmol/L·h) (Fig. 3B). Due to its high oral bioavailability and solubility, TAF generates sufficient mass flux across the intestine to saturate efflux transport and reduce intestinal metabolisms44. In HIV-negative patients receiving TAF 25 mg, the pharmacokinetic parameters of Cmax and AUCinf were two times higher in patients with severe renal impairment than those with normal renal function (Cmax: 364 vs 199 ng/mL, AUCinf: 513 vs 267 ng·h/mL)45. The plasma exposure of its active metabolite tenofovir was significantly increased in individuals with severe renal impairment versus those with normal renal function (Cmax: 26.4 vs 9.5 ng/mL, AUCinf: 2070 vs 343 ng·h/mL)45.
The plasma concentration of TAF and its metabolites can be altered by the coadministration of other drugs that affect P-glycoprotein and/or breast cancer resistance protein transporters46. Pharmacokinetic boosters such as ritonavir and cobicistat inhibit the intestinal P-glycoprotein transporter to improve the intestinal absorption of TAF from the gastrointestinal tract47. In the presence of cobicistat, TAF could be reduced from 25 to 10 mg due to the boosting effects48. In the absence of any booster, TAF and TDF offered similar clinical efficacies and safety profiles in virologically suppressed adults with HIV-148, and both exert an equivalent impact on the immune activation and inflammation in treatment-naïve adults49.
3.3. Efficacy and safety of TAF-based combination regimens
3.3.1. TAF 25 mg + emtricitabine 200 mg (Descovy®)
In October 2019, the once-daily, fixed-dose, single-tablet of TAF 25 mg plus FTC 200 mg was approved by the FDA for at-risk HIV-negative adults (body weight ≥35 kg) to reduce the risk of HIV-1 infection from sexual acquisitions. The efficacy and safety of TAF + FTC in HIV-negative or transgender men who have sex with men (MSM) were evaluated by a phase three study called DISCOVER50. This randomized, double-blind, non-inferiority study reported the comparable incidence rates of HIV-1 infection between the TAF + FTC arm and the TDF + FTC arm (0.16 versus 0.34 infections per 100 person-years, P-value > 0.05), but the TAF-based regimen offered better safety on the bone mineral density and renal function50. Of note, the efficacy and safety of TDF + FTC versus the placebo group were previously confirmed51 (Table 2).
Table 2.
Efficacy of approved therapies for HIV-1 pre-exposure prophylaxis.
| Clinical trial (phase) | Recruited individuals | Trial arm | HIV infection no./patient no. | Person-yearsa | Incidence rateb | P-value | Ref. |
|---|---|---|---|---|---|---|---|
| DISCOVER (phase 3) | HIV-negative or transgender MSM | TAF + FTC | 7/2694 | 8756 | 0.16% | >0.05 | 50 |
| TDF + FTC | 15/2693 | 0.34% | |||||
| iPrEx (phase 3) | HIV-negative or transgender MSM | TDF + FTC | 38/1251 | 3324 | NA | NA | 51 |
| Placebo | 72/1248 | NA | |||||
| MTN-020–ASPIRE (phase 3) | HIV-negative women (18–45 years) | Dapivirine vaginal ring | 71/1313 | 4280 | 3.3% | 0.046 | 77 |
| Placebo | 97/1316 | 4.5% | |||||
| Ring (phase 3) | HIV-negative women (18–45 years) | Dapivirine vaginal ring | 77/1307 | 1888 | 4.1% | 0.04 | 78 |
| Placebo | 56/652 | 6.1% |
MSM: men who have sex with men.
The person-years of follow-up are summarized for individual studies.
Incidence rate equals the number of HIV-1 infections divided by person-years of follow-up. P-value indicates the statistical difference of incidence rates between the intervention arm and the placebo arm.
Due to the success of TDF + FTC in the pre-exposure prophylaxis of HIV-1 infections, TAF was considered for the replacement of TDF to develop the TAF + FTC regimen. Similar to TDF + FTC, TAF + FTC cannot be used in HIV-1-infected adults with the estimated creatinine clearance <30 mL/min. Ongoing clinical trials (e.g., NCT04616963, NCT04742491) will evaluate the efficacy and safety of TAF + FTC to prevent HIV-1 transmission in transgender-identifying or gender non-binary individuals.
3.3.2. TAF 25 mg + emtricitabine 200 mg + rilpivirine 25 mg (Odefsey®)
In March 2016, the FDA approved the three-drug, once-daily, fixed-dose tablet of TAF + FTC + RPV as the initiating therapy for treatment-naïve patients or maintaining therapy for virologically suppressed patients. According to the drug labeling, TAF + FTC + RPV alone is not recommended for HIV-1-infected adults with HBV or creatinine clearance <30 mL/min.
The clinical efficacy and safety of TAF + FTC + RPV were evaluated by two phase 3b, non-inferiority studies (NCT02345226, NCT02345252) (Table 3, Supporting Information Table S1). Based on the merged data from the two studies above, TAF + FTC + RPV maintained the virological suppression (HIV-1 RNA <50 copies/mL at Week 48) in virologically suppressed adults infected with HIV-1. The drug-associated adverse events (e.g., upper respiratory tract infection, nasopharyngitis, headache, and diarrhea) were observed in 10.1% (76/754) of patients who received TAF + FTC + RPV.
Table 3.
Clinical efficacy of approved anti-HIV regimens in phase 2/3 clinical trials.
| Brand name | Prior treatment | Arm | Efficacya | P-value | Study (phase) | Ref. |
|---|---|---|---|---|---|---|
| Biktarvy® | Treatment-naive | TAF + FTC + BIC | 97% (63/65) | 0.17 | NCT02397694 (phase 2) | 178 |
| TAF + FTC + DTG | 91% (30/33) | |||||
| Treatment-naive | TAF + FTC + BIC | 89% (286/320) | 0.12 | NCT02607956 (phase 3) | 179 | |
| TAF + FTC + DTG | 93% (302/325) | |||||
| Treatment-naive | TAF + FTC + BIC | 92% (290/314) | 0.78 | NCT02607930 (phase 3) | 180 | |
| ABC + 3TC + DTG | 93% (293/315) | |||||
| Virologically suppressed | TAF + FTC + BIC | 94% (264/282) | 0.59 | NCT02603120 (phase 3) | 181 | |
| ABC + 3TC + DTG | 95% (267/281) | |||||
| Virologically suppressed | TAF + FTC + BIC | 92% (267/290) | 0.20 | NCT02603107 (phase 3) | 182 | |
| PI-based regimen | 89% (255/287) | |||||
| Virologically suppressed | TAF + FTC + BIC | 96% (224/234) | 1.00 | NCT02652624 (phase 3) | 183 | |
| Baseline regimen | 95% (225/236) | |||||
| Virologically suppressed | TAF + FTC + BIC | 93% (265/284) | 0.28 | NCT03110380 (phase 3) | 184 | |
| TAF + FTC + DTG | 91% (256/281) | |||||
| Odefsey® | Virologically suppressed | TAF + FTC + RPV | 90% (394/438) | 0.35 | NCT02345226 (phase 3) | 185 |
| TDF + FTC + EFV | 92% (402/437) | |||||
| Virologically suppressed | TAF + FTC + RPV | 94% (296/316) | 1.00 | NCT02345252 (phase 3) | 186 | |
| TDF + FTC + RPV | 94% (294/313) | |||||
| Acriptega® | Treatment-naive | TAF + FTC + DTG | 84% (294/351) | 0.07 | NCT03122262 (phase 3) | 187 |
| TDF + FTC + DTG | 85% (298/351) | |||||
| TDF + FTC + EFV | 79% (276/351) | |||||
| Symtuza® | Treatment-naive | TAF + FTC + DRV/c | 77% (79/103) | 0.41 | NCT01565850 (phase 2) | 188 |
| TDF + FTC + DRV/c | 84% (42/50) | |||||
| Treatment-naïve | TAF + FTC + DRV/c | 91% (331/362) | <0.0001 | NCT02431247 (phase 3) | 189 | |
| TDF + FTC + DRV/c | 88% (321/363) | |||||
| Virologically suppressed | TAF + FTC + DRV/c | 95% (724/763) | 0.39 | NCT02269917 (phase 3) | 190 | |
| TDF + FTC + PI | 94% (354/378) | |||||
| Genvoya® | Treatment-naive | TAF + FTC + EVG/c | 88% (99/112) | 0.84 | NCT01497899 (phase 2) | 191 |
| TDF + FTC + EVG/c | 88% (51/58) | |||||
| Treatment-naive | TAF + FTC + EVG/c | 92% (800/866) | 0.17 |
NCT01780506 NCT01797445 (phase 3) |
40 | |
| TDF + FTC + EVG/c | 90% (784/867) | |||||
| Virologically suppressed | TAF + FTC + EVG/c | 97% (932/959) | 0.0002 | NCT01815736 (phase 3) | 192 | |
| TDF-based regimen | 93% (444/477) | |||||
| Virologically suppressed | TAF + FTC + EVG/c | 94% (150/159) | 0.13 | NCT01705574 (phase 3) | 193 | |
| TDF + FTC + ATV/r | 87% (46/53) | |||||
| Virologically suppressed | TAF + FTC + EVG/c | 94% (102/109) | 1.0 | NCT02616783 (phase 3) | 194 | |
| TDF + FTC + EVG/c | 95% (52/55) |
Clinical efficacy was defined by HIV-1 RNA <50 copies/mL at Week 48.
3.3.3. TAF 25 mg + emtricitabine 200 mg + bictegravir 50 mg (Biktarvy®)
In February 2018, the FDA approved the three-drug fixed-dose regimen of TAF + FTC + BIC as initiating therapy for treatment-naïve patients or maintaining therapy for virologically suppressed patients. According to the drug labeling, TAF + FTC + BIC alone is not recommended for the treatment of HIV-1-infected adults with HBV, severe hepatic impairment, or creatinine clearance <30 mL/min.
The clinical efficacy and safety of TAF + FTC + BIC were evaluated by one phase two study and seven phase three studies (Table 3). For treatment-naïve adults, 91.4% (639/699) achieved the virological suppression after the 48-week treatment of TAF + FTC + BIC, and drug-associated adverse events were reported in 21.9% (139/634) participants. The 48-week treatment of TAF + FTC + BIC maintained HIV-1 RNA <50 copies/mL in 93.6% (1020/1090) patients of treatment-experienced adults who had been virologically suppressed for at least 3 months, while drug-associated adverse events were reported in 12.7% (138/1090) participants. In HIV-1-treatment-naïve patients, the pre-existing resistance substitutions did not affect the virological outcomes of TAF + FTC + BIC, and no treatment-emergent resistance was observed after the 144-week treatment of TAF + FTC + BIC52. Ongoing clinical trials will evaluate the efficacy and safety of Biktarvy® in adolescents (NCT02881320), elderly patients (NCT04222283), HIV-positive renal transplant patients (NCT04530630), patients within 100 days post HIV infections (NCT04483674), and patients co-infected with tuberculosis (NCT04734652).
3.3.4. TAF 25 mg + emtricitabine 200 mg + dolutegravir 50 mg
The tentative approval of TAF + FTC + DTG was granted by the FDA in December 2020, and its fixed-dose, single-tablet is now commercialized with the brand name of Acriptega® in India. Although it has not been marketed in the USA, TAF + FTC + DTG is currently recommended by the IAS-USA and the EACS guidelines10,11. Of note, TAF + FTC + DTG alone is not recommended for HIV-1-infected patients with body weight <40 kg, HBV infections, or creatinine clearance <30 mL/min.
The efficacy and safety of TAF + FTC + DTG were reported by four clinical studies (Table 3). In treatment-naïve patients who received TAF + FTC + DTG, 88.3% (626/709) achieved the virological response of HIV-1 RNA <50 copies/mL at Week 48. In virologically suppressed patients who received TAF + FTC + DTG, 91% (256/281) maintained the virological response of HIV-1 RNA <50 copies/mL at Week 48. The drug-associated adverse events were reported in 10% (28/281) patients (Table S1).
3.3.5. TAF 10 mg + emtricitabine 200 mg + elvitegravir 150 mg + cobicistat 150 mg (Genvoya®)
In November 2015, the FDA approved the once-daily, fixed-dose, single-tablet of TAF + FTC + EVG/c as initiating therapy and maintaining therapy to treat HIV-1-infected patients with no history of resistance to TAF, FTC, or EVG. According to the drug labeling, TAF + FTC + EVG/c alone is not recommended for HIV-1-infected patients with HBV infections, severe hepatic impairment, or creatinine clearance <30 mL/min. The clinical efficacy and safety of TAF + FTC + EVG/c were evaluated by one phase two study and four phase three studies (Table 3). The virological response of HIV-1 RNA <50 copies/mL at Week 48 was observed in 91.9% (899/978) of treatment-naïve patients who received TAF + FTC + EVG/c (Table 3). By merging data from three phase three studies, this regimen offered the virological response up to 96.5% (1184/1227) of virologically suppressed patients (Table 3). Drug-associated adverse events were observed in 19.9% (244/1228) of patients receiving TAF + FTC + EVG/c.
A switch from TDF-based regimens to Genvoya® improved the bone mineral density in virologically suppressed patients ≥60 years (Table 3). Genvoya® also improved the bone mineral density in HIV-infected adults with renal impairment53 or with end-stage renal diseases on chronic hemodialysis54. In a retrospective study of 6704 treatment-naive patients, HIV-1 RT amino acid substitutions such as T215 A/C/D/E/G/H/I/L/N/P/Q/R/S/V showed no measurable impact on virological responses to TAF + FTC + EVG/c55. A low prevalence of resistant RT mutations, including K65 R/N (2 cases, 0.2%) and M184V/I (11 cases, 1.3%) was observed in 866 treatment-naïve adults who received TAF + FTC + EVG/c for 144 weeks56.
3.3.6. TAF 10 mg + emtricitabine 200 mg + darunavir 800 mg + cobicistat 150 mg (Symtuza®)
In July 2018, the FDA approved the once-daily fixed-dose regimen of TAF + FTC + DRV/c as initiating therapy and maintaining therapy to treat HIV-1-infected patients with no history of resistance to TAF, FTC, or DRV. According to the drug labeling, TAF + FTC + DRV/c alone is not recommended for HIV-1-infected patients with body weight <40 kg, HBV infections, severe hepatic impairment, or creatinine clearance <30 mL/min.
The clinical efficacy and safety of TAF + FTC + DRV/c were evaluated by one phase two study and two phase three studies: AMBER and EMERALD (Table 3, Table S1). After the 48-week treatment with TAF + FTC + DRV/c, the virological response of HIV-1 RNA <50 copies/mL was observed in 88.2% (410/465) of treatment-naïve adults and 95% (724/763) of virologically suppressed adults with virological suppression ≥6 months (Table 3). The most common drug-associated adverse event was upper respiratory tract infection (10.6%, 81/763) and nasopharyngitis (10.6%, 81/763) (Table S1).
4. Dapivirine
Dapivirine is a potent NNRTI against HIV-1 infections. On 24 July 2020, the European Medicines Agency approved dapivirine vaginal ring (a silicone elastomer dapivirine vaginal ring containing dapivirine 25 mg) for the pre-exposure prophylaxis of HIV-1 infection. The monthly application of dapivirine vaginal ring can be administered to reduce the risk of HIV-1 infection through vaginal intercourse in sexually active HIV-negative women (≥18 years) in combination with safer sex practices when oral pre-exposure prophylaxis is not/cannot be used or is not available.
4.1. Drug discovery
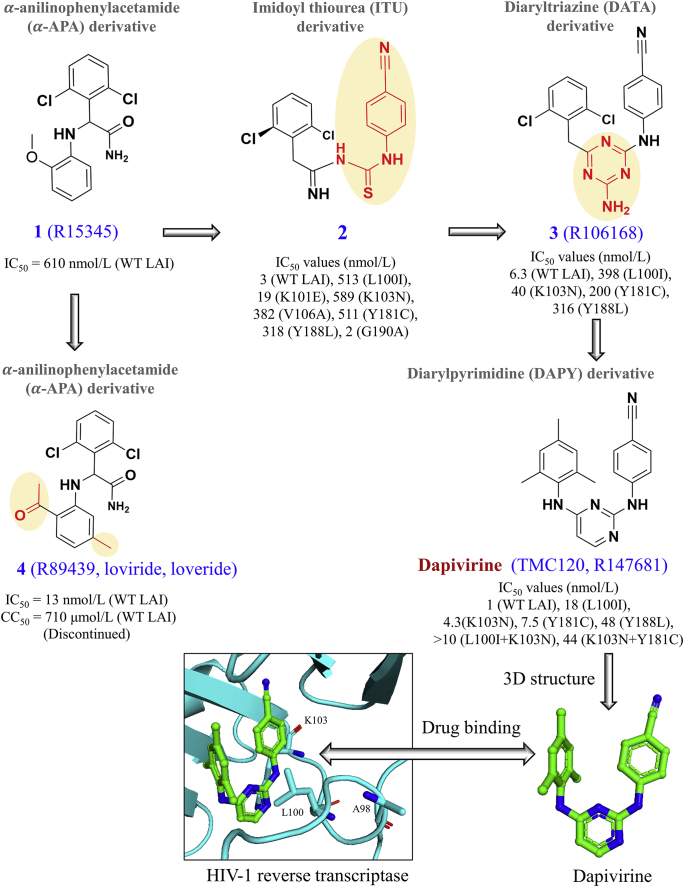
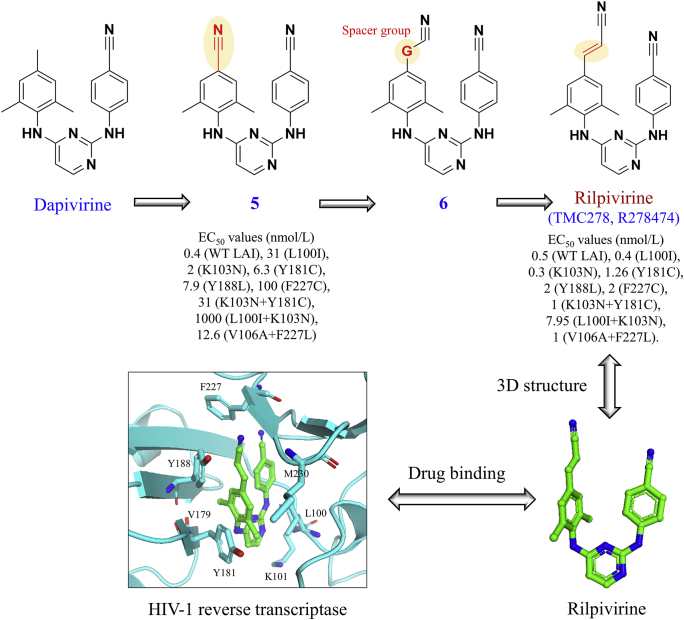
As shown in Fig. 4, the discovery of dapivirine can be traced back to the development of the alpha-anilinophenylacetamide (-APA) derivatives57, the imidoyl thiourea (ITU) derivatives58, the diaryltriazine (DATA) derivatives59, and the diarylpyrimidine (DAPY) derivatives60 based on the multidisciplinary collaborations between our Rega Institute in Belgium61, Janssen Pharmaceutica in Belgium, and Rutgers University in the USA62. In 1991, Pauwels et al.57 from our Rega Institute used a high-flux screening system to screen the Janssen library of 2000 different compounds for their cytotoxicity and capacity to inhibit HIV-1 replication in MT-4 cells. This led to the discovery of an alpha-anilinophenylacetamide lead called R1534557. Further optimization led to R89439 (loviride, loveride) that effectively inhibited HIV-1 strains in vitro at the nanomolar concentration (IC50: 13 nmol/L)57. However, R89439 was later discontinued because it failed to show sufficient potency in clinical trials. Stemming from alpha-anilinophenylacetamide derivatives, imidoyl thiourea analogues with a unique diarylated imidoylthiourea structure was subsequently synthesized58. However, these candidates were not further pursued due to the hydrolytic instability of the imidoyl thiourea functionality58. During the synthesis of the imino-N-cyanoguanidine derivatives of imidoyl thiourea analogues, a ring closure was unexpectedly identified62, leading to the first compound of the DATA derivatives with improved stability59. However, the DATA derivatives such as R106168 showed suboptimal activities against HIV-1 strains with the double mutant L100I + K103N59. After the exploration of the structure‒activity and structure–metabolism relationships, the replacement of the central aminotriazine ring of DATA with a pyrimidine ring was evaluated, leading to the class of diarylpyrimidine derivatives such as dapivirine (TMC120, R147681)63 and etravirine (TMC125, R165335, Intelence®, approved by the FDA in 2008)64.
Figure 4.
Discovery of dapivirine from the alpha-anilinophenylacetamide (-APA) derivatives to imidoyl thiourea derivatives, the diaryltriazine derivatives, and the diarylpyrimidine derivatives. Drug binding pocket of dapivirine is highlighted in HIV-1 reverse transcriptase (PDB code: 1S6Q). Amino acid positions with drug resistant residues are highlighted. Three amino acid substitutions (L100I, K103N, A98G) may cause resistance to dapivirine74,78.
A vaginal microbicide of dapivirine was later considered because dapivirine has long half-life time, high potency, and low cytotoxicity65,66. Although the applications of vaginally administered gel, film, tablet, soft gel capsules, and ring were considered to deliver dapivirine, the vaginal ring was eventually selected due to its easy use and a low frequency of the monthly administration67. To prevent the male-to-female transmission of HIV-1 infections, the silicone elastomer intravaginal ring could provide the sustained and controlled delivery of dapivirine into cervicovaginal fluids and vaginal tissues68. Other drug delivery methods such as gel products, fast-dissolving insert, lubricants and douches are still under investigation69.
4.2. Antiviral activity, drug resistance, and pharmacokinetics
Dapivirine effectively prevented the HIV-1-induced syncytium formation (EC50: 1 nmol/L, IC50: 24 nmol/L) in CEM T cells infected with HIV-1 strain HTLV-IIIB63. A 24-h treatment of dapivirine to the MO–DC/CD4+ T-cell cocultures also prevented the HIV-1 proviral integration (EC50: 4 nmol/L, range: 0.5–15 nmol/L)70. Over a 28-day use of the vaginal ring containing dapivirine 25 mg, dapivirine distributed throughout the lower genital tract of HIV-negative women at a concentration of 4000 times higher than the required EC50 against wildtype HIV-1 strains in MT4 cells71. After the ring insertion for 1.5 h, the Cmax value of dapivirine was the highest near the ring (79.90 ± 23.20 μg/g), followed by the cervix (66.61 ± 20.12 μg/g) and the introitus (31.38 ± 10.95 μg/g) in healthy women72.
A high concentration of dapivirine in the genital tract maintained a strong inhibition against HIV-1 resistant viruses including those with K103N and/or Y191C73. However, L100I and/or K103N mutations may cause significant resistance to dapivirine74. Under suboptimal concentrations in vitro, drug resistance mutations were less frequent in the dapivirine + tenofovir group than in the dapivirine alone75. A significant decrease of fasting lipids (e.g., cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol) was observed in doravirine-treated patients76.
4.3. Efficacy and safety of dapivirine vaginal ring
Based on two large-scale phase three studies77,78, the monthly application of dapivirine vaginal ring was evaluated for the pre-exposure prophylaxis of HIV-1 transmission in sexually active HIV-negative women (Table 2). The ASPIRE study, which was conducted between August 2012 and June 2015, reported the reduced incidence of HIV-1 infections at month 33 in the dapivirine-ring arm compared with the placebo arm (3.3 versus 4.5 seroconversions per 100 person-years, P-value = 0.046)77. Adverse events were 14% (180/1313) in the dapivirine-ring arm77. A follow-up study called HOPE was conducted between 16 July 2016 and 10 October 2018, and this study reported the reduced HIV-1 incidences of 2.7 per 100 person-years in the dapivirine-ring arm compared with the expected incidence of 4.4 per 100 person-years from the ASPIRE study79. Moreover, the dapivirine ring made of the flexible silicone matrix polymers unlikely causeed cervical cytology abnormalities80. In the ring study, the overall incidence rate of HIV-1 infections in the dapivirine arm was significantly lower than that in the placebo arm (4.1 versus 6.1 seroconversions per 100 person-years, P-value = 0.04)78. Three NNRTI resistance mutations were identified, including E138A (11.7%, 9/77), A98G (3.9%, 3/77), and K103N (3.9%, 3/77)78. As an open-label extension of the Ring study, the DREAM study supported the clinical use of the dapivirine ring with low HIV-1 incidences and improved adherence81. Ongoing open-label studies will evaluate the dapivirine-containing vaginal ring in young women between 16 and 21 years (NCT03593655), pregnant women (NCT03965923), and women who are breastfeeding (NCT04140266).
5. Rilpivirine (Edurant®, Rekambys®)
Rilpivirine (other names: TMC278, R278474) is a diarylpyrimidine NNRTI approved by the FDA in 2011. Rilpivirine tablets (Edurant®) and injection (Rekambys®) are both marketed. According to the FDA labeling, the once-daily tablet of rilpivirine 25 mg in combination with other antiretroviral drugs can be orally taken with a meal to treat HIV-1 infections in treatment-naïve patients (>12 years) with bodyweight ≥35 kg, CD4+ cell count >200 cells/mm3, and HIV-1 RNA ≤100,000 copies/mL. According to the drug labeling, rilpivirine should not be coadministered with cytochrome P450 inducers (e.g., rifabutin, rifampin, rifapentine) or drugs (e.g., cimetidine, famotidine, nizatidine) that increase gastric pH, because these drugs may reduce the plasma concentration of rilpivirine and cause the reduced virologic response and possible drug resistance.
5.1. Drug discovery
Rilpivirine is the E-isomer of the p-cyanovinyl analogue of dapivirine. As shown in Fig. 5, the discovery of rilpivirine began with the prototype of the diarylpyrimidine dapivirine62. Molecular modeling analyses suggested that the para substitutions on the trisubstituted phenyl ring of compound 5 may improve the drug interactions with the conserved W229 region in the drug-binding pocket of HIV-1 RT, therefore benefiting its antiviral activity against HIV-1 mutants82. This hypothesis led to the introduction of a spacer group G between the trisubstituted phenyl ring and the cyano group in the 4-position of compound 6 (Fig. 5). A series of substitutions at the G position was subsequently evaluated, leading to the discovery of rilpivirine with the promising potency against single- and double-mutant HIV-1 strains in vitro82.
Figure 5.
Discovery of rilpivirine based on the template of dapivirine. EC50 values are obtained from Ref. 82. Drug binding pocket of rilpivirine is highlighted in HIV-1 reverse transcriptase (PDB code: 3MEG). Amino acid positions with known resistant residues are highlighted.
5.2. Antiviral activity, drug resistance, and pharmacokinetics
Rilpivirine exhibits broad-spectrum antiviral activities against viral strains from HIV-1 genotypes (A1, C, D, F1, G, H) and CRFs (AE, AG, BG) with the EC50 values ranging from 0.13 to 0.44 nmol/L83,84. Based on in vitro experiments, rilpivirine actively inhibits HIV-1 group O isolates (EC50: 2.88–8.45 nmol/L)83. According to the IAS-USA guideline, resistance mutations to rilpivirine include L100I, K101 E/P, E138 A/G/K/Q/R, V179L, Y181C/I/V, Y188L, H221Y, F227C, and M230I/L25. Importantly, common NNRTI-associated resistance mutations (e.g., V106 and G190) do not decrease the sensitivity to rilpivirine83.
In a phase 2a study of treatment-naïve patients (n = 9) who received rilpivirine 25 mg for 7 days, the pharmacokinetic parameters of rilpivirine included mean Cmax: 263 ng/mL, Tmax: 4 h, t1/2: 34–55 h, and AUC24 h: 3659 ± 885 ng h/mL85. Rilpivirine is largely insoluble in water and oils, supporting its development as a nanosuspension86. In HIV-infected adults who received the monthly injection of rilpivirine plus cabotegravir, the total drug concentration was 134 (83–187) ng/mL of rilpivirine and 3.02 (2.37–5.10) μg/mL of cabotegravir in plasma that were collected 7 (±3) days post-injection87.
5.3. Efficacy and safety of rilpivirine-based combination regimens
5.3.1. Rilpivirine 25 mg + TDF 300 mg + emtricitabine 200 mg (Complera®)
In August 2011, the FDA approved the regimen of RPV + TDF + FTC as the initial therapy for treatment-naïve patients and the maintaining therapy for virologically-suppressed patients. According to the drug labeling, TDF + FTC + RPV alone is not recommended for HIV-1-infected adults with HBV, bodyweight <35 kg, or creatinine clearance <50 mL/min. The efficacy and safety of TDF + FTC + RPV were evaluated by at least three phase three studies (Table 4). When TDF + FTC + RPV was used as the initial therapy, 84.5% (625/740) of treatment-naïve patients achieved HIV-1 RNA <50 copies/mL at Week 48. When this regimen was applied as the maintaining therapy, 93.9% (294/313) of virologically-suppressed patients maintained HIV-1 RNA <50 copies/mL at Week 48. The drug-associated adverse events were reported in 11.8% (37/314) of patients who received TDF + FTC + RPV.
Table 4.
Clinical efficacy of approved anti-HIV regimens in phase 2/3 clinical trials.
| Brand name | Prior treatment | Arm | Efficacya | P-value | Study (phase) | Ref. |
|---|---|---|---|---|---|---|
| Complera® | Treatment-naive | RPV + TDF + FTC | 83% (287/346) | 0.17 | NCT00540449 (phase 3) | 195 |
| EFV + TDF + FTC | 83% (285/344) | |||||
| Treatment-naive | RPV + TDF + FTC | 86% (338/394) | 0.12 | NCT01309243 (phase 3b) | 196 | |
| EFV + TDF + FTC | 82% (320/394) | |||||
| Juluca® | Virologically suppressed | RPV + DTG | 95% (486/513) | 0.90 |
NCT02429791 NCT02422797 (phase 3) |
88 |
| ART regimen | 95% (485/511) | |||||
| Cabenuva® | Virologically suppressed | RPV + CAB (monthly) | 91% (105/115) | 0.82 | NCT02120352 (phase 2b) | 197 |
| RPV + CAB (bimonthly) | 92% (106/115) | |||||
| ABC + 3TC + CAB | 89% (50/56) | |||||
| Virologically suppressed | RPV + CAB (monthly) | 94% (265/283) | 0.87 | NCT02938520 (phase 3) | 198 | |
| ABC + 3TC + DTG | 93% (264/283) | |||||
| Virologically suppressed | RPV + CAB (monthly) | 93% (285/308) | 0.13 | NCT02951052 (phase 3) | 199 | |
| ART regimen | 96% (294/308) | |||||
| Virologically suppressed | RPV + CAB (monthly) | 93% (489/523) | 0.61 | NCT03299049 (phase 3b) | 200 | |
| RPV + CAB (bimonthly) | 94% (492/522) | |||||
| Delstrigo™ | Treatment-naive | DOR + TDF + 3TC | 84% (307/364) | 0.24 | NCT02403674 (phase 3) | 201 |
| EFV + TDF + FTC | 81% (294/364) | |||||
| Treatment-naive | DOR + TDF + 3TC | 83% (278/333) | 0.34 | NCT02275780 (phase 3) | 202 | |
| DOR + ABC + 3TC | 86% (43/50) | |||||
| PI-based regimen | 80% (306/383) | |||||
| Virologically suppressed | DOR + TDF + 3TC | 91% (406/447) | 0.12 | NCT02397096 (phase 3) | 203 | |
| Baseline regimen | 95% (211/223) |
Clinical efficacy was defined by HIV-1 RNA <50 copies/mL at Week 48.
5.3.2. Rilpivirine 25 mg + dolutegravir 50 mg (Juluca®)
In November 2017, the two-drug complete regimen of RPV + DTG was approved as the maintaining therapy to treat virologically suppressed patients who had no history of treatment failure and no drug resistance profiles associated with RPV or DTG. The efficacy and safety of RPV + DTG were reported by one phase three study88. The virological suppression of HIV-1 RNA <50 copies/mL at Week 48 was identified in 94.7% (486/513) of virologically suppressed patients (Table 4). The drug-associated adverse events were reported in 19% (97/513) of patients who received RPV + DTG.
5.3.3. Rilpivirine 300 mg/mL + cabotegravir 200 mg/mL (Cabenuva®)
In January 2021, the FDA approved the monthly intramuscular injection of rilpivirine (RPV) plus cabotegravir (CAB) extended-release injectable suspension for virologically-suppressed patients with no history of treatment failure and with no known resistance to RPV or CAB. According to the drug labeling, the tolerability of RPV and CAB should be assessed before the initiating therapy of RPV + CAB. Due to drug–drug interactions, the coadministration of RPV + CAB plus other drugs with a known risk of Torsade de Pointes should be cautious.
The efficacy and safety of the RPV + CAB injection was evaluated by one phase two study called LATTE-2 and three phase three clinical trials called FLAIR, ATLAS, and ATLA-2M (Table 4). By merging the data from the four studies above, the virological response of HIV-1 RNA <50 copies/mL at Week 48 was maintained in 93.1% (1144/1229) of virologically suppressed patients who received the monthly intramuscular injection of RPV + CAB. The drug-associated adverse events were reported in 28.3% (167/591) of patients who received RPV + CAB. The 96-week findings from the FLAIR study reaffirmed the durability of RPV + CAB in virologically suppressed adults89. Ongoing clinical trials will evaluate the oral and long-acting injectable regimen of rilpivirine plus cabotegravir in virologically suppressed children and adolescents (NCT03497676), patients across European countries (NCT04399551), and patients treated with the RPV + CAB injection every 2 months (NCT03299049).
6. Doravirine (DOR, Pifeltro™)
Doravirine, also named MK-1439, is an FDA-approved NNRTI with the brand name Pifeltro™ (Table 1). According to the drug labeling, the oral use of doravirine 100 mg should be combined with other antiretroviral drugs for the treatment of HIV-1 infections in treatment-naïve adults.
6.1. Drug discovery
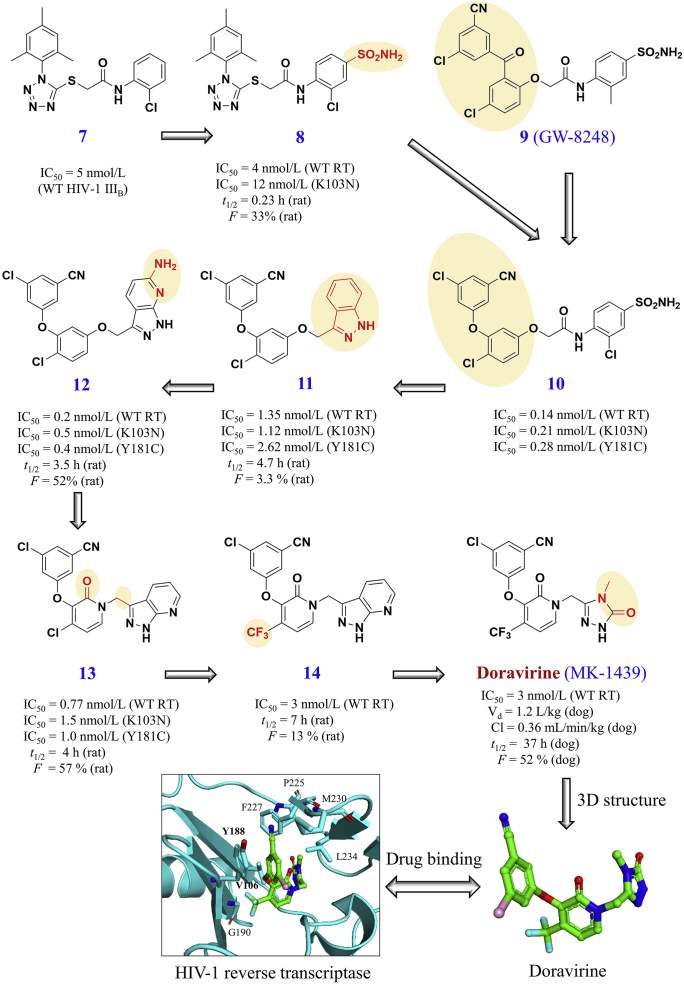
As shown in Fig. 6, the discovery of doravirine began with the high-throughput screening that identified the lead tetrazole thioacetanilide inhibitor (compound 7) of HIV-1 RT using a cell-based assay90. Subsequent optimizations were conducted by the substitution of the labile 2-nitroanilide with the more stable anilide structure (compound 8), thereby improving the oral bioavailability and decreasing the plasma clearance90. However, these tetrazole thioacetanilide analogues showed suboptimal pharmacokinetic profiles (t1/2 ≤ 1.1 h) in animal models91. Inspired by the structural comparisons of compounds 8 and 9, a series of diaryl ether analogues such as compound 10 was synthesized because the diaryl ether may improve the drug binding to inhibit HIV-1 mutant strains with better pharmacokinetic results91. Further analysis of structure–activity relationship led to the optimization of a diaryl ether/indazole compound 11 that inhibited HIV-1 wildtype and mutant strains with L100I, K103N, Y181C and/or G190 A/S (IC50 range: 2.6–171 nmol/L)91.
Figure 6.
Discovery of doravirine based on a template of the lead tetrazole thioacetanilide inhibitor 7. Drug binding pocket of rilpivirine is highlighted in HIV-1 reverse transcriptase (PDB code: 4NCG). Amino acid positions with known resistant residues are highlighted.
Due to the low solubility and poor oral bioavailability of compound 11 (F: 3.3% in rats), a pyridine ring was considered to modify the phenyl ring of the indazole moiety in compound 11, leading to the development of compound 12 with better pharmacokinetic profiles (e.g., F: 52%)92. Next, the aryl ether core of compound 12 was replaced by a pyridone ring to increase the polarity and the binding affinity, thereby identifying the pyridone compound 13 with better antiviral activity against HIV-1 strains with the K103N + Y181C mutation93. To improve the plasma half-life, the replacement of chlorine in compound 13 (t1/2: 2 h) with the strong electron withdrawing CF3 group led to compound 14, resulting in a longer elimination half-life (t1/2: 7 h in rats)94. The alkylated pyridone in compound 14 was modified to disrupt the strong donor–acceptor hydrogen bonds to improve aqueous solubility, eventually leading to the discovery of doravirine with potent pharmacokinetic and antiviral activities against HIV-1 strains with the K103N + Y181C mutation94.
6.2. Antiviral activity, drug resistance, and pharmacokinetics
Drug resistance to doravirine is mostly associated with three primary RT mutations (V106 A/M, Y188L) and 11 secondary RT mutations (V106T/I, Y188 C/H, G190E, P225H, F227 C/L/R, M230L, L234I)25. In vitro assays supported the antiviral potency of doravirine against a broad range of different HIV-1 subtype strains and mutant viruses with K101E, K103N, E138K, Y181C, M184V/I, and/or G190A95, 96, 97, 98. Doravirine-associated resistance mutations were very rare (1.4%; 137/9764) according to a large-scale cohort of 9764 treatment-naïve patients from Greece, Italy, and France99. A significant increase of doravirine-associated resistance mutations (V106 A/M, V108I, Y188 L/C/H, G190E, F227L, M230L, K103N + P225H) was observed in a large cohort of 6893 patients treated with other NNRTIs (delavirdine, efavirenz, nevirapine, etravirine, rilpivirine)100.
Doravirine 100 mg/day can be orally taken with or without food and its clinical potency is unlikely altered by host factors (e.g., gender, age, race)101, severe renal impairment102, and modest hepatic impairment103. In six healthy males, the pharmacokinetic profiles of single-dose doravirine 100 mg were estimated, including mean Cmax: 1713.17 nmol/L, C24 h: 593.43 nmol/L, AUCinf: 38.32 μmol/L·h, and AUC24 h: 22.84 μmol/L·h104. In 12 healthy males who received the intravenous injection of a single-dose doravirine 100 μg, doravirine metabolites were mostly distributed in feces (84.1% of the dose recovered up to 168 h) and urine (2.2% of the dose recovered up to 96 h)105.
According to the drug labeling, doravirine should not be co-administered with strong CYP3A4 inducers (e.g., efavirenz, rifampin) that significantly decrease the plasma concentration of doravirine, resulting in the reduced virological response and possible drug resistance. However, doravirine is unlikely involved with other CYP enzymes (e.g., CYP3A5, CYP1A2, CYP2B6, CYP2C8) and drug transporters (e.g., organic anion transporting polypeptide 1B1)106,107. Moreover, doravirine unlikely interacts with other drugs such as TDF, lamivudine, atorvastatin, methadone, elbasvir, grazoprevir, ledipasvir, and sofosbuvir108,109.
6.3. Efficacy and safety of doravirine-based combination regimens
6.3.1. Doravirine 100 mg + TDF 300 mg + lamivudine 300 mg (Delstrigo™)
In August 2018, the FDA approved the once-daily fixed-dose regimen of TDF + 3TC + DOR for treatment-naïve adults infected with HIV-1 infections. According to the drug labeling, TDF + 3TC + DOR alone is not recommended for HIV-1-infected adults with HBV or creatinine clearance <50 mL/min. The clinical efficacy and safety of TDF + 3TC + DOR were evaluated by one phase two study110 and three phase three studies: DRIVE-AHEAD, DRIVE-FORWARD, and DRIVE-SHIFT (Table 4 and Table S1). In treatment-naïve patients who received TDF + 3TC + DOR for 48 weeks, the clinical efficacy of HIV-1 RNA <50 copies/mL at Week 48 was 83.5% (278/333) in the DRIVE-FORWARD study, 84.3% (307/364) in the DRIVE-AHEAD study, and 100% (8/8) in the single-arm, open-label, phase two study110. Drug-associated adverse events were observed in 31% (113/364) of patients receiving TDF + 3TC + DOR. The most common adverse events include dizziness, abnormal dreams, and nausea. In the DRIVE-SHIFT study of treatment-experienced patients with the virological suppression for ≥6 months, treatment switching from the baseline regimen to TDF+3TC + DOR maintained the virological suppression of HIV-1 RNA <50 copies/mL at Week 48 in 90.8% (406/447) patients (Table 4). Ongoing clinical trials will report the potential application of doravirine 100 mg plus TAF 25 mg and FTC 200 mg to maintain the viral suppression in treatment-experienced adults (NCT04079452, NCT04097925).
6.3.2. Doravirine 100 mg + islatravir 0.75 mg + lamivudine 300 mg (novel regimen)
Doravirine was evaluated with the once-daily combination of lamivudine and islatravir for the treatment of previously untreated adults with HIV-1 infections based on a phase 2b, randomized, double-blind study111. At Week 48, the virological suppression of HIV-1 RNA <50 copies/mL was observed in 90% (27/30) of participants receiving the regimen of doravirine 100 mg + islatravir 0.75 mg + lamivudine 300 mg111. The clinical efficacy was 84% (26/31) in the control group of doravirine 100 mg + TDF 300 mg + lamivudine 300 mg111. The ongoing phase three studies will evaluate doravirine 100 mg plus islatravir 0.75 mg in treatment-naïve adults (NCT04233879), virologically suppressed adults (NCT04223778, NCT04223791), pediatric patients (NCT04295772), and heavily treatment-experienced adults (NCT04233216).
7. Azvudine (FNC)
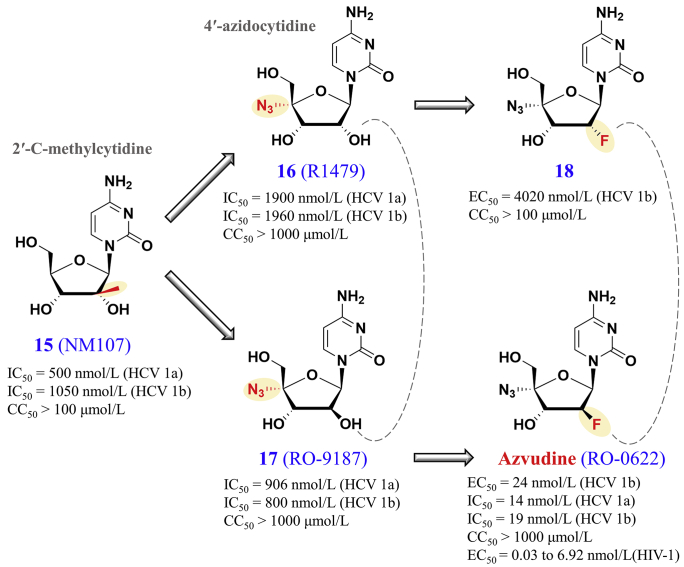
On 2021 July 21, azvudine was conditionally approved by the National Medical Products Administration in China. As shown in Fig. 7, the discovery of azvudine (2′-deoxy-2′-β-fluoro-4′-azidocytidine) began with the initial search of antiviral inhibitors that target HCV RNA-dependent RNA polymerase112. In 2001, 2′-C-methylcytidine (compound 15, NM107) was discovered as a potent nucleoside inhibitor of Flaviviridae virus in cell cultures, but its further development was hampered by its low oral bioavailability113. Subsequent design of 4′-substituted cytidine analogues led to the synthesis of 4′-azidocytidine (compound 16, R1479) to inhibit HCV chain elongation112. With the inversion of the 2′-hydroxyl group, the 4′-azido-arabinocytidine (compound 17, RO-9187) showed better phosphorylation efficiency than compound 16114. Because fluoronucleosides are often good substrates of RNA polymerases, the monofluoro and difluoro analogues of 4′-azidocytidine were subsequently designed, leading to the optimization of azvudine (RO-0622) and compound 18113. In the HCV replicon system, azvudine (EC50: 24 nmol/L) showed better antiviral potency than compound 18 (EC50: 4020 nmol/L)113. Moreover, azvudine exhibited broad-spectrum antiviral activities against HIV115, HBV116, and SARS-CoV-2117, but not respiratory syncytial virus or influenza A virus118. In cell cultures, azvudine inhibited various strains from HIV-1 subtype B and recombinants B/C and CRF01_AE (EC50: 0.03–6.92 nmol/L)115. Regarding its mechanisms of action, azvudine may inhibit HIV-1 RT and target the Vif-containing E3 ubiquitin ligase complex to block the Vif-induced degradation of APOBEC3G119. Moreover, azvudine is mainly metabolized by CYP3A120, and its absolute oral bioavailability is 82.7% in dog plasmas121.
Figure 7.
Discovery of azvudine. Synthesis pathways and in vitro results can be found in Refs. 112, 113, 114, 115.
A phase two study evaluated the safety and efficacy of azvudine (NCT04109183, completion date: 6 March 2019), but its clinical results have not been published as of today. An ongoing phase three trial will evaluate the efficacy and safety of azvudine 3 mg plus TDF 300 mg and EFV 200 mg in treatment-naive patients infected with HIV-1 (NCT04303598). Future studies also need to address the advantage of azvudine over FDA-approved NRTIs such as TAF.
8. Elsulfavirine (Elpida®)
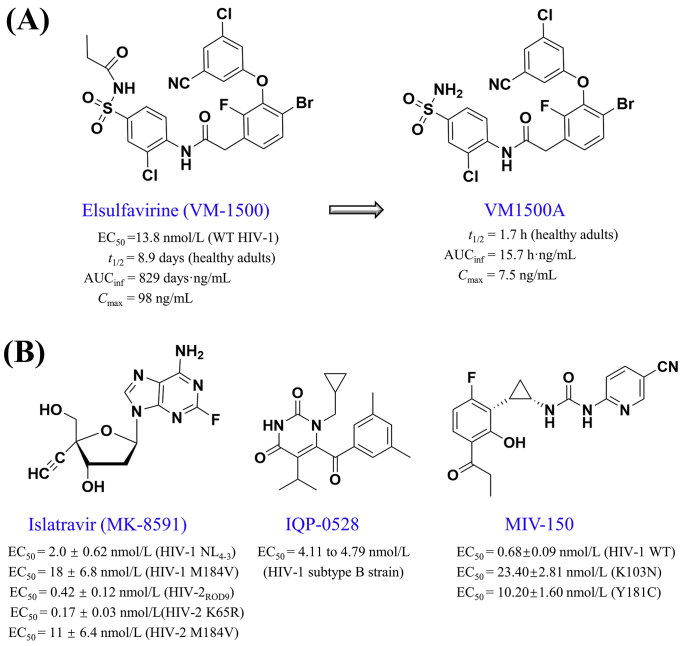
Elsulfavirine, the prodrug of the active NNRTI VM1500A (Fig. 8A), was approved by the Russian Ministry of Health in 2017. Although the discovery of elsulfavirine has not been documented yet in peer-reviewed literature, pharmacokinetic features of elsulfavirine 20 mg were characterized by a pre-clinical study122. In four healthy individuals treated with a single-dose of elsulfavirine 20 mg, the VM-1500A metabolite was measured with a mean half-life t1/2: 8.9 ± 2 days, Tmax: 0.15 ± 0.04 days, Cmax: 98 ± 74.3 ng/mL, and AUCinf: 829 ± 507 ng·h/mL122. In HIV-infected patients receiving elsulfavirine 20 mg for 7 days, the pharmacokinetic parameters of the VM-1500A metabolite included (i) t1/2: 7.4 ± 1.6 days, (ii) Tmax: 6.3 ± 0.5 days, (iii) Cmax: 148 ± 8 ng/mL, and (iv) AUCinf: 2123 ± 266 ng·h/mL122.
Figure 8.
Chemical structures of elsulfavirine, islatravir, IQP-0528, and MIV-150.
Elsulfavirine 20 mg is now considered with the once-daily combination of TDF 300 mg and emtricitabine 200 mg. A phase 2b study evaluated the efficacy and safety of elsulfavirine 20 mg versus efavirenz 600 mg in combination with TDF + FTC123. In treatment-naïve patients, the efficacy of HIV-1 plasma RNA <50 copies/mL at Week 48 was higher in the elsulfavirine arm (81%, 44/55) than in the elsulfavirine arm (74%, 35/47)123. A follow-up study reported the virological suppression of HIV RNA <50 copies/mL at week 96 in 83.9% (73/87) of treatment-naïve patients who continued the treatment of elsulfavirine 20 mg plus TDF + FTC124. The drug-associated adverse events were reported in 23.3% (14/60)124. Ongoing trials will report the drug–drug interactions (NCT03709355) and the feasibility of the once-weekly administration of elsulfavirine (NCT03730311).
9. Development of novel NRTIs and NNRTIs
A large number of novel NRTIs and NNRTIs have been reported in the literature6,125, 126, 127, 128, but only a few candidates are currently evaluated by clinical trials. Most candidates were discontinued because of their limited efficacy, unfavorable side effects, and/or high toxicity. For example, (i) lersivirine (UK-453061) was discontinued in 2013 because lersivirine showed no advantage over the existing NNRTIs; (ii) fosdevirine was halted in 2014 due to unexpected side effects such as seizures; (iii) censavudine was discontinued in 2015 due to the success of tenofovir alafenamide with better clinical efficacy and safety profiles129; and (iv) apricitabine was discontinued in 2016 due to a lack of funding and interests from its sponsor. Here, we summarize the recent development of three NRTIs (islatravir, MK-8504, MK-8583) and three NNRTIs (MK-8507, IQP-0528, MIV-150) that have been evaluated by phase one clinical trials.
9.1. Islatravir (MK-8591, EFdA)
Islatravir, known as MK-8591 or EFdA (4′-ethynyl-2-fluoro-2′-deoxyadenosine), is a deoxyadenosine nucleoside analogue (Fig. 8B) that targets the reverse transcriptase of HIV-1, HIV-2, and SIV130, 131, 132, 133. Unlike the conventional chain-terminating NRTIs, islatravir acts as an effective immediate or delayed chain terminator depending on the sequence of the nucleic acid substrate134. The biocatalytic cascade synthesis of islatravir could be efficiently made by five engineered enzymes with four auxiliary enzymes135. Islatravir offers potent antiviral activities (EC50 ≤ 11 nmol/L) against broad-spectrum viral isolates with multi-drug resistant mutations (e.g., K65R, Q151M, M184V)133. The EC50 values of islatravir were less than 21 nmol/L against a variety of HIV-1 strains (e.g., HIV-1NL4-3, HIV-1MDR, HIV-1Ba-L)136. In cell-based assays, an apparent synergism of islatravir plus rilpivirine may enhance the antiviral and prophylactic activity against HIV-1137. Pharmacokinetics features such as Cmax: 3.2 nmol/mL, Tmax: 0.5 h, and AUC12 h: 9.5 nmol/h/mL were measured after the oral administration of islatravir in 25 humanized mice infected with HIV-1NL4-3138.
The once-weekly oral dosing of islatravir (1.3 or 0.43 mg/kg/week for 6 weeks) protected all male rhesus macaques (n = 8) from intrarectal challenges with SIV and HIV139. A single subcutaneous administration of islatravir-eluting implants in rodents and rhesus macaques sustained the drug release at clinically relevant concentrations for at least 6 months140. In a phase one study (NCT02217904), a single dose of islatravir 0.5 mg suppressed HIV-1 RNA levels by more than one log on Day 7 in 30 treatment-naïve adults141. The prophylaxis activity of islatravir is currently evaluated by phase three clinical studies of cisgender women (NCT04644029) and men and transgender women who have sex with men (NCT04652700).
9.2. MK-8504 and MK-8583
Similar to TAF, MK-8504 and MK-8583 are prodrugs of tenofovir that can be intracellularly converted to the active form of tenofovir-diphosphate142. Two phase one studies evaluated the single-dose pharmacokinetics, antiviral activities, and safety of MK-8504 (NCT03188523) and MK-8583 (NCT03552536). In HIV-negative patients, MK-8504 and MK-8583 were rapidly absorbed (Tmax: 0.5 h) and eliminated from plasma142. Both compounds were well-tolerated with only mild to moderate adverse events142. The efficacy and safety of MK-8503 and MK-8583 in once-weekly regimens are yet to be clarified.
9.3. MK-8507
As a novel NNRTI with two trifluoromethyl groups, MK-8507 could be a potential once-weekly oral regimen against HIV-1 infection143. In healthy adults without HIV infection, a long terminal half-life of MK-8507 (t1/2 range: 58–84 h) was observed across different doses and MK-8507 was unlikely a strong inducer of CYP3A4144. In treatment-naïve patients infected with HIV-1, the single-dose monotherapy of MK-8507 (80 mg) reduced the plasma HIV-1 RNA by −1.5 (−1.8 to −1.19) log10 copies/mL at 7-day post-dose and its plasma pharmacokinetics were measured as follows: terminal t1/2: 69.4 h, Tmax: 2.0 h, Cmax: 2.11 μmol/L and AUC0-inf: 129 μmol/L·h145. In vitro IC50 of MK-8507 was 51.3 nmol/L based on the Monogram's PhenoSense assay145. Moreover, MK-8507 maintained the in vitro potency against NNRTI resistant-associated variants such as K103N, Y181C and G190A145. A phase 2 study (NCT04564547) was planned to evaluate the once-weekly two-drug regimen of MK-8507 (100, 200, 400 mg) plus islatravir 20 mg for the treatment of HIV-1 infection. On 18 November 2021, however, the development of MK-8507 was temporarily paused by Merck due to side effects (e.g., decreases in total lymphocyte and CD4+ T-cell counts) in the phase 2 study.
9.4. IQP-0528
IQP-0528 (SJ-3991) is a novel NNRTI that inhibits the viral reverse transcription and the viral entry of HIV-1 and HIV-2146. A dual chamber vaginal/rectal microbicide gel called DuoGel™ inhibited the CCR5-tropic subtype B strain HIV-1US/92/727 with the EC50 values of 4.11–4.79 nmol/L147. IQP-0528 gel is a promising candidate to protect human polarized ectocervical tissues against HIV-1 infections, because of its dual mechanisms of action and its minimal toxicity to vaginal cells and natural flora148. In a phase one study, seven HIV-negative individuals received one 10 mL rectal dose of 1% IQP-0528 gel, and no drug-associated adverse event was observed (NCT03082690). However, the application of IQP-0528 is limited because of its rapid clearance and its inability to penetrate vaginal tissues following the rectal dosing149.
9.5. MIV-150
MIV-150 (Fig. 8B) is a novel NNRTI with antiviral activities against HIV-1 replications73. Although the oral use of MIV-150 was discontinued because of its poor systemic absorption and rapid systemic clearance150, a novel core-matrix intravaginal ring called PC-1005 was proposed in the combination of (i) MIV-150 plus zinc acetate to prevent HSV-2 infections; (ii) MIV-150 plus carrageenan to prevent the infections of HSV-2 and human papillomavirus; and (iii) MIV-150 plus levonorgestrel to prevent the HIV-1, HSV-2, human papillomavirus, and unintended pregnancy151. In a phase one study (NCT02033109), the vaginal use of PC-1005 was well-tolerated with low systemic levels in sexually abstinent, HIV-negative women152. Another phase one study will evaluate PC-1005 as a rectal microbicide in HIV-negative adults with a history of consensual anal intercourse (NCT03408899).
10. Conclusions
In this study, we provide a comprehensive review of approved RT inhibitors and their combination regimens in the past decade. Although the development of each approved RT inhibitor had a different story, their drug discovery all requires coordinated multidisciplinary efforts from medicinal chemists, virologists, pharmacists, crystallographers, and many others153. Tenofovir alafenamide was discovered as the phenyl monoester isopropyl alaninyl phosphoramidate of tenofovir based on an unexpected observation of the mixture GS-7171, while its clinical approval was only granted 15 years later after its discovery. Although rilpivirine and dapivirine were both originated from the -APA derivatives, rilpivirine was developed as once-daily oral tablets and dapivirine was applied as the vaginal ring application due to their different oral bioavailability and half-life time. Elsulfavirine has been approved only in Russian, while its clinical advantage requires further investigations. Moreover, many drugs (e.g., doravirine) were developed with a series of structure-guided optimizations, thereby highlighting the importance of crystallography in antiviral drug discovery.
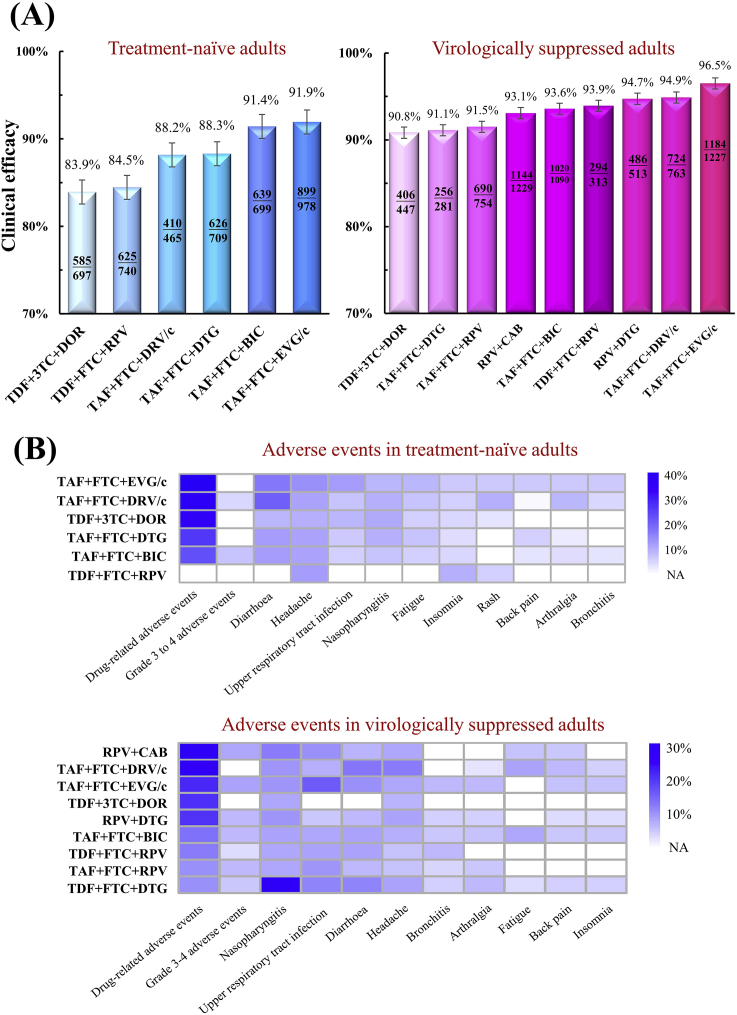
Our review summarized the clinical efficacy and safety of approved anti-HIV regimens based on randomized controlled trials. In clinical studies of HIV-1 pre-exposure prophylaxis, TAF + FTC and dapivirine vaginal ring significantly reduced the incidence rate of HIV infections (Table 2). To treat HIV-1 infections in treatment-naïve patients, the highest efficacy was observed in the initial therapy of TAF + FTC + EVG/c (91.9%, 899/978), followed by 3TC + DTG (91.5%, 655/716), TAF + FTC + BIC (91.4%, 639/699), ABC+3TC + DTG (90.1%, 657/729), TDF + FTC + EVG/c (89.5%, 1456/1626), TAF + FTC + DTG (88.3%, 626/709), TAF + FTC + DRV/c (88.2%, 410/465), TDF + FTC + RPV (84.5%, 625/740), and TDF+3TC + DOR (83.9%, 585/697) (Fig. 9A). Drug-associated adverse events of preceding regimens included diarrhea, headache, and nasopharyngitis (Fig. 9B). In the treatment of virologically suppressed patients with HIV-1 infections, the highest efficacy was observed in the maintaining therapy of TAF + FTC + EVG/c (96.5%, 1184/1227), followed by TAF + FTC + DRV/c (94.9%, 724/763), RPV + DTG (94.7%, 486/513), TDF + FTC + EVG/c (94.5%, 52/55), TDF + FTC + RPV (93.9%, 294/313), TAF + FTC + BIC (93.6%, 1020/1090), RPV + CAB (93.1%, 1144/1229), 3TC + DTG (93.0%, 384/413), TAF + FTC + RPV (91.5%, 690/754), TAF + FTC + DTG (91.1%, 256/281), TDF+3TC + DOR (90.8%, 406/447), and ABC+3TC + DTG (90.3%, 758/839) (Fig. 9A). The drug-associated adverse events of the preceding regimens include nasopharyngitis, diarrhea, and headache (Fig. 9B). Among these regimens, only two NRTI-free regimens (RPV + DTG, RPV + CAB) have been approved, but they are often used as maintaining therapies rather than initial therapies.
Figure 9.
Efficacy and safety of approved antiretroviral regimens in the treatment of treatment-naïve and virologically-suppressed adults with HIV-1 infections. (A) Clinical efficacy was defined by the proportion of patients achieving HIV-1 RNA <50 copies/mL at Week 48. Data from Table 3, Table 4 were merged to demonstrate the clinical efficacy of approved antiretroviral regimens. (B) Proportions of adverse events during the treatment of approved antiretroviral regimens in randomized clinical trials. Missing data are indicated by white-colored cells. Original data are available in Table S1.
In clinical practice, once-daily fixed-dose regimens of two NRTIs (TAF + FTC, TDF + FTC, TDF+3TC) plus another drug (e.g., integrase inhibitors) have been widely applied as the standard of care for most patients infected with HIV-1 infections. Of note, TAF is now gradually replacing TDF as the backbone in the once-daily, fixed-dose, single-tablet regimens such as Odefsey®, Genvoya®, and Descovy® because TAF offers high potency and safety profiles in the life-long treatment of HIV infections154. NNRTIs are now gradually replaced by HIV integrase inhibitors in the first-line combination regimens according to the IAS-USA and the EACS guidelines10,11. First, NNRTIs are only limited to the treatment of HIV-1 but not HIV-21. Second, many NNRTIs have clinically significant drug–drug and drug–food interactions since they are the substrates (e.g., doravirine, rilpivirine) or inducers (e.g., efavirenz) of cytochrome P450 3A4 (CYP3A4)–an important oxidizing enzyme in humans. Third, NNRTIs usually have low genetic barriers to resistance and there is an increasing prevalence of NNRTI-associated transmitted drug resistance in treatment-naïve patients from North America, Latin America/Caribbean, Sub-Saharan Africa, South/Southeast Asia, and Upper-Income Asian Countries155. Fourth, transmitted NNRTI resistance before first-line treatment is potentially associated with the long-term failure of first-line regimens containing integrase inhibitors such as dolutegravir156. Fifth, NNRTIs may cause neuropsychiatric adverse effects and these drug-related adverse effects should be closely monitored in clinical practice157.
Despite the success of many approved RT inhibitors, HIV strains evolve fast especially under the drug selective pressure158,159 and mutated residues near the drug-binding pocket of HIV RT may reduce the drug-target binding affinity5. To encounter emerging drug resistance mutations, many strategies have been proposed to develop the next generation of RT inhibitors with better drug resistance profiles5,6,8,160. (i) Multi-target strategy that designs novel RT inhibitors to target evolutionarily conserved binding pockets, divalent metal binding pockets, the substrate-envelope pocket, and/or the RNase H domain8. (ii) Conformation-based strategy that designs novel RT inhibitors with conformational flexibility to target ever-changing binding pockets with multiple drug positioning, covalent bonding, multivalent binding, and van der Waals forces5,6,8,161. (iii) RT inhibitors without overlapping resistance profiles could be combined to act synergistically for the maximize suppression against diverse strains of the highly mutable HIV5,6,8. Additionally, novel antiretroviral agents such as lenacapavir (GS-6207) that targets the viral capsid162 may provide alternative options in the long-acting fixed-dose regimens.
HIV RT inhibitors can be repurposed for the treatment of other human diseases. (i) Three NRTIs (TAF, TDF, lamivudine) have also been approved and recommended for the treatment of HBV infections163, 164, 165. (ii) Anti-inflammasome NRTIs such as lamivudine may improve insulin sensitivity and prevent the development of type two diabetes166. (iii) NRTIs may reduce the risk of age-related macular degeneration that causes blindness in humans167. (iv) NRTIs such as lamivudine might be repurposed as anti-cancer drugs168. (v) HIV RT inhibitors have been initially proposed to treat coronavirus disease 2019 (COVID-19)168,169, which has caused a global pandemic with severe morbidity and mortality since the end of 2019170, 171, 172, 173, 174. As of today, no anti-HIV drug has been approved for the treatment of COVID-19175, whereas TDF + FTC could reduce the risk of COVID-19 and hospitalization in HIV-positive patients176.
Future development of antiretroviral regimens could be conceived as follows: (i) the development of novel inhibitors with better potency and safety profiles, and longer half-life for long-acting application; (ii) new applications of approved inhibitors in two-drug regimens, long-acting formulations, and once-daily, fixed-dose, single-tablet regimens; and (iii) weekly or monthly applications of novel regimens in the HIV pre-exposure prophylaxis. Novel extended-release delivery devices such as vaginal rings, nanoformulations, and subcutaneous implants also provide new options to deliver antiretroviral drugs weekly, monthly, and maybe even yearly177. In the foreseeable future, antiretroviral regimens will still play a significant role in saving the lives of millions of human beings worldwide.
Acknowledgments
This work was funded by the National Nature Science Foundation of China (31871324, 81730064, 31571368, China); the Hunan Youth Elite Project (2018RS3006, China); the National Science and Technology Major Project (2018ZX10715004, China). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2021.11.009.
Contributor Information
Guangdi Li, Email: liguangdi.research@gmail.com.
Yali Wang, Email: wangyali@csu.edu.cn.
Erik De Clercq, Email: erik.declercq@kuleuven.be.
Author contributions
Guangdi Li and Yali Wang performed the literature search and data collection. Erik De Clercq conceived the concept of the study. Guangdi Li, Yali Wang and Erik De Clercq prepared and revised the manuscript. All authors read and approved the final paper.
Conflicts of interest
Prof. Dr. Erik De Clercq was the co-inventor of tenofovir. We declare no competing interests.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.De Clercq E., Li G. Approved antiviral drugs over the past 50 years. Clin Microbiol Rev. 2016;29:695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Clercq E. Fifty years in search of selective antiviral drugs. J Med Chem. 2019;62:7322–7339. doi: 10.1021/acs.jmedchem.9b00175. [DOI] [PubMed] [Google Scholar]
- 3.De Clercq E. Role of tenofovir alafenamide (TAF) in the treatment and prophylaxis of HIV and HBV infections. Biochem Pharmacol. 2018;153:2–11. doi: 10.1016/j.bcp.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y., De Clercq E., Li G. Current and emerging non-nucleoside reverse transcriptase inhibitors (NNRTIs) for HIV-1 treatment. Expet Opin Drug Metabol Toxicol. 2019;15:813–829. doi: 10.1080/17425255.2019.1673367. [DOI] [PubMed] [Google Scholar]
- 5.Ma Y., Frutos-Beltran E., Kang D., Pannecouque C., De Clercq E., Menendez-Arias L., et al. Medicinal chemistry strategies for discovering antivirals effective against drug-resistant viruses. Chem Soc Rev. 2021;50:4514–4540. doi: 10.1039/d0cs01084g. [DOI] [PubMed] [Google Scholar]
- 6.Zhuang C., Pannecouque C., De Clercq E., Chen F. Development of non-nucleoside reverse transcriptase inhibitors (NNRTIs): our past twenty years. Acta Pharm Sin B. 2020;10:961–978. doi: 10.1016/j.apsb.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song L., Ding S., Ge Z., Zhu X., Qiu C., Wang Y., et al. Nucleoside/nucleotide reverse transcriptase inhibitors attenuate angiogenesis and lymphangiogenesis by impairing receptor tyrosine kinases signalling in endothelial cells. Br J Pharmacol. 2018;175:1241–1259. doi: 10.1111/bph.14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cilento M.E., Kirby K.A., Sarafianos S.G. Avoiding drug resistance in HIV reverse transcriptase. Chem Rev. 2021;121:3271–3296. doi: 10.1021/acs.chemrev.0c00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ha B., Larsen K.P., Zhang J., Fu Z., Montabana E., Jackson L.N., et al. High-resolution view of HIV-1 reverse transcriptase initiation complexes and inhibition by NNRTI drugs. Nat Commun. 2021;12:2500. doi: 10.1038/s41467-021-22628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saag M.S., Gandhi R.T., Hoy J.F., Landovitz R.J., Thompson M.A., Sax P.E., et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. J Am Med Assoc. 2020;324:1651–1669. doi: 10.1001/jama.2020.17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryom L., Cotter A., De Miguel R., Beguelin C., Podlekareva D., Arribas J.R., et al. 2019 update of the European AIDS Clinical Society Guidelines for treatment of people living with HIV version 10.0. HIV Med. 2020;21:617–624. doi: 10.1111/hiv.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu S.X., Xiao T., Zhu Y.Y., Liu G.Y., Chen F. Recent progress in HIV-1 inhibitors targeting the entrance channel of HIV-1 non-nucleoside reverse transcriptase inhibitor binding pocket. Eur J Med Chem. 2019;174:277–291. doi: 10.1016/j.ejmech.2019.04.054. [DOI] [PubMed] [Google Scholar]
- 13.Wang L., Sarafianos S.G., Wang Z. Cutting into the substrate dominance: pharmacophore and structure-based approaches toward inhibiting human immunodeficiency virus reverse transcriptase-associated ribonuclease H. Acc Chem Res. 2020;53:218–230. doi: 10.1021/acs.accounts.9b00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin Y.H., Park C.M., Yoon C.H. An overview of human immunodeficiency virus-1 antiretroviral drugs: general principles and current status. Infect Chemother. 2021;53:29–45. doi: 10.3947/ic.2020.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcus J.L., Neugebauer R.S., Leyden W.A., Chao C.R., Xu L., Quesenberry C.P.J., et al. Use of abacavir and risk of cardiovascular disease among HIV-infected individuals. J Acquir Immune Defic Syndr. 2016;71:413–419. doi: 10.1097/QAI.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 16.Dalwadi D.A., Ozuna L., Harvey B.H., Viljoen M., Schetz J.A. Adverse neuropsychiatric events and recreational use of efavirenz and other HIV-1 antiretroviral drugs. Pharmacol Rev. 2018;70:684–711. doi: 10.1124/pr.117.013706. [DOI] [PubMed] [Google Scholar]
- 17.Casado J.L. Renal and bone toxicity with the use of tenofovir: understanding at the end. AIDS Rev. 2016;18:59–68. [PubMed] [Google Scholar]
- 18.Li G., Xu M., Yue T., Gu W., Tan L. Life-long passion for antiviral research and drug development: 80th birthday of Prof. Dr. Erik De Clercq. Biochem Pharmacol. 2021;185:114485. doi: 10.1016/j.bcp.2021.114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G., Yue T., Zhang P., Gu W., Gao L.J., Tan L. Drug discovery of nucleos(t)ide antiviral agents: dedicated to Prof. Dr. Erik De Clercq on occasion of his 80th birthday. Molecules. 2021;26:923. doi: 10.3390/molecules26040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendrix C.W. HIV Antiretroviral pre-exposure prophylaxis: development challenges and pipeline promise. Clin Pharmacol Ther. 2018;104:1082–1097. doi: 10.1002/cpt.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slagman S., Fessner W.D. Biocatalytic routes to anti-viral agents and their synthetic intermediates. Chem Soc Rev. 2021;50:1968–2009. doi: 10.1039/d0cs00763c. [DOI] [PubMed] [Google Scholar]
- 22.Vanangamudi M., Kurup S., Namasivayam V. Non-nucleoside reverse transcriptase inhibitors (NNRTIs): a brief overview of clinically approved drugs and combination regimens. Curr Opin Pharmacol. 2020;54:179–187. doi: 10.1016/j.coph.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Gu S.X., Zhu Y.Y., Wang C., Wang H.F., Liu G.Y., Cao S., et al. Recent discoveries in HIV-1 reverse transcriptase inhibitors. Curr Opin Pharmacol. 2020;54:166–172. doi: 10.1016/j.coph.2020.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Miao M., De Clercq E., Li G. Clinical significance of chemokine receptor antagonists. Expet Opin Drug Metabol Toxicol. 2020;16:11–30. doi: 10.1080/17425255.2020.1711884. [DOI] [PubMed] [Google Scholar]
- 25.Wensing A.M., Calvez V., Ceccherini-Silberstein F., Charpentier C., Gunthard H.F., Paredes R., et al. 2019 update of the drug resistance mutations in HIV-1. Top Antivir Med. 2019;27:111–121. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M., Li G., Shang J., Pan C., Zhang M., Yin Z., et al. Rapidly decreased HBV RNA predicts responses of pegylated interferons in HBeAg-positive patients: a longitudinal cohort study. Hepatol Int. 2020;14:212–224. doi: 10.1007/s12072-020-10015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G., De Clercq E. Current therapy for chronic hepatitis C: the role of direct-acting antivirals. Antivir Res. 2017;142:83–122. doi: 10.1016/j.antiviral.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miao M., Jing X., De Clercq E., Li G. Danoprevir for the treatment of hepatitis C virus infection: design, development, and place in therapy. Drug Des Dev Ther. 2020;14:2759–2774. doi: 10.2147/DDDT.S254754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balzarini J., Holý A., Jindrich J., Naesens L., Snoeck R., Schols D., et al. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob Agents Chemother. 1993;37:332–338. doi: 10.1128/aac.37.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ying C., De Clercq E., Neyts J. Lamivudine, adefovir and tenofovir exhibit long-lasting anti-hepatitis B virus activity in cell culture. J Viral Hepat. 2000;7:79–83. doi: 10.1046/j.1365-2893.2000.00192.x. [DOI] [PubMed] [Google Scholar]
- 31.Cundy K.C., Sueoka C., Lynch G.R., Griffin L., Lee W.A., Shaw J.P. Pharmacokinetics and bioavailability of the anti-human immunodeficiency virus nucleotide analog 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Antimicrob Agents Chemother. 1998;42:687–690. doi: 10.1128/aac.42.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deeks S.G., Barditch-Crovo P., Lietman P.S., Hwang F., Cundy K.C., Rooney J.F., et al. Safety, pharmacokinetics, and antiretroviral activity of intravenous 9-[2-(R)-(phosphonomethoxy)propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob Agents Chemother. 1998;42:2380–2384. doi: 10.1128/aac.42.9.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cihlar T. Nucleotide HIV reverse transcriptase inhibitors: tenofovir and beyond. Curr Opin HIV AIDS. 2006;1:373–379. doi: 10.1097/01.COH.0000239849.20828.09. [DOI] [PubMed] [Google Scholar]
- 34.Serpi M., Pertusati F. An overview of ProTide technology and its implications to drug discovery. Expet Opin Drug Discov. 2021;16:1149–1161. doi: 10.1080/17460441.2021.1922385. [DOI] [PubMed] [Google Scholar]
- 35.Mehellou Y., Rattan H.S., Balzarini J. The ProTide prodrug technology: from the concept to the clinic. J Med Chem. 2018;61:2211–2226. doi: 10.1021/acs.jmedchem.7b00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapman H., Kernan M., Rohloff J., Sparacino M., Terhorst T. Purification of PMPA amidate prodrugs by SMB chromatography and X-ray crystallography of the diastereomerically pure GS-7340. Nucleos Nucleot Nucleic Acids. 2001;20:1085–1090. doi: 10.1081/NCN-100002495. [DOI] [PubMed] [Google Scholar]
- 37.Chapman H., Kernan M., Prisbe E., Rohloff J., Sparacino M., Terhorst T., et al. Practical synthesis, separation, and stereochemical assignment of the PMPA pro-drug GS-7340. Nucleos Nucleot Nucleic Acids. 2001;20:621–628. doi: 10.1081/NCN-100002338. [DOI] [PubMed] [Google Scholar]
- 38.Lee W.A., He G.X., Eisenberg E., Cihlar T., Swaminathan S., Mulato A., et al. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother. 2005;49:1898–1906. doi: 10.1128/AAC.49.5.1898-1906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Callebaut C., Stepan G., Tian Y., Miller M.D. In vitro virology profile of tenofovir alafenamide, a novel oral prodrug of tenofovir with improved antiviral activity compared to that of tenofovir disoproxil fumarate. Antimicrob Agents Chemother. 2015;59:5909–5916. doi: 10.1128/AAC.01152-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sax P.E., Wohl D., Yin M.T., Post F., DeJesus E., Saag M., et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385:2606–2615. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- 41.Wang H., Lu X., Yang X., Xu N. The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy: meta-analysis. Medicine (Baltim) 2016;95:e5146. doi: 10.1097/MD.0000000000005146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fletcher C.V., Podany A.T., Thorkelson A., Winchester L.C., Mykris T., Anderson J., et al. The lymphoid tissue pharmacokinetics of tenofovir disoproxil fumarate and tenofovir alafenamide in HIV-infected persons. Clin Pharmacol Ther. 2020;108:971–975. doi: 10.1002/cpt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruane P.J., DeJesus E., Berger D., Markowitz M., Bredeek U.F., Callebaut C., et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63:449–455. doi: 10.1097/QAI.0b013e3182965d45. [DOI] [PubMed] [Google Scholar]
- 44.Babusis D., Phan T.K., Lee W.A., Watkins W.J., Ray A.S. Mechanism for effective lymphoid cell and tissue loading following oral administration of nucleotide prodrug GS-7340. Mol Pharm. 2013;10:459–466. doi: 10.1021/mp3002045. [DOI] [PubMed] [Google Scholar]
- 45.Custodio J.M., Fordyce M., Garner W., Vimal M., Ling K.H., Kearney B.P., et al. Pharmacokinetics and safety of tenofovir alafenamide in HIV-uninfected subjects with severe renal impairment. Antimicrob Agents Chemother. 2016;60:5135–5140. doi: 10.1128/AAC.00005-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Begley R., Das M., Zhong L., Ling J., Kearney B.P., Custodio J.M. Pharmacokinetics of tenofovir alafenamide when coadministered with other HIV antiretrovirals. J Acquir Immune Defic Syndr. 2018;78:465–472. doi: 10.1097/QAI.0000000000001699. [DOI] [PubMed] [Google Scholar]
- 47.Lepist E.I., Phan T.K., Roy A., Tong L., Maclennan K., Murray B., et al. Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro. Antimicrob Agents Chemother. 2012;56:5409–5413. doi: 10.1128/AAC.01089-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill A., Hughes S.L., Gotham D., Pozniak A.L. Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety?. J Virus Erad. 2018;4:72–79. doi: 10.1016/S2055-6640(20)30248-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Funderburg N.T., McComsey G.A., Kulkarni M., Bannerman T., Mantini J., Thornton B., et al. Equivalent decline in inflammation markers with tenofovir disoproxil fumarate vs. tenofovir alafenamide. EBioMedicine. 2016;13:321–327. doi: 10.1016/j.ebiom.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayer K.H., Molina J.M., Thompson M.A., Anderson P.L., Mounzer K.C., De Wet J.J., et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet. 2020;396:239–254. doi: 10.1016/S0140-6736(20)31065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grant R.M., Lama J.R., Anderson P.L., McMahan V., Liu A.Y., Vargas L., et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acosta R.K., Chen G.Q., Chang S., Martin R., Wang X., Huang H., et al. Three-year study of pre-existing drug resistance substitutions and efficacy of bictegravir/emtricitabine/tenofovir alafenamide in HIV-1 treatment-naive participants. J Antimicrob Chemother. 2021;76:2153–2157. doi: 10.1093/jac/dkab115. [DOI] [PubMed] [Google Scholar]
- 53.Pozniak A., Arribas J.R., Gathe J., Gupta S.K., Post F.A., Bloch M., et al. Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected patients with renal impairment: 48-week results from a single-arm, multicenter, open-label phase 3 study. J Acquir Immune Defic Syndr. 2016;71:530–537. doi: 10.1097/QAI.0000000000000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eron J.J., Jr., Lelievre J.D., Kalayjian R., Slim J., Wurapa A.K., Stephens J.L., et al. Safety of elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide in HIV-1-infected adults with end-stage renal disease on chronic haemodialysis: an open-label, single-arm, multicentre, phase 3b trial. Lancet HIV. 2018;6:e15–e24. doi: 10.1016/S2352-3018(18)30296-0. [DOI] [PubMed] [Google Scholar]
- 55.Margot N.A., Wong P., Kulkarni R., White K., Porter D., Abram M.E., et al. Commonly transmitted HIV-1 drug resistance mutations in reverse-transcriptase and protease in antiretroviral treatment-naive patients and response to regimens containing tenofovir disoproxil fumarate or tenofovir alafenamide. J Infect Dis. 2017;215:920–927. doi: 10.1093/infdis/jix015. [DOI] [PubMed] [Google Scholar]
- 56.Margot N., Cox S., Das M., McCallister S., Miller M.D., Callebaut C. Rare emergence of drug resistance in HIV-1 treatment-naive patients receiving elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide for 144 weeks. J Clin Virol. 2018;103:37–42. doi: 10.1016/j.jcv.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Pauwels R., Andries K., Debyser Z., Van Daele P., Schols D., Stoffels P., et al. Potent and highly selective human immunodeficiency virus type 1 (HIV-1) inhibition by a series of alpha-anilinophenylacetamide derivatives targeted at HIV-1 reverse transcriptase. Proc Natl Acad Sci U S A. 1993;90:1711–1715. doi: 10.1073/pnas.90.5.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ludovici D.W., Kukla M.J., Grous P.G., Krishnan S., Andries K., de Bethune M.P., et al. Evolution of anti-HIV drug candidates. Part 1: from alpha-anilinophenylacetamide (alpha-APA) to imidoyl thiourea (ITU) Bioorg Med Chem Lett. 2001;11:2225–2228. doi: 10.1016/s0960-894x(01)00410-3. [DOI] [PubMed] [Google Scholar]
- 59.Ludovici D.W., Kavash R.W., Kukla M.J., Ho C.Y., Ye H., De Corte B.L., et al. Evolution of anti-HIV drug candidates. Part 2: diaryltriazine (DATA) analogues. Bioorg Med Chem Lett. 2001;11:2229–2234. doi: 10.1016/s0960-894x(01)00411-5. [DOI] [PubMed] [Google Scholar]
- 60.Ludovici D.W., De Corte B.L., Kukla M.J., Ye H., Ho C.Y., Lichtenstein M.A., et al. Evolution of anti-HIV drug candidates. Part 3: diarylpyrimidine (DAPY) analogues. Bioorg Med Chem Lett. 2001;11:2235–2239. doi: 10.1016/s0960-894x(01)00412-7. [DOI] [PubMed] [Google Scholar]
- 61.De Clercq E. An Odyssey in antiviral drug development-50 years at the Rega Institute: 1964‒2014. Acta Pharm Sin B. 2015;5:520–543. doi: 10.1016/j.apsb.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Janssen P.A., Lewi P.J., Arnold E., Daeyaert F., de Jonge M., Heeres J., et al. In search of a novel anti-HIV drug: multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2-pyrimidinyl]amino]benzonitrile (R278474, rilpivirine) J Med Chem. 2005;48:1901–1909. doi: 10.1021/jm040840e. [DOI] [PubMed] [Google Scholar]
- 63.Van Herrewege Y., Michiels J., Van Roey J., Fransen K., Kestens L., Balzarini J., et al. In vitro evaluation of nonnucleoside reverse transcriptase inhibitors UC-781 and TMC120-R147681 as human immunodeficiency virus microbicides. Antimicrob Agents Chemother. 2004;48:337–339. doi: 10.1128/AAC.48.1.337-339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andries K., Azijn H., Thielemans T., Ludovici D., Kukla M., Heeres J., et al. TMC125, a novel next-generation nonnucleoside reverse transcriptase inhibitor active against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2004;48:4680–4686. doi: 10.1128/AAC.48.12.4680-4686.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malcolm R.K., Woolfson A.D., Toner C.F., Morrow R.J., McCullagh S.D. Long-term, controlled release of the HIV microbicide TMC120 from silicone elastomer vaginal rings. J Antimicrob Chemother. 2005;56:954–956. doi: 10.1093/jac/dki326. [DOI] [PubMed] [Google Scholar]
- 66.Chen B.A., Panther L., Marzinke M.A., Hendrix C.W., Hoesley C.J., van der Straten A., et al. Phase 1 safety, pharmacokinetics, and pharmacodynamics of dapivirine and maraviroc vaginal rings: a double-blind randomized trial. J Acquir Immune Defic Syndr. 2015;70:242–249. doi: 10.1097/QAI.0000000000000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Devlin B., Nuttall J., Wilder S., Woodsong C., Rosenberg Z. Development of dapivirine vaginal ring for HIV prevention. Antivir Res. 2013;100(Suppl):S3–S8. doi: 10.1016/j.antiviral.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 68.Romano J., Variano B., Coplan P., Van Roey J., Douville K., Rosenberg Z., et al. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Hum Retrovir. 2009;25:483–488. doi: 10.1089/aid.2008.0184. [DOI] [PubMed] [Google Scholar]
- 69.Baeten J.M., Hendrix C.W., Hillier S.L. Topical microbicides in HIV prevention: state of the promise. Annu Rev Med. 2020;71:361–377. doi: 10.1146/annurev-med-090518-093731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Herrewege Y., Vanham G., Michiels J., Fransen K., Kestens L., Andries K., et al. A series of diaryltriazines and diarylpyrimidines are highly potent nonnucleoside reverse transcriptase inhibitors with possible applications as microbicides. Antimicrob Agents Chemother. 2004;48:3684–3689. doi: 10.1128/AAC.48.10.3684-3689.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nel A., Smythe S., Young K., Malcolm K., McCoy C., Rosenberg Z., et al. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J Acquir Immune Defic Syndr. 2009;51:416–423. doi: 10.1097/qai.0b013e3181acb536. [DOI] [PubMed] [Google Scholar]
- 72.Nel A., Haazen W., Nuttall J., Romano J., Rosenberg Z., van Niekerk N. A safety and pharmacokinetic trial assessing delivery of dapivirine from a vaginal ring in healthy women. AIDS. 2014;28:1479–1487. doi: 10.1097/QAD.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 73.Giacobbi N.S., Sluis-Cremer N. In vitro cross-resistance profiles of rilpivirine, dapivirine, and MIV-150, nonnucleoside reverse transcriptase inhibitor microbicides in clinical development for the prevention of HIV-1 infection. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.00277-17. e00277-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Penrose K.J., Wallis C.L., Brumme C.J., Hamanishi K.A., Gordon K.C., Viana R.V., et al. Frequent cross-resistance to dapivirine in HIV-1 subtype C-infected individuals after first-line antiretroviral therapy failure in South Africa. Antimicrob Agents Chemother. 2017;61:e01805–e01816. doi: 10.1128/AAC.01805-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schader S.M., Oliveira M., Ibanescu R.I., Moisi D., Colby-Germinario S.P., Wainberg M.A. In vitro resistance profile of the candidate HIV-1 microbicide drug dapivirine. Antimicrob Agents Chemother. 2012;56:751–756. doi: 10.1128/AAC.05821-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thompson M., Orkin C., Molina J.M., Sax P., Cahn P., Squires K., et al. Once-daily doravirine for initial treatment of adults living with human immunodeficiency virus-1: an integrated safety analysis. Clin Infect Dis. 2020;70:1336–1343. doi: 10.1093/cid/ciz423. [DOI] [PubMed] [Google Scholar]
- 77.Baeten J.M., Palanee-Phillips T., Brown E.R., Schwartz K., Soto-Torres L.E., Govender V., et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375:2121–2132. doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nel A., van Niekerk N., Kapiga S., Bekker L.G., Gama C., Gill K., et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med. 2016;375:2133–2143. doi: 10.1056/NEJMoa1602046. [DOI] [PubMed] [Google Scholar]
- 79.Baeten J.M., Palanee-Phillips T., Mgodi N.M., Mayo A.J., Szydlo D.W., Ramjee G., et al. Safety, uptake, and use of a dapivirine vaginal ring for HIV-1 prevention in African women (HOPE): an open-label, extension study. Lancet HIV. 2021;8:e87–e95. doi: 10.1016/S2352-3018(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reddy K., Kelly C., Brown E.R., Jeenarain N., Naidoo L., Siva S., et al. Use of the dapivirine vaginal ring and effect on cervical cytology abnormalities. AIDS. 2020;34:559–567. doi: 10.1097/QAD.0000000000002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nel A., van Niekerk N., Van Baelen B., Malherbe M., Mans W., Carter A., et al. Safety, adherence, and HIV-1 seroconversion among women using the dapivirine vaginal ring (DREAM): an open-label, extension study. Lancet HIV. 2021;8:e77–e86. doi: 10.1016/S2352-3018(20)30300-3. [DOI] [PubMed] [Google Scholar]
- 82.Guillemont J., Pasquier E., Palandjian P., Vernier D., Gaurrand S., Lewi P.J., et al. Synthesis of novel diarylpyrimidine analogues and their antiviral activity against human immunodeficiency virus type 1. J Med Chem. 2005;48:2072–2079. doi: 10.1021/jm040838n. [DOI] [PubMed] [Google Scholar]
- 83.Azijn H., Tirry I., Vingerhoets J., de Béthune M.P., Kraus G., Boven K., et al. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother. 2010;54:718–727. doi: 10.1128/AAC.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mordant C., Schmitt B., Pasquier E., Demestre C., Queguiner L., Masungi C., et al. Synthesis of novel diarylpyrimidine analogues of TMC278 and their antiviral activity against HIV-1 wild-type and mutant strains. Eur J Med Chem. 2007;42:567–579. doi: 10.1016/j.ejmech.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 85.Goebel F., Yakovlev A., Pozniak A.L., Vinogradova E., Boogaerts G., Hoetelmans R., et al. Short-term antiviral activity of TMC278―a novel NNRTI―in treatment-naive HIV-1-infected subjects. AIDS. 2006;20:1721–1726. doi: 10.1097/01.aids.0000242818.65215.bd. [DOI] [PubMed] [Google Scholar]
- 86.van 't Klooster G., Hoeben E., Borghys H., Looszova A., Bouche M.P., van Velsen F., et al. Pharmacokinetics and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation. Antimicrob Agents Chemother. 2010;54:2042–2050. doi: 10.1128/AAC.01529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Letendre S.L., Mills A., Hagins D., Swindells S., Felizarta F., Devente J., et al. Pharmacokinetics and antiviral activity of cabotegravir and rilpivirine in cerebrospinal fluid following long-acting injectable administration in HIV-infected adults. J Antimicrob Chemother. 2020;75:648–655. doi: 10.1093/jac/dkz504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Llibre J.M., Hung C.C., Brinson C., Castelli F., Girard P.M., Kahl L.P., et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391:839–849. doi: 10.1016/S0140-6736(17)33095-7. [DOI] [PubMed] [Google Scholar]
- 89.Orkin C., Oka S., Philibert P., Brinson C., Bassa A., Gusev D., et al. Long-acting cabotegravir plus rilpivirine for treatment in adults with HIV-1 infection: 96-week results of the randomised, open-label, phase 3 FLAIR study. Lancet HIV. 2021;8:e185–e196. doi: 10.1016/S2352-3018(20)30340-4. [DOI] [PubMed] [Google Scholar]
- 90.Muraglia E., Kinzel O.D., Laufer R., Miller M.D., Moyer G., Munshi V., et al. Tetrazole thioacetanilides: potent non-nucleoside inhibitors of WT HIV reverse transcriptase and its K103N mutant. Bioorg Med Chem Lett. 2006;16:2748–2752. doi: 10.1016/j.bmcl.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 91.Tucker T.J., Saggar S., Sisko J.T., Tynebor R.M., Williams T.M., Felock P.J., et al. The design and synthesis of diaryl ether second generation HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) with enhanced potency versus key clinical mutations. Bioorg Med Chem Lett. 2008;18:2959–2966. doi: 10.1016/j.bmcl.2008.03.064. [DOI] [PubMed] [Google Scholar]
- 92.Tucker T.J., Sisko J.T., Tynebor R.M., Williams T.M., Felock P.J., Flynn J.A., et al. Discovery of 3-{5-[(6-amino-1H-pyrazolo[3,4-b]pyridine-3-yl)methoxy]-2-chlorophenoxy}-5-chloro benzonitrile (MK-4965): a potent, orally bioavailable HIV-1 non-nucleoside reverse transcriptase inhibitor with improved potency against key mutant viruses. J Med Chem. 2008;51:6503–6511. doi: 10.1021/jm800856c. [DOI] [PubMed] [Google Scholar]
- 93.Gomez R., Jolly S., Williams T., Tucker T., Tynebor R., Vacca J., et al. Design and synthesis of pyridone inhibitors of non-nucleoside reverse transcriptase. Bioorg Med Chem Lett. 2011;21:7344–7350. doi: 10.1016/j.bmcl.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 94.Cote B., Burch J.D., Asante-Appiah E., Bayly C., Bedard L., Blouin M., et al. Discovery of MK-1439, an orally bioavailable non-nucleoside reverse transcriptase inhibitor potent against a wide range of resistant mutant HIV viruses. Bioorg Med Chem Lett. 2014;24:917–922. doi: 10.1016/j.bmcl.2013.12.070. [DOI] [PubMed] [Google Scholar]
- 95.Feng M., Sachs N.A., Xu M., Grobler J., Blair W., Hazuda D.J., et al. Doravirine suppresses common nonnucleoside reverse transcriptase inhibitor-associated mutants at clinically relevant concentrations. Antimicrob Agents Chemother. 2016;60:2241–2247. doi: 10.1128/AAC.02650-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lai M.T., Feng M., Falgueyret J.P., Tawa P., Witmer M., DiStefano D., et al. In vitro characterization of MK-1439, a novel HIV-1 nonnucleoside reverse transcriptase inhibitor. Antimicrob Agents Chemother. 2014;58:1652–1663. doi: 10.1128/AAC.02403-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feng M., Wang D., Grobler J.A., Hazuda D.J., Miller M.D., Lai M.T. In vitro resistance selection with doravirine (MK-1439), a novel nonnucleoside reverse transcriptase inhibitor with distinct mutation development pathways. Antimicrob Agents Chemother. 2015;59:590–598. doi: 10.1128/AAC.04201-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith S.J., Pauly G.T., Akram A., Melody K., Ambrose Z., Schneider J.P., et al. Rilpivirine and doravirine have complementary efficacies against NNRTI-resistant HIV-1 mutants. J Acquir Immune Defic Syndr. 2016;72:485–491. doi: 10.1097/QAI.0000000000001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soulie C., Santoro M.M., Charpentier C., Storto A., Paraskevis D., Di Carlo D., et al. Rare occurrence of doravirine resistance-associated mutations in HIV-1-infected treatment-naive patients. J Antimicrob Chemother. 2019;74:614–617. doi: 10.1093/jac/dky464. [DOI] [PubMed] [Google Scholar]
- 100.Sterrantino G., Borghi V., Callegaro A.P., Bruzzone B., Saladini F., Maggiolo F., et al. Prevalence of predicted resistance to doravirine in HIV-1-positive patients after exposure to non-nucleoside reverse transcriptase inhibitors. Int J Antimicrob Agents. 2019;53:515–519. doi: 10.1016/j.ijantimicag.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 101.Yee K.L., Ouerdani A., Claussen A., de Greef R., Wenning L. Population pharmacokinetics of doravirine and exposure-response analysis in individuals with HIV-1. Antimicrob Agents Chemother. 2019;63:e02502–e02518. doi: 10.1128/AAC.02502-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ankrom W., Yee K.L., Sanchez R.I., Adedoyin A., Fan L., Marbury T., et al. Severe renal impairment has minimal impact on doravirine pharmacokinetics. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.00326-18. e00326-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khalilieh S., Yee K.L., Liu R., Fan L., Sanchez R.I., Auger P., et al. Moderate hepatic impairment does not affect doravirine pharmacokinetics. J Clin Pharmacol. 2017;57:777–783. doi: 10.1002/jcph.857. [DOI] [PubMed] [Google Scholar]
- 104.Anderson M.S., Gilmartin J., Cilissen C., De Lepeleire I., Van Bortel L., Dockendorf M.F., et al. Safety, tolerability and pharmacokinetics of doravirine, a novel HIV non-nucleoside reverse transcriptase inhibitor, after single and multiple doses in healthy subjects. Antivir Ther. 2015;20:397–405. doi: 10.3851/IMP2920. [DOI] [PubMed] [Google Scholar]
- 105.Sanchez R.I., Fillgrove K.L., Yee K.L., Liang Y., Lu B., Tatavarti A., et al. Characterisation of the absorption, distribution, metabolism, excretion and mass balance of doravirine, a non-nucleoside reverse transcriptase inhibitor in humans. Xenobiotica. 2019;49:422–432. doi: 10.1080/00498254.2018.1451667. [DOI] [PubMed] [Google Scholar]
- 106.Bleasby K., Fillgrove K.L., Houle R., Lu B., Palamanda J., Newton D.J., et al. In vitro evaluation of the drug interaction potential of doravirine. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.02492-18. e02492-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khalilieh S.G., Yee K.L., Sanchez R.I., Fan L., Anderson M.S., Sura M., et al. Doravirine and the potential for CYP3A-mediated drug‒drug interactions. Antimicrob Agents Chemother. 2019;63:e02016–e02018. doi: 10.1128/AAC.02016-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ankrom W., Sanchez R.I., Yee K.L., Fan L., Mitra P., Wolford D., et al. Investigation of pharmacokinetic interactions between doravirine and elbasvir-grazoprevir and ledipasvir-sofosbuvir. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.02491-18. e02491-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Anderson M.S., Gilmartin J., Fan L., Yee K.L., Kraft W.K., Triantafyllou I., et al. No meaningful drug interactions with doravirine, lamivudine and tenofovir disoproxil fumarate coadministration. Antivir Ther. 2019;24:443–450. doi: 10.3851/IMP3324. [DOI] [PubMed] [Google Scholar]
- 110.Wong A., Goldstein D., Mallolas J., DeJesus E., Johnson M., Molina J.M., et al. Efficacy and safety of doravirine/lamivudine/tenofovir disoproxil fumarate (DOR/3TC/TDF) in treatment-naive adults with HIV-1 and transmitted nonnucleoside reverse transcriptase inhibitor resistance mutations. J Acquir Immune Defic Syndr. 2019;82:e47–e49. doi: 10.1097/QAI.0000000000002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Molina J.M., Yazdanpanah Y., Afani Saud A., Bettacchi C., Chahin Anania C., DeJesus E., et al. Islatravir in combination with doravirine for treatment-naive adults with HIV-1 infection receiving initial treatment with islatravir, doravirine, and lamivudine: a phase 2b, randomised, double-blind, dose-ranging trial. Lancet HIV. 2021;8:e324–e333. doi: 10.1016/S2352-3018(21)00021-7. [DOI] [PubMed] [Google Scholar]
- 112.Smith D.B., Martin J.A., Klumpp K., Baker S.J., Blomgren P.A., Devos R., et al. Design, synthesis, and antiviral properties of 4′-substituted ribonucleosides as inhibitors of hepatitis C virus replication: the discovery of R1479. Bioorg Med Chem Lett. 2007;17:2570–2576. doi: 10.1016/j.bmcl.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 113.Smith D.B., Kalayanov G., Sund C., Winqvist A., Maltseva T., Leveque V.J., et al. The design, synthesis, and antiviral activity of monofluoro and difluoro analogues of 4ʹ-azidocytidine against hepatitis C virus replication: the discovery of 4ʹ-azido-2ʹ-deoxy-2ʹ-fluorocytidine and 4ʹ-azido-2ʹ-dideoxy-2ʹ,2ʹ-difluorocytidine. J Med Chem. 2009;52:2971–2978. doi: 10.1021/jm801595c. [DOI] [PubMed] [Google Scholar]
- 114.Klumpp K., Kalayanov G., Ma H., Le Pogam S., Leveque V., Jiang W.R., et al. 2ʹ-Deoxy-4ʹ-azido nucleoside analogs are highly potent inhibitors of hepatitis C virus replication despite the lack of 2ʹalpha-hydroxyl groups. J Biol Chem. 2008;283:2167–2175. doi: 10.1074/jbc.M708929200. [DOI] [PubMed] [Google Scholar]
- 115.Wang R.R., Yang Q.H., Luo R.H., Peng Y.M., Dai S.X., Zhang X.J., et al. Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. PLoS One. 2014;9:e105617. doi: 10.1371/journal.pone.0105617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou Y., Zhang Y., Yang X., Zhao J., Zheng L., Sun C., et al. Novel nucleoside analogue FNC is effective against both wild-type and lamivudine-resistant HBV clinical isolates. Antivir Ther. 2012;17:1593–1599. doi: 10.3851/IMP2292. [DOI] [PubMed] [Google Scholar]
- 117.Ren Z., Luo H., Yu Z., Song J., Liang L., Wang L., et al. A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv Sci. 2020;7:2001435. doi: 10.1002/advs.202001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu N., Yang J., Zheng B., Zhang Y., Cao Y., Huan C., et al. The pyrimidine analog FNC potently inhibits the replication of multiple enteroviruses. J Virol. 2020;94:e00204–e00220. doi: 10.1128/JVI.00204-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun L., Peng Y., Yu W., Zhang Y., Liang L., Song C., et al. Mechanistic insight into antiretroviral potency of 2ʹ-Deoxy-2′-beta-fluoro-4′-azidocytidine (FNC) with a long-lasting effect on HIV-1 prevention. J Med Chem. 2020;63:8554–8566. doi: 10.1021/acs.jmedchem.0c00940. [DOI] [PubMed] [Google Scholar]
- 120.Liu Y., Liu B., Zhang Y., Peng Y., Huang C., Wang N., et al. Intestinal absorption mechanisms of 2′-deoxy-2′-beta-fluoro-4′-azidocytidine, a cytidine analog for AIDS treatment, and its interaction with P-glycoprotein, multidrug resistance-associated protein 2 and breast cancer resistance protein. Eur J Pharmaceut Sci. 2017;105:150–158. doi: 10.1016/j.ejps.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 121.Peng Y., Cheng T., Dong L., Zhang Y., Chen X., Jiang J., et al. Quantification of 2′-deoxy-2′-beta-fluoro-4′-azidocytidine in rat and dog plasma using liquid chromatography-quadrupole time-of-flight and liquid chromatography-triple quadrupole mass spectrometry: application to bioavailability and pharmacokinetic studies. J Pharmaceut Biomed Anal. 2014;98:379–386. doi: 10.1016/j.jpba.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 122.Ratanasuwan W., Werarak P., Koryakova A., Berzins B., Bichko V., Murphy R. 20th international AIDS conference. 20‒25 July 2014. Parmacokinetics of VM-1500 20 mg and 40 mg in healthy and HIV-infected patients.https://www.natap.org/2014/IAC/IAC_108.htm Melbourne, Australia. Available from: [Google Scholar]
- 123.Murphy R., Kravchenko A.V., Orlova-Morozova E., Nagimova F., Kozirev O., Shimonava T., et al. Conference on retroviruses and opportunistic infection (CROI) 13‒16 February 2017. Elsulfavirine as compared to efavirenz in combination with TDF/FTC: 48-week study.https://www.croiconference.org/abstract/elsulfavirine-compared-efavirenz-combination-tdfftc-48-week-study/ Seattle, USA. Available from: [Google Scholar]
- 124.Murphy R., Kravchenko A., Orlova-Morozova E., Nagimova F., Kozirev O., Shimonova T., et al. 22nd international AIDS conference. 23‒27 July 2018. Elsulfavirine-based antiretroviral treatment in combination with two NRTIs: 96 weeks.http://programme.aids2018.org/Abstract/Abstract/7018 Amsterdam, Netherlands. Available from: [Google Scholar]
- 125.Kang D., Feng D., Ginex T., Zou J., Wei F., Zhao T., et al. Exploring the hydrophobic channel of NNIBP leads to the discovery of novel piperidine-substituted thiophene[3,2-d]pyrimidine derivatives as potent HIV-1 NNRTIs. Acta Pharm Sin B. 2020;10:878–894. doi: 10.1016/j.apsb.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jin K., Liu M., Zhuang C., De Clercq E., Pannecouque C., Meng G., et al. Improving the positional adaptability: structure-based design of biphenyl-substituted diaryltriazines as novel non-nucleoside HIV-1 reverse transcriptase inhibitors. Acta Pharm Sin B. 2020;10:344–357. doi: 10.1016/j.apsb.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao C., Weber S., Schols D., Balzarini J., Meier C. Prodrugs of gamma-alkyl-modified nucleoside triphosphates: improved inhibition of HIV reverse transcriptase. Angew Chem Int Ed Engl. 2020;59:22063–22071. doi: 10.1002/anie.202003073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ding L., Pannecouque C., De Clercq E., Zhuang C., Chen F. Improving druggability of novel diarylpyrimidine NNRTIs by a fragment-based replacement strategy: from biphenyl-DAPYs to heteroaromatic-biphenyl-DAPYs. J Med Chem. 2021;64:10297–10311. doi: 10.1021/acs.jmedchem.1c00708. [DOI] [PubMed] [Google Scholar]
- 129.Boyd M.A., Cooper D.A. Novel antiretroviral agents and universal access to HIV care. Lancet HIV. 2016;3:e2–3. doi: 10.1016/S2352-3018(15)00244-1. [DOI] [PubMed] [Google Scholar]
- 130.Kawamoto A., Kodama E., Sarafianos S.G., Sakagami Y., Kohgo S., Kitano K., et al. 2′-Deoxy-4′-C-ethynyl-2-halo-adenosines active against drug-resistant human immunodeficiency virus type 1 variants. Int J Biochem Cell Biol. 2008;40:2410–2420. doi: 10.1016/j.biocel.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 131.Njenda D.T., Aralaguppe S.G., Singh K., Rao R., Sönnerborg A., Sarafianos S.G., et al. Antiretroviral potency of 4′-ethnyl-2′-fluoro-2′-deoxyadenosine, tenofovir alafenamide and second-generation NNRTIs across diverse HIV-1 subtypes. J Antimicrob Chemother. 2018;73:2721–2728. doi: 10.1093/jac/dky256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Murphey-Corb M., Rajakumar P., Michael H., Nyaundi J., Didier P.J., Reeve A.B., et al. Response of simian immunodeficiency virus to the novel nucleoside reverse transcriptase inhibitor 4′-ethynyl-2-fluoro-2′-deoxyadenosine in vitro and in vivo. Antimicrob Agents Chemother. 2012;56:4707–4712. doi: 10.1128/AAC.00723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu V.H., Smith R.A., Masoum S., Raugi D.N., Ba S., Seydi M., et al. MK-8591 (4′-ethynyl-2-fluoro-2′-deoxyadenosine) exhibits potent activity against HIV-2 isolates and drug-resistant HIV-2 mutants in culture. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.00744-17. e00744-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Michailidis E., Huber A.D., Ryan E.M., Ong Y.T., Leslie M.D., Matzek K.B., et al. 4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) inhibits HIV-1 reverse transcriptase with multiple mechanisms. J Biol Chem. 2014;289:24533–24548. doi: 10.1074/jbc.M114.562694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huffman M.A., Fryszkowska A., Alvizo O., Borra-Garske M., Campos K.R., Canada K.A., et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science. 2019;366:1255–1259. doi: 10.1126/science.aay8484. [DOI] [PubMed] [Google Scholar]
- 136.Nakata H., Amano M., Koh Y., Kodama E., Yang G., Bailey C.M., et al. Activity against human immunodeficiency virus type 1, intracellular metabolism, and effects on human DNA polymerases of 4′-ethynyl-2-fluoro-2′-deoxyadenosine. Antimicrob Agents Chemother. 2007;51:2701–2708. doi: 10.1128/AAC.00277-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hachiya A., Reeve A.B., Marchand B., Michailidis E., Ong Y.T., Kirby K.A., et al. Evaluation of combinations of 4′-ethynyl-2-fluoro-2′-deoxyadenosine with clinically used antiretroviral drugs. Antimicrob Agents Chemother. 2013;57:4554–4558. doi: 10.1128/AAC.00283-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stoddart C.A., Galkina S.A., Joshi P., Kosikova G., Moreno M.E., Rivera J.M., et al. Oral administration of the nucleoside EFdA (4′-ethynyl-2-fluoro-2′-deoxyadenosine) provides rapid suppression of HIV viremia in humanized mice and favorable pharmacokinetic properties in mice and the rhesus macaque. Antimicrob Agents Chemother. 2015;59:4190–4198. doi: 10.1128/AAC.05036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Markowitz M., Gettie A., St Bernard L., Andrews C.D., Mohri H., Horowitz A., et al. Once-weekly oral dosing of MK-8591 protects male rhesus macaques from intrarectal challenge with SHIV109CP3. J Infect Dis. 2020;221:1398–1406. doi: 10.1093/infdis/jiz271. [DOI] [PubMed] [Google Scholar]
- 140.Barrett S.E., Teller R.S., Forster S.P., Li L., Mackey M.A., Skomski D., et al. Extended-duration MK-8591-eluting implant as a candidate for HIV treatment and prevention. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.01058-18. e01058-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schurmann D., Rudd D.J., Zhang S., De Lepeleire I., Robberechts M., Friedman E., et al. Safety, pharmacokinetics, and antiretroviral activity of islatravir (ISL, MK-8591), a novel nucleoside reverse transcriptase translocation inhibitor, following single-dose administration to treatment-naive adults infected with HIV-1: an open-label, phase 1b, consecutive-panel trial. Lancet HIV. 2020;7:e164–e172. doi: 10.1016/S2352-3018(19)30372-8. [DOI] [PubMed] [Google Scholar]
- 142.Matthews R.P., Hsieh S.J., Nussbaum J.C., Jajamovich G.H., Zhao T., Selverian D., et al. Conference on retroviruses and opportunistic infection (CROI) 2020. 8‒11 March 2020. MK-8504 and MK-8583 (tenofovir prodrugs) single-dose PK and antiviral activity in HIV infection.https://www.croiconference.org/abstract/mk-8504-and-mk-8583-tenofovir-prodrugs-single-dose-pk-and-antiviral-activity-in-hiv/ Boston, USA. Available from: [Google Scholar]
- 143.Gillespie G., Rudd D.J., Zhang S., Schaeffer A., Tomek C., Larson P., et al. Fluoride pharmacokinetics in urine and plasma following multiple doses of MK-8507, an investigational, oral, once-weekly non-nucleoside reverse transcriptase inhibitor. J Clin Pharmacol. 2022;62:199–205. doi: 10.1002/jcph.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ankrom W., Jackson Rudd D., Schaeffer A., Panebianco D., Friedman E.J., Tomek C., et al. Pharmacokinetic and safety profile of the novel HIV non-nucleoside reverse transcriptase inhibitor MK-8507 in adults without HIV. Antimicrob Agents Chemother. 2021;65:e0093521. doi: 10.1128/AAC.00935-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schürmann D., Rudd D.J., Schaeffer A., De Lepeleire I., Friedman E.J., Robberechts M., et al. Single oral doses of MK-8507, a novel non-nucleoside reverse transcriptase inhibitor, suppress HIV-1 RNA for a week. J Acquir Immune Defic Syndr. 2022;89:191–198. doi: 10.1097/QAI.0000000000002834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Buckheit R.W., Jr., Hartman T.L., Watson K.M., Chung S.G., Cho E.H. Comparative evaluation of the inhibitory activities of a series of pyrimidinedione congeners that inhibit human immunodeficiency virus types 1 and 2. Antimicrob Agents Chemother. 2008;52:225–236. doi: 10.1128/AAC.00972-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ham A.S., Nugent S.T., Peters J.J., Katz D.F., Shelter C.M., Dezzutti C.S., et al. The rational design and development of a dual chamber vaginal/rectal microbicide gel formulation for HIV prevention. Antivir Res. 2015;120:153–164. doi: 10.1016/j.antiviral.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mahalingam A., Simmons A.P., Ugaonkar S.R., Watson K.M., Dezzutti C.S., Rohan L.C., et al. Vaginal microbicide gel for delivery of IQP-0528, a pyrimidinedione analog with a dual mechanism of action against HIV-1. Antimicrob Agents Chemother. 2011;55:1650–1660. doi: 10.1128/AAC.01368-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Al-Khouja A., Shieh E., Fuchs E.J., Marzinke M.A., Bakshi R.P., Hummert P., et al. Examining the Safety, Pharmacokinetics, and pharmacodynamics of a rectally administered IQP-0528 gel for HIV pre-exposure prophylaxis: a first-in-human study. AIDS Res Hum Retrovir. 2021;37:444–452. doi: 10.1089/aid.2020.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Singer R., Derby N., Rodriguez A., Kizima L., Kenney J., Aravantinou M., et al. The nonnucleoside reverse transcriptase inhibitor MIV-150 in carrageenan gel prevents rectal transmission of simian/human immunodeficiency virus infection in macaques. J Virol. 2011;85:5504–5512. doi: 10.1128/JVI.02422-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ugaonkar S.R., Wesenberg A., Wilk J., Seidor S., Mizenina O., Kizima L., et al. A novel intravaginal ring to prevent HIV-1, HSV-2, HPV, and unintended pregnancy. J Contr Release. 2015;213:57–68. doi: 10.1016/j.jconrel.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Friedland B.A., Hoesley C.J., Plagianos M., Hoskin E., Zhang S., Teleshova N., et al. First-in-human trial of MIV-150 and zinc acetate coformulated in a carrageenan gel: safety, pharmacokinetics, acceptability, adherence, and pharmacodynamics. J Acquir Immune Defic Syndr. 2016;73:489–496. doi: 10.1097/QAI.0000000000001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li G., De Clercq E. A medicinal chemist who reshaped the antiviral drug industry: John Charles Martin (1951–2021) Med Res Rev. 2022;42:647–653. doi: 10.1002/med.21858. [DOI] [PubMed] [Google Scholar]
- 154.De Clercq E. Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF) Biochem Pharmacol. 2016;119:1–7. doi: 10.1016/j.bcp.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 155.Rhee S.Y., Kassaye S.G., Barrow G., Sundaramurthi J.C., Jordan M.R., Shafer R.W. HIV-1 transmitted drug resistance surveillance: shifting trends in study design and prevalence estimates. J Int AIDS Soc. 2020;23:e25611. doi: 10.1002/jia2.25611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Siedner M.J., Moorhouse M.A., Simmons B., de Oliveira T., Lessells R., Giandhari J., et al. Reduced efficacy of HIV-1 integrase inhibitors in patients with drug resistance mutations in reverse transcriptase. Nat Commun. 2020;11:5922. doi: 10.1038/s41467-020-19801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Senneker T., Tseng A. An update on neuropsychiatric adverse effects with second-generation integrase inhibitors and nonnucleoside reverse transcriptase inhibitors. Curr Opin HIV AIDS. 2021;16:309–320. doi: 10.1097/COH.0000000000000705. [DOI] [PubMed] [Google Scholar]
- 158.Li G., Piampongsant S., Faria N.R., Voet A., Pineda-Pena A.C., Khouri R., et al. An integrated map of HIV genome-wide variation from a population perspective. Retrovirology. 2015;12:18. doi: 10.1186/s12977-015-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Biswas A., Haldane A., Arnold E., Levy R.M. Epistasis and entrenchment of drug resistance in HIV-1 subtype B. Elife. 2019;8:e50524. doi: 10.7554/eLife.50524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ding L., Zhuang C., Chen F. Druggability modification strategies of the diarylpyrimidine-type non-nucleoside reverse transcriptase inhibitors. Med Res Rev. 2021;41:1255–1290. doi: 10.1002/med.21760. [DOI] [PubMed] [Google Scholar]
- 161.Jin X., Piao H.R., Pannecouque C., De Clercq E., Zhuang C., Chen F.E. Design of the naphthyl-diarylpyrimidines as potent non-nucleoside reverse transcriptase inhibitors (NNRTIs) via structure-based extension into the entrance channel. Eur J Med Chem. 2021;226:113868. doi: 10.1016/j.ejmech.2021.113868. [DOI] [PubMed] [Google Scholar]
- 162.Link J.O., Rhee M.S., Tse W.C., Zheng J., Somoza J.R., Rowe W., et al. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature. 2020;584:614–618. doi: 10.1038/s41586-020-2443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Terrault N.A., Lok A.S.F., McMahon B.J., Chang K.M., Hwang J.P., Jonas M.M., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 165.Sarin S.K., Kumar M., Lau G.K., Abbas Z., Chan H.L., Chen C.J., et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ambati J., Magagnoli J., Leung H., Wang S.B., Andrews C.A., Fu D., et al. Repurposing anti-inflammasome NRTIs for improving insulin sensitivity and reducing type 2 diabetes development. Nat Commun. 2020;11:4737. doi: 10.1038/s41467-020-18528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fukuda S., Varshney A., Fowler B.J., Wang S.B., Narendran S., Ambati K., et al. Cytoplasmic synthesis of endogenous Alu complementary DNA via reverse transcription and implications in age-related macular degeneration. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2022751118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Garcia-Trejo J.J., Ortega R., Zarco-Zavala M. Putative repurposing of lamivudine, a nucleoside/nucleotide analogue and antiretroviral to improve the outcome of cancer and COVID-19 patients. Front Oncol. 2021;11:664794. doi: 10.3389/fonc.2021.664794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 170.Li G., Liu Y., Jing X., Wang Y., Miao M., Tao L., et al. Mortality risk of COVID-19 in elderly males with comorbidities: a multi-country study. Aging. 2020;13:27–60. doi: 10.18632/aging.202456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jiang C., Wang Y., Hu M., Wen L., Wen C., Wang Y., et al. Antibody seroconversion in asymptomatic and symptomatic patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Transl Immunol. 2020;9:e1182. doi: 10.1002/cti2.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhou Z., Zhang M., Wang Y., Zheng F., Huang Y., Huang K., et al. Clinical characteristics of older and younger patients infected with SARS-CoV-2. Aging. 2020;12:11296–11305. doi: 10.18632/aging.103535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Miao M., Clercq E., Li G. Genetic diversity of SARS-CoV-2 over a one-year period of the COVID-19 pandemic: a global perspective. Biomedicines. 2021;9:412. doi: 10.3390/biomedicines9040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen Z., Du R., Galvan Achi J.M., Rong L., Cui Q. SARS-CoV-2 cell entry and targeted antiviral development. Acta Pharm Sin B. 2021;11:3879–3888. doi: 10.1016/j.apsb.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Baraniuk C. Where are we with drug treatments for COVID-19?. BMJ. 2021;373:n1109. doi: 10.1136/bmj.n1109. [DOI] [PubMed] [Google Scholar]
- 176.Del Amo J., Polo R., Moreno S., Diaz A., Martinez E., Arribas J.R., et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study. Ann Intern Med. 2020;173:536–541. doi: 10.7326/M20-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sang Y., Ding L., Zhuang C., Chen F. Design strategies for long-acting anti-HIV pharmaceuticals. Curr Opin Pharmacol. 2020;54:158–165. doi: 10.1016/j.coph.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 178.Sax P.E., DeJesus E., Crofoot G., Ward D., Benson P., Dretler R., et al. Bictegravir versus dolutegravir, each with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection: a randomised, double-blind, phase 2 trial. Lancet HIV. 2017;4:e154–e160. doi: 10.1016/S2352-3018(17)30016-4. [DOI] [PubMed] [Google Scholar]
- 179.Sax P.E., Pozniak A., Montes M.L., Koenig E., DeJesus E., Stellbrink H.J., et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390:2073–2082. doi: 10.1016/S0140-6736(17)32340-1. [DOI] [PubMed] [Google Scholar]
- 180.Gallant J., Lazzarin A., Mills A., Orkin C., Podzamczer D., Tebas P., et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390:2063–2072. doi: 10.1016/S0140-6736(17)32299-7. [DOI] [PubMed] [Google Scholar]
- 181.Molina J.M., Ward D., Brar I., Mills A., Stellbrink H.J., Lopez-Cortes L., et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV. 2018;5:e357–e365. doi: 10.1016/S2352-3018(18)30092-4. [DOI] [PubMed] [Google Scholar]
- 182.Daar E.S., DeJesus E., Ruane P., Crofoot G., Oguchi G., Creticos C., et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2018;5:e347–e356. doi: 10.1016/S2352-3018(18)30091-2. [DOI] [PubMed] [Google Scholar]
- 183.Kityo C., Hagins D., Koenig E., Avihingsanon A., Chetchotisakd P., Supparatpinyo K., et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide (B/F/TAF) in virologically suppressed HIV-1 infected women: a randomized, open-label, multicenter, active-controlled, phase 3, noninferiority trial. J Acquir Immune Defic Syndr. 2019;82:321–328. doi: 10.1097/QAI.0000000000002137. [DOI] [PubMed] [Google Scholar]
- 184.Sax P.E., Rockstroh J.K., Luetkemeyer A.F., Yazdanpanah Y., Ward D., Trottier B., et al. Switching to bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with HIV. Clin Infect Dis. 2020;73:e485–e493. doi: 10.1093/cid/ciaa988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.DeJesus E., Ramgopal M., Crofoot G., Ruane P., LaMarca A., Mills A., et al. Switching from efavirenz, emtricitabine, and tenofovir disoproxil fumarate to tenofovir alafenamide coformulated with rilpivirine and emtricitabine in virally suppressed adults with HIV-1 infection: a randomised, double-blind, multicentre, phase 3b, non-inferiority study. Lancet HIV. 2017;4:e205–e213. doi: 10.1016/S2352-3018(17)30032-2. [DOI] [PubMed] [Google Scholar]
- 186.Orkin C., DeJesus E., Ramgopal M., Crofoot G., Ruane P., LaMarca A., et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide coformulated with rilpivirine and emtricitabine in virally suppressed adults with HIV-1 infection: a randomised, double-blind, multicentre, phase 3b, non-inferiority study. Lancet HIV. 2017;4:e195–e204. doi: 10.1016/S2352-3018(17)30031-0. [DOI] [PubMed] [Google Scholar]
- 187.Venter W.D.F., Moorhouse M., Sokhela S., Fairlie L., Mashabane N., Masenya M., et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381:803–815. doi: 10.1056/NEJMoa1902824. [DOI] [PubMed] [Google Scholar]
- 188.Mills A., Crofoot G., Jr., McDonald C., Shalit P., Flamm J.A., Gathe J., Jr., et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate in the first protease inhibitor-based single-tablet regimen for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr. 2015;69:439–445. doi: 10.1097/QAI.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 189.Eron J.J., Orkin C., Gallant J., Molina J.M., Negredo E., Antinori A., et al. A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients. AIDS. 2018;32:1431–1442. doi: 10.1097/QAD.0000000000001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Orkin C., Molina J.M., Negredo E., Arribas J.R., Gathe J., Eron J.J., et al. Efficacy and safety of switching from boosted protease inhibitors plus emtricitabine and tenofovir disoproxil fumarate regimens to single-tablet darunavir, cobicistat, emtricitabine, and tenofovir alafenamide at 48 weeks in adults with virologically suppressed HIV-1 (EMERALD): a phase 3, randomised, non-inferiority trial. Lancet HIV. 2018;5:e23–e34. doi: 10.1016/S2352-3018(17)30179-0. [DOI] [PubMed] [Google Scholar]
- 191.Sax P.E., Zolopa A., Brar I., Elion R., Ortiz R., Post F., et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr. 2014;67:52–58. doi: 10.1097/QAI.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 192.Mills A., Arribas J.R., Andrade-Villanueva J., DiPerri G., Van Lunzen J., Koenig E., et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16:43–52. doi: 10.1016/S1473-3099(15)00348-5. [DOI] [PubMed] [Google Scholar]
- 193.Hodder S., Squires K., Kityo C., Hagins D., Avihingsanon A., Kido A., et al. Brief report: efficacy and safety of switching to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide (E/C/F/TAF) in virologically suppressed women. J Acquir Immune Defic Syndr. 2018;78:209–213. doi: 10.1097/QAI.0000000000001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Maggiolo F., Rizzardini G., Raffi F., Pulido F., Mateo-Garcia M.G., Molina J.M., et al. Bone mineral density in virologically suppressed people aged 60 years or older with HIV-1 switching from a regimen containing tenofovir disoproxil fumarate to an elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide single-tablet regimen: a multicentre, open-label, phase 3b, randomised trial. Lancet HIV. 2019;6:e655–e666. doi: 10.1016/S2352-3018(19)30195-X. [DOI] [PubMed] [Google Scholar]
- 195.Molina J.M., Cahn P., Grinsztejn B., Lazzarin A., Mills A., Saag M., et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet. 2011;378:238–246. doi: 10.1016/S0140-6736(11)60936-7. [DOI] [PubMed] [Google Scholar]
- 196.Cohen C., Wohl D., Arribas J.R., Henry K., Van Lunzen J., Bloch M., et al. Week 48 results from a randomized clinical trial of rilpivirine/emtricitabine/tenofovir disoproxil fumarate vs. efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV-1-infected adults. AIDS. 2014;28:989–997. doi: 10.1097/QAD.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 197.Margolis D.A., Gonzalez-Garcia J., Stellbrink H.J., Eron J.J., Yazdanpanah Y., Podzamczer D., et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390:1499–1510. doi: 10.1016/S0140-6736(17)31917-7. [DOI] [PubMed] [Google Scholar]
- 198.Orkin C., Arasteh K., Górgolas Hernández-Mora M., Pokrovsky V., Overton E.T., Girard P.M., et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. 2020;382:1124–1135. doi: 10.1056/NEJMoa1909512. [DOI] [PubMed] [Google Scholar]
- 199.Swindells S., Andrade-Villanueva J.F., Richmond G.J., Rizzardini G., Baumgarten A., Masia M., et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med. 2020;382:1112–1123. doi: 10.1056/NEJMoa1904398. [DOI] [PubMed] [Google Scholar]
- 200.Overton E.T., Richmond G., Rizzardini G., Jaeger H., Orrell C., Nagimova F., et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet. 2021;396:1994–2005. doi: 10.1016/S0140-6736(20)32666-0. [DOI] [PubMed] [Google Scholar]
- 201.Orkin C., Squires K.E., Molina J.M., Sax P.E., Wong W.W., Sussmann O., et al. Doravirine/lamivudine/tenofovir disoproxil fumarate is non-inferior to efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive adults with human immunodeficiency virus-1 infection: week 48 results of the DRIVE-AHEAD trial. Clin Infect Dis. 2019;68:535–544. doi: 10.1093/cid/ciy540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Molina J.M., Squires K., Sax P.E., Cahn P., Lombaard J., DeJesus E., et al. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 48-week results of a randomised, double-blind, phase 3, non-inferiority trial. Lancet HIV. 2018;5:e211–e220. doi: 10.1016/S2352-3018(18)30021-3. [DOI] [PubMed] [Google Scholar]
- 203.Johnson M., Kumar P., Molina J.M., Rizzardini G., Cahn P., Bickel M., et al. Switching to doravirine/lamivudine/tenofovir disoproxil fumarate (DOR/3TC/TDF) maintains HIV-1 virologic suppression through 48 weeks: results of the DRIVE-SHIFT trial. J Acquir Immune Defic Syndr. 2019;81:463–472. doi: 10.1097/QAI.0000000000002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.