Abstract
Discovery of drugs rapidly and effectively is an important aspect for Alzheimer's disease (AD). In this study, a novel high-throughput screening (HTS) method aims at screening the small-molecules with amyloid-β (Aβ) binding affinity from natural medicines, based on the combinational use of biolayer interferometry (BLI) and ultra-high-performance liquid chromatography coupled with diode-array detector and quadrupole/time-of-flight tandem mass spectrometry (UHPLC−DAD-Q/TOF-MS/MS) has been firstly developed. Briefly, the components in natural medicines disassociated from biotinylated Aβ were collected to analyze their potential Aβ binding affinity by UHPLC−DAD-Q/TOF-MS/MS. Here, baicalein was confirmed to exhibit the highest binding affinity with Aβ in Scutellaria baicalensis. Moreover, polyporenic acid C (PPAC), dehydrotumulosic acid (DTA), and tumulosic acid (TA) in Kai-Xin-San (KXS) were also identified as potent Aβ inhibitors. Further bioactivity validations indicated that these compounds could inhibit Aβ fibrillation, improve the viability in Aβ-induced PC-12 cells, and decrease the Aβ content and improve the behavioral ability in Caenorhabditis elegans. The molecular docking results confirmed that PPAC, DTA, and TA possessed good binding properties with Aβ. Collectively, the present study has provided a novel and effective HTS method for the identification of natural inhibitors on Aβ fibrillation, which may accelerate the process on anti-AD drugs discovery and development.
KEY WORDS: Alzheimer's disease, High-throughput screening, Biolayer interferometry, UHPLC−DAD-Q/TOF-MS/MS, Kai-Xin-San
Graphical abstract
A high-throughput screening (HTS) method based on the combinational use of biolayer interferometry (BLI) and UHPLC‒DAD-Q/TOF-MS/MS is developed. PPAC, DTA, and TA are identified from Kai-Xin-San as potent Aβ inhibitors.
1. Introduction
Alzheimer's disease (AD), an age-related neurodegenerative disease, has become the most common dementia and accounted for almost 60%–80% of all cases1. The epidemiological data indicated that the prevalence of AD is increasing with the world's population ageing. Currently, there are approximately 35.6 million AD patients worldwide2,3. The clinical symptoms of AD manifest mainly as progressive memory loss, cognitive dysfunction, distraction, mood disorder, and personality change4. At present, AD is still an irreversible and incurable neurodegenerative disease. The mechanistic studies indicated that the pathology of AD is closely related the occurrence of neuroinflammation and neuronal death induced by the increasingly accumulated misfolded protein aggregates, such as β-amyloid (Aβ) and Tau5. Among them, Aβ is generated from amyloid precursor protein (APP) through the sequential cleavage of β- and γ-secretase6. The generated fragments including Aβ1‒40 and Aβ1‒42 aggregates are recognized to be the most toxic types, which are accumulated extracellularly to form Aβ fibrils and ultimately form senile plaque in the brain of AD patients7. In the early state of AD, Aβ can still be cleared through the autophagy‒lysosome pathway (ALP)8 and the ubiquitin−proteasome pathway (UPP) in neurons or engulfed by microglia9. However, both ALP and UPP are dysregulated with age, and the clearance of Aβ cannot be executed effectively10. Therefore, targeting inhibition of Aβ fibrils generation has become a promising therapeutic strategy for AD.
It has been proved for a long time that natural medicines such as traditional Chinese medicines (TCMs) are safe and exhibit the favorable characteristics, including multi-components, multi-efficacies, and multi-targets11. In addition, natural medicines have rich resources for the discovery of new drugs12. Many natural medicines or their ingredients were found to exhibit potent neuroprotective effects in multiple models of AD13, 14, 15, 16, 17. For example, Aegle marmelos extract18, lychee seed fraction19, Cirsium japonicum var. maackii extract20, curcumin, ferulic acid21, quercetin13, chlorogenic acid22, and polysaccharides in Lycium barbarum23 were reported to inhibit Aβ-induced cytotoxicity and ameliorate the Aβ pathology in AD animals. However, there are still very few promising compounds identified from natural medicines for the treatment of AD. This may be due to the discovery of bioactive components from natural medicines such as Chinese medical herbs or formulas with complex chemical constituents is time-consuming and laborious24. Therefore, it is very important to develop an effective, time-saving, and reliable method for the screening of small-molecule inhibitors of Aβ from natural medicines. To date, several screening methods reported, including cell membrane chromatography (CMC)25, network pharmacology and molecular docking26, affinity ultrafiltration with drug targets of interest coupled to high-performance liquid chromatography−mass spectrometry (AUF−HPLC−MS/MS)27, bubble-generating magnetic liposomes coupled with liquid chromatography−mass spectrometry (LC−MS)28, etc. In general, most of them are developed based the application of advanced analytical instruments, such as HPLC, MS, and nuclear magnetic resonance (NMR)29,30.
In our previous studies, we employed CMC, target-fishing approach, and protein-molecule interaction coupled with ultra-HPLC coupled with diode-array detector and time of flight/mass spectrometry (UHPLC−DAD-TOF/MS) to identify the bioactive components from Chinese medical herbs31. Here, we developed a more rapid and reliable high-throughput screening (HTS) method for the discovery of inhibitors of Aβ fibrillation from TCMs by the combinational use of biolayer interferometry (BLI) technology and UHPLC−DAD and quadrupole with a time of flight tandem mass spectrometry (UHPLC−DAD-Q/TOF-MS/MS). Scutellaria baicalensis (SB) is widely reported to inhibit Aβ both in vitro and in vivo32. In addition, we previously identified that baicalein and baicalin exhibited binding affinity with Aβ31. Therefore, SB was further selected to validate the feasibility of the present screening method. Similarly, baicalein and baicalin were the components in SB that showed the best binding characteristics with Aβ31. Moreover, we further employed this method to identify the Aβ fibrilization inhibitor from Kai-Xin-San (KXS), a classical Chinese medical formula composed of Ginseng Radix, Polygalae Radix, Acori Tatarinowii Rhizoma, and Poria. KXS is commonly used for the treatment of dementia in ancient China and also in modern Chinese medicine hospitals33. Finally, three compounds in Poria, including polyporenic acid C (PPAC), dehydrotumulosic acid (DTA), and tumulosic acid (TA), were identified. The bioassay results showed that PPAC, DTA, and TA significantly inhibited Aβ fibrils formation, improved the viability of Aβ1–42-treated PC-12 cells, as well as decreased the Aβ content and improved behavioral abilities in Caenorhabditis elegans of AD. The molecular docking results indicated that PPAC, DTA, and TA possessed good binding property with Aβ. Collectively, the current study presents a novel and effective HTS method for the natural inhibitors of Aβ fibrillation from TCMs based on the combinational use of BLI and UHPLC−DAD-Q/TOF-MS/MS, which accelerates the discovery and development of anti-AD drugs in the future.
2. Materials and methods
2.1. Chemicals and reagents
3-(4,5-Dimethyl-thiazol-2-yl)-2,5-dimethyl-tetrazolium bromide (MTT, M2128) and Thioflavin T (ThT, 596200) were acquired from Sigma–Aldrich (St. Louis, USA). Water used for experiment was prepared using the Milli-Q integral system (Millipore, Billerica, MA, USA). Acetonitrile reagent was bought from Anaqua Chemicals Supply (ACS, Houston, TX, USA). Aβ1–42 peptide was from China Peptides Co., Ltd. (Shanghai, China). EZ-Link NHS-LC-LC-Biotin was purchased from Thermo Fisher Scientific Inc., (Waltham, MA, USA). Streptavindin (SA) biosensors used for BLI analysis were obtained from PALL ForteBio (NY, USA). Hoechst 33342 (B2261) and propidium iodide (PI, P4170) were bought from Sigma (St. Louis, MO, USA). The Annexin V-FITC/PE Apoptosis Detection Kit was purchased from BD Biosciences (San Jose, CA, USA). The antibodies including 6E10 (803001; BioLegend), A11 (AHB0052; Invitrogen), and OC (ab201062; Abcam) were used in this study. Ponceau S was purchased from Saint-Bio (Shanghai, China).
2.2. Cell culture
PC-12 cells were purchased from American Type Culture Collection (ATCC; Rockville, MD, USA) and maintained in Dulbecco's modified Eagle's medium (DMEM) culture medium which was supplemented with 10% horse serum (HS), 5% fetal bovine serum (FBS), and 1% penicillin/streptomycin (PS). PC-12 cells used for the in vitro experiments were cultured in an incubator with 5% humidified CO2 and 37 °C.
2.3. Extract preparation of natural medicines
Chinese medical herbs including Scutellaria Radix and Poria, as well as the Chinese medical formula KXS composed of Ginseng Radix, Polygalae Radix, Acori Tatarinowii Rhizoma, and Poria were weighed and crushed, respectively. Then, the crude powders were extracted by reflux method using 10 times of its volume of water at boiling temperature for 1 h. After 3 repeat extractions, the water extract solutions were combined, filtered, and concentrated. The dried extract was quantified and dissolved by DMSO to the stock concentration of 200 mg/mL. All the extracts were stored at −80 °C until further experiments.
2.4. Preparation of Aβ1–42 peptide
Aβ1–42 peptide was prepared as described previously31. To begin with, Aβ1–42 peptide (5 mg) was dissolved with appropriate volume of hexafluoroisopropanol (HFIP, Sigma) solution. The HFIP solution was then aliquoted and dried under the nitrogen flow. The generated thin Aβ1–42 peptide film was then stored at −80 °C until further BLI analysis, Western blot analysis, and the evaluation of cell viability experiments.
2.5. Isolations of PPAC, DTA, and TA
The crude powder of Poria was extracted by ethyl acetate reagent to obtain the extract for the isolation of PPAC, DTA, and TA. In brief, 100 g of Poria dry extract was loaded onto the open glass column filled with silica gel particles. The components in Poria ethyl acetate extract were eluted by the reagent system consisting of methanol and dichloromethane. Then, the components were collected for the further purification on a reversed-phase YMC-Triart C18 column (250 mm × 10 mm, S-5 μm, 12 nm, YMC Co., Ltd. Kyoto, Japan) which was equipped on a Shimadzu (Kyoto, Japan) Prominence HPLC system. This HPLC system consists of two LC-20AD pumps, a SIL-20ACHT autosampler, a CBM-20A communications bus module, an SPD-M20A diode array detector, and a CTO-20AC column heater-cooler. The column temperature was maintained at 30 °C. The mobile phase was composed of methanol and 0.1% formic acid in water (80:20, v/v), and the flow rate was set at 4 mL/min. Three main peaks corresponding to PPAC, DTA, and TA were collected. Then, the collected solution was evaporated by the rotary vacuum concentration instrument.
2.6. BLI analysis
The preparation of biotinylated Aβ1–42 and the BLI analysis were performed according to the reported literatures34,35. In brief, the above generated Aβ1–42 peptide film was dissolved in PBS to make a concentration of 25 μg/mL, which was subjected to the biotinylation with EZ-Link NHS-LC-LC-Biotin. After pre-wetting the SA biosensor with PBS to record the baseline, the biotinylated Aβ1–42 in 96-well black F-bottom plates (655209, Greiner, Germany) were directly immobilized on the SA biosensor. The extract of natural medicines or single compounds were diluted by PBS (containing 15% DMSO and 0.02% Tween 20) to the appropriate concentrations with the final volume of 200 μL per well. At the same time, an equal volume of PBS (containing 15% DMSO and 0.02% Tween 20) was added to wells and set as the control group. A total of 4 major steps, including loading for 300 s, baseline for 60 s, association for 120 s, and dissociation for 120 s, are repeated circularly. The data were acquired and analyzed by ForteBio Octet® Data Acquisition and Data Analysis software (Port Washington, NY, USA).
2.7. UHPLC−DAD-Q/TOF-MS/MS conditions
Analyses of all samples were performed on a UHPLC system (Shimadzu, Kyoto, Japan) which is composed of a solvent delivery system LC-3AD, an autosampler SIL30ACXR, a column oven CTO-30AC, a degasser DGU-20A3, and a controller CBM-20A. The separation was conducted on an UHPLC Agilent column Zorbax EcLipse Plus C18 at a flow rate of 0.3 mL/min (1.8 μm, 100 mm × 2.1 mm). During the analysis, the column temperature was maintained at 40 °C. The mobile phase is made of water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B). The elution program was set as follows: 0–15 min, 10%–95% B; 15.01–19 min, 10% B. A triple TOFM X500R system equipped with a Duo Spray source (AB SCIEX, Foster City, CA, USA) was used to conduct the MS analysis in the negative electrospray ion mode. The parameters of electrospray ionization (ESI) were set as follows: ion spray voltage: −4500 V; curtain gas: 35 psi; ion source temperature: 550 °C; declustering potential (DP): −100 V, nebulizer gas (GS 1): 55 psi, and heater gas (GS 2): 55 psi. m/z 100–1600 Da was used for the MS scan. Data analysis were performed by the Peak View® 1.4 software (AB SCIEX Foster City, CA, USA).
2.8. Calculation of the relative binding amount
Calculation of the relative binding amount (RBA) of the compounds with Aβ in TCMs was performed according to the following procedures. In brief, after BLI analysis, the samples, including the dissociation buffer without extract of natural medicines (S1), the dissociation buffer with extract of natural medicines (S2), and the extract of natural medicines used for the BLI assay (S3), were collected for the analysis by the UHPLC−DAD-Q/TOF-MS/MS instrument. Through comparison of the identified compounds in the above samples, the compounds that could be detected in S2 and S3 but cannot be detected in S1 were recognized to be the potential compounds with Aβ binding affinity. Then, the relative binding amount (RBA) of compounds was calculated using Eq.(1):
| (1) |
2.9. MTT assay
100 μL of PC-12 cells were seeded into 96-well plate with a density of 30,000 cells/mL. On the second day, PC-12 cells were treated with the test drugs in the absence or presence of Aβ1–42 for 24 h. After treatment, 10 μL of MTT solution (5 mg/mL) was added to the wells, which was followed by an incubation at 37 °C for 4 h. Then, the solution was discarded and the blue formazan was dissolved by 100 μL of DMSO. The optical density (OD) value of the solution was read by a microplate spectrophotometer (BioTek, VT Lab, USA) with a wavelength of 570 nm. The cell viability was calculated according to Eq.(2):
| (2) |
2.10. ThT assay
2 μL of freshly prepared 1 mmol/L of Aβ1–42 was mixed with PBS in the presence or absence of the test drugs including PPAC, DTA, TA, curcumin (Cur), Poria or KXS extract. The final volume of above solutions was set to 100 μL36. At the incubation time points of 0, 24, and 48 h, 50 μL of ThT (20 μmol/L) was diluted with PBS (pH = 7.4) and mixed with 100 μL of the above solutions. Then, the mixture solutions were transferred into black 96-well plates for the reading of OD values by a microplate spectrophotometer (BioTek, VT Lab, USA) with an excitation wavelength of 450 nm and an emission wavelength of 490 nm.
2.11. Detection of Aβ structural species by Western blot
Aβ1–42 peptide film was dissolved in PBS to make a concentration of 20 μmol/L, which was incubated at 37 °C without or with the test drugs. After 24 h, 2 μL of each mixture was diluted with PBS and incubated with 0.01% glutaraldehyde (v/v) for 10 min. The crosslinking reaction was then terminated with 5.0 μL 5 × loading buffer containing 5% β-mercaptoethanol (Beyotime, Shanghai, China). All samples were subjected to sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) electrophoresis without boiling. Subsequently, the peptide was transferred to polyvinylidene difluoride (PVDF) membranes (PALL, USA). After blocking with 5% milk in PBST, the membranes were incubated with the primary anti-Aβ monoclonal antibody 6E10 (1:2000, Biolegend) at 4 °C overnight. Then, the membranes were incubated with horseradish peroxidase (HRP)-linked secondary antibodies (1:2000) at room temperature for 1 h. Protein bands were detected by the UltraSignal™ ECL Substrate kit (4A Biotech Co., Ltd., Beijing, China) and visualized by BIO-RAD ChemiDoc™ Imaging System (Hercules, USA). Quantification of band intensity was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.12. Flow cytometry analysis
In addition to MTT assay, the viability of PC-12 cells was also evaluated by flow cytometry using the Annexin V-FITC/PE apoptosis detection kit (BD Biosciences, San Jose, CA, USA)37. Briefly, Aβ1–42-induced PC-12 cells were treated with or without test drugs. After 24 h, cells were collected for centrifugation at a speed of 2000 rpm. The cell pellets were re-suspended and stained in 250 μL of 1 × Annexin V solution composed of 2 μL propidium iodide (PI) and 1 μL fluoresceine isothiocyanate (FITC) reagent in the dark for 15 min. Then, the analysis of cell death was performed on a flow cytometer (FACSverse, BD Biosciences, USA). Data analysis were performed with Flowjo 7.6.1 software (Tree Star, San Carlos, CA, USA).
2.13. Hoechst 33342/PI double staining assay
Moreover, cell death was also examined measured by the Hoechst 33342/PI staining method as described previously38. Briefly, Aβ1–42-induced PC-12 cells on the coverslips were treated with or without test drugs. After 24 h, cells were subjected to fixation with 4% paraformaldehyde (PFA) reagent and PBS washing for 3 times, which was followed by a staining with 5 mg/L of Hoechst 33342 reagent and 5 mg/L of PI reagent. After incubation for 5 min, the representative images of cells at the same field were captured and merged by ImageXpress Micro4 (Molecular Devices, USA). Then, the death of PC-12 cells was measured by calculating the ratio of PI stained cells to the Hoechst 33342 stained cells.
2.14. C. elegans strains and maintenance conditions
The worm strains including N2 (wild type); CL4176, dvIs27 [myo-3p::A-Beta (1–42)::let-851 3′UTR) + rol-6(su1006)] X; CL2122, dvIs15 [(pPD30.38) unc-54(vector) + (pCL26) mtl-2::GFP]; CL2355, dvIs50 [pCL45 (snb-1::Abeta 1–42::3′ UTR(long) + mtl-2::GFP] I; and CL2331, dvIs37 [myo-3p::GFP::A-Beta (3–42) + rol-6(su1006)] were from Caenorhabditis Genetics Center (CGC, University of Minnesota, USA). All worms were cultured on plates covered with nematode growth media (NGM) and feed with Escherichia coli OP50 in an incubator at 20 °C unless otherwise noted.
2.15. Paralysis assay
The behavioral improvement effect of the test drugs in vivo was performed by measuring the paralysis ratio in the C. elegans models of AD39. In brief, CL4176 nematodes containing a heat-inducible human Aβ1–42 transgene expressed in muscle cells were transferred to NGM and treated with the test drugs. The temperature of incubator was shifted from 15 to 25 °C so as to stimulate Aβ expression and aggregation. After exposure to 25 °C for 36 h, the paralysis of worms was scored. The performance of nematodes which failed to move their bodies when touched or exhibited a “halo” of cleared bacterial lawn while feeding was considered to be paralysis.
2.16. Food-searching behavior assay
CL2355 strains which express the human Aβ1–42 and its vector control CL2122 strains were used to investigate the improvement effect of the test drugs on the food-sensing behavior. In brief, the plates were spread with E. coli OP50 in a ring with an outer diameter of 8 cm and an inner diameter of 1 cm40. After treatment with PPAC, DTA, TA, Cur or Poria extract for 48 h, worms were washed off and collected with M9 buffer and moved to an NGM agar plate which was pre-spotted with or without OP50 E. coli lawn. After 5 min, body-bends of worms were counted for 20 s intervals. The slowing of the body bending rate was calculated according to Eq.(3):
| (3) |
where N indicates the numbers of body-bends of nematodes.
2.17. Preparation of worm proteins
After treatment, worms in the plates were collected into tubes with M9 buffer, which was followed by a centrifugation and washing to remove bacteria. The worms were then resuspended with 100 μL lysis buffer (50 mmol/L HEPES, 75 mmol/L sucrose, 1 mmol/L EDTA, 6 mmol/L MgCl2, 1 mmol/L DTT, 25 mmol/L benzamidine, and 1% Triton X-100) in the presence of protease inhibitors. The lysis solution was sonicated for 3 times for 15 s on ice, which was followed by the centrifugation for 10 min at 14,000 rpm. Then, the supernatant was collected for the measurement of protein concentration using the Bio-RAD Bradford Protein Assay.
2.18. Detection of Aβ content in worms by dot blot analysis
Equal amount of worm protein was spotted on a 0.22 mm2 PVDF membrane. Then, 5% nonfat milk was used to block the membrane for 1 h at room temperature. After wash with PBST, the membrane was incubated with the primary anti-Aβ monoclonal antibodies including 6E10, A11, and OC at 4 °C overnight. Then, the membrane was washed and subsequently incubated with the HRP-conjugated secondary antibody at room temperature. The antibody binding was detected using the UltraSignal™ ECL Substrate kit and visualized by a ChemiDoc™ Imaging System. Meanwhile, ponceau S (0.1%, w/v) solution (Saint-Bio, Shanghai, China) was used to stain the membrane for the visualization of total proteins.
2.19. Detection of Aβ content in worms by Western blot
Equal amount of worm protein in loading buffer (100 mmol/L DTT, 50 mmol/L Tris-HCl, pH 6.8, 10% glycerol, 5% SDS, 5% β-mercaptoethanol, and 0.02% bromophenol blue) was heated to 99 °C for 5 min. The electrophoresis was performed on a Tris-tricine SDS-PAGE using a constant voltage (150 V). Then, the proteins on gel were transferred to PVDF membranes. The membranes were blocked with 5% milk for 1 h at room temperature and followed by an overnight incubation with the primary anti-Aβ monoclonal antibody 6E10 (1:2000, Biolegend) at 4 °C overnight. Then, the membranes were incubated with the HRP-conjugated secondary antibody (1:2000) at room temperature for 1 h. After washing with TBST for 3 times, the membranes were detected by the UltraSignal™ ECL Substrate Kit and visualized by a BIO-RAD ChemiDoc™ Imaging System. Quantification of band was performed as described above.
2.20. Aβ3–42 aggregation analysis
Aggregation of Aβ3–42 was studied using CL2331 strains which temperature-sensitively express GFP bound to human Aβ3–42 in body wall muscle41. Briefly, worms treated with PPAC, DTA, TA or Poria extract were incubated at 23 °C to induce Aβ3–42 aggregation until day 2 of the adult worms. After treatment, the worms were mounted on glass slides containing 0.1% NaN3. The representative images of worms were captured by a fluorescence microscope (Leica DM6B, Leica Microsystems GmbH, Germany). The total number of Aβ deposits in the anterior area was then calculated.
2.21. Molecular docking
Due to the lacking of crystal structure mono Aβ1–42, a template-free modeling approach was used from a predicted distance/orientation matrix by deep residual neural networks training to the 3D model of human Aβ1–42 in trRosetta, which used multiple sequence information from a multiple sequence alignment in the Protein Data Bank database. Following trRosetta, the inter-residue distance and orientation constraints minimization of 3D models of Aβ1–42 was generated by neural network. Finally, the best fitted structure of Aβ1–42 satisfying the restraints was selected as the initial model for further ligand-docking according to Rosetta energy score.
To further explore the interaction mechanism and the binding modes of PPAC, DTA, or TA with Aβ1–42, a molecular docking study was performed using the DockThor protein−ligand docking program42. The SDF files of the ligands, including PPAC (CAS: 465-18-9), DTA (CAS: 6754-16-1), and TA (CAS: 508-24-7) were prepared from the NCBI Pubchem database. Then, the potential interaction modes between Aβ1–42 and PPAC, DTA or TA were investigated, and the docked conformation was visualized by Pymol program.
2.22. Statistical analysis
All data were obtained from three or more independent experiments and presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) followed by the post-Tukey test was conducted to analyze the significance of data using the GraphPad Prism 8.0 (San Diego, CA, USA). P < 0.05 was considered to have statistical significance among the compared groups.
3. Results
3.1. Screening of Aβ binding components in KXS
In our previous study, baicalin and baicalein were identified to be the potential inhibitors of Aβ fibrilization in SB by the UHPLC−DAD-Q/TOF-MS/MS analysis coupled with the pre-incubation of Aβ1–42 with SB extract31. In this study, we established a novel HTS method for the screening of Aβ fibrilization inhibitors from natural medicines based on the combinational use of BLI and UHPLC−DAD-Q/TOF-MS/MS, and used SB to validate the feasibility of this method. After the dissociation of SB from the SA biosensor, the components in the dissociation buffer were then subjected to detection by UHPLC−DAD-Q/TOF-MS/MS, and the RBA of the components in SB was determined. The curves indicating the association/dissociation binding of SB with Aβ and the kinetic constants suggested that the interaction of SB with Aβ was direct and reversible (Supporting Information Fig. S1A and Table S1). After dissociation of SB (400 μg/mL) from the biosensor for 5 times, the dissociation buffer with or without SB were collected and dried under the flow of nitrogen gas, respectively. At the same time, an equal volume of SB extract (400 μg/mL) used for the BLI assay was prepared in parallel. After analysis by the UHPLC−DAD-Q/TOF-MS/MS instrument. The representative total ion chromatograms (TICs) in Supporting Information Fig. S1B showed that some compounds in the dissociation buffer were detected when compared to SB extract alone. In addition, the Supporting Information Table S2 and Fig. S1C showed that baicalin and baicalein had the RBA in SB. Taken together, this method is a more effective, rapid, and reliable method for the screening of Aβ fibrillization inhibitor from natural medicines.
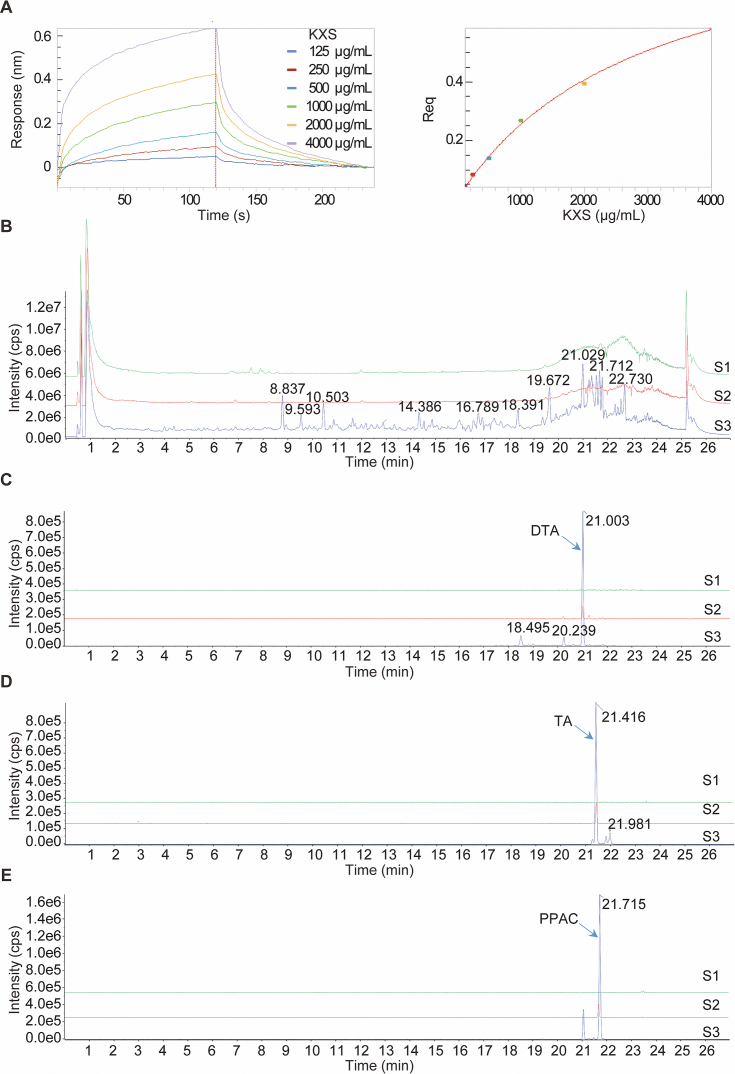
To further validate the feasibility of this newly developed screening method in more complex system, we employed it to screen the potential inhibitors of Aβ fibrillization from KXS, a classical formula widely used for the treatment of dementia, depression, and ageing. At first, the binding effect of KXS to Aβ1–42 was investigated by employing increasing concentrations of KXS extract (125, 250, 500, 1000, 2000, 4000 μg/mL) to monitor the real-time association/dissociation of KXS with biotinylated Aβ1–42. The result showed that KXS had a direct and reversible interaction with Aβ1–42, which was revealed by the concentration-dependent increase in response indicating the optical thickness (nm) on the sensor layer (Fig. 1A). In addition, the Table 1 showed that the kinetic constants including dissociation affinity (KD) association rate (Kon), and dissociation rate constant (Kdis) calculated by the ForteBio data analysis software were 170 μg/mL, 1.15 × 102 L/mol∙s, and 2.6 × 10−2 1/s, respectively. Then, the dissociation solutions with or without KXS extract were collected for the UHPLC−DAD-Q/TOF-MS/MS analysis. After analysis of the representative TICs in Fig. 1B, the information including the retention time, accurate mass, molecular weight, chemical name, formula, and RBA of the detected components in KXS extract were obtained in Table 2. Among the binding components, three compounds in Poria, including polyporenic acid C (PPAC), dehydrotumulosic acid (DTA), and tumulosic acid (TA), showed the strongest binding affinity towards Aβ, which were demonstrated by the significant increased peak area and RBA in the dissociation buffer with KXS extract (Fig. 1C–E and Table 2). Collectively, the above data suggested that PPAC, DTA, and TA in Poria may be the main inhibitors of Aβ fibrillization that contribute to the neuroprotective effect of KXS in AD.
Figure 1.
Screening of Aβ binding components in KXS by the combinational use of BLI and UHPLC−DAD-Q/TOF-MS/MS. (A) Real-time kinetic binding sensorgrams of different concentrations of KXS increasing from 125 to 4000 μg/mL are shown. Response (nm) indicates the optical thickness on the SA biosensor layer. The equilibrium binding signal (Req) revealed by the flattened curve is reached. (B) The TICs were recorded by UHPLC−DAD-Q/TOF-MS/MS. S1: The dissociation buffer without KXS extract; S2: The dissociation buffer with KXS extract; S3: KXS extract used for the BLI assay. (C) The extracted ion chromatograms (EICs) of dehydrotumulosic acid (DTA) in S1, S2, and S3. (D) The EICs of tumulosic acid (TA) in S1, S2, and S3. (E) The EICs of polyporenic acid C (PPAC) in S1, S2, and S3.
Table 1.
The binding affinity (KD), association rate constant (Kon) and dissociation rate constant (Kdis) of KXS to Aβ1–42.
| TCM | KD (μg/mL) | Kon (L/mol∙s) | Kdis (1/s) |
|---|---|---|---|
| KXS extract | 170 | 1.15 × 102 | 2.6 × 10−2 |
Table 2.
The main detected components in KXS and their RBA (%) with Aβ.
| Compd. | RT (min) | [M‒H]− or [M+HCOOH]‒ | Formula | MW | Chemcial name | RBA (%) |
|---|---|---|---|---|---|---|
| 1 | 2.889 | 461.1143 | C19H26O13 | 462 | Sibiricose A3 | 0.15 |
| 2 | 3.303 | 443.1772 | C20H28O11 | 444 | Sibiricaphenone | 0.04 |
| 3 | 3.409 | 431.1422 | C24H32O7 | 432 | Acortatarinowin E | 0.13 |
| 4 | 3.824 | 451.1092 | C21H24O11 | 452 | Telephenone B | 0.18 |
| 5 | 4.053 | 517.1389 | C22H30O14 | 518 | Sibiricose A5 | 0.17 |
| 6 | 4.246 | 429.1259 | C19H26O11 | 430 | Polygalatenoside B | 0.11 |
| 7 | 4.44 | 547.1481 | C23H32O15 | 548 | Sibiricose A6 | 0.18 |
| 8 | 5.435 | 547.1481 | C23H32O15 | 548 | Sibiricose A1 | 0.12 |
| 9 | 6.229 | 537.1063 | C24H26O14 | 538 | Sibiricaxanthone B | 0.20 |
| 10 | 6.416 | 567.1163 | C25H28O15 | 568 | Polygalaxanthone III | 0.11 |
| 11 | 6.425 | 537.1063 | C24H26O14 | 538 | Sibiricaxanthone A | 0.11 |
| 12 | 6.531 | 607.1685 | C24H34O15 | 608 | Sibiricose A2 | 0.14 |
| 13 | 6.773 | 567.1163 | C25H28O15 | 568 | Polygalaxanthone VIII | 0.19 |
| 14 | 6.963 | 567.1163 | C25H28O15 | 568 | Polygalaxanthone XI | 0.18 |
| 15 | 7.043 | 549.1429 | C26H30O13 | 550 | Telephiose F | 0.22 |
| 16 | 7.421 | 667.1659 | C30H36O17 | 668 | Tenuifoliside B | 0.12 |
| 17 | 7.638 | 607.1685 | C24H34O15 | 608 | Dalmaisione D | 0.11 |
| 18 | 7.676 | 753.1995 | C34H42O19 | 754 | 3′,4-Disinapoylsucrose | 0.05 |
| 19 | 7.897 | 607.1685 | C24H34O15 | 608 | Glomeratose A | 0.05 |
| 20 | 8.098 | 521.185 | C24H26O13 | 522 | Tricornoside C | 0.11 |
| 21 | 8.34 | 521.185 | C24H26O13 | 522 | Tricornoside F | 0.10 |
| 22 | 8.839 | 753.1995 | C34H42O19 | 754 | 3,6′-Disinapoylsucrose | 0.21 |
| 23 | 8.996 | 723.1903 | C33H40O18 | 724 | 3′-O-Feruloyl-6-O sinapoylsucrose | 0.16 |
| 24 | 9.361 | 429.1264 | C19H26O11 | 430 | Polygalatenoside C | 0.00 |
| 25 | 9.601 | 681.1806 | C31H38O17 | 682 | Tenuifoliside A | 0.18 |
| 26 | 9.946 | 977.4995 | C47H80O18 | 933 | Notoginsenoside R1 | 0.16 |
| 27 | 10.115 | 493.2122 | C25H36O7 | 448 | Asarolignan D | 0.12 |
| 28 | 10.507 | 845.4609 | C42H72O14 | 846 | Ginsenoside Rg1 | 0.12 |
| 29 | 10.768 | 783.2088 | C42H72O13 | 784 | Ginsenoside Rg2 | 0.10 |
| 30 | 10.92 | 767.2175 | C35H44O19 | 768 | Tenuifoliside C | 0.17 |
| 31 | 11.593 | 1307.3448 | C59H72O33 | 1308 | Tenuifoliose I/J | 0.05 |
| 32 | 11.674 | 1265.5381 | C57H70O32 | 1267 | Tenuifoliose K | 0.07 |
| 33 | 11.792 | 1337.353 | C60H74O34 | 1338 | Tenuifoliose B | 0.11 |
| 34 | 12.039 | 1235.529 | C65H88O23 | 1237 | Arillatanoside A | 0.03 |
| 35 | 12.237 | 1103.4905 | C53H84O24 | 1104 | Polygalaxanthone XXVIII | 0.12 |
| 36 | 12.242 | 1307.3448 | C59H72O33 | 1308 | Tenuifoliose I/J | 0.02 |
| 37 | 12.479 | 1337.353 | C60H74O34 | 1338 | Tenuifoliose D | 0.12 |
| 38 | 12.717 | 1349.3529 | C61H74O34 | 1350 | Tenuifoliose H | 0.09 |
| 39 | 12.928 | 1379.3626 | C62H76O35 | 1380 | Tenuifoliose A | 0.16 |
| 40 | 13.119 | 1409.3733 | C63H78O36 | 1410 | Tenuifoliose N | 0.00 |
| 41 | 13.38 | 1307.3448 | C61H96O30 | 1308 | Polygalasaponin XXXIV | 0.00 |
| 42 | 13.389 | 845.4609 | C42H72O14 | 846 | Pseudoginsenoside F11 | 0.07 |
| 43 | 13.675 | 1337.353 | C60H74O34 | 1338 | Senegose L | 0.00 |
| 44 | 13.845 | 1307.3448 | C61H96O30 | 1308 | Polygalasaponin XXXVII | 0.00 |
| 45 | 13.93 | 815.4517 | C41H70O13 | 771 | Notoginsenoside R2 | 0.05 |
| 46 | 14.387 | 1107.56 | C54H92O23 | 1109 | Ginsenoside Rb1 | 0.05 |
| 47 | 14.586 | 1195.5702 | C56H94O24 | 1151 | Quinquenoside R1 | 0.01 |
| 48 | 14.594 | 1193.5564 | C57H94O26 | 1195 | Malonyl-ginsenoside Rb1 | 0.01 |
| 49 | 14.78 | 1077.5495 | C53H90O22 | 1079 | Ginsenoside Rc | 0.04 |
| 50 | 14.78 | 1123.5539 | C54H92O24 | 1125 | Koryoginsenoside Rg2 | 0.03 |
| 51 | 14.908 | 955.4588 | C48H76O19 | 956 | Ginsenoside Ro | 0.21 |
| 52 | 14.994 | 1119.5584 | C55H92O23 | 1121 | Ginsenoside Rs1 | 0.00 |
| 53 | 14.994 | 1163.5472 | C56H92O25 | 1165 | Ginsenoside mRc | 0.00 |
| 54 | 15.121 | 1077.5495 | C53H90O23 | 1079 | Ginsenoside Rb2 | 0.03 |
| 55 | 15.121 | 1123.5539 | C54H92O24 | 1125 | Notoginsenoside A | 0.02 |
| 56 | 15.308 | 1119.5584 | C55H92O23 | 1121 | Ginsenoside Rs2 | 0.00 |
| 57 | 15.308 | 1163.5472 | C56H92O25 | 1165 | Ginsenoside mRb3 | 0.00 |
| 58 | 15.544 | 1617.6449 | C76H114O37 | 1619 | Onjisaponin R | 0.00 |
| 59 | 15.566 | 1195.5702 | C56H94O24 | 1151 | Yesanchinoside F | 0.00 |
| 60 | 15.773 | 1455.595 | C70H104O32 | 1457 | Onjisaponin G | 0.00 |
| 61 | 15.901 | 1761.6844 | C82H122O41 | 1763 | Onjisaponin V | 0.00 |
| 62 | 16.011 | 1119.5584 | C55H92O23 | 1121 | Pseudoginsenoside F8 | 0.00 |
| 63 | 16.085 | 1485.606 | C71H106O33 | 1487 | Onjisaponin E | 0.10 |
| 64 | 16.118 | 1659.6386 | C78H116O38 | 1661 | Polygalasaponin XLV | 0.00 |
| 65 | 16.237 | 1425.5885 | C69H102O31 | 1427 | E-Senegasaponin B | 0.00 |
| 66 | 16.329 | 1119.5584 | C55H92O23 | 1121 | Pseudoginsenoside F8 | 0.00 |
| 67 | 16.334 | 1455.595 | C70H104O32 | 1457 | Senegin II | 0.00 |
| 68 | 16.343 | 1587.6325 | C75H112O36 | 1589 | Polygalasaponin XXX | 0.03 |
| 69 | 16.408 | 1703.6772 | C80H120O39 | 1705 | Onjisaponin A | 0.04 |
| 70 | 16.596 | 1847.7135 | C86H128O43 | 1849 | Onjisaponin L | 0.00 |
| 71 | 16.598 | 1587.6325 | C75H112O36 | 1589 | Onjisaponin Wg | 0.00 |
| 72 | 16.667 | 1599.6327 | C76H112O36 | 1601 | Onjisaponin Gg | 0.00 |
| 73 | 16.687 | 1731.6713 | C81H120O40 | 1733 | Onjisaponin W | 0.07 |
| 74 | 16.688 | 1631.6568 | C77H116O37 | 1633 | Onjisaponin O | 0.01 |
| 75 | 16.951 | 1617.643 | C76H114O37 | 1619 | Polygalasaponin XLIV | 0.03 |
| 76 | 16.995 | 1571.6369 | C75H112O35 | 1573 | Onjisaponin B | 0.01 |
| 77 | 17.112 | 1455.595 | C70H104O32 | 1457 | Z-Senegin II | 0.00 |
| 78 | 17.194 | 1541.6315 | C74H110O34 | 1543 | Onjisaponin H | 0.00 |
| 79 | 17.242 | 1761.6775 | C82H122O41 | 1763 | Onjisaponin Vg | 0.00 |
| 80 | 17.252 | 1673.6672 | C79H118O38 | 1675 | Polygalasaponin XXXII | 0.04 |
| 81 | 17.385 | 1469.61 | C71H106O32 | 1471 | Onjisaponin Z | 0.02 |
| 82 | 17.39 | 1659.6386 | C78H116O38 | 1661 | Polygalasaponin XLVI | 0.00 |
| 83 | 17.399 | 1685.6665 | C80H118O38 | 1687 | Onjisaponin Ng | 0.00 |
| 84 | 17.427 | 1425.5885 | C69H102O31 | 1427 | Myrtifolioside C1 | 0.00 |
| 85 | 17.446 | 1817.7057 | C85H126O42 | 1819 | Onjisaponin J | 0.00 |
| 86 | 17.493 | 1791.691 | C83H124O42 | 1793 | Onjisaponin T | 0.00 |
| 87 | 17.677 | 1587.6325 | C75H112O36 | 1589 | Onjisaponin F | 0.01 |
| 88 | 17.691 | 1455.595 | C70H104O32 | 1457 | Onjisaponin TH | 0.00 |
| 89 | 17.743 | 1409.5909 | C69H102O30 | 1411 | Onjisaponin Y | 0.00 |
| 90 | 17.859 | 1425.5885 | C69H102O31 | 1427 | Z-Senegasaponin B | 0.07 |
| 91 | 17.995 | 1731.6713 | C81H120O40 | 1733 | Onjisaponin Fg | 0.01 |
| 92 | 18.007 | 1599.6327 | C76H112O36 | 1601 | Onjisaponin K | 0.00 |
| 93 | 18.391 | 499.3252 | C31H48O5 | 500 | Poricoic acid GM | 0.22 |
| 94 | 19.404 | 513.3043 | C31H46O6 | 514 | Poricoic acid HM | 0.23 |
| 95 | 19.681 | 497.3099 | C31H46O5 | 498 | 16α,25-Dihydroxydehydroeburi conic acid | 0.26 |
| 96 | 21.003 | 483.2946 | C30H44O5 | 484 | 3-Epidehydrotumulosic acid | 0.22 |
| 97 | 21.048 | 541.3348 | C33H50O6 | 542 | 6-Hydroxydehydropachymic acid | 0.27 |
| 98 | 21.277 | 483.3306 | C30H44O5 | 484 | Dehydrotumulosic acid | 5.95 |
| 99 | 21.343 | 497.3099 | C31H46O5 | 498 | Poricoic acid A | 0.32 |
| 100 | 21.415 | 485.3461 | C31H50O4 | 486 | Tumulosic acid | 6.23 |
| 101 | 21.474 | 469.316 | C30H46O4 | 470 | 3β,16α-Dihydroxy-lanosta-7,9(11),24-trien-21-oic acid | 0.71 |
| 102 | 21.714 | 481.3159 | C31H46O4 | 482 | Polyporenic acid C | 2.23 |
| 103 | 22.317 | 511.3247 | C32H48O5 | 512 | Poricoic acid AM | 0.34 |
| 104 | 22.357 | 525.3395 | C33H50O5 | 526 | Dehydropachymic acid | 0.69 |
| 105 | 22.47 | 513.3407 | C31H46O6 | 514 | 3-O-Acetyl-16a-hydroxytrametenolic acid | 0.31 |
| 107 | 22.585 | 525.3395 | C33H50O5 | 526 | Poricoic acid AE | 0.29 |
| 108 | 22.731 | 527.3554 | C33H52O5 | 528 | Pachymic acid | 0.26 |
Note: RT, retention time; MW, molecular weight; RBA, relative binding amount.
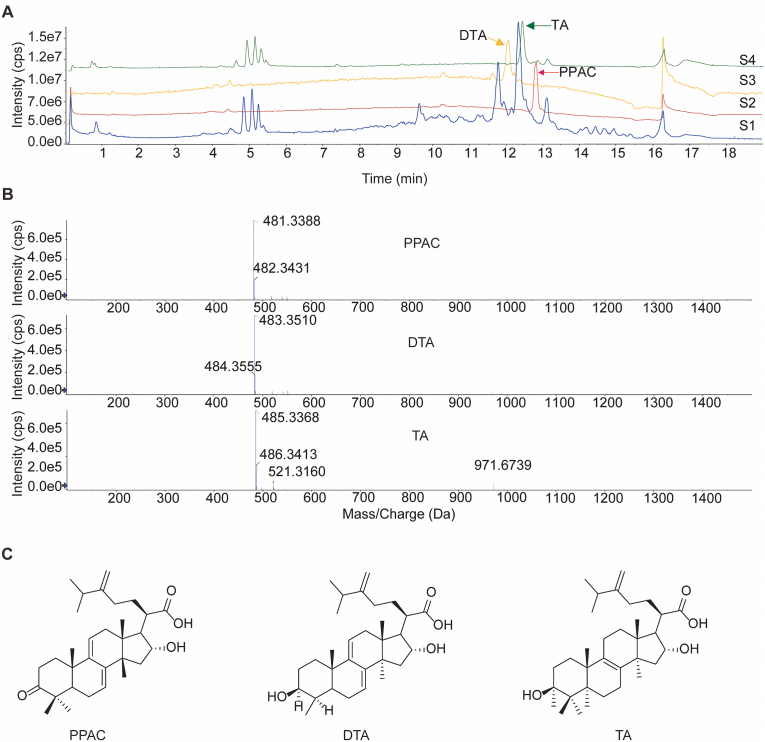
3.2. Isolation and purification of PPAC, DTA, and TA from Poria
Based on the above screening results, we aimed to isolate PPAC, DTA, and TA for the bioassay validation in vitro and in vivo. To begin, Poria crude powder were extracted with ethyl acetate reagent for three times using the reflux method. The dried extract was then subjected to separation by an open glass column filled with silica gel, and the components were eluted by the reagent system of methanol with dichloromethane. Then, the collected fractions were analyzed by UHPLC−DAD-Q/TOF-MS/MS and further purified by the pre-HPLC instrument. Finally, three compounds including PPAC, DTA, and TA were successfully isolated. The representative TIC of PPAC, DTA, TA, and Poria extract was acquired by UHPLC−DAD-Q/TOF-MS/MS instrument (Fig. 2A). The measured accurate mass of PPAC, DTA, and TA were [M‒H]− 481.3388, [M‒H]− 483.3510, and [M‒H]− 485.3368, respectively (Fig. 2B), and their molecular structures were showed in Fig. 2C, which were consistent with the reported compounds43.
Figure 2.
Isolation and purification of PPAC, DTA, and TA. (A) The TICs of PPAC, DTA, TA, and Poria extract. S1: Poria extract; S2: PPAC; S3: DTA; S4: TA. (B) The mass spectrums of PPAC, DTA, and TA. All the samples were analyzed by UHPLC−DAD-Q/TOF-MS/MS according to the chromatography condition in the method section. (C) The molecular structures of PPAC, DTA, and TA.
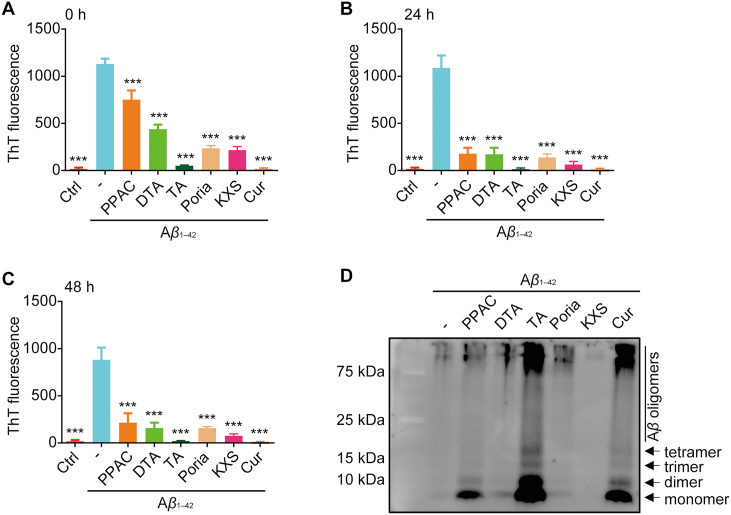
3.3. PPAC, DTA, and TA inhibit Aβ1–42 fibrils formation
To validate the feasibility and precision of this screening method, we firstly investigated the inhibitory effect of PPAC, DTA, and TA on the formation of Aβ1–42 fibrils by ThT assay. Meanwhile, the extracts of Poria and KXS, and Cur were employed for comparison. It is well-known that the binding of ThT to Aβ1–42 gives a relatively strong fluorescence signal at an emission wavelength of 482 nm which reflects the formation Aβ fibril31. At the time points of 0, 24, and 48 h, the fluorescence intensity of the solutions was detected by a spectrophotometer. As showed in Fig. 3A–C, Aβ1–42 dramatically increased the ThT fluorescence, while the treatment of PPAC, DTA, TA, Cur, or the extract of Poria and KXS could decrease the intensity of fluorescence. Among these tested drugs, TA and Cur exhibited the best inhibitory effect on the formation Aβ fibrils. In addition, we detected the Aβ isoforms by Western blot using an anti-Aβ antibody (6E10). As shown in Fig. 3D, incubation with Aβ1–42 alone led to the decreased level of both monomeric and oligomeric species. In the presence of PPAC, TA and Cur, monomeric (4 kDa), along with the dimeric, trimeric, tetrameric, and oligomeric (around 20 kDa) species were observed at the bottom of the gel. Therefore, the above results suggested that PPAC, TA and Cur inhibited Aβ fibrils formation and caused the accumulation of several intermediates including larger oligomeric to smaller monomer species. Taken together, PPAC, DTA, and TA can inhibit the formation of Aβ1–42 fibrils.
Figure 3.
PPAC, DTA, and TA inhibit the formation of Aβ fibrils. 20 μmol/L Aβ1–42 was incubated without or with Poria extract, KXS exctrat, PPAC, DTA, TA or Cur at 37 °C. At the time points of 0 h (A), 24 h (B), and 48 h (C), the solutions were mixed with 20 μmol/L of ThT solution. Then, the OD value of solutions was read by the spectrophotometer. The bar chart indicates the intensity of ThT fluorescence. (D) 20 μmol/L Aβ1–42 peptide was incubated without or with Poria extract, KXS extract, PPAC, DTA, TA or Cur for 24 h. Then, the proteins were subjected to the crosslinking reaction and Western blot analysis using an anti-Aβ antibody (6E10). The arrows indicated Aβ monomer, dimer, trimer, tetramer, and oligomers. All data are representative of at least three independent experiments and are presented as mean ± SD, n = 3. ∗∗∗P < 0.001. Poria extract: 20 μg/mL; KXS extract: 20 μg/mL; PPAC: 0.5 μmol/L; DTA: 0.5 μmol/L; TA: 0.5 μmol/L; Cur: 0.5 μmol/L.
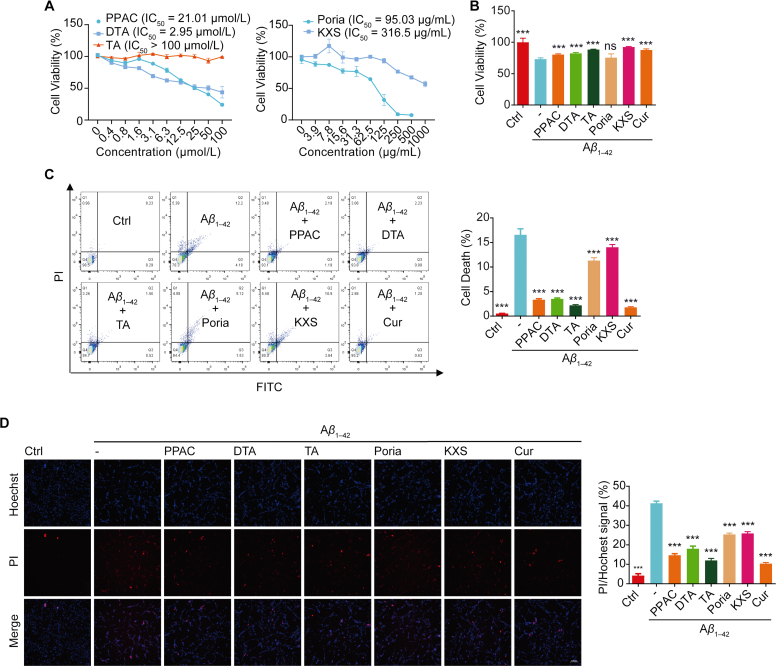
3.4. PPAC, DTA, and TA inhibit the cell death in Aβ1–42-treated PC-12 cells
Emerging evidence indicated that the extracellular Aβ fibril accumulates increasingly and induces cell death of neurons44. In current experiments, the improvement effect of PPAC, DTA, and TA on the cell viability of Aβ1–42-induced PC-12 cells was investigated. At first, the cytotoxicity of PPAC, DTA, TA, Poria or KXS extract in PC-12 cells was measured by MTT method (Fig. 4A). Then, their effects on the cell viability were investigated in Aβ1–42-treated PC-12 cells. As shown in Fig. 4B, PPAC, DTA, TA, Cur, and KXS extract significantly increased the cell viability of PC-12. Furthermore, by employing the flow cytometer and the Annexin V-FITC/PE apoptosis detection kit, we found that Poria extract, PPAC, DTA, TA, and Cur significantly decreased the cell death of Aβ1–42-treated PC-12 cells (Fig. 4C). Moreover, the Hoechst/PI staining result indicated that Poria extract, PPAC, DTA, TA, and Cur could inhibit the cell death as revealed by the decreased percentage of cells with PI signal (Fig. 4D). Taken together, the above data indicated that PPAC, DTA, and TA inhibited the cells death of PC-12 induced by Aβ1–42.
Figure 4.
PPAC, DTA, and TA decrease the cytotoxicity in Aβ1–42-induced PC-12 cells. (A) Cell viability of PC-12 cells treated with different concentrations of PPAC, DTA, TA, Poria or KXS extract at 24 h was examined using MTT assay. (B) PC-12 cells induced by Aβ1–42 were treated without or with PPAC, DTA, TA, Cur, Poria or KXS extract for 48 h. After treatment, the cell viability was measured by MTT assay. The bar chart indicates the viability of PC-12 cells. (C) PC-12 cells induced by Aβ1–42 were treated without or with PPAC, DTA, TA, Cur, Poria or KXS extract for 48 h. After treatment, cells were collected for the analysis of cell death by flow cytometer using a Annexin V-FITC/PE apoptosis kit. The bar chart represents the cell death of PC-12 cells. (D) PC-12 cells induced by Aβ1–42 were treated without or with PPAC, DTA, TA, Cur, Poria or KXS extract. After 48 h, cells were subjected to the staining with Hoechst33324/PI reagent for 5 min. The representative images were captured and merged at 10×magnification (Scale bar: 100 μm). The bar chart represents the ratio of PI/Hoechst signal of PC-12 cells. All data are representative of at least three independent experiment and are presented as mean ± SD, n = 3. ns: not significant, ∗∗∗P < 0.001. Poria extract: 20 μg/mL; KXS extract: 20 μg/mL; PPAC: 0.5 μmol/L; DTA: 0.5 μmol/L; TA: 0.5 μmol/L; Cur: 0.5 μmol/L.
3.5. PPAC, DTA, and TA improve the behavioral ability in C. elegans
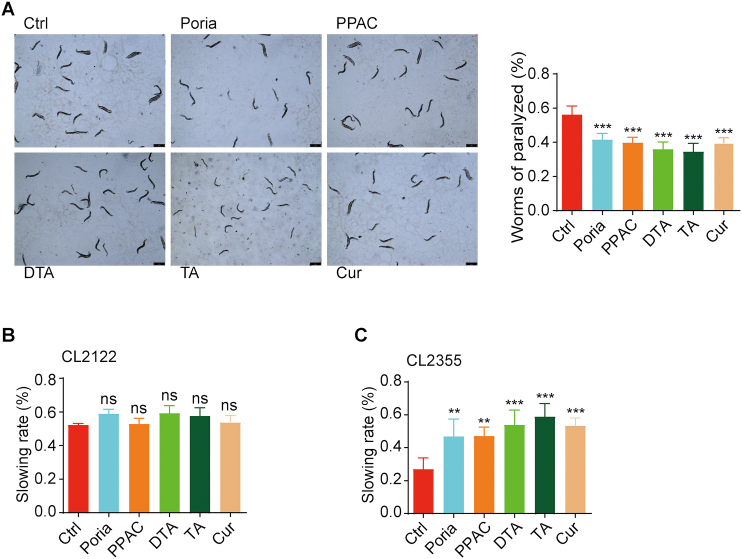
To investigate the neuroprotective effect of PPAC, DTA, and TA in the in vivo model of AD, C. elegans, a widely used model organism in neurodegenerative diseases, were used. As showed in Fig. 5A, Poria extract, and the single compounds including PPAC, DTA, TA, and Cur significantly decreased the percentage of worms of paralyzed in the transgenic C. elegans CL4176 strain, a nematode strain with temperature-induced expression of the human Aβ peptide in muscle cells. In addition, Aβ-induced C. elegans CL2355 and its control strain CL2122 were used for the study of the food-searching behavior. As shown in Fig. 5B, there were no significance difference observed among the control and treated groups in the CL2122 strain. However, the treatments of Poria extract, PPAC, DTA, TA, and Cur could significantly increase the slowing rate in the CL2355 strain. Taken together, the above data suggested that PPAC, DTA, and TA improved the behavioral function in the C. elegans models of AD.
Figure 5.
PPAC, DTA, and TA improve the behavioral function in C. elegans. (A) Aβ1–42-induced CL4176 worms were treated without or with Poria extract, PPAC, DTA, TA or Cur for 72 h. After treatment, the representative images of worms were captured by a microscope at 10×magnification (Scale bars: 100 μm). The bar chart represents the quantification of worms (n > 60) that were not paralyzed. (B, C) Aβ-induced C. elegans CL2355 and its control strain CL2122 were treated with Poria extract, PPAC, DTA, TA or Cur for 72 h. After treatment, the food-sensing behavior was evaluated by counting the body bends of worms per 20 s on NGM plates in the absence or presence of food. The bar charts indicate the slowing rate of worms (n = 20). All data are representative of at least three independent experiment and are presented as mean ± SD. ns: not significant, ∗∗P < 0.01, ∗∗∗P < 0.001. Poria extract: 500 μg/mL; PPAC: 100 μmol/L; DTA: 100 μmol/L; TA: 100 μmol/L; Cur: 100 μmol/L.
3.6. PPAC, DTA, and TA reduce Aβ species in C. elegans
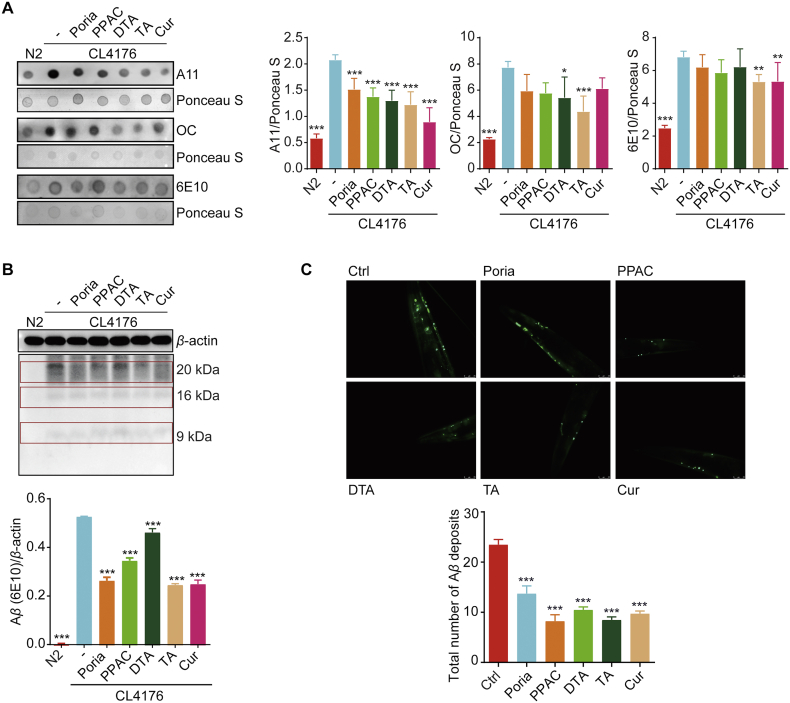
To determine if the effect of PPAC, DTA, and TA on delayed onset of paralysis was associated with a decrease in Aβ accumulation in transgenic CL4176, dot blot analysis was performed to detect the different isoforms of Aβ including prefibrillar oligomers (A11), fibrillary conformers (OC) and N terminus of Aβ (6E10) using different specific antibodies. As shown in Fig. 6A, PPAC, DTA, TA, Cur or Poria extract significantly decreased the expression of Aβ prefibrillar oligomers. Meanwhile, TA also dramatically decreased the level of Aβ fibrillar conformers and total Aβ. In addition, the Western blot analysis showed that the intensity of bands (9–20 kDa) representing Aβ oligomeric species was also reduced by PPAC, DTA, TA, Cur or Poria extract (Fig. 6B). Furthermore, we measured the Aβ deposits in the C. elegans CL2331 strain which temperature-sensitively expressed human Aβ1–42 conjugated with GFP in the body wall muscle cells. As shown in Fig. 6C, Poria extract, PPAC, DTA, TA, and Cur significantly reduced the total number of Aβ deposits in the anterior area. Collectively, PPAC, DTA, and TA could inhibit Aβ accumulation both in vitro and in vivo.
Figure 6.
PPAC, DTA, and TA reduce Aβ species in C. elegans. (A) Synchronized L1 CL4176 worms were treated without or with Poria extract, PPAC, DTA, TA or Cur at 16 °C for 36 h, which was followed by an incubation for 36 h at 25 °C to induce the expression of Aβ. After treatment, worm proteins were collected for dot blot analysis of Aβ using antibodies including A11, OC, and 6E10 followed by Ponceau S staining. The bar chart indicates the ratio of A11/Ponceau S, OC/Ponceau S, and 6E10/Ponceau S in worms (n ≥ 1000), respectively. N2: wild type worms. (B) After treatment, worm proteins were collected and subjected to the analysis of Aβ by Western blot using an anti-Aβ antibody (6E10). The bar chart indicates the ratio of Aβ oligomers/β-actin (n ≥ 1000). N2: wild type worms. (C) CL2331 worms were treated with Poria extract, PPAC, DTA, TA or Cur for 72 h. After treatment, the representative images of the anterior area were captured using a fluorescence microscope at 40×magnification (scale bars: 25 μm). The bar chart indicates the total number of Aβ deposits in the anterior area of CL2331 worms (n = 20). All data are representative of at least three independent experiment and are presented as mean ± SD. ns: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Poria extract: 500 μg/mL; PPAC: 100 μmol/L; DTA: 100 μmol/L; TA: 100 μmol/L; Cur: 100 μmol/L.
3.7. PPAC, DTA, and TA exhibit binding affinity towards Aβ1–42
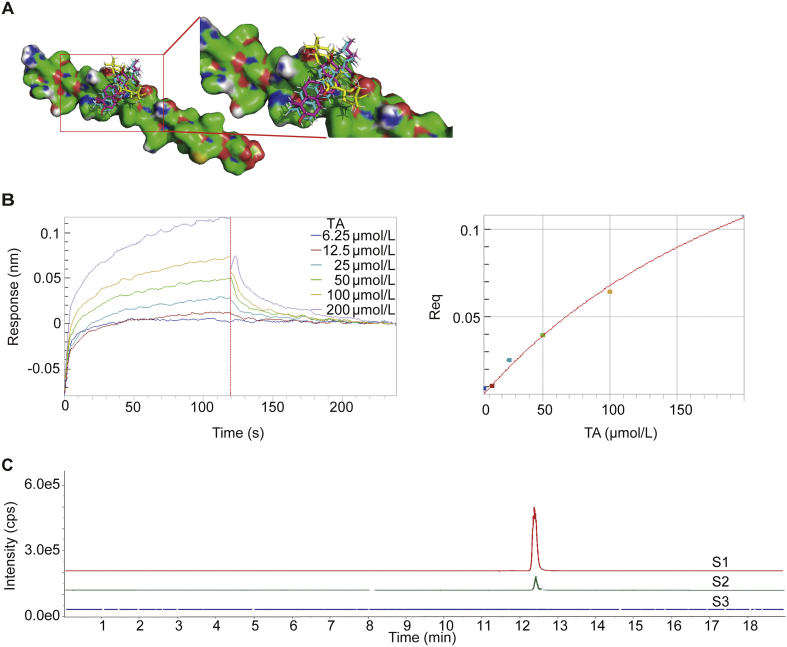
The value of template modeling score of Aβ1–42 model is 0.727 by the trRosetta45, which identified that the confidence of the predicted model of Aβ1–42 is high. To identify a more desired binding site and the theoretical binding mode of PPAC, DTA, or TA on Aβ1–42 protein, we performed molecular docking-based calculations using DockThor service46. The docking-based calculations binding energy of PPAC, TA, and DTA on Aβ1–42 protein is −8.056, −7.656 and −7.359 kJ/mol (Fig. 7A and Table 3) as revealed by multiple solutions genetic algorithm, respectively. Besides, the total energy of PPAC, TA, and DTA on Aβ1–42 protein is 40.647, 43.795 and 43.053 kJ/mol as confirmed by MMFF94S force field methods. Therefore, PPAC, DTA, and TA were confirmed to have strong binding properties to Aβ. Based on the above in vitro and in vivo results, we further selected TA to validate its binding affinity with Aβ1‒42 using BLI technology. As shown in Fig. 7B, TA exhibited a direct and reversible interaction with Aβ1–42 as revealed by the concentration-dependent increase in response indicating the optical thickness (nm) on the sensor layer. In addition, the kinetic constants including dissociation affinity (KD), association rate (Kon), and dissociation rate (Kdis) were 49.9 μmol/L, 1.49 × 10+2 L/mol∙s, and 7.42 × 10−3 1/s, respectively (Table 4). Moreover, the UHPLC−DAD-Q/TOF-MS/MS analysis result indicated that TA could be detected in the dissociated solution (Fig. 7C). Collectively, PPAC, DTA, and TA exhibited the strongest binding affinity with Aβ among the screened compounds presented in KXS.
Figure 7.
The binding of PPAC, DTA, and TA with Aβ1–42. (A) Predicted binding modes of PPAC, TA and DTA against Aβ1–42 conformations. PPAC, TA and DTA are represented as cyan sticks, red sticks and yellow sticks, respectively. (B) Real-time kinetic binding sensorgrams of different concentrations of TA (12.5–400 μmol/L) are shown. Response (nm) indicates the optical thickness on the SA biosensor layer. The equilibrium binding signal (Req) revealed by the flattened curve is reached. (C) UHPLC‒DAD-Q/TOF-MS/MS analysis of Mass spectrogram of TA: S1 represents TA, S2 represents the dissociated buffer, S3 represents the blank buffer.
Table 3.
Molecular docking of PPAC, TA and DTA on Aβ1–42 using DockThor Server.
| Chemical | Binding energy (kJ/mol) | Total energy (kJ/mol) |
|---|---|---|
| PPAC | −8.056 | 40.647 |
| TA | −7.656 | 43.795 |
| DTA | −7.359 | 43.053 |
Table 4.
The binding affinity (KD), association rate constant (Kon), and dissociation rate constant (Kdis) of TA to Aβ1–42.
| Compd. | KD (μmol/L) | Kon (L/mol·s) | Kdis (1/s) |
|---|---|---|---|
| TA | 49.9 | 1.49 × 10+2 | 7.42 × 10−3 |
4. Discussion
AD is a progressive neurodegenerative disease with insidious onset. Clinically, the main symptoms are characterized by the memory impairment, executive dysfunction, impairment of visual spatial skills, personality and behavior changes47. In addition, AD is an age-related disease and has become the most common type of neurodegenerative disease, which increases exponentially with age and accounts for 60%–80% of all cases48. Emerging evidence indicates that accumulations of extracellular Aβ and hyperphosphorylation of intracellular Tau are recognized as two pathological characteristics of AD5. Among them, Aβ is mainly generated from amyloid precursor protein (APP) with the cleavage actions of β- and γ-secretase49, and with the most common types as Aβ40 and Aβ42. It is reported that Aβ aggregation can enhance the phosphorylation of Tau and facilitate tau seeding by promoting the internalization of the seeds50. In addition, the over-generation of Aβ produces a larger amount of reactive oxygen species (ROS), which results in neuronal death and microglial over-activation51. In addition, Aβ can promote the depolarization of the synaptic membrane, excessive influx of calcium and mitochondrial impairment. Moreover, there is a growing of evidence showing that amyloid deposition starts before the appearance of AD symptoms52. Therefore, the Aβ hypothesis is still prevailing in the mechanistic study of AD.
To date, although there are currently no drugs developed to cure AD, several prescription drugs are still approved by the US Food and Drug Administration (FDA) to alleviate the clinical symptoms of AD patients53. The clinical drugs mainly include the cholinesterase inhibitors (galantamine, rivastigmine, and donepezil) and N-methyl d-aspartate (NMDA) antagonists (namantine)54. However, there are still no drugs targeting Aβ or Tau approved to treat AD. Recently, most drugs for AD have failed due to its high difficulty of research and development and complexity of molecular mechanism. Therefore, most of the identified compounds are still in the stage of preclinical trial and discovery55,56. Of noted, FDA has just accepted the listing application of aducanumab, a monoclonal antibody targeting Aβ, based on the phase III clinical data on Alzheimer's early-stagy patients57. This application was recognized to be a landmark for new drugs. Therefore, targeting Aβ may be a promising strategy for the treatment of AD, and the discovery of Aβ aggregation inhibitors has become an important issue.
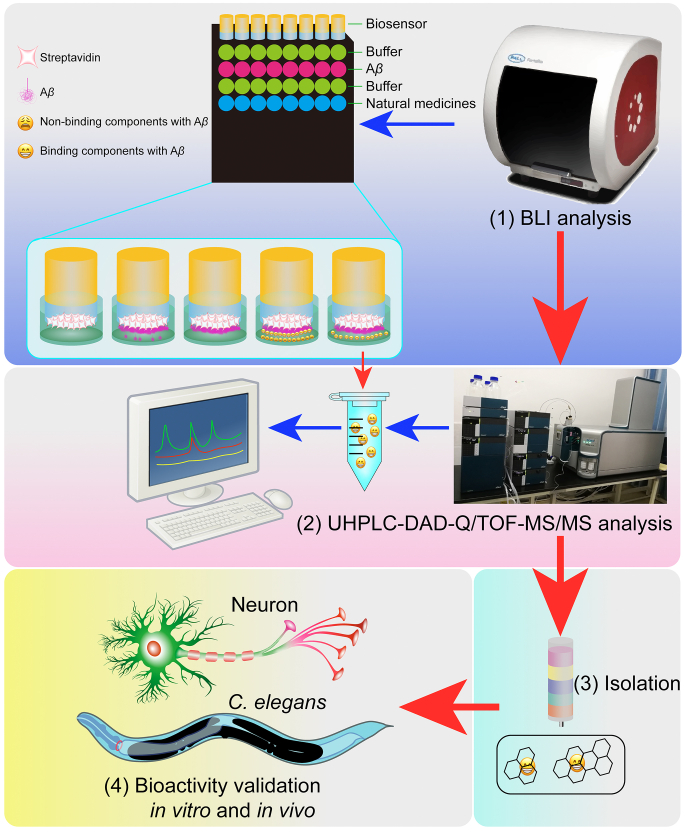
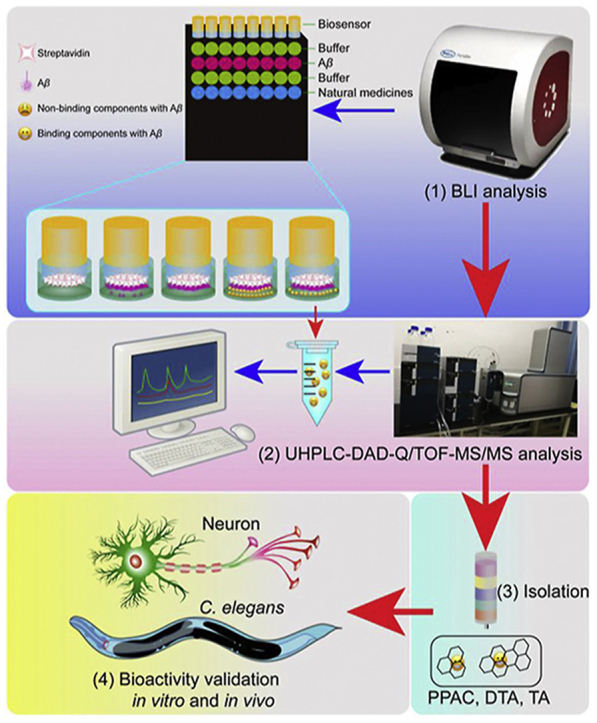
TCMs have a long history in the prevention and treatment of various diseases in China, which have been demonstrated to be safe and effective in the treatment of neurodegenerative diseases58. At present, EGb 761 (the extract of Ginkgo biloba L.) and huperzine A (an alkaloid of Huperzia serrata) have been widely used in clinical for the treatment of AD59. Although TCMs are the rich source of chemical compounds, the complexed components in a medical herb or even a Chinese medical formula hinder the process of the discovery of bioactive compounds. In general, the traditional bioactivity guided isolation and identification of the potential compounds from TCMs is time-consuming and laborious24. To date, there are many screening methods developed for the rapid discovery of bioactive compounds in the inhibition of Aβ based on the application of modern analytical instruments, including HPLC, MS, and NMR29,30. In our previous study, after incubation of the extract of Polygonum cuspidatum with methylglyoxal solution, two compounds, including polydatin and resveratrol, were identified as the natural methylglyoxal scavengers by UHPLC−DAD-MS hyphenated technique31. In addition, we employed cell membrane chromatography to identify 17 pentacyclic triterpenoid saponins from Radix Polygalae as the potent autophagy enhancers for the degradation of misfolded proteins associated with Parkinson's disease (PD) and Huntingtin's disease (HD). Moreover, we have recently developed a screening method to discover the Aβ inhibitors from SB based on the analysis of UHPLC−DAD-TOF/MS after the co-incubation of Aβ with SB extract. Through the comparison of the peak area between the solution with SB extract and the solution with SB and Aβ, we have successfully identified the compounds with decreased peak area as the Aβ inhibitors31. However, the physical adsorption of the components cannot be ruled out completely. Therefore, a more specific and reliable screening method for Aβ inhibitors is essential. BLI is a label-free technology for measuring the interaction of protein with protein or small molecule60. The target protein was first immobilized on the biosensor tips, then the small molecule or protein bound to the biosensor tip caused a shift that can be measured in real-time (Fig. 8). In this study, we hypothesized that the components acting as Aβ inhibitors in the extract of TCMs could bind onto the biotinylated Aβ1–42, while the components without biding property were washed away. Therefore, we used the ForteBio Octet® RED96e system to collect the components that were dissociated from the SA biosensor biotinylated with Aβ1–42. Then, the components with binding affinity were collected and subjected to the UHPLC−DAD-Q/TOF-MS/MS analysis. Meanwhile, the dissociation buffer without natural medicine extract was set blank control, and the solution with natural medicine extract prepared for the BLI analysis was set reference for the detection of the components in natural medicines (Fig. 8). Finally, the RBA of the detected components in the dissociation solution were calculated, and the components with high RBA were proposed to be the potent inhibitors of Aβ fibrillization. In this study, SB was selected to validate the screening method, and we found that baicalein and baicalin were the potent Aβ inhibitors, which were consistent with our previous results31. KXS is an ancient Chinese medical formula that is composed of Radix Polygalae, Radix Ginseng, Poria, and Rhizoma Acori Tatarinowii. It is widely used to treat dementia and forgetfulness33. The modern pharmacological researches indicated that the KXS extract and its bioactive components exhibit potent neuroprotective effect in various cellular and animal models61. However, there are still no report on the systematic screening of Aβ inhibitors from KXS. In the present study, the potential Aβ inhibitors including DTA, TA, and PPAC were successfully screened out from KXS, which demonstrated that our currently developed screening method was high-throughput, feasible, and reliable. Moreover, both the in vitro and in vivo bioassays indicated that DTA, TA, and PPAC could inhibit Aβ fibrils formation, decrease the cytotoxicity of Aβ in PC-12 cells, and improve the behavioral abilities in C. elegans. In the future, this method will also be useful for the screening of the potential inhibitors from natural medicine that target other pathological proteins, such as Tau, α-synuclein, and huntingtin (HTT) associated with different neurodegenerative diseases.
Figure 8.
Schematic diagram of this HTS method and bioactivity validation of Aβ binding small molecules from natural medicines. (1) BLI analysis by Octet® RED96e system. (2) Identification by UHPLC−DAD-Q/TOF-MS/MS. (3) Isolation of the target ingredients from natural medicines. (4) Bioactivity validation of the screened Aβ binding small-molecules in vitro and in vivo.
5. Conclusions
This study presents an effective, specific and reliable HTS method that is developed based on the combinational use of BLI and UHPLC−DAD-Q/TOF-MS/MS, which accelerates the discovery of natural small-molecule inhibitors of Aβ fibrils from natural medicines and the development of novel anti-AD drugs.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81903829, 81801398). Department of Science and Technology of Sichuan Province, China (2018JY0474, 2019JDPT0010, 2021YJ0180, 21RCYJ0021, 202086, 2019YFSY0014). The Joint project of Luzhou Municipal People’s Government and Southwest Medical University, China (2019LZXNYDJ02, 2019LZXNYDJ05, 2020LZXNYDJ37). The Science and Technology Planning Programs of Luzhou (2018-JYJ-34, China).
Author contributions
Anguo Wu, Xiaogang Zhou and Dalian Qin supervised the work. Anguo, Wu, Minsong Guo, Fengdan Zhu and Wenqiao Qiu performed all experiments. Min-Song Guo, Fengdan Zhu, Anguo Wu wrote the manuscript. Gan Qiao conducted the molecular docking. Yong Tang, Lu Yu and Jianming Wu provided the technical support. Chonglin Yu and Betty Yuen-Kwan Law helped with editing and English writing. Anguo Wu, Min-Song Guo, Fengdan Zhu and Betty Yuen-Kwan Law helped with manuscript revision and data collection.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2021.08.030.
Contributor Information
Dalian Qin, Email: dalianqin@swmu.edu.cn.
Xiaogang Zhou, Email: zxg@swmu.edu.cn.
Anguo Wu, Email: wuanguo@swmu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Tezel G., Timur S.S., Bozkurt İ., Türkoğlu Ö.F., Eroğlu İ., Nemutlu E., et al. A snapshot on the current status of Alzheimer's disease, treatment perspectives, in-vitro and in-vivo research studies and future opportunities. Chem Pharm Bull (Tokyo) 2019;67:1030–1041. doi: 10.1248/cpb.c19-00511. [DOI] [PubMed] [Google Scholar]
- 2.Porteri C. Advance directives as a tool to respect patients' values and preferences: discussion on the case of Alzheimer's disease. BMC Med Ethics. 2018;19:9. doi: 10.1186/s12910-018-0249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann B., Woehrer A., Ricken G., Augustin M., Mitter C., Pircher M., et al. Visualization of neuritic plaques in Alzheimer's disease by polarization-sensitive optical coherence microscopy. Sci Rep. 2017;7:43477. doi: 10.1038/srep43477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao Y.C., Ho P.C., Tu Y.K., Jou I.M., Tsai K.J. Lipids and Alzheimer's disease. Int J Mol Sci. 2020;21:1505. doi: 10.3390/ijms21041505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grosso C., Valentão P., Ferreres F., Andrade P.B. Bioactive marine drugs and marine biomaterials for brain diseases. Mar Drugs. 2014;12:2539–2589. doi: 10.3390/md12052539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richter M., Mewes A., Fritsch M., Krügel U., Hoffmann R., Singer D. Doubly phosphorylated peptide vaccines to protect transgenic P301S mice against Alzheimer's disease like tau aggregation. Vaccines (Basel) 2014;2:601–623. doi: 10.3390/vaccines2030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaręba N., Kepinska M. The function of transthyretin complexes with metallothionein in Alzheimer's disease. Int J Mol Sci. 2020;21:9003. doi: 10.3390/ijms21239003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitroi D.N., Karunakaran I., Gräler M., Saba J.D., Ehninger D., Ledesma M.D., et al. SGPL1 (sphingosine phosphate lyase 1) modulates neuronal autophagy via phosphatidylethanolamine production. Autophagy. 2017;13:885–899. doi: 10.1080/15548627.2017.1291471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nury T., Lizard G., Vejux A. Lipids nutrients in Parkinson and Alzheimer's diseases: cell death and cytoprotection. Int J Mol Sci. 2020;21:2501. doi: 10.3390/ijms21072501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S., Chen S.T., Sun Y., Xu Z., Wang Y., Yao S.Y., et al. Fibroblast growth factor 21 ameliorates neurodegeneration in rat and cellular models of Alzheimer's disease. Redox Biol. 2019;22:101133. doi: 10.1016/j.redox.2019.101133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu S., Tan G., Dong X., Zhu Z., Li W., Lou Z., et al. Metabolic profiling provides a system understanding of hypothyroidism in rats and its application. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohamad Zobir S.Z., Mohd Fauzi F., Liggi S., Drakakis G., Fu X., Fan T.P., et al. Global mapping of traditional Chinese medicine into bioactivity space and pathways annotation improves mechanistic understanding and discovers relationships between therapeutic action (Sub)classes. Evid Based Compl Alternat Med. 2016;2016:2106465. doi: 10.1155/2016/2106465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiménez-Aliaga K., Bermejo-Bescós P., Benedí J., Martín-Aragón S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci. 2011;89:939–945. doi: 10.1016/j.lfs.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Jiang W., Luo T., Li S., Zhou Y., Shen X.Y., He F., et al. Quercetin protects against okadaic acid-induced injury via MAPK and PI3K/Akt/GSK3β signaling pathways in HT22 hippocampal neurons. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong Y.T., Cao K., Tan L.C., Wang X.L., Qi X.L., Xiao Y., et al. Stimulation of SIRT1 attenuates the level of oxidative stress in the brains of APP/PS1 double transgenic mice and in primary neurons exposed to oligomers of the amyloid-β peptide. J Alzheimers Dis. 2018;63:283–301. doi: 10.3233/JAD-171020. [DOI] [PubMed] [Google Scholar]
- 16.Xiong R., Zhou X.G., Tang Y., Wu J.M., Sun Y.S., Teng J.F., et al. Lychee seed polyphenol protects the blood‒brain barrier through inhibiting Aβ(25-35)-induced NLRP3 inflammasome activation via the AMPK/mTOR/ULK1-mediated autophagy in bEnd.3 cells and APP/PS1 mice. Phytother Res. 2021;35:954–973. doi: 10.1002/ptr.6849. [DOI] [PubMed] [Google Scholar]
- 17.Qiu W.Q., Pan R., Tang Y., Zhou X.G., Wu J.M., Yu L., et al. Lychee seed polyphenol inhibits Aβ-induced activation of NLRP3 inflammasome via the LRP1/AMPK mediated autophagy induction. Biomed Pharmacother. 2020;130:110575. doi: 10.1016/j.biopha.2020.110575. [DOI] [PubMed] [Google Scholar]
- 18.Keowkase R., Kijmankongkul N., Sangtian W., Poomborplab S., Santa-ardharnpreecha C., Weerapreeyakul N., et al. Protective effect and mechanism of fruit extract of Aegle marmelos against amyloid-β toxicity in a transgenic Caenorhabditis elegans. Nat Prod Commun. 2020;15:1–12. [Google Scholar]
- 19.Tang Y., Yu C., Wu J., Chen H., Zeng Y., Wang X., et al. Lychee seed extract protects against neuronal injury and improves cognitive function in rats with type II diabetes mellitus with cognitive impairment. Int J Mol Med. 2018;41:251–263. doi: 10.3892/ijmm.2017.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim M.J., Kim J.H., Kim J.H., Lee S., Cho E.J. Amelioration effects of Cirsium japonicum var. maackii extract/fractions on amyloid beta25-35-induced neurotoxicity in SHSY5Y cells and identification of the main bioactive compound. Food Funct. 2020;11:9651–9661. doi: 10.1039/d0fo01041c. [DOI] [PubMed] [Google Scholar]
- 21.Cui L., Zhang Y., Cao H., Wang Y., Teng T., Ma G., et al. Ferulic acid inhibits the transition of amyloid-β42 monomers to oligomers but accelerates the transition from oligomers to fibrils. J Alzheimers Dis. 2013;37:19–28. doi: 10.3233/JAD-130164. [DOI] [PubMed] [Google Scholar]
- 22.Ștefănescu B.E., Szabo K., Mocan A., Crişan G. Phenolic compounds from five Ericaceae species leaves and their related bioavailability and health benefits. Molecules. 2019;24:2046. doi: 10.3390/molecules24112046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye M., Moon J., Yang J., Lim H.H., Hong S.B., Shim I., et al. The standardized Lycium chinense fruit extract protects against Alzheimer's disease in 3xTg-AD mice. J Ethnopharmacol. 2015;172:85–90. doi: 10.1016/j.jep.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 24.Yuan X.Y., Wang M., Lei S., Yang Q.X., Liu Y.Q. Rapid screening of active components with an osteoclastic inhibitory effect in herba epimedii using quantitative pattern-activity relationships based on joint-action models. Molecules. 2017;22:1767. doi: 10.3390/molecules22101767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan X., Wang S., Yu A., Shen X., Zheng H., Wang L. Cell chromatography-based screening of the active components in buyang huanwu decoction promoting axonal regeneration. BioMed Res Int. 2019;2019:6970198. doi: 10.1155/2019/6970198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu L., Hong F., Fan K., Zhao L., Zhang C., Yu B., et al. Integrated network pharmacology analysis and pharmacological evaluation to explore the active components and mechanism of Abelmoschus manihot (L.) Medik. on renal fibrosis. Drug Des Dev Ther. 2020;14:4053–4067. doi: 10.2147/DDDT.S264898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao Y., Chen Z., Zhang Y., Wang Y., Cheng Y. Immobilized magnetic beads based multi-target affinity selection coupled with high performance liquid chromatography-mass spectrometry for screening anti-diabetic compounds from a Chinese medicine "Tang-Zhi-Qing". J Pharmaceut Biomed Anal. 2013;78–79:190–201. doi: 10.1016/j.jpba.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Gu X., Wang D., Wang X., Liu Y., Di X. Fast screening of biomembrane-permeable compounds in herbal medicines using bubble-generating magnetic liposomes coupled with LC-MS. Molecules. 2021;26:1742. doi: 10.3390/molecules26061742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M., Liu Y., Liu M., Liu B., Li N., Dong X., et al. UHPLC‒QTOF/MS-based metabolomics investigation for the protective mechanism of Danshen in Alzheimer's disease cell model induced by Aβ1-42. Metabolomics. 2019;15:13. doi: 10.1007/s11306-019-1473-x. [DOI] [PubMed] [Google Scholar]
- 30.Ciaramelli C., Palmioli A., De Luigi A., Colombo L., Sala G., Salmona M., et al. NMR-based Lavado cocoa chemical characterization and comparison with fermented cocoa varieties: insights on cocoa's anti-amyloidogenic activity. Food Chem. 2021;341:128249. doi: 10.1016/j.foodchem.2020.128249. [DOI] [PubMed] [Google Scholar]
- 31.Yu L., Wu A.G., Wong V.K., Qu L.Q., Zhang N., Qin D.L., et al. The new application of UHPLC‒DAD-TOF/MS in identification of inhibitors on β-amyloid fibrillation from Scutellaria baicalensis. Front Pharmacol. 2019;10:194. doi: 10.3389/fphar.2019.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S.Q., Obregon D., Ehrhart J., Deng J., Tian J., Hou H., et al. Baicalein reduces β-amyloid and promotes nonamyloidogenic amyloid precursor protein processing in an Alzheimer's disease transgenic mouse model. J Neurosci Res. 2013;91:1239–1246. doi: 10.1002/jnr.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Y., Duan X., Cheng X., Cheng X., Li X., Zhang L., et al. Kai-Xin-San, a standardized traditional Chinese medicine formula, up-regulates the expressions of synaptic proteins on hippocampus of chronic mild stress induced depressive rats and primary cultured rat hippocampal neuron. J Ethnopharmacol. 2016;193:423–432. doi: 10.1016/j.jep.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 34.Heller G.T., Aprile F.A., Michaels T.C.T., Limbocker R., Perni M., Ruggeri F.S., et al. Small molecule sequestration of amyloid-β as a drug discovery strategy for Alzheimer's disease. Sci Adv. 2020;6 doi: 10.1126/sciadv.abb5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein A.N., Ziehm T., Tusche M., Buitenhuis J., Bartnik D., Boeddrich A., et al. Optimization of the all-D peptide D3 for abeta oligomer elimination. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu J., Cao Q., Wang C., Zheng J., Luo F., Xie J., et al. Structure-based peptide inhibitor design of amyloid-β aggregation. Front Mol Neurosci. 2019;12:54. doi: 10.3389/fnmol.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L., Yang Y., Di L., Li J.L., Li N. Erxian decoction, a famous Chinese medicine formula, antagonizes corticosterone-induced injury in PC12 cells, and improves depression-like behaviours in mice. Pharm Biol. 2020;58:498–509. doi: 10.1080/13880209.2020.1765812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y.B., Zhao H., Mu D.L., Zhang W., Cui J., Wu L., et al. Dexmedetomidine inhibits astrocyte pyroptosis and subsequently protects the brain in in vitro and in vivo models of sepsis. Cell Death Dis. 2019;10:167. doi: 10.1038/s41419-019-1416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saraceno C., Musardo S., Marcello E., Pelucchi S., Di Luca M. Modeling Alzheimer's disease: from past to future. Front Pharmacol. 2013;4:77. doi: 10.3389/fphar.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohankumar A., Shanmugam G., Kalaiselvi D., Levenson C., Nivitha S., Thiruppathi G., et al. East indian sandalwood (Santalum album L.) oil confers neuroprotection and geroprotection in Caenorhabditis elegans via activating SKN-1/Nrf2 signaling pathway. RSC Adv. 2018;8:33753–33774. doi: 10.1039/c8ra05195j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aprile F.A., Sormanni P., Podpolny M., Chhangur S., Needham L.M., Ruggeri F.S., et al. Rational design of a conformation-specific antibody for the quantification of Aβ oligomers. Proc Natl Acad Sci U S A. 2020;117:13509–13518. doi: 10.1073/pnas.1919464117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sankaranarayanan N.V., Bi Y., Kuberan B., Desai U.R. Combinatorial virtual library screening analysis of antithrombin binding oligosaccharide motif generation by heparan sulfate 3-O-sulfotransferase 1. Comput Struct Biotechnol J. 2020;18:933–941. doi: 10.1016/j.csbj.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu L.X., Xu J., Wang R.J., Li H.X., Tan Y.Z., Chen H.B., et al. Correlation between quality and geographical origins of Poria cocos revealed by qualitative fingerprint profiling and quantitative determination of triterpenoid acids. Molecules. 2018;23:2200. doi: 10.3390/molecules23092200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Söllvander S., Nikitidou E., Gallasch L., Zyśk M., Söderberg L., Sehlin D., et al. The Aβ protofibril selective antibody mAb158 prevents accumulation of Aβ in astrocytes and rescues neurons from Aβ-induced cell death. J Neuroinflammation. 2018;15:98. doi: 10.1186/s12974-018-1134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang J., Anishchenko I., Park H., Peng Z., Ovchinnikov S., Baker D. Improved protein structure prediction using predicted interresidue orientations. Proc Natl Acad Sci U S A. 2020;117:1496–1503. doi: 10.1073/pnas.1914677117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santos K.B., Guedes I.A., Karl A.L.M., Dardenne L.E. Highly flexible ligand docking: benchmarking of the DockThor program on the LEADS-PEP protein-peptide data set. J Chem Inf Model. 2020;60:667–683. doi: 10.1021/acs.jcim.9b00905. [DOI] [PubMed] [Google Scholar]
- 47.Xu F., Na L., Li Y., Chen L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020;10:54. doi: 10.1186/s13578-020-00416-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Al Rihani S.B., Darakjian L.I., Kaddoumi A. Oleocanthal-rich extra-virgin olive oil restores the blood‒brain barrier function through NLRP3 inflammasome inhibition simultaneously with autophagy induction in TgSwDI mice. ACS Chem Neurosci. 2019;10:3543–3554. doi: 10.1021/acschemneuro.9b00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen G.F., Xu T.H., Yan Y., Zhou Y.R., Jiang Y., Melcher K., et al. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin. 2017;38:1205–1235. doi: 10.1038/aps.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin W.S., Di J., Cao Q., Li B., Seidler P.M., Murray K.A., et al. Amyloid β-protein oligomers promote the uptake of tau fibril seeds potentiating intracellular tau aggregation. Alzheimer's Res Ther. 2019;11:86. doi: 10.1186/s13195-019-0541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H.J., Shen Y.C., Shiao Y.J., Liou K.T., Hsu W.H., Hsieh P.H., et al. Multiplex brain proteomic analysis revealed the molecular therapeutic effects of buyang huanwu decoction on cerebral ischemic stroke mice. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bodart-Santos V., de Carvalho L.R.P., de Godoy M.A., Batista A.F., Saraiva L.M., Lima L.G., et al. Extracellular vesicles derived from human Wharton's jelly mesenchymal stem cells protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. Stem Cell Res Ther. 2019;10:332. doi: 10.1186/s13287-019-1432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iranshahy M., Javadi B. Diet therapy for the treatment of Alzheimer's disease in view of traditional Persian medicine: a review. Iran J Basic Med Sci. 2019;22:1102–1117. doi: 10.22038/ijbms.2019.36505.8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker V.M., Davies N.M., Kehoe P.G., Martin R.M. What is the impact of regulatory guidance and expiry of drug patents on dementia drug prescriptions in England? A trend analysis in the Clinical Practice Research Datalink. Alzheimer's Res Ther. 2018;10:51. doi: 10.1186/s13195-018-0379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mancuso C., Gaetani S. Preclinical and clinical issues in Alzheimer's disease drug research and development. Front Pharmacol. 2014;5:234. doi: 10.3389/fphar.2014.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colligris P., Perez de Lara M.J., Colligris B., Pintor J. Ocular Manifestations of Alzheimer's and other neurodegenerative diseases: the sprospect of the eye as a tool for the early diagnosis of Alzheimer's disease. J Ophthalmol. 2018;2018:8538573. doi: 10.1155/2018/8538573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanchez L., Richter J., Cho H.J., Jagannath S., Madduri D., Parekh S., et al. Subcutaneous daratumumab and hyaluronidase-fihj in newly diagnosed or relapsed/refractory multiple myeloma. Ther Adv Hematol. 2021;12:1–12. doi: 10.1177/2040620720987075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang X.H., Liang L.N., Zhan L.B., Lu X.G., Shi X., Qi X., et al. The effect of Chinese Jinzhida recipe on the hippocampus in a rat model of diabetes-associated cognitive decline. BMC Compl Alternative Med. 2013;13:161. doi: 10.1186/1472-6882-13-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howes M.R., Fang R., Houghton P.J. Effect of Chinese herbal medicine on Alzheimer's disease. Int Rev Neurobiol. 2017;135:29–56. doi: 10.1016/bs.irn.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 60.Rosen O., Ozeri E., Barnea A., David A.B., Zichel R. Development of an innovative in vitro potency assay for anti-botulinum antitoxins. Toxins (Basel) 2016;8:276. doi: 10.3390/toxins8100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo S., Wang J., Xu H., Rong W., Gao C., Yuan Z., et al. Classic prescription, Kai-Xin-San, ameliorates Alzheimer's disease as an effective multitarget treatment: from neurotransmitter to protein signaling pathway. Oxid Med Cell Longev. 2019;2019:9096409. doi: 10.1155/2019/9096409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.