Abstract
Objective
A considerable proportion of patients with irritable bowel syndrome (IBS) may be wheat-sensitive and respond to a gluten-free diet (GFD) although they do not have coeliac disease. However, a diagnostic test for wheat sensitivity (WS) is missing. Our study evaluated the diagnostic accuracy (sensitivity and specificity) of confocal laser endomicroscopy (CLE) for the identification of WS as primary outcome.
Design
In this prospective, double-blind diagnostic study 147 non-coeliac patients fulfilling the Rome III criteria for IBS were tested by CLE for duodenal changes after wheat (index test), soy, yeast or milk exposure. Patients with IBS responding to 2 months of GFD were classified as having WS (reference test) using response criteria recommended by regulatory bodies for pharmaceutical trials of patients with IBS. After 2 months, CLE results were unblinded and patients were advised to exclude those food components that had led to a positive CLE reaction. The clinical response was assessed at follow-up after 6 and 12 months.
Results
Of 130 patients who completed the study per protocol, 74 (56.9%) responded to GFD and were classified as WS after 2 months, and 38 of these 74 patients were correctly identified by CLE (sensitivity 51.4%; 97.5% CI: 38.7% to 63.9%). A total of 38 of 56 patients without WS were correctly identified by CLE (specificity 67.9%; 97.5% CI: 52.9% to 79.9%). At 6 months follow-up, CLE correctly identified 49 of 59 food-sensitive patients (sensitivity 83.1%; 97.5% CI: 69.9% to 91.3%) but specificity was only 32% (97.5% CI: 15.7% to 54.3%).
Conclusion
In light of the high proportion of patients with IBS responding to GFD, the diagnostic accuracy of CLE is too low to recommend widespread use of this invasive procedure.
Trail registration number
This study was registered as clinical trial in the German Registry for Clinical Studies (DRKS00010123).
Keywords: gluten, irritable bowel syndrome
Significance of this study.
What is already known on this subject?
Recent studies suggested that confocal laser endomicroscopy (CLE) may be useful for the detection of hypersensitivities or atypical allergies to food in patients with irritable bowel syndrome (IBS).
What are the new findings?
Our prospective, double-blind multicentre diagnostic study aimed to determine the diagnostic accuracy of CLE for the diagnosis of wheat sensitivity in patients with IBS.
Results of CLE as index test were compared to the response to a gluten-free diet (GFD) as reference standard for the diagnosis of wheat sensitivity.
More than half of our patients with IBS including those with constipation responded to GFD. However, not only CLE-positive patients, but a large proportion of CLE-negative patients also responded to GFD. Thus, the diagnostic accuracy of CLE for wheat sensitivity was unsatisfactory.
How might it impact on clinical practice in the foreseeable future?
In light of the high proportion of patients with IBS responding to GFD, the diagnostic accuracy of CLE is too low to recommend widespread use of this invasive procedure.
A rather practical approach with an 8-week GFD period may be more beneficial to patients with IBS suspicious of suffering from food intolerances.
Introduction
The irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder characterised by abdominal pain or discomfort in combination with altered bowel movements and a lack of biochemical or structural abnormalities when applying conventional diagnostic procedures.1 Systematic reviews reported an IBS prevalence of 10%–15% in the general population2 and about 12% in Northern Europe.3 In Germany, 12.5% of the population complain about IBS symptoms and half of those affected seek medical help.4 Incidence rates of IBS are largely unknown, a 12-year survey from the USA estimated an annual incidence between 1% and 2%.5 After exclusion of structural and systemic gastrointestinal pathologies, an empiric therapy is introduced to relieve symptoms in patients with IBS but results are often unsatisfactory,6 although numerous studies evaluated treatment options such as cognitive behavioural therapies as well as classical pharmacological therapies with a variety of substances in the treatment of IBS.7
Over the last decades, the relation between gastrointestinal symptoms in patients without coeliac disease and symptom relief after gluten-free diet (GFD) was examined in several trials.8 9 In 2001, we reported symptomatic improvement after GFD in a subgroup of patients with IBS.10 This condition, initially described as non-coeliac gluten sensitivity (NCGS) in 1978,11 could be the most common gluten-related disorder and is now widely discussed as wheat sensitivity (WS) and several other designations.12 13 Reliable biomarkers for WS, however, are still missing and it is clear that the response to GFD could also result from reduced intake of wheat ingredients apart from gluten, for example, the pest resistance molecules amylase trypsin inhibitors14 and fructans as poorly absorbed fermentable, oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) which may induce symptoms in susceptible patients.13 In fact, both, a low FODMAP diet (LFD) and GFD have been considered as dietary treatment of IBS.15
Confocal laser endomicroscopy (CLE) generates high-resolution images of the gastrointestinal tract after intravenous injection of fluorescein during ongoing endoscopy. CLE was introduced in 200416 and demonstrated clinical impact in a variety of gastrointestinal diseases.17–20
In 2014, a pilot study reported the accurate identification by CLE of patients with IBS responding to specific exclusion diets, in particular 13 of 13 patients with IBS responding to dietary wheat exclusion.21 In a later study the same group found CLE-reactions to food antigens in 76 (70%), and to wheat in 46 (43%) of 108 patients with IBS.22 Thus, CLE may represent a diagnostic tool to identify wheat-sensitive patients and could improve IBS therapy by offering causative treatment options.
Based on these uncontrolled data, our prospective, double-blind multicentre diagnostic study therefore examined whether CLE can identify patients responding to wheat exclusion by means of a standard GFD using response criteria recommended by European Medical Agency (EMA) for pharmaceutical trials of patients with IBS.23 Results of CLE as index test were compared with the response to GFD as a reference standard for the diagnosis of WS. We found evidence for a high proportion of patients with IBS with WS, however, the diagnostic accuracy of CLE was unsatisfactory.
Methods
Patients/inclusion criteria
Outpatients suffering from IBS with daily symptoms were invited to participate. The presence of the Rome III criteria was determined and a run-in observation period of 4 weeks was used to ascertain daily symptoms and to exclude pre-intervention improvement. Eligible patients were screened and at the final visit the following inclusion criteria had to be met: Age ≥18 years, fulfilment of Rome III criteria for IBS, daily symptoms worsening after meals, score ≥50 of the IBS-36 questionnaire, ≥175 points of the IBS-Symptom Severity Scale (IBS-SSS), normal gastroscopy including duodenal histology, assessment and confirmation of duodenal histology by a reference pathology centre, normal colonoscopy within the last 5 years, ability and will to follow an 8-week GFD period, capability of understanding and availability of a signed informed consent form. In addition, the following conditions had to be excluded: Chronic gastrointestinal or pancreatic disease, elevated anti-tissue transglutaminase IgA (or, in case of IgA deficiency, anti-tissue transglutaminase IgG), elevated wheat-specific IgE, abnormal lactose hydrogen breath test (25 g lactose), antidepressant drug treatment, involuntary weight loss of more than 10% of body weight within the last 6 months, known allergy against fluorescein, fever (body temperature >38°C), erythrocyte sedimention rate >30 mm/hour, C-reactive protein >5 mg/L, white blood cell count ≥10∧9/L, elevated levels for serum creatinine, serum lipase, thyroid stimulating hormone, lowered faecal elastase, chronic heart failure (NYHA III and IV), pregnancy. Expression of the coeliac disease-associated alleles HLA-DQ2 and -DQ8 was determined in all study patients.
Baseline measurements of the IBS-SSS,24 IBS Quality of Life (IBS-QoL) Score, European Quality of Life 5 Dimensions (EQ-5D) Score, stool frequency, stool consistency and type,25 flatulence, pain, bowel movement were collected.
Informed consent in writing was obtained from all patients.
Index CLE with duodenal antigen provocation
Patients included into the study were examined with CLE (EG-3870CIK, EC-3870CIFK, Pentax, Tokyo, Japan) under propofol sedation. The endoscope was introduced to the duodenum. After injection of 2.5 mL 10% fluorescein intravenously (Fluorescein, Alcon Pharma) confocal images were recorded to determine a baseline status. Standardised suspensions of commercially available food antigens (wheat flour DIN 10355 type 405 (Weizenmehl Type 405, Aurora Mühlen, Hamburg, Germany), dry yeast (Bio Hefe, RUF Lebensmittelwerk KG, Quakenbrück, Germany), skimmed milk powder (Magermilchpulver, J. M. Gabler-Saliter Milchwerk, Obergünzburg, Germany) and natural soy flour (Bio Soja-Mehl, Berief Food, Beckum, Germany)) were then applied through the working channel of the endoscope in the specified order: 3 g wheat flour, 3 g soy, 1 g yeast and 1.5 g milk, each diluted in 30 mL 0.9% sodium chloride solution. The centres received tubes with dry substances and diluted them immediately before endoscopy. Any remaining particles were resuspended by vigorous shaking immediately before use. The first substance was applied in the distal part of the descending duodenum and for the application of consecutively applied substances the endoscope was retracted approximately 5 cm to ensure that the mucosa was not in contact with high dosages of food before. Five minutes after application of each substance re-evaluation of the mucosa with CLE continued with documentation of at least four different sites with 10–20 pictures for each site according to the protocol described elsewhere21 (online supplemental figure). If the CLE reaction was positive as described below, the operating physician terminated the examination and no further antigen solutions were applied during the same CLE session. Because WS was our primary research question, wheat was always applied as the first food substance. To avoid bias, the pictures were recorded to hard disk and separately documented in pseudonymised folders. All participating study centres sent their image folders with the CLE pictures to the study centre in Berlin for blinded evaluation. All authors had access to the study data and reviewed and approved the final manuscript.
gutjnl-2021-325181supp001.pdf (2MB, pdf)
During the baseline CLE and after food provocation testing, we used the following criteria for defining the CLE test result as previously described by Fritscher-Ravens et al 21: Fluorescein leakage was a major criterion and increased intraepithelial lymphocytes (IEL) and intervillous spaces were minor criteria. A positive CLE test result (CLE+) was stated, if at least one major and one minor criterion were documented. Following these criteria,21 the quantification of the density of IEL during ongoing endoscopy was not found suitable for determination during the first 20 index endoscopies and therefore was not used for further CLE evaluations.
The findings of both blinded reviewers were required to be concordant in order to be defined as positive. If the results of both reviewers were discordant an open discussion with re-evaluation of the CLE pictures was done. The results were then marked as concordant otherwise as unclear. The result of the CLE index test was not disclosed to the patient or other study personnel in contact with the patient.
Trial visits after inclusion
Following CLE, patients received a detailed 1-hour dietary counselling in the hospital by a trained nutritionist and were started on a GFD as established for coeliac disease, that is, a diet free of wheat, rye and barley.
After 2 weeks, another dietary counselling ascertained that patients have been following the GFD and if required further dietary guidance was provided. During the next 8 weeks of GFD weekly interviews were performed to evaluate symptoms and assess improvements, and patients were asked to provide a stool and symptoms diary. The study visit after 2 months of GFD served to determine the presence or absence of WS as primary endpoint and to apply secondary outcome measures as described below.
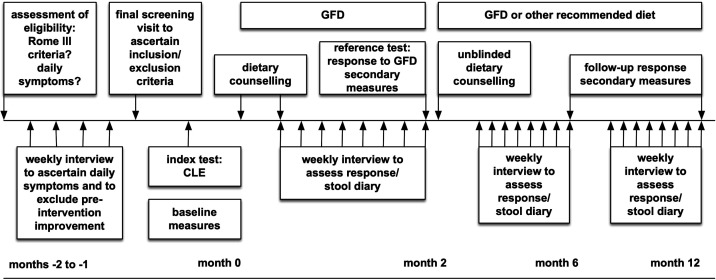
At this time point, CLE results were unblinded and patients received another dietary counselling including recommendations to specifically exclude those additional food components that had led to a positive reaction in CLE. The patients’ diet was recorded and the clinical response as defined below was assessed at follow-up visits after 6 and 12 months. A full overview of the screening and study visits is shown in figure 1.
Figure 1.
Time schedule for patient’s study visits. CLE, confocal laser endomicroscopy; GFD, gluten-free diet.
Primary and secondary outcomes
The diagnostic accuracy (sensitivity and specificity) of CLE for the identification of WS was the primary outcome. Index test: Patients who were CLE-positive after administration of wheat topically were classified as WS by CLE. Reference standard: There is no standard for the diagnosis of WS. Double-blind placebo-controlled challenges with wheat or gluten have been suggested but have not been standardised, and several studies applying food challenges have revealed conflicting results with highly variable response rates to placebo and verum.26 27 Because it is not even clear at present which wheat ingredients cause symptoms in sensitive patients, the clinical response to a GFD served as reference test to identify WS patients using response criteria recommended by the EMA for pharmaceutical trials of patients with IBS.23 Patient-reported outcomes have also been recommended by the FDA for IBS studies.28 Thus, patients with IBS responding clinically to a GFD were classified as having WS.
A patient-defined global assessment of IBS symptoms in combination with an abdominal pain score is recommended as primary endpoint for studies examining more than one IBS subtype.23 Accordingly, our primary outcome measurement was first the patient’s global assessment of improvement using a seven-point scale.29 This measure is a validated tool for the primary assessment of outcome in clinical studies of IBS.30 It consists of one question (‘Please consider how you felt this past week with regard to your IBS, in particular your overall wellbeing, and symptoms of abdominal discomfort, pain and altered bowel habit. Compared with the way you usually felt before entering the study, how would you rate your relief of symptoms during the past week?’) and offers seven different answers: (1) completely relieved, (2) considerably relieved, (3) somewhat relieved, (4) unchanged, (5) somewhat worse, (6) considerably worse or (7) extremely worse. Second, abdominal pain was evaluated using an 11-point Numeric Rating Scale that has been partially validated31 and is therefore currently recommended.23
Patients who reported (1) the highest two improvement grades and (2) an abdominal pain score that improved at least 30% compared with baseline on at least 50% of weeks over the last 2 months of treatment were defined as responders.
Changes in QoL were evaluated by specific (IBS-QoL) and general (EQ-5D) scales and the severity of gastrointestinal symptoms in all patients was evaluated by the IBS-SSS.29 Patients were asked to provide a daily stool diary to give information on stool form (Bristol Stool Scale25) and pain perception, urgency of defecation and bloating to analyse frequency of defecation, stool consistency, and number of days with pain, urgency or bloating in patients according to their IBS subtype as recommended.32
Statistics
The determination of the diagnostic accuracy of CLE for the diagnosis of WS was the main aim of this study. Although 13/13 patients who were CLE-positive after wheat challenge showed a long-term response to an exclusion diet in the feasibility study,21 we conservatively assumed a specificity of approximately 90%.
In our previous study 34% (12/35) non-constipated patients with IBS responded to GFD,33 and an Italian study found WS in 30% (276/920) patients with IBS.26 In the feasibility study, 36% (13/36) patients were CLE-positive after wheat challenge21 suggesting that the majority of WS patients with IBS was identified by CLE.
A diagnostic test with sensitivity and specificity above 80% can be considered a good test for discrimination of WS patients. Thus, a 20% width of the confidence intervals for the expected sensitivity and specificity was chosen to assure the discriminative ability of CLE. A total number of 130 patients were required to simultaneously construct a 97.5% CI with length 20% for an expected sensitivity and specificity of 90% and a prevalence of WS of approximately 35%.32 Based on a drop-out rate of 10%, 144 patients had to be included into the study while 179 patients were required to be assessed for eligibility when assuming a 20% screening failure rate.
For evaluation of the predictive value of CLE in the diagnosis of WS, sensitivity and specificity including 97.5% CIs as well as positive and negative predictive value, and positive and negative likelihood ratio including 95% CIs were determined. Descriptive statistics were used to define the proportion of patients with and without WS. Further variables (eg, changes in IBS-QOL score, EQ-5D score, IBS-SSS, stool frequency, stool consistency) were analysed using appropriate parametric and non-parametric statistical methods.
Results
Patients
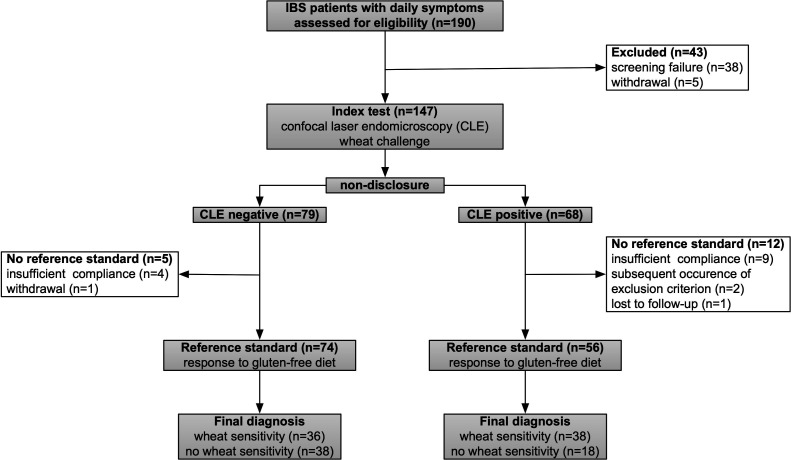
A total of 190 patients entered the screening process and 147 (77%) of them were finally recruited (figure 2). Of 147 study patients 34 (23%) were male, 113 (77%) were female, 72 (49%) had IBS-M, 60 (41%) IBS-D and 15 (10%) IBS-C.
Figure 2.
Diagram of the patient flow through the study. One hundred and forty-seven patients were examined by CLE after wheat challenge (index test), and the responder status after 2 months of GFD (reference test) was available in 130 patients. GFD, gluten-free diet; IBS, irritable bowel syndrome.
The age of the study patients among IBS-subtypes was similar (mean age ranging from 34.5 to 36.5). Details on patient demographics including lifestyle habits and social status are depicted in table 1.
Table 1.
Patients’ demographics
| IBS mixed subtype (IBS-M) |
IBS with diarrhea (IBS-D) |
IBS with constipation (IBS-C) |
Total | |
| IBS type (n, %) | 72 (49) | 60 (41) | 15 (10) | 147 (100) |
| Age (years) | ||||
| Mean±SD | 35.3±9.6 | 37±10 | 34±10 | 35.7±9.9 |
| Range | 22–59 | 21–60 | 20–53 | 19–60 |
| Gender (n, %) | ||||
| Male | 17 (24) | 15 (25) | 2 (13) | 34 (23) |
| Female | 55 (76) | 45 (75) | 13 (87) | 113 (77) |
| Lifestyle habits (n/total, %) | ||||
| Smoker | 11/72 (15) | 13/59 (22) | 0/15 (0) | 24/146 (16) |
| Ex-smoker | 2/72 (3) | 6/59 (10) | 1/15 (7) | 9/146 (6.2) |
| Non-smoker | 59/72 (82) | 40/59 (68) | 14/15 (93) | 113/146 (77) |
| Coffee consumption | 55/68 (81) | 37/51 (73) | 11/14 (79) | 103/133 (77) |
| No coffee consumption | 13/68 (19) | 14/51 (27) | 3/14 (21) | 30/133 (23) |
| Alcohol consumption | 36/72 (50) | 32/58 (55) | 5/15 (33) | 73/145 (50) |
| No alcohol consumption | 36/72 (50) | 26/58 (45) | 10/15 (67) | 72/145 (50) |
| Employment (n/total, %) | ||||
| Full-time job | 42/72 (58) | 42/59 (71) | 10/15 (67) | 94/146 (64) |
| Part-time job | 10/72 (14) | 7/59 (12) | 3/15 (20) | 20/146 (14) |
| Incapable of working | 2/72 (3) | 0/59 (0) | 0/15 (0) | 2/146 (1.4) |
| Unemployed | 4/72 (6) | 1/59 (2) | 0/15 (0) | 5/146 (3.4) |
| Student | 14/72 (19) | 9/59 (15) | 2/15 (13) | 25/146 (17) |
IBS, irritable bowel syndrome.
CLE performance and safety
CLE as index test was safely performed in all 147 patients. Minor adverse events were documented in five patients (tonsillitis n=2, exanthema n=1, common cold n=1, enteritis n=1, 3.4%) and medium adverse events in two patients (serology positive for rheumatoid factor, Yersinia spp and rotavirus n=1, influenza n=1, 1.4%) were observed after CLE. None of these adverse events were classified as related to CLE or dietary intervention.
Positive CLE reactions were usually clear, and there were no discordant results in blinded review. Positive reactions were observed after topical application of wheat in 68 (46.3%) out of 147 patients, soy in 20 (19.4%) out of 103 patients, yeast in 20 (22%) of 91 patients and milk in nine (12.2%) out of 74 patients.
Wheat sensitivity
Blinded to the CLE findings patients with IBS followed a GFD for 2 months. In 17 out of 147 patients, no reference standard was achievable, and therefore, 130/147 (88%) patients finally completed the study per protocol (figure 2). Seventy-four (57%) out of 130 patients fulfilled the response criteria after 2 months of GFD and were classified as having WS. Gender, IBS-type and HLA-DQ 2 or 8 were not significantly associated with the proportion of responders to GFD (table 2).
Table 2.
Responders to GFD after 2, 6 and 12 months in relation to gender, IBS types (IBS mixed subtype, IBS-M; IBS with diarrhea IBS-D; IBS with constipation IBS-C) and HLA-DQ2/8 expression
|
|
Responders to GFD after | |||||
| 2 months | 6 months | 12 months | ||||
| No | Yes | No | Yes | No | Yes | |
| N (%) | 56 (43) | 74 (57) | 25 (30) | 59 (70) | 21 (24) | 67 (76) |
| Gender* | ||||||
| Female, n (%) | 40 (40) | 60 (60) | 18 (28) | 47 (72) | 19 (27) | 52 (73) |
| Male, n (%) | 16 (53) | 14 (47) | 7 (37%) | 12 (63) | 2 (12) | 15 (88) |
| IBS type* | ||||||
| IBS-M, n (%) | 33 (49) | 34 (51) | 18 (38) | 29 (62) | 15 (33) | 31 (67) |
| IBS-D, n (%) | 16 (32) | 34 (68) | 6 (19) | 26 (81) | 5 (15) | 29 (85) |
| IBS-C, n (%) | 7 (54) | 6 (46) | 1 (20) | 4 (80) | 1 (13) | 7 (87) |
| HLA-DQ2 or -DQ8 expression* | ||||||
| DQ2/DQ8 negative, n (%) | 36 (54) | 31 (46) | 16 (35) | 30 (65) | 14 (32) | 30 (68) |
| DQ2/DQ8 positive, n (%) | 20 (37) | 34 (63) | 9 (27) | 24 (73) | 7 (19) | 30 (81) |
*No significant differences in the proportion of responders.
GFD, gluten-free diet; IBS, irritable bowel syndrome.
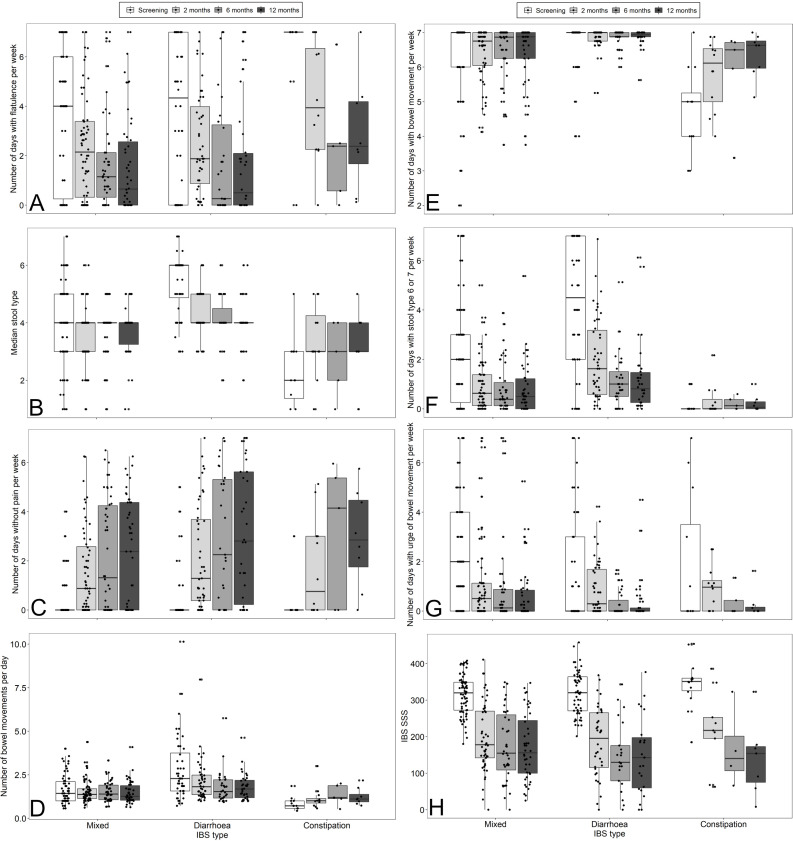
In addition to the composite endpoint, secondary endpoints assessing improvement in several gastrointestinal symptoms in response to GFD were determined and are shown in figure 3. After 2 months of GFD, the median number of days with flatulence was lower in all IBS subtypes (figure 3A), the median stool type according to Bristol Stool Scale decreased from six to four in IBS-D and increased from two to three in IBS-C (figure 3B), the median number of days without abdominal pain increased in all IBS-subtypes (figure 3C). The median number of bowel movements per day decreased in IBS-D and increased in IBS-C (figure 3D), the median number of days with bowel movement increased in IBS-C (figure 3E), the median number of days with stool type 6 or 7 decreased in IBS-M and IBS-D (figure 3F) and the median number of days with urge of bowel movements per week was reduced in IBS-M (figure 3G). Overall, these changes demonstrate specific symptomatic improvements in all IBS subtypes and support the high proportion of patients with IBS classified as responders to GFD by the composite endpoint.
Figure 3.
Evaluation of secondary study endpoints after screening and at 2, 6 and 12 months according to IBS type. (A) Number of days with flatulence/bloating per week, (B) median stool type (according to Bristol Stool Scale), (C) number of days without pain per week, (D) number of bowel movements per day, (E) number of days with bowel movements per week, (F) number of days with stool type 6 or 7 (according to Bristol Stool Scale), (G) number of days with urge of bowel movement per week. (H) IBS-SSS. IBS-SSS, irritable bowel syndrome-Symptom Severity Scale.
IBS-SSS did not differ significantly between IBS-types. After 2, 6 and 12 months, IBS-SSS was significantly reduced in all IBS-types (figure 3H).
Interestingly, symptomatic improvement was most prominent during the first 2 months of blinded GFD. Only minor additional improvements which can be attributed to specific exclusion diets were seen after 6 months in CLE-positive patients.
Accuracy of CLE for WS
Diagnostic accuracy measures of CLE for the diagnosis of WS are presented in table 3. Of the 74 patients with WS at the primary endpoint after 2 months of GFD, 38 were correctly identified by CLE resulting in a sensitivity of 51.4% (97.5% CI: 38.7% to 63.9%). A total of 38/56 patients without WS were correctly identified by CLE showing a specificity of 67.9% (97.5% CI: 52.9% to 79.9%). Both diagnostic accuracy values were below the prespecified limit of 80%. The accuracy parameters of CLE assessed by the two blinded reviewers and by the CLE performing physicians for the detection of WS were not statistically different (table 3). The proportion of patients with WS in CLE-positive patients was 67.9% (95% CI: 54.8% to 78.6%) while the proportion of patients without WS in CLE-negative patients was 51.4% (95% CI: 40.2% to 62.4%). Of 79 patients who were negative for wheat in CLE 36 patients (46%) responded to GFD, 38 patients (48%) were considered as non-responders and 5 patients (6%) were lost to follow-up. Accuracy of CLE for WS was also determined at 6 and 12 months of follow-up, excluding patients who changed their diets after 2 months due to CLE reactions to other food antigens. Compared with the primary endpoint, sensitivities at follow-up were higher, but specificities were lower (table 3). Overall, patients with IBS who were CLE-positive after application of wheat were about twice as likely to have WS compared with those who were CLE negative after wheat exposure (positive likelihood ratio: 1.65, 95% CI: 1.04 to 2.6 vs negative likelihood ratio: 0.72, 95% CI: 0.54 to 0.96).
Table 3.
Accuracy of CLE for the detection of wheat sensitivity and for any food sensitivity after 2, 6 and 12 months
| Time point | Accuracy of CLE | |||||
| For wheat sensitivity | For any food sensitivity | |||||
| 2 months | 6 months |
12 months |
6 months | 12 months | ||
| Blinded CLE evaluation | CLE evaluation by examiner | |||||
| Sensitivity (97.5% CI) | 51.4% (38.7% to 63.9%) | 50% (37.4% to 62.6%) | 72.2% (53.6% to 85.4%) | 70% (52.3% to 83.2%) | 83.1% (69.6% to 91.3%) | 82.1% (69.5% to 90.2%) |
| Specificity (97.5% CI) | 67.9% (52.9% to 79.9%) | 69.6% (54.7% to 81.3%) | 40% (20% to 64%) | 36.8% (17.4% to 61.8%) | 32% (15.7% to 54.3%) | 33.3% (15.6% to 57.5%) |
| Positive predictive value (95% CI) | 67.9% (54.8% to 78.6%) | 68.5% (55.3% to 79.3%) | 68.4% (52.5% to 80.9%) | 70% (54.6% to 81.9%) | 74.2% (62.6% to 83.3%) | 79.7% (68.8% to 87.5%) |
| Negative predictive value (95% CI) | 51.4% (40.2% to 62.4%) | 51.3% (40.3% to 62.2%) | 44.4% (24.6% to 66.3%) | 36.8% (19.1% to 59%) | 44.4% (24.6% to 66.3%) | 36.8% (19.1% to 59%) |
| Positive likelihood ratio (95% CI) | 1.6 (1.03 to 2.48) | 1.65 (1.04 to 2.6) | 1.2 (0.8 to 1.82) | 1.11 (0.74 to 1.65) | 1.22 (0.91 to 1.64) | 1.23 (0.89 to 1.7) |
| Negative likelihood ratio (95% CI) | 0.72 (0.53 to 0.96) | 0.72 (0.54 to 0.96) | 0.69 (0.33 to 1.47) | 0.81 (0.38 to 1.73) | 0.53 (0.24 to 1.18) | 0.54 (0.24 to 1.19) |
CLE, confocal laser endomicroscopy.;.
Food sensitivities
After 2 months of GFD, CLE results were unblinded, patients received recommendations to specifically exclude additional food components that led to a positive reaction in CLE, and the patients who responded to the recommended exclusion diet after six and twelve months (figure 2) were classified as food sensitive. After 6 months, 59 (70.2%) of 84 patients and after twelve months 67 (76.1%) of 88 patients had responded to the dietary intervention. There was no significant association of the proportion of food sensitive patients at 6 or 12 months with gender or IBS-type (table 2).
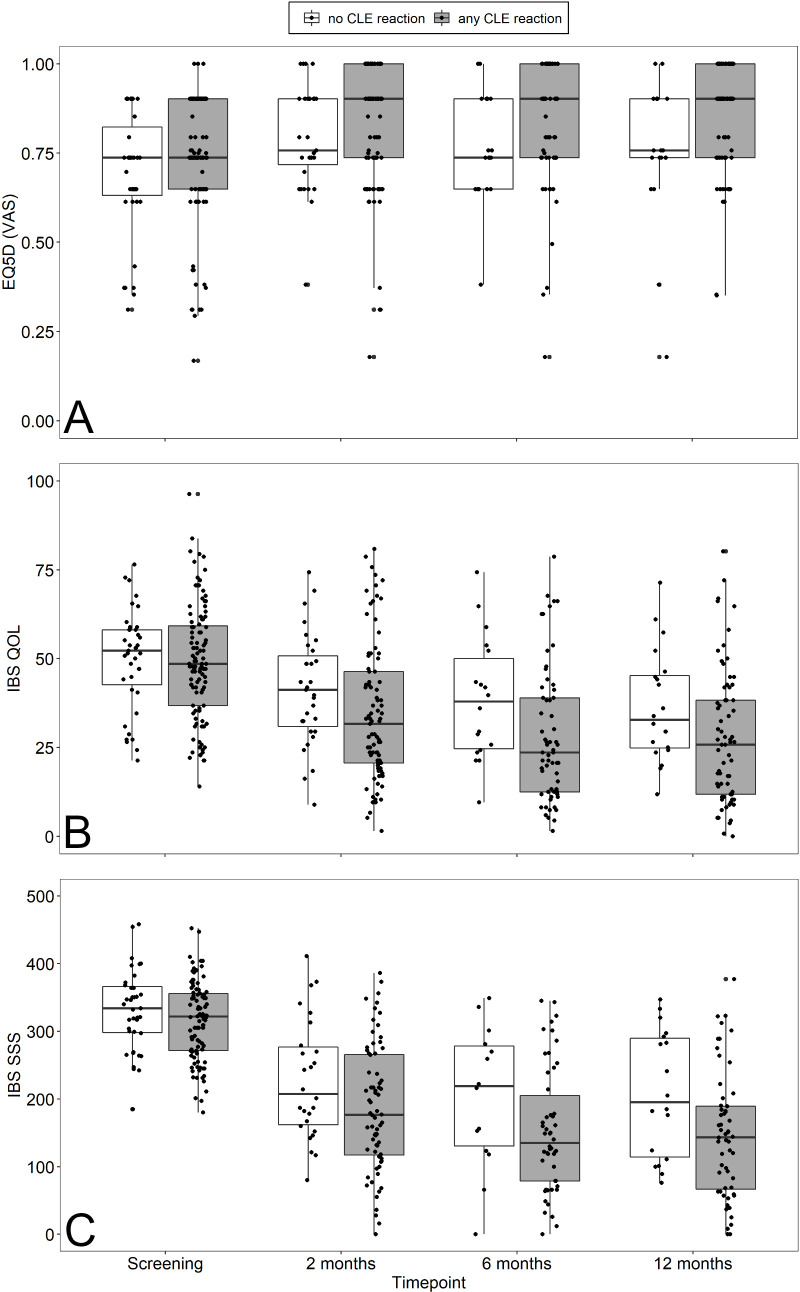
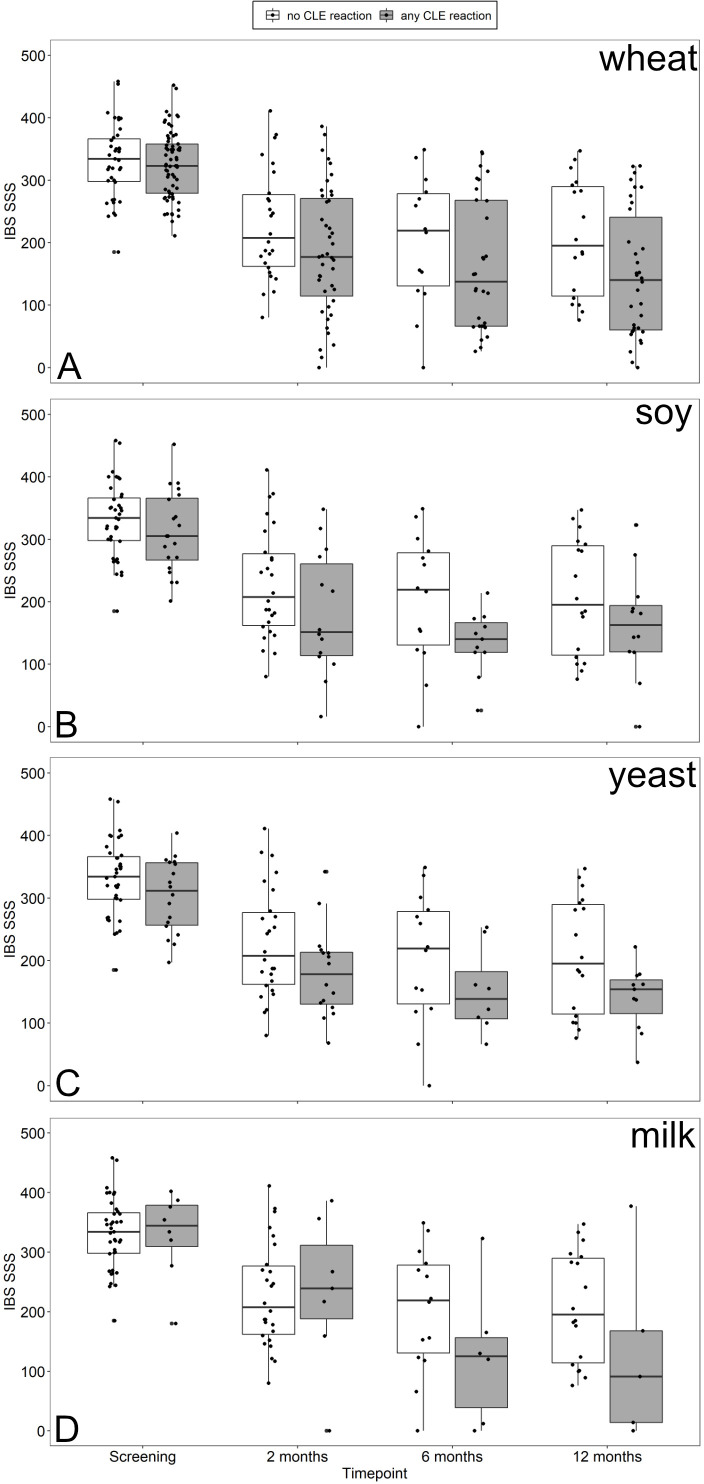
IBS-SSS and IBS-QoL decreased in both, patients with any or no CLE reaction to food antigens mainly after 2 months of GFD and, to a lesser extent, in CLE-positive patients at follow-up (figure 4). EQ5D increased only in CLE-positive patients after 2 months of GFD and remained stable afterwards (figure 4). A specific dietary response should have become visible by symptomatic improvements following unblinding and exclusion of food antigens inducing a CLE reaction. Such an effect may be seen in patients who showed reactions to milk. Only minor decreases of IBS-SSS were observed at 6 and 12 months of follow-up in patients who were CLE-positive after wheat, soy, of yeast challenge (figure 5).
Figure 4.
Box plots of EQ-5D (A), (B) and IBS-SSS (C) at screening and 2, 6 and 12 months after initiating dietary treatment (GFD) in patients with (grey) or without (white) any CLE reaction to food antigens. CLE, confocal laser endomicroscopy; EQ-5D, European Quality of Life 5 Dimensions; GFD, gluten-free diet; IBS-SSS, irritable bowel syndrome-Symptom Severity Scale; QoL, quality of life; VAS, visual analogue scale.
Figure 5.
Box plots of IBS-SSS at screening and 2, 6 and 12 months after initiating dietary treatment comparing patients with CLE reactions (grey) to wheat (A), soy (B), yeast (C) or milk (D) to patients without (white) any CLE reaction to food antigens. CLE, confocal laser endomicroscopy; IBS-SSS, irritable bowel syndrome-Symptom Severity Scale.
Accuracy data of CLE for the diagnosis of any food sensitivity (ie, wheat, soy, yeast or milk) are presented in table 3. The proportion of food sensitive patients correctly identified by CLE was 49/59 patients (sensitivity 83.1%; 97.5% CI: 69.9% to 91.3%) at 6 months and 55/67 patients (sensitivity 82.1%; 97.5% CI: 69.5% to 90.2%) at 12 months. However, high proportions of CLE-negative patients were also found to be food sensitive at 6 months (10/18, 55.6%) and at 12 months (12/19, 63.2%) of follow-up, resulting in low sensitivities of the CLE.
Discussion
WS has been described as NCGS more than 40 years ago.11 As a possible cause of widespread gastrointestinal symptoms, it has received considerable attention in recent years.8 13 26 IBS is a frequent gastrointestinal disorder and more than 80% of patients report symptoms related to food ingestion34 indicating an overlap between WS and IBS. In fact, about one-third of patients with IBS seem to respond to GFD,26 33 and 50%–75% to a LFD.35 Self-reported WS is also common and associated with IBS.36
In the absence of objective diagnostic criteria for WS an expert group suggested 2015 a double-blind gluten challenge to clearly establish the diagnosis.12 In such a controlled study 14% of patients responding to GFD showed a relapse of symptoms during gluten challenge and were identified as having NCGS.37 However, a Norwegian study of patients with self-reported gluten sensitivity showed considerable overlap in their responses to fructan, gluten or placebo bars indicating that gluten challenge may not be a reliable diagnostic method to identify WS in patients with IBS.38
CLE was proposed as a diagnostic instrument to demonstrate food-related mucosal reactions in patients with IBS.21 22 However, the extraordinary diagnostic performance of CLE for detecting food sensitivities reported in these studies was not confirmed in our prospective controlled diagnostic multicentre study. Sensitivity and specificity for WS were well below the threshold of 80%, which we deemed acceptable for such an invasive procedure. CLE has not been proposed to identify gluten sensitivity, so our study did not address the question whether patients had NCGS as defined by the Salerno criteria,12 13 or whether the response to GFD was due to reduced intake of other wheat components as FODMAPs like fructans or ATIs. Wheat FODMAPS, such as fructans, are not expected to elicit any of the changes detected on CLE, so FODMAP-sensitive patients with IBS would probably respond to GFD in the absence of CLE reactivity to wheat and may in part explain this disappointing result. Diagnostic sensitivity for any of the four investigated food sensitivities was found to be >80% at follow-up after 6 and 12 months. However, at these time points specificities were <35% and therefore too low as to recommend widespread use or general diagnostic application of such technique.
To define a positive CLE reaction, we used the criteria described previously21 except the real-time quantification of IEL. In our experience semiquantitative assessment of IEL requires topical acriflavine application.39 Another study also showed that CLE alone was not able to identify IEL.20
More than half of our patients with IBS including those with constipation responded to GFD. This proportion was even higher than reported earlier33 and well in line with responses to LFDs which have been recommended by some associations for patients with IBS,15 although a German study reported low patient adherence.40 The detection rate of WS among patients with IBS in our study is in good accordance with the proportion of patients described elsewhere.21 22 However, not only CLE-positive patients, but a large proportion of CLE-negative patients also responded to GFD in our study.
Although CLE did not seem to significantly contribute to the final diagnosis of WS, the large proportion of responders to GFD may justify the practical approach recommending this diet to patients with IBS symptoms. The efficacy of a GFD should certainly be examined in further controlled clinical studies.
The underlying pathophysiology of food-related duodenal alterations is mostly unknown so far. Whereas an increase in paracellular permeability might be a possible pathway for fluorescein leakage in the small intestinal mucosa,41 42 transcellular permeability changes43 or an impairment of the gut vascular barrier with downregulation of the endothelial barrier may also play a role.44 Recent data identified claudin-2 increase, occludin decrease and eosinophil degranulation, indicating an atypical food intolerance, as causes for the barrier defects in patients with food-related positive CLE findings.22
To summarise, CLE cannot currently be recommended as an initial diagnostic test in patients with IBS suspicious of having WS. Nevertheless, CLE may help to further elucidate the underlying pathomechanism contributing to mucosal and submucosal changes during food-induced alterations in the gastrointestinal tract. A rather practical approach with an 8-week GFD period may be more beneficial to patients with IBS.
Acknowledgments
The authors gratefully acknowledge study nurse and nutritionist Cornelia Krohn for excellent assistance throughout the whole study period. Professor Dr. H. J. Glaser from Vitalisklinik Bad Hersfeld, Germany, supported the study by the generous donation of his Pentax Endomicroscopy System ISC-1000 including an EC3870-CIK confocal laser endomicroscope to one participating study centre. Dr. Miranda Lomer from King’s College London provided helpful dietary information on yeast free diet. Professor Dr. Axel Dignass, Frankfurt, Professor Dr. Stefan Müller-Lissner, Berlin and Professor Dr. Wolfgang Kruis provided valuable support as members of our data monitoring board.
Footnotes
Contributors: CBo, MS and RU: study concept and design, study centre guidance and supervision, manuscript writing; CBo, MS, RK, ME and CS: CLE performance; PT, JB, CBo, MS, RK, ME and CS: screening and study visits; CBo, CBa, MS and PT: blinded assessment of CLE images. RR: statistical advice, supervision and final statistical evaluation; CL: reference pathologist for all upper GI biopsies; all authors: manuscript editing, scientific supervision.
Funding: This study was supported by grants from the German Research Foundation (DFG Bo 1775/3-1, INST 335/534-1 FUGG).
Competing interests: CBo, MS and RU received research grants from Dr. Schär AG. ME obtained consulting and lecture fees from Maunakeatech, Boston Scientific, Takeda, Abbvie, Janssen. CS received research grants and lecture fees from Olympus and Pentax. AS obtained consulting fees from Abbvie, Amgen, Astellas, Biogen, Celltrion, Consal, CSL Behring, Galapagos, Gilead, Institut Allergosan, Janssen, MSD, Norgine, Pfizer Pharma, Roche, Shire and Takeda, lecture fees and travel support from Abbvie, Astellas, Celltrion, Falk Foundation, Ferring, Janssen, MSD, Recordati Pharma and Takeda, and research support from Abbvie. SD obtained lecture fees from BMS, Recordati, Amgen and Falk. BS has served as consultant for Abbvie, Arena, BMS, Boehringer, Celgene, Falk, Janssen, Lilly, Pfizer, Prometheus and Takeda and received speaker’s fees from Abbvie, CED Service, Falk, Ferring, Janssen, Novartis, Pfizer, Takeda (served as representative of the Charité).
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All authors had full access to the study data and have reviewed and approved the final manuscript.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was performed in compliance with the Helsinki declaration and approved by the local ethics committee of the Charité Universitätsmedizin Berlin (approval no. EA4/078/15).
References
- 1. Layer P, Andresen V, Pehl C, et al. [Irritable bowel syndrome: German consensus guidelines on definition, pathophysiology and management]. Z Gastroenterol 2011;49:237–93. 10.1055/s-0029-1245976 [DOI] [PubMed] [Google Scholar]
- 2. Usai-Satta P, Bassotti G, Bellini M, et al. Irritable bowel syndrome and Gluten-Related disorders. Nutrients 2020;12:1117. 10.3390/nu12041117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012;10:712–21. 10.1016/j.cgh.2012.02.029 [DOI] [PubMed] [Google Scholar]
- 4. Icks A, Haastert B, Enck P, et al. Prevalence of functional bowel disorders and related health care seeking: a population-based study. Z Gastroenterol 2002;40:177–83. 10.1055/s-2002-22324 [DOI] [PubMed] [Google Scholar]
- 5. Halder SLS, Locke GR, Schleck CD, et al. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology 2007;133:799–807. 10.1053/j.gastro.2007.06.010 [DOI] [PubMed] [Google Scholar]
- 6. Ford AC, Sperber AD, Corsetti M, et al. Irritable bowel syndrome. Lancet 2020;396:1675–88. 10.1016/S0140-6736(20)31548-8 [DOI] [PubMed] [Google Scholar]
- 7. Gendi R, Jahan N. Pharmacological and non-pharmacological treatments of irritable bowel syndrome and their impact on the quality of life: a literature review. Cureus 2020;12:e9324. 10.7759/cureus.9324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fasano A, Sapone A, Zevallos V, et al. Nonceliac gluten sensitivity. Gastroenterology 2015;148:1195–204. 10.1053/j.gastro.2014.12.049 [DOI] [PubMed] [Google Scholar]
- 9. Sergi C, Villanacci V, Carroccio A. Non-Celiac wheat sensitivity: rationality and irrationality of a gluten-free diet in individuals affected with non-celiac disease: a review. BMC Gastroenterol 2021;21:5. 10.1186/s12876-020-01568-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wahnschaffe U, Ullrich R, Riecken EO, et al. Celiac disease–like abnormalities in a subgroup of patients with irritable bowel syndrome. Gastroenterology 2001;121:1329–38. 10.1053/gast.2001.29572 [DOI] [PubMed] [Google Scholar]
- 11. Ellis A, Linaker BD. NON-CŒLIAC gluten sensitivity? The Lancet 1978;311:1358–9. 10.1016/S0140-6736(78)92427-3 [DOI] [PubMed] [Google Scholar]
- 12. Catassi C, Elli L, Bonaz B, et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015;7:4966–77. 10.3390/nu7064966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Catassi C, Alaedini A, Bojarski C, et al. The overlapping area of non-celiac gluten sensitivity (NCGS) and Wheat-Sensitive irritable bowel syndrome (IBS): an update. Nutrients 2017;9:1268. 10.3390/nu9111268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Junker Y, Zeissig S, Kim S-J, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of Toll-like receptor 4. J Exp Med 2012;209:2395–408. 10.1084/jem.20102660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dionne J, Ford AC, Yuan Y, et al. A systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low FODMAPS diet in treating symptoms of irritable bowel syndrome. Am J Gastroenterol 2018;113:1290–300. 10.1038/s41395-018-0195-4 [DOI] [PubMed] [Google Scholar]
- 16. Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology 2004;127:706–13. 10.1053/j.gastro.2004.06.050 [DOI] [PubMed] [Google Scholar]
- 17. Kiesslich R, Duckworth CA, Moussata D, et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut 2012;61:1146–53. 10.1136/gutjnl-2011-300695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bojarski C, Günther U, Rieger K, et al. In vivo diagnosis of acute intestinal graft-versus-host disease by confocal endomicroscopy. Endoscopy 2009;41:433–8. 10.1055/s-0029-1214604 [DOI] [PubMed] [Google Scholar]
- 19. Kiesslich R, Goetz M, Vieth M, et al. Technology insight: confocal laser endoscopy for in vivo diagnosis of colorectal cancer. Nat Clin Pract Oncol 2007;4:480–90. 10.1038/ncponc0881 [DOI] [PubMed] [Google Scholar]
- 20. Leong RWL, Nguyen NQ, Meredith CG, et al. In vivo confocal endomicroscopy in the diagnosis and evaluation of celiac disease. Gastroenterology 2008;135:1870–6. 10.1053/j.gastro.2008.08.054 [DOI] [PubMed] [Google Scholar]
- 21. Fritscher-Ravens A, Schuppan D, Ellrichmann M, et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014;147:1012–20. 10.1053/j.gastro.2014.07.046 [DOI] [PubMed] [Google Scholar]
- 22. Fritscher-Ravens A, Pflaum T, Mösinger M, et al. Many patients with irritable bowel syndrome have atypical food allergies not associated with immunoglobulin E. Gastroenterology 2019;157:109–18. 10.1053/j.gastro.2019.03.046 [DOI] [PubMed] [Google Scholar]
- 23. European Medicines Agency, Committee for Medicinal Products for Human use (CHMP) . Guideline on the evaluation of medicinal products for the treatment of irritable bowel syndrome. CPMP/EWP/785/97 Rev 1, 2014. Available: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-medicinal-products-treatment-irritable-bowel-syndrome-revision-1_en.pdf
- 24. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395–402. 10.1046/j.1365-2036.1997.142318000.x [DOI] [PubMed] [Google Scholar]
- 25. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920–4. 10.3109/00365529709011203 [DOI] [PubMed] [Google Scholar]
- 26. Carroccio A, Mansueto P, Iacono G, et al. Non-Celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am J Gastroenterol 2012;107:1898–906. quiz 907. 10.1038/ajg.2012.236 [DOI] [PubMed] [Google Scholar]
- 27. Biesiekierski JR, Newnham ED, Irving PM, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol 2011;106:508–14. quiz 15. 10.1038/ajg.2010.487 [DOI] [PubMed] [Google Scholar]
- 28. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) . Guidance for Industry Irritable Bowel Syndrome - Clinical Evaluation of Drugs for Treatment, 2012. Available: https://www.fda.gov/media/78622/download
- 29. Miller LE. Study design considerations for irritable bowel syndrome clinical trials. Ann Gastroenterol 2014;27:338–45. [PMC free article] [PubMed] [Google Scholar]
- 30. Müller-Lissner S, et al. Subject’s Global Assessment of Relief: An appropriate method to assess the impact of treatment on irritable bowel syndrome-related symptoms in clinical trials. J Clin Epidemiol 2003;56:310–6. 10.1016/S0895-4356(03)00027-1 [DOI] [PubMed] [Google Scholar]
- 31. Spiegel B, Bolus R, Harris LA, et al. Measuring irritable bowel syndrome patient-reported outcomes with an abdominal pain numeric rating scale. Aliment Pharmacol Ther 2009;30:1159–70. 10.1111/j.1365-2036.2009.04144.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krummenauer F, Kauczor HU. [Sample size determination in reference-controlled diagnostic trials]. Rofo 2002;174:1438–44. 10.1055/s-2002-35346 [DOI] [PubMed] [Google Scholar]
- 33. Barmeyer C, Schumann M, Meyer T, et al. Long-Term response to gluten-free diet as evidence for non-celiac wheat sensitivity in one third of patients with diarrhea-dominant and mixed-type irritable bowel syndrome. Int J Colorectal Dis 2017;32:29–39. 10.1007/s00384-016-2663-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Böhn L, Störsrud S, Törnblom H, et al. Self-Reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol 2013;108:634–41. 10.1038/ajg.2013.105 [DOI] [PubMed] [Google Scholar]
- 35. Rej A, Avery A, Ford AC, et al. Clinical application of dietary therapies in irritable bowel syndrome. J Gastrointestin Liver Dis 2018;27:307–16. 10.15403/jgld.2014.1121.273.avy [DOI] [PubMed] [Google Scholar]
- 36. Potter MDE, Walker MM, Jones MP, et al. Wheat intolerance and chronic gastrointestinal symptoms in an Australian population-based study: association between wheat sensitivity, celiac disease and functional gastrointestinal disorders. Am J Gastroenterol 2018;113:1036–44. 10.1038/s41395-018-0095-7 [DOI] [PubMed] [Google Scholar]
- 37. Elli L, Tomba C, Branchi F, et al. Evidence for the presence of non-celiac gluten sensitivity in patients with functional gastrointestinal symptoms: results from a multicenter randomized double-blind placebo-controlled gluten challenge. Nutrients 2016;8:84. 10.3390/nu8020084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Skodje GI, Sarna VK, Minelle IH, et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology 2018;154:529–39. 10.1053/j.gastro.2017.10.040 [DOI] [PubMed] [Google Scholar]
- 39. Günther U, Daum S, Heller F, et al. Diagnostic value of confocal endomicroscopy in celiac disease. Endoscopy 2010;42:197–202. 10.1055/s-0029-1243937 [DOI] [PubMed] [Google Scholar]
- 40. Frieling T, Heise J, Krummen B, et al. Tolerability of FODMAP - reduced diet in irritable bowel syndrome - efficacy, adherence, and body weight course. Z Gastroenterol 2019;57:740–4. 10.1055/a-0859-7531 [DOI] [PubMed] [Google Scholar]
- 41. Zakrzewski SS, Richter JF, Krug SM, et al. Improved cell line IPEC-J2, characterized as a model for porcine jejunal epithelium. PLoS One 2013;8:e79643. 10.1371/journal.pone.0079643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schumann M, Richter JF, Wedell I, et al. Mechanisms of epithelial translocation of the alpha(2)-gliadin-33mer in coeliac sprue. Gut 2008;57:747–54. 10.1136/gut.2007.136366 [DOI] [PubMed] [Google Scholar]
- 43. Ayala-Torres C, Krug SM, Schulzke JD, et al. Tricellulin effect on paracellular water transport. Int J Mol Sci 2019;20:5700. 10.3390/ijms20225700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spadoni I, Fornasa G, Rescigno M. Organ-Specific protection mediated by cooperation between vascular and epithelial barriers. Nat Rev Immunol 2017;17:761–73. 10.1038/nri.2017.100 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2021-325181supp001.pdf (2MB, pdf)
Data Availability Statement
Data are available on reasonable request. All authors had full access to the study data and have reviewed and approved the final manuscript.