Abstract
Objectives
Myeloid cell activation by antineutrophil cytoplasmic antibody (ANCA) is pivotal for necrotising vasculitis, including necrotising crescentic glomerulonephritis (NCGN). In contrast to neutrophils, the contribution of classical monocyte (CM) and non-classical monocyte (NCM) remains poorly defined. We tested the hypothesis that CMs contribute to antineutrophil cytoplasmic antibody-associated vasculitis (AAV) and that colony-stimulating factor-2 (CSF2, granulocyte-macrophage colony-stimulating factor (GM-CSF)) is an important monocyte-directed disease modifier.
Methods
Myeloperoxidase (MPO)-immunised MPO−/− mice were transplanted with haematopoietic cells from wild-type (WT) mice, C–C chemokine receptor 2 (CCR2)−/− mice to abrogate CM, or transcription factor CCAAT–enhancer-binding protein beta (C/EBPβ)−/− mice to reduce NCM, respectively. Monocytes were stimulated with CSF2, and CSF2 receptor subunit beta (CSF2rb)-deficient mice were used. Urinary monocytes and CSF2 were quantified and kidney Csf2 expression was analysed. CSF2-blocking antibody was used in the nephrotoxic nephritis (NTN) model.
Results
Compared with WT mice, CCR2−/− chimeric mice showed reduced circulating CM and were protected from NCGN. C/EBPβ−/− chimeric mice lacked NCM but developed NCGN similar to WT chimeric mice. Kidney and urinary CSF2 were upregulated in AAV mice. CSF2 increased the ability of ANCA-stimulated monocytes to generate interleukin-1β and to promote TH17 effector cell polarisation. CSF2rb−/− chimeric mice harboured reduced numbers of kidney TH17 cells and were protected from NCGN. CSF2 neutralisation reduced renal damage in the NTN model. Finally, patients with active AAV displayed increased urinary CM numbers, CSF2 levels and expression of GM-CSF in infiltrating renal cells.
Conclusions
CMs but not NCMs are important for inducing kidney damage in AAV. CSF2 is a crucial pathological factor by modulating monocyte proinflammatory functions and thereby TH17 cell polarisation.
Keywords: Autoantibodies, Autoimmune Diseases, Granulomatosis with polyangiitis, Systemic vasculitis, Inflammation
Key messages.
What is already known about this subject?
Antineutrophil cytoplasmic antibody-associated vasculitis is a myeloid cell-mediated vascular inflammation and glomerulonephritis. However, the detailed pathogenic contribution of distinct monocyte subsets is not clear.
What does this study add?
We identified C–C chemokine receptor 2 (CCR2)-positive classical monocytes as essential components for induction of antineutrophil cytoplasmic antibody-induced glomerulonephritis. Furthermore, colony-stimulating factor-2 (CSF2) and its receptor CSF2 receptor subunit beta are important for activation of monocytes and subsequent regulation of T cells.
How might this impact on clinical practice or future developments?
Direct targeting of monocytes (CCR2 antibodies) or CSF2 (granulocyte-macrophage colony-stimulating factor) could be a novel treatment strategy in human crescentic glomerulonephritis.
Introduction
Antineutrophil cytoplasmic antibody-associated vasculitis (AAV) is a systemic autoimmune disease featuring inflammation of small blood vessels and multiorgan damage, including necrotising crescentic glomerulonephritis (NCGN).1 2 Antineutrophil cytoplasmic antibody (ANCA) recognises either myeloperoxidase (MPO) or proteinase 33 exclusively expressed by neutrophils and monocytes. ANCAs bind to their cell surface-presented antigens and activate both myeloid cell types.4 The contribution of neutrophils to AAV is well documented, whereas the mechanistic role of monocytes remains incompletely understood.5–7
Murine monocytes are classified into distinct Ly6Chi inflammatory or classical monocytes (CMs) and Ly6Clo patrolling or non-classical monocytes (NCMs).8 Corresponding human subsets are CD14+CD16− and CD14loCD16+ monocytes, respectively.9 CMs are released from the bone marrow and give rise to circulating blood NCM.10 Murine Ly6Cint and human CD14+CD16+ intermediary monocytes (IMs) provide a third subset that possibly represents a maturation stage between CM and NCM. The CM to NCM conversion depends on the transcription factor CCAAT–enhancer-binding protein beta (C/EBPβ).11 Similar to neutrophils, ANCAs induce monocyte activation resulting in reactive oxygen species (ROS) production12 and release of proinflammatory cytokines, such as interleukin (IL)-6,13 monocyte chemoattractant protein-1 (MCP-1)14 and IL-1β, that are crucial for AAV induction in mice.15 16 Monocyte exposure to MPO–ANCA promotes survival and macrophage differentiation.17 Soluble CD163 (sCD163) shed from activated monocytes may provide a biomarker of renal flares in AAV.18 Unselective monocyte depletion protected mice from anti-MPO-induced AAV.7 Information on the mechanistic contribution of monocyte subsets in AAV is not available.
The colony stimulating factor 2 (CSF2), also known as granulocyte-macrophage colony-stimulating factor (GM-CSF), is produced by various cells, including myeloid, lymphoid and non-haematopoietic cells.19 CSF2 binds to its receptor CSF2R (GM-CSFR) constituted by a ligand-binding α-chain and a signal-transducing β-chain (GM-CSF2Rβ or CSF2 receptor subunit beta (CSF2Rb)).20 21 CSF2 was initially described as a haematopoietic growth factor for myeloid cell development. However, recent studies found a normal myelopoiesis in mice deficient either for CSF2 or for its receptor.19 Instead, CSF2 was suggested as a modifier of myeloid cell activation in inflammatory conditions, including a murine experimental autoimmune encephalomyelitis (EAE) model where mice with a specific deletion of the GM-CSFRβ subunit (Csf2rb−/−) in C–C chemokine receptor 2 (CCR2)+ monocytes were protected.22 Clinical studies indicated that CSF2 levels were strongly increased in patients with active AAV compared with patients on remission (Rem) and healthy controls (HCs).23 We have previously shown that monocytes are important mediators of kidney damage in ANCA-induced vasculitis.7 We now investigated the specific contribution of CM and NCM and explored whether CSF2 controls disease-mediating monocyte functions.
Methods
Animal experiments
MPO−/− mice were immunised intraperitoneally with murine MPO in complete Freund’s adjuvant, boosted intraperitoneally after 4 weeks with murine MPO in incomplete Freund’s adjuvant, lethally irradiated and subsequently transplanted intravenously with bone marrow cells (1.5×107) from either C57BL/6 J wild type (WT) (The Jackson Laboratory), CCR2−/−, C/EBPβ−/− or Csf2rb−/− mice. Nephrotoxic nephritis (NTN) was induced by intraperitoneal injection of 10 µL/g BW NTS (Probetex, San Antonio, Texas, USA), and mice were sacrificed on day 6.
A detailed method section is provided in the online supplemental file 2.
annrheumdis-2021-221984supp002.pdf (162.3KB, pdf)
Results
CMs are essential for anti-MPO-induced glomerulonephritis
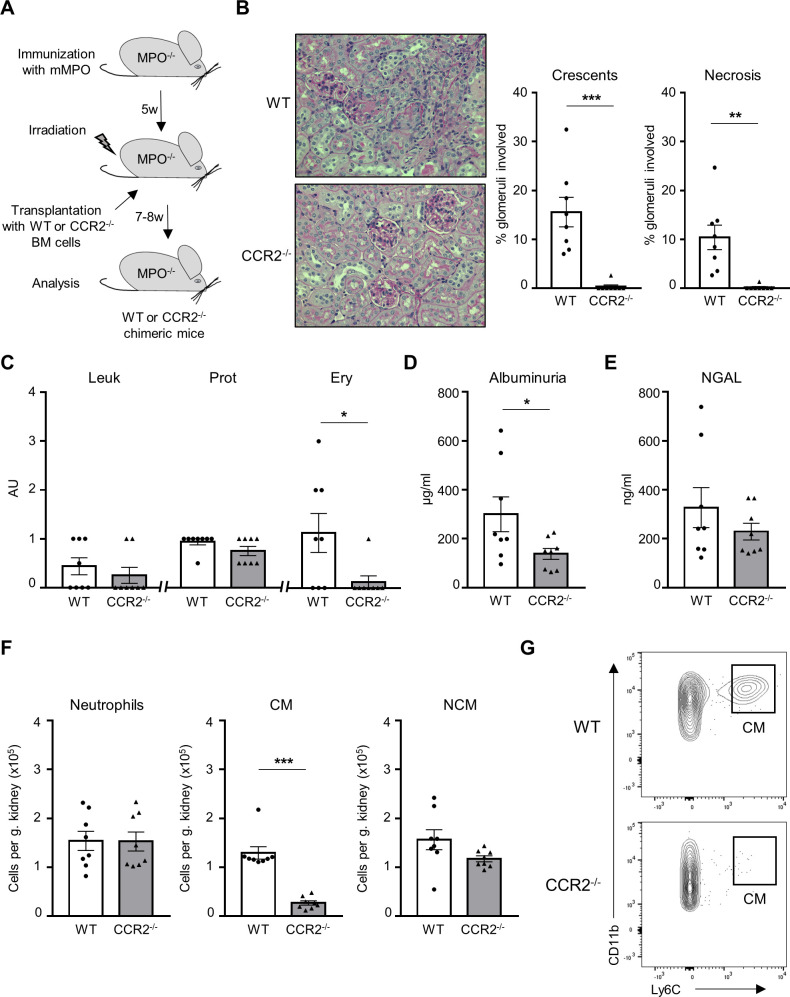
We first explored whether CMs mediate anti-MPO ANCA-induced glomerulonephritis in an MPO–ANCA mouse model. MPO-deficient mice were immunised with murine MPO, irradiated and transplanted with bone marrow (BM) cells from either WT or CCR2-deficient (CCR2−/−) animals to generate MPO−/−/WT mice and MPO−/−/CCR2−/− chimeric mice, respectively (figure 1A). Mice were euthanised 7–8 weeks after transplantation, and blood, serum, urine, spleens and kidneys were analysed. Blood analysis was performed by flow cytometry with an adapted gating strategy to characterise neutrophils and both monocyte subsets (online supplemental figure 1A). The percentage of CD11b+Ly6G+ neutrophils was similar in both groups. CD11b+CD115+Ly6Chi CMs were almost absent in CCR2−/− chimeric mice (online supplemental figure 1B). Consequently, a non-significant marginal reduction of CD11b+CD115+Ly6Clo NCMs was observed. Renal histology revealed protection from kidney injury in CCR2−/− chimeric mice with a significantly reduced percentage of crescentic and necrotic glomeruli (figure 1B). Correspondingly, erythrocyturia and albuminuria were also significantly decreased (figure 1C, D). Urinary neutrophil gelatinase-associated lipocalin (NGAL) was similar in both groups (figure 1E), suggesting tubular injury. We analysed renal infiltrating inflammatory cells by flow cytometry to identify neutrophils and monocyte subsets (online supplemental figure 2). We observed strongly reduced numbers of kidney-infiltrating CD11b+Ly6Chi CM, whereas both CD11b+Ly6C−MHCII−CD11cmid/hi NCM and CD11b+Ly6G+ neutrophil influx were not altered (figure 1F, G and online supplemental figure 3). These experiments firmly establish the central role of CM in mediating anti-MPO-induced NCGN.
Figure 1.
CCR2−/− chimeric mice are protected from anti-MPO-induced NCGN. (A) Experimental protocol describing the induction of NCGN in MPO−/−. Chimeric mice were analysed 7–8 weeks following BM transplantation. (B) CCR2−/− chimeric mice showed reduced renal damage compared with WT chimeric mice with significant reduction of crescentic and necrotic glomeruli. For each group, a representative image of a kidney section stained with PAS at high magnification (×40) is shown. (C) CCR2−/− chimeric mice displayed reduced Ery by dipstick. Leu and Prot were similar in both groups. (D) Reduction in albuminuria by ELISA in CCR2−/− chimeric mice. (E) Urinary NGAL levels by ELISA were similar in CCR2−/− and WT chimeric mice. (F) Renal CD11b+Ly6G+ neutrophils, CD11b+Ly6G−Ly6C+ CMs and CD11b+Ly6G−Ly6C−MHC-II−CD11cmid/+ NCMs were analysed by flow cytometry. Infiltration of CM was strongly reduced in CCR2−/− chimeric mice, whereas infiltration of neutrophils and NCMs was similar in both groups. The number of immune cells is expressed per gram kidney and calculated using counting beads. (G) Representative flow cytometry plot showing that the renal infiltration of CM is strongly reduced in CCR2−/− chimeric mice. *P<0.05, **P<0.01, ***P<0.001. CCR2, C–C chemokine receptor 2; AU, arbitrary unit; CM, classical monocyte; Ery, erythrocyturia; Leu, leukocyturia; mMPO, murine MPO; MPO, myeloperoxidase; NCGN, necrotising crescentic glomerulonephritis; NCM, non-classical monocyte; NGAL, neutrophil gelatinase-associated lipocalin; PAS, periodic acid-Schiff; Prot, proteinuria; WT, wild type.
annrheumdis-2021-221984supp001.pdf (1.3MB, pdf)
NCMs are dispensable for anti-MPO-induced glomerulonephritis
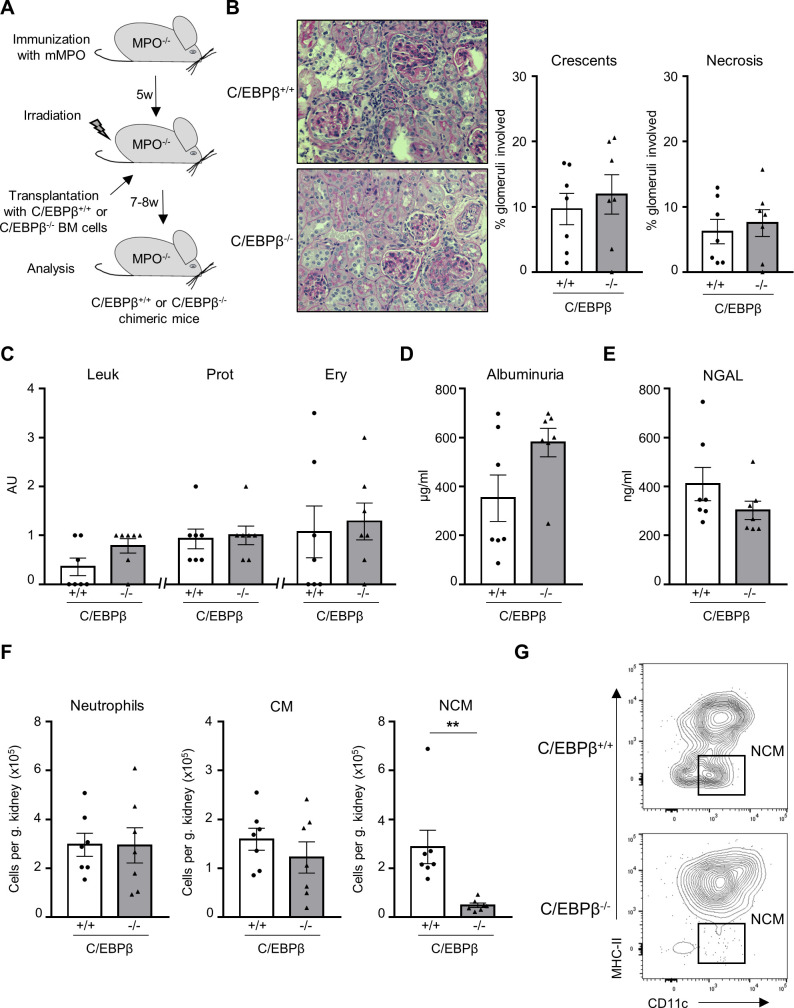
Next, we studied whether NCM contribute to disease induction as previous data suggested that NCM are essential in mediating glomerular neutrophil influx.24 We took advantage of mice deficient in the transcription factor C/EBPβ, which is essential for NCM Ly6C− monocyte development and survival.11 MPO-deficient mice were immunised with murine MPO, irradiated and transplanted with BM cells from either C/EBPβ-positive (C/EBPβ+/+) or C/EBPβ-deficient (C/EBPβ−/−) animals to generate MPO−/− C/EBPβ+/+ mice (WT) and MPO−/− C/EBPβ−/− chimeric (NCM-deficient) mice, respectively (figure 2A). Both chimeric mice had similar numbers of circulating neutrophils (online supplemental figure 1A, C). As expected, C/EBPβ−/− chimeric mice displayed an almost total absence of circulating NCM together with a marginal, non-significant reduction of blood CM. Renal histology showed no differences in glomerular crescents and necrosis between WT and NCM-deficient mice (figure 2B). Urinalysis did not show differences by dipstick analysis, albuminuria and urinary NGAL concentration (figure 2C–E). We observed a profound reduction in NCM influx, whereas CM and neutrophil influx were not affected (figure 2). These data establish that NCMs are dispensable for anti-MPO induced NCGN.
Figure 2.
Absence of NCMs in C/EBPβ−/− chimeric mice does not affect anti-MPO-induced NCGN. (A) Experimental scheme describing the induction of NCGN in MPO−/− mice. (B) No difference in histological renal damage between C/EBPβ−/− and WT chimeric mice. For each group, a representative image of a kidney section stained with PAS at high magnification (×40) is shown. (C) C/EBPβ−/− chimeric mice showed no difference in Leu, Prot and Ery by dipstick compared with C/EBPβ+/+ chimeric mice. (D) Albuminuria by ELISA was similar in both groups. (E) Urinary NGAL levels by ELISA were similar in both groups. (F) Renal CD11b+Ly6G+ neutrophils, CD11b+Ly6G−Ly6C+ CMs and CD11b+Ly6G−Ly6C−MHC-II−CD11cmid/+ NCMs were analysed by flow cytometry. Infiltration of NCM was strongly reduced in C/EBPβ−/− chimeric mice, whereas infiltration of neutrophils and CM was similar in both groups. The number of immune cells is expressed per gram kidney and calculated using counting beads. (G) Representative flow cytometry plot showing that the renal infiltration of NCM is strongly reduced in C/EBPβ−/− chimeric mice. **P<0.01. AU, arbitrary unit; C/EPBβ, CCAAT–enhancer-binding protein beta; CM, classical monocyte; Ery, erythrocyturia; Leu, leukocyturia; MPO, myeloperoxidase; mMPO, murine MPO; NCGN, necrotising crescentic glomerulonephritis; NCM, non-classical monocyte; NGAL, neutrophil gelatinase-associated lipocalin; PAS, periodic acid-Schiff; Prot, proteinuria; WT, wild type.
CSF2 is increased in kidneys and urine of AAV mice, and CSF2rb deletion in haematopoietic cells protects from anti-MPO induced glomerulonephritis
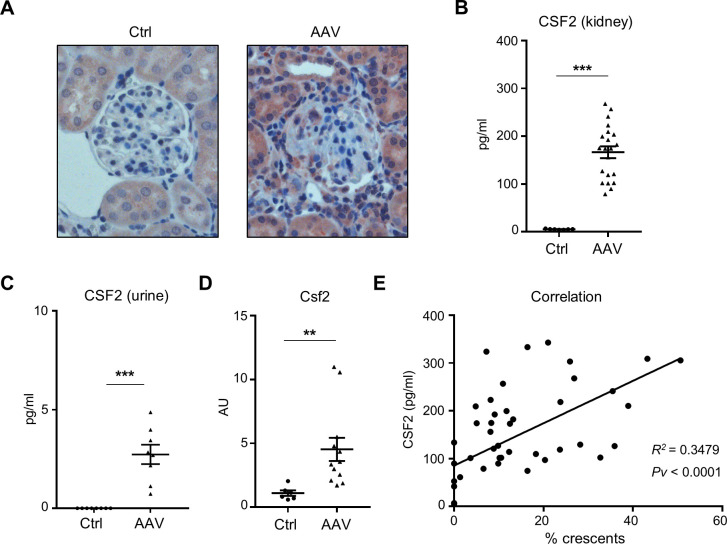
We next investigated CSF2 as a monocyte-targeting disease modifier in AAV. Using immunohistochemistry, we found that CSF2 protein was expressed in kidney sections from AAV but not HC mice (figure 3A). We observed strong CSF2 expression in tubular cells and in some glomerulus-infiltrating leucocytes. We confirmed these results on CSF2 protein assessing kidney lysates by ELISA. High CSF2 protein was detectable in AAV but not in control mice (figure 3B). Moreover, urinary CSF2 was strongly upregulated in AAV mice but undetectable in urine from healthy mice (figure 3C). In parallel, kidney Csf2 mRNA expression was strongly upregulated, confirming that CSF2 is locally expressed (figure 3D). Finally, we found a positive correlation between CSF2 protein level and the percentage of renal crescents (figure 3E). These results indicate that CSF2 is strongly upregulated in kidneys of AAV mice. We next explored the hypothesis that CSF2 is a critical mediator of AAV.
Figure 3.
CSF2 expression is increased in kidneys from mice with anti-MPO-induced NCGN. (A) Immunofluorescence images show strong CSF2 expression in kidney sections from mice with AAV compared with Ctrl mice. A representative image at magnification ×40 is shown for each group. 4',6-Diamidin-2-phenylindol (DAPI) was used to stain nuclei (blue). (B) CSF2 level in urine by ELISA is increased in AAV mice compared with Ctrl mice. (C) CSF2 level by ELISA in renal lysates is increased in AAV mice compared with Ctrl mice. (D) Csf2 mRNA by RT-PCR expression in kidney lysates is increased in AAV mice compared with Ctrl mice. (E) Correlation between the percentage of crescents and the amount of renal CSF2. **P<0.01, ***P<0.001. AAV, antineutrophil cytoplasmic antibody-associated vasculitis; AU, arbitrary unit; CSF2, colony-stimulating factor-2; Ctrl, control; MPO, myeloperoxidase; NCGN, necrotising crescentic glomerulonephritis.
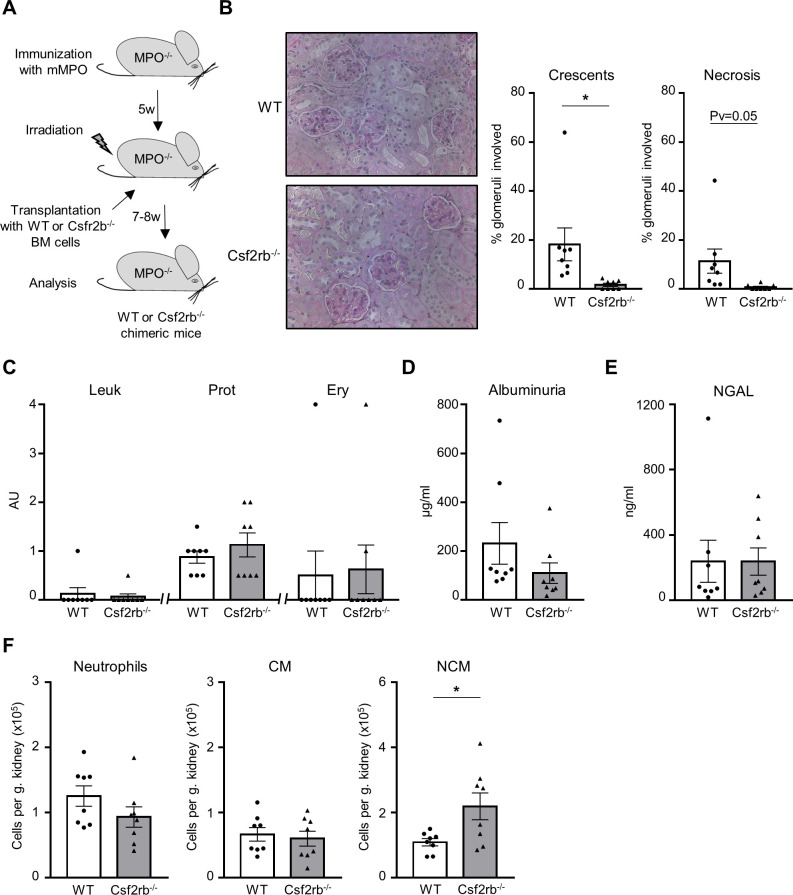
To study the mechanistic role of CSF2 in AAV, MPO-deficient mice were immunised with murine MPO, irradiated and transplanted with BM cells from either C57BL/6J WT (WT) or CSF2rb-deficient (CSF2rb−/−) animals to generate MPO−/− CSF2rb+/+ mice (WT) and MPO−/− CSF2rb−/− chimeric (CSF2rb−/−) mice, respectively (figure 4A). Anti-MPO titres and blood cell counts were similar in both animal groups (online supplemental figure 4). Renal histology revealed strongly reduced crescent formation in CSF2rb−/− chimeric mice compared with the WT group (figure 4B). Urine analysis did not show group differences by dipstick analysis, albumin ELISA or NGAL ELISA (figure 4C–E). Remarkably, quantification of kidney-infiltrating leucocytes revealed no difference in either neutrophil, CM or in NCM influx (figure 4F and online supplemental figure 3). These data demonstrate that CSF2 and its interaction with its CSF2rb receptor on myeloid cells are essential for crescent formation and that the effect is not mediated by reduced myeloid cell influx. Thus, we next explored CSF2 effects on monocyte functions in the presence of MPO–ANCA.
Figure 4.
Csf2rb−/− chimeric mice are protected from anti-MPO-induced NCGN. (A) Experimental settings describing the induction of NCGN. (B) Csf2rb−/− chimeric mice developed less renal damage compared with WT chimeric mice with a reduction of crescentic and necrotic glomeruli. A representative image of a kidney section stained with PAS at high magnification (×40) is shown for each group. (C) Leu, Prot and Ery by urine dipstick are similar in both groups. (D) Albuminuria and (E) NGAL urine levels by ELISA were similar in both groups. (F) Renal infiltration of immune cells was analysed by flow cytometry. Infiltration of CD11b+Ly6G+ neutrophils and CD11b+Ly6G−Ly6C+ CMs was similar in both groups, whereas CD11b+Ly6G-Ly6C−MHC-II−CD11cmid/+ NCM infiltration was slightly increased in Csf2rb−/− chimeric mice compared with WT chimeric mice. The number of immune cells is expressed per gram kidney and calculated using counting beads. *P<0.05. AU, arbitrary unit; CM, classical monocyte; Csf2rb, CSF2 receptor subunit beta; Ery, erythrocyturia; Leu, leukocyturia; MPO, myeloperoxidase; NCGN, necrotising crescentic glomerulonephritis; NCM, non-classical monocyte; NGAL, neutrophil gelatinase-associated lipocalin; PAS, periodic acid-Schiff; Prot, proteinuria; WT, wild type.
CSF2 modulates ANCA-stimulated monocytes
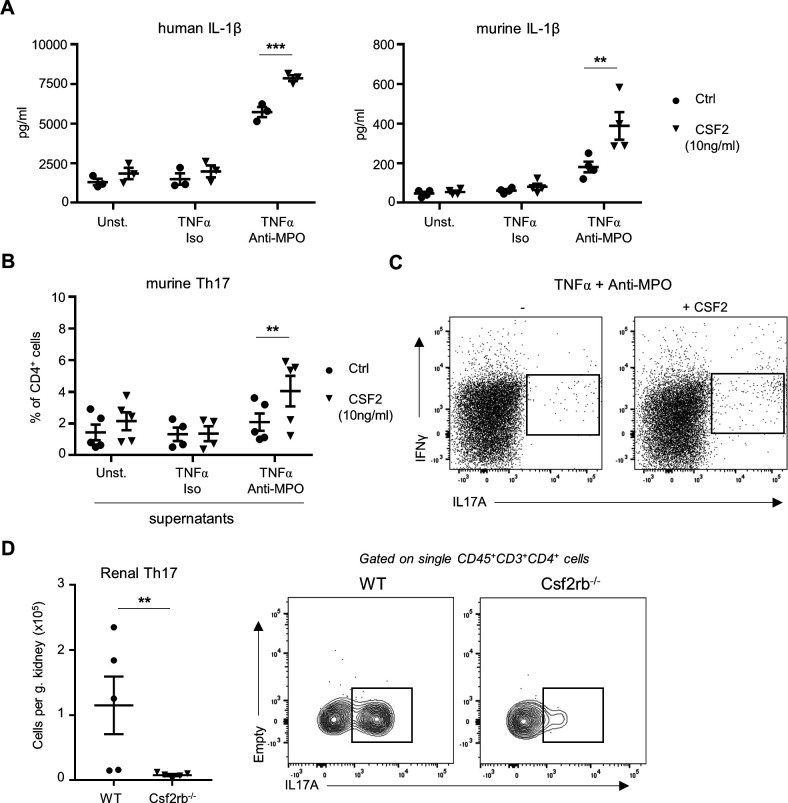
Monocytes were isolated from human blood and murine BM. First, we analysed the effect of CSF2 priming on anti-MPO IgG-induced IL-1β generation. We confirmed that anti-MPO IgG-stimulated human and murine monocytes released IL-1β (figure 5A, B).15 16 Importantly, CSF2 priming significantly increased the IL-1β release by MPO-ANCA. We next tested the capacity of supernatants from ANCA-stimulated murine monocytes to promote the polarisation of CD4+ T cells into IL-17A-producing T-helper cells (TH17), which are known to be an important contributor to kidney injury in AAV. Monocyte supernatants were added to CD4+ T cells from murine spleens. After 5 days, TH17 cells were detected by flow cytometry. Supernatants from anti-MPO IgG-stimulated monocytes did not induce TH17 polarisation, whereas supernatants from anti-MPO IgG-stimulated monocytes that were primed with CSF2 strongly induced TH17 polarisation in vitro (figure 5B, C). Importantly, we found a reduction in numbers of kidney-infiltrating TH17 cell in MPO−/− CSF2rb−/− chimeric mice compared with MPO−/− WT mice, suggesting that this polarisation effect occurs also in vivo (figure 5D). Together, these data establish that CSF2 increases proinflammatory monocyte functions and promote the subsequent polarisation of CD4+ T cells. Importantly, human monocyte-derived macrophages lost MPO protein expression during differentiation with either GM-CSF or macrophage colony-stimulating factor (M-CSF) (online supplemental figure 5A, B) and could not be activated by anti-MPO ANCA, thereby excluding a direct role of macrophages in this context (online supplemental figure 5C, D).
Figure 5.
CSF2 increases the capacity of anti-MPO stimulated monocytes to release IL-1β and polarise TH17 CD4+ T cells. (A) TNFα-primed human or murine neutrophils were isolated and stimulated with either isotype IgG or anti-MPO IgG. Unstimulated cells were used as negative controls. Addition of recombinant murine or human CSF2 (10 ng/mL) increases IL-1β generation by TNFα-primed-monocytes stimulated with anti-MPO as measured in culture medium by ELISA. (B, C) CD4+ T cells were sorted from murine spleen and cultured in medium containing anti-IFN-γ, anti-IL-4, anti-IL-2 antibodies, together with supernatants from TN-α-primed monocytes stimulated with either isotype IgG or anti-MPO IgG. Supernatants from unstimulated monocytes were used as negative control. Addition of recombinant murine CSF2 (10 ng/mL) increases the capacity of TNFα-primed anti-MPO stimulated monocytes to induce TH17 polarisation. A representative flow cytometry plot is shown. (D) Renal TH17 CD4+ T cells were analysed by flow cytometry in WT and Csf2rb−/− chimeric mice. **P<0.01, ***P<0.001. CSF2, colony-stimulating factor-2; Csf2rb, CSF2 receptor subunit beta; Ctrl, control; IFN-γ, interferon gamma; IL, interleukin; MPO, myeloperoxidase; TNFα, tumour necrosis factor alpha; WT, wild type.
Patients with ANCA-induced NCGN display elevated renal CSF2 level and urinary myeloid cells
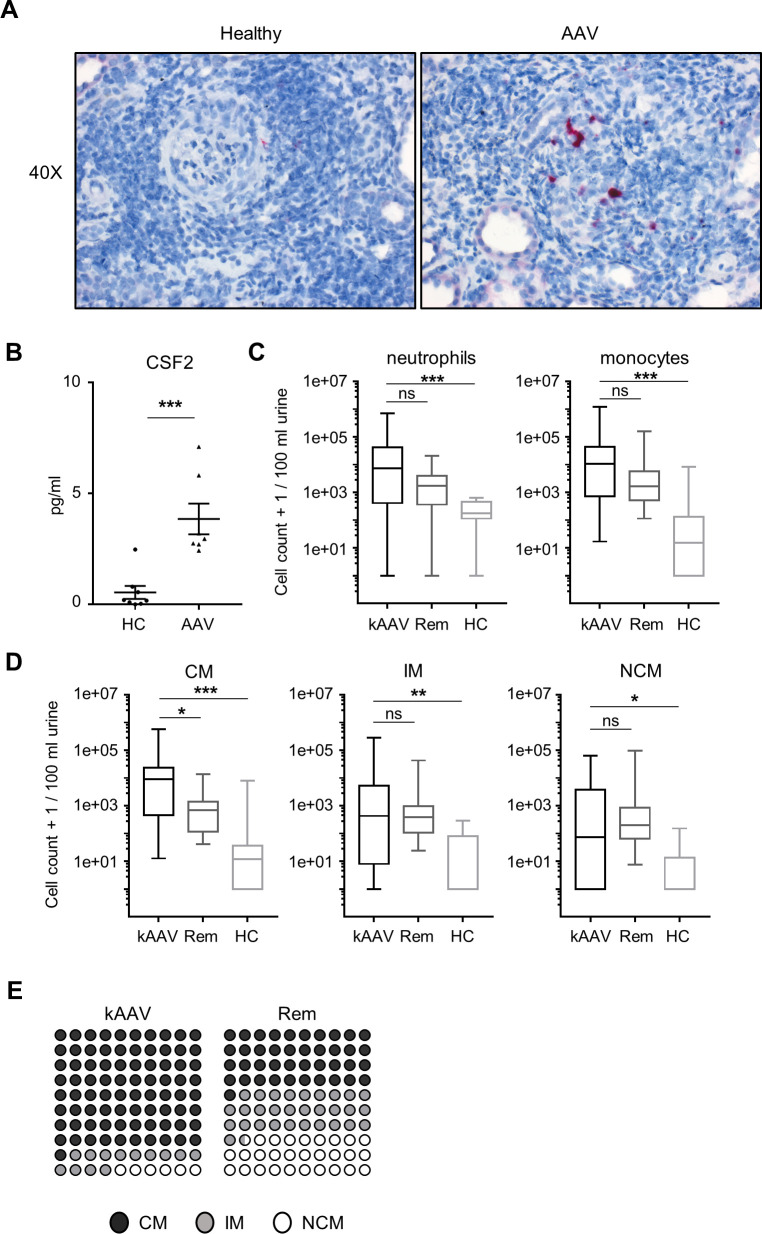
Next, we analysed CSF2 expression by in situ hybridisation in kidney biopsies from patients with active AAV and controls (figure 6A). We observed strong upregulation of CSF2 expression in glomerulus-infiltrating cells and detected increased CSF2 in urine from patients with active AAV (figure 6B). Finally, we analysed urinary myeloid cells by flow cytometry in 59 subjects, 31 with active antineutrophil cytoplasmic antibody-associated vasculitis kidney manifestation (kAAV), 20 patients in stable AAV Rem but previous AAV kidney manifestation and 8 HCs. Patient characteristics are given in the online supplemental table 1. The gating strategy for the distinct myeloid cell populations is shown in online supplemental figure 6. We found significantly increased urinary neutrophil and monocyte cell counts in kAAV (median: 17599, IQR 5459–72 194) in comparison to all other groups (Rem: 1116, IQR 638–2376; HC: 1263, IQR 406–2064) (figure 6C). Analysis of distinct monocyte subpopulations by surface staining showed that classical, intermediate and NCMs were strongly increased in patients with kAAV compared with HCs (figure 6D). Moreover, urinary CMs were increased in patients with kAAV compared with patients in Rem, while NCMs were similar (figure 6D, E).
Figure 6.
CSF2 and monocyte subsets in patients with AAV and HCs. (A) Human kidney biopsies from patients with AAV were analysed for in situ expression of Csf2 usingRNAscope Probe-Hs-CSF2 mRNA and appropriate positive and negative controls. Counterstaining was done with haematoxylin. A representative picture at ×40 magnification is shown for both groups (left: negative control, right: RNAscope for Csf2). (B) CSF2 protein in human urines was measured by ELISA. CSF2 was increased in urine from patients with active AAV compared with HCs. (C, D) Myeloid cells in urines from patients with active AAV with kidney involvement (kAAV), patients in Rem and HCs were analysed by flow cytometry. Neutrophils, monocytes and the three different monocyte subsets were quantified and expressed as cell number per 100 mL urine. Total neutrophil and monocyte numbers are significantly higher in patients with kAVV compared with HCs. CD14+CD16− CMs, CD14+CD16+ IMs and CD14−CD16+ NCMs are significantly increased in urine from patients with kAAV compared with HC. In addition, CMs are increased in urine from patients with kAAV compared with patients with AAV in Rem. (E) Dot plots representing the distribution of the different monocyte subsets in urine from patients with kAAV and Rem. *P<0.05, **P<0.01, ***P<0.001. AAV, antineutrophil cytoplasmic antibody-associated vasculitis; CM, classical monocyte; CSF2, colony-stimulating factor-2; HC, healthy control; IM, intermediary monocyte; kAAV, antineutrophil cytoplasmic antibody-associated vasculitis kidney manifestation; NCM, non-classical monocyte; ns, not significant; Rem, remission.
CSF2 blockade reduces kidney damage in crescentic glomerulonephritis
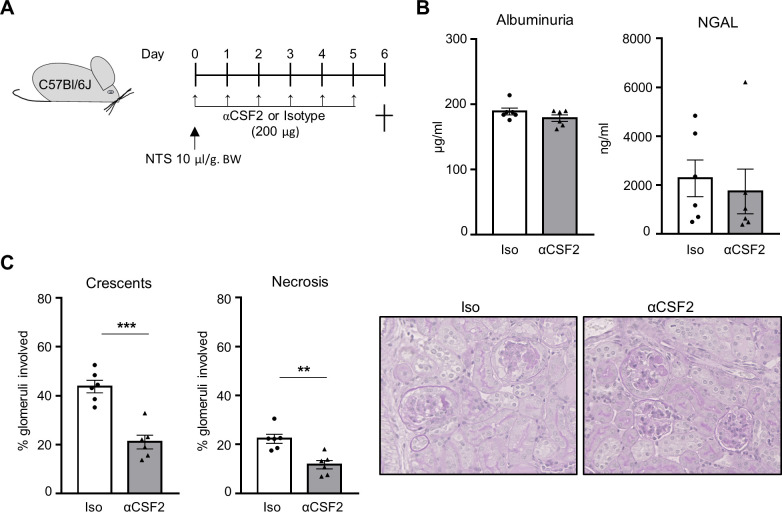
Finally, we performed an animal experiment to address the potential of CSF2 neutralisation as therapeutic option for crescentic glomerulonephritis. To this purpose, we used the nephrotoxic nephritis model (figure 7A). One group of mice received daily injection of anti-CSF2 antibody, whereas control mice received the same amount of isotype IgG. Although mice from both groups developed high albuminuria and urine NGAL levels (figure 7B), CSF2 blockade attenuated the development of renal damage as the amount of crescentic and necrotic glomeruli was decreased in treated mice compared with control mice injected with isotype IgG (figure 7C).
Figure 7.
CSF2 neutralisation attenuates crescentic glomerulonephritis. (A) Experimental protocol describing the induction of cGN in C57Bl/6J mice. Animals were sacrificed 6 days after the induction of cGN with NTS. Anti-CSF2 antibodies were injected daily. Control mice received the same amount of isotype. (B) Albuminuria and urine NGAL were determined by ELISA. (C) Mice treated with anti-CSF2 antibodies showed reduced renal damage compared with control mice with significant reduction of crescentic and necrotic glomeruli. For each group, a representative image of a kidney section stained with PAS at high magnification (×40) is shown. **P<0.01, ***P<0.001. cGN, crescentic glomerulonephritis; CSF2, colony-stimulating factor-2; NGAL, neutrophil gelatinase-associated lipocalin; NTS, nephrotoxic serum; PAS, periodic acid-Schiff.
Discussion
We studied the mechanistic role of monocytes and their subsets in inducing ANCA vasculitis. First, our study shows that CMs play an important non-redundant role in AAV, whereas NCMs are dispensable. Second, we demonstrate the importance of renal CSF2 as a monocyte-targeting disease modifier. Renal CSF2 was increased in mice and patients with AAV and accelerated ANCA-induced monocyte activation, IL-1β release and monocyte-mediated TH17 polarisation. CSF2rb deficiency reduced the kidney-infiltrating TH17 cells and protected from NCGN. Third, we show that patients with active AAV with NCGN feature key findings characterised in the mechanistic murine studies, namely, increased renal and urine CSF2, and increased urinary CM numbers. Fourth, we could demonstrate that CSF2 blockade attenuated the development of renal damage in the NTN model of crescentic glomerulonephritis.
The central role of neutrophils in the pathogenesis of AAV is supported by a multitude of in vitro and in vivo studies. For example, Xiao et al showed that neutrophil depletion with the NIMP-R14 monoclonal antibody protected mice. However, as this antibody targets the Gr-1 antigen, also known as Ly-6G/Ly6-C, that is expressed by both neutrophils and monocytes, the contribution of monocytes could not be completely ruled out.25 Conceivably, both monocytes and neutrophils are essential for NCGN induction with distinct time-specific roles. Our results do not exclude a role of neutrophil in the development of AAV but rather suggest that either monocytes modulate neutrophil functions or both cell subsets work in concert to promote vasculitis and renal damage. Our data now firmly establish that CMs are important contributors to MPO–ANCA-induced vasculitis, whereas NCMs are dispensable. Although we cannot completely exclude that minimal residual NCM in C/EBPβ−/− chimeric mice played a minor role, the almost unaffected circulating NCMs in CCR2−/− chimeric mice and the even increased infiltration of NCM in kidneys of CSF2rb−/− chimeric mice that displayed strongly reduced renal damage further support our conclusion derived from C/EBPβ−/− mice. Using ANCA unrelated animal models, NCMs were described to perform patrolling functions, thereby controlling both CM and neutrophil influx. Using imaging studies, Finsterbusch et al demonstrated in a murine NTN model that NCMs continuously traffic along the glomerular endothelium and that CX3CR1 expressed by NCM is crucial for this process.24 Turner-Stokes et al showed in a rat NTN model that monocytes infiltrate the inflamed glomerulus in a two-wave recruitment process.26 The investigators showed that NCMs enter the glomerulus initially, whereas CMs follow at later time points. However, NTN does not feature characteristics of human ANCA-induced NCGN, and our murine and human data support the notion that CMs rather than NCMs are important in inducing ANCA-mediated pauci-immune NCGN. Importantly, although NCMs have been described to play an important role in the earlier phase of disease induction in the NTN model, our overall injury data at later time points clearly demonstrate that CMs are the major inducer of cumulative kidney damage in an anti-MPO IgG-induced NCGN. Conceivably, with single-cell studies, the distinction of monocytes into three subsets may become obsolete. These techniques will provide a better tool to characterise additional monocyte and monocyte-derived cell populations in physiological and pathological conditions.27–30
Our data provide a mechanistic explanation how monocytes contribute to vasculitis and the subsequent kidney damage. We previously showed that ANCA-stimulated monocytes released significantly more IL-1β compared with neutrophils and that blocking the IL-1β receptor protected mice from AAV.15 31 Now, we show that increased CSF2 augmented the capacity of ANCA-stimulated monocytes to release IL-1β and subsequently to induce TH17 polarisation in vitro. TH17 cells play an important role in the pathogenesis of NCGN,32 including NCGN induced by ANCA.33 Conceivably, additional monocyte-mediated disease mechanisms are at work. For example, monocytes release extracellular traps in response to ANCA that can directly induce endothelial cell damage (personal data). In addition, the absence of CM likely reduces the amount of circulating IM. The contribution of IM in AAV was suggested but remains controversial.16 34 Moreover, the local renal environment of monocyte-derived macrophages and dendritic cells is likely altered by the absence of CM. In summary, we believe that CMs predominantly mediate their pathogenic roles by indirect effects by modulating CD4+ T-cell polarisation rather than by directly damaging effects.
CSF2 has evolved as a key proinflammatory cytokine involved in the pathogenesis of several inflammatory and autoimmune disorders, including experimental autoimmune encephalomyelitis and arthritis.35 CSF2 was produced by resident renal cells in an NTN model,36 and CSF2 released by renal and immune cells contributed to renal injury in a murine anti-GBM glomerulonephritis model.37 38 Interestingly, CSF2 induced a pathogenic signature in Ly6Chi CCR2+ monocytes and monocyte-derived dendritic cells that was characterised by an increased IL-1β generation.22 Our data now establish a mechanistic role of CSF2 in regulating monocyte activation in AAV. We demonstrated that CSF2 is not only increased in kidney and urine of mice with AAV but also augments the capacity of ANCA-activated monocytes to release IL-1β and to induce TH17 polarisation. We confirmed the mechanistic CSF2 role in vivo by showing that mice lacking CSF2rb in their haematopoietic cells were protected from the development of ANCA-induced NCGN and had reduced numbers of kidney-infiltrating TH17 cells. Importantly, we found increased CSF2 expression in kidney biopsies and increased CSF2 protein in the urine from active AAV patients with NCGN. Importantly, CSF2 neutralisation attenuated kidney damage in the NTN model of crescentic glomerulonephritis. Together with the finding that urinary CMs were increased in active, but not patients with AAV on Rem, we confirmed key elements of the mechanistic CSF2-CSF2rb-CM concept in the human AAV disease. Of note, other cytokines (such as tumour necrosis factor alpha or interferon gamma among others) besides CSF2 can potentially activate, modulate or even dampen monocyte functions and thereby can also be involved in the regulation of renal injury in AAV. Our results may encourage future studies exploring CSF2 and its receptor interaction as a potential therapeutic target in AAV, particularly since compounds targeting either CSF2 or its receptor are currently tested in clinical trials (reviewed in Ingelfinger et al 35).
Acknowledgments
We thank Sylvia Lucke, Susanne Rolle, Tanja Filipowski, Eva Vonbrunn and Stefan Söllner for excellent technical assistance.
Footnotes
Handling editor: Josef S Smolen
Contributors: AS is responsible for the overall content as the guarantor. AR, AM and AS designed the study; AR, JS, DL, KA, LK and AS carried out the experiments; AR, AM, JS, DL and AS analysed the data; AS, US and MB provided human samples; AR, JS, KA and AS generated the figures; AR, RK and AS drafted the manuscript; and all authors revised the paper and approved the final version of the manuscript.
Funding: This work was supported by grants from the Deutsche Forschungsgemeinschaft (KE 576/10-1) to RK, the Deutsche Forschungsgemeinschaft (grant SCHR 771/8-1) to AS, the Deutsche Forschungsgemeinschaft (grant 394046635–SFB 1365) to RK and AS, the Deutsche Forschungsgemeinschaft (grant 387509280, SFB 1350) to KA, and ECRC grants to AS and RK.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Ethics Charité (EA3/011/08, EA3/011/06, EA2/103/17 and EA1/034/10), Ethics Erlangen (reference number 4415). Participants gave informed consent to participate in the study before taking part.
References
- 1. Falk RJ, Jennette JC. Anti-Neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med 1988;318:1651–7. 10.1056/NEJM198806233182504 [DOI] [PubMed] [Google Scholar]
- 2. van der Woude FJ. Anticytoplasmic antibodies in Wegener's granulomatosis. Lancet 1985;2:48. 10.1016/s0140-6736(85)90105-9 [DOI] [PubMed] [Google Scholar]
- 3. Kitching AR, Anders H-J, Basu N, et al. Anca-Associated vasculitis. Nat Rev Dis Primers 2020;6:71. 10.1038/s41572-020-0204-y [DOI] [PubMed] [Google Scholar]
- 4. Schreiber A, Choi M. The role of neutrophils in causing antineutrophil cytoplasmic autoantibody-associated vasculitis. Curr Opin Hematol 2015;22:60–6. 10.1097/MOH.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 5. Brunini F, Page TH, Gallieni M, et al. The role of monocytes in ANCA-associated vasculitides. Autoimmun Rev 2016;15:1046–53. 10.1016/j.autrev.2016.07.031 [DOI] [PubMed] [Google Scholar]
- 6. Ferrario F, Rastaldi MP. Necrotizing-crescentic glomerulonephritis in ANCA-associated vasculitis: the role of monocytes. Nephrol Dial Transplant 1999;14:1627–31. 10.1093/ndt/14.7.1627 [DOI] [PubMed] [Google Scholar]
- 7. Rousselle A, Kettritz R, Schreiber A. Monocytes promote crescent formation in anti-myeloperoxidase antibody-induced glomerulonephritis. Am J Pathol 2017;187:1908–15. 10.1016/j.ajpath.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 8. Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003;19:71–82. 10.1016/S1074-7613(03)00174-2 [DOI] [PubMed] [Google Scholar]
- 9. Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989;74:2527–34. 10.1182/blood.V74.7.2527.2527 [DOI] [PubMed] [Google Scholar]
- 10. Yona S, Kim K-W, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013;38:79–91. 10.1016/j.immuni.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mildner A, Schönheit J, Giladi A, et al. Genomic Characterization of Murine Monocytes Reveals C/EBPβ Transcription Factor Dependence of Ly6C- Cells. Immunity 2017;46:849–62. 10.1016/j.immuni.2017.04.018 [DOI] [PubMed] [Google Scholar]
- 12. Weidner S, Neupert W, Goppelt-Struebe M, et al. Antineutrophil cytoplasmic antibodies induce human monocytes to produce oxygen radicals in vitro. Arthritis Rheum 2001;44:1698–706. [DOI] [PubMed] [Google Scholar]
- 13. Hattar K, Bickenbach A, Csernok E, et al. Wegener's granulomatosis: antiproteinase 3 antibodies induce monocyte cytokine and prostanoid release-role of autocrine cell activation. J Leukoc Biol 2002;71:996–1004. [PubMed] [Google Scholar]
- 14. Casselman BL, Kilgore KS, Miller BF, et al. Antibodies to neutrophil cytoplasmic antigens induce monocyte chemoattractant protein-1 secretion from human monocytes. J Lab Clin Med 1995;126:495–502. [PubMed] [Google Scholar]
- 15. Schreiber A, Luft FC, Kettritz R. Phagocyte NADPH oxidase restrains the inflammasome in ANCA-induced GN. J Am Soc Nephrol 2015;26:411–24. 10.1681/ASN.2013111177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Brien EC, Abdulahad WH, Rutgers A, et al. Intermediate monocytes in ANCA vasculitis: increased surface expression of ANCA autoantigens and IL-1β secretion in response to anti-MPO antibodies. Sci Rep 2015;5:11888. 10.1038/srep11888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Popat RJ, Hakki S, Thakker A, et al. Anti-myeloperoxidase antibodies attenuate the monocyte response to LPS and shape macrophage development. JCI Insight 2017;2:e87379. 10.1172/jci.insight.87379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Reilly VP, Wong L, Kennedy C, et al. Urinary soluble CD163 in active renal vasculitis. J Am Soc Nephrol 2016;27:2906–16. 10.1681/ASN.2015050511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Becher B, Tugues S, Greter M. Gm-Csf: from growth factor to central mediator of tissue inflammation. Immunity 2016;45:963–73. 10.1016/j.immuni.2016.10.026 [DOI] [PubMed] [Google Scholar]
- 20. Kitamura T, Hayashida K, Sakamaki K, et al. Reconstitution of functional receptors for human granulocyte/macrophage colony-stimulating factor (GM-CSF): evidence that the protein encoded by the AIC2B cDNA is a subunit of the murine GM-CSF receptor. Proc Natl Acad Sci U S A 1991;88:5082–6. 10.1073/pnas.88.12.5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tavernier J, Devos R, Cornelis S, et al. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific alpha chain and a beta chain shared with the receptor for GM-CSF. Cell 1991;66:1175–84. 10.1016/0092-8674(91)90040-6 [DOI] [PubMed] [Google Scholar]
- 22. Croxford AL, Lanzinger M, Hartmann FJ, et al. The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity 2015;43:502–14. 10.1016/j.immuni.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 23. Kronbichler A, Kerschbaum J, Gründlinger G, et al. Evaluation and validation of biomarkers in granulomatosis with polyangiitis and microscopic polyangiitis. Nephrol Dial Transplant 2016;31:930–6. 10.1093/ndt/gfv336 [DOI] [PubMed] [Google Scholar]
- 24. Finsterbusch M, Hall P, Li A, et al. Patrolling monocytes promote intravascular neutrophil activation and glomerular injury in the acutely inflamed glomerulus. Proc Natl Acad Sci U S A 2016;113:E5172–81. 10.1073/pnas.1606253113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xiao H, Heeringa P, Liu Z, et al. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol 2005;167:39–45. 10.1016/S0002-9440(10)62951-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Turner-Stokes T, Garcia Diaz A, Pinheiro D, et al. Live imaging of monocyte subsets in immune complex-mediated glomerulonephritis reveals distinct phenotypes and effector functions. J Am Soc Nephrol 2020;31:2523–42. 10.1681/ASN.2019121326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Menezes S, Melandri D, Anselmi G, et al. The Heterogeneity of Ly6Chi Monocytes Controls Their Differentiation into iNOS+ Macrophages or Monocyte-Derived Dendritic Cells. Immunity 2016;45:1205–18. 10.1016/j.immuni.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Satoh T, Nakagawa K, Sugihara F, et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature 2017;541:96–101. 10.1038/nature20611 [DOI] [PubMed] [Google Scholar]
- 29. Villani A-C, Satija R, Reynolds G, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017;356:eaah4573. 10.1126/science.aah4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yáñez A, Coetzee SG, Olsson A, et al. Granulocyte-Monocyte progenitors and Monocyte-Dendritic cell progenitors independently produce functionally distinct monocytes. Immunity 2017;47:890–902. 10.1016/j.immuni.2017.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schreiber A, Pham CTN, Hu Y, et al. Neutrophil Serine Proteases Promote IL-1 β Generation and Injury in Necrotizing Crescentic Glomerulonephritis. JASN 2012;23:470–82. 10.1681/ASN.2010080892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krebs CF, Schmidt T, Riedel J-H, et al. T helper type 17 cells in immune-mediated glomerular disease. Nat Rev Nephrol 2017;13:647–59. 10.1038/nrneph.2017.112 [DOI] [PubMed] [Google Scholar]
- 33. Schreiber A, Rousselle A, Klocke J, et al. Neutrophil Gelatinase-Associated Lipocalin Protects from ANCA-Induced GN by Inhibiting TH17 Immunity. J Am Soc Nephrol 2020;31:1569–84. 10.1681/ASN.2019090879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tarzi RM, Liu J, Schneiter S, et al. CD14 expression is increased on monocytes in patients with anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis and correlates with the expression of ANCA autoantigens. Clin Exp Immunol 2015;181:65–75. 10.1111/cei.12625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ingelfinger F, De Feo D. Becher B: GM-CSF: master regulator of the T cell-phagocyte interface during inflammation. Semin Immunol 2021;101518. [DOI] [PubMed] [Google Scholar]
- 36. Melderis S, Hagenstein J, Warkotsch MT, et al. Amphiregulin Aggravates Glomerulonephritis via Recruitment and Activation of Myeloid Cells. J Am Soc Nephrol 2020;31:1996–2012. 10.1681/ASN.2019111215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Timoshanko JR, Kitching AR, Semple TJ, et al. Granulocyte macrophage colony-stimulating factor expression by both renal parenchymal and immune cells mediates murine crescentic glomerulonephritis. J Am Soc Nephrol 2005;16:2646–56. 10.1681/ASN.2004121107 [DOI] [PubMed] [Google Scholar]
- 38. Kitching AR, Ru Huang X, Turner AL, et al. The requirement for granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in leukocyte-mediated immune glomerular injury. J Am Soc Nephrol 2002;13:350–8. 10.1681/ASN.V132350 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2021-221984supp002.pdf (162.3KB, pdf)
annrheumdis-2021-221984supp001.pdf (1.3MB, pdf)
Data Availability Statement
Data are available upon reasonable request.