Abstract
Background
There have been no studies examining differences in clinical manifestations and prognosis between second and third generation coronavirus disease 2019 (COVID-19) patients. Our object was to analyze the epidemiological data and correlation between clinical types and COVID-19 generations.
Methods
Older than 18 years COVID-19 patients who met two of the three items listed in COVID-19 Diagnosis Protocol were enrolled and divided into two groups based on epidemiological history. Clinical characteristics (age, gender, body mass index, course), disease severity, laboratory results (platelets, white blood cells, lymphocytes, inflammatory biomarkers, alanine aminotransferase, lactate dehydrogenase, creatine kinase, myoglobin, troponin, D-dimer blood biochemical indexes), clinical types were analyzed. Two groups were compared by chi-square test, group means were compared by t test, correlation between COVID-19 generations and clinical severity and clinical types were examined by Spearman correlation analysis.
Results
There were no significant differences in gender composition (P=0.488), A-DROP scores (P=0.079) nor BMI (P=0.532) between the two generations. The number of second generation patients over 60 years was significantly greater than that in third generation (P<0.001). Creatine kinase levels of third generation patients were significantly higher than those of second generation patients at admission (P=0.009) and during hospitalization (P=0.023). The troponin levels of third generation patients were significantly higher than those of second generation patients at admission (P=0.020). At discharged, the creatine kinase and troponin levels were not significantly different between the two generations. Rate of severe (P=0.130) and critical cases (P=0.314) in second generation COVID-19 patients was not significantly different from that of third generation patients. Age (ρ=0.224, P<0.001), duration (ρ=0.317, P<0.001), transmission generation (ρ=0.269, P<0.001), serum creatine kinase (ρ=0.240, P<0.001), troponin (ρ=0.296, P<0.001), C-reaction protein (ρ=0.278, P<0.001), procalcitonin levels (ρ=0.221, P=0.001), lymphocyte count (ρ=−0.245, P<0.001), and platelet count (ρ=−0.265, P<0.001) of COVID-19 patients were significantly s correlated with clinical types.
Conclusions
Increased virulence may occur in specific tissues and organs during intergenerational transmission of COVID-19 virus. COVID-19 virus virulence in different regions is different. The clinical prognosis of COVID-19 patients is closely related to age, course, transmission generations, and some laboratory indicators. Transmission generation, regional differences, and laboratory indicators may have certain potential value in predicting prognosis and treatment.
Keywords: Coronavirus disease 2019 (COVID-19), transmission generation, clinical characteristics, prognosis
Introduction
COVID-19 is caused by a novel coronavirus that is closely related to severe acute respiratory syndrome-associated coronavirus (SARS-CoV). It has spread globally and become a critical health burden worldwide (1,2). As of December 2021, a total of 244 million cases have been reported globally, and ~5 million deaths (3). As an acute respiratory disease, COVID-19 is characterized by rapid transmission, strong infectivity, long incubation period, and widespread susceptibility (4-6). Therefore, early diagnosis, self-isolation, and effective treatments are critical to prevent infections. In the Diagnosis and Treatment Protocol for COVID-19 Patients (Trial eighth edition) issued by the National Health Commission (NHC) (7), a patient who meets two of the three criteria of epidemiological history and clinical manifestations, based on etiology, is a confirmed COVID-19 case. Due to the incidence of false negative molecular test results, chest computed tomography (CT) scans play a vital role in preclinical screening and is strongly recommended for investigating possible COVID-19 cases (8).
COVID-19 can be easily transmitted directly from person to person, resulting in its rapid spread across the globe (9). Therefore, imported cases from overseas can lead to second or third generation transmission. The first generation COVID-19 patients refer to those who are first exposed to the environment carrying novel coronavirus and infected. While the second generation COVID-19 patients refer to those who are not exposed to the polluted environment or are not infected with novel coronavirus in the polluted environment, but are infected by the first generation COVID-19 patients. The third generation COVID-19 patients refer to those who are infected after close contact with the second generation COVID-19 patients. The rate of viral infections and transmissions during large outbreaks have become an immense challenge to Chinese society. To date, there have been less studies examining the differences in clinical manifestations and prognosis between second and third generation COVID-19 patients.
Sixty COVID-19 patients in Qingdao from January 21 to March 3, 2020 were analyzed and there were three generations COVID-19 patients, the number of patients decreased generation by generation, and the basic reproduction number (R0) values of the local first generation, second generation and third generation are 1.38, 1.53 and 1.56 respectively (10). A study (11) about an aggregated epidemic caused by novel coronavirus super carriers showed that there were significant differences in alanine aminotransferase, aspartate aminotransferase, hydroxybutyrate dehydrogenase, serum creatinine and uric acid between second and third generation patients (P<0.05). In this study, patients infected with COVID-19 received first-line treatment. The severity of COVID-19 patients varied with the progression of the generations. Therefore, it is crucial to explore the differences in clinical characteristics and prognosis between different generations of COVID-19 patients. By analyzing and comparing the basic clinical characteristics, prognosis, outcome, cure rate, and other clinical data of COVID-19 patients from different generations, the association between severity and prognosis of COVID-19 patients of different generations may be clarified. These results may provide a reference guide for evaluating the prognosis of COVID-19 patients. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1819/rc).
Methods
Subjects
Patients with COVID-19 who were admitted to Central South University Xiangya School of Medicine Affiliated Haikou Hospital, Shishou People’s Hospital, and Sanya Central Hospital from January 2020 to April 2020 were retrospectively included into this multi-center study. The inclusion criteria were as follows: (I) patients who met two of the three items listed in the COVID-19 Diagnosis Protocol (Trial version 8) issued by the NHC (7), including fever and/or respiratory symptoms, imaging features of pneumonia, and normal or reduced white blood cell count or lymphocyte count; (II) positive novel coronavirus tests based on respiratory tract samples using second generation sequencing or real-time polymerase chain reaction (PCR); (III) patients were at the acute stage of the disease and were not receiving other treatment at the time of enrollment; (IV) patients aged greater than 18 years; and (V) patients provided consent for venous blood collection, complete clinical data, and signed an informed consent form. The following exclusion criteria were applied: (I) patients with other cardiovascular, cerebrovascular, liver, or kidney diseases; (II) patients who were complicated with systemic infection and malignant tumors; (III) patients who presented with infectious diseases associated with nervous system, blood system, urinary system, etc.; and (IV) patients who were pregnant or lactating. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Haikou Hospital affiliated to Xiangya Medical College, Central South University (No. 2020-022) and informed consent was taken from all the patients. Shishou People’s hospital, and Sanya Central Hospital were informed and agreed with this study. All enrolled patients received lopinavir ritonavir tablets (400/100 mg orally, twice daily, for not more than 10 days) and antiviral therapy with interferon α (5 million U atomization, twice daily). Antibiotic anti-infection therapy was provided if there was co-infection. Methylprednisolone (1–2 mg/kg/d, 3–5 days) was used for severe cases. Other treatments consisted of symptomatic supportive treatment.
Research methods
General data
The general information of the subjects, including age, sex, and body mass index (BMI), were collated.
Laboratory tests
Blood routine tests, inflammatory indicators, liver enzymes, muscle enzymes, d-dimer, and other laboratory tests were performed for patients infected with COVID-19. Information related to hospital admission, length of hospital stay, and hospital discharge were collated. Routine blood tests including platelets, white blood cells, lymphocytes, inflammatory biomarkers (c-reactive protein, calcitonin, blood sedimentation), alanine aminotransferase, lactate dehydrogenase, creatine kinase, myoglobin, troponin, and D-dimer blood biochemical indexes were analyzed.
Clinical severity and prognosis of different cases
The A-DROP score was performed for each patient upon admission to assess the disease severity of COVID-19 (12). The A-DROP score considers the patient’s age, urea nitrogen, SpO2/PaO2, state of consciousness, and blood pressure. Patients were divided into asymptomatic, mild, ordinary, severe, and critical types according to their clinical symptoms at admission. The diagnostic criteria for severe COVID-19 included the following (13): (I) patients with respiratory distress with respiratory rate not less than 30 times/min; (II) the patient’s finger oxygen saturation in the resting state is less than 93%; (III) the arterial oxygen partial pressure/oxygen absorption concentration is less than 300, and/or lung infiltration is greater than 50% within 24–48 hours. The diagnostic criteria for critical COVID-19 were (13): (I) respiratory failure; (II) symptoms such as shock; (III) complications such as multiple organ failure. The cases of severe and critical COVID-19 patients in different COVID-19 generations were recorded and their ratios were calculated.
Statistical analysis
Statistical analyses were performed using SPSS 26.0 software. The Kolmogorov-Smirnov test was employed to evaluate data normality. Continuous variables were presented as mean ± standard deviation or median with interquartile range, as appropriate. Categorical variables were expressed as number and percentage, and compared with the Chi-squared test. Comparisons between 2 groups were performed with Student’s t test or Mann-Whitney U test for continuous data with normal and non-normal distribution, respectively. The Spearman correlation analysis was utilized to evaluate the correlations between 2 ordinal variables. A two-sided P value <0.05 was considered statistically significant.
Results
The general clinical data of patients with different generations of COVID-19
A total of 218 COVID-19 patients were included in this study, including 68 cases from Haikou city, 55 cases from Sanya city, and 95 cases from Shishou city. There were 107 second generation cases and 111 third generation cases. Demographic data (age, sex, BMI, and course of disease) and A-DROP scores of the COVID-19 patients are shown in Table 1. The results showed that the age distribution of second generation patients was significantly different from that of third-generation patients, and the proportion of patients over 60 years old with third generation COVID-19 was significantly higher than that in second generation patients (P<0.001). There were no significant differences in gender composition (P=0.488) nor BMI (P=0.532) between the second and third generations of patients. There was no significant difference in A-DROP scores between the second and third generation patients (P=0.079).
Table 1. The clinical data of patients with different generations of COVID-19.
| Clinical characteristics | Second generation patients (n=107) | Third generation patients (n=111) | P value |
|---|---|---|---|
| Age, n (%) | <0.001 | ||
| <60 years old | 85 (79.4) | 32 (28.8) | |
| ≥60 years old | 22 (20.6) | 79 (71.2) | |
| Gender, n (%) | 0.488 | ||
| Female | 48 (44.9) | 55 (49.5) | |
| Male | 59 (55.1) | 56 (50.5) | |
| BMI (kg/m2), n (%) | 0.532 | ||
| <25.0 | 67 (62.6) | 74 (66.7) | |
| ≥25.0 | 40 (37.4) | 37 (33.3) | |
| Course, n (%) | 0.473 | ||
| <21 days | 88 (82.2) | 87 (78.4) | |
| ≥21 days | 19 (17.8) | 24 (21.6) | |
| A-DROP score, n (%) | 0.079 | ||
| <3.0 | 87 (81.3) | 79 (71.2) | |
| ≥3.0 | 20 (18.7) | 32 (28.8) | |
BMI, body mass index.
Laboratory results of patients with different generations of COVID-19
The laboratory test results of different generations of COVID-19 patients during admission, hospitalization, and discharge are shown in Table 2. The creatine kinase levels of third generation patients at admission (P=0.009) and hospitalization (P=0.029) were significantly higher than that of second generation patients, while there was no significant difference between second generation and third generation patients at discharge (P=0.872). The troponin levels of the patients in the third generation was significantly higher than that of the patients in the second generation at admission (P=0.020). However, no significant difference was observed between the second and third generation patients during hospitalization (P=0.266) and at discharge (P=0.452). At discharge, the platelet value of third generation patients was significantly lower than that of second generation patients (P=0.001), while the erythrocyte sedimentation rate (ESR) of third generation patients was significantly higher than that of second generation patients (P=0.032), however, there were no significant differences between the two groups at admission (platelet: P=0.241; ESR: P=0.698) and during hospitalization (platelet: P=0.934; ESR: P=0.358). There were no significant differences in other laboratory indicators between the second and third generation patients at different time points (P>0.05).
Table 2. The laboratory test results of COVID-19 patients from three regions of China.
| Laboratory result | At admission | During hospitalization | At discharge | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Second generation (n=107) | Third generation (n=111) | P value | Second generation (n=106) | Third generation (n=110) | P value | Second generation (n=103) | Third generation (n=106) | P value | |||
| White blood cell (109/L) | 5.04±2.26 | 5.19±2.54 | 0.642 | 9.54±36.36 | 6.26±2.58 | 0.346 | 5.44±1.50 | 5.81±2.75 | 0.237 | ||
| Lymphocyte (109/L) | 1.34±0.52 | 1.41±0.58 | 0.273 | 1.53±0.63 | 1.47±0.58 | 0.464 | 1.67±0.45 | 1.74±0.54 | 0.244 | ||
| Blood platelet (109/L) | 202±82.71 | 189.84±69.04 | 0.241 | 214.82±81.29 | 213.91±81.13 | 0.934 | 220.23±67.85 | 190.66±58.41 | 0.001 | ||
| CRP (mg/L) | 15.80 (6.90–30.90) | 15.00 (7.29–34.20) | 0.847 | 5.38 (2.92–11.97) | 4.56 (2.30–11.90) | 0.466 | 4.00 (2.12–6.70) | 3.60 (2.40–6.43) | 0.884 | ||
| GPT (U/L) | 36.00 (21.30–46.70) | 32.50 (28.00–42.30) | 0.717 | 32.40 (21.00–47.10) | 35.40 (26.80–49.80) | 0.181 | 34.00 (21.60–43.20) | 32.40 (22.20–44.40) | 0.925 | ||
| LDH (U/L) | 171.30±53.74 | 193.09±105.09 | 0.057 | 176.84±62.44 | 197.84±127.69 | 0.128 | 162.68±38.77 | 172.41±63.36 | 0.183 | ||
| CK (U/L) | 77.20±40.85 | 99.11±75.46 | 0.009 | 78.22±38.18 | 100.47±96.98 | 0.029 | 91.26±39.13 | 92.17±42.17 | 0.872 | ||
| Myoglobin (ng/mL) | 42.88±21.22 | 41.50±18.74 | 0.612 | 34.50 (20.98–51.25) | 34.00 (20.90–41.20) | 0.088 | 37.82±12.96 | 35.75±23.22 | 0.429 | ||
| CTn (ng/mL) | 0.010 (0.009–0.021) | 0.013 (0.010–0.049) | 0.020 | 0.010 (0.010–0.027) | 0.013 (0.010–0.040) | 0.266 | 0.017 (0.010–0.054) | 0.019 (0.010–0.056) | 0.452 | ||
| PCT (ng/mL) | 0.068 (0.033–0.270) | 0.090 (0.039–0.330) | 0.197 | 0.012 (0.049–0.230) | 0.101 (0.049–0.190) | 0.319 | 0.100 (0.054–0.211) | 0.140 (0.070–0.210) | 0.3929 | ||
| D-dimer (ug/mL) | 0.42 (0.23–0.57) | 0.54 (0.43–0.73) | 0.062 | 0.45 (0.21–0.70) | 0.48 (0.31–0.71) | 0.461 | 0.41 (0.24–0.67) | 0.46 (0.25–0.80) | 0.170 | ||
| ESR (mm/h) | 29.41±19.69 | 30.76±21.84 | 0.698 | 28.05±21.02 | 31.97±28.38 | 0.358 | 19.74±8.77 | 25.2±18.12 | 0.032 | ||
Data were presented as mean ± standard deviation or median with interquartile range, as appropriate. CRP, C-reactive protein; GPT, glutamic-pyruvic transaminase; LDH, lactic dehydrogenase; CK, creatine kinase; CTn, troponin; PCT, procalcitonin; ESR, erythrocyte sedimentation rate.
The laboratory indicators of the second and third generation patients in Shishou (Table 3), Haikou (Table 4), and Sanya (Table 5) were further compared at three different time points. According to the results, the creatine kinase levels of third generation patients in the Shishou area was significantly higher than that of second generation patients at admission (P=0.009), during hospitalization (P=0.023), and at discharge (P=0.011). The troponin values of third generation patients at admission were also significantly higher than that of second generation patients (P=0.041). However, there was no significant difference in creatine kinase expression between the second and third generation patients in Sanya at admission (P=0.056), during hospitalization (P=0.076), and at discharge (P=0.925). Similarly, there were no significant differences in troponin levels between the second and third generation patients at admission (P=0.067), during hospitalization (P=0.067), and at discharge (P=0.459). There was no significant difference in creatine kinase expression between second and third generation patients at admission (P=0.580) and during hospitalization (P=0.439). However, at discharge, the creatine kinase levels of second generation patients were higher than those of third generation patients (P<0.001). There was no significant difference in troponin levels at admission (P=0.995) and discharge (P=0.776). During hospitalization, the troponin levels in second generation patients were higher than that of third generation patients (P<0.001). In addition, the glutamic-pyruvate transaminase (P=0.022) and lactate dehydrogenase (P=0.026) levels of third generation COVID-19 patients in the Shishou area were also significantly higher than those of second generation COVID-19 patients at admission. However, there were no significant differences in laboratory indexes between second generation and third generation COVID-19 patients in different regions and at different time points (P>0.05).
Table 3. The laboratory results of COVID-19 patients from Shishou at different times.
| Laboratory result | At admission | During hospitalization | At discharge | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Second generation (n=28) | Third generation (n=26) | P value | Second generation (n=36) | Third generation (n=58) | P value | Second generation (n=27) | Third generation (n=24) | P value | |||
| White blood cell(109/L) | 5.48±3.06 | 4.68±1.80 | 0.11 | 7.03±4.06 | 6.30±2.80 | 0.306 | 5.45±1.39 | 5.25±0.93 | 0.417 | ||
| Lymphocyte (109/L) | 1.33±0.47 | 1.45±0.57 | 0.263 | 1.25±0.56 | 1.32±0.52 | 0.542 | 6.86±30.06 | 1.76±0.42 | 0.206 | ||
| Blood Platelet (109/L) | 204.40±89.63 | 185.5±64.13 | 0.234 | 194.14±72.26 | 201.29±77.66 | 0.657 | 215.12±57.57 | 178.89±52.56 | 0.003 | ||
| CRP (mg/L) | 20.70 (8.05–41.87) | 10.65 (6.98–28.33) | 0.299 | 3.30 (1.80–19.45) | 3.20 (1.98–13.80) | 0.960 | 3.30 (2.40–4.93) | 3.45 (2.40–6.60) | 0.635 | ||
| GPT (U/L) | 29.92±13.81 | 36.00±11.43 | 0.022 | 35.33±26.22 | 29.22±11.60 | 0.219 | 29.22±11.60 | 34.10±19.19 | 0.184 | ||
| LDH (U/L) | 160.30±47.45 | 189.43±67.92 | 0.026 | 177.74±83.26 | 184.34±74.57 | 0.691 | 171.66±36.59 | 171.21±53.34 | 0.965 | ||
| CK (U/L) | 62.86±29.99 | 91.68±60.69 | 0.009 | 78.37±41.05 | 104.31±58.99 | 0.023 | 92.19±33.61 | 112.33±36.97 | 0.011 | ||
| Myoglobin (ng/mL) | 43.87±19.99 | 43.63±18.93 | 0.953 | 24.40±10.64 | 25.13±11.55 | 0.760 | 32.98±7.49 | 28.74±7.82 | 0.013 | ||
| CTn (ng/mL) | 0.018 (0.010–0.034) | 0.036 (0.013–0.073) | 0.041 | 0.020 (0.013–0.079) | 0.038 (0.018–0.068) | 0.694 | 0.020 (0.009–0.074) | 0.032 (0.010–0.070) | 0.914 | ||
| PCT (ng/mL) | 0.240 (0.060–0.390) | 0.270 (0.090–0.380) | 0.652 | 0.170 (0.100–0.228) | 0.130 (0.078–0.210) | 0.162 | 0.180 (0.100–0.230) | 0.170 (0.100–0.208) | 0.463 | ||
| D-dimer (ug/mL) | 0.40 (0.25–0.54) | 0.44 (0.35–0.75) | 0.090 | 0.47 (0.23–0.76) | 0.54 (0.33–0.72) | 0.643 | 0.45 (0.29–0.78) | 0.63 (0.43–0.83) | 0.115 | ||
| ESR (mm/h) | 34.7±18.15 | 33.95±22.08 | 0.848 | 33.51±22.62 | 38.24±30.85 | 0.433 | 22.35±10.76 | 26.28±18.15 | 0.255 | ||
Data were presented as mean ± standard deviation or median with interquartile range, as appropriate. CRP, C-reactive protein; GPT, glutamic-pyruvic transaminase; LDH, lactic dehydrogenase; CK, creatine kinase; CTn, troponin; PCT, procalcitonin; ESR, erythrocyte sedimentation rate.
Table 4. The laboratory examination results of COVID-19 patients from Haikou at different time points.
| Laboratory result | At admission | During hospitalization | At discharge | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Second generation (n=42) | Third generation (n=26) | P value | Second generation (n=42) | Third generation (n=26) | P value | Second generation (n=42) | Third generation (n=26) | P value | |||
| White blood cell (109/L) | 4.88±1.47 | 5.30±1.81 | 0.279 | 5.62±2.91 | 5.68±2.50 | 0.926 | 5.56±1.50 | 5.70±1.38 | 0.700 | ||
| Lymphocyte (109/L) | 1.13±0.37 | 1.31±0.40 | 0.065 | 1.61±0.65 | 1.71±0.59 | 0.503 | 1.59±0.36 | 1.71±0.55 | 0.279 | ||
| Blood Platelet (109/L) | 216.43±80.14 | 206.92±65.09 | 0.612 | 228.43±80.75 | 236.23±61.92 | 0.675 | 233.76±81.24 | 213.85±49.28 | 0.264 | ||
| CRP (mg/L) | 15.62 (8.55–31.14) | 13.54 (8.90–26.30) | 0.682 | 9.29 (5.70–24.09) | 9.20 (4.48–9.41) | 0.108 | 6.37 (4.58–8.74) | 6.33 (4.14–7.61) | 0.331 | ||
| GPT (U/L) | 44.48±33.84 | 35.06±12.26 | 0.178 | 47.05±51.93 | 41.22±13.89 | 0.578 | 39.94±14.60 | 42.71±25.50 | 0.571 | ||
| LDH (U/L) | 188.24±63.23 | 207.27±140.10 | 0.447 | 191.55±56.16 | 195.62±62.63 | 0.782 | 166.42±43.02 | 172.12±58.07 | 0.645 | ||
| CK (U/L) | 93.24±46.23 | 101.04±69.60 | 0.582 | 87.43±35.37 | 109.15±175.82 | 0.439 | 102.71±40.66 | 68.15±19.70 | <0.001 | ||
| Myoglobin (ng/mL) | 49.94±17.26 | 48.76±12.87 | 0.766 | 50.91±13.12 | 45.10±11.13 | 0.065 | 48.14±11.32 | 48.27±12.44 | 0.964 | ||
| CTn (ng/mL) | 0.012 (0.005–0.018) | 0.009 (0.004–0.023) | 0.995 | 0.015 (0.003–0.026) | 0.008 (0.003–0.015) | <0.001 | 0.033 (0.009–0.059) | 0.040 (0.011–0.058) | 0.776 | ||
| PCT (ng/mL) | 0.071 (0.044–0.228) | 0.071 (0.043–0.099) | 0.710 | 0.189 (0.057–0.392) | 0.078 (0.047–0.227) | 0.004 | 0.098 (0.059–0.388) | 0.137 (0.048–0.402) | 0.686 | ||
Data were presented as mean ± standard deviation or median with interquartile range, as appropriate. CRP, C-reactive protein; GPT, glutamic-pyruvic transaminase; LDH, lactic dehydrogenase; CK, creatine kinase; CTn, troponin; PCT, procalcitonin; ESR, erythrocyte sedimentation rate.
Table 5. The laboratory examination results of COVID-19 patients from Sanya at different time points.
| Laboratory result | At admission | During hospitalization | At discharge | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Second generation (n=28) | Third generation (n=26) | P value | Second generation (n=28) | Third generation (n=26) | P value | Second generation (n=27) | Third generation (n=24) | P value | |||
| White blood cell (109/L) | 4.70±1.99 | 6.21±3.96 | 0.079 | 6.25±1.66 | 6.74±2.09 | 0.354 | 5.26±1.66 | 7.24±5.25 | 0.070 | ||
| Lymphocyte (109/L) | 1.66±0.63 | 1.43±0.74 | 0.227 | 1.78±0.55 | 1.57±0.61 | 0.192 | 1.74±0.61 | 1.74±0.76 | 0.998 | ||
| Blood Platelet (109/L) | 177.18±3.62 | 182.46±82.23 | 0.804 | 221±90.17 | 219.73±100.94 | 0.961 | 205.63±53.80 | 193±73.35 | 0.483 | ||
| CRP (mg/L) | 11.25 (0.50–23.23) | 24.83 (5.94–41.50) | 0.129 | 3.95 (1.62–6.65) | 3.71 (1.13–13.61) | 0.481 | 1.92 (1.15–1.92) | 2.74 (1.73–3.60) | 0.096 | ||
| GPT (U/L) | 44.36±32.70 | 0.453 | 37.57±24.02 | 0.342 | 33.92±27.53 | 53.67±85.12 | 0.259 | ||||
| LDH (U/L) | 160.32±39.10 | 187.08±133.15 | 0.314 | 153.61±22.12 | 230.19±230.15 | 0.085 | 145.56±29.11 | 175.54±88.56 | 0.103 | ||
| CK(U/L) | 72.11±37.38 | 113.77±105.97 | 0.056 | 64.21±35.35 | 83.23±41.68 | 0.076 | 72.26±37.10 | 71.12±48.20 | 0.925 | ||
| Myoglobin (ng/mL) | 30.98±23.59 | 29.46±18.22 | 0.795 | 28.91±14.32 | 45.10±11.13 | 0.142 | 27.60 (21.80–31.20) | 28.70 (21.43–40.08) | 0.467 | ||
| CTn (ng/mL) | 0.010 (0.010–0.010) | 0.010 (0.010–0.010) | 0.067 | 0.010 (0.010–0.010) | 0.010 (0.010–0.010) | 0.067 | 0.010 (0.010–0.010) | 0.010 (0.010–0.010) | 0.459 | ||
| PCT (ng/mL) | 0.028 (0.019–0.038) | 0.032 (0.021–0.077) | 0.349 | 0.043 (0.019–0.120) | 0.059 (0.024–0.124) | 0.431 | 0.034 (0.024–0.098) | 0.052 (0.028–0.158) | 0.196 | ||
| D-dimer (ug/mL) | 0.43 (0.23–0.67) | 0.52 (0.34–0.68) | 0.239 | 0.43 (0.16–0.69) | 0.38 (0.29–0.60) | 0.897 | 0.37 (0.21–0.62) | 0.54 (0.22–0.70) | 0.246 | ||
| ESR (mm/h) | 22.32±19.69 | 23.65±19.92 | 0.806 | 21.21±16.83 | 0.65±1.04 | 0.453 | 16.44±3.22 | 22.67±18.20 | 0.087 | ||
Data were presented as mean ± standard deviation or median with interquartile range, as appropriate. CRP, C-reactive protein; GPT, glutamic-pyruvic transaminase; LDH, lactic dehydrogenase; CK, creatine kinase; CTn, troponin; PCT, procalcitonin; ESR, erythrocyte sedimentation rate.
Clinical types of patients with different generations of COVID-19
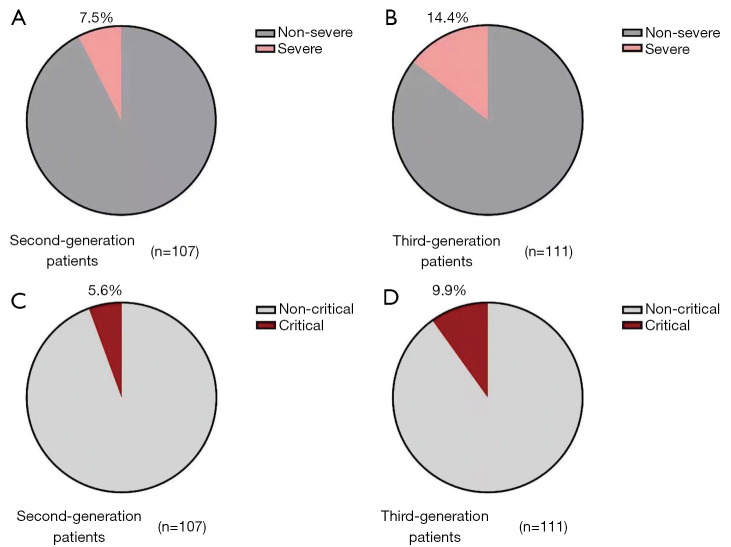
According to clinical symptoms, the spectrum of COVID-19 infections ranged from asymptomatic infection to mild, ordinary, severe, and critical illness. The clinical characteristics of patients with different generations of COVID-19 were further recorded and compared (Table 2). There were 4 (3.7%) asymptomatic second generation COVID-19 patients and 4 (3.6%) asymptomatic third generation COVID-19 patients. No significant difference was observed (P>0.999). There were 6 (5.6%) second generation COVID-19 patients and no third generation patients with mild illness (P=0.013). The proportion of patients with ordinary illness was comparable between the 2 groups, including 83 (77.6%) second generation patients and 80 (72.1%) third generation patients (P=0.436). Among second-generation COVID-19 cases, 8 (7.5%) were severe and 11 (5.6%) were critical. This was not significantly different from the third generation COVID-19 cases, in which there were 19 severe (14.4%; P=0.130) and 11 critical (9.9%; P=0.314) cases (Figure 1).
Figure 1.
The severity of cases in the different generations of COVID-19 patients.
Correlation between age, course of disease, disease generation, laboratory indicators, and clinical types in COVID-19 patients
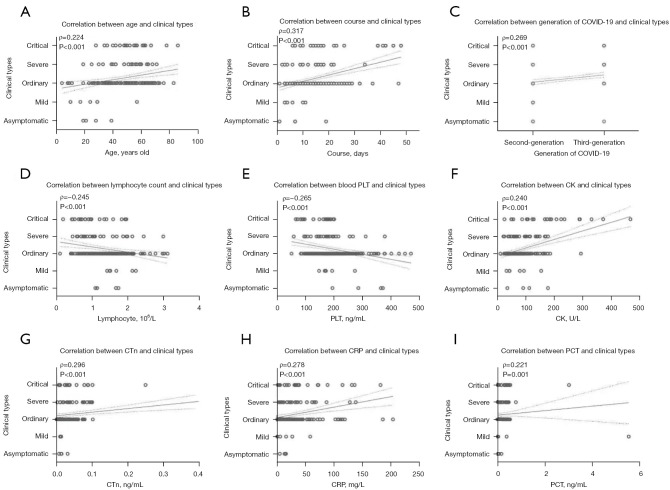
The correlation between patient clinical types and age, course of disease, COVID-19 generation, whole blood, and biochemical indicators (including lymphocyte count, platelet, creatine kinase, troponin, C-reactive protein, and procalcitonin levels) was assessed (Figure 2). Spearman correlation analysis suggested that age (ρ=0.224, P<0.001), course of disease (ρ=0.317, P<0.001), COVID-19 generation (ρ=0.269, P<0.001), creatine kinase (ρ=0.240, P<0.001), troponin (ρ=0.296, P<0.001), C-reactive protein (ρ=0.278, P<0.001), and procalcitonin levels (ρ=0.221, P=0.001) were all significantly positively correlated with the clinical types of the patients, while the blood lymphocyte count (ρ=−0.245, P<0.001) and platelet levels (ρ=−0.265, P<0.001) were negatively correlated with clinical types.
Figure 2.
The correlation between clinical staging and the clinical features, transmission generations, and laboratory test indicators of COVID-19 patients. PLT, platelet; CK, creatine kinase; CTn, troponin; CRP, C-reactive protein; PCT, procalcitonin.
Discussion
The COVID-19 pandemic has become a major threat to global health and economy. Understanding the clinical features and developing effective early treatments is imperative for improving prognosis. However, current studies have mostly focused on the characteristics of the disease itself, with few reports comparing the clinical characteristics and prognosis of COVID-19 patients from different viral generations. Considering the characteristics of human-to-human transmission of COVID-19 and the shortage of hospital resources, it is vital to compare and understand the clinical characteristics and prognosis of patients from different COVID-19 generations, so as to allocate medical resources and develop treatment plans effectively.
Approved and promulgated by the Japan Respiratory Disease Society in 2005, the A-DROP score is an objective indicator used to evaluate the severity of clinical symptoms in patients with pneumonia, with particular regard to clinical and laboratory indicators (12,13). Nevertheless, few studies have used the A-DROP score to evaluate COVID-19 symptoms in China. This current study demonstrated that the general clinical characteristics and severity of COVID-19 cases in different generations are not identical. Indeed, the proportion of third generation patients aged 60 years and over is higher than that of second generation patients. Compared with patients with second generation COVID-19, patients with third generation COVID-19 also tended to have A-DROP scores greater than or equal to 3, and a higher incidence of severe or critical disease. Over the age of 60 years, the majority of cases were complicated with underlying diseases such as diabetes and hypertension. Previous studies have reported that age and comorbidities are associated with the severity of COVID-19 (14). The mortality of elderly patients with COVID-19 is generally high, and elderly patients usually have severe clinical symptoms (15). Although there were no significant statistical differences in A-DROP scores and the rate of severe or critical illness between the second and third generation patients, it cannot be ruled out that the statistical analyses were limited by the small sample size. Therefore, future studies should include a greater number of severe and critical cases. Furthermore, multiple regression analyses should be conducted to investigate the correlation between virus generations and the clinical rates of severe and critical cases.
Laboratory results from different generations of COVID-19 patients suggested that creatine kinase and troponin levels in third generation COVID-19 patients were significantly higher on admission compared to second generation COVID-19 patients. However, there was no difference between the 2 generations at discharge. This result suggested that the injury of the myocardium, skeletal muscle, or smooth muscle in patients with third generation COVID-19 may be higher at onset compared to that in patients with second generation COVID-19. It is possible that the virulence of COVID-19, which does not decrease significantly as the number of virus transmission increases, may increase in some target organs. In addition, classification analysis revealed that patients from Haikou and Sanya showed no significant difference in myocardial injury caused by different viral generations, while in Shishou, there was a significant difference in the severity of myocardial injury between patients with different generations of COVID-19. This may suggest that the novel coronavirus strain from Shishou is different from the strains in Haikou and Sanya in terms of virulence. Previous studies have shown functional impairment of liver, kidney, and myocardium in COVID-19 patients (16,17). However, to date, there have been no reports comparing liver, kidney, myocardium, skeletal muscle, or smooth muscle injury between different generations of SARS-Cov-2 virus. Our study further indicated that different organs may be affected by different strains of SARS-Cov-2 virus in different regions.
In addition, the laboratory test results of COVID-19 patients were correlated with prognosis. The reduction of circulating lymphocyte count and the increase in C-reactive protein expression in COVID-19 patients have been shown to be positively correlated with disease severity of disease (18,19). Our correlation analyses suggested that age, course of disease, viral generation, and some laboratory indicators (such as lymphocyte count, platelet count, creatine kinase, troponin, C-reactive protein and procalcitonin levels) are closely correlated with clinical types and clinical prognosis. Both C-reactive protein and procalcitonin were positively correlated with the severity of COVID-19 patients, while lymphocyte count was negatively correlated with clinical types, suggesting that both propagation generation and laboratory indicators should be considered together when predicting patient prognosis.
This current investigation demonstrated that the myocardial injury of third generation patients with COVID-19 was greater than that of second generation patients. Indeed, the virulence of COVID-19 does not necessarily decline when it is transmitted between different generations. The virulence may increase in some specific tissues and organs due to viral mutations. Furthermore, the virulence of the COVID-19 virus varies from region to region. Although the rate of severe and critically ill patients in third generation COVID-19 cases was higher than that in second generation patients, no statistical difference was observed. Further analysis with a larger cohort is warranted to confirm these results.
Supplementary
The article’s supplementary files as
Acknowledgments
We acknowledge Central South University Xiangya School of Medicine Affiliated Haikou Hospital, and Shishou People’s Hospital, and Sanya Central Hospital for providing data of the patients.
Funding: The study was supported by Hainan Science and Technology Project (No. ZDYFXGFY2020007).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Haikou Hospital affiliated to Xiangya Medical College, Central South University (No. 2020-022) and informed consent was taken from all the patients. Shishou People’s hospital, and Sanya Central Hospital were informed and agreed with this study.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1819/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1819/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1819/coif). The authors have no conflicts of interest to declare.
References
- 1.Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 2020;382:727-33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paules CI, Marston HD, Fauci AS. Coronavirus Infections-More Than Just the Common Cold. JAMA 2020;323:707-8. 10.1001/jama.2020.0757 [DOI] [PubMed] [Google Scholar]
- 3.Premeaux TA, Yeung ST, Bukhari Z, et al. Emerging Insights on Caspases in COVID-19 Pathogenesis, Sequelae, and Directed Therapies. Front Immunol 2022;13:842740. 10.3389/fimmu.2022.842740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet 2020;395:470-3. 10.1016/S0140-6736(20)30185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020;395:514-23. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Health Commission of the People’s Republic of China. Notice on the issuance of COVID-19 Diagnosis and Treatment Protocol (Trial version 8). Available online: http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=0a7bdf12bd4b46e5bd28ca7f9a7f5e5a
- 8.Chen Z, Wang RF. Application value of CT in diagnosis and differential diagnosis of COVID-19. Computerized Tomography Theory and Applications 2020;29:273-9. [Google Scholar]
- 9.Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med 2020;382:1199-207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu XN, Sun X, Jia SJ, et al. Epidemiologyica characteristics of COVID-19 in Qingdao. Chinese Journal of Microecology 2020;32:586-90. [Google Scholar]
- 11.Du Y, Xu YF, Zheng JJ. Analysis of transmission chain and clinical characteristics of a case of novel coronavirus super carrier. The Journal of Medical Theory and Practice 2021;34:1393-5. [Google Scholar]
- 12.Li Q, Hou J, Huang R, et al. Comparison of A-DROP and CURB-65 scoring systems for the diagnosis of severe; community-acquired pneumonia. Chinese Journal of Coal Industry Medicine 2015;18:1146-9. [Google Scholar]
- 13.Yu HJ. Study of A-DROP score on severity of community-acquired pneumonia. Guizhou Medical University, 2016. [Google Scholar]
- 14.Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020;75:1730-41. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 15.Epidemiology Group, COVID-19 Emergency Response Mechanism, Chinese Center for Disease Control and Prevention . Analysis of epidemiological characteristics of COVID-19. Chinese Journal of Epidemiology 2020;41:141-51. [Google Scholar]
- 16.Huang Y, Cheng SQ, Ma HQ, et al. Clinical characteristics of 330 COVID-19 ordinary patients and analysis of part laboratory test results of liver,renal,and cardiac functions. Journal of Guizhou Medical University 2021;46:739-44. [Google Scholar]
- 17.Zhou YP, Zhu CX, Gong JF, et al. Analysis of Clinical Laboratory Test Results in Patients with Novel Coronavirus Pneumonia. Journal of Modern Laboratory Medicine 2020;35:83-7. [Google Scholar]
- 18.Liu M, He P, Liu HG, et al. Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:E016. [DOI] [PubMed] [Google Scholar]
- 19.Gao L, Liu X, Zhang D, et al. Early diagnosis of bacterial infection in patients with septicopyemia by laboratory analysis of PCT, CRP and IL-6. Exp Ther Med 2017;13:3479-83. 10.3892/etm.2017.4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as