Abstract
Background
There remains a disproportionally high tobacco smoking rate in low-income populations. Multicomponent tobacco dependence interventions in theory are effective. However, which intervention components are necessary to include for low socioeconomic status (SES) populations is still unknown.
Objective
To assess the effectiveness of multicomponent tobacco dependence interventions for low SES and create a checklist tool examining multicomponent interventions.
Methods
EMBASE and MEDLINE databases were searched to identify randomised controlled trials (RCTs) published with the primary outcome of tobacco smoking cessation measured at 6 months or post intervention. RCTs that evaluated tobacco dependence management interventions (for reduction or cessation) in low SES (experience of housing insecurity, poverty, low income, unemployment, mental health challenges, illicit substance use and/or food insecurity) were included. Two authors independently abstracted data. Random effects meta-analysis and post hoc sensitivity analysis were performed.
Results
Of the 33 included studies, the number of intervention components ranged from 1 to 6, with smoking quit rates varying between 1% and 36.6%. Meta-analysis revealed that both the 6-month and 12-month outcome timepoints, multicomponent interventions were successful in achieving higher smoking quit rates than the control (OR 1.64, 95% Cl 1.41 to 1.91; OR 1.74, 95% Cl 1.30 to 2.33). Evidence of low heterogeneity in the effect size was observed at 6-month (I2=26%) and moderate heterogeneity at 12-month (I2=56%) outcomes.
Conclusion
Multicomponent tobacco dependence interventions should focus on inclusion of social support, frequency and duration of components. Employing community-based participatory-action research approach is essential to addressing underlying psychosocioeconomic-structural factors, in addition to the proven combination pharmacotherapies.
PROSPERO registration number
CRD42017076650.
Keywords: tobacco; behavior, addictive; homeless persons; prevention; economics
WHAT IS ALREADY KNOWN ON THIS TOPIC
Multicomponent intervention programmes are recommended as they are known to increase smoking abstinence in comparison to single-component interventions. Many studies in the literature are tailoring tobacco dependence interventions to vulnerable population’s day-to-day challenges, including lack of social support, financial stress, coaddictions, and low self-efficacy and life opportunities. However, which intervention components are necessary to include for low socioeconomic populations is still unknown. This review will assist in developing a targeted and multifaceted tobacco cessation strategy for systematically disadvantaged populations.
WHAT THIS STUDY ADDS
This review and meta-analysis provide a step towards evidence-based findings that will encourage future equity-focused research for tobacco dependence in low socioeconomic status populations considering the growing implementation of tailored, multicomponent interventions. The customised checklist tool offers an innovative whole-person perspective at examining multicomponent interventions and has the potential to be generalised to other chronic conditions such as diabetes, chronic obstructive pulmonary disease and asthma.
Background
Globally, tobacco smoking causes more than eight million deaths each year, where 80% of people who use tobacco live in low-income and middle-income countries.1 Nicotine dependence is the leading cause of preventable deaths and diseases in Canada, responsible for 45 464 deaths annually. People who smoke have approximately 30% greater hospitalisation costs compared with people who are non-smokers.2 The total economic costs of tobacco smoking are more than US$300 billion a year.3 Despite a steady decrease of tobacco smoking in the general Canadian population over the last decade, there remains a disproportionally high tobacco smoking rate in low-income populations of Canada who experience homelessness, are at risk of homelessness and face challenges with substance use and mental health. For instance, there is a tobacco smoking rate of 23.4% among the lowest-income quintile compared with 12% among Canada’s highest-income quintile.4 A study conducted in Ottawa, Canada, found that 96% of the city’s homeless or at risk for homelessness residents reported having smoked tobacco in the past year.5 There are similar trends in the USA, with prevalence ranging between 70% and 80%.6 7 Furthermore, smoking-related illnesses are the leading cause of diseases and deaths in homeless and shelter-housed populations in Canada.8 Several factors create challenges for reducing tobacco use in vulnerably housed and low socioeconomic status (SES) populations in comparison to the general population, including the higher burden of nicotine dependence and resulting chronic respiratory diseases, as well as psychiatric symptoms, coexisting substance-use disorders and chronic diseases, and the daily challenges with street life.4 9–11
Multicomponent intervention programmes are known to increase smoking abstinence compared with single-component interventions in general populations.12 13 There are three main reasons for the improved effectiveness of multicomponent interventions for tobacco dependence. (1) Each component targets a different aspect of recovery. For instance, pharmacotherapy aims to relieve nicotine withdrawal symptoms, whereas behavioural counselling focuses on developing coping mechanisms. (2) Each intervention component impacts individuals differently, allowing participants to discover preferred treatment methods, encouraging patient-centred approaches. (3) Multiple intervention components enhance overall treatment adherence and retention.14 However, generic multicomponent interventions do not address the peculiar needs of the most socioeconomically disadvantaged populations, causing a recent shift in the literature towards tailoring these interventions to this population’s day-to-day challenges, including lack of social support, financial stress, coaddictions, and low self-efficacy and life opportunities.5 15–18 Such tailored whole-person approaches, in theory, are expected to reduce health inequities. Previous reviews have suggested that tailoring smoking dependence interventions for socioeconomically disadvantaged groups (ie, disadvantaged in terms of race, caste, social class, income, unemployment or residential neighbourhood) are necessary to improve their overall well-being.19–21
Tobacco dependence is multifaceted, involving biological, psychological, environmental, and social factors.22–24 Despite the progress towards understanding tobacco dependence as a complex health inequity issue, current tobacco dependence management programmes for low SES populations have not comparably evolved. Despite well-established smoking dependence and management programmes existing, very few considered the difficulties this vulnerable population faces, such as access to transportation, nutritious food, stable housing and other social determinants of health.25 To our knowledge, a comprehensive guideline to assist healthcare professionals, policymakers and community programmes in determining which intervention components are necessary to include for low SES populations does not exist. A comprehensive, tailored and multifaceted approach is required at the individual, community and population level to improve the disproportionate burden of tobacco inequity among low SES populations. The objective of this paper is to conduct a systematic review to assess the efficacy of multicomponent tobacco dependence interventions for low SES populations using a whole-person approach (defined as a comprehensive, holistic approach to care considering an individual’s emotional, physical, spiritual, social, physiological and financial well-being). Our review will assist in developing a targeted and multifaceted tobacco cessation strategy for this systematically disadvantaged population.
Methods
Study design
We conducted a systematic review using a prospective protocol available on PROSPERO.26 We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 Checklist for study reporting guidelines.27
The review’s primary outcome was to analyse the effectiveness of multicomponent tobacco dependence interventions by comparing the smoking cessation/reduction rate between the control and intervention study arms at 6-month and 12-month outcomes. Smoking reduction rate was assessed as point prevalence of abstinence or smoking quit rate at a specific timepoint. The secondary outcome was to create a customised checklist tool using a whole-person perspective examining multicomponent interventions, with the intention to be generalisable to other chronic conditions such as diabetes, chronic obstructive pulmonary disease and asthma.
Eligibility criteria
This review only included studies that evaluated the effectiveness of tobacco dependence management interventions with low SES outpatient populations with outcomes as tobacco smoking cessation and/or reduction at 6 or 12 months. We defined low SES populations as vulnerable adults who experienced homelessness, at risk of homelessness, poverty, unemployment, mental health challenges, illicit substance use, food insecurity, self-identify as an ethnic minority and/or a low income (as defined by the authors of the article). This definition was operationalised as the following search terms: homeless, vulnerable, marginalized, poverty, unemployment, low-income, low socioeconomic status and food insecurity (online supplemental appendix B). Further, this review included studies reporting only randomised controlled trials, available in the full-text, English language only and had an endpoint of quitting or reducing tobacco smoking. Eligible comparator arms for studies included usual care or enhanced usual care which is defined as the standard of care the target population would expect to receive, including brief quit advice, pharmacotherapy, pamphlets or self-quit booklets and counselling. In most cases, the intervention arm involved usual/enhanced care plus the intervention. We excluded secondary analyses, study protocols and inpatient populations in treatment centres (eg, hospital or rehabilitation centres). Biochemically validated abstinence at 6 months is considered the gold standard for smoking tobacco dependence interventions.28 Therefore, we only included studies that had an outcome timepoint of 6 or 12 months, and any intermediate timepoints between were not included. Article selection criteria are further described in online supplemental appendix A.
jech-2021-216783supp001.pdf (317.6KB, pdf)
Search strategy
We conducted searches in EMBASE and MEDLINE databases in November 2016 and updated in April 2017, May 2018, October 2019, February 2019 and September 2020 to include studies published from any year. Eligible studies were also identified through article reference lists. Further details regarding the search strategy and keywords are provided in online supplemental appendix B.
Data collection
Pairs of reviewers (NH and ST, OB and TH, PA and CC) collected data from each study independently, and a third reviewer (SP or SJ) resolved any discrepancies. We used a customised data extraction table for data collection. Data of interest included study location, study type, participant characteristics, intervention and control treatment component(s), duration and smoking cessation outcomes for self-reported and biochemically verified outcomes(s). For the studies with more than two interventions, we considered the arm with the greatest number of components as the intervention arm.
Checklist tool development
We co-created a customised reporting data collection sheet titled ‘A Checklist for a Comprehensive Community-based Chronic Disease Management Program for Marginalized Populations: Example Tobacco Dependence (Checklist Tool)’ to capture the key elements of the multicomponent tobacco dependence interventions used. Three authors (SP, NH, ST) who have methodological expertise and extensive experience working with the low SES populations in Ottawa, Canada, created the checklist in collaboration with two community peer researchers (TB, TH), who have relevant lived and living experience.29 30 Detailed discussions among all authors led to the formation of seven main sections to emphasise a whole-person comprehensive assessment. The seven sections included: social support, social-economic support, counselling, follow-up, pharmacotherapy, compensation (monetary and non-monetary) and project site and approach (community-based, community-placed, or institutional). Each section was further evaluated based on the following components: (1) who conducted the intervention (community peer researcher, healthcare professional, research staff), (2) location (on site or referral), (3) intervention delivery method (in person, multimedia, telephone, printed materials) and (4) frequency of intervention offered (daily, weekly, monthly, quarterly, biannually). The checklist with associated definitions of each of the seven sections is explained in online supplemental appendix F.
Risk of bias assessment
Two independent reviewers (TH, PA, NH or ST) assessed the risk of bias for all included studies using the Cochrane risk of bias tool (RoB) V.1 and rated each RoB domain as ‘high’, ‘low’ or ‘unclear’.31 A third reviewer (SP or SJ) oversaw the process and resolved discrepancies if any. Following the Cochrane RoB tool, the overall risk of bias was classified as low if the majority of domains were at low risk of bias, the same approach was used for classifying unclear risk of bias.31 The overall risk of bias was classified as high if more than one domain were at high risk of bias, as any source of bias in a trial is problematic, and one domain should not be prioritised over the others. As it is difficult to conduct a true blinding of participants and research personnel for complex interventions delivered in person, the domain was not considered during the overall risk of bias.31 32
Statistical analysis
We used RevMan software (V.5.4) to perform random effects meta-analysis and presented the 6-month and 12-month outcomes.33 Using the Mantel-Haenszel method, we calculated the estimated pooled effect size for tobacco quitting at the longest follow-up using OR with corresponding 95% CIs. Further, we summarised the total number and type of intervention components used. We quantified heterogeneity of effect size at the 6-month and the 12-month outcomes using Higgin’s I2 statistics. To evaluate any publication bias, we visually inspected the funnel plots, and any plot asymmetry was further evaluated using Egger’s regression test.34 We conducted a post hoc sensitivity analysis for 6-month and 12-month outcomes by excluding studies that were classified as high overall risk of bias as it may pose risk to the validity of study findings.
Patient and public involvement
Two community peer researchers (TB, TH), individuals of lived and living experience of low income, substance use, food insecurity and tobacco dependence, were involved in design and development of the checklist tool and research question to ensure the review was representative of low SES population’s perspective and priorities. As well, community peer researchers (TB and others) co-created the definition of low SES used in this study and worked on the literature review informing this review. Community researchers were from The Bridge Engagement Centre (The Bridge), a community-based research centre under the auspices of the Ottawa Hospital Research Institute, Canada, and are trained in ethics, research methods, recruitment and community engagement using the Community Peer Researchers’ Training Material and The Bridge Model.29 35
Results
Study characteristics
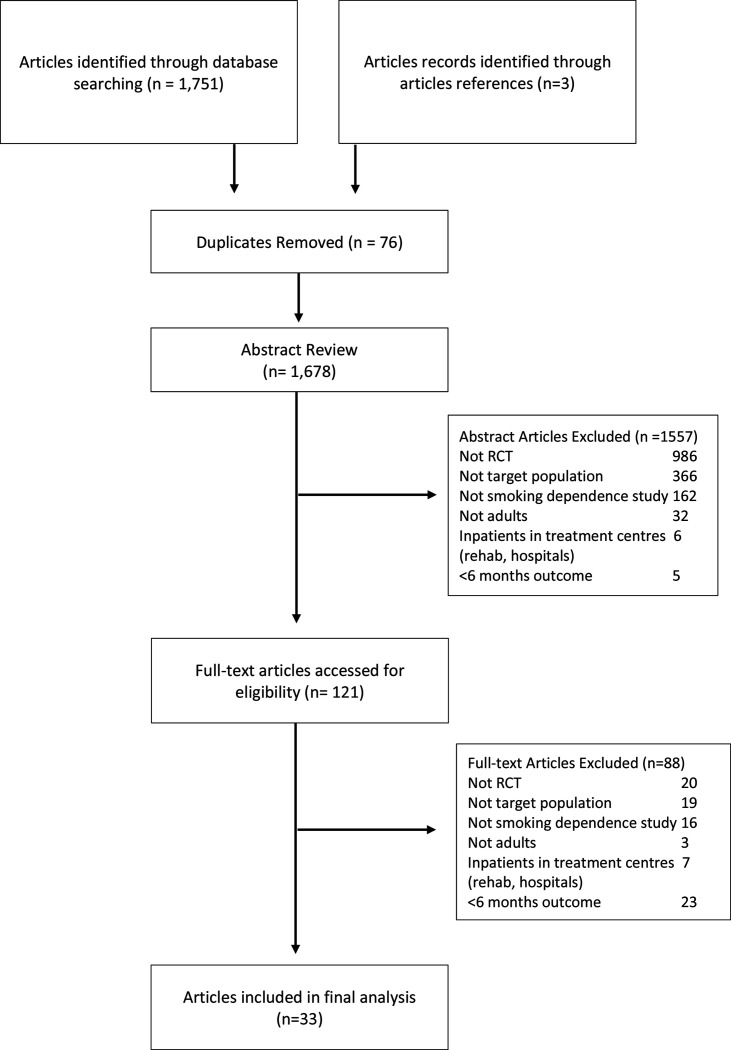
We identified 1751 studies published between 1946 and 2020. Of the 1751 studies, 121 full-text articles were screened for eligibility and 33 studies met eligibility criteria for this review (figure 1; table 1). The average age of the total study population (17 040) was 36.7 years, with 33.6% identifying as male and 66.4% as female. In total, 6721 (39.4%) were identified as black, 6476 (38.0%) were identified as Caucasian, 629 (3.7%) were identified as Hispanic, 11 (0.06%) were identified as Asian, 310 (1.8%) were identified as of Indigenous descent and 974 (5.7%) as unclear. Four (12%) studies involved only black participants36–39 and nine (27%) studies recruited only women participants.40–49 In total, 14 (42.4%) of the included studies indicated that the participants had histories of mental health disorders such as depression and anxiety,38–41 45 46 48 50–55 whereas 9 (27.3%) studies indicated participants had used illicit/non-illicit substances within the past year.41 48 50–52 54–57 Twenty-four studies (72.7%) reported on participant’s education status as less than high school.
Figure 1.
Consolidated Standards of Reporting Trials diagram of search strategy. RCT, randomised controlled trial.
Table 1.
Descriptive summary of intervention and control components of included articles
| Author(s) | Average no. of cigarettes/day | Total no. randomised | Intervention component(s)* |
Frequency/duration of Intervention | Control component(s)* |
Tobacco smoking outcome | Biochemical verification | Intervention tobacco smoking quit rate (%) |
| Froelicher et al.,201038 | 11.3 | 60 |
|
Weekly for 5 weeks |
|
7-day point prevalence (6 and 12 months) | Yes | 6 months: 13.5 12 months:15.7 |
| Andrews et al., 201648 | 12.7 | 409 |
|
24 weeks |
|
7-day point prevalence (6 and12 months) | Yes | 6 months: 10 12 months:12 |
| Brooks et al., 201761 | <10 | 250 |
|
9 sessions over 6 months |
|
7-day point prevalence (12 months) | Yes | 16.5 |
| McBride et al., 200239 | 15.5 | 557 |
|
10 weeks |
|
7-day point prevalence (6 and 12 months) | Yes | 6 months: 19 12 months:14 |
| Alaniz et al., 202049 | Unclear | 185 |
|
4 sessions over 6 months |
|
Smoking abstinence at 6 months post partum | Yes | 36.6 |
| Bonevski et al., 201854 | 15 | 431 |
|
5 sessions |
|
7-day point prevalence (6 months) | Yes | 1 |
| Brunette et al., 201763 | 17.3 | 661 |
|
12 sessions |
|
7-day point prevalence (6 and 12 months) | Yes | 6 months: 16 12 months: 12.5 |
| Lasser et al., 201760 | 15 | 352 |
|
4 hours over 6 months |
|
6- and 12-month smoking abstinence | Yes | 6 months:9.6 12 months:11.9 |
| Okuyemi et al., 201351 | 19.3 | 430 |
|
6 sessions, 15–20 min each |
|
7-day point prevalence (6 months) | Yes | 9.3 |
| Okuyemi et al., 200768 | 17.5 | 173 |
|
5 sessions over 20 weeks |
|
7-day point prevalence (6 months) | Yes | 7.6 |
| Bock et al., 201465 | Unclear | 846 |
|
3 sessions |
|
7-day point prevalence (6 and 12 months) | Yes | 6 months: 24 12 months: 29 |
| Bullock et al., 200947 | Unclear | 695 |
|
Weekly for 8 months |
|
Point prevalence (32 weeks gestation and 6 weeks post delivery) | Yes | 32 weeks: 17 Post delivery: 12.5 |
| Coleman-Cowger et al.,201859 | 8.6 | 128 |
|
10 calls over 6 months |
|
7-day point prevalence (6 months post partum) | Yes | 24 |
| Curry et al., 200346 | 12.1 | 303 |
|
3 sessions | Unclear | 7-day point prevalence (12 months) | Yes | 14 |
| Fraser et al. 201764 | 17.2 | 1900 |
|
5 sessions |
|
7-day point prevalence (6 months) | Yes | 21.6 |
| Fu et al., 201658 | 13.6 | 2406 |
|
Unclear | Usual care | 6-month smoking abstinence (12 months) | No | 16.5 |
| Gielen et al.,199744 | 8.6 | 246 |
|
1 session (15 min) | Usual care | 6 months postpartum smoking abstinence | Yes | 6.2 |
| Lepore et al., 201853 | 11.5 | 327 |
|
5 sessions over 12 weeks |
|
7-day point prevalence (12 months) | Yes | 15.2 |
| Marks and Sykes et al., 200267 | 25 | 260 |
|
10 sessions over 3 months |
|
7-day point prevalence (12 months) | Yes | 17.2 |
| McClure et al., 201853 | 19.1 | 718 |
|
16 text messages, 5 counselling sessions |
|
7-day point prevalence (6 months) | No | 30.3 |
| Okuyemi et al., 200650 | 15.3 | 46 |
|
5 sessions over 20 weeks |
|
7-day point prevalence (6 months) | Yes | 17.4 |
| Rash et al.,201854 | 15.4 | 70 |
|
4 sessions over 24 weeks |
|
4-week smoking abstinence (6 months) | Yes | 10 |
| Resenicow et al., 199734 | 15.9 | 1244 |
|
6 months | 1. Non-formal education (multimedia, printed) | 6-month smoking abstinence | No | 8.9 |
| Solomon et al., 200043 | 23 | 214 |
|
Weekly to biweekly calls for 3 months | 1. Pharmacotherapy | 6-month smoking abstinence | Yes | 20 |
| Sykes et al., 200164 | 25 | 214 |
|
3 months | 1. Non-formal education (printed) | 6-month smoking abstinence | Yes | 17.2 |
| Lipkus et al., 199935 | <11 | 160 |
|
1–2 calls for 1 year |
|
16-month smoking abstinence | No | 13.2 |
| Gritz et al.,201348 | 19.1 | 474 |
|
Unclear | 1. Non-formal education (printed) | 7-day point prevalence (12 months) | Yes | 20 |
| Baker et al., 201841 | <20 | 1014 |
|
8 sessions over 6 months | 1. Individual counselling (in person) | 7-day point prevalence (6 months) | Yes | 14.65 |
| Mayer et al., 199040 | 19.9 | 219 |
|
1 session (20 min) | 1. Individual counselling (in person) | 9 months postpartum smoking abstinence | Yes | 7 |
| Sarkar et al., 201760 | Unclear | 1213 |
|
1 session | 1. Individual counselling: brief quit advice (in person) | 6-month smoking abstinence (7 months) | Yes | 2.6 |
| Solomon et al., 200538 | 23.6 | 330 |
|
12 calls over 4 months | 1. Pharmacotherapy | 6-month smoking abstinence | No | 38 |
| Wagner et al., 201655 | Unclear | 400 |
|
12 sessions | 1. Group counselling (in person) | 9-month smoking abstinence | Yes | 8.9 |
| Dornelas et al., 200639 | Less than 10 | 105 |
|
1 session (90 min) | 1. Non-formal education (printed) | 7-day point prevalence (end of pregnancy and 6 months post partum) | Yes | End of pregnancy: 28.3 Post partum: 9.4 |
*Intervention and control components derived from the customised data collection sheet titled ‘A Checklist for a Comprehensive Community-based Chronic Disease Management Program for Marginalized Populations: Example Tobacco Dependence’.
This review contained a wide range of publication dates, with 15 (45.5%) studies published in the last 5 years,43 48 49 53–64 4 (12.1%) published in the last 10 years,38 50 51 65 and 14 (42.4%) published over 10 years ago.36 37 39–42 44–46 52 66–68 Majority (87.9%) were completed in the USA,36–53 55–57 59–61 63–65 68 and four (12.1%) were completed internationally.54 62 66 67
Participants were recruited from different locations: 9 (27.3%) used primary care clinics located in low-income areas,37–39 46 53 54 57 65–67 5 (15.2%) used low-income neighbourhoods,36 45 62 64 67 7 (21.2%) used prenatal clinics located in low-income areas,40–44 47 59 1 (3%) used primary care clinics located in non-low-income areas,60 3 (9%) used homeless shelters,51 52 56 1 (3%) used HIV/AIDS clinics,50 2 (6%) used an insurance database58 63 and 3 (9%) used public/subsidised housing neighbourhoods.48 61 68 The most frequent methods of recruitment were clinic visits and flyers.
Study measurements were conducted differently across each study, with self-report and expired carbon monoxide being the two most common: 11 (33%) studies used salivary cotinine, 22 (66.7%) studies used expired carbon monoxide, 25 (75.8%) studies used self-report and 2 (6%) study used urine cotinine.
Intervention components
The number of intervention components ranged from one to six components: 1 study had six intervention components (3%),38 3 studies had five intervention components (9%),39 48 61 6 studies had four intervention components (18%),49 51 54 60 63 68 15 studies had three intervention components (45%),36 42 44–47 52 53 56 58 59 64–67 7 studies had two intervention components (21%)37 40 42 43 50 57 62 and 1 study had one intervention component (3%) (tables 1 and 2; online supplemental appendix C).41
Table 2.
Summary of intervention components
| Components* | Total (n=33) | Intervention, n (%) | Control, n (%) | ||
| Social support | 7 (21.2) | Peer support | 4 (12.1) | Peer support | 1 (3) |
| Social capital | 3 (9) | Social capital | 0 | ||
| Socioeconomic supports | 22 (66.7) | Non-formal education | 22 (66.7) | Non-formal education | 16 (48.5) |
| Counselling | 32 (96.9) | Individual | 29 (87.9) | Individual | 11 (33.3) |
| Group | 5 (15.2) | Group | 3 (9) | ||
| Pharmacotherapy | 20 (60) | NRT | 20 (57.8) | NRT | 7 (21.2) |
| Varenicline | 2 (6) | Varenicline | 1 (3) | ||
| Bupropion | 2 (6) | Varenicline | 1 (3) | ||
| Compensation | 17 (51) | Monetary | 17 (51.2) | Monetary | 7 (21.2) |
| Non-monetary | 7 (21.2) | Non-monetary | 7 (21.2) | ||
| Project site and approach | Institution: 17(52) Community placed: 11(33) Community based: 4 (12.1) Virtual: 1 (3) |
||||
*Intervention components derived from the customised data collection sheet titled, ‘A Checklist for a Comprehensive Community-based Chronic Disease Management Program for Marginalized Populations: Example Tobacco Dependence’.
NRT, nicotine replacement therapy.
Counselling typically occurred through telephone,36–41 43–46 49 50 53–55 58–61 64 65 68 in person41–44 46 49 51 52 54 56 57 60–62 66 68 and through multimedia platforms36 55 64 or through a combination of two or more delivery mechanisms. Usually, counselling included one or more sessions with a health professional or research assistant who already provided smoking cessation support as part of their position or were specifically trained for this role. Five studies (15.2%) conducted counselling sessions within a group setting38 48 52 66 67 and two studies (6%) offered both individual and group sessions.38 68 A variety of counselling techniques were administered: motivational interviewing, cognitive behavioural methods, digital behaviour support and brief quit advice from health professionals.
Twenty studies (60%) used pharmacotherapy as an intervention,38–40 45 48 51–54 56–58 60 61 65–68 the most common being nicotine replacement therapy (NRT) as patches. The NRT was either mailed, provided in person during counselling sessions or prescribed through healthcare providers. Pharmacotherapy was typically offered for a duration of four to 4–8 weeks and did not span the entire duration of the follow-up period. Only two studies offered varenicline and bupropion in addition to NRT.60
This review defines non-formal education as education programmes that take place outside the school system and do not have a structured curriculum. Twenty-two (66.7%) studies included non-formal education as an intervention component, most of which occurred in person, often as printed handouts or booklets with approaches for relapse prevention and quitting strategies.36–39 42 44 47 49–51 53 55 58–61 64 66–68 Some non-formal education interventions were tailored towards the participant’s literacy level or cultural beliefs.
Seven (21.2%) studies included a social support component within the intervention focused on providing physical and emotional comfort to improve participants' social capital and smoking outcomes.38 44 47–49 61 Individuals in the community who had lived experience with smoking dependency, also named as patient navigators, Smoke-Free Mom counsellors and women ex-smokers, often provided the social support component.44 45 60 Examples of social support interventions used include: neighbourhood level anti-smoking events, access to a peer who connected participants to existing smoking cessation resources in the community and offering enrolment in a text-based smoking cessation programme for household members who smoke.48 49 61 The social network of low SES populations, defined in this review as the social interactions and relationships within an individual’s social structure, was not discussed in any social support components.
Just over half of the included studies compensated participants in a monetary or non-monetary form to acknowledge their participation in the study. Monetary forms typically included an honorarium,38 46–49 51 52 59–61 68 gift card or contingency management strategies.39 43 49 56 64 Very few studies offered non-monetary items to mitigate accessibility barriers such as transportation, food insecurity and childcare services.36 38 46 52 56 59 68
Intervention delivery
More than half of the included studies’ interventions (17, 51%) took place in an academic or clinical setting.37 40–47 53 55 59 60 64–67 Eleven (33%) study interventions used a community-placed setting36 39 49–51 54 56 61–63 68 (defined as a site located in the community it serves to help, but without involvement of community in the design, implementation or dissemination of research programmes).17 Only four (12.1%) studies used a community-based participatory action approach,38 48 52 57 defined as a joint partnership with communities they serve to help, who are equal partners and are involved in every step of the research project from study design to knowledge dissemination. One (3%) study completed intervention entirely through phone and mail.58
Research staff and healthcare professionals frequently administered intervention components. These professionals included counsellors, nurses, physicians and social workers who were either trained or already provided these services. Very few interventions were administrated by individuals in the community with lived or living experience. On average, these individuals received 8 hours of training to deliver social support or counselling interventions.
We observed significant variations in the frequency and duration of intervention components. The shortest intervention duration consisted of one 15–20 min brief quit counselling session provided by a healthcare professional over 6 months.42 Whereas the longest intervention duration included weekly telephone support calls by a nurse for 8 months.47
Efficacy of multicomponent interventions
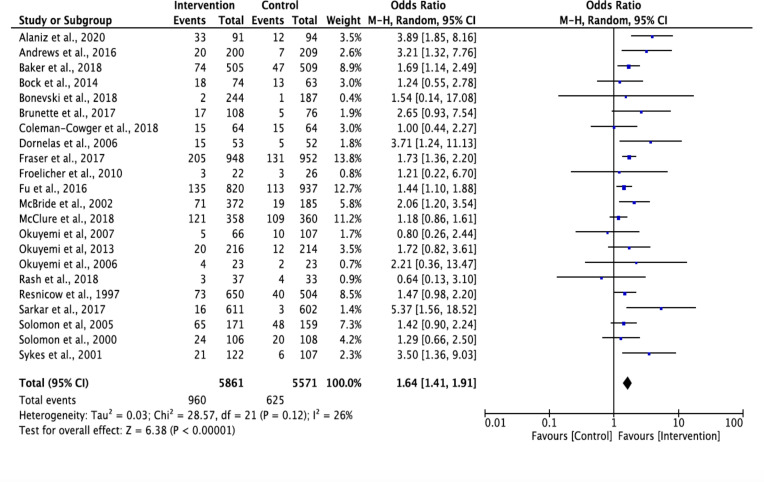
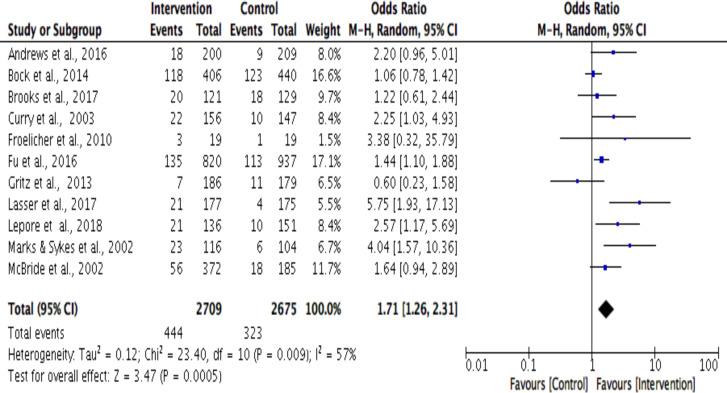
The random effects meta-analysis revealed that for both the 6-month and 12-month outcome timepoints, multicomponent interventions had 1.65 times higher odds than the control arm of being effective (OR 1.64, 95% Cl 1.41 to 1.91; OR 1.74, 95% Cl 1.30 to 2.33). The OR for individual trials ranged from 0.64 to 5.75.55 60 Higher the I2 values, greater the heterogeneity69; 6-month outcome’s forest plot shows evidence of low (I2=26%) heterogeneity in the effect size between studies, while 12-month outcome’s forest plot demonstrates moderate (I2=56%) heterogeneity (figures 2 and 3). Based on visual inspection of the funnel plots, heterogeneity did not appear to be related to the timing of the outcome assessment (online supplemental appendix E).
Figure 2.
Smoking cessation outcomes at 6-month outcome timepoint using Mantel-Haenszel (M-H) method.
Figure 3.
Smoking cessation outcomes at 12-month outcome timepoint using Mantel-Haenszel (M-H) method.
Risk of bias
Majority of the included studies were classified as low or unclear risk of bias in each RoB domain. The main issues for high risk of bias were lack of blinding of participants and study personnel, lack of blinding of outcome assessment and incomplete outcome data. Information about random sequence generation and allocation concealment were unclear or low for most studies. Overall, two of the included studies were classified as low risk of bias on all domains of the Cochrane RoB tool and seven studies were classified as overall high risk of bias (online supplemental appendix D).
Post hoc sensitivity analysis
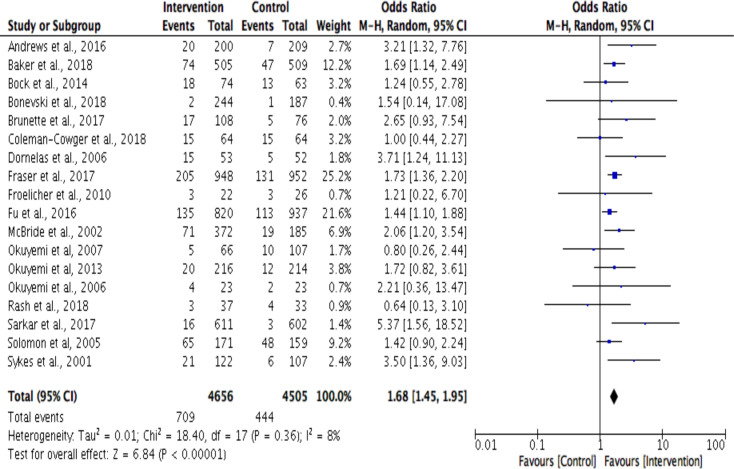
The 6-month outcome post hoc sensitivity analysis (n=18) explored the effect of excluding four studies that were classified as high overall risk of bias. Blinding of outcome assessment and incomplete outcome data were the two RoB domains that greatly contributed to the overall high risk of bias for the excluded studies. The exclusion of the studies further reduced heterogeneity from 26% to 8% and multicomponent interventions were found to be significantly effective in reducing smoking dependence (OR 1.68, 95% Cl 1.45 to 1.95) (figure 4). A post hoc sensitivity analysis was not conducted at the 12-month outcome as all included studies were classified as low overall risk of bias.
Figure 4.
Sensitivity analysis for smoking cessation outcomes at 6-month outcome timepoint using Mantel-Haenszel (M-H) method.
Discussion
This review provides insight into the types of multicomponent tobacco dependence interventions that are suggested to positively impact smoking cessation outcomes for low SES populations. Smoking quit rates were highly variable and ranged from 1% to 36.6% at the 6-month outcome. There was evidence to suggest that multicomponent interventions were more successful in achieving smoking cessation than controls, and there was low and moderate heterogeneity between effect sizes at the 6-month and 12-month outcomes. Differences in choice of cessation outcome (eg, 7-day point prevalence or smoking abstinence at 6 months) and study measures (eg, self-report or biochemically verified) contributed to the heterogeneity. The meta-analysis results demonstrate the complex relationship low SES populations have with tobacco dependence. Single timepoint objective measurements fail to capture the complexity of smoking cessation patterns over time as it fluctuates depending on socioeconomic factors such as access to food, healthcare and social support.70 71 Many researchers recognise the challenges low SES populations face with tobacco dependence and use multicomponent interventions to mitigate barriers due to health inequities. However, similar adaptations in measures of success are not seen in research. Researchers should consider capturing assessments of person-centred successes in addition to standard smoking cessation outcomes to better inform programmes and policy decisions.
Furthermore, studies that were classified as overall high risk of bias displayed poor reporting quality, where implementation details of the intervention such as location, who administered the intervention and outcome measures were not mentioned. The ambiguity in reporting makes it difficult to interpret results and research findings. However, as demonstrated in the post hoc sensitivity analysis such effects are minimal and did not impact the overall bias of our results.72 The majority of studies were classified as low risk of bias and we did not observe any publication bias in the funnel plots. Further research should focus on developing a reporting guideline for multicomponent (tobacco dependence) interventions to improve clarity in reporting to better inform policy decisions.
The included studies respond to the evidence of the higher smoking prevalence and health inequity among racial or ethnic minorities and women.69 73 74 Literature has shown that women may be more susceptible to smoking-related morbidity and mortality than men.75–77 Historically, racial or ethnic minorities, including black and Latin populations in America, have been disproportionately targeted by tobacco companies, resulting in increased disease burden and deaths.19 78 Among the studies' participants, most were women and the most common racial identity for males was black (eg, African, Caribbean). Within the nine studies that recruited only female participants, seven of them were pregnancy-related interventions. This suggests researchers are tailoring tobacco dependence interventions to target populations most at risk.
Tailored approaches are expected to play a fundamental role in reducing health inequities by simultaneously addressing the target population’s unique, competing needs. Majority of interventions used combinations of pharmacotherapy, individual counselling and non-formal education intervention components. Despite the strong evidence for using varenicline as the most effective pharmacotherapy strategy to manage tobacco dependence,79 only two studies offered varenicline.60 A significant number of studies used a targeted approach for literacy level, patient-driven counselling topics and incorporating aspects of culture into the interventions.37–39 47 48 However, the support of this tailored approach contradicts a recent review that showed that tailoring individual-level interventions for people who smoke in a lower SES position were not significantly different in comparison to non-socioeconomic position tailored interventions.19 This variance should be interpreted with caution as Kock et al. excluded community or population level delivered interventions, whereas this review did.19
Success of targeted interventions is dependent on the frequency of engagement to reiterate social support. Studies with a higher smoking quit rate incorporated a longer intervention period that had weekly to biweekly touchpoints with participants to provide social support and encouragement.47 Some studies mentioned that participant engagement often tapered off towards the end of the study due to loss of interest and concerns, such as transportation cost, safety to and from evening sessions, unmet expectations with incentives, a distrust with healthcare providers and food security.38 41 44 47 52 66 Recruiting and engaging low SES populations is a commonly reported challenge in research,5 29 suggesting a need for tailored interventions designed in partnership to understand the factors impacting retention and access to interventions.
Social support is a critical component of smoking dependency that needs to be systematically included in interventions. The literature has demonstrated that poor social networks and low social support are more common in low SES populations, as they face a greater number of triggers and facilitators to smoking uptake than more advantaged socioeconomic groups, including higher nicotine dependency, positive social norms, day-to-day stress and challenging life situations.20 80 Recent studies have shown that family and social networks can positively influence smoking habits and cessation efforts.81–83 However, low SES populations may have a complicated relationship with family and their social networks due to the challenges associated with social determinants of health.84 Additional research is needed to understand how low SES populations define social networks to include said networks in designing smoking tobacco reduction interventions, or for any other chronic disease-related interventions.
One approach to improve social support and provide appropriate, tailored interventions is participatory action research. Participatory methods are in practice since the 1940s in diverse fields and can address the lack of distrust low SES populations have towards authorities, especially in healthcare and research.85 86 Related, community-based participatory action research approach (CBPAR) can address inequities by exploring local knowledge and perceptions,85 87 empowering the community to be agents of change87 88 and aligning research objectives with community’s needs and interests.85 87 Within this review, only four studies employed CBPAR,38 48 52 57 where community members were part of the formal decision-making process at every stage of the study through a community advisory board or partnering with community leaders. Studies that employed a CBPAR did not show superior smoking quit rates compared with studies that did not, as challenges with retention and engagement of participants persisted despite well-established relationships with communities. Limitations in success were most likely related to long follow-up periods with infrequent social engagement touchpoints with participants. Additional research is needed to overcome the ethical, cultural and scientific concerns communities still have with researchers and institutions.89
Strengths and limitations
To our knowledge, no previous reviews have assessed the effectiveness of multicomponent tobacco dependence interventions at 6 months or later for low SES populations. This analysis provides a first step towards understanding evidence-based findings that will encourage future equity-focused research (for tobacco dependence or other chronic diseases) in low SES populations considering the growing application of tailored, multicomponent interventions. The customised checklist tool offers an innovative whole-person perspective at examining multicomponent interventions and has the potential to be generalised to other chronic disorders such as diabetes, chronic obstructive pulmonary disease and asthma.
The review is not without limitations. The statistical contribution of each intervention component was not analysed. Additionally, we did not create a comprehensive weighted tool and included only English language literature and full-text articles that were accessible without contacting the author(s), potentially narrowing this review’s scope. Finally, this review also did not account for between-country or between-study differences in how low SES is defined and experienced and the potential impact on quit rates.90 There is a drastic difference between people experiencing homelessness and at risk of homelessness, imposing different socioeconomic positions and levels of deprivation across populations. Accordingly, studies have used varying indicators to define low SES. However, it is rare for these definitions to consider the realities of people with lived and living experience and their understanding of low SES, which can differ from expert understanding of low SES,91 leading to findings that are not relevant to laypeople. As such, we co-created a list of indicators to define low SES over numerous months of discussion with community peer researchers on their experiences of low SES in Canada and common indicators identified through literature reviews. With this definition of low SES, we believe we appropriately achieved this study’s aims. Yet, we still recognise there are limitations to our definition of low SES, and there remains a potential of missing studies in our review due to our definition of low SES.
For example, an important measure of low SES is (low) educational attainment. Through our conversations with community peers, we found that high-educational attainment did not always prevent individuals from experiencing poverty, precarious housing and/or substance use later in life. For example, 31.3% of the The Participatory Research in Ottawa: Management and Point-of-Care for Tobacco Dependence (PROMPT) cohort had some or full postsecondary school (ie, university or college) completed.5 As a result, we did not explicitly include education in the eligibility criteria. This is a limitation that needs to be recognised as we may have missed studies that use education as the defining indicator for low SES. However, it is unlikely that studies solely focused on education while defining low SES since 28% of our included studies did not report any level of education, and majority of our included studies (72%) reported participants’ education level and one other indicator of low SES.
A similar approach was taken with income, another foundational indicator of SES. Through our co-creation process, we learnt that income can be very fluid and as a result, our target population may not be captured if the income cut-off was lowered too much or set a fixed amount. Given this heterogeneity in income criteria, we used income in conjunction with other vulnerability measures. We believe this helped us keep studies that might have used higher income thresholds than ours or used no income thresholds while defining low SES. As a result of this approach, a substantial number of studies in our sample (48%) did not report on income. Those who have reported income used different thresholds of average annual or monthly income while defining low SES. Overall, despite the limitations of our definition of low SES, we believe our focus on broader vulnerabilities has helped capture a wide net of studies that explored multicomponent tobacco dependence interventions for low SES populations.
Conclusion
In this systematic review, we examined a variety of multicomponent interventions that aimed to reduce smoking dependency among low SES populations. To reduce smoking prevalence, implementation of multicomponent interventions is critical, in particular the inclusion of social support, employing community-based participatory approaches to develop person-centred tailored approaches, frequency and duration of components, in addition to effective combination pharmacotherapies with varenicline and NRT.79 Future research should focus on how low SES populations define social networks and employ innovative methods to partner and build trust within said populations.
Acknowledgments
The authors are indebted to The Bridge community peer researchers. Without their trust in the research team, partnership and dedication towards a harm reduction approach, this project would not be possible, or to complete the work at The Bridge. The authors also wish to thank local allies: Oasis, Sandy Hill Community Health Centre (CHC) and Somerset West CHC, without their support it would not have been possible to implement the project. The Community Advisory Committee at The Bridge, comprised of Dave, Susie, Ted, Kelly and Terry (community peer researchers and participants), R. Boyd (Oasis, Sandy Hill CHC), S. Willmott (Somerset CHC), J. Haddad (CMHA), Ticket Defense Program (Suzanne Bouclin) and S. Pakhale (The Bridge lead scientist) meet quarterly and as needed at The Bridge and guide the mission and vision of the space along with overseeing the smooth functioning of projects. The authors are indebted to the vision, dedication and passion of The Bridge CAC. The authors are also grateful to two of their dedicated student volunteers, Avanti Garde and Ferdousa Ibrahim, who helped them in data extraction in the early stages of the project. The authors are thankful to the Ottawa Hospital, the Ottawa Hospital Research Institute and the administrative staff for their support. The authors thank all the anonymous reviewers, as their comments improved the quality of this review.
Footnotes
Contributors: All authors were actively involved in the conception and design of the review, have given final approval of the version of the manuscript submitted for publication, agree to be accountable for all aspects of the work, made substantial contributions to acquisition of the data, screened trials for eligibility, assessed the quality of the trials and assisted with data analysis. NH, ST, TH, OB, CC, PA, SJ and SP contributed to drafting the manuscript or the revisions made to the manuscript. SP as the guarantor had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. World Health Organization . Tobacco, 2020. Available: https://www.who.int/news-room/fact-sheets/detail/tobacco [Accessed 7 Jan 2021].
- 2. Isaranuwatchai W, de Oliveira C, Mittmann N, et al. Impact of smoking on health system costs among cancer patients in a retrospective cohort study in Ontario, Canada. BMJ Open 2019;9:e026022. 10.1136/bmjopen-2018-026022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. CDC . Economic Trends in Tobacco | Smoking & Tobacco Use. Available: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/economics/econ_facts/index.htm [Accessed 29 Aug 2021].
- 4. Government of Canada . Smoking. Ottawa, 2016. [Google Scholar]
- 5. Pakhale S, Kaur T, Charron C, et al. Management and point-of-care for tobacco dependence (prompt): a feasibility mixed methods community-based participatory action research project in Ottawa, Canada. BMJ Open 2018;8:e018416. 10.1136/bmjopen-2017-018416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Szerlip MI, Szerlip HM. Identification of cardiovascular risk factors in homeless adults. Am J Med Sci 2002;324:243–6. 10.1097/00000441-200211000-00002 [DOI] [PubMed] [Google Scholar]
- 7. Lee TC, Hanlon JG, Ben-David J, et al. Risk factors for cardiovascular disease in homeless adults. Circulation 2005;111:2629–35. 10.1161/CIRCULATIONAHA.104.510826 [DOI] [PubMed] [Google Scholar]
- 8. Hwang SW, Wilkins R, Tjepkema M, et al. Mortality among residents of shelters, rooming houses, and hotels in Canada: 11 year follow-up study. BMJ 2009;339:b4036. 10.1136/bmj.b4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The Conference Board of Canada . The Costs of Tobacco Use in Canada, 2012 - Canada.ca, 2017. [Google Scholar]
- 10. Leone FT, Carlsen K-H, Folan P, et al. An official American thoracic Society research statement: current understanding and future research needs in tobacco control and treatment. Am J Respir Crit Care Med 2015;192:e22–41. 10.1164/rccm.201506-1081ST [DOI] [PubMed] [Google Scholar]
- 11. Pakhale S, Tariq S, Huynh N, et al. Prevalence and burden of obstructive lung disease in the urban poor population of Ottawa, Canada: a community-based mixed-method, observational study. BMC Public Health 2021;21:1–11. 10.1186/s12889-021-10209-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grandes G, Sanchez A, Cortada JM, et al. Is integration of healthy lifestyle promotion into primary care feasible? discussion and consensus sessions between clinicians and researchers. BMC Health Serv Res 2008;8:213. 10.1186/1472-6963-8-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hall SM. Extended nortriptyline and psychological treatment for cigarette smoking. Am J Psychiatry 2004;161:2100–7. 10.1176/appi.ajp.161.11.2100 [DOI] [PubMed] [Google Scholar]
- 14. Cofta-Woerpel L, Wright KL, Wetter DW. Smoking cessation 3: multicomponent interventions. Behav Med 2007;32:135–49. 10.3200/BMED.32.4.135-149 [DOI] [PubMed] [Google Scholar]
- 15. Canada PHA of . Life and breath: respiratory disease in Canada, 2007. Available: http://www.phac-aspc.gc.ca/publicat/2007/lbrdc-vsmrc/index-eng.php
- 16. Canada G of . Suspected opioid-related overdoses in jurisdictions across Canada based on emergency medical services data.
- 17. Burns JC, Cooke DY, Schweidler C. A short guide to community based participatory action research. Adv Proj City 2011:1–18. [Google Scholar]
- 18. PARC . The Co-op Cred program at the Parkdale Activity-Recreation centre, 2017. Available: http://parc.on.ca/programs/coop-cred-program/
- 19. Kock L, Brown J, Hiscock R, et al. Individual-Level behavioural smoking cessation interventions tailored for disadvantaged socioeconomic position: a systematic review and meta-regression. Lancet Public Health 2019;4:e628–44. 10.1016/S2468-2667(19)30220-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith C, Hill S, Amos A. Stop smoking inequalities: a systematic review of socioeconomic inequalities in experiences of smoking cessation interventions in the UK, 2018. Available: http://www.cancerresearchuk.org/ [Accessed 20 Aug 2020].
- 21. Bryant J, Bonevski B, Paul C, et al. A systematic review and meta-analysis of the effectiveness of behavioural smoking cessation interventions in selected disadvantaged groups. Addiction 2011;106:1568–85. 10.1111/j.1360-0443.2011.03467.x [DOI] [PubMed] [Google Scholar]
- 22. Heishman S. Behavioral and cognitive effects of smoking: relationship to nicotine addiction. Nicotine Tob Res 1999;1:143–7. 10.1080/14622299050011971 [DOI] [PubMed] [Google Scholar]
- 23. Fiore MC, Jaen CR, Tb B. Treating tobacco use and dependence: 2008 update. Rockville, MD: US Dep Heal Hum Serv, 2008. [Google Scholar]
- 24. Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in Tobacco-Dependent adults. An official American thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2020;202:e5–31. 10.1164/rccm.202005-1982ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Echenberg H, Jensen H. Defining and enumerating homelessness in Canada, 2008. [Google Scholar]
- 26. Charron C. A systematic review of the effectiveness of smoking cessation interventions in low income populations. Available: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=76650 [Accessed 30 Aug 2021].
- 27. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. W64. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 28. West R, Hajek P, Stead L, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299–303. 10.1111/j.1360-0443.2004.00995.x [DOI] [PubMed] [Google Scholar]
- 29. Pakhale S, Kaur T, Florence K, et al. The Ottawa citizen engagement and action model (OCEAM): a citizen engagement strategy Operationalized through the participatory research in Ottawa, management and point-of-care of tobacco (prompt) study: a community based participatory action research project in inner City Ottawa. Res Involv Engagem 2016;2:20. 10.1186/s40900-016-0034-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. The bridge – connect. engage. research – for better health. Available: http://bridgeengagement.ca/ [Accessed 24 Aug 2020].
- 31. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grant SP, Mayo-Wilson E, Melendez-Torres GJ, et al. Reporting quality of social and psychological intervention trials: a systematic review of reporting guidelines and trial publications. PLoS One 2013;8:e65442. 10.1371/journal.pone.0065442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. The Cochrane Collaboration . RevMan5, 2020. [Google Scholar]
- 34. de Bruin M, Viechtbauer W, Eisma MC, et al. Identifying effective behavioural components of intervention and comparison group support provided in smoking cEssation (IC-SMOKE) interventions: a systematic review protocol. Syst Rev 2016;5:77. 10.1186/s13643-016-0253-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Charron CB, Hudani A, Kaur T, et al. Assessing community (peer) researcher’s experiences with conducting spirometry and being engaged in the ‘Participatory Research in Ottawa: Management and Point-of-care for Tobacco-dependence’ (PROMPT) project. Res Involv Engagem 2018;4:43. 10.1186/s40900-018-0125-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Resnicow K, Vaughan R, Futterman R, et al. A self-help smoking cessation program for inner-city African Americans: results from the Harlem health connection project. Health Educ Behav 1997;24:201–17. 10.1177/109019819702400208 [DOI] [PubMed] [Google Scholar]
- 37. Lipkus IM, Lyna PR, Rimer BK. Using tailored interventions to enhance smoking cessation among African-Americans at a community health center. Nicotine Tob Res 1999;1:77–85. 10.1080/14622299050011181 [DOI] [PubMed] [Google Scholar]
- 38. Froelicher ES, Doolan D, Yerger VB, et al. Combining community participatory research with a randomized clinical trial: the protecting the hood against tobacco (PhAT) smoking cessation study. Heart Lung 2010;39:50–63. 10.1016/j.hrtlng.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 39. McBride CM, Bepler G, Lipkus IM, et al. Incorporating genetic susceptibility feedback into a smoking cessation program for African-American smokers with low income. Cancer Epidemiol Biomarkers Prev 2002;11:521–8. [PubMed] [Google Scholar]
- 40. Solomon LJ, Marcy TW, Howe KD, et al. Does extended proactive telephone support increase smoking cessation among low-income women using nicotine patches? Prev Med 2005;40:306–13. 10.1016/j.ypmed.2004.06.005 [DOI] [PubMed] [Google Scholar]
- 41. Dornelas EA, Magnavita J, Beazoglou T, et al. Efficacy and cost-effectiveness of a clinic-based counseling intervention tested in an ethnically diverse sample of pregnant smokers. Patient Educ Couns 2006;64:342–9. 10.1016/j.pec.2006.03.015 [DOI] [PubMed] [Google Scholar]
- 42. Mayer JP, Hawkins B, Todd R. A randomized evaluation of smoking cessation interventions for pregnant women at a WIC clinic. Am J Public Health 1990;80:76–8. 10.2105/AJPH.80.1.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baker TB, Fraser DL, Kobinsky K, et al. A randomized controlled trial of financial incentives to low income pregnant women to engage in smoking cessation treatment: effects on post-birth abstinence. J Consult Clin Psychol 2018;86:464–73. 10.1037/ccp0000278 [DOI] [PubMed] [Google Scholar]
- 44. Gielen AC, Windsor R, Faden RR, et al. Evaluation of a smoking cessation intervention for pregnant women in an urban prenatal clinic. Health Educ Res 1997;12:247–54. 10.1093/her/12.2.247 [DOI] [PubMed] [Google Scholar]
- 45. Solomon LJ, Scharoun GM, Flynn BS, et al. Free nicotine patches plus proactive telephone peer support to help low-income women stop smoking. Prev Med 2000;31:68–74. 10.1006/pmed.2000.0683 [DOI] [PubMed] [Google Scholar]
- 46. Curry SJ, Ludman EJ, Graham E, et al. Pediatric-Based smoking cessation intervention for low-income women. Arch Pediatr Adolesc Med 2003;157:295–302. 10.1001/archpedi.157.3.295 [DOI] [PubMed] [Google Scholar]
- 47. Bullock L, Everett KD, Mullen PD, et al. Baby BEEP: a randomized controlled trial of nurses' individualized social support for poor rural pregnant smokers. Matern Child Health J 2009;13:395–406. 10.1007/s10995-008-0363-z [DOI] [PubMed] [Google Scholar]
- 48. Andrews JO, Mueller M, Dooley M, et al. Effect of a smoking cessation intervention for women in subsidized neighborhoods: a randomized controlled trial. Prev Med 2016;90:170–6. 10.1016/j.ypmed.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alaniz K, Christiansen B, Sullivan T, et al. Addressing postpartum smoking relapse among low-income women: a randomized control trial. J Patient Cent Res Rev 2019;6:233–42. 10.17294/2330-0698.1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gritz ER, Danysh HE, Fletcher FE, et al. Long-Term outcomes of a cell Phone–Delivered intervention for smokers living with HIV/AIDS. Clin Infect Dis 2013;57:608–15. 10.1093/cid/cit349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Okuyemi KS, Goldade K, Whembolua G-L, et al. Motivational interviewing to enhance nicotine patch treatment for smoking cessation among homeless smokers: a randomized controlled trial. Addiction 2013;108:1136–44. 10.1111/add.12140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Okuyemi KS, Thomas JL, Hall S, et al. Smoking cessation in homeless populations: a pilot clinical trial. Nicotine Tob Res 2006;8:689–99. 10.1080/14622200600789841 [DOI] [PubMed] [Google Scholar]
- 53. Lepore SJ, Collins BN, Coffman DL, et al. Kids safe and Smokefree (kiss) multilevel intervention to reduce child tobacco smoke exposure: long-term results of a randomized controlled trial. Int J Environ Res Public Health 2018;15:1239. 10.3390/ijerph15061239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bonevski B, Twyman L, Paul C, et al. Smoking cessation intervention delivered by social service organisations for a diverse population of Australian disadvantaged smokers: a pragmatic randomised controlled trial. Prev Med 2018;112:38–44. 10.1016/j.ypmed.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 55. McClure JB, Bush T, Anderson ML, et al. Oral health promotion and smoking cessation program delivered via tobacco quitlines: the oral health 4 life trial. Am J Public Health 2018;108:689–95. 10.2105/AJPH.2017.304279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rash CJ, Petry NM, Alessi SM. A randomized trial of contingency management for smoking cessation in the homeless. Psychol Addict Behav 2018;32:141–8. 10.1037/adb0000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wagner FA, Sheikhattari P, Buccheri J, et al. A community-based participatory research on smoking cessation intervention for urban communities. J Health Care Poor Underserved 2016;27:35–50. 10.1353/hpu.2016.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fu SS, van Ryn M, Nelson D, et al. Proactive tobacco treatment offering free nicotine replacement therapy and telephone counselling for socioeconomically disadvantaged smokers: a randomised clinical trial. Thorax 2016;71:446–53. 10.1136/thoraxjnl-2015-207904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Coleman-Cowger VH, Mark KS, Rosenberry ZR, et al. A pilot randomized controlled trial of a Phone-based intervention for smoking cessation and relapse prevention in the postpartum period. J Addict Med 2018;12:193–200. 10.1097/ADM.0000000000000385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lasser KE, Quintiliani LM, Truong V, et al. Effect of patient navigation and financial incentives on smoking cessation among primary care patients at an urban safety-net Hospital. JAMA Intern Med 2017;177:1798–807. 10.1001/jamainternmed.2017.4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brooks DR, Burtner JL, Borrelli B, et al. Twelve-month outcomes of a group-randomized community health advocate-led smoking cessation intervention in public housing. Nicotine Tob Res 2018;20:1434–41. 10.1093/ntr/ntx193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sarkar BK, West R, Arora M, et al. Effectiveness of a brief community outreach tobacco cessation intervention in India: a cluster-randomised controlled trial (the BABEX trial). Thorax 2017;72:167–73. 10.1136/thoraxjnl-2016-208732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brunette MF, Pratt SI, Bartels SJ, et al. Randomized trial of interventions for smoking cessation among Medicaid beneficiaries with mental illness. Psychiatr Serv 2018;69:274–80. 10.1176/appi.ps.201700245 [DOI] [PubMed] [Google Scholar]
- 64. Fraser DL, Fiore MC, Kobinsky K, et al. A randomized trial of incentives for smoking treatment in Medicaid members. Am J Prev Med 2017;53:754–63. 10.1016/j.amepre.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bock BC, Papandonatos GD, de Dios MA, et al. Tobacco cessation among low-income smokers: motivational enhancement and nicotine patch treatment. Nicotine Tob Res 2014;16:413–22. 10.1093/ntr/ntt166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sykes CM, Marks DF. Effectiveness of a cognitive behaviour therapy self-help programme for smokers in London, UK. Health Promot Int 2001;16:255–60. 10.1093/heapro/16.3.255 [DOI] [PubMed] [Google Scholar]
- 67. Marks DF, Sykes CM. Randomized controlled trial of cognitive behavioural therapy for smokers living in a deprived area of London: outcome at one-year follow-up. Psychol Health Med 2002;7:17–24. 10.1080/13548500120101513 [DOI] [Google Scholar]
- 68. Okuyemi KS, James AS, Mayo MS, et al. Pathways to health: a cluster randomized trial of nicotine gum and motivational interviewing for smoking cessation in low-income housing. Health Educ Behav 2007;34:43–54. 10.1177/1090198106288046 [DOI] [PubMed] [Google Scholar]
- 69. Waldron I. Patterns and causes of gender differences in smoking. Soc Sci Med 1991;32:989–1005. 10.1016/0277-9536(91)90157-8 [DOI] [PubMed] [Google Scholar]
- 70. Reid JL, Hammond D, Boudreau C, et al. Socioeconomic disparities in quit intentions, quit attempts, and smoking abstinence among smokers in four Western countries: findings from the International tobacco control four country survey. Nicotine Tob Res 2010;12 Suppl:S20–33. 10.1093/ntr/ntq051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Twyman L, Bonevski B, Paul C, et al. Perceived barriers to smoking cessation in selected vulnerable groups: a systematic review of the qualitative and quantitative literature. BMJ Open 2014;4:6414. 10.1136/bmjopen-2014-006414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Walters SJ. Therapist effects in randomised controlled trials: what to do about them. J Clin Nurs 2010;19:1102–12. 10.1111/j.1365-2702.2009.03067.x [DOI] [PubMed] [Google Scholar]
- 73. Healton C, Nelson K. Reversal of misfortune: viewing tobacco as a social justice issue. Am J Public Health 2004;94:186–91. 10.2105/AJPH.94.2.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Drope J, Liber AC, Cahn Z, et al. Who's still smoking? disparities in adult cigarette smoking prevalence in the United States. CA Cancer J Clin 2018;68:106–15. 10.3322/caac.21444 [DOI] [PubMed] [Google Scholar]
- 75. Haghani A, Arpawong TE, Kim JK, et al. Female vulnerability to the effects of smoking on health outcomes in older people. PLoS One 2020;15:e0234015. 10.1371/journal.pone.0234015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rahmanian SD, Diaz PT, Wewers ME. Tobacco use and cessation among women: research and treatment-related issues. J Womens Health 2011;20:349–57. 10.1089/jwh.2010.2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Allen AM, Oncken C, Hatsukami D. Women and smoking: the effect of gender on the epidemiology, health effects, and cessation of smoking. Curr Addict Rep 2014;1:53–60. 10.1007/s40429-013-0003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pakhalé S, Folan P, Neptune E. Retail tobacco sale in the community. should pharmacies sell tobacco products? 2015. [DOI] [PubMed] [Google Scholar]
- 79. Smith C, Hill S, Amos A. Stop smoking inequalities: a systematic review of socioeconomic inequalities in experiences of smoking cessation interventions in the UK. Addiction 2011. [Google Scholar]
- 80. Weyers S, Dragano N, Möbus S, et al. Low socio-economic position is associated with poor social networks and social support: results from the Heinz Nixdorf recall study. Int J Equity Health 2008;7:1–7. 10.1186/1475-9276-7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rüge J, Ulbricht S, Schumann A, et al. Intention to quit smoking: Is the partner’s smoking status associated with the smoker’s intention to quit? Int J Behav Med 2008;15:328–35. 10.1080/10705500802365607 [DOI] [PubMed] [Google Scholar]
- 82. Hubbard G, Gorely T, Ozakinci G, et al. A systematic review and narrative summary of family-based smoking cessation interventions to help adults quit smoking. BMC Fam Pract 2016;17:73. 10.1186/s12875-016-0457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Whitehouse E, Lai J, Golub JE, et al. A systematic review of the effectiveness of smoking cessation interventions among patients with tuberculosis. Public Health Action 2018;8:37–49. 10.5588/pha.18.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Conger RD, Conger KJ, Martin MJ. Socioeconomic status, family processes, and individual development. J Marriage Fam 2010;72:685–704. 10.1111/j.1741-3737.2010.00725.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Israel BA, Schulz AJ, Parker EA, et al. Community-Based participatory research: policy recommendations for promoting a partnership approach in health research. Educ Health 2001;14:182–97. 10.1080/13576280110051055 [DOI] [PubMed] [Google Scholar]
- 86. Neill SJ. Developing children's nursing through action research. J Child Health Care 1998;2:11–15. 10.1177/136749359800200103 [DOI] [PubMed] [Google Scholar]
- 87. Stevens PE, Hall JM. Participatory action research for sustaining individual and community change: a model of HIV prevention education. AIDS Educ Prev 1998;10:387–402. [PubMed] [Google Scholar]
- 88. Webb C. Partners in research. Nurs Times 1990;86:40–4. [PubMed] [Google Scholar]
- 89. Holkup PA, Tripp-Reimer T, Salois EM, et al. Community-Based participatory research: an approach to intervention research with a native American community. ANS Adv Nurs Sci 2004;27:162–75. 10.1097/00012272-200407000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Galobardes B, Shaw M, Lawlor DA, et al. Indicators of socioeconomic position (Part 1). J Epidemiol Community Health 2006;60:7–12. 10.1136/jech.2004.023531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shim JK. Heart-sick : the politics of risk, inequality, and heart disease, 2014: 1–277. https://books.google.com/books/about/Heart_Sick.html?id=FG0TCgAAQBAJ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jech-2021-216783supp001.pdf (317.6KB, pdf)
Data Availability Statement
Data are available upon request.