Abstract
Fibroblast-like synoviocytes or synovial fibroblasts (FLS) are important cellular components of the inner layer of the joint capsule, referred to as the synovial membrane. They can be found in both layers of this synovial membrane and contribute to normal joint function by producing extracellular matrix components and lubricants. However, under inflammatory conditions like in rheumatoid arthritis (RA), they may start to proliferate, undergo phenotypical changes and become central elements in the perpetuation of inflammation through their direct and indirect destructive functions. Their importance in autoimmune joint disorders makes them attractive cellular targets, and as mesenchymal-derived cells, their inhibition may be carried out without immunosuppressive consequences. Here, we aim to give an overview of our current understanding of the target potential of these cells in RA.
Keywords: Fibroblasts; Arthritis, Rheumatoid; Inflammation
Introduction
Rheumatoid arthritis (RA) is an autoimmune disorder that affects 0.5%–1% of the Western world population.1 There are several cell types that have been implicated in the pathogenesis of RA, among which both immune and non-immune cells can be found.1 The prominent role of immune cells in autoimmune arthritis has been based on a plethora of experimental findings as well the successful use of immunosuppressive therapies in clinical practice. Particularly, novel targeted therapies that interfere with specific pathways of the inflammatory and immune response such as antibodies against inflammatory cytokines or against surface molecules on immune cells have revolutionised the therapy of RA. Nonetheless, still a considerable proportion of patients with RA do not respond adequately to available therapies.2 In other words, although with the introduction of biological and targeted synthetic disease-modifying antirheumatic drugs (DMARDs), the treatment of RA improved significantly, a notable part of patients remains symptomatic. These patients might be considered as having ‘difficult-to-treat’ or ‘refractory’ RA, which categories represent great therapeutic challenges both to the medical teams and to the patients. In addition to new management strategies, the optimal care of these patients require new therapeutic drug targets. Meanwhile, there is an increasing body of evidence to suggest a crucial role for mesenchymal cell-derived cells, particularly fibroblast-like synoviocytes (FLS) in mediating both direct tissue injury and perpetuation of the complex disease process in autoimmune joint disorders like RA.1
FLS represent one key cell type of the synovial membrane and can be found in both the lining and the sublining layers of the membrane.3 The normal function of FLS is essential for maintaining the homeostasis of the diarthrodial joints, where they are responsible for the production of the extracellular matrix, but also regulate their environment by releasing matrix metalloproteinases (MMPs).3 Importantly, FLS in the lining layer cannot be considered as conventional barrier-forming cells at the border of the synovium and the synovial fluid, as they have a pronounced linking rather than a blocking feature and provide the joint cavity and the adjacent cartilage with lubricating molecules such as hyaluronic acid as well as with nutritive plasma. This is due to a peculiarity of the synovial membrane that unlike other border membranes lacks an organised basement membrane, and thus, the classical architecture of an epithelium. Several studies have demonstrated that although cellular contacts between the fibroblast-like synoviocytes lack tight junctions and desmosomes, there are specific adhesion molecules such as cadherin-11, which mediate a strong homophilic adhesion between synoviocytes through interaction with organisers of the cytoskeleton and that are largely responsible for their organisation into a tissue.4 5
Under inflammatory conditions such as in RA, FLS can undergo both morphological and phenotypical changes and transform into essential cellular component of the disease process.1 6 7 These changes have been described in detail in a number of reviews and comprise a complex set of cellular changes that eventually result in the proliferation and differentiation into distinct subpopulations.8 9 During this process, some FLS acquire invasive, tumour cell-like destructive characteristics through epigenetic and metabolic changes as well as through interaction with both themselves and different immune cells like lymphocytes or macrophages.5 10 As a consequence of their transformation, some FLS subsets contribute prominently to the destruction of articular structures while others have been hypothesised to exert more regulatory functions.6 These effector functions and orchestrating roles of FLS in autoimmune joint disorders, particularly in RA, make them attractive potential cellular targets with the possibility to promote disease control without immunosuppression in RA. In this review, we aim to give an overview of the different potential routes of intervention to influence the fate and function of RA-FLS (figures 1 and 2, table 1). Except for some selected cases, we do not discuss the effects of currently used conventional synthetic or biological DMARDs (cs-DMARDs or bDMARDs).
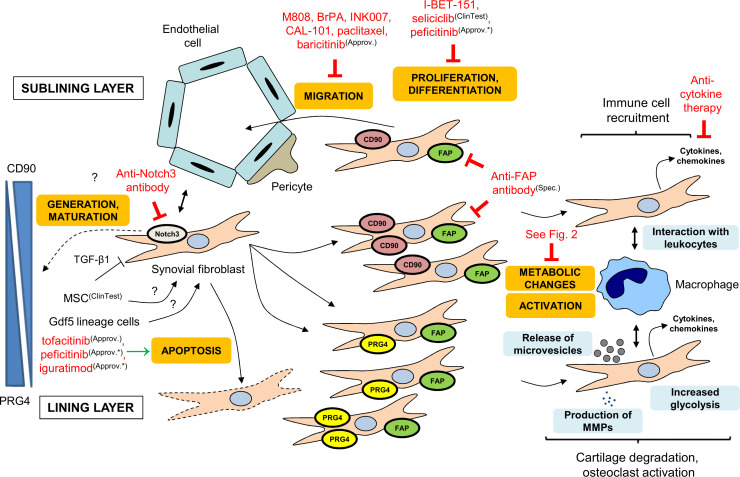
Figure 1.
Overview of targeting RA-FLS. RA-FLS can be influenced at different points of their life cycle and activation. For example, the generation and proliferation, the migration, the direct destructive functions and the interactions with leukocytes can be targeted. Approv., approved in the European Union (EU); Approv.*, approved outside of the EU; BET, bromodomain and extraterminal protein; ClinTest, clinically tested; FAP, fibroblast activation protein; FLS, fibroblast-like synoviocytes; MMP, matrix metalloproteinase; MSC, mesenchymal stem cell; PRG4, proteoglycan 4; RA, rheumatoid arthritis; Spec., specific targeting molecule; TGF-β1, transforming growth factor β1.
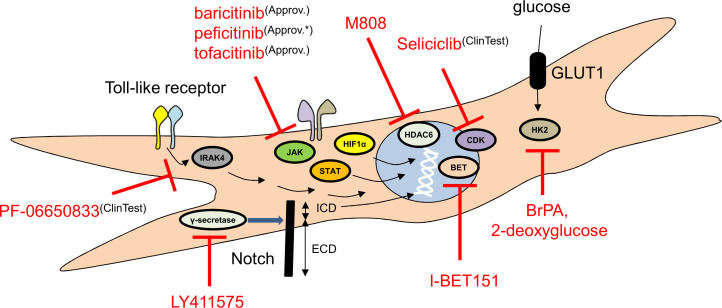
Figure 2.
Potential intracellular molecular targets of RA-FLS. There are several attractive intracellular molecules that can be blocked in RA-FLS: inhibitors of proteins participating in epigenetic changes, in signal transduction or in proliferation may have beneficial effects in RA. Approv., approved in the European Union (EU); Approv.*, approved outside of the EU; BET, bromodomain and extraterminal protein; BrPA, 3-bromopyruvate; ClinTest, clinically tested; FLS, fibroblast-like synoviocytes; ICD, intracellular domain; RA, rheumatoid arthritis.
Table 1.
Drugs and interventions in the pipeline aiming to control FLS in RA
| Target/aim | Drug/intervention name | Company | Mouse (M) or human (H) data (clinical study phase) | Comments | Reference(s) |
| Notch3 | Anti-Notch3 antibody | – | M | An anti-Notch3 antibody decreased inflammation in a mouse arthritis model. | 10 |
| SSAT-1 | Diminazene aceturate | Sigma-Aldrich | H | The use of the SSAT-1 inhibitor diminazene aceturate decreased the expression of ß1 integrin, CXCL12 (stromal cell-derived factor 1, SDF-1) and MMP1 of RA-FLS; however, the reduction was more pronounced when the inhibitor was used with S-adenosyl methionine supplementation. | 21 |
| HDAC6 | M808 | ? | M | M808 could decrease the MMP and cytokine release of RA-FLS, while attenuating their migration. Meanwhile, M808 had a positive effect in the rodent adjuvant-induced arthritis model. | 23 |
| BET proteins | I-BET151 | GlaxoSmithKline | H | I-BET151 could significantly reduce the MMP1, MMP3, IL-6 and IL-8 production of TNFα-, IL-1ß- or Toll-like receptor agonist-activated synovial fibroblasts, while the proliferation rate was also affected. | 25 |
| Hexokinase 2 |
|
Sigma-Aldrich | M, H |
|
27 28 |
| Notch | LY411575 | Selleckchem | M | LY411575, an inhibitor of Notch cleavage, could effectively attenuate the inflammation, the cartilage and bone destruction in a rodent model of RA. | 36 |
| Cyclin-dependent kinases (eg, CDK2, CDK7, CDK9) |
Seliciclib | Cyclacel Pharmaceuticals | H (phase 1b) |
It is tested in TNF-inhibitor-refractory patients with RA (TRAFIC trial). | 38 |
| PDGFR, PI3K or GSK-3 |
PDGFR, PI3K or GSK-3 inhibitors |
Merck Millipore, ChemScene, Active Biochem, Sigma-Aldrich | M | These inhibitors were shown to decrease the invasive characteristics of RA-FLS under in vitro circumstances. | 47 |
| IRAK4 | PF-06650833 | Pfizer | M, H (phase 2) |
|
55 |
| FAPα | Depletion of FAPα-positive FLS by targeted photodynamic therapy | ? | M | The conjugated antibody-treated animals showed a decreased inflammation score in the collagen-induced arthritis model. | 59 |
| Syndecan-4 | Anti-syndecan-4 antibody | – | M | The antibody directed against the dimerisation domain of syndecan-4 could effectively decrease the expression of IL-1 receptor on FLS and reduced the pannus formation, the cartilage destruction and the MMP3 content of the affected joints in the TNF transgenic mouse arthritis model. | 81 |
| RA-FLS signalling and function | Mesenchymal stem cell therapy | Different sources | M, H (phase 1 and 2) |
There are several ongoing clinical trials (see ClinicalTrials.gov identifiers NCT03618784, NCT03333681 or NCT03691909). | 82 83 |
BET, bromodomain and extraterminal protein; BrPA, 3-bromopyruvate; FAPα, fibroblast activation protein-α; FLS, fibroblast-like synoviocytes; GSK-3, glycogen synthase kinase 3; HDAC6, histone deacetylase 6; IL-1, interleukin 1; MMP, matrix metalloproteinase; PDGFR, platelet-derived growth factor receptor; PI3K, phosphoinositide 3-kinase.; RA, rheumatoid arthritis; SSAT-1, spermidine/spermine-N 1-acetyltransferase; TGF-β1, transforming growth factor β1; TNF, tumour necrosis factor.
Targeting the generation and differentiation of FLS
The exact origin and pathways of transformation of RA-FLS is still a matter of debate, but understanding their origin is crucial for therapeutic strategies to interfere with their specific phenotypes. While it has been believed that RA-FLS are generated in the inflamed synovium through transformation or differentiation of existing synovial fibroblasts, recent interest has expanded towards the role of mesenchymal progenitors as well as potential transdifferentiation events.
There is recent evidence that the adult synovium harbours unique joint morphogenic cells that expand in response to injury and may give rise to FLS. It has been shown through lineage tracing experiments that Gdf5-lineage cells persist as mesenchymal stem cells (MSCs) in the adult synovium and that FLS can be differentiated from these Gdf5-positive interzone MSCs.11 Based on the observation that these FLS are negative for nestin, leptin receptor and gremlin-1, it has further been hypothesised that Gdf5 lineage cells in the adult synovium differ and are distinct from skeletal stem cells found in other locations such as in the bone marrow. Moreover, joint surface injury by medial parapatellar arthrotomy led to a proliferative response in these Gdf5 lineage cells that clearly contributed to synovial hyperplasia. Most interestingly, conditional knockout of YAP1 in Gdf5-lineage cells prevented synovial hyperplasia after cartilage injury pointing to one targetable pathway involved in the specific response of these cells.11
These findings are of high interest for RA and related conditions because it has been hypothesised that cartilage damage is an early and central triggering factor for the recruitment and activation of RA-FLS.12 13 Consequently, it may be speculated that during RA, quiescent Gdf5 lineage cells in response to inflammatory cartilage damage along with concomitant immune activation re-enter the cell cycle and differentiate into specific RA-FLS populations. Pathways relevant for initiating this differentiation, such as the YAP pathway along with a more precise description of these progenitors in the future may, therefore, serve as a basis for therapeutically reducing the number of RA-FLS in the diseased synovium. In addition, platelet-derived growth factor receptor α-expressing Gdf5 lineage cells were found to expand during experimental arthritis and the deletion of YAP from these cells reduced arthritis severity.14 However, it is important to emphasise that the role of these Gdf5 lineage cells in inflammatory arthritis needs further investigations.
The differentiation of FLS can be mediated by cells of the vessel wall through a JAG1/DLL4-Notch3 interaction.10 In a recent paper, Notch3-positive and Notch-activated FLS have been found to be upregulated in the RA synovium compared with osteoarthritic (OA) tissues and Notch3 was identified as a central element of the differentiation of CD90-positive perivascular and sublining FLS.10 It was shown that in the synovium, the Notch ligands JAG1 and DLL4 had higher expression in the arterial than in the venous endothelial cells and Notch3 mRNA/protein levels were elevated in mural cells (like pericytes and vascular smooth muscle cells) and sublining FLS.10 These data may indicate that the positional identity of CD90-positive sublining FLS is orchestrated by arterial endothelial cells through a JAG1/DLL4-Notch3 interaction.10 Consistent with this, elevated levels of Notch3 intracellular domain (Notch3-ICD) were detected in JAG1- or DLL4-activated FLS.10 While Notch3-deficient mice exhibited normal joint structure, those animals showed a significantly reduced arthritis severity compared with wild type ones.10 Moreover, the pharmacological intervention by a Notch3-targeting antibody was able to attenuate the extent of joint inflammation and the appearance of bone erosions pointing to the effect of FLS on osteoclast activation.10
However, gene regulation and signalling behind the differentiation of synovial fibroblasts remain mainly undiscovered in contrast to the fact that it could be a robust and effective area, where FLS may be targeted to block the development of an aggressive and destructive phenotype in RA. This is highlighted by the finding that FLS can account for a significant percentage of RA heritability, and the function of some putative causal genes may be modified in the future.15
Controlling the aggressively imprinted phenotype
Under non-infectious inflammation, RA-FLS undergo phenotypical changes and acquire an aggressive phenotype. Their destructive behaviour (which affects cartilage and bone structures) has been suggested to be due at least partly to epigenetic and metabolic changes. The consecutive higher proliferation rate and invasive migratory characteristics make them important effectors and ideal cellular targets in RA.
Epigenetic changes of FLS in the focus of intervention
Epigenetic changes result in the modification of gene expression without the alteration of the original DNA base sequence. There are several mechanisms of epigenetics like DNA methylation, histone modifications (eg, acetylation, ubiquitination, phosphorylation or methylation) or the transcription of non-coding RNA molecules.16 In RA-FLS, DNA hypomethylation and subsequently increased gene expression could be observed with pathways mediating cell migration or extracellular matrix interactions pointing to a possible role of these changes in the pathogenesis of RA.17 In line with this, it has been found that RA-FLS have decreased levels of DNA methyltransferase 1 (Dnmt1) compared with OA synovial fibroblasts, which correlated with the hypomethylation of DNA in RA-FLS.18 Polyamines are important regulators of cell growth and their metabolism is largely affected by the activity of spermidine/spermine-N 1-acetyltransferase (SSAT).19 It has been shown that the expression of SSAT-1 in RA-FLS was higher than in OA synovial fibroblasts and has been proposed that the elevated polyamine metabolism could be linked to a decrease in the level of the methyl donor S-adenosyl methionine, thus contributing to DNA hypomethylation in RA-FLS.20 The use of the SSAT-1 inhibitor diminazene aceturate decreased the expression of ß1 integrin, CXCL12 (stromal cell-derived factor 1, SDF-1) and MMP1 of RA-FLS; however, the reduction was more pronounced when the inhibitor was used with S-adenosyl methionine supplementation.21 Moreover, diminazene aceturate, S-adenosyl methionine or their combination could effectively decrease the invasion of FLS to the cartilage.21 On the other hand, the inhibition of Dnmt1 by 5-azacytidine resulted in an RA-FLS-like phenotype of normal synovial fibroblasts, including elevated expression of cytokines, growth factors or MMPs.18 However, both the hypothesis of a general hypomethylation and the question of how important DNA hypomethylation is in mediating the aggressive phenotype of FLS are still being discussed controversially.
Histone modifications are crucial molecular events of epigenetic changes, where acetylation is mediated by histone acetyltransferases (HATs), which make the histone-DNA interaction less tight and the transcription of the affected genes more intense.22 On the other hand, the catalytic activity of histone deacetylases (HDACs) suppresses gene expression and an imbalance has been found between the expression and the activity of HATs and HDACs in RA with a HDAC predominance.22 M808, a specific inhibitor of HDAC 6 (HDAC6), could inhibit the MMP and the cytokine release of interleukin 1ß (IL-1ß)-activated RA FLS.23 HDAC6 inhibition also decreased the IL-1ß-induced migration of the cells, probably due to altering the reorganisation of F-actin.23 Moreover, in the murine adjuvant-induced arthritis model, M808 could dose-dependently decrease the macroscopic signs of joint inflammation, while attenuating the extent of cartilage damage and bone erosions.23 Bromodomain and extraterminal (BET) proteins recognise acetylated lysines in histones and transcription factors and regulate gene expression.24 BET proteins can recruit HATs but can also contain HAT domains influencing the chromatin structure and transcription.25 The BET family proteins BRD2, BRD3 and BRD4 have been shown to be upregulated in the RA synovium and the inhibitor of the molecules, I-BET151 could significantly reduce the MMP1, MMP3, IL-6 and IL-8 production of tumour necrosis factor α (TNFα)-, IL-1ß- or Toll-like receptor (TLR) agonist-activated FLS.25 Moreover, the proliferation rate of RA-FLS was also decreased in the presence of the inhibitor.25
Metabolic changes as therapeutic targets
RA-FLS have an increased rate of metabolism in general including accelerated glucose metabolism, features that are common in tumour cells and which most likely help to support the augmented energy demand of proliferative FLS.9 In line with this, in a recent metabolomic study, it was found that RA-FLS in terms of metabolism had a tumour cell-like phenotype with disturbances in glycolysis, glyconeogenesis or the pentose phosphate pathway, which resulted in a decrease in glycose and amino acid levels, while promoted the production of nucleotide precursors that are essential in proliferating cells.26 Garcia-Carbonell and his colleagues found that the mRNA level of the main glycose transporter on synovial fibroblasts, glucose transporter 1 (GLUT1) and the expression of hexokinase 2 (HK2) was upregulated in RA-FLS compared with OA-FLS, and the former was correlated with a higher baseline MMP3 production.27 The authors also detected that the attenuation of glycolysis by 3-bromopyruvate (BrPA), a HK2 inhibitor resulted in a reduced PDGF-induced RA-FLS migration, lipopolysaccharide-triggered IL-6 production and MMP3 production, while the in vivo administration of BrPA led to the attenuation of arthritis severity in a mouse model of RA, similarly when given at the time of induction or later when the joint inflammation already developed.27 Moreover, the administration of 2-deoxyglucose, another inhibitor of HK2 could decrease the cytokine and MMP release and production of TNF-stimulated healthy and non-activated RA-FLS.28 Interestingly, treatment with the JAK (Janus kinase) inhibitor tofacitinib decreased the extent of glycolysis partially by downregulating the expression of GLUT1 and HK2 in the RA synovium.29 Interestingly, primed synovial fibroblasts seem to require the complement factor 3 and its receptor to mediate metabolic changes in response to repeated inflammatory stimuli.30 These results point towards the target potential of the molecules that are involved in the metabolic changes of FLS in RA.
Modulating hypoxia-induced factors
Hypoxia is well established in the RA synovium, and it was observed that both macroscopic and microscopic inflammation showed a correlation with the severity of hypoxia, which raised the possibility that reduced oxygen tension may alter the inflammatory process.31 In line with these findings, FLS migration was enhanced at lower pO2 levels and hypoxia induced the production of the chemotactic and angiogenic cytokine CXCL12 and vascular endothelial growth factor (VEGF) by FLS.31 32 Under hypoxic conditions, the transcription modulator hypoxia-inducible factor 1α (HIF-1α) escapes the effects of hydroxylation of a proline residue by prolyl hydroxylases (which normally leads to the ubiquitination and degradation of the molecule), makes a heterodimer with the constitutively expressed HIF-1β and translocates to the nucleus, where it binds to the DNA.33 On binding, HIF-1α promotes the production of different molecules from the epigenetic modificating histone demethylases to VEGF, p53, Notch or MMPs, while it can also interact with non-coding RNA molecules, as well.33 34 Moreover, HIF-1α has influences on the cytokine production of RA synovial fibroblasts, for example, the expression of IL-6 and IL-8 was decreased in HIF-1α small interfering RNA- (siRNA-)treated RA-FLS, while the overexpression of HIF-1α resulted in the enhancement of cytokine production.35 The modulation of HIF-1α expression also had effects on RA-FLS-mediated Th1 and Th17 responses, as well as on B-cell activation and antibody production.35 In a recent study, both Notch1 and Notch3 ICD were found to be overexpressed in the RA synovium compared with OA samples and under hypoxia, HIF-1α could bind to the Notch1 and Notch3 promoter sites, suggesting that under hypoxic conditions, HIF-1α could regulate the expression of Notch1 and Notch3.36 The authors found that Notch1 and Notch3 augmented the invasion and Notch1 promoted the migration of RA-FLS under hypoxia, while Notch3 had antiapoptotic effect at lower oxygen tensions.36 In line with these findings, LY411575, an inhibitor of the cleavage of Notch ICD, substantially suppressed the inflammatory and structural signs of collagen-induced arthritis in rats.36
Blocking proliferation and induction of apoptosis
RA-FLS show a strong tendency to proliferate and in the affected RA synovium, some populations of FLS have been shown to expand.10 37 Seliciclib (R-roscovitine), a cyclin-dependent kinase inhibitor blocking cell proliferation and partially acting on FLS has been found to be safe in a phase 1b clinical trial in TNF-inhibitor-refractory RA patients, giving way to further studies.38 In line with the importance of JAK-STAT signalling in arthritic FLS functions (see below), the pan-JAK inhibitor peficitinib has been shown to modulate synovial fibroblast proliferation in a concentration close to the in vivo well-tolerated values.39
There are several ways to induce apoptosis in FLS. MicroRNAs (miRNAs) are small, non-coding gene silencing RNA molecules. A regulatory molecule, miR-140-5 p had decreased expression in the rheumatoid synovium in contrast to healthy synovial tissue and RA-FLS had a lower miR-140-5 p level compared with healthy FLS.40 The overexpression of miR-140-5 p in RA-FLS decreased survival and proliferation, while downregulating proinflammatory cytokine production, which all seemed to be dependent on the effect of miR-140-5 p on the expression of signal transducer and activator of transcription 3 (STAT3).40 Similarly, the level of miR-431-5 p was lower in the RA synovium and in RA-FLS, and while the overexpression of mIR-431-5 p induced apoptosis, its inhibition resulted in a prolonged survival.41 Interestingly, treatment with iguratimod, a novel antirheumatic agent approved in Japan and China, increased the level of another miRNA, miRNA-146a and induced apoptosis in the arthritic synovium, which could be reversed by miR-146a inhibition in the collagen-induced arthritis model.42 Moreover, Fas expression was found to be elevated in FLS and apoptosis could be triggered by an anti-Fas antibody.43 Furthermore, JAK inhibitors like tofacitinib or peficitinib effectively promoted RA-FLS apoptosis.44 In a recent article, methotrexate and theaflavin-3-3′-digallate decreased the production of proinflammatory and angiogenic markers, while the combination of the two drugs led to the restoration of the balance between autophagy and apoptosis in RA-FLS.45
These results indicate that blocking the proliferation and/or the apoptosis of synovial fibroblasts could help to control autoimmune joint inflammation.
Interference with migration
RA-FLS have aggressive migratory characteristics that contribute to bone and cartilage destruction in RA. In an RA-FLS-cartilage implantation model, where the sponge-cartilage was implanted with fibroblasts to SCID (severe combined immunodeficient) mice subcutaneously and a cell-free cartilage-sponge complex contralaterally, the cartilage destruction could not only be observed in the coimplantation (ipsilateral) site but also on the synovial fibroblast-free contralateral area, indicating that the RA-FLS were able to migrate actively from one site to the other.46 The phoyphorylation rate of the receptor tyrosine kinase PDGFR has been observed to be elevated in the rheumatoid synovium and PDGFR inhibitors were found to dose-dependently inhibit the in vitro migration of human FLS.47 Moreover, the inhibition of PI3Kδ (by INK007) could decrease PDGF-induced migration of RA-FLS (both when investigated in the wound-healing assay or in the Boyden chamber), while invasion of the cells was also affected by the presence of the PI3K inhibitors INK007 and CAL-101.48 Paclitaxel, a known anticancer drug was shown to inhibit the in vitro migration of RA-FLS and could reduce the severity of joint inflammation in mice in the collagen-induced arthritis model.49 However, the paclitaxel-induced decrease of the production of inflammatory mediators by RA-FLS can also contribute to the in vivo phenotype, while the inhibitor itself has a considerable toxicity, which requires further investigation.49 Meanwhile, there are more and more data on the altered metabolism of RA-FLS (see above) and its effects on the aggressive behaviour of these cells, which is nicely highlighted with the massive inhibitory effect of glycogen synthase kinase 3 (GSK-3) inhibitors on the migratory capacity of RA-FLS.47 In a recent paper, Orange and colleagues found elevated levels of a special mesenchymal cell type with shared features of inflammatory synovial fibroblasts, namely the CD45/CD31/Podoplanin triple positive preinflammatory mesenchymal (PRIME) cells in the blood by transcriptional analysis just before RA flares (and after B-cell activation in the circulation).50 These PRIME cells have been proposed to migrate to the affected joints and participate in the initiation of the inflammatory process.50 The potential blockade of these cells may serve as a beneficial target option in the prevention of RA flares.
Focusing on signal transduction
Targeting signal transduction pathways is an emerging therapeutic strategy in RA. Type I and II cytokine receptors (eg, the IL-6, the granulocyte-macrophage colony-stimulating factor (GM-CSF) or the interferon-γ (IFN-γ) receptors) signal through the tyrosine kinase JAK and the transcription factor STAT (JAK-STAT pathway). JAK1 is a central molecule in autoimmune arthritis, and it seems that inhibitors of JAK1 partially exert their effects by modulating synovial fibroblast function.51 Tofacitinib, a JAK inhibitor mainly targeting JAK1 and JAK3, was found to attenuate the production of IL-6 and monocyte chemoattractant protein-1 in oncostatin M-treated RA-FLS.29 Moreover, tofacitinib reduced the level of IL-1β, IL-6, IL-8 and soluble intercellular adhesion molecule (sICAM) in ex vivo synovial explants.29 JAK2 has been shown to participate in the IFN-γ-induced migration of FLS, and baricitinib was able to decrease the invasion of these cells.52 Tofacitinib has been also shown to influence the invasion of RA-FLS and the outgrowth of RA-FLS from RA explants in Matrigel.29 However, JAK inhibitors are not selective for FLS and have immunosuppressive effects in patients with RA due to the inhibition of different immune cell functions.
FLS express several types of the pattern recognition receptor TLRs, some of which signal through IRAK4.53 54 The inhibition of IRAK4 by a specific molecule called PF-06650833 reduced the cytokine and MMP release of human RA-FLS when stimulated by TLR1/2, TLR4 or TLR5 agonists under in vitro conditions.55 Moreover, the in vivo administration of PF-06650833 could attenuate the severity of collagen-induced arthritis in rats.55 Based on the effectiveness of the molecule in preclinical models, PF-06650833 was and is being tested in phase 2 trials in patients with RA (ClinicalTrials.gov Identifiers: NCT02996500 and NCT04413617).
FLS express different types of heterodimer integrins (like β1 and β3 integrins) on their cell surface that mainly mediate cell-extracellular matrix (eg, FLS-collagen, FLS-laminin and FLS-fibronectin) interactions in RA.56 β1 integrins were shown to signal through the Syk tyrosine kinase in airway epithelial cells that raises the possibility of a similar signalling machinery in FLS.57 However, while the administration of R406, a so-called Syk (but rather a non-selective tyrosine kinase) inhibitor had effects on RA-FLS activity, in the absence of data regarding the impact of a specific Syk inhibitor, the role of Syk in RA-FLS is still largely unknown.58
A real-time cell analysis system mainly based on a wound-healing assay and intracellular phosphorylation of human FLS pointed towards the effectiveness of the inhibitors of PDGFR, Akt, PI3K or GSK-3 in controlling the invasive capacity of RA-FLS.47 However, more detailed studies are needed to test these inhibitors in the control of experimental and human autoimmune arthritis.
Attempts to deplete synovial fibroblasts
The depletion of FLS would be an ideal selective therapy in RA without immunosuppressive consequences. In RA, the synovial hyperplasia does not affect the different layers equally: the sublining area expands much more than the lining layer, which suggests that different types of FLS exist. Fibroblast activation protein-α (FAPα), a cell surface marker of activated stromal fibroblasts was upregulated in the RA synovium, suggesting that it may be linked to an inflammatory behaviour.6 Similar density augmentation could be observed in a murine model of arthritis, where the deletion of FAPα reduced the severity of joint inflammation in both the acute and the chronic arthritis forms and attenuated the development of bone erosions, cartilage destruction and immune cell infiltration.6 Within the FAPα-positive group, arthritic FLS seemed to form various subpopulations with dedicated functions: while lining layer-resident FAPα single positive cells were found to be essential in mediating cartilage and bone destruction, FAPα/CD90 double-positive FLS recruited immune cells to the inflamed joints.6 In line with these findings, arthritic synovium-derived CD90-negative fibroblasts expressed higher amounts of RANKL and MMPs, while cytokine and chemokine production was much more robust in the CD90-expressing cells.6 Intra-articular injection of CD90-positive FLS resulted in a significant increase of ankle thickness and recruitment of leukocytes like neutrophils to the synovial area.6 Another group developed a new therapeutic strategy by the selective ablation of FAPα-positive cells by targeted photodynamic therapy, where an anti-FAPα antibody was conjugated to a photosensitiser.59 The authors found that the conjugated antibody-treated animals showed a decreased inflammation score in the collagen-induced arthritis model, further highlighting the importance of FAPα in the pathogenesis of autoimmune joint disease and providing a selective pharmacological method to block the proinflammatory and tissue destructive functions of FLS by depleting them.59
However, the picture seems to be even more complex as in another work, Mizoguchi and his colleagues identified seven different synovial fibroblast groups in the RA synovium by bulk and single-cell transcriptomics.37 They found that CD90, podoplanin and cadherin-11 triple-positive FLS — which localise in the perivascular zone, secrete cytokines and show a proliferative and invasive potential — are upregulated in the joints of patients with RA, which may lead to the development and use of bispecific antibodies against this harmful group of cells.37
Blocking cellular and cell-matrix interactions of synovial fibroblasts
Interference with endothelial cells in the focus
E-selectin is an adhesion molecule expressed by activated endothelial cells that mediate cellular interactions mainly with leukocytes.60 In a recent study, the authors showed that E-selectin could mediate the adhesion and rolling of FLS under flow conditions and RA-FLS had increased binding capacity towards TNFα-stimulated endothelial cells.61 It was also detected that the E-selectin ligand CD15s was expressed in RA-FLS in the human synovium, while E-selectin and P-selectin-deficient mice showed a decreased invasion score in the contralateral site in the cartilage implantation model pointing at the importance of selectins in mediating synovial fibroblast migration in the circulation.61 Interestingly, different adipokines (like visfatin or resistin) were found to be able to increase the adherence of RA-FLS to endothelial cells under flow conditions, which could be attenuated by the application of corticosteroids.60 These data raise the possibility to target molecules that participate in the adhesion and migration of FLS in RA by blocking endothelial cell-RA-FLS interactions.
Targeting interactions with leukocytes and platelets
Several articles have been published on how FLS communicate with recruited leukocytes — especially with lymphocytes or macrophages — in the synovial area. The levels of the Th1 cell-recruiting CXCR3 ligands (CXCL9, CXCL10 and CXCL11) were found to be elevated in the synovial fluid of patients with RA, and inflammatory cytokines like TNFα or IFN-γ were shown to stimulate the expression and production of these chemokines from RA synovial fibroblasts, pointing at an important interaction between the two cell types.62 Furthermore, the fractalkine receptor CX3CR1 was found to be upregulated on peripheral T cells of patients with RA and the synovium contained significant amount of CX3CR1-positive T cells, while fractalkine is thought to be produced by RA-FLS and endothelial cells.63 However, the treatment with a monoclonal anti-fractalkine antibody (E6011) did not show efficacy in a phase 2 clinical trial in patients with RA.64 FLS can also upregulate major histocompatibility complex class II molecules on their cell surface when stimulated by Th1 or Th17 cells and are thought to be able to present antigens to T cells.65 FLS can interact with B cells in the rheumatoid synovium: they may promote maturation, differentiation or antibody class-switching through the production of IL-6 or BAFF/APRIL.66 67
One important source of IL-6 in RA is the FLS and the production of IL-6 is greatly enhanced by TNFα that is mainly derived from macrophages.68 On the other hand, FLS produce macrophage colony-stimulating factor and GM-CSF, which are important in the local expansion of macrophages.69 In a recent study, the inflammatory heparin binding EGF-like growth factor- (HBEGF-)positive macrophages were found to be shaped by FLS and in turn, these macrophages were able to promote the invasive characteristics of synovial fibroblasts, while several currently used drugs could reduce the HBEGF-positive macrophage cluster with a potential alteration of the macrophage-FLS axis.70 In addition to communicating with macrophages, RA-FLS also contribute to osteoclastogenesis as synovial fibroblast-specific receptor activator of nuclear factor κB ligand (RANKL) expression was found to mediate bone erosions through promoting osteoclast differentiation under experimental conditions.71 Here, using the Cre-Lox system, the authors showed that the deletion of the RANKL gene from FLS and chondrocytes did not influence the arthritis severity in the collagen antibody-induced arthritis model, but decreased the number of osteoclasts and bone erosions in contrast to animals with chondrocyte- and T-cell specific deletion of the molecule, suggesting that bone destruction can be independent of the macroscopic inflammatory phenotype and targeting synovial fibroblast functions can result in the preservation of the original bone structure.71
Podoplanin (gp38) has been shown to be upregulated in the RA synovium, especially in cases with lymphoid neogenesis or seropositivity, and was sensitive to anti-TNF therapy.72 Podoplanin on FLS was further analysed in fibroblast-platelet cocultures, where its binding to CLEC2 was associated with increased production of IL-6 and IL-8.72 It is an interesting finding that podoplanin-positive FLS express the transcription factor autoimmune regulator (Aire) in a cytokine-dependent manner, which seems to mediate the enhanced production of various proinflammatory cytokines and an IFN-γ signature after TNFα- and/or IL-1ß stimulation, without mediating the transcription of tissue restricted antigens, further highlighting the immune reaction-orchestrating role of RA-FLS.73
Targeting other cell-cell and cell-matrix interactions of FLS
FLS express several molecules that mediate their adhesion to other cells, to themselves or the extracellular matrix. Identifying important unique molecules in these processes could lead to the selective targeting of FLS. Cadherin-11, one of the major adhesion molecules of synovial fibroblasts, was found to be essential in regulating synovial architecture as its absence resulted in a hypoplastic synovium with the loss of an evident lining layer, meanwhile cadherin-11-deleted mice showed a decreased arthritis severity compared with the wild-type controls in an experimental mouse model.4 Cadherin-11 was also found to be an important molecule in mediating the invasiveness of FLS and participating in the development of cartilage erosions in vivo.4 In line with its proposed role in RA, cadherin-11 mRNA transcripts were detected in the majority of patients with RA in comparison with control subjects and cadherin-11-positivity correlated with polyarthritis.74 In accordance with the above findings, treatment with a cadherin-11-Fc fusion protein or an anti-cadherin-11 antibody resulted in a decreased arthritis severity in a mouse model of autoimmune joint inflammation.4 However, the treatment with a humanized monoclonal antibody against cadherin-11 (RG6125) failed to show efficacy in patients with RA inadequately responding to anti-TNFα therapy in a phase 2 clinical trial and led to the withdrawal of this synovial fibroblast-selective intervention from the list of potential future therapies of RA.75 Meanwhile, in a recent article, the LIM and SH3 domain protein 1 (Lasp1) was found to be upregulated in the RA synovium and was described as an important mediator of the cell responses (eg, migration) of arthritic FLS.5 Further studies revealed that the loss of Lasp1 resulted in abrogated cadherin-11-containing cell-cell contacts and decreased the severity of experimental arthritis in mice, pointing at its target potential in the therapy of RA.5
As mentioned before, integrins are heterodimer adhesion molecules, which are crucial in several processes from embryogenesis to immune functions. It has been shown that the α9ß1 integrin had higher levels in the rheumatoid synovium compared with the synovial tissue of patients with OA and stimulation of RA-FLS with tenascin-C, an α9ß1 ligand resulted in elevated MMP and IL-6 expression, which could be downregulated in the presence of an anti-α9 monoclonal antibody.76 Moreover, anti-α9 treatment could decrease arthritis severity in the collagen-induced arthritis model without a major influence on systemic immunomodulation.77 78 Interestingly, ASP5094, a humanized monoclonal antibody against integrin α9, did not show efficacy in patients with RA with a therapeutic refractory to methotrexate.79
Syndecan-4 is a cell surface molecule that can interact with extracellular matrix components and can mediate various cell responses in synovial fibroblasts. In a recently published paper, the authors found that syndecan-4 was an essential regulator molecule in the pathogenesis of RA and was important in mediating nitric oxide, reactive oxygen species, IL-1ß, IL-6 and TNFα production in RA-FLS, while contributing to the resistance to apoptosis.80 In line with these findings, an antibody directed against the dimerisation domain of syndecan-4 could effectively decrease the expression of the IL-1 receptor on FLS and reduced the pannus formation, the cartilage destruction and the MMP3 content of the affected joints in the TNF transgenic mouse arthritis model.81
Mesenchymal stem cells as therapeutic mediators
MSC-based therapeutic approaches have gained significant attention in the past decade for the control of autoimmune arthritis. The administration of bone marrow-derived MSCs has been shown to ameliorate the signs of collagen-induced arthritis, to decrease histological scores, the levels of proinflammatory cytokines and to upregulate anti-inflammatory molecules like IL-10 or transforming growth factor β1 (TGF-ß1).82 Mechanistic studies revealed that nuclear factor kappa B (NF-κB) activity was downregulated in synovial fibroblasts, perhaps due to the lowered miR-548e levels provoked by TGFß1.82 In line with these findings, the cotransplantation of MSCs and miR-548e viruses to arthritic mice abolished the effect of MSC transplantation alone, pointing at a possible scenario, where MSCs exert their therapeutic effect by increasing TGFß1 levels and attenuating NF-κB signalling by decreasing miR-548e expression in FLS.82 In another study, umbilical cord-derived MSCs were found to decrease the cadherin-11 upregulation of RA-FLS (and of FLS of the synovium of collagen-induced arthritis in rats), mainly through the production of the anti-inflammatory cytokine IL-10.83 The promising results with the administration of MSCs in autoimmune joint inflammation led to the launch of several clinical trials in RA, for instance the effect of intravenous administration of allogeneic umbilical cord-derived MSCs is investigated in a phase 1/2 trial (NCT03618784; for further studies, visit ClinicalTrials.gov (identifiers NCT03333681 or NCT03691909).
Discussion and concluding remarks
FLS are crucial cellular elements in RA and are promising targets of arthritis therapy in the future. There are many ways where these mesenchymal cells could be influenced: their origin and differentiation, their epigenetics and metabolism, their proliferation and apoptosis, their signal transduction and their interactions with other cells (eg, with immune cells) or the extracellular matrix can be targeted resulting in the decrease of different cell responses like migration and invasive tendencies or the production of MMPs.
The specificity of RA synovial fibroblast therapy can be a real challenge as altering the fate or functions of regular fibroblasts at other sites may cause side effects (eg, reduced wound healing). Buechler and colleagues—based on the development of fibroblast atlases by integrating single-cell transcriptomic data—showed that there are only two universal fibroblast transcriptional subtypes in mice, which serve as sources for different fibroblast variants in various organs and diseases, with high similarity to human fibroblasts.84 In addition, in a recent study, the expansion of two clusters of FLS (a CXCL10+CCL19+ ‘immune interacting’ and a SPARC+COL3A1+ ‘vascular interacting’) were identified in patients with inflamed tissues of RA, inflammatory bowel disease, interstitial lung disease and Sjögren’s syndrome by single-cell RNA sequencing (Korsunsky et al, bioRxiv, 2021). However, several FLS subsets have been described in the last years (eg, by integrating single cell transcriptomics and mass spectometry) and the recognition of this heterogeneity may support the development of novel therapies (perhaps by aiming selective depletion, etc.), which could significantly decrease the off-target effects of FLS-based treatments.85 In a recently published paper, the different FLS subsets have been associated with distinct clinical and laboratory alterations, raising the possibility of FLS-based personalized treatments in the future.86 So far, however, there are no well-working FLS-specific therapies in RA and several of the above-mentioned therapeutic approaches can modify the function of other cell types, as well.
The development and successful use of drugs acting on RA synovial fibroblasts, which can decrease the imprinted aggressive behaviour, can potentiate the use of novel treatments in other fibroblast-mediated diseases like systemic autoimmune disease-associated pulmonary fibrosis or in skin and internal organ involvement in systemic sclerosis. Meanwhile, the mesenchymal cell origin could be the basis of the development of promising non-immunosuppressive future therapies in RA, which is not a negligible factor in these years of a global pandemic.
Footnotes
Handling editor: Josef S Smolen
Contributors: TN, GN and TP wrote the manuscript together.
Funding: This work was funded and supported by the Hungarian National Research, Development and Innovation Office (No. FK 132251 to TN, No. K 131479 to GN and No. TKP2021-EGA-29 to TN and GN), the European Union’s H2020 IMI2 program (RTCure project; No. 777357), the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (to TN) and the New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund (No. UNKP 20-5-SE-4 and UNKP-21-5-SE-2 to TN).
Competing interests: TP is an associate editor of Annals of the Rheumatic Diseases.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: We apologise for not including further studies in our review due to the focus of our paper and to space limitations.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Not applicable.
References
- 1. Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers 2018;4:18001. 10.1038/nrdp.2018.1 [DOI] [PubMed] [Google Scholar]
- 2. Nagy G, Roodenrijs NMT, Welsing PMJ, et al. EULAR points to consider for the management of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis 2022;81:20–33. 10.1136/annrheumdis-2021-220973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pap T, Dankbar B, Wehmeyer C, et al. Synovial fibroblasts and articular tissue remodelling: role and mechanisms. Semin Cell Dev Biol 2020;101:140–5. 10.1016/j.semcdb.2019.12.006 [DOI] [PubMed] [Google Scholar]
- 4. Lee DM, Kiener HP, Agarwal SK, et al. Cadherin-11 in synovial lining formation and pathology in arthritis. Science 2007;315:1006–10. 10.1126/science.1137306 [DOI] [PubMed] [Google Scholar]
- 5. Beckmann D, Römer-Hillmann A, Krause A, et al. Lasp1 regulates adherens junction dynamics and fibroblast transformation in destructive arthritis. Nat Commun 2021;12:3624. 10.1038/s41467-021-23706-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Croft AP, Campos J, Jansen K, et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 2019;570:246–51. 10.1038/s41586-019-1263-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lochhead RB, Ordoñez D, Arvikar SL, et al. Interferon-Gamma production in Lyme arthritis synovial tissue promotes differentiation of fibroblast-like synoviocytes into immune effector cells. Cell Microbiol 2019;21:e12992. 10.1111/cmi.12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bartok B, Firestein GS. Fibroblast-Like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev 2010;233:233–55. 10.1111/j.0105-2896.2009.00859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mousavi MJ, Karami J, Aslani S, et al. Transformation of fibroblast-like synoviocytes in rheumatoid arthritis; from a Friend to foe. Auto Immun Highlights 2021;12:3. 10.1186/s13317-020-00145-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wei K, Korsunsky I, Marshall JL, et al. Notch signalling drives synovial fibroblast identity and arthritis pathology. Nature 2020;582:259–64. 10.1038/s41586-020-2222-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roelofs AJ, Zupan J, Riemen AHK, et al. Joint morphogenetic cells in the adult mammalian synovium. Nat Commun 2017;8:15040. 10.1038/ncomms15040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Korb-Pap A, Stratis A, Mühlenberg K, et al. Early structural changes in cartilage and bone are required for the attachment and invasion of inflamed synovial tissue during destructive inflammatory arthritis. Ann Rheum Dis 2012;71:1004–11. 10.1136/annrheumdis-2011-200386 [DOI] [PubMed] [Google Scholar]
- 13. Hillen J, Geyer C, Heitzmann M, et al. Structural cartilage damage attracts circulating rheumatoid arthritis synovial fibroblasts into affected joints. Arthritis Res Ther 2017;19:40. 10.1186/s13075-017-1245-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Symons RA, Colella F, Collins FL, et al. Targeting the IL-6-Yap-Snail signalling axis in synovial fibroblasts ameliorates inflammatory arthritis. Ann Rheum Dis 2022;81:214–24. 10.1136/annrheumdis-2021-220875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ge X, Frank-Bertoncelj M, Klein K, et al. Functional genomics atlas of synovial fibroblasts defining rheumatoid arthritis heritability. Genome Biol 2021;22:247. 10.1186/s13059-021-02460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mazzone R, Zwergel C, Artico M, et al. The emerging role of epigenetics in human autoimmune disorders. Clin Epigenetics 2019;11:34. 10.1186/s13148-019-0632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakano K, Whitaker JW, Boyle DL, et al. DNA methylome signature in rheumatoid arthritis. Ann Rheum Dis 2013;72:110–7. 10.1136/annrheumdis-2012-201526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karouzakis E, Gay RE, Michel BA, et al. DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 2009;60:3613–22. 10.1002/art.25018 [DOI] [PubMed] [Google Scholar]
- 19. Pegg AE. Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator. Am J Physiol Endocrinol Metab 2008;294:E995–1010. 10.1152/ajpendo.90217.2008 [DOI] [PubMed] [Google Scholar]
- 20. Karouzakis E, Gay RE, Gay S, et al. Increased recycling of polyamines is associated with global DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 2012;64:1809–17. 10.1002/art.34340 [DOI] [PubMed] [Google Scholar]
- 21. Neidhart M, Karouzakis E, Jüngel A, et al. Inhibition of spermidine/spermine N1-acetyltransferase activity: a new therapeutic concept in rheumatoid arthritis. Arthritis Rheumatol 2014;66:1723–33. 10.1002/art.38574 [DOI] [PubMed] [Google Scholar]
- 22. Karami J, Aslani S, Tahmasebi MN, et al. Epigenetics in rheumatoid arthritis; fibroblast-like synoviocytes as an emerging paradigm in the pathogenesis of the disease. Immunol Cell Biol 2020;98:171–86. 10.1111/imcb.12311 [DOI] [PubMed] [Google Scholar]
- 23. Park JK, Shon S, Yoo HJ, et al. Inhibition of histone deacetylase 6 suppresses inflammatory responses and invasiveness of fibroblast-like-synoviocytes in inflammatory arthritis. Arthritis Res Ther 2021;23:177. 10.1186/s13075-021-02561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morgado-Pascual JL, Rayego-Mateos S, Tejedor L, et al. Bromodomain and extraterminal proteins as novel epigenetic targets for renal diseases. Front Pharmacol 2019;10:1315. 10.3389/fphar.2019.01315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klein K, Kabala PA, Grabiec AM, et al. The bromodomain protein inhibitor I-BET151 suppresses expression of inflammatory genes and matrix degrading enzymes in rheumatoid arthritis synovial fibroblasts. Ann Rheum Dis 2016;75:422–9. 10.1136/annrheumdis-2014-205809 [DOI] [PubMed] [Google Scholar]
- 26. Ahn JK, Kim S, Hwang J, et al. GC/TOF-MS-based metabolomic profiling in cultured fibroblast-like synoviocytes from rheumatoid arthritis. Joint Bone Spine 2016;83:707–13. 10.1016/j.jbspin.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 27. Garcia-Carbonell R, Divakaruni AS, Lodi A, et al. Critical role of glucose metabolism in rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheumatol 2016;68:1614–26. 10.1002/art.39608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koedderitzsch K, Zezina E, Li L, et al. TNF induces glycolytic shift in fibroblast like synoviocytes via GLUT1 and HIF1A. Sci Rep 2021;11:19385. 10.1038/s41598-021-98651-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGarry T, Orr C, Wade S, et al. JAK/STAT blockade alters synovial bioenergetics, mitochondrial function, and proinflammatory mediators in rheumatoid arthritis. Arthritis Rheumatol 2018;70:1959–70. 10.1002/art.40569 [DOI] [PubMed] [Google Scholar]
- 30. Friščić J, Böttcher M, Reinwald C, et al. The complement system drives local inflammatory tissue priming by metabolic reprogramming of synovial fibroblasts. Immunity 2021;54:e10:1002–21. 10.1016/j.immuni.2021.03.003 [DOI] [PubMed] [Google Scholar]
- 31. Ng CT, Biniecka M, Kennedy A, et al. Synovial tissue hypoxia and inflammation in vivo. Ann Rheum Dis 2010;69:1389–95. 10.1136/ard.2009.119776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hitchon C, Wong K, Ma G, et al. Hypoxia-Induced production of stromal cell-derived factor 1 (CXCL12) and vascular endothelial growth factor by synovial fibroblasts. Arthritis Rheum 2002;46:2587–97. 10.1002/art.10520 [DOI] [PubMed] [Google Scholar]
- 33. Choudhry H, Harris AL. Advances in hypoxia-inducible factor biology. Cell Metab 2018;27:281–98. 10.1016/j.cmet.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 34. Liu W, Shen S-M, Zhao X-Y, et al. Targeted genes and interacting proteins of hypoxia inducible factor-1. Int J Biochem Mol Biol 2012;3:165–78. [PMC free article] [PubMed] [Google Scholar]
- 35. Hu F, Liu H, Xu L, et al. Hypoxia-Inducible factor-1α perpetuates synovial fibroblast interactions with T cells and B cells in rheumatoid arthritis. Eur J Immunol 2016;46:742–51. 10.1002/eji.201545784 [DOI] [PubMed] [Google Scholar]
- 36. Chen J, Cheng W, Li J. Notch-1 and Notch-3 mediates hypoxia-induced synovial fibroblasts activation in rheumatoid arthritis. Arthritis Rheumatol 2021. [DOI] [PubMed] [Google Scholar]
- 37. Mizoguchi F, Slowikowski K, Wei K, et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat Commun 2018;9:789. 10.1038/s41467-018-02892-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pratt AG, Siebert S, Cole M, et al. Targeting synovial fibroblast proliferation in rheumatoid arthritis (TRAFIC): an open-label, dose-finding, phase 1B trial. Lancet Rheumatol 2021;3:e337–46. 10.1016/S2665-9913(21)00061-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Diller M, Hasseli R, Hülser M-L, et al. Targeting activated synovial fibroblasts in rheumatoid arthritis by Peficitinib. Front Immunol 2019;10:541. 10.3389/fimmu.2019.00541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu J, Wang J, Huang J, et al. MicroRNA-140-5p regulates the proliferation, apoptosis and inflammation of RA FLSs by repressing STAT3. Exp Ther Med 2021;21:171. 10.3892/etm.2020.9602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y, Zhang K, Yuan X, et al. miR-431-5p regulates cell proliferation and apoptosis in fibroblast-like synoviocytes in rheumatoid arthritis by targeting XIAP. Arthritis Res Ther 2020;22:231. 10.1186/s13075-020-02328-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kong R, Gao J, Ji L, et al. Iguratimod ameliorates rheumatoid arthritis progression through regulating miR-146a mediated IRAK1 expression and TRAF6/JNK1 pathway: an in vivo and in vitro study. Clin Exp Rheumatol 2021;39:289–303. [PubMed] [Google Scholar]
- 43. Firestein GS, Yeo M, Zvaifler NJ. Apoptosis in rheumatoid arthritis synovium. J Clin Invest 1995;96:1631–8. 10.1172/JCI118202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Emori T, Kasahara M, Sugahara S, et al. Role of JAK-STAT signaling in the pathogenic behavior of fibroblast-like synoviocytes in rheumatoid arthritis: effect of the novel JAK inhibitor peficitinib. Eur J Pharmacol 2020;882:173238. 10.1016/j.ejphar.2020.173238 [DOI] [PubMed] [Google Scholar]
- 45. Misra S, Bagchi A, Sarkar A, et al. Methotrexate and theaflavin-3, 3'-digallate synergistically restore the balance between apoptosis and autophagy in synovial fibroblast of RA: an ex vivo approach with cultured human RA FLS. Inflammopharmacology 2021;29:1427-1442. 10.1007/s10787-021-00857-0 [DOI] [PubMed] [Google Scholar]
- 46. Lefèvre S, Knedla A, Tennie C, et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat Med 2009;15:1414–20. 10.1038/nm.2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sugiura T, Kamino H, Nariai Y, et al. Screening of a panel of low molecular weight compounds that inhibit synovial fibroblast invasion in rheumatoid arthritis. J Immunol 2020;205:3277–90. 10.4049/jimmunol.1901429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bartok B, Hammaker D, Firestein GS. Phosphoinositide 3-kinase δ regulates migration and invasion of synoviocytes in rheumatoid arthritis. J Immunol 2014;192:2063–70. 10.4049/jimmunol.1300950 [DOI] [PubMed] [Google Scholar]
- 49. Chen X, Lin H, Chen J, et al. Paclitaxel inhibits synoviocyte migration and inflammatory mediator production in rheumatoid arthritis. Front Pharmacol 2021;12:714566. 10.3389/fphar.2021.714566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Orange DE, Yao V, Sawicka K, et al. Rna identification of prime cells predicting rheumatoid arthritis flares. N Engl J Med 2020;383:218–28. 10.1056/NEJMoa2004114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Spinelli FR, Colbert RA, Gadina M. Jak1: number one in the family; number one in inflammation? Rheumatology 2021;60:ii3–10. 10.1093/rheumatology/keab024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karonitsch T, Beckmann D, Dalwigk K, et al. Targeted inhibition of Janus kinases Abates interfon gamma-induced invasive behaviour of fibroblast-like synoviocytes. Rheumatology 2018;57:572–7. 10.1093/rheumatology/kex426 [DOI] [PubMed] [Google Scholar]
- 53. Ospelt C, Brentano F, Rengel Y, et al. Overexpression of Toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: Toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum 2008;58:3684–92. 10.1002/art.24140 [DOI] [PubMed] [Google Scholar]
- 54. Kawasaki T, Kawai T. Toll-Like receptor signaling pathways. Front Immunol 2014;5:461. 10.3389/fimmu.2014.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Winkler A, Sun W, De S, et al. The interleukin-1 receptor-associated kinase 4 inhibitor PF-06650833 blocks inflammation in preclinical models of rheumatic disease and in humans enrolled in a randomized clinical trial. Arthritis Rheumatol 2021;73:2206-2218. 10.1002/art.41953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lowin T, Straub RH. Integrins and their ligands in rheumatoid arthritis. Arthritis Res Ther 2011;13:244. 10.1186/ar3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ulanova M, Marcet-Palacios M, Muñoz S, et al. Involvement of Syk kinase in TNF-induced nitric oxide production by airway epithelial cells. Biochem Biophys Res Commun 2006;351:431–7. 10.1016/j.bbrc.2006.10.073 [DOI] [PubMed] [Google Scholar]
- 58. Cha H-S, Boyle DL, Inoue T, et al. A novel spleen tyrosine kinase inhibitor blocks c-Jun N-terminal kinase-mediated gene expression in synoviocytes. J Pharmacol Exp Ther 2006;317:571–8. 10.1124/jpet.105.097436 [DOI] [PubMed] [Google Scholar]
- 59. Dorst DN, Rijpkema M, Boss M, et al. Targeted photodynamic therapy selectively kills activated fibroblasts in experimental arthritis. Rheumatology 2020;59:3952–60. 10.1093/rheumatology/keaa295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hasseli R, Frommer KW, Schwarz M, et al. Adipokines and inflammation alter the interaction between rheumatoid arthritis synovial fibroblasts and endothelial cells. Front Immunol 2020;11:925. 10.3389/fimmu.2020.00925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zimmermann-Geller B, Köppert S, Kesel N, et al. Interactions between rheumatoid arthritis synovial fibroblast migration and endothelial cells. Immunol Cell Biol 2019;97:178–89. 10.1111/imcb.12208 [DOI] [PubMed] [Google Scholar]
- 62. Ueno A, Yamamura M, Iwahashi M, et al. The production of CXCR3-agonistic chemokines by synovial fibroblasts from patients with rheumatoid arthritis. Rheumatol Int 2005;25:361–7. 10.1007/s00296-004-0449-x [DOI] [PubMed] [Google Scholar]
- 63. Nanki T, Imai T, Nagasaka K, et al. Migration of CX3CR1-positive T cells producing type 1 cytokines and cytotoxic molecules into the synovium of patients with rheumatoid arthritis. Arthritis Rheum 2002;46:2878–83. 10.1002/art.10622 [DOI] [PubMed] [Google Scholar]
- 64. Tanaka Y, Takeuchi T, Yamanaka H, et al. A phase 2 study of E6011, an anti-Fractalkine monoclonal antibody, in patients with rheumatoid arthritis inadequately responding to biological disease-modifying antirheumatic drugs. Mod Rheumatol 2021;31:783–9. 10.1080/14397595.2020.1868675 [DOI] [PubMed] [Google Scholar]
- 65. Kato H, Endres J, Fox DA. The roles of IFN-γ versus IL-17 in pathogenic effects of human Th17 cells on synovial fibroblasts. Mod Rheumatol 2013;23:1140–50. 10.1007/s10165-012-0811-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bombardieri M, Kam N-W, Brentano F, et al. A BAFF/APRIL-dependent TLR3-stimulated pathway enhances the capacity of rheumatoid synovial fibroblasts to induce aid expression and Ig class-switching in B cells. Ann Rheum Dis 2011;70:1857–65. 10.1136/ard.2011.150219 [DOI] [PubMed] [Google Scholar]
- 67. Hunter CA, Jones SA. Il-6 as a keystone cytokine in health and disease. Nat Immunol 2015;16:448–57. 10.1038/ni.3153 [DOI] [PubMed] [Google Scholar]
- 68. Tu J, Hong W, Zhang P, et al. Ontology and function of fibroblast-like and macrophage-like synoviocytes: how do they talk to each other and can they be targeted for rheumatoid arthritis therapy? Front Immunol 2018;9:1467. 10.3389/fimmu.2018.01467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yoshitomi H. Regulation of immune responses and chronic inflammation by fibroblast-like synoviocytes. Front Immunol 2019;10:1395. 10.3389/fimmu.2019.01395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kuo D, Ding J, Cohn IS, et al. HBEGF+ macrophages in rheumatoid arthritis induce fibroblast invasiveness. Sci Transl Med 2019;11. 10.1126/scitranslmed.aau8587. [Epub ahead of print: 08 05 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Danks L, Komatsu N, Guerrini MM, et al. Rankl expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann Rheum Dis 2016;75:1187–95. 10.1136/annrheumdis-2014-207137 [DOI] [PubMed] [Google Scholar]
- 72. Del Rey MJ, Faré R, Izquierdo E, et al. Clinicopathological correlations of podoplanin (gp38) expression in rheumatoid synovium and its potential contribution to fibroblast platelet crosstalk. PLoS One 2014;9:e99607. 10.1371/journal.pone.0099607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bergström B, Lundqvist C, Vasileiadis GK, et al. The rheumatoid arthritis risk gene AIRE is induced by cytokines in fibroblast-like synoviocytes and augments the pro-inflammatory response. Front Immunol 2019;10:1384. 10.3389/fimmu.2019.01384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sfikakis PP, Christopoulos PF, Vaiopoulos AG, et al. Cadherin-11 mRNA transcripts are frequently found in rheumatoid arthritis peripheral blood and correlate with established polyarthritis. Clin Immunol 2014;155:33–41. 10.1016/j.clim.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 75. Finch R, Sostelly A, Sue-Ling K. Results of a phase 2 study of RG6125, an anti-Cadherin-11 monoclonal antibody, in rheumatoid arthritis patients with an inadequate response to anti-TNFα therapy. Annals of the Rheumatic Diseases 2019;78:189. [Google Scholar]
- 76. Asano T, Iwasaki N, Kon S, et al. α9β1 integrin acts as a critical intrinsic regulator of human rheumatoid arthritis. Rheumatology 2014;53:415–24. 10.1093/rheumatology/ket371 [DOI] [PubMed] [Google Scholar]
- 77. Kanayama M, Morimoto J, Matsui Y, et al. α9β1 integrin-mediated signaling serves as an intrinsic regulator of pathogenic Th17 cell generation. J Immunol 2011;187:5851–64. 10.4049/jimmunol.1101524 [DOI] [PubMed] [Google Scholar]
- 78. Sugahara S, Hanaoka K, Yamamoto N. Integrin, alpha9 subunit blockade suppresses collagen-induced arthritis with minimal systemic immunomodulation. Eur J Pharmacol 2018;833:320–7. 10.1016/j.ejphar.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 79. Takeuchi T, Tanaka Y, Erdman J, et al. ASP5094, a humanized monoclonal antibody against integrin alpha-9, did not show efficacy in patients with rheumatoid arthritis refractory to methotrexate: results from a phase 2A, randomized, double-blind, placebo-controlled trial. Arthritis Res Ther 2020;22:252. 10.1186/s13075-020-02336-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cai P, Lu Z, Jiang T, et al. Syndecan-4 involves in the pathogenesis of rheumatoid arthritis by regulating the inflammatory response and apoptosis of fibroblast-like synoviocytes. J Cell Physiol 2020;235:1746–58. 10.1002/jcp.29093 [DOI] [PubMed] [Google Scholar]
- 81. Godmann L, Bollmann M, Korb-Pap A, et al. Antibody-mediated inhibition of syndecan-4 dimerisation reduces interleukin (IL)-1 receptor trafficking and signalling. Ann Rheum Dis 2020;79:481–9. 10.1136/annrheumdis-2019-216847 [DOI] [PubMed] [Google Scholar]
- 82. Yan X, Cen Y, Wang Q. Mesenchymal stem cells alleviate experimental rheumatoid arthritis through microRNA-regulated IκB expression. Sci Rep 2016;6:28915. 10.1038/srep28915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhao C, Zhang L, Kong W, et al. Umbilical cord-derived mesenchymal stem cells inhibit cadherin-11 expression by fibroblast-like synoviocytes in rheumatoid arthritis. J Immunol Res 2015;2015:137695. 10.1155/2015/137695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Buechler MB, Pradhan RN, Krishnamurty AT, et al. Cross-tissue organization of the fibroblast lineage. Nature 2021;593:575–9. 10.1038/s41586-021-03549-5 [DOI] [PubMed] [Google Scholar]
- 85. Zhang F, Wei K, Slowikowski K, et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol 2019;20:928–42. 10.1038/s41590-019-0378-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Micheroli R, Elhai M, Edalat S, et al. Role of synovial fibroblast subsets across synovial pathotypes in rheumatoid arthritis: a deconvolution analysis. RMD Open 2022;8. 10.1136/rmdopen-2021-001949 [DOI] [PMC free article] [PubMed] [Google Scholar]