Abstract
The European Society of Gynaecological Oncology (ESGO), the International Society for the Study of Vulvovaginal Disease (ISSVD), the European College for the Study of Vulval Disease (ECSVD), and the European Federation for Colposcopy (EFC) developed consensus statements on pre-invasive vulvar lesions in order to improve the quality of care for patients with vulvar squamous intraepithelial neoplasia, vulvar Paget disease in situ, and melanoma in situ. For differentiated vulvar intraepithelial neoplasia (dVIN), an excisional procedure must always be adopted. For vulvar high-grade squamous intraepithelial lesion (VHSIL), both excisional procedures and ablative ones can be used. The latter can be considered for anatomy and function preservation and must be preceded by several representative biopsies to exclude malignancy. Medical treatment (imiquimod or cidofovir) can be considered for VHSIL. Recent studies favor an approach of using imiquimod in vulvar Paget’s disease. Surgery must take into consideration that the extension of the disease is usually wider than what is evident in the skin. A 2 cm margin is usually considered necessary. A wide local excision with 1 cm free surgical margins is recommended for melanoma in situ. Following treatment of pre-invasive vulvar lesions, women should be seen on a regular basis for careful clinical assessment, including biopsy of any suspicious area. Follow-up should be modulated according to the risk of recurrence (type of lesion, patient age and immunological conditions, other associated lower genital tract lesions).
Keywords: Vulvar and Vaginal Cancer; Vulvar Neoplasms; Melanoma; Paget Disease, Extramammary
Background
The European Society of Gynaecological Oncology (ESGO), the International Society for the Study of Vulvovaginal Disease (ISSVD), the European College for the Study of Vulval Disease (ECSVD), and the European Federation for Colposcopy (EFC) are leading international societies among gynecologists, pathologists, dermatologists, and related disciplines. One of their aims is to promote the highest quality of care for women with pre-invasive and invasive gynecological neoplasia through prevention, advancing treatment, excellence in care, and high-quality research and education.
ECSVD, EFC, ESGO, and ISSVD collaborated to develop a consensus statement on pre-invasive vulvar lesions.
Methods
The ESGO, ISSVD, ECSVD, and EFC executive councils nominated selected specialists from their membership bodies with well-recognized expertise, clinical and research activity, and leadership in the field as surrogate markers for their continuous effort in improving the quality of care for patients with vulvar and vaginal pre-invasive lesions.
A systematic literature review of studies published from January 2000 to March 2021 was carried out using the MEDLINE database. Search indexing terms and criteria are listed in an additional file (see online supplemental appendix 1). The literature search was limited to publications in English, Italian, Spanish, Portuguese, German, and French. The search strategy excluded editorials, case reports, letters, and in vitro studies.
ijgc-2021-003262supp001.pdf (26.3KB, pdf)
A total number of 192 articles were retrieved; 89 were on squamous vulvar intraepithelial neoplasia (VIN), 33 on vulvar Paget’s disease, and 26 on vulvar melanoma in situ. A further 12 articles with more than one pre-invasive disease and 32 reviews were considered.
Data extraction was performed for all articles dealing with treatment by two independent teams and was double-checked. Tables with the most relevant clinical outcomes were completed and summarized in the text (see online supplemental appendices 2 and 3).
ijgc-2021-003262supp002.xlsx (20.3KB, xlsx)
Evidence-based consensus statements were also developed on the management of patients with pre-invasive vulvar lesions, chaired by professors Mario Preti and Murat Gultekin. The chairs were responsible for drafting corresponding preliminary statements based on the review of the relevant literature (residents assisted in preparing data extraction and analyses: FB, NG, BEE, BET). These were then sent to the group of selected specialists. A first round of binary voting (agree/disagree) was carried out for each potential statement. The participants took part in each vote, but they were permitted to abstain from voting if they felt they had insufficient expertise to agree/disagree with the statement or if they had a conflict of interest that could be considered to influence their vote. The voters had the opportunity to provide comments/suggestions with their votes. The chairs then discussed the results of this first round of voting and revised the statements if necessary. The voting results and the revised version of the statements were again sent to the whole group, and another round of binary voting was organized according to the same rules, to allow the whole group to evaluate the revised statements. The statements were finalized based on the results of this second round of voting. The group achieved consensus on 12 statements. One of the authors (FP) provided the methodology support for the entire process and did not participate in voting for statements.
Two external independent reviewers (MVB, MB), who have been internationally acknowledged for their research in vulvar pre-invasive lesions, reviewed the final manuscript.
Evolution of terminology and classification
The two carcinogenic pathways of vulvar squamous cell neoplasia were reflected in the 19861 and again in the 20042 ISSVD classifications. They included two vulvar intraepithelial neoplasia (VIN) groups: ‘VIN, usual type, HPV related’ and ‘VIN, differentiated type, HPV unrelated’.
The 2013 Lower Anogenital Squamous Terminology (LAST) unifies the nomenclature of human papillomavirus (HPV)-associated squamous lesions of the entire lower anogenital tract and uses a two-tier terminology: ‘low-grade squamous intraepithelial lesion (LSIL)’ and ‘high-grade squamous intraepithelial lesion (HSIL)’ for the vulva as well as other genital organs.3 The absence of reference to differentiated vulvar intraepithelial neoplasia (dVIN), despite its malignant potential, and the inclusion of vulvar LSIL (low-grade squamous intraepithelial lesion), recreating the potential for overdiagnosis and overtreatment of benign and usually self-limiting lesions, are the main limitations of the LAST classification.
The 2018 International classification of diseases for mortality and morbidity statistics, 11th revision (ICD-11) system4 still uses the term ‘carcinoma in situ’ of the vulva for both squamous and non-squamous pre-invasive lesions (Paget’s disease), where the implication of impending cancer may lead to unnecessary radical excisions of every intraepithelial neoplastic lesion.
The current 2015 ISSVD terminology does contain the terms LSIL (low-grade squamous intraepithelial lesion) and HSIL (high-grade squamous intraepithelial lesion) (box 1)5; however, the word ‘neoplasia’ was replaced by ‘lesion’, and it was stated that the meaning of LSIL (low-grade squamous intraepithelial lesion) was the manifestation of a productive HPV infection, a flat condyloma, or HPV effect. ‘Vulvar intraepithelial neoplasia differentiated’ was the third category, just as in the previous ISSVD terminologies.
Box 1. 2015 International Society for the Study of Vulvovaginal Disease terminology of vulvar squamous intraepithelial lesions.
LSIL of the vulva (vulvar LSIL, flat condyloma, or HPV effect)
HSIL of the vulva (vulvar HSIL ((VHSIL)), VIN usual type)
dVIN
The World Health Organization (WHO) in 2014 used LSIL (low-grade squamous intraepithelial lesion), HSIL (high-grade squamous intraepithelial lesion), and ‘VIN-differentiated type’,6 while the 2020 WHO classification of tumors7 divides the vulvar lesions into ‘HPV-associated squamous intraepithelial lesions’ and ‘HPV-independent VIN’ (box 2). Along with dVIN, differentiated exophytic vulvar intraepithelial lesion (DEVIL) and vulvar acanthosis with altered differentiation (VAAD) have been described as subtypes of HPV-independent VIN.
Box 2. 2020 WHO terminology.
HPV-associated squamous intraepithelial lesions: low-grade squamous intraepithelial lesion of the vulva (LSIL); high-grade squamous intraepithelial lesion of the vulva (HSIL)
HPV-independent VIN: differentiated vulvar intraepithelial neoplasia (dVIN); differentiated exophytic vulvar intraepithelial lesion (DEVIL); vulvar acanthosis with altered differentiation (VAAD)
In 1986, the ISSVD classified vulvar Paget’s disease as an in situ adenocarcinoma of the vulvar skin.1 In 2001, Wilkinson et al proposed a histopathological classification of vulvar Paget’s disease that distinguished primary, of cutaneous origin, vulvar Paget’s disease (type 1) as arising within the vulvar epithelium, from secondary/non-cutaneous vulvar Paget’s disease (type 2), that originates from the spread of an internal malignancy (anorectal adenocarcinoma or urothelial carcinoma of the bladder or urethra, to the vulvar epithelium).8 Type 1 vulvar Paget’s disease is further divided into 1a-intraepithelial, 1b-invasive, and 1c-manifestation of an underlying vulvar adenocarcinoma. Vulvar Paget’s disease is a subset of extramammary Paget’s disease.
Even if the 2014 WHO tumors classification6 no longer supports Wilkinson classification, current literature often refers to that classification, mainly based on the histopathologic features of vulvar Paget’s disease. The 2014 WHO tumors classification defines vulvar Paget’s disease as intraepithelial neoplasm of epithelial origin expressing apocrine or eccrine glandular-like features and characterized by distinctive large cells with prominent cytoplasm, referred to as Paget cells. This definition was reiterated by the 2020 WHO tumors classification that considers vulvar Paget’s disease an in situ adenocarcinoma of the vulvar skin, with or without underlying invasive adenocarcinoma.7 Secondary involvement of vulvar skin by carcinoma of rectal, bladder, and cervical origin is defined as ‘secondary Paget disease’.
Melanoma in situ was originally included in the 1986 ISSVD classification as non-squamous intraepithelial neoplasia.1 Cutaneous melanoma is staged using the American Joint Committee on Cancer melanoma staging system for melanoma of the skin.9 This staging system has been validated for vulvar melanoma and melanoma in situ. Melanoma in situ represents stage Ia.
Epidemiology
Vulvar condyloma/condylomatous low-grade squamous intraepithelial lesions (LSIL) are usually associated with low-risk HPV infections (HPV 6 or 11 in 90% of cases).10 They do not progress to invasive cancer and are common in the general population with a prevalence of around 107–229 per 100 000 women.11 12
Vulvar high-grade squamous intraepithelial lesions (VHSIL) are seen with an incidence of 2.5 to 8.8 per 100 000 women/year and may have a risk of transforming into an invasive carcinoma.10 13 14 Differentiated vulvar intraepithelial neoplasia (dVIN) represent less than 10% of all the squamous vulvar intraepithelial lesions15 16 and has potential for malignant transformation greater than that of VHSIL (32.8% in elderly women with dVIN vs 5.7% in VHSIL seen in young patients).17 In a recent Dutch study, the overall European Standardized Rate of high-grade VIN without concurrent vulvar squamous cell carcinoma was 2.99 per 100 000 woman-years: 2.95 for VHSIL and 0.05 for dVIN. This rate has increased for VHSIL from 2.39 between 1991–1995 to 3.26 between 2006–2011 (+36.4%) and from 0.02 to 0.08 (+300.0%) for dVIN.15 Using the Surveillance, Epidemiology, and End Results (SEER) databases, between 1973 and 2004, the incidence of VIN and vulvar squamous cell carcinoma increased 3.5% and 1.0% per year, respectively, in the USA, and the largest increase was seen in younger patients.18
Despite the rarity of anal cancer at the population level (1–2 cases per 100 000 person-years), due to the HPV field infection, VHSIL patients are at increased risk for anal squamous cell carcinoma and precursors. A recent meta-analysis showed an incidence ratio of anal cancer of 42 per 100 000 person-years (95% CI 33 to 52) in women diagnosed with VHSIL,19 that is the third-highest risk for anal cancer after HIV-positive men who have sex with men ≥30 years old and transplanted women ≥10 years post-transplant. The mean time interval between the incidence of VIN and anal cancer diagnosis was 8.9 years.20
Extramammary Paget’s disease accounts for about 1–10% of all cases of Paget’s disease with an incidence estimated at around 0.6/100 000 people per year in Europe.21 22 Among female patients, more than 80% of extramammary Paget’s disease are located in the vulva.21 Of all primary vulvar Paget’s disease cases, vulvar Paget’s disease with invasive adenocarcinoma is reported in 16–19% and vulvar Paget’s disease as a manifestation of an underlying vulvar adenocarcinoma is reported in 4–17% of all cases.23–25
Vulvar melanoma accounts for 6% to 10% of vulvar malignancies and only about 3% of all melanomas.26–28 An analysis of the National Cancer Database showed that melanoma in situ is less frequent than vulvar melanoma, with a median age at diagnosis of 63 and 66 years, respectively.29
Molecular etiology
VHSIL is the precursor of HPV-related invasive carcinoma and it is caused by high-risk HPVs (HPV 16 in >70% of cases),16 30 with smoking and immunosuppression as additional risk factors.31 VHSIL oncogenesis is comparable to that of high-grade squamous intraepithelial lesion (HSIL) of the cervix, vagina, and anus. Molecular heterogeneity is observed among anogenital HSIL. High host-cell DNA methylation levels in VHSIL32 seems to reflect a high cancer risk, which might be relevant when conservative management for VHSIL is considered. Using whole-genome shallow sequencing, a chromosome 1pq gain was identified as another strong indicator for the risk of HPV-positive VIN to progress to vulvar squamous cell carcinoma.33
The HPV-independent pathway is less well understood and, although approximately 80% of vulvar carcinomas in Europe are HPV-negative, less than 10% of vulvar pre-invasive lesions are differentiated VIN.15 16
dVIN and HPV-negative vulvar squamous cell carcinoma arise mostly in a field of lichen sclerosus or lichen planus, chronic inflammatory lymphocyte-mediated skin diseases.34
In dVIN TP53 mutations are frequently identified. Cyclin D1 amplification and copy number variations in chromosomes 3, 8, and 11q13 have been reported in HPV-negative VIN, similarly to HPV-negative vulvar squamous cell carcinoma.33 35
A subset of the HPV-independent precursors was found to be TP53 wild-type with somatic mutations in PIK3CA, NOTCH1, and HRAS suggesting a third, not-previously described, molecular subtype.36–38
The proteomic analysis points at inflammation as a driver of progression39: the chronic inflammatory environments in lichen sclerosus and lichen planus are considered the main contributory factors for oxidative damage and local immune dysregulation.40–47
Vulvovaginal microbiome disturbances seem also to be a trigger for the inflammatory response altering the balance in the host’s commensal microbes.39
Vulvar Paget’s disease type Ia is an in situ adenocarcinoma of the vulvar skin, which may give rise to invasive adenocarcinoma.7 Vulvar Paget’s disease arises from intraepidermal pluripotent stem cells in the infundibulo-sebaceous unit of hair follicles and adnexal structures.7 48 The reported frequency of HER2 oncogene amplification varies.22 49–52 Mutations in genes encoding the PIK3/AKT cascade have been found to significantly correlate with CDH1 hypermethylation.53 54 Amplification at chromosomes Xcent-q21 and 19, as well as loss at 10q24-qter, have been reported.55
Cutaneous and mucosal vulvar melanomas arise from melanocytes. Melanoma in situ consists of malignant melanocytes that spread along the epidermis but do not extend into the papillary dermis. Vulvar melanomas may develop de novo, or from pre-existing benign or atypical pigmented lesions. The etiology and pathogenesis are largely unknown. Ultraviolet radiations are unlikely to be involved since most tumors arise on surfaces not exposed to sun.56
Clinical aspects
There is no single pathognomonic clinical feature of vulvar SIL. Approximately 60% of patients report itching and/or irritation, pain, or bleeding along with visible vulvar lesions.57 In others, lesions are diagnosed incidentally during a routine gynecological examination. It is difficult to distinguish among various types of vulvar lesions based only on macroscopical aspects and the distribution of vulvar changes. Clinical aspects of vulvar SIL are variable with significant differences in number, size, shape, color, surface, thickness, and topography. Lesions may be solitary or multiple. They are characteristically papular, raised, with sharp borders and a keratotic, roughened surface. Their color may range from white to red, gray, blue, or brown. Magnification of the vulvar skin with lens or colposcope after thorough naked eye examination may allow (a) a better definition of the extent of the lesion, (b) the direction of biopsies to the area(s) of most clinically severe abnormality, and (c) direct treatment by visualizing anatomic landmarks.
Three percent to 5% acetic acid can be applied by expert hands58 when HPV-associated SIL is suspected: sharply demarcated and raised acetowhite epithelium generally corresponds to VHSIL, whereas dVIN generally does not react to acetic acid. It should be kept in mind that acetic acid in vulvoscopy should be used only in experienced hands, considering the high false-positive rate.58
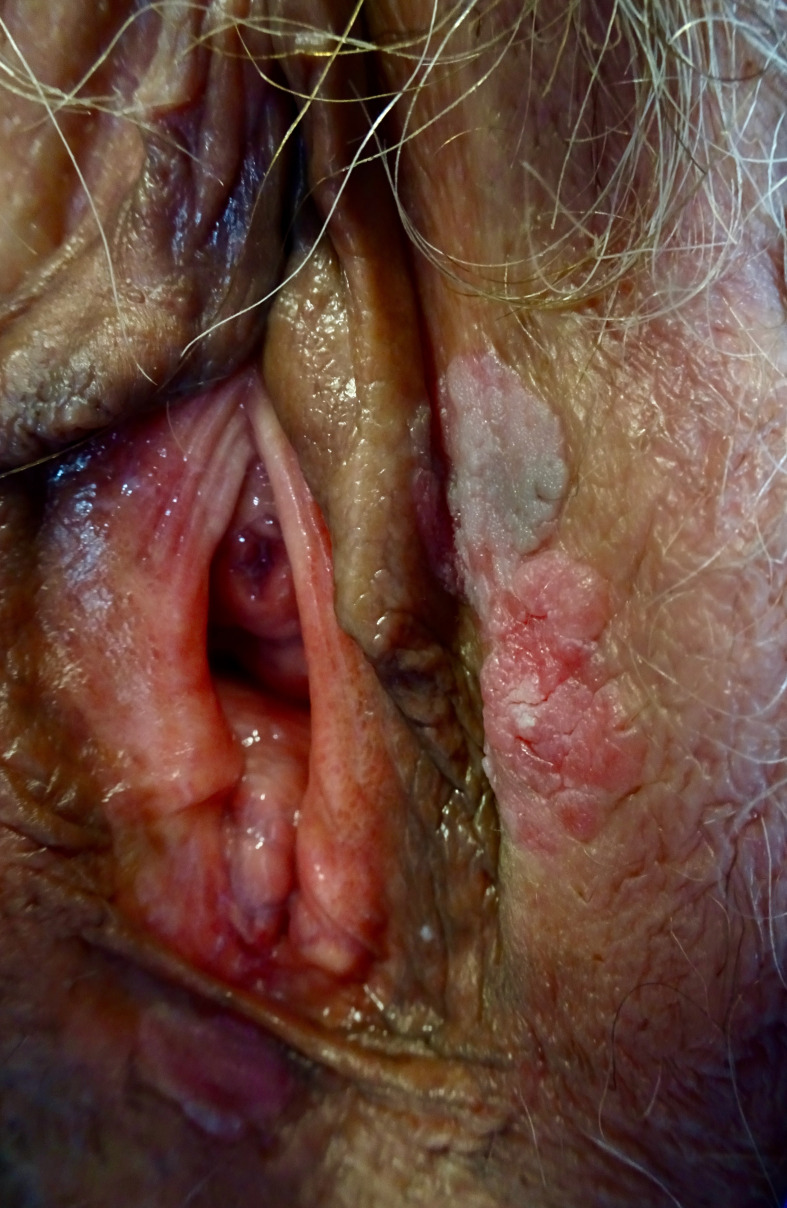
VHSIL tends to occur in young women and it is usually multifocal, located around the introitus, and often involving the labia minora (Figure 1). Multicentric/multizonal disease often presents in cases with VHSIL, and may involve cervical, vaginal, perianal, or anal squamous epithelium. A careful examination of the whole vulva, perineum, perianal, and anal areas, including the cervix and vagina, is mandatory. There are not enough data to screen all VHSIL patients with high-resolution anoscopy, and anal cytology sensitivity seems to be low in women with VHSIL.59 In the meantime, accurate anal squamous cell carcinoma symptom questioning should be performed in this group of patients.
Figure 1.
Vulvar high grade squamous intraepithelial lesion; brownish and erythematous poorly marginated plaques on the inner side of left labium.
The clinical approach to dVIN patients is completely different in that it is seen primarily in older women (median age 67.0 years vs 47.8 years in VHSIL).15 17 Clinically, dVIN is sometimes difficult to distinguish from the associated dermatosis, in particular lichen sclerosus involving the adjacent skin, and usually it appears as unifocal and unicentric poorly demarcated pink or gray-white (hyperkeratotic) rough plaques60–62 (Figure 2). Long-lasting symptoms and treatment-resistant dermatoses need to be carefully inspected to rule out dVIN and to promptly biopsy.
Figure 2.
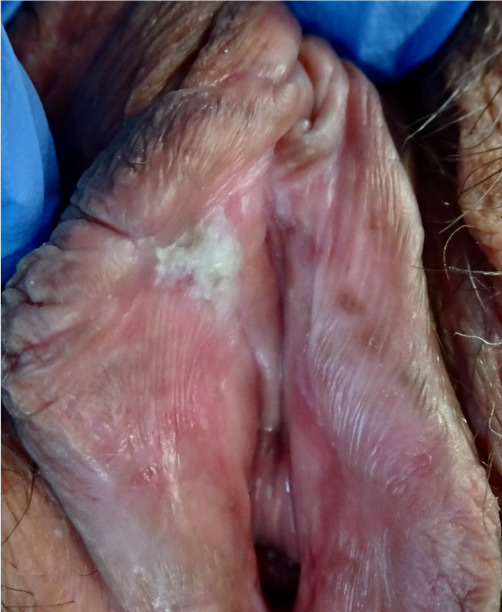
Differentiated vulvar intraepithelial neoplasia; whitish poorly marginated plaque on internal side of right labium minus in a field of lichen sclerosus.
An underlying early invasive squamous cancer may be present in up to 20% of VHSIL patients63 64 and this percentage is even higher in dVIN.
For a definitive diagnosis of a vulvar lesion, a biopsy needs to be performed. As many vulvar cancers are missed and have delayed diagnosis due to biopsies not having been taken, a biopsy should be performed of any suspicious lesion identified with multiple biopsies performed for lesions of multiple colors, large lesions, and multicentric lesions.
Punch/incision biopsy establishes the diagnosis. All multiple lesions should be biopsied separately and mapped.
Differential diagnosis
Due to the variation in the clinical features of vulvar SILs, these lesions can mimic different diseases: lichen simplex chronicus, lichen sclerosus, lichen planus, psoriasis, contact dermatitis, and more.
Paget’s disease
Vulvar Paget’s disease is considered the great mimic of vulvar pathology. Its lesions can be mistaken for chronic dermatitis or dermatosis, and so delay the histological diagnosis of the disease. In the ISSVD Terminology and classification of vulvar dermatologic disorders (2011), vulvar Paget’s disease is assigned to the morphological group 2, ‘Red lesions, patches and plaques’, and to subgroup B, ‘Red patches and plaques (no epithelial disruption)’.65
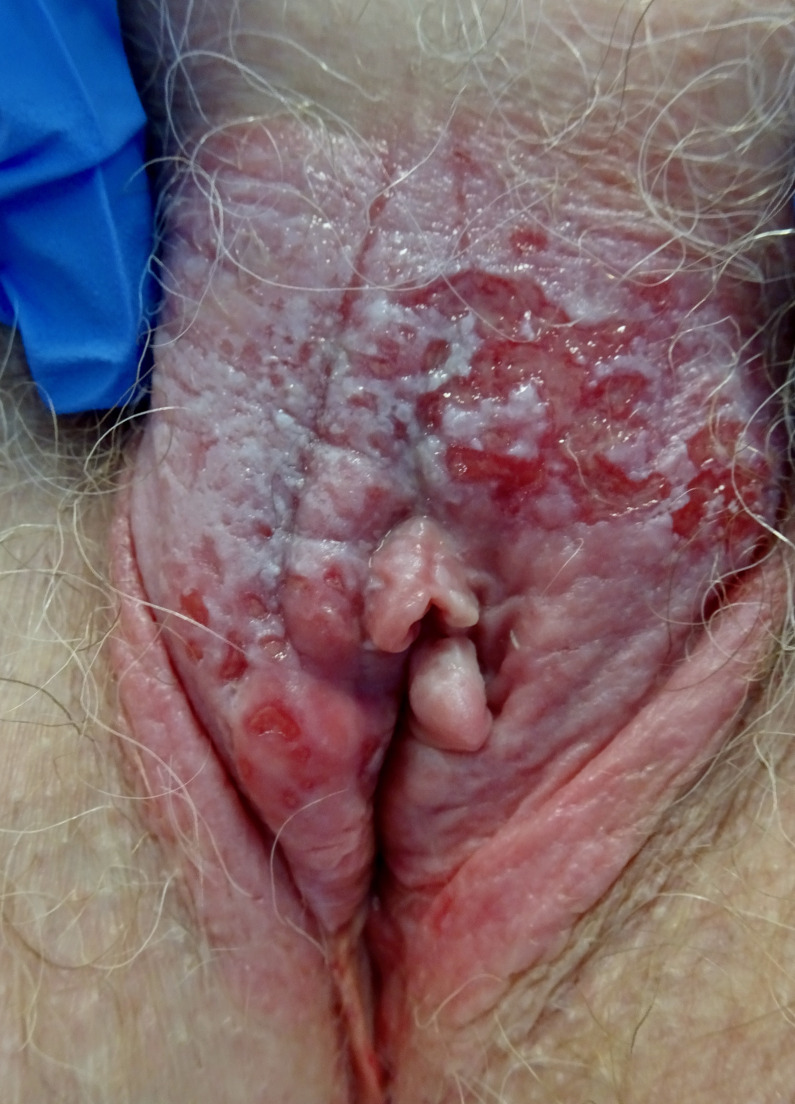
On inspection, the lesion may look red or exhibit different shades of white and gray, usually eczematous, ulcerated, or with a crusty appearance, but it is seldom pigmented (Figure 3). Most of the lesions are found on the labia majora and vary in size. However, vulvar Paget’s disease can involve labia minora, clitoris, inguinal folds, urinary meatus, and perineum.66 67
Figure 3.
Vulvar Paget disease in situ; erythematous and white lesion involving whole vulva with superficial erosions.
The visible borders are mostly irregular, slightly elevated, and sharply demarcated; the disease often extends the macroscopic margins. With periurethral and perianal lesions, an involvement of the skin by a non-cutaneous underlying neoplasm must be excluded.
Differential diagnosis
Lichen sclerosus, dermatophytosis, candidiasis, contact dermatitis, psoriasis, seborrheic dermatitis, and squamous VIN are among the differential diagnoses. Finding similar lesions elsewhere on the body and a biopsy including the derma with appropriate use of immunohistochemistry will confirm a vulvar Paget’s disease diagnosis.
Melanoma in situ
Biopsy including the derma allows diagnosis of melanoma in situ, which is an uncommon pigmented vulvar lesion often clinically indistinguishable from the more common benign pigmented lesions, such as melanosis (Figure 4). Asymmetry, indistinct borders, variegated color, and a large diameter (>6 mm) are similar in both lesions. Consequently, a biopsy is necessary for diagnosis, and the threshold to biopsy a genital pigmented lesion should be low.68 69
Figure 4.
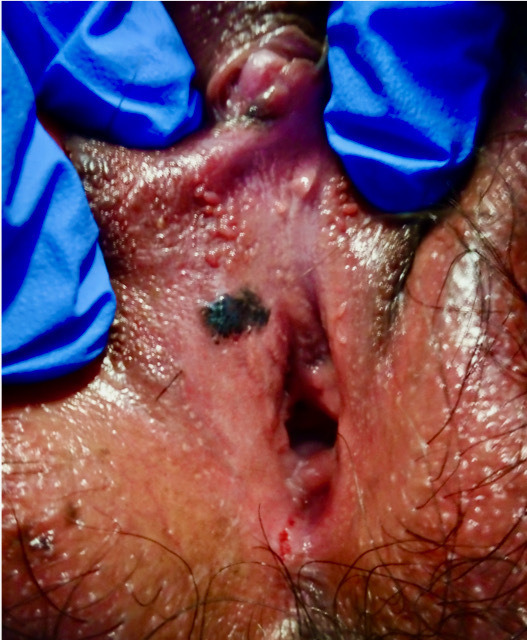
Melanoma in situ; black poorly marginated oval smooth lesion on the right superior vestibule.
Differential diagnosis
Physiologic hyperpigmentation, congenital adrenal hyperplasia, Addison’s or Cushing’s disease, postinflammatory hyperpigmentation, acanthosis nigricans, seborrheic keratosis, vulvar melanosis/lentiginosis, melanocytic nevi (pigmented nevi, nevocellular nevi, common nevi), pigmented condylomata acuminata, pigmented basal cell carcinoma, pigmented VIN, and squamous cell carcinoma should be considered among the differential diagnoses.
Histopathology
Accurate histological diagnosis is crucial for appropriate treatment; histological assessment of vulvar intraepithelial lesions requires pathologists dealing with high-volume vulvar biopsies. Inter-observer agreement was demonstrated low for VHSIL70 and it is even worse for dVIN diagnosis71 72 where associated dermatoses complicate the histological pattern.73
The recommendation for tissue sampling of suspected precursor lesions is to obtain optimal specimens with a minimum 4 mm width with 5 mm depth for hair-bearing skin and 3 mm depth for hairless skin and mucosal sites, achieved with punch, cold knife, or suture-assisted snip. In the case of ulcer or fissure, biopsy should be performed where there is intact epithelium.74
In non-invasive lesions of the vulva, immunohistochemistry is helpful in distinguishing difficult cases (Table 1).
Table 1.
Immunohistochemistry in vulvar pre-invasive lesions
| Lesion | Immunohistochemistry | Comment |
| VHSIL (VIN 2/3) | P16 block positivity, ki-67 extends above basal layers through entire epithelium | Ki-67 will stain above the basal layers in LSIL as well and cannot be used to distinguish LSIL from VHSIL. P16 is more useful in this distinction and can be occasionally positive in LSIL |
| dVIN | Aberrant p53 staining patterns. P16 not block positive. Ki-67 confined to basal layers | A panel of p53, p16, and ki-67 helpful in distinguishing VHSIL from dVIN |
| Vulvar Paget’s disease | Cells contain mucine (PAS-D or alcian blue), mucicarmine, CK 7, GCDFP-15, GATA377 | Stains to distinguish secondary Paget’s disease of urothelial (including uroplakin200) or anorectal origin (including CDX-2, CK20201) should be considered in appropriate cases |
| Melanoma in situ | Positivity with s100, Melan-A, and HMB 45202 | A panel to distinguish melanoma in situ from Paget’s disease can be helpful |
dVIN, differentiated-type vulvar intraepithelial neoplasia; LSIL, low-grade squamous intraepithelial lesions; VHSIL, vulvar high-grade squamous intraepithelial lesions.
VLSIL shows abnormal maturation and dysplastic features up to the lower third of the epithelium, while in VHSIL these abnormal features extend above the lower third of the epithelium (Figure 5). Immunohistochemistry with p16 can be of help to distinguish VLSIL from VHSIL, or atrophy from VHSIL, as VHSIL shows block positivity compared with mimics.3
Figure 5.
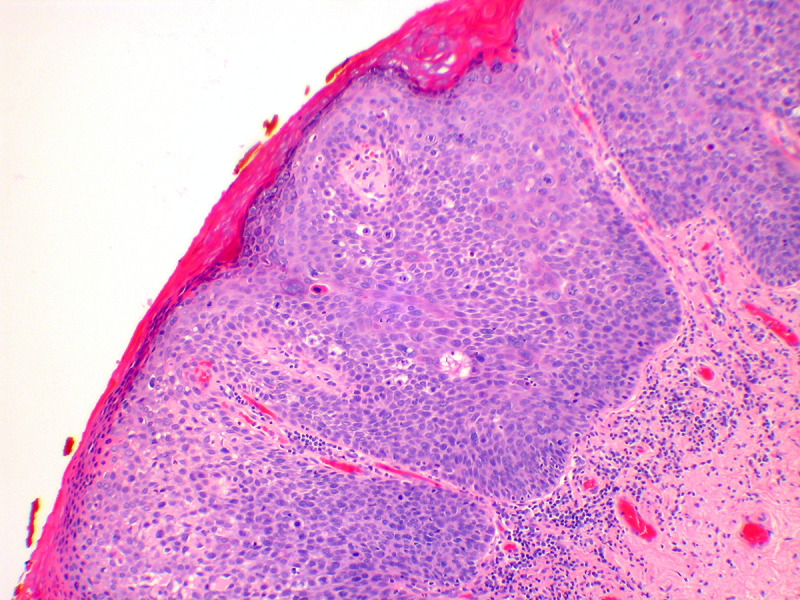
Vulvar high-grade squamous intraepithelial lesion; the lesion shows full thickness abnormality of maturation, and acanthosis (hematoxylin and eosin, x 10 magnification).
The histologic features of dVIN can be subtle, and the histological diagnosis may be further complicated by coexisting conditions such as lichen sclerosus. dVIN underdiagnoses could be partially explained by misclassification as reported by Van de Nieuwenhof et al, who found that 42% of the biopsies initially diagnosed as lichen sclerosus were reclassified as dVIN after review.73 75
dVIN shows basal atypia with abrupt (premature) maturation (hypereosinophylic keratinocytes), basal spongiosis, absence of granular layer, and parakeratosis (Figure 6). Nuclear atypia with enlarged and angulated hyperchromatic nuclei and increased mitotic activity together with premature keratinization with hypereosinophylic keratinocytes may be seen. Other common features in dVIN are squamous hyperplasia with elongation of rete ridges and pronounced intercellular bridges in the lower part of the epithelium and absence of the granular layer in combination with hyperkeratosis with parakeratosis. P53 often shows an aberrant staining pattern in the dysplastic cells of dVIN.38 74 76
Figure 6.
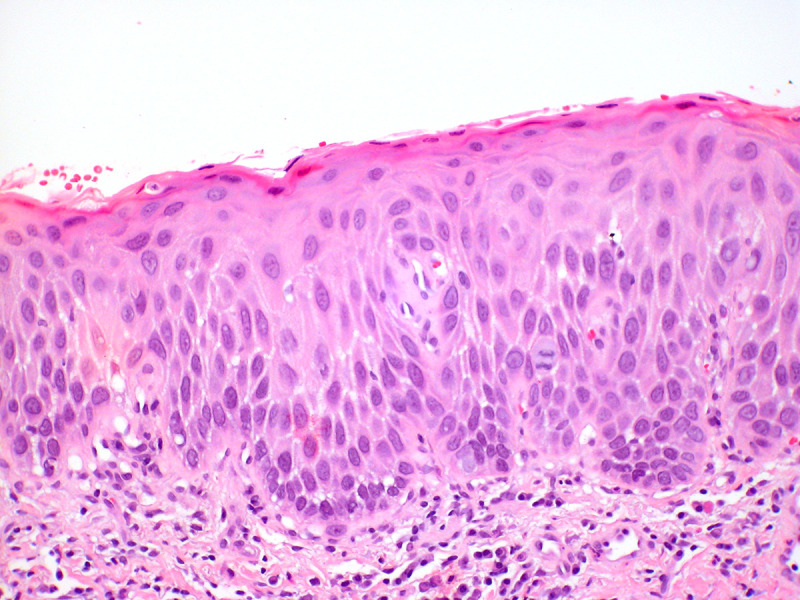
Differentiated vulvar intraepithelial neoplasia (dVIN). The histologic changes of dVIN are very subtle, and may be missed. Here there is basal atypia and acanthosis, but overall maturation is maintained. P53 and Ki-67 showed increased basal activity, and p16 was not block-positive, not shown (hematoxylin and eosin, x 20 magnification)
Vulvar Paget’s disease is usually an intraepithelial lesion. Histologically, the Paget cells are seen predominantly at the dermal–epidermal junction, percolating up the epithelium as individual cells in what has been called ‘Pagetoid spread’ (Figure 7). Paget cells are large and have prominent eosinophilic, basophilic, amphophilic or clear cytoplasm and vesicular nuclei with prominent nucleoli.77
Figure 7.
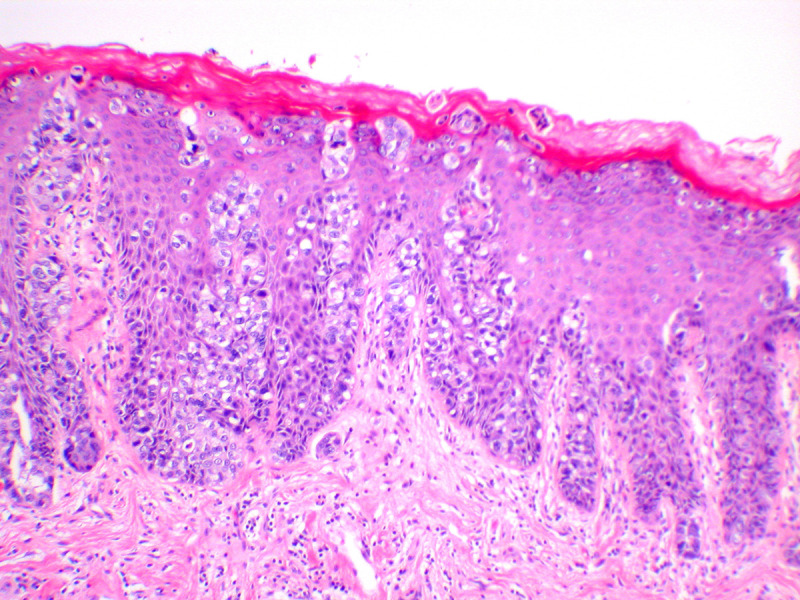
Vulvar Paget disease. The large cells of Paget’s disease are seen predominantly in the basal epithelium, but percolate up the epithelium in what is known as ‘Pagetoid spread’. Negative markers of melanoma, and positive markers of Paget, such as periodic acid Schiff stain with diastase (PAS-D), and breast markers such as Gata-3, are helpful in making this diagnosis (hematoxylin and eosin, x 10 magnification)
Melanoma in situ of the vulva is rare.67 It must be distinguished from Paget’s disease, as the atypical melanocytes arise at the dermal–epidermal junction, as individual cells and clusters, and spread upwards in the epithelium by ‘Pagetoid spread’ (Figure 8). Melanoma in situ will stain for markers of melanoma, including s100, Melan-A, and HMB 45.
Figure 8.
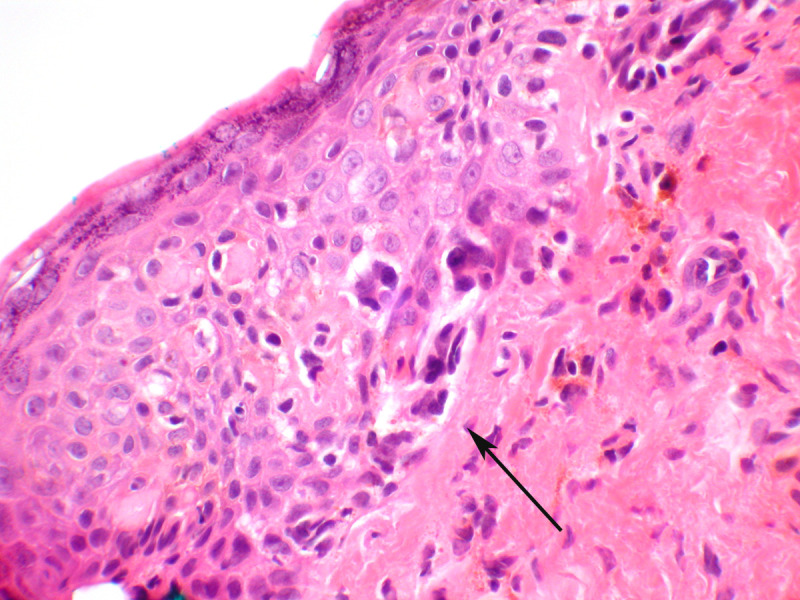
Melanoma in situ. Atypical melanocytes are seen predominantly in the basal portion of the epithelium (arrow) and will stain for melanocytic markers, which helps distinguish this lesion from Paget’s disease, which can be architecturally similar. This lesion did show pigmentation (hematoxylin and eosin, x 40 magnification)
Immunology
The promising clinical results of immunotherapy in VHSIL treatment proceeded in parallel with the studies on immunology and VHSIL microenvironment.78 79
Persistent HPV infection in VHSIL is able to induce a local immunosuppressive microenvironment, with upregulation of T-regulatory cells, increased infiltration with CD4+ (T helper cells), and decreased number of CD8+ (cytotoxic T cells).78–80
The presence and clinical impact of different myeloid cell populations in patients with non-recurrent and recurrent VHSIL were studied,81 showing the highest number of intraepithelial CD14+ (marker for monocytes) in the non-responding group. In VHSIL the population of M2 macrophages exceeds the M1 macrophages by at least four times, suggesting an immunosuppressive environment in the VHSIL epithelium.81
Some VHSIL lesions are infiltrated by high numbers of regulatory T cells (Tregs) that may induce an immunosuppressive microenvironment.82 Clinical response to immunotherapy in VHSIL is associated with an increase in intralesional CD8 +T cells as well as low numbers of Tregs.78 83 Indeed normalization of CD4+, CD8 +T cell counts in the epidermis and clearance of HPV is correlated with histological regression of VHSIL.78
HPV clearance after VHSIL treatment with imiquimod was also associated with a decreased number of intraepithelial CD14 +cells and an increased number of CD1a+Langerhans cells.78 On the other hand, the increase in CD14 +myeloid cells characterizes a progressive course of vulvar neoplasia84 and it is an independent prognostic factor for decreased recurrence-free survival.81
Complete responders to HPV therapeutic vaccination showed significantly stronger response of interferon (IFN)-γ-associated proliferative CD4 +T cells and a broad response of CD8 +IFN-γ T cells than did non-responders.85 86
Thus, an estimation of the number of intraepithelial immune cells may help in stratifying the prognosis of patients diagnosed with VHSIL and serve as a predictive biomarker for clinical response of VHSIL to immunotherapy and therapeutic vaccination.
Tumor microenvironment in vulvar Paget’s disease has been scantly studied. Tregs in vulvar Paget’s disease are frequently found at the epidermal–dermal junction,87 while healthy surrounding skin is negative for Tregs. Increased Tregs infiltrate was associated with more frequent positive surgical margins and recurrence of disease.88
It has been hypothesized that this is due to both local immunity suppression and lack of recognition of the Paget cells by the immune system as malignant or aberrant cells.89
Future research will focus on the changes in the immune infiltrate in vulvar Paget’s disease, clarifying clinical outcomes after imiquimod treatment.
Management
Vulvar squamous intraepithelial lesions
For dVIN, an excisional procedure must always be adopted.
For VHSIL, both excisional procedures and ablative ones can be used. The latter can be considered for anatomy and function preservation and must be preceded by several representative biopsies to exclude malignancy.
Medical treatment (imiquimod or cidofovir) can be considered for VHSIL.
In the past, extensive surgery with the intent to eradicate disease was the standard of therapy. The current aims are now prevention of progression to vulvar squamous cell carcinoma, preservation of normal anatomy, symptom relief, and maintenance of quality of life and sexual function with individualized treatments.
In a long-term follow-up study, median progression time to cancer ranged from 0.3 to 24.2 years after VIN diagnosis: 4.1 years for VHSIL and 1.4 years for dVIN.15 A 2016 Cochrane review reported a rate of progression to squamous cell cancer in 15% of women treated surgically for VHSIL over a median of 71.5 months.90
The increased risk of women with vulvar squamous cell carcinoma arising in a field of lichen sclerosus (through a dVIN pathway)91–93 is reduced by treatment with high-potency topical corticosteroids91 94 and should be recommended in these patients.
Surgical interventions
Because of the risk of progression to invasive vulvar squamous cell carcinoma from dVIN with a short interval,17 there is no role for medical treatment or ablation of dVIN, and therapy is conservative excision with negative surgical margins followed by continuous follow-up.95 96
Surgical interventions for VHSIL include both surgical excision (from wide local excision to superficial vulvectomy) and ablative therapy (carbon dioxide (CO2) laser vaporization, argon beam coagulation, cavitational ultrasonic surgical aspiration). Choosing the latter treatment must be preceded by representative biopsies to exclude malignancies before treatment as there is a risk of unexpected stromal invasion.63 In case of positive margins after surgical excisional treatment of VHSIL, if clinical inspection does not show a residual lesion, patients must be followed, and immediate re-excision is not recommended. Surgeries resulting in significant impairment should be discouraged and, when it is occasionally necessary to perform a large resection, the use of reconstructive techniques in experienced hands is required.
Despite treatment, VIN recurrence rate ranges from 6% to 50% post treatment,14 97–118 and it is influenced by margins status, duration of follow-up, patient-related factors (multifocality of disease, immunosuppression, and smoking), and VIN type (even if disease outcome between VHSIL and dVIN is not always detailed). In addition, methodological limitations and statistical analysis differences between studies contribute to the wide range reported. Fifty percent of recurrences are reported within 16.9 months requiring closer follow-up during the first 2 years after surgery, particularly in patients over the age of 50.111
In this context, the duration of follow-up is fundamental when comparing the reported rates of recurrence: 6.8% at the 6 month mark101 and up to 50% by the 14th year of follow-up.14 Immunosuppression exemplifies another important confounding factor both for recurrence (51.5% in HIV+ vs 27% in HIV− over 32 months) and progression to invasion (15.2% HIV+ vs 1.6% HIV− over median 32 months follow-up).98
No randomized controlled trials were performed comparing surgery with CO2 laser vaporization, and the available clinical data provided low-quality evidence. Leufflen et al reported 91.0% recurrence-free survival at 1 year for surgery and 65.2% for the laser vaporization groups (p<0.01).106 The mean time to recurrence following either treatment was 21.7 months. With a median follow-up of 4.4 years (range 0.8–18.4 years), the rate of progression to invasive disease was 2%.
Hillemanns et al reported a recurrence rate of 40.4% for CO2 laser vaporization compared with 41.7% for cold knife excision, 48.1% for photodynamic therapy, and 0% for vulvectomy, with a mean follow-up of 53.7 months.103
Van Esch et al reported a lower recurrence rate of surgically treated women (48.8%) compared with patients treated with laser ablation (56.0%) or combined laser and excision (66.7%).113 Also, Wallbillich et al reported a higher recurrence rate associated with laser ablation (45%) compared with cold knife excision (26.7%).116
Fehr et al100 and Van Esch et al113 reported a rate of progression of 6.1% and 15.1%, respectively, with mean time to invasion of 82 months100 and 71.5 months.113 The type of first treatment showed no differences in progression-free survival in the univariate Cox analysis.113
Only one paper compared114 loop electrosurgical excision procedure (LEEP, n=20), cold knife surgery (n=22), and laser vaporization (n=20): recurrences after the first procedure were significantly fewer with LEEP (15%) and wide local excision (10%) than with laser ablation (50%).
Argon beam coagulation was evaluated in VIN3 (VHSIL) treatment, with a recurrence rate of 48.3% and a mean time to recurrence of 23.2 months.105 The main advantage of this treatment modality is preservation of vulvar anatomy and the ability to perform multiple treatments.
CO2 laser vaporization was compared with cavitational ultrasonic aspiration (CUSA) in a single randomized controlled trial. No statistical difference in recurrence was reported at 12 months follow-up, with CUSA being reported as causing less pain and less scarring than laser.115 Investigating CUSA alone in VIN treatment, a recurrence rate of 35% after a median interval of 16 months and a progression rate of 3% after 33 months of median follow-up was reported.108
Medical interventions
Medical therapy is a therapeutic option suitable for VHSIL to preserve normal vulvar anatomy and to avoid mutilation. On the other hand, medical therapies do not provide histological specimens with the risk of missing early invasion foci. Consequently, several biopsies are needed prior to medical treatment.
Imiquimod is an immune response modifier directed to TLR-7 and stimulates dendritic cell secretion of pro-inflammatory cytokines, thereby eliciting strong immune infiltration.119 After 87% complete or partial response in patients enrolled in a pilot study,120 two randomized controlled trials121 122 compared imiquimod with placebo. The complete response for imiquimod-treated women was 81% for Mathiesen et al121 and 35% for Van Seters et al122 from 2 to 5 months after treatment. Only Van Seters et al122 reported 12 months follow-up data with 35% complete responders (n=9) in the imiquimod arm compared with 0% in the placebo group; and no difference in rates of progression to invasive disease between the two arms (1/26 vs 2/26). Long-term follow-up of the initial cohort from Van Seters was available123 and eight out of nine initial complete responders were disease-free after a median follow-up period of 7.2 years. The lesion sizes of long-term complete imiquimod-responders were significantly smaller than those of patients with residual and/or recurrent disease.
One randomized controlled trial with 180 patients enrolled evaluated topical 5% imiquimod cream versus 1% cidofovir gel and found no difference in terms of complete response (46% for both arms).124 At 12 months follow-up, the complete responders showed sustained results in 87% of cidofovir complete responders and 78% in the imiquimod arm. After 18 months follow-up of the same group of patients,125 cidofovir complete responders had a 6% recurrence rate compared with 28.4% of the imiquimod arm.
HPV E2 DNA methylation demonstrated to be a predictive biomarker for successful response in VIN treatment with cidofovir.126 Two other non-randomized controlled trials of imiquimod as single therapy were available and reported a range of recurrence 20.5–27% after 16–21 months of follow-up.127 128
Combining cold knife surgery and imiquimod cream as adjuvant does not seem to offer advantages in terms of lower recurrence rate,102 but may allow less extensive excisions and better preservation of the anatomy and function.
Photodynamic therapy
Photodynamic therapy uses a topical photosensitizer, 5-aminolevulinic acid, in combination with non-thermal light of appropriate wavelength to induce oxidation reactions that lead to cell apoptosis. The overall clinical response varies from 31.2% to 56%,83 118 129 and it seems to be comparable to laser ablation.129 130 The recurrence rate ranges from 14.3%129 at a mean 13 months to 48%103 after a mean 53.7 months of follow-up. Only one paper reported a 9.4% rate of invasion after treatment.83
Therapeutic vaccine
Therapeutic vaccine against HPV-16 E6 and E7 oncoprotein has been investigated, and an observational phase II study showed promising results.85 At 12 months of follow-up, 47% of patients showed complete response and 32% partial response; complete responders were still free of disease at 24 months.
Follow-up of women with vulvar intraepithelial neoplasia
Following treatment of VIN, women should be seen on a regular basis for careful clinical assessment, including biopsy of any suspicious area. Follow-up should be modulated according to the risk of recurrence (type of lesion, patient age and immunological conditions, other associated lower genital tract lesions).
The reported risk of progression to malignancy varies widely but appears to be around 10% for VHSIL and up to 50% in dVIN.13–15 131 The risk is higher in untreated women. Age (HR 2.3, 95% CI 1.5 to 3.4) and lichen sclerosus (3.1, 95% CI 1.8 to 5.3) are also independent risk factors for progression.15 Women treated surgically for VIN still have a residual risk of developing invasive cancer in the order of 2–4%.13
The risk for recurrence of VIN is up to 60%, independent of the surgical approach.14 About 25% of recurrences are late (more than 44 months after initial diagnosis) in one large long-term observational study.111 Women need clear information regarding signs and symptoms (such as pain or ulcers) that should prompt an earlier review. There is less evidence on long-term clinical outcomes and the risk of invasion following a full clinical response to topical medical treatments, but it may be similar to surgical treatment.
At least 4% (up to 25%) of women diagnosed with VIN will have intraepithelial neoplasia at other lower genital tract sites,132 133 and accurate inspection of lower genital tract sites including cervix, vagina, vulvar, and perianal skin is mandatory during follow-up. Similar rates of VHSIL were found in one study whether or not the woman had a previous hysterectomy, indicating that surveillance of the vagina is still required.134 Initiatives for anal squamous cell carcinoma screening in HPV-related VIN and vulvar squamous cell carcinoma patients are needed.19
Data suggest that dVIN carries a higher risk of progression and recurrence than VHSIL62 73 and closer follow-up is recommended after dVIN treatment.
Vulvar Paget’s disease
Recent studies favor an approach of using imiquimod. Surgery must take into consideration that the extension of the disease is usually wider than what is evident in the skin. A 2 cm margin is usually considered necessary.
Surgery is the cornerstone of vulvar Paget’s disease treatment in the published literature (ranging from 58.6% to 100% in published papers). Surgical options vary from local wide excision to radical vulvectomy with or without inguinal lymphadenectomy. If there is no underlying invasive disease (intraepithelial disease; 1 a), a wide resection with 2 cm clear margins is the most reported surgical treatment. Frozen section may be useful to achieve margin-free surgical excisions as disease often extends past what is visible to the eye.135–138 However, there is no clear demonstration that there should be a minimal distance to resection margins for vulvar Paget’s disease and the level of evidence is not very high to support this statement. Re-excision to achieve larger margins with ‘mutilation’ could not be of benefit. In cases with invasive disease or an underlying adenocarcinoma, a more radical approach (both in extension and in depth of excision) should be considered135 137 with lymphadenectomy,135 137 139 as there are not enough data for sentinel node in invasive vulvar Paget’s disease.
Topical 5% imiquimod cream has also been shown to be a safe conservative treatment option for in situ vulvar Paget’s disease with minimal adverse effects. Complete response rates have been reported with a range from 22% to 90% of cases.22 140 141 This allows a chance for the anatomical and functional conservation of vulvar structures. Treatment schedule varies among different studies (1–5 times a week, from a minimum of 3 weeks to an entire year). A total treatment duration of 16 weeks seems to be commonly used.22 140
Photodynamic therapy is not curative at all but can be used for symptom control.142
Radiotherapy can be considered when there is lymph node positivity or positive surgical margin in situations with associated invasive disease where there are contraindications for surgery or inoperable situations. There has still been no standard dose or schedule for the radiotherapy, so larger case series are warranted.
Melanoma in situ
A wide local excision with 1 cm free surgical margins is recommended.
Melanoma in situ is rarely seen in the vulva and appears to progress gradually to invasive melanoma.143 144 In some reports, association with lichen sclerosus is detected during the in situ phase, which usually disappears at later invasive stages.145
An excisional biopsy is the preferred method for diagnosis in small lesions with complete excision and depth to rule out invasion.146 A punch biopsy can also be used for large lesions, targeting the thickest area of the lesion.146 147 A wide local excision with 1 cm free surgical margins is considered curative.148 There is no need for lymph node assessment. Prognosis is usually excellent, being slightly better for melanoma in situ developing from melanocytic nevi, compared with those de novo.149
Only one study reported details of patients with vulvar melanoma in situ. The study evaluated 394 patients with a median age of 63. The 5 year overall survival rate was 74.4%. Vulvar melanoma in situ and invasive melanoma show worse overall survival compared with non-vulvar melanomas.28
Prevention
Most of the vulvar LSIL and VHSIL are HPV-related; the predominant HPV types are HPV 6 and 11 in LSIL, HPV 16 in VHSIL,150 and HPV 16 and 33 in HPV-related invasive vulvar cancer.16 The HPV vaccines are highly effective in preventing lesions related to the vaccine types.151 152 Approximately 90% of these lesions are related to HPV genotypes included in the 9-valent HPV vaccine.
Women with HPV-related vulvar disease are at high risk for contracting subsequent or recurrent disease.
Published studies show reduced VHSIL recurrence when HPV vaccines are administered before or after treatment153 154; HPV vaccination may be beneficial, and further studies are necessary to support these findings. Early prophylactic vaccination is recommended to every girl and woman according to national guidelines.
Women with lichen sclerosus showed a risk of cancer of 3.5% (incidence rate of 8.1:1000 person-years), increasing with advancing age.155 156 A recent Dutch study analyzing the incidence rate of vulvar squamous cell carcinoma in patients with VIN (median follow-up time 13.9 years, range 0.3–27.4 years) demonstrated in multivariate Cox regression analysis that type of VIN, age, and lichen sclerosus were independent risk factors for vulvar squamous cell carcinoma, with hazard ratios of, respectively, 3.0 for dVIN (vs VHSIL), 2.3 for age >50 years (vs <50 years), and 3.1 for lichen sclerosus (vs no lichen sclerosus).15
Women with lichen sclerosus who are compliant with topical steroid use have a much lower rate of vulvar cancer and better symptom control.94 The current belief is that women should continue regular use of topical steroids, even if asymptomatic, at least weekly and have lifelong regular check-ups (at least every 6–12 months, or when symptoms do not improve with adequate treatment, or new lesions are identified). Well-controlled patients can have these follow-up visits with their primary care physicians.157 Long-term follow-up is also advised for those who had the diagnosis during childhood, even if they experienced significant improvement during adolescence.158 No response to treatment or suspicious lesions (persistent erosions, tumors, and hyperkeratosis) should promptly be biopsied. Women with vulvar cancer and lichen sclerosus are often not offered topical steroids post-treatment of the cancer, but their use may reduce the recurrence risk to nearly a half (27% vs 44–47%).91
Immunosuppressed patients
The immunosuppressed population includes HIV-infected women, solid organ transplant recipients, as well as women undergoing immunosuppressing treatments for rheumatologic or autoimmune diseases. Evidence suggests that immunosuppression is a risk factor for development of HPV-related pre-invasive lesions and invasive cancers.
HPV and HIV have tight immune interactions, the latter facilitating HPV infection through the disruption of epithelial tight junctions.159 In addition, immune system defects such as CD4 +lymphocyte loss may contribute to impaired clearance or reactivation of latent HPV infections.159 160
HIV-infected women have higher incidence rates of VIN at a younger age and frequently have multifocal and multicentric HPV-related lesions.98 107 161–164 Indeed high-grade cervico-vaginal cytology was reported following treatment for VIN or vulvar cancer with OR 3.4 for immunodeficiency (95% CI 1.3 to 8.8).132
The recurrence and progression rates are far higher and with a shorter disease-free interval for HIV+ women than HIV− women,98 162 with a lower CD4 +lymphocyte count linked to shorter time to recurrence.107 162 Highly active antiretroviral therapy may decrease the incidence of condyloma and LSIL but appears to have no impact on VHSIL.165–167
Immunosuppressive drugs for renal transplant recipients may increase the risk of HPV carcinogenesis.168 169 Renal transplant recipients are at higher risk of VHSIL within 20 years after transplantation (5–12% vs 0.2–0.4% of female non-renal transplant recipients).170 One systematic review reported a higher Standardized Incidence Ratio of HPV-associated cancers in transplant patients compared with the general population: 2.1 (95% CI 1.37 to 3.30) for cervical cancer, and 22.8 (95% CI 15.8 to 32.7) for vulvar and vaginal cancer.171 A 41-fold increased risk for vulvar cancer and a 122-fold increased risk for anal cancer among renal transplant recipients were also reported in a Dutch study. Interestingly, 100% of vulvar cancer in this population were HPV+, compared with as low as 4.9% in immunocompetent patients.172–175
Thus, immunosuppressed patients should undergo a complete lower ano-genital tract examination as a part of routine screening and be appropriately managed by the multidisciplinary team.
Education and information
The adherence to follow-up after VHSIL treatment is essential, due to the risk of recurrence; however, no study was performed with this aim. Thus, there is no evidence about effective interventions for enhancing patients’ adherence to follow-up. Providing patients oral and written information on their medical situation appears, however, to be justified as it might improve patients’ awareness of symptoms and the need for regular clinical vulvar examination.176 When considering patients’ adherence to prescribed medication, current intervention methods seem to be not very effective, but are likely to be more successful when repeated.177 178 This suggests that information delivered to these affected patients should be multimedial, using various supports, and repeated over time.
Reconstructive surgery
Limited evidence is available regarding indications for reconstructive surgery and procedure selection for patients diagnosed with vulvar precancer lesions, and generally comes from retrospective, observational, and descriptive studies.90 179
Therefore, patients should be consulted before surgery by a team experienced in the field of vulvar and reconstructive surgery, with all members using consistent terminology based on well-defined and reproducible anatomic landmarks.180 In general, premalignant vulvar lesions are excised in a conservative fashion, preserving as much of the vulvar anatomy and function as possible. Surgery ranges from a local excision to skinning (superficial) vulvectomy with the removal of the clitoral hood. The majority of wounds after being locally excised, if not distorting the local anatomy, are closed primarily and do not require reconstructive surgery. The larger the size of the excision of a vulvar premalignant lesion, the more the quality of life and sexual function decreases without reconstruction.181 Therefore, the method of reconstruction should be individually tailored to the size and site of the vulvar defect. Reconstructive procedures are aimed at tension-free skin closure, maintenance of vulvovaginal anatomy, and appearance without shrinkage of vaginal and urethral introitus. It is important to avoid their lateral displacement and preserve cosmesis, sensation, and sexual function.182 Skills in basic plastic surgery procedures are consequently required.
Where a primary closure without tension is not possible, the defect may be closed by rotated or transposed local cutaneous flaps, although wound size exceeding 5 cm might be a limiting factor.183–185
Superficial (skinning) vulvectomy with subsequent grafting of split or full thickness skin can be applied in a limited group of patients with confluent multifocal lesions or involving clitoris, urethra, vaginal introitus, or anus not responding to medical therapy. Skin grafts are usually taken from the groin, mons pubis, or inner thigh. Recently, dermal substitutes less prone to wound contraction and more pliable than grafts are starting to be applied in reconstructive surgery.186 Dermal substitutes are collagen-based regenerative matrices, either acellular or synthetic, placed in direct contact with the wound and promoting autologous and spontaneous skin regeneration. These procedures allow the preservation of the shape and functional integrity of the vulva.187–191
Where extensive excision is performed, traditional fasciocutaneous and myocutaneous local or regional advancement flaps remain the best choice, and more advanced perforator flaps are usually not needed.179 185 192–195
Teleconsulting
Telemedicine is broadly defined as the ‘use of electronic information and communication technologies to provide and support healthcare when distance separates the patient and the healthcare professional’.196 In the last 30 years, this field has undergone a huge expansion and many subspecialties are trusting this type of healthcare (eg, telecolposcopy).197 Vulvar pathology could follow the example of tele-dermatoscopy, in which patients send digital photographs to their physician, who can examine skin lesions without visiting the patient. The follow-up of vulvar dermatoses (eg, lichen sclerosus) could be carried out using teleconsulting; some dermatologists are already doing so.198 Furthermore, to achieve an effective vulvar examination, patients would need to collect images of their external genitalia, improving the vulvar self-examination, which could lead to an early diagnosis and treatment of vulvar pathologies.176
Quality of life and psychological sequelae of vulvar pre-invasive lesion treatment
Pre-invasive vulvar lesions deserve specific attention because they affect not only functionality and body image but also psychosexual factors. Symptoms of intraepithelial neoplasia (ie, burning and itching), together with a change in appearance of vulvar skin, may cause dyspareunia and feelings of being less attractive. Additionally, concern of infecting the partner in HPV-related VIN and the potential effect on future pregnancy might contribute to the emotional burden. Surgery may exacerbate, rather than relieve, sexual dysfunction due to postoperative scarring and anxiety of revealing their body. Usually, these women have a fear of recurrence or development of cancer. Overall, a lower quality of life was reported in women with VIN.199 Education and psychological support by gynecologists, psychiatrists, or psychologists, together with partner counseling, could help regain sexual confidence, restore sexual functioning, and increase quality of life.
Consensus statements
In the following pre-invasive lesions of vulva, immunohistochemistry is recommended in distinguishing difficult cases: p16, ki-67 p53 (squamous lesions), PAS-D, mucicarmine, CK 7, GCDFP-15, GATA3 (Paget’s disease of the vulva), s100, Melan-A, HMB 45 (melanoma in situ). Consensus: 100%
dVIN complete surgical excision of visible lesions is recommended to treat the lesion and to exclude invasive disease. Consensus: 93.3%
After dVIN excision, treatment of associated Lichen sclerosus and Lichen Planus with topical high potency corticosteroids is recommended to reduce the risk of recurrence/progression. Consensus: 100%
Colposcopy of cervix and vagina and inspection of the entire lower genital tract, including vulvar, perianal and anal region, is recommended in women diagnosed for VHSIL. Consensus: 93.3%
Multiple representative biopsies are recommended to exclude invasion before VHSIL non-excisional treatments (medical treatment, LASER vaporization, CUSA, PDT). Consensus: 100%
Imiquimod should be considered as a therapeutic option to preserve normal vulvar anatomy in VHSIL patients. Consensus: 100%
In case of positive margins after surgical excisional treatment of VHSIL, if clinical inspection doesn’t show a residual lesion, patients must be followed, and immediate re-excision is not recommended. Consensus: 100%
HPV vaccination adjuvant to surgical treatment may be considered with the aim to reduce VHSIL recurrences. Consensus: 846%
In patients treated for VHSIL, life-long surveillance for HPV related carcinomas is recommended. Consensus: 93.3%
In case of positive margins after surgical excisional treatment of vulvar Paget disease, if clinical inspection doesn’t show a residual lesion, patients must be followed, and immediate re-excision is not recommended. Consensus: 92.9%
In vulvar pre-invasive lesions treatment, surgeries resulting in significant distortion of the vulvar anatomy should be discouraged. Consensus: 92.9%
After vulvar pre-invasive lesions treatment, follow up should be modulated according to the risk of recurrence (Type of lesion, patients’ age and immunological conditions, other associated lower genital tract lesions). Consensus: 93.3%
ijgc-2021-003262supp003.xlsx (28.6KB, xlsx)
Footnotes
Presented at: The present document developed by ESGO, ISSVD, ECSVD, and EFC was presented at the 22nd European Gynaecological Oncology Congress of the European Society of Gynaecological Oncology, October 23rd–25th 2021 in Prague, Czech Republic, and is submitted to the International Journal of Gynecological Cancer and Journal of Lower Genital Tract Disease.
This article has been co-published with permission in Journal of Lower Genital Tract Disease and International Journal of Gynecological Cancer. All rights reserved in respect of Journal of Lower Genital Tract Disease, © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the ASCCP, and in respect of International Journal of Gynecological Cancer, © IGCS and ESGO 2022. Re-use permitted under CC BY. The articles are identical except for minor stylistic and spelling differences in keeping with each journal’s style. Either citation can be used when citing this article.
Contributors: The development group (including all authors) is collectively responsible for the decision to submit the paper for publication. All authors contributed to manuscript drafting under the lead of MP. All contributors have actively given personal input, reviewed the manuscript, and have given final approval before submission. MVB, MB independently reviewed the final manuscript.
Funding: All costs relating to the development process were covered by ESGO, ISSVD, ECSVD, and EFC funds.
Competing interests: CC: advisory boards for GSK and MSD, support for clinical research from Roche and TherAguiX; DQ: advisory boards for Mimark; EJ: advisory boards for MSD and Roche Diagnostics, grants for traveling from MSD; JB support for clinical research from Merck (Galilee Medical Center Research Fund), member of speakers’ bureau for MSD Israel. BET, BEE, CS, DH, FB, FP, JZ, LW, MB, MEC, MG, MP, MVB, NG, OR, PVB, VK, XC: no conflict of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Wilkinson EJ, Kneale B, Lynch PJ. Report of the ISSVD terminology committee. J Reprod Med Obstet Gynecol 1986;31:973–4. [Google Scholar]
- 2. Sideri M, Jones RW, Wilkinson EJ. Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD vulvar oncology subcommittee. J Reprod Med 2005;50:807–10 http://www.ncbi.nlm.nih.gov/pubmed/16419625 [PubMed] [Google Scholar]
- 3. Darragh TM, Colgan TJ, Cox JT, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis 2012;16:205–42. 10.1097/LGT.0b013e31825c31dd [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . International classification of diseases for mortality and morbidity statistics (11th Revision), 2018. Available: https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/913387730
- 5. Bornstein J, Bogliatto F, Haefner HK, et al. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) terminology of vulvar squamous intraepithelial lesions. J Low Genit Tract Dis 2016;20:11–14. 10.1097/LGT.0000000000000169 [DOI] [PubMed] [Google Scholar]
- 6. Crum C, Herrington C, McCluggage W. Tumours of the vulva; epithelial tumors. In: WHO classification of tumours of female reproductive organs. Lyon: IARC Press, 2014. [Google Scholar]
- 7. WHO Classification of Tumours Editorial Board . Female genital tumours. 5 edn. Lyon, 2020. https://tumourclassification.iarc.who.int/chapters/34 [Google Scholar]
- 8. Wilkinson EJ, Brown HM. Vulvar Paget disease of urothelial origin: a report of three cases and a proposed classification of vulvar Paget disease. Hum Pathol 2002;33:549–54. 10.1053/hupa.2002.124788 [DOI] [PubMed] [Google Scholar]
- 9. Gershenwald JE, Scolyer RA. Melanoma staging: American Joint Committee on Cancer (AJCC) 8th edition and beyond. Ann Surg Oncol 2018;25:2105–10. 10.1245/s10434-018-6513-7 [DOI] [PubMed] [Google Scholar]
- 10. Lebreton M, Carton I, Brousse S, et al. Vulvar intraepithelial neoplasia: classification, epidemiology, diagnosis, and management. J Gynecol Obstet Hum Reprod 2020;49. 10.1016/j.jogoh.2020.101801 [DOI] [PubMed] [Google Scholar]
- 11. Lukasiewicz E, Aractingi S, Flahault A. Incidence et prise en charge des condylomes acuminés externes en médecine générale. Ann Dermatol Venereol 2002;129:991–6 http://www.ncbi.nlm.nih.gov/pubmed/12442095 [PubMed] [Google Scholar]
- 12. Monsonégo J, Breugelmans J-G, Bouée S. Incidence, prise en charge et coût des condylomes acuminés anogénitaux chez les femmes consultant leur gynécologue en France. Gynécologie Obs Fertil 2007;35:107–13. 10.1016/j.gyobfe.2006.12.010 [DOI] [PubMed] [Google Scholar]
- 13. van Seters M, van Beurden M, de Craen AJM. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol Oncol 2005;97:645–51. 10.1016/j.ygyno.2005.02.012 [DOI] [PubMed] [Google Scholar]
- 14. Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol 2005;106:1319–26. 10.1097/01.AOG.0000187301.76283.7f [DOI] [PubMed] [Google Scholar]
- 15. Thuijs NB, Beurden M, Bruggink AH, et al. Vulvar intraepithelial neoplasia: incidence and long‐term risk of vulvar squamous cell carcinoma. Int J Cancer 2021;148:90–8. 10.1002/ijc.33198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Sanjosé S, Alemany L, Ordi J, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer 2013;49:3450–61. 10.1016/j.ejca.2013.06.033 [DOI] [PubMed] [Google Scholar]
- 17. van de Nieuwenhof HP, Massuger LFAG, van der Avoort IAM, et al. Vulvar squamous cell carcinoma development after diagnosis of VIN increases with age. Eur J Cancer 2009;45:851–6. 10.1016/j.ejca.2008.11.037 [DOI] [PubMed] [Google Scholar]
- 18. Bodelon C, Madeleine MM, Voigt LF, et al. Is the incidence of invasive vulvar cancer increasing in the United States? Cancer Causes Control 2009;20:1779–82. 10.1007/s10552-009-9418-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clifford GM, Georges D, Shiels MS, et al. A meta‐analysis of anal cancer incidence by risk group: toward a unified anal cancer risk scale. Int J Cancer 2021;148:38–47. 10.1002/ijc.33185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saleem AM, Paulus JK, Shapter AP, et al. Risk of anal cancer in a cohort with human papillomavirus-related gynecologic neoplasm. Obstet Gynecol 2011;117:643–9. 10.1097/AOG.0b013e31820bfb16 [DOI] [PubMed] [Google Scholar]
- 21. van der Zwan JM, Siesling S, Blokx WAM, et al. Invasive extramammary Paget’s disease and the risk for secondary tumours in Europe. Eur J Surg Oncol 2012;38:214–21. 10.1016/j.ejso.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 22. van der Linden M, Meeuwis KAP, Bulten J, et al. Paget disease of the vulva. Crit Rev Oncol Hematol 2016;101:60–74. 10.1016/j.critrevonc.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 23. Mendivil AA, Abaid L, Epstein HD, et al. Paget's disease of the vulva: a clinicopathologic institutional review. Int J Clin Oncol 2012;17:569–74. 10.1007/s10147-011-0325-0 [DOI] [PubMed] [Google Scholar]
- 24. Karam A, Dorigo O. Treatment outcomes in a large cohort of patients with invasive extramammary Paget's disease. Gynecol Oncol 2012;125:346–51. 10.1016/j.ygyno.2012.01.032 [DOI] [PubMed] [Google Scholar]
- 25. Niikura H, Yoshida H, Ito K, et al. Paget's disease of the vulva: clinicopathologic study of type 1 cases treated at a single institution. Int J Gynecol Cancer 2006;16:1212–5. 10.1136/ijgc-00009577-200605000-00040 [DOI] [PubMed] [Google Scholar]
- 26. Sanchez A, Rodríguez D, Allard CB, et al. Primary genitourinary melanoma: epidemiology and disease-specific survival in a large population-based cohort. Urol Oncol 2016;34:166.e7–166.e14. 10.1016/j.urolonc.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 27. Gungor T, Altinkaya SO, Ozat M, et al. Primary malignant melanoma of the female genital tract. Taiwan J Obstet Gynecol 2009;48:169–75. 10.1016/S1028-4559(09)60281-3 [DOI] [PubMed] [Google Scholar]
- 28. Behbahani S, Malerba S, Warren CJ, et al. Melanoma in situ and invasive melanoma of the vulva: an analysis of the National Cancer Database. J Am Acad Dermatol 2021;84:1744–9. 10.1016/j.jaad.2020.09.036 [DOI] [PubMed] [Google Scholar]
- 29. Mert I, Semaan A, Winer I, et al. Vulvar/vaginal melanoma: an updated surveillance epidemiology and end results database review, comparison with cutaneous melanoma and significance of racial disparities. Int J Gynecol Cancer 2013;23:1118–25. 10.1097/IGC.0b013e3182980ffb [DOI] [PubMed] [Google Scholar]
- 30. Faber MT, Sand FL, Albieri V, et al. Prevalence and type distribution of human papillomavirus in squamous cell carcinoma and intraepithelial neoplasia of the vulva. Int J Cancer 2017;141:1161–9. 10.1002/ijc.30821 [DOI] [PubMed] [Google Scholar]
- 31. Rakislova N, Saco A, Sierra A, et al. Role of human papillomavirus in vulvar cancer. Adv Anat Pathol 2017;24:201–14. 10.1097/PAP.0000000000000155 [DOI] [PubMed] [Google Scholar]
- 32. Thuijs NB, Berkhof J, Özer M, et al. DNA methylation markers for cancer risk prediction of vulvar intraepithelial neoplasia. Int J Cancer 2021;148:2481–8. 10.1002/ijc.33459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Swarts DRA, Voorham QJM, van Splunter AP, et al. Molecular heterogeneity in human papillomavirus-dependent and -independent vulvar carcinogenesis. Cancer Med 2018;7:4542–53. 10.1002/cam4.1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2016;25:1224–30. 10.1158/1055-9965.EPI-16-0019 [DOI] [PubMed] [Google Scholar]
- 35. del Pino M, Rodriguez-Carunchio L, Ordi J. Pathways of vulvar intraepithelial neoplasia and squamous cell carcinoma. Histopathology 2013;62:161–75. 10.1111/his.12034 [DOI] [PubMed] [Google Scholar]
- 36. Nooij LS, Ter Haar NT, Ruano D, et al. Genomic characterization of vulvar (pre)cancers identifies distinct molecular subtypes with prognostic significance. Clin Cancer Res 2017;23:6781–9. 10.1158/1078-0432.CCR-17-1302 [DOI] [PubMed] [Google Scholar]
- 37. Watkins JC, Howitt BE, Horowitz NS, et al. Differentiated exophytic vulvar intraepithelial lesions are genetically distinct from keratinizing squamous cell carcinomas and contain mutations in PIK3CA. Mod Pathol 2017;30:448–58. 10.1038/modpathol.2016.187 [DOI] [PubMed] [Google Scholar]
- 38. Tessier-Cloutier B, Kortekaas KE, Thompson E, et al. Major p53 immunohistochemical patterns in in situ and invasive squamous cell carcinomas of the vulva and correlation with TP53 mutation status. Mod Pathol 2020;33:1595–605. 10.1038/s41379-020-0524-1 [DOI] [PubMed] [Google Scholar]
- 39. Fatalska A, Rusetska N, Bakuła‐zalewska E. Inflammatory proteins HMGA2 and PRTN3 as drivers of vulvar squamous cell carcinoma progression. Cancers 2021;13:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hoang LN, Park KJ, Soslow RA, et al. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology 2016;48:291–302. 10.1016/j.pathol.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Regauer S, Reich O, Eberz B. Vulvar cancers in women with vulvar lichen planus: a clinicopathological study. J Am Acad Dermatol 2014;71:698–707. 10.1016/j.jaad.2014.05.057 [DOI] [PubMed] [Google Scholar]
- 42. Halonen P, Jakobsson M, Heikinheimo O, et al. Cancer risk of lichen planus: a cohort study of 13,100 women in Finland. Int J Cancer 2018;142:18–22. 10.1002/ijc.31025 [DOI] [PubMed] [Google Scholar]
- 43. Lewis FM, Harrington CI. Squamous cell carcinoma arising in vulval lichen planus. Br J Dermatol 1994;131:703–5. 10.1111/j.1365-2133.1994.tb04987.x [DOI] [PubMed] [Google Scholar]
- 44. Zaki I, Dalziel KL, Solomonsz FA, et al. The under-reporting of skin disease in association with squamous cell carcinoma of the vulva. Clin Exp Dermatol 1996;21:334–7. 10.1111/j.1365-2230.1996.tb00117.x [DOI] [PubMed] [Google Scholar]
- 45. Jones RW, Rowan DM, Kirker J, et al. Vulval lichen planus: progression of pseudoepitheliomatous hyperplasia to invasive vulval carcinomas. BJOG 2001;108:665–6. 10.1111/j.1471-0528.2001.00134.x [DOI] [PubMed] [Google Scholar]
- 46. Derrick EK, Ridley CM, Kobza-Black A, et al. A clinical study of 23 cases of female anogenital carcinoma. Br J Dermatol 2000;143:1217–23. 10.1046/j.1365-2133.2000.03891.x [DOI] [PubMed] [Google Scholar]
- 47. Regauer S, Reich O, Beham-Schmid C. Monoclonal γ-T-cell receptor rearrangement in vulvar lichen sclerosus and squamous cell carcinomas. Am J Pathol 2002;160:1035–45. 10.1016/S0002-9440(10)64924-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Regauer S. Extramammary Paget’s disease-a proliferation of adnexal origin? Histopathology 2006;48:723–9. 10.1111/j.1365-2559.2006.02405.x [DOI] [PubMed] [Google Scholar]
- 49. Reich O, Liegl B, Tamussino K, et al. p185HER2 overexpression and HER2 oncogene amplification in recurrent vulvar Paget's disease. Mod Pathol 2005;18:354–7. 10.1038/modpathol.3800243 [DOI] [PubMed] [Google Scholar]
- 50. Horn L-C, Purz S, Krumpe C, et al. COX-2 and Her-2/neu are overexpressed in Paget’s disease of the vulva and the breast: results of a preliminary study. Arch Gynecol Obstet 2008;277:135–8. 10.1007/s00404-007-0434-1 [DOI] [PubMed] [Google Scholar]
- 51. Tessier-Cloutier B, Asleh-Aburaya K, Shah V, et al. Molecular subtyping of mammary-like adenocarcinoma of the vulva shows molecular similarity to breast carcinomas. Histopathology 2017;71:446–52. 10.1111/his.13239 [DOI] [PubMed] [Google Scholar]
- 52. Garganese G, Inzani F, Mantovani G, et al. The vulvar immunohistochemical panel (VIP) project: molecular profiles of vulvar Paget's disease. J Cancer Res Clin Oncol 2019;145:2211–25. 10.1007/s00432-019-02975-3 [DOI] [PubMed] [Google Scholar]
- 53. Kang Z, Xu F, Zhang Q-an, et al. Oncogenic mutations in extramammary Paget's disease and their clinical relevance. Int J Cancer 2013;132:824–31. 10.1002/ijc.27738 [DOI] [PubMed] [Google Scholar]
- 54. Konstantinova AM, Shelekhova KV, Imyanitov EN, et al. Study of selected BRCA1, BRCA2, and PIK3CA mutations in benign and malignant lesions of anogenital mammary-like glands. Am J Dermatopathol 2017;39:358–62. 10.1097/DAD.0000000000000725 [DOI] [PubMed] [Google Scholar]
- 55. Lee M-W, Jee K-J, Gong G-Y, et al. Comparative genomic hybridization in extramammary Paget’s disease. Br J Dermatol 2005;153:290–4. 10.1111/j.1365-2133.2005.06589.x [DOI] [PubMed] [Google Scholar]
- 56. Gadducci A, Carinelli S, Guerrieri ME, et al. Melanoma of the lower genital tract: prognostic factors and treatment modalities. Gynecol Oncol 2018;150:180–9. 10.1016/j.ygyno.2018.04.562 [DOI] [PubMed] [Google Scholar]
- 57. Preti M, Igidbashian S, Costa S, et al. VIN usual type-from the past to the future. Ecancermedicalscience 2015;9:531. 10.3332/ecancer.2015.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van Beurden M, van der Vange N, de Craen AJ, et al. Normal findings in vulvar examination and vulvoscopy. Br J Obstet Gynaecol 1997;104:320–4. 10.1111/j.1471-0528.1997.tb11461.x [DOI] [PubMed] [Google Scholar]
- 59. Albuquerque A, Rios E, Schmitt F. Recommendations favoring anal cytology as a method for anal cancer screening: a systematic review. Cancers 2019;11. 10.3390/cancers11121942. [Epub ahead of print: 04 12 2019]. 10.3390/cancers11121942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Preti M, Scurry J, Marchitelli CE, et al. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol 2014;28:1051–62. 10.1016/j.bpobgyn.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 61. Hart WR. Vulvar intraepithelial neoplasia: historical aspects and current status. Int J Gynecol Pathol 2001;20:16–30. 10.1097/00004347-200101000-00003 [DOI] [PubMed] [Google Scholar]
- 62. Jin C, Liang S. Differentiated vulvar intraepithelial neoplasia: a brief review of clinicopathologic features. Arch Pathol Lab Med 2019;143:768–71. 10.5858/arpa.2018-0019-RS [DOI] [PubMed] [Google Scholar]
- 63. Preti M, Bucchi L, Ghiringhello B, et al. Risk factors for unrecognized invasive carcinoma in patients with vulvar high-grade squamous intraepithelial lesion at vulvoscopy-directed biopsy. J Gynecol Oncol 2017;28:e27. 10.3802/jgo.2017.28.e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maclean AB. Vulval cancer: prevention and screening. Best Pract Res Clin Obstet Gynaecol 2006;20:379–95. 10.1016/j.bpobgyn.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 65. Lynch PJ, Moyal-Barracco M, Scurry J. ISSVD terminology and classification of vulvar dermatological disorders: an approach to clinical diagnosis. J Low Genit Tract Dis 2011;2012:339–44. [DOI] [PubMed] [Google Scholar]
- 66. Preti M, Micheletti L, Massobrio M, et al. Vulvar Paget disease: one century after first reported. J Low Genit Tract Dis 2003;7:122–35. 10.1097/00128360-200304000-00009 [DOI] [PubMed] [Google Scholar]
- 67. Terlou A, Blok LJ, Helmerhorst TJM, et al. Premalignant epithelial disorders of the vulva: squamous vulvar intraepithelial neoplasia, vulvar Paget’s disease and melanoma in situ. Acta Obstet Gynecol Scand 2010;89:741–8. 10.3109/00016341003739575 [DOI] [PubMed] [Google Scholar]
- 68. Sadownik LA, Crawford RI. Post-surgical treatment of melanoma in situ of the vulva with imiquimod. J Obstet Gynaecol Can 2010;32:771–4. 10.1016/S1701-2163(16)34619-9 [DOI] [PubMed] [Google Scholar]
- 69. Venkatesan A. Pigmented lesions of the vulva. Dermatol Clin 2010;28:795–805. 10.1016/j.det.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 70. Preti M, Mezzetti M, Robertson C, et al. Inter-observer variation in histopathological diagnosis and grading of vulvar intraepithelial neoplasia: results of a European collaborative study. BJOG 2000;107:594–9. 10.1111/j.1471-0528.2000.tb13298.x [DOI] [PubMed] [Google Scholar]
- 71. Dasgupta S, de Jonge E, Van Bockstal MR. Histological interpretation of differentiated vulvar intraepithelial neoplasia (dVIN) remains challenging—observations from a bi-national ring-study. Virchows Arch (Published Online First: 8 March 2021). [DOI] [PMC free article] [PubMed]
- 72. Singh N, Leen SL, Han G, et al. Expanding the morphologic spectrum of differentiated VIN (dVIN) through detailed mapping of cases with p53 loss. Am J Surg Pathol 2015;39:52–60. 10.1097/PAS.0000000000000291 [DOI] [PubMed] [Google Scholar]
- 73. van de Nieuwenhof HP, Bulten J, Hollema H, et al. Differentiated vulvar intraepithelial neoplasia is often found in lesions, previously diagnosed as lichen sclerosus, which have progressed to vulvar squamous cell carcinoma. Mod Pathol 2011;24:297–305. 10.1038/modpathol.2010.192 [DOI] [PubMed] [Google Scholar]
- 74. Heller DS, Day T, Allbritton JI, et al. Diagnostic criteria for differentiated vulvar intraepithelial neoplasia and vulvar aberrant maturation. J Low Genit Tract Dis 2021;25:57–70. 10.1097/LGT.0000000000000572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Day T, Wilkinson E, Rowan D, et al. Clinicopathologic diagnostic criteria for vulvar lichen planus. J Low Genit Tract Dis 2020;24:317–29. 10.1097/LGT.0000000000000532 [DOI] [PubMed] [Google Scholar]
- 76. Dasgupta S, Ewing-Graham PC, Swagemakers SMA, et al. Precursor lesions of vulvar squamous cell carcinoma - histology and biomarkers: a systematic review. Crit Rev Oncol Hematol 2020;147:102866. 10.1016/j.critrevonc.2020.102866 [DOI] [PubMed] [Google Scholar]
- 77. Konstantinova AM, Kazakov DV. Extramammary Paget disease of the vulva. Semin Diagn Pathol 2021;38:62–70. 10.1053/j.semdp.2020.09.007 [DOI] [PubMed] [Google Scholar]
- 78. Terlou A, van Seters M, Kleinjan A, et al. Imiquimod-induced clearance of HPV is associated with normalization of immune cell counts in usual type vulvar intraepithelial neoplasia. Int J Cancer 2010;127:2831–40. 10.1002/ijc.25302 [DOI] [PubMed] [Google Scholar]
- 79. van Seters M, Beckmann I, Heijmans-Antonissen C, et al. Disturbed patterns of immunocompetent cells in usual-type vulvar intraepithelial neoplasia. Cancer Res 2008;68:6617–22. 10.1158/0008-5472.CAN-08-0327 [DOI] [PubMed] [Google Scholar]
- 80. Singh K, Yeo Y, Honest H, et al. Antigen processing and correlation with immunological response in vulval intraepithelial neoplasia--a study of CD1a, CD54 and LN3 expression. Gynecol Oncol 2006;102:489–92. 10.1016/j.ygyno.2006.01.036 [DOI] [PubMed] [Google Scholar]
- 81. van Esch EMG, van Poelgeest MIE, Trimbos JBMZ, et al. Intraepithelial macrophage infiltration is related to a high number of regulatory T cells and promotes a progressive course of HPV-induced vulvar neoplasia. Int J Cancer 2015;136:E85–94. 10.1002/ijc.29173 [DOI] [PubMed] [Google Scholar]
- 82. Uyttenhove C, Pilotte L, Théate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003;9:1269–74. 10.1038/nm934 [DOI] [PubMed] [Google Scholar]
- 83. Abdel-Hady ES, Martin-Hirsch P, Duggan-Keen M. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res 2001;61:192–6 http://www.ncbi.nlm.nih.gov/pubmed/11196160 [PubMed] [Google Scholar]
- 84. Abdulrahman Z, Kortekaas KE, De Vos Van Steenwijk PJ, et al. The immune microenvironment in vulvar (pre)cancer: review of literature and implications for immunotherapy. Expert Opin Biol Ther 2018;18:1223–33. 10.1080/14712598.2018.1542426 [DOI] [PubMed] [Google Scholar]
- 85. Kenter GG, Welters MJP, Valentijn ARPM, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med 2009;361:1838–47. 10.1056/NEJMoa0810097 [DOI] [PubMed] [Google Scholar]
- 86. van Poelgeest MIE, Welters MJP, Vermeij R, et al. Vaccination against oncoproteins of HPV16 for noninvasive vulvar/vaginal lesions: lesion clearance is related to the strength of the T-cell response. Clin Cancer Res 2016;22:2342–50. 10.1158/1078-0432.CCR-15-2594 [DOI] [PubMed] [Google Scholar]
- 87. Press JZ, Allison KH, Garcia R, et al. FOXP3+ regulatory T-cells are abundant in vulvar Paget’s disease and are associated with recurrence. Gynecol Oncol 2011;120:296–9. 10.1016/j.ygyno.2010.10.019 [DOI] [PubMed] [Google Scholar]
- 88. Fujimura T, Kambayashi Y, Hidaka T, et al. Comparison of Foxp3+ regulatory T cells and CD163+ macrophages in invasive and non-invasive extramammary Paget's disease. Acta Derm Venereol 2012;92:625–8. 10.2340/00015555-1453 [DOI] [PubMed] [Google Scholar]
- 89. van der Linden M, van Esch E, Bulten J, et al. The immune cell infiltrate in the microenvironment of vulvar Paget disease. Gynecol Oncol 2018;151:453–9. 10.1016/j.ygyno.2018.09.026 [DOI] [PubMed] [Google Scholar]
- 90. Lawrie TA, Nordin A, Chakrabarti M, et al. Medical and surgical interventions for the treatment of usual-type vulval intraepithelial neoplasia. Cochrane Database Syst Rev 2016;2016. 10.1002/14651858.CD011837.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chin S, Scurry J, Bradford J, et al. Association of topical corticosteroids with reduced vulvar squamous cell carcinoma recurrence in patients with vulvar lichen sclerosus. JAMA Dermatol 2020;156:813–4. 10.1001/jamadermatol.2020.1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Te Grootenhuis NC, Pouwer A-FW, de Bock GH, et al. Prognostic factors for local recurrence of squamous cell carcinoma of the vulva: a systematic review. Gynecol Oncol 2018;148:622–31. 10.1016/j.ygyno.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 93. Yap JKW, Fox R, Leonard S, et al. Adjacent lichen sclerosis predicts local recurrence and second field tumour in women with vulvar squamous cell carcinoma. Gynecol Oncol 2016;142:420–6. 10.1016/j.ygyno.2016.06.019 [DOI] [PubMed] [Google Scholar]
- 94. Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol 2015;151:1061–7. 10.1001/jamadermatol.2015.0643 [DOI] [PubMed] [Google Scholar]
- 95. Bigby SM, Eva LJ, Fong KL. The natural history of vulvar intraepithelial neoplasia, differentiated type: evidence for progression and diagnostic challenges. Int J Gynecol Pathol 2016;35:574–84. [DOI] [PubMed] [Google Scholar]
- 96. Regauer S. Residual anogenital lichen sclerosus after cancer surgery has a high risk for recurrence: a clinicopathological study of 75 women. Gynecol Oncol 2011;123:289–94. 10.1016/j.ygyno.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 97. Athavale R, Naik R, Godfrey KA, et al. Vulvar intraepithelial neoplasia--the need for auditable measures of management. Eur J Obstet Gynecol Reprod Biol 2008;137:97–102. 10.1016/j.ejogrb.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 98. Bradbury M, Cabrera S, García-Jiménez A. Vulvar intraepithelial neoplasia: clinical presentation, management and outcomes in women infected with HIV. Aids 2016;30:859–67. [DOI] [PubMed] [Google Scholar]
- 99. Bruchim I, Gotlieb WH, Mahmud S, et al. HPV-related vulvar intraepithelial neoplasia: outcome of different management modalities. Int J Gynecol Obstet 2007;99:23–7. 10.1016/j.ijgo.2007.03.023 [DOI] [PubMed] [Google Scholar]
- 100. Fehr MK, Baumann M, Mueller M, et al. Disease progression and recurrence in women treated for vulvovaginal intraepithelial neoplasia. J Gynecol Oncol 2013;24:236–41. 10.3802/jgo.2013.24.3.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Frega A, Sopracordevole F, Scirpa P, et al. The re-infection rate of high-risk HPV and the recurrence rate of vulvar intraepithelial neoplasia (VIN) usual type after surgical treatment. Med Sci Monit 2011;17:CR532–5. 10.12659/MSM.881941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gentile M, Bianchi P, Sesti F, et al. Adjuvant topical treatment with imiquimod 5% after excisional surgery for VIN 2/3. Eur Rev Med Pharmacol Sci 2014;18:2949–52 http://www.ncbi.nlm.nih.gov/pubmed/25339491 [PubMed] [Google Scholar]
- 103. Hillemanns P, Wang X, Staehle S, et al. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO(2) laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecol Oncol 2006;100:271–5. 10.1016/j.ygyno.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 104. Kesterson JP, Lele S. Vulvar intraepithelial neoplasia 3 in women less than 35 years. J Low Genit Tract Dis 2009;13:196–9. 10.1097/LGT.0b013e318196bd23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kushnir CL, Fleury AC, Hill MC, et al. The use of argon beam coagulation in treating vulvar intraepithelial neoplasia III: a retrospective review. Gynecol Oncol 2013;131:386–8. 10.1016/j.ygyno.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 106. Leufflen L, Baermann P, Rauch P, et al. Treatment of vulvar intraepithelial neoplasia with CO(2) laser vaporization and excision surgery. J Low Genit Tract Dis 2013;17:446–51. 10.1097/LGT.0b013e318284c1ed [DOI] [PubMed] [Google Scholar]
- 107. Massad LS, Xie X, Darragh T. Genital warts and vulvar intraepithelial neoplasia: natural history and effects of treatment and human immunodeficiency virus infection. Obstet Gynecol 2011;118:831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Miller BE. Vulvar intraepithelial neoplasia treated with cavitational ultrasonic surgical aspiration. Gynecol Oncol 2002;85:114–8. 10.1006/gyno.2001.6577 [DOI] [PubMed] [Google Scholar]
- 109. Polterauer S, Catharina Dressler A, Grimm C, et al. Accuracy of preoperative vulva biopsy and the outcome of surgery in vulvar intraepithelial neoplasia 2 and 3. Int J Gynecol Pathol 2009;28:559–62. 10.1097/PGP.0b013e3181a934d4 [DOI] [PubMed] [Google Scholar]
- 110. Ribeiro F, Figueiredo A, Paula T. Vulvar intraepithelial neoplasia: evaluation of treatment modalities. J Low Genit Tract Dis 2012;16:313–7. [DOI] [PubMed] [Google Scholar]
- 111. Satmary W, Holschneider CH, Brunette LL, et al. Vulvar intraepithelial neoplasia: risk factors for recurrence. Gynecol Oncol 2018;148:126–31. 10.1016/j.ygyno.2017.10.029 [DOI] [PubMed] [Google Scholar]
- 112. Sykes P, Smith N, McCormick P, et al. High-grade vulval intraepithelial neoplasia (VIN 3): a retrospective analysis of patient characteristics, management, outcome and relationship to squamous cell carcinoma of the vulva 1989-1999. Aust N Z J Obstet Gynaecol 2002;42:75–80. 10.1111/j.0004-8666.2002.00075.x [DOI] [PubMed] [Google Scholar]
- 113. van Esch EMG, Dam MCI, Osse MEM, et al. Clinical characteristics associated with development of recurrence and progression in usual-type vulvar intraepithelial neoplasia. Int J Gynecol Cancer 2013;23:1476–83. 10.1097/IGC.0b013e3182a57fd6 [DOI] [PubMed] [Google Scholar]
- 114. Vlastos A-T, Levy LB, Malpica A, et al. Loop electrosurgical excision procedure in vulvar intraepithelial neoplasia treatment. J Low Genit Tract Dis 2002;6:232–8. 10.1097/00128360-200210000-00008 [DOI] [PubMed] [Google Scholar]
- 115. von Gruenigen VE, Gibbons HE, Gibbins K, et al. Surgical treatments for vulvar and vaginal dysplasia: a randomized controlled trial. Obstet Gynecol 2007;109:942–7. 10.1097/01.AOG.0000258783.49564.5c [DOI] [PubMed] [Google Scholar]
- 116. Wallbillich JJ, Rhodes HE, Milbourne AM, et al. Vulvar intraepithelial neoplasia (VIN 2/3): comparing clinical outcomes and evaluating risk factors for recurrence. Gynecol Oncol 2012;127:312–5. 10.1016/j.ygyno.2012.07.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gu Y, Zhu L, Li X, et al. Surgical treatment of usual type vulvar intraepithelial neoplasia: a study at three academic hospitals. Chin Med J 2014;127:784–6. [PubMed] [Google Scholar]
- 118. Zawislak AA, Price JH, Dobbs SP, et al. Contemporary experience with the management of vulval intraepithelial neoplasia in Northern Ireland. Int J Gynecol Cancer 2006;16:780–5. 10.1111/j.1525-1438.2006.00375.x [DOI] [PubMed] [Google Scholar]
- 119. de Witte CJ, van de Sande AJM, van Beekhuizen HJ, et al. Imiquimod in cervical, vaginal and vulvar intraepithelial neoplasia: a review. Gynecol Oncol 2015;139:377–84. 10.1016/j.ygyno.2015.08.018 [DOI] [PubMed] [Google Scholar]
- 120. van Seters M, Fons G, van Beurden M. Imiquimod in the treatment of multifocal vulvar intraepithelial neoplasia 2/3. Results of a pilot study. J Reprod Med 2002;47:701–5 http://www.ncbi.nlm.nih.gov/pubmed/12380448 [PubMed] [Google Scholar]
- 121. Mathiesen O, Buus S, Cramers M. Topical imiquimod can reverse vulvar intraepithelial neoplasia: a randomised, double-blinded study. Gynecol Oncol 2007;107:219–22. 10.1016/j.ygyno.2007.06.003 [DOI] [PubMed] [Google Scholar]
- 122. van Seters M, van Beurden M, ten Kate FJW, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med 2008;358:1465–73. 10.1056/NEJMoa072685 [DOI] [PubMed] [Google Scholar]
- 123. Terlou A, van Seters M, Ewing PC, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod: seven years median follow-up of a randomized clinical trial. Gynecol Oncol 2011;121:157–62. 10.1016/j.ygyno.2010.12.340 [DOI] [PubMed] [Google Scholar]
- 124. Tristram A, Hurt CN, Madden T, et al. Activity, safety, and feasibility of cidofovir and imiquimod for treatment of vulval intraepithelial neoplasia (RT³VIN): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol 2014;15:1361–8. 10.1016/S1470-2045(14)70456-5 [DOI] [PubMed] [Google Scholar]
- 125. Hurt CN, Jones SEF, Madden T-A, et al. Recurrence of vulval intraepithelial neoplasia following treatment with cidofovir or imiquimod: results from a multicentre, randomised, phase II trial (RT3VIN). BJOG 2018;125:1171–7. 10.1111/1471-0528.15124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Jones SEF, Hibbitts S, Hurt CN, et al. Human papillomavirus DNA methylation predicts response to treatment using cidofovir and imiquimod in vulval intraepithelial neoplasia 3. Clin Cancer Res 2017;23:5460–8. 10.1158/1078-0432.CCR-17-0040 [DOI] [PubMed] [Google Scholar]
- 127. Westermann C, Fischer A, Clad A. Treatment of vulvar intraepithelial neoplasia with topical 5% imiquimod cream. Int J Gynecol Obstet 2013;120:266–70. 10.1016/j.ijgo.2012.09.020 [DOI] [PubMed] [Google Scholar]
- 128. Le T, Menard C, Hicks-Boucher W, et al. Final results of a phase 2 study using continuous 5% imiquimod cream application in the primary treatment of high-grade vulva intraepithelial neoplasia. Gynecol Oncol 2007;106:579–84. 10.1016/j.ygyno.2007.05.019 [DOI] [PubMed] [Google Scholar]
- 129. Fehr MK, Hornung R, Degen A, et al. Photodynamic therapy of vulvar and vaginal condyloma and intraepithelial neoplasia using topically applied 5-aminolevulinic acid. Lasers Surg Med 2002;30:273–9. 10.1002/lsm.10048 [DOI] [PubMed] [Google Scholar]
- 130. Zawislak A, Donnelly RF, McCluggage WG, et al. Clinical and immunohistochemical assessment of vulval intraepithelial neoplasia following photodynamic therapy using a novel bioadhesive patch-type system loaded with 5-aminolevulinic acid. Photodiagnosis Photodyn Ther 2009;6:28–40. 10.1016/j.pdpdt.2009.03.004 [DOI] [PubMed] [Google Scholar]
- 131. Jones RW, MacLean AB. Re: ‘Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. ’ Gynecol Oncol 2006;101:371–2. [DOI] [PubMed] [Google Scholar]
- 132. Buchanan TR, Zamorano AS, Massad LS, et al. Risk of cervical and vaginal dysplasia after surgery for vulvar intraepithelial neoplasia or cancer: a 6 year follow-up study. Gynecol Oncol 2019;155:88–92. 10.1016/j.ygyno.2019.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ait Menguellet S, Collinet P, Debarge VH, et al. Management of multicentric lesions of the lower genital tract. Eur J Obstet Gynecol Reprod Biol 2007;132:116–20. 10.1016/j.ejogrb.2006.04.011 [DOI] [PubMed] [Google Scholar]
- 134. Kuroki LM, Frolova AI, Wu N, et al. Yield of cytology surveillance after high-grade vulvar intraepithelial neoplasia or cancer. J Low Genit Tract Dis 2017;21:193–7. 10.1097/LGT.0000000000000321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Cai Y, Sheng W, Xiang L, et al. Primary extramammary Paget’s disease of the vulva: the clinicopathological features and treatment outcomes in a series of 43 patients. Gynecol Oncol 2013;129:412–6. 10.1016/j.ygyno.2013.02.029 [DOI] [PubMed] [Google Scholar]
- 136. De Magnis A, Checcucci V, Catalano C, et al. Vulvar Paget disease: a large single-centre experience on clinical presentation, surgical treatment, and long-term outcomes. J Low Genit Tract Dis 2013;17:104–10. 10.1097/LGT.0b013e31826569a9 [DOI] [PubMed] [Google Scholar]
- 137. Black D, Tornos C, Soslow RA, et al. The outcomes of patients with positive margins after excision for intraepithelial Paget’s disease of the vulva. Gynecol Oncol 2007;104:547–50. 10.1016/j.ygyno.2006.09.017 [DOI] [PubMed] [Google Scholar]
- 138. Liu G, Yuan B, Wang Y, et al. Clinicopathologic study of vulvar Paget’s disease in China. J Low Genit Tract Dis 2014;18:281–4. 10.1097/LGT.0b013e3182a64a58 [DOI] [PubMed] [Google Scholar]
- 139. Borghi C, Bogani G, Ditto A, et al. Invasive Paget disease of the vulva. Int J Gynecol Cancer 2018;28:176–82. 10.1097/IGC.0000000000001131 [DOI] [PubMed] [Google Scholar]
- 140. van der Linden M, Meeuwis K, van Hees C, et al. The Paget trial: a multicenter, observational cohort intervention study for the clinical efficacy, safety, and immunological response of topical 5% imiquimod cream for vulvar Paget disease. JMIR Res Protoc 2017;6:e178. 10.2196/resprot.7503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Marchitelli C, Peremateu MS, Sluga MC, et al. Treatment of primary vulvar Paget disease with 5% imiquimod cream. J Low Genit Tract Dis 2014;18:347–50. 10.1097/LGT.0000000000000053 [DOI] [PubMed] [Google Scholar]
- 142. Fontanelli R, Papadia A, Martinelli F, et al. Photodynamic therapy with M-ALA as non surgical treatment option in patients with primary extramammary Paget's disease. Gynecol Oncol 2013;130:90–4. 10.1016/j.ygyno.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 143. Kingston NJ, Jones RW, Baranyai J. Recurrent primary vulvovaginal malignant melanoma arising in melanoma in situ - the natural history of lesions followed for 23 years. Int J Gynecol Cancer 2004;14:628–32. [DOI] [PubMed] [Google Scholar]
- 144. Bartoli C, Bono A, Clemente C, et al. Clinical diagnosis and therapy of cutaneous melanoma in situ. Cancer 1996;77:888–92. [DOI] [PubMed] [Google Scholar]
- 145. Heinzelmann-Schwarz VA, Nixdorf S, Valadan M, et al. A clinicopathological review of 33 patients with vulvar melanoma identifies c-kit as a prognostic marker. Int J Mol Med 2014;33:784–94. 10.3892/ijmm.2014.1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Abbasi NR, Shaw HM, Rigel DS, et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA 2004;292:2771–6. 10.1001/jama.292.22.2771 [DOI] [PubMed] [Google Scholar]
- 147. Tran KT, Wright NA, Cockerell CJ. Biopsy of the pigmented lesion—when and how. J Am Acad Dermatol 2008;59:852–71. 10.1016/j.jaad.2008.05.027 [DOI] [PubMed] [Google Scholar]
- 148. ACOG practice Bulletin No. 93: diagnosis and management of vulvar skin disorders. Obstet Gynecol 2008;111:1243–54. 10.1097/AOG.0b013e31817578ba [DOI] [PubMed] [Google Scholar]
- 149. Moxley KM, Fader AN, Rose PG, et al. Malignant melanoma of the vulva: an extension of cutaneous melanoma? Gynecol Oncol 2011;122:612–7. 10.1016/j.ygyno.2011.04.007 [DOI] [PubMed] [Google Scholar]
- 150. Garland SM, Joura EA, Ault KA, et al. Human papillomavirus genotypes from vaginal and vulvar intraepithelial neoplasia in females 15-26 years of age. Obstet Gynecol 2018;132:261–70. 10.1097/AOG.0000000000002736 [DOI] [PubMed] [Google Scholar]
- 151. Giuliano AR, Joura EA, Garland SM, et al. Nine-valent HPV vaccine efficacy against related diseases and definitive therapy: comparison with historic placebo population. Gynecol Oncol 2019;154:110–7. 10.1016/j.ygyno.2019.03.253 [DOI] [PubMed] [Google Scholar]
- 152. Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet 2007;369:1693–702. 10.1016/S0140-6736(07)60777-6 [DOI] [PubMed] [Google Scholar]
- 153. Joura EA, Garland SM, Paavonen J, et al. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ 2012;344:e1401. 10.1136/bmj.e1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ghelardi A, Marrai R, Bogani G, et al. Surgical treatment of vulvar HSIL: adjuvant HPV vaccine reduces recurrent disease. Vaccines 2021;9. 10.3390/vaccines9020083. [Epub ahead of print: 25 01 2021]. 10.3390/vaccines9020083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis 2016;20:180–3. 10.1097/LGT.0000000000000186 [DOI] [PubMed] [Google Scholar]
- 156. Corazza M, Borghi A, Gafà R, et al. Risk of vulvar carcinoma in women affected with lichen sclerosus: results of a cohort study. JDDG - J Ger Soc Dermatology 2019;17:1069–71. 10.1111/ddg.13961 [DOI] [PubMed] [Google Scholar]
- 157. Lewis FM, Tatnall FM, Velangi SS, et al. British Association of Dermatologists guidelines for the management of lichen sclerosus, 2018. Br J Dermatol 2018;178:839–53. 10.1111/bjd.16241 [DOI] [PubMed] [Google Scholar]
- 158. Nerantzoulis I, Grigoriadis T, Michala L. Genital lichen sclerosus in childhood and adolescence-a retrospective case series of 15 patients: early diagnosis is crucial to avoid long-term sequelae. Eur J Pediatr 2017;176:1429–32. 10.1007/s00431-017-3004-y [DOI] [PubMed] [Google Scholar]
- 159. Brickman C, Palefsky JM. Human papillomavirus in the HIV-infected host: epidemiology and pathogenesis in the antiretroviral era. Curr HIV/AIDS Rep 2015;12:6–15. 10.1007/s11904-014-0254-4 [DOI] [PubMed] [Google Scholar]
- 160. Konopnicki D, De Wit S, Clumeck N. HPV and HIV coinfection: a complex interaction resulting in epidemiological, clinical and therapeutic implications. Future Virol 2013;8:903–15. 10.2217/fvl.13.69 [DOI] [Google Scholar]
- 161. Smith AJB, Varma S, Rositch AF, et al. Gynecologic cancer in HIV-positive women: a systematic review and meta-analysis. Am J Obstet Gynecol 2019;221:194–207. 10.1016/j.ajog.2019.02.022 [DOI] [PubMed] [Google Scholar]
- 162. Ayakannu T, Murugesu S, Taylor AH, et al. The impact of focality and centricity on vulvar intraepithelial neoplasia on disease progression in HIV+ patients: a 10-year retrospective study. Dermatology 2019;235:327–33. 10.1159/000500469 [DOI] [PubMed] [Google Scholar]
- 163. Conley LJ, Ellerbrock TV, Bush TJ, et al. HIV-1 infection and risk of vulvovaginal and perianal condylomata acuminata and intraepithelial neoplasia: a prospective cohort study. Lancet 2002;359:108–13. 10.1016/S0140-6736(02)07368-3 [DOI] [PubMed] [Google Scholar]
- 164. Toby M, Conway K, Sethi G, et al. Usual vulval intraepithelial neoplasia in HIV-positive women – a case series. Int J STD AIDS 2016;27:1253–6. 10.1177/0956462415611513 [DOI] [PubMed] [Google Scholar]
- 165. Blitz S, Baxter J, Raboud J, et al. Evaluation of HIV and highly active antiretroviral therapy on the natural history of human papillomavirus infection and cervical cytopathologic findings in HIV-positive and high-risk HIV-negative women. J Infect Dis 2013;208:454–62. 10.1093/infdis/jit181 [DOI] [PubMed] [Google Scholar]
- 166. Massad LS, Silverberg MJ, Springer G, et al. Effect of antiretroviral therapy on the incidence of genital warts and vulvar neoplasia among women with the human immunodeficiency virus. Am J Obstet Gynecol 2004;190:1241–8. 10.1016/j.ajog.2003.12.037 [DOI] [PubMed] [Google Scholar]
- 167. Franzetti M, Adorni F, Parravicini C. Trends and predictors of non-AIDS-defining cancers in men and women with HIV infection: a single-institution retrospective study before and after the introduction of HAART. J Acquir Immune Defic Syndr 2013;62:414–20. [DOI] [PubMed] [Google Scholar]
- 168. Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant 2017;17:1563–73. 10.1111/ajt.14181 [DOI] [PubMed] [Google Scholar]
- 169. Meeuwis KAP, Hilbrands LB, IntHout J, et al. Cervicovaginal HPV infection in female renal transplant recipients: an observational, self-sampling based, cohort study. Am J Transplant 2015;15:723–33. 10.1111/ajt.13053 [DOI] [PubMed] [Google Scholar]
- 170. Reinholdt K, Thomsen LT, Dehlendorff C, et al. Human papillomavirus‐related anogenital premalignancies and cancer in renal transplant recipients: a Danish nationwide, registry‐based cohort study. Int J Cancer 2020;146:2413–22. 10.1002/ijc.32565 [DOI] [PubMed] [Google Scholar]
- 171. Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59–67. 10.1016/S0140-6736(07)61050-2 [DOI] [PubMed] [Google Scholar]
- 172. Preti M, Rotondo JC, Holzinger D, et al. Role of human papillomavirus infection in the etiology of vulvar cancer in Italian women. Infect Agent Cancer 2020;15:20. 10.1186/s13027-020-00286-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Meeuwis KAP, Melchers WJG, Bouten H, et al. Anogenital malignancies in women after renal transplantation over 40 years in a single center. Transplantation 2012;93:914–22. 10.1097/TP.0b013e318249b13d [DOI] [PubMed] [Google Scholar]
- 174. Chin-Hong PV. Human papillomavirus in kidney transplant recipients. Semin Nephrol 2016;36:397–404. 10.1016/j.semnephrol.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Chin-Hong PV, Reid GE, AST Infectious Diseases Community of Practice . Human papillomavirus infection in solid organ transplant recipients: guidelines from the American Society of Transplantation infectious diseases community of practice. Clin Transplant 2019;33:e13590. 10.1111/ctr.13590 [DOI] [PubMed] [Google Scholar]
- 176. Preti M, Selk A, Stockdale C, et al. Knowledge of vulvar anatomy and self-examination in a sample of Italian women. J Low Genit Tract Dis 2021;25:166–71. 10.1097/LGT.0000000000000585 [DOI] [PubMed] [Google Scholar]
- 177. Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2014;22. 10.1002/14651858.CD000011.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Conn VS, Ruppar TM. Medication adherence outcomes of 771 intervention trials: systematic review and meta-analysis. Prev Med 2017;99:269–76. 10.1016/j.ypmed.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Höckel M, Dornhöfer N. Vulvovaginal reconstruction for neoplastic disease. Lancet Oncol 2008;9:559–68. 10.1016/S1470-2045(08)70147-5 [DOI] [PubMed] [Google Scholar]
- 180. Micheletti L, Haefner H, Zalewski K, et al. The International Society for the Study of Vulvovaginal Disease Surgical Oncological Procedure Definitions Committee “Surgical Terminology for Vulvar Cancer Treatment”. J Low Genit Tract Dis 2020;24:62–8. 10.1097/LGT.0000000000000501 [DOI] [PubMed] [Google Scholar]
- 181. Likes WM, Stegbauer C, Tillmanns T, et al. Correlates of sexual function following vulvar excision. Gynecol Oncol 2007;105:600–3. 10.1016/j.ygyno.2007.01.027 [DOI] [PubMed] [Google Scholar]
- 182. Salgarello M, Farallo E, Barone-Adesi L, et al. Flap algorithm in vulvar reconstruction after radical, extensive vulvectomy. Ann Plast Surg 2005;54:184–90. 10.1097/01.sap.0000141381.77762.07 [DOI] [PubMed] [Google Scholar]
- 183. Reid R. Local and distant skin flaps in the reconstruction of vulvar deformities. Am J Obstet Gynecol 1997;177:1372–84. 10.1016/S0002-9378(97)70078-4 [DOI] [PubMed] [Google Scholar]
- 184. Julian CG, Callison J, Woodruff JD. Plastic management of extensive vulvar defects. Obstet Gynecol 1971;38:193–8 http://www.ncbi.nlm.nih.gov/pubmed/4327054 [PubMed] [Google Scholar]
- 185. Narayansingh GV, Cumming GP, Parkin DP, et al. Flap repair: an effective strategy for minimising sexual morbidity associated with the surgical management of vulval intra epithelial neoplasia. J R Coll Surg Edinb 2000;45:81–4. [PubMed] [Google Scholar]
- 186. Lucich EA, Rendon JL, Valerio IL. Advances in addressing full-thickness skin defects: a review of dermal and epidermal substitutes. Regen Med 2018;13:443–56. 10.2217/rme-2017-0047 [DOI] [PubMed] [Google Scholar]
- 187. Terlou A, Hage JJ, van Beurden M. Skinning clitorectomy and skin replacement in women with vulvar intra-epithelial neoplasia. J Plast Reconstr Aesthetic Surg 2009;62:341–5. 10.1016/j.bjps.2008.11.073 [DOI] [PubMed] [Google Scholar]
- 188. Rutledge F, Sinclair M. Treatment of intraepithelial carcinoma of the vulva by skin excision and graft. Am J Obstet Gynecol 1968;102:806–18. 10.1016/0002-9378(68)90508-5 [DOI] [PubMed] [Google Scholar]
- 189. Ayhan A, Tancer ZS, Doǧan L. Skinning vulvectomy for the treatment of vulvar intraepithelial neoplasia 2-3: a study of 21 cases. Eur J Gynaecol Oncol 1998;19:508–10 http://www.ncbi.nlm.nih.gov/pubmed/9863927 [PubMed] [Google Scholar]
- 190. Caglar H, Delgado G, Hreshchyshyn MM. Partial and total skinning vulvectomy in treatment of carcinoma in situ of the vulva. Obstet Gynecol 1986;68:504–7. [PubMed] [Google Scholar]
- 191. Rettenmaier MA, Berman ML, DiSaia PJ. Skinning vulvectomy for the treatment of multifocal vulvar intraepithelial neoplasia. Obstet Gynecol 1987;69:484–50. 10.1016/0020-7292(87)90073-7 [DOI] [PubMed] [Google Scholar]
- 192. Hage J, Beurden M, van BM. Reconstruction of acquired perineovulvar defects: a proposal of sequence. Semin Plast Surg 2011;25:148–54. 10.1055/s-0031-1281484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Tan B-K, Kang GC-W, Tay EH, et al. Subunit principle of vulvar reconstruction: algorithm and outcomes. Arch Plast Surg 2014;41:379–86. 10.5999/aps.2014.41.4.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Negosanti L, Sgarzani R, Fabbri E, et al. Vulvar reconstruction by perforator flaps: algorithm for flap choice based on the topography of the defect. Int J Gynecol Cancer 2015;25:1322–7. 10.1097/IGC.0000000000000481 [DOI] [PubMed] [Google Scholar]
- 195. Gentileschi S, Servillo M, Garganese G, et al. Surgical therapy of vulvar cancer: how to choose the correct reconstruction? J Gynecol Oncol 2016;27:e60. 10.3802/jgo.2016.27.e60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Weinstein RS, Krupinski EA, Doarn CR. Clinical examination component of telemedicine, telehealth, mHealth, and connected health medical practices. Med Clin North Am 2018;102:533–44. 10.1016/j.mcna.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 197. Ferris DG, Macfee MS, Miller JA, et al. The efficacy of telecolposcopy compared with traditional colposcopy. Obstet Gynecol 2002;99:248–54. 10.1016/s0029-7844(01)01671-4 [DOI] [PubMed] [Google Scholar]
- 198. Ekeland AG, Bowes A, Flottorp S. Effectiveness of telemedicine: a systematic review of reviews. Int J Med Inform 2010;79:736–71. 10.1016/j.ijmedinf.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 199. Kobleder A, Nikolic N, Hechinger M, et al. Perceived health-related quality of life in women with vulvar neoplasia: a cross sectional study. Int J Gynecol Cancer 2016;26:1313–9. 10.1097/IGC.0000000000000770 [DOI] [PubMed] [Google Scholar]
- 200. Brown HM, Wilkinson EJ. Uroplakin-III to distinguish primary vulvar Paget disease from Paget disease secondary to urothelial carcinoma. Hum Pathol 2002;33:545–8. 10.1053/hupa.2002.124787 [DOI] [PubMed] [Google Scholar]
- 201. Liao X, Liu X, Fan X, et al. Perianal Paget’s disease: a clinicopathological and immunohistochemical study of 13 cases. Diagn Pathol 2020;15:29. 10.1186/s13000-020-00952-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Parra-Medina R, Morales SD. Diagnostic utility of epithelial and melanocitic markers with double sequential immunohistochemical staining in differentiating melanoma in situ from invasive melanoma. Ann Diagn Pathol 2017;26:70–4. 10.1016/j.anndiagpath.2016.07.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ijgc-2021-003262supp001.pdf (26.3KB, pdf)
ijgc-2021-003262supp002.xlsx (20.3KB, xlsx)
ijgc-2021-003262supp003.xlsx (28.6KB, xlsx)