Abstract
Objective
To quantify sex differences in activity and severity of multiple sclerosis (MS) and how it depends on disease duration and time since clinical onset.
Methods
All Danish citizens with onset of relapsing MS since 1996 who have received disease-modifying therapy have been followed with annual or biannual control visits with mandatory notification of the Danish Multiple Sclerosis Registry. Men and women were compared by the inverse probability of being female. Relapse rates and changes in the Expanded Disability Status Scale (EDSS) scores were analysed with weighted general linear models, and we used weighted Cox regression for HRs between men and women for different EDSS endpoints.
Results
We included 3028 men and 6619 women. The weighted female:male relapse rate ratio was 1.16 (95% CI: 1.10 to 1.22) but after age 50 years, the difference disappeared. The annualised increase in EDSS was 0.07 in men (95% CI: 0.05 to 0.08) and 0.05 in women (95% CI: 0.04 to 0.06); p=0.017. With women as reference, the HR for reaching EDSS 4 was 1.34 (95% CI: 1.23 to 1.45; p<0.001), and for reaching EDSS 6 it was 1.43 (95% CI: 1.28 to 1.61; p<0.001). The diagnostic delay did not differ significantly between the sexes.
Conclusion
Women have more inflammatory disease activity in terms of relapses than men up to the age of menopause indicating that sex hormones may play a role. Men are more subject to the neurodegenerative component of MS than women, particularly after the age of 45 years.
Keywords: MULTIPLE SCLEROSIS, NEUROEPIDEMIOLOGY, CLINICAL NEUROLOGY, EPIDEMIOLOGY
Key messages.
What is already known on this topic
Multiple sclerosis (MS) is more prevalent in women than men and sex differences are shown in the disease course.
What this study adds
In a nationwide population-based cohort study, we quantified sex differences in the inflammatory and neurodegenerative component of MS development. The sex differences disappear after the age of 50 years indicating that the differences between younger men and women are primarily attributable to sex hormones.
How this study might affect research, practice or policy
Sex differences are important for the personalised management of MS, for scientists and healthcare providers.
Introduction
Multiple sclerosis (MS) is an immunological disease of the central nervous system predominantly affecting women with a female:male incidence ratio which has increased to about 2.1 2 The disease is characterised by attacks of localised white matter inflammation causing neurological symptoms with a tendency for remission and, later in the course of the disease, progressive symptoms caused by chronic smouldering inflammation also involving grey matter. The spectrum of severity of the disease is broad, ranging from severe disability within a few years to being free of disability or symptoms for decades or even life-long. Female patients with MS tend to show more disease activity in terms of relapses,3 and male patients with MS accrue more disability with time,4–6 and have a higher excess death rate than women.7 The sex hormones may play a role in this difference, but genes on the X chromosome may also exert effect on the immune system independent of the sex hormones.8 The hormonal component of a sex effect on MS in women is expected to fade away with increasing age, particularly after menopause.9
In the first part of the study, we aim to quantify the sex differences in terms of relapse rates and disability accumulation, and how they depend on time since onset, on current age and on age at onset of MS using a complete nationwide cohort of patients who have been treated with disease-modifying therapy (DMT) for relapsing-remitting MS (RRMS) or clinical isolated syndrome (CIS).
In the second and retrospective part of the study, we look at how the delay of diagnosis has changed through the last 70 years in men and women and changes over time in proportion of male and female patients with non-motor symptoms at onset.
Subjects and methods
The data source is the nationwide Danish MS Registry (DMSR) in which all known cases of MS among Danish citizens since 1948 have been recorded with identity, diagnostic classification, year of onset and diagnosis, and initial course of MS. DMSR is based on clinical records sent from all Danish departments of neurology with backup from the Danish National Patient Registry,10 in which all inpatient or outpatient admissions to Danish hospitals are registered with dates, ID and and diagnosis codes of the International Classification of Diseases, version 10, ensuring high completeness of DMSR. Since 1996, DMSR has also recorded information about relapses and disability at each obligatory biannual or annual control visit for all patients under DMT. DMSR is now notified directly online from the 13 neurological departments that classify the patients according to the current diagnostic criteria, at present the McDonald-2017 criteria.11 The DMSR has been presented in detail elsewhere.12
Design
The first part of the study is a cohort follow-up study. We have restricted this part of the study to patients who have had clinical onset of relapsing MS or CIS since 1 January 1996 and have received DMT. Patients with progressive onset were not treated with DMT and were not included in this part of the study. We have selected this cohort for the follow-up study because it has been followed with regular control visits. We have indexed patients by date of birth, date of onset and date of entry, which is the date of the commencement of the first DMT. From this time, relapses and Expanded Disability Status Scale (EDSS) scores have been recorded systematically. End of follow-up was 23 July 2021 or at the final discontinuation of DMT, whichever came first. Patients were followed irrespective of temporary discontinuation of treatment.
The second, retrospective and descriptive part of the study includes all patients with MS in Denmark who were prevalent in 1949, and all patients with onset or diagnosis of MS from 1948 onwards.
Methods: the follow-up study
The endpoints of the follow-up part of the study are (1) sex differences in annualised relapse rates (ARRs) stratified by time since clinical onset and by current age; (2) sex differences in recorded EDSS scores stratified by time since entry and by current age; and (3) sex differences in cumulative probability of reaching the disability endpoints EDSS 3, EDSS 4, EDSS 6 and a score 3 in Pyramidal and Cerebellar Functional System since onset, given that the score did not revert within the remaining observation time; and if it happened near the end of follow-up, it should be confirmed by at least one subsequent observation.
Methods: the descriptive study
In this study, we investigate how diagnostic delays have changed over 70 years by sex, and how the proportion of men and women with sensory symptoms at onset varies through the decades. To avoid right truncation, we calculated the delay of diagnosis as the time from diagnosis backwards to clinical onset.
Statistical analyses
Men and women differ modestly as to clinical and demographic baseline variables. To make them comparable, we, in the follow-up study, used stabilised inverse probability treatment weights (sIPTWs) truncated by the 1/99 percentiles. In this context, ‘treatment’ means exposure by being a female. We calculated sIPTW using propensity scores for being a female given the other background variables: (1) age and disease duration at entry; (2) number of relapses in 24 months prior to entry; (3) diagnostic delay; (4) the first EDSS assessment after entry; (5) first symptom (motor or sphincter vs sensory or brainstem); and (6) the percentage of the observation time in which the patient has been treated with high-efficacy DMT. ARRs were analysed using weighted general linear models (GENLIN) with sex as an independent variable and counts of relapses as dependent variables, and logarithmic-transformed observation time since entry as offset, and the results were expressed as estimated marginal means. We also analysed annualised increase of EDSS using weighted GENLIN with sex as an independent variable, expressed as estimated marginal means. In the analyses of the subcohort with onset at or after age 50 years, we calculated the subcohort’s own sIPTWs. For data management, we used Visual Basic and SPSS V.25, and for statistical analyses we used SPSS V.25, but we performed the weighted Cox regressions using the statistical package R V.3.6.1. The curves in figure 1 tare sIPT-weighted Kaplan-Meier plots13 in which 95% CIs of the cumulative probabilities were calculated by the exponential variant of the method of Greenwood.14 The figures were created using R. The 95% CI of the time from diagnosis back to onset in figure 2, left panel, was obtained by bootstrapping with 1000 random samples per analysis.
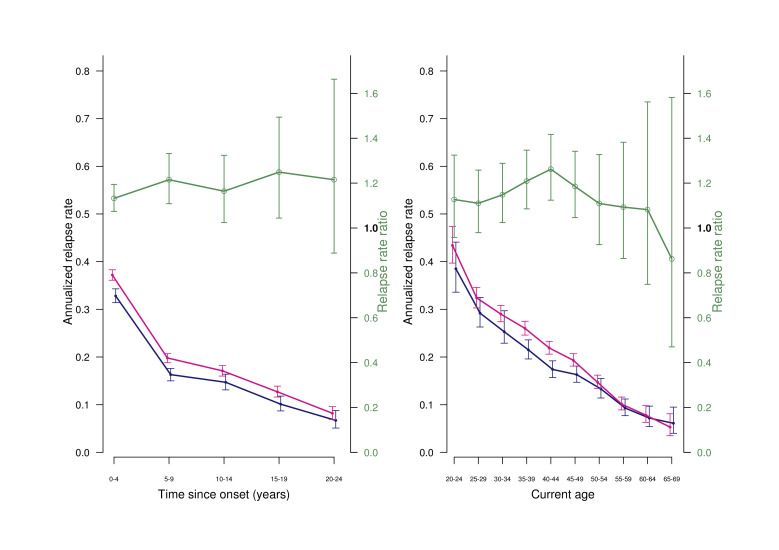
Figure 1.
Weighted estimated marginal means of annualized relapse rates and female: male relapse rate ratios in patients still under treatment, by sex. Left panel by time since onset. Right panel by current age, with 95% CI. Blue: men; red, women; green: relapse rare ratios. Vertical bars: 95% CI
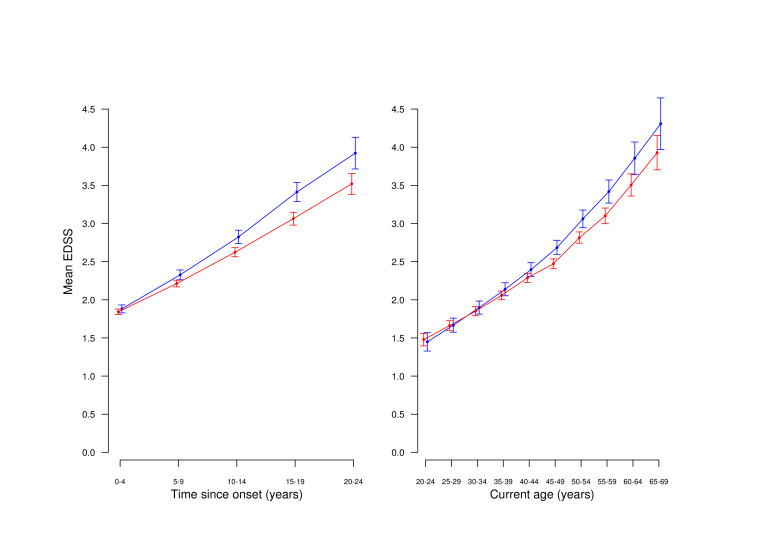
Figure 2.
Weighted estimated marginal mean EDSS in patients still under treatment, by sex. Left panel: by time since onset. Right panel: by current age. Blue: men; red: women.
Sensitivity analysis
Where sIPTW from propensity scores adjusts for the association between the covariates and the exposure in question, traditional analysis adjusts for the effect of the covariates on the endpoints. We repeated some of the key analyses without weighting but with adjustment for the same covariates that we used for computing the propensity scores (see above).
Results
The follow-up study
In the DMSR, 13 645 patients had been registered with onset of MS or CIS since 1 January 1996 (4494 men and 9151 women; female:male ratio 2.03). The proportion of patients with primary progressive MS (PPMS) was 12.0% (15.8% among men and 12.0% among women) with a decreasing trend. Among them, we extracted 10 770 patients with an approved diagnosis of relapsing-remitting MS, secondary progressive MS, or CIS who had started DMT after 1 June 1996 (3397 men and 7373 women; female:male ratio 2.17). Among them, we included 9647 (3028 men and 6619 women; female:male ratio 2.19) who had been followed with relapses and EDSS for at least 1 year and at least two control visits. The baseline characteristics before and after weighting are shown in table 1 from which it appears that the two sexes even before weighting were well balanced as to the demographical and clinical parameters. Of the patients, 461 (185 men and 276 women) were censored because of final discontinuation of treatment before follow-up in the sense that they did not take up again treatment within the follow-up period. The remaining patients were still under treatment at follow-up by July 2021. Men who finally stopped treatment before the end of follow-up had a mean of last recorded EDSS score of 3.62 as opposed to 2.76 in men who were still on treatment at follow-up date. The corresponding mean EDSS scores for women were 3.50 and 2.54.
Table 1.
Baseline variables in the subgroup of patients with onset from 1996 treated with DMT
| Variable | Unweighted | sIPT-weighted | ||
| Men | Women | Men | Women | |
| N=3028 | N=6619 | N~3018 | N~6625 | |
| Mean age at onset (SD) | 34.3 (10.5) | 33.5 (10.2) | 33.8 (10.4) | 33.8 (10.3) |
| Mean age at entry (SD) | 37.7 (11.2) | 37.2 (10.8) | 37.4 (11.1) | 37.4 (10.8) |
| Mean disease duration at entry (SD) | 2.91 (3.74) | 3.17 (4.05) | 3.06 (3.86) | 3.09 (3.99) |
| Mean diagnostic delay, years (SD) | 2.49 (3.02) | 2.70 (3.32) | 2.62 (3.16) | 2.63 (3.24) |
| Mean EDSS score at entry (SD) | 2.19 (1.48) | 2.09 (1.41) | 2.18 (1.47) | 2.09 (1.41) |
| Mean relapses 24 months before entry (SD) | 1.63 (1.03) | 1.71 (1.09) | 1.68 (1.07) | 1.68 (1.06) |
| % with motor symptom at onset | 32.2 | 26.7 | 29.5 | 28.0 |
| Mean observation time (years) | ||||
| IQR | 8.52 (3.99–12.26) | 8.75 (4.17–12.48) | 8.67 (4.06–12.49) | 8.70 (4.12–12.38) |
| % of observation time with HE DMT* | 41.9 | 39.5 | 40.3 | 40.3 |
| Mean number of treatment switches per year of observation | 0.059 | 0.062 | 0.057 | 0.063 |
*HE DMT: high-efficacy DMT (natalizumab, fingolimod, ofatuzumab, alemtuzumab, cladribine, mitoxantrone, ocrelizumab, rituximab).
DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; sIPT, stabilised inverse probability treatment.
Sex differences in relapse rates
With sIPT-weighted GENLIN analyses, the female:male relapse rate ratio was 1.16 (95% CI: 1.10 to 1.22). The estimated marginal means of ARRs were 0.32 for women (95% CI: 0.31 to 0.33) and 0.28 for men (95% CI: 0.27 to 0.29). The difference was statistically significant (p<0.001).
In both men and women, the ARR decreased with time since onset, and the difference was maintained throughout the observation period (figure 1, left panel). ARR also decreased with current age in both men and women, but after age 50 years, the sex difference disappeared; (figure 1, right panel).
In the subgroup of patients with clinical onset at age 50 years or later, there were no differences between men and women. The female:male relapse rate ratio was 1.08 (95% CI: 0.94 to 1.26); p=0.26.
Increase of EDSS by time and age
The weighted analyses of estimated marginal means of EDSS worsening showed that through the observation time, men deteriorated by 0.065 EDSS points per year (95% CI: 0.054 to 0.075), and women deteriorated less, with 0.049 EDSS points per year (95% CI: 0.042 to 0.056). The male:female ratio was 1.0216 (95% CI: 1.003 to 1.029). The difference was statistically significant (p=0.0017).
In both sexes, mean values of EDSS score increased with time since onset as well as by current age. Figure 2, left panel, shows the mean EDSS by time since onset of the disease, and the right panel by time since birth (starting with age 20 years). Only after age 45 years men seem to accrete more disability than women.
In the subgroup of patients with clinical onset at age 50 years or later, there were no differences between men and women. The mean annualised increase in EDSS was 0.154 in women and 0.139 in men, and the male:female ratio (exponentiated regression coefficient) was 0.986 (95% CI: 0.936 to 1.038; p=0.58).
EDSS milestones
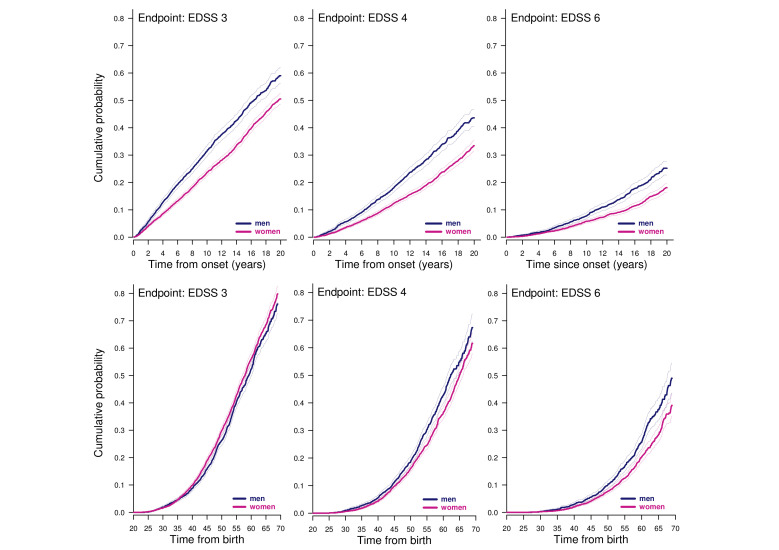
Whether using onset or age 20 years as a starting point, the cumulative probability of reaching the confirmed and sustained endpoints EDSS 3, EDSS 4, EDSS 6, and a score of 3 in Pyramidal and Cerebellar Functional Systems was higher in men than in women. Table 2 shows the HRs with women as reference for all the endpoints and onset as starting point, while table 3 has age 20 years as starting point. In both settings, the HRs for all endpoints were statistically significantly above unity. From the tables it appears that HRs were higher for more remote endpoints. figure 3 shows the weighted Kaplan-Meier curves for the EDSS endpoints with onset (upper panels) and birth as starting point (lower panels) left truncated at age 20 with 95% CIs. It appears from the figures that the proportionality assumption was not met for the closest endpoint EDSS 3.
Table 2.
Weighted Cox regression analyses of time from onset to irreversibly reaching disability milestones
| Endpoint | Events men | Censored men | Events women | Censored women | HR*, unweighted | HR*, weighted | 95% CI | Cox p value |
| EDSS 3† | 1854 | 988 | 3900 | 2568 | 1.300 | 1.227 | 1.138 to 1.323 | <0.001 |
| EDSS 4 | 774 | 2068 | 1322 | 4846 | 1.446 | 1.364 | 1.240 to 1.502 | <0.001 |
| EDSS 6 | 443 | 2399 | 701 | 5467 | 1.627 | 1.529 | 1.342 to 1.743 | <0.001 |
| Pyramidal FS score 2 | 619 | 2223 | 1041 | 5127 | 1.430 | 1.337 | 1.209 to 1.479 | <0.001 |
| Cerebellar FS score 2 | 195 | 2647 | 254 | 5914 | 1.872 | 1.796 | 1.483 to 2.174 | <0.0001 |
*HR with women as reference.
†Proportionality not met.
EDSS, Expanded Disability Status Scale; FS, Functional System.
Table 3.
Weighted Cox regression analyses of time from birth to irreversibly reaching disability milestones
| Endpoint | Events men | Censored men | Events women | Censored women | HR*, unweighted | HR*, weighted | 95% CI | Cox p value |
| EDSS 3† | 1854 | 988 | 3900 | 2568 | 1.152 | 1.175 | 1.090 to 1.266 | <0.01 |
| EDSS 4 | 774 | 2068 | 1322 | 4846 | 1.274 | 1.310 | 1.193 to 1.440 | <0.001 |
| EDSS 6 | 443 | 2399 | 701 | 5467 | 1.440 | 1.488 | 1.311 to 1.690 | <0.001 |
| Pyramidal FS score 2 | 619 | 2223 | 1041 | 5127 | 1.268 | 1.295 | 1.173 to 1.429 | <0.001 |
| Cerebellar FS score 2 | 195 | 2647 | 254 | 5914 | 1.520 | 1.586 | 1.322 to 1.902 | <0.0001 |
*HR with women as reference.
†Proportionality not met.
EDSS, Expanded Disability Status Scale; FS, Functional System.
Figure 3.
sIPTW-weighted ‘one-minus-survival’ Kaplan-Meier plots of with onset (upper panels) and birth (lower panels) as starting points. Shaded areas: 95% CI.
If we define ‘benign MS’ by escaping EDSS 2 within 20 years from onset, there was a significant difference between women and men. The cumulative probability of not reaching EDSS 2 by year 20 after onset was 22.5% in women (95% CI: 21.0 to 24.0) and 13.1% in men (95% CI: 10.7 to 15.4). Hence, the general finding of higher hazards for disability in men was also reflected in the lower probability of remaining ‘benign’ after 20 years of onset in this cohort of patients with relapsing MS selected by being treated with DMT.
Effect of stabilised probability treatment weighting
It appears from table 1 that the demographic and clinical background variables are well balanced between the two sexes even before weighting. As a result, the differences between weighted and unweighted analyses were small. In the GENLIN analyses of relapse rates, the unweighted and unadjusted exponentiated regression coefficient with men as reference was 1.153 and the weighted ditto was 1.159. Results from the unweighted Cox regressions are listed in tables 2 and 3, and they differ little from the results of the weighted analyses.
Sensitivity analysis
In the unweighted but fully adjusted GENLIN analysis of relapse rates in the two sexes, the exponentiated female:male regression coefficient was 1.15, indeed close to 1.16 in the weighted unadjusted analysis. We also performed the Cox regression analysis with onset as starting point and EDSS 4 as endpoint in the unweighted but fully adjusted analysis which was also adjusted for treatment (high or medium efficacy) as a time-dependent covariate. In this analysis, HR was 1.32 as compared with 1.34 in the weighted analysis (table 2). In all the analyses, the p value was <0.001. Hence, the results seem largely invariant to the method used for controlling confounding.
The descriptive study
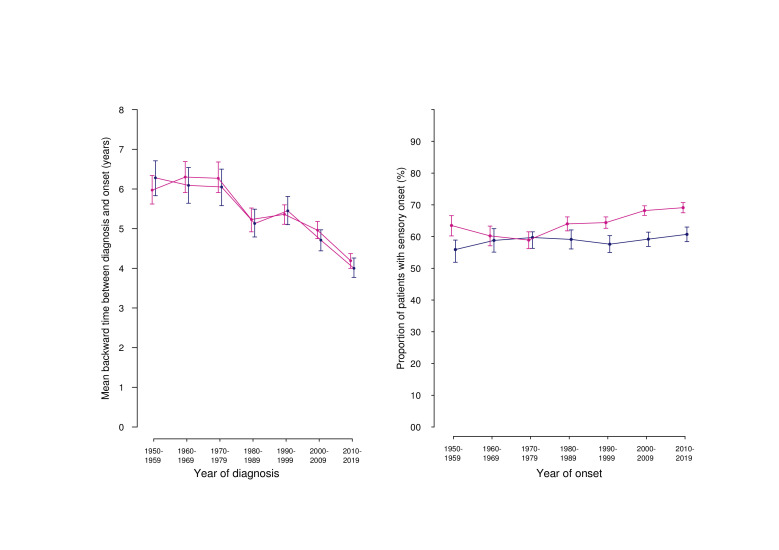
In the DMSR, we have since 1948 registered 31 175 records of patients with onset of MS according to the current diagnostic criteria. In 12 624 patients from the DMSR, the diagnosis had been classified as ‘Definite MS’ but the initial course was unknown. The initial course of the remaining patients with MS was relapsing in 87.9% (men 83.9%, women 89.9%) and primary progressive in 12.1% of the cases (men 16.1%; women 10.1%). In 29.8% of the cases (men 32.1; women 28.5), the initial symptom was classified as either pyramidal, cerebellar or sphincteric, and in 50.1% the presenting symptom was either sensory including optic nerve or related to the brainstem (men 45.2%; women 52.9%). In the remaining 20.1%, the presenting symptom was unknown or otherwise classified (men 22.7%; women 18.6%). Figure 4, right panel, shows that the proportion of women with sensory (non-motor) symptoms at onset has increased since the 1970s but has remained virtually constant in men. This parallels the increasing MS incidence in women in the same periods.15
Figure 4.
Left panel: mean retrospective time from diagnosis back to onset with 95% CI (obtained by bootstrapping). Right panel: Proportion of patients with sensory presenting symptoms with 95% CI. Blue: men; red: women.
The retrospective mean time from diagnosis back to onset was for the whole cohort and period 5.03 years in men (95% CI: 4.92 to 5.15) and 5.10 years in women (95% CI: 5.00 to 5.18), but this diagnostic delay decreased with time, and for patients diagnosed in the period 2010–2019, it was 4.00 years in men (95% CI: 3.77 to 4.26) and 4.19 years in women (95% CI: 4.00 to 4.37). The reason why it is higher in this part of the study than in the follow-up study (see table 1) is that we also included patients with PPMS and those who had not received DMT for other reasons. Figure 4, left panel, shows that there was no difference between men and women, over time.
Discussion
In this nationwide study with virtually complete follow-up of all patients with MS in Denmark with onset of relapsing MS or CIS since 1996, who have been subjected to DMT, we have found modest but statistically significant differences between the sexes as to clinical disease activity and progression of disability. We found that women have more inflammatory disease activity clinically manifested as 16% higher relapse rates than men. Men, on the other hand, accrue more disability with time than women. The difference between men and women in this respect is most conspicuously shown by the HRs for the EDSS milestones, ranging from 1.23 for EDSS 3 to 1.53 for EDSS 6. We also found that the difference in inflammatory disease activity between men and women faded away after the age of 50 years, whereas the sex difference in neurodegenerative component of MS became apparent after the age of 45 years and deepened with older age.
A shorter time to EDSS endpoints in men compared with women has been shown in several studies,4 5 16–19 but not in PPMS.20 However, Debouverie found no significant differences between male and female patients with MS after adjustments.6 Male patients with MS are more subject to cognitive decline than female patients with MS.21–24 In accordance with higher relapse rates in our study in women, also found by others,3 women with CIS revert sooner than men to MS.25 The rate of EDSS worsening increased significantly in women after menopause.26 The faster disability progression in men is in accordance with our previous study where we found that men with MS had a higher excess mortality than women with MS when compared with the background population. The excess death rate for men was 6.82 extra deaths per 1000 person-years. For women, it was 4.63.7
Male patients with MS had fewer contrast-enhancing lesions on MRI than women but a tendency to have a higher proportion of lesions evolved into black holes,27 and there was more grey matter atrophy in male patients with MS,28 29 and more white matter atrophy in female patients with MS.28 The finding of more contrast-enhancing lesions in female patients with MS27 could not be confirmed in a study of a large dataset from the Sylvia Lawry Centre of patients originally eligible for randomised clinical trials, where there was no predictive value of sex for the presence of gadolinium enhancement.30
The effects of sex on disease progression can be associated with genetic factors, such as genes on the X chromosomes or by sex hormones, or both, as reviewed by Voskuhl.31 The disappearance of sex differences in disease activity after age 50 years in our study indicates that sex hormones play an important role.
The main limitation of our study is that it only concerns patients with relapsing MS during DMT. Patients with PPMS were not included, because we less systematically collected information about EDSS in this group. The same is true for patients with relapsing MS who have never received DMT or after their final discontinuation of treatment, where they may have been lost for regular follow-up. Another limitation is that we have not included MRI results. That information has only been systematically recorded in recent years. However, they are based on routine–structured–descriptions from different MRI departments. In future studies, we will be able to include MRI data. Because we only analysed men and women while they were treated with DMT, the question arises whether the sex differences could be attributed to differences in how men and women respond to DMT. A large study involving pooled data from five clinical studies of patients with CIS treated with intramuscular interferon β-1a. showed no sex differences in efficacy of the treatment.32 The increasing number of available drugs may also have affected men and women differently.
The strength of the study is that it includes virtually all patients with MS in the whole country with onset since 1996 who have received DMT.
In summary, women are more subject to the inflammatory component of MS, while men are more subject to the neurodegenerative component. For the inflammatory component, the sex difference disappears after the age of 50 years, indicating that the differences between younger men and women may, to some extent, be attributable to sex hormones, but further investigations of their role are needed.
Footnotes
Contributors: MM was the guarantor and conceived the study, collected data from its sources, drafted and critically revised and interpreted the results. NK-H was responsible for data management and statistical analyses, contributed to the design and critically revised the manuscript and interpreted the results.
Funding: The DMSR is funded by the Danish Multiple Sclerosis Society.
Competing interests: MM has served on scientific advisory board, as consultant for, received support for congress participation or speaker honoraria from Biogen, Sanofi, Roche, Novartis, Merck, Alexion and Bristol Myers Squibb. The Danish MS Registry received research support from Biogen, Genzyme, Roche, Merck and Novartis. NK-H has nothing to declare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplemental information. The data underlying this article cannot be shared publicly due to data protection regulation. Data are accessible to authorised researchers after application to the Danish Health Data Authority and the board of the Danish Multiple Sclerosis Registry.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Ethics approval and informed consent are not required in Denmark for studies based on national health registries, as data are fully anonymised. Approval to store and analyse data was given by the Danish Data Protection Agency (reference number: P-2021-391).
References
- 1. Alonso A, Hernán MA. Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology 2008;71:129–35. 10.1212/01.wnl.0000316802.35974.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koch-Henriksen N, Magyari M. Apparent changes in the epidemiology and severity of multiple sclerosis. Nat Rev Neurol 2021;17:676–88. 10.1038/s41582-021-00556-y [DOI] [PubMed] [Google Scholar]
- 3. Kalincik T, Vivek V, Jokubaitis V, et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain 2013;136:3609–17. 10.1093/brain/awt281 [DOI] [PubMed] [Google Scholar]
- 4. Weinshenker BG, Rice GP, Noseworthy JH, et al. The natural history of multiple sclerosis: a geographically based study. 3. multivariate analysis of predictive factors and models of outcome. Brain 1991;114:1045–56. 10.1093/brain/114.2.1045 [DOI] [PubMed] [Google Scholar]
- 5. Runmarker B, Andersen O. Prognostic factors in a multiple sclerosis incidence cohort with twenty-five years of follow-up. Brain 1993;116:117–34. 10.1093/brain/116.1.117 [DOI] [PubMed] [Google Scholar]
- 6. Debouverie M. Gender as a prognostic factor and its impact on the incidence of multiple sclerosis in Lorraine, France. J Neurol Sci 2009;286:14–17. 10.1016/j.jns.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 7. Koch-Henriksen N, Laursen B, Stenager E, et al. Excess mortality among patients with multiple sclerosis in Denmark has dropped significantly over the past six decades: a population based study. J Neurol Neurosurg Psychiatry 2017;88:626–31. 10.1136/jnnp-2017-315907 [DOI] [PubMed] [Google Scholar]
- 8. Voskuhl RR, Sawalha AH, Itoh Y. Sex chromosome contributions to sex differences in multiple sclerosis susceptibility and progression. Mult Scler 2018;24:22–31. 10.1177/1352458517737394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bove R, Okai A, Houtchens M, et al. Effects of menopause in women with multiple sclerosis: an evidence-based review. Front Neurol 2021;12:554375. 10.3389/fneur.2021.554375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–73. 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 12. Magyari M, Joensen H, Laursen B, et al. The Danish multiple sclerosis registry. Brain Behav 2021;11:e01921. 10.1002/brb3.1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004;75:45–9. 10.1016/j.cmpb.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 14. Hosmer D, Lemeshow S. Applied survival analysis: regression modeling of time to event data. New York: John Wiley & Sons, 1999. [Google Scholar]
- 15. Koch-Henriksen N, Thygesen LC, Stenager E, et al. Incidence of MS has increased markedly over six decades in Denmark particularly with late onset and in women. Neurology 2018;90:e1954–63. 10.1212/WNL.0000000000005612 [DOI] [PubMed] [Google Scholar]
- 16. Runmarker B, Andersson C, Odén A, et al. Prediction of outcome in multiple sclerosis based on multivariate models. J Neurol 1994;241:597–604. 10.1007/BF00920623 [DOI] [PubMed] [Google Scholar]
- 17. Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain 2003;126:770–82. 10.1093/brain/awg081 [DOI] [PubMed] [Google Scholar]
- 18. Achiron A, Gurevich M. Gender effects in relapsing-remitting multiple sclerosis: correlation between clinical variables and gene expression molecular pathways. J Neurol Sci 2009;286:47–53. 10.1016/j.jns.2009.06.038 [DOI] [PubMed] [Google Scholar]
- 19. Alroughani RA, Akhtar S, Ahmed SF, et al. Clinical predictors of disease progression in multiple sclerosis patients with relapsing onset in a nation-wide cohort. Int J Neurosci 2015;125:831–7. 10.3109/00207454.2014.976641 [DOI] [PubMed] [Google Scholar]
- 20. Ribbons KA, McElduff P, Boz C, et al. Male sex is independently associated with faster disability accumulation in relapse-onset MS but not in primary progressive MS. PLoS One 2015;10:e0122686. 10.1371/journal.pone.0122686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beatty WW, Aupperle RL. Sex differences in cognitive impairment in multiple sclerosis. Clin Neuropsychol 2002;16:472–80. 10.1076/clin.16.4.472.13904 [DOI] [PubMed] [Google Scholar]
- 22. Schoonheim MM, Hulst HE, Landi D, et al. Gender-Related differences in functional connectivity in multiple sclerosis. Mult Scler 2012;18:164–73. 10.1177/1352458511422245 [DOI] [PubMed] [Google Scholar]
- 23. Donaldson E, Patel VP, Shammi P, et al. Why sex matters: a cognitive study of people with multiple sclerosis. Cogn Behav Neurol 2019;32:39–45. 10.1097/WNN.0000000000000188 [DOI] [PubMed] [Google Scholar]
- 24. Chaves AR, Kenny HM, Snow NJ, et al. Sex-specific disruption in corticospinal excitability and hemispheric (a)symmetry in multiple sclerosis. Brain Res 2021;1773:147687. 10.1016/j.brainres.2021.147687 [DOI] [PubMed] [Google Scholar]
- 25. Dobson R, Ramagopalan S, Giovannoni G. The effect of gender in clinically isolated syndrome (cis): a meta-analysis. Mult Scler 2012;18:600–4. 10.1177/1352458511426740 [DOI] [PubMed] [Google Scholar]
- 26. Bove R, Healy BC, Musallam A, et al. Exploration of changes in disability after menopause in a longitudinal multiple sclerosis cohort. Mult Scler 2016;22:935–43. 10.1177/1352458515606211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pozzilli C, Tomassini V, Marinelli F, et al. ‘Gender gap’ in multiple sclerosis: magnetic resonance imaging evidence. Eur J Neurol 2003;10:95–7. 10.1046/j.1468-1331.2003.00519.x [DOI] [PubMed] [Google Scholar]
- 28. Antulov R, Weinstock-Guttman B, Cox JL, et al. Gender-Related differences in MS: a study of conventional and nonconventional MRI measures. Mult Scler 2009;15:345–54. 10.1177/1352458508099479 [DOI] [PubMed] [Google Scholar]
- 29. Voskuhl RR, Patel K, Paul F, et al. Sex differences in brain atrophy in multiple sclerosis. Biol Sex Differ 2020;11:49. 10.1186/s13293-020-00326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barkhof F, Held U, Simon JH, et al. Predicting gadolinium enhancement status in MS patients eligible for randomized clinical trials. Neurology 2005;65:1447–54. 10.1212/01.wnl.0000183149.87975.32 [DOI] [PubMed] [Google Scholar]
- 31. Voskuhl RR. The effect of sex on multiple sclerosis risk and disease progression. Mult Scler 2020;26:554–60. 10.1177/1352458519892491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rudick RA, Kappos L, Kinkel R, et al. Gender effects on intramuscular interferon beta-1a in relapsing-remitting multiple sclerosis: analysis of 1406 patients. Mult Scler 2011;17:353–60. 10.1177/1352458510384605 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplemental information. The data underlying this article cannot be shared publicly due to data protection regulation. Data are accessible to authorised researchers after application to the Danish Health Data Authority and the board of the Danish Multiple Sclerosis Registry.