Abstract
Purpose
Staphylococcus aureus, cause a range of ocular diseases in humans, including noninfectious corneal infiltrative events (niCIE), infectious conjunctivitis and sight threatening microbial keratitis (MK). This study aimed to determine the possession of known virulence genes of S. aureus associated with MK and conjunctivitis, in strains isolated from these conditions and niCIE.
Methods
Sixty-three S. aureus strains—23 from MK, 26 from conjunctivitis, and 14 from niCIE—were evaluated for possession of genes. Polymerase chain reaction was used for the detection of mecA and 10 known virulence genes involved in MK (clfA, fnbpA, eap, coa, scpA, sspB, sspA, hla, hld, and hlg), 2 associated with conjunctivitis (pvl and seb).
Results
mecA was present in 35% of infections and 7% of niCIE strains (P = 0.05). It was not seen in infection strains from Australia. Adhesion genes were found in all strains except clfA, which was found in 75% of infection and 93% of niCIE strains. Invasion genes were found in higher frequency in infections strains—hlg (100% vs. 85%; P = 0.04) and hld (94% vs. 50%; P = 0.005)—compared with niCIE strains. Evasion genes were common in infection strains except scpA, which was found at a significantly higher frequency in niCIE strains (86%) compared with infection strains (45%; P = 0.001).
Conclusions
The higher rates of hlg and hld in strains isolated from infections than niCIE may have a role in pathogenesis, whereas scpA may be an important virulence factor during niCIEs.
Translational Relevance
This study has identified virulence factors involved in the ocular pathogenesis of S. aureus infections and niCIE.
Keywords: virulence determinants/genes, pathogenesis, ocular conditions
Introduction
Staphylococcus aureus is an opportunistic human pathogen causing both community and hospital acquired infections. Approximately 30% of humans are asymptomatic carriers and these are at higher risk of infection as well as being a source of infection for others.1,2 S. aureus can cause infection of various ocular sites such as keratitis,3 blepharitis,4 and conjunctivitis.5 S. aureus can also cause infectious6 and noninfectious or inflammatory contact lens–related keratitis,7 the later are collectively called noninfectious corneal infiltrative events (niCIE).8
S. aureus secretes toxins and other virulence determinants that play an important role in the pathogenesis of infections.9 S. aureus virulence factors are categorized based on their biological activities and include products involved in adhesion to host tissues or fomites (adhesins), evasion of host defense systems (evasins), and invasion of host tissue (invasins). Adhesins called microbial surface components recognizing adhesive matrix molecules recognize extracellular components.10,11 For S. aureus, these include fibronectin-binding proteins, collagen-binding proteins, clumping factors, and coagulase.12,13
Evasion of the host immune system is facilitated by collagen-binding proteins, staphylokinase, enterotoxins, toxic shock protein toxin, lipase, protein A, v8 protease, and leucocidin.14–16 The toxins alpha, beta, and gamma hemolysins; exfoliative toxins A and B; enterotoxin B; staphopain A and B; phospholipase; Panton-Valentine leukocidin; and hyaluronidase are involved in the invasion of cells and tissues.17–23 Some of these virulence factors can function within multiple biological activities, such as collagen-binding protein that is involved both in adhesion and invasion.24 Alpha toxin and gamma toxin are involved in evasion and invasion.25,26
The virulence factors of S. aureus that have been associated with microbial keratitis (MK) include genes encoding adhesins clfA,27 fnbpA,18,28,29 eap,18 and coa24 and evasins scpA, sspB,18,30, hla,20,21 sspA,31 and invasins coa24 hlg.25,26,32 The virulence factor of S. aureus that has been associated with conjunctivitis is pvl23; seb is reported to be involved in contact lens corneal infiltrative events.19
Most research has focused on investigating the virulence determinants associated with keratitis, with less information on those associated with conjunctivitis or niCIEs. Therefore, the aim of this study was to explore previously known virulence factors of S. aureus isolated from keratitis, conjunctivitis, and niCIE.
Methods
S. aureus Isolates
Sixty-three S. aureus clinical isolates recovered from ocular diseases were evaluated (Supplementary Table S1). Strains from the Bascom Palmer Institute (Miami, FL) was kindly provided by Dr Darlene Miller; those from Prince of Wales Hospital (Australia) were kindly provided by Dr Monica Lahra. All strains were donated without identifiable patient data. All strains were stored at −80°C in the culture collection of the School of Optometry and Vision Science at the University of New South Wales (UNSW). The genera and species of each strain was confirmed using the automated identification system VITEK 2 for gram-positive bacteria (BioMérieux, Baulkham Hills, NSW, Australia) according to the manufacturer's instructions.
Virulence Factors of S. aureus Strains
Genomic DNA from each S. aureus strain was extracted using QIAGEN DNeasy blood and tissue extraction kit (Hilden, Germany). The quantity of the extracted DNA was assessed using a spectrophotometer (Nanodrop ND-1000, ThermoFisher Scientific, Waltham, MA). The eluted DNA was stored at −20°C. Polymerase chain reaction (PCR) amplification and detection of the virulence genes was carried out using gene specific primers (Supplementary Table S2) as described previously.19,24,26,27,32,34–46,73 PCR was performed in a 25-µL reaction mix, containing 10 to 15 ng of template DNA. PCR amplification reactions were carried out using the PCR Master mix (ThermoFisher Scientific, Vilnius, Lithuania). The thermocycler conditions for amplification were initial denaturation at 94°C for 5 minutes for each primer, with various annealing temperatures and cycles specific for each primer (Supplementary Table S2) and final extension at 72°C for 2 minutes. Synthesized DNA fragments were visualized on 1.0% to 1.5% agarose gel containing GelRed (Biotium, Fremont, CA).
Bands of PCR products in agarose gels after electrophoresis were randomly sampled and sent to the Ramaciotti Centre for Genomic (UNSW, Sydney) for Sanger sequencing using their forward primers to confirm the gene sequences (Supplementary Table S2). Amplified PCR products were cleaned using Exosap-IT kit (ThermoFisher Scientific, Victoria, Australia) with BigDye v3.1 (ThermoFisher Scientific, Victoria, Australia) using Applied Biosystems 3730 DNA analyzer for Sanger sequencing, at a standard annealing temperature (50°C). The sequencing reaction clean-up was carried out using BigDye Xterminator Purification (Life Technologies, Vitoria, Australia). Fast Qc version 0.117 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc) was used to assess the quality of sequenced nucleotides using raw reads. Sequences were used for basic local alignment search tools searches conducted against the National Center for Biotechnology Information database to examine the similarity of sequences with available genes sequences of S. aureus.
Statistical Analysis
Differences in virulence gene between the disease groups; infection strains (MK+ conjunctivitis) and noninfectious (niCIE) were analyzed using the χ2 test (GraphPad Prism 2019, v8.0.2.263). Correlations between possession of mecA and oxacillin resistance or possession of virulence genes or being multidrug resistant and possession of virulence genes were analyzed using Spearman's rho. For all analyses, a P value of less than 0.05 was considered statistically significant.
Results
The genes randomly selected for Sanger sequencing were fnbpA, pvl, sspB, and clfA and were confirmed to belong to their respective genes in three US conjunctivitis (100, 102, 103) and three Australian niCIE (12, 20, and 24) strains.
Methicillin Resistance Gene (mecA)
In S. aureus isolates, possession of the methicillin resistance gene (mecA) was determined using PCR. A higher frequency of mecA gene was observed in infection strains 35% (MK, 22%; conjunctivitis, 46%) than niCIE strains (7%) (P = 0.05). However, mecA was not seen in any infection strains from Australia (0%) vs infection strains from the United States (35%; P = 0.0001) (Supplementary Table S3). Oxacillin susceptibility of these strains have been previously published,33 and there was a significant correlation between possession of mecA and oxacillin resistance (correlation coefficient, rs = 0.48, P = 0.005). The only other correlation was a negative association between possession of mecA and scpA (rs = −0.40, P = 0.001).
As previously reported, 87% of the isolates were multidrug resistant (MDR); that is, resistant to three or more antibiotics from different antibiotic groups. The following positive correlations between MDR and possession of virulence genes were found: coa (rs = 0.34, P = 0.007) and hlg (rs = 0.29, two-tailed P = 0.023), but there was a negative correlation between MDR and possessing seb (rs = –0.28; P = 0.025). Not surprisingly, there was a strong correlation between possession of mecA and oxacillin resistance (s = 0.48; P = 0.005). The only other correlation with mecA was for possession of scpA which was negatively correlated (rs = −0.402; P = 0.001).
The correlation between possession of mecA and oxacillin resistance was expected. The negative association between possession of mecA and scpA reflected the sensitivity of the niCIE strains to oxacillin and their possession of scpA and might suggest some incompatibility between possession of these genes or their associated mobile genetic elements, and this should be examined in future studies.
Adhesin Genes
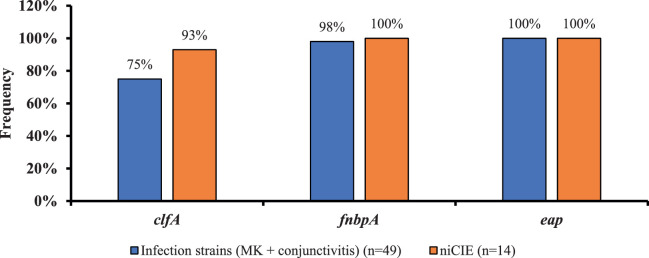
Figure 1 shows the possession of adhesin genes. Most genes were present in all strains. However, there was a difference in the frequency of possession of clfA, which was present in 75% of infection strains (MK, 69%; conjunctivitis, 85%) and 93% of niCIE strains; however, this difference was not significant (P = 0.26) (Supplementary Table S3).
Figure 1.
Frequency of three virulence genes involved in bacterial adhesion in ocular conditions (differences in frequency of possession of these genes were not significant between isolates from infections or niCIE).
Evasin Genes
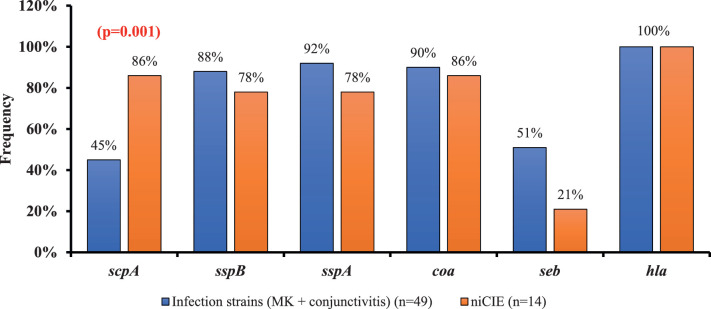
Most of the evasin genes (Fig. 2) were more common in infection strains than niCIE strains, except scpA. For seb, there was a trend (P = 0.06) for possession of this gene to be at a higher frequency in infection compared with niCIE isolates. For strains from infections, 45% (MK, 48%; conjunctivitis, 42%) possessed scpA, whereas 86% of niCIE strains possessed this gene (P = 0.001); scpA was more frequently observed in infection strains from Australia 83% (MK, 78%; conjunctivitis, 100%) than infection strain from the United States 22% (MK, 0%; conjunctivitis, 68%; P = 0.0001) (Supplementary Table S3). Infection strains (MK, 35%; conjunctivitis, 65%) had a higher frequency of seb than niCIE strains (21%; P = 0.0060 (Fig. 2). Most strains (84%) possessed sspB and, although there was a slightly higher frequency of sspB in the US MK strains (100%) compared with the Australian MK strains (78%), this difference was not significant (P = 0.22) (Supplementary Table S3). The gene sspA that encodes the v8 protease was present in infection strains 94% (MK, 100%; conjunctivitis, 82%), which was slightly more common than in niCIE strains (78%; P = 0.17) (Supplementary Table S3). Most infection strains (90%) (MK, 100%; conjunctivitis, 77%) possessed coa, as did most niCIE strains (86%; P = 0.6).
Figure 2.
Frequency of virulence genes involved in bacterial evasion in ocular conditions, gene showing P values, indicates a significant difference between strains isolated from infections and niCIE.
Invasin Genes
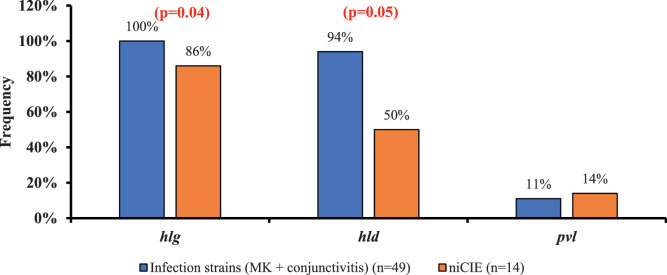
The genes hla and eap, which are involved in invasion of host tissues, were present in all strains, whereas there were differences in possession of hlg and hld (Fig. 3). Most infection strains (94%) (MK, 100%; conjunctivitis, 86%) possessed hld, whereas only 50% of niCIEs strains possessed this gene (P = 0.005) (Fig. 3). Similarly, 100% of infection strains (MK, 100%; conjunctivitis, 100%) possessed hlg, whereas only 86% of niCIEs strains possessed this gene (P = 0.04). Possession of pvl varied with condition and origin of the isolates, but those differences did not reach statistical significance (16% of US infections vs. 0% of Australian infection strains; P = 0.14 [Supplementary Table S3]; 14% of niCIE strains from Australia vs. 0% of infection strains from Australia; P = 0.18).
Figure 3.
Frequency of S. aureus virulence genes involved in bacterial invasion in ocular conditions, genes showing P values indicates a significant difference between strains isolated from infections or niCIE.
Discussion
This study has demonstrated distinct differences in S. aureus virulence determinants in strains isolated from ocular infections and niCIE. The hypothesis of the current study was that there would be more potential virulence determinants produced by infection strains than noninfectious strains. The hypothesis was confirmed, with PCR of infection strains frequently possessing the virulence genes scpA, seb, hld, and hlg compared with the niCIE strains.
Previous studies have reported the virulence factors of S. aureus that have been associated with corneal infections such as clfA as an important virulence factor in skin and soft tissue infections, fnbpA18,28 and coa24 described as mediating adhesion and initiating keratitis. Similarly, eap was shown as an important virulence determinant in a clinical S. aureus keratitis isolate.18 Alpha-toxin (hla) was shown as major virulence factor in both rabbit and murine models of keratitis.21 SspA/v8 caused severe pathology in rabbit corneas.31 Another study showed involvement of sspA and sspB in adhesion and invasion of human corneal epithelial cells.35 Similarly hlg was reported to contribute in corneal virulence,25,32 whereas pvl23 and seb19 were reported to contribute in conjunctivitis and contact lens infiltrative events virulence, respectively.
All ocular strains in the current study possessed the adhesins eap and fnbpA and most possessed clfA. The product of extracellular adhesion protein (eap) is an important virulence determinant of S. aureus, promoting adhesion and internalization of the organism into epithelial47 and other mammalian cells,48 and binding to plasma proteins including fibrinogen and fibronectin.49,58 Most (97.9%) of the S. aureus possess eap, and it is important in the colonization of cornea in S. aureus keratitis.50 So, the possession by all strains in the current study reinforces its role in adhesion and in ocular infections and inflammatory conditions. Most strains in the current study (98%) also possessed fnbpA. The product of fnbpA, fibronectin binding protein A, promotes adhesion to mammalian cells and initiation of biofilm formation.51 FnbpA and eap play contributary roles in the internalization of S. aureus.52 A study comparing a clinical S. aureus isolate with a less virulent laboratory strain identified fnbpA and eap as important virulence factors. The clinical S. aureus isolate produced more potentially important virulence factors than the less virulent laboratory strain, accounting for the ability of the clinical S. aureus isolate to cause more severe keratitis.18 Ocular surface cell injury, which may occur during contact lens wear53,54 or ocular surface disease,17 increases the presence of fibronectin on the ocular surface.34 This property may enhance the ability of S. aureus to cause ocular infection or inflammation. Similarly, the high frequency of possession of clfA, the product of which is a fibrinogen binding protein of S. aureus, suggests an important role for this gene/protein in ocular surface disease. Clumping factor is an important virulence factor of S. aureus in other skin and soft tissue infections55,56 and blood,57 often by increasing bacterial survival in the bloodstream.
Most strains possessed all the genes associated with evasion of the host defense systems (sspB, sspA, coa, and hla). The product of hla, namely, alpha hemolysin, is a major virulence factor of S. aureus in rabbit models of keratitis21,36 and is associated with corneal damage. Although an alpha toxin deficient strain of S. aureus can still produce the niCIE contact lens peripheral ulcers in a rabbit model,37 the gene seems to have been retained by strains causing niCIE and so it may be needed for survival of these strains before them being isolated from niCIE. The product of sspA, a serine protease (also called v8 protease), after maturation targets the Fc regions of immunoglobulins leading to partial loss of antigenic determinants, interfering in the interaction between cell surface antigens and immune effector cells mediated by immunoglobulins.56 This serine protease is also important in biofilm remodelling.59,60 This serine protease can cause severe pathology in rabbit corneas.36 All strains had one or more of the protease genes scpA (encoding the cysteine protease staphopain A), sspB (encoding the cysteine protease staphopain B), or sspA (encoding the serine protease v8). There is a complex interplay between scpA and sspB in which the product of sspA is important in adhesion and internalization of the bacteria into human corneal epithelial cells by activation of fnbp and assists with evasion by delaying the host immune system, whereas sspB is involved in the modulation of bacterial invasion.30 However for collagen binding adhesin (coa), a study used rabbit model, in which contact lenses soaked in S. aureus strains containing the collagen-binding adhesin (cna+) or its isogenic mutant lacking the adhesin (cna−), were placed on the injured cornea and the outcome showed that cna significantly contributed to bacterial adherence and corneal colonization and produced suppurative inflammation in a rabbit model of soft contact lens–associated bacterial keratitis more often than its collagen binding–negative isogenic mutant, which suggests that collagen-binding adhesin is involved in the pathogenesis of S. aureus keratitis.24 The possession of this gene by most strains in the current study suggests that it may be important for virulence in ocular infection and inflammation.
In the current study, more strains isolated from infections than niCIEs possessed seb (Fig. 2). The product of seb had been previously shown to stimulate high level of cytokines in cultured corneal epithelial cells to produce inflammation, especially interleukin 8, it did not stimulate the production of leukotriene B4 in an in vitro cell culture model.19 Because leukotriene B4 has been associated with contact lens peripheral ulcers,62 which are associated with the adhesion of S. aureus to contact lenses,63 it is perhaps not surprising that possession of seb was low in strains from niCIEs in the current study. S. aureus isolates from various forms of allergic conjunctivitis with concurrent corneal ulceration possessed seb more frequently compared with patients with no ulceration,64 which suggests a role in keratitis. The proinflammatory nature of the S. aureus super antigen seb has been shown to induce conjunctivitis with localized cutaneous swelling in 1 to 6 hours after accidental ocular exposure to seb in three US laboratory workers.61
There were also some differences in the frequency of genes often associated with the invasion of host tissues. All strains from infections possessed hlg, but this rate was significantly lower for strains from niCIEs. Strains deficient in hlg have decreased virulence in a rabbit model of MK25; the injection of gamma-toxin in cornea induced disease and caused corneal pathology,32 but perhaps this toxin is not as important in survival of strains that go on to cause niCIE. Gamma toxin (hlg) and Panton Valentine leucocidin (pvl)23 contribute to corneal virulence. In the current study, only 11% of the strains possessed pvl, and more conjunctivitis strains isolated from the United States (18%) possessed pvl than strains from Australia (0%). The possession of pvl, a bicomponent leukocidin that is responsible for leukocyte death, is often associated with community-acquired methicillin-resistant S. aureus (MRSA) strains.51 In the current study, a higher frequency of the strains from the United States possessed pvl so they were probably more likely to be community acquired. According to literature data pvl gene is present in approximately 2% to 5% of S. aureus isolates.65,66 The detection of pvl gene in strains, and their correlation with methicillin resistance, could be addressed in the future study. Eighty-four percent of all the strains in the current study possessed hld (MK, 100%; conjunctivitis, 88%), but this rate was significantly lower for strains from niCIEs (50%) (Fig. 3). However, strains producing hld, but not other hemolysins, produce minimal corneal virulence, suggesting that hld is not an important virulence factor in keratitis.67 In the current study significantly higher rates of hlg and hld in infections (MK+ conjunctivitis) and lower rates for strains from niCIE (Supplementary Table S3) indicates that hlg and hld may have a role in the pathogenesis of keratitis.
Previously reported antibiotic susceptibility data of these isolates33 demonstrated that although most of the strains were MDR, noninfectious (niCIE) strains were more susceptible to antibiotics than conjunctivitis strains, and conjunctivitis strains were more susceptible than MK strains. MK strains from Australia were more susceptible compared with MK strains from the United States. One MDR strain from niCIE group (S. aureus, 27) had a higher minimum inhibitory concentration to all tested multipurpose disinfectant solutions.33 The correlation between possession of mecA and oxacillin resistance was expected. The negative association between the possession of mecA and scpA might suggest some incompatibility between the possession of these genes or their associated mobile genetic elements, and this finding should be examined in future studies.
The presence of a gene does not necessarily mean the expression of that gene; the expression of S. aureus virulence factors is regulated by a complex regulatory system that enables the bacteria to adapt to different host environments. Several global regulators are influenced by environmental stimuli, such as nutrients and oxygen availability, cell density, pH, and osmolarity.68–71 Moreover, studies showed that host niche–specific factors also have an impact on S. aureus.72 Further gene expression study will help to better understand the S. aureus pathogenicity and the factors influencing the gene expression during ocular infections.
Because S. aureus MRSA strains are more likely to be MDR and difficult to treat, the association between ocular MRSA strains and S. aureus cytolysin (hla, hlg and pvl) has been studied widely.75,77 One study found ocular MRSA population was dominated by two major clonal complexes, CC8 (40%) and CC5 (47%), which are also common causes of MRSA infections in other body sites. Alpha-toxin (hla) secreted by S. aureus is shown to interfere with the corneal epithelial wound healing, it also promotes invasion of pathogen within the inner layers of cornea. The virulence factors hla, hlg, and pvl contribute to ocular tissue damage and inflammation worldwide.23,74 Because most community-acquired MRSA strains possess pvl and pvl has cytotoxic activity against many different types of immune cells,23 ocular S. aureus pvl–positive strains showed worst clinical outcomes (greater treatment time, healing, and ulcer size) and more surgical interventions compared with pvl–negative ocular S. aureus strains.76 Additionally, the prevalence of S. aureus virulence factors such as pvl, enterotoxin E (sea) or leucocidin E (LukE), among ocular isolates, have demonstrated that pvl and lukE were found in majority of ocular strains, whereas sea was less common.78,79 However, a recent study showed that the enrichment of enterotoxin superantigens in S. aureus ocular strains when compared with nonocular S. aureus strains.80
The present study has some limitations. This study used a convenience sample of strains within the culture collection of School of Optometry and Vision Science, UNSW Sydney, Australia. All strains from the United States were isolated in 2004. The Australian isolates were isolated over a longer period, from 1995 to 2018, with the majority from infection being isolated between 2006 and 2018 (17/18). Although the number of virulence genes of the Australian isolates were on average 10 ± 1 between 1995 and 2006 and in 2018 and the types of genes did not change, the determination of virulence genes with additional new S. aureus isolates from the United States might be useful to provide further insights into the involvement of virulence genes in the pathogenesis of keratitis and conjunctivitis and to see if genes change over time. Another limitation that isolates with polymorphism or closely related isolates may show false-negative or false-positive PCR results. This limitation could be addressed in the future study by analyzing single nucleotide polymorphisms in the genomes of the strains. Because these strains have not been genotyped previously, exploring whether these strains possess these known virulence gene would help future studies to explore the genetic relationship of isolates (sequence types or clonal complexes) along with agr profiles, which could also shed light on the pathogenic potential of the isolates from the epidemiologic point of view.
Overall, the findings of this study illustrate that the genes involved in adhesion, except clfA, were observed in most of the strains. Conversely, infection strains had a higher frequency of the genes involved in evasion and invasion compared with niCIEs. Whole-genome sequencing can provide a powerful tool to understand the virulence determinants associated with pathogenesis of infections and noninfectious ocular conditions. Additionally, the presence of a gene does not indicate whether the gene is expressed during ocular infections, and further animal model studies using gene knockout for hlg, hld, pvl, coa, seb, scpA, and sspB and their effect on keratitis could help to understand their involvement in its pathogenesis and potential therapy.
Supplementary Material
Acknowledgments
The authors thank Darlene Miller, Bascom Palmer Institute, Miami (USA), and Monica Lahra, Prince of Wales Hospital Sydney, for providing S. aureus MK strains.
Author Contributions: Conceptualization, M.A., M.D.P.W., F.S., A.K.V.; methodology, M.A., M.D.P.W., F.S., and A.K.V.; writing-original draft preparation, M.A.; writing-review and editing, M.D.P.W., F.S., and A.K.V.; supervision, M.D.P.W., F.S., and A.K.V.; funding acquisition, M.D.P.W. All authors have approved the final article.
Disclosure: M. Afzal, None; A. Vijay, None; F. Stapleton, None; M. Willcox, None
References
- 1. Kluytmans J, van Belkum A, Verbrugh H.. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev . 1997; 10(3): 505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gorwitz RJ, Kruszon-Moran D, McAllister SK., et al.. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis. 2008; 197(9): 1226–1234. [DOI] [PubMed] [Google Scholar]
- 3. Liesegang TJ. Bacterial keratitis. Infect Dis Clin North Am . 1992; 6(4): 815–829. [PubMed] [Google Scholar]
- 4. Shine WE, Silvany R, McCulley JP.. Relation of cholesterol-stimulated Staphylococcus aureus growth to chronic blepharitis. Invest Ophthalmol Vis Sci. 1993; 34(7): 2291–2296. [PubMed] [Google Scholar]
- 5. Azari AA, Barney NP.. Conjunctivitis: a systematic review of diagnosis and treatment. JAMA . 2013; 310(16): 1721–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Snyder C. Infiltrative keratitis with contact lens wear—a review. J Am Optom Assoc. 1995; 66(3): 160–177. [PubMed] [Google Scholar]
- 7. Suchecki JK, Ehlers WH, Donshik PC.. Peripheral corneal infiltrates associated with contact lens wear. Eye Contact Lens . 1996; 22(1): 41–46. [PubMed] [Google Scholar]
- 8. Sweeney DF, Jalbert I, Covey M, et al.. Clinical characterization of corneal infiltrative events observed with soft contact lens wear. Cornea. 2003; 22(5): 435–442. [DOI] [PubMed] [Google Scholar]
- 9. Otto M. Staphylococcus aureus toxins. Curr Opin Microbiol . 2014; 17: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ponnuraj K, Bowden MG, Davis S, et al.. A “dock, lock, and latch” structural model for a staphylococcal adhesin binding to fibrinogen. Cell. 2003; 115(2): 217–228. [DOI] [PubMed] [Google Scholar]
- 11. Patti JM, Höök M.. Microbial adhesins recognizing extracellular matrix macromolecules. Curr Opin Cell Biol . 1994; 6(5): 752–758. [DOI] [PubMed] [Google Scholar]
- 12. Vazquez V, Liang X, Horndahl JK, et al.. Fibrinogen is a ligand for the Staphylococcus aureus microbial surface components recognizing adhesive matrix molecules (MSCRAMM) bone sialoprotein-binding protein (Bbp). J Biol Chem . 2011; 286(34): 29797–29805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McAdow M, DeDent AC, Emolo C, et al.. Coagulases as determinants of protective immune responses against Staphylococcus aureus. Infect Immun . 2012; 80(10): 3389–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bokarewa MI, Jin T, Tarkowski A. Staphylococcus aureus: staphylokinase. Int J Biochem Cell Biol. 2006; 38(4): 504–509. [DOI] [PubMed] [Google Scholar]
- 15. Foster TJ, Geoghegan JA, Ganesh VK, Höök M.. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol . 2014; 12(1): 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jusko M, Potempa Kantyka T, Bielecka E, et al.. Staphylococcal proteases aid in evasion of the human complement system. J Innate Immun . 2014; 6(1): 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Callaghan RJ. The pathogenesis of Staphylococcus aureus eye infections. Pathogens (Basel, Switzerland). 2018; 7(1): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khan S, Cole N, Hume BHE, et al.. Identification of pathogenic factors potentially involved in Staphylococcus aureus keratitis using proteomics. Exp Eye Res. 2016; 8: 151–171. [DOI] [PubMed] [Google Scholar]
- 19. Thakur A, Clegg A, Chauhan A, Willcox M.. Modulation of cytokine production from an epiOcular corneal cell culture model in response to Staphylococcus aureus superantigen. Aust N Z J Ophthalmol. 1997; 25(4): 43–45. [DOI] [PubMed] [Google Scholar]
- 20. Yang J, Ji Y.. Investigation of Staphylococcus aureus adhesion and invasion of host cells. Methicillin-Resistant Staphylococcus aureus (MRSA) Protocols . 2014;187–194. [DOI] [PubMed] [Google Scholar]
- 21. O'Callaghan R, Callegan M, Moreau J, et al.. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect Immun . 1997; 65: 1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Girgis DO, Sloop GD, Reed JM, O'Callaghan RJ. Effects of toxin production in a murine model of Staphylococcus aureus keratitis. Invest Opthalmol Vis Sci. 2005; 46(6): 2064–2070. [DOI] [PubMed] [Google Scholar]
- 23. Zaidi T, Zaidi T, Yoong P, Pier GB.. Staphylococcus aureus corneal infections: effect of the Panton-Valentine Leukocidin (PVL) and antibody to PVL on virulence and pathology. Invest Opthalmol Vis Sci. 2013; 54(7): 4430–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rhem MN, Lech EM, Patti JM, et al.. The collagen-binding adhesin is a virulence factor in Staphylococcus aureus keratitis. Infect Immun . 2000; 68(6): 3776–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dajcs JJ, Thibodeaux BA, Girgis DO, O'Callaghan RJ.. Corneal virulence of Staphylococcus aureus in an experimental model of keratitis. DNA Cell Biol. 2002; 21(5-6): 375–382. [DOI] [PubMed] [Google Scholar]
- 26. Peacock SJ, Moore CE, Justice A, et al.. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect Immun . 2002; 70(9): 4987–4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lacey KA, Mulcahy ME, Towell AM, et al.. Clumping factor B is an important virulence factor during Staphylococcus aureus skin infection and a promising vaccine target. PLoS Pathogens . 2019; 15(4): e1007713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jett BD, Gilmore MS.. Internalization of Staphylococcus aureus by human corneal epithelial cells: Role of bacterial fibronectin-binding protein and host cell factors. Infect Immun . 2002; 70(8): 4697–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maurin C, Courrier E, He Z, et al.. Key role of Staphylococcal fibronectin-binding proteins during the initial stage of Staphylococcus aureus keratitis in humans. Front Cell Infect Microbiol . 2021; 11: 745659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hume E, Cole N, Khan S, Walsh BJ, Willcox M.. The role of staphopain a in Staphylococcus aureus keratitis. Exp Eye Res. 2020; 193: 107994. [DOI] [PubMed] [Google Scholar]
- 31. Caballero AR, Tang A, O'Callaghan R.. Staphylococcus aureus protease activity and ocular virulence. Invest Ophthalmol Vis Sci . 2012; 53(14): 6138. [Google Scholar]
- 32. Bierdeman MA, Torres AM, Caballero AR, Tang A, O'Callaghan RJ.. Reactions with antisera and pathological effects of Staphylococcus aureus gamma-toxin in the cornea. Curr Eye Res. 2017; 42(8): 1100–1107. [DOI] [PubMed] [Google Scholar]
- 33. Afzal M, Vijay AK, Stapleton F, Willcox M.. Susceptibility of ocular Staphylococcus aureus to antibiotics and multipurpose disinfecting solutions. Antibiotics. 2021; 10(10): 1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Atshan S, Shamsudin M, Karunanidhi A, et al.. Quantitative PCR analysis of genes expressed during biofilm development of methicillin resistant Staphylococcus aureus (MRSA). Infect Genet Evol. 2013; 18: 106–112 [DOI] [PubMed] [Google Scholar]
- 35. Cotar A-I, Chifiriuc M-C, Dinu S, et al.. Screening of molecular virulence markers in Staphylococcus aureus and Pseudomonas aeruginosa strains isolated from clinical infections. Int J Mol Sci . 2010; 11(12): 5273–5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoppe P-A, Hanitsch LG, Leistner R, et al.. Periorbital infections and conjunctivitis due to Panton-Valentine Leukocidin (PVL) positive Staphylococcus aureus in children. BMC Infect Dis . 2018; 18(1): 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu PZJ, Zhu H, Thakur A, Willcox M.. Comparison of potential pathogenic traits of staphylococci that may contribute to corneal ulceration and inflammation. Aust N Z J Ophthalmol . 1999; 27(3-4): 234–236. [DOI] [PubMed] [Google Scholar]
- 38. Greene C, McDevitt D, Francois P, et al.. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol Microbiol. 1995; 17(6): 1143–1152. [DOI] [PubMed] [Google Scholar]
- 39. Stephan R, Annemüller C, Hassan AA, Lämmler C.. Characterization of enterotoxigenic Staphylococcus aureus strains isolated from bovine mastitis in north-east Switzerland. Vet Microbiol. 2001; 78(4): 373–382. [DOI] [PubMed] [Google Scholar]
- 40. Reinoso EB, El-Sayed A, Lämmler C, Bogni C, Zschöck M.. Genotyping of Staphylococcus aureus isolated from humans, bovine subclinical mastitis and food samples in Argentina. Microbiol Res . 2008; 163(3): 314–322. [DOI] [PubMed] [Google Scholar]
- 41. Golonka E, Filipek R, Sabat A, Sinczak A, Potempa J.. Genetic characterization of staphopain genes in Staphylococcus aureus. Biol Chem . 2004; 385(11): 1059–6107. [DOI] [PubMed] [Google Scholar]
- 42. Turkey AM, Barzani KK, Suleiman AJ, Abed JJ.. Molecular assessment of accessory gene regulator (agr) quorum sensing system in biofilm forming Staphylococcus aureus and study of the effect of silver nanoparticles on agr system. Iran J Microbiol. 2018; 10(1): 14–21. [PMC free article] [PubMed] [Google Scholar]
- 43. Marconi C, Cunha MLRS, Araújo JP Jr., Rugolo LMSS. Standardization of the PCR technique for the detection of delta toxin in Staphylococcus spp. J Venom Anim Toxins incl Trop Dis. 2005; 11: 117–128. [Google Scholar]
- 44. McClure J-A, Conly JM, Lau V, et al.. Novel multiplex PCR assay for detection of the staphylococcal virulence marker Panton-Valentine leukocidin genes and simultaneous discrimination of methicillin-susceptible from -resistant staphylococci . J Clin Microbiol. 2006; 44(3): 1141–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mehrotra M, Wang G, Johnson WM.. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance . J Clin Microbiol. 2000; 38(3): 1032–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hussain M, von Eiff C, Sinha B, et al.. Eap gene as novel target for specific identification of Staphylococcus aureus. J Clin Microbiol. 2008; 46(2): 470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haggar A, Flock JI, Norrby-Teglund A.. Extracellular adherence protein (Eap) from Staphylococcus aureus does not function as a superantigen. Clin Microbiol Infect . 2010; 16(8): 1155–1158. [DOI] [PubMed] [Google Scholar]
- 48. Herman-Bausier P, El-Kirat-Chatel S, Foster TJ, Geoghegan JA, Dufrêne YF.. Staphylococcus aureus fibronectin-binding protein A mediates cell-cell adhesion through low-affinity homophilic bonds. MBio. 2015; 6(3): e00413–e00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hussain M, Becker K, von Eiff C, Peters G, Herrmann M.. Analogs of Eap protein are conserved and prevalent in clinical Staphylococcus aureus isolates. Clin Diagn Lab Immunol. 2001; 8(6): 1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hume EB, Khan S, Cole N, Willcox M.. Investigating specific virulence factors involved in Staphylococcus aureus keratitis. Invest Opthalmol Vis Sci. 2009; 50(13): 3457. [Google Scholar]
- 51. Melles DC, van Leeuwen WB, Boelens HAM, et al.. Panton-Valentine leukocidin genes in Staphylococcus aureus. Emerg Infect Dis. 2006; 12(7): 1174–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haggar A, Hussain M, Lönnies H, et al.. Extracellular adherence protein from Staphylococcus aureus enhances internalization into eukaryotic cells. Infect Immun. 2003; 71(5): 2310–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Green M, Apel A, Stapleton F.. Risk factors and causative organisms in microbial keratitis. Cornea . 2008; 27(1): 22–27. [DOI] [PubMed] [Google Scholar]
- 54. Ng AL-K, To KK-W, Choi CC-L, et al.. Predisposing factors, microbial characteristics, and clinical outcome of microbial keratitis in a tertiary centre in Hong Kong: a 10-year experience. J Ophthalmol. 2015; 2015: 769436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kwiecinski J, Jin T, Josefsson E.. Surface proteins of Staphylococcus aureus play an important role in experimental skin infection. APMIS. 2014; 122(12): 1240–1250. [DOI] [PubMed] [Google Scholar]
- 56. Li X, Wang X, Thompson CD, et al.. Preclinical efficacy of clumping factor A in prevention of Staphylococcus aureus Infection. MBio . 2016; 7(1): e02232–e02315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McAdow M, Kim HK, Dedent AC, et al.. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog . 2011; 7(10): e1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu PZJ, Zhu H, Stapleton F, et al.. Effects of α-toxin-deficient Staphylococcus aureus on the production of peripheral corneal ulceration in an animal model. Curr Eye Res . 2005; 30(1): 63–70. [DOI] [PubMed] [Google Scholar]
- 59. Martí M, Trotonda MP, Tormo-Más MÁ, et al.. Extracellular proteases inhibit protein-dependent biofilm formation in Staphylococcus aureus. Microbes Infect . 2010; 12(1): 55–64. [DOI] [PubMed] [Google Scholar]
- 60. O'Neill E, Pozzi C, Houston P, et al.. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J Bacteriol. 2008; 190(11): 3835–3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rusnak M, Kortepeter M, Ulrich R, et al.. Laboratory exposures to staphylococcal enterotoxin B. Emerg Infect Dis . 2004; 10(9): 1544–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thakur A, Willcox M.. Cytokine and lipid inflammatory mediator profile of human tears during contact lens associated inflammatory diseases. Exp Eye Res. 1998; 67(1): 9–19. [DOI] [PubMed] [Google Scholar]
- 63. Jalbert I, Willcox M, Sweeney DF.. Isolation of Staphylococcus aureus from a contact lens at the time of a contact lens-induced peripheral ulcer: case report. Cornea . 2000; 19(1): 116–120. [DOI] [PubMed] [Google Scholar]
- 64. Fujishima H, Okada N, Dogru M, et al.. The role of Staphylococcal enterotoxin in atopic keratoconjunctivitis and corneal ulceration. Allergy. 2012; 67(6): 799–803. [DOI] [PubMed] [Google Scholar]
- 65. Prevost G, Couppie P, Prevost P, et al.. Epidemiological data on Staphylococcus aureus strains producing synergohymenotropic toxins. J Med Microbiol. 1995; 42(4): 237–245. [DOI] [PubMed] [Google Scholar]
- 66. Lina G, Piémont Y, Godail-Gamot F, et al.. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999; 29(5): 1128–1132. [DOI] [PubMed] [Google Scholar]
- 67. Willcox M, Sharma S, Naduvilath TJ, et al.. External ocular surface and lens microbiota in contact lens wearers with corneal infiltrates during extended wear of hydrogel lenses. Eye Contact Lens. 2011; 37(2): 90–95. [DOI] [PubMed] [Google Scholar]
- 68. Cheung AL, Bayer AS, Zhang G, Gresham H, Xiong YQ.. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol Med Microbiol. 2004; 40(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 69. Balasubramanian D, Harper L, Shopsin B, Torres VJ.. Staphylococcus aureus pathogenesis in diverse host environments. Pathog Dis . 2017; 75(1): ftx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Haag AF, Bagnoli F.. The role of two-component signal transduction systems in Staphylococcus aureus virulence regulation. Curr Top Microbiolo Immunol . 2017; 409: 145–198. [DOI] [PubMed] [Google Scholar]
- 71. Jenul C, Horswill AR.. Regulation of Staphylococcus aureus virulence. Microbiol Spectr . 2019; 7(2): 10.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rothfork JM, Dessus-Babus S, Van Wamel WJ, Cheung AL, Gresham HD.. Fibrinogen depletion attenuates Staphylococcus aureus infection by preventing density-dependent virulence gene up-regulation. J Immunol . 2003; 171(10): 5389–5395. [DOI] [PubMed] [Google Scholar]
- 73. Zdzalik M, Karim AY, Wolski K, et al.. Prevalence of genes encoding extracellular proteases in Staphylococcus aureus — important targets triggering immune response in vivo. FEMS Immunol Med Microbiol . 2012; 66(2): 220–229. [DOI] [PubMed] [Google Scholar]
- 74. Astley R, Miller FC, Mursalin MH, et al.. An eye on staphylococcus aureus toxins: roles in ocular damage and inflammation. Toxins . 2019; 11(6): 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Putra I, Rabiee B, Anwar KN, et al.. Staphylococcus aureus alpha-hemolysin impairs corneal epithelial wound healing and promotes intracellular bacterial invasion. Exp Eye Res . 2019; 181, : 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sueke H, Shankar J, Neal T, et al.. lukSF-PV in Staphylococcus aureus keratitis isolates and association with clinical outcome. Invest Opthalmol Vis Sci . 2013; 54(5): 3410–3416. [DOI] [PubMed] [Google Scholar]
- 77. Ong SJ, Huang YC, Tan HY, et al.. Staphylococcus aureus keratitis: a review of hospital cases. PloS one . 2013; 8(11): e80119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Peterson JC, Durkee H, Miller D, et al.. Molecular epidemiology and resistance profiles among healthcare-and community-associated Staphylococcus aureus keratitis isolates. Infect Drug Resist . 2019; 12: 831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kłos M, Pomorska-Wesołowska M, Romaniszyn D, et al.. Epidemiology, drug resistance, and virulence of Staphylococcus aureus isolated from ocular infections in polish patients. Pol J Microbiol . 2019; 68(4): 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Johnson WL, Sohn MB, et al.. Genomics of Staphylococcus aureus ocular isolates. PloS One , 2021; 16(5): e0250975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.