Abstract
Background
The selective pressure imposed by chemotherapy creates a barrier to tumor eradication and an opportunity for metastasis and recurrence. As a newly discovered stemness marker of pancreatic ductal adenocarcinoma (PDAC), the impact of CD9 on tumor progression and patient's prognosis remain controversial.
Methods
A total of 179 and 211 PDAC patients who underwent surgical resection with or without neoadjuvant chemotherapy, respectively, were recruited for immunohistochemical analyses of CD9 expression in both tumor and stromal areas prior to statistical analyses to determine the prognostic impact and predictive accuracy of CD9.
Results
The relationship between CD9 and prognostic indicators was not significant in the non‐neoadjuvant group. Nevertheless, CD9 expression in both tumor (T‐CD9) and stromal areas (S‐CD9) was significantly correlated with the clinicopathological features in the neoadjuvant group. High levels of T‐CD9 were significantly associated with worse OS (p = 0.005) and RFS (p = 0.007), while positive S‐CD9 showed the opposite results (OS: p = 0.024; RFS: p = 0.008). Cox regression analyses identified CD9 in both areas as an independent prognostic factor. The T&S‐CD9 risk‐level system was used to stratify patients with different survival levels. The combination of T&S‐CD9 risk level and TNM stage were accurate predictors of OS (C‐index: 0.676; AIC: 512.51) and RFS (C‐index: 0.680; AIC: 519.53). The calibration curve of the nomogram composed of the combined parameters showed excellent predictive consistency for 1‐year RFS. These results were verified using a validation cohort.
Conclusion
Neoadjuvant chemotherapy endows CD9 with a significant prognostic value that differs between tumor and stromal areas in patients with pancreatic cancer.
Keywords: CD9, neoadjuvant chemotherapy, pancreatic cancer, prognostic factor
Neoadjuvant chemotherapy strengthens the expression and prognostic value of CD9 in pancreatic cancer. The prognostic significance of CD9 differs between tumor and stroma areas in pancreatic cancer.

1. INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is a fatal malignancy with an extremely low resection rate. Even for a small group of patients diagnosed with localized and resectable tumors, their prognoses remain poor, with only 20% surviving 5 years after surgery. 1 Accordingly, efforts have been made to improve their prognosis following surgery. The importance of neoadjuvant chemotherapy in the management of pancreatic cancer has been recognized by a majority of researchers. 2 , 3 , 4 , 5 However, the increasingly malignant properties of tumors remain a challenge for most patients receiving long‐term treatment. Although studies have underlined the benefits of adjuvant treatment in patients with pancreatic cancer who received neoadjuvant therapy, 6 , 7 those receiving long‐term chemotherapy usually have difficulty achieving the same beneficial effects as before. Efforts should be made to investigate the effect of neoadjuvant chemotherapy on the characteristics and phenotypes of pancreatic tumors to obtain targeted improvement of clinical treatment.
Studies have indicated that selective pressure exerted by chemotherapy can promote tumor metastasis and recurrence. 8 , 9 , 10 During this process, the treatment‐induced phenotypic conversion of differentiated cancer cells into an immature stemness state creates a barrier to tumor eradication. 11 , 12 , 13 Cancer stem cells (CSCs) are self‐renewing cells that facilitate tumor initiation, relapse, and metastasis, 14 thus posing a high level of intrinsic resistance to a broad range of therapeutic approaches. 15 , 16 Specific cell surface markers facilitate the identification of CSCs. The well‐confirmed markers of pancreatic cancer include CD133, CD44, c‐Met, and Dclk1. 17 , 18 , 19 , 20 A recent study recognized CD9 as a new biomarker of CSC in pancreatic cancer. 21 As a member of the tetraspanin family, CD9 is an integral 24–27‐kDa membrane protein widely expressed in multiple immune and tumor cells, and participates in various cellular activities, including intercellular and cell‐matrix contact, integrin‐mediated cell migration, proliferation, and differentiation. 22 , 23 According to previous studies, CD9 has been identified to have both pro‐ and antitumor properties. 24 Similarly, in pancreatic cancer, the beneficial and disadvantageous effects of CD9 on tumor progression and patient's prognosis have also been reported. 21 , 25 , 26 , 27 , 28 , 29 Accordingly, it is necessary to investigate and validate the impact of CD9 in patients with pancreatic cancer, especially that of alterations in CD9 expression caused by the selective pressure of chemotherapy.
This study aimed to assess the potential significance of CD9 as a marker for predicting recurrence and survival in patients with pancreatic cancer who underwent curative resection with and without neoadjuvant chemotherapy. Furthermore, the results of immunohistochemical (IHC) analyses attempted to reveal the impact of neoadjuvant chemotherapy on cancer cells using this newly identified CSC marker to clarify the post‐chemotherapy risk factors and guide clinical treatment.
2. MATERIALS AND METHODS
2.1. Patients and samples
Three independent cohorts were retrospectively enrolled in the study. A total of 98 patients who were pathologically diagnosed with PDAC, received neoadjuvant chemotherapy, and had undergone radical resection at our institution between 2011 and 2015 were included in the neoadjuvant group Fudan cohort. Another validation cohort comprised 81 patients with PDAC who completed neoadjuvant chemotherapy and surgical resection at an external medical center with pathology consultations performed at our institute between 2010 and 2016. The non‐neoadjuvant group included 211 patients with PDAC who underwent pancreatectomy without preoperative treatment between 2012 and 2014. In the neoadjuvant group, all patients' regimens and doses conformed to the latest version of the NCCN Clinical Practice Guidelines. None of the patients died from postoperative complications within 30 days. The complete perioperative and follow‐up information of each patient were recorded.
Formalin‐fixed and paraffin‐embedded surgical specimens from all patients were obtained and sectioned. The clinicopathological characteristics, such as age, gender, tumor location, tumor size, tumor differentiation, lymph node involvement, and vascular and perineural invasion, were retrospectively retrieved from the clinical records. Tumors were staged based on the Tumor‐Nodes‐Metastases (TNM) staging system, according to the eighth edition of the American Joint Committee on Cancer. Recurrence‐free survival and overall survival were defined as the period from the date of surgery to the date of tumor recurrence and patient's death or last follow‐up. This study was approved by the Human Research Ethics Committee of Fudan University Shanghai Cancer Center and was conducted in accordance with the tenets of the World Medical Association Declaration of Helsinki.
2.2. Immunohistochemical assessment
Formalin‐fixed and paraffin‐embedded pathological sections of the surgical specimens from the two cohorts were used for immunohistochemistry. Primary antibodies composed of monoclonal mouse antihuman CD9 (anti‐CD9 [C‐4], sc‐13,118, diluted in 1:100 ratio; Santa Cruz Biotechnology) were used. The immunostaining images of the whole section of each specimen were observed under a microscope at low‐power magnification (×100). The hotspot area was defined as the area with the most immunostained cells in the tumor. Under high‐power magnification (×200), five representative photographs of each hotspot area were taken to evaluate the level of CD9 expression in the intratumoral area and peritumoral stroma.
With regard to the CD9 expression in tumor sites (T‐CD9), an immunoreactivity score (IS) was produced based on the intensity and extent of CD9 staining, as described previously. 28 , 30 The intensity scores ranged from 0 to 3: with 0 as negative, 1 as faintly positive, 2 as moderately positive, and 3 as intensively positive. The extent of staining was determined as the percentage of CD9‐stained tumor cells in the entire tumor site: 0% (0), 1%–25% (1), 26%–50% (2), 51%–75% (3), and > 75% (4). The ultimate IS of T‐CD9 was generated by multiplying the intensity and extent scores (0–12). With regard to the CD9 expression in the stroma (S‐CD9), the percentage of the stromal area occupied by CD9‐positive cells was calculated. The T‐CD9 and S‐CD9 scores were subsequently dichotomized according to previous studies 28 , 30 based on the mean value (IS of 4 for T‐CD9) and cutoff point (1% for S‐CD9), which rendered the most meaningful outcomes using the Kaplan–Meier method and log‐rank test. Two blinded independent observers (X. Han and WH. Zhang) rated the stained sections, and a third observer (WQ. Wang) validated the level of CD9 expression in cases of disagreement between the two observers.
2.3. Statistical analyses
Statistical analyses were performed using SPSS (version 26.0, IBM) and R software (version 4.1.0, R Core Team). The correlations between CD9 expression and clinicopathological parameters were analyzed using the chi‐square or Fisher's exact test. The statistical differences in overall survival (OS) and recurrence‐free survival (RFS) were evaluated using the Kaplan–Meier method and log‐rank test. Univariate and multivariate Cox regression analyses were used to identify the independent prognostic factors for recurrence and survival. The Akaike information criterion (AIC) value and concordance index (C‐index) were calculated to compare the accuracy of the different predictive models. A nomogram was created to predict the 1‐year RFS, and a calibration curve of the actual risk proportion and predicted risk probability determined using the nomogram was applied to demonstrate the predictive effect of the prognostic models. All tests were two sided, and a p‐value of <0.05 was considered significant.
3. RESULTS
3.1. Expression pattern of CD9 in the tumor and stromal area
The expression patterns of CD9 vary in different tumor specimens. The representative immunohistochemical staining patterns of the subsites of pancreatic cancer are shown in Figure 1. Immunofluorescence staining of these pathological sections was also performed, which showed that a few CD9‐high cancer cells co‐expressed CD133, a classic CSC marker, confirming the findings of the previous study; 21 that is, the expression of CD9 and CD133 may not overlap and are representative of different CSC types. The distribution of patients based on the immunoreactivity score (IS) of T‐CD9 expression and the proportion of stromal area occupied by S‐CD9 are listed in Figure S1. In the neoadjuvant group, high expression of T‐CD9 (IS ≥4) was reported in 51 (52.0%) and 37 (45.7%) patients in the Fudan and validation cohort, respectively. In the non‐neoadjuvant group, 80 (37.9%) patients showed high T‐CD9 expression. With regard to S‐CD9, positive expression (staining stroma area ≥1%) was observed in 47 (48.0%) and 35 (43.2%) patients from the Fudan and validation cohort of the neoadjuvant group, respectively, whereas 66 (31.3%) patients in the non‐neoadjuvant group had positive S‐CD9 expression.
FIGURE 1.
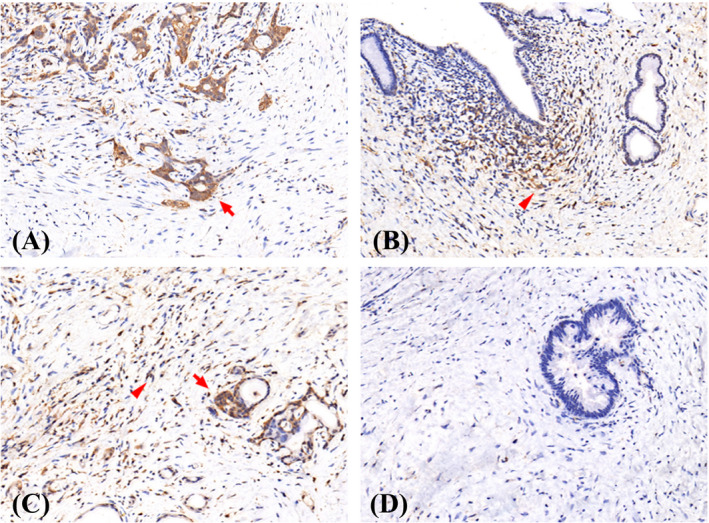
Representative microphotographs of CD9 staining in tumor (arrow) and stroma (arrowhead) area. (A) tumor+stroma‐, (B) tumor‐stroma+, (C) tumor+stroma+, (D) tumor‐stroma‐
3.2. Correlation of T‐ and S‐CD9 expression with clinicopathological parameters
The clinicopathological parameters of the three cohorts are shown in Table S1. The median age of patients in the non‐neoadjuvant group was 61 years, while that of patients in both neoadjuvant cohorts was 60 years. More than half of the patients in all cohorts were men. In the non‐neoadjuvant group, 106 patients had tumors at the head or neck of the pancreas, while the other 105 patients had tumors located at either the body or tail of the pancreas. By contrast, the number of patients with tumors at the head or neck of the pancreas was lower than that of the other patients in both neoadjuvant cohorts. As for pathological parameters, the median tumor diameter in the non‐neoadjuvant group was larger than that in the neoadjuvant Fudan and validation cohort (3.5 cm vs. 3.2 cm vs. 3.4 cm, respectively). A total of 97/39/31, 75/43/29, 152/78/56, and 125/53/37 patients in the non‐neoadjuvant group, and the neoadjuvant group Fudan cohort and validation cohort had poor differentiation, vascular invasion, perineural invasion, and lymph node involvement, respectively.
The T‐CD9 and S‐CD9 expression in the two groups demonstrated different correlations with the clinicopathological parameters. As shown in Table 1, neither the clinical nor the pathological characteristics showed a statistical association with CD9 expression (all p > 0.05) in patients who did not receive neoadjuvant chemotherapy. However, the high expression of T‐CD9 was significantly associated with poor tumor differentiation (p = 0.012) and higher TNM stage (p = 0.044), whereas positive S‐CD9 expression showed a correlation with relatively low TNM stage (p = 0.021) in patients who received neoadjuvant chemotherapy from the Fudan cohort. In the validation cohort, the high expression of T‐CD9 was significantly associated with poor tumor differentiation (p = 0.007), vascular invasion (p = 0.032), involvement of more than 1 lymph node (p = 0.033), and higher TNM stage (p = 0.046), whereas positive S‐CD9 expression was correlated with smaller tumor size (p = 0.037) and lower TNM stage (p = 0.028).
TABLE 1.
Correlation between CD9 expression in tumor/stroma and clinicopathologic characteristics of PDAC patients in the non‐neoadjuvant and neoadjuvant group
| Non‐neoadjuvant group (n = 211) | Neoadjuvant group ‐ Fudan cohort (n = 98) | Neoadjuvant group ‐ validation cohort (n = 81) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD9 expression in tumor | p | CD9 expression in stroma | p | CD9 expression in tumor | p | CD9 expression in stroma | p | CD9 expression in tumor | p | CD9 expression in stroma | p | |||||||
| Low | High | Negative | Positive | Low | High | Negative | Positive | Low | High | Negative | Positive | |||||||
| Age | 0.736 | 0.398 | 0.535 | 0.667 | 0.311 | 0.565 | ||||||||||||
| <Median | 64 | 41 | 75 | 30 | 21 | 25 | 25 | 21 | 24 | 16 | 24 | 16 | ||||||
| ≥Median | 67 | 39 | 70 | 36 | 27 | 25 | 26 | 26 | 20 | 21 | 22 | 19 | ||||||
| Gender | 0.638 | 0.295 | 0.310 | 0.222 | 0.232 | 0.704 | ||||||||||||
| Male | 78 | 45 | 88 | 35 | 27 | 23 | 23 | 27 | 24 | 25 | 27 | 22 | ||||||
| Female | 53 | 35 | 57 | 31 | 21 | 27 | 28 | 20 | 20 | 12 | 19 | 13 | ||||||
| Tumor location | 0.365 | 0.217 | 0.563 | 0.940 | 0.965 | 0.175 | ||||||||||||
| Head&neck | 69 | 37 | 77 | 29 | 21 | 19 | 21 | 19 | 20 | 17 | 18 | 19 | ||||||
| Body&tail | 62 | 43 | 68 | 37 | 27 | 31 | 30 | 28 | 24 | 20 | 28 | 16 | ||||||
| Tumor differentiation | 0.229 | 0.092 | 0.012 | 0.903 | 0.007 | 0.855 | ||||||||||||
| Well to moderate | 75 | 39 | 84 | 30 | 35 | 24 | 31 | 28 | 33 | 17 | 28 | 22 | ||||||
| Poor | 56 | 41 | 61 | 36 | 13 | 26 | 20 | 19 | 11 | 20 | 18 | 13 | ||||||
| Vascular invasion | 0.291 | 0.633 | 0.098 | 0.140 | 0.032 | 0.236 | ||||||||||||
| No | 88 | 48 | 95 | 41 | 31 | 24 | 25 | 30 | 32 | 20 | 27 | 25 | ||||||
| Yes | 43 | 32 | 50 | 25 | 17 | 26 | 26 | 17 | 12 | 17 | 19 | 10 | ||||||
| Perineural invasion | 0.143 | 0.609 | 0.269 | 0.480 | 0.243 | 0.697 | ||||||||||||
| No | 32 | 27 | 39 | 20 | 12 | 8 | 9 | 11 | 16 | 9 | 15 | 10 | ||||||
| Yes | 99 | 53 | 106 | 46 | 36 | 42 | 42 | 36 | 28 | 28 | 31 | 25 | ||||||
| Tumor size stage | 0.147 | 0.740 | 0.281 | 0.057 | 0.590 | 0.037 | ||||||||||||
| 1 | 38 | 16 | 35 | 19 | 16 | 10 | 13 | 13 | 9 | 10 | 6 | 13 | ||||||
| 2 | 59 | 34 | 66 | 27 | 22 | 25 | 20 | 27 | 24 | 16 | 25 | 15 | ||||||
| 3 | 34 | 30 | 44 | 20 | 10 | 15 | 18 | 7 | 11 | 11 | 15 | 7 | ||||||
| Lymph node stage | 0.807 | 0.834 | 0.051 | 0.143 | 0.033 | 0.070 | ||||||||||||
| 0 | 55 | 31 | 58 | 28 | 28 | 17 | 19 | 26 | 29 | 15 | 20 | 24 | ||||||
| 1 | 52 | 31 | 59 | 24 | 15 | 26 | 26 | 15 | 13 | 15 | 19 | 9 | ||||||
| 2 | 24 | 18 | 28 | 14 | 5 | 7 | 6 | 6 | 2 | 7 | 7 | 2 | ||||||
| TNM stage | 0.687 | 0.602 | 0.044 | 0.021 | 0.046 | 0.028 | ||||||||||||
| I | 47 | 25 | 47 | 25 | 22 | 11 | 11 | 22 | 21 | 10 | 12 | 19 | ||||||
| II | 60 | 37 | 70 | 27 | 21 | 32 | 34 | 19 | 21 | 20 | 27 | 14 | ||||||
| III | 24 | 18 | 28 | 14 | 5 | 7 | 6 | 6 | 2 | 7 | 7 | 2 | ||||||
3.3. Prognostic significance of T‐CD9 and S‐CD9 expression
Our Kaplan–Meier survival analyses demonstrated that patients with high levels of T‐CD9 expression in the non‐neoadjuvant chemotherapy group tended to have shorter OS and RFS than those with lower T‐CD9 expression, although the difference was not significant (p = 0.144 and 0.280) (Figure S2A,C). By contrast, positive S‐CD9 expression was associated with longer OS and RFS, although no marked difference was observed (p = 0.300 and 0.215, respectively) (Figure S2B,D). However, in patients who received neoadjuvant chemotherapy from the Fudan cohort, high levels of T‐CD9 expression were significantly associated with worse OS and RFS (p = 0. 005 and 0.007, respectively) (Figure 2A,C), indicating that the presence of CD9 at tumor sites was a disadvantage. Furthermore, patients with positive stromal CD9 expression showed longer OS and RFS than those with negative S‐CD9 expression (p = 0. 024 and 0.008, respectively) (Figure 2B,D), revealing the variable prognostic value of CD9 in different subsites. These results were confirmed in the validation cohort (Figure 2E–H), which showed that neoadjuvant chemotherapy significantly strengthened the prognostic value of CD9 compared with the results from the non‐neoadjuvant chemotherapy group.
FIGURE 2.
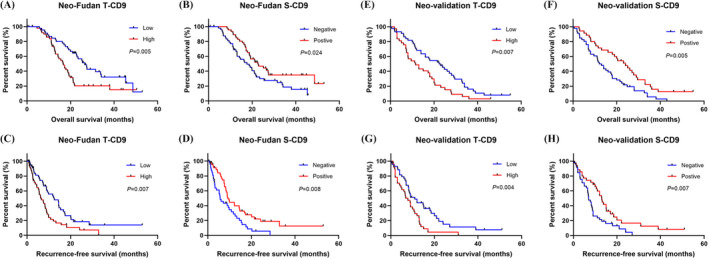
Survival analyses for OS and RFS of patients from neoadjuvant chemotherapy group with different CD9 expression in tumor and stroma. Kaplan–Meier survival curves for OS of patients in the Fudan cohort with different CD9 expression in tumor (A) and stroma (B). Kaplan–Meier survival curves for RFS of patients in the Fudan cohort with different CD9 expression in tumor (C) and stroma (D). Kaplan–Meier survival curves for OS of patients in the validation cohort with different CD9 expression in tumor (E) and stroma (F). Kaplan–Meier survival curves for RFS of patients in the validation cohort with different CD9 expression in tumor (G) and stroma (H)
According to the results of univariate and multivariate Cox regression analyses in Table 2, T‐CD9 and S‐CD9 expression along with tumor differentiation and TNM stage were independent predictors of OS (hazard ratio [HR] = 1.849, 95% confidence interval [CI]: 1.108–3.086, p = 0.019; HR = 0.559, 95% CI: 0.337–0.926, p = 0.024; HR = 1.779, 95% CI: 1.062–2.982, p = 0.029; HR = 1.739, 95% CI: 1.141–2.650, p = 0.010, respectively) and RFS (HR = 1.668, 95% CI: 1.013–2.747, p = 0.044; HR = 0.481, 95% CI: 0.294–0.788, p = 0.004; HR = 1.671, 95% CI: 1.003–2.786, p = 0.049; HR = 1.575, 95% CI: 1.057–2.347, p = 0.026, respectively) in the neoadjuvant group Fudan cohort and similar results were obtained in the external validation cohort (Table 2).
TABLE 2.
Univariate and multivariate analyses of overall survival and recurrence‐free survival with CD9 expression levels and clinicopathological characteristics of the non‐neoadjuvant and neoadjuvant group
| Non‐neoadjuvant group (n = 211) | Neoadjuvant group ‐ Fudan cohort (n = 98) | Neoadjuvant group ‐ validation cohort (n = 81) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analyses | Multivariate analyses | Univariate analyses | Multivariate analyses | Univariate analyses | Multivariate analyses | |||||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| OS | ||||||||||||
| Age (</> = median) | 1.060 (0.800,1.403) | 0.686 | 1.069 (0.662,1.727) | 0.784 | 1.084 (0.683,1.721) | 0.732 | ||||||
| Gender (male/female) | 1.035 (0.779,1.375) | 0.812 | 0.824 (0.511,1.328) | 0.426 | 0.802 (0.498,1.291) | 0.364 | ||||||
| Tumor location (head/others) | 1.164 (0.879,1.542) | 0.289 | 1.176 (0.717,1.927) | 0.521 | 1.575 (0.982,2.527) | 0.060 | ||||||
| Tumor differentiation (well to moderate/poor) | 1.666 (1.250,2.220) | <0.001 | 1.537 (1.149,2.056) | 0.004 | 1.924 (1.165,3.176) | 0.011 | 1.779 (1.062,2.982) | 0.029 | 1.791 (1.112,2.883) | 0.016 | 1.665 (1.027,2.702) | 0.039 |
| Vascular invasion (no/yes) | 1.268 (0.947,1.698) | 0.111 | 1.496 (0.920,2.432) | 0.104 | 1.489 (0.924,2.401) | 0.102 | ||||||
| Perineural invasion (no/yes) | 1.257 (0.919,1.719) | 0.152 | 1.328 (0.735,2.397) | 0.347 | 1.431 (0.860,2.381) | 0.167 | ||||||
| Tumor size stage (1/2/3/) | 1.505 (1.238,1.830) | <0.001 | 1.508 (1.082,2.101) | 0.015 | 1.499 (1.051,2.138) | 0.025 | ||||||
| Lymph node stage (0/1/2) | 1.551 (1.276,1.886) | <0.001 | 2.117 (1.468,3.052) | <0.001 | 1.993 (1.376,2.887) | <0.001 | ||||||
| TNM stage (I/II/III) | 1.651 (1.349,2.021) | <0.001 | 1.590 (1.296,1.949) | <0.001 | 2.186 (1.462,3.269) | <0.001 | 1.739 (1.141,2.650) | 0.010 | 2.219 (1.484,3.318) | 0.000 | 1.652 (1.057,2.582) | 0.028 |
| Tumor‐CD9 group (low/high) | 1.235 (0.927,1.646) | 0.149 | 1.991 (1.222,3.245) | 0.006 | 1.849 (1.108,3.086) | 0.019 | 1.874 (1.172,2.997) | 0.009 | 1.855 (1.107,3.109) | 0.019 | ||
| Stroma‐CD9 group (negative/positive) | 0.855 (0.632,1.155) | 0.306 | 0.575 (0.354,0.936) | 0.026 | 0.559 (0.337,0.926) | 0.024 | 0.509 (0.316,0.822) | 0.006 | 0.533 (0.314,0.904) | 0.020 | ||
| RFS | ||||||||||||
| Age (</> = median) | 0.995 (0.741,1.335) | 0.973 | 0.866 (0.547,1.372) | 0.540 | 1.104 (0.685,1.781) | 0.685 | ||||||
| Gender (male/female) | 0.928 (0.687,1.255) | 0.629 | 0.892 (0.561,1.419) | 0.630 | 0.618 (0.373,1.024) | 0.062 | ||||||
| Tumor location (head/others) | 1.078 (0.804,1.446) | 0.615 | 1.466 (0.898,2.393) | 0.126 | 1.389 (0.853,2.263) | 0.187 | ||||||
| Tumor differentiation (well to moderate/poor) | 1.519 (1.118,2.062) | 0.007 | 1.399 (1.027,1.907) | 0.033 | 1.889 (1.171,3.048) | 0.009 | 1.671 (1.003,2.786) | 0.049 | 1.837 (1.116,3.026) | 0.017 | 1.803 (1.077,3.018) | 0.025 |
| Vascular invasion (no/yes) | 1.246 (0.912,1.703) | 0.168 | 1.473 (0.920,2.357) | 0.107 | 1.583 (0.958,2.617) | 0.073 | ||||||
| Perineural invasion (no/yes) | 1.302 (0.933,1.816) | 0.120 | 1.378 (0.788,2.410) | 0.261 | 1.487 (0.880,2.512) | 0.138 | ||||||
| Tumor size stage (1/2/3/) | 1.392 (1.132,1.712) | 0.002 | 1.796 (1.245,2.590) | 0.002 | 1.590 (1.097,2.304) | 0.014 | ||||||
| Lymph node stage (0/1/2) | 1.503 (1.225,1.843) | <0.001 | 1.771 (1.247,2.517) | 0.001 | 1.791 (1.227,2.614) | 0.003 | ||||||
| TNM stage (I/II/III) | 1.532 (1.239,1.895) | <0.001 | 1.481 (1.195,1.836) | <0.001 | 2.012 (1.394,2.904) | <0.001 | 1.575 (1.057,2.347) | 0.026 | 2.171 (1.463,3.223) | 0.000 | 1.603 (1.027,2.502) | 0.038 |
| Tumor‐CD9 group (low/high) | 1.178 (0.871,1.592) | 0.288 | 1.887 (1.182,3.011) | 0.008 | 1.668 (1.013,2.747) | 0.044 | 2.005 (1.226,3.280) | 0.006 | 1.885 (1.072,3.313) | 0.028 | ||
| Stroma‐CD9 group (negative/positive) | 0.821 (0.598,1.127) | 0.222 | 0.532 (0.333,0.850) | 0.008 | 0.481 (0.294,0.788) | 0.004 | 0.515 (0.313,0.847) | 0.009 | 0.497 (0.286,0.865) | 0.013 | ||
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival; RFS, recurrence‐free survival.
p <0.05 is deemed significant and marked in bold.
A risk‐level rank was also established, and the survival differences of patients in the three cohorts were analyzed based on the level of CD9 expression in both tumor and stromal areas. All patients who received neoadjuvant chemotherapy were divided into three subgroups: low‐risk group I, with low levels of T‐CD9 and positive S‐CD9 expression; moderate‐risk group II, with high levels of T‐CD9 and positive S‐CD9 expression or low levels of T‐CD9 and negative S‐CD9 expression; and high‐risk group III, with high levels of T‐CD9 and negative S‐CD9 expression. The OS and RFS of patients who received neoadjuvant chemotherapy in the three risk groups were stratified significantly (Figure 3A–D).
FIGURE 3.
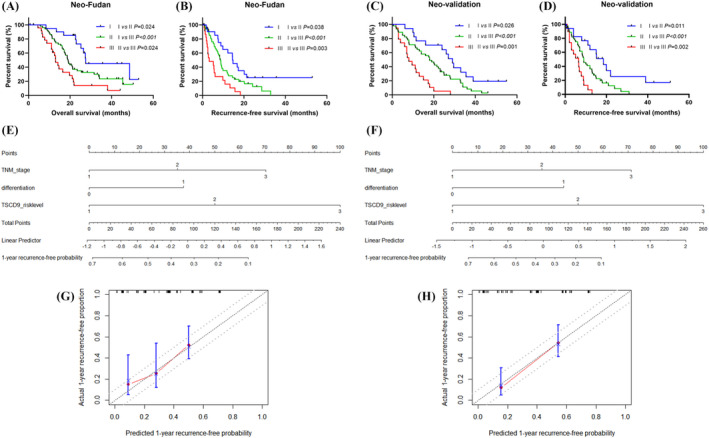
Establishment and validation of a new predictive model for PDAC patients receiving neoadjuvant chemotherapy by combining expression patterns of CD9 in both tumor and stroma. Survival analyses for OS and RFS of patients from neoadjuvant chemotherapy group with different CD9 risk levels. Kaplan–Meier survival curves for OS (A) and RFS (B) of patients in the Fudan cohort with different CD9 risk levels. Kaplan–Meier survival curves for OS (C) and RFS (D) of patients in the validation cohort with different CD9 risk levels. The nomogram (E, F) for 1‐year recurrence‐free predictive probability and the calibration curve (G, H) were established based on the Fudan (E, G) and validation (F, H) cohort
3.4. Extension of the TNM stage prognostic model with T‐ and S‐CD9
According to the results of the multivariate analysis, a new prognostic model was established to determine the OS and RFS of patients with PDAC receiving neoadjuvant chemotherapy based on the TNM stage and the T‐ and S‐CD9 (Table 3). This newly established model composed of the TNM staging system and T&S‐CD9 risk level had a higher C‐index (0.676 vs. 0.624) and lower AIC (512.51 vs. 518.79) in predicting the overall survival compared with the model composed of TNM stage alone. In terms of predicting the recurrence‐free survival, this new model produced similar results with a higher C‐index (0.680 vs. 0.628) and lower AIC (519.53 vs. 527.28) compared with the TNM stage. The external validation cohort yielded similar results (Table 3). Subsequently, a nomogram incorporating the independent prognostic factors included in the multivariate analyses was constructed to predict the 1‐year recurrence‐free probability in patients receiving neoadjuvant chemotherapy (Figure 3E,F). The calibration curve of our nomogram displayed excellent consistency between the actual and predicted 1‐year recurrence‐free probability, which centralized in the 10% margin of error (Figure 3G,H).
TABLE 3.
Comparison of the prognostic accuracies of combinational models for overall and recurrence‐free survival in the neoadjuvant group
| Model | Neoadjuvant group ‐ Fudan cohort (n = 98) | Neoadjuvant group ‐ validation cohort (n = 81) | ||
|---|---|---|---|---|
| C‐index | AIC | C‐index | AIC | |
| OS | ||||
| Tumor‐CD9 | 0.604 | 525.52 | 0.588 | 518.49 |
| Stroma‐CD9 | 0.579 | 528.24 | 0.592 | 517.30 |
| Tumor&Stroma‐CD9 risk level | 0.643 | 518.86 | 0.642 | 506.00 |
| TNM stage | 0.624 | 518.79 | 0.604 | 510.02 |
| Tumor&Stroma‐CD9 risk level + TNM stage | 0.676 | 512.51 | 0.666 | 502.91 |
| RFS | ||||
| Tumor‐CD9 | 0.593 | 533.42 | 0.583 | 484.63 |
| Stroma‐CD9 | 0.590 | 533.64 | 0.588 | 485.21 |
| Tumor&Stroma‐CD9 risk level | 0.643 | 524.59 | 0.637 | 472.79 |
| TNM stage | 0.628 | 527.28 | 0.606 | 477.45 |
| Tumor&Stroma‐CD9 risk level + TNM stage | 0.680 | 519.53 | 0.665 | 470.73 |
Abbreviations: AIC, Akaike information criterion; C‐index, concordance index; OS, overall survival; RFS, recurrence‐free survival.
4. DISCUSSION
Our research demonstrated that CD9 was differentially expressed in tumor sites and stroma, and that their distinct expression patterns exhibited different correlations with the clinicopathological characteristics of patients with pancreatic cancer. Moreover, neoadjuvant chemotherapy significantly endowed CD9 with a prognostic value. Both T‐ and S‐CD9 were independent prognostic indicators, with opposite meanings for OS and RFS. Only in pancreatic cancer cells has the prognostic impact of CD9 expression been reported previously. Inspired by Kwon's work on breast cancer, 30 the prognostic value of CD9 expression was explored based on the different tumor compartments and pre‐surgical treatment of pancreatic cancer.
The malignant behavior of CD9 in pancreatic cancer, such as manipulating tumor metabolism, has been confirmed in previous studies. It has been reported to increase the glutamine uptake in pancreatic cancer cells by facilitating plasma membrane localization of ASCT2, a glutamine transporter. 21 Another study by Lu et al. found that CD9 regulated cell surface trafficking of alpha‐secretase, such as ADAM10, thus leading to the activation of Notch signaling. 29 In particular, Wang et al. identified and characterized a tumor‐initiating cell group marked by abundant CD9 on the cell surface, which is required for the efficient tumorigenesis of PDAC. 21 However, the proportion of tumor cells with high CD9 expression was relatively low, accounting for approximately 10% in human PDAC samples and 5% in mouse models and tumor organoids, 21 which potentially explained the lack of significant prognostic indicative effect of this marker in the non‐neoadjuvant group.
These tumor‐initiating cells make up a small proportion of the bulk of tumors, which is probably attributable to the dormancy of CSCs, indicating that these cell groups can remain quiescent and exhibit few tumor‐initiating properties for some time. 31 However, the activation of these dormant cells tends to occur after an external stimulation represented, such as chemotherapy. Under the selective pressure of chemotherapy, these drug‐resistant cell groups survive and boom to dominate the bulk of the tumor. 32 , 33 Furthermore, chemotherapy induces stemness in cancer cells. Wiechert et al. reported that cisplatin provided inductive stress for the stem cell state in ovarian cancer cells. 11 Auffinger et al. found that the clinical doses of temozolomide significantly expanded the glioma stem cell population both in vitro and in vivo, which could be attributed to the phenotypic conversion of the non‐CSC population to a stemness state, as revealed by lineage‐tracing analysis. This newly converted stem cell group exhibited typical markers related to stemness and pluripotency, such as CD133. 13 Notably, CD9 has also been identified as a marker of CSCs in glioma. 34 , 35 Therefore, cancer treatment using a standard regimen frequently ends up in the emergence of drug‐resistant cell populations, ultimately resulting in therapeutic failure and tumor relapse. Similar phenomena have also been observed in pancreatic cancer. Neoadjuvant chemotherapy left residual cancer tissues rich in drug‐refractory CSCs marked with CD44 as reported in Tajima's study. 12 Whole‐genome sequencing revealed post‐treatment genomic evolution with an increased mutational burden in recurrent PDAC tissues. 9 With the constantly evolving heterogeneous tumor, systemic therapy is a form of selective pressure, and additional mutations drive the development of therapy‐resistant subclones. 8 Based on the above‐mentioned evidence and the role of CD9 as a stemness marker, the significant correlation between CD9 expression in tumor sites and poor tumor differentiation in the neoadjuvant group in our results can be explained, and it is reasonable to infer that the impact of neoadjuvant chemotherapy on primary tumors preserves and induces the presence of CD9‐expressing populations so that they can provide a significant prognostic value.
A group of CD9‐stained immune cells was also observed within the stroma, which had an opposite prognostic effect compared with tumor cells stained with CD9. The infiltration of stromal immune cells indicated a host immune reaction against the tumor, which has been confirmed associated with a better prognosis in patients with pancreatic cancer. Kobayashi et al. found that CD9 was preferentially expressed in the human CD4+ CD45RA+ naive T‐cell subgroup and was involved in both self‐ and recall antigen‐triggered T‐cell activation. 36 This study revealed the potential impact of CD9 on antitumor immunity and offered a theoretical basis for the relationship between stromal CD9 expression and the immune infiltration level. Furthermore, Ferrone et al. reported that neoadjuvant chemotherapy stimulated an antitumor immune response in pancreatic cancer with increased stromal CD4+ and CD8+ cell densities and decreased regulatory T cell and M2 macrophage infiltration. 37 Similar results were observed in Demir's research. 38 Neoadjuvant therapy in pancreatic cancer led to the selective depletion of pro‐tumorigenic immune cells, reshaping the microenvironment, increasing the level of intratumoral CD4+ T cells, and improving the clinical outcomes. Accordingly, the association between CD9 expression in the stroma strengthened by neoadjuvant chemotherapy and a favorable outcome is understandable.
Although several markers are currently considered to have potential prognostic ability in postoperative pathological examination of pancreatic cancer, CD9 still has its unique superiority over other indicators. As a stemness marker of PDAC and other types of malignancy, the expression and prognostic value of CD9 in both tumor and stromal areas were significantly strengthened by the selective pressure of chemotherapy, as shown in our research; this means that the application of CD9 as a prognostic indicator is more accurate and targeted toward a specific group of patients receiving neoadjuvant chemotherapy. Furthermore, most molecular markers clinically used for predicting recurrence and survival of patients are expressed in a single locus, whereas this marker is expressed in both tumor and stromal areas; this enables pathologists to assess a single marker stained in a specimen from different aspects, thus improving its practical value. This study also has a few limitations. Although the above‐mentioned results were achieved in a relatively large cohort, the retrospective nature of the study with a limited sample size from a single medical center may restrict its clinical application. Hence, a multicenter cooperation is necessary to validate these results. In addition, this study did not find a correlation between CD9 expression in tumor sites and that in the stroma, which requires further research. Based on our findings and those of previous studies, it is understandable to regard CD9 as a therapeutic target. Targeting CD9 may help to avoid the risk of stemness acquisition caused by chemotherapy. However, it is possible that CD9 expression in stromal immune cells is simultaneously inhibited. Strategies used in differentially treating CD9 at tumor and stroma sites require further investigation.
In summary, CD9 could serve as a postsurgical prognostic indicator in patients with pancreatic cancer receiving neoadjuvant chemotherapy. CD9 expression at tumor and stromal sites showed an opposite association with prognosis. Consequently, compartment‐specific analyses of CD9 expression using IHC are necessary to evaluate the prognostic impact of CD9 expression in pancreatic cancer tissues.
AUTHOR CONTRIBUTION
Xuan Han, Wen‐Quan Wang, Liang Liu, and Xian‐Jun Yu provided the original conception of this study. Xuan Han, Wu‐Hu Zhang, and Tian‐Jiao Li completed cohort recruitment, specimen collection, and experiment implementation. Xuan Han, Hua‐Xiang Xu, and Hao Li performed statistical analyses. He‐Li Gao, Peng‐Cheng Li, and Xu Wang offered an interpretation of the data. Xuan Han completed the writing of this article. All of the authors reviewed the entire manuscript, discussed the results, and gave ultimate approval for publication.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
INFORMED CONSENT
All of the patients from our cohorts were informed of the implementation of this study and gave approval for the utilization of their clinical data and materials. All of the authors gave approval for the publication of this manuscript.
Supporting information
Figure S1
Figure S2
Table S1
Han X, Zhang W‐H, Gao H‐L, et al. Neoadjuvant chemotherapy endows CD9 with prognostic value that differs between tumor and stromal areas in patients with pancreatic cancer. J Clin Lab Anal. 2022;36:e24517. doi: 10.1002/jcla.24517
Xuan Han and Wu‐Hu Zhang contributed equally to this work.
Funding information
This work was funded by grants from the National Science Foundation for Distinguished Young Scholars of China (81625016), the National Natural Science Foundation of China (81,871,941, 81,872,366, 81,827,807, 81,802,675, 81,701,630, and 81,702,341), the National Key R&D Program of China (2019YFC1315900), the Scientific Innovation Project of Shanghai Education Committee (2019‐01‐07‐00‐07‐E00057), the Natural Science Foundation of Shanghai (19ZR1410800), the Clinical and Scientific Innovation Project of Shanghai Hospital Development Center (SHDC12018109), and the Clinical Research Plan of Shanghai Hospital Development Center (SHDC2020CR1006A). The funding agencies above had no involvement in study design, data collection, and analysis, decision to publish, or preparation of the manuscript
Contributor Information
Xian‐Jun Yu, Email: yuxianjun@fudanpci.org.
Wen‐Quan Wang, Email: wenquanwang09@fudan.edu.cn.
Liang Liu, Email: liuliang.zlhospital@fudan.edu.cn.
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395(10242):2008‐2020. [DOI] [PubMed] [Google Scholar]
- 2. Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol. 2017;35(5):515‐522. [DOI] [PubMed] [Google Scholar]
- 3. Jang JY, Han Y, Lee H, et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open‐label, multicenter phase 2/3 trial. Ann Surg. 2018;268(2):215‐222. [DOI] [PubMed] [Google Scholar]
- 4. Janssen QP, Buettner S, Suker M, et al. Neoadjuvant FOLFIRINOX in patients with borderline resectable pancreatic cancer: a systematic review and patient‐level meta‐analysis. J Natl Cancer Inst. 2019;111(8):782‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020;38(16):1763‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2018;4(7):963‐969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perri G, Prakash L, Qiao W, et al. Postoperative chemotherapy benefits patients who received preoperative therapy and pancreatectomy for pancreatic adenocarcinoma. Ann Surg. 2020;271(6):996‐1002. [DOI] [PubMed] [Google Scholar]
- 8. Bednar F, Pasca di Magliano M. Chemotherapy and tumor evolution shape pancreatic cancer recurrence after resection. Cancer Discov. 2020;10(6):762‐764. [DOI] [PubMed] [Google Scholar]
- 9. Sakamoto H, Attiyeh MA, Gerold JM, et al. The evolutionary origins of recurrent pancreatic cancer. Cancer Discov. 2020;10(6):792‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xie IY, Gallinger S. The genomic landscape of recurrent pancreatic cancer is modified by treatment. Nat Rev Gastroenterol Hepatol. 2020;17(7):389‐390. [DOI] [PubMed] [Google Scholar]
- 11. Wiechert A, Saygin C, Thiagarajan PS, et al. Cisplatin induces stemness in ovarian cancer. Oncotarget. 2016;7(21):30511‐30522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tajima H, Ohta T, Kitagawa H, et al. Neoadjuvant chemotherapy with gemcitabine for pancreatic cancer increases in situ expression of the apoptosis marker M30 and stem cell marker CD44. Oncol Lett. 2012;3(6):1186‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Auffinger B, Tobias AL, Han Y, et al. Conversion of differentiated cancer cells into cancer stem‐like cells in a glioblastoma model after primary chemotherapy. Cell Death Differ. 2014;21(7):1119‐1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645‐648. [DOI] [PubMed] [Google Scholar]
- 15. Clara JA, Monge C, Yang Y, Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells ‐ a clinical update. Nat Rev Clin Oncol. 2020;17(4):204‐232. [DOI] [PubMed] [Google Scholar]
- 16. Chen P, Hsu WH, Han J, Xia Y, DePinho RA. Cancer stemness meets immunity: from mechanism to therapy. Cell Rep. 2021;34(1):108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313‐323. [DOI] [PubMed] [Google Scholar]
- 18. Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030‐1037. [DOI] [PubMed] [Google Scholar]
- 19. Li C, Wu JJ, Hynes M, et al. C‐met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141(6):2218‐27 e5. [DOI] [PubMed] [Google Scholar]
- 20. Bailey JM, Alsina J, Rasheed ZA, et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146(1):245‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang VM, Ferreira RMM, Almagro J, et al. CD9 identifies pancreatic cancer stem cells and modulates glutamine metabolism to fuel tumour growth. Nat Cell Biol. 2019;21(11):1425‐1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kersey JH, LeBien TW, Abramson CS, et al. P‐24: a human leukemia‐associated and lymphohemopoietic progenitor cell surface structure identified with monoclonal antibody. J Exp Med. 1981;153(3):726‐731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lorico A, Lorico‐Rappa M, Karbanova J, et al. CD9, a tetraspanin target for cancer therapy? Exp Biol Med (Maywood). 2021;1535370220981855:1121‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hemler ME. Tetraspanin proteins promote multiple cancer stages. Nat Rev Cancer. 2014;14(1):49‐60. [DOI] [PubMed] [Google Scholar]
- 25. Sho M, Adachi M, Taki T, et al. Transmembrane 4 superfamily as a prognostic factor in pancreatic cancer. Int J Cancer. 1998;79(5):509‐516. [DOI] [PubMed] [Google Scholar]
- 26. Gronborg M, Kristiansen TZ, Iwahori A, et al. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics. 2006;5(1):157‐171. [DOI] [PubMed] [Google Scholar]
- 27. Tang M, Yin G, Wang F, et al. Downregulation of CD9 promotes pancreatic cancer growth and metastasis through upregulation of epidermal growth factor on the cell surface. Oncol Rep. 2015;34(1):350‐358. [DOI] [PubMed] [Google Scholar]
- 28. Khushman M, Patel GK, Laurini JA, et al. Exosomal markers (CD63 and CD9) expression and their prognostic significance using immunohistochemistry in patients with pancreatic ductal adenocarcinoma. J Gastrointest Oncol. 2019;10(4):695‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu W, Fei A, Jiang Y, Chen L, Wang Y. Tetraspanin CD9 interacts with alpha‐secretase to enhance its oncogenic function in pancreatic cancer. Am J Transl Res. 2020;12(9):5525‐5537. [PMC free article] [PubMed] [Google Scholar]
- 30. Kwon HJ, Choi JE, Kang SH, Son Y, Bae YK. Prognostic significance of CD9 expression differs between tumour cells and stromal immune cells, and depends on the molecular subtype of the invasive breast carcinoma. Histopathology. 2017;70(7):1155‐1165. [DOI] [PubMed] [Google Scholar]
- 31. Phan TG, Croucher PI. The dormant cancer cell life cycle. Nat Rev Cancer. 2020;20(7):398‐411. [DOI] [PubMed] [Google Scholar]
- 32. De Angelis ML, Francescangeli F, La Torre F, et al. Stem cell plasticity and dormancy in the development of cancer therapy resistance. Front Oncol. 2019;9:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Talukdar S, Bhoopathi P, Emdad L, et al. Dormancy and cancer stem cells: an enigma for cancer therapeutic targeting. Adv Cancer Res. 2019;141:43‐84. [DOI] [PubMed] [Google Scholar]
- 34. Podergajs N, Motaln H, Rajcevic U, et al. Transmembrane protein CD9 is glioblastoma biomarker, relevant for maintenance of glioblastoma stem cells. Oncotarget. 2016;7(1):593‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shi Y, Zhou W, Cheng L, et al. Tetraspanin CD9 stabilizes gp130 by preventing its ubiquitin‐dependent lysosomal degradation to promote STAT3 activation in glioma stem cells. Cell Death Differ. 2017;24(1):167‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kobayashi H, Hosono O, Iwata S, et al. The tetraspanin CD9 is preferentially expressed on the human CD4(+)CD45RA+ naive T cell population and is involved in T cell activation. Clin Exp Immunol. 2004;137(1):101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Michelakos T, Cai L, Villani V, et al. Tumor microenvironment immune response in pancreatic ductal adenocarcinoma patients treated with neoadjuvant therapy. J Natl Cancer Inst. 2020;113:182‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mota Reyes C, Teller S, Muckenhuber A, et al. Neoadjuvant therapy remodels the pancreatic cancer microenvironment via depletion of Protumorigenic immune cells. Clin Cancer Res. 2020;26(1):220‐231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Table S1
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author on reasonable request.


