Abstract
Background
T‐helper (Th) cells regulate inflammation and immunity, which is implicated in psychological disorders. The current study aimed to explore the clinical role of blood Th1, Th2, and Th17 cells and their main secreted cytokines in postpartum depression (PPD) and postpartum anxiety (PPA).
Methods
A total of 226 postpartum women were included. At 6 weeks postpartum, Edinburgh Postnatal Depression Scale (EPDS) and State Trait Anxiety Inventory 6 item version (STAI6) scores were assessed; meanwhile, blood Th1, Th2, and Th17 cells were detected by flow cytometry, serum interferon‐gamma (IFN‐γ), interleukin‐4 (IL‐4), and IL‐17A were detected by enzyme‐linked immunosorbent assay.
Results
The incidence of PPD and PPA were 24.3% and 27.9%, respectively. Th17 cells and IL‐17A were positively correlated with EPDS score and STAI6 score (all p < 0.001). Besides, Th17 cells (p < 0.001) and IL‐17A (p = 0.002) were increased in PPD cases vs. non‐PPD cases, and they were also elevated in PPA cases vs. non‐PPA cases (both p < 0.05). However, Th1 cells, Th2 cells, IFN‐γ, and IL‐4 were not linked with EPDS score or STAI6 score (all p > 0.05); besides, they did not vary in PPD cases vs. non‐PPD cases or in PPA cases vs. non‐PPA cases (all p > 0.05). Multivariate logistic regression model analysis showed that Th17 cells were independently associated with an elevated risk of PPD (odds ratio [OR] = 1.600, p = 0.001) and PPA (OR = 1.371, p = 0.022).
Conclusion
Blood Th17 cells and IL‐17A are positively linked with the risk of PPD and PPA, indicating which may be involved in the development of PPD and PPA.
Keywords: cytokines, postpartum anxiety, postpartum depression, risk factors, T‐helper cells
The current study aimed to explore the clinical role of blood Th1, Th2, and Th17 cells and their main secreted cytokines in postpartum depression (PPD) and postpartum anxiety (PPA). At 6‐week postpartum, Edinburgh Postnatal Depression Scale (EPDS) and State Trait Anxiety Inventory 6 item version (STAI6) scores were assessed, and Th cells and their main secreted cytokines were detected among 226 postpartum women. The data revealed that the incidence of PPD and PPA was 24.3% and 27.9%, respectively. Blood Th17 cells and IL‐17A were positively correlated with EPDS score and STAI6 score; meanwhile, they were increased in PPD cases vs. non‐PPD cases and elevated in PPA cases vs. non‐PPA cases. Besides, Th17 cells were independently correlated with an elevated risk of PPD and PPA. However, Th1 cells, Th2 cells, IFN‐γ, and IL‐4 were not linked with EPDS score, STAI6 score, PPD, or PPA. Our discoveries indicate the involvement of Th17 cells and IL‐17A in PPD and PPA development.

1. INTRODUCTION
The transition of motherhood is a challenge for women, which results in not only a physical burden but also mental disorders. 1 , 2 , 3 , 4 Postpartum depression (PPD) and postpartum anxiety (PPA) are common psychological problems that occur in up to approximately 20% of women, which result in detrimental effects on postpartum women 5 , 6 ; the common symptoms include insomnia, mood swings, loss of appetite, irritability, and even thoughts of self‐harm and suicide. 7 , 8 In addition, PPD and PPA also have an adverse effect on the neurodevelopment of children, subsequently resulting in physical and psychological problems among children of women with PPD and PPA. 9 , 10 Thus, it is vital to explore factors affecting PPD and PPA to promote their management.
The abnormal differentiation of T cells plays a crucial clinical role in anxiety and depression by dysregulating the immune system. 11 , 12 , 13 , 14 , 15 For instance, the T‐helper 1 (Th1)/Th2 cells ratio is elevated in people with depression 13 ; moreover, it also has been reported that Th17 cells are increased in people with depression 16 ; furthermore, another interesting study also presents that Th17 cell‐secreted cytokine (interleukin‐17 [IL‐17]) are positively correlated with the severity of anxiety and depression among lung cancer survivors. 15 Notably, accumulating studies have shown that Th1, Th2, and Th17 cells are abnormally regulated during the postpartum period. 17 , 18 Taken together, we deduced that Th1, Th2, and Th17 might play an important clinical role in PPD and PPA, while related information is scarce.
Thus, the current study aimed to explore the correlation of blood Th1, Th2, and Th17 cells, as well as their main secreted cytokines with PPD and PPA.
2. METHODS
2.1. Participants
Between March 2021 and October 2021, 226 postpartum women were enrolled in this study. The inclusion criteria (1) aged ≥18 years; (2) within 6‐week postpartum; (3) voluntarily participated and was able to complete the questionnaire or scale independently; (4) willing to provide peripheral blood (PB) samples for scientific research. The exclusion criteria (1) had complications within 6‐week postpartum; (2) history of mental disorders before pregnancy; (3) history of severe central nervous system disease; (4) history of psychoactive drug abuse; (5) with insufficiency of important organs or malignant tumors. The study ethics was approved by the Institutional Review Board of Shanghai Key Laboratory of Maternal Fetal Medicine, Shanghai First Maternity and Infant Hospital, School of Medicine, Tongji University. All patients signed the informed consent before enrolment.
2.2. Demographic and perinatal characteristics
After enrollment, demographic and perinatal characteristics were recorded by case report form. The demographic features included the following: age; smoke history (yes/no); drink history (yes/no); education level (primary school or less, high school, undergraduate, graduate or above); annual family income; previous gravidity number; previous birth number; previous abortion number. The perinatal features included the following: gestational week; unplanned pregnancy (yes/no); planned maternity leave time (≤128 days or >128 days); satisfaction of labor experience (unsatisfied or satisfied); breastfeeding difficulty (yes/no); number of caregivers (<2 or ≥2).
2.3. PPD and PPA evaluation
The participants assessed PPD and PPA at 6‐week (±1 week) postpartum. The Edinburgh Postnatal Depression Scale (EPDS) was used to evaluate PPD. The EPDS included 10 items, and the total score of EPDS ranged from 0 to 30. The EPDS score > 12 was considered as PPD. 19 , 20 The State Trait Anxiety Inventory 6 item version (STAI6) was used to evaluate PPA. The STAI6 included 6 items, and the total score of STAI6 ranges from 0 to 24. The STAI6 score > 15 was considered as PPA. 19
2.4. Sample detections
A total of 226 PB samples of participants were collected at 6‐week postpartum. Within 24 h after collection, 226 fresh PB samples were analyzed using flow cytometry to detect Th1 cells (%, in CD4+ T cells), T‐helper 2 (Th2) cells (%, in CD4+ T cells), and T‐helper 17 (Th17) cells (%, in CD4+ T cells) with the employment of BD Pharmingen™ Human Th1/Th2/Th17 Phenotyping Kit (BD Biosciences, San Diego, CA, USA). Serum was isolated from 226 PB samples by centrifuge and stored in liquid nitrogen for further detection. The levels of interferon‐gamma (IFN‐γ), interleukin‐4 (IL‐4), and interleukin‐17A (IL‐17A) in serum were detected in batch by enzyme‐linked immunosorbent assay (ELISA) Kit, Human IL‐4 ELISA Kit, and Human IL‐17A ELISA Kit (BD Biosciences, San Diego, CA, USA), respectively. All procedures were strictly implemented according to the product protocol.
2.5. Statistical analysis
The distributions of continuous variables were assessed by Kolmogorov–Smirnov (KS) test. With a p < 0.05 for the KS test, the variables were non‐normally distributed. The correlation of T helper cells and their related cytokines with EPDS score and STAI6 score was determined using Spearman's rank correlation test. The comparison of T helper cells and their related cytokines between two groups was evaluated using the Mann–Whitney U test. Multivariate logistic regression analysis was used to assess relating factors of PPD and PPA, with all potential factors were included by the step forward method. Statistical analysis was performed by SPSS V.22.0 (IBM Corp., Armonk, NY, USA), and figures were plotted by GraphPad Prism V.7.02 (GraphPad Software Inc., San Diego, CA, USA). The p < 0.05 was considered as statistical significance.
3. RESULTS
3.1. Study flow
A total of 323 women within 6‐week postpartum were screened for eligibility, then 97 women were excluded (including 46 women who did not meet inclusion criteria or met exclusion criteria, and 51 women who declined to participate). Subsequently, 226 women were eligible, then PPD evaluation (using EPDS scale) and PPA evaluation (using STAI6 scale) were conducted at 6‐week (±1 week) postpartum among them. Then, PB samples of participants were collected at 6‐week postpartum, and serum was isolated from PB samples; afterward, Th1 cells, Th2 cells, and Th17 cells in PB were detected by flow cytometry; IFN‐γ, IL‐4, and IL‐17A in serum were detected by ELISA. Finally, all 226 women were included in the analysis (Figure 1).
FIGURE 1.
Study flow chart
3.2. Clinical characteristics
Among 226 participants, the mean age was 27.7 ± 3.6 years; meanwhile, 32 (14.2%) and 50 (22.1%) participants had a smoke history and drink history, respectively. Furthermore, the mean previous gravidity, birth, and abortion numbers were 1.4 ± 0.9, 0.3 ± 0.5, and 1.1 ± 0.7, accordingly. Moreover, 30 (13.3%) participants had unplanned pregnancies; meanwhile, 64 (28.3%) participants had planned maternity leave time > 128 days; besides, there were 173 (76.5%) participants with satisfied labor experience; furthermore, 153 (67.7%) participants had caregivers≥2 (Table 1).
TABLE 1.
Characteristics of the participants
| Items | Participants (N = 226) |
|---|---|
| Age (years), mean ± SD | 27.7 ± 3.6 |
| Smoke history, n (%) | |
| No | 194 (85.8) |
| Yes | 32 (14.2) |
| Drink history, n (%) | |
| No | 176 (77.9) |
| Yes | 50 (22.1) |
| Education level, n (%) | |
| Primary school or less | 7 (3.1) |
| High school | 94 (41.6) |
| Undergraduate | 114 (50.4) |
| Graduate or above | 11 (4.9) |
| Annual family income (CNY), n (%) | |
| <100,000 | 9 (4.0) |
| 100,000–199,999 | 86 (38.1) |
| 200,000–299,999 | 93 (41.2) |
| ≥300,000 | 38 (16.8) |
| Previous gravidity number, mean ± SD | 1.4 ± 0.9 |
| Previous birth number, mean ± SD | 0.3 ± 0.5 |
| Previous abortion number, mean ± SD | 1.1 ± 0.7 |
| Gestational week, mean ± SD | 39.1 ± 1.9 |
| Unplanned pregnancy, n (%) | |
| No | 196 (86.7) |
| Yes | 30 (13.3) |
| Planned maternity leave time, n (%) | |
| ≤128 days | 162 (71.7) |
| >128 days | 64 (28.3) |
| Satisfaction of labor experience, n (%) | |
| Unsatisfied | 53 (23.5) |
| Satisfied | 173 (76.5) |
| Breastfeeding difficulty, n (%) | |
| No | 146 (64.6) |
| Yes | 80 (35.4) |
| Number of caregivers, n (%) | |
| <2 | 73 (32.3) |
| ≥2 | 153 (67.7) |
Abbreviations: CNY, China Yuan; SD, standard deviation.
3.3. PPD and PPA evaluation
The mean ± standard deviation (SD), median (interquartile range [IQR]), and range of EPDS score were 9.2 ± 4.6, 8.0 (6.0–12.0), and 0.0–25.0, respectively (Figure 2A); besides, the rate of PPD was 24.3% (Figure 2B). In addition, the mean ± SD, median (IQR), and range of STAI6 score were 13.5 ± 3.4, 13.0 (11.0–16.0), and 6.0–23.0, accordingly (Figure 2C); besides, the rate of PPA was 27.9% (Figure 2D).
FIGURE 2.
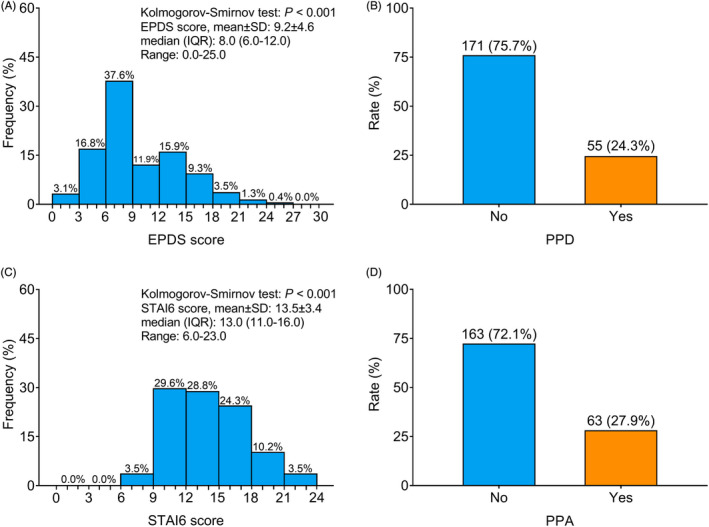
Incidence of PPD and PPA. Frequency of different EPDS score (A), rate of PPD (B), frequency of different STAI6 score (C) and rate of PPA (D)
3.4. Correlation of T helper cells and their related cytokines with EPDS score
Th1 cells were not correlated with EPDS score (r s = 0.097, p = 0.147) (Figure 3A). Th2 cells were not correlated with EPDS score either (r s = −0.062, p = 0.355) (Figure 3B). Th17 cells were positively correlated with EPDS score (r s = 0.306, p < 0.001) (Figure 3C). Furthermore, no correlation was found in IFN‐γ (r s = 0.104, p = 0.118) (Figure 3D) or IL‐4 (r s = −0.061, p = 0.359) (Figure 3E) with EPDS score. While positive correlation was found in IL‐17A with EPDS score (r s = 0.259, p < 0.001) (Figure 3F).
FIGURE 3.
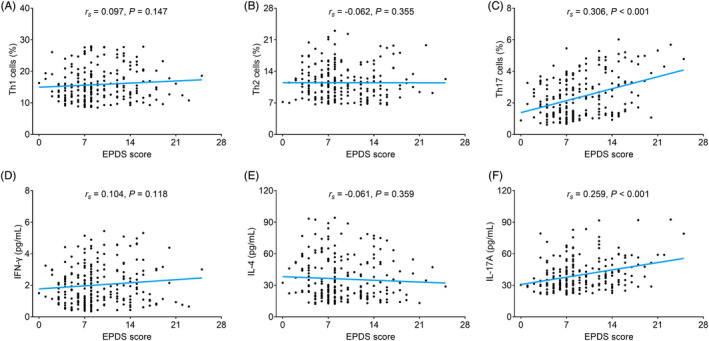
Th17 cells and IL‐17A were positively correlated with EPDS score. Correlation of Th1 cells (A), Th2 cells (B), Th17 cells (C), IFN‐γ (D), IL‐4 (E), and IL‐17A (F) with EPDS score
3.5. Comparison of T helper cells and their related cytokines in participants with and without PPD
No difference of Th1 cells (p = 0.243) (Figure 4A) or Th2 cells (p = 0.749) (Figure 4B) was found between participants with and without PPD. However, Th17 cells (median (IQR): 3.2 (1.5–4.5)% vs. 1.8 (1.2–2.9)%) were elevated in participants with PPD compared to those without PPD (p < 0.001) (Figure 4C). Moreover, no difference of IFN‐γ (p = 0.425) (Figure 4D) or IL‐4 (p = 0.918) (Figure 4E) was found between participants with and without PPD. While IL‐17A (median (IQR): 41.8 (33.1–54.3) pg/ml vs. 34.3 (27.8–45.1) pg/ml) was increased in participants with PPD compared to those without PPD (p = 0.002) (Figure 4F).
FIGURE 4.
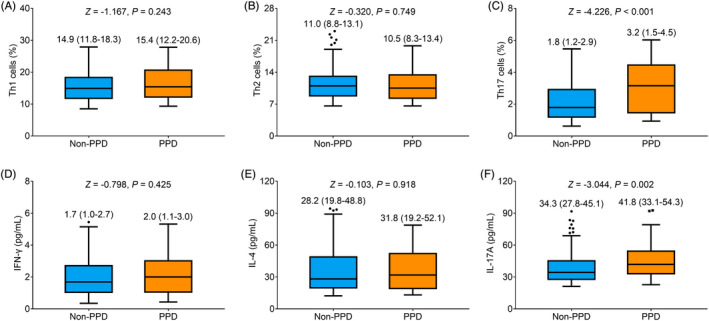
Th17 cells and IL‐17A were elevated in PPD women vs. non‐PPD women. Comparison of Th1 cells (A), Th2 cells (B), Th17 cells (C), IFN‐γ (D), IL‐4 (E), and IL‐17A (F) in PPD women vs. non‐PPD women
3.6. Correlation of T helper cells and their related cytokines with STAI6 score
Th1 cells (r s = 0.113, p = 0.090) (Figure 5A) and Th2 cells (r s = −0.077, p = 0.252) (Figure 5B) were not correlated with STAI6 score. While Th17 cells were positively correlated with STAI6 score (r s = 0.235, p < 0.001) (Figure 5C). Furthermore, no correlation was found in IFN‐γ (r s = 0.111, p = 0.095) (Figure 5D) or IL‐4 (r s = −0.090, p = 0.180) (Figure 5E) with STAI6 score, while positive correlation was found in IL‐17A with STAI6 score (r s = 0.240, p < 0.001) (Figure 5F).
FIGURE 5.
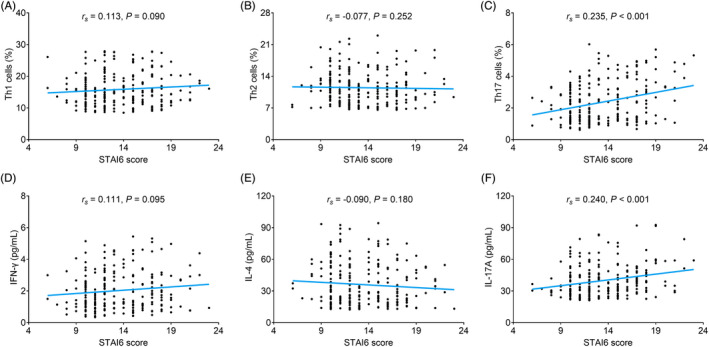
Th17 cells and IL‐17A were positively correlated with STAI6 score. Correlation of Th1 cells (A), Th2 cells (B), Th17 cells (C), IFN‐γ (D), IL‐4 (E), and IL‐17A (F) with STAI6 score
3.7. Comparison of T helper cells and their related cytokines in participants with and without PPA
No difference of Th1 cells (p = 0.190) (Figure 6A) or Th2 cells (p = 0.518) (Figure 6B) was found between participants with and without PPA. However, Th17 cells (median (IQR): 2.8 (1.3–4.2)% vs. 1.9 (1.3–3.0)%) were elevated in participants with PPA compared to those without PPA (p = 0.004) (Figure 6C). Moreover, no difference in IFN‐γ (p = 0.292) (Figure 6D) or IL‐4 (p = 0.316) (Figure 6E) was found between participants with and without PPA. While IL‐17A (median (IQR): 40.6 (33.7–52.6) pg/ml vs. 33.1 (27.6–44.2) pg/ml) was increased in participants with PPA compared to those without PPA (p = 0.001) (Figure 6F).
FIGURE 6.
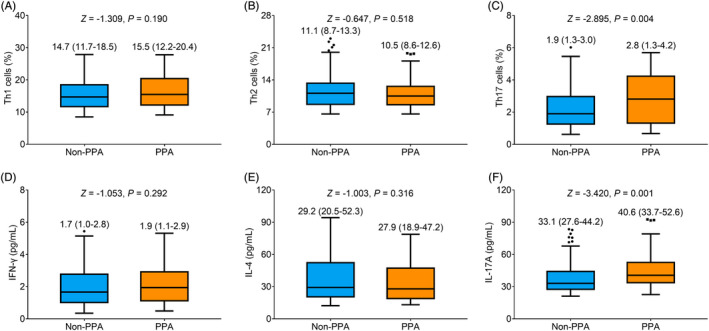
Th17 cells and IL‐17A were increased in PPA women vs. non‐PPA women. Comparison of Th1 cells (A), Th2 cells (B), Th17 cells (C), IFN‐γ (D), IL‐4 (E), and IL‐17A (F) in PPA women vs. non‐PPA women
3.8. Independent factors for PPD and PPA
Multivariate logistic regression model analysis presented that Th17 cells were independently correlated with an elevated risk of PPD (odds ratio [OR] = 1.600, p = 0.001), while annual family income (OR = 0.283, p < 0.001), the satisfaction of labor experience (vs. unsatisfaction) (OR = 0.239, p = 0.001), and caregiver ≥2 (vs. <2) (OR = 0.248, p = 0.001) were independently correlated with declined risk of PPD. Moreover, Th17 cells were independently correlated with an increased risk of PPA (OR = 1.371, p = 0.022), while annual family income (OR = 0.231, p < 0.001), previous gravidity number (OR = 0.644, p = 0.029), planned maternity leave time > 128 days (vs. ≤128 days) (OR = 0.332, p = 0.011), the satisfaction of labor experience (vs. unsatisfaction) (OR = 0.260, p = 0.001), and caregiver ≥2 (vs. <2) (OR = 0.151, p < 0.001) were independently correlated with declined risk of PPA (Table 2).
TABLE 2.
Factors related to PPD and PPA by multivariate logistic regression model analysis
| Items | p Value | OR | 95%CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| PPD | ||||
| Th17 cells (%) | 0.001 | 1.600 | 1.216 | 2.104 |
| Annual family income | <0.001 | 0.283 | 0.163 | 0.490 |
| Satisfaction of labor experience (Satisfied vs. Unsatisfied) | 0.001 | 0.239 | 0.105 | 0.544 |
| Number of caregivers (≥2 vs. <2) | 0.001 | 0.248 | 0.112 | 0.546 |
| PPA | ||||
| Th17 cells (%) | 0.022 | 1.371 | 1.047 | 1.796 |
| Annual family income | <0.001 | 0.231 | 0.131 | 0.407 |
| Previous gravidity number | 0.029 | 0.644 | 0.434 | 0.956 |
| Planned maternity leave time (>128 days vs. ≤128 days) | 0.011 | 0.332 | 0.142 | 0.775 |
| Satisfaction of labor experience (Satisfied vs. Unsatisfied) | 0.001 | 0.260 | 0.115 | 0.590 |
| Number of caregivers (≥2 vs. <2) | <0.001 | 0.151 | 0.067 | 0.340 |
Abbreviations: CI, confidence interval; OR, odds ratio; PPA, postpartum anxiety; PPD, postpartum depression; Th17 cells, T‐helper 17 cells.
4. DISCUSSION
Regarding the incidence of PPD and PPA, it has been reported that 19.2% women suffer from PPD during the first 3 months after giving birth 6 ; furthermore, 19% Sweden women experience PPD after 2 months postpartum 5 ; additionally, another interesting study also illustrated that the incidence of PPD ranges from 9% to 12% among women during postpartum. 21 In the current study, the incidence of PPD and PPA were 24.3% and 27.9%, respectively, which was numerically higher than in previous studies. 5 , 6 , 21 The possible explanations might be that (1) different types of participants (such as those coming from different countries and different ages) might result in the different incidence of PPD and PPA 5 , 21 ; (2) different assessment criteria and different evaluation time points led to a different assessment result of the incidence of PPD and PPA. 5 , 6 , 21 In the current study, EPDS and STAI6 scores were used to evaluate PPD and PPA at 6 weeks after women gave birth, among which, EPDS score was the most common worldwide used screening tool for PPD, and the STAI6 score was convenient to evaluate PPA. 19 , 20 , 22
In terms of the association of T‐helper cells and their related cytokines with depression and anxiety, it has been reported that Th1 and Th2 cells are dysregulated in patients with depression 23 ; besides, an interesting study illustrates that Th17 cells are increased in depressed patients 11 ; meanwhile, it also has been presented that IL‐17A is elevated but IFN‐γ and IL‐4 are normal in depressive patients. 24 However, the data about the correlation of Th1, Th2, and Th17 cells and their related cytokines with PPD and PPA is limited. In the current study, we found that Th17 cells and IL‐17A were positively correlated with PPD and PPA. The possible explanations might be that (1) Th17 cells might modulate the function of the brain through several methods, including regulating hippocampal neurogenesis, psychological stress resilience, and brain tissue homeostasis, which led to the development of PPD and PPA 25 , 26 , 27 , 28 , 29 ; (2) Th17 cells might take part in the regulation of brain inflammation through secreting proinflammatory cytokines such as IL‐17A, which resulted in the development of PPD and PPA. 11 , 30 Thus, Th17 cells and IL‐17A were positively correlated with PPD and PPA.
As for factors to predict the risk of PPD and PPA, it has been reported that exposure of sulfur dioxide is correlated with an elevated risk of PPD 31 ; in addition, the mothers with worse sleep quality during pregnancy have more possibilities to experience PPD and PPA. 32 In the current study, we found that Th17 cells were independently correlated with an elevated risk of PPD and PPA, which might be explained by that Th17 might take part in the modulation of the central nervous system, such as regulation of brain function and brain inflammation, which could result in the occurrence of PPD and PPA. 11 , 25 , 26 , 27 , 28 , 29 , 30 , 33 In addition, we also found that annual family income, the satisfaction of labor experiments, previous gravidity number, caregivers number, and planned maternity leave time were independently correlated with the risk of PPD or PPA, indicating these factors should be paid more attention among pregnant women to potentially avoid PPD and PPA.
From the previous studies, 5 , 20 PPA and PPD were commonly assessed at 6‐week postpartum, thus we also used inclusion “within 6 weeks postpartum” in the current study to make sure PPA and PPD could be assessed at 6‐week postpartum. Meanwhile, the planned maternity leave time normally was 128 days in China, thus, we applied “128 days” as the cutoff time in the current study. However, there exited several limitations in the current study: (1) the mechanism of Th17 cells in the development of PPD and PPA could be explored; (2) the monitoring of Th1, Th2, and Th17 cells, as well as their related cytokines at multiple time points in women with PPD and PPA was needed; (3) questionnaire or scale of EPDS and STAI6 assessment was completed by participants themselves, which might exist bias.
To be conclusive, blood Th17 cells and IL‐17A are positively linked with the risk of PPD and PPA, indicating which may be involved in the development of PPD and PPA.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Min Z, Li Y, Ying H. Blood T‐helper 17 cells and interleukin‐17A correlate with the elevated risk of postpartum depression and anxiety. J Clin Lab Anal. 2022;36:e24559. doi: 10.1002/jcla.24559
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Daly D, Moran P, Wuytack F, et al. The maternal health‐related issues that matter most to women in Ireland as they transition to motherhood ‐ a qualitative study. Women Birth. 2022;35(1):e10‐e18. [DOI] [PubMed] [Google Scholar]
- 2. Asadi M, Noroozi M, Alavi M. Identifying women's needs to adjust to postpartum changes: a qualitative study in Iran. BMC Pregnancy Childbirth. 2022;22(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katou Y, Okamura M, Ohira M. Transition to motherhood for Japanese primiparas from delivery to 6 months postpartum: a qualitative study. Nurs Open. 2022;9(1):490‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Delgado‐Perez E, Yuste‐Sanchez MJ, Perez‐Martin Y, Abuin‐Porras V, Rodriguez‐Costa I. New motherhood concepts, implications for healthcare. A qualitative study. Int J Environ Res Public Health. 2021;18(24):13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hildingsson I, Rubertsson C. Depressive symptoms during pregnancy and after birth in women living in Sweden who received treatments for fear of birth. Arch Womens Ment Health. 2022;25(2):473‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gavin NI, Gaynes BN, Lohr KN, Meltzer‐Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071‐1083. [DOI] [PubMed] [Google Scholar]
- 7. Newman DM, Boyarsky M, Mayo D. Postpartum depression. JAAPA. 2022;35(4):54‐55. [DOI] [PubMed] [Google Scholar]
- 8. Wan Mohamed Radzi C, Salarzadeh Jenatabadi H, Samsudin N. Postpartum depression symptoms in survey‐based research: a structural equation analysis. BMC Public Health. 2021;21(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tainaka H, Takahashi N, Nishimura T, et al. Long‐term effect of persistent postpartum depression on children's psychological problems in childhood. J Affect Disord. 2022;305:71‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kingston D, Kehler H, Austin MP, et al. Trajectories of maternal depressive symptoms during pregnancy and the first 12 months postpartum and child externalizing and internalizing behavior at three years. PLoS One. 2018;13(4):e0195365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beurel E, Medina‐Rodriguez EM, Jope RS. Targeting the adaptive immune system in depression: focus on T helper 17 cells. Pharmacol Rev. 2022;74(2):373‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Osborne LM, Gilden J, Kamperman AM, et al. T‐cell defects and postpartum depression. Brain Behav Immun. 2020;87:397‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Xiao B, Qiu W, et al. Altered expression of CD4(+)CD25(+) regulatory T cells and its 5‐HT(1a) receptor in patients with major depression disorder. J Affect Disord. 2010;124(1–2):68‐75. [DOI] [PubMed] [Google Scholar]
- 14. Balderas‐Vazquez CL, Bernal‐Morales B, Garcia‐Montalvo EA, et al. Association between socio‐affective symptoms and glutathione and CD4 and CD8 lymphocytes in college students. Front Psychol. 2021;12:666347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu M, Li Y, Liu X. Serum tumor necrosis factor‐alpha, interleukin‐1beta, interleukin‐6, and interleukin‐17 relate to anxiety and depression risks to some extent in non‐small cell lung cancer survivor. Clin Respir J. 2022;16(2):105‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ghosh R, Kumar PK, Mitra P, Purohit P, Nebhinani N, Sharma P. Circulating T helper 17 and IFN‐gamma positive Th17 cells in major depressive disorder. Behav Brain Res. 2020;394:112811. [DOI] [PubMed] [Google Scholar]
- 17. Groer ME, Jevitt C, Ji M. Immune changes and dysphoric moods across the postpartum. Am J Reprod Immunol. 2015;73(3):193‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shimaoka Y, Hidaka Y, Tada H, et al. Changes in cytokine production during and after normal pregnancy. Am J Reprod Immunol. 2000;44(3):143‐147. [DOI] [PubMed] [Google Scholar]
- 19. Slykerman RF, Hood F, Wickens K, et al. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double‐blind placebo‐controlled trial. EBioMedicine. 2017;24:159‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y, Guo N, Li T, Zhuang W, Jiang H. Prevalence and associated factors of postpartum anxiety and depression symptoms among women in Shanghai, China. J Affect Disord. 2020;274:848‐856. [DOI] [PubMed] [Google Scholar]
- 21. Woody CA, Ferrari AJ, Siskind DJ, Whiteford HA, Harris MG. A systematic review and meta‐regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;219:86‐92. [DOI] [PubMed] [Google Scholar]
- 22. Nakic Rados S, Tadinac M, Herman R. Anxiety during pregnancy and postpartum: course, predictors and comorbidity with postpartum depression. Acta Clin Croat. 2018;57(1):39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Myint AM, Leonard BE, Steinbusch HW, Kim YK. Th1, Th2, and Th3 cytokine alterations in major depression. J Affect Disord. 2005;88(2):167‐173. [DOI] [PubMed] [Google Scholar]
- 24. Alvarez‐Mon MA, Gomez‐Lahoz AM, Orozco A, et al. Expansion of CD4 T lymphocytes expressing interleukin 17 and tumor necrosis factor in patients with major depressive disorder. J Pers Med. 2021;11(3):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Niebling J, Runker AE, Schallenberg S, Kretschmer K, Kempermann G. Myelin‐specific T helper 17 cells promote adult hippocampal neurogenesis through indirect mechanisms. F1000Res. 2014;3:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zeng J, Ji Y, Luan F, et al. Xiaoyaosan ethyl acetate fraction alleviates depression‐like behaviors in CUMS mice by promoting hippocampal neurogenesis via modulating the IGF‐1Rbeta/PI3K/Akt signaling pathway. J Ethnopharmacol. 2022;288:115005. [DOI] [PubMed] [Google Scholar]
- 27. Poletti S, de Wit H, Mazza E, et al. Th17 cells correlate positively to the structural and functional integrity of the brain in bipolar depression and healthy controls. Brain Behav Immun. 2017;61:317‐325. [DOI] [PubMed] [Google Scholar]
- 28. Wolf SA, Steiner B, Wengner A, Lipp M, Kammertoens T, Kempermann G. Adaptive peripheral immune response increases proliferation of neural precursor cells in the adult hippocampus. FASEB J. 2009;23(9):3121‐3128. [DOI] [PubMed] [Google Scholar]
- 29. Cohen H, Ziv Y, Cardon M, et al. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J Neurobiol. 2006;66(6):552‐563. [DOI] [PubMed] [Google Scholar]
- 30. Worthen RJ, Beurel E. Inflammatory and neurodegenerative pathophysiology implicated in postpartum depression. Neurobiol Dis. 2022;165:105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duan CC, Li C, Xu JJ, et al. Association between prenatal exposure to ambient air pollutants and postpartum depressive symptoms: a multi‐city cohort study. Environ Res. 2022;209:112786. [DOI] [PubMed] [Google Scholar]
- 32. Gueron‐Sela N, Shahar G, Volkovich E, Tikotzky L. Prenatal maternal sleep and trajectories of postpartum depression and anxiety symptoms. J Sleep Res. 2021;30(4):e13258. [DOI] [PubMed] [Google Scholar]
- 33. Park BV, Pan F. The role of nuclear receptors in regulation of Th17/Treg biology and its implications for diseases. Cell Mol Immunol. 2015;12(5):533‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


