Abstract
Objective
This case‐control study was designed to compare the composition of the predominant oral bacterial microbiome in Alzheimer's disease (AD) and control group.
Subject
A total of 30 adult participants (15 AD and 15 healthy individuals) were entered in this study. The composition of oral bacterial microbiome was examined by quantitative real‐time polymerase chain reaction (qPCR) using bacterial 16S rDNA gene. The levels of systemic inflammatory cytokines in both groups were assessed using enzyme‐linked immunosorbent assays (ELISA).
Results
The loads of Porphyromonas gingivalis, Fusobacterium nucleatum, and Prevotella intermedia were significantly more abundant in the AD compared to the control group (p < 0.05). Although Aggregatibacter actinomycetemcomitans and Streptococcus mutans were relatively frequent in the AD group, no significance difference was observed in their copy number between two groups. Although the concentrations of IL‐1, IL‐6, and TNF‐α were higher in the AD group, there was a significant difference in their levels between the two groups (p < 0.05). Finally, there was a significant relationship between increased number of pathogenic bacteria in oral microbiome and higher concentration of cytokines in patient's blood.
Conclusion
Our knowledge of oral microbiome and its exact association with AD is rather limited; our study showed a significant association between changes in oral microbiome bacteria, increased inflammatory cytokines, and AD.
Keywords: Alzheimer's disease, inflammatory cytokines, neurological disease, oral microbiome, periodontitis, qPCR
1. INTRODUCTION
Alzheimer's disease (AD) is considered as a devastating neurodegenerative disease and the most common type of dementia that is associated with both central and peripheral immune dysregulations. It represents 60%–80% of dementia cases and has a multifactorial etiology. 1 , 2 Although it has been researched for decades, the exact cause of the disease is unknown and no definitive prevention or treatment has been proposed. 3 Even though the causes of AD are shrouded in obscurity, aging is one of the most important risk factors. The increasing life expectancy has led to population aging,as a consequence, it is likely that AD will quadruple over the next 40 years. 4 A number of other factors such as lifestyle, genetic signature, and environmental parameters have been contributed to AD besides aging. 5 It is estimated that 13–14 million people in the United States are likely to suffer from AD with a treatment cost of more than 1 trillion dollars. In 2015, 47 million AD patients were diagnosed worldwide with the prediction that the number of patients would reach 75 million by 2030 and 132 million by 2050. 6 Familial‐early‐onset and sporadic‐late‐onset are two main forms of AD based on pathogenic features which comprise around 2% and 98% of all cases, respectively. Mutations in PSEN1 (Presenilin 1), and PSEN2 (Presenilin 2) as amyloid precursor protein genes, are a reason of familial‐early‐onset form which occurs earlier in life with an extensive amyloid beta (Aβ) deposits and functional loss. 7 On the contrary, the inheritance of Apolipoprotein ɛ4 (APOEɛ4) gene which is considered as the main cause for the late‐onset form has an indispensable environmental role in expression of APOEɛ4. 8 Moreover, hyperphosphorylated tau (HPtau), introduced as another cerebral feature of AD, has proven to be responsible for progressive cognitive impairment. AD progression are split into three (A–C) and six (I–VI) stages according to Aβ and HPtau movements in the brain, respectively. 9 The Aβ deposition are usually found in the isocortex of the cerebral cortex and are not equal in size and shape as well as show inter‐individual variations at early stages. Aβ plaques develop before the HPtau formation, and the development of Aβ plaques does not mean HPtau lesion will certainly develop. 10 It is worth mentioning that in AD patients, chronic inflammation and dysfunction of the immune system occur several decades before cognitive impairment as impaired immune regulation is the first sign of AD progression. 11 An influential factor responsible for chronic inflammation is microbiome changes, which is one of the most important issues in various diseases. Researchers are conducting various studies on the relationship between oral microbiome changes and various diseases. 12 , 13 , 14 , 15 The population of microbes which live in commensalism or symbiosis form with human is described as the “human microbiome”. 16 Oral cavity has an intricate environment in which various microbial populations including bacteria, viruses, fungi, archaea, and protozoa live. 17 Perturbation in the oral microbiota enhances the risk of periodontitis consequently raising Aβ peptide plaques in the brains. On the other hand, a close correlation has been declared between chronic periodontitis and intensification of systemic inflammation and reduction of cognitive functions. 18 , 19 In addition to neuro‐inflammation by bacterial products and Aβ deposition in the brain, there are other mechanisms for microbiome changes that progress AD, including dysbiosis in the gut microbiota, damage to oral mucosal barriers, and microbes entering brain through bloodstream. 20 Therefore, among the essential risk factors involved in exacerbation of AD, include change in the microbiome, especially the oral microbiome. In the current study, the relationship between perturbation of inflammatory cytokines and microbiome oral bacteria was compared in Alzheimer patients and healthy individuals.
2. MATERIAL AND METHODS
2.1. Subjects and sample preparation
This cross‐sectional study was carried out during 18 months from April 2020 to October 2021 by convenient sampling method on elderly individuals hospitalized in the neurology department of Poursina hospital of Rasht city which is the provisional capital of Guilan province in Iran. All sampling methods and techniques of this study were approved by the ethics committee of Iran University of Medical Sciences (IR.IUMS.FMD.REC.1399.553). Demographic questionnaires were used for collecting data related to all variants. The mini‐mental state examination (MMSE) was used by a neurologist as one of the most common methods of measuring general cognitive function of participants in the study. Finally, brain magnetic resonance imaging (MRI) examination and blood tests were conducted for definitive diagnosis of hospitalized Alzheimer patients. The inclusion criteria for AD group included age of 60 years or above, accurate diagnosis of AD by a neurologist, and voluntary participation. The exclusion criteria included the elderly over 60 years without teeth, people despite the consent of companions who did not allow sampling due to neurological disorders, COVID‐19 positivity, and people with severe oral and maxillofacial injuries (Figure 1). Following diagnosis of AD by a neurologist and obtaining informed consent, as part of a protocol approved by the ethics committee of Iran University of Medical Sciences, 5 ml of venous peripheral blood was collected from the AD and control individuals (volunteers over 60 years of age, with teeth, without neurological disease, and COVID‐19 negative). The blood samples were then centrifuged (10 min at 2500 g) and sera were isolated and stored at −20°C for further examination. Oral specimens were sampled from mucosa, teeth, supra‐ and sub‐gingival spaces, tongue, and keratinized gingiva using sterile paper points according to the instructions of Santigli et al. 21 All oral samples were fixed in phosphate‐buffered saline (PBS), then snap‐frozen and stored at −70°C until DNA extraction.
FIGURE 1.
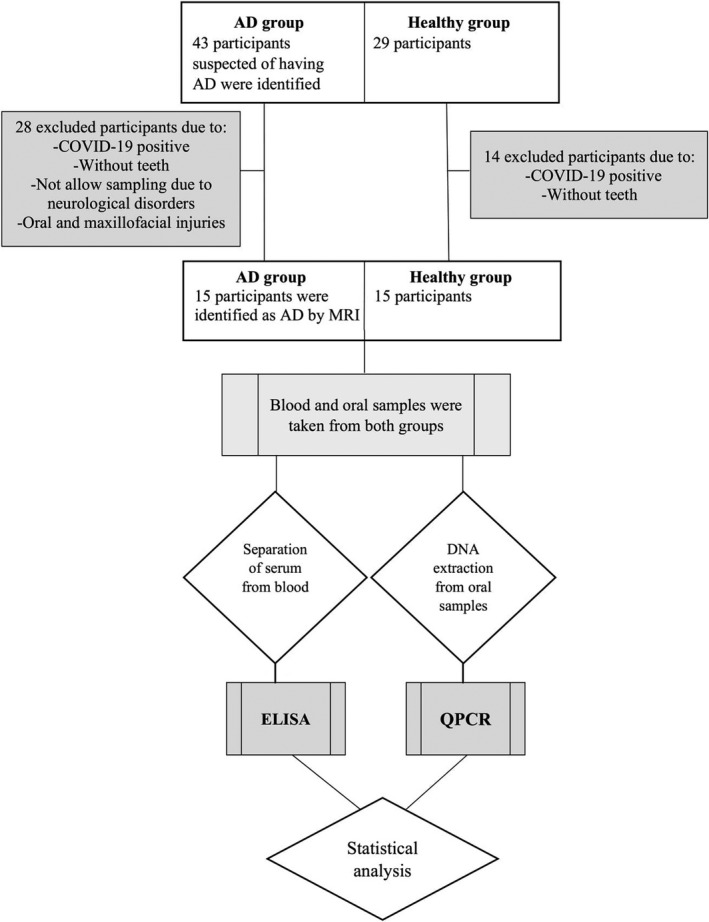
A schematic diagram of the experimental design
2.2. DNA extraction
Each paper point sample was included in 2 ml microcentrifuge tube containing 200 µl of sterile 1X PBS which was vortexed (LABINCO, L46, Netherlands) for 2 min at 300 g in order to release microbial cells. Thereafter, tubes were centrifuged at 1200 g for 10 min and supernatant was carefully removed. DNA extraction was performed from the deposits. Genomic DNA of all oral samples was extracted according to the instructions of QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany). The quantity and quality of the extracted DNA were evaluated in OD 260/280 nm as well as on agarose gel (1.5%) and the confirmed DNA was stored at −20°C for further analysis.
2.3. Primers and probes design
TaqMan probes and primers were used for the detection of 16S rDNA gene sequence of five main oral microbiome, including Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, Prevotella intermedia, and Streptococcus mutans. Primer Express software V.3.0 (Applied Biosystems) was used to design the probes and primers, based on research conducted by Shariati et al. 22 Table 1 exhibits the sequences and detailed characteristics of the probes and primers used in this study. Notably, 3′ and 5′ ends of all TaqMan probes were labeled with the quencher dye BHQ and reporter dye molecule FAM, respectively. In addition, to prevent the probe extension in the PCR process, the 3′ ends of each probe were phosphorylated. Finally, BLAST analysis was applied to evaluate the specificity of the sequences of primers and probes (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
TABLE 1.
The sequence of primers designed in this study to identify the composition of bacteria in the oral microbiome
| Target bacteria | Primer/probe | Oligonucleotide sequence (50e30) | Product size (bp) | Reference |
|---|---|---|---|---|
| Porphyromonas gingivalis | Primer F | TCGTCGAAACGATCGAAACC | 162 | This study |
| Primer R | GCAGAGCGGTGTAATACGTC | |||
| Probe | TTCGCGGTATCTTGCCGGCC | |||
| Aggregatibacter actinomycetemcomitans | Primer F | CCACGCCGTTAATGTTCCAT | 120 | This study |
| Primer R | GCCCGTAAGCCTTGCTATTC | |||
| Probe | AAACGCCTGTGTGCCGCGCC | |||
| Fusobacterium nucleatum | Primer F | AGCTACAAGAGAAGAAAATGAAAATGG | 105 | This study |
| Primer R | CCAACTCCTACAAATCCAGTAACC | |||
| Probe | TTACTTCATACCATACACGAGGATCTACTT | |||
| Prevotella intermedia | Primer F | AAGACCGTGTTCAACCAACG | 102 | This study |
| Primer R | TGTCATCACTTCCTGCTCGT | |||
| Probe | CTGGCGCAGGCTTACTCGCA | |||
| Streptococcus mutans | Primer F | TGGAACAATCTCACCAGCCA | 112 | This study |
| Primer R | TCGTCAGTTCTTCACCACGA | |||
| Probe | TGCTGCTTCCAAGGCTTGTTCCAGC |
2.4. Quantitative real‐time PCR (qPCR)
Specific primers and probes designed for 16SrDNA and TaqMan qPCR were used to compare the number of oral microbiome bacteria between the patient and healthy groups. The final volume of amplification reaction was 20 ml in each qPCR including a mixture of 9 µl of Universal Probe Ex Taq PCR Master Mix (Ampliqon), 0.25 µM of the probe, 0.5 µM of each forward and reverse primer, 20 ng of extracted DNA, and 5.8 ml demineralized water. The qPCR thermal‐cycling condition were performed in a Rotor‐Gene 6000 real‐time PCR cycler (Qiagen Corbett) as follows: an initial denaturation at 95°C for 15 min, annealing of 40 cycle for 15 s at 58°C, and extension at 58°C for 30 s. Non‐template control (NTC) reactions were conducted in all tests and consisted of all the substances of the amplification reaction except the template DNA. In addition, final analysis of the results was carried out on the mean values of duplicate qPCR tests on the six studied genes. Standard curves were obtained applying serially diluted four dilutions of genomic DNA according to positive control strain for each qPCR assay. In the next stage, absolute quantification was carried out via a standard curve for definition of the copy number concentration of F. nucleatum ATCC 25586, S. mutans PTCC 1683, A. actinomycetemcomitans ATCC 33384, P. gingivalis ATCC 33227, and P. intermedia ATCC 25671. In this way, threshold cycle values (CT) were applied to measuring absolute bacterial concentration from each oral sample and reported as quantity of copy number of each bacterium per sample. 23
2.5. Inflammatory cytokine measurements
Human serum inflammatory cytokine levels of Interleukin‐1 (IL‐1β), Interleukin‐6 (IL‐6), and tumor necrosis factor‐α (TNF‐α) were quantified using specific sandwich enzyme‐linked immunosorbent assays (ELISA). ELISA were performed with the Human kit (R&D, Bio‐Techne kit) based on the procedures recommended by the manufacturer. All collected sera from both groups were assayed on duplicate and the yielded concentrations were expressed as pg/mL, according to calibration curves prepared with cytokine standards contained in the kits. The fluorescence intensity of ELISA plates was measured at 450 nm absorbance.
2.6. Statistical analysis
All statistical analysis were performed using SPSS‐22 (SPSS incorporate) at a two‐tailed p value of ≤0.05. Normal distribution of data was determined using the Shapiro–Wilk's test. Mann–Whitney U test was used to define between‐group differences of the three cytokines. A chi‐squared (X 2) test was also used to assess the differences of demographic characteristics between the groups. Moreover, independent samples t‐tests were applied to evaluate significance between the groups for the microbiome bacteria. Effect size statistics including Cohen's d (2 × ) and r () were calculated for the t‐test and Mann–Whitney U test, respectively, presenting 0.1 (small), 0.3 (medium), and 0.5 (large). 24 We also used Spearman's rank correlation coefficient (ρ) to determine if there was a relationship between the number of pathogenic bacteria in the oral microbiome and the concentration of cytokines.
3. RESULTS
3.1. 3–1 Subjects
The current study included a total of 30 participants distributed in the following groups: AD group (n = 15) and healthy group (n = 15). The AD group included 8 (53.3%) male and 7 (46.7%) female which were diagnosed with the disease. In interpreting the demographic information, different characteristics of the participants and their relationship with AD were analyzed. Table 2 shows the details of the differences between the control and AD groups. The mean age of participants in the control group was 64.33 ± 3.73, but in the AD group the mean age of patients was 69.47 ± 6.88. The age of the subjects in the AD group was higher than the control group. Statistical analysis showed that there was a significant difference between the control and AD groups in terms of age (p < 0.01). The incidence of diabetes, hypertension, and hyperlipidemia was significantly higher in the AD group than in healthy individuals. In addition, level of physical activity and education were two of the most influential characteristics in AD, which were lower in the AD group than control group. It was also found that more than half of the people in the AD group had a family history of the disease. All participants in both groups were married. The results also confirmed that there was a significant difference between the two groups in terms of hypertension, diabetes, hyperlipidemia, family history, education status, and level of physical activity (Table 2). On the other hand, there was no significant difference between BMI, sex, smoking, dentistry services, and occupation between AD and control groups (p > 0.05).
TABLE 2.
Comparison of frequency and significance level of different characteristics between AD and control groups
| Characteristics | Healthy group (n = 15) | AD group (n = 15) | p value |
|---|---|---|---|
| Mean ± SD | |||
| Age | 64.33 ± 3.73 | 69.47 ± 6.88 | 0.019 |
| BMI | 25.88 ± 3.26 | 28.44 ± 5.66 | 0.147 |
| Frequency (%) | |||
| Hypertension | 4 (26.7) | 11 (73.3) | 0.027 |
| Diabetes | 4 (26.7) | 11 (73.3) | 0.027 |
| Hyperlipidemia | 4 (26.7) | 10 (66.7) | 0.028 |
| Sex | |||
| Male | 8 (53.3) | 8 (53.3) | >0.5 |
| Female | 7 (46.7) | 7 (46.7) | |
| Family history | 0 (0.0) | 8 (53.3) | 0.001 |
| Smoking | |||
| Smoker | 2 (13.3) | 2 (13.3) | 0.64 |
| Non‐smoker | 9 (60.0) | 11 (73.3) | |
| Ex‐smoker | 4 (26.7) | 2 (13.3) | |
| Level of education | |||
| Illiterate | 0 (0.0) | 7 (46.7) | 0.009 |
| High school | 5 (33.3) | 6 (40.0) | |
| Diploma | 7 (46.7) | 1 (6.7) | |
| Associate degree | 1 (6.7) | 1 (6.7) | |
| Bachelor's degree | 2 (13.3) | 0 (0.0) | |
| Dentistry services | |||
| Low | 10 (66.7) | 12 (80.0) | 0.69 |
| Moderate | 3 (20.0) | 2 (13.3) | |
| High | 2 (13.3) | 1 (6.7) | |
| Marital status | |||
| Married | 15 (100) | 15 (100) | – |
| Bachelor | 0 (0.0) | 0 (0.0) | |
| Living area | |||
| Urban | 14 (93.3) | 9 (60.0) | 0.031 |
| Rural | 1 (6.7) | 6 (40.0) | |
| Inactive | 0 (0.0) | 10 (66.7) | <0.001 |
| Sedentary | 0 (0.0) | 4 (26.7) | |
| Low | 10 (66.7) | 1 (6.7) | |
| Moderate | 5 (33.3) | 0 (0.0) | |
| Occupation | |||
| Unemployed | 0 (0.0) | 1 (6.7) | 0.66 |
| Self‐employment | 5 (33.3) | 3 (20.0) | |
| Employee | 5 (33.3) | 5 (33.3) | |
| Homemaker | 5 (33.3) | 6 (40.0) | |
3.2. Bacterial quantification
In this case‐control study, qPCR analysis was carried out to compare differences between composition of the most important oral microbiome bacteria in the two groups. Statistical analysis illustrated significant differences in abundance of three bacteria in oral microbiome between AD and healthy groups (Figure 2). Quantification of bacterial genera showed P. gingivalis was more frequent in the AD compared to the control group (t = 3.19, p = 0.003, Cohen's d = 1.21). The quantity of F. nucleatum was higher in the oral microbiota of AD group than control group, and there was significant difference in F. nucleatum abundance between the AD and healthy individuals (t = 2.362, p = 0.002, Cohen's d = 0.89). One other bacterium of the oral microbiome which showed sharply higher abundance in the AD group compared to the healthy group included P. intermedia (t = 3.831, p < 0.001, Cohen's d = 1.45). Besides that, although A. actinomycetemcomitans was higher in the AD population, there was no significant difference compared to the healthy individuals (t = 1.50, p = 0.143). Similarly, even though S. mutans was relatively frequent in the AD group, there was no meaningful difference in its copy number between two groups (t = 1.153, p = 0.259).
FIGURE 2.
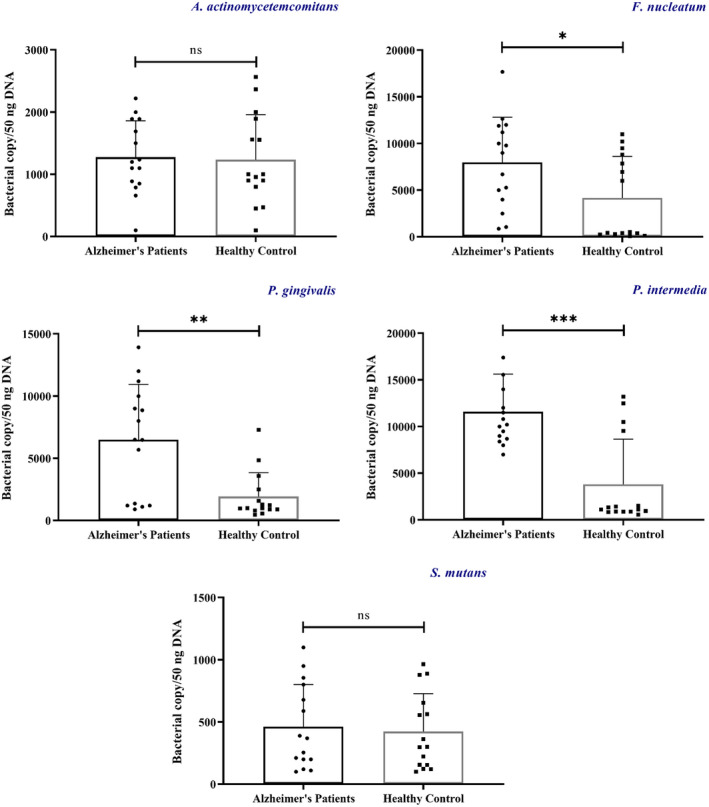
The t‐test shows significance difference in the copy number of bacteria in AD and control group. *p < 0.05, **p < 0.01, ***≤0.001
3.3. Cytokine assay
The ELISA assay results have indicated that the amounts of all three cytokines were higher in AD as compared to the healthy subjects (Figures 3 and 4). Comparison of IL‐1 concentration in the two groups showed that the concentration of this cytokine in the AD group was significantly different compared to the control group (U = 62.5, p = 0.038). On the other hand, the level of IL‐6 in the AD group was higher than healthy individuals, which was a significant difference in concentration between the two groups (U = 64, p = 0.044). Not only the concentration of TNF‐α in the AD group was higher than the control group, but also statistical analysis shows a significant level for this concentration difference between the two groups (U = 26, p < 0.001).
FIGURE 3.
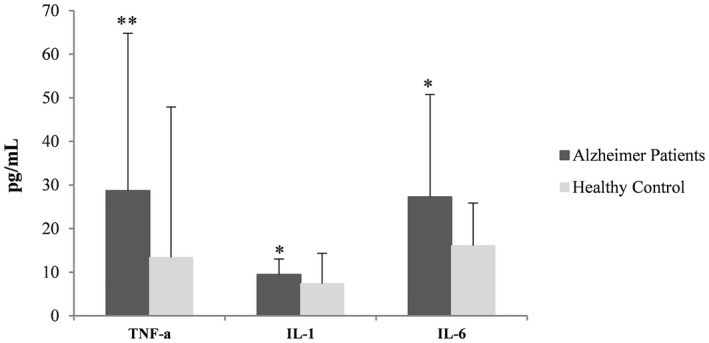
Differences in cytokine concentrations between AD and healthy groups. *Indicates the significant level of difference p < 0.05; **Indicates the significant level of difference p < 0.001
FIGURE 4.
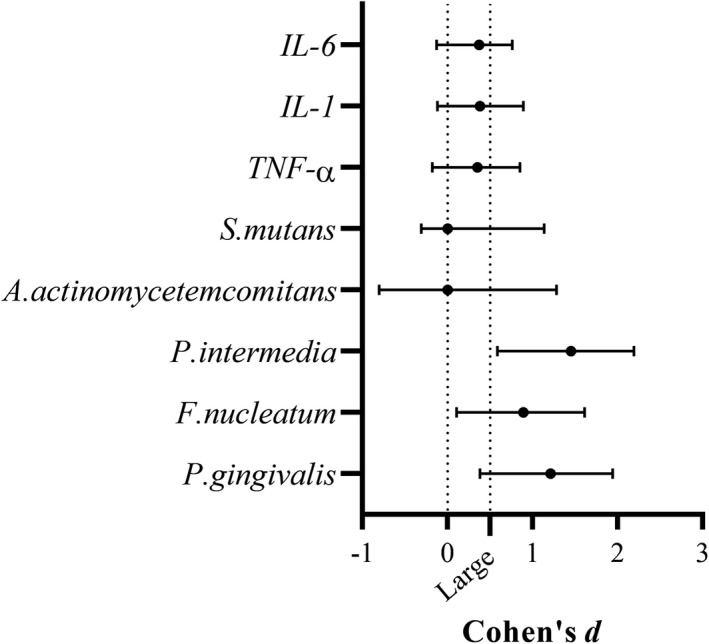
Effect of size estimates for the three cytokines (r) and the five studied oral microbiome bacteria (Cohen's d) in AD and control group
3.4. Correlation between bacterial load and cytokines
Statistical analysis of the correlation between increasing the number of bacterial microbiome and increasing the concentration of cytokines in the blood of patients showed that there was a significant relationship in this regard (Figure 5). Higher numbers of P. gingivalis in the oral cavity of AD group showed a significant relationship with increased serum levels of IL‐1 (ρ = 0.65, p = 0.008) and IL‐6 (ρ = 0.60, p = 0.018). Similarly, the increase in quantity of P. intermedia was associated with increased serum concentration of all three cytokines (TNF‐α: ρ = 0.67, p = 0.006, IL‐1: ρ = 0.55, p = 0.032, IL‐6: ρ = 0.82, p < 0.001) in Alzheimer's patients. In addition, higher loads of F. nucleatum in AD group was associated with increased serum levels of IL‐1 (ρ = 0.68, p = 0.005) and IL‐6 (ρ = 0.64, p = 0.009). However, there was no significant relationship between increasing the number of A. actinomycetemcomitans and S. mutans and serum concentrations of the three cytokines in AD group (p > 0.05). There was no association between oral bacteria in the control group and increased cytokines in the blood.
FIGURE 5.
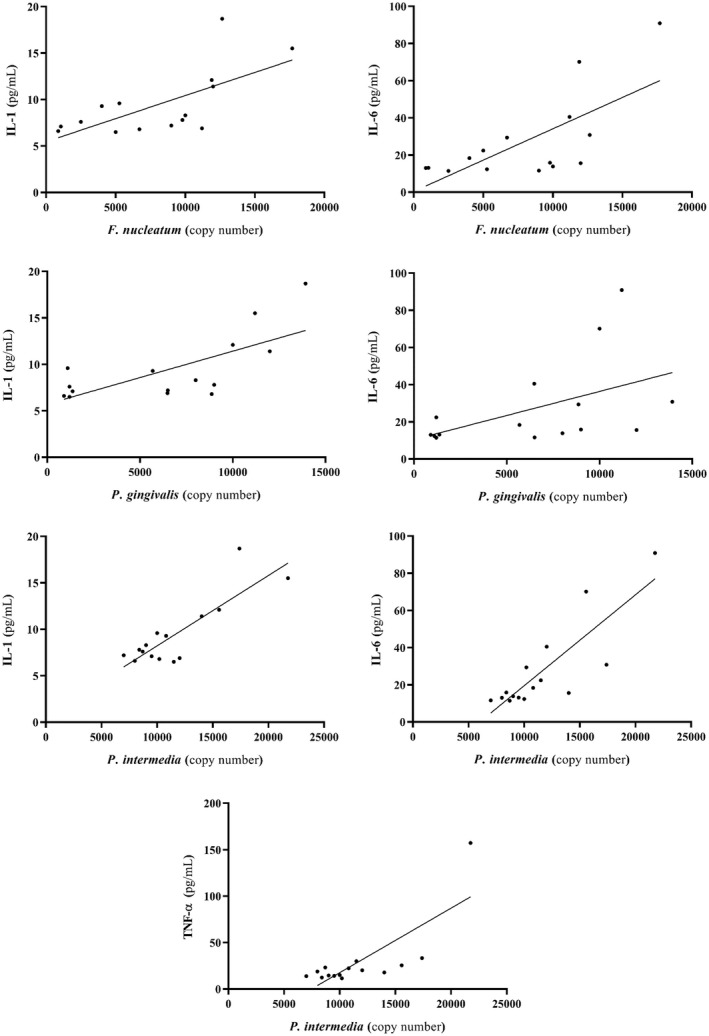
Correlation between copy number of each oral microbiome bacterium and the three inflammatory cytokines
4. DISCUSSION
Emerging evidence suggested an association between change in human oral microbiota and AD, such that utilization of antibiotics may help to improve AD situation. 18 In this study, a significant difference in various factors such as hypertension, diabetes, hyperlipidemia, family history, level of education, living area, and level of physical activity was noted between AD and control groups. In line with the results of our study, van Loenhoud et al. reported a significant relationship between AD with age, diabetes, blood pressure, literacy level, and BMI. On the other hand, they stated that there is no significant link between AD with alcohol use, smoking, and hyperlipidemia. 25 Another study found that although literacy levels were not associated with AD, BMI, smoking, alcohol intake, and hypertension were significantly associated with AD. 26 Scarmeas et al 27 reported that although low exercise activity, BMI, ethnicity, age, and literacy levels significantly contributed to AD, smoking and sex did not show any association with it. In another study, it was reported that diabetes, level of education, type of occupation were significantly involved in AD, but no significant difference in BMI and hypertension was observed between Alzheimer's and healthy individuals. 28 The results of these studies indicate that the underlying factors involved in AD may vary in different geographical areas according to people's lifestyle, ethnicity, and traditions.
In this study, for the first time, we compared the differences and changes of oral microbiome bacteria in healthy and AD groups using the qPCR technique, as well as although there is no study that compared oral microbiome bacteria by direct oral sampling in AD and healthy individuals, studies have shown an increase or decrease antigens of oral microbiota main bacteria in the blood or brain tissue of both groups. Results of present study showed that the frequency of the five oral microbiome bacteria were higher in Alzheimer's patients than in healthy individuals. The difference was significant for P. gingivalis, F. nucleatum, and P. intermedia. Although there have been very few studies looking at changes in the oral microbiota in AD, the limited studies have pointed to a significant association. Our observation was in agreement with findings of Stein et al. who screened serum samples of AD patients for specific IgG antibodies against P. gingivalis, A. actinomycetemcomitans, P. intermedia, and F. nucleatum. Antibodies against P. gingivalis, F. nucleatum, and P. intermedia were remarkably higher in Alzheimer's patients in comparison with healthy subjects. Also, there was no significant difference between the two groups in regards to A. actinomycetemcomitans. 29 Consistently, a study was conducted to assess interactive correlation between different genera of oral microbiome bacteria and the incidence of AD which reported that there was no correlation between S. mutans and incidence of AD. However, F. nucleatum and P. intermedia were predisposing pathogenic bacteria in the incidence of AD. 30 Our results are in line with the study conducted by Kamer et al 31 who compared the levels P. gingivalis between AD and normal subjects, however, in contrast to our results, they showed A. actinomycetemcomitans‐specific antibody in Alzheimer's patients were significantly higher than those in healthy individuals. Na et al 32 reported that the diversities of Fusobacterium and Porphyromonas genera in the oral cavity, as conducted by genome sequencing, were higher in Alzheimer's patients in comparison with those in healthy individuals. Similarly, according to the results of Noble et al, 33 high levels of IgG against P. gingivalis were correlated to cognitive impairment. Similar to our findings, in a pilot study it was reported that P. gingivalis was a key bacterium associated with cognitive conditions,however, there was no significance difference in terms of P. intermedia in the two groups. On the other hand, A. actinomycetemcomitans had no role in progression of AD. 34 In another study, there was a correlation between major oral bacteria and AD. They noticed presence of P. gingivalis LPS in the brain of Alzheimer's patients which may have been linked to an inflammatory state. 35 In accordance with the results of our study, Kamer et al. reported the only shift in P. gingivalis counts in the AD patients among different oral pathogenic bacteria including A. actinomycetemcomitans, T. denticola, T. forsythia, P. intermedia, and P. gingivalis. 18 It can be stipulated that the increase of pathogenic bacteria in the composition of oral microbiome causes these bacteria to invade the brain. This may be a probable mechanism for etiology of AD. However, it is not yet clear how oral bacteria can gain access to brain and spread. There are some suggested pathways such as direct infection and damage to endothelial cells protecting the blood–brain barrier, infection and spreading through cranial nerves (e.g., olfactory or trigeminal) to the brain, and infection of monocytes followed by brain recruitment. After accessing the brain, bacteria such as P. gingivalis can spread slowly over many years from neuron to neuron along anatomically connected pathways. 36
In this study, the inflammatory reactions induced by different cytokines including IL‐1, IL‐6, and TNF‐α in the serum of Iranian AD patients compared to healthy individuals showed that all three cytokines were meaningfully higher in Alzheimer's patients. Similar to the results of our study, Babić Leko et al 37 reported that TNF‐α, IL‐1, and IL‐6 levels were significantly higher in the CSF of AD group than in the healthy individuals. In another study, it was reported that although serum levels of TNF‐α and IL‐1 in Alzheimer's patients were significantly higher than in healthy individuals, there was no significant difference in IL‐6 between the two groups (Demirci et al 38 ). In line with the results of our study, Azzam et al 39 declared that serum concentrations of IL‐6 and TNF‐α in Alzheimer's patients significantly increased compared to those in healthy individuals. In another study in 2009, it was reported that although serum levels of TNF‐α were significantly higher in Alzheimer's patients, there was no significant increase in IL‐1 and IL‐6 levels in the patients. Finally, it was stipulated that IgG antibodies against periodontal pathogens such as P. gingivalis, A. actinomycetemcomitans, and T. forsythia lead to high levels of TNF‐α in plasma of patients with AD. 31 In contrast, Lanzrein et al 40 did not observe a significant difference in serum concentrations of IL‐1, IL‐6, and TNF‐α between patients with AD and healthy individuals. Increased inflammation by cytokines is one of the mechanisms of perturbation of oral microbiome bacteria that lead to nerve and brain damage resulting in AD. 20 However, the difference between some of the results of our study and other studies may be due to differences in sample size.
5. CONCLUSION
These results suggest that an increase in the number of some oral pathogenic bacteria and consequently an increase in systemic inflammation may be one of the factors associated with AD and may contribute to a better clinical diagnosis of AD in the future. These initial observations require further extensive studies to evaluate more extensive genera of oral bacteria, their virulence factors, and possible diffusion of biological products into brain and nerve tissue. Finally, due to the inability of existing medical or psychological treatments for AD, the discovery of changes in the oral microbiome and cytokines involved in systemic inflammation, as new modulators and mediators of AD, could lead to the development of new preventive and therapeutic strategies.
AUTHOR CONTRIBUTIONS
Majid Taati Moghadam and Aref Shariati developed the idea and drafted the manuscript. Nour Amirmozafari, Ali Mojtahedi, Babak Bakhshayesh, and Faramarz Masjedian Jazi co‐wrote, developed, and edited the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest relevant to this article.
ACKNOWLEDGMENTS
In this study, we are grateful to Dr. Behzad Taati who helped in the field of statistical analysis in this study. The authors of this article are grateful to residents at the Neurology Department of Poursina Hospital in Rasht for their help in collecting samples. We also thank the medical laboratory research technicians in Tehran and Guilan University of Medical Sciences for their laboratory assistance.
Taati Moghadam M, Amirmozafari N, Mojtahedi A, Bakhshayesh B, Shariati A, Masjedian Jazi F. Association of perturbation of oral bacterial with incident of Alzheimer's disease: A pilot study. J Clin Lab Anal. 2022;36:e24483. doi: 10.1002/jcla.24483
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Gaugler JE, Burgio LD. Caregiving for individuals with Alzheimer’s disease and related disorders. The Spectrum of Family Caregiving for Adults and Elders with Chronic Illness, 2016;15‐57.
- 2. Lai KSP, Liu CS, Rau A, et al. Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta‐analysis of 175 studies. J Neurol Neurosurg Psychiatry. 2017;88:876‐882. [DOI] [PubMed] [Google Scholar]
- 3. Vogt NM, Kerby RL, Dill‐Mcfarland KA, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olsen I, Singhrao SK. Can oral infection be a risk factor for Alzheimer's disease? J Oral Microbiol. 2015;7:29143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moreno‐Arribas M, Bartolome B, Peñalvo JL, Perez‐Matute P, Motilva MJ. Relationship between wine consumption, diet and microbiome modulation in Alzheimer’s disease. Nutrients. 2020;12:3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Global Action Plan on the Public Health Response to Dementia. World Health Organization;2017:2017‐2025. [Google Scholar]
- 7. Shao W, Peng D, Wang X. Genetics of Alzheimer’s disease: from pathogenesis to clinical usage. J Clin Neurosci. 2017;45:1‐8. [DOI] [PubMed] [Google Scholar]
- 8. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921‐923. [DOI] [PubMed] [Google Scholar]
- 9. Swarbrick S, Wragg N, Ghosh S, Stolzing A. Systematic review of miRNA as biomarkers in Alzheimer’s disease. Mol Neurobiol. 2019;56:6156‐6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Braak H, Braak E. Neuropathological staging of Alzheimer‐related changes. Acta Neuropathol. 1991;82:239‐259. [DOI] [PubMed] [Google Scholar]
- 11. Fox M, Knorr DA, Haptonstall KM. Alzheimer’s disease and symbiotic microbiota: an evolutionary medicine perspective. Ann NY Acad Sci. 2019;1449:3‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mihaila D, Donegan J, Barns S, et al. The oral microbiome of early stage Parkinson’s disease and its relationship with functional measures of motor and non‐motor function. PLoS One. 2019;14:e0218252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sampaio‐Maia B, Caldas I, Pereira M, Perez‐Mongiovi D, Araujo R. The oral microbiome in health and its implication in oral and systemic diseases. Adv Appl Microbiol. 2016;97:171‐210. [DOI] [PubMed] [Google Scholar]
- 14. Shoemark DK, Allen SJ. The microbiome and disease: reviewing the links between the oral microbiome, aging, and Alzheimer's disease. J Alzheimers Dis. 2015;43:725‐738. [DOI] [PubMed] [Google Scholar]
- 15. Xu Y, Teng F, Huang S, et al. Changes of saliva microbiota in nasopharyngeal carcinoma patients under chemoradiation therapy. Arch Oral Biol. 2014;59:176‐186. [DOI] [PubMed] [Google Scholar]
- 16. He J, Li Y, Cao Y, Xue J, Zhou X. The oral microbiome diversity and its relation to human diseases. Folia Microbiol. 2015;60:69‐80. [DOI] [PubMed] [Google Scholar]
- 17. Zhang Y, Wang X, Li H, Ni C, Du Z, Yan F. Human oral microbiota and its modulation for oral health. Biomed Pharmacother. 2018;99:883‐893. [DOI] [PubMed] [Google Scholar]
- 18. Leblhuber F, Huemer J, Steiner K, Gostner JM, Fuchs D. Knock‐on effect of periodontitis to the pathogenesis of Alzheimer’s disease? Wien Klin Wochenschr. 2020;132:493‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parra‐Torres V, Melgar‐Rodríguez S, Muñoz‐Manríquez C, et al. Periodontal bacteria in the brain—Implication for Alzheimer's disease: a systematic review. Oral Dis. 2021;27:1593‐1853. [DOI] [PubMed] [Google Scholar]
- 20. Sureda A, Daglia M, Castilla SA, et al. Oral microbiota and Alzheimer’s disease: do all roads lead to Rome? Pharmacol Res. 2020;151:104582. [DOI] [PubMed] [Google Scholar]
- 21. Santigli E, Koller M, Klug B. Oral biofilm sampling for microbiome analysis in healthy children. J vis Exp. 2017:56320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shariati A, Razavi S, Ghaznavi‐Rad E, et al. Association between colorectal cancer and Fusobacterium nucleatum and Bacteroides fragilis bacteria in Iranian patients: a preliminary study. Infect Agent Cancer. 2021;16:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sedighi M, Razavi S, Navab‐Moghadam F, et al. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb Pathog. 2017;111:362‐369. [DOI] [PubMed] [Google Scholar]
- 24. Cohen J. Statistical power analysis for the behavioral sciences. Academic press; 2013. [Google Scholar]
- 25. van Loenhoud AC, de Boer C, Wols K, et al. High occurrence of transportation and logistics occupations among vascular dementia patients: an observational study. Alzheimers Res Ther. 2019;11:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ott A, Stolk R, van Harskamp F, Pols H, Hofman A, Breteler M. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology. 1999;53:1937. [DOI] [PubMed] [Google Scholar]
- 27. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302:627‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Santabárbara J, Gracía‐Rebled AC, López‐Antón R, et al. The effect of occupation type on risk of Alzheimer’s disease in men and women. Maturitas. 2019;126:61‐68. [DOI] [PubMed] [Google Scholar]
- 29. Stein PS, Steffen MJ, Smith C, et al. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimer's & Dementia. 2012;8:196‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beydoun MA, Beydoun HA, Weiss J, Hossain S, El‐Hajj ZW, Zonderman AB. Helicobacter pylori, periodontal pathogens, and their interactive association with incident all‐cause and Alzheimer’s disease dementia in a large national survey. Mol Psychiatry. 2020;26(10):6038‐6053. [DOI] [PubMed] [Google Scholar]
- 31. Kamer AR, Craig RG, Pirraglia E, et al. TNF‐α and antibodies to periodontal bacteria discriminate between Alzheimer's disease patients and normal subjects. J Neuroimmunol. 2009;216:92‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Na HS, Jung N‐Y, Choi S, et al. (2020). Analysis of oral microbiome in chronic periodontitis with Alzheimer’s disease: Pilot study.
- 33. Noble JM, Borrell LN, Papapanou PN, Elkind M, Scarmeas N, Wright CB. Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES‐III. J Neurol Neurosurg Psychiatry. 2009;80:1206‐1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leblhuber F, Huemer J, Steiner K, Fuchs D. On the potential role of periodontitis in the pathogenesis of Alzheimer’s. Acta Microbiol Bulg. 2020;30. [Google Scholar]
- 35. Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean S. Determining the presence of periodontopathic virulence factors in short‐term postmortem Alzheimer's disease brain tissue. J Alzheimers Dis. 2013;36:665‐677. [DOI] [PubMed] [Google Scholar]
- 36. Dominy SS, Lynch C, Ermini F, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small‐molecule inhibitors. Science Advances. 2019;5:eaau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Babić Leko M, Nikolac Perković M, Klepac N, et al. IL‐1β, IL‐6, IL‐10, and TNF α single nucleotide polymorphisms in human influence the susceptibility to Alzheimer’s disease pathology. J Alzheimers Dis. 2020;75:1029‐1047. [DOI] [PubMed] [Google Scholar]
- 38. Demirci S, Aynalı A, Demirci K, Demirci S, Arıdoğan BC. The serum levels of resistin and its relationship with other proinflammatory cytokines in patients with Alzheimer’s disease. Clinical Psychopharmacology and Neuroscience. 2017;15:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Azzam EZ, Elneily D, Elgayar N, Elfatatry A, Saad M. Serum levels of resistin and its relationship with some pro‐inflammatory cytokines in a cohort of Egyptian patients with Alzheimer's disease. Endocrine and Metabolic Science. 2020;1:100054. [Google Scholar]
- 40. Lanzrein A‐S, Johnston CM, Perry VH, Jobst KA, King EM, Smith AD. Longitudinal study of inflammatory factors in serum, cerebrospinal fluid, and brain tissue in Alzheimer disease: interleukin‐1beta, interleukin‐6, interleukin‐1 receptor antagonist, tumor necrosis factor‐alpha, the soluble tumor necrosis factor receptors I and II, and alpha1‐antichymotrypsin. Alzheimer Dis Assoc Disord. 1998;12:215‐227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


