Abstract
Background
T‐helper (Th) cells regulate immunity and inflammation, and modulate cognitive impairment in both cardio‐cerebrovascular and neurological diseases. This study aimed to explore the correlation of longitudinal change of Th1/2/17 cells with cognitive impairment and prognosis in acute ischemic stroke (AIS).
Methods
Th1/2/17 cells were detected by flow cytometry in peripheral blood samples from 150 AIS patients at admission (baseline), Day (D)1, D3, and D7 after admission, and from 30 controls. Mini‐Mental State Examination (MMSE) score among AIS patients at discharge was assessed. Stroke recurrence and mortality were evaluated.
Results
Th1 (p = 0.013) and Th17 cells (p < 0.001) but not Th2 cells (p = 0.105) were elevated in AIS patients versus controls. Th1 cells (p = 0.027) and Th17 cells (p < 0.001) but not Th2 cells (p = 0.227) were positively correlated with NIHSS score in AIS patients. Furthermore, Th1 and Th17 cells elevated from baseline to D3 and then decreased on D7 after AIS onset, while Th2 cells illustrated an opposite trend (all p < 0.001). Th17 cells on D1 (p = 0.011), D3 (p = 0.014), and D7 (p < 0.001) were correlated with lower MMSE score, and their levels on D3 (p = 0.033) and D7 (p = 0.004) were related to elevated cognitive impairment. Th1 and Th2 cells were not related to cognitive function (all p > 0.05). Additionally, Th17 cells at baseline, D1, D3, and D7 (all p < 0.05) were increased in recurrent patients versus non‐recurrent patients, and in survived patients versus dead patients, but Th1 or Th2 cells did not vary (all p > 0.05).
Conclusion
Th17 cells correlate with increased cognitive impairment, stroke recurrence, and mortality among AIS patients.
Keywords: acute ischemic stroke, cognitive impairment, mortality, recurrence, Th1/Th2/Th17 cells
This study aimed to explore the correlation of longitudinal change of T‐helper (Th)1/2/17 cells with cognitive impairment and prognosis in acute ischemic stroke (AIS). Th1/2/17 cells were detected from 150 AIS patients at admission (baseline), Day (D)1, D3, and D7 after admission, and from 30 controls. Th1 and Th17 but not Th2 cells were increased in AIS patients and positively correlated with the National Institutes of Health Stroke Scale score. Moreover, Th1 and Th17 cells elevated from baseline to D3 and then decreased on D7 after AIS onset, while Th2 cells illustrated an opposite trend. Furthermore, Th17 cells on D1, D3, or D7 were correlated with lower Mini‐Mental State Examination score and elevated cognitive impairment at discharge, while Th1 and Th2 cells were not related to cognitive function. Additionally, Th17 cells at baseline, D1, D3, and D7 were positively correlated with stroke recurrence and mortality, whereas Th1 or Th2 cells were not.
1. INTRODUCTION
Acute ischemic stroke (AIS) is a cerebrovascular disorder caused by arterial stenosis or occlusion, which is one of the major causes of neurological deaths globally. 1 , 2 Currently, great progress has been made in treatments of AIS (including thrombolytic treatment, revascularization therapy, and neuroprotective treatment), while there exist a proportion of AIS patients facing long‐term adverse outcomes. 3 , 4 , 5 Cognitive impairment is a common undesirable outcome of AIS patients that is correlated with permanent disability and even death, which results in a huge economical and humanistic burden. 5 , 6 , 7 In view of the challenge in the management of AIS, the exploration of factors affecting cognitive impairment and prognosis of AIS is an urgent issue.
The dysregulation of T‐helper (Th) cells plays an important role in the development of cardio‐cerebrovascular diseases. For instance, it has been reported that the imbalance of Th1 and Th2 cells promote atherosclerotic plaque formation in atherosclerosis model mice 8 ; furthermore, suppressed Th2 cells and increased Th17 cells are able to accelerate ischemic brain injury in middle cerebral artery occlusion mice. 9 In addition, the measurement of Th cells also has a crucial clinical value in cardio‐cerebrovascular disease supervision. For example, Th2 and Th17 cells are varied between ischemic stroke patients and healthy populations 10 , 11 ; moreover, Th17 cells are positively correlated with the atherosclerosis severity in coronary artery disease patients 12 ; furthermore, increased Th17 cells but not Th1 cells are related to higher recurrence‐free survival among AIS patients. 13 Apart from that, Th cells are involved in the progression of cognitive impairment in neurological diseases 14 , 15 ; meanwhile, Th17 cells are also negatively correlated with cognitive impairment among patients with Alzheimer's disease and Parkinson's disease. 16 , 17 However, few studies have explored the clinical implication of longitudinal change of Th1, Th2, and Th17 cells in AIS.
Thus, the current study aimed to explore the longitudinal change of Th1, Th2, and Th17 cells, and their correlation between cognitive impairment and prognosis in AIS patients, aiming to promote the management of AIS.
2. METHODS
2.1. Participants
This study consecutively recruited 150 first‐ever stroke AIS patients hospitalized and was registered at Jing'an District Central Hospital between March 2018 and July 2021. The inclusion criteria for AIS patients were as follows: (1) Newly diagnosed as AIS according to the American Stroke Association Guideline 18 ; (2) aged ≥18 years; and (3) admitted to the hospital within 24 hours after the episode of symptoms. The exclusion criteria for AIS patients were as follows: (1) had intracranial hemorrhage; (2) history of stroke; (3) history of head surgery; (4) accompanied by inflammatory disease or immune system disease; (5) history of cancers or malignancies; and (6) pregnancy or lactation. Besides, the study enrolled 30 subjects during the same period as controls. The inclusion criteria for controls were as follows: (1) with ≥2 risk factors of stroke 19 and (2) aged ≥18 years. The exclusion criteria for controls were the same as the AIS patients. The Ethical Committee of Jing'an District Central Hospital approved the study. Each subject or corresponding guardian provided written informed consent.
2.2. Clinical data and blood sample collection
After enrollment, the demographics, medical history, disease features, and treatment were recorded by case report form. The demographics included the following: Age; gender (female or male); body mass index (BMI); and smoke history (yes/no). The medical history included the following: hypertension (yes/no); hyperlipidemia (yes/no); diabetes mellitus (yes/no); and chronic kidney disease (CKD) (yes/no). The disease features included the following: National Institutes of Health Stroke Scale (NIHSS) score; and Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification. 20 Treatment included thrombolysis and mechanical thrombectomy. The peripheral blood (PB) samples of AIS patients were collected at the following time points: at admission (baseline), D1, D3, and D7. The PB samples of controls were also collected after enrollment. The PB samples were used to detect Th cells.
2.3. Assessment of cognitive function
The patient's cognitive function was assessed by the Mini‐Mental State Examination (MMSE) at discharge from the hospital. MMSE includes 11 items and five facets (space and time, registration, attention and calculation, recall, and language). The total score of MMSE ranged from 0 to 30. Lower scores represent a worse cognitive function. The MMSE score ≤26 was considered cognitive impairment. 21
2.4. Th cell determination
Within 24 h after collection, fresh PB samples were analyzed using flow cytometry to detect Th1 cells (%, in CD4+ T cells), Th2 cells (%, in CD4+ T cells), and Th17 cells (%, in CD4+ T cells) with the employment of Human Th1/Th2/Th17 staining kit (MultiSciences). The flow cytometry used was FACSCanto™ II (BD Biosciences). Then, FlowJo (Tree Star, Ashland, Oregon, United States) was used for analyzing flow cytometry data. All procedures were strictly implemented according to the product protocol.
2.5. Follow‐up
AIS patients were closely followed up according to the AIS Guideline. 18 The last follow‐up date was October 31, 2021. Then, the recurrence of stroke and patient's death were recorded. The median follow‐up duration was 18.0 months with a range from 2.0 to 41.0 months. The recurrence and death of patients were processed as censored data if lost to follow‐up. Seventeen AIS patients were lost to follow‐up during the study.
2.6. Statistics
The statistical analyses were performed by SPSS V.26.0 (IBM Corp.). The figures were plotted by GraphPad Prism V.7.02 (GraphPad Software Inc.). The comparison of Th cells between the two groups was evaluated using the Mann–Whitney U test. The repeated‐measures analysis of Th cells was assessed using the Friedman test. The correlation between two variables was analyzed by Spearman's rank correlation test. The p < 0.05 was considered statistical significance.
3. RESULTS
3.1. Study flow
Between March 2018 and July 2021, 167 patients with first‐onset AIS were screened for eligibility; then, 17 patients were excluded (including 12 patients who did not meet inclusion criteria or met exclusion criteria, and 5 patients who disagreed with informed consent). Thus, 150 patients were eligible for recruitment. Then, PB samples of 150 patients were collected at baseline, D1, D3, and D7; meanwhile, Th1, Th2, and Th17 cells from PB samples were detected by flow cytometry. Besides, cognitive function was assessed by MMSE at discharge from the hospital. The follow‐up was managed until October 2021 (the last follow‐up date was October 31, 2021). During the follow‐up period, 17 patients experienced AIS recurrence and 7 patients died. Finally, all 150 patients were included in the analysis according to the intention to treat principle (Figure 1).
FIGURE 1.
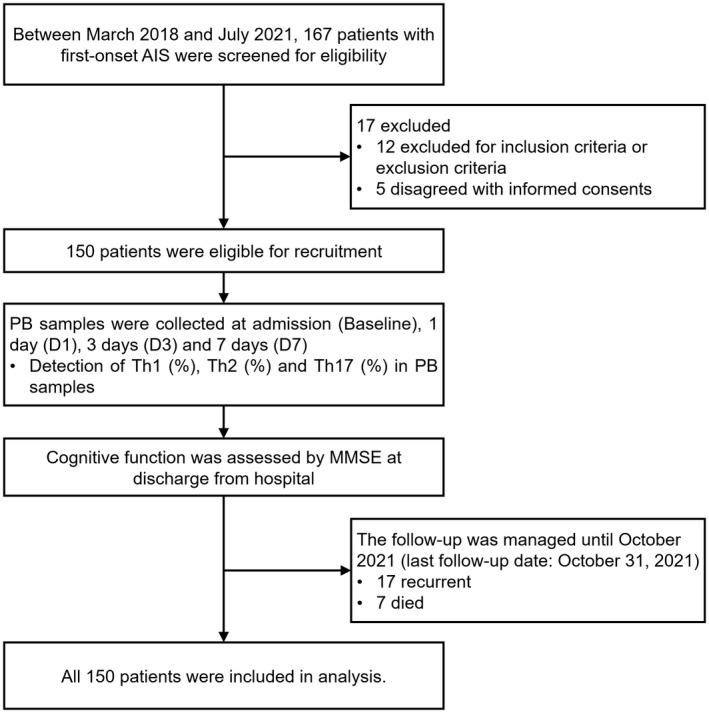
Study flowchart. AIS, acute ischemic stroke; MMSE, Mini‐Mental State Examination; PB, peripheral blood; Th, T helper
3.2. Characteristics of AIS patients
Among 150 patients, the mean age was 63.8 ± 9.2 years; meanwhile, there exited 47 (31.3%) females and 103 (68.7%) males. Furthermore, there were 79 (52.7%) patients with smoke history, 129 (86.0%) patients with hypertension, 73 (48.7%) patients with hyperlipidemia, 27 (18.0%) patients with diabetes mellitus, and 28 (18.7%) patients with CKD. According to the TOAST classification, 62 (41.3%), 38 (25.3%), 24 (16.0%), 16 (10.7%), and 10 (6.7%) patients were classified into large artery atherosclerosis (LAA), small artery occlusion (SAA), cardioembolism (CE), stroke of undetermined etiology (SUE), and stroke of other determined etiology (SOE), respectively. In addition, the median (interquartile range [IQR]) of the NIHSS score on the first day was 6.0 (4.0–9.0). Besides, 121 (80.7%) and 29 (19.3%) patients received thrombolysis and mechanical embolectomy, respectively (Table 1).
TABLE 1.
Characteristics of AIS patients
| Items | AIS patients (N = 150) |
|---|---|
| Age (years), mean ± SD | 63.8 ± 9.2 |
| Gender, n (%) | |
| Female | 47 (31.3) |
| Male | 103 (68.7) |
| BMI (kg/m2), mean ± SD | 24.4 ± 2.7 |
| Smoke history, n (%) | |
| No | 71 (47.3) |
| Yes | 79 (52.7) |
| Hypertension, n (%) | |
| No | 21 (14.0) |
| Yes | 129 (86.0) |
| Hyperlipidemia, n (%) | |
| No | 77 (51.3) |
| Yes | 73 (48.7) |
| Diabetes mellitus, n (%) | |
| No | 123 (82.0) |
| Yes | 27 (18.0) |
| CKD, n (%) | |
| No | 122 (81.3) |
| Yes | 28 (18.7) |
| TOAST classification, n (%) | |
| LAA | 62 (41.3) |
| SAA | 38 (25.3) |
| CE | 24 (16.0) |
| SUE | 16 (10.7) |
| SOE | 10 (6.7) |
| NIHSS score, median (IQR) | 6.0 (4.0–9.0) |
| Treatment, n (%) | |
| Thrombolysis | 121 (80.7) |
| Mechanical embolectomy | 29 (19.3) |
Abbreviations: AIS, acute ischemic stroke; BMI, body mass index; CE, cardioembolism; CKD, chronic kidney disease; IQR, interquartile range; LAA, large artery atherosclerosis; NIHSS, National Institute Health of Stroke Scale; SAA, small artery occlusion; SD, standard deviation; SOE, stroke of other determined etiology; SUE, stroke of undetermined etiology; TOAST, Trial of Org 10172 in acute stroke treatment.
3.3. Comparison of Th cells between AIS patients and controls
Th1 cells (median (IQR): 15.4 (12.7–20.1) % vs. 13.8 (10.9–16.1) %) were elevated in AIS patients compared with controls (p = 0.013) (Figure 2A). However, no difference was found in Th2 cells (median (IQR): 10.5 (8.0–13.1) % vs. 11.6 (9.4–15.6) %) between AIS patients and controls (p = 0.105) (Figure 2B). In addition, Th17 cells (median (IQR): 5.8 (4.4–7.5) % vs. 3.3 (2.6–3.7) %) were increased in AIS patients compared with controls (p < 0.001) (Figure 2C).
FIGURE 2.
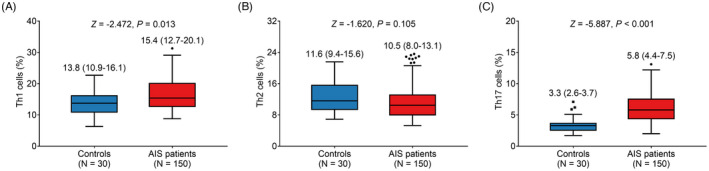
Th cells in AIS patients and controls. Comparison between Th1 cells (A), Th2 cells (B), and Th17 cells (C) between AIS patients and controls. AIS, acute ischemic stroke; Th, T helper
3.4. Correlation of Th cells with NIHSS score and treatment in AIS patients
Th1 cells were positively linked with NIHSS score (r s = 0.181, p = 0.027) (Figure 3A). However, no correlation was found in Th2 cells with NIHSS score (r s = −0.099, p = 0.227) (Figure 3B). Additionally, Th17 cells were positively related to NIHSS score (r s = 0.282, p < 0.001) (Figure 3C). In addition, Th1 cells (Figure S1A), Th2 cells (Figure S1B), and Th17 cells (Figure S1C) from baseline to D7 were not linked with treatment options (thrombolysis or mechanical embolectomy) (all p > 0.05).
FIGURE 3.
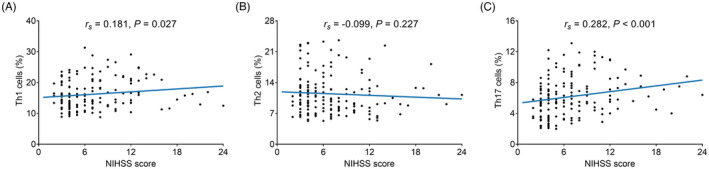
Association of Th cells with NIHSS score in AIS patients. Association of Th1 cells (A), Th2 cells (B), and Th17 cells (C) with NIHSS score. NIHSS, National Institutes of Health Stroke Scale; Th, T helper
3.5. Change of Th cells from baseline to D7 in AIS patients
Th1, Th2, and Th17 cells all varied from baseline to D7 (all p < 0.001). In specific, Th1 cells presented an elevated trend from baseline to D3, but were declined at D7 (Figure 4A). Th2 cells illustrated a decreased trend from baseline to D3, but were elevated at D7 (Figure 4B). Additionally, Th17 cells showed a rising trend from baseline to D3, but were decreased at D7 (Figure 4C).
FIGURE 4.
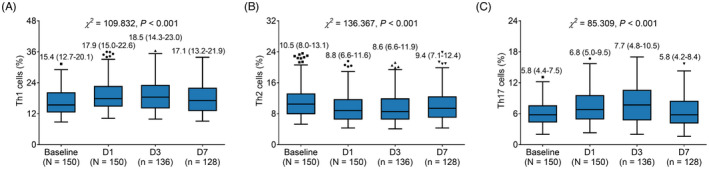
Th cells at different time points in AIS patients after onset. Change of Th1 cells (A), Th2 cells (B), and Th17 cells (C) at baseline, D1, D3, and D7. D, day; Th, T helper
3.6. Association of Th cells at different time points with cognitive function in AIS patients
Th1 and Th2 cells at baseline, D1, D3, and D7 were not associated with MMSE score (all p > 0.05). Moreover, Th17 cells at baseline were not linked to MMSE score (r s = −0.144, p = 0.079), while Th17 cells at D1 (r s = −0.206, p = 0.011), D3 (r s = −0.211, p = 0.014), and D7 (r s = −0.304, p < 0.001) were negatively correlated with MMSE score (Table 2).
TABLE 2.
Correlation of Th cells with MMSE score in AIS patients
| Items | MMSE score at discharge | |
|---|---|---|
| Statistic (r s ) | p value | |
| Baseline | ||
| Th1 cells (%) | 0.015 | 0.856 |
| Th2 cells (%) | −0.033 | 0.688 |
| Th17 cells (%) | −0.144 | 0.079 |
| D1 | ||
| Th1 cells (%) | 0.003 | 0.970 |
| Th2 cells (%) | <0.001 | 0.998 |
| Th17 cells (%) | −0.206 | 0.011 |
| D3 | ||
| Th1 cells (%) | −0.015 | 0.864 |
| Th2 cells (%) | 0.076 | 0.380 |
| Th17 cells (%) | −0.211 | 0.014 |
| D7 | ||
| Th1 cells (%) | −0.011 | 0.900 |
| Th2 cells (%) | 0.050 | 0.576 |
| Th17 cells (%) | −0.304 | <0.001 |
Abbreviations: AIS, acute ischemic stroke; MMSE, Mini‐Mental State Examination; Th, T helper; Th1, T helper 1; Th17, T helper 17; Th2, T helper 2.
Bold value means it has statistical significance.
Then, cognitive impairment was assessed by MMSE score ≤26, which illustrated that Th1 and Th2 cells at baseline, D1, D3, and D7 were not linked to cognitive impairment (all p > 0.05). In addition, Th17 cells at baseline (p = 0.114) and D1 (p = 0.066) were not related to cognitive impairment, while Th17 cells at D3 (p = 0.033) and D7 (p = 0.004) were related to elevated cognitive impairment (Table 3).
TABLE 3.
Correlation of Th cells with cognitive impairment in AIS patients
| Items | Cognitive impairment at discharge, median (IQR) | Statistic (Z) | p Value | |
|---|---|---|---|---|
| No (n = 112) | Yes (n = 38) | |||
| Baseline | ||||
| Th1 cells (%) | 15.7 (12.7–20.5) | 14.3 (12.6–18.1) | −0.756 | 0.449 |
| Th2 cells (%) | 10.1 (7.9–13.0) | 10.8 (8.2–14.6) | −0.506 | 0.613 |
| Th17 cells (%) | 5.8 (3.9–7.5) | 6.3 (4.9–8.8) | −1.582 | 0.114 |
| D1 | ||||
| Th1 cells (%) | 18.8 (15.2–22.7) | 17.5 (14.3–20.5) | −0.694 | 0.488 |
| Th2 cells (%) | 8.8 (6.6–11.2) | 9.4 (6.7–12.0) | −0.523 | 0.601 |
| Th17 cells (%) | 6.7 (4.7–9.3) | 7.5 (5.4–10.3) | −1.839 | 0.066 |
| D3 | ||||
| Th1 cells (%) | 18.6 (14.5–23.1) | 17.9 (13.5–22.0) | −0.632 | 0.527 |
| Th2 cells (%) | 8.8 (6.6–11.7) | 8.0 (6.1–12.4) | −0.541 | 0.589 |
| Th17 cells (%) | 7.0 (4.5–9.9) | 8.8 (5.4–12.9) | −2.137 | 0.033 |
| D7 | ||||
| Th1 cells (%) | 17.8 (13.3–22.1) | 15.8 (12.8–21.4) | −0.626 | 0.532 |
| Th2 cells (%) | 9.5 (7.2–12.0) | 9.2 (7.1–13.0) | −0.032 | 0.974 |
| Th17 cells (%) | 5.5 (3.8–7.9) | 7.5 (5.3–10.9) | −2.890 | 0.004 |
Abbreviations: AIS, acute ischemic stroke; IQR, interquartile range; Th, T helper; Th1, T helper 1; Th17, T helper 17; Th2, T helper 2.
Bold value means it has statistical significance.
3.7. Correlation of Th cells at different time points with AIS recurrence and mortality in AIS patients
Th1 cells on D3 were elevated in recurrent patients compared with non‐recurrent patients (p = 0.048), but did not vary at baseline, D1, or D7 (all p > 0.05) (Figure 5A). No difference was found in Th2 cells at each time point between recurrent patients and non‐recurrent patients (all p > 0.05) (Figure 5B). Inspiringly, Th17 cells at baseline (p = 0.002), D1 (p = 0.015), D3 (p = 0.006), and D7 (p = 0.002) were all increased in recurrent patients compared with non‐recurrent patients (Figure 5C).
FIGURE 5.
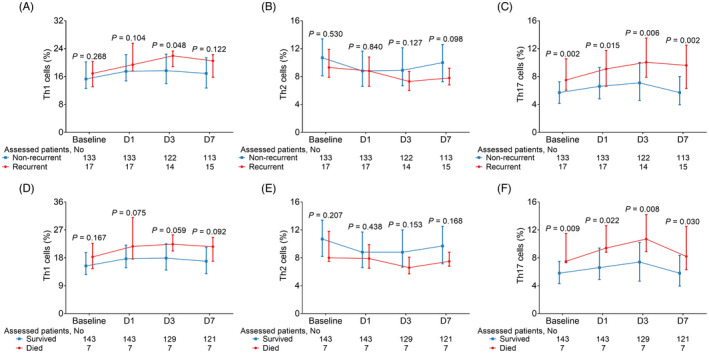
Th cells in recurrent and non‐recurrent AIS patients, and survived and dead AIS patients. Comparison of Th1 cells (A), Th2 cells (B), and Th17 cells (C) at baseline, D1, D3, and D7 between recurrent patients and non‐recurrent patients; comparison of Th1 cells (D), Th2 cells (E), and Th17 cells (F) at baseline, D1, D3, and D7 between survived patients and dead patients. D, day; Th, T helper
In addition, Th1 cells (Figure 5D) and Th2 cells (Figure 5E) at baseline, D1, D3, and D7 did not vary between survived patients and dead patients (all p > 0.05). It is important that Th17 cells at baseline (p = 0.009), D1 (p = 0.022), D3 (p = 0.008), and D7 (p = 0.030) were all elevated in dead patients compared with survived patients (Figure 5F).
4. DISCUSSION
The dysregulation of Th cells in cardio‐cerebrovascular diseases is of interest. For instance, it has been reported that Th17 cells are increased in ischemic stroke patients compared with healthy populations 10 ; moreover, an increment of Th17 cells is found in patients with atherosclerosis compared to healthy controls 22 ; furthermore, Th1 cells and Th17 cells are elevated, while Th2 cells are declined in aortic dissection patients compared with controls. 23 In the current study, we found that Th1 and Th17 cells but not Th2 cells were elevated in AIS patients compared with controls; meanwhile, Th1 and Th17 cells but not Th2 cells were positively correlated with disease severity of AIS. The potential explanation might be that Th1 and Th17 cells could secret pro‐inflammatory cytokines to increase neuroinflammation, which accelerated the pathogenesis and severity of AIS. 24 , 25 However, Th2 cells might be not involved in the development of AIS; thus, they were not varied between AIS patients and controls, as well as did not correlate with disease severity among AIS patients.
Apart from that, we also explored the longitudinal change of Th1, Th2, and Th17 cells in AIS, which showed that Th1 and Th17 cells presented an elevated trend from baseline to D3, and they were decreased at D7, while Th2 cells illustrated an opposite trend. The potential explanation might be that Th1 and Th17 cells were able to secret pro‐inflammatory cytokines, while Th2 could secret anti‐inflammation cytokines, indicating their expressions could reflect inflammation levels 24 , 25 ; meanwhile, from admission to D3, the neuroinflammation was elevated among AIS patients; thus, Th1 and Th17 cells were increased, while Th2 cells were decreased from admission to D3 among AIS patients. In addition, the neuroinflammation was alleviated along with the treatment at D7; thus, Th1 and Th17 cells were declined, while Th2 cells were elevated at D7 among AIS patients.
It has been presented that Th cells take part in cognitive impairment in neurological diseases. For instance, Th1, Th2, and Th17 cells are able to modulate cognitive impairment in Alzheimer's disease and Parkinson's disease model mice 14 , 26 ; moreover, it has been reported that Th17 cells are negatively correlated with 1‐year, 2‐year, and 3‐year MMSE scores in Alzheimer's disease patients. 16 However, the correlation of Th1, Th2, and Th17 cells with cognitive impairment in AIS is unclear. In the current study, we discovered that Th1 and Th2 cells at any time point were not related to cognitive function. However, Th17 cells on D1, D3, and D7 were negatively associated with MMSE score; meanwhile, Th17 cells on D3 and D7 were also related to increased cognitive impairment (MMSE score <26) among AIS patients. The potential explanation might be that Th17 cells might modulate cognitive function through several methods, including regulating hippocampal neurogenesis, brain tissue integrity, and neuroinflammation 24 , 27 , 28 ; thus, Th17 cells were associated with cognitive impairment among AIS patients.
The correlation of Th cells with long‐term clinical outcomes in patients with the cardio‐cerebrovascular disease has been explored. For example, the ratio of Th1 to Th2 cells is correlated with shorter event‐free survival among acute myocardial infarction patients 29 ; moreover, interleukin‐17A (the main secreted cytokines of Th17 cells) is related to declined recurrence‐free survival among AIS patients. 13 However, the data about the correlation of longitudinal change of Th cells with prognosis among AIS patients are scarce. In the current study, Th17 cells but not Th1 and Th2 cells at baseline, D1, D3, and D7 were positively correlated with AIS recurrence and mortality. The potential explanations might be that: (1) Th17 cells were able to promote immune dysregulation and neuroinflammation, as well as accelerate atherosclerosis 24 , 25 , 30 ; therefore, AIS patients with increased Th17 cells might face more risk of recurrence and death; (2) Th17 cells could promote brain injury through regulating neurovascular dysfunction and blood–brain barrier disruption, which lead to poor prognosis in AIS patients 5 , 31 ; (3) Th1 cells might be weakly correlated with neuroinflammation in AIS 24 ; thus, they were not correlated with prognosis among AIS patients, which needed further exploration; and (4) Th2 cells were not related to disease severity and cognitive impairment (above‐mentioned); therefore, they were not associated with prognosis among AIS patients. Thus, Th17 cells but not Th1 or Th2 cells were elevated in recurrent and dead AIS patients.
According to our discovery, Th17 might be a potential biomarker among Th cells for stroke cognitive impairment prediction, as well as stroke recurrence and mortality estimation among AIS patients. However, there were some limitations in the current study: (1) The underlying mechanism of Th17 cells in the progression of AIS could be explored in the future; (2) the enrolled patients were first‐episode AIS patients; hence, the correlation of Th cells with cognitive impairment and prognosis in recurrent AIS could be further explored; (3) the variation of Th1/2/17 cells in longer time could be explored in the forthcoming study; and (4) the follow‐up duration was relatively short, which could be prolonged in the future.
To be conclusive, Th1/2/17 cells are varied within 7 days after AIS onset, among which Th17 cells are related to increased cognitive impairment, stroke recurrence, and mortality among AIS patients, indicating their monitoring may help promote the management of AIS.
CONFLICT OF INTEREST
The authors have no relevant financial or non‐financial interests to disclose.
Supporting information
Figure S1
ACKNOWLEDGEMENT
None.
Yu S, Cui W, Han J, Chen J, Tao W. Longitudinal change of Th1, Th2, and Th17 cells and their relationship between cognitive impairment, stroke recurrence, and mortality among acute ischemic stroke patients. J Clin Lab Anal. 2022;36:e24542. doi: 10.1002/jcla.24542
Shijian Yu and Wei Cui contributed equally to this work.
Funding information
This research is supported by Grant No. 2018YFC2002400 from the National Key R&D Program of China
Contributor Information
Jiawei Chen, Email: Jiawei_chen@hotmail.com.
Weiping Tao, Email: 13331901392@189.cn.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Petty K, Lemkuil BP, Gierl B. Acute ischemic stroke. Anesthesiol Clin. 2021;39(1):113‐125. [DOI] [PubMed] [Google Scholar]
- 2. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139‐e596. [DOI] [PubMed] [Google Scholar]
- 3. Rabinstein AA. Update on treatment of acute ischemic stroke. Continuum (Minneap Minn). 2020;26(2):268‐286. [DOI] [PubMed] [Google Scholar]
- 4. Herpich F, Rincon F. Management of acute ischemic stroke. Crit Care Med. 2020;48(11):1654‐1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rost NS, Brodtmann A, Pase MP, et al. Post‐stroke cognitive impairment and dementia. Circ Res. 2022;130(8):1252‐1271. [DOI] [PubMed] [Google Scholar]
- 6. Verdelho A, Wardlaw J, Pavlovic A, et al. Cognitive impairment in patients with cerebrovascular disease: a white paper from the links between stroke ESO Dementia Committee. Eur Stroke J. 2021;6(1):5‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sexton E, McLoughlin A, Williams DJ, et al. Systematic review and meta‐analysis of the prevalence of cognitive impairment no dementia in the first year post‐stroke. Eur Stroke J. 2019;4(2):160‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang Y, Hu H, Liu L, et al. Interleukin‐12p35 deficiency reverses the Th1/Th2 imbalance, aggravates the Th17/Treg imbalance, and ameliorates atherosclerosis in ApoE−/− Mice. Mediators Inflamm. 2019;2019:3152040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luo Y, Zhou Y, Xiao W, et al. Interleukin‐33 ameliorates ischemic brain injury in experimental stroke through promoting Th2 response and suppressing Th17 response. Brain Res. 2015;1597:86‐94. [DOI] [PubMed] [Google Scholar]
- 10. Dolati S, Ahmadi M, Khalili M, et al. Peripheral Th17/Treg imbalance in elderly patients with ischemic stroke. Neurol Sci. 2018;39(4):647‐654. [DOI] [PubMed] [Google Scholar]
- 11. Theodorou GL, Marousi S, Ellul J, et al. T helper 1 (Th1)/Th2 cytokine expression shift of peripheral blood CD4+ and CD8+ T cells in patients at the post‐acute phase of stroke. Clin Exp Immunol. 2008;152(3):456‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Potekhina AV, Pylaeva E, Provatorov S, et al. Treg/Th17 balance in stable CAD patients with different stages of coronary atherosclerosis. Atherosclerosis. 2015;238(1):17‐21. [DOI] [PubMed] [Google Scholar]
- 13. Chen X, Zhang X, Lan L, Xu G, Li Y, Huang S. MALT1 positively correlates with Th1 cells, Th17 cells, and their secreted cytokines and also relates to disease risk, severity, and prognosis of acute ischemic stroke. J Clin Lab Anal. 2021;35(9):e23903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williams GP, Schonhoff AM, Jurkuvenaite A, Gallups NJ, Standaert DG, Harms AS. CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson's disease. Brain. 2021;144(7):2047‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Browne TC, McQuillan K, McManus RM, O'Reilly JA, Mills KH, Lynch MA. IFN‐gamma production by amyloid beta‐specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer's disease. J Immunol. 2013;190(5):2241‐2251. [DOI] [PubMed] [Google Scholar]
- 16. Zeng J, Liu J, Qu Q, Zhao X, Zhang J. JKAP, Th1 cells, and Th17 cells are dysregulated and inter‐correlated, among them JKAP and Th17 cells relate to cognitive impairment progression in Alzheimer's disease patients. Ir J Med Sci. 2021. doi: 10.1007/s11845-021-02749-2. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 17. Magistrelli L, Storelli E, Rasini E, et al. Relationship between circulating CD4+ T lymphocytes and cognitive impairment in patients with Parkinson's disease. Brain Behav Immun. 2020;89:668‐674. [DOI] [PubMed] [Google Scholar]
- 18. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870‐947. [DOI] [PubMed] [Google Scholar]
- 19. Mi T, Sun S, Zhang G, et al. Relationship between dyslipidemia and carotid plaques in a high‐stroke‐risk population in Shandong Province, China. Brain Behav. 2016;6(6):e00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Madden KP, Karanjia PN, Adams HP Jr, Clarke WR. Accuracy of initial stroke subtype diagnosis in the TOAST study. Trial of ORG 10172 in Acute Stroke Treatment. Neurology. 1995;45(11):1975‐1979. [DOI] [PubMed] [Google Scholar]
- 21. Cumming TB, Churilov L, Linden T, Bernhardt J. Montreal Cognitive Assessment and Mini‐Mental State Examination are both valid cognitive tools in stroke. Acta Neurol Scand. 2013;128(2):122‐129. [DOI] [PubMed] [Google Scholar]
- 22. Wang B, Wang X, Sun H, Hu L, Gao J. The effects of T helper 17 and regulatory T cells on patients with carotid atherosclerosis. Pak J Pharm Sci. 2017;30(5[Supplementary]):1923‐1928. [PubMed] [Google Scholar]
- 23. Ye J, Wang Y, Wang Z, et al. Circulating Th1, Th2, Th9, Th17, Th22, and Treg levels in aortic dissection patients. Mediators Inflamm. 2018;2018:5697149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jin R, Liu L, Zhang S, Nanda A, Li G. Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res. 2013;6(5):834‐851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Machhi J, Yeapuri P, Lu Y, et al. CD4+ effector T cells accelerate Alzheimer's disease in mice. J Neuroinflammation. 2021;18(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Niebling J, Rünker AE, Schallenberg S, Kretschmer K, Kempermann G. Myelin‐specific T helper 17 cells promote adult hippocampal neurogenesis through indirect mechanisms. F1000Res. 2014;3:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poletti S, de Wit H, Mazza E, et al. Th17 cells correlate positively to the structural and functional integrity of the brain in bipolar depression and healthy controls. Brain Behav Immun. 2017;61:317‐325. [DOI] [PubMed] [Google Scholar]
- 29. Li C, Zong W, Zhang M, et al. Increased ratio of circulating t‐helper 1 to t‐helper 2 cells and severity of coronary artery disease in patients with acute myocardial infarction: a prospective observational study. Med Sci Monit. 2019;25:6034‐6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wei S, Sun J, Li Y, Xu K, Wang M, Zhang Y. Losartan attenuates atherosclerosis in uremic mice by regulating Treg/Th17 balance via mediating PTEN/PI3K/Akt pathway. Nephron. 2022;1‐11. doi: 10.1159/000521770. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 31. Cipollini V, Anrather J, Orzi F, Iadecola C. Th17 and cognitive impairment: possible mechanisms of action. Front Neuroanat. 2019;13:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


