Abstract
Gastric cancer (GC) is the fifth most common cancer and the third leading cause of cancer-related deaths worldwide. Currently, surgery is the treatment of choice for GC. However, the associated expenses and post-surgical pain impose a huge burden on these patients. Furthermore, disease recurrence is also very common in GC patients, thus necessitating the discovery and development of other potential treatment options. A growing body of knowledge about ferroptosis in different cancer types provides a new perspective in cancer therapeutics. Ferroptosis is an iron-dependent form of cell death. It is characterized by intracellular lipid peroxide accumulation and redox imbalance. In this review, we summarized the current findings of ferroptosis regulation in GC. We also tackled on the action of different potential drugs and genes in inducing ferroptosis for treating GC and solving drug resistance. Furthermore, we also explored the relationship between ferroptosis and the tumor microenvironment in GC. Finally, we discussed areas for future studies on the role of ferroptosis in GC to accelerate the clinical utility of ferroptosis induction as a treatment strategy for GC.
Keywords: ferroptosis, iron, gastric cancer, ROS, microenvironment, drug resistance
Introduction
Gastric cancer (GC) is the fifth most common cancer and the third leading cause of cancer-related deaths worldwide (Smyth et al., 2020). Some of the risk factors for this disease include Helicobacter pylori infection, age, high salt intake, and unhealthy diet (Chen et al., 2021a). GC is commonly treated with surgery (Mihmanli et al., 2016). However, the associated expenses and post-surgical pain impose a huge burden on GC patients. Several studies which focused on identifying molecular signatures and genetic alterations in GC in order to improve treatment selection and aid drug development have already been conducted (Lordick et al., 2017). However, the underlying mechanisms in disease progression are still unclear. Thus, an in-depth understanding of the GC pathobiology will not only facilitate the identification of new drug targets but also provide help in the development of new clinical treatment strategies.
Ferroptosis is a relatively new form of programmed cell death, which was first described in 2012 (Dixon et al., 2012). Several studies have implicated the contribution of ferroptosis in the progression of multiple diseases (Galluzzi et al., 2018; Stockwell et al., 2020), including GC (Jiang et al., 2021a; Liu G. et al., 2021). In this review, we summarized the relationship between ferroptosis and gastric cancer. Furthermore, we suggested that effective regulation of iron metabolism may provide a novel strategy for treating gastric cancer.
Iron Metabolism in Gastric Cancer
Iron is an indispensable molecule in almost all living organisms. Iron-containing enzymes are involved in many physiological activities (Dixon and Stockwell, 2014), such as cellular metabolism, oxygen transport, DNA synthesis, energy production, and cellular respiration. Aside from its roles in various life processes, the catalytic form of iron can also catalyze the formation of reactive oxygen species (ROS) in oxygen-rich environments. Interestingly, iron and ROS can initiate and mediate cell death in several organisms and disease states (Fischbacher et al., 2017). In addition, ROS can also affect several processes, such as cell survival, proliferation, and differentiation through multiple signaling pathways (Lambeth and Neish, 2014). Although low cellular ROS levels are beneficial to some extent, it has also been found to result in base modification and DNA strand breaks (Inoue and Kawanishi, 1987; Dizdaroglu et al., 1991). These findings hint at the potential contribution of free radical-induced DNA damage in the etiology of numerous diseases, including cancer (Dizdaroglu and Jaruga, 2012). Consistent with this idea, extensive studies have shown that poor regulation of iron metabolism is associated with many diseases, including atherosclerosis, neurodegenerative disorders, and cancer (Lambeth and Neish, 2014; Zhou et al., 2018; Vinchi et al., 2019).
Iron levels and stomach health are closely interrelated. For example, several iron-related conditions, such as unexplained iron deficiency, idiopathic thrombocytopenic purpura, and anemia, were found to be associated with H. pylori infection (Hagymási and Tulassay, 2014; Durazzo et al., 2021). Iron homeostasis has also been implicated in cancer development. In one study, iron oxidation has been shown to contribute to tumor formation and subsequent cancer development (Torti and Torti, 2013). On the other hand, several studies reported that iron deficiency may enhance the risk of developing cancer (Janssen et al., 2020). Anemia, low serum ferritin levels, and autoimmune gastritis–related iron malabsorption were identified as risk factors associated with gastrointestinal tumors and GC (Nomura et al., 1992; Cover et al., 2013; Kamada et al., 2021). Consistent with these studies, in vivo data from rodent models show that iron deficiency may contribute to early progression of gastrointestinal tumors (Prá et al., 2009). Taken together, these studies highlight the significant role of iron in the development of various gastrointestinal malignancies, and the potential value of iron regulation as a treatment strategy (Palzer et al., 2021).
Mechanism of Ferroptosis
Ferroptosis is a unique form of cell death (Kerr et al., 1972; Jacobson and Raff, 1995; Christofferson and Yuan, 2010; Shimada et al., 2016). Some protein modulators, such as p53, can exert their physiologic functions either through apoptosis or ferroptosis (Jiang et al., 2015). Similarly, several small molecules can initiate cell death via specific molecular events related to either ferroptosis, apoptosis, or necrosis (Kerr et al., 1972; Schweichel and Merker, 1973; Yang and Stockwell, 2008; Dixon et al., 2012; Shimada et al., 2016). The activation of distinct pathways suggests that the molecular mechanism involved in ferroptosis differs from those of apoptosis and necrosis (Dixon et al., 2012; Dong et al., 2015; Shimada et al., 2016; Torii et al., 2016).
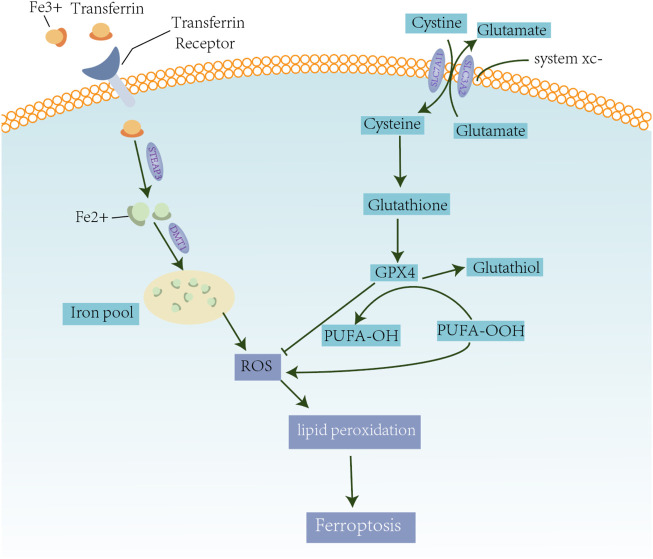
Iron accumulation is the first step in ferroptosis (Galluzzi et al., 2015). Free ferric iron (Fe3+) in the blood conjugates with transferrin proteins. The iron-bound transferrin molecules are then captured by the transferrin receptors present on the cell membrane and enter the cell through endocytosis (Gao et al., 2019). Reducing proteins, such as six transmembrane proteins of prostate 3 (STEAP3), reduces Fe3+ to its highly reactive ferrous ion (Fe2+) form. Upon conversion, Fe2+ is transported from the endosomes to the cytoplasm and is included to the labile iron pool. To protect the cells and tissues from iron-mediated damage, excess Fe2+ in the iron pool is stored in ferritin, while the remaining Fe2+ can be pumped out of the cell through ferroportin molecules on the cell membrane (Yang et al., 2016; Hassannia et al., 2019). Under normal conditions, intracellular iron concentrations remain stable (Yamaguchi et al., 2021). However, in cases of iron overload, excessive Fe2+ is produced within the cell. The accumulation of intracellular Fe2+ further leads to the production of Fe3+ and ROS through the Fenton chemical reaction (Talvenmäki et al., 2019). In addition, excess Fe3+ can also be reduced to Fe2+ through the Haber–Weiss reaction (Kehrer, 2000). Furthermore, under stress conditions, ferritin can self-degrade into Fe2+ through iron autophagy (Talvenmäki et al., 2019). Collectively, these processes can lead to ferroptosis, which in turn induces the formation of more ROS. Excessive ROS can damage biofilms, proteins, and nucleic acids eventually leading to cell death (Kehrer, 2000).
The high intracellular ROS and free radical levels are usually controlled by cells through the actions of antioxidants such as glutathione (GSH) and glutathione peroxidase 4 (GPX4) (Aldini et al., 2018). However, in some cases, glutathione and GPX4 are used up by the cells in other processes, such as in regulating amino acid metabolism. The intracellular levels of glutathione are also affected by amino acid availability. Increased glutamine decomposition may affect the synthesis of glutathione and cause a cell death event similar to a GSH consumption–induced ferroptosis (Linkermann et al., 2014a; Yang et al., 2014). Furthermore, since GPX4 converts the potentially toxic lipid hydroperoxides (L-OOH) to non-toxic lipid alcohols (L-OH) (Ursini et al., 1982), the inactivation of GPX4 can ultimately cause cell death (Linkermann et al., 2014b) (Figure 1).
FIGURE 1.
Mechanisms of ferroptosis. Excess iron is related to lipid peroxidation and abnormal iron metabolism of mercaptan, which induces the production of ROS. On the one hand, circulating iron in the form of Fe3+ binds to the transferrin receptor and enters the cell. Iron oxide reductase, STEAP3, reduces Fe3+ to Fe2 +, which is transported to the iron pool through DMT1 to induce the formation of ROS. Finally, it promotes lipid peroxidation and causes ferroptosis. On the other hand, the Xc system transports intracellular Glu to the extracellular space and extracellular cystine simultaneously into the cells, which is then transformed into cysteine for GSH synthesis. GPX4 converts -OOH to -OH in polyunsaturated fatty acid (PUFA) to reduce ROS accumulation.
Ferroptosis in Gastric Cancer
Proliferation, Invasion, and Metastasis of Gastric Cancer
The proliferation, invasion, and metastasis of tumor cells are crucial events in the occurrence and development of malignant tumors. These cell activities lead to varying degrees of clinical responses (Machlowska et al., 2018). Like in other cancer types, GC develops from preneoplastic and early neoplastic precursor lesions (Song et al., 2015). These lesions may develop into tumors when the rate of cell proliferation is faster than cell death (Ginsberg et al., 1996; Li et al., 2018). Early stages of GC are characterized by good prognosis with 5-year survival rates reaching >90%; however, most patients are already in the advanced stages of the disease upon initial diagnosis (Tan, 2019). To date, curative surgical resection procedure is the only available treatment for GC (Santoro et al., 2014). Unfortunately, the metastasis of malignant tumors often causes treatment failure (Coburn et al., 2018; Hatta et al., 2020). Tumor invasion and metastasis refer to cellular events when malignant tumor cells continue to grow from the primary site into other sites through lymphatic, vascular, or the body cavity routes. The origin of tumor cells, genetic variations, circulatory mode, and the physiological structure of the metastatic organ determine the specific sites for distant metastasis (Jin et al., 2014).
Several studies try to identify other potential candidates for GC treatment (Table 1). One of these substances, Tanshinone IIA, can inhibit tumor proliferation and metastasis by increasing the level of lipid peroxides and decreasing that of glutathione in the GC cells (Ni et al., 2021a). Another extract, Actinidia chinensis (Planch) exerts anti-proliferation and anti-migration effects on GC cells. Additionally, it can significantly downregulate the expression of GPX4 in a dose-dependent manner (Gao et al., 2020). On the other hand, physcion 8-O-β-glucopyranoside displays antitumor effects in several cancer types, and it induces ferroptosis by regulating the miR-103a-3p/GLS2 axis in GC (Niu et al., 2019).
TABLE 1.
Candidate substances and genes for inducing ferroptosis in gastric cancer.
| Substances and Genes | Target/Function | Mechanism |
|---|---|---|
| Actinidia chinensis Planch (Gao et al., 2020) | GPx4, SLC7A11 | Induces ROS accumulation |
| Tanshinone IIA (Guan et al., 2020) | Ptgs2, Chac1, p53, xCT | Tanshinone IIA upregulates p53 expression and downregulates xCT expression; Tan IIA decreases intracellular glutathione and cysteine levels and increases the levels of intracellular ROS. |
| Tanshinone IIA (Ni et al., 2021a) | SLC7A11 | Induces ROS accumulation |
| Physcion 8-O-β-glucopyranoside (Niu et al., 2019) | GLS2 | Induces ROS accumulation |
| Erastin (Sun et al., 2020) | Mitochondrial dysfunction | Induces ROS accumulation |
| Erastin (Chen L. et al., 2020; Mao et al., 2021) | SLC7A11 | Induces ROS accumulation |
| Cysteine Dioxygenase 1 | GPX4, maintains stability of mitochondrial morphology | Mediates erastin (Hao et al., 2017); induces ROS accumulation |
| Exosomes miR-522 (Zhang et al., 2020) | ALOX15 | Leads to ALOX15 suppression, decreased lipid-ROS accumulation in cancer cells, and ultimately results in decreased chemosensitivity |
| SIRT6 (Cai et al., 2021) | GPX4 | Inhibits GPX4 activity, induces ROS accumulation |
| CPEB1 (Wang J. et al., 2021) | Gpx4 | Induces ROS accumulation |
| MiR-375 (Ni et al., 2021b) | SLC7A11 | Induces ROS accumulation |
Consistent with the studies on the relationship of iron and GC, ferroptosis has also been found to be closely related to the proliferation, invasion, and metastasis in GC (Chen et al., 2021a; Huang et al., 2021). However, the predictive role of ferroptosis in GC remains elusive (Shao et al., 2021). Thus, understanding the processes underlying ferroptosis is promising for the development of cancer treatment strategies.
Tumor Microenvironment in Gastric Cancer
A tumor is closely connected with where it arises and develops in the organism (Chen et al., 2015). Tumor cells in tumor microenvironment (TME) play an active role in the disease progression (Bubnovskaya and Osinsky, 2020; Jiang et al., 2020). The TME favors the growth and expansion of cancer cells (Oya et al., 2020; Rojas et al., 2020). Interestingly, tumor cells and their surrounding microenvironment can be shaped by varying degrees of ferroptosis activation (Zavros, 2017; Xiao et al., 2021). It has been found that ferroptosis serves as an important factor in the formation of the TME in GC (Jiang et al., 2021b; Wang F. et al., 2021; Chen et al., 2021b). In addition, numerous studies have demonstrated that dying cells, including ferroptotic cancer cells, communicate with the immune cells in the TME via a series of signals (Friedmann Angeli et al., 2019). These signals produced during cell death allow the recruitment and activation of immune cells, such as macrophages, regulatory T cell, and neutrophils (Matsushita et al., 2015; Klöditz and Fadeel, 2019; Li et al., 2019), which regulate the growth and expansion of other cancer cells.
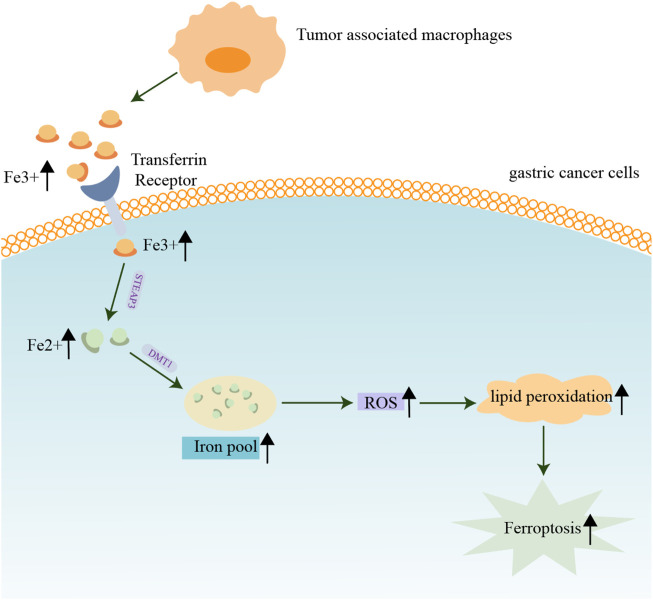
Tumor-associated macrophages are emerging as key players in the development of GC (Gambardella et al., 2020). Aside from its role in phagocytosis of foreign antigens, another physiological function of macrophages is to maintain the iron balance in human tissues. Iron homeostasis should be tightly maintained since excess labile iron is toxic (Henle and Linn, 1997; Muckenthaler et al., 2017). Surprisingly, malignant cells can evade the deleterious effect of excessive iron and require high amounts of these reactive ions for their proliferation (Pfeifhofer-Obermair et al., 2018). Depending on the circumstances, increased iron traffic by tumor-associated macrophages either promotes tumor progression or tumor protraction (Soares and Hamza, 2016). Therefore, the detection of macrophages and iron levels in the TME may provide a basis for predicting tumor progression (Liu S. J. et al., 2021; Xiang et al., 2021) (Figure 2). To better understand the functional role of ferroptosis and immune cells in TME, a comprehensive investigation of ferroptosis-related signals and the immune responses they trigger is warranted.
FIGURE 2.
Contribution of iron and macrophages in the tumor microenvironment of gastric cancer. Macrophages maintain iron balance in human tissues. The proliferation of GC cells requires a large amount of iron, and the increased iron flow from tumor-associated macrophages promotes tumor progression or tumor protraction.
Despite of reduction in the incidence of GC and the development of novel therapeutic strategies, the prognosis of GC remains poor (Lazăr et al., 2018). Biomarkers for the characterization of the tumor immune microenvironment may add to the predictive value of the current staging system (Jiang et al., 2019). In recent decades, large-scale clinical trans-omics studies allowed the identification of some crucial ferroptosis-related genes as reliable biomarkers to describe the tumor immune microenvironment landscape and predict response to antitumor therapy (Liu S. J. et al., 2021; Shao et al., 2021).
Drug Resistance in Gastric Cancer
Resistance to cisplatin and paclitaxel has become increasingly severe in GC patients (Shao et al., 2019; Zhai et al., 2019). This proves to be a major hurdle in clinical oncology and leads to poor prognosis (Chen Z. et al., 2020; Wei et al., 2020). Resistance to chemotherapy is usually related to mutations in genes regulating cell apoptosis and increased levels of glutathione (Silva et al., 2019). Interestingly, ferroptosis inducers may help in overcoming drug resistance and warrants further investigation (Shin et al., 2018).
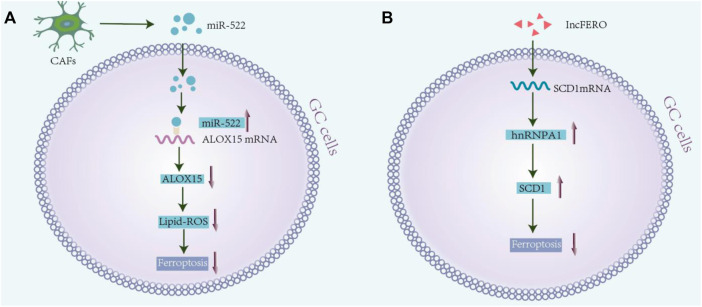
Owing to genetic alterations and abnormal growth, cancer cells have higher oxidative tolerance from ROS than non-malignant cells. This ability is attributed to the maintenance of high levels of the antioxidant GSH, which is essential for cell survival and proliferation (Cramer et al., 2017). Studies show that blocking CAF-exosomes–mediated lipid-ROS inhibition leads to increased levels of ferroptosis in cancer cells, which in turn enhances cell sensitivity towards chemotherapy (Zhang et al., 2020). Another potential target for GC therapy is through the blockage of the ROS-activated GCN2-eIF2α-ATF4-xCT pathway, a signaling cascade leading to mitochondrial dysfunction-enhanced cisplatin resistance (Wang et al., 2016). In addition, regulating ROS levels may serve as another novel therapeutic strategy, since ROS can disturb the cellular oxidative environment and induce cell death (Dharmaraja, 2017). In line with this, studies have shown that the antioxidant enzyme, peroxiredoxin 2, significantly sensitizes the AGS and SNU-1 cells towards cisplatin treatment by regulating the level of ROS (Wang et al., 2020). As chronic and exorbitant ROS levels instigate drug resistance (Liao et al., 2019; Xu et al., 2020; Zhu et al., 2020), regulating ferroptosis may be a useful strategy for targeting the drug-resistant tumor cells (Yang et al., 2017; Huang et al., 2019; Choi et al., 2020; Zhang et al., 2021). A possible relevant mechanism is presented in Figure 3.
FIGURE 3.
Drug resistance and ferroptosis in gastric cancer. (A) Exosomal mir-522 secreted from cancer-associated fibroblasts (CAFs) enter the GC cells and bind to ALOX15 mRNA, resulting in ALOX15 inhibition and reduction in lipid-ROS accumulation in cancer cells. It inhibits ferroptosis in GC cells, and finally reduces chemosensitivity (Namee and O'Driscoll, 2018). (B) Exosomal lnc-ENDOG-1:1 from GC cells can promote the expression of SCD1 by directly interacting with the SCD1 mRNA in GC cells and recruiting heterogenous ribonucleoprotein A1 (hnRNPA1), thereby leading to the inhibition of ferroptosis in GC cells.
Conclusion
Gastric adenocarcinoma is a common disease worldwide. Currently, surgery is the only considered effective treatment strategy. However, disease recurrence is very common even after complete resection (Johnston and Beckman, 2019). Interestingly, ferroptosis has been found to have a very vital role in several cancer types, especially in GC (Lee et al., 2020). As a relatively new discovered mode of cell death, the field of ferroptosis is a research hotspot. Although numerous studies have examined the biological mechanisms underlying ferroptosis, its relationship to tumor progression remains to be poorly understood.
In this review, we have highlighted the importance of iron metabolism and ferroptosis in GC. Iron is an important nutrient in humans (Tan et al., 1997; Goddard et al., 2011). However, iron oxidation also contributes to tumor formation and development of cancer (Doll et al., 2019). In addition, iron in macrophages of the tumor microenvironment is an important index for predicting and detecting GC as well as for evaluating the clinical utility of the related gene signature. Meanwhile, ferroptosis is an iron-dependent form of cell death, which is often characterized by the accumulation of lipid peroxidation products in a cellular-iron–dependent manner (Stockwell et al., 2017; Tang et al., 2021). It functions through two main pathways: iron metabolism and Xc system–induced ROS production (Zheng and Conrad, 2020). Different inducers can affect different steps in ferroptosis to regulate GC proliferation, invasion, and metastasis. Furthermore, the development of drug resistance in GC cells poses a major hurdle. As chronic and exorbitant ROS levels instigate drug resistance, ROS homeostasis may provide a useful treatment strategy for targeting the drug-resistant tumor cells.
Taken together, this review tries to elucidate the relationship between ferroptosis and GC, based on available research findings. We summarized the known ferroptosis processes mediated by gastric cancer-related biomolecules and discussed the actions of some drugs in the different pathways involved in ferroptosis. Lastly, this may serve as a reference for future studies on the mechanism of ferroptosis and the treatment of GC.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This project was supported in part by the National Natural Science Foundation of China (82074312).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CAFs, cancer-associated fibroblasts; DMT1, divalent metal transporter 1; GC, gastric cancer; Gpx4, glutathione peroxidase 4; ncRNAs, noncoding RNAs; PUFA, polyunsaturated fatty acid; ROS, reactive oxygen species; STEAP3, six transmembrane protein of prostate 3.
References
- Aldini G., Altomare A., Baron G., Vistoli G., Carini M., Borsani L., et al. (2018). N-acetylcysteine as an Antioxidant and Disulphide Breaking Agent: the Reasons Why. Free Radic. Res. 52 (7), 751–762. 10.1080/10715762.2018.1468564 [DOI] [PubMed] [Google Scholar]
- Bubnovskaya L., Osinsky D. (2020). Tumor Microenvironment and Metabolic Factors: Contribution to Gastric Cancer. Exp. Oncol. 42 (1), 2–10. 10.32471/exp-oncology.2312-8852.vol-42-no-1.14056 [DOI] [PubMed] [Google Scholar]
- Cai S., Fu S., Zhang W., Yuan X., Cheng Y., Fang J. (2021). SIRT6 Silencing Overcomes Resistance to Sorafenib by Promoting Ferroptosis in Gastric Cancer. Biochem. Biophysical Res. Commun. 577, 158–164. 10.1016/j.bbrc.2021.08.080 [DOI] [PubMed] [Google Scholar]
- Chen D., Chen H., Chi L., Fu M., Wang G., Wu Z., et al. (2021). Association of Tumor-Associated Collagen Signature with Prognosis and Adjuvant Chemotherapy Benefits in Patients with Gastric Cancer. JAMA Netw. Open 4 (11), e2136388. 10.1001/jamanetworkopen.2021.36388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Zhuang X., Lin L., Yu P., Wang Y., Shi Y., et al. (2015). New Horizons in Tumor Microenvironment Biology: Challenges and Opportunities. BMC Med. 13, 45. 10.1186/s12916-015-0278-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Qiao L., Bian Y., Sun X. (2020). GDF15 Knockdown Promotes Erastin-Induced Ferroptosis by Decreasing SLC7A11 Expression. Biochem. Biophysical Res. Commun. 526 (2), 293–299. 10.1016/j.bbrc.2020.03.079 [DOI] [PubMed] [Google Scholar]
- Chen X., Zeh H. J., Kang R., Kroemer G., Tang D. (2021a). Cell Death in Pancreatic Cancer: from Pathogenesis to Therapy. Nat. Rev. Gastroenterol. Hepatol. 18 (11), 804–823. 10.1038/s41575-021-00486-6 [DOI] [PubMed] [Google Scholar]
- Chen X., Zhu Z., Li X., Yao X., Luo L. (2021b). The Ferroptosis-Related Noncoding RNA Signature as a Novel Prognostic Biomarker in the Tumor Microenvironment, Immunotherapy, and Drug Screening of Gastric Adenocarcinoma. Front. Oncol. 11, 778557. 10.3389/fonc.2021.778557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Li Y., Tan B., Zhao Q., Fan L., Li F., et al. (2020). Progress and Current Status of Molecule-Targeted Therapy and Drug Resistance in Gastric Cancer. Drugs Today 56 (7), 469–482. 10.1358/dot.2020.56.7.3112071 [DOI] [PubMed] [Google Scholar]
- Choi H.-J., Jhe Y.-L., Kim J., Lim J. Y., Lee J. E., Shin M.-K., et al. (2020). FoxM1-dependent and Fatty Acid Oxidation-Mediated ROS Modulation Is a Cell-Intrinsic Drug Resistance Mechanism in Cancer Stem-like Cells. Redox Biol. 36, 101589. 10.1016/j.redox.2020.101589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofferson D. E., Yuan J. (2010). Necroptosis as an Alternative Form of Programmed Cell Death. Curr. Opin. Cel Biol. 22 (2), 263–268. 10.1016/j.ceb.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn N., Cosby R., Klein L., Knight G., Malthaner R., Mamazza J., et al. (2018). Staging and Surgical Approaches in Gastric Cancer: A Systematic Review. Cancer Treat. Rev. 63, 104–115. 10.1016/j.ctrv.2017.12.006 [DOI] [PubMed] [Google Scholar]
- Cover T. L., Peek, Jr R. M., Jr (2013). Diet, Microbial Virulence, andHelicobacter Pylori-Induced Gastric Cancer. Gut Microbes 4 (6), 482–493. 10.4161/gmic.26262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer S. L., Saha A., Liu J., Tadi S., Tiziani S., Yan W., et al. (2017). Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat. Med. 23 (1), 120–127. 10.1038/nm.4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmaraja A. T. (2017). Role of Reactive Oxygen Species (ROS) in Therapeutics and Drug Resistance in Cancer and Bacteria. J. Med. Chem. 60 (8), 3221–3240. 10.1021/acs.jmedchem.6b01243 [DOI] [PubMed] [Google Scholar]
- Dixon S. J., Lemberg K. M., Lamprecht M. R., Skouta R., Zaitsev E. M., Gleason C. E., et al. (2012). Ferroptosis: an Iron-dependent Form of Nonapoptotic Cell Death. Cell 149 (5), 1060–1072. 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon S. J., Stockwell B. R. (2014). The Role of Iron and Reactive Oxygen Species in Cell Death. Nat. Chem. Biol. 10, 9–17. 10.1038/nchembio.1416 [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M., Jaruga P. (2012). Mechanisms of Free Radical-Induced Damage to DNA. Free Radic. Res. 46 (4), 382–419. 10.3109/10715762.2011.653969 [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M., Rao G., Halliwell B., Gajewski E. (1991). Damage to the DNA Bases in Mammalian Chromatin by Hydrogen Peroxide in the Presence of Ferric and Cupric Ions. Arch. Biochem. Biophys. 285 (2), 317–324. 10.1016/0003-9861(91)90366-q [DOI] [PubMed] [Google Scholar]
- Doll S., Freitas F. P., Shah R., Aldrovandi M., da Silva M. C., Ingold I., et al. (2019). FSP1 Is a Glutathione-independent Ferroptosis Suppressor. Nature 575 (7784), 693–698. 10.1038/s41586-019-1707-0 [DOI] [PubMed] [Google Scholar]
- Dong T., Liao D., Liu X., Lei X. (2015). Using Small Molecules to Dissect Non-apoptotic Programmed Cell Death: Necroptosis, Ferroptosis, and Pyroptosis. Chembiochem 16 (18), 2557–2561. 10.1002/cbic.201500422 [DOI] [PubMed] [Google Scholar]
- Durazzo M., Adriani A., Fagoonee S., Saracco G. M., Pellicano R. (2021). Helicobacter pylori and Respiratory Diseases: 2021 Update. Microorganisms 9 (10), 2033. 10.3390/microorganisms9102033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbacher A., von Sonntag C., Schmidt T. C. (2017). Hydroxyl Radical Yields in the Fenton Process under Various pH, Ligand Concentrations and Hydrogen peroxide/Fe(II) Ratios. Chemosphere 182, 738–744. 10.1016/j.chemosphere.2017.05.039 [DOI] [PubMed] [Google Scholar]
- Friedmann Angeli J. P., Krysko D. V., Conrad M. (2019). Ferroptosis at the Crossroads of Cancer-Acquired Drug Resistance and Immune Evasion. Nat. Rev. Cancer 19 (7), 405–414. 10.1038/s41568-019-0149-1 [DOI] [PubMed] [Google Scholar]
- Galluzzi L., Bravo-San Pedro J. M., Vitale I., Aaronson S. A., Abrams J. M., Adam D., et al. (2015). Essential versus Accessory Aspects of Cell Death: Recommendations of the NCCD 2015. Cell Death Differ 22 (1), 58–73. 10.1038/cdd.2014.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., Vitale I., Aaronson S. A., Abrams J. M., Adam D., Agostinis P., et al. (2018). Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cel Death Differ 25 (3), 486–541. 10.1038/s41418-017-0012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella V., Castillo J., Tarazona N., Gimeno-Valiente F., Martínez-Ciarpaglini C., Cabeza-Segura M., et al. (2020). The Role of Tumor-Associated Macrophages in Gastric Cancer Development and Their Potential as a Therapeutic Target. Cancer Treat. Rev. 86, 102015. 10.1016/j.ctrv.2020.102015 [DOI] [PubMed] [Google Scholar]
- Gao M., Yi J., Zhu J., Minikes A. M., Monian P., Thompson C. B., et al. (2019). Role of Mitochondria in Ferroptosis. Mol. Cel 73 (2), 354–363. e3. 10.1016/j.molcel.2018.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Deng G., Li Y., Huang H., Sun X., Shi H., et al. (2020). Actinidia Chinensis Planch Prevents Proliferation and Migration of Gastric Cancer Associated with Apoptosis, Ferroptosis Activation and Mesenchymal Phenotype Suppression. Biomed. Pharmacother. 126, 110092. 10.1016/j.biopha.2020.110092 [DOI] [PubMed] [Google Scholar]
- Ginsberg G. G., Al-Kawas F. H., Fleischer D. E., Reilly H. F., Benjamin S. B. (1996). Gastric Polyps: Relationship of Size and Histology to Cancer Risk. Am. J. Gastroenterol. 91 (4), 714–717. [PubMed] [Google Scholar]
- Goddard A. F., James M. W., McIntyre A. S., Scott B. B. British Society of Gastroenterology (2011). Guidelines for the Management of Iron Deficiency Anaemia. Gut 60 (10), 1309–1316. 10.1136/gut.2010.228874 [DOI] [PubMed] [Google Scholar]
- Guan Z., Chen J., Li X., Dong N. (2020). Tanshinone IIA Induces Ferroptosis in Gastric Cancer Cells through P53-Mediated SLC7A11 Down-Regulation. Biosci. Rep. 40 (8), BSR20201807. 10.1042/BSR20201807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagymási K., Tulassay Z. (2014). Helicobacter Pyloriinfection: New Pathogenetic and Clinical Aspects. Wjg 20 (21), 6386–6399. 10.3748/wjg.v20.i21.6386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S., Yu J., He W., Huang Q., Zhao Y., Liang B., et al. (2017). Cysteine Dioxygenase 1 Mediates Erastin-Induced Ferroptosis in Human Gastric Cancer Cells. Neoplasia 19 (12), 1022–1032. 10.1016/j.neo.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassannia B., Vandenabeele P., Vanden Berghe T. (2019). Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 35 (6), 830–849. 10.1016/j.ccell.2019.04.002 [DOI] [PubMed] [Google Scholar]
- Hatta W., Gotoda T., Koike T., Masamune A. (2020). History and Future Perspectives in Japanese Guidelines for Endoscopic Resection of Early Gastric Cancer. Dig. Endosc. 32 (2), 180–190. 10.1111/den.13531 [DOI] [PubMed] [Google Scholar]
- Henle E. S., Linn S. (1997). Formation, Prevention, and Repair of DNA Damage by Iron/hydrogen Peroxide. J. Biol. Chem. 272 (31), 19095–19098. 10.1074/jbc.272.31.19095 [DOI] [PubMed] [Google Scholar]
- Huang R., Chen H., Liang J., Li Y., Yang J., Luo C., et al. (2021). Dual Role of Reactive Oxygen Species and Their Application in Cancer Therapy. J. Cancer 12 (18), 5543–5561. 10.7150/jca.54699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T., Song C., Zheng L., Xia L., Li Y., Zhou Y. (2019). The Roles of Extracellular Vesicles in Gastric Cancer Development, Microenvironment, Anti-cancer Drug Resistance, and Therapy. Mol. Cancer 18 (1), 62. 10.1186/s12943-019-0967-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Kawanishi S. (1987). Hydroxyl Radical Production and Human DNA Damage Induced by Ferric Nitrilotriacetate and Hydrogen Peroxide. Cancer Res. 47 (24 Pt 1), 6522–6527. [PubMed] [Google Scholar]
- Jacobson M. D., Raff M. C. (1995). Programmed Cell Death and Bcl-2 protection in Very Low Oxygen. Nature 374 (6525), 814–816. 10.1038/374814a0 [DOI] [PubMed] [Google Scholar]
- Janssen H. J. B., Fransen L. F. C., Ponten J. E. H., Nieuwenhuijzen G. A. P., Luyer M. D. P. (2020). Micronutrient Deficiencies Following Minimally Invasive Esophagectomy for Cancer. Nutrients 12 (3), 778. 10.3390/nu12030778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Kon N., Li T., Wang S.-J., Su T., Hibshoosh H., et al. (2015). Ferroptosis as a P53-Mediated Activity during Tumour Suppression. Nature 520 (7545), 57–62. 10.1038/nature14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Yan Q., Xie L., Xu S., Jiang K., Huang J., et al. (2021). Construction and Validation of a Ferroptosis-Related Prognostic Model for Gastric Cancer. J. Oncol. 2021, 6635526. 10.1155/2021/6635526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Liu F., Liu P., Yan Y., Lan S., Zhuang K., et al. (2021). Ferroptosis Patterns Correlate with Immune Microenvironment Characterization in Gastric Cancer. Ijgm Vol. 14, 6573–6586. 10.2147/ijgm.s331291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Xie J., Huang W., Chen H., Xi S., Han Z., et al. (2019). Tumor Immune Microenvironment and Chemosensitivity Signature for Predicting Response to Chemotherapy in Gastric Cancer. Cancer Immunol. Res. 7 (12), 2065–2073. 10.1158/2326-6066.CIR-19-0311 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Wang H., Wu J., Chen C., Yuan Q., Huang W., et al. (2020). Noninvasive Imaging Evaluation of Tumor Immune Microenvironment to Predict Outcomes in Gastric Cancer. Ann. Oncol. 31 (6), 760–768. 10.1016/j.annonc.2020.03.295 [DOI] [PubMed] [Google Scholar]
- Jin X., Zhu Z., Shi Y. (2014). Metastasis Mechanism and Gene/protein Expression in Gastric Cancer with Distant Organs Metastasis. Bull. Cancer 101 (1), E1–E12. 10.1684/bdc.2013.1882 [DOI] [PubMed] [Google Scholar]
- Johnston F. M., Beckman M. (2019). Updates on Management of Gastric Cancer. Curr. Oncol. Rep. 21 (8), 67. 10.1007/s11912-019-0820-4 [DOI] [PubMed] [Google Scholar]
- Kamada T., Maruyama Y., Monobe Y., Haruma K. (2021). Endoscopic Features and Clinical Importance of Autoimmune Gastritis. Dig. Endosc. (Online ahead of print). 10.1111/den.14175 [DOI] [PubMed] [Google Scholar]
- Kehrer J. P. (2000). The Haber-Weiss Reaction and Mechanisms of Toxicity. Toxicology 149 (1), 43–50. 10.1016/s0300-483x(00)00231-6 [DOI] [PubMed] [Google Scholar]
- Kerr J. F. R., Wyllie A. H., Currie A. R. (1972). Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics. Br. J. Cancer 26 (4), 239–257. 10.1038/bjc.1972.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöditz K., Fadeel B. (2019). Three Cell Deaths and a Funeral: Macrophage Clearance of Cells Undergoing Distinct Modes of Cell Death. Cel Death Discov. 5, 65. 10.1038/s41420-019-0146-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth J. D., Neish A. S. (2014). Nox Enzymes and New Thinking on Reactive Oxygen: a Double-Edged Sword Revisited. Annu. Rev. Pathol. Mech. Dis. 9, 119–145. 10.1146/annurev-pathol-012513-104651 [DOI] [PubMed] [Google Scholar]
- Lazăr D. C., Avram M. F., Romoșan I., Cornianu M., Tăban S., Goldiș A. (2018). Prognostic Significance of Tumor Immune Microenvironment and Immunotherapy: Novel Insights and Future Perspectives in Gastric Cancer. World J. Gastroenterol. 24 (32), 3583–3616. 10.3748/wjg.v24.i32.3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-Y., Nam M., Son H. Y., Hyun K., Jang S. Y., Kim J. W., et al. (2020). Polyunsaturated Fatty Acid Biosynthesis Pathway Determines Ferroptosis Sensitivity in Gastric Cancer. Proc. Natl. Acad. Sci. U.S.A. 117 (51), 32433–32442. 10.1073/pnas.2006828117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Xu L., Run Z.-C., Feng W., Liu W., Zhang P.-J., et al. (2018). Multiple Cytokine Profiling in Serum for Early Detection of Gastric Cancer. Wjg 24 (21), 2269–2278. 10.3748/wjg.v24.i21.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Feng G., Gauthier J. M., Lokshina I., Higashikubo R., Evans S., et al. (2019). Ferroptotic Cell Death and TLR4/Trif Signaling Initiate Neutrophil Recruitment after Heart Transplantation. J. Clin. Invest. Feb 26 129 (6), 2293–2304. 10.1172/jci126428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X., Fan Y., Hou J., Chen X., Xu X., Yang Y., et al. (2019). Identification of Chaetocin as a Potent Non-ROS-mediated Anticancer Drug Candidate for Gastric Cancer. J. Cancer 10 (16), 3678–3690. 10.7150/jca.32803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkermann A., Skouta R., Himmerkus N., Mulay S. R., Dewitz C., De Zen F., et al. (2014). Synchronized Renal Tubular Cell Death Involves Ferroptosis. Proc. Natl. Acad. Sci. U.S.A. 111 (47), 16836–16841. 10.1073/pnas.1415518111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkermann A., Skouta R., Himmerkus N., Mulay S. R., Dewitz C., De Zen F., et al. (2014). Synchronized Renal Tubular Cell Death Involves Ferroptosis. Proc. Natl. Acad. Sci. U.S.A. 111 (47), 16836–16841. 10.1073/pnas.1415518111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Ma J.-y., Hu G., Jin H. (2021). Identification and Validation of a Novel Ferroptosis-Related Gene Model for Predicting the Prognosis of Gastric Cancer Patients. PLoS One 16 (7), e0254368. 10.1371/journal.pone.0254368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. J., Yang Y. B., Zhou J. X., Lin Y. J., Pan Y. L., Pan J. H. (2021). A Novel Ferroptosis-Related Gene Risk Signature for Predicting Prognosis and Immunotherapy Response in Gastric Cancer. Dis. Markers 2021, 2385406. 10.1155/2021/2385406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lordick F., Shitara K., Janjigian Y. Y. (2017). New Agents on the Horizon in Gastric Cancer. Ann. Oncol. 28 (8), 1767–1775. 10.1093/annonc/mdx051 [DOI] [PubMed] [Google Scholar]
- Machlowska J., Maciejewski R., Sitarz R. (2018). The Pattern of Signatures in Gastric Cancer Prognosis. Ijms 19 (6), 1658. 10.3390/ijms19061658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S.-H., Zhu C.-H., Nie Y., Yu J., Wang L. (2021). Levobupivacaine Induces Ferroptosis by miR-489-3p/SLC7A11 Signaling in Gastric Cancer. Front. Pharmacol. 12, 681338. 10.3389/fphar.2021.681338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M., Freigang S., Schneider C., Conrad M., Bornkamm G. W., Kopf M. (2015). T Cell Lipid Peroxidation Induces Ferroptosis and Prevents Immunity to Infection. J. Exp. Med. Apr 6 212 (4), 555–568. 10.1084/jem.20140857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihmanli M., Ilhan E., Idiz U. O., Alemdar A., Demir U. (2016). Recent Developments and Innovations in Gastric Cancer. Wjg 22 (17), 4307–4320. 10.3748/wjg.v22.i17.4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckenthaler M. U., Rivella S., Hentze M. W., Galy B. (2017). A Red Carpet for Iron Metabolism. Cell 168 (3), 344–361. 10.1016/j.cell.2016.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namee N. M., O'Driscoll L. (2018). Extracellular Vesicles and Anti-cancer Drug Resistance. Biochim. Biophys. Acta (Bba) - Rev. Cancer 1870 (2), 123–136. 10.1016/j.bbcan.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Ni H., Qin H., Sun C., Liu Y., Ruan G., Guo Q., et al. (2021). MiR-375 Reduces the Stemness of Gastric Cancer Cells through Triggering Ferroptosis. Stem Cel Res Ther 12 (1), 325. 10.1186/s13287-021-02394-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H., Ruan G., Sun C., Yang X., Miao Z., Li J., et al. (2021). Tanshinone IIA Inhibits Gastric Cancer Cell Stemness through Inducing Ferroptosis. Environ. Toxicol. 37 (2), 192–200. 10.1002/tox.23388 [DOI] [PubMed] [Google Scholar]
- Niu Y., Zhang J., Tong Y., Li J., Liu B. (2019). RETRACTED: Physcion 8-O-β-Glucopyranoside Induced Ferroptosis via Regulating miR-103a-3p/GLS2 axis in Gastric Cancer. Life Sci. 237, 116893. 10.1016/j.lfs.2019.116893 [DOI] [PubMed] [Google Scholar]
- Nomura A., Chyou P. H., Stemmermann G. N. (1992). Association of Serum Ferritin Levels with the Risk of Stomach Cancer. Cancer Epidemiol. Biomarkers Prev. 1 (7), 547–550. [PubMed] [Google Scholar]
- Oya Y., Hayakawa Y., Koike K. (2020). Tumor Microenvironment in Gastric Cancers. Cancer Sci. 111 (8), 2696–2707. 10.1111/cas.14521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palzer J., Eckstein L., Slabu I., Reisen O., Neumann U. P., Roeth A. A. (2021). Iron Oxide Nanoparticle-Based Hyperthermia as a Treatment Option in Various Gastrointestinal Malignancies. Nanomaterials 11 (11), 3013. 10.3390/nano11113013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifhofer-Obermair C., Tymoszuk P., Petzer V., Weiss G., Nairz M. (2018). Iron in the Tumor Microenvironment-Connecting the Dots. Front. Oncol. 8, 549. 10.3389/fonc.2018.00549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prá D., Rech Franke S. I., Pêgas Henriques J. A., Fenech M. (2009). A Possible Link between Iron Deficiency and Gastrointestinal Carcinogenesis. Nutr. Cancer 61 (4), 415–426. 10.1080/01635580902803701 [DOI] [PubMed] [Google Scholar]
- Rojas A., Araya P., Gonzalez I., Morales E. (2020). Gastric Tumor Microenvironment. Adv. Exp. Med. Biol. 1226, 23–35. 10.1007/978-3-030-36214-0_2 [DOI] [PubMed] [Google Scholar]
- Santoro R., Ettorre G. M., Santoro E. (2014). Subtotal Gastrectomy for Gastric Cancer. Wjg 20 (38), 13667–13680. 10.3748/wjg.v20.i38.13667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweichel J. U., Merker H. J. (1973). The Morphology of Various Types of Cell Death in Prenatal Tissues. Teratology 7 (3), 253–266. 10.1002/tera.1420070306 [DOI] [PubMed] [Google Scholar]
- Shao L., Chen Z., Soutto M., Zhu S., Lu H., Romero-Gallo J., et al. (2019). Helicobacter pylori ‐induced miR‐135b‐5p Promotes Cisplatin Resistance in Gastric Cancer. FASEB j. 33 (1), 264–274. 10.1096/fj.201701456rr [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Jia H., Li S., Huang L., Aikemu B., Yang G., et al. (2021). Comprehensive Analysis of Ferroptosis-Related Markers for the Clinical and Biological Value in Gastric Cancer. Oxid Med. Cel Longev 2021, 7007933. 10.1155/2021/7007933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Hayano M., Pagano N. C., Stockwell B. R. (2016). Cell-Line Selectivity Improves the Predictive Power of Pharmacogenomic Analyses and Helps Identify NADPH as Biomarker for Ferroptosis Sensitivity. Cel Chem. Biol. 23 (2), 225–235. 10.1016/j.chembiol.2015.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D., Kim E. H., Lee J., Roh J.-L. (2018). Nrf2 Inhibition Reverses Resistance to GPX4 Inhibitor-Induced Ferroptosis in Head and Neck Cancer. Free Radic. Biol. Med. 129, 454–462. 10.1016/j.freeradbiomed.2018.10.426 [DOI] [PubMed] [Google Scholar]
- Silva M. M., Rocha C. R. R., Kinker G. S., Pelegrini A. L., Menck C. F. M. (2019). The Balance between NRF2/GSH Antioxidant Mediated Pathway and DNA Repair Modulates Cisplatin Resistance in Lung Cancer Cells. Sci. Rep. 9 (1), 17639. 10.1038/s41598-019-54065-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth E. C., Nilsson M., Grabsch H. I., van Grieken N. C., Lordick F. (2020). Gastric Cancer. The Lancet 396 (10251), 635–648. 10.1016/s0140-6736(20)31288-5 [DOI] [PubMed] [Google Scholar]
- Soares M. P., Hamza I. (2016). Macrophages and Iron Metabolism. Immunity 44 (3), 492–504. 10.1016/j.immuni.2016.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B., Yu J., Wu T. S. (2015). Correlation between C-Erbb-2 with Gastric Mucosal Atypical Hyperplasia and Gastric Carcinoma. J. Biol. Regul. Homeost Agents 29 (2), 471–477. [PubMed] [Google Scholar]
- Stockwell B. R., Friedmann Angeli J. P., Bayir H., Bush A. I., Conrad M., Dixon S. J., et al. (2017). Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171 (2), 273–285. 10.1016/j.cell.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell B. R., Jiang X., Gu W. (2020). Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cel Biol. 30 (6), 478–490. 10.1016/j.tcb.2020.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Deng R., Zhang C. (2020). Erastin Induces Apoptotic and Ferroptotic Cell Death by Inducing ROS Accumulation by Causing Mitochondrial Dysfunction in Gastric Cancer Cell HGC-27. Mol. Med. Rep. 22 (4), 2826–2832. 10.3892/mmr.2020.11376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talvenmäki H., Lallukka N., Survo S., Romantschuk M. (2019). Fenton's Reaction-Based Chemical Oxidation in Suboptimal Conditions Can lead to Mobilization of Oil Hydrocarbons but Also Contribute to the Total Removal of Volatile Compounds. Environ. Sci. Pollut. Res. 26 (33), 34670–34684. 10.1007/s11356-019-06547-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W. C., Krasner N., O'Toole P., Lombard M. (1997). Enhancement of Photodynamic Therapy in Gastric Cancer Cells by Removal of Iron. Gut 41 (1), 14–18. 10.1136/gut.41.1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z. (2019). Recent Advances in the Surgical Treatment of Advanced Gastric Cancer: A Review. Med. Sci. Monit. 25, 3537–3541. 10.12659/msm.916475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Chen X., Kang R., Kroemer G. (2021). Ferroptosis: Molecular Mechanisms and Health Implications. Cell Res 31 (2), 107–125. 10.1038/s41422-020-00441-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii S., Shintoku R., Kubota C., Yaegashi M., Torii R., Sasaki M., et al. (2016). An Essential Role for Functional Lysosomes in Ferroptosis of Cancer Cells. Biochem. J. 473 (6), 769–777. 10.1042/bj20150658 [DOI] [PubMed] [Google Scholar]
- Torti S. V., Torti F. M. (2013). Iron and Cancer: More Ore to Be Mined. Nat. Rev. Cancer 13, 342–355. 10.1038/nrc3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini F., Maiorino M., Valente M., Ferri L., Gregolin C. (1982). Purification from Pig Liver of a Protein Which Protects Liposomes and Biomembranes from Peroxidative Degradation and Exhibits Glutathione Peroxidase Activity on Phosphatidylcholine Hydroperoxides. Biochim. Biophys. Acta (Bba) - Lipids Lipid Metab. 710 (2), 197–211. 10.1016/0005-2760(82)90150-3 [DOI] [PubMed] [Google Scholar]
- Vinchi F., Porto G., Simmelbauer A., Altamura S., Passos S. T., Garbowski M., et al. (2019). Atherosclerosis Is Aggravated by Iron Overload and Ameliorated by Dietary and Pharmacological Iron Restriction. Eur. Heart J. 00, 1–16. 10.1093/eurheartj/ehz112 [DOI] [PubMed] [Google Scholar]
- Wang F., Chen C., Chen W. P., Li Z. L., Cheng H. (2021). Development and Validation of a Novel Ferroptosis-Related Gene Signature for Predicting Prognosis and the Immune Microenvironment in Gastric Cancer. Biomed. Res. Int. 2021, 6014202. 10.1155/2021/6014202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang T., Zhang Y., Liu J., Song J., Han Y., et al. (2021). CPEB1 Enhances Erastin‐induced Ferroptosis in Gastric Cancer Cells by Suppressing Twist1 Expression. IUBMB Life 73 (9), 1180–1190. 10.1002/iub.2525 [DOI] [PubMed] [Google Scholar]
- Wang S.-F., Chen M.-S., Chou Y.-C., Ueng Y.-F., Yin P.-H., Yeh T.-S., et al. (2016). Mitochondrial Dysfunction Enhances Cisplatin Resistance in Human Gastric Cancer Cells via the ROS-Activated GCN2-eIF2α-ATF4-xCT Pathway. Oncotarget 7 (45), 74132–74151. 10.18632/oncotarget.12356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Chen Z., Zhu S., Lu H., Peng D., Soutto M., et al. (2020). PRDX2 Protects against Oxidative Stress Induced by H. pylori and Promotes Resistance to Cisplatin in Gastric Cancer. Redox Biol. 28, 101319. 10.1016/j.redox.2019.101319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Sun J., Zhang N., Zheng Y., Wang X., Lv L., et al. (2020). Noncoding RNAs in Gastric Cancer: Implications for Drug Resistance. Mol. Cancer 19 (1), 62. 10.1186/s12943-020-01185-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang R., Song W., Ren J., Wu J., Fu J., Fu T. (2021). Identification of Stem Cell-Related Subtypes and Risk Scoring for Gastric Cancer Based on Stem Genomic Profiling. Stem Cel Res Ther 12 (1), 563. 10.1186/s13287-021-02633-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Liu X., Yuan L., Chen X., Wang F. (2021). Expression of Ferroptosis-Related Genes Shapes Tumor Microenvironment and Pharmacological Profile in Gastric Cancer. Front. Cel Dev. Biol. 9, 694003. 10.3389/fcell.2021.694003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Feng J., Li Y., Guan D., Chen H., Zhai X., et al. (2020). The Vicious Cycle between Ferritinophagy and ROS Production Triggered EMT Inhibition of Gastric Cancer Cells Was through p53/AKT/mTor Pathway. Chemico-Biological Interactions 328, 109196. 10.1016/j.cbi.2020.109196 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Hamano T., Oka T., Doi Y., Kajimoto S., Shimada K., et al. (2021). Mean Corpuscular Hemoglobin Concentration: an Anemia Parameter Predicting Cardiovascular Disease in Incident Dialysis Patients. J. Nephrol. 35 (2), 535–544. 10.1007/s40620-021-01107-w [DOI] [PubMed] [Google Scholar]
- Yang W., Ma J., Zhou W., Cao B., Zhou X., Yang Z., et al. (2017). Molecular Mechanisms and Theranostic Potential of miRNAs in Drug Resistance of Gastric Cancer. Expert Opin. Ther. Targets 21 (11), 1063–1075. 10.1080/14728222.2017.1389900 [DOI] [PubMed] [Google Scholar]
- Yang W. S., Kim K. J., Gaschler M. M., Patel M., Shchepinov M. S., Stockwell B. R. (2016). Peroxidation of Polyunsaturated Fatty Acids by Lipoxygenases Drives Ferroptosis. Proc. Natl. Acad. Sci. U S A. 113 (34), E4966–E4975. 10.1073/pnas.1603244113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. S., SriRamaratnam R., Welsch M. E., Shimada K., Skouta R., Viswanathan V. S., et al. (2014). Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 156 (1-2), 317–331. 10.1016/j.cell.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. S., Stockwell B. R. (2008). Synthetic Lethal Screening Identifies Compounds Activating Iron-dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem. Biol. 15 (3), 234–245. 10.1016/j.chembiol.2008.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavros Y. (2017). Initiation and Maintenance of Gastric Cancer: A Focus on CD44 Variant Isoforms and Cancer Stem Cells. Cell Mol. Gastroenterol. Hepatol. 4 (1), 55–63. 10.1016/j.jcmgh.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J., Shen J., Xie G., Wu J., He M., Gao L., et al. (2019). Cancer-associated Fibroblasts-Derived IL-8 Mediates Resistance to Cisplatin in Human Gastric Cancer. Cancer Lett. 454, 37–43. 10.1016/j.canlet.2019.04.002 [DOI] [PubMed] [Google Scholar]
- Zhang H., Deng T., Liu R., Ning T., Yang H., Liu D., et al. (2020). CAF Secreted miR-522 Suppresses Ferroptosis and Promotes Acquired Chemo-Resistance in Gastric Cancer. Mol. Cancer 19 (1), 43. 10.1186/s12943-020-01168-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Wang M., He Y., Deng T., Liu R., Wang W., et al. (2021). Chemotoxicity-induced Exosomal lncFERO Regulates Ferroptosis and Stemness in Gastric Cancer Stem Cells. Cell Death Dis 12 (12), 1116. 10.1038/s41419-021-04406-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Conrad M. (2020). The Metabolic Underpinnings of Ferroptosis. Cel Metab. 32 (6), 920–937. 10.1016/j.cmet.2020.10.011 [DOI] [PubMed] [Google Scholar]
- Zhou L., Zhao B., Zhang L., Wang S., Dong D., Lv H., et al. (2018). Alterations in Cellular Iron Metabolism Provide More Therapeutic Opportunities for Cancer. Ijms 19 (5), 1545. 10.3390/ijms19051545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q., Guo Y., Chen S., Fu D., Li Y., Li Z., et al. (2020). Irinotecan Induces Autophagy-dependent Apoptosis and Positively Regulates ROS-Related JNK- and P38-MAPK Pathways in Gastric Cancer Cells. Ott Vol. 13, 2807–2817. 10.2147/ott.s240803 [DOI] [PMC free article] [PubMed] [Google Scholar]