ABSTRACT
We report treating a term neonate with tuberous sclerosis and giant rhabdomyomas who presented with incessant supraventricular tachycardia with Everolimus. The treatment was efficient in reducing tumor size and assisted as an adjunct therapy in controlling arrhythmia and limiting preexcitation. Treatment was challenged by difficulty to achieve stable drug level and limited by neutropenia as a serious side effect.
Keywords: Everolimus, rhabdomyomas, Wolff-Parkinson-White
INTRODUCTION
Rhabdomyomas are the most common cardiac tumors in infants and children, commonly presenting in neonates with tuberous sclerosis complex (TSC). These tumors involute spontaneously and do not require treatment or intervention unless presenting with significant complications (cardiac dysfunction or arrhythmia). Recent case series and case reports describe the safety of the mTOR-inhibitor, Everolimus, and its efficacy in promoting tumor regression. However, its clinical use may still be limited as very little pharmacologic data for its use in neonates are available. We present the case of a newborn who was treated with Everolimus for giant rhabdomyomas with Wolff-Parkinson-White (WPW) syndrome and incessant re-entrant tachycardia. The case is discussed to present both the use of Everolimus when the primary indication is arrhythmia management and reporting side effect in a neonate.
CASE REPORT
This male neonate was followed prenatally for family history of TSC and diagnosed with large cardiac rhabdomyomas. Genetic testing after birth confirmed the diagnosis with a pathogenic TSC1 gene mutation. He was born full term through spontaneous delivery and was immediately noted to be in narrow complex tachycardia to 293 bpm and was transferred to our institution. Echocardiogram on admission showed four rhabdomyomas in the apex and outflow tracts of both ventricles with no evidence of obstruction to flow. He had a normal baseline electrocardiogram (ECG) but recurrent supraventricular tachycardia (SVT) episodes that responded to adenosine and arrhythmias controlled were achieved with sotalol (120 mg/m2/day) on day of life 2. On day of life 6, his episodes of SVT recurred and were again prolonged and incessant. A change in his ECG while in sinus rate was noted with a WPW pattern and evidence of a left-sided accessory pathway [Figure 1]. A repeat echocardiogram the next day showed an interval growth in tumor size [Figure 2]. These changes suggested that the increase in tumor size may contribute to worsening arrhythmias and we chose to use Everolimus to achieve tumor size reduction. We started with daily doses of 0.125 mg on day of life 8, achieving a trough blood level of 7.4 ng/ml after 48 h. To achieve better control over SVT, we also changed his anti-arrhythmic therapy from sotalol to flecainide that was titrated up to 130 mg/m2/day. SVT control was achieved, and within a few days, the WPW pattern on ECG resolved as well. For ECG evidence of flecainide toxicity (wide QRS without evidence of preexcitation) and short bursts of atrial ectopy, the flecainide dose was decreased (82 mg/m2/day) and propranolol was added (2 mg/kg/day). Echocardiogram at day of life 15 showed a significant decrease in the size of his tumors that continued through the time of his therapy [Figure 2]. We aimed for Everolimus blood level of 5–10 ng/ml and had wide fluctuations from 4.4 to 11.4 ng/ml. Fluctuations were related, initially, to changes in feeding status while treating arrhythmias, but he continued to require close monitoring and dosing adjustments through the admission and after hospital discharge. Cell count and liver functions were monitored routinely as outpatient: A slow decrease in absolute neutrophil count was noted down to 666/mm3 by the end of 5 weeks of treatment. In consultation with Pediatric Hematology, we elected to stop the treatment for the concern of hematologic toxicity and risk for immunecompromise. His counts recovered when checked 1 week after medication discontinuation.
Figure 1.
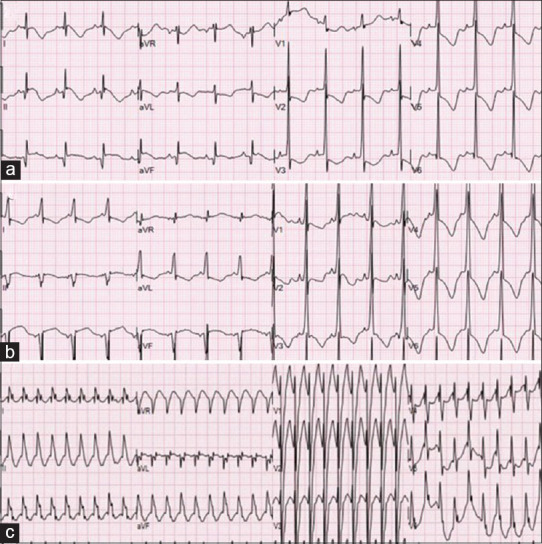
12-lead electrocardiograms: (a) Day of life 1: Sinus rhythm at 108 bpm with no evidence of preexcitation, abnormal repolarization pattern suggestive of myocardial strain. (b) Day of life 7: Sinus rhythm at a rate of 124 bpm with preexcitation pattern suggestive of a left-sided accessory pathway. (c) Day of life 10: Wide-complex tachycardia at a rate of 227 bpm, likely AVT with aberrant interventricular conduction
Figure 2.
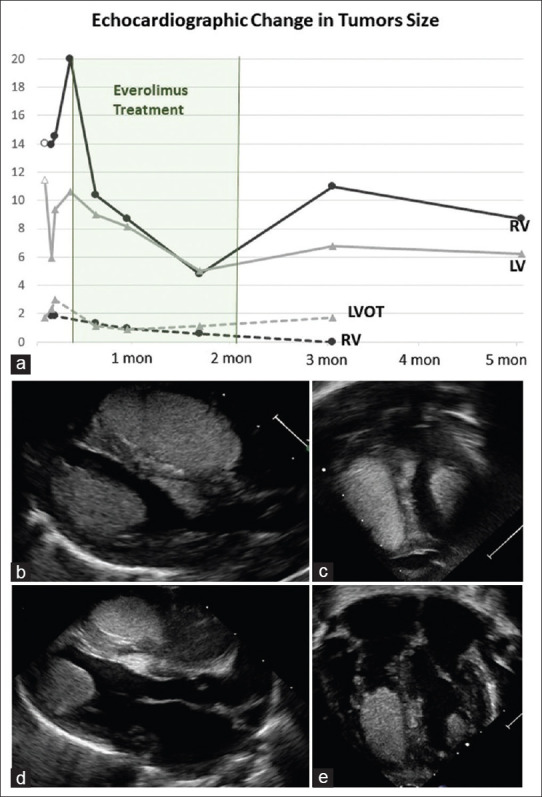
Echocardiographic monitoring of tumor size: (a) Tumor size charted based on ellipse area calculation (A = π ab in cm2). The right ventricular and left ventricular apical tumors are measured from the apical 4-chamber view. The right ventricular outflow tract and left ventricular outflow tract tumors are measured from anterior apical or subcoastal views. The first time-point for right ventricular and left ventricular is from the fetal echocardiogram. Parasternal long axis view showing right and left apical rhabdomyomas (b; where the smaller left ventricular outflow tract tumor is seen as well) and the apical view (c) at end diastole on Day of life 7, before treatment with Everolimus. Echo images after 2 weeks of treatment with Everolimus show reduction in tumor size from the parasternal long (d) and apical (e) views
DISCUSSION
Tuberous sclerosis causing mutations result in upregulation of the mTOR protein associated with tumor formation in early and adult life, including cardiac rhabdomyomas. Everolimus is a direct mTOR inhibitor that was proved effective in treating tumors and epilepsy in TSC patients but only small case series and single case reports are published for treating infantile tumors.[1,2,3] These reports consistently show that Everolimus is effective in accelerating size reduction in cardiac rhabdomyomas, when tumor-related cardiac dysfunction is the indication for treatment.[1,4] For addressing arrhythmia, the relationship between cardiac tumors, WPW and SVT is well established.[5] Accessory pathways are thought to originate from tumor sites, suggested to comprise of conducting tumor cells, and thus often regress as the tumors involute.[6] Miyake et al.[5] found that 24% of children with cardiac tumors have clinically significant arrhythmias. While ventricular tumors are more commonly associated with ventricular tachycardia, in patients with rhabdomyomas, the predominant arrhythmia is SVT and 10% of patients have preexcitation. Two previous case reports discussed the effect of Everolimus on SVT: Öztunç et al.[7] reported arrhythmia control after 8 days of treatment and Saffari et al.[1] reported success within 1 month, both also in association with reduction in tumor size. In our case, rapid tumor growth in the 1st week of life coincided with the manifestation of new antegrade preexcitation, a changed or new accessory pathway. We hypothesize that reduction in tumor size using Everolimus, which resulted in regression of antegrade preexcitation, helped in achieving arrhythmia control together with antiarrhythmic medications. This was particularly helpful given the drug toxicity the patient experienced, that limited dosing escalation of anti-arrhythmic medications.
The commonly reported side effects of Everolimus include stomatitis, vomiting or diarrhea, nasopharyngitis, upper respiratory tract infection, rash, and headaches. In neonates treated for rhabdomyomas, the reviewed literature reports side effects of stomatitis, mucositis and mouth ulcers, hyperlipidemia, and lymphopenia[8] at drug levels of 5–15 ng/ml, increased frequency of preexisting ventricular tachycardia[9] and liver dysfunction or pulmonary hemorrhage at supra-therapeutic levels.[10] It was thus somewhat disappointing that in our patient, at goal drug levels, neutropenia limited the use of the medication that was discontinued after 5 weeks.
CONCLUSION
Everolimus is an effective therapy for accelerating size reduction of cardiac rhabdomyomas in infants with TSC. We suggest that it may have a role in addressing tumor-related SVT. While case reports and case series suggest relative safety for the use of Everolimus in neonates, a stable medication blood level is difficult to achieve and potentially life-threatening adverse events do occur. Hopefully, more information about the pharmacodynamics and pharmacokinetics of the medication will be collected as clinicians continue report their experience.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Saffari A, Brösse I, Wiemer-Kruel A, Wilken B, Kreuzaler P, Hahn A, et al. Safety and efficacy of mTOR inhibitor treatment in patients with tuberous sclerosis complex under 2 years of age-a multicenter retrospective study. Orphanet J Rare Dis. 2019;14:96. doi: 10.1186/s13023-019-1077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahdah N. Everolimus for the treatment of tuberous sclerosis complex-related cardiac rhabdomyomas in pediatric patients. J Pediatr. 2017;190:21–6.e7. doi: 10.1016/j.jpeds.2017.06.076. [DOI] [PubMed] [Google Scholar]
- 3.Kuki I, Kawawaki H, Okazaki S, Ehara E, Yoshida Y, Kunihiro N, et al. Efficacy and safety of everolimus in patients younger than 12 months with congenital subependymal giant cell astrocytoma. Brain Dev. 2018;40:415–20. doi: 10.1016/j.braindev.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Aw F, Goyer I, Raboisson MJ, Boutin C, Major P, Dahdah N. Accelerated cardiac rhabdomyoma regression with everolimus in infants with tuberous sclerosis complex. Pediatr Cardiol. 2017;38:394–400. doi: 10.1007/s00246-016-1528-y. [DOI] [PubMed] [Google Scholar]
- 5.Miyake CY, Del Nido PJ, Alexander ME, Cecchin F, Berul CI, Triedman JK, et al. Cardiac tumors and associated arrhythmias in pediatric patients, with observations on surgical therapy for ventricular tachycardia. J Am Coll Cardiol. 2011;58:1903–9. doi: 10.1016/j.jacc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 6.O’Callaghan FJ, Clarke AC, Joffe H, Keeton B, Martin R, Salmon A, et al. Tuberous sclerosis complex and Wolff-Parkinson-White syndrome. Arch Dis Child. 1998;78:159–62. doi: 10.1136/adc.78.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Öztunç F, Atik SU, Güneş AO. Everolimus treatment of a newborn with rhabdomyoma causing severe arrhythmia. Cardiol Young. 2015;25:1411–4. doi: 10.1017/S1047951114002261. [DOI] [PubMed] [Google Scholar]
- 8.Wagner R, Riede FT, Seki H, Hornemann F, Syrbe S, Daehnert I, et al. Oral everolimus for treatment of a giant left ventricular rhabdomyoma in a neonate-rapid tumor regression documented by real time 3d echocardiography. Echocardiography. 2015;32:1876–9. doi: 10.1111/echo.13015. [DOI] [PubMed] [Google Scholar]
- 9.Davis KA, Dodeja AK, Clark A, Hor K, Baker P, Cripe LH, et al. Use of cardiac MRI to assess antitumor EFFICACY of everolimus in sporadic cardiac rhabdomyoma. Pediatrics. 2019;143:8. doi: 10.1542/peds.2018-2495. [DOI] [PubMed] [Google Scholar]
- 10.Shibata Y, Maruyama H, Hayashi T, Ono H, Wada Y, Fujinaga H, et al. Effect and complications of everolimus use for giant cardiac rhabdomyomas with neonatal tuberous sclerosis. AJP Rep. 2019;9:e213–7. doi: 10.1055/s-0039-1692198. [DOI] [PMC free article] [PubMed] [Google Scholar]


