Abstract
Background
The upper belt of Azad Kashmir is a hilly, mountainous, and remote area where the indigenous communities mainly believe in traditional medicines for the treatment of different ailments. This study aimed to conserve scientifically and culturally important medicinal knowledge of Primula species in Azad Kashmir, Western Himalaya, Pakistan. The additional objective was to evaluate the antibacterial activity of these plants against pathogenic bacteria.
Methods
The ethnomedicinal data of Primula species was explored by conducting structured interviews with 40 informants of the study area, especially asking about the medicinal uses of Primula species. The indigenously used Primula species were further analyzed for their antibacterial activity against both gram-positive and gram-negative bacteria by using disc diffusion assay supplemented with a more robust minimum inhibitory concentration assay.
Results
Ethnomedicinal data revealed that indigenous communities living in upper regions of Azad Kashmir use 5 Primula species for the treatment of various disorders. The highly cited disease category was ophthalmic disorders. P. denticulata and P. macrophylla were the most cited plant species with higher use reports such as 104 and 93, respectively. One or more extracts of different parts of Primula species showed a noteworthy antibacterial activity against one or more tested bacteria.
Conclusion
This study provides novel information regarding several categories of traditional uses and antibacterial activity of Primula species in Azad Kashmir, Western Himalaya. The need for novel and more effective drugs derived from natural products is more important than ever, making future studies on herbal remedies both justified and urgently required.
Keywords: Indigenous knowledge, Primula denticulata, Gram-positive, Traditional uses
1. Introduction
Plants have been used by mankind for treating various ailments and as a source of natural remedies in different parts of the world, and medicinal plant use is still the predominant form of primary healthcare services (Kigen et al., 2019). In Latin America, the Middle East, Asia, and Africa, over 85 percent of the populations mainly rely on traditional medicine, especially on herbal medicines, for their health ailments. Approximately, 100 million people in the European Union and up to 90% of the inhabitants of several countries still use traditional and herbal medicines (Jamshidi-Kia et al., 2018) and about 20% of the total plants identified so far are used as herbal remedies (Khan et al., 2015). Due to the peculiar phytogeography and varied climatic conditions, Pakistan is rich in the diversity of medicinal and aromatic plants. It has been estimated that about 75% of people of Pakistan use plants to fulfill their medicinal needs and more than 600 plants are being used traditionally or medicinally in Pakistan (Shinwari and Qaisar, 2011, Hamayun et al., 2006). However, the major number of medicinal plants is confined to the Himalayan region of Pakistan, having about 8000 flowering plant species and considered to be a biodiversity hub for a wide range of medicinal plants (Malik et al., 2015, Ballabha and Chaurasia, 2009). The Azad Kashmir, where this study took place, being a part of the western Himalayas has a very rich biodiversity with a unique bio-geographical location. The people living in this region depend on traditional healers for basic health care because trained physicians are almost absent in the area. Although WHO recommends the trained physician-to-population ratio of 1:1000 for rural areas (WHO, 2016). Alternatively, the healthcare needs of the people are addressed by traditional healers in a more culturally appropriate manner. Indeed, due to the poor transport system, difficult access to urban areas and health care centers as well as high prices of allopathic drugs, people of this area still use plants to meet their primary healthcare. However, it is very important to conserve this traditional knowledge before it is disappearing from oral history because it has been reported that traditional knowledge lose (Pieroni et al., 2002) due to the absence of transmission of information between older generations and younger ones and fashions towards adaptation of modern lifestyles.
From the beginning, the records of traditional knowledge of medicinal uses of plants create a modern system biology-based approach to health and healing which provided many important drugs of modern-day (Lemonnier et al., 2017). With the wealth of advanced technology, together with chemo-diversity, biological diversity, great innovations in evolutionary techniques, and a wealth of knowledge about natural products, it is necessary to establish a drug library from the screening of plant products. Humankind needs to learn more from natural products derived from plants and traditional medicines (Yuan et al., 2016). As it is noted in the last few decades, infectious diseases have increased to a great extent in countries with poor living style, poor sanitation, and hygiene, undernutrition, unsafe water, iron deficiency, indoor solid smoke, and antibiotics resistance effects become an ever-growing therapeutic problem (Brundtland, 2002). Therefore, the use of medicinal plants as accessible and efficient remedies, with antimicrobial properties, is still the best alternative particularly in developing countries (Girish and Satish, 2008). Plants contain natural products as the main source of antimicrobial agents for treating bacterial infectious diseases (Barbour et al., 2004). Therefore, it is of great interest to carry out a screening of these traditionally used medicinal plants to ratify their use in the drug industry. This may result in the discovery of novel active compounds (Nitta et al., 2002).
Primula, commonly known as Primroses, belongs to family Primulaceae and grows in cooler and humid regions of the northern hemisphere (Richards, 2003). It is a habitat-sensitive and low-emerging herb that bears colorful and attractive flowers that bloom in April, June, and July. The Primula plants are well known among the inhabitants who cultivate them for medicinal and ornamental values (Başbülbül et al., 2008, Fico et al., 2007). Primula species have a very extensive history of traditional uses and have been mainly used in treating conditions like cramps, paralysis, rheumatic pain, and insomnia in children (Majid et al., 2014). The decoction made from these plants is used to cure catarrhs, cough, bronchitis, nervousness, headache, diaphoretic, rheumatism, gout, and diuretic (Langer, 2012). Leaves of Primula are known as a remedy against wounds, fevers, ulcers, and sores; moreover, their oral extracts have been used for the treatment of diarrhea, sore mouth, and internal bleeding (Shaheen et al., 2012).
Even though some ethnobotanical surveys on medicinal plants have been conducted in the Neelum valley (Ahmad et al., 2017, Ghufran et al., 2007, Dar, 2003), but no study focused on the use of this particular taxon 'Primula ', having deep-rooted traditional values among the local communities. Besides, no research was found that combines the ethnobotanical characteristics and antibacterial properties of Primula plants used by a local community. Based on these considerations, this study aimed to provide the first ethnobotanical report and antibacterial activity of Primula plants in an attempt to explore and search the healing properties and useful potential of this indigenous herbal plant. Thus, this documentation of traditional knowledge concerning the use of Primula plants in the upper part of Azad Kashmir can play a role in the conservation of traditional knowledge for future generations.
2. Methods
2.1. Study area
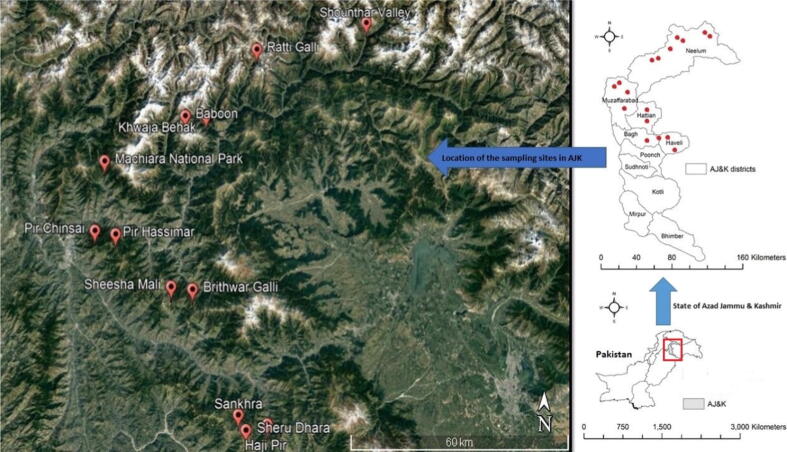
The state of Azad Jammu and Kashmir lies between longitude 730–750 and latitude 330 – 360 that comprises an area of 5134 square miles (13,297 square kilometers). The topography of the area is mainly hilly and mountainous with valleys and stretches of plains. The average annual rainfall is 1300 mm. The elevation ranges from 360 m in the south to 6325 m in the North. This study was conducted in 22 villages/sites of upper regions (temperate to alpine) of Azad Jammu and Kashmir with an altitudinal range of 2195–4017 m (Fig. 1), specifically where the Primula plants grow. The names of the study villages/sites along with approximate coordinates are provided in Table 1.
Fig. 1.
Map of the study area. In the right lower corner, the map of Pakistan. In the upper right corner, the state of Azad Jammu and Kashmir and on the left side, google map of study sites from Azad Jammu and Kashmir (Western Himalaya).
Table 1.
The study locations where the ethnobotanical survey and collection of plants carried out.
| S. NO. | Site name | District | Geo-coordinates |
||
|---|---|---|---|---|---|
| Elevation (m) | Longitude E | Latitude N | |||
| 17 | Haji Peer | Bagh | 2940 | 74.06 | 33.96 |
| 18 | Shairo Dhara | Bagh | 3020 | 74.00 | 33.95 |
| 19 | Sankra | Bagh | 2939 | 73.99 | 33.95 |
| 12 | Seher Machyara Top | Hattian Bala | 3370 | 73.50 | 34.56 |
| 13 | Sheesha Mali | Hattian Bala | 3100 | 73.79 | 34.26 |
| 14 | Brithwaar Gali North | Leepa | 3320 | 73.85 | 34.25 |
| 15 | Brithwaal Gali Base | Leepa | 3002 | 73.51 | 34.15 |
| 16 | Brithwaar Top | Leepa | 3580 | 73.86 | 34.24 |
| 20 | Dao Khan | Leepa | 2490 | 73.47 | 34.15 |
| 10 | Machyara National Park | Muzaffarabad | 2195 | 73.40 | 34.55 |
| 11 | Dana Machyara Behak | Muzaffarabad | 3050 | 73.56 | 34.58 |
| 21 | Peer Hassimar | Muzaffarabad | 3080 | 73.54 | 34.41 |
| 22 | Peer Chinasi | Muzaffarabad | 2980 | 73.36 | 34.23 |
| 1 | Shonther Valley | Neelum | 3200 | 74.30 | 34.58 |
| 2 | Shonther Spoon Lake | Neelum | 3150 | 74.47 | 34.97 |
| 3 | Ratti Gali Base | Neelum | 3715 | 74.39 | 34.49 |
| 4 | Ratti Gali Top | Neelum | 3900 | 73.59 | 34.51 |
| 5 | Khawaja Behak | Neelum | 3069 | 74.06 | 34.46 |
| 6 | Kilwan Behak | Neelum | 3070 | 74.06 | 34.46 |
| 7 | Baboon Valley | Neelum | 3600 | 73.49 | 34.41 |
| 8 | Baboon Behak | Neelum | 3230 | 73.83 | 34.70 |
| 9 | Jabri Behak | Neelum | 4017 | 73.48 | 34.41 |
2.2. Demography and data collection
For the collection of ethnomedicinal data, multiple field surveys were conducted from 2018 to 2019 during early snow melting seasons in 22 villages/sites such as Shonther Valley, Shonther Spoon Lake, Ratti Gali Base, Ratti Gali Top, Khawaja Behak, Kilwan Behak, Baboon Valley, Baboon Behak, Jabri Behak, Machyara National Park, Dana Machyara Behak, Seher Machyara Top, Sheesha Mali, Brithwaar Gali North, Brithwaal Gali Base, Brithwaar Top, Haji Peer, Shairo Dhara, Sankra, Dao Khan, Peer Hassimar and Peer Chinasi. Data were collected in two steps (a) observation, formal and informal interviews of local inhabitants of both genders male and female to inquire about the medicinal utilization of plants, that helped in the formulation of a questionnaire (b) Open-ended questionnaire designed to obtain the information regarding plants local name, part of the plant utilized, the process of remedy preparation and medicinal uses from each informant (Weckerle et al., 2018). A total of 40 informants were interviewed with 10% males and 90% females. Age wise, 20% of the informants had 30–50 years of age and 80% informants had an age of about 50 years. This shows that older women have more traditional knowledge than young ones. Women are responsible to collect plants for firework and other domestic uses, so the knowledge of women is more than men (Tabuti et al., 2003). Before taking the ethnomedicinal survey, all the participants were informed and explained the aims of the study and what would be involved in obtaining the data followed by obtaining a written prior informed consent. A sample of the questionnaire is provided in Supplementary file 1.
2.3. Plant material, collection, and identification
All the species of Primula, grow wild in nature, were collected using the standard methodology of Martin (2004). Plant specimens have been collected, shad-dried, pressed, mounted, and labeled on herbarium sheets by using the protocol of Seshagirirao et al. (2016). Identification of all species was done by using the Flora of Pakistan (Ali and Qaiser, 1993–2007., Ali and Nasir, 1989–1992, Nasir and Ali, 1970–1979) and scientific names were confirmed with http://www.theplantlist.org. The identified specimens along with their vernacular name were deposited in AKASH Herbarium, Department of Botany, University of Azad Jammu and Kashmir Muzaffarabad.
2.4. Ethnobotanical data analysis
After completing the fieldwork, all the questionnaires were brought to the Lab and processed for data analysis, i.e., data was transferred to excel sheets. All the parameters were analyzed through descriptive statistics. Firstly, the frequency of citation (FC) for each Primula species was calculated, conversing the transparency of this study. FC is simply the total number of participants interviewed that use a certain Primula species for a specific event or disease (Schultz et al., 2020). The value of the FC varies from 0 (The Primula is not used by any informant for a specific event) to 40 (The Primula species is used by all the informants interviewed). The use reports and consensus upon the uses, applications, and plant parts were calculated by following the methods adopted by Carabajal et al. (2020). To find which Primula species are recognized as therapeutic ones for specific medicinal uses, a use-report (UR) for each species was calculated as individual reports for a single species being useful for certain symptoms or diseases (URsp). The consensus upon the use of plants to the disease categories (CUuse) was calculated by dividing the number of informants that mentioned the use of each Primula species per disease category to the total informants interviewed × 100. The use-report for each ailment (URail) was defined as the number of informants that mentioned the ailments (nail) that had been treated with these plants. Use-report for each disease category (URuse) was defined as the total sum of the nail into the same use-category. The consensus of uses for the method of application (CUapp) in each use-category was calculated as use-reports for each method of application (URapp) divided by the total informants interviewed × 100. URapp is defined as the number of informants that mentioned the method of application that had been used for the treatment of each use category. The same formula was used for the calculation of plant parts used (CUparts).
2.5. Preparation of extracts
Freshly harvested plant parts such as roots, leaves, and flowers of 5 Primula species were dried in shade at room temperature and ground into a fine powder using an electrical grinder. The extracts of each plant part were prepared non-subsequentially using 5 solvents; distilled water, methanol, ethanol, acetone, and petroleum ether purchased from local markets (Merck KGaA Germany). For extraction, the ground plant material and solvent, in a ratio of 2 g/45 mL, the mixture was vortexed, followed by sonication (RK 510H model: Bandelin, Berlin, Germany) in a cold-water bath for 1hr. After that, the mixture was filtered through Whatman no. 42 filter paper and extracts were then concentrated under reduced pressure in a rotary evaporator (Base-Hel- Vap, Germany). The concentrated extracts were transferred into pre-weighed glass vials and used for antibacterial testing.
2.6. Microorganisms and inoculum preparation
The antibacterial activity was examined against two Gram-positive bacteria (Staphylococcus aureus, Bacillus cereus) and three Gram-negative bacteria (Achromobacter xyloxidans, Escherichia coli, Pseudomonas aeruginosa). The choice of these microbes was attributed to their significance as pathogens cause human disorders. A ubiquitous bacterium P. aeruginosa causes infection in the lungs, burn wounds, and eyes (Lyczak et al., 2000). A. xylosoxidans is considered as a potential pathogen in patients with skin and soft tissue infections (Tena et al., 2014). S. aureus release toxins on the skin surface, cause cutaneous infections, and create skin blisters (Ghalehnoo, 2018). E. coli causes bacterial infections, urinary tract infections, diarrhea, phenomena, and intestinal infections (Johnson et al., 2010). B. cereus is associated mainly with food poisoning, eye infections, anthrax, and fulminant sepsis (Bottone, 2010). All these bacterial strains were collected and identified from the Laboratory of Mayo Hospital Lahore. A loop of isolated bacteria was separately streaked on sterilized agar medium and incubated at 37 ˚C for 24 h before experiments.
2.7. Antibacterial activity
The antibacterial activity of root, leaf, and flower extracts of 5 Primula species was determined using the agar disc diffusion assay (Ibraheem et al., 2016). Nutrient agar media were prepared by suspended 28 g of nutrient agar in 1000 mL of sterile distilled water. The mixture was gently stirred to dissolve all components, and then autoclaved the whole mixture at 121 °C for 15 min. The autoclaved nutrient agar was allowed to cool and poured into sterilized Petri dishes (9 cm diameter) under aseptic conditions in laminar airflow. One loop full of 24 h old pure each bacterial colony was transferred into distilled water aseptically and vortexed for obtaining suspension. A 100 µL bacterial suspension was then inoculated into Petri plates followed by the addition of liquid nutrient agar. The Petri dishes were then allowed to cool until the medium was solidified. The filter paper discs (6 mm) were impregnated with plant extracts (1 mg/mL) and placed on the agar medium in the Petri dishes at their marked places. The paper disc of antibiotic Ampicillin (10 µg/disc) was used as a positive control. All the plates were allowed to diffuse the extracts for 1 h and then were incubated for 24 h at 37 °C. The diameter of the zone of inhibition was measured in millimeter (mm) using a ruler (Prabhu et al., 2010). All the tests were performed in triplicates.
2.8. Minimum inhibitory concentration (MIC)
The MIC of each plant extract was determined through the nutrient broth micro-dilution method as described by Asadollahi et al. (2019) with some modifications. The plant extracts were diluted into 5 serial dilutions such as 1, 0.5, 0.25, 0.125 and 0.0625 mg/mL. An aliquot of 5 µL of each dilution (plant extract) was added to fresh sterile media (95 µL) followed by the addition of bacterial sus = pension (100 µL) into different wells of 96-well microplate. The microplate was placed in a shaking incubator at 37 °C for 24 h and then antibacterial activity was detected using a colorimetric method by the addition 10 µL of 0.5% aqueous solution of iodonitrotetrazolium chloride (INT) and placed further at 37 °C for 30 min. The MIC was defined as the lowest concentration of plant extracts that inhibited the growth of bacteria, indicated by a change in color of the INT treated media from purple to pale yellow. The experiment was conducted thrice.
3. Results
3.1. Collection and identification of Primula species
A total of seven Primula species were collected from the Western Himalayan region (Azad Kashmir) of Pakistan but only five species were recognized by the local informants that have ethnomedicinal uses. The scientific names, life-forms, local names, and detail of voucher specimens of those five species are listed in Table 2. The voucher specimens of all the identified species were deposited in the Herbarium of the University of Azad Jammu and Kashmir Muzaffarabad. The voucher specimen numbers are provided in Table 2.
Table 2.
List of the Primula plants used as ethnomedicinal and related frequency of citation, confirming the traditional use of these species in rural communities of Western Himalaya, Azad Kashmir, Pakistan.
| Botanical name | Local name | Voucher number | Part (s) used | Mode of preparation | Ethno-medicinal uses | Use report for species | Frequency of citation |
|---|---|---|---|---|---|---|---|
| Primula denticulata Sm. | Mameera | SK001 | Root, leaves, flower | The stem of a plant is used, leaves infusion, leaves, and roots are used to make tea, powder of the whole plant is mixed with Sinopodophyllum hexandrum (Royle) T.S. Ying and taken as orally |
Insomnia, Urinary infection, (UTI) Wound healings, Eye disorders Cough and cold, Dysuria, Red urination in animals, Bronchitis |
104 | 32 |
| Primula elliptica Royle | SK005 | Mucilage of root | Toothache, Sleeplessness, Eye ailments |
19 | 14 | ||
| Primula macrophylla D. Don | Chit patra, Khakhri Thandi jari |
SK003 | Flower, root, leaves |
The powdered form of leaf is mixed with kohl (eye cosmetic) | Increased bronchial secretion, making phlegm less thick, asthma, red urination in animals, and joint pain. Given to children in fever, diarrhea, and to increase eyesight |
93 | 27 |
| Primula rosea Royle | Meo | SK002 | Whole plant | Powdered root mixed with honey | Blood purifier, eye ailments, Pain of muscles Cramps |
53 | 27 |
| Primula stuartii Wall. | SK004 | Leaves | Leaf powder | Narcotic action, kidney pain |
13 | 6 |
3.2. Ethnobotanical knowledge
We identified five species of Primula from the study area that are being used as ethnomedicine for different disease categories. The number of informants (FC) that claim the use of particular species at all are shown in Table 2, verifying the traditional use of selected medicinal plants. According to FC, P. denticulata was used medicinally by 32 participants (80 %). P. rosea and P. macrophylla were mentioned by 27 participants each (67.5%). P. elliptica and P. stuartii were used by less than half of the informants as 14 (35%) and 6 (15%) participants, respectively.
By compiling the information about the particular use of each species, we have identified nine disease categories being treated with these plants. These disease categories are named as diseases of the digestive system, diseases of the respiratory system, diseases of the circulatory system, diseases of the kidney and urinary system, musculoskeletal, diseases of neurological (insomnia, headache), Injury, poisoning and certain other consequences of external causes, ophthalmic and general and unspecified (cold fever, toothache). For the determination of predominant species being used for the treatment of these disease categories, a use report for each species (URsp) was calculated as shown in Table 2. P. denticulata and P. macrophylla were the dominant species that exhibited a higher number of use reports such as 104 and 93, respectively. The high use reports reflect the frequent use of these plants for the treatment of a large variety of diseases and medical disorders. A reasonable number of use reports were also reported for P. rosea and P. elliptica i.e., 53 and 19, respectively. The lowest number of use reports were recorded for P. stuartti.
When we calculated the use reports per ailment (URail), the ailments related to eyes (ophthalmic) gained the highest use reports followed by insomnia (URail 24) as shown in Table 3. It is worth mentioning that ophthalmic, disease of the urinary system, and diseases of the respiratory system were the most cited disease categories among the participants. A consensus was made upon the use of each plant species (CUuse) for the treatment of a particular disease category and results are shown in Table 4. According to consensus, P. denticulata, P. rosea, and P. macrophylla were mainly used for the treatment of ophthalmic diseases. Among them, P. denticulata was the most cited remedy for ophthalmic diseases with the highest CUuse values of 70%. It was also mentioned as a potent remedy in diseases of the kidney and urinary system, injury, poisoning and certain other consequences of external causes, and disease of respiratory system with the CUuse values of 60, 42.5, and 37.5%, respectively.
Table 3.
Use reports for each ailment and disease categories. The disease categories were following International Classification of Primary Care-2 with some modifications (n = 40).
| Use-Report per ailment (URail) | ||
|---|---|---|
| Disease-Categories | General and unspecified (cold fever, toothache) | Toothache (5), Cough and cold (10), Fever in kids (9) |
| Diseases of the digestive system | Diarrhea (6) | |
| Diseases of the respiratory system | Making phlegm less thick (19), Asthma (14), Bronchial secretion (10) | |
| Diseases of the circulatory system | Blood purifier (9) | |
| Diseases of the urinary system | Urinary infections (23), Red urination in animals (21) | |
| Musculoskeletal | muscles cramps (17), Joint pain (7) | |
| Diseases of neurological | Insomnia (24), headache (6) | |
| Ophthalmic | To increase eyesight (27), Eye disorders (28), Eye infections (25) | |
| Injury, poisoning and certain other consequences of external causes | Wound healing and disinfectant (17) |
Table 4.
Use reports for uses (URuse) and consensus upon the uses of plants against each disease category (CUuse) according to informants (n = 40).
|
P. denticulata |
P. rosea |
P. macrophylla |
P. stuartii |
P. elliptica |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Disease category | URuse | CUuse | URuse | CUuse | URuse | CUuse | URuse | CUuse | URuse | CUuse |
| General and unspecified (cold fever. Toothache) | 10 | 25% | 9 | 22.5% | 2 | 5% | 5 | 12.5% | ||
| Diseases of the Digestive system | 6 | 15% | ||||||||
| Diseases of the Respiratory system | 15 | 37.5% | 14 | 35% | ||||||
| Diseases of the circulatory system | 9 | 22.5% | ||||||||
| Diseases of the urinary system | 24 | 60% | 21 | 52.5% | 5 | 12.5% | ||||
| Musculoskeletal | 17 | 42.5% | 7 | 17.5% | ||||||
| Diseases of neurological (insomnia, headache) | 10 | 25% | 6 | 15% | 14 | 35% | ||||
| Ophthalmic | 28 | 70% | 27 | 67.5% | 25 | 62.5% | ||||
| Injury, poisoning and certain other consequences of external causes | 17 | 42.5% | 1 | 2.5% | ||||||
Based upon the consensus of the informants, tea/decoction, powder and mucilage were the most popular methods of preparation (Table 5). Making tea/decoction is mainly used against the disease of the respiratory system with CUapp value of 30%. Powder is mainly used against diseases of the digestive system, musculoskeletal, and diseases of neurological (insomnia, headache) with CUapp value of 17.5% each. The treatment mucilage is commonly used against diseases of neurological (insomnia, headache), general and unspecified (cold fever and toothache) and Injury, poisoning and certain other consequences of external causes with CUapp values of 37.5, 32.5, and 30%, respectively. The detailed recipe for the preparation of treatment is provided in Table 6.
Table 5.
Consensus upon the use of a method of application (CUapp) and plant parts used (CUparts) against each disease category mentioned by informants (n = 40).
| Methods of Application |
Plant Parts Used |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease-Categories | Tea |
Powder |
Mucilage |
Roots |
Leaves |
Flowers |
||||||
| URapp | CUapp | URapp | CUapp | URapp | CUapp | URparts | CUparts | URparts | CUparts | URparts | CUparts | |
| General and unspecified (cold fever. toothache) | 3 | 7.5% | 7 | 17.5% | 13 | 32.5% | 3 | 7.5% | 1 | 2.5% | ||
| Diseases of the Digestive system | 1 | 2.5% | 7 | 17.5% | 2 | 5% | ||||||
| Diseases of the Respiratory system | 12 | 30% | 4 | 10% | 2 | 5% | 22 | 55% | ||||
| Diseases of the circulatory system | 7 | 17.5% | 2 | 5% | 9 | 22.5% | 4 | 10% | ||||
| Diseases of the urinary system | 3 | 7.5% | 2 | 5% | 10 | 25% | 3 | 7.5% | ||||
| Musculoskeletal | 5 | 12.5% | 7 | 17.5% | 1 | 2.5% | 10 | 25% | 5 | 12.5% | ||
| Diseases of neurological (insomnia, headache) | 1 | 2.5% | 7 | 17.5% | 15 | 37.5% | 19 | 47.5% | ||||
| Ophthalmic | 2 | 5% | 9 | 22.5% | 1 | 2.5% | 1 | 2.5% | ||||
| Injury, poisoning and certain other consequences of external causes | 4 | 10% | 1 | 2.5% | 12 | 30% | 11 | 27.5% | 5 | 12.5% | ||
Table 6.
The traditional mode of preparation for herbal remedies.
| Application | Therapeutic Procedure |
|---|---|
| Tea/infusion | Add fresh leaves, flower or powdered form into boiling water add sugar and drink as a tea |
| Powder | Make a paste by using the powder form of root, leaf or flower and honey and apply in eyes preventing infections |
| Mucilage | Make a paste in simple water or oil for wound healing |
A consensus was also developed upon the use of plant parts for the treatment of particular disease categories and results are shown in Table 5. According to consensus, roots, leaves, and flowers of the plants were being used for the treatment of different ailments. The roots were mainly used in the treatment of disease of the respiratory system, disease of neurological (insomnia and headache), musculoskeletal, disease of the urinary system, and disease of the circulatory system with CUparts values of 55, 47.5, 25, 25, and 22.5%, respectively. The leaves and flowers were largely used against injury, poisoning, and certain other consequences of external causes with CUparts values of 27.5, and 12.5%, respectively.
3.3. Antibacterial activity
The antibacterial activity was evaluated by disc diffusion assay supplemented with a more robust MIC assay. The five extracts such as aqueous, methanol, ethanol, acetone, and pet ether of roots, leaves, and flowers of five Primula species were evaluated against five bacteria, including both gram-negative and gram-positive bacterial strains (Table 7). According to MIC values, a varying antibacterial activity of different extracts of different parts of different plants was observed against all the tested bacteria. The MIC values of 0.0625 mg/mL were marked as noteworthy antibacterial activity in this study. The extracts showing MIC values of 0.125 mg/mL were also considered as effective antibacterial while the extracts having MIC values more than 0.5 mg/mL were considered as poor antibacterial. In the case of P. denticulata, the noteworthy antibacterial activity (MIC = 0.0625 mg/mL) was observed for methanol extract of leaves against B. cereus, an aqueous extract of flowers against E. coli, A. xyloxidans, B. cereus, and P. aeruginosa and methanol and ethanol extract of flowers against E. coli. The aqueous extract of flowers of P. denticulata was found active against all the tested bacteria.
Table 7.
Antimicrobial activity in the form of the zone of inhibition (ZOI) and minimum inhibitory concentration (MIC) against gram-positive and gram-negative bacterial strains.
| Solvent extracts |
B. cereus |
S. aureus |
A. xyloxidans |
E. coli |
P. aeruginosa |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZOI (mm) | MIC (mg/mL) | ZOI (mm) |
MIC (mg/mL) | ZOI (mm) | MIC (mg/mL) | ZOI (mm) |
MIC (mg/mL) | ZOI (mm) | MIC (mg/mL) | |||
| P. denticulata | Root | Aqueous | 8 | 0.25 | 7.3 | 0.25 | 7.3 | 0.25 | – | – | ||
| Methanol | 11.33 | 0.125 | 4.6 | 0.5 | 12.33 | 0.125 | 12.4 | 0.125 | 4.7 | 0.5 | ||
| Ethanol | 12 | 0.125 | 8.3 | 0.5 | 12 | 0.125 | 10 | 0.125 | 4.7 | 0.5 | ||
| Acetone | 8 | 0.25 | 10.66 | 0.25 | 10.66 | 0.125 | 11.66 | 0.125 | 5 | 0.25 | ||
| Pet ether | – | 4.6 | 0.5 | – | – | – | ||||||
| Leaves | Aqueous | – | – | – | 7.33 | 0.5 | – | |||||
| Methanol | 11 | 0.0625 | 8.7 | 0.25 | 12.3 | 0.125 | 13.3 | 0.125 | 12.3 | 0.25 | ||
| Ethanol | 9.66 | 0.25 | 7.3 | 0.25 | 10 | 0.125 | 11 | 0.125 | 7.33 | 0.5 | ||
| Acetone | 10 | 0.125 | 10.33 | 0.25 | 9.66 | 0.125 | 10.66 | 0.25 | – | |||
| Pet ether | 7 | 1 | 5 | 1 | 7.33 | 0.5 | 7.3 | 1 | – | |||
| Flowers | Aqueous | 12.7 | 0.0625 | 13.7 | 0.125 | 14.33 | 0.0625 | 14.7 | 0.0625 | 16.7 | 0.0625 | |
| Methanol | 11.7 | 0.125 | 9.3 | 0.25 | 11.7 | 0.125 | 12.7 | 0.0625 | 12 | 0.25 | ||
| Ethanol | 9 | 0.125 | 9.7 | 0.25 | 11.7 | 0.125 | 12 | 0.0625 | 10.7 | 0.125 | ||
| Acetone | 10 | 0.125 | 8.7 | 0.5 | 10.66 | 0.25 | 11 | 0.125 | 8.33 | 0.5 | ||
| Pet ether | 7.7 | 0.5 | 4.6 | 1 | 7.6 | 1 | 11.7 | 0.25 | – | |||
| P. elliptica | Root | Aqueous | – | – | – | – | – | |||||
| Methanol | 7.7 | 1 | 9 | 0.25 | 10.3 | 0.125 | 10.7 | 0.25 | 10.7 | 0.25 | ||
| Ethanol | 11.7 | 0.5 | – | 15 | 0.0625 | 9.6 | 0.25 | 13.3 | 0.125 | |||
| Acetone | 13.3 | 0.25 | 8 | 1 | 10.6 | 0.5 | 10.7 | 0.25 | 10.7 | 0.25 | ||
| Pet ether | – | – | – | – | – | |||||||
| Leaves | Aqueous | – | – | – | – | – | ||||||
| Methanol | 8 | 0.5 | 7.3 | 1 | 9 | 0.25 | 11 | 0.125 | 8.7 | 0.5 | ||
| Ethanol | 12.3 | 0.25 | 4.7 | 1 | 10 | 0.25 | 10.6 | 0.25 | 13 | 0.125 | ||
| Acetone | – | – | 13 | 0.125 | – | 13 | 0.125 | |||||
| Pet ether | – | – | – | – | – | |||||||
| Flowers | Aqueous | – | – | 11 | 0.25 | 10.7 | 0.5 | – | ||||
| Methanol | 7.6 | 0.5 | 8.3 | 0.5 | 11 | 0.25 | 11 | 0.25 | 9.7 | 0.5 | ||
| Ethanol | 4.3 | 1 | – | 12.3 | 0.125 | 9.7 | 0.25 | 11.7 | 0.25 | |||
| Acetone | 11.3 | 0.125 | 5.7 | 1 | 11 | 0.25 | 9 | 0.5 | 12.3 | 0.25 | ||
| Pet ether | 11.3 | 0.25 | – | 13.3 | 0.25 | – | 13.3 | 0.25 | ||||
| P. macrophylla | Root | Aqueous | 7 | 1 | 11.7 | 0.25 | 7.7 | 1 | 8 | 1 | 10 | 0.5 |
| Methanol | 7 | 0.5 | 7.7 | 0.5 | 10 | 0.25 | 2.7 | 1 | 7.3 | 0.5 | ||
| Ethanol | 11.3 | 0.25 | 11.3 | 0.25 | 10.3 | 0.25 | 11.6 | 0.125 | 7.7 | 0.5 | ||
| Acetone | 7 | 1 | 8.7 | 0.5 | 7.6 | 0.5 | 6.7 | 1 | 7.3 | 1 | ||
| Pet ether | 4.8 | 1 | – | – | – | 6.7 | 1 | |||||
| Leaves | Aqueous | 7 | 1 | 9 | 0.5 | 7 | 1 | 8.3 | 0.5 | 10 | 0.25 | |
| Methanol | 16.7 | 0.06225 | 12.3 | 0.25 | 13.3 | 0.125 | 8.3 | 0.5 | 8.7 | 0.5 | ||
| Ethanol | 12.3 | 0.125 | 11 | 0.5 | 7.7 | 0.5 | 11.7 | 0.25 | 11.7 | 0.25 | ||
| Acetone | 2 | 1 | 12 | 0.25 | 7 | 1 | 8.7 | 0.5 | 9.3 | 0.5 | ||
| Pet ether | 6.3 | 1 | 8.7 | 0.5 | 8.7 | 1 | – | 7.3 | 1 | |||
| Flowers | Aqueous | 9.7 | 0.5 | 7.3 | 1 | 6.7 | 1 | 8.7 | 1 | 10.3 | 0.25 | |
| Methanol | 13.3 | 0.125 | 12 | 0.1225 | 11.7 | 0.125 | 7.7 | 0.5 | 13.3 | 0.125 | ||
| Ethanol | 15 | 0.0625 | 13.6 | 0.25 | 10.3 | 0.25 | 12.7 | 0.25 | 8 | 0.5 | ||
| Acetone | 13.3 | 0.125 | 12.3 | 0.25 | 11 | 0.25 | 8.7 | 1 | 10.3 | 0.5 | ||
| Pet ether | 4.6 | 1 | 9 | 0.5 | 8.7 | 0.5 | – | 7.3 | 1 | |||
| P. rosea | Root | Aqueous | 11.3 | 0.25 | – | 8.7 | 0.25 | 10.7 | 0.5 | 12.3 | 0.25 | |
| Methanol | 13 | 0.125 | 12.3 | 0.25 | 9.3 | 0.25 | 11.7 | 0.25 | 14.3 | 0.125 | ||
| Ethanol | 15.7 | 0.0625 | 10.3 | 0.25 | 13 | 0.125 | 11.3 | 0.25 | 14.33 | 0.0625 | ||
| Acetone | 8.33 | 0.5 | 8 | 1 | 7.66 | 0.5 | 8 | 0.5 | 10 | 0.5 | ||
| Pet ether | 10 | 0.25 | – | 2.33 | 1 | 10 | 0.5 | 12 | 0.125 | |||
| Leaves | Aqueous | 9 | 0.5 | 7.3 | 1 | – | 8.6 | 1 | 11.3 | 0.25 | ||
| Methanol | 10.7 | 0.125 | 10.3 | 0.25 | 10.7 | 0.25 | 9.7 | 0.25 | 15.7 | 0.0625 | ||
| Ethanol | 13.3 | 0.125 | 10.3 | 0.5 | 12 | 0.125 | 12.7 | 0.25 | 14.3 | 0.125 | ||
| Acetone | 7.66 | 0.5 | 8 | 1 | 8.33 | 0.5 | 8 | 1 | 7.7 | 0.5 | ||
| Pet ether | 9.33 | 0.5 | – | – | 10.7 | 0.35 | 8.6 | 1 | ||||
| Flowers | Aqueous | 10 | 0.5 | 7 | 1 | – | 7 | 1 | 14 | 0.25 | ||
| Methanol | 13 | 0.125 | 11 | 0.25 | 11 | 0.25 | 15 | 0.125 | 16 | 0.125 | ||
| Ethanol | 11.3 | 0.25 | 10 | 0.25 | 12.33 | 0.125 | 12.3 | 0.25 | 16 | 0.25 | ||
| Acetone | 2.33 | 1 | 4.6 | 1 | 10.66 | 0.25 | 9.33 | 0.5 | 5.33 | 1 | ||
| Pet ether | 4.7 | 1 | – | – | 10.3 | 0.5 | 12.7 | 0.25 | ||||
| P. stuartii | Root | Aqueous | – | 7.3 | 0.5 | 2.7 | 1 | 10.3 | 0.5 | – | ||
| Methanol | 13.33 | 0.125 | 10 | 0.125 | 11.7 | 0.125 | 12.33 | 0.125 | 9 | 0.5 | ||
| Ethanol | 12.3 | 0.125 | 14.7 | 0.125 | 12.6 | 0.25 | 12.3 | 0.25 | 14 | 0.125 | ||
| Acetone | 9.7 | 0.5 | 11 | 0.5 | 10.3 | 0.25 | 11.7 | 0.25 | 10 | 0.25 | ||
| Pet ether | 10.33 | 0.25 | 7.8 | 1 | 5.7 | 0.5 | – | – | ||||
| Leaves | Aqueous | – | – | 7 | 1 | 7.3 | 1 | – | ||||
| Methanol | 13.7 | 0.125 | 12.3 | 0.25 | 9.7 | 0.125 | 13.6 | 0.125 | 10.3 | 0.25 | ||
| Ethanol | 13.3 | 0.125 | 18 | 0.0625 | 12 | 0.25 | 11 | 0.5 | 16.3 | 0.125 | ||
| Acetone | 11 | 0.25 | 12 | 0.125 | 8.7 | 0.5 | 10.7 | 0.25 | 10.7 | 0.5 | ||
| Pet ether | 7.7 | 1 | 7.33 | 0.5 | 8.7 | 1 | 7.3 | 1 | – | |||
| Flowers | Aqueous | – | 9.3 | 1 | – | 9 | 0.5 | 7.3 | 1 | |||
| Methanol | 12 | 0.25 | 11.3 | 0.25 | 9 | 0.25 | 14 | 0.125 | 12 | 0.125 | ||
| Ethanol | 14 | 0.125 | 10.3 | 0.25 | 17.3 | 0.0625 | 13.3 | 0.125 | 13 | 0.25 | ||
| Acetone | 11 | 0.25 | 11 | 0.25 | 11.6 | 0.25 | aq111 | 0.25 | 11.7 | 0.5 | ||
| Pet ether | 6 | 1 | 9.7 | 0.5 | 9 | 0.5 | 11 | 0.25 | – | |||
| Ampicillin | 13.3 | 12.7 | 14 | 14.3 | 13.7 | |||||||
For P. rosea, a noteworthy antibacterial activity (MIC = 0.0625 mg/mL) was recorded for ethanol extract of roots against B. cereus and P. aeruginosa and methanol extract of flowers against P. aeruginosa. When different extracts of roots, leaves, and flowers of P. macrophylla were tested, a noteworthy antibacterial activity was demonstrated only by ethanol extract of flowers (MIC = 0.0625 mg/mL) against B. cereus. Some of the other extracts also displayed effective antibacterial activity (MIC = 0.125 mg/mL) as shown in Table 6. For P. stuartii, the ethanol extract of leaves and flowers was found to have noteworthy antibacterial activity (MIC = 0.0625 mg/mL) against B. cereus and A. xyloxidans respectively. In the case of P. elliptica, a noteworthy antibacterial activity (MIC = 0.0625 mg/mL) was observed for ethanol extract of roots against A. xyloxidans.
4. Discussion
4.1. Ethnobotanical knowledge
The occurrence of wild medicinal plant knowledge, particularly in the high altitudinal areas may be associated with the cultural difference and topography of these areas. While the disappearance of indigenous knowledge from the urban areas has been associated with urbanization and industrialization (Łuczaj et al., 2012). The strong traditional knowledge in our study area may be linked to the following reasons. Firstly, the area is comprising of different ethnic groups having different descendent that possess diverse traditional knowledge. Secondly, the study area is one of the remote areas of Azad Kashmir with no infrastructure of roads, hospitals, and access to allopathic medicines is very limited, because people are living in far-flung rural areas. It was observed that local communities are well aware of the medicinal uses of the herbal plants, and they use herbal remedies to combat infectious and non-infectious diseases. In this study, we have collected seven species of Primula from the study area where they grow wild in nature. Only five species were identified by all the 40 informants, used as ethnomedicine. Two species were found unknown to all the informants and therefore not considered for further study. The remaining five species focused in this study have already been reported to be used as ethnomedicine in different parts of the world (Sher et al., 2020, Bano et al., 2014, Khan et al., 2013, Shaheen et al., 2012, Haq, 2012, Sekar and Rawat, 2011, Hassan and Mohammad, 2010, Najmus-Saqib et al., 2009, Kala, 2005, Chopra et al., 1986), however, we have reported some new uses of these species as well. The selection of these species as study objects was done before the survey and based on the formal/verbal interviews from the traditional healers of the study area. The genus Primula was chosen because it was cited as playing a very important role in local traditional medicines. The survey results confirmed the accuracy of pre-assessment that five species of Primula were found to have ethnomedicinal uses in the study area. Among the five species, the highest use reports were recorded for P. denticulata and P. macrophylla. The highest use reports were expected for P. denticulata, P. macrophylla, and P. rosea as these are frequently used in different parts of the world. However, an unexpected result was obtained in terms of use reports for P. stuartii because no particular medicinal use of this species was found in previous studies. This might be the first report of medicinal uses of P. stuartii.
A total of nine disease categories were found to treat with these five Primula species as shown in results (Table 3). Particularly, P. denticulata was the most cited remedy for ophthalmic diseases with the highest CUuse values of 70%. P. denticulata had already been documented as ethnomedicine against cough and cold (Kala, 2005), ophthalmic, wound healing and urinary infection, air tonic, red urination in animals, fever, dysentery, and hepatic fever (Bano et al., 2014, Khan et al., 2013, Haq, 2012). We first time reported the use of P. denticulata in insomnia. However, in literature, P. macrophylla is found to be used in insomnia (Najmus-Saqib et al., 2009, Chopra et al., 1986). This substitution might be due to different distribution of plants in a region with different climate and vegetation and local people can always choose a substitute plant to mitigate the problem of distribution. Because both these plants belong to the same genus and have high morphological similarity, they can easily be interchangeable. P. rosea was the second most cited species against ophthalmic disease with CUuse value of 67.5%. It was also mentioned as a potential remedy against musculoskeletal with CUuse value of 42.5%. The use of P. rosea in ophthalmic was also reported by Sher et al. (2020) along with some other uses such as wound healing, cough in the child, and high fever. The evidence of its use in musculoskeletal is also provided by Kala (2005). The other most mentioned species against the ophthalmic diseases was the P. macrophylla with CUuse value of 62.5%. P. macrophylla was also used frequently for the treatment of kidney and urinary diseases and diseases of the respiratory system with CUuse value of 52.5 and 35%, respectively. In literature, the medicinal uses of P. macrophylla are found against cough, joint pain, and asthma (Kala, 2005). There is a common consensus that exists upon its use in insomnia (Najmus-Saqib et al., 2009, Chopra et al., 1986) but in our study, none of the participants cited P. macrophylla against insomnia. Diseases of neurological (insomnia, headache) were treated with only three species such as P. elliptica P. denticulata and P. stuartii with CUuse values of 35, 25, and 15%, respectively. The role of P. elliptica and P. stuartii in treating insomnia is not reported previously, however, P. elliptica is documented to be used for pimples and to kill lice (Sher et al., 2020, Sekar and Rawat, 2011). The ethnomedicinal uses of P. stuartii are not mentioned previously, suggesting a novel species with therapeutic uses.
4.2. Antibacterial activity
In this study, an effort was also made to investigate the antibacterial activity of five ethnomedicinally used Primula species. The antibacterial results revealed the different levels of activities according to plant species, plant parts, and extraction solvents tested. For example, the aqueous extract of flowers of P. denticulata was found active against all the tested bacteria which support the traditional use of flowers of P. denticulata as herbal tea (Başbülbül et al., 2008). The antibacterial activity of this plant has already been reported. Shafi et al. (2016) reported a strong antibacterial activity of ethyl acetate extract against E. coli and Aslam et al. (2015) reported a strong antibacterial activity of ethanol extract of P. denticulata against both gram-positive and gram-negative pathogens.
For P. rosea, a noteworthy antibacterial activity (MIC = 0.0625 mg/mL) was recorded for ethanol extract of roots against B. cereus and P. aeruginosa and methanol extract of flowers against P. aeruginosa. The results are well supported by previous work that ethanol and methanol extracts exhibit more antibacterial activity (Majhenic et al., 2007). When different extracts of roots, leaves, and flowers of P. macrophylla were tested, a noteworthy antibacterial activity was demonstrated only by ethanol extract. Previously, Najmus-Saqib et al. (2009) carried out an antibacterial activity of different extracts of P. macrophylla and found no antibacterial activity. In contrast to this, we reported a considerable antibacterial activity of this plant. This difference may be due to the different protocols adopted during these two studies. Firstly, they have used only the ethanol extract, and secondly, they have used the whole plant. Moreover, the different environmental and experimental conditions can also affect the antibacterial activity of the plant extracts. No previous record of antibacterial activity of P. stuartii and P. elliptica was found and we are reporting the first time in this paper.
Overall, among all the tested bacterial strains, B. cereus, a gram-positive bacterium, was found the more sensitive bacterial strains. This result is in agreement with previous reports that gram-positive bacteria are more sensitive than gram-negative bacteria (Chariandy et al., 1999, Rabe and van Staden, 1997) because the outer membrane of gram-negative bacteria presents a barrier to various antibacterial molecules (Sleigh and Timbury, 1998). B. cereus is an infectious bacterium associated with food poisoning and eye infections (Bottone, 2010), supporting the use of Primula species in ophthalmic diseases as described in ethnobotanical knowledge of Primula.
5. Conclusions
This study documented the ethnomedicinal knowledge and antibacterial activity of five Primula species from western Himalayan regions (Azad Kashmir) of Pakistan. The selection of these species was made based on a prior survey of the area as well as ethnobotanical studies already conducted in the area. We observed that Primula plants are widely used among the local communities against various disorders and previously no study has reported their uses in our study area. The ethnomedicinal data confirmed the uses of Primula species in different disease categories, most significantly in ophthalmic disorders. The different extracts of plants exhibited effective antibacterial activity against both gram-positive and gram-negative bacteria with more evident activity against B. cereus (MIC 0.0625 mg/mL). This traditional medicinal knowledge can help in conservation and future drug discovery endeavors of five reported Primula species. Further biological assays and phytochemical screening of these plant will be performed for better scientific interpretation of traditional knowledge of them.
Ethics approval and consent to participate
All participants gave full oral consent for the study, including presentation of data in a formal publication. Plant specimens were collected for herbarium voucher deposit at Herbarium of Department of Botany, University of Azad Jammu and Kashmir Muzaffarabad following standard collection guidelines.
Author’s contribution
S. K: study design, field surveys and manuscript preparation. H. S: study design and supervision. A. M: data analysis, editing and revision. S. N: manuscript revision. T. K: editing and revision. All authors read and approved the final manuscript.
Funding
This study did not receive funding from any organization or institution
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are extremely thankful to all 40 informants who participated in the survey and provided information about the medicinal uses of Primula. Special thanks to the Microbiology Laboratory of Mayo Hospital for providing bacterial strains.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2022.01.048.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Ahmad K.S., Hamid A., Nawaz F., Hameed M., Ahmad F., Deng J., Akhtar N., Wazarat A., Mahroof S. Ethnopharmacological studies of indigenous plants in Kel village, Neelum Valley, Azad Kashmir, Pakistan. J. Ethnobiol. Ethnomed. 2017;13:68. doi: 10.1186/s13002-017-0196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, S.I., Nasir, Y.J., 1989–1992. Flora of Pakistan. Nos. 191-204. Islamabad, Karachi.
- Ali, S.I., Qaiser, M., 1993–2007. Flora of Pakistan. No. 191-215. Islamabad, Karachi.
- Asadollahi M., Firuzi O., Jamebozorgi F.H., Alizadeh M., Jassbi A.R. Ethnopharmacological studies, chemical composition, antibacterial and cytotoxic activities of essential oils of eleven Salvia in Iran. J. Herb. Med. 2019;17–18:100250. [Google Scholar]
- Aslam K., Nawchoo I.A., Ganai B.A. In vitro antioxidant, antibacterial activity and phytochemical studies of Primula denticulata an important medicinal plant of Kashmir Himalaya. Int. J. Pharmacol. Res. 2015;5(3):49–56. [Google Scholar]
- Ballabha B., Chaurasia O.P. Medicinal plants of cold desert Ladakh used in the treatment of stomach disorders. Indian J. Tradit. Knowl. 2009;8:185–190. [Google Scholar]
- Bano A., Ahmad M., Hadda T.B., Saboor A., Sultana S., Zafar M., Khan M.P.Z., Arshad M., Ashraf M.A. Quantitative ethnomedicinal study of plants used in the skardu valley at high altitude of Karakoram-Himalayan range, Pakistan. J. Ethnobiol. Ethnomed. 2014;10(1):43. doi: 10.1186/1746-4269-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour E.K., Al Sharif M., Sagherian V.K., Habre A.N., Talhouk R.S., Talhouk S.N. Screening of selected indigenous plants of Lebanon for antimicrobial activity. J. Ethnopharmacol. 2004;93(1):1–7. doi: 10.1016/j.jep.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Başbülbül G., Özmen A., Biyik H.H., Şen Ö. Antimitotic and antibacterial effects of the Primula veris L. flower extracts. Caryologia. 2008;61(1):88–91. [Google Scholar]
- Bottone E.J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010;23(2):382–398. doi: 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundtland G.H. Reducing risks to health, promoting healthy life. Jama. 2002;288(16) doi: 10.1001/jama.288.16.1974. 1974-1974. [DOI] [PubMed] [Google Scholar]
- Carabajal M.P.A., Perea M.C., Isla M.I., Zampini I.C. The use of Jarilla native plants in a Diaguita-Calchaquí indigenous community from northwestern Argentina: an ethnobotanical, phytochemical and biological approach. J. Ethnopharmacol. 2020;247 doi: 10.1016/j.jep.2019.112258. [DOI] [PubMed] [Google Scholar]
- Chariandy C.M., Seaforth C.E., Phelps R.H., Pollard G.V., Khambay B.P.S. Screening of medicinal plants from Trinidad and Tobago for antimicrobial and insecticidal properties. J. Ethnopharmacol. 1999;64(3):265–270. doi: 10.1016/s0378-8741(98)00130-5. [DOI] [PubMed] [Google Scholar]
- Chopra, R.N., Nayar, S.L., Chopra, I.C., 1986. Glossary of Indian Medicinal Plants. CSIR, Delhi.
- Dar E.I. Ethnobotanical uses of plants of Lawat district Muzaffarabad, Azad Jammu and Kashmir. Asian J. Plant Sci. 2003;2(9):680–682. [Google Scholar]
- Fico G., Rodondi G., Flamini G., Passarella D., Tomé F. Comparative phytochemical and morphological analyses of three Italian Primula species. Phytochemistry. 2007;68(12):1683–1691. doi: 10.1016/j.phytochem.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Ghalehnoo Z.R. Diseases caused by Staphylococcus aureus. Int. J. Med. Health Res. 2018;4(11):65–67. [Google Scholar]
- Ghufran M.A., Ghafar S.A., Qureshi R.A. Ethnobotanical study of the alpine subalpine flora of Neelum Valley, Azad Jammu and Kashmir. Pak. J. Sci. lnd. Res. 2007;50(4):278–283. [Google Scholar]
- Girish H.V., Satish S. Antibacterial activity of important medicinal plants on human pathogenic bacteria-a comparative analysis. World Appl. Sci. J. 2008;5:267–271. [Google Scholar]
- Hamayun M., Khan S.A., Kim H.Y., Leechae I.J. Traditional knowledge and ex-situ conservation of some threatened medicinal plants of Swat Kohistan. Pak. J. Bot. 2006;38:205–209. [Google Scholar]
- Haq F. The ethno botanical uses of medicinal plants of Allai Valley, Western Himalaya Pakistan. Int. J. Plant Res. 2012;2(1):21–34. [Google Scholar]
- Hassan S., Mohammad A. Economically and ecologically important plant communities in high altitude coniferous forest of Malam Jabba, Swat, Pakistan. Saudi J. Biol. Sci. 2010;18:53–61. doi: 10.1016/j.sjbs.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibraheem I.B.M., Abd-Elaziz B.E.E., Saad W.F., Fathy W.A. Green biosynthesis of silver nanoparticles using marine red algae Acanthophora specifera and its antimicrobial activity. J. Nanomed. Nanotechnol. 2016;7(409):1–4. [Google Scholar]
- Jamshidi-Kia F., Lorigooini Z., Amini-Khoei H. Medicinal plants: past history and future perspective. J. Herbmed Pharmacol. 2018;7(1):1–7. [Google Scholar]
- Johnson J., Johnston B., Clabots C., Kuskowski M., Castanheira M. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin. Infect. Dis. 2010;51(3):286–294. doi: 10.1086/653932. [DOI] [PubMed] [Google Scholar]
- Kala C.P. Indigenous uses, population density, and conservation of threatened medicinal plants in protected areas of the Indian Himalayas. Conservat. Biol. 2005;19(2):368–378. [Google Scholar]
- Khan M.P.Z., Ahmad M., Zafar M., Sultana S., Ali M.I., Sun H. Ethnomedicinal uses of edible wild fruits (EWFs) in Swat Valley, Northern Pakistan. J. Ethnopharmacol. 2015;173:191–203. doi: 10.1016/j.jep.2015.07.029. [DOI] [PubMed] [Google Scholar]
- Khan S.M., Page S., Ahmad H., Shaheen H., Ullah Z., Ahmad M., Harper D.M. Medicinal flora and ethnoecological knowledge in the Naran Valley, Western Himalaya, Pakistan. J. Ethnobiol. Ethnomed. 2013;9(1):4. doi: 10.1186/1746-4269-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigen G., Kamuren Z., Njiru E., Wanjohi B., Kipkore W. Ethnomedical survey of the plants used by traditional healers in Narok county, Kenya. Evid. Compl. Alternat. Med. 2019;2019:1–8. doi: 10.1155/2019/8976937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer, R., 2012. Assessment Report on Primula veris L. and/or Primula elatior (L.) Hill, Radix. European Medicines Agency.
- Lemonnier N., Zhou G.B., Prasher B., Mukerji M., Chen Z., Brahmachari S.K., Noble D., Auffray C., Sagner M. Traditional knowledge-based medicine: a review of history, principles, and relevance in the present context of P4 systems medicine. Prog. Prev. Med. 2017;2(7):e0011. [Google Scholar]
- Łuczaj Ł., Pieroni A., Tardío J., Pardo-de-Santayana M., Sõukand R., Svanberg I., Kalle R. Wild food plant use in 21st century Europe: the disappearance of old traditions and the search for new cuisines involving wild edibles. Acta Soc. Bot. Pol. 2012;81(4):359–370. [Google Scholar]
- Lyczak J.B., Cannon C.L., Pier G.B. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2(9):1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- Majhenic L., Kerget M.S., Knez Z. Antioxidant and antimicrobial of guarana seed extracts. Food Chem. 2007;104:1258–1268. [Google Scholar]
- Majid A., Hassan S., Hussain W., Khan A., Hassan A., Khan A., Khan T., Ahmad T., Ur-Rehman M. In vitro approaches of Primula vulgaris leaves and roots extraction against human pathogenic bacterial strains. World Appl. Sci. J. 2014;30(5):575–580. [Google Scholar]
- Malik Z.A., Bhat J.A., Ballabha R., Bussmann R.W., Bhatt A.B. Ethnomedicinal plants traditionally used in health care practices by inhabitants of western Himalaya. J. Ethnopharmacol. 2015;172:133–144. doi: 10.1016/j.jep.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Martin G.J. Routledge; 2004. Ethnobotany: A Methods Manual. People and Plants International Conservation; p. 296. [Google Scholar]
- Najmus-Saqib Q., Alam F., Ahmad M. Antimicrobial and cytotoxicity activities of the medicinal plant Primula macrophylla. J. Enzyme Inhib. Med. Chem. 2009;24(3):697–701. doi: 10.1080/14756360802333406. [DOI] [PubMed] [Google Scholar]
- Nasir, E., Ali, S.I., 1970–1979. Flora of Pakistan. Nos. 1-131. Islamabad, Karachi.
- Nitta T., Arai T., Takamatsu H., Inatomi Y., Murata H., Iinuma M., Tanaka T., Ito T., Asai F., Ibrahim I., Nakanishi T., Watabe K., Nakanishi T. Antibacterial activity of extracts prepared from tropical and subtropical plants on methicillin-resistant Staphylococcus aureus. J. Health Sci. 2002;48(3):273–276. [Google Scholar]
- Pieroni A., Nebel S., Quave C., Münz H., Heinrich M. Ethnopharmacology of liakra: traditional weedy vegetables of the Arbëreshë of the Vulture area in southern Italy. J. Ethnopharmacol. 2002;81(2):165–185. doi: 10.1016/s0378-8741(02)00052-1. [DOI] [PubMed] [Google Scholar]
- Prabhu N., Raj D.T., Yamuna G.K., Ayisha S.S., Puspha J., Innocent D. Synthesis of silver phyto nanoparticles and their antibacterial efficacy. Dig. J. Nanomater. Biostruct. 2010;5(1):185–189. [Google Scholar]
- Rabe T., van Staden J. Antibacterial activity of South African plants used for medicinal purposes. J. Ethnopharmacol. 1997;56(1):81–87. doi: 10.1016/s0378-8741(96)01515-2. [DOI] [PubMed] [Google Scholar]
- Richards J. Primula: Illustrated by B. Edwards. Portland: Timber Press; 2003. [Google Scholar]
- Schultz F., Anywar G., Wack B., Quave C.L., Garbe L.-A. Ethnobotanical study of selected medicinal plants traditionally used in the rural Greater Mpigi region of Uganda. J. Ethnopharmacol. 2020;256:112742. doi: 10.1016/j.jep.2020.112742. [DOI] [PubMed] [Google Scholar]
- Sekar K.C., Rawat B. Diversity, utilization and conservation of ethnomedicinal plants in Devikund - a high altitude, sacred wetland of Indian Himalaya. Med. Plants. 2011;3(2):105–112. [Google Scholar]
- Seshagirirao K., Harikrishnanaik L., Venumadhav K., Nanibabu B., Jamir K., Ratnamma B.K., Kunal D. Preparation of herbarium specimen for plant identification and voucher number. Roxburghia. 2016;6(1–4):111–119. [Google Scholar]
- Shafi S., Ishaq F., Kamli A.N., Ganie B.A. Evaluation of antimicrobial activity of Primula denticulata. Adv. Biomed. Pharm. 2016;3(5):270–278. [Google Scholar]
- Shaheen H., Shinwari Z.K., Qureshi R.A., Ullah Z. Indigenous plant resources and their utilization practices in village populations of Kashmir Himalayas. Pak. J. Bot. 2012;44(2):739–745. [Google Scholar]
- Sher H., Inamuddin I., Khan Z., Bussmann R.W., Rahman I.U. Medicinal plant diversity of Hindubaig Mountain, Lalku Valley, District Swat, Pakistan. Ethnobot. Res. Appl. 2020;20:1–13. [Google Scholar]
- Shinwari Z.K., Qaisar M. Efforts on conservation and sustainable use of medicinal plants of Pakistan. Pak. J. Bot. 2011;43:5–10. [Google Scholar]
- Sleigh J.D., Timbury M.C. fifth ed. Churchill; Livingstone, Edinburgh: 1998. Notes on Medical Bacteriology. [Google Scholar]
- Tabuti J.R.S., Dhillion S.S., Lye K.A. Firewood use in Bulamogi county, Uganda: species selection, harvesting and consumption patterns. Biomass Bioen. 2003;25(6):581–596. [Google Scholar]
- Tena D., Martínez N.M., Losa C., Solís S. Skin and soft tissue infection caused by Achromobacter xylosoxidans: report of 14 cases. Scandinavian J. Infect. Dis. 2014;46(2):130–135. doi: 10.3109/00365548.2013.857043. [DOI] [PubMed] [Google Scholar]
- Weckerle C.S., de Boer H.J., Puri R.K., van Andel T., Bussmann R.W., Leonti M. Recommended standards for conducting and reporting ethnopharmacological field studies. J. Ethnopharmacol. 2018;210:125–132. doi: 10.1016/j.jep.2017.08.018. [DOI] [PubMed] [Google Scholar]
- WHO, 2016. World Health Organization's global health workforce statistics, OECD, supplemented by country data. Available from: https://data.worldbank.org/indicator/SH.MED.PHYS.ZS. (accessed date: 25 January 2020).
- Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.