Abstract
Hyperuricemia is defined as a metabolic abnormality that occurs when serum uric acid (UA) level is abnormally high in the body. We previously reported that A. longiloba possesses various important phytochemicals and in vitro xanthine oxidase activity. Despite A. longiloba ethnomedicinal benefits, its toxicity and anti-hyperuricemic effects have not been reported. The present study was carried out to ensure the safety and investigate the anti-hyperuricemic effects of A. longiloba fruit and petiole ethanolic extracts on rats. In the acute toxicity study, extracts were orally administered at a dose of 2000 mg/kg bodyweight and closely monitored for 2-week for any toxicity effects. The rats were then sacrificed and samples were collected and analyzed for hematological, biochemical, and histopathological parameters. The anti-hyperuricemic effect of A. longiloba fruit or petiole extract was investigated through determination of UA levels on potassium oxonate (PO)-induced hyperuricemic rats. Extracts or standard drug treatments were orally administrated 1-h after PO administration for 14-day. Animals were euthanized and samples were collected for further experiments. The toxicity results show, no significant changes were observed in behavioral, bodyweight changes in experimental groups compared to the control. Moreover, there were no significant changes in hematological, biochemical, and histological parameters between extracts treated and control group. In the anti-hyperuricemia study, the fruit and petiole extracts treatments significantly reduced the level of UA in serum compared to the hyperuricemic model group. This study demonstrated that the extracts of A. longiloba have anti-hyperuricemic activity and was found to be non-toxic to rats in acute toxicity test.
Keywords: Alocasia longiloba, Keladi candik, Herbal medicine, Drug discovery, Uric acid, Hyperuricemia, Gout, Acute oral toxicity
1. Introduction
Over the last decades, hyperuricemia (HU) has become a serious global health issue due to its increased prevalence and the associated risk of several diseases including cardiovascular, inflammatory arthritis, renal, and heart disease (Huang et al., 2020, Leander et al., 2021, Russo et al., 2021). HU is an abnormal metabolic condition that results from higher levels of serum uric acid (UA) in the human blood exceeding 6.8 mg/dL (female) and 7.0 mg/dL (male) (Chen et al., 2016, Benn et al., 2018, Barkas et al., 2018, Seong et al., 2021). The global prevalence of HU has increased significantly over the years with the highest prevalence were reported in the United States of America (>21%), followed by Korea (>10%), Taiwan (10%), and in Malaysia (5%) (Liu et al., 2015, Hafez et al., 2017, Ali et al., 2018, Gaur et al., 2018) and is most prevalent among men (Richette et al., 2014, Shahin et al., 2021). UA is the waste material of purine metabolism in humans and is produced by the oxidation of xanthine oxidase (XOD). The source of UA has been identified by several scientists (Miao et al., 2009, Ali et al., 2018, Lee et al., 2020). Approximately one-third of serum UA is endogenously-produced and the rest is produced during purines metabolism (Cornelis et al., 2011, Mallat et al., 2016, Srivastava et al., 2018). In humans, about 70% of urate is removed by the kidney and the remainder by the gastrointestinal tract (Tan et al., 2016). While, in rodents (rats and mice), the uricase enzyme further converts UA into allantoin, which is more efficiently excreted by the kidney and eliminated from the body through urine (Desideri et al., 2014, Chen et al., 2016, Braga et al., 2020, An et al., 2021). However, human often lacks the uricase enzyme and therefore abnormal UA may be accumulated in the blood (Riches et al., 2009, Richette and Bardin, 2010, Winder et al., 2021).
The main pharmacological agents used for the treatment of HU is mainly by using UA synthesis inhibitor and excretion promoter drugs, such as allopurinol, colchicine, and NSAIDs (Kostić et al., 2015, Rahmi et al., 2020). XOD inhibitors reduce UA concentrations by inhibiting UA synthesis. However, drugs used to treat HU have shown adverse effects include skin rash, diarrhea, and liver damage (Hafez et al., 2017, Srivastava et al., 2018). Therefore, natural medicine that helps reduction of UA concentration in the human body, become top global priority and a hotspot of metabolic disorder research.
Medicinal plants or herbs have long been utilized in folk medicines to treat several ailments in several parts of the world (Okoye et al., 2014, Oladeji, 2016, Ferid et al., 2020, Nur-Izzati et al., 2021). Ifeoma and Oluwakanyinsol (2013), reported that approximately one-fourth of pharmaceutically prescribed in the world contains at least one plant-based constituent. It is well known that medicinal plants are rich sources of various secondary metabolites (SM’s) which are responsible for their biological effects in mammals (Sarkar et al., 2021). SM’s also called phytochemicals include phenolic, flavonoids, tannins, and others. These important natural chemicals have been widely reported to have biological effects that are important for drug manufacturing (Kabir et al., 2016, Maher et al., 2021a, Maher et al., 2021b, Hazra et al., 2020, Bharadwaj et al., 2021a, Bharadwaj et al., 2021b, Bharadwaj et al., 2021c). In recent times, treating disease using plant-derived products has gained significant attention worldwide due to several reasons, including efficacy, availability, affordability, considered fewer/no side effects (Mintah et al., 2019, Abdulhafiz et al., 2020a, Mohammadi et al., 2020, Mohammadi et al., 2022, Roumeliotis et al., 2021).
Although therapy involves herbal medicines have shown promising results in treating several diseases, there is limited information concerning the potential toxicology effects that plant may cause to the users (Ekor, 2014). Medicinal plants can produce toxic and poisonous substances that may affect the entire human organ system, with some substances produced from plants could be deadly too (Reduan et al., 2020a). Hence, toxicity study of plants is crucial to examine the risks that may be related to the use of plants, plants parts, or plant-derived products, therefore it can help to avoid the possible side effects when used as medication.
A. longiloba is a medicinal herb and is locally known as “Keladi candik” or “Keladi Rimau” in Kelantan, Malaysia (Yusoff et al., 2013, Ridley and Curtis, 1902, Nur-Hadirah et al., 2021, Zulhazman et al., 2021). A. longiloba species is widely distributed in tropical Asia countries such as Cambodia, Laos, Vietnam, South West China, India, Peninsular Malaysia, Thailand, and Singapore. Mostly found in the rainforest and grow understory, in swampy areas and well-drained slopes, occasionally on rocks at the altitude of 0–500 m above sea level (Nur-Alya et al., 2021). In Malaysia, the plant has been used traditionally (mostly fruit and petiole) to treat wounds and inflammation (Hamzah et al., 2019). It has also been used for cough and high-fever treatment in India (Das, 2018). Our previous studies show that A. longiloba has in vitro XOD inhibitory, high antioxidant and wound healing activities (Hamzah et al., 2019, Abdulhafiz et al., 2020a, Abdulhafiz et al., 2020b). Hence, the present study was carried out to ensure the safety and investigate the anti-hyperuricemic effects of A. longiloba EtOH fruit and petiole extracts in rats.
2. Materials and methods
2.1. Chemicals and apparatus
Ethanol 95% and Dimethyl sulfoxide (DMSO) 99% was procured from R&M chemicals Co. Ltd (United Kingdom). Formaldehyde (37% formalin), Blood Collection Tubes (EDTA tubes), normal tubes, syringe, needles, stainless steel oral gavage needle, plastic cassettes, paraffin wax, haematoxylin, eosin, and microscopic slides were procured from a local vendor. Potassium oxonate (PO) and UA assay kit (ab65344) (Abcam, Cambridge, United Kingdom). All the chemical and reagents used in the present studies were analytical grade. For blood analysis, RANDOX Blood Analyzer (Crumlin, United Kingdom) was used. A compound microscope (Olympus CX-21, Tokyo, Japan) was used for the histological study for the microscopic observations during histological study. Thin section preparations were done using a rotary microtome (Leica RM2245, Buffalo Grove, United States).
2.2. Collection of plant and extract preparation
The fruit and petiole of A. longiloba were collected from Sering, Kelantan, Malaysia (6.128920° N, 102.323295° E) between January and February 2020. The plant samples were authenticated by Dr. Zulhazman Hamzah at Universiti Malaysia Kelantan, Campus Jeli. The authenticity of the plant was also verified with previously published articles (Hamzah et al., 2019; Nur-Alya et al., 2021). The fruit and petiole parts were detached from the mother plant, followed by washing with running water. The samples were then dried on oven-drier at the temperature of 40 °C for a 72-h. Subsequently, the dried sample was pounded into a fine powder and kept at 8 °C for further use. The powder was then extracted with EtOH (95%) using Soxhlet extraction apparatus (1:10 w/v) for 6-round and then the solvent was separated from the crude compound using a vacuum evaporator. The extracts were then kept in the fume hood to allow the remaining solvents to evaporate for 24 h and then the extracts paste were kept at 8 °C until further experiment.
2.3. Animal ethics and preparation
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC), Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Kota Bahru, Malaysia (Approval number: UMK/FPV/ACUC/PG/1/2020). Healthy Sprague Dawley (SD) rats were purchased from ARASC. In toxicity assessment, a total of 15, 6-week-old SD male rats (180–200 g) were used and the rats were randomly selected, labelled, and kept in the polycarbonate rat cage (410 × 282 × 153 mm) for 1 week prior to dose administration to get adapted to the laboratory conditions. The rats were kept in an animal rooms (Department of Paraclinical Study, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan) with an air-conditioned environment at 22–25 °C in 12/12 h light/dark condition and humidity at the range of 60–65%. The animal cage and water bottles were cleaned every 3 days. In the anti-hyperuricemia study, a total of 35, 6-week-old SD male rats (180–200 g) were used.
2.4. Acute oral toxicity assessment
2.4.1. Animal group and dosage preparation
Toxicity assessment was carried out in accordance with the guideline of the Organization for Economic Co-operation and Development (O.E.C.D). The rats were randomly grouped into three groups (n = 5); group A, B, and C. Group A were treated with deionized water (control), while group B and C were given a single dose of 2000 mg/kg body weight of fruit and petiole extracts orally, respectively. The rats were fasted for 3-h prior to plant extracts treatment following the protocol of Aliyu et al. (2020a). Thereafter, all animals were maintained on standard animal feed (rat chow) and sterile water ad libitum until the end-point of the experiment
2.4.2. General assessments and bodyweight
After administration of A. longiloba extracts, the rats were closely monitored for the first 4 h and then every day for 2 weeks. The observed parameters include behavioral signs (restlessness, dullness, and distress), respiratory pattern, eyes, skin, diarrhea, mortality, and any other sign of toxicity. The food and water intake of the rats were checked during the observational period. The body weight of all animals was measured weekly for 2 weeks using digital scaling.
2.4.3. Euthanasia and necropsy
All rats were fasted for 15-h prior to post-mortem examination. The rats were euthanized humanly followed by cervical dislocation according to Aliyu et al. (2020a). All rats were subjected to gross necropsy and organs are taken out for macroscopic examination. Absolute and relative organ weights were measured. The relative organ weights were calculated as the weight of each organ (absolute organ weight) divided by the bodyweight of the corresponding rat in each group. The collected organs were then kept in formalin solution until further use.
2.4.4. Haematological analysis
The blood samples from animals were collected from the inferior vena cava into clean ethylenediaminetetraacetic acid (EDTA) bottles from each necropsied for hematological investigation. Blood samples were also kept in plain tubes for serum chemistry. The parameters investigated were as follows; total red blood cells (RBC), hemoglobin (Hb) concentration, hematocrit (HCT), mean cell hemoglobin (MCH), mean corpuscular hemoglobin concentrations (MCHC), total white blood cells (WBC), red cell distribution width (RDW), granulocytes (GRA), monocytes (MON), lymphocytes (LYM), platelet (PLT), plateletcrit (PCT), platelet distribution width (PDW), and mean platelet volume (MPV) count. The analyses were carried out using an automated hematology analyzer (RANDOX Blood Analyzer, Crumlin, United Kingdom).
2.4.5. Serum biochemistry
The blood samples collected in tubes were allowed to clot for about 45 min and then spun at 2500 rpm for 15 min to get the serum. The serum obtained was kept at −20 °C until further analysis. The serum was then further analyzed for blood urea nitrogen (BUN), creatinine (CR), creatine kinase (CK), total protein (TP), albumin (ALB), globulin (GB), and alanine aminotransferase (ALT) using an automatic biochemical analyzer (RANDOX, United Kingdom).
2.4.6. Histopathological examination
Histopathological study was conducted on organ samples of the kidneys and liver. The organs were harvested and fixed in 10% buffered formalin for 48-h (Kaid et al., 2019). The formalin-fixed tissues were then sliced to 0.6 cm and arranged in the cassette. The tissues were then further processed using automatic “Dip and dunk” tissue processors (Leica TP1020, Buffalo Grove, United States) for 15 h, followed by paraffin embedding, trimming and sectioning. A 4 µm thickness tissue section was obtained on a rotary microtome (Leica RM2245, Buffalo Grove, United States). The tissue sections were then mounted on a microscope slide and placed on an oven at 42 °C and allowed to dry overnight. Consequently, the material was stained with hematoxylin and eosin stain to detect any abnormality lesions formed by the administration of plant extracts. The histological examination of the samples was conducted using a compound light microscope (Olympus CX-21, Tokyo, Japan). Lesions in liver and kidneys were scored as 0 (no lesion detected), 1 (mild = <10%), 1.5 (mid-moderate = 10–30%), 2 (moderate = 30–50%), 2.5 (moderate-severe = 50–70%) and 3 (severe = more than 70%) following the protocol of Nurul et al. (2018).
2.5. Anti-hyperuricemia activity of A. Longiloba in the rat model
2.5.1. Hyperuricemia development and treatments
For HU development in rats, the uricase inhibitor drug (PO) was administered following Tang et al. (2017), with some modifications. The rats were grouped into seven groups (n = 5). The control (healthy group) was given 0.5% carboxymethyl cellulose sodium solution (0.5% CMC-Na) orally. Treatment groups II-VII were intraperitoneally injected with PO (250 mg/kg b.wt), 1 h before plant extract administration. Group II served as the HU model group. Group III-VII were treated with allopurinol or plant extracts orally. Thereafter, all rats were accessed water and food ad libitum throughout the study. All the PO, 0.5% CMC-Na, and plant extracts were prepared freshly and given for 14 consecutive days. All the PO, 0.5% CMC-Na, and plant extracts were prepared freshly.
2.5.2. Sample collection and uric acid assay
All rats were fasted for 15-h prior to post-mortem examination. The rats were euthanized humanly followed by cervical dislocation. The blood samples were collected from the posterior vena cava into clean ethylenediaminetetraacetic acid (EDTA) bottles from each necropsied rats. The blood sample was allowed to clot approximately 1 h at room temperature and then centrifuged at 10,000×g for 15 min to obtain the serum. The serum sample was kept at − 20 °C until used. Serum UA level was measured by an enzymatic colorimetric method using a UA assay (ab65344). The procedures were per the kit manufacturer’s instructions.
2.6. Statistical analysis
The data were analyzed by one-way Analysis of Variance (ANOVA) followed by Duncan multiple range test (DMRT) using SPSS v. 21.0 for windows. All data were presented as mean ± standard error. The statistical significance level was established at p < 0.05.
3. Results
3.1. Acute oral toxicity effects of A. Longiloba extracts
3.1.1. Effects of the ethanolic fruit and petiole extracts on the behavioral pattern of rats
In the current study, a single dose of EtOH fruit or petiole extracts (2000 mg/kg) of A. longiloba was administered orally to male Sprague-Dawley rats to evaluate toxicity effects. During the experimental period, behavioral or physical condition changes were not observed in the extract-treated groups. In addition, no significant changes were observed in food and water consumption compared with the control group.
3.1.2. Effects of ethanolic fruit and petiole extracts on body weight of rats
The effect of oral administration of EtOH fruit or petiole extracts on the average body weight gain over the study period is presented in Fig. 1. The results show no significant differences between the bodyweight of the plant extracts treated group compared to that of the control.
Fig. 1.
Acute oral toxicity effects of ethanolic fruit and petiole extracts of A. longiloba on body weight after 14-day observation. Group (a) control (treated distilled water), (b) treated fruit extract at the dose of 2000 mg/kg and (c) treated petiole extract at 2000 mg/kg.
3.1.3. Effects of ethanolic fruit and petiole extracts on organ weight of rats
The effects of EtOH fruit and petiole extracts on the absolute and relative organs weight of male Sprague-Dawley rats are displayed in Table 1. The absolute weights of all organs of the plant extracts test groups were within the normal ranges and no statistically significant alterations were shown between the selected organs weights of treated groups and the control group. Likewise, the relative organ weight of all selected organs of the test group was not statistically different compared to the control.
Table 1.
The effect of A. longiloba ethanolic extracts on the organ weights of SD rats after 14-day.
| Organs | Study Groups |
||
|---|---|---|---|
| A | B | C | |
| Absolute organ weight (g) | |||
| Liver | 10.24 ± 0.31a | 9.74 ± 0.55a | 10.06 ± 0.38a |
| Heart | 1.06 ± 0.08a | 0.98 ± 0.04a | 0.94 ± 0.05a |
| Lungs | 2.72 ± 0.16a | 2.34 ± 0.10a | 2.04 ± 0.19a |
| Right kidney | 1.24 ± 0.08a | 1.12 ± 0.03a | 1.16 ± 0.04a |
| Left kidney | 1.26 ± 0.11a | 1.12 ± 0.03a | 1.10 ± 0.04a |
| Spleen | 0.98 ± 0.05a | 0.84 ± 0.06a | 0.94 ± 0.06a |
| Relative organ weight (g) | |||
| Liver | 0.038 ± 0.001a | 0.035 ± 0.004a | 0.030 ± 0.001a |
| Heart | 0.004 ± 0.00a | 0.0035 ± 0.00a | 0.0033 ± 0.00a |
| Lungs | 0.010 ± 0.000a | 0.008 ± 0.000a | 0.007 ± 0.000a |
| Right kidney | 0.0046 ± 0.00a | 0.0041 ± 0.00a | 0.0041 ± 0.00a |
| Left kidney | 0.0047 ± 0.00a | 0.0041 ± 0.00a | 0.0039 ± 0.00a |
| Spleen | 0.003 ± 0.000a | 0.003 ± 0.000a | 0.003 ± 0.000a |
Same letters within the same row is not significantly different. Data are expressed as mean ± SEM. group A (control group), group B (fruit extract) and group C (petiole extract).
3.1.4. Effects of the ethanolic fruit and petiole extracts on hematological parameters
The hematological analysis of fruit and petiole extracts treated rats show no significant alterations in any hematological parameters compared to control (Table 2). A little increase in WBC count and platelets were observed in test groups (B and C), which are no-significant compared to the control group. The mean platelet volume (MPV) in group B showed significance difference when compared with the value of 5.96 ± 0.11. However, the rest of the parameters exhibited negligible variations mostly not significant.
Table 2.
Effects of the fruit and petiole extracts of A. longiloba on hematological parameters in SD rats after 14 days observation.
| Parameter | Study Groups |
||
|---|---|---|---|
| A | B | C | |
| WBC (×103/µl) | 5.62 ± 1.56a | 7.26 ± 1.93a | 6.82 ± 1.19a |
| MON (×109/L) | 0.52 ± 0.13a | 0.56 ± 0.17a | 0.56 ± 0.11a |
| GRA (×103/µl) | 0.36 ± 0.10a | 0.44 ± 0.12a | 0.38 ± 0.06a |
| LYM (×109/L) | 4.72 ± 1.35a | 6.26 ± 1.65a | 5.86 ± 1.03a |
| LYM (%) | 82.24 ± 2.34a | 85.96 ± 0.98a | 86.26 ± 0.21a |
| MON (%) | 10.10 ± 1.39a | 7.92 ± 0.71a | 8.30 ± 0.53a |
| GRA (%) | 7.66 ± 1.08a | 6.12 ± 0.31a | 5.44 ± 0.41a |
| RBC (×106/µl) | 5.18 ± 0.18a | 5.43 ± 0.28a | 5.10 ± 0.36a |
| Hb (g/dL) | 15.08 ± 0.36a | 14.52 ± 0.71a | 14.72 ± 1.01a |
| HCT (%) | 30.98 ± 0.84a | 33.00 ± 1.56a | 30.56 ± 2.16a |
| MCV (fL) | 59.90 ± 0.71a | 60.80 ± 0.47a | 59.84 ± 0.29a |
| MCH (pg) | 29.16 ± 0.47a | 27.18 ± 2.28a | 28.84 ± 0.24a |
| MCHC (g/dL) | 48.74 ± 0.85a | 44.68 ± 3.59a | 48.20 ± 0.26a |
| RDW (%) | 13.58 ± 0.38a | 13.74 ± 0.29a | 13.60 ± 0.24a |
| PLT (×109/L) | 530.6 ± 140.7a | 320.4 ± 44.4a | 478.4 ± 98.9a |
| PDW (fL) | 13.64 ± 1.17a | 13.22 ± 0.68a | 13.34 ± 1.40a |
| PCT | 0.32 ± 0.08a | 0.20 ± 0.02a | 0.28 ± 0.05a |
| MPV (fL) | 5.96 ± 0.11a | 6.32 ± 0.12b | 5.98 ± 0.09ab |
Data are presented as mean ± SEM, as compared to group A (control group), group B (fruit extract) and group C (petiole extract). Same letters within the same row is not significantly different.
3.1.5. Effects of the ethanolic fruit and petiole extracts on biochemical parameters
The results showed no significant alteration (p < 0.05) in the extract-treated groups compared with the control. Nevertheless, there was a significant rise in creatine kinase in group B (fruit extract treated rats) compared with control. The rest of the liver biochemical parameters displayed slight insignificant changes in the extracts test groups compared to control, showing the extracts has no significant toxicity effects on biochemical parameters (Table 3).
Table 3.
Effects of the fruit and petiole extracts of A. longiloba on serum chemistry in SD rats after 14 days.
| Biochemical Parameters | Study Groups |
||
|---|---|---|---|
| Group A | Group B | Group C | |
| TP (g/dL) | 7.34 ± 0.07a | 7.44 ± 0.05a | 7.24 ± 0.17a |
| ALB (g/L) | 3.31 ± 0.03a | 3.36 ± 0.05a | 3.33 ± 0.05a |
| Globulins (g/L) | 4.03 ± 0.04a | 4.07 ± 0.09a | 3.90 ± 0.14a |
| ALT (U/L) | 66.82 ± 4.21a | 68.54 ± 2.50a | 79.9 ± 10.0a |
| BUN (mmol/L) | 4.64 ± 0.24a | 4.80 ± 0.29a | 4.92 ± 0.22a |
| Creatinine (μmol/L) | 1.03 ± 0.09 a | 1.14 ± 0.13a | 1.14 ± 0.08a |
| CK (U/L) | 12.00 ± 0.20a | 13.14 ± 0.16b | 11.56 ± 0.24a |
Data are presented as mean ± SEM, as compared to group A (control group), group B (fruit extract) and group C (petiole extract). Same letter within the same row is not significantly different.
3.1.6. Effects of the ethanolic fruit and petiole extracts on histology of rats
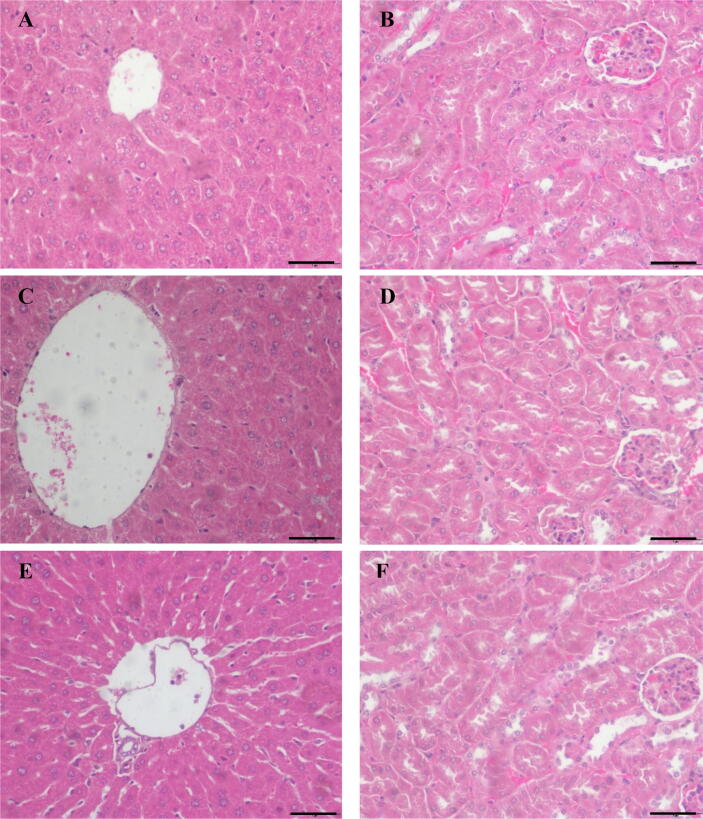
In histopathological studies, there are no major changes in the kidney and liver were found. No liver and renal lesions were observed (Fig. 2). This indicates that the extracts have no significant toxic effects on those organs. The scoring result also shows no significant difference (data not presented).
Fig. 2.
The light microscopic observations of liver and kidney tissues in control and treated groups in acute toxicity study of ethanol extracts of A. longiloba fruit and petiole sacrificed at the end of 14-day study period (a) The liver section of a control group (b) kidney section of a control group (c) Liver section of a fruit extract treated group (Note: no significance histopathological changes were observed) (d) The kidney section of a fruit extract treated groups (no significant histopathological alterations) (e) The liver section of a petiole extract treated group with no significance histopathological changes were observed. (f) The kidney section of a petiole extract-treated group (no significant histopathological lesions observed). (H & E, observed under 40x magnification, Bar = 50 µm).
3.2. Effect of A. Longiloba extracts on serum UA levels of PO-induced rats
The A. longiloba extracts at 250 mg/kg and 500 mg/kg were treated for 14 days on PO-induced hyperuricemia rats and serum UA levels were measured. As presented in Table 4, in comparison to the UA levels of the normal rats (1.13 mg/dL), the uric acid level of the hyperuricemic rats (1.57 ± 0.015 mg/dL) was significantly (p < 0.05) was significantly elevated by the injection of PO (p < 0.05). In contrast, administration of the fruit and petiole extracts treatment at doses of 250 mg/kg and 500 mg/kg b.wt significantly lowered the level of uric acid of hyperuricemic rats to normal ranges. Allopurinol drug used as a standard treatment at the dose of 5 mg/kg b.wt also caused a significant reduction in the serum uric acid level to 1.08 mg/dL (p < 0.05). Interestingly, both plant extracts had an almost similar uric acid-reductive effect to that of allopurinol and no significant differences were found between the effects of allopurinol and plant extracts treatment.
Table 4.
Effects of A. longiloba extracts on serum UA levels in PO-induced hyperuricemic rats.
| Treatment | Doses (mg/kg b.wt) | Serum uric acid levels (mg/dL) | Inhibition(%) |
|---|---|---|---|
| Normal | 1.13 ± 0.014 | ||
| Hyperuricemia model (control) | 1.57 ± 0.015# | ||
| Fruit | 250 | 1.16 ± 0.040* | 26.27 |
| 500 | 1.15 ± 0.015* | 27.00 | |
| Petiole | 250 | 1.14 ± 0.001* | 27.33 |
| 500 | 1.10 ± 0.040* | 28.8 | |
| Allopurinol (Positive control) | 5 | 1.08 ± 0.041* | 31.11 |
Values are displayed as mean ± SEM *p < 0.05 vs. hyperuricemic rats (control); # p < 0.05 vs. normal rats. Five animals used per group and all except normal rats received potassium oxonate injection (250 mg/kg b.wt, body weight) for inducing hyperuricemia.
4. Discussion
Global health is facing many challenges caused by various non-communicable diseases, including metabolic disorders (hyperuricemia, gout, and diabetes), cancer, and chronic heart disease (Excler et al., 2021). They are responsible for more than 70% of all deaths globally, and the increase of these diseases has been reported over the last few years (Excler et al., 2021, Habib and Saha, 2010, Lozano et al., 2012). HU is a metabolic disorder and is becoming a serious public-health challenge worldwide (Cao et al., 2017, Zhang et al., 2018). Previous studies have reported that HU is a risk factor for several diseases such as arthritis, diabetes and, stroke (Wahyuningsih and Sukandar, 2016; Li et al., 2017, Yu et al., 2018, Rahmi et al., 2020). These often lead to increased economic burden and disability especially in developed countries. Besides, the toxicity effect of available treatment has made the situation more difficult. In recent years, several attempts have been made to discover new therapeutics to overcome this problem using plant-based products/compounds isolated from medicinal plants. Most recent studies have shown that the use of herbal therapy or plant-based drugs for the treatment of several diseases is increasing (Rahmi et al., 2020; Chen et al., 2020). It is estimated that there are 450,000 plant species in the world, and more than 50,000 plant species are being used for their therapeutics values (Dal Toso and Melandri, 2011). Of which about 95% of the medicinal plants are collected from wild resources. Furthermore, up to 80% of the global population depends on medicinal plants or plant-based products for maintaining their primary healthcare and over 25% of the drugs currently produced in the world markets are derived from wild plant species.
Before administering any substance or plant-based medicine to laboratory animals, its toxicity effects should examined to make sure the substance does not have toxicity effect on animals. Toxicity study examine the risks that maybe related to the use of the products, therefore it can help to avoid the possible side effects when used as medication (Saiyed et al., 2015, Aamir et al., 2019 Aliyu et al., 2020b). In the present study, the acute toxicity effects of ethanolic fruit and petiole extracts were assessed. The aim of conducting this experiment was to determine whether the extracts are safe to move forward to perform in vivo studies on animal model. All experiments were carried out in accordance with relevant guidelines and regulations.
A study on the effect of A. longiloba extracts on the behavioral pattern and physical appearance shows that both fruit and petiole extracts did not cause any changes in the behavioral pattern and physical appearance of the animals at 2000 mg/kg b.wt. Our findings are in line with Islam et al. (2013), who previously conducted the acute oral toxicity test on different species in this genus (Alocasia indica Schott). Their report shows a non-toxcity effects of Alocasia indica Schott at different doses tested on Swiss albino mice. Another study by Aliyu et al. (2020a) shows a non-significant effect of EtOH leaf extract of Clinacanthus nutans on behavioral activities of treated animals with 2000 mg/kg body weight of the animal. In the present, the results demonstrated that the fruit and petiole extracts did not cause any changes in behavioral and physical parameters.
All animals gained normal body weight without a noticeable increase. The feed and water consumption patterns of the rats were normal and consistent throughout the study period. The weekly bodyweight of experimental animals did not display any significant changes after administration of the EtOH fruit and petiole extracts compared to control. A minor increase in bodyweight was witnessed in all experimental groups and this effect increased continuously over time in all groups. Nevertheless, insignificant body weight change effects usually occur at the endpoint of toxicity study as the age and feed intake is increase and considered as a normal physiological condition. A decrease in body weight after administration of plant extracts may result in suppression of animal's appetite for food (Raza et al., 2002, Nurul et al., 2018 Reduan et al., 2020b; Mohd et al., 2021). Throughout this study, regardless of the type of plant part used (fruit and petiole), A. longiloba extracts had no significant effect on body weight.
Analysis of organ weight in toxicology assessment is an essential measurement to evaluate the animal’s health condition. Whereas, relative organ weight is vital to detect organs injuries. The kidney, liver, heart, spleen and lung are the organs most affected by the metabolic reactions instigated by toxic agents (Mohamed et al., 2011, Rajeh et al., 2012). In the current study, the weights of selected organs (liver, kidney, lung, spleen and heart) between the test and the control were not statistically different (p < 0.05), indicating that both fruit and petiole extracts were non-toxic in these selected organs.
In addition to the above parameters, the hematology parameters are important indicators to determine the toxicity effect of the herbal extracts on animal blood (Yakubu et al., 2007). In contrast, the hematological study conducted by Adedapo et al. (2004), showed a significant reduction in RBC and hemoglobin concentration of Euphorbia balsamifera aqueous extract treated SD rats at the dose of 1 g/100 g b.wt. This may cause a complication such as anemia due to a significant reduction in the concentration of RBC and hemoglobin. Nevertheless, A. longiloba extracts in the present study did not cause any major changes in the hematological parameters however few changes were detected which may carry little or no toxicological significance and therefore, the EtOH fruit and petiole extracts can be considered safe to the hematological parameters. Similar results were reported by Awe and Banjoko (2013), a lower concentration of plant extracts (<2000 mg/kg) did not affect the serum biochemical parameters. However, they noted that EtOH leaf extract of Petroselinum crispum was hepato and nephron-toxic at more than 2000 mg/kg.
The measurement of biochemical parameters is a significant biomarker for toxicology study as both liver and kidneys are essential organs for organism survival. The liver and kidney are the primary organs and site of drug metabolism and excretion, and increase/decrease of these biomarkers such as TP, ALB, GB, ALT, BUN, and CR, can help determine whether the plant extracts are hepato and reno-toxic or protective in nature. Higher concentrations of ALT, AST, and CR are reported in liver disease. While the reduction in TP, ALB, and GLB is an indication reduced liver function (Yuet Ping et al., 2013, Adeyemi et al., 2014, Chuffa et al., 2014; Brautbar et al., 2020). The results were consistent with normal serum biochemical parameters of all treated rats when compared with control. In contrast, the histology study conducted by Aliyu et al. (2020a) using Clinacanthus nutans EtOH extract showed no statistically significant (p > 0.05) differences in the kidney and liver lesion when compared with control at the dose of 2000 mg/kg b.wt. Meanwhile, the other study by Nurul et al. (2018) using M. christia vespertillionis extract showed (karyolysis, eosinophilic cytoplasm and pyknotic cells), degeneration and inflammations. These results indicated that the plant extracts could cause deleterious effects to the organs, mainly on the liver and kidney. Therefore, it is expedient for the consumer to take extra caution in the usage of any medicinal plants to avoid overdosing and side effects. In summary, administration of EtOH extracts of A. longiloba fruit and petiole did not induce any major toxicity effects in Sprague-Dawley male rats. Furthermore, almost all the hematology, biochemical and histopathological parameters were normal, however insignificant changes in some parameters were noted which could or could not be associated with treatments, thus carrying slight or no toxicological significance.
In the present study, HU was induced by administering PO, uricase-inhibitor drug (250 mg/kg) to normal Sprague-Dawley rats, indicating the successful establishment of the model. HU is characterized by a high concentration of UA in the blood. It is documented that excessive production and under-excretion of UA could lead to several diseases, including HU, gout, and many other metabolic disorders (Haidari et al., 2009, Kuo et al., 2015, Meng et al., 2014, Chen et al., 2019). HU is also been reported to cause the kidney and heart diseases (Sánchez-Lozada et al., 2002, Mazzali et al., 2001, Zhang et al., 2018, Huang et al., 2020).
Hyperuricemia and gout disorders are mostly controlled by reducing UA production. In man, UA is created as the major waste-product of purine metabolism in the liver and excreted by the kidneys. The key enzyme in this process is XOD (Yisireyili et al., 2017, Ojha et al., 2017, Cicero et al., 2021). XOD enzyme catalyzes the conversion of hypoxanthine to xanthine, which is then further converted to UA. Therefore, the inhibition of XOD can be used as an effective therapeutic approach to treat HU (Haidari et al., 2009, Meng et al., 2014, Sarvaiya et al., 2015 Wahyuningsih and Sukandar, 2016; Li et al., 2017, Rahmi et al., 2020).
Due to some serious side effects of XOD inhibitory drugs which are used for the treatment of HU, scientists are now focusing their efforts on finding a safer alternative for XOI drugs, particularly from natural sources (Haidari et al., 2009; Rahmi et al., 2020; Chen et al., 2020). In the present study, it was shown that the administration of fruit and petiole as a phytochemical-rich medicinal plant could reduce the elevated UA levels in HU rats. Interestingly, both fruit and petiole extracts and allopurinol had significant reductive effects on the serum UA levels in the HU rats.
Our results are in line with Haidari et al. (2009) who reported the UA inhibitory effect of Allium cepa extract, which had almost similar effect to that of allopurinol. Chen et al. (2019) have also reported the extracts of the rhizomes of Curcuma longa decreased the levels of UA in serum and showed almost similar hyperuricemic action to that of allopurinol. In addition, they have explained that the hyperuricemic property of extracts was due to its inhibitory effect on XOD activity. Similar findings have been reported by others (Yan et al., 2013, Meng et al., 2014, Sarvaiya et al., 2015 Wahyuningsih and Sukandar, 2016; Li et al., 2017, Rahmi et al., 2020). The mechanism in which the extracts of A. longiloba reduced the serum uric level in rats might be by inhibiting the activity of XOD. Our results suggest that both fruit and petiole extracts could alleviate PO-induced HU, which has a similar effect as compared to allopurinol, a standard drug commonly utilized to treat hyperuricemia.
5. Conclusion
In conclusion, the present study demonstrated that the fruit and petiole extracts of A. longiloba have anti-hyperuricemia activity by reducing the serum uric acid level in the serum. The extracts were safer and non-toxic to rats. The present study may provide insights and scientific evidence on the use of Alocasia longiloba fruit and petiole as a traditional and complementary medicine to prevent hyperuricemia and associated inflammations.
Funding
This research was supported by a grant from Universiti Malaysia Kelantan (grant number: R/SGJP/A0700/00710A/005/2019/00609) and Ministry of Higher Education, Malaysia (grant number: R/FRGS/ A07.00/00710A/002/2016/000374).
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committee (IACUC), Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Kota Bahru, Malaysia (Approval number: UMK/FPV/ACUC/PG/1/2020). All procedures were carried out following the relevant guidelines and regulations.
CRediT authorship contribution statement
Ferid Abdulhafiz: Conceptualization, Methodology, Performed the experiments, Writing - original draft, Writing – review & editing. Mohd Farhan Hanif Reduan: Methodology, Data curation, Validation, Investigation, Supervision, Project administration, Writing – review & editing. Zulhazman Hamzah: Writing - review & editing. Zulhisyam Abdul Kari: Writing – review & editing. Mahmoud A.O. Dawood: Writing – review & editing. Arifullah Mohammed: Funding acquisition, Investigation, Supervision, Project administration, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Universiti Malaysia Kelantan for funding and providing the research facilities. The first author would like to sincerely thank Universiti Malaysia Kelantan for the award of Zamalah fellowship. We also extend our sincere thanks to Dr. Fathin Faahimaah, Dr. Amalina and Dr. Athira for their assistance.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aamir K., Khan H.U., Hossain C.F., Afrin M.R., Shaik I., Salleh N., Giribabu N., Arya A. Oral toxicity of arjunolic acid on hematological, biochemical and histopathological investigations in female Sprague Dawley rats. PeerJ. 2019;7:e8045. doi: 10.7717/peerj.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulhafiz F., Mohammed A., Kayat F., Bhaskar M., Hamzah Z., Podapati S.K., Reddy L.V. Xanthine Oxidase Inhibitory Activity, Chemical Composition, Antioxidant Properties and GC-MS Analysis of Keladi Candik (Alocasia longiloba Miq) Molecules. 2020;25(11):2658. doi: 10.3390/molecules25112658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulhafiz F., Mohammed A., Kayat F., Zakaria S., Hamzah Z., Reddy Pamuru R., Gundala P.B., Reduan M.F.H. Micropropagation of Alocasia longiloba Miq and Comparative Antioxidant Properties of Ethanolic Extracts of the Field-Grown Plant, In Vitro Propagated and In Vitro-Derived Callus. Plants. 2020;9(7):816. doi: 10.3390/plants9070816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adedapo A.A., Abatan M.O., Olorunsogo O.O. Toxic effects of some plants in the genus Euphorbia on haematological and biochemical parameters of rats. Vet. Arh. 2004;74(1):53–62. [Google Scholar]
- Adeyemi D.O., Ukwenya V.O., Obuotor E.M., Adewole S.O. Anti-hepatotoxic activities of Hibiscus sabdariffa L. in animal model of streptozotocin diabetes-induced liver damage. BMC Complement. Altern. Med. 2014;14(1):1–11. doi: 10.1186/1472-6882-14-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N., Perveen R., Rahman S., Mahmood S., Rahman S., Islam S., Haque T., Sumon A.H., Kathak R.R., Molla N.H., Islam F., Mohanto N.C., Nurunnabi S.M., Ahmed S., Rahman M., Bjornstad P. Prevalence of hyperuricemia and the relationship between serum uric acid and obesity: A study on Bangladeshi adults. PLoS ONE. 2018;13(11):e0206850. doi: 10.1371/journal.pone.0206850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An M.F., Wang M.Y., Shen C., Sun Z.R., Zhao Y.L., Wang X.J., Sheng J. Isoorientin exerts a urate-lowering effect through inhibition of xanthine oxidase and regulation of the TLR4-NLRP3 inflammasome signaling pathway. J. Nat. Med. 2021;75(1):129–141. doi: 10.1007/s11418-020-01464-z. [DOI] [PubMed] [Google Scholar]
- Awe E.O., Banjoko S.O. Biochemical and haematological assessment of toxic effects of the leaf ethanol extract of Petroselinum crispum (Mill) Nyman ex A.W. Hill (Parsley) in rats. BMC. Complement. Altern. Med. 2013;13:75. doi: 10.1186/1472-6882-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliyu A., Shaari M.R., Ahmad Sayuti N.S., Reduan M.F.H., Sithambaram S., Noordin M.M., Shaari K., Hamzah H. Subacute Oral Administration of Clinacanthus nutans Ethanolic Leaf Extract Induced Liver and Kidney Toxicities in ICR Mice. Molecules. 2020;25(11):2631. doi: 10.3390/molecules25112631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliyu A., Shaari M.R., Ahmad Sayuti N.S., Reduan M.F.H., Sithambaram S., Noordin M.M., Shaari K., Hamzah H. N-Ethyl-n-Nitrosourea Induced Leukaemia in a Mouse Model through Upregulation of Vascular Endothelial Growth Factor and Evading Apoptosis. Cancers. 2020;12(3):678. doi: 10.3390/cancers12030678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkas F., Elisaf M., Liberopoulos E., Kalaitzidis R., Liamis G. Uric acid and incident chronic kidney disease in dyslipidemic individuals. Curr. Med. Res. Opin. 2018;34(7):1193–1199. doi: 10.1080/03007995.2017.1372157. [DOI] [PubMed] [Google Scholar]
- Benn C.L., Dua P., Gurrell R., Loudon P., Pike A., Storer R.I., Vangjeli C. Physiology of hyperuricemia and urate-lowering treatments. Front. Med. 2018;5:160. doi: 10.3389/fmed.2018.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj K.K., Rabha B., Choudhury B.K., Rosalin R., Sarkar T., Baishya D., Chanu N.B., Singh Y.D., Panda M.K., Pati S. Current strategies in inhibiting biofilm formation for combating Urinary tract infections: special focus on peptides, nano-particles and phytochemicals. Biocatal. Agric. Biotechnol. 2021;38:102209. doi: 10.1016/j.bcab.2021.102209. [DOI] [Google Scholar]
- Bharadwaj K.K., Rabha B., Pati S., Choudhury B.K., Sarkar T., Gogoi S.K., Kakati N., Baishya D., Kari Z.A., Edinur H.A. Green Synthesis of Silver Nanoparticles Using Diospyros malabarica Fruit Extract and Assessments of Their Antimicrobial, Anticancer and Catalytic Reduction of 4-Nitrophenol (4-NP) Nanomaterials. 2021;11(8):1999. doi: 10.3390/nano11081999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj K.K., Rabha B., Pati S., Sarkar T., Choudhury B.K., Barman A., Bhattacharjya D., Srivastava A., Baishya D., Edinur H.A., Abdul Kari Z., Mohd Noor N.H. Green Synthesis of Gold Nanoparticles Using Plant Extracts as Beneficial Prospect for Cancer Theranostics. Molecules. 2021;26(21):6389. doi: 10.3390/molecules26216389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga T.T., Foresto-Neto O., Camara N.O.S. The role of uric acid in inflammasome-mediated kidney injury. Curr. Opin. Nephrol. Hypertens. 2020;29(4):423–431. doi: 10.1097/MNH.0000000000000619. [DOI] [PubMed] [Google Scholar]
- Cao J., Wang C., Zhang G., Ji X., Liu Y., Sun X., Xue F. Incidence and simple prediction model of Hyperuricemia for urban Han Chinese adults: a prospective cohort study. Int. J. Environ. Res. Public Health. 2017;14(1):67. doi: 10.5812/numonthly.7(3)2015.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Li C., Duan S., Yuan X., Liang J., Hou S. Curcumin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Biomed. Pharmacother. 2019;118:109195. doi: 10.1016/j.biopha.2019.109195. [DOI] [PubMed] [Google Scholar]
- Chen C., Lü J.M., Yao Q. Hyperuricemia-related diseases and xanthine oxidoreductase (XOR) inhibitors: an overview. Med. Sci. Monitor: Int. Med. J. Exp. Clin. Res. 2016;22:2501. doi: 10.12659/MSM.899852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.-D., Zhao Y.-L., Sun W.-J., He Y.-J., Liu Y.-P., Jin Q., Yang X.-W., Luo X.-D. “Kidney Tea” and Its Bioactive Secondary Metabolites for Treatment of Gout. J. Agric. Food Chem. 2020;68(34):9131–9138. doi: 10.1021/acs.jafc.0c0384810.1021/acs.jafc.0c03848.s001. [DOI] [PubMed] [Google Scholar]
- Chuffa L.G.A., Fioruci-Fontanelli B.A., Bordon J.G., Pires R.B., Braga C.P., Seiva F.R., Fernandes A.A.H. Rutin ameliorates glycemic index, lipid profile and enzymatic activities in serum, heart and liver tissues of rats fed with a combination of hypercaloric diet and chronic ethanol consumption. Indian J. Biochem. Biophy. 2014:215–222. http://nopr.niscair.res.in/handle/123456789/29087 [PubMed] [Google Scholar]
- Cicero A.F., Fogacci F., Cincione R.I., Tocci G., Borghi C. Clinical effects of xanthine oxidase inhibitors in hyperuricemic patients. Med. Princ. Pract. 2021;30(2):122–130. doi: 10.1159/000512178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis T., Odutayo A., Keunen J., Hladunewich M. The Kidney in Normal Pregnancy and Preeclampsia. Semin. Nephrol. 2011;31(1):4–14. doi: 10.1016/j.semnephrol.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Das B.N. Karyomorphological Studies in Three Species of Alocasia (Schott.) G. Don.-An Ethno-medicinally and Economically Important Genus. Int. J. Life. Sci. Scienti. Res. 2018;2455(1716):1716. doi: 10.21276/ijlssr.2018.4.6.8. [DOI] [Google Scholar]
- Desideri G., Castaldo G., Lombardi A., Mussap M., Testa A., Pontremoli R., Punzi L., Borghi C. Is it time to revise the normal range of serum uric acid levels? Eur. Rev. Med. Pharmacol. Sci. 2014;18(9):1295–1306. [PubMed] [Google Scholar]
- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excler J.L., Saville M., Berkley S., Kim J.H. Vaccine development for emerging infectious diseases. Nat. Med. 2021;27(4):591–600. doi: 10.1038/s41591-021-01301-0. [DOI] [PubMed] [Google Scholar]
- Ferid A., Mohammed A., Khalivulla S.I., Korivi M., Razab M.K.A.A. IOP Conference Series: Earth and Environmental Science. Vol. 596. IOP Publishing; 2020. Plant Cell and Callus Cultures as an Alternative Source of Bioactive Compounds with Therapeutic Potential against Coronavirus Disease (COVID-19) p. 012099. [DOI] [Google Scholar]
- Gaur V., Malik P., Paul O. Prevalence of Hyperuricemia & relation of Serum uric Acid in Burn Patients amongst Different Gender and Age Groups. Int. J. Biochem. 2018;1(1):16–19. [Google Scholar]
- Habib S.H., Saha S. Burden of non-communicable disease: global overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2010;4(1):41–47. doi: 10.1016/j.dsx.2008.04.005. [DOI] [Google Scholar]
- Hafez R.M., Abdel-Rahman T.M., Naguib R.M. Uric acid in plants and microorganisms: Biological applications and genetics-A review. J. Adv. Res. 2017;8(5):475–486. doi: 10.1016/j.jare.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidari F., Keshavarz S.A., Rashidi M.R., Shahi M.M. Orange juice and hesperetin supplementation to hyperuricemic rats alter oxidative stress markers and xanthine oxidoreductase activity. J. Clin. Biochem. Nutr. 2009;45(3):285–291. doi: 10.3164/jcbn.09-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzah N.H.C., Mohammed A., Sirajudeen K.N.S., Asari M.A., Hamzah Z., Shaik I.K. Keladi candik (Alocasia longiloba Miq.) petiole extracts promote wound healing in a full thickness excision wound model in rats. Asian Pac. J. Trop. Biomed. 2019;9(4):140. [Google Scholar]
- Hazra S.K., Sarkar T., Salauddin M., Sheikh H.I., Pati S., Chakraborty R. Characterization of phytochemicals, minerals and in vitro medicinal activities of bael (Aeglemarmelos L.) pulp and differently dried edible leathers. Heliyon. 2020;6(10):e05382. doi: 10.1016/j.heliyon.2020.e05382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G., Xu J., Zhang T., Cai L., Liu H., Yu X., Wu J. Hyperuricemia is associated with metabolic syndrome in the community very elderly in chengdu. Sci. Rep. 2020;10(1):1–7. doi: 10.1038/s41598-020-65605-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifeoma O., Oluwakanyinsol S. New Insights into Toxicity and Drug Testing. InTech; 2013. [DOI] [Google Scholar]
- Islam M.K., Mahmud I., Saha S., Sarker A.B., Mondal H., Monjur-Al-Hossain A.S.M., Anisuzzman M. Preliminary pharmacological evaluation of Alocasia indica Schott tuber. J. Integr. Med. 2013;11(5):343–351. doi: 10.3736/jintegrmed2013045. [DOI] [PubMed] [Google Scholar]
- Kabir M.S.H., Hossain M.M., Kabir M.I., Rahman M.M., Hasanat A., Bin Emran T., Rahman M.A. Phytochemical screening, Antioxidant, Thrombolytic, alpha-amylase inhibition and cytotoxic activities of ethanol extract of Steudnera colocasiifolia K. Koch leaves. J. Young. Pharm. 2016;8(4):391–397. doi: 10.5530/jyp.2016.4.15. [DOI] [Google Scholar]
- Kaid F., Alabsi A.M., Alafifi N., Ali-Saeed R., Ameen Al-koshab M., Ramanathan A., Ali A.M. Histological, biochemical, and hematological effects of Goniothalamin on selective internal organs of male Sprague-Dawley rats. J. Toxicol. 2019;2019:1–13. doi: 10.1155/2019/6493286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostić D.A., Dimitrijević D.S., Stojanović G.S., Palić I.R., Đorđević A.S., Ickovski J.D. Xanthine oxidase: isolation, assays of activity, and inhibition. J. Chem. 2015;2015:1–8. doi: 10.1155/2015/294858. [DOI] [Google Scholar]
- Kuo C.C., Weaver V., Fadrowski J.J., Lin Y.S., Guallar E., Navas-Acien A. Arsenic exposure, hyperuricemia, and gout in US adults. Environ. Int. 2015;76:32–40. doi: 10.1016/j.envint.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Leander J., Sunnåker M., Rekić D., Aksenov S., Eriksson U.G., Johansson S., Parkinson J. A semi-mechanistic exposure–response model to assess the effects of verinurad, a potent URAT1 inhibitor, on serum and urine uric acid in patients with hyperuricemia-associated diseases. J. Pharmacokinet Pharmacodyn. 2021;48(4):525–541. doi: 10.1007/s10928-021-09747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., Oh B.K., Sung K.C. Uric acid and cardiometabolic diseases. J. Clin. Hypertens. 2020;26(1):1–7. doi: 10.1186/s40885-020-00146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Teng M., Liu Y., Qu Y., Zhang Y., Lin F., Wang D.i. Anti-gouty arthritis and antihyperuricemia effects of sunflower (Helianthus annuus) head extract in gouty and hyperuricemia animal models. Biomed. Res. Int. 2017;2017:1–9. doi: 10.1155/2017/5852076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Han C., Wu D.i., Xia X., Gu J., Guan H., Shan Z., Teng W. Prevalence of Hyperuricemia and Gout in Mainland China from 2000 to 2014: A Systematic Review and Meta-Analysis. Biomed. Red. Int. 2015;2015:1–12. doi: 10.1155/2015/762820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y., AlMazroa M.A., Alvarado M., Anderson H.R., Anderson L.M., Andrews K.G., Atkinson C., Baddour L.M., Barker-Collo S., Bartels D.H., Bell M.L., Benjamin E.J., Bennett D., Bhalla K., Bikbov B., Abdulhak A.B., Birbeck G., Blyth F., Bolliger I., Boufous S., Bucello C., Burch M., Burney P., Carapetis J., Chen H., Chou D., Chugh S.S., Coffeng L.E., Colan S.D., Colquhoun S., Colson K.E., Condon J., Connor M.D., Cooper L.T., Corriere M., Cortinovis M., de Vaccaro K.C., Couser W., Cowie B.C., Criqui M.H., Cross M., Dabhadkar K.C., Dahodwala N., De Leo D., Degenhardt L., Delossantos A., Denenberg J., Des Jarlais D.C., Dharmaratne S.D., Dorsey E.R., Driscoll T., Duber H., Ebel B., Erwin P.J., Espindola P., Ezzati M., Feigin V., Flaxman A.D., Forouzanfar M.H., Fowkes F.G.R., Franklin R., Fransen M., Freeman M.K., Gabriel S.E., Gakidou E., Gaspari F., Gillum R.F., Gonzalez-Medina D., Halasa Y.A., Haring D., Harrison J.E., Havmoeller R., Hay R.J., Hoen B., Hotez P.J., Hoy D., Jacobsen K.H., James S.L., Jasrasaria R., Jayaraman S., Johns N., Karthikeyan G., Kassebaum N., Keren A., Khoo J.-P., Knowlton L.M., Kobusingye O., Koranteng A., Krishnamurthi R., Lipnick M., Lipshultz S.E., Ohno S.L., Mabweijano J., MacIntyre M.F., Mallinger L., March L., Marks G.B., Marks R., Matsumori A., Matzopoulos R., Mayosi B.M., McAnulty J.H., McDermott M.M., McGrath J., Memish Z.A., Mensah G.A., Merriman T.R., Michaud C., Miller M., Miller T.R., Mock C., Mocumbi A.O., Mokdad A.A., Moran A., Mulholland K., Nair M.N., Naldi L., Narayan K.M.V., Nasseri K., Norman P., O'Donnell M., Omer S.B., Ortblad K., Osborne R., Ozgediz D., Pahari B., Pandian J.D., Rivero A.P., Padilla R.P., Perez-Ruiz F., Perico N., Phillips D., Pierce K., Pope C.A., Porrini E., Pourmalek F., Raju M., Ranganathan D., Rehm J.T., Rein D.B., Remuzzi G., Rivara F.P., Roberts T., De León F.R., Rosenfeld L.C., Rushton L., Sacco R.L., Salomon J.A., Sampson U., Sanman E., Schwebel D.C., Segui-Gomez M., Shepard D.S., Singh D., Singleton J., Sliwa K., Smith E., Steer A., Taylor J.A., Thomas B., Tleyjeh I.M., Towbin J.A., Truelsen T., Undurraga E.A., Venketasubramanian N., Vijayakumar L., Vos T., Wagner G.R., Wang M., Wang W., Watt K., Weinstock M.A., Weintraub R., Wilkinson J.D., Woolf A.D., Wulf S., Yeh P.-H., Yip P., Zabetian A., Zheng Z.-J., Lopez A.D., Murray C.JL. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher T., Ahmad Raus R., Daddiouaissa D., Ahmad F., Adzhar N.S., Latif E.S., Abdulhafiz F., Mohammed A. Medicinal Plants with Anti-Leukemic Effects: A Review. Molecules. 2021;26(9):2741. doi: 10.3390/molecules26092741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher T., Kabbashi N.A., Mirghani M.E.S., Alam M.Z., Daddiouaissa D., Abdulhafiz F., Reduan M.F.H., Omran J.I., Abdul Razab M.K.A., Mohammed A. Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Acacia Seyal Gum Using Response Surface Methodology and Their Chemical Content Identification by Raman, FTIR, and GC-TOFMS. Antioxidants. 2021;10(10):1612. doi: 10.3390/antiox10101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat S.G., Al Kattar S., Tanios B.Y., Jurjus A. Hyperuricemia, hypertension, and chronic kidney disease: an emerging association. Curr. Hypertens. Rep. 2016;18(10):1–6. doi: 10.1007/s11906-016-0684-z. [DOI] [PubMed] [Google Scholar]
- Mazzali M., Hughes J., Kim Y.-G., Jefferson J.A., Kang D.-H., Gordon K.L., Lan H.Y., Kivlighn S., Johnson R.J. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- Meng Z.-Q., Tang Z.-H., Yan Y.-X., Guo C.-R., Cao L., Ding G., Huang W.-Z., Wang Z.-Z., Wang K.D.G., Xiao W., Yang Z.-L. Study on the anti-gout activity of chlorogenic acid: Improvement on hyperuricemia and gouty inflammation. Am. J. Chin. Med. 2014;42(06):1471–1483. doi: 10.1142/S0192415X1450092X. [DOI] [PubMed] [Google Scholar]
- Miao Z.-M., Zhao S.-H., Yan S.-L., Li C.-G., Wang Y.-G., Meng D.-M., Zhou L.i., Mi Q.-S. NALP3 inflammasome functional polymorphisms and gout susceptibility. Cell Cycle. 2009;8(1):27–30. doi: 10.4161/cc.8.1.7325. [DOI] [PubMed] [Google Scholar]
- Mintah S.O., Asafo-Agyei T., Archer M.A., Junior P.A.A., Boamah D., Kumadoh D., Agyare C. Medicinal plants for treatment of prevalent diseases. Pharmacogn. Med. Plants. 2019;1–19 doi: 10.5772/intechopen.82049. [DOI] [Google Scholar]
- Mohamed E.A.H., Lim C.P., Ebrika O.S., Asmawi M.Z., Sadikun A., Yam M.F. Toxicity evaluation of a standardised 50% ethanol extract of Orthosiphon stamineus. J. Ethnopharmacol. 2011;133(2):358–363. doi: 10.1016/j.jep.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Mohammadi G., Rafiee G., El Basuini M.F., Van Doan H., Ahmed H.A., Dawood M.A.O., Abdel-Latif H.M.R. Oregano (Origanum vulgare), St John's-wort (Hypericum perforatum), and lemon balm (Melissa officinalis) extracts improved the growth rate, antioxidative, and immunological responses in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquac. Rep. 2020;18:100445. doi: 10.1016/j.aqrep.2020.100445. [DOI] [Google Scholar]
- Mohammadi G., Hafezieh M., Karimi A.A., Azra M.N., Van Doan H., Tapingkae W., Abdelrahman H.A., Dawood M.A.O. The synergistic effects of plant polysaccharide and Pediococcus acidilactici as a synbiotic additive on growth, antioxidant status, immune response, and resistance of Nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila. Fish Shellfish Immunol. 2022;120:304–313. doi: 10.1016/j.fsi.2021.11.028. [DOI] [PubMed] [Google Scholar]
- Mohd F.H.R., Mohd R.S., Sayuti N.S.A., Aliyu A., Md N.M.M., Bakar Z.A., Hamzah H. Evaluation of dermal toxicity study of ethanolic extract of Morinda citrifolia fruit in Spraque Dawley rats. Thai. J. Vet. Med. 2021;51(1):101–109. doi: 10.14456/tjvm.2021.14. [DOI] [Google Scholar]
- Nur-Alya I.S., Aurifullah M., Nazahatul A.A., Srisawat T., Permpoonpattana P., Norhazlini M.Z., Suhaimi O., Zulhazman H. Synergistic effect of Alocasia longiloba fruit’s extract with ampicilin and tetracycline against bacteria. IOP Conf. Ser.: Earth Environ. Sci. 2021;842(1):012065. doi: 10.1088/1755-1315/842/1/012065. [DOI] [Google Scholar]
- Nur-Hadirah, K., Arifullah, M., Nazahatul, A.A., Klaiklay, S., Chumkaew, P., Norhazlini, M.Z., Zulhazman, H., 2021. Total phenolic content and antioxidant activity of an edible Aroid, Colocasia esculenta (L.) Schott. In IOP Conference Series: Earth and Environmental Science (Vol. 756, No. 1, p. 012044). IOP Publishing. 10.1088/1755-1315/756/1/012044. [DOI]
- Nur-Izzati M., Arifullah M., Nazahatul A.A., Klaiklay S., Chumkaew P., Norhazlini M.Z., Abdulhafiz F., Zulhazman H. Elucidation of total phenolic content and antioxidant activity in medicinal Aroid, Alocasia longiloba Miq. IOP Conf. Ser.: Earth Environ. Sci. 2021;756(1):012043. doi: 10.1088/1755-1315/756/1/012043. [DOI] [Google Scholar]
- Nurul S.A.S., Hazilawati H., Mohd R.S., Mohd F.H.R., Noordin M.M., Norhaizan M.E. Subacute oral toxicity assesment of ethanol extract of Mariposa christia vespertilionis leaves in male Sprague Dawley rats. Toxicol. Res. 2018;34(2):85–95. doi: 10.5487/TR.2018.34.2.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha R., Singh J., Ojha A., Singh H., Sharma S., Nepali K. An updated patent review: xanthine oxidase inhibitors for the treatment of hyperuricemia and gout (2011–2015) Expert Opin. Ther. Pat. 2017;27(3):311–345. doi: 10.1080/13543776.2017.1261111. [DOI] [PubMed] [Google Scholar]
- Okoye N.N., Ajaghaku D.L., Okeke H.N., Ilodigwe E.E., Nworu C.S., Okoye F.B.C. beta-Amyrin and alpha-amyrin acetate isolated from the stem bark of Alstonia boonei display profound anti-inflammatory activity. Pharm. Biol. 2014;52(11):1478–1486. doi: 10.3109/13880209.2014.898078. [DOI] [PubMed] [Google Scholar]
- Oladeji O. The characteristics and roles of medicinal plants: Some important medicinal plants in Nigeria. Indian J. Nat. Prod. 2016;12(3):102. [Google Scholar]
- Rahmi E.P., Kumolosasi E., Jalil J., Husain K., Buang F., Abd. Razak A.F., Jamal J.A. Anti-hyperuricemic and anti-inflammatory effects of Marantodes pumilum as potential treatment for gout. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.0028910.3389/fphar.2020.00289.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeh M.A.B., Kwan Y.P., Zakaria Z., Latha L.Y., Jothy S.L., Sasidharan S. Acute toxicity impacts of Euphorbia hirta L extract on behavior, organs body weight index and histopathology of organs of the mice and Artemia salina. Pharmacogn. Rese. 2012;4(3):170. doi: 10.4103/0974-8490.99085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza M., Al-Shabanah O.A., El-Hadiyah T.M., Al-Majed A.A. Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Sci. Pharm. 2002;70:135–145. doi: 10.3797/scipharm.aut-02-16. [DOI] [Google Scholar]
- Riches P.L., Wright A.F., Ralston S.H. Recent insights into the pathogenesis of hyperuricaemia and gout. Hum. Mol. Genet. 2009;18(R2):R177–R184. doi: 10.1093/hmg/ddp369. [DOI] [PubMed] [Google Scholar]
- Richette P., Bardin T. Colchicine for the treatment of gout. Expert Opin. Pharmacother. 2010;11(17):2933–2938. doi: 10.1517/14656566.2010.529432. [DOI] [PubMed] [Google Scholar]
- Richette P., Perez-Ruiz F., Doherty M., Jansen T.L., Nuki G., Pascual E., Punzi L., So A.K., Bardin T. Improving cardiovascular and renal outcomes in gout: what should we target? Nat. Rev. Rheumatol. 2014;10(11):654–661. doi: 10.1038/nrrheum.2014.124. [DOI] [PubMed] [Google Scholar]
- Reduan F.H., Shaari R.M., Sayuti N.S.A., Mustapha N.M., Abu Bakar M.Z., Sithambaram S., Hamzah H. Acute and subacute dermal toxicity of ethanolic extract of Melastoma malabathricum leaves in Sprague-Dawley rats. Toxicol. Res. 2020;36(3):203–210. doi: 10.1007/s43188-019-00013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reduan M.F.H., Hamid F.F.A., Nordin M.L., Shaari R., Hamdan R.H., Chung E.L.T., Noralidin N. Acute oral toxicity study of ethanol extract of Oroxylum indicum leaf in mice. Thai. J. Vet. Med. 2020;50(4):573–581. [Google Scholar]
- Ridley H.N., Curtis C. Malay plant names. J. Straits Branch Roy. Asiat. Soc. 1902;38:39–122. [Google Scholar]
- Roumeliotis C., Siomos A.S., Gerasopoulos D. Comparative Nutritional and Antioxidant Compounds of Organic and Conventional Vegetables during the Main Market Availability Period. Nitrogen. 2021;2(1):18–29. doi: 10.3390/nitrogen2010002. [DOI] [Google Scholar]
- Russo E., Verzola D., Cappadona F., Leoncini G., Garibotto G., Pontremoli R., Viazzi F. The role of uric acid in renal damage-a history of inflammatory pathways and vascular remodeling. Vessel Plus. 2021;5 doi: 10.20517/2574-1209.2021.11. [DOI] [Google Scholar]
- Saiyed Z.M., Sengupta K., Krishnaraju A.V., Trimurtulu G., Lau F.C., Lugo J.P. Safety and toxicological evaluation of Meratrim®: An herbal formulation for weight management. Food Chem. Toxicol. 2015;78:122–129. doi: 10.1016/j.fct.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Sánchez-Lozada L.G., Tapia E., Avila-Casado C., Soto V., Franco M., Santamaría J., Nakagawa T., Rodríguez-Iturbe B., Johnson R.J., Herrera-Acosta J. Mild hyperuricemia induces glomerular hypertension in normal rats. Am. J. Physiol. Renal Physiol. 2002;283(5):F1105–F1110. doi: 10.1152/ajprenal.00170.2002. [DOI] [PubMed] [Google Scholar]
- Sarkar T., Salauddin M., Pati S., Sheikh H.I., Chakraborty R. Application of raw and differently dried Pineapple (Ananas comosus) pulp on Rasgulla (sweetened Casein Ball) to enhance its phenolic profile, shelf life, and in-vitro digestibility characteristics. J. Food Process. Preserv. 2021;45(3) doi: 10.1111/jfpp.15233. [DOI] [Google Scholar]
- Sarvaiya V.N., Sadariya K.A., Pancha P.G., Thaker A.M., Patel A.C., Prajapati A.S. Evaluation of antigout activity of Phyllanthus emblica fruit extracts on potassium oxonate-induced gout rat model. Vet. World. 2015;8(10):1230. doi: 10.14202/vetworld.2015.1230-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong J.M., Park C.E., Gi M.Y., Cha J.A., Jung E.Y., Lee J.H., Sung H.H., Yang S.B., Lee B., Lim J.H., Yoon H. Relationship between uric acid and lipid accumulation product index by gender in Korean adults: The 2016 Korean National Health and Nutrition Examination Survey. Primary Care Diabetes. 2021;15(3):541–547. doi: 10.1016/j.pcd.2020.12.001. [DOI] [PubMed] [Google Scholar]
- Shahin L., Patel K.M., Heydari M.K., Kesselman M.M. Hyperuricemia and Cardiovascular Risk. Cureus. 2021;13(5) doi: 10.7759/cureus.14855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Kaze A.D., McMullan C.J., Isakova T., Waikar S.S. Uric acid and the risks of kidney failure and death in individuals with CKD. Am. J. Kidney Dis. 2018;71(3):362–370. doi: 10.1053/j.ajkd.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P.K., Farrar J.E., Gaucher E.A., Miner J.N. Coevolution of URAT1 and Uricase during Primate Evolution: Implications for Serum Urate Homeostasis and Gout. Mol. Biol. Evol. 2016;33(9):2193–2200. doi: 10.1093/molbev/msw116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D.H., Ye Y.S., Wang C.Y., Li Z.L., Zheng H., Ma K.L. Potassium oxonate induces acute hyperuricemia in the tree shrew (tupaia belangeri chinensis) Exp. Anim. 2017;66(3):209–216. doi: 10.1538/expanim.16-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahyuningsih S., Sukandar E. In Vitro Xanthine Oxidase Inhibitor Activity of Ethanol Extract and Fraction Roselle Calyx (Hibiscus sabdariffa L.) Int. J. Pharma. Clin. Res. 2016;8(6):619–622. [Google Scholar]
- Winder M., Owczarek A.J., Mossakowska M., Broczek K., Grodzicki T., Wierucki Ł., Chudek J. Prevalence of Hyperuricemia and the Use of Allopurinol in Older Poles—Results From a Population-Based PolSenior Study. Int. J. Environ. Res. Public Health. 2021;18(2):387. doi: 10.3390/ijerph18020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubu M.T., Akanji M.A., Oladiji A.T. Hematological evaluation in male albino rats following chronic administration of aqueous extract of Fadogia agrestis stem. Pharmacogn. Mag. 2007;3(9):34. [Google Scholar]
- Yan J., Zhang G., Hu Y., Ma Y. Effect of luteolin on xanthine oxidase: inhibition kinetics and interaction mechanism merging with docking simulation. Food Chem. 2013;141(4):3766–3773. doi: 10.1016/j.foodchem.2013.06.092. [DOI] [PubMed] [Google Scholar]
- Yisireyili M., Hayashi M., Wu H., Uchida Y., Yamamoto K., Kikuchi R., Shoaib Hamrah M., Nakayama T., Wu Cheng X., Matsushita T., Nakamura S., Niwa T., Murohara T., Takeshita K. Xanthine oxidase inhibition by febuxostat attenuates stress-induced hyperuricemia, glucose dysmetabolism, and prothrombotic state in mice. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-01366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K.-H., Chen D.-Y., Chen J.-H., Chen S.-Y., Chen S.-M., Cheng T.-T., Hsieh S.-C., Hsieh T.-Y., Hsu P.-F., Kuo C.-F., Kuo M.-C., Lam H.-C., Lee I.-T., Liang T.-H., Lin H.-Y., Lin S.-C., Tsai W.-P., Tsay G.J., Wei J.-C., Yang C.-H., Tsai W.-C. Management of gout and hyperuricemia: multidisciplinary consensus in Taiwan. Int. J. Rheum Dis. 2018;21(4):772–787. doi: 10.1111/1756-185X.13266. [DOI] [PubMed] [Google Scholar]
- Yuet Ping K., Darah I., Chen Y., Sreeramanan S., Sasidharan S. Acute and Subchronic Toxicity Study of Euphorbia hirta L. Methanol Extract in Rats. Biomed. Res. Int. 2013;2013:1–14. doi: 10.1155/2013/182064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusoff N.Y., Hamzah Z., Kayat F., Zulhisyam A.K. Assessment on diversity and abundance of araceae in limestone and pyroclastics areas in Gua Musang, Kelantan, Malaysia. J. Trop. Resour. Sustain. Sci. 2013;1(1):16–24. doi: 10.47253/jtrss.v1i1.665. [DOI] [Google Scholar]
- Zhang X., Meng Q., Feng J., Liao H., Shi R., Shi D.i., Renqian L., Langtai Z., Diao Y., Chen X. The prevalence of hyperuricemia and its correlates in Ganzi Tibetan Autonomous Prefecture, Sichuan Province. China. Lipids Health Dis. 2018;17(1) doi: 10.1186/s12944-018-0882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulhazman H., Fizree M.A., Azahar A.M., Fadzelly A.M., Anis A.N. Vol. 736, No. 1. IOP Publishing; 2021. p. 012076. (A Survey on Edible Aroids Consumed by Locals in Kelantan, Peninsular Malaysia. In IOP Conference Series: Earth and Environmental Science). [DOI] [Google Scholar]
Further Reading
- Abu Bakar F.I., Abu Bakar M.F., Abdullah N., Endrini S., Fatmawati S. Optimization of Extraction Conditions of Phytochemical Compounds and Anti-Gout Activity of Euphorbia hirta L. (Ara Tanah) Using Response Surface Methodology and Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis. Evid.-Based Complement. Altern. Med. 2020;2020:1–13. doi: 10.1155/2020/4501261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajose F.O.A. Some Nigerian plants of dermatologic importance. Int. J. Dermatol. 2007;46(s1):48–55. doi: 10.1111/j.1365-4632.2007.03466.x. [DOI] [PubMed] [Google Scholar]
- Awodele O., Oreagba I.A., Odoma S., da Silva J.A.T., Osunkalu V.O. Toxicological evaluation of the aqueous leaf extract of Moringa oleifera Lam. (Moringaceae) J. Ethnopharmacol. 2012;139(2):330–336. doi: 10.1016/j.jep.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Aydιn A., Aktay G., Yesilada E. A guidance manual for the toxicity assessment of traditional herbal medicines. Nat. Prod. Commun. 2016;11(11) doi: 10.1177/1934578X1601101131. 1934578X1601101131. [DOI] [PubMed] [Google Scholar]
- Azubike N.C., Okwuosa C.N., Achukwu P.U., Maduka T.C., Chike O. Acute toxicity and histopathological effects of crude aqueous extract of Jatropha curcas leaves in mice. Res. J. Med. Plants. 2015;9(7):340–346. doi: 10.3923/rjmp.2015.340.346. [DOI] [Google Scholar]
- Brautbar N., Williams J., II Industrial solvents and liver toxicity: risk assessment, risk factors and mechanisms. Int. J. Hyg. Environ. Health. 2002;205(6):479–491. doi: 10.1078/1438-4639-00175. [DOI] [PubMed] [Google Scholar]
- Conen D., Wietlisbach V., Bovet P., Shamlaye C., Riesen W., Paccaud F., Burnier M. Prevalence of hyperuricemia and relation of serum uric acid with cardiovascular risk factors in a developing country. BMC Public Health. 2004;4(1):1–9. doi: 10.1186/1471-2458-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djerrou Z., Djaalab H., Riachi F., Serakta M., Chettoum A., Maameri Z., Hamdi-Pacha Y. Irritantcy potential and sub-acute dermal toxicity study of Pistacia lentiscus fatty oil as a topical traditional remedy. Afr. J. Tradit. Complement. Altern. Med. 2013;10:480–489. doi: 10.4314/ajtcam.v10i3.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D., Mondal S., Ramakrishna K. Acute and sub-acute (30-day) toxicity studies of Aegialitis rotundifolia Roxb, leaves extract in Wistar rats: safety assessment of a rare mangrove traditionally utilized as pain antidote. Clin. Phytoscience. 2019;5(1):13. doi: 10.1186/s40816-019-0106-2. [DOI] [Google Scholar]
- Glantzounis G.K., Tsimoyiannis E.C., Kappas A.M., Galaris D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005;11(32):4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- Harvey A.L. Natural products in drug discovery. Drug. Discov. Today. 2008;13(19–20):894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Kang D.H., Nakagawa T., Feng L., Watanabe S., Han L., Mazzali M., Johnson R.J. A role for uric acid in the progression of renal disease. J. Am. Soc. Nephrol. 2002;13(12):2888–2897. doi: 10.1097/01.ASN.0000034910.58454.FD. [DOI] [PubMed] [Google Scholar]
- Kasolo J.N., Bimenya G.S., Ojok L., Ogwal-Okeng J.W. Sub-acute toxicity evaluation of Moringa oleifera leaves aqueous and ethanol extracts in Swiss Albino rats. Int. J. Med. Plant. Res. 2012;1(6):075–081. [Google Scholar]
- Lorke D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983;54(4):275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- Nokhala A., Siddiqui M.J. Phytochemicals and biological activity of Tetracera scandens Linn. Merr. (Dilleniaceae): A short review. J. Pharm. Bioallied Sci. 2020;12(3):217. doi: 10.4103/jpbs.JPBS_192_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O.E.C.D. Guidelines for the Testing of Chemicals. Test Guideline. 1981;201 doi: 10.1016/0045-6535(81)90245-9. [DOI] [Google Scholar]
- Pan M.H., Lai C.S., Ho C.T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010;1(1):15–31. doi: 10.1039/c0fo00103a. [DOI] [PubMed] [Google Scholar]
- Pariyani R., Safinar Ismail I., Azam A.A., Abas F., Shaari K., Sulaiman M.R. Phytochemical screening and acute oral toxicity study of Java tea leaf extracts. Biomed Res. Int. 2015;2015:1–8. doi: 10.1155/2015/742420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salawu O.A., Chindo B.A., Tijani A.Y., Obidike I.C., Salawu T.A., Akingbasote A.J. Acute and sub-acute toxicological evaluation of the methanolic stem bark extract of Crossopteryx febrifuga in rats. Afr. J. Pharm. Pharmacol. 2009;3(12):621–626. doi: 10.5897/AJPP.9000013. [DOI] [Google Scholar]
- Serfilippi L.M., Stackhouse Pallman D.R., Russell B., Spainhour C.B. Serum clinical chemistry and hematology reference values in outbred stocks of albino mice from three commonly used vendors and two inbred strains of albino mice. J. Am. Assoc. Lab. Anim. Sci. 2003;42(3):46–52. [PubMed] [Google Scholar]
- Strazzullo P., Puig J.G. Uric acid and oxidative stress: relative impact on cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 2007;17(6):409–414. doi: 10.1016/j.numecd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Teke G.N., Kuete V. Toxicological survey of African medicinal plants. Elsevier Inc.; London: 2014. Acute and subacute toxicities of African medicinal plants; pp. 63–98. [DOI] [Google Scholar]
- Wahyuningsih S., Sukandar E.Y., Sukrasno L.D. Antihyperuricemia activity of the ethanol extract of Roselle calyx and its fraction (Hibiscus sabdariffa Linn) on male wistar rats. Int. J. Pharm. Pharm, Sci. 2016;8:278–280. [Google Scholar]