Abstract
Cervical cancer (CCa) is the second most frequent carcinoma in females and human papilloma virus (HPV) oncoproteins are regarded as one of the critical etiological agent. Despite recent advances in screening and management of CCa, still it remains the deadliest carcinoma as advanced and metastatic stages are mostly incurable. This urges for the development of newer therapeutic interventions. The current was aimed to investigate the antiproliferative and apoptotic potential of glycyrrhizin (Gly) against HPV16+ CaSki CCa cells. Our findings substantiated that Gly exerted antiproliferative effects on the CaSki cells by obstructing their proliferation rate. Gly substantially enhanced apoptosis in Caski cells in a dose-dependent manner via augmenting the generation of ROS, DNA fragmentation and disruption of the mitochondrial membrane potential. Gly mediated apoptosis in CaSki cells was found to be due to activation of caspase-8 and capsase-9 along with the modulation of pro-and anti-apoptotic gene expression. Moreover, Gly halts the progression of CaSki cells at G0/G1 phase which was found to be due to reduced expression of cyclin D1 and cyclin-dependent kinase 4 (CDK4) along with the enhanced expression of CDK inhibitor p21Cip1. Further, Gly downregulates the expression of HPV oncoproteins (E6 & E7) along with the inhibition of Notch signaling pathway. Taken together, Gly represents as a potential therapeutic modality for CCa which could rapidly be translated for clinical studies.
Keywords: Glycyrrhizin, Cervical cancer, HPV oncoproteins, Apoptosis, Cell cycle, Notch signaling pathway
1. Introduction
Globally, CCa is the most commonly occuring cancer among different types of cancers in females (Sun et al., 2021). According to the global cancer observatory report, CCa was accountable for 604,127 new cases in 2021, which corresponds to 3.1% of the total reported 19,292,789 cancer casesworldwide, whereas the count of fatalities was 341,831 or 3.3% of total cancer-related causalities (Global Cancer Observatory, 2021). Intriguingly, it is estimated that 85% of cumulative CCa cases are reported from the underdeveloped economies of the world, where it is responsible for approximately 12% of altogether female cancers. Among various others, infection of HPV is a ubiquitous risk factor which is related with CCa in approximately 90% of all diagnosed cases (Aftab et al., 2021). Nevertheless, the occurrence of CCa is multifactorial as it develop in a minority of women affected with HPV16 and HPV18 (high-risk CCa associated HPV strains) (Zur Hausen, 1996).
Screening initiatives have contributed substantially in reducing the overall incidence and mortalities associated with CCa however, the persistence of this precarious disease within the young female population still remains a major concern (Doorbar, 1979). However, administration of standard chemotherapeutic drugs and induction of drug resistance are associated with chronic side effects resulting in undesired gynecological and obstetric related consequences (Moon et al., 2017, Lin et al., 2016). Moreover, the efficacy of the drug is affected by surgical resection and systemic chemotherapy. Inevitably, chemotherapeutics that exert adverse effects on CCa patients are the present options available to cure the affected tissues of mucosal along with epithelial surfaces of the tumor. The therapeutical management of CCa depends largely upon the usage of Doxorubicin, although its use is restricted by its inferior bioavailability, high toxicity index, and unpropitious side effects (Moss and Kaye, 2002, Ulbrich et al., 2011, Jeong et al., 2011).
Evading apoptosis represents a peculiar attribute of cancers, and thus, is most commonly targeted non-surgical intervention against cancer as it is not specific to the type or cause of cancer. Dependening upon the apoptotic impetus, apoptosis can occur either through mithondria-dependent intrinsic pathway or death receptor-dependent extrinsic apoptotic pathway (Du et al., 2013). Bcl-2 protein family members activate the intrinsic apoptosis pathway and do not require receptor-mediated signal transduction (Du et al., 2013). Interestingly, members of TNF family also known as the “death domain receptors” mediate the activation of extrinsic apoptotic pathways (Pfeffer and Singh, 2018).
HPV oncoprotein E6 primarily regulates two different apoptosis pathways such as inactivating p53 and blockade of apoptosis (Howie et al., 2009). Initially, inactivated p53 plausibly instigate E6–mediated apoptosis inhibition. p53 activation promptly triggers the downstream activation of DNA repair and apoptosis; however, HPV E6 oncoprotein obstructs the p53–instigated signaling and programmed cell death. Furthermore, p53–independent apoptosis is also another apoptosis pathway which is responsible for the eradication of cancer cells, whereas E6 can obstruct apoptosis in vitro and p53 knock out mice (Aylon and Oren, 2011).
Notch signaling pathway represents a highly conserved developmental signaling cascade which is a prerequisite for determination of cell fate, cell proliferation and apoptosis during vertebrate development processes (Engel-Pizcueta and Pujades, Aug. 2021). Several investigations in the past have delineated that Notch signaling pathway acts as suppressors or promotors of HPV-associated CCa (Aster et al., 2017). Abnormal activation of Notch pathway also associates with various cancers including neck, prostate, head, breast and glioma. The pathway is comprises of Notch receptors (1–4; expressed in humans), membrane-spanning proteins and ligands, namely Jagged-1, −2 and delta-like ligand 1, 3 and 4. (Li,). Notch-1 and −3 are reported to play a critical role with exert the synergistic effect of HPV infection in mediating the proliferation and development of CCa by deregulation of Notch signaling (Talora et al., 2002).
Glycyrrhizin (Gly), is a representative of gluco-conjugated triterpenoids and is isolated from the Glycyrrhiza glabra roots. Gly is also known chmenically as 20-β-carboxy-11-oxo-30-norolean-12-en-3β-yl-2-O-β-d-glucopyranurosyl-α-d-glucopyranosiduronic acid (C42H62O16, MW 822.92) and is a member of triterpenoide family whose major constituent is glycyrrhetinic acid or O-β-d-glucuronosyl-(1′→2)-β-d-glucuronic acid. During its initial investigations, Gly was documented for its potent anti-viral effects and was further used as herbal medication for patients exhibiting symptoms of chronic infections of hepatitis-B and/or –C owing to its anti-inflammatory attributes (Sen et al., 2011). Subsequently, Gly is also reported to possess several pharmacological characteristics (Ahmad and Ansari, 2021). However, still there is a lacuna of studies focusing on anticancer efficacy of Gly against Caski cells. Thus, in the present report, the authors tried to investigate the anticancer and antiapoptotic efficacy of Gly and its correlation with downregulation of Notch signaling pathway in CCa.
2. Materials and methods
2.1. Materials
Glycyrrhizin (Gly), 2, 7-dichlorodihydrofluorescein diacetate (DCFH-DA), propidium iodide (PI), Hoechst 33342 and caspase-8, −9 and −3 inhibitors (Z-IETD-FMK, Z-LEHD-FMK, and Z-DEVD-FMK) were from Sigma (St. Louis, MO, USA). RPMI-1640 medium, fetal bovine serum (FBS), antibiotic–antimycotic solution, RNase A, HiPurATM Total RNA Miniprep Purification Kit, and MTT dye, N-Acetyl cysteine (NAC) were purchased from Himedia India, Ltd., Mumbai, India. NIR Mitochondrial Membrane Potential Assay Kit (Microplate), Verso cDNA synthesis kit, and DyNAmoColorFlash SYBR Green qPCR Kit were purchased from Abcam and Thermo-Scientific, USA, respectively. FITC Annexin V Apoptosis Detection Kit was obtained from BD Bioscience, PharMingen (San Diego, CA, USA). Caspase-3, −8 and −9 activity assay kits were purchased from BioVision, U.S.A. All the primer sequences utilized during the study were procured from IDT, USA.
2.2. Methods
2.2.1. Cell culture conditions
Human CCa CaSki cells (HPV16+) were acquired from the repository of the National Centre for Cell Sciences (NCCS), Pune, India. These were subsequently maintained in RPMI-1640 completed with FBS (10%) and antibiotic–antimycotic solution (1%) in a controlled humidified atmosphere with at least 5% CO2 at 37 °C.
2.2.2. Assessment of cell viability
To determine cytotoxicity of Gly on CCa cells, CaSki cells were co-cultured with varying Gly concentrations, and their viability was further evaluated using MTT dye (Ahmad and Ansari, 2021). Briefly, 5X103 cells/well was seeded in a 96-well plate and incubated overnight in the humidified atmosphere for adherence. Cells were exposed to varying doses of Gly (20–320 μM) and further incubated for 24 and 48 h under standard conditions. Gly purchased from Sigma Aldrich in our present study is in powder form, and it was dissolved in DMSO in order to perform our experimental studies. After the incubation, MTT dye (10 μl; 5 mg/ml) was supplemented in all the wells and incubated for an additional 4 h (37 °C). Eventually, the formazan or purple-colored precipitate was solubilized by supplementing 100 μl DMSO. Thereafter each well was assessed for absorbance at 490 nm using a spectrophotometer (Bio-Rad, California, USA), and the survival of cells was interpolated as percent (%) cell viability in comparison with cells which did not reveived Gly treatment (control).
2.2.3. Evaluation of cell death by trypan assay
Percentage of cell death in Gly-treated CaSki cells were analyzed by performing trypan blue dye exclusion assay. Briefly, 7 × 104 cells/well were overnight incubated in a 12-well plate under standard conditions. Thereafter, cells were treated with various concentrations of Gly and incubated for another 24 and 48 h. Cells were harvested after the treatment schedule and centrifuged at 1500 rpm for 5 min. Finally, the pellet was resuspended in 0.4% trypan blue (10 μl) and counted by using hemocytometer carefully under the light microscope.
2.2.4. Evaluation of nuclear morphological changes
Morphological changes within apoptotic CaSki cells were further evaluated using Hoechst-33342 dye (Ansari et al., 2021). CaSki cells (5 X 103 cells/well) were incubated overnight for adherence and thereafter exposed to Gly treatment (24 h) at different concentrations, as mentioned earlier, and incubated under standard conditions. Thereafter, media was decanted, and cells were further stained with Hoechst 33342 dye (2 μg/ml; 10 min) under standard conditions. Lastly, fluorescent nuclei of treated cells were visualized, and photomicrographs were captured using blue fluorescence channel (Excitation: 390/40 nm–Emission: 446/33 nm) of FLoid imaging station (Thermo Scientific, US).
2.2.5. Quantitative assessment of apoptosis in Gly-treated CaSki cells
Annexin V-FITC/PI apoptosis kit (BD Biosciences, San Diego, CA, USA) in accordance with manufacturer’s protocol was performed to quantify the amount of apoptosis induced within Gly-treated CaSki cells. Approximately, 4 X 105 cells/well were dosed with different concentration of Gly followed by further incubation of 24 h. After the incubation cells were given treatment with 1X binding buffer as per manufacturer’s protocol. Finally, around 1 × 105 cells (∼100 μl of suspension) was separately mixed with Annexin V-FITC and PI (5 μl each) and incubated briefly in dark (15 min; 37 °C). After increasing the total volume of suspension by adding 400 µl binding buffer, the suspension was evaluated through FACS Calibur (BD Biosciences, CA, USA).
2.2.6. Evaluation of caspases activity in Gly-treated CaSki cells
Colorimetric assay kits were used to determine the caspase activities in Gly-treated human CCa cells in accordance with the manufacturer’s protocol. Post-treatment with Gly (concentrations as stated), CaSki cells (3 × 106) were lysed using chilled lysis buffer (50 μl) along with 10 min incubation on ice. The resultant cell suspension was centrifuged (10,000g for 1 min; 4 °C), and the supernatant was collected and placed on ice. Thereafter, 50 μl/well of lysate was added in a 96 well along with 50 μl reaction buffer constituted by DTT (10 mM). Subsequently, DEVD-pNA/LEHD-pNA/IETD-pNA substrates (4 mM) were supplemented in each well and the plate was further incubated for 10 min. At the end of incubation, the absorbance of the plate was recorded at 405 nm through a microtiter plate reader. The result was interpretated as percent (%) caspases activity relative to untreated control.
2.2.7. Assessment of the effect of different caspase inhibitors on Gly treated cervical cancer cells
Gly mediated cytotoxicity on CaSki cells was characterized by using caspase inhibitors. Briefly, CaSki cells were pre-treated (2 h) with 50 μM each of Z-LEHD-FMK, Z-IETD-FMK, and Z-DEVD-FMK (inhibitors of caspase-9, −8 and −3 respectively). Subsequently, cells were re-treated with different concentrations of Gly (20–320 μM) and were additionally incubated for 24 h. The viability of CaSki cells was evaluated using MTT as mentioned in Section 2.2.2.
2.2.8. Quantitative estimation of mitochondrial membrane potential (ΔΨm)
Mitochondrial viability within Gly-treated CASKI cells was estimated by using commercially available kit as per the supplier’s manual. The intensity of fluorescence (Mito-NIR) Dye was assessed 30 min post-addition of assay buffer-B through a microplate reader at excitation/emission = 640/680 nm.
2.2.9. Quantitative assessment of oxidative stress in Gly-reated caski cells
Induction of ROS post Gly treatment was evaluated both qualitatively and quantitatively using DCFH-DA dye as described earlier (Tiwari et al., 2021). Briefly for microscopic evaluation, cells (2 X 104 cells/well) were allowed overnight adherence in a 12 well plate under standard conditions. After incubation cells were dosed with different concentrations of Gly as above and were subsequently incubated for another 12 h under standard conditions. Thereafter, media was decanted and cells were re-treated with DCFH-DA (10 μM; 30 min in the dark at 37 °C). Finally, to remove the surplus stain the cells were washed using PBS if any prior to imaging using green fluorescence channel (Excitation:482/18 nm–Emission:532/59 nm) of Floid imaging station (Thermo-Scientific, USA). The same protocol was followed for quantitative assessment of intracellular ROS during which CaSki cells at the aforesaid quantity were used in a back bottom 96 well plate followed by Gly treatment and 12 h incubation. Post-incubation cells were re-treated with DCFH-DA (10 μM; 30 min) in the dark at RT. Finally, DCF-DA mediated fluorescence intensity was recorded (Excitation: 485 nm and Emission: 528 nm) by fluorescent microplate reader Synergy H1 Hybrid Reader, BioTek, USA. Results were plotted as percentage of fluorescence intensity in comparison with untreated control.
2.2.10. Evaluation of the effect of NAC on Gly-treated CaSki cells
To confirm whether Gly treatment-induced ROS generation within CaSki human CCa cells, N-acetyl-L-cysteine (NAC), a potent ROS inhibitor, was used. CaSki cells were prior treated with NAC (10 mM; 2 h) under standard conditions and then subsequently followed by dosage with Gly (at different concentrations). Post-PBS washing CaSki cells were treated with DCFH-DA (10 μM; 30 min) in the absence of light at RT. Eventually, DCF-DA mediated fluorescence was quantified through micro-plated reader. Furthermore, with the intent of deciphering the role of augmented intracellular ROS generation on initiation of apoptosis within Gly-treated CaSki cells, MTT assay was undertaken to substantiate Gly mediated toxic ramifications on CaSki cells pre-treated with NAC as elaborated in Section 2.2.1.
2.2.11. Cell cycle
Cell cycle assay was performed to study the effect of Gly on the progression of cell cycle phases in CaSki cells as described previously (Ahmad and Ansari, 2021). Concisely, 5 X 105 CaSki cells/well were allowed overnight adherence and afterwards dosed with aforesaid concentrations of Gly and incubated additionally for next 24 h. Cells were then harvested, subjected to centrifugation (500 rpm; 5 min) and incubated briefly with RNase A (50 μg/ml; 30 min) at RT. Subsequently, these were fixed using ice-cold ethanol succeeded by overnight incubation at −20 °C. Finally, Gly pretreated cells were re-treated with PI (25 μg/ml; 15 min) at RT and evaluated using FACS Calibur (BD Biosciences, San Diego, CA, USA).
2.2.12. qPCR analysis
Approximately 106 CaSki cells/well were allowed overnight adherence under standard conditions. After incubation, these were subjected to Gly treatment (above stated concentrations) and DMSO control for an additional 24 h. Thereafter total RNA from respective Gly treated cells was extricated through HiPurATM Total RNA Miniprep Purification Kit (Himedia, India). Initially, 2 µg of RNA extracted from Caski cells post-treatment with Gly was used for preparation of cDNA using commercially available kit. Primers for genes studied herewith were previously described (Park et al., 2014, Zhang et al., 2018, Tsikitis et al., 2005, Sun et al., 2015, Choi et al., 2009, Elumalai et al., 2012, Bordigoni et al., 2021, Liu and Zhou, Jul. 2017) and are mentioned in Table 1. Subsequently, qRT-PCR was performed using qPCR kit (Thermo Scientific, Massachusetts, USA). All quantifications were normalized to the housekeeping glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene. ΔΔCt method was used to calculate fold change in the gene expression.
Table 1.
List of primers used for qPCR.
| S. No. | Target Gene | Sequence of Primers |
|
|---|---|---|---|
| Forward (5′-3′) | Reverse (3′-5′) | ||
| 1. | GAPDH | CGACCACTTTGTCAAGCTCA | CCCCTCTTCAAGGGGTCTAC |
| 2. | Bax | GCTGGACATTGGACTTCCTC | CTCAGCCCATCTTCTTCCAG |
| 3. | Bad | CCTCAGGCCTATGCAAAAAG | AAACCCAAAACTTCCGATGG |
| 4. | Bcl2 | ATTGGGAAGTTTCAAATCAGC | TGCATTCTTGGACGAGGG |
| 5. | CDK4 | CCTGGCCAGAATCTACAGCTA | ACATCTCGAGGCCAGTCATC |
| 6. | p21Cip1 | TGTCCGTCAGAACCCATG | GTGGGAAGGTAGAGCTTGG |
| 7. | CyclinD1 | CTTCCTCTCCAAAATGCCAG | AGAGATGGAAGGGGGAAAGA |
| 8. | HPV E6 | AAT GTT TCA GGA CCC ACA GG | GTT GCT TGC AGT ACA CAC ATTC |
| 9. | HPV E7 | TCA GAG GAG GAG GAT GAA ATAGA | GCA CAA CCG AAG CGT AGA |
| 10. | P53 | TGCGTGTGGAGTATTTGGATG | TGGTACAGTCAGAGCCAACCTC |
| 11. | Hes1 | GAGCAGAGAAAGTCATCAAAGC | ATTTCCAGAATGTCCGCCTTC |
| 12. | Jagged1 | GAAGCAGAACACGGGCGTT | CAGGTCACGCGGATCTGAT |
| 13. | Notch1 | TCAGCGGGATCCACTGTGAG | ACACAGGCAGGTGAACGAGTTG |
2.2.13. Statistical analysis
Data represent mean ± SEM of three independent experiments, each performed in triplicates. Significance among different dosage groups were determined by GraphPad Prism (Ver. 5) using one-way ANOVA followed by Dunnett post-hoc test and two-tailed, paired Student’s t-test. p value < 0.05 was considered significant. *p < 0.05 (significant), **p < 0.01 (very significant) and ***p < 0.001 (highly significant).
3. Results
3.1. Gly treatment suppresses proliferation and promotes cell death
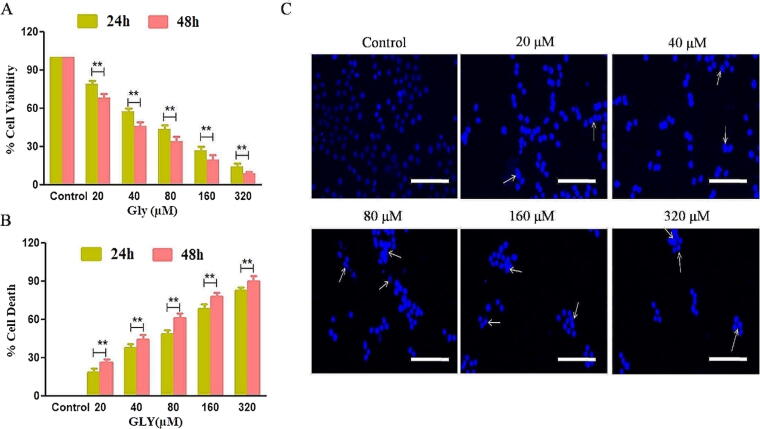
To evaluate the anticancer potential of Gly on the proliferation of cervical cancer cells, we performed MTT assay after treatment of CaSki (HPV16+) cells with Gly for 24 and 48 h. As depicted in Fig. 1A the viability of CaSki cells was reduced significantly in a dose- and time-related manner after treatment with increasing concentrations of Gly (20, 40, 80, 160 and 320 μM) for 24 and 48 h. The cell viability of CaSki cells was decreased to 79.46 ± 2.35%, 57.42 ± 2.63%, 43.73 ± 3.01%, 26.92 ± 2.95% & 14.25 ± 2.68 at 24 h and 68.23 ± 2.97%, 46.00 ± 3.01%, 34.56 ± 3.05%, 19.85 ± 3.70% & 9.30 ± 1.14 at 48 h of treatment with Gly (Fig. 1A).
Fig. 1.
Gly mediated cytotoxic effects on CaSki cells (A) cellular viability, (B) cell death percentage of CaSki cells post-exposure with Gly and (C)Gly induced nuclear condensation and fragmentation in Hoechst33342-stained fluorescent micrographs. Scale = 100 µm.
Similarly, we also reaffirmed the cytotoxic effects of Gly through dye exclusion assay. We observed a substantial amount of cell death by 18.92 ± 2.79%, 38.04 ± 2.77%, 48.89 ± 2.73%, 68.85 ± 2.95% & 82.61 ± 2.72%; 23.61 ± 2.68%, 44.77 ± 3.06%, 61.24 ± 3.72%, 77.87 ± 3.33% & 90.31 ± 4.25 (Fig. 1B) in Gly-treated CaSki cells at mentioned concentrations after 24 and 48 h, respectively.
3.2. Gly promotes apoptosis through extrinsic and intrinsic pathway
To assess the functional impact of Gly treatment, we performed fluorescence microscopic assay by Hoechst 33342 stain to detect nuclear condensation and apoptotic body formations, both of which are peculiar characteristics of apoptosis (Häcker, 2000). We concluded that CaSki cells exhibited bright blue fluorescence directly proportional to the concentration of Gly for 24 h indicating chromatin condensation, nuclear shrinkage, and apoptotic body formation, which are distinctive of early and late apoptosis, whereas untreated or control cells, stained nuclei exhibited flattened morphology with diffuse blue fluorescence as marked by white arrows in the Fig. 1C suggesting the efficacy of Gly in inducing apoptosis and DNA damage in CaSki CCa cells.
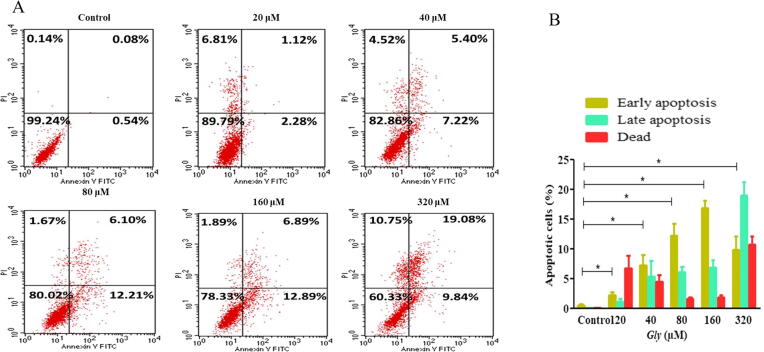
Additionally, these findings were further substantiated by quantitatively estimating the activation of apoptotic pathways by Gly-treatment in CaSki cells. As evident through Fig. 2A, Gly treatment demonstrated enhanced positivity of Annexin V and PI against the ascending range of Gly doses after 24 h. It was observed that Gly exposure resulted in dose-proportional increase of early apoptosis 1.12 ± 0.48% (20 µM), 5.40 ± 2.59% (40 µM), 6.10 ± 0.96% (80 µM), 6.89 ± 1.24% (160 µM) and 19.08 ± 3.89% (320 µM) in contrast with the untreated or control cells (Fig. 2B).
Fig. 2.
Quantification of Gly mediated apoptosis in CaSki cells after 24 h. Within the histogram reported herewith, LR or lower right quadrant marked CaSki cells in early apoptotic phase, the UR (upper right) section elucidates late apoptotic CaSki cells and (B) bar graph of apoptotic cell percentage (%) during flow cytometry evaluation.
Apoptosis or programmed cell death is primarily regulated through Bcl-2 and caspase protein family and is sub-divided into extrinsic- or intrinsic-apoptotic pathways. Consequently, we performed caspase assay on Gly-treated CaSki cells, the findings demonstrated that Gly (20–320 μM) treatment substantially enhanced the caspase-8, −9 & −3 activity by 16.87 ± 4.33%, 32.10 ± 3.93%, 59.05 ± 4.72%, 100.30 ± 3.81% & 149.68 ± 4.64%; 32.65 ± 4.76, 57.06 ± 5.02%, 85.25 ± 4.78%, 119.18 ± 3.55 & 163.21 ± 5.14% and 38.36 ± 3.75%, 65.79 ± 3.18%, 97.87 ± 3.18%, 135.33 ± 3.39 & 198.46 ± 4.96% respectively (Fig. 3A).
Fig. 3.
Gly treatment induced consequences on activation of caspases-8, −9, and −3 within CaSki cells (A) percent (%) modulation of caspase-8, −9, and −3 activities post-Gly exposure at 20–320 µM concentrations, (B-D) inhibitory effects of caspase-8, −9 & −3 inhibitors on viability of Gly-treated CaSki cells, (E) decrease in NIR fluorescence in Gly-treated cells after staining with Mito-NIR dye suggesting depolarization of ΔΨm, (F-H) mRNA expression levels of pro- and anti-apoptotic genes within Gly-treated CaSki cells.
Furthermore, to re-affirm the involvement of caspases in Gly-mediated apoptosis, CaSki cells were pretreated with respective inhibitors of caspase-9, −8 and −3 for 2 h before Gly treatment. The results exhibited that inhibitors pretreatment has entirely abrogated the Gly-mediated apoptosis in CaSki cells (Fig. 3B, C & D), substantiating that Gly induced apoptosis within these cells strictly correlates with activation of caspase.
Mitochondria play an imperative role in mediating apoptosis; altered mitochondrial membrane potential (MMP) indicates apoptosis induction via the mitochondrial-mediated intrinsic pathway. Fig. 3E indicated decrease in NIR fluorescence was evident in Gly-treated cells after staining with Mito-NIR dye, suggesting depolarization of mitochondria comparatively with control where the mitochondria were totally intact. Thus, Gly treatment strongly altered the MMP directly depending upon Gly concentration in cervical cancer CaSki cells.
Moreover, we assessed the mRNA levels of Bax, Bad (pro-apoptotic genes) and Bcl-2 (anti-apoptotic genes) which critically regulate in the mitochondrial-mediated intrinsic pathway. We observed Bax, Bad & Bcl-2 mRNA levels in Gly-treated CaSki cells as measured by qRT-PCR analysis. Intriguingly, Bax and Bad mRNA expression escalated up to 1.85 ± 0.10, 2.69 ± 0.28, 4.05 ± 0.28 & 5.13 folds and 1.61 ± 0.29, 2.09 ± 0.26, 3.38 ± 0.26 & 4.34 ± 0.30 folds respectively, comparatively with untreated control (Fig. 3F & G). Furthermore, the Bcl-2 mRNA levels of was 0.92 ± 0.01, 0.61 ± 0.11, 0.46 ± 0.07 & 0.36 ± 0.07 folds relatively to the control (Fig. 3H). Thus, Gly treatment enhanced pro-apoptotic genes expression with concomitant downregulation of anti-apoptotic genes in CaSki CCa cells.
3.3. Gly aggravates intracellular oxidative stress and modulates GSH/GSSH levels
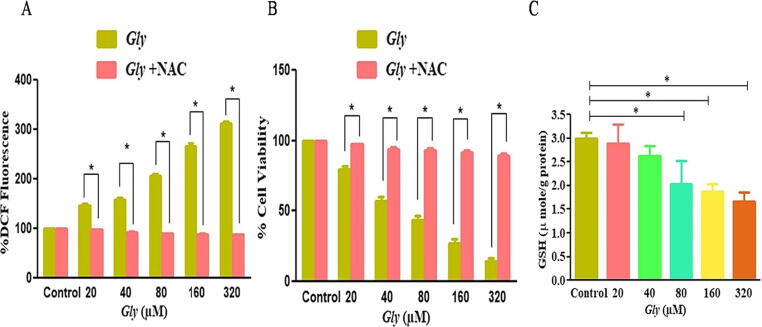
Drug or radiation mediated stress incduced by cancer therapeutics are established ROS inducer. ROS generation is considered as a primitive signal for initiation of apoptosis (Pizzino et al., 2017). Thus, DCFH-DA staining was performed for ascertaining ROS production levels after Gly-treatment in CaSki cells. As depicted in Fig. 4A, increasing the concentration of Gly exacerbated ROS in a dose-related manner within CaSki cells.
Fig. 4.
Augmentation of intracellular ROS within CaSki cells post-Gly exposure (A) fluorescent photomicrographs indicate augmented ROS by DCFH-DA staining post-Gly treatment at different doses (20–320 µM) within CaSki cells after 12 h (scale bar = 100 µm) and (B) quantitative assessment of ROS level as percent (%) DCF-DA fluorescence.
Furthermore, the quantitative estimation of ROS generation was performed to validate above results. As observed, in CaSki cells, intracellular level of ROS was enhanced by 46.71 ± 4.35%, 57.35 ± 4.62%, 105.42 ± 4.85%, 166.88 ± 4.44% and 211.53 ± 4.27% in CaSki cells at the concentrations of 40, 80, 160 and 320 µM Gly, respectively (Fig. 4B). Therefore, these findings substantiated the above finding that Gly escalated ROS generation within CCa cells in a dose-dependent fashion (Fig. 4B).
The findings of our above results suggested that Gly-treatment in CaSki cells mediates ROS generation which might be the center stage for instigation of apoptosis. For evaluating this notion, CaSki cells were pre-treated with ROS inhibitor NAC, before treatment of cells with Gly. Post-incubation a considerable attenuation in ROS production implicating that Gly could enhance the ROS generation and mediates apoptosis (Fig. 5A & B).
Fig. 5.
(A) Estimation of ROS within NAC pre-treated CaSki cells followed by treatment with Gly at varying concentrations (B) Percentage (%) vilability of NAC pre-treated CaSki cells dosed subsequently with Gly and (C) alterations within Glutathione levels in Gly-treated cells. Statistical significance was estimated therough one-way Anova using student’s paired t-test for (A) and (B).
Reduced glutathione (GSH) is a well-known member of antioxidant family which are important for imparting protection against cellular injury induced by excess ROS (Nur et al., 2011, Mizuashi et al., 2005). Conversion of GSH into GSSG (oxidized) is an established marker for portraying the redox environment within a cell (Janicek and Averette, 2001). The GSH levels were quantified to delineate the effect of the Gly-treatment on oxidative stress. Fig. 5C confirmed that Gly-treatment induced a substantial decrease of total GSH levels within every treatment group comparatively with untreated CaSki cells.
3.4. Gly obstructs cell cycle progression at G0/G1 phase
Gly instigated effects modulated the proliferative abilities of CaSki cells was assessed through cytometric cell cycle evaluation. As depicted in Fig. 6A, co-culturing CaSki cells with different doses of Gly quantitatively led to the elevated cells at G0/G1 phase by 57.74 ± 4.06%, 64.84 ± 3.63%, 70.94 ± 3.13%, 78.58 ± 4.54% & 89.26 ± 3.47% comparably with control cells (53.97 ± 3.25) (Fig. 6B).
Fig. 6.
Abrogation of cell cycle progression in CaSki cells exposed with varying Gly doses (A) distribution of PI-stained CaSki cells during cell cycle treated post-Gly exposure and (B) cell cycle distribution elucidated as percent (%) of CaSki cells in different phases of cell cycle.
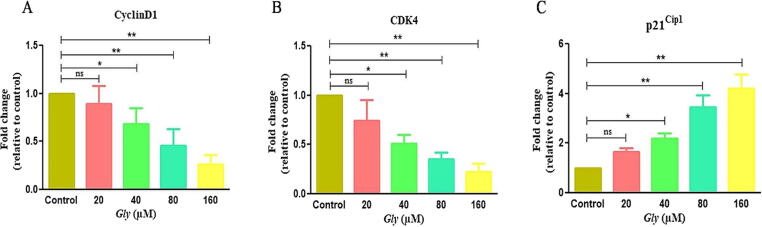
To gain mechanistic insights within quantitative elevation of cells at G0/G1 induced by Gly, expression of G1-phase specific cyclinD1, p21Cip1 and CDK4 genes were evaluated. Firstly, we elaborated Gly-effect on cyclin D1 expression, an important protein governing G1 phase transitions, in CCa cells. Gly-dosage curtailed substantially cyclin D1 mRNA expression to 0.89 ± 0.15, 0.68 ± 0.13, 0.46 ± 0.13 and 0.26 ± 0.07 folds comparatively with the control (Fig. 7A).
Fig. 7.
Effect of Gly on mRNA expression of genes playing important regulatory roles in progression of cell cycle as analyzed by qRT-PCR.
Furthermore, we scrutinized Gly instigated effects on mRNA level of CDK4 in CCa cells. As shown in Fig. 7B, post-Gly exposure in CaSki cells, significant decreased in CDK4 mRNA expression was confirmed viz. 0.74 ± 0.16, 0.51 ± 0.07, 0.35 ± 0.05 and 0.22 ± 0.11 folds, relative to the control. Eventually, the regulatory effect of Gly on p21Cip1 mRNA expression, within CaSki cells was also assessed through qRT-PCR after 24 h of treatment. Our results illustrated that Gly increased p21Cip1 mRNA expression proportionally to its concentration within CCa cell. As shown in Fig. 7C with Gly-treatment substantialy increased p21Cip1 mRNA expression to 1.65 ± 0.13, 2.19 ± 0.18, 3.45 ± 0.39 and 4.21 ± 0.44 folds, at respective concentrations, relatively to the untreated cells.
3.5. Gly downregulates HPV oncoproteins (E6 and E7) expression, restores levels of p53 and inhibits Notch cascade
Notch cascade represents a highly conserved signaling cascade implicated in regulating key stages governing cellular differentiation and proliferation. Notch-1 receptor expression is increased when cervical intraepithelial lesions (CIN) is progressed into metastatic cervical carcinoma. Moreover, HPV encoded E6 and E7 oncoproteins are main causative agent of CCa. Finally, we studied the effect of Gly on p53 expression. Gly-treatment increased p53 mRNA expression to 2.19 ± 0.37, 2.48 ± 0.37, 3.72 ± 0.08 and 5.16 ± 0.47 folds, respectively, in comparison with control cells (Fig. 8A).
Fig. 8.
Effects of Gly treatment on mRNA expression levels of (A) tumor suppressor genes (p53), (B & C) HPV oncogenes and components of Notch signaling cascade (D, E & F).
Furthermore, we also studied the expression of HPV oncoproteins mRNA particularily E6 along with E7 on Gly-treated CaSki CCa cells. Evidently, it was observed that Gly impeded HPV E6 expression by 0.84 ± 0.11, 0.57 ± 0.07, 0.42 ± 0.07 and 0.29 ± 0.08 folds; and E7 by 0.64 ± 0.12, 0.42 ± 0.04, 0.27 ± 0.05 and 0.18 ± 0.04 folds, at 20, 40, 80 and 160 μM, respectively (Fig. 8B & C).
Moreover, we outlined the Gly effect on Notch signaling molecules and its subsequent target namely Hes-1 within CaSki cells. As exhibited in the Fig. 6 and Fig. 8D, Gly lowered Notch-1 mRNA expression within CaSki cells; however, the reduction in Notch-1 receptor RNA profoundly depended on Gly concentration. As observed, Gly reduced Notch-1 mRNA expression to 0.82 ± 0.15, 0.49 ± 0.12, 0.34 ± 0.12 and 0.16 ± 0.03 folds, relatively to the untreated CaSki cells (Fig. 6) (Fig. 8D). Moreover, it was also proved that Gly-treatment abated mRNA expression of Jagged-1 and Notch-1 to 0.89 ± 0.18, 0.64 ± 0.16, 0.46 ± 0.09, and 0.28 ± 0.04; 0.85 ± 0.13, 0.54 ± 0.14, 0.31 ± 0.09 and 0.17 ± 0.03 folds, respectively, relative to the control (Figs. 6 and 9) (Fig. 8E & F).
Fig. 9.
Flowchart representation of the mechanism of action of Gly against CCa. Gly suppressed the proliferation of CaSki cells and significantly inhibited the Notch pathway along with the downregulation of HPV E6/E7 oncogenes resulting in apoptosis of cervical cancer cells.
4. Discussion
In spite of the availability of a number of preventive modalities such as vaccines and screening programs, CCa still regarded as the deadliest malignancy of females (Mizuashi et al., 2005). HPV infection is regarded to be the most crucial impetus behind the occurrence and prevalence of CCa (Janicek and Averette, 2001, Sung et al., 2021). Among all HPVs, HPV-16 and HPV-18 are documented in pathogenesis of 70% CCa (Moore et al., 2012). Recurrening and metastatic cinical presentation of the disease is the leading cause of CCa related fatalities (Lui et al., 2009, Janicek and Averette, 2001).
Advanced stages of CCa are often remains untreatable due to several alterations occurring at cellular and molecular level which consequently aggravates the invasiveness and resistance towards chemo and radio-therapy (Carter and Downs Jr, 2011, Galluzzi et al., 2018). Thus, newer therapeutic modalities are the need of the hour to evade the resistance developed against chemo- and/or radio-therapeutics. During past few decades, the natural or bioactive compounds have gained considerable attention due to their reduced toxicity levels as compared with the current chemotherapeutic drugs (Ahmad and Ansari, 2021). Gly has substantial nutraceutical potential partially evident by its gentle woody flavor, limiting its use as a sweetening indegredient. Furthermore, it is a common flavouring agent which potentially balances the bitter flavor. Earlier reports have elucidated glycyrrhizin mediated medicinal attributes including its anti-inflammatory, anti-viral and anti-cancer among several others (Kao et al., 2014).
In the present report, the authors provided evidences demonstrating anticancer and antiapoptotic potential of Gly in HPV-16+ human CCa cells (CaSki) as an in vitro system. The observations indicated that Gly suppressed the growth of CaSki cells along with its proliferation. A drug can affect a cancer cell by various mechanisms like autophagy, apoptosis and necrosis. Among them, apoptosis involve the killing of cancer cells (Bhakkiyalakshmi et al., 2016). Thus, instigation of apoptotic mechanisms upon treatment of a plausible therapeutic candidate is considered to be a potent strategy for eliminating the cancer cells. A number of previous studies suggest the potential of triterpenes for its anticancer efficacy against several in vitro systems by instigating apoptosis (Liang et al., 2013); however, studies demonstrating the mechanism behind the anticancer potential of Gly in CCa are limited. Earlier reports of our group succeeded in delineating the anticancer and antiapoptosis potential of Gly in C33A (HPV−) CCa cells through inhibition of Notch signaling cascade (Ahmad et al., 2021). Here we have subsequently investigated the antiproliferative and apoptosis inducing efficacy of Gly by studying its role on Notch pathway and HPV oncoproteins (E6 and E7).
Determination of cytotoxicity is a primary step to establish the anticancer efficacy of any plausible anti-cancer therapeutics. We observed reduced viability of CaSki cells by MTT assay when treated with Gly and the result was in accordance with trypan blue assay. This was in line with previous findings which also showcased anticancer efficacy of Gly in several in vitro models of cancers (Taylor et al., 2008, Nica et al., 2008). Abrupt nuclear morphology are the trademarks of apoptotic cell death. Our results showed an increased chromatin condensation by Hoechst-33342 stain. Moreover, the apoptotic potential of Gly in CaSki cells was further confirmed through FITC-Annexin V/PI mediated cytometric assay. It was observed that Gly significantly amplified the percentage of cells stained positively for Annexin-V in a dose-dependent manner within CCa cells suggesting the apoptotic potential of Gly.
Caspases are the critical modulators of apoptosis and are family members of the cysteine proteases (Kaufmann and Hengartner, 2001). During apoptosis the activation of caspases is mediated by the death-receptor pathway in which caspase-8 acts as an instigator. It also mediates downstream activation of mitochondria-dependent apoptotic pathway resulting from accumulation of cytosolic cytochrome-c level (Ahmad et al., 2021, Nita and Grzybowski, 2016). However, cumulative activation of these pathways eventually results in cleavage-dependent caspase-3 activation. Our findings were also in accordance with the above stated mechanism since initiation of apoptosis, after treatment with Gly, was associated with augmented ROS, dissipation of ΔΨm, elevated activities of caspases along with the modulation in the expression of proteins associated with apoptosis. Therefore it can be implicated that Gly-mediated of apoptosis involved activation of caspases. Moreover, Gly-induced cytotoxicity was substantially ameliorated by caspase-8, −9, and −3 inhibitors repectively, thereby delineating the importance of caspase- 8, −9, and −3 activation in apoptosis upon treatment with Gly.
Mitochondria are primary cellular sites involved in generation of ROS leading to mitochondrial dysfunction and ΔΨm dissipation, leading to cytochrome-c release (Poot et al., 1995). We observed a marked difference in ΔΨm post-Gly exposure, thereby activating caspase-3, which is indespensible for its role of activating endonucleases along with several other cellular proteins during apoptosis endonucleases (Gamcsik et al., 2012). We found that the Gly enhances the level of ROS subsequently instigating apoptosis. Furthermore, we investigated whether elevation in ROS production was linked with dissipation of ΔΨm and apoptosis post-Gly exposure. Augmentation of intracellular ROS within Gly-pretreated CaSki cells was evident which could be correlated with mitochondrial pathway of apoptosis.
GSH belonging to non-peptide thiol group is a predominant antioxidant system commonly expressed in several cells of mammalian origin and also in malignant cells. Elevated GSH level correlates with enhanced proliferation within several cell types (Estrela et al., 1995). Generally, the level of GSH in cancerous cells is elevated comparatively than the non-cancerous cells and thus malignant cells exhibit drug resistance (Circu and Aw, 2012). In the present report, endogenous level of GSH declined significantly post-Gly exposure within CaSki cells. Accumulating research provided compelling evidence to suggest that the reduction of GSH is regarded as a marker of apoptosis induction in response multiple proapoptotic stimuli (Thayyullathil et al., 2011). Therefore, our findings suggested that the Gly-mediated apoptosis induction in CaSki CCa is in line with the notion that GSH depletion favors programmed cell death.
Abrupt notch cascade is reported in several malignancies of human such as breast, cancer, lung and cervical among others. Moreover, elevated levels of Notch-1 receptor are documented for its direct correlation with progression of CCa grading (Yang et al., 2020, Sun et al., 2015). Our present report hypothesized that Gly may obstruct the notch cascade within CCa in vitro. Our data signified that treatment of Gly downregulated Notch cascade via Notch-1 and Jagged-1 inhibition within treated CCa cells. Also, Gly inhibited the expression of Hes-1 mRNA which is a crucial Notch-1 downstream target. Furthermore, we demonstrated that the treatment of Gly arrested the progression of CaSki cells at G0/G1 phase accompanied by inhibition of Notch pathway. Our findings suggested that Gly exerted significant anti-proliferative effects on CCa cells by restraining the progession of cell cycle at G0/G1 phase. Cyclins and their dependent kinases also known as CDKs are responsible for homeostatic regulation of cell cycle whereas inactivation of CDKs is responsible for cell cycle arrest. We showed inhibitory effect of Gly on cyclin D1 and CDK4 in CCa cells implicating its involvement in regulation of proteins involved during cell cycle. The inhibitors of CDKs including p21/WAF1 and p27/KIP1 family members regulate the activities of CDKs. The reported findings demonstrated that Gly increased the expression of p21 mRNA within CCa cells. Therefore, all these findings suggested that Gly has potential of arresting the cell cycle progression via regulating the cell cycle regulatory protein expression to suppress the growth of CCa cells. Altogether, our data concluded that apoptosis induction and growth suppression in Gly-treated CaSki cells were associated with the Notch pathway's inhibition. The plausible mechanistic action of Gly against CCa cells is summarized in Fig. 9.
Furthermore, we have also studied the mechanistic details of Gly mediated antiproliferative efficacy by investigating its consequences on HPV-associated oncogenes particularily E6 and E7. It is previously reported that E6 and E7 oncoproteins exerts their effects on p53 and pRb by escalating their degradation mediated by ubiquitin (Scheffner et al., 1991). Our data demonstrated that Gly impeded the expression of E6 and E7 oncoproteins mRNA within CaSki CCa cells. Therefore, it could be anticipated that agents with the ability to suppress these specific oncogenes may lead to re-activation of pathways culminating in suppression of tumor growth and have effective therapeutical implications against CCa. Our results substantiated that the treatment of Gly downregulated E6 and E7 expression along with restoring p53expression in CCa cells.
5. Conclusion
To conclude, the inhibitory efficacy of Gly on CCa cells resulted from multiple factors. It was observed that Gly exerted substantial cytotoxic effect in CCa cells (CaSki). Furthermore, results from various experiments established that the cytotoxic efficacy of Gly was due to the involvement of both extrinsic and intrinsic apoptotic pathways along with disruption of cell cycle progression. Gly treatment mediates the inhibition of notch pathway along with the downregulation HPV E6 & E7oncoproteins in CCa cells. Thus, we could conclude that Gly suppressed CCa proliferation in vitro via lowering HPV oncoproteins expression and notch pathway. Thus, Gly is a plausible natural chemotherapeutic compound for the treatment and management of CCa, and its anticancer mechanism deserves further investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
This research was funded by the Ministry of Education in KSA, grant number IFP-KKU-2020/14. Kindly please add funding statement after declarartion of competing interest statement.
Acknowledgments
The authors extend their appreciation to the Ministry of Education in KSA for funding this research through project number IFP-KKU-2020/14.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohd Saeed, Email: Mo.saeed@uoh.edu.sa.
Irfan Ahmad Ansari, Email: ahmadirfan.amu@gmail.com.
References
- Aftab M., Poojary S.S., Seshan V., Kumar S., Agarwal P., Tandon S., Zutshi V., Das B.C. Urine miRNA signature as a potential non-invasive diagnostic and prognostic biomarker in cervical cancer. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-89388-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Afza, et al. Glycyrrhizin Mediates Downregulation of Notch Pathway Resulting in Initiation of Apoptosis and Disruption in the Cell Cycle Progression in Cervical Cancer Cells. Nutr. Cancer. 2021:1–18. doi: 10.1080/01635581.2021.1895234. [DOI] [PubMed] [Google Scholar]
- Ahmad A., Ansari I.A. Intervening Stemness and Angiogenesis in Cervical Carcinoma: Revisiting the Efficacy of Natural Compounds. EMIDDT. 2021;21(10):1709–1712. doi: 10.2174/1871530321999201211214608. [DOI] [PubMed] [Google Scholar]
- Ahmad A., Ansari I.A. Carvacrol Exhibits Chemopreventive Potential against Cervical Cancer Cells via Caspase-Dependent Apoptosis and Abrogation of Cell Cycle Progression. ACAMC. 2021;21(16):2224–2235. doi: 10.2174/1871520621999201230201258. [DOI] [PubMed] [Google Scholar]
- Ahmad A., Tiwari R.K., Almeleebia T.M., Al Fayi M.S., Alshahrani M.Y., Ahmad I., Abohassan M.S., Saeed M., Ansari I.A. Swertia chirayita suppresses the growth of non-small cell lung cancer A549 cells and concomitantly induces apoptosis via downregulation of JAK1/STAT3 pathway. Saudi J. Biol. Sci. 2021;28(11):6279–6288. doi: 10.1016/j.sjbs.2021.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari I.A., Ahmad A., Imran M.A., Saeed M., Ahmad I. Organosulphur Compounds Induce Apoptosis and Cell Cycle Arrest in Cervical Cancer Cells via Downregulation of HPV E6 and E7 Oncogenes. ACAMC. 2021;21(3):393–405. doi: 10.2174/1871520620999200818154456. [DOI] [PubMed] [Google Scholar]
- Aster J.C., Pear W.S., Blacklow S.C. The Varied Roles of Notch in Cancer. Annu. Rev. Pathol. Mech. Dis. 2017;12(1):245–275. doi: 10.1146/annurev-pathol-052016-100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylon Y., Oren M. p53: guardian of ploidy. Mol. Oncol. 2011;5(4):315–323. doi: 10.1016/j.molonc.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakkiyalakshmi E., Suganya N., Sireesh D., Krishnamurthi K., Saravana Devi S., Rajaguru P., Ramkumar K.M. Carvacrol induces mitochondria-mediated apoptosis in HL-60 promyelocytic and Jurkat T lymphoma cells. Eur. J. Pharmacol. 2016;772:92–98. doi: 10.1016/j.ejphar.2015.12.046. [DOI] [PubMed] [Google Scholar]
- Bordigoni A., Motte A., Tissot-Dupont H., Colson P., Desnues C. Development and validation of a multiplex qPCR assay for detection and relative quantification of HPV16 and HPV18 E6 and E7 oncogenes. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-83489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J.S., Downs Jr L.S. Cervical Cancer Tests and Treatment. Female Pat. 2011;36(1):34–37. [PMC free article] [PubMed] [Google Scholar]
- Choi K., Ahn Y.-H., Gibbons D.L., Tran H.T., Creighton C.J., Girard L., Minna J.D., Qin F.-F., Kurie J.M. Distinct biological roles for the notch ligands Jagged-1 and Jagged-2. J. Biol. Chem. 2009;284(26):17766–17774. doi: 10.1074/jbc.M109.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circu M.L., Aw T.Y. Glutathione and modulation of cell apoptosis. BBA. 2012;1823(10):1767–1777. doi: 10.1016/j.bbamcr.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin. Sci. (London, England) 1979;110(5) doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- Du C., Deng D., Shan L., Wan S., Cao J., Tian J., Achilefu S., Gu Y. A pH-sensitive doxorubicin prodrug based on folate-conjugated BSA for tumor-targeted drug delivery. Biomaterials. 2013;34(12):3087–3097. doi: 10.1016/j.biomaterials.2013.01.041. [DOI] [PubMed] [Google Scholar]
- Elumalai P., Gunadharini D.N., Senthilkumar K., Banudevi S., Arunkumar R., Benson C.S., Sharmila G., Arunakaran J. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol. Lett. 2012;215(2):131–142. doi: 10.1016/j.toxlet.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Engel-Pizcueta C., Pujades C. Interplay Between Notch and YAP/TAZ Pathways in the Regulation of Cell Fate During Embryo Development. Front. Cell Dev. Biol. 2021;9(711531):19. doi: 10.3389/fcell.2021.711531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrela J.M., Obrador E., Navarro J., De La Vega M.C.L., Pellicer J.A. Elimination of Ehrlich tumours by ATP-induced growth inhibition, glutathione depletion and X-rays. Nat. Med. 1995;1(1):84–88. doi: 10.1038/nm0195-84. [DOI] [PubMed] [Google Scholar]
- Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., Annicchiarico-Petruzzelli M., Antonov A.V., Arama E., Baehrecke E.H., Barlev N.A., Bazan N.G., Bernassola F., Bertrand M.J.M., Bianchi K., Blagosklonny M.V., Blomgren K., Borner C., Boya P., Brenner C., Campanella M., Candi E., Carmona-Gutierrez D., Cecconi F., Chan F.-M., Chandel N.S., Cheng E.H., Chipuk J.E., Cidlowski J.A., Ciechanover A., Cohen G.M., Conrad M., Cubillos-Ruiz J.R., Czabotar P.E., D’Angiolella V., Dawson T.M., Dawson V.L., De Laurenzi V., De Maria R., Debatin K.-M., DeBerardinis R.J., Deshmukh M., Di Daniele N., Di Virgilio F., Dixit V.M., Dixon S.J., Duckett C.S., Dynlacht B.D., El-Deiry W.S., Elrod J.W., Fimia G.M., Fulda S., García-Sáez A.J., Garg A.D., Garrido C., Gavathiotis E., Golstein P., Gottlieb E., Green D.R., Greene L.A., Gronemeyer H., Gross A., Hajnoczky G., Hardwick J.M., Harris I.S., Hengartner M.O., Hetz C., Ichijo H., Jäättelä M., Joseph B., Jost P.J., Juin P.P., Kaiser W.J., Karin M., Kaufmann T., Kepp O., Kimchi A., Kitsis R.N., Klionsky D.J., Knight R.A., Kumar S., Lee S.W., Lemasters J.J., Levine B., Linkermann A., Lipton S.A., Lockshin R.A., López-Otín C., Lowe S.W., Luedde T., Lugli E., MacFarlane M., Madeo F., Malewicz M., Malorni W., Manic G., Marine J.-C., Martin S.J., Martinou J.-C., Medema J.P., Mehlen P., Meier P., Melino S., Miao E.A., Molkentin J.D., Moll U.M., Muñoz-Pinedo C., Nagata S., Nuñez G., Oberst A., Oren M., Overholtzer M., Pagano M., Panaretakis T., Pasparakis M., Penninger J.M., Pereira D.M., Pervaiz S., Peter M.E., Piacentini M., Pinton P., Prehn J.H.M., Puthalakath H., Rabinovich G.A., Rehm M., Rizzuto R., Rodrigues C.M.P., Rubinsztein D.C., Rudel T., Ryan K.M., Sayan E., Scorrano L., Shao F., Shi Y., Silke J., Simon H.-U., Sistigu A., Stockwell B.R., Strasser A., Szabadkai G., Tait S.W.G., Tang D., Tavernarakis N., Thorburn A., Tsujimoto Y., Turk B., Vanden Berghe T., Vandenabeele P., Vander Heiden M.G., Villunger A., Virgin H.W., Vousden K.H., Vucic D., Wagner E.F., Walczak H., Wallach D., Wang Y., Wells J.A., Wood W., Yuan J., Zakeri Z., Zhivotovsky B., Zitvogel L., Melino G., Kroemer G. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamcsik M.P., Kasibhatla M.S., Teeter S.D., Colvin O.M. Glutathione levels in human tumors. Biomarkers. 2012;17(8):671–691. doi: 10.3109/1354750X.2012.715672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Cancer Observatory. https://gco.iarc.fr/today/fact-sheets-cancers (Accessed on 18 September, 2021).
- Häcker G. The morphology of apoptosis. Cell Tissue Res. 2000;301(1):5–17. doi: 10.1007/s004410000193. [DOI] [PubMed] [Google Scholar]
- Howie H.L., Katzenellenbogen R.A., Galloway D.A. Papillomavirus E6 proteins. Virology. 2009;384(2):324–334. doi: 10.1016/j.virol.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicek M.F., Averette H.E. Cervical cancer: prevention, diagnosis, and therapeutics. CA Cancer J. Clin. 2001;51(2):92–114. doi: 10.3322/canjclin.51.2.92. quiz 115–8. [DOI] [PubMed] [Google Scholar]
- Janicek M.F., Averette H.E. “Cervical cancer: prevention, diagnosis, and therapeutics”. CA: a cancer journal for clinicians. CA Cancer J. Clin. 2001;51(2):92–114. doi: 10.3322/canjclin.51.2.92. [DOI] [PubMed] [Google Scholar]
- Jeong H., Huh MyungSook, Lee S.J., Koo H., Kwon I.C., Jeong S.Y., Kim K. Photosensitizer-Conjugated Human Serum Albumin Nanoparticles for Effective Photodynamic Therapy. Theranostics. 2011;1:230–239. doi: 10.7150/thno/v01p0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao T.-C., Wu C.-H., Yen G.-C. Bioactivity and Potential Health Benefits of Licorice. J. Agric. Food Chem. 2014;62(3):542–553. doi: 10.1021/jf404939f. [DOI] [PubMed] [Google Scholar]
- Kaufmann S.H., Hengartner M.O. Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 2001;11(12):526–534. doi: 10.1016/s0962-8924(01)02173-0. [DOI] [PubMed] [Google Scholar]
- Li D. The Notch ligand Jagged1 as a target for anti-tumor therapy. Front. Oncol. 2014;4 doi: 10.3389/fonc.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W.-Z., Chou C.-T., Lu T.i., Chi C.-C., Tseng L.-L., Pan C.-C., Lin K.-L., Kuo C.-C., Jan C.-R. The mechanism of carvacrol-evoked [Ca2+]i rises and non-Ca2+-triggered cell death in OC2 human oral cancer cells. Toxicology. 2013;303:152–161. doi: 10.1016/j.tox.2012.10.026. [DOI] [PubMed] [Google Scholar]
- Lin C.-J., Kuan C.-H., Wang L.-W., Wu H.-C., Chen Y., Chang C.-W., Huang R.-Y., Wang T.-W. Integrated self-assembling drug delivery system possessing dual responsive and active targeting for orthotopic ovarian cancer theranostics. Biomaterials. 2016;90:12–26. doi: 10.1016/j.biomaterials.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Liu H., Zhou M. Evaluation of p53 gene expression and prognosis characteristics in uveal melanoma cases. OncoTarg. Ther. 2017;10(3429–3434):12. doi: 10.2147/OTT.S136785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui Vivian W.Y., et al. Cucurbitacin I elicits anoikis sensitization, inhibits cellular invasion and in vivo tumor formation ability of nasopharyngeal carcinoma cells. Carcinogenesis. 2009;30(12):2085–2094. doi: 10.1093/carcin/bgp253. [DOI] [PubMed] [Google Scholar]
- Mizuashi M., Ohtani T., Nakagawa S., Aiba S. Redox Imbalance Induced by Contact Sensitizers Triggers the Maturation of Dendritic Cells. J. Invest. Dermatol. 2005;124(3):579–586. doi: 10.1111/j.0022-202X.2005.23624.x. [DOI] [PubMed] [Google Scholar]
- Moon E.-K., Oh C.-M., Won Y.-J., Lee J.-K., Jung K.-W., Cho H., Jun J.K., Lim M.C., Ki M. Trends and Age-Period-Cohort Effects on the Incidence and Mortality Rate of Cervical Cancer in Korea. Cancer Res. Treat. 2017;49(2):526–533. doi: 10.4143/crt.2016.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E.E., Wark J.D., Hopper J.L., Erbas B., Garland S.M. The roles of genetic and environmental factors on risk of cervical cancer: a review of classical twin studies. Twin Res. Hum. Gen. 2012;15(1):79–86. doi: 10.1375/twin.15.1.79. [DOI] [PubMed] [Google Scholar]
- Moss C., Kaye S.B. Ovarian cancer: progress and continuing controversies in management. Eur. J. Cancer (Oxford, England) 2002;38(13):1701–1707. doi: 10.1016/s0959-8049(02)00161-2. [DOI] [PubMed] [Google Scholar]
- Nica A.F., Tsao C.C., Watt J.C., Jiffar T., Kurinna S., Jurasz P., Konopleva M., Andreeff M., Radomski M.W., Ruvolo P.P. Ceramide promotes apoptosis in chronic myelogenous leukemia-derived K562 cells by a mechanism involving caspase-8 and JNK. Cell Cycle. 2008;7(21):3362–3370. doi: 10.4161/cc.7.21.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nita M., Grzybowski A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longevity. 2016;2016:1–23. doi: 10.1155/2016/3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nur E., Verwijs M., de Waart D.R., Schnog J.-J., Otten H.-M., Brandjes D.P., Biemond B.J., Elferink R.P.J.O. Increased efflux of oxidized glutathione (GSSG) causes glutathione depletion and potentially diminishes antioxidant defense in sickle erythrocytes. BBA. 2011;1812(11):1412–1417. doi: 10.1016/j.bbadis.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Park Y.J., Kim E.K., Moon S., Hong D.P., Bae J.Y., Kim J. Human telomerase reverse transcriptase is a promising target for cancer inhibition in squamous cell carcinomas. Anticancer Res. 2014;34(11):6389–6395. [PubMed] [Google Scholar]
- Pfeffer C., Singh A. Apoptosis: A Target for Anticancer Therapy. IJMS. 2018;19(2):448. doi: 10.3390/ijms19020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longevity. 2017;2017:1–13. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poot M., Teubert H., Rabinovitch P.S., Kavanagh T.J. De novo synthesis of glutathione is required for both entry into and progression through the cell cycle. J. Cell. Physiol. 1995;163(3):555–560. doi: 10.1002/jcp.1041630316. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Munger K., Byrne J.C., Howley P.M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc. Natl. Acad. Sci. 1991;88(13):5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen Subhrojit, et al. Ameliorative effects of glycyrrhizin on streptozotocin-induced diabetes in rats. J. Pharm. Pharmacol. 2011;63(2):287–296. doi: 10.1111/j.2042-7158.2010.01217.x. [DOI] [PubMed] [Google Scholar]
- Sun Yan, et al. Overexpression of Notch1 is associated with the progression of cervical cancer. Oncol. Lett. 2015;9(6):2750–2756. doi: 10.3892/ol.2015.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Xu H., Dai T., Xie L., Zhao Q., Hao X., Sun Y., Wang X., Jiang N., Sang M. Alantolactone inhibits cervical cancer progression by ownregulating BMI1. Sci. Rep. 2021;11(1):9251. doi: 10.1038/s41598-021-87781-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D.W., Zhang H.D., Mao L., Mao C.F., Chen W., Cui M., Ma R., Cao H.X., Jing C.W., Wang Z., Wu J.Z., Tang J.H. Luteolin Inhibits Breast Cancer Development and Progression In Vitro and In Vivo by Suppressing Notch Signaling and Regulating MiRNAs. Cell. Physiol. Biochem. 2015;37(5):1693–1711. doi: 10.1159/000438535. [DOI] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Talora C., Sgroi D.C., Crum C.P., Dotto G.P. Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation. Genes Dev. 2002;16(17):2252–2263. doi: 10.1101/gad.988902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R.C., Cullen S.P., Martin S.J. Apoptosis: controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008;9(3):231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- Thayyullathil F., Chathoth S., Kizhakkayil J., Galadari A., Hago A., Patel M., Galadari S. Glutathione selectively inhibits Doxorubicin induced phosphorylation of p53Ser15, caspase dependent ceramide production and apoptosis in human leukemic cells. Biochem. Biophys. Res. Commun. 2011;411(1):1–6. doi: 10.1016/j.bbrc.2011.05.156. [DOI] [PubMed] [Google Scholar]
- Tiwari R.K., Singh S., Gupta C.L., Pandey P., Singh V.K., Sayyed U., Shekh R., Bajpai P. Repolarization of glioblastoma macrophage cells using non-agonistic Dectin-1 ligand encapsulating TLR-9 agonist: plausible role in regenerative medicine against brain tumor. Int. J. Neurosci. 2021;131(6):591–598. doi: 10.1080/00207454.2020.1750393. [DOI] [PubMed] [Google Scholar]
- Tsikitis M., Zhang Z., Edelman W., Zagzag D., Kalpana G.V. Genetic ablation of Cyclin D1 abrogates genesis of rhabdoid tumors resulting from Ini1 loss. Proc. Natl. Acad. Sci. USA. 2005;102(34):12129–12134. doi: 10.1073/pnas.0505300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrich K., Michaelis M., Rothweiler F., Knobloch T., Sithisarn P., Cinatl J., Kreuter J. Interaction of folate-conjugated human serum albumin (HSA) nanoparticles with tumour cells. Int. J. Pharm. 2011;406(1-2):128–134. doi: 10.1016/j.ijpharm.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Yang L., Shi P., Zhao G., Xu J., Peng W., Zhang J., Zhang G., Wang X., Dong Z., Chen F., Cui H. Targeting cancer stem cell pathways for cancer therapy. Sig. Transduct. Target Ther. 2020;5(1) doi: 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.-L., Liu G.-C., Peng L.i., Zhang C., Jia Y.-M., Yang W.-H., Mao L. Effect of PAK1 gene silencing on proliferation and apoptosis in hepatocellular carcinoma cell lines MHCC97-H and HepG2 and cells in xenograft tumor. Gene Ther. 2018;25(4):284–296. doi: 10.1038/s41434-018-0016-9. [DOI] [PubMed] [Google Scholar]
- Zur Hausen H. Papillomavirus infections–a major cause of human cancers. BBA. 1996;1288(2):F55–F78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]