Abstract
Background
Tranexamic acid (TXA) is an antifibrinolytic agent applied in orthopedic surgery and has been proven to reduce post-surgery infection rates. We previously showed that TXA also had an additional direct antimicrobial effect against planktonic bacteria. Therefore, we aimed to evaluate whether it has a synergistic effect if in combination with antibiotics.
Materials and Methods
Three ATCC and seven clinical strains of staphylococci were tested against serial dilutions of vancomycin and gentamicin alone and in combination with TXA at 10 and 50 mg/ml. The standardized microtiter plate method was used. Minimal inhibitory concentrations (MICs) were calculated by standard visualization of well turbidity (the lowest concentration at which complete absence of well bacterial growth was observed by the researcher) and using the automated method (the lowest concentration at which ≥80% reduction in well bacterial growth was measured using a spectrophotometer).
Results
Tranexamic acid-10 mg/ml reduced the MIC of vancomycin and gentamicin with both the standard method (V: 1-fold dilution, G: 4-fold dilutions) and the automated turbidity method (vancomycin: 8-fold dilutions, gentamicin: 8-fold dilutions). TXA-50 mg/ml reduced the MIC of gentamicin with both the standard turbidity method (6-fold dilutions) and the automated turbidity method (1-fold dilutions). In contrast, for vancomycin, the MIC remained the same using the standard method, and only a 1-fold dilution was reduced using the automated method.
Conclusion
Ours was a proof-of-concept study in which we suggest that TXA may have a synergistic effect when combined with both vancomycin and gentamicin, especially at 10 mg/ml, which is the concentration generally used in clinical practice.
Keywords: tranexamic acid, bacterial growth, antimicrobial effect, synergy, in vitro model
Introduction
Periprosthetic joint infections (PJIs) are caused by bacterial biofilm that adheres to the prosthesis and hampers eradication of infection once established (Josse et al., 2019; Shoji and Chen, 2020). Therefore, preventive strategies are necessary to reduce the frequency of PJIs at the time of surgery (Kapadia et al., 2016; Romanò et al., 2017).
Tranexamic acid (TXA) is an antifibrinolytic agent used in daily clinical practice as it binds to plasminogen and enables blood clotting (Jennings et al., 2016; Draxler et al., 2019). Numerous studies support the use of this drug in surgical interventions, specifically in orthopedic surgery and traumatology, as it has improved the postoperative management of patients undergoing both elective and emergency surgeries without leading to increase in complications among the healthy population (Melvin et al., 2015; Jennings et al., 2016; Waddell et al., 2016). Some authors hypothesize that as TXA reduces local hematoma, it could indirectly reduce infection rates (Ruder and Springer, 2017; Klement et al., 2020; Lacko et al., 2020; Sukeik et al., 2020; Yazdi et al., 2020).
The hypothesis of whether, in addition to its anti-fibrinolytic action, TXA could have a direct antibiotic effect similar to that observed with hyaluronic acid is an interesting area of research (Romanò et al., 2017). Therefore, given that this effect may be related to biofilm formation and have an antibacterial effect against planktonic cells under experimental conditions (Zhang et al., 2021; Benjumea et al., 2022), we assessed whether TXA could have a synergistic effect when in combination with vancomycin and gentamicin, which are two of the most commonly used antibiotics in orthopedic surgery (Ratto et al., 2016; Myers and Lipof, 2020; Indelli et al., 2021; Parvizi, 2021).
Materials and Methods
Ours was an in vitro experimental study carried out at Hospital General Universitario Gregorio Marañón, Madrid, Spain (Supplementary Material 1; Behzadi and Gajdács, 2021).
We included 10 strains: three American Type Culture Collection (ATCC) strains (methicillin-susceptible Staphylococcus aureus [MSSA; ATCC29213], methicillin-resistant Staphylococcus aureus [MRSA; ATCC43300], and methicillin-resistant Staphylococcus epidermidis [MRSE; ATCC35984]), and seven high-biofilm-producing clinical strains of MSSA (n = 3), MRSA (n = 1), and MRSE (n = 3) isolated from patients with PJIs.
Tranexamic Acid at Increasing Concentrations
We first tested serial dilutions of TXA from 5 to 50 mg/ml diluted in sterile water to identify which TXA concentration/s best reduced bacterial turbidity. The solutions were kept at 2–4°C until use.
The procedure was performed following CLSI guidelines and using a microtiter plate (Clinical and Laboratory Standards Institute, 2018). The wells of a 96-well plate were inoculated with 50 μl of a suspension of 106 cfu/ml of each microorganism in Müller-Hinton broth and treated with serial dilutions (from 5 to 50 mg/ml) of TXA (50 μl of twice the final dose in the well). Positive controls (bacterial suspension) were treated with 100 μl of sterile water, and negative controls (only Müller-Hinton broth) were treated with 100 μl of TXA 50 mg/ml. All the experiments were performed in triplicate. Plates were incubated at 37°C for 24 h.
Calculation of Percentage Reduction in Turbidity
We used a spectrophotometer (492 nm) to calculate the percentage reduction in turbidity of each well at each TXA concentration compared to the positive control.
Tranexamic Acid Combined With Vancomycin and Gentamicin
We then tested the effect of TXA at extreme concentrations where similar results were observed according to reduction in bacterial turbidity (10 and 50 mg/ml) in combination with serial dilutions of vancomycin (Merck KGaA, Darmstadt, Alemania) (from 0.03 to 16 mg/L) and gentamicin (Merck KGaA, Darmstadt, Alemania) (from 0.06 to 32 mg/L), which were also tested separately in parallel.
The procedure was performed following CLSI guidelines (32nd edition, 2018) and using a microtiter plate (Clinical and Laboratory Standards Institute, 2018). The wells of a 96-well plate were inoculated with 5 μl of a suspension of 107 cfu/ml of each microorganism in a Müller-Hinton broth and treated with the following: 100 μl of vancomycin (from 0.03 to 16 mg/L) alone (100 μl) and in combination (50 μl of twice the final dose in the well) with 50 μl of TXA (at 10 and 50 mg/ml) and gentamicin (from 0.06 to 32 mg/l) alone (100 μl) and in combination (50 μl of twice the final dose in the well) with 50 μl of TXA (at 10 and 50 mg/ml). Positive controls (bacterial suspension) were treated with 100 μl of sterile water, and negative controls (only Müller-Hinton broth) were treated with 100 μl of each highest antibiotic concentration. All the experiments were performed in triplicate. Plates were incubated at 37°C for 24 h.
Calculation of Minimal Inhibitory Concentration
We defined the Minimal Inhibitory Concentration (MIC) of each antibiotic tested, alone or in combination with TXA, using both the standard visualization of well turbidity and the automated turbidity method (Clinical and Laboratory Standards Institute, 2018).
For the standard visualization method, MIC was defined as the lowest concentration at which complete absence of well bacterial growth (compared to the positive control) was observed by the researcher (Clinical and Laboratory Standards Institute, 2018).
For the automated turbidity method, MIC was defined as the lowest concentration at which ≥80% reduction in well bacterial growth (compared to the positive control) was measured using a spectrophotometer at 492 nm.
As our objective was not to identify whether the strains were susceptible or resistant but to assess the possible synergistic effect of TXA, we did not take into consideration the lack of correlation between the MICs obtained by one method or the other. We used the automated turbidity method in addition to the standard visualization method, because it provided a higher MIC, which enabled us to better detect the synergistic effect of TXA starting from higher MICs than with the standard method.
Data Analysis
The effect of serial dilutions of TXA against planktonic bacteria was expressed as the mean percentage reduction in well turbidity compared to the positive control.
Minimal Inhibitory Concentration was expressed as the geometric mean (MIC50) with the corresponding ranges.
Data were expressed by combining the results from all species together and separately according to species (Staphylococcus aureus, N = 6, and Staphylococcus epidermidis, N = 4).
The data are collected in the repository C.0001228 of the ISCIII.
Results
Percentage Reduction in Well Turbidity When Tranexamic Acid Serial Dilutions Were Tested Against Planktonic Bacteria
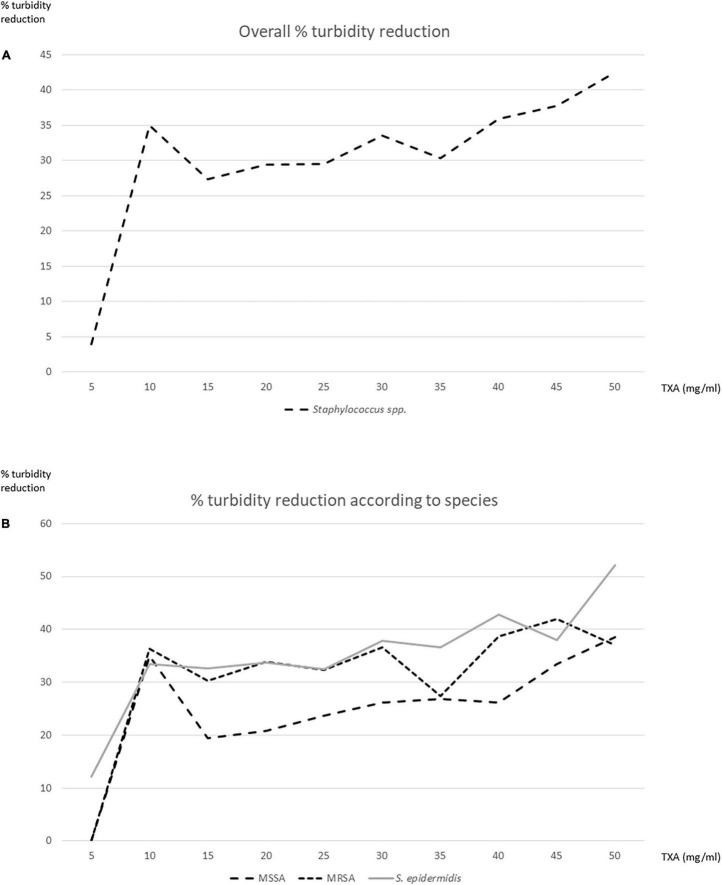
Overall, TXA at 5 mg/ml showed almost no antibacterial effect (mean percentage reduction in turbidity 4%), especially for S. aureus (0%) (Figures 1A,B).
FIGURE 1.
Percentage reduction in well turbidity after testing tranexamic acid (TXA) serial dilutions against planktonic bacteria. (A) Overall. (B) According to species.
Above 5 mg/ml, the mean percentage reduction in well turbidity ranged from 27.4 to 42.5% (Figures 1A,B).
In order to select the extreme concentrations at which similar percentage reductions were observed, we tested TXA 10 (35%) and 50 mg/ml (42.5%) in subsequent experiments.
The mean percentage reduction in well turbidity for TXA 10 mg/ml in the S. aureus strains and the S. epidermidis strains was, respectively, 35.7 and 33.5%. The mean percentage reduction in well turbidity for TXA 50 mg/ml in the S. aureus strains and the S. epidermidis strains was, respectively, 37.8 and 52.2% (Figures 1A,B).
Minimal Inhibitory Concentration of Vancomycin and Gentamicin Alone and in Combination With Tranexamic Acid Calculated Using the Standard Visualization Method
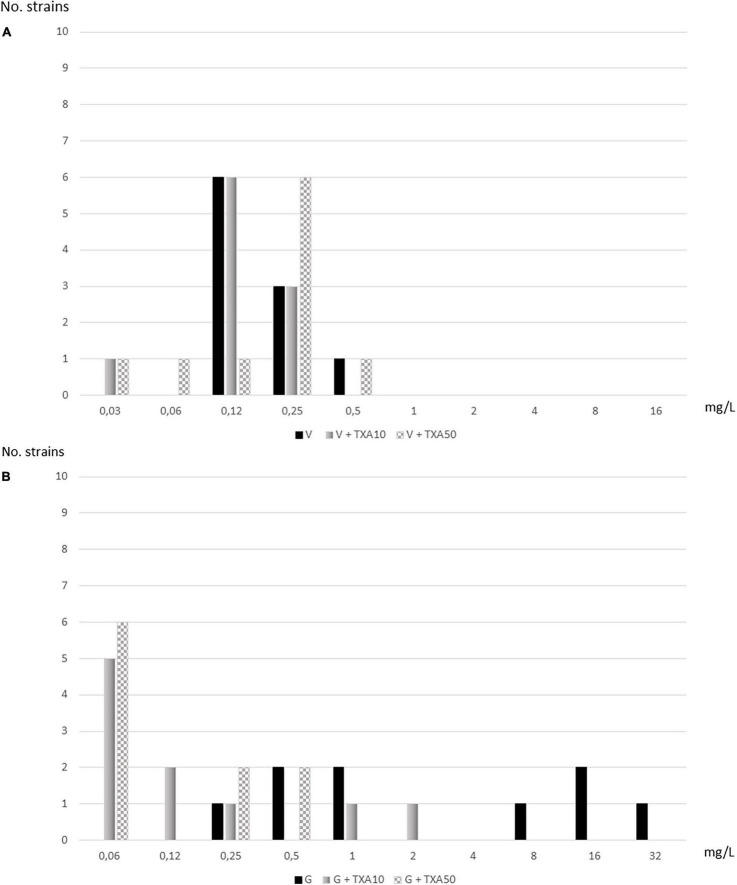
Using the standard visualization method, TXA-10 mg/ml reduced the MIC50 of vancomycin and gentamicin by 1- and 4-fold, respectively: TXA10-V, 0.12 mg/L vs. V, 0.25 mg/L and TXA10-G, 0.5 mg/L vs. G, 8 mg/L. TXA-50 mg/ml reduced the MIC50 of gentamicin (TXA50-G, 0.12 mg/L vs. G, 8 mg/L) by 6-fold, whereas the MIC50 of vancomycin remained unchanged (0.25 mg/L) (Table 1 and Figures 2A,B).
TABLE 1.
MIC50 for vancomycin and gentamicin alone and in combination with tranexamic acid (TXA) using the standard visualization method.
| Treatment |
Staphylococcus aureus MIC50 (ranges) mg/L |
MIC50 fold reductions (no.) |
Staphylococcus epidermidis MIC50 (ranges) mg/L |
MIC50 fold reductions (no.) | Overall MIC50 (ranges) mg/L |
MIC50 fold reductions (no.) |
| Vancomycin | 0.12 (0.12–0.25) | 0.25 (0.12–0.5) | 0.25 (0.12–0.5) | |||
| Vancomycin + TXA 10 mg/ml | 0.12 (0.12–0.25) | 0 | 0.12 (0.03–0.25) | 1 | 0.12 (0.03–0.25) | 1 |
| Vancomycin + TXA 50 mg/ml | 0.25 (0.12–0.25) | NA* | 0.25 (0.03–0.5) | 0 | 0.25 (0.03–0.5) | 0 |
| Gentamicin | 4 (0.25–16) | 16 (0.5–32) | 8 (0.25–32) | |||
| Gentamicin + TXA 10 mg/ml | 0.12 (0.06–0.25) | 5 | 1 (0.06–2) | 4 | 0.5 (0.06–2) | 4 |
| Gentamicin + TXA 50 mg/ml | 0.12 (0.06–0.5) | 5 | 0.25 (0.06–0.5) | 6 | 0.12 (0.06–0.5) | 6 |
TXA, tranexamic acid; MIC, minimal inhibitory concentration; NA, not applicable. *1-fold increase was observed.
FIGURE 2.
Distribution of strains according to serial dilutions of vancomycin and gentamicin alone and in combination with tranexamic acid (TXA) using the standard visualization method. (A) Vancomycin. (B) Gentamicin.
Minimal Inhibitory Concentration of Vancomycin and Gentamicin Alone and in Combination With Tranexamic Acid Calculated Using the Automated Turbidity Method
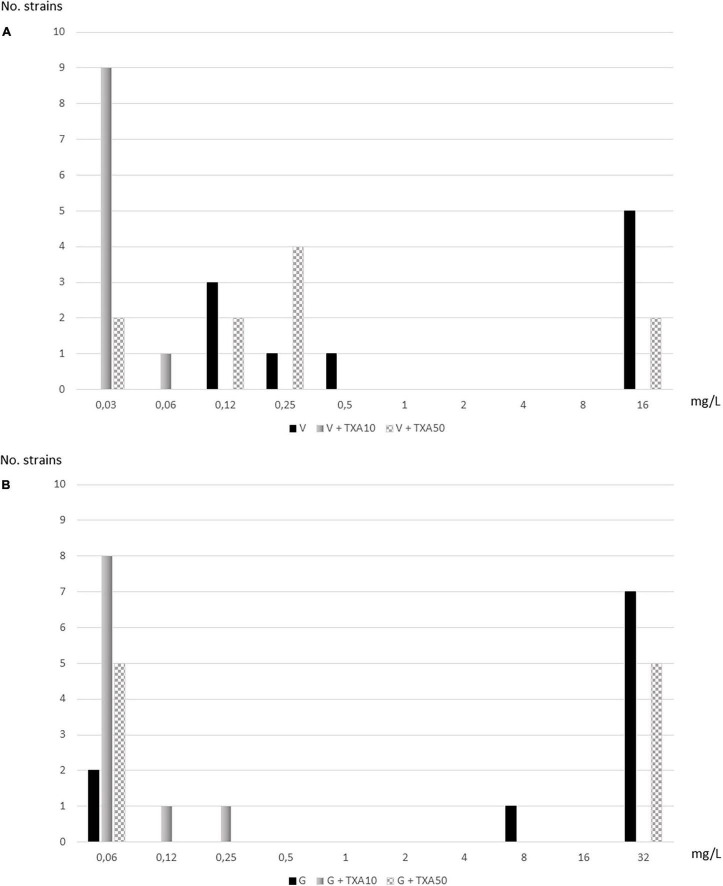
Using the automated turbidity method, TXA-10 mg/ml reduced the MIC50 of vancomycin and gentamicin by 8-fold: TXA10-V, 0.03 mg/L vs. V, 8 mg/L and TXA10-G, 0.12 mg/L vs. G, >32 mg/L. TXA-50 mg/ml reduced the MIC50 of vancomycin and gentamicin 1-fold: TXA50-V, 4 mg/L vs. V, 8 mg/L and TXA50-G, 16 mg/L vs. G, >32 mg/L (Table 2 and Figures 3A,B).
TABLE 2.
MIC50 for vancomycin and gentamicin alone and in combination with tranexamic acid (TXA) using the automatized turbidity method.
| Treatment |
Staphylococcus aureus MIC50 (ranges) mg/L |
MIC50 fold reductions (no.) |
Staphylococcus epidermidis MIC50 (ranges) mg/L |
MIC50 fold reductions (no.) | Overall MIC50 (ranges) mg/L |
MIC50 fold reductions (no.) |
| Vancomycin | 4 (0.12–16) | 16 (16–16) | 8 (0.12–16) | |||
| Vancomycin + TXA 10 mg/ml | 0.03 (0.03–0.06) | 8 | 0.03 (0.03–0.03) | 8 | 0.03 (0.03–0.06) | 8 |
| Vancomycin + TXA 50 mg/ml | 0.12 (0.03–0.25) | 1 | 8 (0.03–16) | 1 | 4 (0.03–16) | 1 |
| Gentamicin | 16 (0.06–32) | 32 (32–32) | 32 (0.06–32) | |||
| Gentamicin + TXA 10 mg/ml | 0.06 (0.06–0.12) | 7 | 0.12 (0.06–0.25) | 8 | 0.12 (0.06–0.25) | 8 |
| Gentamicin + TXA 50 mg/ml | 8 (0.06–32) | 1 | 16 (0.06–32) | 1 | 16 (0.06–32) | 1 |
TXA, tranexamic acid; MIC, minimal inhibitory concentration.
FIGURE 3.
Distribution of strains according to serial dilutions of vancomycin and gentamicin alone and in combination with tranexamic acid (TXA) using the automated turbidity method. (A) Vancomycin. (B) Gentamicin.
Discussion
In combination with vancomycin and gentamicin, TXA showed a significant synergistic effect against the staphylococcal strains.
As we previously demonstrated that the effect of TXA on reduction in PJI rates was due to its direct effect by reducing bacterial growth (Benjumea et al., 2022), in addition to its indirect effect by reducing hematoma (Lohinai et al., 2015; Ruder and Springer, 2017; Draxler et al., 2019; Perez-Roman et al., 2019; Klement et al., 2020; Lacko et al., 2020; Sukeik et al., 2020; Yazdi et al., 2020), we further investigated whether it could also have a synergistic effect if in combination with antibiotics. Therefore, we decided to test it with vancomycin and gentamicin, which are two of the most commonly used antibiotics in orthopedic surgery (Ratto et al., 2016; Gandhi et al., 2018; Myers and Lipof, 2020; Indelli et al., 2021; Parvizi, 2021).
We also previously observed that percentage reductions in bacterial growth were generally better when the concentration of TXA was lower (Benjumea et al., 2022). Therefore, in the present study, we tested the effect of TXA at serial dilutions. We demonstrated that the percentage reduction in well turbidity was similar between TXA concentrations of 10 and 50 mg/ml (range 35–42.5%). Also interesting was the finding that TXA at 5 mg/ml did not have an antimicrobial effect (4% reduction in well turbidity). Thus, we confirm that if the final volume of TXA was finally reached and absorbed in the joint did not reach at least 10 mg/ml, TXA would not have any antimicrobial effect at all. However, this is not a likely scenario, as the dose of TXA used by most authors in the literature is between 1 and 3 g. Therefore, if the joint volume of one knee is estimated at 131 (±53) ml, the intra-articular TXA dilution would be approximately 10–30 mg/ml (Hewitt and Stringer, 2008; Maniar et al., 2012; Matziolis et al., 2015; Jennings et al., 2016).
Although toxicity has been reported at TXA concentrations of >20 mg/ml (Parker et al., 2018; Sukur and Kucukdurmaz, 2018; McLean et al., 2019; Mortazavi et al., 2020; Bolam et al., 2021; González Osuna et al., 2021; Ma et al., 2021; Touzopoulos et al., 2021), we decided to include a concentration of 50 mg/ml in our experiments in order to demonstrate that the synergistic effect was not dose-dependent. Using the standard visualization method, TXA 50 mg/ml reached higher fold reductions in gentamicin MIC50 in S. epidermidis (6 vs. 4 with TXA 10 mg/ml), although the same fold reduction in gentamicin MIC50 was observed for S. aureus (5 with TXA 10 and 50 mg/ml). Moreover, no synergistic effect was observed when TXA 50 mg/ml was combined with vancomycin on S. aureus or on S. epidermidis, whereas TXA 10 mg/ml reduced by 1-fold the vancomycin MIC50 in S. epidermidis. In addition, using the automated turbidity method, TXA 50 mg/ml reduced both the vancomycin and gentamicin MIC50 only by 1-fold compared with the 8-fold reduction for vancomycin and gentamicin with TXA 10 mg/ml.
Regarding the possible risk of using an anti-fibrinolytic drug in combination with an antibiotic during staphylococcal infection, to our knowledge, there are no studies answering the question of whether TXA combined with an antibiotic could produce or increase an adverse effect other than that which these drugs can produce by themselves. However, in orthopedic surgery, the use of systemic antibiotic treatment as prophylaxis of periprosthetic infection is widespread, including the use of antibiotic-loaded cement. On the other hand, TXA is commonly used both systemically and locally concomitantly in the same surgeries. In our clinical experience, we have not found an increase in local and/or systemic adverse effects (Wong et al., 2010; Poeran et al., 2014; Franchini et al., 2018).
Therefore, we can conclude that TXA 10 mg/ml showed a better synergistic effect with either vancomycin or gentamicin than TXA 50 mg/ml. However, whether the TXA mechanisms of action are direct or indirect is still unknown. Some studies have tried to answer this question. On the one hand, there is research that indicates the inhibitory effect of TXA, a lysine analog, on certain enzymes that favors biofilm formation (Lohinai et al., 2015). On the other hand, Draxler et al. (2019) conclude in their study on TXA and immune response that TXA has an immunomodulatory effect, altering the study of plasma cytokine levels, the number of immune cells, and the expression of multiple immunological markers.
The main limitation of the study is that we only tested 6 and 4 strains of S. aureus and S. epidermidis, respectively. Future studies are needed to validate our findings in a large sample size of clinical staphylococci strains. Furthermore, as we only tested a single dose, we do not know whether continuous doses could affect our results, as observed in the in vivo study by Zhang et al. (2021). In addition, we did not run growth curves or time-kill kinetic assays.
Conclusion
Our results suggest that TXA may reduce the MIC of vancomycin and gentamicin against staphylococci in an experimental planktonic model at the concentration generally used in clinical practice (10 mg/ml). This proof-of-concept study provides new insights regarding the preventive role of TXA in clinical practice.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
MG and FC were responsible for the organization and coordination of the trial. FC, JV, PM, and MG were the chief investigator and responsible for the data analysis. AB, MD-N, RH, EC, and MS-S developed the trial design and data collection. All authors contributed to wrote the final manuscript and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Thomas O’Boyle for his help in the preparation of the manuscript.
Funding Statement
MG was supported by the Miguel Servet Program (ISCIII-MICINN, MSII18/00008) from the Health Research Fund (FIS) of the Carlos III Health Institute (ISCIII), Madrid, Spain. MD-N was supported by the Consejería de Educación, Juventud y Deporte de la Comunidad de Madrid and Fondo Social Europeo(PEJD-2020-AI_BMD-17971). The study was partially funded by grants from the Fundación Mutua Madrileña (MM21/01) and by grants from the FIS of the ISCIII (PI21/00344).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.935646/full#supplementary-material
References
- Behzadi P., Gajdács M. (2021). Writing a strong scientific paper in medicine and the biomedical sciences: a checklist and recommendations for early career researchers. Biol. Futur. 72 395–407. 10.1007/s42977-021-00095-z [DOI] [PubMed] [Google Scholar]
- Benjumea A., Díaz-Navarro M., Hafian R., Sánchez-Somolinos M., Vaquero J., Chana F., et al. (2022). Effect of Tranexamic Acid against Staphylococcus spp. and Cutibacterium acnes Associated with Peri-Implant Infection: Results from an In Vitro Study. Microbiol. Spectr. 10:e0161221. 10.1128/spectrum.01612-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam S. M., O’Regan-Brown A., Paul Monk A., Musson D. S., Cornish J., Munro J. T. (2021). Toxicity of tranexamic acid (TXA) to intra-articular tissue in orthopaedic surgery: a scoping review. Knee Surg. Sports Traumatol. Arthrosc. 29 1862–1871. 10.1007/s00167-020-06219-7 [DOI] [PubMed] [Google Scholar]
- Draxler D. F., Yep K., Hanafi G., Winton A., Daglas M., Ho H., et al. (2019). Tranexamic acid modulates the immune response and reduces postsurgical infection rates. Blood Adv. 3 1598–1609. 10.1182/bloodadvances.2019000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini M., Mengoli C., Cruciani M., Bergamini V., Presti F., Marano G., et al. (2018). Safety and efficacy of tranexamic acid for prevention of obstetric haemorrhage: an updated systematic review and meta-analysis. Blood Transfus. 16 329–337. 10.2450/2018.0026-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi R., Backstein D., Zywiel M. G. (2018). Antibiotic-laden Bone Cement in Primary and Revision Hip and Knee Arthroplasty. J. Am. Acad. Orthop. Surg. 26 727–734. 10.5435/JAAOS-D-17-00305 [DOI] [PubMed] [Google Scholar]
- González Osuna A., Rojas L. F., Valencia W. G., Atehortúa L., Urrea A. I., Fister A. S. (2021). Population Pharmacokinetics of Intra-articular and Intravenous Administration of Tranexamic Acid in Patients Undergoing Total Knee Replacement. Clin. Pharmacokinet. 61 83–95. 10.1007/s40262-021-01043-9 [DOI] [PubMed] [Google Scholar]
- Hewitt K. M., Stringer M. D. (2008). Correlation between the surface area of synovial membrane and the surface area of articular cartilage in synovial joints of the mouse and human. Surg. Radiol. Anat. 30 645–651. 10.1007/s00276-008-0399-1 [DOI] [PubMed] [Google Scholar]
- Indelli P. F., Iannotti F., Ferretti A., Valtanen R., Prati P., Pérez Prieto D., et al. (2021). Recommendations for periprosthetic joint infections (PJI) prevention: the European Knee Associates (EKA)-International Committee American Association of Hip and Knee Surgeons (AAHKS)-Arthroplasty Society in Asia (ASIA) survey of members. Knee Surg. Sports Traumatol. Arthrosc. [Epub ahead of print]. 10.1007/s00167-021-06742-1 [DOI] [PubMed] [Google Scholar]
- Jennings J. D., Solarz M. K., Haydel C. (2016). Application of Tranexamic Acid in Trauma and Orthopedic Surgery. Orthop. Clin. North Am. 47 137–143. 10.1016/j.ocl.2015.08.014 [DOI] [PubMed] [Google Scholar]
- Josse J., Valour F., Maali Y., Diot A., Batailler C., Ferry T., et al. (2019). Interaction Between Staphylococcal Biofilm and Bone: How Does the Presence of Biofilm Promote Prosthesis Loosening? Front. Microbiol. 10:1602. 10.3389/fmicb.2019.01602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia B. H., Berg R. A., Daley J. A., Fritz J., Bhave A., Mont M. A. (2016). Periprosthetic joint infection. Lancet 387 386–394. 10.1016/S0140-6736(14)61798-0 [DOI] [PubMed] [Google Scholar]
- Klement M. R., Padua F. G., Li W. T., Detweiler M., Parvizi J. (2020). Tranexamic Acid Reduces the Rate of Periprosthetic Joint Infection After Aseptic Revision Arthroplasty. J. Bone Jt. Surg. Am. Volume 102 1344–1350. 10.2106/JBJS.19.00925 [DOI] [PubMed] [Google Scholar]
- Lacko M., Jarèuška P., Schreierova D., Lacková A., Gharaibeh A. (2020). Tranexamic acid decreases the risk of revision for acute and delayed periprosthetic joint infection after total knee replacement. Jt. Dis. Relat. Surg. 31 8–13. 10.5606/ehc.2020.72061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohinai Z., Keremi B., Szöko E., Tábi T., Szabo C., Tulassay Z., et al. (2015). Biofilm Lysine Decarboxylase, a New Therapeutic Target for Periodontal Inflammation. J. Periodontol. 86 1176–1184. 10.1902/jop.2015.140490 [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (2018). Performance Standards for Antimicrobial Susceptibility Testing, 32nd Edn. Wayne, PA: Clinical and Laboratory Standards Institute, 362. [Google Scholar]
- Ma J., Lu H., Chen X., Wang D., Wang Q. (2021). The efficacy and safety of tranexamic acid in high tibial osteotomy: a systematic review and meta-analysis. J. Orthop. Surg. Res. 16:373. 10.1186/s13018-021-02512-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniar R. N., Kumar G., Singhi T., Nayak R. M., Maniar P. R. (2012). Most effective regimen of tranexamic acid in knee arthroplasty: a prospective randomized controlled study in 240 patients. Clin. Orthop. Relat. Res. 470 2605–2612. 10.1007/s11999-012-2310-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matziolis G., Roehner E., Windisch C., Wagner A. (2015). The volume of the human knee joint. Arch. Orthop. Trauma Surg. 135 1401–1403. 10.1007/s00402-015-2272-0 [DOI] [PubMed] [Google Scholar]
- McLean M., McCall K., Smith I. D. M., Blyth M., Kitson S. M., Crowe L. A. N., et al. (2019). Tranexamic acid toxicity in human periarticular tissues. Bone Jt. Res. 8 11–18. 10.1302/2046-3758.81.BJR-2018-0181.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin J. S., Stryker L. S., Sierra R. J. (2015). Tranexamic Acid in Hip and Knee Arthroplasty. J. Am. Acad. Orthop. Surg. 23 732–740. 10.5435/JAAOS-D-14-00223 [DOI] [PubMed] [Google Scholar]
- Mortazavi S. J., Sattartabar B., Moharrami A., Kalantar S. H. (2020). Intra-articular versus Intravenous Tranexamic Acid in Total Knee Arthroplasty: A Randomized Clinical Trial. Arch. Bone Jt. Surg. 8 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers T. G., Lipof J. S. (2020). Antibiotic Stewardship for Total Joint Arthroplasty in 2020. J. Am. Acad. Orthop. Surg. 28:e793–e802. 10.5435/JAAOS-D-19-00850 [DOI] [PubMed] [Google Scholar]
- Parker J. D., Lim K. S., Kieser D. C., Woodfield T. B. F., Hooper G. J. (2018). Is tranexamic acid toxic to articular cartilage when administered topically? What is the safe dose? Bone Jt. J. 100 404–412. 10.1302/0301-620X.100B3.BJJ-2017-1135.R1 [DOI] [PubMed] [Google Scholar]
- Parvizi J. G. T. (2021). Consenso Internacional Sobre Infecciones Musculoesqueléticas. Parte II Cadera y Rodilla. Madrid: Imaidea Interactiva, S.L. [Google Scholar]
- Perez-Roman R. J., Lugo-Pico J. G., Burks J. D., Madhavan K., Sheinberg D., Green B. A., et al. (2019). Short-term safety of tranexamic acid use in posterior cervical decompression and fusion surgery. J. Clin. Neurosci. 66 41–44. 10.1016/j.jocn.2019.05.029 [DOI] [PubMed] [Google Scholar]
- Poeran J., Rasul R., Suzuki S., Danninger T., Mazumdar M., Opperer M., et al. (2014). Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ 349:g4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratto N., Arrigoni C., Rosso F., Bruzzone M., Dettoni F., Bonasia D. E., et al. (2016). Total knee arthroplasty and infection: how surgeons can reduce the risks. EFORT Open Rev. 1 339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanò C. L., De Vecchi E., Bortolin M., Morelli I., Drago L. (2017). Hyaluronic Acid and Its Composites as a Local Antimicrobial/Antiadhesive Barrier. J. Bone Jt. Infect. 2 63–72. 10.7150/jbji.17705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruder J. A., Springer B. D. (2017). Treatment of Periprosthetic Joint Infection Using Antimicrobials: Dilute Povidone-Iodine Lavage. J. Bone Jt. Infect. 2 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M. M., Chen A. F. (2020). Biofilms in Periprosthetic Joint Infections: A Review of Diagnostic Modalities, Current Treatments, and Future Directions. J. Knee Surg. 33 119–131. 10.1055/s-0040-1701214 [DOI] [PubMed] [Google Scholar]
- Sukeik M., Alshryda S., Powell J., Haddad F. S. (2020). The effect of tranexamic acid on wound complications in primary total Hip Arthroplasty: a meta-analysis. Orthop. Surg. 18 53–61. [DOI] [PubMed] [Google Scholar]
- Sukur E., Kucukdurmaz F. (2018). Comparison of Cytotoxic Effects of Intra-Articular Use of Tranexamic Acid versus Epinephrine on Rat Cartilage. Med. Sci. Monit. 24 1166–1170. 10.12659/msm.908560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzopoulos P., Arvanitidis K., Filidou E., Tilkeridis K., Karanikas M., Kolios G., et al. (2021). Is serum gentamicin concentration modified with autologous cell-saved blood transfusion after total knee arthroplasty using tranexamic acid? A randomised control trial. Orthop. Traumatol. 107:102794. 10.1016/j.otsr.2020.102794 [DOI] [PubMed] [Google Scholar]
- Waddell B. S., Zahoor T., Meyer M., Ochsner L., Chimento G. (2016). Topical Tranexamic Acid Use in Knee Periprosthetic Joint Infection Is Safe and Effective. J. Knee Surg. 29 423–429. 10.1055/s-0035-1564599 [DOI] [PubMed] [Google Scholar]
- Wong J., Abrishami A., El Beheiry H., Mahomed N. N., Roderick Davey J., Gandhi R., et al. (2010). Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J. Bone Jt. Surg. Am. 92 2503–2513. [DOI] [PubMed] [Google Scholar]
- Yazdi H., Klement M. R., Hammad M., Inoue D., Xu C., Goswami K., et al. (2020). Tranexamic Acid Is Associated With Reduced Periprosthetic Joint Infection After Primary Total Joint Arthroplasty. J. Arthroplasty 35 840–844. 10.1016/j.arth.2019.10.029 [DOI] [PubMed] [Google Scholar]
- Zhang F. D. W., Wang F., Yu J., Jiang F., Tang J., Qian Y., et al. (2021). The Topical Tranexamic Acid Have Potential Hazard of Promoting Biofilm Formation of Staphylococcus aureus in Microenviroment of the Prosthetic Joint. Biomed Res. Int. 2021:5748069. 10.1155/2021/5748069 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.