Abstract
Probiotics such as Lactobacillus spp. play an important role in human health as they embark beneficial effect on the human gastrointestinal microflora composition and immune system. Dysbiosis in the gastrointestinal microbial composition has been identified as a major contributor to chronic inflammatory conditions, such as inflammatory bowel disease (IBD). Higher prevalence of IBD is often recorded in most of the developed Western countries, but recent data has shown an increase in previously regarded as lower risk regions, such as Japan, Malaysia, Singapore, and India. Although the IBD etiology remains a subject of speculation, the disease is likely to have developed because of interaction between extrinsic environmental elements; the host’s immune system, and the gut microbial composition. Compared to conventional treatments, probiotics and probiotic-based interventions including the introduction of specific prebiotics, symbiotic and postbiotic products had been demonstrated as more promising therapeutic measures. The present review discusses the association between gut dysbiosis, the pathogenesis of IBD, and risk factors leading to gut dysbiosis. In addition, it discusses recent studies focused on the alteration of the gastrointestinal microbiome as an effective therapy for IBD. The impact of the COVID-19 pandemic and other viral infections on IBD are also discussed in this review. Clinical and animal-based studies have shown that probiotic-based therapies can restore the gastrointestinal microbiota balance and reduce gut inflammations. Therefore, this review also assesses the status quo of these microbial-based therapies for the treatment of IBD. A better understanding of the mechanisms of their actions on modulating altered gut microbiota is required to enhance the effectiveness of the IBD therapeutics.
Keywords: Probiotics, Prebiotics, Synbiotics, Postbiotics, Inflammatory bowel disease, Gastrointestinal dysbiosis
1. Introduction
IBD, globally known as inflammatory bowel disease are referred to the chronic inflammatory conditions of the human gastrointestinal tract (GI). The inflammation affects the function of the gastrointestinal tract including food digestion, absorption of nutrients, and excretion of undigested materials (Darb Emamie et al., 2021, Desai et al., 2016, Spiller and Major, 2016). IBD causes symptoms such as abdominal pain, diarrhea, rectal bleeding, fatigue, and weight loss. In addition, it has been linked to other inevitable convolutions such as abscesses, fistulas, stenoses, colitis-associated neoplasias, and colorectal cancers (Asha and Khalil, 2020, Spiller and Major, 2016). Confusion with symptoms between IBD and Irritable bowel syndrome (IBS) exists in some cases. Both are gastrointestinal disorders were 0.3 to 0.5% and 7 to 21% of the world populations affected by IBD and IBS, respectively (Ananthakrishnan, 2015, Kaplan, 2015, Spiller and Major, 2016). Both diseases impose a large burden on patients, affecting the quality of living, also increasing the financial burden, directly on health care cost and indirectly in the overall economic growth of a nation (Alatab et al., 2020, Ananthakrishnan, 2015). Despite sounding similar, IBS has its distinguishable features compared to IBD. Both are chronic conditions that lead to similar manifestations including severe abdominal pain and cramping that cause an urgent bowel movement. However, the gastrointestinal disorder of IBS affects the lower region of the gastrointestinal tract, particularly in the small and large bowel (Casen et al., 2015; Darb Emamie et al., 2021). Patients with IBS often experience chronic diarrhea, constipation, and bloating symptoms. Meanwhile, IBD is characterized by gut inflammation. Severe inflammation results in worse symptoms including bloody stools, loss of appetite, massive weight loss, and could lead eventually to colonic cancer (Casen et al., 2015). Apart from environmental factors, recent data reported that the host’s genetic factors play an important role during the progressive development of IBD, either through microbial dysbiosis or influence over the host’s immune system (Casén et al., 2015, Darb Emamie et al., 2021, Spiller and Major, 2016).
IBD is generally diagnosed into two branches which are Crohn’s disease (CD) and Ulcerative colitis (UC). Men or women at any age can develop CD or UC (Casén et al., 2015, Spiller and Major, 2016). Despite similar pathogenesis, CD and UC have some distinguishable features which are the key differences that affect the treatments (Hazel and O’Connor, 2020, Kaplan, 2015). Unlike UC, the CD can affect any part of the digestive system from the mouth to anus. Crohn’s disease can affect any part of the intestine, often in a discontinuous pattern. It can be associated with intestinal granulomas, strictures, and/or fistulas. On the other hand, UC typically starts in the rectum and can involve the whole colon in an uninterrupted pattern (Lewis et al., 2015, Spiller and Major, 2016). Histological analysis shows the formation of thickened submucosa, necrotizing granulomas, transmural inflammation, fissuring ulceration during the development of CD. In ulcerative colitis, inflammatory changes are more superficial and limited to the mucosa and submucosa with cryptitis and crypt abscesses (Ananthakrishnan, 2015, Wang et al., 2017).
Pathogenesis of IBD is still not well understood to date, yet it is often linked to the collective effectiveness of microbial infections, diet, immune system aberrant reactions, inherited genes, and environmental factors (Ananthakrishnan, 2015, Bajinka et al., 2020). The maintenance of homeostasis being the important key factor, especially in epithelial barrier function and its interaction with the pathogenic microorganisms and innate immune system, can be a key factor (Kim et al., 2015). However, the disruption of the intestinal mucosal barrier is believed to be a frequent event and most often the immune system is not stimulated (Franzosa et al., 2019, Goodrich et al., 2014, Michielan and D’Incà, 2015). This indicates none of the identified risk factors alone are adequate to drive the progression of IBD (Ananthakrishnan, 2015). In 2001, the first CD-associated gene was described, and UC-associated genes started to be characterized (Kim et al., 2015, Michielan and D’Incà, 2015). This has marked a new turnover in our understanding of factors causing the pathogenesis of IBD. The human gastrointestinal microbiome has begun to be recognized for its vital role and as a possible therapeutic solution for IBD (Hoffmann et al., 2016). The treatment goals for IBD usually include induction of remission, and prevention of relapse (Ananthakrishnan, 2015, Hazel and O’Connor, 2020). Some of these therapeutics offer a lower efficacy rate with higher adverse side effects (Casén et al., 2015, Hazel and O’Connor, 2020). Growing understanding of the synergy between a host’s genetics, gut microbiome, and external environment has opened a new framework to seek alternative effective therapies (Bajinka et al., 2020, Lewis et al., 2015).
Gut dysbiosis would exhibit a major decline in the population of commensal microbial populations, functional diversity, and stability in the gut lumen (Alhagamhmad et al., 2016, Franzosa et al., 2019). This alteration of the microbiome affects the interaction of the host’s homeostatic systems with lumen stimuli and eventually perpetuates uncontrolled inflammation in the intestinal mucosa that potentially leads to IBD (Alhagamhmad et al., 2016, Hoffmann et al., 2016, Michielan and D’Incà, 2015). Numerous external factors including antibiotic therapy and unhealthy dietary patterns are often considered as a cause for intestinal dysbiosis and lead to the development of IBD (Hoffmann et al., 2016). Several studies have recently begun to highlight that, the pandemic Coronavirus disease 2019 (COVID-19) severity has also been associated with alteration of gut microbiota (Liang et al., 2020, Ong et al., 2020, Segal et al., 2020). Despite its severe respiratory illness, COVID-19 patients also reported gastrointestinal disorders including diarrhea, nausea, and vomiting (Franzosa et al., 2019). A study by Liang et al. (2020) had raised concern that the alteration and gut microbiota and its associated diarrhea could be an underestimated symptom of COVID-19. Patients had a detectable level of virus in the feces, which proved its involvement in the severity of gastrointestinal tract disease (Liang et al., 2020, Yeoh et al., 2021). In another study, the occurrence of gut dysbiosis was confirmed with the depletion of symbionts and enrichment of opportunistic pathogens in the fecal samples of COVID-19 patients (Zuo et al., 2020). Additionally, recent data reported that the gut microbiome is significantly affected in patients with COVID-19 and the alteration was found to remain even after complete clearance of the virus (Yeoh et al., 2021). Even after 30 days of the post-disease resolution, some post-COVID-19 patients are diagnosed with a relatively reduced abundance of gastrointestinal commensal microbial populations, including Faecalibacterium prausnitzii. The study also reported an elevated concentration of inflammatory cytokines among recovered COVID-19 patients (Yeoh et al., 2021, Zuo et al., 2020). The post-COVID-19 symptoms were identified to cause microbial dysbiosis in the gastrointestinal environment and might lead to chronic IBD symptoms, as the patients’ immune responses are still at a dysfunctional state (Liang et al., 2020, Segal et al., 2020, Zuo et al., 2020).
There is no known complete cure for IBD (Hazel and O’Connor, 2020). Current therapies practiced are based on pharmacological approaches to reduce inflammation, disease relapse and promote clinical remission (Casén et al., 2015, Zimmermann and Curtis, 2019). The traditional medicines used for IBD treatment are amino salicylates, corticosteroids, thiopurines, and folic acid antagonists (Hazel and O’Connor, 2020). Selection criteria for alternative effective therapy must be concerned with the frequency of disease relapse, disease severity, and the disease complication level (Asha and Khalil, 2020, Mahlich et al., 2017). However, the efficiency of current treatments is temporary and relieves symptomatic complications due to a remarkably high dosage (Asha and Khalil, 2020, Darb Emamie et al., 2021). Additionally, the lesion site and clinical history of the patient also influence the therapeutic management. Although UC and CD share common clinical diagnostics, the site and characteristics of inflammations distinguish them (Spiller and Major, 2016). This clinical difference, together with differences in the host’s genetic and environmental make-up, as well as the immune state, affects the patient’s response towards therapy. This explains the need for more clinical studies and medical interventions to achieve the most effective and promising therapy for UC or CD (Ananthakrishnan, 2015, Spiller and Major, 2016). The most extensively investigated hypothesis for the development of IBD includes an altered immune system and a disrupted host’s gut microbiome (Franzosa et al., 2019, Li et al., 2015, Zuo et al., 2018). The human body together with its gastrointestinal microbial community is regarded as a “superorganism”. The human gut microbiome has been estimated to be composed of one hundred times greater number of genes than the host’s genome (Bäckhed et al., 2015, Casén et al., 2015). Clarke et al. (2014) proposed the gut microbiota as an unrecognized “human metabolic organ” due to its multiple roles in digestion, modulation, and physical differentiation of human tissue. Therefore, it would be a possible cure for IBD if the dysbiotic gut microbiota could revert to its original state, without adverse side effects (Bäckhed et al., 2015, Li et al., 2015). Several studies have demonstrated alternative therapies using probiotics and specific dietary supplements such as prebiotics to modulate the gastrointestinal microbiome (Altun et al., 2019, Amoroso et al., 2020, Naseer et al., 2020). Additional studies confirmed the efficacy of utilizing the commensal microbiota to stimulate immune function and mucosal integrity (Chen et al., 2020, Kim et al., 2020). Recent well-designed randomized clinical trials had extended the promising impacts of probiotics, prebiotics, and synbiotic therapies for IBD treatments in patients (Franzosa et al., 2019, Goodrich et al., 2014). Therefore, here we present the most current research on the role of the human gastrointestinal microbiome and factors causing gut dysbiosis that leads to IBD, and studies that support the role of probiotics, prebiotic, and synbiotic therapies in combating IBD.
2. Global prevalence of IBD
For many decades, IBD had been referred to as a Western disease and thought to be infrequent in the Eastern region (Ananthakrishnan, 2015, Chiba et al., 2019). Howbeit, growing neoteric data has suggested that the prevalence of both UC and CD of the Eastern countries continues to rise in parallel to rapid urbanization culture (Chiba et al., 2019, Mak et al., 2020). In 2013, a large-scale population study was performed comprising eight Asia-Pacific countries from Mainland of China, Macau, Hong Kong to ASEAN countries of Thailand, Malaysia, Singapore and Indonesia, and Sri Lanka (Ng et al., 2013). The epidemiology study also compared the incidence of IBD in Australia. Based on the study, the recorded Asian incidences were 1.37 per 100 k individuals, in comparison to 23.67 incidences per 100 k individuals in Australia. The mainland of China recorded the highest incidence 3.44 per 100,000in Asia (Ng et al., 2013, Ng et al., 2016). Although it is still lower compared to Western countries, the prevalence of IBD in the Asia region has doubled in the last decade (Mak et al., 2020). Despite the variations in the numbers, UC is more prevalent in Asian countries compared to CD (Ng et al., 2016). Yet, present-day data begins a contrasting trend where the prevalence of CD is revolting among the developed Asian countries including South Korea, Hong Kong, and Japan (Ong et al., 2018). Recent reports revealed that Asian IBD patients share many similarities with their Western counterparts. Kim et al. (Kim et al., 2015) had demonstrated that the prevalence of UC and CD had elevated to 5.0 and 3.6 per 100,000 individuals respectively from the year 2006 to 2012, based on a large population epidemiological study conducted in South Korea. This incidence has risen 10-fold from 1986 to 1990 (Kim et al., 2015). Meanwhile, the neighboring country Japan recorded a prevalence of UC at 140,000 compared to 40,000 of CD, based on records from the Ministry of Health Labor and Welfare, Japan. A Japanese study reported that the significant burden of IBD suffered by Japanese patients is four times higher risk of unemployment issues in the Japanese population (Mahlich et al., 2017).
A study by Ng et al. (2013) deduced that the rise of IBD incidences does not have similar circumstances in all Asian countries. The study demonstrated some metropolitanized Asian countries like Malaysia and Singapore recorded fewer incidences of IBD compared to the others. The prevalence of IBD recorded in Malaysia and Singapore were 0.94 and 1.06 per 100,000 persons, respectively (Ng et al., 2013). However, in a recent record at the National University Hospital of Singapore, the number has increased eight times in 2018, compared to the year 2013 (Ong et al., 2018). Similarly, a recent Malaysian meta-analysis study has demonstrated a steady increase in IBD incidences over the last three decades. In comparison, the prevalence of IBD among Malaysians had doubled to 1.46 per 100,000 population from 2010 to 2018 (Mokhtar et al., 2019). A significant rise in the CD was also recorded among Malaysians with a reduction in the ratio of UC to CD from 5:1 (in the year 1990 to 1999) to the ratio of 1.7:1 (in the year 2010 to 2018). In the year 2018, Malaysia recorded the prevalence rate of IBD, UC, and CD at 23.0; 15.67, and 7.36 per 100,000 individuals, respectively (Mokhtar et al., 2019). Malaysia and Singapore being multi-racial countries, are populated by three main ethnicities which are the Malays, Chinese, and Indians. This multiracial diversity has made its unique way of combating the growing incidence of IBD in this region (Mokhtar et al., 2019, Ong et al., 2018). Although the incidence numbers differ across the demographic differential categories, a limited number of studies that documented the trend of IBD incidences over the last four decades, were available (Mak et al., 2020). Overall, few studies have documented the incidences in developing countries and require further work (Zuo et al., 2018).
As previously noted, the highest rates of IBD tend to be in high-income countries, namely Europe and North America (Ananthakrishnan, 2015). Additionally, most of the studies reported the highest incidence rates in West regions (Chiba et al., 2019, Desai et al., 2016). Based on recent meta-analysis prevalence rates for both sexes from 1990 to 2017, was 422 per 100,000 population. The study also reported the highest age-standardized mortality rate in the year 2017 in western Europe, which was 0.97 per 100 000 population. Further, the study reported a prevalence rate of 0.83 per 100,000 population by the higher-income regions of North America (Alatab et al., 2020, Mak et al., 2020). The higher incidence of IBD in high-income regions has been noted since the industrial revolution in the 1700 s, with the transformation from rural to urban living believed to be a major driver for the increased incidence of IBD. This societal transformation comprises increased pollution rates and dietary changes involving reduced plant-fiber consumption (Chiba et al., 2019, Desai et al., 2016). Over the decades, the Western world experienced stabilized incidence numbers but recorded a steadily rising prevalence (Alatab et al., 2020).
3. A gut feeling: Is microbial dysbiosis the main cause of IBD or post-symptom?
3.1. Understanding of the human gut microbiome.
Clarke et al. (2014) proposed the human gut microbiome should be recognized as a human vital organ due to its role not only in the gut but in other systems as well. The number of cells is estimated to be far greater than the number of host cells, with a ratio of 10:1 and an approximate weight of 1 to 2 kg in an average adult (Chiba et al., 2019, Clarke et al., 2014). The estimates of human and bacterial cells found in a healthy human body had been revised recently where the microbe in the human body outnumbers the human own cells by about 10:1. The actual numbers of human microbiota were estimated to be 1013 to 1014 with around a 1:1 ratio of microbes to human cells. The study derived the new ratio based on the total microbial cells found in the colon of a healthy human. A healthy human gut is composed of a diverse and complex microbiome, and it varies in concentration by the site. In the colon, the population reaches 1011 to 1012 cells per gram of the luminal content (Bäckhed et al., 2015, Clarke et al., 2014, Goodrich et al., 2014, Selvamani et al., 2021). It was estimated that the gut microbiome harbor 100-fold more genes than humans. These genes mostly code for enzymatic proteins not found in the host to facilitate the metabolic process. They also regulate the physiology of the host, especially the immune system (Chang and Kao, 2019). Human gut microbiota develops from a low diversity at birth into an overly complex and matured community, after the introduction of solid foods. The microbiota achieves stability by 9 to 12 months of age (Bäckhed et al., 2015, Selvamani et al., 2021). The gut microbiota becomes resilient to perturbations including changes in the types of food and exposure to antibiotics (Lange et al., 2016, Zimmermann and Curtis, 2019). Overall, the predominant gastrointestinal microbial composition belongs to the phyla of Firmicutes, Bacteroidetes, Actinobacteria, Fusobacteria, Proteobacteria, and Verrucomicrobiota (Bäckhed et al., 2015, Franzosa et al., 2019, Selvamani et al., 2021). The phyla of Firmicutes comprises the largest genera including Lactobacillus, Bacillus, Clostridium, Enterococcus, and Ruminococcus. Members of the Clostridium genera represent almost 95% of the gastrointestinal Firmicutes. Meanwhile, Bacteroidetes are predominantly composed of genera such as Bacteroides and Prevotella. In addition, the phyla of Actinobacteria are proportionally lower in abundance and mostly represented by the Bifidobacterium (Amoroso et al., 2020, Bäckhed et al., 2015, Casén et al., 2015, Chang and Kao, 2019, Clarke et al., 2014, Selvamani et al., 2021, Sokol et al., 2017).
The advent of genetic tools and the revolution of metagenomic studies over the last two decades revealed that abnormal alterations occurred in the human gastrointestinal microbiota framework and functions (Selvamani et al., 2021, Xie et al., 2016). The recent high-throughput DNA sequencing technology has not only helped in the understanding of the complex microbiome in the human body, but also for characterizing disease-associated microbiota changes, together with precisely specifying the altered microbial species (Casen et al., 2015). The traditional culture-based analysis suspected that IBD was solely caused by single pathogenic microorganisms, like Mycobacterium avium subspecies paratuberculosis (MAP); adherent-invasive Escherichia coli strains (AIEC), or Clostridium difficile (Li et al., 2015). However, advancing genomic studies in the present day has depicted a more precise and thorough understanding of the collective role of the gut microbiome. Among many genomic studies that have been reported, the integrative Human Microbiome Project (HMP) is one of the excellent resources that evaluates the core microbiome and its transitions from a healthy-balanced state to a dysbiotic state (Parada Venegas et al., 2019).
3.2. Growing evidence on dysbiosis
As mentioned earlier, the complex human gut microbiota varies taxonomically and functionally based on location in the gastrointestinal tract. Within the same individual microbial complexity undergoes variations due to several factors including age transition, gene expressions, food consumption, lifestyle, environmental factors, and medications such as an antibiotic (Bajinka et al., 2020, Lange et al., 2016). However, the multitude of factors that intervene with the gut microbiome also harmed human health. Gastrointestinal dysbiosis is an alteration and disturbance of the diversity of gut microbiota (Carding et al., 2015, Sokol et al., 2017). Numerous human gastrointestinal diseases including diabetes, IBD, and obesity were found to be associated with these abnormal prominent features of the gastrointestinal microbiome (Alhagamhmad et al., 2016, Sasaki and Klapproth, 2012). Accumulating evidence in an animal model and clinical studies had revealed that dysbiotic conditions of the gut microbial populations are linked to IBD or its associated symptoms. Numerous animal studies used germ-free mice (GF) to evaluate gastrointestinal dysbiosis associated with IBD. GF mice were inoculated with gastrointestinal microbiota collected from IBD patients. This induced expression of pro-inflammatory genes in GF mice models, compared to control study which is inoculated with healthy gastrointestinal microbiota. These studies also revealed that GF mice inoculated with IBD microbiota developed severe colitis (DeGruttola et al., 2016, Singh et al., 2016). The term “Dysbiosis” refers to a disproportion condition in the gut microbial populations and this disparity is often associated with indisposition of health state (Carding et al., 2015, Casén et al., 2015, Sokol et al., 2017). The microbial disparity could be a loss or introduction of microbial population in the healthy state composition. Therefore, gut dysbiosis could be from three causes: (1) reduction and insufficient population of commensal microflora; (2) loss of diversity in commensal microbiota; and (3) great competition between the commensal microbiome and pathogenic flora (Casén et al., 2015, Singh et al., 2016, Zuo et al., 2018).
In IBD, a decrease in α-diversity of gut microbiome such as Faecalibacterium spp. and Roseburia spp. was consistently observed (Bäckhed et al., 2015, Korpela et al., 2016). These patients are often diagnosed with an associated increase in the relative abundance of Bacteroidetes and other facultative anaerobic bacteria such as the genera of Enterobacteriaceae. Despite the overlap in microbial dysbiotic changes among CD and UC, there are significant differences in the mucosal and fecal microbial composition (Amoroso et al., 2020). In CD, the predominant dysbiosis is associated with microbial alteration of more than five microbial populations such as a decline in abundance of Faecalibacterium prausnitzii, Bifidobacterium adolescentis, and Dialister invisus; as well as a sudden increase in the population of Ruminococcus spp. and pathogenic Clostridium cluster XIV (Pascal et al., 2017). Gevers et al. reported an elevation in the gastrointestinal microbiota among newly diagnosed pediatric CD patients in a recent cohort study. The study also reported a significant increase in the population of Pasteurellaceae, Enterobacteriaceae, Veillonellaceae, and Fusobacteriaceae, whereas a decline was found for the relative abundance of Bacteroidales, Clostridiales, and Erysipelotrichales. The study compared microbial disparity of the pediatric IBD patients with healthy controls (Gevers et al., 2014). In addition, another recent study by Pascal et al. (2017) also reported a greater incidence of gastrointestinal dysbiosis in CD compared to UC (Pascal et al., 2017). The study reported enrichment of six microbial genera among the CD patients compared to only two in UC patients and one in healthy controls. The study also confirmed that CD patients also had a decline in the relative abundance of Faecalibacterium spp., in contrast to UC patient samples which do not show the absence of this bacterium (Pascal et al., 2017). Thus, these findings discriminate microbial composition of dysbiosis in CD with UC (Gevers et al., 2014).
A Study conducted by Pascal et al. (2017) also reported that loss of beneficial microbes was more abundant in CD patients, and such organisms are involved in butyrate production (Pascal et al., 2017). These bacterial metabolites are well-known for their role in reducing pro-inflammatory cytokines during anti-inflammatory (Goodrich et al., 2014). Meanwhile, the members of Firmicutes are the primary producers for butyrate and short-chain-fatty acids (SCFA) in the human gastrointestinal tract (Parada Venegas et al., 2019). Reduction in the SCFA increases inflammation and affects the colonic barrier functions (Chen et al., 2020, Desai et al., 2016). The impaired gut barrier leads to the invasion of pathogenic microorganisms that increases the IBD severity. Elevation of pathogenic microbes in the gastrointestinal environment such as the species of Enterobacteriaceae and Bacteroides fragilis was found to be a secondary inflammation state in the gastrointestinal tract of IBD (Carding et al., 2015, Li et al., 2015). High endotoxic LPS was found in the outer membrane of both pathogenic microorganisms. These endotoxic LPS were found to exhibit the suppressive impact of the regulation of T-lymphocytes or in the activation of the helper-T cells via the host’s TLR signaling pathway (Parada Venegas et al., 2019). The endotoxic expression from these opportunistic pathogens was evaluated with capabilities to induce inflammation in the gut and leads to a progression of colitis in animal models (Carding et al., 2015, Parada Venegas et al., 2019). In another related study, distinctive adhesive characteristics were reported in the E. coli samples isolated from CD and UC patients (Lee et al., 2019). In comparison to CD biopsy samples, the isolates of E. coli associated with UC were found to harbor more adhesive and virulent determinants. The UC-associated E. coli isolate was positive for pathogenicity factors such as OmpA, AfaE, and USP (Lee et al., 2019, Sasaki and Klapproth, 2012). The virulent E. coli isolates are originated from phylotype B2 and D, which can cause inflammatory effects. The in vitro studies using these E. coli isolates in macrophage cultures had demonstrated a reconcilable increase in the UC pathogenicity (Lee et al., 2019). Thus, these findings strongly indicate dysbiosis serves as a factor of gastrointestinal inflammation that contributes to IBD (Carding et al., 2015, Casén et al., 2015, DeGruttola et al., 2016). Takashi et al. (2016) characterized a decline in the abundance of butyrate-producing bacteria during dysbiosis of CD patients. The decline was observed in the genera of Faecalibacterium, Eubacterium, Bacteroides, and Ruminococcus in CD patients. However, the contrast found was that the genera of Actinomyces and Bifidobacterium increased their population in the gastrointestinal environment of CD patients (Takahashi et al., 2016). The study reported the occurrence of gut dysbiosis in CD patients with significant reduction of butyrate-producing bacterial species including Bacteroides uniforms, Blautia feces, Roseburia inulinivorans, Ruminococcus torques, Clostridium lavalense, and F. prausnitzii (Rivière et al., 2016, Takahashi et al., 2016).
3.3. The origin of dysbiosis
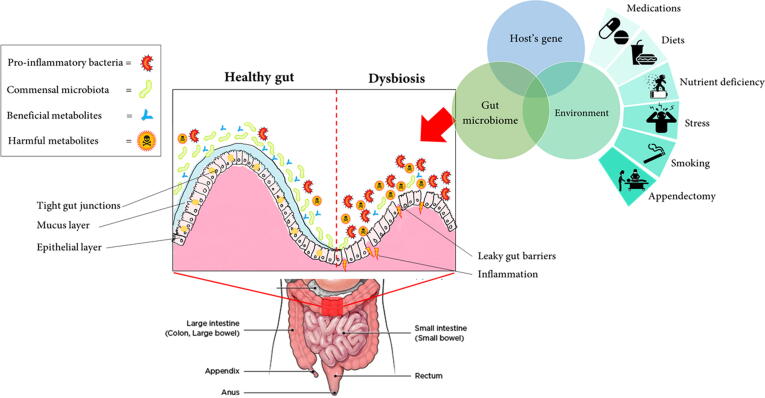
As gut dysbiosis is anticipated as a key factor for the pathogenesis of IBD, the most vexing question is: What would be the origin of gastrointestinal dysbiosis? Bujinkan et al. (2020) had highlighted that various extrinsic factors lead to gut dysbiosis which is extensively studied but poorly linked the varying degree of consequences to respective factors (Bajinka et al., 2020). Notably, there are several studies that discussed the factors that cause human gastrointestinal dysbiosis, based on previously published works (DeGruttola et al., 2016, Pittayanon et al., 2020). Specifically, Bajinka et al. (2020) had reviewed extrinsic factors that are associated with dysbiosis including mode of delivery; effect of dietary (high fat; fiber; animal fat and amino acids; gluten; sucralose); different types of diet therapy (Mediterranean or Vegan diet); the effects of antibiotics and drugs; the effects of prebiotics; oxidative stress, and the impacts of socioeconomic status (Chiba et al., 2019, Racine et al., 2016). Fig. 1 summarizes the different lumen conditions between healthy gut and dysbiosis, together with factors that possibly lead to gut dysbiotic conditions.
Fig. 1.
Schematic representation of the healthy intestinal lumen and in dysbiotic conditions. The correlation between host gene, gut microbiome, and environmental factors are identified to lead the development of IBD. However, none of the risk factors alone are sufficient for the progression of IBD.
In a recent population-based analysis conducted in Canada, a significant IBD increase in children aged 0–5 years old was reported and the incidence remained stable in children older than 6 years (Pittayanon et al., 2020). The poor development of the human gastrointestinal microbiome in early life was caused by the cesarean delivery and formulated milk feedings mode (Bäckhed et al., 2015). Severe disruption of the gastrointestinal microbiome was also reported in infants born by cesarean sections which then affects the development of balanced immune systems and gut microbiome which later leads to other complications (Selvamani et al., 2021). In comparison to natural delivery, the cesarean section delivered infants experience lower diversity of Actinobacteria, Bacteroides, and Bifidobacterium spp. since birth to a minimum of 90 days of life (Rutayisire et al., 2016). A decline in the probiotic Bifidobacteria populations leads to a significant increase of Clostridia spp. in the cesarean section-infants was explained as the impact of antibiotic use (Lange et al., 2016, Yoon and Yoon, 2018). Another study conducted on cesarean section-born infants also reported a disproportionate representation of Klebsiella and Veillonella spp. in. Mothers undergoing cesarean section surgery need to take antibiotics before, during, and after delivery which subsequently alters and cause dysbiosis in their new-born (Clarke et al., 2014, Rutayisire et al., 2016). In addition, mothers’ breast milk also influences the framework of microbial composition in the newborn gastrointestinal environment during the early stages of life. The unique and diverse composition of microbial, nutrient, and other valuable components of human milk has a great influence on shaping the gut microbiome structure (Selvamani et al., 2021). However, formula feeding had been found to lower the abundance of Bifidobacteria, in contracts with the normal gut microbial establishment by breastfeeding infants. Human milk oligosaccharides act as prebiotics that flourishes the growth of the intestinal microbiome (Selvamani et al., 2021).
Another factor that largely contributes to gut dysbiosis and further leads to IBD would be the type of diet (Chiba et al., 2019). Several clinical studies and reviews have reported the impact of diets during progressive inflammation of the gastrointestinal tract at the chronic level (Desai et al., 2016, Singh et al., 2017). Recent research reported that gut dysbiosis caused by Western-based diets had increased susceptibility to IBD (Chiba et al., 2019). Westernized diets were found to cause poor production of microbial metabolites, promote the proliferation of microbes, and accelerate the mucus degradation that had resulted in the disrupted barrier functions (Mak et al., 2020). Under the incessant deficiency of dietary fibers, the gastrointestinal microbiota started to utilize the host secreted mucous glycoproteins as their metabolic nutrients. This eventually brings the disintegration of the gastrointestinal mucosal barrier, and this condition also promotes greater invasion by mucosal pathogenic microbial populations, such as Citrobacter rodentium (Ananthakrishnan et al., 2013, Desai et al., 2016). Racine et al. (Racine et al., 2016) reported that malnourishment with a large intake of sugar and carbonated beverages had caused rising UC incidents among Europeans. The study also reported the consumed meals lack of vegetables and fibers. Another two recent meta-analyses also showed that larger consumption of carbonated drinks and their simple sugars such as sucrose also linked with the progression of UC (Nie and Zhao, 2017, Wang et al., 2017). Meanwhile, the development of CD was found associated more with an insufficient dietary fiber intake, especially under malabsorption conditions. A study by Ananthakrishnan et al. (2013) reported that prolonged consumption of dietary fibers derived from fruits can lower the risk of CD, notwithstanding for UC (Ananthakrishnan et al., 2013). Furthermore, patients suffering from ileal CD and ileal resection were found to be experiencing a deficiency of vitamin B12 (Ward et al., 2015). With rising obesity and IBD on a global scale, low-calorie diets such as low-calorie sweeteners had become popular ‘healthier’ diets recently. However, a recent study by Suez and colleagues reported that these artificial sweeteners also had significantly disrupted gut microbiota in both animal and human experimental interventions (Suez et al., 2014).
Moreover, antibiotics represent a long-time extrinsic factor that is associated with gut dysbiosis. Antibiotics are highly prescribed drugs on a global scale that saved millions of lives since their discovery (Lange et al., 2016). However, broad-spectrum antibiotics such as clindamycin had severely reduced bacterial diversity in the human gut which subsequently increased the relative abundance of opportunistic pathogens (Lange et al., 2016, Lewis et al., 2015). In a recent Finnish pediatric cohort study, antibiotic macrolides triggered a prolonged transposition in the gastrointestinal microbiome, with significant depletion of the relative abundance of Actinobacteria, Bifidobacterium, and Lactobacilli (Korpela et al., 2016). Macrolides are effectively used for bacterial infections, including in nonspecific inflammatory bowel diseases, especially CD, and the eradication of Helicobacter pylori. Ciprofloxacin and metronidazole are considered frontline antibiotics prescribed for CD and found to cause gastrointestinal disturbances, stunted growth of cartilages and rupture of tendons in the fetus, oral candidiasis, and a prolonged consumption could lead to permanent damage of the peripheral nerves (Lange et al., 2016, Lewis et al., 2015). Antibiotic therapy also increases antibiotic resistance in the gastrointestinal microflora. A recent study reported the development of ciprofloxacin resistance in about 66.7% of the Gram-negative bacterial isolates isolated from abdominal abscess samples collected from CD patients (Yoon and Yoon, 2018). In addition, recent findings also highlighted the side impact of non-antibiotic drugs which increase the severity of dysbiosis in the gut lumen (Zimmermann and Curtis, 2019). In a recent systematic review, the prescription of non-antibiotic drugs also causes intestinal dysbiosis (Le Bastard et al., 2018). The study reported that the non-antibiotic medications including anti-psychotic drugs and proton-pump inhibitors lowered the α diversity of the gastrointestinal microbiota. In addition, oral diabetes medicine – metformin; non-steroid-based anti-inflammatory drugs, and the broad group of pain-relieving drugs – opioids were also found to trigger gut dysbiosis by increasing the relative abundance of Enterobacter, Escherichia, Klebsiella, and Citrobacter (Le Bastard et al., 2018).
Moreover, other extrinsic factors such as exercise, oxidative stress, quality of water, and socio-economic status of an individual also significantly alter the framework of gut microbiota (Bajinka et al., 2020). Notwithstanding, Chang and Kao (Chang and Kao, 2019) reviewed that the impact of host factors should not be neglected on gut dysbiosis. Despite the compelling evidence on the extrinsic factors, the study highlighted the importance of host genetic factors that are responsible for the modulation of gut microbial compositions (Goodrich et al., 2014, Xie et al., 2016). A comprehensive understanding of host genetics could open the window of opportunity for alternative and effective therapeutic strategies by manipulating the gastrointestinal microbiome (Xie et al., 2016). Goodrich et al. (2016) evaluated the association between hosts’ genes in the modulation of the gastrointestinal microbiome by performing a comparative study in fecal samples of 416 twin pairs (Goodrich et al., 2016). The study reported that the relative abundance of commensal microbiota in the gut was influenced by the host’s genetics. This group of researchers had expanded the investigation with 1126 twins and successfully reported the involvement of notable host genes associated with microbial diversity (Goodrich et al., 2016). The study demonstrated that the relative abundance of Bifidobacterium in the gastrointestinal environment is based on the association between the bacterial lactase gene with the host’s ALDH1L1 gene locus. This has explained the existence of a co-evolution link of a host’s blood pressure with formate production by the gut bacterium. The study also projected the association of other genes that were involved in the barrier defense, metabolism, and diet-sensing (Goodrich et al., 2014, Goodrich et al., 2016).
3.4. The grey spot
Despite the accumulating evidence, there are more profound utterances that misapprehended the concept of gut dysbiosis with IBD. One of the key questions that arise is: what time does the gastrointestinal microflora become dysbiotic – before or after IBD? Is gut dysbiosis is the actual cause of IBD or it is just a secondary symptom of IBD? As mentioned earlier, there are several extrinsic factors and host factors that cause gut dysbiosis. Among them, antibiotics are generally known to kill broad microbial components. Despite the benefits of antibiotics, their usage has been linked to short- and long-term health issues. Antibiotic administration was found to cause perturbations in the intestinal microbial compositions (Lange et al., 2016, Yoon and Yoon, 2018). The world’s first true antibiotic – penicillin was found to have less impact on the microbial composition (Zimmermann and Curtis, 2019) However, amoxicillin which is widely prescribed for skin, throat, and urinary tract infections were found to increase the abundance of Enterobacteriaceae, mainly Citrobacter spp., Enterobacter spp., and Klebsiella spp. Similar perturbations were also observed as side effects of macrolides, ketolides, clindamycin, tigecycline, Fosfomycin, cephalosporins, and clavulanate (Zimmermann and Curtis, 2019). Many antibiotics induced a decline in the relative abundance of butyrate-synthesizing microflora in the gut environment (Takahashi et al., 2016).
Butyrate is important evidence for the existence of a ‘symbiotic’ relationship, a shared existence that benefits both the microbial population and the host cells. Butyrate is an SCFA produced by gastrointestinal microbes that supports host immune system functions and protects against several diseases of the digestive tract (Rivière et al., 2016, Takahashi et al., 2016). Additionally, butyrate regulates the hypoxia conditions of the gut environment to ensure the sustainability of the gastrointestinal microbiome (Takahashi et al., 2016). However, antibiotic therapy has been shown to increase oxygen content in the lumen of the gut tract (Rivera-Chávez et al., 2016). Consumption of antibiotics decreases the abundance of Clostridia spp. which is a well-known butyrate producer. This increases the oxidative reactions in the intestinal environment which subsequently promotes the diffusion of oxygen in the lower regions of the gut (Lewis et al., 2015, Yoon and Yoon, 2018). The transformation of the hypoxia state of the gut is a major drawback for the survival of anaerobic intestinal microbiota. The oxidative state exacerbates the condition by endorsing the growth of aerobic or facultative microflora such as Actinobacteria and Proteobacteria. This leads to gastrointestinal dysbiosis (Rivera-Chávez et al., 2016, Rivière et al., 2016, Takahashi et al., 2016). Nonetheless, several animal model studies also found that the pathogenic microorganisms also promote the oxidative activity of the epithelial layer and increase oxygen concentration in the mucosal surfaces (Rivera-Chávez et al., 2016). This finding proves the hypothesis of the impact of antibiotic treatment for regular infectious disease on gut dysbiosis which then leads to the IBD symptoms (Lange et al., 2016, Lewis et al., 2015, Zimmermann and Curtis, 2019).
Despite the pathogenesis, antibiotic treatment for IBD was found to induce severe dysbiotic conditions, after the patient’s diagnosis of IBD (Lewis et al., 2015, Zimmermann and Curtis, 2019). Some of the IBD-associated antibiotic therapy such as metronidazole and ciprofloxacin were proven to cause severe aftereffects including vomiting, abdominal pain, diarrhea, headache, and nausea, as well as anxiety and confusion (Yoon and Yoon, 2018). Another evidence for the occurrence of dysbiosis after IBD could be the rising of Clostridium difficile infection (CDI) incidences which are common in all antibiotic therapies. The cornerstones of IBD therapies are being causative agents to increase CDI incidences and worsen the clinical course (Berg et al., 2012). The impact of CDI on IBD patients was characterized in several reports (Berg et al., 2012, Rivera-Chávez et al., 2016). In contrast with antibiotics as therapy for IBD, it has been related to the pathogenesis of IBD through gut dysbiosis. Patients diagnosed with IBD, especially CD, must be prescribed antibiotics for a longer time such as 2 to 5 years, compared to a healthy human. This prescription was found to alter the intestinal microflora composition, decreasing luminal bacteria concentration, and leading to gut dysbiosis (Korpela et al., 2016). However, these conditions give rise to another argument that gut dysbiosis is just a secondary phenomenon and not the cause of IBD. It will be a giant leap in the development of an effective and more promising strategy, not merely for treating IBD, but also for other gastrointestinal-associated disorders such as obesity and diabetes (Nie and Zhao, 2017). In a recent review by Brüssow (2020) a concern had been raised that human microbiome research and medical understanding are still in a descriptive phase (Brüssow, 2020). The study evaluated that the existing number of microbiome studies resulted in datasets which statistically underpowered and require more sophisticated analysis (Brüssow, 2020). Ni and Zhao (2017) highlighted those existing reports clarified the role of dysbiosis as causative for IBD are mostly based on animal models. Extensive animal model studies also enlightened the role of diverse and sustained gastrointestinal microbiome in the healthy state and during illness. However, metamorphosing the scientific understanding of the microbiome into a feasible and productive therapy is a challenge (DeGruttola et al., 2016, Nie and Zhao, 2017).
Thus, scientific investigations must be expanded in numbers and scope to delineate the interaction of the host and its microbiome. The transition of experimental data to causal relationships with the disease is required to fulfill Koch’s postulates (Singh et al., 2016). Koch’s postulates are a set of standards that define a reliable role of an organism in a disease. Four postulates must be fulfilled where (1) identified organism must be present in all cases of the study; (2) the organism must be isolated from infected or diseased patients; (3) the organism must be able to cause the same disease/symptoms when reintroduced to a healthy susceptible animal model; and (4) the organism can be isolated again from the new host (Singh et al., 2017). As the gut dysbiosis that leads to IBD is caused by several organisms, Koch's postulates have been modified to accommodate new understandings of microorganisms (Nie and Zhao, 2017, Singh et al., 2016). Singh et al. (2017) proposed a modified Koch’s postulate which comprises: (1) re-define single organism to several possible pathobionts; (2) acceptance of molecular microbial characterization of dysbiosis; (3) allow the introduction of several possible dysbiotic communities in the re-introduction; and finally, the re-isolation analysis must require re-characterization of the dysbiotic community by molecular methods (Singh et al., 2016, Singh et al., 2017).
The modification of Koch’s postulates and the development of advanced methods to distinguish a healthy microbiome from dysbiosis conditions are required. This is as important as the existence of a substantial inter-individual variability and manipulation by the confounding factors (DeGruttola et al., 2016, Singh et al., 2016). In addition, most studies focused on the correlation between inflammation and the gastrointestinal microbiome. Indeed, the human gut microbiome is also comprised of fungi and viruses. Successful therapy in animal models failed to reduce or control fungal colonization. This has raised questions on the causative role of fungi in the healthy gut microbiome and inflammation. Antibiotic exposure in IBD treatment was found to promote the expansion of fungal taxa (Sokol et al., 2017). A recent study, involving adults experiencing IBD symptoms, showed a differential relative abundance of fungi compared to healthy individuals (Yeoh et al., 2021). Analogously, the perspicuous role of viruses in the IBD is still not well understood to date (Liang et al., 2020).
4. Defining Pro- and prebiotic mechanisms in IBD
4.1. Probiotics – Beyond ‘just a living cell’
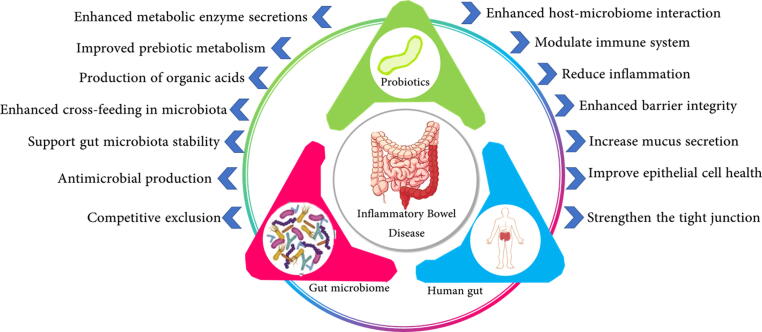
Historically, the term Probiotic was derived from Greek, meaning “for life”. In 1954, Ferdinand Vergin who studied the destructive side-effects of antibiotics and other anti-microbial agents on the gastrointestinal microbial populations first introduced the term ‘Probiotics’ (Brüssow, 2020, Naseer et al., 2020). However, the term was subsequently changed over the purpose and benefits. The WHO and FDA had defined Probiotics as live microorganisms that can be administered in an adequate amount to confer health benefits (Asha and Khalil, 2020). Since 2013, this definition was maintained by the International Scientific Association for Probiotics and Prebiotics (ISAPP) (Darb Emamie et al., 2021, Palumbo et al., 2016). The commonly used probiotics comprise the genera of Lactobacillus, Bifidobacteria, and Saccharomyces yeasts (Selvamani et al., 2021). The efficacy of probiotics mostly depends on the microbiological and physiological properties of the selected strains (Darb Emamie et al., 2021). Briefly, the probiotics must be able to withstand human consumption, transit through differential conditions of the gastrointestinal tract, and they should produce a significant physiological impact on the host’s microbial ecosystem and immune system (Asha and Khalil, 2020). Many studies have reported the mechanism of action of probiotics and mostly they are species or strain specific. Appropriate selection of the probiotic strain is paramount for the promising success of the therapy (Altun et al., 2019). Therefore, to evaluate the effectiveness of each probiotic action, the mechanism underlying the probiotic action must be well understood. Recent studies have demonstrated the potential mechanisms of action underlying the therapeutic action of probiotics in patients with IBD (Altun et al., 2019, Fedorak et al., 2015, Matsuoka et al., 2018). Among the potential mechanisms that could be linked to probiotic action (Fig. 2) is the alteration of the gastrointestinal microbiome, modulation of intestinal barrier function, and modulation of the host’s immune response.
Fig. 2.
The mechanism of actions of probiotics in combating IBD symptoms. The probiotics were identified to interact with both host tissue and resident microbiota to drive the probiotic benefits.
Several studies have reported that probiotic therapy significantly induced changes over the gastrointestinal microbial composition and diversity (Chen et al., 2020, Liu et al., 2020a, Liu et al., 2020b, Xia et al., 2020). Recently in 2016, Yasuda and colleagues demonstrated a successful modulation of gut microbiota using a probiotic strain, Clostridium butyricum MIYAIRI (CBM). The CBM probiotic preparation was used safely in Japan over four decades and MIYAVI-CBM was clinically targeted for adverse conditions of IBD. Even though the study did not have a placebo, it had proven that the probiotic preparation of CBM can alter the microbiome by inhibiting putrefying microflora and enriching the population of probiotics including Bifidobacterium and Lactobacillus (Yasueda et al., 2016).
These findings also reported a significant decline in the relative abundance of Escherichia during the probiotic therapy. Higher levels of E. coli were always linked to gastrointestinal tract inflammation in patients with IBD (Yasueda et al., 2016). In patients of UC, consumption of a mixture of Lactobacillus and Bifidobacteria reported a significant increase in the population of genera of Proteobacteria and reduction in Gram-negative rods. Supplementation of the probiotic mixture had a significant effect on the alteration of the gastrointestinal microbiome (Pilarczyk-Żurek et al., 2017). Similarly, several other studies also reported that consumption of probiotics successfully restores and modulates a healthy microbial ecosystem as a treatment for IBD in patients (Liu et al., 2020a, Liu et al., 2020b, Xia et al., 2020). Moreover, Chen et al. (2020) demonstrated probiotic action in animal studies (Chen et al., 2020). The study reported that probiotic mixture is given to experimentally colitis-induced DSS mice was productive as it could restore the relative abundance of gut microbiota such as Lactobacillus, Bifidobacteria, Bacteroides, and Akkermansia (Chen et al., 2020). Although recent works had demonstrated the numerous probiotic strain’s potential to alter the intestinal microbiota, it remains unclear which strain could specifically drive the induction of gastrointestinal homeostasis in IBD patients. Another probiotic action that is always linked to the efficacy of IBD treatment would be the modulation of the host’s intestinal barrier. Several studies reported IBD patients to display increased paracellular permeability on the intestinal tight junction abnormalities (Komaki et al., 2020). The disruption of the mucosal barrier was found to initiate and accelerate inflammation of intestinal linings during the progression of IBD (Amoroso et al., 2020). A reduced number of goblet cells were observed in UC patients. This reduced the thick mucosal layers that cover the intestinal lining and altered the mucus composition, especially lacking antimicrobial mucins, phosphatidylcholine, and glycoproteins (Chen et al., 2020, Michielan and D’Incà, 2015).
Probiotic strains of Lactobacillus and Bifidobacterium strains were elevated to express goblet cells and contributed to the inhibition of pathogenic bacterial adherence at intestinal epithelial cells and ultimately play a crucial role in the anti-inflammatory actions (Ivanovska et al., 2017). Chen et al. (2020) demonstrated that the consumption of a mixture of the quadruple probiotic mixture (P-qua) improved the functions of the disrupted intestinal barrier. The preparation of P-qua comprises gastrointestinal commensal microflora such as B. infantis, L. acidophilus, Enterococcus faecalis and Bacillus cereus. The induced UC studies evaluated the efficacy of the probiotic therapy where the integrity of the mucosal layer improved; the trans-epithelial electrical resistances had enhanced, and the permeability of epithelium and endothelium towards macromolecules had decreased (Chen et al., 2020, Naseer et al., 2020). The lactic acid probiotic strains were found to have a direct impact on the intestinal epithelial barrier by upregulating the expression of tight-junction proteins. These effects were mediated through probiotic fermentation in the gastrointestinal environment which produced SCFAs, including acetate, propionate, butyrate (Chen et al., 2020, Nataraj et al., 2020).
Probiotics are also well-known for modulating the host’s immune responses and several studies reported this effect in probiotic therapy for IBD (Darb Emamie et al., 2021, Singh et al., 2016). Probiotic treatment was proven to downregulate the expression of proinflammatory cytokines like tumor necrosis factor-α (TNFα), interleukin-6 (IL-6), and interferon-gamma, inducible nitric oxide synthase, and matrix metalloproteinase activity in inflamed mucosa of active IBD or experimental ulcerative colitis (Ananthakrishnan, 2015, Chen et al., 2020). The host’s immune cells secrete excessive amounts of inflammatory substances, including cytokines and active oxides during adverse IBD conditions. The pathogenic infections also caused the secretion of IL8 and TNFα from intestinal epithelial cells (D’Incà et al., 2011). Recent works have evaluated the impact of probiotic therapy on immunomodulation in IBD patients. Xia et al. (2020) demonstrated that probiotic therapy over the DSS-induced colitis model has proven to suppress the NF-κB signaling pathway (Xia et al., 2020). Supporting this, another animal model and the in-vitro study reported that probiotic therapy using Bifidobacterium lactis decreased the NF-κB signaling during the pro-inflammatory expression (Tamaki et al., 2016). A study by Fedorak et al. (Fedorak et al., 2015) had demonstrated that the VSL which is a commercial probiotic preparation composed of one strain of Streptococcus thermophilous, three Bifidobacteria strains and four different strains of Lactobacilli, had decreased the inflammatory cytokines, effectively in the intestinal mucosa. Similarly, in another study, a mix of 11 strains of Lactobacillus and Bifidobacterium were tested, and they found 5 strains that had high potential for the management of IBD through immunomodulation in the host’s body (Chen et al., 2020).
4.2. Prebiotics – The ‘booster’ of gastrointestinal microbiota
The health benefits of prebiotics and dietary fibers being part of the human meal down have been well described before civilization. The great Greek physician - Hippocrates had described in 450 BCE, that coarse wheat attributes of laxation compared to refined variety (Asha and Khalil, 2020). Scientifically, the term ‘prebiotics’ is firstly defined as “a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus improves host health” (Darb Emamie et al., 2021). Later in the year 2004, ’prebiotics’ was further defined with additional criteria such as 1) being impervious to adverse gastric conditions and mammalian enzymatic degradation as well as intestinal absorption; 2) being able to be fermented by the gastrointestinal microflora, and 3) selective enrichment of growth or activity of gastrointestinal microflora to enhance health benefits (Asha and Khalil, 2020, Darb Emamie et al., 2021). Now, the International Scientific Association of Probiotics and Prebiotics (ISAPP) has redefined the term of prebiotics as “a substrate which selectively fermented by the gut microflora and bestowed health benefits to the host”. This new definition allows non-carbohydrate substances also recognized as prebiotics and application prebiotics are not only restricted to the gastrointestinal tract (Naseer et al., 2020). The example of prebiotics is numerous. The majority of the prebiotics belong to carbohydrates under oligosaccharide subsets. Fibers and carbohydrate-based prebiotics are distinguished by two measures. Firstly, the degree of polymerization (DP) where fibers have DP of 3 or higher. Secondly, fibers are indigestible by endogenous enzymes compared to prebiotic oligosaccharides (Ananthakrishnan et al., 2013). The most common prebiotics known for their beneficial impacts would be fructooligosaccharides (FOS), galactooligosaccharides (GOS), starch-derived oligosaccharides, and other oligosaccharides such as pectin, galacturonic acid, and arabinose (Naseer et al., 2020). Vegetables and fruits are the natural sources of prebiotics such as asparagus, sugar beet, garlic, chicory, onion, Jerusalem artichoke, wheat, honey, banana, barley, tomato, rye, soybean, human and cow milk, peas, beans, and recently the seaweeds and microalgae also were reported (Desai et al., 2016) However, natural sources/foods often contained in low concentrations of prebiotics. Thus, prebiotics is also manufactured on large industrial capacity from raw materials such as lactose, sucrose, or starch (Kanwal et al., 2020, Li et al., 2020a, Li et al., 2020b).
FOS is most frequently utilized in human prebiotic therapy compared to other prebiotics. FOS is an oligosaccharide with a fructose-based linear chain with β (2 → 1) linkage, where inulin has higher DP compared to the rest of the FOS (Benjamin et al., 2011). The length of fructans chains is being an important criterion to determine which bacteria can ferment these prebiotics (Benjamin et al., 2011, Halfvarson et al., 2017). GOS is another prebiotic which widely found in many probiotic-associated food products. GOS composed of lactose residues that are composed of galactose in β (1 → 6), β(1 → 3), and β(1 → 4) linkages. The beta galactosidases of different origins result in the formation of GOS that differs in the amount, glycosidic linkages, and DPs (Azpiroz et al., 2017, Ishikawa et al., 2011). Both FOS and GOS type prebiotics are synthesized by various methods including chemical synthesis using specific transferase enzymes or through microbial fermentations. Although chemical reactions are costly, the concentrations of end products are low (Naseer et al., 2020). Therefore, microbial fermentation using similar probiotic strains including Bifidobacterium, Lactobacillus spp., or other strains such as Aspergillus, Aureobasidium pullulans, were exploited to produce therapeutic potential prebiotics (Rogha et al., 2014, Shavakhi et al., 2014). Prebiotics are indigestible by the host’s enzyme but will be fermented selectively by gastrointestinal microbiota. When this prebiotic reaches the colonic environment, the host’s gut microflora release various enzymes to convert higher DP prebiotics into simpler derivatives. Prebiotics acts as an anchor to establish a unique ecosystem in the lumen of the gastrointestinal tract (Kim et al., 2020). Several studies have shown that prebiotics promotes intestinal microflora shift by stimulating the growth of commensal gut microbiota such as Bifidobacteria, Lactobacilli, and non-pathogenic E. coli. This has enhanced gut resistance towards colonization by disease-causing microflora (Benjamin et al., 2011, De Preter et al., 2007). Despite some selective fermentations, prebiotic stimulation of gut microbiota is also non-selective species basis and could promote the growth of non-common probiotics such as Eubacteria rectale, Clostridium coccoides, and Roseburia inulinivorans (Niv et al., 2016).
Prebiotic fermentations by specific microflora of the gut produce SCFAs, lactic, acetic, propionic, and butyrate acids. These SCFAs are produced through several systemic and colon-specific pathways (Berni Canani et al., 2017). Acetate is widely used up as cell fuel to generate for muscles and colon. Propionic acid is utilized during the synthesis of cholesterol molecules. Butyrate receives particular attention exerts various benefits to the host, such as improving metabolism, promoting anti-inflammatory actions, and modulating the host’s immune system (Takahashi et al., 2016). Therefore, prebiotic consumption was proven to enhance the host’s immune function, strengthen colonic integrity, decrease infection rate, down-regulate allergic responses, and improve the digestion process. However, these effects are not directly imposed upon prebiotic ingestion. A recent review suggested that the benefits of prebiotics are attained indirectly, as the prebiotic fermentations alter the gastrointestinal microbiota compositions (Darb Emamie et al., 2021, Naseer et al., 2020). Numerous animal model studies have shown that prebiotics had improved over the mucosal barriers by enhancing the growth of probiotics that are capable of upregulating epithelial defense mechanisms (Asha and Khalil, 2020, Naseer et al., 2020).
5. Arising probiotic, prebiotic and synbiotic therapy for IBD
Large numbers of clinical studies were previously conducted regarding antibiotic therapy for IBD (Lange et al., 2016, Zimmermann and Curtis, 2019). As the current understanding of the pathogenesis of IBD also involved intestinal microflora, antibiotics are extensively prescribed to reduce IBD-associated microbial concentration in the host’s gut. However, antibiotic treatment can be associated with negative outcomes as well (Lewis et al., 2015). In addition, antibiotic treatment does not always result in a uniform outcome. Studies have shown the adverse impact of antibiotics altering gut microbial populations. The perpetuated consumption of antibiotics with higher concentrations had impacted gastrointestinal microfloral activity (Yoon and Yoon, 2018). The growing evidence on the role of gastrointestinal microbiota in combating IBD could be utilized to manipulate the microenvironment and cure the inflammatory process (Casen et al., 2015). Rapid advancement in metagenomics and molecular studies does not only describes the pathogenesis of IBD but also helped to explore alternative remedies for IBD by alteration of the gastrointestinal microbiome (Xie et al., 2016). Probiotics, prebiotics, and their synbiotic approaches are being emerging strategies that target the gut microbiome, slow the progression of IBD, and restore intestinal health (Asha and Khalil, 2020). Although the beneficial impacts of probiotics, prebiotics, and synbiotics therapies have been established, the collective data is still insufficient to support the impact on restoration and recovery of the host’s gut microbiome. Therefore, further studies particularly in IBD are needed to evaluate and understand the potential therapies in the amelioration of gastrointestinal disorders.
5.1. Probiotic therapy in animal models and human studies
As the enteric microflora become more aberrant in IBD patients, a significant decline of probiotics especially Bifidobacterium and Lactobacillus spp. was observed in many studies (Matsuoka et al., 2018, van der Waal et al., 2019). Pathogenesis of IBD might be engendered by the rising of abnormal microflora in the gut. Oral administration of probiotics is widely practiced for various health improvements, including as therapies for gut-related disorders (Altun et al., 2019, Pilarczyk-Żurek et al., 2017, van der Waal et al., 2019). Studies conducted to evaluate the efficiency of probiotics in the IBD, that demonstrated in animal models are summarized in Table 1 and the clinical intervention are summarized in Table 2.
Table 1.
Summary of animal models’ studies of using probiotics.
| Reference | Type of treatment | Model | Dose | Intake | Duration | Parameters analyzed | Conclusion |
|---|---|---|---|---|---|---|---|
| Chen et al. 2020 | Bifidobacterium breve CCFM683 | C57BL/6J mice | 0.2 mL (106 cfu/day CCFM683, 107 cfu/day CCFM683, 108 cfu/day CCFM683, 109 cfu/day CCFM683, 1010 cfu/day CCFM683) |
Once daily | 2 weeks | weight loss, stool consistency and fecal blood | Effective (Improved intestinal epithelial barriers, protecting the intestinal mucus layer, restoring gut microbiota, and downregulating the inflammatory cytokines) |
| Chen et al. 2020 | Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis with (quadruple probiotics, P-qua) or without (triple probiotics, P-tri) aerobic Bacillus cereus | C57BL/6 mice | B. infantis, L. acidophilus, and E. faecalis (1.5 × 109 CFU respectively) in 200ul PBS and B. cereus (0.5 × 108 CFU) in 200ul | Once-daily | 3 cycles (45 days) | Intestinal inflammation and functions of multiple barriers, including the mucus barrier, epithelial barrier, and endothelial barrier known as a gut-vascular barrier (GVB) | Effective (Aerobe-contained P-qua was a powerful adjuvant therapy for chronic colitis, via restoring the intestinal microflora and recovering the multi-barriers in the inflamed gut) |
| Kim et al. 2020 | Lactobacillus plantarum CBT LP3 (KCTC 10782BP) | male C57BL/6 mice | 1 × 108 bacteria in 0.1 mL PBS | Once-daily | 16 days | Disease activity index (DAI), analysis of macrophages and T cell subsets gene expression and cytokine profiles | Effective (showed anti-inflammatory effects, with increased induction of regulatory T cells and type 2 helper T cells in splenocytes and restoration of goblet cells accompanied by suppression of proinflammatory cytokine expressions |
| Komaki et al. 2020 | Lactococcus lactis subsp. lactic JCM5805 | Mice |
1 mg, 5 mg, 10 mg, 15 mg, or 20 mg | Once daily | 1 week | The survival rate, length, histopathological parameters of the colon, and concentrations of inflammatory cytokines in serum | Effective (High-dose administration of L. lactis deteriorates intestinal inflammation) |
| Liu et al. 2020 | Clostridium butyricum | male C57BL/6 mice | 2 × 108 CFU bacteria in 200ul physiological saline | 3 times per week | 30 days | Detect severity of colitis, tumorigenesis, and cytokines including TNF-a, IL-6, and Cyclo-oxygenase-2 (COX-2) | Effective (Regulate structure and composition of gut microbiota) |
| Sanders et al. 2020 | Weissella paramesenteroides WpK4 | C57BL/6J female mice | Approximately 108 CFU | Once-daily | 10 days | Acute colitis assay, intestinal permeability, histological analysis, assessment of social, anxiety, depressive-like behaviors, cytokines, and inducible nitric oxide synthase | Effective (Promoted the epithelial barrier, reducing gut leakage) |
| Silveira et al. 2020 | Lactobacillus bulgaricus | C57BL/6 mice | 1 × 109 CFU were diluted in 200 μL of PBS | 3times per week | 12 weeks | Intestinal inflammation, cytokines levels were determined from colon and/or tumor | Effective (Regulates the inflammatory response and preventing Colitis-associated cancer, CAC) |
| Xia et al. 2020 | Lactobacillus plantarum AR113 | male C57BL/6J | 1 × 109 CFU | Once-daily | 2 weeks | Disease activity index (DAI) scores, colon morphology, colonic myeloperoxidase (MPO) activity, microbiota, and gene expression analysis | Effective (mitigate dysbiosis of gut microbiota) |
| Rodríguez-Nogales et al. 2018 | Saccharomyces boulardii | Male C57BL/6 J mice | 5 × 109 CFU in 200 μL PBS | Once daily | 26 days | Disease Activity Index (DAI), expression of inflammatory markers, micro-RNAs, and microbiota populations | Effective (showed intestinal anti-inflammatory effects) |
| Souza et al. 2016 | Escherichia coli strain Nissle 1917 (EcN) | female BALB/c mice |
0.1 mL (9.0 log10 c.f.u. EcN ml − 1) | Once-daily | 10 days | Animal body weight, feces consistency, presence of blood in feces, histology, measurement of myeloperoxidase (MP0), eosinophil peroxidase (EPO), and cytokine levels (KC/CXCL-1, eotaxin/CCL11, and IL-1β) in the intestinal tissue | Effective (reduced inflammation) |
| Thakur et al. 2016 | Lactobacillus casei Lbs2 | Balb/c mice | 1 × 109 CFU | Once-daily | 2 weeks | Macroscopic and microscopic inflammation scoring, Myeloperoxidase (MPO) activity, and In-vitro T-cells suppression assay | Effective (suppressed lipopolysaccharide-induced pro-inflammatory cytokine (TNF-alpha, IL-6) secretion) |
| Lim et al. 2016 | Bifidobacterium longum CH57 and Lactobacillus brevis CH23 | Male C57BL/6 | 2 × 109 CFU/0.2 mL | Once-daily | 3 days | Histological examination, an assay of myeloperoxidase activity and determine Th17 and Treg cells in the lamina propria of colons | Effective (probiotic mixture inhibiting macrophage activation and restoring Th17/Treg balance) |
| Oliva et al. 2012 | L. reuteri ATCC 55,730 | Balb/c mice | Enema solution, 1010 CFU | – | 8 weeks | Clinical endoscopic and histological scores as well as rectal mucosal expression levels of IL-10, IL-1b, TNFa and IL-8 |
Effective (improving mucosal inflammation and changing mucosal expression levels of some cytokines) |
Table 2.
Summary of randomized clinical intervention of probiotics in patients with IBD.
| References | Treatment & Composition | Dose | Intake | Duration | Parameters analyzed | Conclusion |
|---|---|---|---|---|---|---|
| Altun et al. 2019 | Enterococcus faecium, Lactobacillus Plantarum, Streptococcus thermophilus, Bifidobacterium lactis, Lactobacillus acidophilus, Bifidobacterium long, and fructooligosaccharide | six probiotic strains (3x109 CFU) and fructooligosaccharide (225 mg/ tablet) | 1 tablet for twice a day |
8 weeks | Hemoglobin, leukocyte, neutrophil-to-lymphocyte ratio, sedimentation, and C-reactive protein (CRP) values, clinical and endoscopic activity indices | Effective |
| van der Waal et al. 2019 | Nine bacterial strains (Bifidobacterium bifidum W23; Bifidobacterium lactis W51; Bifidobacterium lactis W52; Lactobacillus acidophilus W22; Lactobacillus casei W56; Lactobacillus paracasei W20; Lactobacillus plantarum W62; Lactobacillus salivarius W24; Lactobacillus lactis W19) | 7.5 × 10^9 CFU per 3 g in powder form | Once-daily | 6 weeks | Quality of life from a patient perspective (semi-structured interviews) | Effective |
| Matsuoka et al. 2018 | Bifidobacterium breve strain Yakult and Lactobacillus acidophilus | 10 billion bacteria of Bifidobacterium breve and 1 billion bacteria of Lactobacillus acidophilus | One pack per day | 48 weeks | Clinical procedure (change in abdominal symptom score from baseline, Sutherland DAI subscore, change in abdominal symptom scores (passage of flatus and bloating), and intestinal microbiota | Less significant effect |
| Sun et al. 2018 | Clostridium butyricum | 420 mg per capsule, 1.5 × 107 CFU /g | 3 capsules 3 times a day | 4 weeks | Change from baseline in IBD symptoms, quality of life, stool consistency, and frequency | Effective |
| Pilarczyk-Żurek et al. 2017 | Lactobacillus plantarum PL02, Lactobacillus rhamnosus KL 53A, and Bifidobacterium longum PL03 | one sachet, mixture of three viable strains, with a total of ≥ 1 × 108 CFU | once a day | 2 months | colonoscopy, including colon biopsies for histopathology and microbiology, collection of a stool sample for microbiology as at the initial visit | Effective |
| Palumbo et al. 2016 | Lactobacillus salivarius, Lactobacillus acidophilus, Bifidobacterium bifidum strain BGN4 | Not mentioned | Two | 24 months | stool frequency, endoscopy, rectal bleeding | Effective |
| Fedorak et al. 2015 | VSL#3 (900 billion viable bacteria, comprising 4 strains of Lactobacillus, 3 strains of Bifidobacterium, and 1 strain of Streptococcus salivarius subspecies thermophilus) | One sachet (3 g) | Twice daily | One year | Crohn’s Disease Activity Index (CDAI), inflammatory bowel disease questionnaire, and mucosal cytokine measurements | No significant effect (no statistical differences) |
| Shadnoush et al. 2015 | Yogurt (Lactobacillus acidophilus La-5 and Bifidobacterium BB-12) | 106 CFU g/yogurt | 250 g yogurt per day | 8 weeks | Stool specimens measured by Taqman real-time PCR method. | Improve intestinal function by increasing probiotic bacteria |
| Tamaki et al. 2016 | Bifidobacterium longum 536 | One sachet, 2–3 × 1011 freeze‐dried viable BB536 | 1 for three times/day | 8 weeks | Primary endpoints & secondary endpoints | Effective |
| Yasuda et al. 2016 | Clostridium butyricum MIYAIRI | 20 mg of CBM, | 3 for three times/day | 24 months | Endoscopic examination, blood tests, fecal microbiota, | Effective with minimal side effects |
| Yoshimatsu et al. 2015 | Bio-Three [Streptococcus faecalis + Clostridium faecalis and Bacillus mesentricus] | 2 mg of lactogen (Streptococcus faecalis T-110), 10 mg of Clostridium butyricum TO-A, and 10 mg of Bacillus mesenteric TO-A | 3 tablets 3 time/daily | 12 months | Analysis of intestinal microflora, HPLC analysis of fecal organic acids | Effective |
| Petersen et al. 2014 | EcN, Escherichia coli Nissle 1917 | 100 mg EcN | 1 tablet for 4 days then 2 d | 7 weeks | clinical activity index (CAI)-score, blood, and stool samples for future analysis | Less significant effect |
| Bourreille et al. 2013 | Saccharomyces boulardii (yeast) | 1 g/day | Once-daily | 52 weeks | CDAI scores, full blood counts, C-reactive protein (CRP), and erythrocyte sedimentation rates (ESRs) | No effect |
| Yoon et al. 2014 | LacClean Gold-S® (Cell Biotech, Co. Ltd., Gimpo, Korea) | 500 mg/capsule (5 × 109 viable cells) |
One capsule daily | 4 weeks | The intensity of abdominal pain/discomfort, bloating, stool frequency/consistency, alterations in fecal microflora | Significant (increased significantly in the probiotics groups) |
|
Wildt et al. 2011 |
Probio-Tec AB-25, Lactobacillus acidophilus La-5, and B. animalis subsp. lactis BB-12 | One capsule, 2.5 × 1010 CFU | 3 times/ day | 52 weeks | Quantification of LA-5 and BB-12 in feces by qPCR | Less significant effect |
| Ng et al. 2010 | VSL#3 | One sachet contains 900 billion viable lyophilized bacteria | 2 sachets twice per day | 8 weeks | Rectal biopsies, Myeloid colonic DC, surface expression of activation markers | Effective |
| Tursi et al. 2010 | VSL#3 | One sachet contains 900 billion viable lyophilized bacteria | 2 sachets twice per day | 8 weeks | Endoscopic examination, bowel frequency, rectal bleeding, physician's rating of severity | Effective |
| Matthes et al. 2010 | Escherichia coli Nissle 1917 (EcN) | 40-, 20-, or 10-ml enemas containing 10^8 EcN/ml | Once-daily | 8 weeks | Clinical DAI (stool frequency, rectal bleeding, assessment of disease activity by physician) | Effective |
|
D’Incà et al. 2011 |
Lactobacillus casei DG | 8 × 108 CFU | Twice daily | 8 weeks | Biopsies from sigmoid region to culture mucosal-associated microbes and to assess cytokine and TLR messenger RNA (mRNA) levels by quantitative real-time polymerase chain reaction (RT-PCR) | Effective |
The effect of probiotic administration is different in both animal models and clinical studies, as the probiotics are used at different types, doses, frequencies, and duration of administration. Generally, there are either a single stain or a consortium of probiotic strains was utilized for the probiotic therapy in both animal and human trials. The commercial probiotic preparations of VSL#3 are one of the common probiotics analyzed in human trials and it is composed of four different strains of Lactobacillus spp., three strains of Bifidobacterium spp., and a sole strain of Streptococcus spp. (Fedorak et al., 2015, Ng et al., 2010, Tursi et al., 2010). However, several recent studies also demonstrated the effectiveness of a single strain. Chen et al. (2020) had reported the effectiveness of Bifidobacterium breve CCFM683 therapy in restoring gut microbial population in C57BL/6J mice model.
One of the common questions that will arise in probiotic therapy will be the effective doses. Many commercially available probiotic supplements contain one to 10 billion CFU per dose (Fedorak et al., 2015, Yoshimatsu et al., 2015). This number of doses is generally recommended in probiotic therapy as the live cells must pass through the adverse conditions of the alimentary canal and sufficient in numbers to provide their probiotic effects. Thus, a range of 106 to 1011cfu of probiotics was commonly used in all animal studies and human trials (Kim et al., 2020). In both animal and human studies, probiotics are consumed at least once a day to a maximum of three times per day. However, the total duration of the probiotic therapy is varying among the studies. The minimal duration would be seven days (Komaki et al. 2020). Fedorak et al. (2015) suggested that probiotics should be consumed at least for one year continuously to observe their effectiveness (Fedorak et al., 2015).
Although probiotics are known as GRASS and the efficacy of the different dosages and types had been proven in human volunteers, the animal model studies are crucial in the investigation to understand the chronological genesis of IBD (Chen et al., 2020, Komaki et al., 2020). Even though not all mechanisms that would occur in humans are presented, animal models are still the most effective way to understand the pathogenesis of IBD, compared to others (Lim et al., 2016). The common probiotic strains or preparations were used for both animal studies and clinical trials (Table 1 and Table 2) belonged to genera of Lactobacillus (Komaki et al., 2020, Silveira et al., 2020, Xia et al., 2020) and Bifidobacterium (Chen et al., 2020, Lim et al., 2016). Besides these genera, other probiotics that were evaluated for their therapeutic potentials are Escherichia coli Nissle 1917 (Matthes et al., 2010, Souza et al., 2016), Clostridium butyricum (Liu et al., 2020a, Liu et al., 2020b, Sun et al., 2018, Yasueda et al., 2016), and Weissella paramesenteroides WpK4 (Sandes et al., 2020).
Therapeutic potential of Saccharomyces boulardii was studied in animal model and human clinical trial (Bourreille et al., 2013, Rodríguez-Nogales et al., 2018). This strain was originally isolated from a cholera epidemic. Later, it had been characterized as non-pathogenic and only yeast that has been verified and recognized with probiotic properties (Rodríguez-Nogales et al., 2018). The efficacy of this human probiotic yeast has been demonstrated widely in gut-associated disorders, such as diarrhea associated with antibiotics, traveler’s diarrhea, or in CDI incidences (Bourreille et al., 2013, Rodríguez-Nogales et al., 2018). In a dysbiotic animal model study, S. boulardii was found to upregulate two endothelial genes during angiogenesis, in germ-free mice models. Angiogenesis causes chronic inflammations in the gut that leads to IBD. The study demonstrated that probiotic therapy using a preparation of probiotic yeast can prevent angiogenesis that reduces inflammations (Sokol et al., 2017). However, in another study, probiotic therapy using probiotic yeast S. boulardii was reported as not effective in clinical trials. To date, there are only four clinical trials that have reported using S. boulardii as probiotic therapy for IBD were, three works reported the efficacy (Bourreille et al., 2013, Rodríguez-Nogales et al., 2018). The probiotic therapy using probiotic yeasts such as S. boulardii could be a plausible treatment for clinal studies, yet it requires several placebo studies to validate the efficacy reported in the animal models.
Several studies reported the usage of multi-strain probiotic preparations and commercial probiotic preparations (Altun et al., 2019, Chen et al., 2020, Mardini and Grigorian, 2014). Among them, VSL#3 is well known for its effectiveness and there are numerous supportive data for use in IBD (dos Santos Cruz et al., 2020, Fedorak et al., 2015, Mardini and Grigorian, 2014, Tursi et al., 2010). This commercial probiotic mixture comprises three genera of bacteria with a total of 8 different strains that exert different probiotic effects. The VSL#3 contains L. acidophilus, L. Plantarum, L. casei, L. delbrueckii subspecies bulgaricus, B. breve, B. longum, B. infantis, and S. salivarius (Fedorak et al., 2015, Mardini and Grigorian, 2014). This commercial probiotic preparation has been widely used for UC, and other IBD symptoms to restore the microflora, attenuate inflammation and reduce microbial translocation. The probiotic strains in VSL#3 participate in various health regulation mechanisms which have been appraised in-depth, by recently published review works (dos Santos Cruz et al., 2020, Mardini and Grigorian, 2014). Tursi et al. (2010) reported a significant cure of UC with long-term consumption of this commercial VSL#3 probiotic preparations, compared to placebo (Tursi et al., 2010). In addition, Mardini and Grigorian (2014) reported a meta-analysis that stated consumption of VSL#3 in combination with standard therapy is significantly more curative compared to standard therapy alone in UC associated patients (Mardini and Grigorian, 2014). However, one of the probiotic formulations was administrated to prevent the recurrence of CD after surgery. Fedorak et al. (2015) published the first assessment on the prevention of recurrence of endoscopic CD by using VSL#3. These findings showed long-term consumption of VSL#3 is highly linked to promising and effective outcomes to reduce IBD (Fedorak et al., 2015).
5.2. Prebiotic therapy in animal models and human studies
Prebiotic therapy was proven to alter and restore the gastrointestinal microbiome by flourishing the growth of commensal microbiota. These prebiotics were also found to enhance resistance towards disease-inducing microorganisms (Liu et al., 2016, Morse et al., 2010, Naseer et al., 2020; Sakena et al., 2020). Surprisingly, more studies were performed in animal models compared to human trials, in the reduction of IBD (Table 3 and Table 4). A low number of significant prebiotic-associated human clinical trials was also reported in other recent reviews and meta-analyses (Naseer et al., 2020). A limited number of studies is a major drawback and not sufficiently conclusive to support the utilization of prebiotics to treat IBD. Most of the prebiotics used for the animal study were polysaccharides extracted from vegetables such as kale (de Albuquerque et al., 2010; yam (Li et al., 2020a, Li et al., 2020b), from fruits such as grapes (Li et al., 2020a, Li et al., 2020b), from mushrooms such as Ganoderma lucidum (Xie et al., 2019), and herbs (Gou et al., 2019). In contrast, most of the human clinical trials reported usage of fructo-oligosaccharides for prebiotic therapy of IBD (Benjamin et al., 2011, De Preter et al., 2007, Kim et al., 2020, Morse et al., 2010). Available data had demonstrated that FOS-enriched inulin supplementation for patients with CD, had shown elevated levels of acetaldehyde and butyrate productions (De Preter et al., 2007, Morse et al., 2010). The fructooligosaccharides (FOS) are the short-sugar polymers that contain fructose residues (Naseer et al., 2020). Most of the gut microflora is characterized by an ability to break down the FOS compounds. Therefore, FOS are mainly required to increase the population of endogenous microflora, especially Lactobacillus and Bifidobacterium (Azpiroz et al., 2017). Fermentation of FOS in the gastrointestinal environment enhanced SCFA productions, as well as organic acids and butyrate which are required to enhance immunomodulation in the gut (Takahashi et al., 2016, Zha et al., 2020). These could be the reason behind the extensive applications of FOS as prebiotic therapy for IBD. Prebiotics generally have no life-threatening or severe side effects. The human genome does not encode the digestive enzymes for prebiotics. Naturally, the human gastrointestinal environment is rich with prebiotic fermenting microflora. Only the prebiotic chain length could be an influential parameter to develop any side effects or failure in fermentation (Naseer et al., 2020). FOS supplemented to IBD patients in a range of 2.5 to 15 g per day, either one- or two-time consumption (Benjamin et al., 2011, Morse et al., 2010). Azpiroz et al. (2017) reported that increasing doses of FOS supplementation was associated with an increase in fecal Bifidobacterium (Azpiroz et al., 2017). Additionally, in vitro and animal studies also revealed the effect of FOS on the enrichment of other bacterial abundance such as the major butyrate-producing species in the human gut (Rivera-Chávez et al., 2016, Rivière et al., 2016).
Table 3.
Summary of animal models’ studies of using prebiotics.
| Reference | Type of treatment | Composition | Model | Dose | Intake | Duration | Parameters analyzed | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Cui et al. 2021 | Polysaccharide from Scutellaria baicalensis Georgi, SP2-1 | Mannose, ribose, rhamnose, glucuronic acid, glucose, xylose, arabinose, fucose Colitis inducer: 3% dextran sodium sulfate (DSS) |
C57BL/6J mice | 50 and 200 mg/kg | Once-daily | 10 days | r body weight, loose stools, morbidity, and hematochezia. The disease activity index (DA Evaluated body weight, loose stools, morbidity, hematochezia, and the disease activity index |
Effective (attenuated body weight loss, reduced DAI, ameliorated colonic pathological damage, and decreased MPO activity) |
| Kanwal et al. 2020 | Dictyophora indusiate polysaccharide (DIP) | Glucose 59.84%, mannose 23.55%, and galactose 12.95% Colitis inducer: dextran sodium sulfate (DSS) |
low dose 10 mg/kg and (high dose 33 mg/kg | Once-daily | 2 weeks | Assessment of disease activity index, histological, analysis of goblet cells and mucus layer thickness, cytokines by ELISA and Immunoblotting assay | Effective | |
| Sakena et al. 2020 | Gracilaria fisheri oligosaccharides (GFO) | monosaccharide composition in the GFO was D-galactose. Colitis inducer: acetic acid (AA)-induced colitis mice |
100, 500, or 1000 mg/kg | Once-daily | 2 weeks | Gastrointestinal (GI) transit time, ex vivo propulsive motility, in vitro colonic smooth muscle contractility, the composition of colonic microbiota, and production of SCFAs | Effective (prevent and attenuate colitis symptoms and GI dysmotility & reducing populations of harmful bacteria and increasing SCFAs | |
| Li et al. 2020 | Freeze-dried muscadine grapes (FMG) or dealcoholized muscadine wine (DMW) | FMG: Fructose 34.7 %Glucose 31 %, sucrose 9.9% DMW: Fructose, sucrose, and glucose not detected Colitis inducer: 3% dextran sodium sulfate (DSS) |
C57BL/6J mice | FMG (7%, w/w) or DMW (5.5%, v/w) | NM | 3 weeks | Bodyweight, stool consistency and bleeding, Disease activity index (DAI), short-chain fatty acids in feces, and Mucin 2 and IgA in feces | Effective (Reduced dysbiosis in the colon.) |
| Li et al. 2020 | CYP-1 (Chinese yam polysaccharide) | CYP-1, mannoglucan of 1,4-α-linked Glcp branched at O-2, O-3, and O-6 position by t-α-linked Map Colitis inducer: dextran sodium sulfate (DSS) | 300 mg/kg body weight | Once-daily | 1 week | Histological, measurement of cytokines, gut microbiota analysis, and colonic transcriptomic | Effective | |
| Liu et al. 2020 | α-D-glucan from marine fungus Phoma herbarum YS4108 | Polysaccharide with backbone possessed most likely a linear α-(1 → 4) bonded glucopyranose main chain co-bearing through side α-(1 → 6) | Male C57BL/6 mice | 40 mg/kg/day | Once-daily | 1 week | Disease activity index (DAI) scores, histology immunohistochemistry analysis, evaluation of SOD and MDA activities, and determinations of inflammatory cytokines | Effective (significantly increased butyrate, isovaleric acid levels, and prominent alterations on specific microbiota) |
| Gou et al. 2019 | WPSPP-1 | Glucose and mannose in the molar ratio of 20.44:1.00 |
400 mg/kg body weight | NM | 4 weeks | Histological, gut microbiota, and biochemical analysis | Effective | |
| Xie et al. 2019 | Ganoderma lucidum polysaccharide (GLP) | β-glucan (>90%) that contained a 1,6-linked β-D-Glcp backbone | male Wistar rats | NM | NM | 3 weeks | Determination of the disease activity index, body weight, short-chain fatty acids in cecal samples, | Effective (immunity enhancement, inflammatory response alleviation & colon cancer risk reduction) |
| Zha et al. 2020 | xylan butyrate ester, Xylo |
xylose, arabinose, and glucose at a ratio of 1:5:1 | C57BL/6 mice | 50 and 200 mg/kg | NM | 2 weeks | body weights, feces, and physical activity of the mice, Compositional analysis of the gut microbiota, | Effective (reduces inflammatory intestinal damage) |
| Liu et al. 2016 | konjac oligosaccharide (KOS) | Glucose and mannose residues at a molar ratio of 1:1.6 joined through the β1,4-glucosidic link Colitis inducer: 2,4,6-trinitrobenzenesulfonic acid (TNBS) |
1 g and 4 g/kg | Once-daily | 2 weeks | Bacteria profile, short-chain fatty acids (SCFAs) production in feces, colon damage by macroscopic and histological | Effective | |
| de Albuquerque et al. 2010 | Kale and papaya |
Kale (60%) + papaya (40%) Colitis inducer: tri-nitro-benzenesulfonic acid (TNBS) |
500 mg/kg per rat weight | NM | 3 weeks | Clinical endoscopic and histological scores as well as rectal mucosal expression levels of IL-10, IL-1b, TNFa and IL-8 Evaluation of the intestinal anti-inflammatory effects and microbiological studies of intestine luminal content |
Effective |
Table 4.
Summary of randomized clinical intervention of prebiotics in patients with IBD.
| Reference | Type of treatment | Composition | Dose | Intake | Duration | Parameters analyzed | Conclusion |
|---|---|---|---|---|---|---|---|
| Azpiroz et al. 2017 | Short-chain fructooligosaccharides (scFOS) | 95% of pure scFOS & 5% of simple sugars (sucrose, fructose, and glucose) | 5 g per sachet | Twice daily in powder sachets | 4 weeks | rectal sensitivity, anxiety/depression, quality of life scores, and composition of fecal microbiota | Less significant (scFOS on rectal sensitivity may require higher doses and may depend on the subgroup) |
| Niv et al. 2016 | Partially hydrolyzed guar gum (PHGG) | galactomannan | 6 g PHGG | Once-daily in powder sachets | 18 weeks | Francis Severity score and quality-of-life scores | Less significant (Improvement of bloating score, however, no effect on other journal reported symptoms) |
| De Preter et al. 2007 | OF-IN (ORAFTISynergy-1) | 1:1 mixture of inulin and oligofructose. | 10 g OF-IN | Twice daily | 4 weeks | Fecal metabolites before and after treatment | Effective |
| Benjamin et al. 2011 | Fructo-oligosaccharides (FOS). | Synergy1, Beneo-Orafti, Belgium | 7.5 g × two sachets per day | Twice daily | 4 weeks | Crohn’s Disease Activity Index (CDAI), cytokine production by intestinal DCs, quantified fecal bifidobacteria and Faecalibacterium prausnitzii | No significant (no significant differences in the fecal concentration of bifidobacteria and F. prausnitzii |
| Morse et al. 2010 | A mixture of Inulin Plus Oligofructose | inulin plus oligofructose 1:1 mixture | 7.5 or 15 g | Once-daily | 9 weeks | stool analyzed for microflora composition | Not clear |
Recently, mushrooms and their polysaccharides are being recognized as a promising source of prebiotic fibers. The mushroom polysaccharides are widely used as prebiotic fibers with various beneficial impacts on the human gut microbiota (Liu et al., 2020a, Liu et al., 2020b, Xie et al., 2019). β-glucans are the most notable mushroom polysaccharide which regarded as medicinal prebiotics. However, mushrooms contain different types of polysaccharides which have similar prebiotic potentials as β-glucans and these include α-glucans, chitin, mannans, xylans, and Galatians (Liu et al., 2020a, Liu et al., 2020b). Xie et al. (2019) reported that the Ganoderma lucidum polysaccharide (GLP) prebiotics bring down the disease activity index significantly and increase SCFA production with an enrichment of SCFA producing bacteria, including Ruminococcus. The study also reported a reduction of pathogenic microflora such as Escherichia and Shigella spp. in the rat model (Xie et al., 2019).
5.3. Synbiotic therapy
Synbiotics refer to preparations composed of both prebiotics and probiotics (Darb Emamie et al., 2021, Rogha et al., 2014). However, DeGrutelle et al. (2016) had the term ‘symbiotic’ to refer to such preparations. Symbiotics usually referred to a type of relationship that exists between two living organisms or groups of organisms in a niche that could be harmful or beneficial to each other. However, Darb Emamie et al. (2021) re-defined such preparations with the term ‘synbiotics’- referring to the synergistic conditions where the probiotics metabolize the supplemented prebiotics to stimulate specific refabrications of dysbiotic gut and hosts health. The synergistically acting probiotics and prebiotics stimulate the growth of selective microorganisms or activate specific metabolism by gut microflora (Darb Emamie et al., 2021, DeGruttola et al., 2016). Table 5 summarizes the administration of synbiotics in the animal model studies and Table 6 summarizes clinical intervention for IBD treatment in patients by using selected synbiotics. These studies revealed a significant improvement over inflammations through modulation of the immune system. A clinical trial with a random placebo control study evaluated the efficacy of synbiotic therapy for UC patients (Altun et al., 2019). The patients consumed synbiotic preparations that consists of six probiotic strains and prebiotic FOS. The findings revealed a significant reduction of inflammations and showed an amelioration of the disease status (Altun et al., 2019, Liang et al., 2021). The study by Liang et al. (2021) demonstrated the efficiency of synbiotic preparations in reducing IBD-linked diarrhea symptoms, through a double-blind random placebo clinical trial. The synbiotic preparation was composed of prebiotic FOS with B. lactis DSMZ 32269; B. bifidum DSMZ 32403; B. longum DSMZ 32946; L. acidophilus DSMZ 32418, and L. rhamnosus FloraActiveTM 19070–2. The feelings of incomplete bowel movements, pain at the rectal, flatulence, and diarrhea were significantly ameliorated in the patients compared to placebos (Liang et al., 2021).
Table 5.
Summary of animal models’ studies of using synbiotics.
| Reference | Probiotics | Prebiotics | Model | Dose | Intake | Duration | Parameters analyzed | Conclusion |
|---|---|---|---|---|---|---|---|---|
| dos Santos Cruz et al. 2020 | VSL#3 | Yacon (6% Fructooligosaccharide + inulin) | Interleukin-10-knockout mice | VSL#3® (109 CFU/day) + PBY (6% FOS and inulin) | NM | 13 weeks | Manifestations of colitis, colon histology, expression of antioxidant enzymes, production of organic acids, and intestinal microbiota | Preservation of intestinal architecture, improve intestinal integrity, increased expression of antioxidant enzymes and concentration of organic acids |
| Seong et al. 2020 | L. paracasei | Opuntia humifusa extract (mucilage + pectin) | male Wistar rats |
L. paracasei (1 × 1010 cfu/g) & (10.0 / 30.0 mg%, w/w) of Opuntia humifusa extract IBS/IBD inducer: Chronic restraint stress |
Once daily | 4 weeks | Fecal microbial analysis, serum corticosterone levels, tumor necrosis factor-α (TNF-α) levels in the colon tissue, and expression of tight junction proteins | Effective (Greater abundance of L. paracasei in fecal microbial analysis, lower serum corticosterone levels, lower TNF-α levels in the colon tissue, and higher expression of tight junction proteins) |
| Son et al. 2019 | L. rhamnosus strain GG (LGG) | Tagatose | Female BALB/c mice | 109 cfu/mL of LGG and 25 mg of tagatose Colitis inducer: dextran sodium sulfate (DSS) |
Two days once | 3 weeks | Measure body weight, food intake, noting rectal bleeding, stool conditions, blood in stool, expression of proinflammatory cytokines in serum, analysis on gut microbiota | Effective (Gut microbiota composition recovered from the dysbiosis caused by DSS treatment) |
| Ivanovska et al. 2017 | L. Casey 01 | oligofructose-enriched inulin | Female Wistar rats | 1 mL ayran containing non-encapsulated probiotic/prebiotics Colitis inducer: 2,4,6-trinitrobenzenesulfonic acid (TNBS) |
Once-daily | 2 weeks (before induction) & 1 week (after induction) | Assessment of colonic damage, inflammation scoring, myeloperoxidase assay and microbiological studies |
Effective (Significant decline in myeloperoxidase (MPO) activity) |
| Kaur et al. 2017 | L. acidophilus, L. rhamnosus, B. longum and S. boulardi | guar gum and xanthan gum | Wistar albino rats | 23 mg/kg (Prebiotics) + 1 g/day (probiotic) | Once-daily | 17 days | Comparative evaluation of fecal contents, weight gain trend, and histopathological studies | Efficient and cost-effective targeting of the drug to the colon |
Table 6.
Summary of randomized clinical intervention of synbiotics in patients with IBD.
| Reference | Probiotics | Prebiotics | Dose | Intake | Duration | Parameters analyzed | Conclusion |
|---|---|---|---|---|---|---|---|
| Matijašić et al. 2016 | Fermented milk (L. acidophilus La-5, B. animalis ssp. lactis BB-12, St. thermophiles) | dietary fiber (90% inulin, 10% oligofructose) | L. acidophilus La-5 (1.8 × 107 cfu/g) & B. animalis ssp. lactis BB-12 (2.5 × 107 cfu/g); 2% dietary fiber (packed in 180-g) | Twice daily | 2 weeks | Stool samples subjected to real-time PCR and 16S rDNA profiling by next-generation sequencing | Less effective (short-term effect on the amount and proportion of La-5-like strains and B. animalis ssp. lactis in the fecal microbiome) |
| Rocha et al. 2014 | Synbiotic Lactol® Bacillus Coagulans |
Fructo-oligosaccharides | Bacillus Coagulans (15 × 107 Spores) & Fructo-oligosaccharides (100 mg) | Once-daily | 12 weeks | Abdominal pain (scored 1 to 7), diarrhea, and constipation (scored 1 to 5) | Effective (Relieving abdominal pain/discomfort, diarrhea, and beneficial effects remained for at least nine months) |
| Shavakhi et al. 2014 | Balance®(L. casei, L. rhamnosus, L. acidophilus, L/ bulgaricus, B. breve, B. longum and St. thermophiles. | Fructo-oligosaccharides | 1 × 108 CFU/per capsule | Twice daily | 2 weeks | Abdominal pain, distension, symptoms, and quality-of-life | No significant effect (No beneficial effects over placebo) |
| Cappello et al. 2013 | L. plantarum, L. casei subp. Rhamnosus, L. gasseri, B. infantis, B. longum, L. acidophilus, L. salivarus, L. sporogenes and St. termophilus | inulin and 1.3 g of tapioca-resistant starch | 5-g sachets | Twice daily | 4 weeks | Assess abdominal bloating, flatulence, pain, urgency by a 100-mm visual analog scale, stool frequency, and bowel functions quality of life (SF-36) | Less effective (decreasing the severity of flatulence in IBD patients, failed improvement in global satisfactory relief of abdominal flatulence) |
|
Ishikawa et al. 2011 |
BbY, Live B. breve strain Yakult | GOS, galactooligosaccharides | Freeze-dried powder of 1 g (109 CFU/g) & GOS 5.5 g (55% as a gel) | three times a day | one year | Colonoscopy index, amount of myeloperoxidase in a lavage solution, Analysis of Fecal Microorganisms | Effective |
| Steed et al. 2010 | Bifidobacterium longum | Synergy 1 | 2 × 1011 freeze-dried viable B. longum in a gelatin capsule, and a sachet | Twice daily | 6 months | Transcription levels of pro-inflammatory cytokines in mucosal tissue, microbiological analysis of tissue biopsies, and histopathology |
Effective (improvement in their histological scores, and reductions in serum levels of pro-inflammatory TNF-α) |
6. Other microbial based therapies for IBD
6.1. Faecal microbiota transplantation (FMT) in IBD
FMT is an emerging milestone in microbial therapy for patients with metabolic disorders associated with gut dysbiosis. FMT is a technique of transferring healthy fecal microbial preparation to patients with metabolic disorders (Shinde et al., 2019). The technical approaches of FMT include oral capsules; enemas, nasogastric or nasojejunal tubes which are used to restore the healthy gastrointestinal microbiome (Colman et al., 2014; Nicco et al., 2020). The oldest intervention of FMT will be dated back to the 4th century when ‘yellow soup’ - prepared from human stool was consumed to treat severe diarrhea (Zhang et al., 2012). There were historical descriptions of fresh and fermented fecal preparations prescribed for patients experiencing constipation, abdominal pain, diarrhea, dating back until the 6th century of the Ming Dynasty (Wang et al., 2018). Then, FMT was widely used in veterinary fields since the 17th century, to cure gastrointestinal diseases among equines and ruminants (Shinde et al., 2019). In the recent years before 2013, the application of FMT was generally limited to patients with CDI incidence (Zhang et al., 2012). At present, several studies demonstrate successful and efficient therapy using FMT (Colman et al., 2014; Nicco et al., 2020).
Samples of FMT are selected from healthy donors who must undergo a standard screening protocol to avoid the risks of transmitting unknown pathogens from donor to recipient. The donors will be investigated on their historical background which encloses their health state including blood transfusion details, family history on autoimmune response, metabolic disorders, and previous operations. The collected details also include the donor’s travel details, sexual behaviors, and food consumption, especially on alcohol and drugs. Upon selection of a suitable donor, their blood and fecal samples will be screened for the presence of pathogens (Colman et al., 2014; Shinde et al., 2019). Patients undergoing FMT also receive strong support and education before the treatment. The recipient will be ensured not to consume any types of antibiotics in 48 h before the infusion, the gut should be free of fecal materials and some patients consumed loperamide an hour before FMT, to ensure the transplanted feces stay in the gut for at least 4 h long (Evrensel et al., 2016; Hu et al., 2018).
There are several methods of transferring fecal material to the recipients (Selvamani et al., 2021). Currently, the fecal material is given through the upper gastrointestinal route, lower gastrointestinal route, or oral capsules. FMT by an upper gastrointestinal route which would be through esophagogastroduodenoscopy, nasogastric, nasojejunal, or nasoduodenal tube, is performed for patients suffering from an inflamed colon (Wang et al., 2019). The lower gastrointestinal route FMT will be performed through colonoscopy or retention enema (Caldeira et al., 2020). The colonoscopy method is more successful in recolonization of the entire colonic area with favorable microbiota, compared with retention enema which is limited to the distal colon only. Compared to colonoscopy, the retention enema is much more affordable and less invasive than colonoscopy (Selvamani et al., 2021). In most of the reported therapies, patients receive FMT relatively a mean of 25 g of inoculum via the upper gastrointestinal route than a mean of 90 g by colonoscopy (Cammarota et al., 2014; Orenstein et al., 2016). In a randomized clinical study by Cammarota et al. (2014) 90% successful rate of preventing recurrent infection through FMT by colonoscopy was reported using 152 g of fecal samples. In the retention enema method, Lee et al. (2016) reported that consumption of 17 g of frozen-and-thawed or fresh FMT showed efficacy at a 60% rate. FMT therapy via administration of capsules had shown comparative and more effective outcomes than FMT through colonoscopy, in preventing the recurrence of CDI (Lee et al., 2016). The fecal capsules can overcome the microbial loss in the gastrointestinal environment and restore the integrity of the intestinal ecosystem effectively (Orenstein et al., 2016).
Recently, work done by Calderia, and colleagues (2020) reported meta-analysis studies evaluating the effectiveness of FMT in patients diagnosed with IBD, up to April 2020. The study reported that FMT for IBD patients had shown a rate of response at 53.8% with a complete remission rate at 37%. A higher clinical remission rate was recorded for frozen-fecal material compared to fresh FMTs (Calderia et al., 2020). Lee et al. (2016) had compared the effectiveness of FMT therapy using frozen and fresh material to patients suffering from CDI after IBD. The frozen FMT approach also added value of wide applicability in health care settings compared to fresh type (lee et al., 2016). The frozen capsules only contained centrifuged bacterial pellets compared to fresh samples which is a whole fecal suspension. The use of frozen FMT was found to reduce the frequency of pre-screening of fecal samples. This was potentially reduced the FMT preparation costs. In addition, FMT performed through colonoscopy often causes discomfort, severe pain, and increased risks of gastrointestinal bleeding in patients. The oral FMT procedure with frozen samples showed comparable efficacy and safety compared to colonoscopy procedures (Orentein et al., 2016; Selvamani et al., 2021).
There are several systematic reviews published that evaluated the safety criteria and efficacy of FMT as a possible therapy for IBD. The first review was published in 2012, but the information collected was less as the FMT was only used for selected diseases, not for IBD (Cammarota et al., 2014). A recent meta-analysis published in 2020, suggested FMT for treating patients with active UC as a more practical therapy with beneficial and safe outcomes (Caldeira et al., 2020). The pooled results showed that FMT therapy could improve the clinical and endoscopic rates in active UC, compared to controls. The study also suggested further research must focus on understanding the rate of efficacy for fresh and frozen FMT preparations (Caldeira et al., 2020). Despite other FMT trials, one study reported colonoscopy application using fresh material had a higher remission rate compared to the enema method (Anderson et al., 2012). The study demonstrated an effective outcome in the lower gastrointestinal route compared to the upper route. The presence of live bacteria in FMTs leads to more subsequent colonization at lower gastrointestinal regions. The study also found that an adequate FMT dose is required for a successful infusions rate of FMT.
FMT was found to significantly alter the microflora compositions in the UC patients compared to control patients (not administered with FMT) and to healthy donors. Tian et al. (2019) evaluated the Bacteroidetes proportions in patients with UC, which showed a gradual upward trend after treatment. Several genera such as Bacteroides, Proteus, and Prevotella were significantly enriched compared to the original healthy donors. Meanwhile, the population of pathogenic Klebsiella genus and Streptococcus genus before treatment were found to decrease significantly after FMT (Tian et al., 2019). Reduction in the abundance of Prevotella with enriching the proportion of Klebsiella and Streptococcus were important factors to the onset of UC (Pilarczyk-Żurek et al., 2017). Another study also evaluated the remission of active UC linked to several microflorae such as Clostridium cluster IV and XVIII; Barnesiella spp.; Blautia spp.; Parabacteroides spp.; Dorea spp., and Ruminococcus spp. The study, which used colonoscopy infusion reported that microflora such as Fusobacterium spp. and Sutterella spp. were linked to no remissions (Sood et al., 2019). Unfortunately, some studies reported conflicting results where FMT therapy failed to ameliorate disorder and restore gut dysbiosis (Khoruts et al., 2016; Sood et al., 2019). The disease was found to flare following the FMT, compared to placebo. Rossen et al. (2015) reported no significant differences among UC patients for both clinical and endoscopic remissions. Khoruts et al. (2016) reported that more than a quarter of analyzed IBD patients had disease flare following the FMT. In this context, several studies found that the effectiveness of FMT to treat IBD is not predictable (Borody et al., 2013; Cammarota et al., 2014). Therefore, it is still unknown whether FMT fits the treatment paradigm. In addition, all reported systematic and meta-analyses have highlighted a low number of thorough investigations of FMT (Borody et al., 2013; Sood et al., 2019). Despite reported significant and positive taxonomic alteration in the gut of patients diagnosed with FMT, the observations are still inconsistent and their function, as well as the metabolic consequences, are not well documented (Sood et al., 2019). FMT could be a promising therapy for UC but CD and other IBDs including chronic constipation, pouchitis, and irritable bowel syndrome, the data collected were too limited to predict better conclusions (Borody et al., 2013; Paramsothy et al., 2017).
6.2. Postbiotics in IBD
The term ‘biotic’ originates from a Greek word -‘biōtikós’, – which is defined as ‘about life’ referring to a biological system composed of living organisms with an interaction with the external environment. This term is currently being used in the ‘biotics’ family terms after pro-, pre-and syn-biotics (Aguilar-Toalá et al., 2018, Żółkiewicz et al., 2020). The types and functional characteristics of bioactive compounds secreted during microbial fermentation are enclosed under this ‘postbiotic’ criteria (Aguilar-Toalá et al., 2018, Amoroso et al., 2020). Recently several investigators proposed different descriptions to define these new interventions under the ‘biotics’ family. According to Nataraj et al. (2020), ‘postbiotics’ refer to preparations made of metabolic products or non-viable products synthesized by commensal microorganisms. The study refers to microorganisms that originate from the host and are known to produce specific biological products in the host’s body (Nataraj et al., 2020). Although various definitions have been proposed and used widely, hitherto none of the international regulatory bodies or scientific or research associations have recognized a specific definition (Aguilar-Toalá et al., 2018, Żółkiewicz et al., 2020).
The postbiotics comprise SCFAs, enzymes, peptides, vitamins, peptidoglycans, endo-polysaccharides, exopolysaccharides, plasmalogens, organic acids that are produced from microbial fermentation (Nataraj et al., 2020). The viability of microbial cells is no longer paramount in the criteria for postbiotics. The efficiency of postbiotic therapy is based on the metabolites or complex molecules synthesized during microbial fermentation (Aguilar-Toalá et al., 2018). Two recent reviews have highlighted several pharmacodynamic features that must be standardized in postbiotics therapy (Nataraj et al., 2020, Żółkiewicz et al., 2020). Based on these reviews, the postbiotic preparations must exert: (1) zero risk on translocation of microflora from the gastrointestinal environment to blood as this will be of risk to vulnerable and immunocompromised subjects; (2) zero risk on acquisition and transfer of antibiotic resistance; (3) standardized and natural extract with good transport and stored; (4) the beneficial impact of postbiotics should not change during the process of extraction and/or separation from microbial cells, and (5) an enhanced interaction between released molecules from disrupted cells with epithelial cells (Nataraj et al., 2020, Żółkiewicz et al., 2020).
Rising concern on a healthy lifestyle has drawn more innovative applications in functional food productions. Several studies reported the effectiveness of probiotics, such as fermented milk products, in the reduction of numerous infectious diseases (Matijašić et al., 2016; Shadnoush et al., 2015). Probiotic L. paracasei fermented milk was reported to reduce infections in respiratory and gastrointestinal tracts of young children (Berni Canani et al., 2017). The bacterium was heat-killed, yet it has shown positive alteration in the human gut, similar to performed by living cells. Viable cells in fermented products will be inactivated at the time of consumption. However, the postbiotics mechanisms are still not described clearly, even so, a scientific hypothesis has been made as microbial fermented foods still can perform and may influence similar beneficial functions as the live cells (Aguilar-Toalá et al., 2018, Nataraj et al., 2020, Żółkiewicz et al., 2020). Fermented products may influence microflora compositions and host health (Amoroso et al., 2020). Notwithstanding that several studies have confirmed the potential of postbiotics, it is still poorly evaluated as a novel therapeutic approach in CD or UC. However, several animal models and cell-line-based studies were used to develop postbiotic therapy for IBD (Nataraj et al., 2020). According to Żółkiewicz et al. (Żółkiewicz et al., 2020), postbiotics could be an attractive and modern therapeutic strategy as this method does not contain living microbial cells. All the risks associated with the probiotic and synbiotic therapies would be minimized. The study also suggested that postbiotic therapy could maintain similar efficacy as previous alternative therapies with no serious side effects (Żółkiewicz et al., 2020). In addition, postbiotic therapies also allow researchers to study methods of concentrating or mixing different bioactive components to speed up the recovery process or alteration based on the patient’s requirements. This serves as an attractive strategy for managing IBD among different groups of patients such as age, gender, or even different regions.
7. Conclusions and future perspectives
Human gut microflora plays a crucial role in triggering, progressing, maintaining, and exacerbating IBD. Specific bacterial strains are overrepresented, and some important commensals are suppressed during IBD. A decrease in relative abundance and microbial diversity has been found to dysregulate the host’s immune responses. Several studies have evaluated the factors involved in the alterations of gut microbiota that lead to a dysbiotic state. Gut dysbiosis is highlighted as a key stakeholder associated with IBD. Regrettably, the investigations conducted so far are still unclear regarding the specific core microbiome that is established in a healthy state as well as during IBD pathogenesis. The probiotic, prebiotic, and synbiotic therapies could represent a valid armamentarium to retore the gut dysbiosis, modulate gut microbiota, and eventually might lead to a potential cure for IBD. The probiotic mechanisms of action on IBD are diverse and complex to understand to date. Briefly, probiotic-based therapies can reduce and repair intestinal permeability towards pathogenic microorganisms. The probiotic therapies also induce the intestinal immune system by promoting Th I cell differentiation, gut barrier function, and synthesis of essential proteins. However, several reported significant clinical outcomes are still at low numbers and insufficient to make an effective prediction of future treatments. Therefore, a well-defined and intensive multidisciplinary clinical study must be developed to understand the impact of probiotic, prebiotic, and synbiotic therapy on IBD. Recent publications had opened new opportunities to investigate other interventions such as microbial-derived products (postbiotics) as novel therapies for inflammatory diseases.
Author contributions
SS, VM, KP, TV, HA, ST, and IZ were involved in drafting the manuscript and data collections from different literature sources. HE and BA were involved in the review design, data collection, and interpretation, manuscript drafting. All authors reviewed and approved the final version of the review.
Funding
No funding was associated with this review.
Data availability statement
No data was generated in this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
SS would like to extend this thanks to the School of Postgraduate Studies (SPS), Universiti Teknologi Malaysia (UTM) for the financial support of Zamalah’s Ph.D. Scholarship session 2017/2018. SS also thanks Dr. Daniel JD (Ph.D.) for the technical advice on the construction of data tables. We thank Brittany Lebert for her assistance in the manuscript editing.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Hesham Ali El Enshasy, Email: henshasy@ibd.utm.my.
Theodoros Varzakas, Email: t.varzakas@uop.gr.
Bassam Abomoelak, Email: Bassam.Abomoelak@orlandohealth.com.
References
- Aguilar-Toalá J.E., Garcia-Varela R., Garcia H.S., Mata-Haro V., González-Córdova A.F., Vallejo-Cordoba B., Hernández-Mendoza A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Tech. 2018;75:105–114. [Google Scholar]
- Alatab S., Sepanlou S.G., Ikuta K., Vahedi H., Bisignano C., Safiri S., Sadeghi A., Nixon M.R., Abdoli A., Abolhassani H., Alipour V., Almadi M.A.H., Almasi-Hashiani A., Anushiravani A., Arabloo J., Atique S., Awasthi A., Badawi A., Baig A.A.A., Bhala N., Bijani A., Biondi A., Borzì A.M., Burke K.E., Carvalho F., Daryani A., Dubey M., Eftekhari A., Fernandes E., Fernandes J.C., Fischer F., Haj-Mirzaian A., Haj-Mirzaian A., Hasanzadeh A., Hashemian M., Hay S.I., Hoang C.L., Househ M., Ilesanmi O.S., Jafari Balalami N., James S.L., Kengne A.P., Malekzadeh M.M., Merat S., Meretoja T.J., Mestrovic T., Mirrakhimov E.M., Mirzaei H., Mohammad K.A., Mokdad A.H., Monasta L., Negoi I., Nguyen T.H., Nguyen C.T., Pourshams A., Poustchi H., Rabiee M., Rabiee N., Ramezanzadeh K., Rawaf D.L., Rawaf S., Rezaei N., Robinson S.R., Ronfani L., Saxena S., Sepehrimanesh M., Shaikh M.A., Sharafi Z., Sharif M., Siabani S., Sima A.R., Singh J.A., Soheili A., Sotoudehmanesh R., Suleria H.A.R., Tesfay B.E., Tran B., Tsoi D., Vacante M., Wondmieneh A.B., Zarghi A., Zhang Z.-J., Dirac M., Malekzadeh R., Naghavi M. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020;5(1):17–30. doi: 10.1016/S2468-1253(19)30333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhagamhmad M.H., Day A.S., Lemberg D.A., Leach S.T. An overview of the bacterial contribution to Crohn’s disease pathogenesis. J. Med. Microbiol. 2016;65(10):1049–1059. doi: 10.1099/jmm.0.000331. [DOI] [PubMed] [Google Scholar]
- Altun H.K., Yıldız E.A., Akın M. Effects of synbiotic therapy in mild-to-moderately active ulcerative colitis: A randomized placebo-controlled study. Turk. J. Gastroenterol. 2019;30(4):313. doi: 10.5152/tjg.2019.18356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoroso C., Perillo F., Strati F., Fantini M., Caprioli F., Facciotti F. The role of gut microbiota biomodulators on mucosal immunity and intestinal inflammation. Cells. 2020;9(5):1234. doi: 10.3390/cells9051234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthakrishnan A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015;12(4):205–217. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- Ananthakrishnan A.N., Khalili H., Konijeti G.G., Higuchi L.M., De Silva P., Korzenik J.R., Fuchs C.S., Willett W.C., Richter J.M., Chan A.T. A prospective study of long-term intake of dietary fiber and risk of Crohn's disease and ulcerative colitis. Gastroenterol. 2013;145(5):970–977. doi: 10.1053/j.gastro.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asha M.Z., Khalil S.F.H. Efficacy and Safety of Probiotics, Prebiotics, and Synbiotics in the Treatment of Irritable Bowel Syndrome: A systematic review and meta-analysis. Sultan Qaboos Univ. Med. J. 2020;20(1):13. doi: 10.18295/squmj.2020.20.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiroz F., Dubray C., Bernalier-Donadille A., Cardot J.-M., Accarino A., Serra J., Wagner A., Respondek F., Dapoigny M. Effects of sc FOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: A randomized, double-blind, placebo-controlled study. Neurogastroenterol. Motil. 2017;29(2):e12911. doi: 10.1111/nmo.2017.29.issue-210.1111/nmo.12911. [DOI] [PubMed] [Google Scholar]
- Bäckhed F., Roswall J., Peng Y., Feng Q., Jia H., Kovatcheva-Datchary P., Li Y., Xia Y., Xie H., Zhong H., Khan M., Zhang J., Li J., Xiao L., Al-Aama J., Zhang D., Lee Y., Kotowska D., Colding C., Tremaroli V., Yin Y.e., Bergman S., Xu X., Madsen L., Kristiansen K., Dahlgren J., Wang J. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17(5):690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Bajinka O., Tan Y., Abdelhalim K.A., Özdemir G., Qiu X. Extrinsic factors influence gut microbes, the immediate consequences and restoring eubiosis. AMB Express. 2020;10(1):1–11. doi: 10.1186/s13568-020-01066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin J.L., Hedin C.R.H., Koutsoumpas A., Ng S.C., McCarthy N.E., Hart A.L., Kamm M.A., Sanderson J.D., Knight S.C., Forbes A., Stagg A.J., Whelan K., Lindsay J.O. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn's disease. Gut. 2011;60(7):923–929. doi: 10.1136/gut.2010.232025. [DOI] [PubMed] [Google Scholar]
- Berg A.M., Kelly C.P., Farraye F.A. Clostridium difficile infection in the inflammatory bowel disease patient. Inflamm. Bowel Dis. 2012 doi: 10.1002/ibd.22964. [DOI] [PubMed] [Google Scholar]
- Berni Canani R., De Filippis F., Nocerino R., Laiola M., Paparo L., Calignano A., De Caro C., Coretti L., Chiariotti L., Gilbert J.A., Ercolini D., McBain A.J. Specific signatures of the gut microbiota and increased levels of butyrate in children treated with fermented cow's milk containing heat-killed Lactobacillus paracasei CBA L74. Appl. Environ. Microbiol. 2017;83(19) doi: 10.1128/AEM.01206-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourreille A., Cadiot G., Le Dreau G., Laharie D., Beaugerie L., Dupas J.L., Marteau P., Rampal P., Moyse D., Saleh A. Saccharomyces boulardii does not prevent relapse of Crohn's disease. J. Clin. Gastroenterol. Hepatol. 2013;11(8):982–987. doi: 10.1016/j.cgh.2013.02.021. [DOI] [PubMed] [Google Scholar]
- Brüssow H. Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 2020;13(2):423–434. doi: 10.1111/1751-7915.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello C., Tremolaterra F., Pascariello A., Ciacci C., Iovino P. A randomised clinical trial (RCT) of a symbiotic mixture in patients with irritable bowel syndrome (IBS): effects on symptoms, colonic transit and quality of life. Int. J. Colorectal Dis. 2013;28(3):349–358. doi: 10.1007/s00384-012-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carding S., Verbeke K., Vipond D.T., Corfe B.M., Owen L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015;26(1):26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casén C., Vebø H.C., Sekelja M., Hegge F.T., Karlsson M.K., Ciemniejewska E., Dzankovic S., Frøyland C., Nestestog R., Engstrand L., Munkholm P., Nielsen O.H., Rogler G., Simrén M., Öhman L., Vatn M.H., Rudi K. Deviations in human gut microbiota: a novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment. Pharmacol. Ther. 2015;42(1):71–83. doi: 10.1111/apt.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.-S., Kao C.-Y. Current understanding of the gut microbiota shaping mechanisms. J. Biomed. Sci. 2019;26(1):1–11. doi: 10.1186/s12929-019-0554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang L., Hong G., Huang C., Qian W., Bai T., Song J., Song Y., Hou X. Probiotic mixtures with aerobic constituent promoted the recovery of multi-barriers in DSS-induced chronic colitis. Life Sci. 2020;240:117089. doi: 10.1016/j.lfs.2019.117089. [DOI] [PubMed] [Google Scholar]
- Chiba M., Nakane K., Komatsu M. Westernized diet is the most ubiquitous environmental factor in inflammatory bowel disease. Prem. J. 2019;23 doi: 10.7812/TPP/18-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G., Stilling R.M., Kennedy P.J., Stanton C., Cryan J.F., Dinan T.G. Minireview: gut microbiota: the neglected endocrine organ. J. Mol. Endocrinol. 2014;28(8):1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L.i., Guan X., Ding W., Luo Y.i., Wang W., Bu W., Song J., Tan X., Sun E., Ning Q., Liu G., Jia X., Feng L. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021;166:1035–1045. doi: 10.1016/j.ijbiomac.2020.10.259. [DOI] [PubMed] [Google Scholar]
- D’Incà R., Barollo M., Scarpa M., Grillo A.R., Brun P., Vettorato M.G., Castagliuolo I., Sturniolo G.C. Rectal administration of Lactobacillus casei DG modifies flora composition and Toll-like receptor expression in colonic mucosa of patients with mild ulcerative colitis. Dig. Dis. Sci. 2011;56(4):1178–1187. doi: 10.1007/s10620-010-1384-1. [DOI] [PubMed] [Google Scholar]
- Darb Emamie A., Rajabpour M., Ghanavati R., Asadolahi P., Farzi S., Sobouti B., Darbandi A. The effects of probiotics, prebiotics and synbiotics on the reduction of IBD complications, a periodic review during 2009–2020. J. Appl. Microbiol. 2021;130(6):1823–1838. doi: 10.1111/jam.14907. [DOI] [PubMed] [Google Scholar]
- Lima de Albuquerque C., Comalada M., Camuesco D., Rodríguez-Cabezas M.E., Luiz-Ferreira A., Nieto A., Monteiro de Souza Brito A.R., Zarzuelo A., Gálvez J. Effect of kale and papaya supplementation in colitis induced by trinitrobenzenesulfonic acid in the rat. E Spen. Eur. E. J. Clin Nutr. Metab. 2010;5(3):e111–e116. [Google Scholar]
- De Preter V., Vanhoutte T., Huys G., Swings J., De Vuyst L., Rutgeerts P., Verbeke K. Effects of Lactobacillus casei Shirota, Bifidobacterium breve, and oligofructose-enriched inulin on colonic nitrogen-protein metabolism in healthy humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292(1):G358–G368. doi: 10.1152/ajpgi.00052.2006. [DOI] [PubMed] [Google Scholar]
- DeGruttola A.K., Low D., Mizoguchi A., Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel Dis. 2016;22(5):1137–1150. doi: 10.1097/MIB.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M.S., Seekatz A.M., Koropatkin N.M., Kamada N., Hickey C.A., Wolter M., Pudlo N.A., Kitamoto S., Terrapon N., Muller A., Young V.B., Henrissat B., Wilmes P., Stappenbeck T.S., Núñez G., Martens E.C. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167(5):1339–1353.e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos Cruz B.C., da Silva Duarte V., Giacomini A., Corich V., de Paula S.O., da Silva Fialho L., Guimarães V.M., de Luces Fortes Ferreira C.L., Gouveia Peluzio M.d.C. Synbiotic VSL# 3 and yacon-based product modulate the intestinal microbiota and prevent the development of pre-neoplastic lesions in a colorectal carcinogenesis model. Appl. Microb. Biotechnol. 2020;104(20):8837–8857. doi: 10.1007/s00253-020-10863-x. [DOI] [PubMed] [Google Scholar]
- Fedorak R.N., Feagan B.G., Hotte N., Leddin D., Dieleman L.A., Petrunia D.M., Enns R., Bitton A., Chiba N., Paré P., Rostom A., Marshall J., Depew W., Bernstein C.N., Panaccione R., Aumais G., Steinhart A.H., Cockeram A., Bailey R.J., Gionchetti P., Wong C., Madsen K. The probiotic VSL# 3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn's disease. J Clin. Gastroenterol. Hepatol. 2015;13(5):928–935.e2. doi: 10.1016/j.cgh.2014.10.031. [DOI] [PubMed] [Google Scholar]
- Franzosa E.A., Sirota-Madi A., Avila-Pacheco J., Fornelos N., Haiser H.J., Reinker S., Vatanen T., Hall A.B., Mallick H., McIver L.J. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019;4(2):293–305. doi: 10.1038/s41564-018-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers D., Kugathasan S., Denson L., Vázquez-Baeza Y., Van Treuren W., Ren B., Schwager E., Knights D., Song S., Yassour M., Morgan X., Kostic A., Luo C., González A., McDonald D., Haberman Y., Walters T., Baker S., Rosh J., Stephens M., Heyman M., Markowitz J., Baldassano R., Griffiths A., Sylvester F., Mack D., Kim S., Crandall W., Hyams J., Huttenhower C., Knight R., Xavier R. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15(3):382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich J., Davenport E., Beaumont M., Jackson M., Knight R., Ober C., Spector T., Bell J., Clark A., Ley R. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe. 2016;19(5):731–743. doi: 10.1016/j.chom.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich J., Waters J., Poole A., Sutter J., Koren O., Blekhman R., Beaumont M., Van Treuren W., Knight R., Bell J., Spector T., Clark A., Ley R. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Y., Sun J., Liu J., Chen H., Kan J., Qian C., Zhang N., Jin C. Structural characterization of a water-soluble purple sweet potato polysaccharide and its effect on intestinal inflammation in mice. J. Funct. Foods. 2019;61:103502. doi: 10.1016/j.jff.2019.103502. [DOI] [Google Scholar]
- Halfvarson J., Brislawn C.J., Lamendella R., Vázquez-Baeza Y., Walters W.A., Bramer L.M., D'amato M., Bonfiglio F., McDonald D., Gonzalez A. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2017;2(5):1–7. doi: 10.1038/nmicrobiol.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel, K. and O’Connor, A. 2020. Emerging treatments for inflammatory bowel disease. Ther. Adv. Chronic Dis. 11: 2040622319899297 [DOI] [PMC free article] [PubMed]
- Hoffmann T.W., Pham H.-P., Bridonneau C., Aubry C., Lamas B., Martin-Gallausiaux C., Moroldo M., Rainteau D., Lapaque N., Six A., Richard M.L., Fargier E., Le Guern M.-E., Langella P., Sokol H. Microorganisms linked to inflammatory bowel disease-associated dysbiosis differentially impact host physiology in gnotobiotic mice. ISME J. 2016;10(2):460–477. doi: 10.1038/ismej.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Matsumoto S., Ohashi Y., Imaoka A., Setoyama H., Umesaki Y., Tanaka R., Otani T. Beneficial effects of probiotic bifidobacterium and galacto-oligosaccharide in patients with ulcerative colitis: a randomized controlled study. Digestion. 2011;84(2):128–133. doi: 10.1159/000322977. [DOI] [PubMed] [Google Scholar]
- Ivanovska T.P., Mladenovska K., Zhivikj Z., Pavlova M.J., Gjurovski I., Ristoski T., Petrushevska-Tozi L. Synbiotic loaded chitosan-Ca-alginate microparticles reduces inflammation in the TNBS model of rat colitis. Int. J. Pharm. 2017;527(1-2):126–134. doi: 10.1016/j.ijpharm.2017.05.049. [DOI] [PubMed] [Google Scholar]
- Kanwal S., Joseph T.P., Aliya S., Song S., Saleem M.Z., Nisar M.A., Wang Y., Meyiah A., Ma Y., Xin Y.i. Attenuation of DSS induced colitis by Dictyophora indusiata polysaccharide (DIP) via modulation of gut microbiota and inflammatory related signaling pathways. J. Funct. Foods. 2020;64:103641. doi: 10.1016/j.jff.2019.103641. [DOI] [Google Scholar]
- Kaplan G.G. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015;12(12):720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- Kaur R., Gulati M., Singh S.K. Role of synbiotics in polysaccharide assisted colon targeted microspheres of mesalamine for the treatment of ulcerative colitis. Int. J. Biol. Macromol. 2017;95:438–450. doi: 10.1016/j.ijbiomac.2016.11.066. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Hann H.J., Hong S.N., Kim K.H., Ahn I.M., Song J.Y., Lee S.H., Ahn H.S. Incidence and natural course of inflammatory bowel disease in Korea, 2006–2012: a nationwide population-based study. Inflamm. Bowel Dis. 2015;21(3):623–630. doi: 10.1097/MIB.0000000000000313. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Sohn H.B., Kwon B., Park M.S., Hong S.Y., Nam J.H., Suh J.T., Lee J.N., Kim K.D., Chang D.C. Potential prebiotic effects of yacon extract, a source of fructooligosaccharides, on Bifidobacterium strains. Korean J. Food Sci. Technol. 2020;52(2):149–155. [Google Scholar]
- Komaki S., Haque A., Miyazaki H., Matsumoto T., Nakamura S. Unexpected effect of probiotics by Lactococcus lactis subsp. lactis against colitis induced by dextran sulfate sodium in mice. J. Infect. Chemother. 2020;26(6):549–553. doi: 10.1016/j.jiac.2020.01.006. [DOI] [PubMed] [Google Scholar]
- Korpela K., Salonen A., Virta L.J., Kekkonen R.A., Forslund K., Bork P., De Vos W.M. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat. Commun. 2016;7(1):1–8. doi: 10.1038/ncomms10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange K., Buerger M., Stallmach A., Bruns T. Effects of antibiotics on gut microbiota. J. Dig. Dis. 2016;34(3):260–268. doi: 10.1159/000443360. [DOI] [PubMed] [Google Scholar]
- Le Bastard Q., Al-Ghalith G.A., Grégoire M., Chapelet G., Javaudin F., Dailly E., Batard E., Knights D., Montassier E. Systematic review: human gut dysbiosis induced by non-antibiotic prescription medications. Aliment. Pharmacol. Ther. 2018;47(3):332–345. doi: 10.1111/apt.14451. [DOI] [PubMed] [Google Scholar]
- Lee J.G., Han D.S., Jo S.V., Lee A.R., Park C.H., Eun C.S., Lee Y., Mizoguchi E. Characteristics and pathogenic role of adherent-invasive Escherichia coli in inflammatory bowel disease: Potential impact on clinical outcomes. PloS One. 2019;14(4):e0216165. doi: 10.1371/journal.pone.0216165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J., Chen E., Baldassano R., Otley A., Griffiths A., Lee D., Bittinger K., Bailey A., Friedman E., Hoffmann C., Albenberg L., Sinha R., Compher C., Gilroy E., Nessel L., Grant A., Chehoud C., Li H., Wu G., Bushman F. Inflammation, antibiotics, and diet as environmental stressors of the gut microbiome in pediatric Crohn’s disease. Cell Host Microbe. 2015;18(4):489–500. doi: 10.1016/j.chom.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Butcher J., Mack D., Stintzi A. Functional impacts of the intestinal microbiome in the pathogenesis of inflammatory bowel disease. Inflamm. Bowel Dis. 2015;21(1):139–153. doi: 10.1097/MIB.0000000000000215. [DOI] [PubMed] [Google Scholar]
- Li P., Xiao N., Zeng L., Xiao J., Huang J., Xu Y., Chen Y., Ren Y., Du B. Structural characteristics of a mannoglucan isolated from Chinese yam and its treatment effects against gut microbiota dysbiosis and DSS-induced colitis in mice. Carbohydr. Polym. 2020;250:116958. doi: 10.1016/j.carbpol.2020.116958. [DOI] [PubMed] [Google Scholar]
- Li R., Wang G.P., Whitlock J.A., Zhao S., Yagiz Y., Gu L. Muscadine grapes (Vitis rotundifolia) and dealcoholized muscadine wine alleviated symptoms of colitis and protected against dysbiosis in mice exposed to dextran sulfate sodium. J. Funct. Foods. 2020;65:103746. doi: 10.1016/j.jff.2019.103746. [DOI] [Google Scholar]
- Liang W., Feng Z., Rao S., Xiao C., Xue X., Lin Z., Zhang Q.i., Qi W. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69(6):1141–1143. doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- Liang Y., Liu M., Pu J., Zhu Z., Gao Z., Zhou Q., Gu Q., Li P. Probiotics and Their Metabolites Ameliorate Inflammatory Bowel Disease: A Critical Review. Infect. Microbes Dis. 2021;3(1):4–13. [Google Scholar]
- Lim S.-M., Jeong J.-J., Jang S.-E., Han M.J., Kim D.-H. A mixture of the probiotic strains Bifidobacterium longum CH57 and Lactobacillus brevis CH23 ameliorates colitis in mice by inhibiting macrophage activation and restoring the Th17/Treg balance. J. Funct. Foods. 2016;27:295–309. [Google Scholar]
- Liu M., Xie W., Wan X., Deng T. Clostridium butyricum modulates gut microbiota and reduces colitis associated colon cancer in mice. Int. Immunopharmacol. 2020;88:106862. doi: 10.1016/j.intimp.2020.106862. [DOI] [PubMed] [Google Scholar]
- Liu R., Li Y., Zhang B.o. The effects of konjac oligosaccharide on TNBS-induced colitis in rats. Int. Immunopharmacol. 2016;40:385–391. doi: 10.1016/j.intimp.2016.08.040. [DOI] [PubMed] [Google Scholar]
- Liu W., Tang S., Zhao Q., Zhang W., Li K., Yao W., Gao X. The α-D-glucan from marine fungus Phoma herbarum YS4108 ameliorated mice colitis by repairing mucosal barrier and maintaining intestinal homeostasis. Int. J. Biol. Macromol. 2020;149:1180–1188. doi: 10.1016/j.ijbiomac.2020.01.303. [DOI] [PubMed] [Google Scholar]
- Mahlich J., Matsuoka K., Nakamura Y., Sruamsiri R. The relationship between socio-demographic factors, health status, treatment type, and employment outcome in patients with inflammatory bowel disease in Japan. BMC Public Health. 2017;17(1):1–7. doi: 10.1186/s12889-017-4516-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak W.Y., Zhao M., Ng S.C., Burisch J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020;35(3):380–389. doi: 10.1111/jgh.14872. [DOI] [PubMed] [Google Scholar]
- Mardini H.E., Grigorian A.Y. Probiotic mix VSL# 3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: a meta-analysis. Inflamm. Bowel Dis. 2014;20(9):1562–1567. doi: 10.1097/MIB.0000000000000084. [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Uemura Y., Kanai T., Kunisaki R., Suzuki Y., Yokoyama K., Yoshimura N., Hibi T. Efficacy of Bifidobacterium breve fermented milk in maintaining remission of ulcerative colitis. Dig. Dis. Sci. 2018;63(7):1910–1919. doi: 10.1007/s10620-018-4946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes H., Krummenerl T., Giensch M., Wolff C., Schulze J. Clinical trial: probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN) BMC Complement. Med. Ther. 2010;10(1):1–8. doi: 10.1186/1472-6882-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michielan A., D’Incà R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators of Inflammation. 2015;2015:1–10. doi: 10.1155/2015/628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhtar N.M., Nawawi K.N.M., Verasingam J., Zhiqin W., Sagap I., Azman Z.A.M., Mazlan L., Hamid H.A., Yaacob N.Y., Rose I.M., Den E.L.N., Wan M.S., Raja Ali R.A. A four-decade analysis of the incidence trends, sociodemographic and clinical characteristics of inflammatory bowel disease patients at single tertiary centre, Kuala Lumpur. Malaysia. BMC Public Health. 2019;19(S4) doi: 10.1186/s12889-019-6858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse A.L., Dlusskaya E.A., Valcheva R., Haynes K.M., Gänzle M.G., Dieleman L.A. T2041 prebiotic mixture of inulin plus oligofructose is effective adjunct therapy for treatment of mild to moderate ulcerative colitis. Gastroenterol. 2010;138(5):S-619. doi: 10.1016/S0016-5085(10)62854-5. [DOI] [Google Scholar]
- Naseer M., Poola S., Ali S., Samiullah S., Tahan V. Prebiotics and Probiotics in Inflammatory Bowel Disease: Where are we now and where are we going? Curr. Clin. Pharmacol. 2020;15(3):216–233. doi: 10.2174/1574884715666200312100237. [DOI] [PubMed] [Google Scholar]
- Nataraj B.H., Ali S.A., Behare P.V., Yadav H. Postbiotics-parabiotics: the new horizons in microbial biotherapy and functional foods. Microb. Cell Factories. 2020;19(1):1–22. doi: 10.1186/s12934-020-01426-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S.C., Plamondon S., Kamm M.A., Hart A.L., Al-Hassi H.O., Guenther T., Stagg A.J., Knight S.C. Immunosuppressive effects via human intestinal dendritic cells of probiotic bacteria and steroids in the treatment of acute ulcerative colitis. Inflamm. Bowel Dis. 2010;16(8):1286–1298. doi: 10.1002/ibd.21222. [DOI] [PubMed] [Google Scholar]
- Ng S.C., Tang W., Ching J.Y., Wong M., Chow C.M., Hui A.J., Wong T.C., Leung V.K., Tsang S.W., Yu H.H., Li M.F., Ng K.K., Kamm M.A., Studd C., Bell S., Leong R., de Silva H.J., Kasturiratne A., Mufeena M.N.F., Ling K.L., Ooi C.J., Tan P.S., Ong D., Goh K.L., Hilmi I., Pisespongsa P., Manatsathit S., Rerknimitr R., Aniwan S., Wang Y.F., Ouyang Q., Zeng Z., Zhu Z., Chen M.H., Hu P.J., Wu K., Wang X., Simadibrata M., Abdullah M., Wu J.C., Sung J.J.Y., Chan F.K.L. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterol. 2013;145(1):158–165.e2. doi: 10.1053/j.gastro.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Ng W.K., Wong S.H., Ng S.C. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest. Res. 2016;14(2):111. doi: 10.5217/ir.2016.14.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J.-Y., Zhao Q. Beverage consumption and risk of ulcerative colitis: Systematic review and meta-analysis of epidemiological studies. Medicine. 2017;96(49):e9070. doi: 10.1097/MD.0000000000009070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv E., Halak A., Tiommny E., Yanai H., Strul H., Naftali T., Vaisman N. Randomized clinical study: Partially hydrolyzed guar gum (PHGG) versus placebo in the treatment of patients with irritable bowel syndrome. Nutr. Metab. 2016;13(1):1–7. doi: 10.1186/s12986-016-0070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva S., Di Nardo G., Ferrari F., Mallardo S., Rossi P., Patrizi G., Cucchiara S., Stronati L. Randomised clinical trial: the effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment. Pharmacol. Ther. 2012;35(3):327–334. doi: 10.1111/j.1365-2036.2011.04939.x. [DOI] [PubMed] [Google Scholar]
- Ong C., Aw M.M., Liwanag M.J., Quak S.H., Phua K.B. Rapid rise in the incidence and clinical characteristics of pediatric inflammatory bowel disease in a South-East Asian cohort in Singapore, 1994–2015. J. Dig. Dis. 2018;19(7):395–403. doi: 10.1111/1751-2980.12641. [DOI] [PubMed] [Google Scholar]
- Ong J., Young B.E., Ong S. COVID-19 in gastroenterology: a clinical perspective. Gut. 2020;69(6):1144–1145. doi: 10.1136/gutjnl-2020-321051. [DOI] [PubMed] [Google Scholar]
- Palumbo V.D., Romeo M., Gammazza A.M., Carini F., Damiani P., Damiano G., Buscemi S., Lo Monte A.I., Gerges-Geagea A., Jurjus A., Tomasello G. The long-term effects of probiotics in the therapy of ulcerative colitis: A clinical study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160(3):372–377. doi: 10.5507/bp.2016.044. [DOI] [PubMed] [Google Scholar]
- Parada Venegas D., De la Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G., Harmsen H.J., Faber K.N., Hermoso M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal V., Pozuelo M., Borruel N., Casellas F., Campos D., Santiago A., Martinez X., Varela E., Sarrabayrouse G., Machiels K., Vermeire S., Sokol H., Guarner F., Manichanh C. A microbial signature for Crohn's disease. Gut. 2017;66(5):813–822. doi: 10.1136/gutjnl-2016-313235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A.M., Mirsepasi H., Halkjær S.I., Mortensen E.M., Nordgaard-Lassen I., Krogfelt K.A. Ciprofloxacin and probiotic Escherichia coli Nissle add-on treatment in active ulcerative colitis: a double-blind randomized placebo controlled clinical trial. J. Crohns Colitis. 2014;8(11):1498–1505. doi: 10.1016/j.crohns.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Pilarczyk-Żurek M., Zwolińska-Wcisło M., Mach T., Okoń K., Adamski P., Heczko P., Mikołajczyk-Cichońska A., Stefański G., Strus M. Influence of Lactobacillus and Bifidobacterium combination on the gut microbiota, clinical course, and local gut inflammation in patients with ulcerative colitis: A preliminary, single-center, open-label study. J Prob Health. 2017;5(1):163. [Google Scholar]
- Pittayanon R., Lau J.T., Leontiadis G.I., Tse F., Yuan Y., Surette M., Moayyedi P. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: a systematic review. Gastroenterol. 2020;158(4):930–946.e1. doi: 10.1053/j.gastro.2019.11.294. [DOI] [PubMed] [Google Scholar]
- Racine A., Carbonnel F., Chan S.S.M., Hart A.R., Bueno-de-Mesquita H.B., Oldenburg B., van Schaik F.D.M., Tjønneland A., Olsen A., Dahm C.C., Key T., Luben R., Khaw K.-T., Riboli E., Grip O., Lindgren S., Hallmans G., Karling P., Clavel-Chapelon F., Bergman M.M., Boeing H., Kaaks R., Katzke V.A., Palli D., Masala G., Jantchou P., Boutron-Ruault M.-C. Dietary patterns and risk of inflammatory bowel disease in Europe: results from the EPIC study. Inflamm. Bowel Dis. 2016;22(2):345–354. doi: 10.1097/MIB.0000000000000638. [DOI] [PubMed] [Google Scholar]
- Rivera-Chávez F., Zhang L., Faber F., Lopez C., Byndloss M., Olsan E., Xu G., Velazquez E., Lebrilla C., Winter S., Bäumler A. Depletion of butyrate-producing Clostridia from the gut microbiota drives an aerobic luminal expansion of Salmonella. Cell Host Microbe. 2016;19(4):443–454. doi: 10.1016/j.chom.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivière A., Selak M., Lantin D., Leroy F., De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Nogales A., Algieri F., Garrido-Mesa J., Vezza T., Utrilla M.P., Chueca N., García F., Rodríguez-Cabezas M.E., Gálvez J. Intestinal anti-inflammatory effect of the probiotic Saccharomyces boulardii in DSS-induced colitis in mice: Impact on microRNAs expression and gut microbiota composition. J. Nutr. Biochem. 2018;61:129–139. doi: 10.1016/j.jnutbio.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Rogha M., Esfahani M.Z., Zargarzadeh A.H. The efficacy of a synbiotic containing Bacillus Coagulans in treatment of irritable bowel syndrome: a randomized placebo-controlled trial. Gastroenterol. Hepatol. Bed Bench. 2014;7(3):156. [PMC free article] [PubMed] [Google Scholar]
- Rutayisire E., Huang K., Liu Y., Tao F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants' life: a systematic review. BMC Gastroenterol. 2016;16(1):1–12. doi: 10.1186/s12876-016-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandes S., Figueiredo N., Pedroso S., Sant'Anna F., Acurcio L., Abatemarco Junior M., Barros P., Oliveira F., Cardoso V., Generoso S., Caliari M., Nicoli J., Neumann E., Nunes Á. Weissella paramesenteroides WpK4 plays an immunobiotic role in gut-brain axis, reducing gut permeability, anxiety-like and depressive-like behaviors in murine models of colitis and chronic stress. Food Res. Int. 2020;137:109741. doi: 10.1016/j.foodres.2020.109741. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Klapproth J.-M. The Role of Bacteria in the Pathogenesis of Ulcerative Colitis. Journal of Signal Transduction. 2012;2012:1–6. doi: 10.1155/2012/704953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal, J.P., Mak, J.W., Mullish, B.H., Alexander, J.L., Ng, S.C. and Marchesi, J.R. 2020. The gut microbiome: an under-recognised contributor to the COVID-19 pandemic? Therap. Adv. Gastroenterol. 13: 1756284820974914 [DOI] [PMC free article] [PubMed]
- Selvamani S., Dailin D.J., Gupta V.K., Wahid M., Keat H.C., Natasya K.H., Malek R.A., Haque S., Sayyed R., Abomoelak B. An Insight into Probiotics Bio-Route: Translocation from the Mother’s Gut to the Mammary Gland. Appl. Sci. 2021;11(16):7247. [Google Scholar]
- Seong G., Lee S., Min Y.W., Jang Y.S., Park S.-Y., Kim C.-H., Lee C., Hong S.N., Chang D.K. Effect of a synbiotic containing Lactobacillus paracasei and Opuntia humifusa on a murine model of irritable bowel syndrome. Nutrients. 2020;12(10):3205. doi: 10.3390/nu12103205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadnoush M., Hosseini R.S., Khalilnezhad A., Navai L., Goudarzi H., Vaezjalali M. Effects of probiotics on gut microbiota in patients with inflammatory bowel disease: a double-blind, placebo-controlled clinical trial. Korean J. Gastroenterol. 2015;65(4):215–221. doi: 10.4166/kjg.2015.65.4.215. [DOI] [PubMed] [Google Scholar]
- Shavakhi A., Shavakhi S., Minakari M., Farzamnia S., Peykar MohammadSaleh, Taghipour G., Tayebi A., Hashemi H. The effects of multi-strain probiotic compound on symptoms and quality-of-life in patients with irritable bowel syndrome: A randomized placebo-controlled trial. Adv. Biomed. Res. 2014;3(1):139. doi: 10.4103/2277-9175.135157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira D.S.C., Veronez L.C., Lopes-Júnior L.C., Anatriello E., Brunaldi M.O., Pereira-da-Silva G. Lactobacillus bulgaricus inhibits colitis-associated cancer via a negative regulation of intestinal inflammation in azoxymethane/dextran sodium sulfate model. World J. Gastroenterol. 2020;26(43):6782. doi: 10.3748/wjg.v26.i43.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.K., Chang H.-W., Yan D., Lee K.M., Ucmak D., Wong K., Abrouk M., Farahnik B., Nakamura M., Zhu T.H. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017;15(1):1–17. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V., Proctor S.D., Willing B.P. Koch's postulates, microbial dysbiosis and inflammatory bowel disease. J. Clin. Microbiol. Infect. 2016;22(7):594–599. doi: 10.1016/j.cmi.2016.04.018. [DOI] [PubMed] [Google Scholar]
- Sokol H., Leducq V., Aschard H., Pham H.-P., Jegou S., Landman C., Cohen D., Liguori G., Bourrier A., Nion-Larmurier I., Cosnes J., Seksik P., Langella P., Skurnik D., Richard M.L., Beaugerie L. Fungal microbiota dysbiosis in IBD. Fungal microbiota dysbiosis in IBD. Gut. 2017;66(6):1039–1048. doi: 10.1136/gutjnl-2015-310746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son S.J., Koh J.H., Park M.R., Ryu S., Lee W.J., Yun B., Lee J.-H., Oh S., Kim Y. Effect of the Lactobacillus rhamnosus strain GG and tagatose as a synbiotic combination in a dextran sulfate sodium-induced colitis murine model. J. Dairy Sci. 2019;102(4):2844–2853. doi: 10.3168/jds.2018-15013. [DOI] [PubMed] [Google Scholar]
- Souza É.L., Elian S.D., Paula L.M., Garcia C.C., Vieira A.T., Teixeira M.M., Arantes R.M., Nicoli J.R., Martins F.S. Escherichia coli strain Nissle 1917 ameliorates experimental colitis by modulating intestinal permeability, the inflammatory response and clinical signs in a faecal transplantation model. J. Med. Microbiol. 2016;65(3):201–210. doi: 10.1099/jmm.0.000222. [DOI] [PubMed] [Google Scholar]
- Spiller R., Major G. IBS and IBD—separate entities or on a spectrum? Nat. Rev. Gastroenterol. Hepatol. 2016;13(10):613–621. doi: 10.1038/nrgastro.2016.141. [DOI] [PubMed] [Google Scholar]
- Steed H., Macfarlane G.T., Blackett K.L., Bahrami B., Reynolds N., Walsh S.V., Cummings J.H., Macfarlane S. Clinical trial: the microbiological and immunological effects of synbiotic consumption–a randomized double-blind placebo-controlled study in active Crohn’s disease. Aliment. Pharmacol. Ther. 2010;32(7):872–883. doi: 10.1111/j.1365-2036.2010.04417.x. [DOI] [PubMed] [Google Scholar]
- Suez J., Korem T., Zeevi D., Zilberman-Schapira G., Thaiss C.A., Maza O., Israeli D., Zmora N., Gilad S., Weinberger A., Kuperman Y., Harmelin A., Kolodkin-Gal I., Shapiro H., Halpern Z., Segal E., Elinav E. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- Sun Y.-Y., Li M., Li Y.-Y., Li L.-X., Zhai W.-Z., Wang P., Yang X.-X., Gu X., Song L.-J., Li Z., Zuo X.-L., Li Y.-Q. The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-21241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Nishida A., Fujimoto T., Fujii M., Shioya M., Imaeda H., Inatomi O., Bamba S., Andoh A., Sugimoto M. Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn's disease. Digestion. 2016;93(1):59–65. doi: 10.1159/000441768. [DOI] [PubMed] [Google Scholar]
- Tamaki H., Nakase H., Inoue S., Kawanami C., Itani T., Ohana M., Kusaka T., Uose S., Hisatsune H., Tojo M., Noda T., Arasawa S., Izuta M., Kubo A., Ogawa C., Matsunaka T., Shibatouge M. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: A randomized, double-blinded, placebo-controlled multicenter trial. Dig. Endosc. 2016;28(1):67–74. doi: 10.1111/den.12553. [DOI] [PubMed] [Google Scholar]
- Thakur B.K., Saha P., Banik G., Saha D.R., Grover S., Batish V.K., Das S. Live and heat-killed probiotic Lactobacillus casei Lbs2 protects from experimental colitis through Toll-like receptor 2-dependent induction of T-regulatory response. Int. Immunopharmacol. 2016;36:39–50. doi: 10.1016/j.intimp.2016.03.033. [DOI] [PubMed] [Google Scholar]
- Tursi A., Brandimarte G., Papa A., Giglio A., Elisei W., Giorgetti G.M., Forti G., Morini S., Hassan C., Pistoia M.A., Modeo M.E., Rodino' S., D'Amico T., Sebkova L., Sacca' N., Di Giulio E., Luzza F., Imeneo M., Larussa T., Di Rosa S., Annese V., Danese S., Gasbarrini A. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL# 3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am. J. Gastroenterol. Suppl. 2010;105(10):2218–2227. doi: 10.1038/ajg.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waal M.B., Flach J., Browne P.D., Besseling-van der Vaart I., Claassen E., van de Burgwal L.H.M. Probiotics for improving quality of life in ulcerative colitis: Exploring the patient perspective. PharmaNutrition. 2019;7:100139. doi: 10.1016/j.phanu.2018.100139. [DOI] [Google Scholar]
- Wang F., Feng J., Gao Q., Ma M., Lin X., Liu J., Li J., Zhao Q. Carbohydrate and protein intake and risk of ulcerative colitis: Systematic review and dose-response meta-analysis of epidemiological studies. Clin. Nutr. 2017;36(5):1259–1265. doi: 10.1016/j.clnu.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Ward M.G., Kariyawasam V.C., Mogan S.B., Patel K.V., Pantelidou M., Sobczyńska-Malefora A., Porté F., Griffin N., Anderson S.H., Sanderson J.D. Prevalence and risk factors for functional vitamin B12 deficiency in patients with Crohn's disease. Inflamm. Bowel Dis. 2015;21(12):2839–2847. doi: 10.1097/MIB.0000000000000559. [DOI] [PubMed] [Google Scholar]
- Wildt S., Nordgaard I., Hansen U., Brockmann E., Rumessen J.J. A randomised double-blind placebo-controlled trial with Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp. lactis BB-12 for maintenance of remission in ulcerative colitis. J. Crohns Colitis. 2011;5(2):115–121. doi: 10.1016/j.crohns.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Xia Y., Chen Y., Wang G., Yang Y., Song X., Xiong Z., Zhang H., Lai P., Wang S., Ai L. Lactobacillus plantarum AR113 alleviates DSS-induced colitis by regulating the TLR4/MyD88/NF-κB pathway and gut microbiota composition. J. Funct. Foods. 2020;67:103854. doi: 10.1016/j.jff.2020.103854. [DOI] [Google Scholar]
- Xie H., Guo R., Zhong H., Feng Q., Lan Z., Qin B., Ward K.J., Jackson M.A., Xia Y., Chen X.u., Chen B., Xia H., Xu C., Li F., Xu X., Al-Aama J.Y., Yang H., Wang J., Kristiansen K., Wang J., Steves C.J., Bell J.T., Li J., Spector T.D., Jia H. Shotgun metagenomics of 250 adult twins reveals genetic and environmental impacts on the gut microbiome. Cell Syst. 2016;3(6):572–584.e3. doi: 10.1016/j.cels.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Liu Y., Chen B., Zhang G., Ou S., Luo J., Peng X. Ganoderma lucidum polysaccharide improves rat DSS-induced colitis by altering cecal microbiota and gene expression of colonic epithelial cells. Food. Nutr. Res. 2019;63(0) doi: 10.29219/fnr.v63.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasueda A., Mizushima T., Nezu R., Sumi R., Tanaka M., Nishimura J., Kai Y., Hirota M., Osawa H., Nakajima K., Mori M., Ito T. The effect of Clostridium butyricum MIYAIRI on the prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis. Surg. Today. 2016;46(8):939–949. doi: 10.1007/s00595-015-1261-9. [DOI] [PubMed] [Google Scholar]
- Yeoh Y.K., Zuo T., Lui G.-Y., Zhang F., Liu Q., Li A.YL., Chung A.CK., Cheung C.P., Tso E.YK., Fung K.SC., Chan V., Ling L., Joynt G., Hui D.-C., Chow K.M., Ng S.S.S., Li T.-M., Ng R.WY., Yip T.CF., Wong G.-H., Chan F.KL., Wong C.K., Chan P.KS., Ng S.C. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J.S., Sohn W., Lee O.Y., Lee S.P., Lee K.N., Jun D.W., Lee H.L., Yoon B.C., Choi H.S., Chung W.S. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J. Gastroenterol. Hepatol. 2014;29(1):52–59. doi: 10.1111/jgh.12322. [DOI] [PubMed] [Google Scholar]
- Yoon M.Y., Yoon S.S. Disruption of the gut ecosystem by antibiotics. Yonsei Med. J. 2018;59(1):4–12. doi: 10.3349/ymj.2018.59.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimatsu Y., Yamada A., Furukawa R., Sono K., Osamura A., Nakamura K., Aoki H., Tsuda Y., Hosoe N., Takada N., Suzuki Y. Effectiveness of probiotic therapy for the prevention of relapse in patients with inactive ulcerative colitis. World Journal of Gastroenterology: WJG. 2015;21(19):5985–5994. doi: 10.3748/wjg.v21.i19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha Z., Lv Y., Tang H., Li T., Miao Y., Cheng J., Wang G., Tan Y., Zhu Y., Xing X., Ding K., Wang Y., Yin H. An orally administered butyrate-releasing xylan derivative reduces inflammation in dextran sulphate sodium-induced murine colitis. Int. J. Biol. Macromol. 2020;156:1217–1233. doi: 10.1016/j.ijbiomac.2019.11.159. [DOI] [PubMed] [Google Scholar]
- Zimmermann P., Curtis N. The effect of antibiotics on the composition of the intestinal microbiota-a systematic review. J. Infect. 2019;79(6):471–489. doi: 10.1016/j.jinf.2019.10.008. [DOI] [PubMed] [Google Scholar]
- Żółkiewicz J., Marzec A., Ruszczyński M., Feleszko W. Postbiotics—a step beyond pre-and probiotics. Nutrients. 2020;12(8):2189. doi: 10.3390/nu12082189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T., Kamm M.A., Colombel J.-F., Ng S.C. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018;15(7):440–452. doi: 10.1038/s41575-018-0003-z. [DOI] [PubMed] [Google Scholar]
- Zuo T., Zhang F., Lui G.C.Y., Yeoh Y.K., Li A.Y.L., Zhan H., Wan Y., Chung A.C.K., Cheung C.P., Chen N., Lai C.K.C., Chen Z., Tso E.Y.K., Fung K.S.C., Chan V., Ling L., Joynt G., Hui D.S.C., Chan F.K.L., Chan P.K.S., Ng S.C. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterol. 2020;159(3):944–955.e8. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was generated in this study.