Abstract
The present study assessed the effectiveness of gamma radiation in inducing favorable genetic variability in tomato (Solanum lycopersicum L.). An experiment was conducted in a randomized complete block design to produce M1 generation. Significant differences were observed among the genotypes as well as between the treatments at individual plant level based on observed traits (seed germination percentage, seedling survival, plant height, number of flower clusters plant−1, number of flowers and fruits plant−1). All observed characters in the mutagenized population were adversely affected with increasing radiation dose. Results identified 450 Gy as the most damaging radiation dose followed by 300 Gy and 150 Gy. Moreover, 300 Gy treatment was identified as lethal dose (LD50) as it caused a 50% germination inhibition in almost all the evaluated genotypes. The 150 Gy treatment showed the least damaging impact and induced maximum genetic variability in almost all the genotypes under study. Character association studies were also conducted which could be utilized in the selection of desirable mutants. Correlation studies revealed an altered association among the observed parameters from positive to negative direction in 300 Gy and 450 Gy treatments as compared to control. These deviations in correlation coefficients proved that mutagenesis can break the linkage among specific loci. Furthermore, path coefficient analysis identified the growth attributes with an effective direct and indirect contribution in yield.
Keywords: Tomato, Induced mutation, Gamma radiation, Lethal dose, Genetic variability, Character association studies
1. Introduction
Tomato (Solanum lycopersicum L.) is one of the most important vegetables of the world that originated in South America (Paran and Fallik, 2011). It is considered as model plant species for analyzing fruit developmental phases along with ripening and metabolic activities of several metabolites in other plants having berry fruits (Matsukura et al., 2007). Tomato has been domesticated and selected outside its center of origin in South America in an intentional way to enhance the productivity for human consumption; thus, undergone various genetic and morphological changes (Bergougnoux, 2014, Blanca et al., 2015). It belongs to the nightshade family Solanaceae and exhibits diploid (2n = 24) chromosome number, with a genome size of 950 Mb having heterochromatin (77%) and euchromatin (33%) (Peterson et al., 1996). It is a vital source of nutritional and bioactive antioxidant compounds which play a key role in human health including minerals, vitamin C and E, beta-carotene, lycopene, flavonoids, organic acids, phenolics and chlorophyll (Weisburger, 2002, Siddiqui et al., 2015). Just like other crop species, tomato is also affected by various biotic and abiotic stresses which affect its overall productivity. To overcome this constraint, several techniques have been used to induce genetic variability and to identify the new genes among tomato plants.
Among many, mutations are one of the useful strategies to identify the nature and function of a particular gene which ultimately broaden the genetic base of crop species and help to create raw material for crop improvement (Adamu et al., 2004). Mutation can create variability in both qualitative and quantitative attributes in the target population at already known DNA fragments as well as in the sequence which is not identified earlier, except breaking the linkage among some specific traits (Konzak et al., 1977). It can be induced either to produce direct mutants or utilize these mutants in hybridization to enhance productivity and create favorable agronomic attributes with the least reduction in viability (Ahloowalia et al., 2004).
Induced mutations have great potential and have served as a complementary approach in the genetic improvement of crop plants (Mehandjiev et al., 2001). Over the last 50 years, mutation breeding has significantly contributed to developing high-yielding crop genotypes worldwide. This extensive utilization of induced mutations has led to the development of 3222 varieties of nearly 170 plant species through plant breeding in more than 60 countries (FAO, 2005). With an innovative approach in mutation breeding from conventional mutation research to modern reverse genetics, plant breeders are currently utilizing this approach comparatively more efficiently than ever.
Different mutagenic agents including chemicals and radiations are utilized to produce a desirable mutant population with a higher mutagenic rate. However, gamma radiation and fast neutrons are frequently used mutagenic agents in most crop species. Among these agents, the use of gamma radiation is less damaging which induces point mutations or small deletions while the utilization of fast neutrons results in large deletions, chromosomal loss and translocations (Gupta, 2019) which is lethal in most cases. Until now several commercial cultivars have been produced in various crop plant species, by radiation-induced mutation breeding i.e. brassica oilseed cultivar 'Abasin-95′ (Shah et al., 2001).
Matsukura et al. (2007) used gamma radiation with a dose of 300 Gy to induce genetic variability in the inbred miniature dwarf variety “Micro-Tom”. Total 6301 M2 lines were screened out of which 237 lines were selected as a mutant candidates based on phenotypic and brix aberration. Further selection in the M3 and M4 mutagenized population produced 24 phenotypic mutants and 11 aberrant brix mutants. Thus, it is evident that induction of genetic variability through mutagenesis plays an important role in improving the quality as well as the productivity of crop plants. But still, there is a gap in determining the best range of gamma radiation dose. Therefore, the present study was conducted to optimize the irradiation dose and to assess the effectiveness of different gamma radiation doses in inducing favorable genetic variability in 50 different tomato genotypes.
2. Materials and methods
2.1. Experimental details
Germplasm of 50 different tomato genotypes was procured from National Agriculture Research Institute, Islamabad (NARC) (Table 1). Dry seeds were irradiated with 150 Gy, 300 Gy and 450 Gy doses of gamma rays from a cesium (Cs) source at Nuclear Institute of Agriculture and Biology (NIAB), Faisalabad, Pakistan. Nursery of mutated as well as of normal seeds was raised in the greenhouse under controlled conditions. After 14 days, assessment of germination (%) (Farooq et al., 2019, Farooq et al., 2021), reduction over control (%) and lethal dose (LD50) optimization was carried out in mutagenized population of different genotypes. Seedlings were then transplanted (Onen et al., 2017, Farooq et al., 2017, Ozaslan et al., 2016) after 21 days of sowing on seedbeds prepared in the research area of the department of Plant Breeding and Genetics, University of Agriculture, Faisalabad. Each genotype in all treatments was replicated thrice following randomized complete block design (RCBD). The transplanted mutants of each genotype along with the normal plants were then subjected to morphological characterization based on plant height (cm), number of flower clusters plant−1, number of flowers and fruits plant−1.
Table 1.
List of accessions used in the study.
| Sr. No. | Accession | Sr. No. | Accession | Sr. No. | Accession | Sr. No. | Accession |
|---|---|---|---|---|---|---|---|
| 1 | 10,143 | 14 | 10,137 | 27 | 13,201 | 40 | BPVT-14–1 |
| 2 | 10,164 | 15 | 13,189 | 28 | Tom-15101 | 41 | 13,240 |
| 3 | 10,179 | 16 | Roma | 29 | 10,140 | 42 | TOM-15140 |
| 4 | 13,234 | 17 | 13,210 | 30 | Loo-587 | 43 | Tomato-4 |
| 5 | Pakit | 18 | 13,215 | 31 | Tom-15131 | 44 | 10,186 |
| 6 | Tomato-1 | 19 | 13,232 | 32 | NTH-242 | 45 | Nagina |
| 7 | Tomato-3 | 20 | 13,235 | 33 | 13,226 | 46 | Naqeeb |
| 8 | 10,187 | 21 | 7040 | 34 | 13,201 | 47 | 10,116 |
| 9 | Tom-15111 | 22 | lyp-no-1 | 35 | Rio-Grande | 48 | 13,211 |
| 10 | Loo-602 | 23 | Tom-15146 | 36 | Tomato-2 | 49 | Lo-1214 |
| 11 | Nadir | 24 | Lo-1225 | 37 | 13,231 | 50 | 13,238 |
| 12 | Glacier | 25 | 13,205 | 38 | Tom-15124 | ||
| 13 | Sub-arctic | 26 | 10,170 | 39 | 10,114 |
2.2. Statistical analysis
Statistical analysis including the descriptive statistics (Table 3) and time series plot (Fig. 1) were done by using Minitab 17.1.0 statistical software to analyze the degree of variability among control and mutagenized treatments and to identify the lethal dose (LD50). Analysis of variance (ANOVA), correlation analysis (Table 4) and path coefficient analysis (Table 5) were carried out using R 3.1.0 software for character association studies in control and mutagenized treatments.
Table 3.
Estimates of variability and genetic characters in mutagenized population of 50 different genotypes with different levels.
| Characters | Mean ± S.E | Range | MSS | SD | CV % | Heritability | |
|---|---|---|---|---|---|---|---|
| Control | Plant height (cm) | 57.97 ± 0.78 | 32.9 – 77.3 | 264.4** | 9.59 | 16.55 | 0.92 |
| No. of Flowers Clusters Plant−1 | 8.10 ± 0.09 | 6 – 10 | 2.67** | 1.06 | 13.03 | 0.7 | |
| No. of Flowers Plant−1 | 31.14 ± 0.46 | 21 – 44 | 92.19** | 5.59 | 17.96 | 0.95 | |
| Fruits Plant−1 | 30.62 ± 0.45 | 21 – 44 | 88.19** | 5.55 | 18.13 | 0.91 | |
| 150 Gy | Plant height (cm) | 59 ± 0.54 | 49.5 – 73 | 101.1** | 5.94 | 10.07 | 0.91 |
| No. of Flowers Clusters Plant−1 | 8.71 ± 0.13 | 6 – 13 | 5.19** | 1.40 | 16.06 | 0.81 | |
| No. of Flowers Plant−1 | 34.9 ± 0.39 | 22 – 46 | 52.43** | 4.33 | 12.39 | 0.88 | |
| Fruits Plant−1 | 33.21 ± 0.38 | 21 – 45 | 50.54** | 4.24 | 12.77 | 0.88 | |
| 300 Gy | Plant height (cm) | 49.13 ± 1.19 | 31 – 69.7 | 311.41** | 10.11 | 20.58 | 0.98 |
| No. of Flowers Clusters Plant−1 | 4.73 ± 0.13 | 2 – 7 | 3.36** | 1.13 | 23.77 | 0.80 | |
| No. of Flowers Plant−1 | 18.11 ± 0.54 | 8 – 26 | 64.83** | 4.61 | 25.44 | 0.98 | |
| Fruits Plant−1 | 14.30 ± 0.51 | 5 – 22 | 57.07** | 4.33 | 28.31 | 0.98 | |
| 450 Gy | Plant height (cm) | 46.71 ± 1.72 | 31.4 – 57.8 | 205.43** | 7.90 | 16.90 | 0.98 |
| No. of Flowers Clusters Plant−1 | 3.76 ± 0.17 | 3 – 5 | 1.52** | 0.77 | 20.43 | 0.73 | |
| No. of Flowers Plant−1 | 13.52 ± 0.70 | 9 – 19 | 33.54** | 3.20 | 23.69 | 0.98 | |
| Fruits Plant−1 | 7.09 ± 0.32 | 5 – 9 | 6.63** | 1.48 | 20.86 | 0.88 |
*=Significant (P < 0.05), **=Significant (P < 0.01), NS = Non-Significant (P > 0.05).
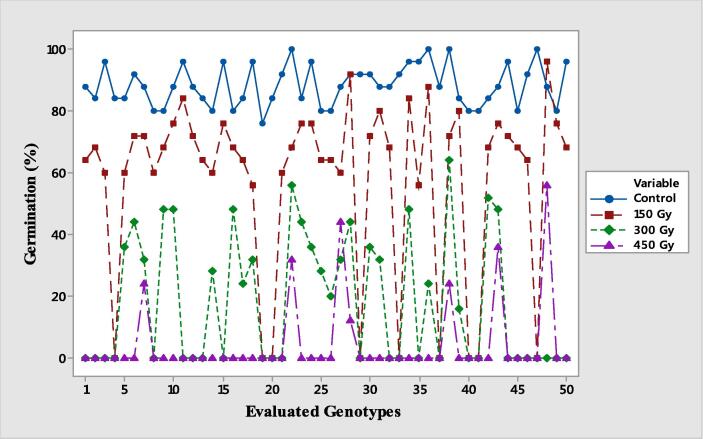
Fig. 1.
Germination % of 50 tomato genotypes in control (untreated) and mutagenized treatments.
Table 4.
Genotypic and phenotypic correlation coefficients among the quantitative traits in gamma radiation treated (150 Gy, 300 Gy and 450 Gy) and control (untreated) population of 50 tomato genotypes.
| Characters | Treatments | Plant Height | No. of Flower Clusters Plant−1 | No. of Flowers Plant−1 | Fruits Plant−1 | |
|---|---|---|---|---|---|---|
| Plant Height | Control | Genotypic | 1 | |||
| Phenotypic | 1 | |||||
| 150 Gy | Genotypic | 1 | ||||
| Phenotypic | 1 | |||||
| 300 Gy | Genotypic | 1 | ||||
| Phenotypic | 1 | |||||
| 450 Gy | Genotypic | 1 | ||||
| Phenotypic | 1 | |||||
| No. of Flower Clusters Plant−1 | Control | Genotypic | 0.55** | 1 | ||
| Phenotypic | 0.43** | 1 | ||||
| 150 Gy | Genotypic | 0.44** | 1 | |||
| Phenotypic | 0.36** | 1 | ||||
| 300 Gy | Genotypic | −0.61** | 1 | |||
| Phenotypic | −0.53** | 1 | ||||
| 450 Gy | Genotypic | 0.29NS | 1 | |||
| Phenotypic | 0.25NS | 1 | ||||
| No. of Flowers Plant−1 | Control | Genotypic | 0.44** | 0.86** | 1 | |
| Phenotypic | 0.41** | 0.77** | 1 | |||
| 150 Gy | Genotypic | 0.74** | 0.71** | 1 | ||
| Phenotypic | 0.67** | 0.69** | 1 | |||
| 300 Gy | Genotypic | −0.40** | 0.91** | 1 | ||
| Phenotypic | −0.40** | 0.85** | 1 | |||
| 450 Gy | Genotypic | 0.21NS | 0.93** | 1 | ||
| Phenotypic | 0.20NS | 0.81** | 1 | |||
| Fruits Plant−1 | Control | Genotypic | 0.46** | 0.86** | 0.99** | 1 |
| Phenotypic | 0.40** | 0.76** | 0.98** | 1 | ||
| 150 Gy | Genotypic | 0.73** | 0.93** | 0.99** | 1 | |
| Phenotypic | 0.66** | 0.85** | 0.97** | 1 | ||
| 300 Gy | Genotypic | −0.40** | 0.97** | 0.99** | 1 | |
| Phenotypic | −0.39** | 0.96** | 0.97** | 1 | ||
| 450 Gy | Genotypic | −0.25NS | 0.96NS | −0.15NS | 1 | |
| Phenotypic | −0.24NS | 0.93NS | −0.10NS | 1 | ||
*=Significant (P < 0.05), **=Significant (P < 0.01), NS = Non-Significant (P > 0.05).
Table 5.
Path co-efficient analysis showing direct (diagonal) and indirect effects of different quantitative traits on no. of fruits/plant in M1 and normal tomato population of 50 different genotypes.
| Control |
150 Gy |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PH | NFC | NF | Correlation with yield | PH | NFC | NF | Correlation with yield | ||
| PH | 0.013 | 0.005 | 0.442 | 0.46 | PH | −0.020 | −0.008 | 0.764 | 0.74 |
| NFC | 0.006 | 0.009 | 0.984 | 0.87 | NFC | −0.015 | −0.012 | 1.025 | 0.71 |
| NF | 0.007 | 0.010 | 0.851 | 1.00 | NF | −0.009 | −0.018 | 0.732 | 1.00 |
| Residual effect = 0.0003 | Residual Effect = 0.0059 | ||||||||
| 300 Gy | Correlation with yield | 450 Gy | Correlation with yield | ||||||
| PH | NFC | NF | PH | NFC | NF | ||||
| PH | −0.005 | 0.001 | −0.405 | −0.41 | PH | −0.443 | 0.655 | −0.464 | −0.25 |
| NFC | 0.002 | −0.002 | 0.990 | 0.91 | NFC | −0.097 | 2.066 | −2.121 | 0.11 |
| NF | 0.003 | −0.002 | 0.905 | 0.99 | NF | −0.131 | 2.217 | −1.976 | −0.15 |
| Residual Effect = 0.0182 | Residual Effect = 0.324 | ||||||||
PH = Plant height, NFC = No. of flower clusters, NF = No. of flowers.
3. Results
3.1. Germination percentage
In the present study, the germination (%) of tomato genotypes in different radiation treatments was calculated by considering control (untreated) as 100% (Table 2). Generally, a substantial decrease in germination (%) was observed among the treatments in comparison to the control. The maximum reduction in germination (%) was observed at 450 Gy treatment (86.96% reduction over control) in genotype Tom-15101 followed by 10,114 (80.95 %) at 300 Gy and Tomato-3 (72.73 %) at 450 Gy radiation. Along with the decrease in germination (%), complete germination inhibition of certain genotypes was also observed in all levels of irradiation. In treatment having 450 Gy, out of total 50 genotypes only seven were germinated with 86 % complete inhibition followed by 300 Gy with 52% and 150 Gy with 18% germination inhibition (Fig. 1).
Table 2.
Effect of mutagenesis on seed germination in M1 generation.
| Sr. No. | Treatment | Mean | % over control | % reduction over control | Sr. No. | Treatment | Mean | % over control | % reduction over control |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Control | 88 | 100.00 | – | 14 | Control | 80 | 100.00 | – |
| 150 Gy | 64 | 72.73 | 27.27 | 150 Gy | 60 | 75.00 | 25.00 | ||
| 300 Gy | – | – | – | 300 Gy | 28 | 35.00 | 65.00 | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 2 | Control | 84 | 100.00 | – | 15 | Control | 96 | 100.00 | – |
| 150 Gy | 68 | 80.95 | 19.05 | 150 Gy | 76 | 79.17 | 20.83 | ||
| 300 Gy | – | – | – | 300 Gy | – | – | – | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 3 | Control | 96 | 100.00 | – | 16 | Control | 80 | 100.00 | – |
| 150 Gy | 60 | 62.50 | 37.50 | 150 Gy | 68 | 85.00 | 15.00 | ||
| 300 Gy | – | – | – | 300 Gy | 48 | 60.00 | 40.00 | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 4 | Control | 84 | 100.00 | – | 17 | Control | 84 | 100.00 | – |
| 150 Gy | – | – | – | 150 Gy | 64 | 76.19 | 23.81 | ||
| 300 Gy | – | – | – | 300 Gy | 24 | 28.57 | 71.43 | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 5 | Control | 84 | 100.00 | – | 18 | Control | 96 | 100.00 | – |
| 150 Gy | 60 | 71.43 | 28.57 | 150 Gy | 56 | 58.33 | 41.67 | ||
| 300 Gy | 36 | 42.86 | 57.14 | 300 Gy | 32 | 33.33 | 66.67 | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 6 | Control | 92 | 100.00 | – | 19 | Control | 76 | 100.00 | – |
| 150 Gy | 72 | 78.26 | 21.74 | 150 Gy | – | – | – | ||
| 300 Gy | 44 | 47.83 | 52.17 | 300 Gy | – | – | – | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 7 | Control | 88 | 100.00 | – | 20 | Control | 84 | 100.00 | – |
| 150 Gy | 72 | 81.82 | 18.18 | 150 Gy | – | – | – | ||
| 300 Gy | 32 | 36.36 | 63.64 | 300 Gy | – | – | – | ||
| 450 Gy | 24 | 27.27 | 72.73 | 450 Gy | – | – | – | ||
| 8 | Control | 80 | 100.00 | – | 21 | Control | 92 | 100.00 | – |
| 150 Gy | 60 | 75.00 | 25.00 | 150 Gy | 60 | 65.22 | 34.78 | ||
| 300 Gy | – | – | – | 300 Gy | – | – | – | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 9 | Control | 80 | 100.00 | – | 22 | Control | 100 | 100.00 | – |
| 150 Gy | 68 | 85.00 | 15.00 | 150 Gy | 68 | 68.00 | 32.00 | ||
| 300 Gy | 48 | 60.00 | 40.00 | 300 Gy | 56 | 56.00 | 44.00 | ||
| 450 Gy | – | – | – | 450 Gy | 32 | 32.00 | 68.00 | ||
| 10 | Control | 88 | 100.00 | – | 23 | Control | 84 | 100.00 | – |
| 150 Gy | 76 | 86.36 | 13.64 | 150 Gy | 76 | 90.48 | 9.52 | ||
| 300 Gy | 48 | 54.55 | 45.45 | 300 Gy | 44 | 52.38 | 47.62 | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 11 | Control | 96 | 100.00 | – | 24 | Control | 96 | 100.00 | – |
| 150 Gy | 84 | 87.50 | 12.50 | 150 Gy | 76 | 79.17 | 20.83 | ||
| 300 Gy | – | – | – | 300 Gy | 36 | 37.50 | 62.50 | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 12 | Control | 88 | 100.00 | – | 25 | Control | 80 | 100.00 | – |
| 150 Gy | 72 | 81.82 | 18.18 | 150 Gy | 64 | 80.00 | 20.00 | ||
| 300 Gy | – | – | – | 300 Gy | 28 | 35.00 | 65.00 | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 13 | Control | 84 | 100.00 | – | 26 | Control | 80 | 100.00 | – |
| 150 Gy | 64 | 76.19 | 23.81 | 150 Gy | 64 | 80.00 | 20 | ||
| 300 Gy | – | – | – | 300 Gy | 20 | 25.00 | 75.00 | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – |
| Sr. No. | Treatment | Mean | % over control | % reduction over control | Sr. No. | Treatment | Mean | % over control | % reduction over control |
|---|---|---|---|---|---|---|---|---|---|
| 27 | Control | 88 | 100.00 | – | 39 | Control | 84 | 100.00 | – |
| 150 Gy | 60 | 68.18 | 31.82 | 150 Gy | 80 | 95.24 | 4.76 | ||
| 300 Gy | 32 | 36.36 | 63.64 | 300 Gy | 16 | 19.05 | 80.95 | ||
| 450 Gy | 44 | 50.00 | 50.00 | 450 Gy | – | – | – | ||
| 28 | Control | 92 | 100.00 | – | 40 | Control | 80 | 100.00 | – |
| 150 Gy | 92 | 100.00 | 0.00 | 150 Gy | – | – | – | ||
| 300 Gy | 44 | 47.83 | 52.17 | 300 Gy | – | – | – | ||
| 450 Gy | 12 | 13.04 | 86.96 | 450 Gy | – | – | – | ||
| 29 | Control | 92 | 100.00 | – | 41 | Control | 80 | 100.00 | – |
| 150 Gy | – | – | – | 150 Gy | – | – | – | ||
| 300 Gy | – | – | – | 300 Gy | – | – | – | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 30 | Control | 92 | 100.00 | – | 42 | Control | 84 | 100.00 | – |
| 150 Gy | 72 | 78.26 | 21.74 | 150 Gy | 68 | 80.95 | 19.05 | ||
| 300 Gy | 36 | 39.13 | 60.87 | 300 Gy | 52 | 61.90 | 38.10 | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 31 | Control | 88 | 100.00 | – | 43 | Control | 88 | 100.00 | – |
| 150 Gy | 80 | 90.91 | 9.09 | 150 Gy | 76 | 86.36 | 13.64 | ||
| 300 Gy | 32 | 36.36 | 63.64 | 300 Gy | 48 | 54.55 | 45.45 | ||
| 450 Gy | – | – | – | 450 Gy | 36 | 40.91 | 59.09 | ||
| 32 | Control | 88 | 100.00 | – | 44 | Control | 96 | 100.00 | – |
| 150 Gy | 68 | 77.27 | 22.73 | 150 Gy | 72 | 75.00 | 25.00 | ||
| 300 Gy | – | – | – | 300 Gy | – | – | – | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 33 | Control | 92 | 100.00 | – | 45 | Control | 80 | 100.00 | – |
| 150 Gy | – | – | – | 150 Gy | 68 | 85.00 | 15.00 | ||
| 300 Gy | – | – | – | 300 Gy | – | – | – | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 34 | Control | 96 | 100.00 | – | 46 | Control | 92 | 100.00 | – |
| 150 Gy | 84 | 87.50 | 12.50 | 150 Gy | 64 | 69.57 | 30.43 | ||
| 300 Gy | 48 | 50.00 | 50.00 | 300 Gy | – | – | – | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 35 | Control | 96 | 100.00 | – | 47 | Control | 100 | 100.00 | – |
| 150 Gy | 56 | 58.33 | 41.67 | 150 Gy | – | – | – | ||
| 300 Gy | – | – | – | 300 Gy | – | – | – | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 36 | Control | 100 | 100.00 | – | 48 | Control | 96 | 100.00 | – |
| 150 Gy | 88 | 88.00 | 12.00 | 150 Gy | 88 | 91.67 | 8.33 | ||
| 300 Gy | 24 | 24.00 | 76.00 | 300 Gy | – | – | – | ||
| 450 Gy | – | – | 450 Gy | 56 | 58.33 | 41.67 | |||
| 37 | Control | 88 | 100.00 | – | 49 | Control | 80 | 100.00 | – |
| 150 Gy | – | – | – | 150 Gy | 76 | 95.00 | 5.00 | ||
| 300 Gy | – | – | – | 300 Gy | – | – | – | ||
| 450 Gy | – | – | – | 450 Gy | – | – | – | ||
| 38 | Control | 100 | 100.00 | – | 50 | Control | 96 | 100.00 | – |
| 150 Gy | 72 | 72.00 | 28.00 | 150 Gy | 68 | 70.83 | 29.17 | ||
| 300 Gy | 64 | 64.00 | 36.00 | 300 Gy | – | – | – | ||
| 450 Gy | 24 | 24.00 | 76.00 | 450 Gy | – | – | – |
3.2. Lethal dose (LD50)
The lethal dose (LD50) is considered as a crucial factor to assess the level of radio-sensitivity in crop plants (Kumar et al., 2013). It quantifies a specific dose or concentration at which 50 percent of the seeds are unable to germinate due to some biochemical and molecular changes. In the present study, estimation of LD50 (Table 2; Fig. 1) in all levels of irradiation revealed that a decrease in 50 % of the seed germination occurred at 300 Gy level in almost all the germinated genotypes except lyp-no-1, Tom-15124 and Tom-15140 (LD50 recorded at 450 Gy). As discussed earlier, mutagenesis also completely inhibited germination in all treatments. Fig. 1 represents the distribution of all genotypes with respect to their germination (%) in each treatment. It indicates the 450 Gy treatment as the most lethal with the highest genotypic inhibition rate followed by 300 Gy. While the treatment having a dose of 150 Gy was observed to be less destructive than the other treatment levels; maximum genetic diversity based on different quantitative traits was observed at that level along with a significant deviation from the normal plants (control). Thus, the dose of 150 Gy is recommended to be utilized for further experiments to generate desirable genetic diversity in tomatoes.
3.3. Radiation-induced genetic variability
Different levels of gamma radiation affect several growth traits of tomato genotypes i.e. plant height, number of flower clusters plant−1, number of flowersplant−1 and fruits yield plant−1. In the case of all 50 tomato genotypes, 450 Gy caused a maximum reduction in plant height (46.71 ± 1.72 cm) followed by 300 Gy (49.13 ± 1.19 cm) (Table 3). The highest plant height was observed in 150 Gy radiation treatment (59 ± 0.54 cm) as compared to control (57.97 ± 0.78 cm) showing the stimulatory effects of low irradiation doses on tomato. A similar trend was observed in other calculated quantitative traits in the study (Table 3). Developing a plant material with an increased number of fruits is an ultimate objective of a plant breeder. Moreover, the plant yield is a quantitative character that can be improved by the manipulation of different component characters.
3.4. Correlation analysis
In the current research, variation in correlation among different yield and yield attributing parameters was observed (Table 4). Correlation studies among different quantitative traits in normal plants (control) showed that plant height is positively correlated on both phenotypic and genotypic basis with number of flower clusters plant−1 (r = 0.55 and 0.43 respectively), number of flowers plant−1 (r = 0.44 and 0.41) and fruits plant−1 (r = 0.46 and 0.40). These associations revealed that increased plant height caused an increase in number of flower clusters and flowers plant−1 which ultimately enhances the fruit yield due to an increased number of fruits plant−1. On the other hand, in the mutated population of different tomato genotypes, the correlation between different quantitative traits had been observed to be altered in comparison with the control from positive to negative direction. This is evidence of the mutagenic effectiveness in breaking the linkage among different traits which are present in normal plants. It resulted in the development of a negative correlation in fruits.plant−1 with plant height (300 Gy and 450 Gy) and with number of flowers plant−1 (450 Gy) due to increased infertility rate in the highest gamma radiation dosage.
3.5. Path coefficient analysis
Path coefficient analysis was conducted (Table 5) to measure the relative contribution of each character in yield. In the control (untreated) population of genotypes, fruits plant−1 was positively and directly affected by number of flowers.plant−1 (0.851) followed by plant height (0.013) and number of flower clusters plant−1 (0.009); these traits also exhibited a positive correlation with yield. Plant height also exhibited a positive indirect effect on number of fruits plant−1 through number of flowers plant−1 (0.007) and number of flower clusters plant−1 (0.006). On the other hand, in mutagenized population, direct and indirect effects were observed to be altered in all levels of irradiation in comparison to control. These deviations might be due to mutagenic interference at the genetic level and linkage breakage. In 150 Gy treatment, fruits plant−1 had a negative direct effect with number of flower clusters plant−1 (−0.012) followed by plant height (−0.020); and a strong positive direct effect with number of flowers plant−1 (0.732). On the other hand, plant height exhibited a negative indirect effect through number of flower clusters plant−1 (−0.015) and number of flowers plant−1 (−0.009). Number of flower clusters plant−1 showed negative indirect effect through plant height (−0.008) and number of flowers plant−1 (−0.018). On the other hand, number of flowers plant−1 had a strong positive indirect effect through plant height (0.764) and number of flower clusters plant−1 (1.025). In 300 Gy treatment, fruits plant−1 was observed to be negatively and directly affected by number of flower clusters plant−1 (−0.002) followed by plant height (−0.005), while positively and directly affected by number of flowers plant−1 (0.905).
Plant height had a positive indirect effect through number of flower clusters plant−1 (0.002) and number of flowers plant−1 (0.003) on fruits plant−1. Number of flower clusters plant−1 showed positive indirect effect through plant height (0.001) and a negative indirect effect through number of flowers plant−1 (−0.002). Number of flowers plant−1 showed a negative indirect effect through plant height (−0.405) and a positive indirect effect through number of flower clusters plant−1 (0.990). In 450 Gy treatment, fruits per plant was positively and directly affected by number of flower clusters plant−1 (2.066); negatively and directly affected by plant height (−0.443) and number of flowers plant−1 (-−1.976). On the other hand, plant height had a negative indirect effect through number of flower clusters plant−1 (−0.097) and number of flowers plant−1 (−0.131) on fruits plant−1. Number of flower clusters plant−1 had a positive indirect effect through plant height (0.655) and number of flowers plant−1 (2.217). Number of flowers plant−1 showed a negative indirect effect through plant height (−0.464) and number of flower clusters plant−1 (−2.121).
4. Discussion
Mutation causes some biological injuries which result in a reduction in seed germination and the development of aberrant phenotypes due to altered genetic makeup. The results of the present study showed a direct relation between the percent (%) germination inhibition rate and the intensity of gamma radiation. Similar results were observed in mungbean by Khan et al. (1998), in lentils by Khan (2002) and in faba beans by Pserveen et al. (2012). On the other hand, Sinha and Godward (1972) observed an increase in the germination of mutagenized lentil seeds at a comparatively low level of mutagen. In the present experiment, the effect of gamma radiation at different levels was observed on 50 different tomato genotypes. In the present study, the germination % was observed to be highly affected at higher doses especially at 450 Gy treatment. These adverse effects might be due to the biochemical changes that occurred in the genes involved in seed germination. Kiong et al. (2008) studied that the tendency of a plant to survive up to maturity depends upon the nature and degree of chromosomal injury due to irradiation dose might be accounted for the decrease in germination, reduced growth of the plant and survival rate. Moreover, Datta (2009) argued that a decrease in germination (%) and complete inhibition of genotypes especially at higher irradiation doses may be due to chromosomal injuries following abnormal mitotic events depicting the radio-sensitivity of plant cells, especially at higher doses.
Plant height along with other growth attributes are generally considered as an index for the assessment of biological damages caused by various physical and chemical mutagenic agents. Wi et al. (2007) argued that a low level of irradiation stimulates growth with an increased rate of hormonal signaling at the cellular level in plants. Similarly, an increase in growth due to low mutagenic dose has been observed in faba beans by Laskar and Khan (2014), in triticale by Trivedi and Dubey (1988) and in lentils by Amin et al. (2015). Contrary to this, a high level of irradiation inhibits the growth rate due to some biological damages that occurred in the cell cycle and the whole genome as well (Preuss et al., 2003).
The mean value of plant height, number of flower clusters plant−1, number of flowers and fruits plant−1 in the mutagenized population varied from the untreated (control) plants. Similar findings were observed by Laskar et al. (2018) in tomato to induce effective mutagenesis. Co-efficient of variation and mean values for the calculated growth attributes indicated that the mutagenesis can change the mean values and generate ample genetic variability to select the best combinations for desirable plant characteristics in tomatoes. Similar results were also observed by Wani et al. (2012) in mungbean, Laskar and Khan (2014) in broad beans, Amin et al. (2015) in lentils and by Djordjevic et al. (2010) and by Ahmad et al. (2011) in tomato.
In general, an overdose of any mutagenic agent possibly causes a maximum reduction in plant growth from germination till the end of its reproductive cycle (Kiong et al., 2008). Hence, the high dose of 450 Gy caused maximum destructive effects on all the genotypes under study especially in the case of germination percentage and no. of fruits developed plant−1. Therefore, the dose of 450 Gy was suggested to be ineffective in inducing desirable genetic diversity, treatment with 300 Gy as LD50 for most of the tested genotypes and 150 Gy treatment-induced favorable mutagenesis for further improvement. Several environmental (non-heritable) factors affect the normal growth of the plant which is clear evidence of a slight reduction in germination in the control/untreated population of genotypes.
The strength of association between two different characters provides the basis of selection, identifying genetic linkage and pleiotropic effects (Sakai and Suzuki, 1964). Studies based on the assessment of association among different agronomic attributes significantly contribute to selecting desirable mutants from the mutagenized population. In addition, it also helps to avoid the selection of combinations having unfavorable linkages. Mutagenic effect on changing, weakening or strengthening correlation among different yield attributing characters was studied in various crop plants (Waghmare and Mehra, 2000, Amin et al., 2015). These variations might be due to the mutagenic effects on altering or weakening the genetic linkage as well as changing the pleiotropic effects of the newly mutated genetic combinations (Amin et al., 2015).
Correlation results in the mutated population clearly showed that the mutagenic agent can break the linkage and can change the strength of association among the studied quantitative characters. Therefore, the development of a high positive correlation in fruits.plant−1 with plant height (150 Gy), no. of flower clusters.plant−1 (300 Gy) and no. of flowers.plant−1 (150 Gy) would facilitate an efficient selection of superior mutants in the successive generations. Association among different studied traits had been strengthened in the mutated genotypic population due to the pleiotropic effects of mutagenesis and might be due to breakdown of linkage as well. Laskar et al. (2018) also observed the effect of mutagen in changing the direction of correlation coefficients among specific combinations might be due to linkage break.
The selection of superior and stable combinations from a mutagenized population is the ultimate objective of mutation breeding. Since plant yield is a very complex quantitative character that depends upon the strength of association among different growth attributes. Association studies based on only correlation analysis may mislead to select the best possible combinations due to its restricted ability of just estimating the association among different variables. While path coefficient analysis measures the relative significance of yield attributing characters by splitting the genotypic correlation into direct and indirect effects.
Path coefficient analysis revealed that characters having high positive direct effects on yield would lead to an effective selection for further improvement in tomato crop. The residual effects described the effectiveness of the independent variable in inducing variability in the dependent variable. In this study, the residual effects of control (0.0003), 150 Gy (0.0059), 300 Gy (0.0182) and 450 Gy (0.324) contribute 99.97 %, 99.41 %, 98.18 % and 67.6 % of variability in fruits/plant respectively. In 450 Gy, a lesser contribution of independent variables to dependent variables was observed which might be due to a non-significant correlation among most of the studied variables.
5. Conclusion
In conclusion, gamma radiation showed effective results in inducing genetic variability with respect to plant height, number of flower clusters, flowers and fruits plant−1. The most effective dose of radiation being 150 Gy generated maximum phenotypic variation. The radio-sensitivity level was optimized by identifying 300 Gy as a lethal dose (LD50) in the studied tomato genotypes. While 450 Gy, showed the most damaging effects with respect to germination % and other traits. Character association studies based on correlation and path coefficient analysis showed that the significant improvement in the association between yield and yield components can be achieved through mutation breeding in tomatoes. Therefore, gamma radiation could be used to generate favorable genetic variability in tomatoes which ultimately enhances the probability of selecting the desirable mutants to improve tomato crop productivity.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Syed Ali Zafar, Email: syedalizafar9@gmail.com.
Ali Tan Kee Zuan, Email: tkz@upm.edu.my.
References
- Adamu A.K., Clung S.S., Abubakar S. Effects of ionizing radiation (gamma-rays) on tomato (Lycopersicon esculentum L.) Nigerian J. Exp. Appl. Bio. 2004;5:185–193. [Google Scholar]
- Ahloowalia B.S., Maluszynski M., Nichterlein K. Global impact of mutation-derived varieties. Euphytica. 2004;135(2):187–204. [Google Scholar]
- Ahmad S., Islam M.S., Hoque M.A. Performances of heat tolerant tomato (Solanum lycopersicum) hybrids during rainy season. Bangladesh J. Agri. Res. 2011;36:189–196. [Google Scholar]
- Amin R., Laskar R.A., Khan S., Moral M.T. Assessment of genetic response and character association for yield and yield components in Lentil (Lens culinaris L.) population developed through chemical mutagenesis. Cogent Food Agri. 2015;1(1):1000715. doi: 10.1080/23311932.2014.1000715. [DOI] [Google Scholar]
- Bergougnoux V. The history of tomato: from domestication to biopharming. Biotech. Adv. 2014;32(1):170–189. doi: 10.1016/j.biotechadv.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Blanca J., Montero-Pau J., Sauvage C., Bauchet G., Illa E., Diez M.J., Francis D., Causse M., Van der Knaap E., Cañizares J. Genomic variation in tomato, from wild ancestors to contemporary breeding accessions. BMC Genom. 2015;16:257. doi: 10.1186/s12864-015-1444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S.K. A report on 36 years of practical work on crop improvement through induced mutagenesis. Induced P. Mutat. Genom. Era. 2009:253–256. [Google Scholar]
- Djordjevic R., Zecevic B., Zdravkovic J., Zivanovic T., Todorovic G. Inheritance of yield components in tomato. Genetika. 2010;42(3):575–583. [Google Scholar]
- FAO/IAEA, Food Agri. Organiz. United Nations; Rome: 2005. Induced plant mutations in the genomics era; pp. 262–265. [Google Scholar]
- Farooq S., Onen H., Tad S., Ozaslan C., Mahmoud S.F., Brestic M., Zivcak M., Skalicky M., El-Shehawi A.M. The influence of environmental factors on seed germination of Polygonum perfoliatum L.: Implications for management. Agronomy. 2021;11(6):1123. [Google Scholar]
- Farooq S., Onen H., Ozaslan C., Baskin C.C., Gunal H. Seed germination niche for common ragweed (Ambrosia artemisiifolia L.) populations naturalized in Turkey. S. Afr. J. Bot. 2019;123:361–371. [Google Scholar]
- Farooq S., Tad S., Onen H., Gunal H., Caldiran U., Ozaslan C. Range expansion potential of two co-occurring invasive vines to marginal habitats in Turkey. Acta Oecol. 2017;84:23–33. [Google Scholar]
- Gupta, S., Schmitt, C., Mahata, K., Shrivastava, A., Sugathan, P., Jhingan, A., ... & Rani, K. (2019). Asymmetric fission around lead: The case of Po 198. Physical Review C, 100(6), 064608.
- Khan, S., 2002. Studies on the differential chemosensitivity in microsperma and macrosperma lentils (Doctoral dissertation, M. Sc. Thesis, Aligarh Muslim University, Aligarh)
- Khan S., Siddiqui B.A., Rehman M.U., Azad S. Response of green gram (Vigna radiate L.) Wilczek to maleic hydrazide. J. Ind. Bot. Soc. 1998;77:95–98. [Google Scholar]
- Kiong A.L.P., Lai A.G., Hussein S., Harun A.R. Physiological responses of Orthosiphon stamineus plantlets to gamma irradiation. American-Eurasian J. Sustain. Agri. 2008;2:135–149. [Google Scholar]
- Konzak C.F., Nilan R.A., Kleinhofs A. Artificial mutagenesis as an aid in overcoming genetic vulnerability of crop plants. Gen. Div. Plants. 1977:163–177. doi: 10.1007/978-1-4684-2886-5_15. [DOI] [PubMed] [Google Scholar]
- Kumar D.P., Chaturvedi A., Sreedhar M., Aparna M., Venu-Babu P., Singhal R.K. Gamma radiosensitivity study on rice (Oryza sativa L.) Asian J. Plant Sci. Res. 2013;3:54–68. [Google Scholar]
- Laskar R.A., Chaudhary C., Khan S., Chandra A. Induction of mutagenized tomato populations for investigation on agronomic traits and mutant phenotyping. J. Saudi Soc. Agri. Sci. 2018;17(1):51–60. [Google Scholar]
- Laskar R.A., Khan S. Mutagenic effects of MH and MMS on induction of variability in broad bean (Vicia faba L.) Ann. Res. Rev. Bio. 2014:1129–1140. [Google Scholar]
- Matsukura C., Yamaguchi I., Inamura M., Ban Y., Kobayashi Y., Yin Y.-G., Saito T., Kuwata C., Imanishi S., Nishimura S. Generation of gamma irradiation-induced mutant lines of the miniature tomato (Solanum lycopersicum L.) cultivar ‘Micro-Tom’. Plant Biotech. 2007;24(1):39–44. [Google Scholar]
- Mehandjiev ATANAS, Kosturkova GEORGINA, Mihov MIHO. Enrichment of Pisum sativum gene resources through combined use of physical and chemical mutagens. Israel J. Pl. Sci. 2001;49(4):280–284. [Google Scholar]
- Onen H., Farooq S., Gunal H., Ozaslan C., Erdem H. Higher tolerance to abiotic stresses and soil types may accelerate common ragweed (Ambrosia artemisiifolia) invasion. Weed Sci. 2017;65(1):115–127. [Google Scholar]
- Ozaslan C., Farooq S., Onen H., Bukun B., Ozcan S., Gunal H., Huzurbazar S. Invasion potential of two tropical Physalis species in arid and semi-arid climates: effect of water-salinity stress and soil types on growth and fecundity. PLoS ONE. 2016;11(10):e0164369. doi: 10.1371/journal.pone.0164369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paran I., Fallik E. Breeding for fruit quality in pepper (Capsicum spp.) Breed. Fruit Qual. 2011:307–322. [Google Scholar]
- Peterson D.G., Stack S.M., Price H.J., Johnston J.S. DNA content of heterochromatin and euchromatin in tomato (Lycopersicon esculentum) pachytene chromosomes. Genome. 1996;39(1):77–82. doi: 10.1139/g96-011. [DOI] [PubMed] [Google Scholar]
- Preuss, S. B., & Britt, A. B. (2003). A DNA-damage-induced cell cycle checkpoint in Arabidopsis. Genetics, 164(1), 323-334. [DOI] [PMC free article] [PubMed]
- Sakai K.-I., Suzuki A. Induced mutation and pleiotropy of genes responsible for quantitative characters in rice. Rad. Bot. 1964;4(2):141–151. [Google Scholar]
- Shah SA, Ali I, Rahman K (2001) ‘Abasin-95’, a new oilseed rape cultivar developed through induced mutations. Mutat Breed News Lett 45:3–4
- Siddiqui M.W., Ayala-Zavala J.F., Dhua R.S. Genotypic variation in tomatoes affecting processing and antioxidant attributes. Critical Rev. Food Sci. Nutrition. 2015;55(13):1819–1835. doi: 10.1080/10408398.2012.710278. [DOI] [PubMed] [Google Scholar]
- Sinha S.S.N., Godward M.B.E. Ranchi Univ; India: 1972. Radiation studies in Lens culinaris. [Google Scholar]
- Trivedi S.C., Dubey D.K. In Rahun. Abstract in Proceeding of the 69th Indian ScienceCongress Part. 1988. Effect of gamma rays on seed germination and seedling growth in Triticale var; pp. 111–240. [Google Scholar]
- Waghmare V.N., Mehra R.B. Induced genetic variability for quantitative characters in grasspea (Lathyrus sativus L.) Indian J. Genet. 2000;60:81–87. [Google Scholar]
- Wani M.R., Khan S., Kozgar M.I. Genetic enhancement of mungbean (Vigna radiata L.) through induced mutagenesis. Crop Res. 2012;43:189–193. [Google Scholar]
- Weisburger J.H. Lycopene and tomato products in health promotion. Exp. Biol. Med. 2002;227(10):924–927. doi: 10.1177/153537020222701014. [DOI] [PubMed] [Google Scholar]
- Wi S.G., Chung B.Y., Kim J.-S., Kim J.-H., Baek M.-H., Lee J.-W., Kim Y.S. Effects of gamma irradiation on morphological changes and biological responses in plants. Micron. 2007;38(6):553–564. doi: 10.1016/j.micron.2006.11.002. [DOI] [PubMed] [Google Scholar]
Further Reading
- Gupta S., Schmitt C., Mahata K., Shrivastava A., Sugathan P., Jhingan A., Rani K. Asymmetric fission around lead: The case of Po 198. Phys. Rev. 2019;100 [Google Scholar]
- Khan S., Wani M.R. Isolation of high yielding mutants in mungbean (Vigna radiata L.) Trop. Agri. 2004;154:51–59. [Google Scholar]
- Perveen R., Alka K.S. Alkylating agent ethyl methane sulphonate (EMS) induced variability in two economically important mutants of Vicia faba L. Int. J. Pharm. Biosci. 2012;3:750–760. [Google Scholar]
- Preuss S.B., Britt A.B. A DNA-damage-induced cell cycle checkpoint in Arabidopsis. Genetics. 2003;164(1):323–334. doi: 10.1093/genetics/164.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scossiroli, R.E., 1966. Investigations on mutability of polygenes and on utilization of induced genetic variability. Investigations on mutability of polygenes and on utilization of induced genetic variability.