Abstract
Perilla frutescens, perilla is a functional food, spice and medicinal herb and ornamental plant in the family of Lamiaceae. Thus, macro-morphological characteristics, phenolic acids, antioxidants of twelve accessions of P. frutescens grown under open field were studied. High polymorphism was found among the perilla accessions and macroscopic features of perilla genotypes showed variable results. Perilla can be classified into two clearly phenotypes green and purple, within these two other colours were appeared. A good level of biomass production was recorded for JTD3, 203P, PS2, 203P respectively. Principal component analysis was performed to cluster phenolic acids. GB phenotype exhibited the major content of polyphenols, followed by JTD3 then J1. Regarding antioxidant capacity, JTD3 showed the highest value followed by 203P and GB respectively. The HPLC analysis showed that the most abundant phenolic acids were ellagic acid which is accumulated in a higher percentage in NP606, 588P and JTD3 cultivars respectively, followed by salicylic acid and gallic acid. This is the first report of cultivation of various Perilla varieties under open field environmental conditions, not only to increase productivity but also to improve the quality. Therefore, the present study results confirm the importance of the Perilla species for human consumption, therapeutic and ornamental purposes.
Keywords: Perilla, Phytochemistry, Functional food, Spice, Morphology
1. Introduction
Perilla frutescens is a functional food, spice and medicinal herb as well as ornamental plant in the family of Lamiaceae. It is a significant therapeutic plant that is generally appropriated in China, India, Korea, Japan, Thailand, Taiwan and Southeast Asia (Ahmed, 2019, Ahmed and Al-Zubaidy, 2020, Gaihre et al., 2021). It is generally called perilla or by different names (perilla mint, Chines basil, Korean perilla, beefsteak plant, purple mint, zisu in China, shiso in Japan, and tia to in Vietnam) and one of the important economic herbs that have been cultivated for more than 2000 years (Asif, 2011, Ahmed, 2019). The main types of perilla are red (purple), green and red/green perilla phenotypes with the important aerial parts used and huge variations in the morphology and taxonomy (Banno et al., 2004, Ahmed, 2019). Perilla also has been considered to be both a nectariferous and a polliniferous plant (Consonni et al., 2013). In China, P. frutescens leaves have been broadly utilized as a culinary spice and flavor and food specialist because of the fragrant taste. Its new leaves are usually utilized for preparing pickles or as an enhancement for crude fish dishes in Japan. It is a mainstream verdant vegetable in Korea, which is by and largely eaten as a pickle or wrapping with broiled meats. The seeds are powdered and added to soup for flavoring in Korea (Huang et al., 2011, Li et al., 2017). The herb has been traditionally used in folk medicine for the treatment of colds, cough, asthma, vomiting, stomach disorders, depression, intoxications with seafood and allergic reactions (Fujiwara et al., 2018, Ahmed and Tavaszi-Sarosi, 2019). The bioactivity of perilla is widely studied and found that it has antioxidant, antimicrobial, antidepressive, anxiolytic, chemopreventive and antitumor, anti-inflammatory activities (Banno et al., 2004, Osakabe et al., 2004, Park et al., 2010), anti-allergic effects (Yang et al., 2021), keratinocyte ageing prevention (Lee and Park, 2021), hyperpigmentation diseases of skin and ageing (Mungmai et al., 2020). A recent study, for the first time, indicates that perilla leaf extracts (PLE) are able to limit the replication of SARS-CoV-2 by disabling the virion. These results could motivate further research into the therapeutic utility of PLE for COVID-19 prevention or treatment (Tang et al., 2021). Numerous studies reported the bioactive metabolites of perilla including phenolic acids, flavonoids, carotenoids, essential oils, fatty acids, triterpenes, phytosterols, tocopherols and policosanols (Meng et al., 2006, Zhou et al., 2014, Ahmed, 2019). The antioxidant content is increased by insect pollination, overall by honey bees, highlighting the important role of this pollination in the quality and nutraceutical value of this medicinal plant (Ferrazzi et al., 2017). Essential oils of perilla leaves are an important resource of natural medicinal products and recorded among commonly safe food flavorings for use in beverages, puddings, frozen dairy products, baked goods and processed vegetables and soups, and they may be mostly liable for the smell and taste of perilla (Chen et al., 2020). Rosmarinic acid is found in many Lamiaceae plant families including perilla, rosemary, sage, basil and mint and plays an anticarcinogenic role by two effects, anti-inflammatory and antioxidative activity (Osakabe et al., 2004).
Many medicinal plants were cultivated under different controlled environmental conditions not only to increase productivity but also to improve the quality. Therefore, in the present study, we examined the macroscopic characteristics, morphology, total polyphenols and phenolic acids, the antioxidant activity of twelve P. frutescens L. Britt accessions grown under open field.
2. Materials and methods
2.1. Reagents
Sodium sulphate (analytical grade), acetic acid (100%), ascorbic acid (C6H8O6), sodium acetate (0.05 M), sodium carbonate (99.5%), Folin-Ciocalteu phenol reagent, (≥95%), 2,4,6-tripyridyl-s-triazine (TPTZ) solution (≥98%), hydrochloric acid (37%), gallic acid (97.5–102.5%), iron-chloride (97%), methanol (99.5%), ethyl acetate (99.5%) were bought from Merck (Darmstadt, Germany). Mueller-Hinton Broth (MHB) and Mueller-Hinton Agar (MHA) were purchased from Difco, Becton Dickinson, Sparks, MD, USA.
2.2. Plant materials and growth conditions
The seeds of Perilla frutescens (L.) Britt varieties were obtained from the gene bank of the Department of Medicinal Plants, Budapest. Seeds were sown in greenhouse in 30/March/2017, using plant seedling tray then the seedlings with two–three leaves were transplanted directly to another tray on 10/May/2017, containing 30 seedlings for each accession named (PS1, PS2, PS3, 203P, 465P, 588P, J1, JTD3, NP-606, RauTiaTo, MP3 and GB) respectively. On 29/5/2017 we transferred the plantlets to the experimental research field plots in sorksor coordinates: 47.398820, 19.149270. The experimental design was a completely randomized block design with six replications for each treatment. The experimental plot area for each accession was 5 m2 consisting of six rows and five lines. The distance between rows was 50 cm and each row contained five plants. The total number of plants in every plot was 30 plants and separated each other by a walking path of 0.5 m width. Plants were irrigated regularly using a sprinkler irrigation system and mechanical weed control was done periodically to maintain vigorous growth. No fertilizer neither organic nor non-organic were applied. Detailed characteristics of the soil are displayed in Table 1. The plant was cultivated in an organic manner no pesticides, fertilizers, genetically modified organisms, antibiotics, or growth hormones have been used.
Table 1.
Physico-chemical properties of open field soil.
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Salt % | 0.039 | Ca% | 0.489 |
| pH | 6.49 | Mg mg kg–1 | 53 |
| Humus% | 1.17 | Fe mg kg–1 | 109 |
| NO3-N mg kg–1 | 1.24 | Mn mg kg–1 | 37.8 |
| P2O5 mg kg–1 | 291 | Zn mg kg–1 | 1.73 |
| K2O mg kg–1 | 36.7 | Cu mg kg–1 | 3.47 |
| CaCO3% | 1 |
2.3. Morphological and macroscopic characteristics
On 15/08/2017 we randomly selected five plants per accession in each plot and took measurements and the harvest processes were conducted by hand in the early morning. We measured plant height on the surface of the soil, a diameter of the plant in the third top of the plant. We noted the appearance of the plant and recorded their characteristics including colour of leaf surface, colour of the reverse side of leaf and colour of the stem (green, bright green, weak green, purple, dark purple, bright purple, weak purple), leaf shape (non-wrinkle, wrinkle, heavily wrinkle), leaf venation (pinnate, palmate, reticulate, dichotomous, parallel), leaf arrangement (alternate, opposite, subopposite, whorled), leaf margin (laciniate, serrate, crisped), degree of leaf pubescence and degree of stem pubescence (lightly pubescent, pubescent, heavily pubescent, more heavily pubescent). Fresh weight and stem weight have been recorded immediately after harvesting the plant before the flowering phase. Plants were dried in the shade at room temperature, and dry weight (drug) was recorded again by an analytical scale. Each measurement was performed in six replications. The voucher specimens of dried perilla herb (No. p-2) were deposited at the herbarium of the research centre. The air-dried plant material was kept in bags at room temperature until laboratory analysis.
2.4. Preparation of extracts
The dried leaves were separated from the stems and ground by means of an electric blender into a fine powder and sifted by using a stainless-steel sieve (500 μm hole size). 50 mL of boiling distilled water was added into 0.5 g of the powder from samples of each accession. The plant test solution was shaken and stored at room temperature for 24 h. The extracts were filtered using filter paper and stored in the refrigerator (4 °C) until the measurements were performed. The preparations were carried out in 6 replications.
2.5. Determination of total polyphenolic content
The total polyphenol content (TPC) of Perilla frutescens accessions was determined using the Folin-Ciocalteu method modified from Ahmed and Sarosi (2019). A stock solution of gallic acid was prepared by dissolving 5.1 mg in 10 mL of distilled water in a 100 mL volumetric flask. 7% Sodium carbonate (Na2CO3) solution was made up by dissolving 7.4 g of sodium carbonate anhydrous in 100 mL distilled water. A serial dilution of the gallic acid stock solution, containing 50 µl, 100 µl, 150 µl, 200 µl, 250 µl was prepared as a standard reference. A blank was also prepared. A portion of 0.5 mL from leaf water extracts was added to a test tube containing 2.5 mL of Folin-Ciocalteu reagent (10% v/v) and shaken well. After 1 mint of incubation, 2 mL of sodium carbonate solution (0.7 M) was added and mixed again. Test tubes were placed into hot water (temperature 50 °C) for 5 min until the blue coloration appeared then the absorbance was recorded at 760 nm by using a Uv–visible spectrophotometer (Thermo Scientific™ Evolution 201/220). Quantification was done with respect to the standard curve of gallic acid and TPC content of samples was expressed as milligram gallic acid equivalent per gram of sample dry matter (mg GAE/g DM).
2.6. Antioxidant iron reduction assay (FRAP)
The ferric reducing antioxidant power (FRAP) assay was performed to determine the antioxidant capacity (AOC) of the perilla accessions according to the described method (Ahmed and Sarosi, 2019) with slight modifications. FRAP reagent solution was prepared freshly by mixing 50 mL acetate-puffer [(3.1) g sodium-acetate was dissolved in16 mL acetic acid, filled with 11 mL distilled water (DW) to make up acetate-puffer] + 5 mL TPTZ solution [2,4,6-tripiridil-S-triazine (TPTZ) solution was prepared by dissolving 0.03123 g TPTZ in 10 mL DW, then 33.6 µl HCl was added to the final solution] + 5 mL iron-chloride solution [0.054 g iron-chloride (FeCl3) dissolved in 10 mL DW] in Florence flask. Serial dilutions of the ascorbic acid solution [prepared by dissolving 0.017613 mg in 10 mL DW, then 100 µl from this was diluted in 900 µl of DW], containing 10 µl, 20 µl, 30 µl, 40 µl, 50 µl was prepared as a standard reference for the calibration curve. Aliquots of 10 µl from test extracts of accessions were added to a test tube containing 1.5 mL FRAP solution plus 40 µl DW. A blank was also prepared with distilled water. Absorbance was read at 596 nm after one minute by using a Thermo Evolution 201 spectrophotometer. The final values were expressed in milligram ascorbic acid equivalent (AAE) per gram of sample dry matter (mg AAE/g DM). Measurements for each sample were carried out in six replications.
2.6.1. Extraction and HPLC analysis
The extraction method utilized for dried examples had as follows: 50 mL of%70 methyl alcohol (Methanol) was added to 4 g of dried sample and left at room temperature for 48 hrs. The filtration has been done using Ederol filter paper (medium pore filtering). The extract was concentrated to satisfactory volume to dispose of alcohol by utilizing an air conditioner. At that point as much as the volume of Petroleum Ether (50–60 boiling point) was added to the item, blended and shacked delicately, set in isolating channel, and left for a while to isolated unmistakably into two-layer. Thereby the major part of chlorophyll was dissolved in the petroleum ether. The upper aqueous was removed, and the bottom clear aqueous (extracts of phenolic compounds) were left then transferred to clean vials and injected into HPLC apparatus.
2.6.2. High performance liquid chromatography (HPLC) analysis
All standards were prepared as stock solutions in methanol. Working standards were made by diluting stock solutions in 62.5% aqueous methanol to yield concentrations ranging between 0.5 and 25 mg/L. The standards of phenolic compounds were Bisphenol, 2,4-Diaminophenol, Ellagic acid, Gallic acid, Salicylic acid, Tannic acid. Stock working solutions of the standards were stored in darkness at −18 °C. The analytical of high-performance liquid chromatography apparatus system was employed. The separation was achieved on the Analytical column: Eurospher 100, C18, 5 µm, 250 × 4.6 mm at ambient temperature. The mobile phase was acetonitrile + acetic acid 1% (40:60 v/v). The flow rate was 1 mL/min and the injection volume was 20 μl. The monitoring wavelength was 280 nm. The identification of each compound was based on a combination of retention time and spectral matching.
2.7. Statistical analysis
The data were analysed using the IBM SPSS Statistics 22 program. The results are reported as mean ± standard deviation (SD) of six replications and one-way analysis of variance (ANOVA) was employed for data comparison, following Tukey’s honestly significant difference (HSD) test. Significant differences (p < 0.05) within rows were represented by different superscript letters. The correlation between the studied variables (antioxidant activity and total polyphenol content in the samples was also determined by a two-tailed Pearson correlation analysis. Principal component analysis (PCA) and the heatmap analysis were performed using Minitab Software V19 (Minitab LLC.) and https://biit.cs.ut.ee/clustvis/.
Caulis Perillae“ and Perilla seed ”Fructus Perillae“.
3. Results
3.1. Macroscopic characteristics and drug yields
The characterization of medical herbs and consumed plants is significant to provide clear data concerning the quality and the intake, by consumers, of pharmaceutically active substances produced by specific cells of those plants. The plant has shown different variety after grown as appeared in Fig. 1, Fig. 2. Perilla can be classified into two clearly phenotypes, green and purple, within these two other colours were appeared. Colour of leaf surface of these accessions displayed bright green such as PS2, 203P, 465P, J1 and NP606 and the only one genotype PS1 showed dark green. On the other hand, the rest taxa appeared to be weak, bright and dark purple. In addition to that, the reverse side of the mentioned leaf colour for the accessions displayed a slightly weak green (PS1, PS2, 203P, 465P, J1, NP606), the other species were bright, weak and dark purple. Regarding the leaf shape of the studied taxa, seven species were grown as non-wrinkle and four as wrinkle taxa. The type of leaf venation of all accessions was reticulate except PS1 and 465P which were pinnate. Leaves were arranged in opposite forms and most of the taxa leaf margin was serrate, two of them PS1 and PS2 were laciniate, while both J1 and NP606 were palmate, crisped respectively. With regard to the colour of stem appears to be purple for all species except two accessions with green stem colour 465P and PS1. Leaves and stem of all the accessions had trichomes but with varying degrees starting from lightly to heavily.
Fig. 1.
Three important parts of Perilla; Perilla leaf “Folium Perillae”, Perilla stalk.
Fig. 2.
Field view of different genotypes of Perilla frutescens grown under open field condition photo (Hiwa M. Ahmed).
The morphological features of Perilla frutescens L. Britt genotypes showed variable results as it is shown in (Fig. 1, Fig. 2 and Table 2). JTD3 recorded the highest length of the plant (70.0 ± 4.47 cm) followed by PS1 (68.20 ± 7.68 cm), and MP3 (67 ± 6.36 cm). Both PS2 and 588P accessions were kept the same level of plant height. On the other hand, NP606 (53.80 ± 7.14 cm) and GB (55 ± 3.35 cm) reached the lowest length of the perilla herb. These accessions showed the widest diameter JTD3 (55.60 ± 4.45 cm), PS2 (52.60 ± 10.05 cm), 203P (52.20 ± 9.70 cm), RauTiato (52.20 ± 4.49 cm), while 465P showed the lowest diameter (41.80 ± 6.05 cm). PS2, 203P and JTD3 produced the maximum fresh yield respectively (312.20 ± 134.68), (296.80 ± 108.34) and (262.80 ± 66.34) g/plant respectively, while others produced less fresh weight such as GB (133.40 g/plant), NP606 (174 g/plant) and PS3 (184.60 g/plant). Regarding the proportion of useful drug, we obtained the height dried yield drug for these phenotypes 203P (83 ± 29.11 g/plant), PS2 (77.60 ± 38.34 g/plant) and PS1 (69.80 ± 31.68 g/plant) whereas PS3 (43.80 ± 18.12 g/plant), GB (32 ± 7.77 g/plant) and NP606 (50 ± 16.59 g/plant) obtained the minimum amount of the herbal drug.
Table 2.
Macroscopic characteristics of perilla accessions after grown in open field condition.
| No | Accessions | Colour of leaf surface {green, bright green, weak green, purple, dark purple, bright purple, weak purple} | Colour of reverse side of leaf {green, bright green, weak green, purple, dark purple, bright purple, weak purple} | Leaf shape (non-wrinkle, wrinkle, heavily wrinkle) | Leaf venation (pinnate, palmate, reticulate, dichotomous, parallel) | Leaf arrangement (alternate, opposite, Subopposite, whorled) | Leaf margin | Colour of stem {green, bright green, weak green, purple, dark purple, bright purple, weak purple} | Degree of leaf pubescence (lightly pubescent, pubescent, heavily pubescent, more heavily pubescent) | Degree of stem pubescence, (lightly pubescent, pubescent, heavily pubescent, more heavily pubescent |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PS1 | Dark green | Weak green | Non-wrinkle | Pinnate | Opposite | Laciniate | Weak green | More heavily pubescent | Heavily pubescent |
| 2 | PS2 | Bright green | Weak green | Wrinkle | Reticulate | Opposite | Laciniate | Purple | Pubescent | Heavily pubescent |
| 3 | PS3 | Dark purple | Bright purple | Non-wrinkle | Reticulate | Opposite | Serrate | Dark purple | Lightly pubescent | Pubescent |
| 4 | 203P | Bright green | Weak green | Wrinkle | Reticulate | Opposite | Serrate | Purple | Pubescent | More heavily pubescent |
| 5 | 465P | Bright green | Slight green | Non-wrinkle | Pinnate | Opposite | Serrate | Bright green | Heavily pubescent | More heavily pubescent |
| 6 | 588P | Weak purple | Bright purple | Non-wrinkle | Reticulate | Opposite | Serrate | Purple | Pubescent | Pubescent |
| 7 | J1 | Bright green | Weak green | Wrinkle | Reticulate | Opposite | Palmate | Weak Purple | Pubescent | More heavily pubescent |
| 8 | JTD3 | Dark purple | Weak purple | Heavily wrinkle | Reticulate | Opposite | Serrate | Dark purple | Pubescent | More heavily pubescent |
| 9 | NP606 | Bright green | Weak green | Non-wrinkle | Reticulate | Opposite | Crisped | Dark purple | Pubescent | Lightly pubescent |
| 10 | RauTiaTo | Bright purple | Bright purple | Non-wrinkle | Reticulate | Opposite | Serrate | Dark purple | Lightly pubescent | More heavily pubescent |
| 11 | MP3 | Bright purple | Weak purple | Non-wrinkle | Reticulate | Opposite | Serrate | Dark purple | Lightly pubescent | Heavily pubescent |
| 12 | GB | Dark purple | Dark purple | Wrinkle | Reticulate | Opposite | Serrate | Bright purple | Lightly pubescent | Lightly pubescent |
3.2. Polyphenolic content and antioxidant capacity
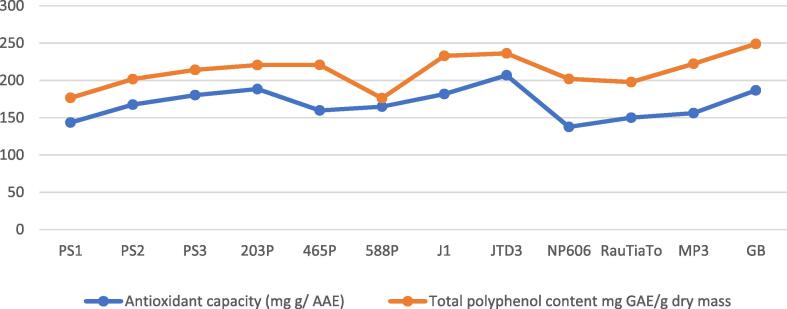
The antioxidant capacity is a common method used to evaluate many medicinal and aromatic plants, foods, crops, fruits, edible plants, vegetables to show their scavenging activities against radical sources concerning phenolic acids to benefit human health. Because of these, we studied the antioxidant capacity of twelve accessions of perilla leaves as well as total polyphenolic content which are considered as drugs and nutraceuticals among many Asian and other communities. The quantitative analytical results are summarized in Table 3. GB (Green/Purple colour) phenotype exhibited the major content of polyphenols with significant difference (p < 0.05) according to Tukey HSD (248.93 ± 13.25 mg GAE/g dry mass), followed by JTD3 (Purple colour) variety which accumulated the second highest content of polyphenols (236.22 ± 3.28 mg GAE/g dry mass) then J1 (Green colour) (232.83 ± 20.55 mg GAE/g dry mass). There is no difference for both (Green colour) accessions 203P and 465P in terms of secondary metabolite accumulation including phenolic compounds and the results of both analyses showed a similar pattern. In contrast, PS1 (Green colour) and 588P (Purple/Green colour) RauTiaTo (Purple colour) were produced of phytochemicals (phenolics) at low content compared to other species.
Table 3.
The phytochemical analysis and morphological features of perilla genotypes grown under open field condition.
| No. | Accessions name | Phenotypes colour | Statistics | Antioxidant capacity (mg AAE/g DM) | Total polyphenol content (mg GAE/g DM). | Height (Cm) | Diameter (Cm) | Stem weight (g/plant) | Leaf weight (g/plant) | Fresh Weight (g/plant) | Dry Weight (g/plant) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PS1 | Green | Mean | 143.50b | 176.54a | 68.20b | 47.20b | 29.60b | 40.20b | 240.60b | 69.80b |
| Std. Deviation | 5.68 | 9.69 | 7.68 | 5.49 | 14.40 | 17.35 | 104.99 | 31.68 | |||
| 2 | PS2 | Green | Mean | 167.52d | 201.74c | 65.40b | 52.60b | 36.60b | 41.00b | 313.20b | 77.60b |
| Std. Deviation | 6.93 | 7.59 | 10.89 | 10.05 | 19.18 | 19.22 | 134.68 | 38.34 | |||
| 3 | PS3 | Purple | Mean | 180.24e | 214.07c | 59.80b | 47.80b | 20.80b | 23.00b | 184.60b | 43.80b |
| Std. Deviation | 2.94 | 5.38 | 7.60 | 4.92 | 10.68 | 7.72 | 85.25 | 18.12 | |||
| 4 | 203P | Green | Mean | 188.36e | 220.56c | 63.00b | 52.20b | 38.20b | 44.80b | 296.80b | 83.00b |
| Std. Deviation | 17.61 | 21.97 | 8.56 | 9.70 | 14.30 | 14.89 | 108.34 | 29.11 | |||
| 5 | 465P | Green | Mean | 159.66c | 220.82c | 58.20b | 41.80a | 24.40b | 29.80b | 236.40b | 54.20b |
| Std. Deviation | 3.38 | 6.28 | 9.74 | 6.05 | 16.85 | 18.88 | 146.24 | 35.54 | |||
| 6 | 588P | Purple/Green | Mean | 164.79d | 176.13a | 65.40b | 49.80b | 25.80b | 30.20b | 210.00b | 56.00b |
| Std. Deviation | 4.14 | 2.16 | 7.71 | 5.53 | 8.59 | 7.93 | 62.65 | 14.41 | |||
| 7 | J1 | Green | Mean | 181.72e | 232.83e | 55.20a | 45.60b | 27.40b | 35.00b | 229.40b | 62.40b |
| Std. Deviation | 12.49 | 20.55 | 4.17 | 3.32 | 13.44 | 10.37 | 96.72 | 23.52 | |||
| 8 | JTD3 | Purple | Mean | 206.83f | 236.22e | 70.00b | 55.60b | 33.00b | 34.20b | 262.80b | 67.20b |
| Std. Deviation | 2.00 | 3.28 | 4.47 | 4.45 | 9.76 | 8.23 | 66.34 | 17.96 | |||
| 9 | NP606 | Green/Purple | Mean | 137.61a | 201.88c | 53.80a | 45.20b | 20.80b | 29.20b | 174.00b | 50.00b |
| Std. Deviation | 7.18 | 17.01 | 7.14 | 4.17 | 7.00 | 9.93 | 54.45 | 16.59 | |||
| 10 | RauTiaTo | Purple | Mean | 149.98c | 197.72b | 59.40b | 52.20b | 24.00b | 30.60b | 194.40b | 54.60b |
| Std. Deviation | 6.78 | 12.55 | 6.62 | 4.49 | 12.43 | 12.45 | 82.83 | 24.77 | |||
| 11 | MP3 | Green/Purple | Mean | 156.09c | 222.25c | 67.00b | 50.20b | 20.60b | 30.00b | 188.00b | 50.60b |
| Std. Deviation | 9.07 | 7.34 | 6.36 | 6.18 | 5.99 | 7.67 | 43.02 | 12.35 | |||
| 12 | GB | Green/Purple | Mean | 186.63e | 248.93e | 55.00a | 47.40b | 13.20a | 18.80a | 133.40a | 32.00a |
| Std. Deviation | 14.46 | 13.25 | 3.35 | 5.08 | 3.66 | 5.34 | 32.52 | 7.77 |
a-f Values with different superscript letters in the same column are significantly different (P < 0.05) according to Tukey HSD.
The antioxidant capacity of perilla accessions exhibited significant differences ranged from (137.61 to 206.83 mg AAE/g DM). JTD3 (Purple colour) showed the highest value (206.83 ± 2 mg AAE/g DM) and statistically significant than all of them (P < 0.05), following 203P (Green colour) and GB (Green/Purple colour) (188.36 ± 17.61 and 186.63 ± 14.46 mg AAE/g DM) respectively. Both taxa J1 (Green colour) and PS3 (Purple) showed almost the same amount (181.72 ± 12.49 and 180.24 ± 2.94 mg AAE/g DM). On the other hand, the mixed phenotype (Green/Purple colour) NP606 showed the lowest antioxidant capacity among all the studied genotypes (137.61 ± 7.18 mg AAE/g DM) following the (Green colour) accession PS1 and (Purple colour) accession RauTiaTo (143.50 ± 5.68 and 149.98 ± 6.78 mg AAE/g DM) individually. Fig. 3 shows the relationship between antioxidant capacity and total polyphenol content of perilla phenotypes.
Fig. 3.
The relationship between antioxidant capacity and total polyphenol content of perilla phenotypes.
3.3. Determination of polyphenolic compounds by HPLC
In this investigation, the concentration of individual components in various perilla accessions was quantified. HPLC chromatogram of different P. frutescens species was performed and found polyphenolic compounds such as (bisphenol, 2,4-Diaminophenol, ellagic acid, gallic acid, salicylic acid, tannic acid) in different percentages as shown in (Table 4). The results showed that the most abundant phenolic acids were ellagic acid which is accumulated in a higher percentage (4.31, 3.8, 3.7 %) in NP606, 588P and JTD3 species respectively. The second most higher phenolic substance was salicylic acid which is accumulated in the perilla cultivars GB, PS3 and NP606 ranged from (3.17, 3.12, 2.97 %) respectively. Two perilla cultivars NP606 and GB seem to had the higher percentage of gallic acid content. In addition, 203P and GB accumulated nearly equal content of tannic acid (1.02%). On the other hand, the level of biphenol and 2,4-Diaminophenol content in all studied perilla taxa was not reached one percentage.
Table 4.
The percentage composition of phenolic acids extracted from Perilla species grown under open field condition using HPLC.
| NO |
Samples |
Bisphenol |
2,4-Diaminophenol |
Ellagic acid |
Gallic acid |
Salicylic acid |
Tannic acid |
|---|---|---|---|---|---|---|---|
| R/t | 8.73 | 8.59 | 3.7 | 1.8 | 3.55 | 4.7 | |
| 1 | PS2 | 0.4 | 0.4 | 0.4 | 0.88 | 2.17 | 0.76 |
| 2 | PS3 | 0.2 | 0.2 | 0.9 | 0.88 | 3.12 | 0.77 |
| 3 | 203P | 0.15 | 0.18 | 1.07 | 0.34 | 2.91 | 1.02 |
| 4 | 465P | 0.4 | 0.42 | 1.23 | 0 | 4.3 | 0.21 |
| 5 | 588P | 0.3 | 0.28 | 3.8 | 2.6 | 2.61 | 0.21 |
| 6 | JTD3 | 0.17 | 0.14 | 3.7 | 0.54 | 2.9 | 0.52 |
| 7 | NP606 | 0.12 | 0.15 | 4.31 | 2.18 | 2.97 | 0.77 |
| 8 | RauTiaTo | 0.16 | 0.19 | 3.4 | 2.37 | 2.11 | 0.83 |
| 9 | MP3 | 0.09 | 0.102 | 1.22 | 0.66 | 2.13 | 0.41 |
| 10 | GB | 0.3 | 0.14 | 3.6 | 2.12 | 3.17 | 1.04 |
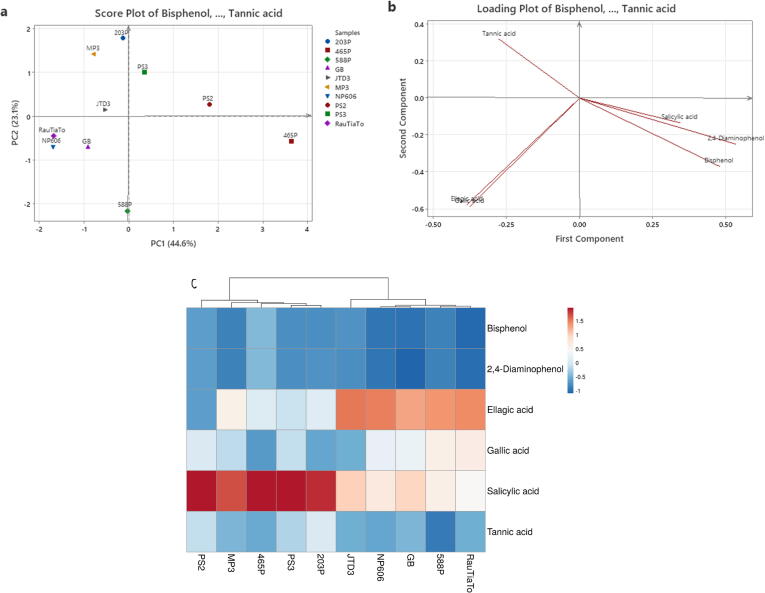
Phenolic acids of perilla genotypes were subjected to principal component analysis (PCA). According to the loading plot obtained by principal component analysis (PCA), a total of 67.7% variance was revealed among original data (Fig. 4a). The first (PCA1) exhibited 44.6% and the second (PCA2) indicated 23.1% variance across perilla phenolic acids. In (Fig. 4a) score plot, phenolic acids are seen significantly distributed with three prominent groupings which are gallic acid and ellagic acid, salicylic acid, and other components. In this (Fig. 4b) loading plot perilla genotypes have large positive loadings on PC1. According to the heatmap analysis (Fig. 4c), the perilla genotypes were clustered into three main groups. Group one (RauTiaTo, 588P, NP606, GB and JTD3) cultivars contained a higher percentage of gallic acid and ellagic acid. Group two (PS2, PS3, 203P and 465p) were rich in salicylic acid and group three contained other phenolic components.
Fig. 4.
Principal component analysis (PCA) Score plot (PC1 × PC2) (a), Loading plot (b) and Heatmap (c) for visualization of ten perilla genotypes (PS2, PS3, 203P, 465P, 588P, JTD3, NP606, RauTiaTo, MP3, GB) based on phenolic compositions (Bisphenol, 2,4-Diaminophenol, Ellagic acid, Gallic acid, Salicylic acid, Tannic acid) by HPLC.
4. Discussion
4.1. Macroscopic characteristics and drug yields
There is not much research addressed to compare the current phenotypes and genotypes of perilla thus in our study, we can obviously classify those accessions into two phenotypes green and purple (red) colour. This was also noted by our previous study carried out on cultivated some varieties under controlled condition Phyto-chamber (Ahmed and Sarosi, 2019). To classify perilla, morphological features are often used as an indicator including e.g. size, shape, and color of the leaves and seeds; the degree of leaf serration; flower color (Hu et al., 2010). There is no doubt, geographic conditions play a major role in determining some of these diverse features (Lee and Ohnishi, 2001). The leaf morphology of perilla seems to be the best taxonomic characteristic to differentiate among cultivars, the morphology of the perilla leaves offered distinct characteristics for each cultivar. In the current results, five taxa were found to be green colour after cultivation in the open field which are (PS1, PS2, 203P, 465P and J1), and three other varieties appeared to be purple colour including (PS3, JTD3 and RauTiaTo), while the rest population appeared to be mixed colour (green/purple or purple/green) which are (PS1, 465P, 588P, NP606, MP3 and GB). Genetic and phenotypic variability, misidentification, instability of extracts, toxic components and contaminants are intrinsic problems associated with medicinal and aromatic herbs (Yi and WetzStein, 2010). Liu et al. (2013) demonstrated different colour obtained from various 12 batches of perilla collected from 8 different regions of China, a similar trend was also observed in the current study. The same author found most of the leaves were small, fragmented, rolled-up, while in the current study no rolled-up leaves were detected. The plant height was not comparatively constant among the studied taxa, only ′JTD3‘reached higher values (70.0 ± 4.47 cm) than others. Rouphael et al. (2019) showed that green perilla genotypes resulted in higher yield and biomass production and higher content of dry matter than red perilla in response to salinity applied as chemical eustressor. Ghimire et al. (2017) showed that the average in Chinese accessions was higher for plant height, leaf length, leaf width than the Japanese accessions. Lin et al., 2020a, Lin et al., 2020b studied red and green perilla phenotypes under temperature and water-stressed conditions and they found a variation in morphological traits, polyphenols and antioxidants with a lower value for all parameters compared to the current study. The morphological and anatomical features are important parts of pharmacobotanical control as the first steps towards the correct identification of medicinal herbs. Even though the phytotherapeutic medicine of the herb depends on the plant material to extract the active component not all the parts of the plant, therefore the morphological descriptions of the herbal drug are crucial to avoid misidentification as it is often seen in the literature and sometimes contradictory and incomplete (de Oliveira et al., 2018). Glandular trichomes might indicate the presence of lipophilic substances on the plant parts. As Zhou et al. (2021) suggest that the active components are produced predominantly in peltate-glandular perilla trichomes. Transcriptomes were used to study genes associated with bioactive component production that served to unravel the biosynthesis of secondary components in this folk medicinal herb (Zhou et al., 2021).
4.2. Polyphenolic content and antioxidant capacity
Numerous studies have shown the health benefits of metabolites, especially polyphenols. Perilla contains considerably high contents of plant-derived bioactive agents which are important for the supplement company with curative and preventive properties for public health, including antioxidant, antidiabetic, anticancer properties (Ahmed, 2019, Igarashi and Miyazaki, 2013). In the current study, there is little higher content was found for JTD3 (Purple colour) phenotype which was cultivated under open field (236.22 ± 3.28 mg GAE/g dry mass), compared to our previous study (234.2 ± 2.723 mg GAE/g DM), where the plant was cultivated in a growth chamber under controlled conditions (Ahmed and Sarosi, 2019). In the current study, the same accession had a paramount antioxidant capacity, this means that AOC is well correlated to TPC as previous studies exhibited a high correlation between TPC and antioxidant activity (Jun et al., 2014, Ahmed and Tavaszi-Sarosi, 2019). Of all fractions, the ethyl acetate fraction of purple Perilla frutescens had the highest antioxidant activity (Jun et al., 2014) while in this research water extract showed a similar pattern for the purple phenotype. The antioxidant properties of green perilla leaves exhibited potent effects based on (DPPH, 86%; ABTS, 90%) at a concentration of 100 μg/mL (Lee et al., 2017). In this study, mixed colour phenotypes showed a higher content of phenolic acids than other perilla phenotypes, as Meng (2009) demonstrated that the antioxidant activity and phenolic compounds of perilla varieties may partly be correlated with the foliage colour. These results propose that the production of phenolic acids in herbal medicines may be affected by a number of factors including environmental stress (light, temperature, location, and moisture) and agronomic conditions (cultivars, years, sowing periods and genetics) (Ahmed and Tavaszi-Sarosi, 2019, Getahun et al., 2019). The plant is cultivated in an organic manner no pesticides, phytohormones, antibiotics, fertilizers, genetically modified organisms have been used, and consequently, the phytochemicals are fully natural compounds without any produced side effects. Rouphael et al. (2019) exhibited that red perilla genotype other than green perilla accumulated higher content of total polyphenols when the plant grown in response to salinity applied as chemical eustressor. The same pattern has been discovered in the present study where mixed and purple perilla genotypes cultivated in open field accumulated much more secondary metabolite polyphenols. Lin et al., 2020a, Lin et al., 2020b studied red and green perilla phenotypes under temperature and water-stressed conditions and they found a variation in polyphenols and antioxidants with a lower value for both phenotypes compared to the current study which we obtained a much higher result.
4.3. Determination of polyphenolic compounds by HPLC
Other studies also found different phenolic acids in perilla leaves. For instance, ethyl acetate fraction of P. frutescens leaves presented high active ingredient content of gallic acid (Wang et al., 2021), while in our study much higher content was detected. Ellagic acid is found in many therapeutic plants and vegetables. It may be found in free form or as complex compounds (ellagitannins), which can be converted to ellagic acid and its metabolites, such as urolithins (Rios et al., 2018). Ellagic Acid has been shown to have antioxidants (Zeb and Akbar, 2018, Tošović and Bren, 2020), osteoarthritis (Lin et al., 2020a, Lin et al., 2020b), diabetes (Ahmad, 2022), anti-inflammatory (Cornélio et al., 2013), antiplatelet activity (Chang, 2013), Neuroprotective (He at al., 2020). Kang and Lee (2011), found three different phenolic acids from purple perilla which were caffeic acid, rosmarinic acid, and rosmarinic acid methylester. Naringin, hesperidin, myricetin, benzoic acid and quercetin were reported to be the major phenolic components in all the Japonica accessions studied by Ghimire et al. (2019). Rosmarinic acid and scutellarin were the most abundant polyphenolic compounds accumulated in higher amounts in the top leaves of the perilla plant than the lower leaves (Gaihre et al., 2021). Rosmarinic acid has been shown to have anti-inflammatory, antiallergic, antioxidant, hepatoprotective, antibacterial, antiviral, and antinociceptive activities (Mikami-konishide et al., 2013, Gaihre et al., 2021). This demonstrates that various cultivars have different phenolics as components. Besides, it has been seen that the phenolic substance of plants can be impacted by different factors, including reaping time, plantation practice, climatic condition, variety, natural conditions, stockpiling time, and temperature (Ahmed and Tavaszi-Sarosi, 2019, Getahun et al., 2019), and genetic background of perilla when grown under the same conditions and periods (Deguchi and Ito 2020).
5. Conclusion
High polymorphism was found among the perilla accessions and macroscopic features of perilla genotypes showed variable results among studied cultivars. The perilla herb has shown different varieties after grown and can be classified into two clearly phenotypes green and purple, within these two other colours are appeared. Regarding the proportion of useful drugs, we obtained the highest drug yield for these green phenotypes 203P, PS2 and PS1 than that of purple phenotypes. GB (Green/Purple colour) phenotype exhibited the major content of polyphenols with significant difference (p < 0.05) according to Tukey HSD (248.93 ± 13.25 mg GAE/g dry mass), followed by JTD3 (Purple colour) variety which accumulated the second highest content of polyphenols then J1 (Green colour). Concerning antioxidant capacity, JTD3 (Purple colour) showed the highest value (206.83 ± 2.0 mg AAE/g DM) and statistically significant than all of them (P < 0.05), followed by 203P (Green colour) and GB (Green/Purple colour) respectively. The HPLC analysis showed that the most abundant phenolic acids were ellagic acid which is accumulated in a higher percentage in NP606, 588P and JTD3 cultivars respectively, followed by salicylic acid and gallic acid. Further study is necessary in future to detect a new molecular mechanism responsible for the development of morphology and biosynthesis of the major chemicals in P. frutescens. These results confirm the importance of perilla cultivars for the purpose of medicinal and aromatic, functional foods and ornamental plants.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Stipendium Scholarship and Newcasle Center for Natural Therapy for fellowships and financial support. The author also thanks Dr. Szilvia Sarosi, Dr Peter Radacsi and Dr. Eva Zambori for helping during the fieldwork and analysis.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad S., Alouffi S., Khan S., Khan M., Akasha R., Ashraf J.M., Farhan M., Shahab U., Khan M.Y., Formanowicz D. Physicochemical Characterization of In Vitro LDL Glycation and Its Inhibition by Ellagic Acid (EA): An In Vivo Approach to Inhibit Diabetes in Experimental Animals. Biomed Res. Int. 2022;2022:1–15. doi: 10.1155/2022/5583298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed H.M. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla frutescens (L.) Britt. Molecules. 2019;24(1):102. doi: 10.3390/molecules24010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed H.M., Tavaszi-Sarosi S. Identification and quantification of essential oil content and composition, total polyphenols and antioxidant capacity of Perilla frutescens (L.) Britt. Food Chem. 2019;275:730–738. doi: 10.1016/j.foodchem.2018.09.155. [DOI] [PubMed] [Google Scholar]
- Ahmed H.M., Al-Zubaidy A.M.A. Exploring natural essential oil components and antibacterial activity of solvent extracts from twelve Perilla frutescens L. Genotypes. Arabian J. Chem. 2020;13(10):7390–7402. [Google Scholar]
- Asif M. Health effects of omega-3, 6, 9 fatty acids: Perilla frutescens is a good example of plant oils. Oriental Pharm. Experimental Med. 2011;11(1):51–59. doi: 10.1007/s13596-011-0002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno N., Akihisa T., Tokuda H., Yasukawa K., Higashihara H., Ukiya M., Watanabe K., Kimura Y., Hasegawa J.-I., Nishino H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci. Biotechnol. Biochem. 2004;68(1):85–90. doi: 10.1271/bbb.68.85. [DOI] [PubMed] [Google Scholar]
- Chang Y., Chen W.F., Lin K.H., Hsieh C.Y., Chou D.S., Lin L.J., Sheu J.R., Chang C.C. Novel Bioactivity of Ellagic Acid in Inhibiting Human Platelet Activation. Evidence-Based Complementary Alternative Med. 2013;2013:1–9. doi: 10.1155/2013/595128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Liu S., Zhao Z., Gao W., Ma Y., Wang X., Luo D. Ultrasound pre-treatment combined with microwave-assisted hydrodistillation of essential oils from Perilla frutescens (L.) Britt. leaves and its chemical composition and biological activity. Ind. Crops Prod. 2020;143 [Google Scholar]
- Consonni R., Cagliani L.R., Docimo T., Romane A., Ferrazzi P. Perilla frutescens (L.) Britton: honeybee forage and preliminary results on the metabolic profiling by NMR spectroscopy. Nat. Prod. Res. 2013;27(19):1743–1748. doi: 10.1080/14786419.2012.751598. [DOI] [PubMed] [Google Scholar]
- Cornélio Favarin D., Martins Teixeira M., Lemos de Andrade E., de Freitas Alves C., Lazo Chica J.E., Artério Sorgi C., Faccioli L.H., Paula Rogerio A. Anti-Inflammatory Effects of Ellagic Acid on Acute Lung Injury Induced by Acid in Mice. Mediators Inflamm. 2013;2013:1–13. doi: 10.1155/2013/164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa V., Borghi A., Mayer J., Sawaya A. Comparison of the Morphology, Anatomy, and Chemical Profile of Mikania glomerata and Mikania laevigata. Planta Med. 2018;84(03):191–200. doi: 10.1055/s-0043-119226. [DOI] [PubMed] [Google Scholar]
- Deguchi Y., Ito M. Caffeic acid and rosmarinic acid contents in genus Perilla. J. Nat. Med. 2020;74(4):834–839. doi: 10.1007/s11418-020-01418-5. [DOI] [PubMed] [Google Scholar]
- Ferrazzi P., Vercelli M., Chakir A., Romane A., Mattana M., Consonni R. Pollination effects on antioxidant content of Perilla frutescens seeds analysed by NMR spectroscopy. Nat. Prod. Res. 2017;31(23):2705–2711. doi: 10.1080/14786419.2017.1292267. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Kono M., Ito A., Ito M. Anthocyanins in perilla plants and dried leaves. Phytochemistry. 2018;147:158–166. doi: 10.1016/j.phytochem.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Gaihre Y.R., Tsuge K., Hamajima H., Nagata Y., Yanagita T. The Contents of Polyphenols in Perilla frutescens (L.) Britton var. frutescens (Egoma) Leaves are Determined by Vegetative Stage, Spatial Leaf Position, and Timing of Harvesting during the Day. J. Oleo Sci. 2021;70(6):855–859. doi: 10.5650/jos.ess20291. [DOI] [PubMed] [Google Scholar]
- Getahun T., Sharma V., Gupta N. The genus Laggera (Asteraceae)–Ethnobotanical and Ethnopharmacological Information, Chemical Composition as well as Biological Activities of Its Essential Oils and Extracts: A Review. Chem. Biodivers. 2019;16(8) doi: 10.1002/cbdv.v16.810.1002/cbdv.201900131. [DOI] [PubMed] [Google Scholar]
- Ghimire B.K., Yoo J.H., Yu C.Y., Chung I.M. GC–MS analysis of volatile compounds of Perilla frutescens Britton var. Japonica accessions: Morphological and seasonal variability. Asian Pacific J. Tropical Med. 2017;10(7):643–651. doi: 10.1016/j.apjtm.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Ghimire B.K., Yoo J.H., Yu C.Y., Kim S.H., Chung I.M. Profiling volatile and phenolic compound composition and characterization of the morphological and biological activities of Perilla frutescence Britton var. Japonica accessions. Acta Physiologiae Plantarum. 2019;41(7):1–16. [Google Scholar]
- He X.M., Zhou Y.Z., Sheng S., Li J.J., Wang G.Q., Zhang F., Izadpanah E. Ellagic Acid Protects Dopamine Neurons via Inhibition of NLRP3 Inflammasome Activation in Microglia. Oxid. Med. Cell. Longevity. 2020;2020:1–13. doi: 10.1155/2020/2963540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Sun L.W., Mokgolodi Neo C., Zhang Y.X., Wen C.X., Xie X.L., Liu Y.J. Primary identifications and palynological observations of Perilla in China. J. Systematics Evolution. 2010;48(2):133–145. [Google Scholar]
- Huang B., Lei Y., Tang Y., Zhang J., Qin L., Liu J. Comparison of HS-SPME with hydrodistillation and SFE for the analysis of the volatile compounds of Zisu and Baisu, two varietal species of Perilla frutescens of Chinese origin. Food Chem. 2011;125(1):268–275. [Google Scholar]
- Igarashi M., Miyazaki Y. A Review on Bioactivities of Perilla: Progress in Research on the Functions of Perilla as Medicine and Food. Evidence-Based Complementary Alternative Med. 2013;2013:1–7. doi: 10.1155/2013/925342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun H.-I., Kim B.-T., Song G.-S., Kim Y.-S. Structural characterization of phenolic antioxidants from purple perilla (Perilla frutescens var. acuta) leaves. Food Chem. 2014;148:367–372. doi: 10.1016/j.foodchem.2013.10.028. [DOI] [PubMed] [Google Scholar]
- Kang N.S., Lee J.H. Characterisation of phenolic phytochemicals and quality changes related to the harvest times from the leaves of Korean purple perilla (Perilla frutescens) Food Chem. 2011;124(2):556–562. [Google Scholar]
- Lee H., Park E. Perilla frutescens Extracts Enhance DNA Repair Response in UVB Damaged HaCaT Cells. Nutrients. 2021;13(4):1263. doi: 10.3390/nu13041263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.K., Ohnishi O. Geographic differentiation of morphological characters among Perilla crops and their weedy types in East Asia. Breeding Sci. 2001;51(4):247–255. [Google Scholar]
- Lee Y.H., Kim B., Kim S., Kim M.-S., Kim H., Hwang S.-R., Kim K., Lee J.H. Characterization of metabolite profiles from the leaves of green perilla (Perilla frutescens) by ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry and screening for their antioxidant properties. J. Food Drug Anal. 2017;25(4):776–788. doi: 10.1016/j.jfda.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Zhang Z., Li M., Li X., Sun Z. Yield, size, nutritional value, and antioxidant activity of oyster mushrooms grown on perilla stalks. Saudi J. Biol. Sci. 2017;24(2):347–354. doi: 10.1016/j.sjbs.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K.-H., Jhou Y.-J., Wu C.-W., Chang Y.-S. Growth, physiological, and antioxidant characteristics in green and red Perilla frutescens varieties as affected by temperature-and water-stressed conditions. Sci. Hortic. 2020;274:109682. doi: 10.1016/j.scienta.2020.109682. [DOI] [Google Scholar]
- Lin Z., Lin C., Fu C., Lu H., Jin H., Chen Q., Pan J. The protective effect of Ellagic acid (EA) in osteoarthritis: An in vitro and in vivo study. Biomed. Pharmacother. 2020;125:109845. doi: 10.1016/j.biopha.2020.109845. [DOI] [PubMed] [Google Scholar]
- Liu J., Wan Y., Zhao Z., Chen H. Determination of the content of rosmarinic acid by HPLC and analytical comparison of volatile constituents by GC-MS in different parts of Perilla frutescens (L.) Britt. Chem. Cent. J. 2013;7(1):61. doi: 10.1186/1752-153X-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L., Lozano Y.F., Gaydou E.M., Li B. Antioxidant activities of polyphenols extracted from Perilla frutescens varieties. Molecules. 2009;14(1):133–140. doi: 10.3390/molecules14010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L., Lozano Y., Bombarda I., Gaydou E., Li B. Anthocyanin and flavonoid production from Perilla frutescens: pilot plant scale processing including cross-flow microfiltration and reverse osmosis. J. Agric. Food. Chem. 2006;54(12):4297–4303. doi: 10.1021/jf0604079. [DOI] [PubMed] [Google Scholar]
- Mikami-konishide I., Murakami S., Nakanishi K., Takahashi Y., Yamaguchi M., Shioya T., Watanabe J., Hino A. Antioxidant capacity and polyphenol content of extracts from crops cultivated in Japan, and the effect of cultivation environment. Food Sci. Technol. Res. 2013;19(1):69–79. [Google Scholar]
- Mungmai L., Preedalikit W., Pintha K., Tantipaiboonwong P., Aunsri N. Collagenase and melanogenesis inhibitory effects of Perilla frutescens pomace extract and its efficacy in topical cosmetic formulations. Cosmetics. 2020;7(3):69. [Google Scholar]
- Osakabe N., Yasuda A., Natsume M., Yoshikawa T. Rosmarinic acid inhibits epidermal inflammatory responses: anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis. 2004;25(4):549–557. doi: 10.1093/carcin/bgh034. [DOI] [PubMed] [Google Scholar]
- Park H.Y., Nam M.H., Lee H.S., Jun W., Hendrich S., Lee K.W. Isolation of caffeic acid from Perilla frutescens and its role in enhancing γ-glutamylcysteine synthetase activity and glutathione level. Food Chem. 2010;119(2):724–730. [Google Scholar]
- Ríos J.-L., Giner R., Marín M., Recio M. A pharmacological update of ellagic acid. Planta Med. 2018;84(15):1068–1093. doi: 10.1055/a-0633-9492. [DOI] [PubMed] [Google Scholar]
- Rouphael Y., Kyriacou M., Carillo P., Pizzolongo F., Romano R., Sifola M. Chemical Eustress Elicits Tailored Responses and Enhances the Functional Quality of Novel Food Perilla frutescens. Molecules. 2019;24(1):185. doi: 10.3390/molecules24010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W.F., Tsai H.P., Chang Y.H., Chang T.Y., Hsieh C.F., Lin C.Y., Horng J.T. Perilla (Perilla frutescens) leaf extract inhibits SARS-CoV-2 via direct virus inactivation. Biomed. J. 2021;44(3):293–303. doi: 10.1016/j.bj.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tošović J., Bren U. Antioxidative action of ellagic acid—a kinetic DFT study. Antioxidants. 2020;9(7):587. doi: 10.3390/antiox9070587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Tu Z., Xie X., Cui H., Kong K.W., Zhang L. Perilla frutescens Leaf extract and fractions: Polyphenol composition, antioxidant, enzymes (α-glucosidase, acetylcholinesterase, and tyrosinase) inhibitory, anticancer, and antidiabetic activities. Foods. 2021;10(2):315. doi: 10.3390/foods10020315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Sun W., Fan Y.-N., Li S.-y., Yuan J.-Q., Zhang Z.-Q., Li X.-y., Lin M.-B., Hou Q.i., Capasso R. Perilla Leaf Extract Attenuates Asthma Airway Inflammation by Blocking the Syk Pathway. Mediators Inflamm. 2021;2021:1–14. doi: 10.1155/2021/6611219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi W., Wetzstein H.Y. Biochemical, biological and histological evaluation of some culinary and medicinal herbs grown under greenhouse and field conditions. J. Sci. Food Agric. 2010;90(6):1063–1070. doi: 10.1002/jsfa.3921. [DOI] [PubMed] [Google Scholar]
- Zeb A., Akbar A. Ellagic acid suppresses the oxidative stress induced by dietary-oxidized tallow. Oxid. Med. Cell. Longevity. 2018;2018:1–10. doi: 10.1155/2018/7408370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yin M., Dai S., Bao K., Song C., Liu C., Wu Q. Multi-omics analysis of the bioactive constituents biosynthesis of glandular trichome in Perilla frutescens. BMC Plant Biol. 2021;21(1):1–15. doi: 10.1186/s12870-021-03069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.-J., Yan L.-L., Yin P.-P., Shi L.-L., Zhang J.-H., Liu Y.-J., Ma C. Structural characterisation and antioxidant activity evaluation of phenolic compounds from cold-pressed Perilla frutescens var. arguta seed flour. Food Chem. 2014;164:150–157. doi: 10.1016/j.foodchem.2014.05.062. [DOI] [PubMed] [Google Scholar]