Abstract
The present study aims to examine PAB culture, synthesizing a significant number of iron-containing enzymes and capable of adhesion. Results show that increased iron concentration increased enzymes activity in all strains studied. An increase of iron ions level increasing up to 0.50–0.60 mg/ml leads to a 1.3-fold and 2-dold increase of catalase and SOD activity respectively, peroxidase activity was virtually unchanged. Optimal iron ions Fe2+ doses to ensure active PAB growth were determined. Of all the cultures studied P. fredenreichii subsp. shermanii AC-2503 has high adhesion: AAI = 5.1; MAI = 5.60; erythrocyte involvement rate = 87%. It was shown that certain iron ion concentrations increased the specific growth rate of PAB (P. freudenrichii subsp. freudenrichii AC-2500 (0.3 mg/ml) and other strains (0.4 mg/ml). A further increase in the iron ions concentration slows bacterial growth, while excessive content inhibits metabolism, including defense mechanisms that offset the negative effects of the metal. Our subsequent studies will focus on the effect of other metal ions on the metabolism of bacteria, mainly lactic acid bacteria, which are important biotechnological objects of the industry similar to propionic acid bacteria.
Keywords: Activity of catalase, Adhesiveness, Extracellular metabolites, Iron ions Fe2+, Peroxidase, Propionic acid bacteria, SOD
1. Introduction
Many studies on the use of Propionibacterium bacteria have been conducted to date. Among other things, it has been shown that these bacteria can biosynthesize valuable metabolites such as propionic acid, vitamin B12, bacteriocins, and trehalose. This suggests that they represent a significant group of microorganisms of future industrial significance. The main advantage of propionic acid bacteria (PAB) is that they can develop and synthesize metabolites on substrates containing various industrial wastes. It greatly increases the economic viability of biotechnology processes (Gonzalez-Garcia et al., 2017).
Propionibacterium metabolites and whole cells are widely used in different industries: food, cosmetic or pharmaceutical (Piwowarek et al., 2018). Furthermore, they are the only food microorganisms capable of generating vitamin B12 (de Assis et al., 2022). Besides, they can be used as feed additives. Propionibacterium spp. have many valuable properties. The most important from a technological point of view are the following: they can use as a carbon source milk sugar and lactic acid salts, secrete intracellular peptidases and cell wall associated proteases, synthesize compounds with conservative properties (bacteriocins, propanoic acid, acetic acid), produce taste compound (proline aminopeptidase). In addition, they can convert free amino acids into flavoring compounds (Fang et al., 2017). Propionibacterium freudenreichii bacteria also play an essential role in the bio-enrichment of yogurt with propionic acid (Zahed et al., 2021). Representatives of the genus Propionibacterium are highly diverse. They vary from “dairy propionibacteria,” which are widely known for their capability of forming flavor qualities in cheese production, to “cutaneous propionibacteria,” which are mainly associated with human skin (Bücher et al., 2021).
However, the efficacy of industrial propionibacterium production is limited by its sensitivity to high concentrations of propionic acid excreted in the culture medium. Therefore, the development of new biotechnological processes and strains able to overcome this limitation and increase the profitability of microbiological production remains a pressing issue. The following factors also act as inhibitors to PAB: high values of acidity, salt concentration and temperature, and water activity. Their adaptation to one of the stressors, as mentioned above, increases their resistance to other parameters (Gaucher et al., 2019).
PABs have significant growth preferences. Their main carbon sources are mono- and disaccharides (e.g., glucose, lactose, fructose, ribose, and galactose) or lactic acid and its salts. Bacteria can obtain nitrogen from organic (peptides and amino acids) or inorganic (ammonia salts, and amines) sources. The presence of asparagus acid in the medium promotes the growth of bacteria and enhances their fermentation efficiency and carbon dioxide production (Yin et al., 2017). Besides the substances needed for their growth (carbon and nitrogen source), they also require appropriate micronutrient supplements (Fe, Mg, Co, Mn, Cu, vitamins B7 and B5), which is particularly important for bacteria because of functional and nonfunctional proteins contain different amount of metal ions (Valasatava et al., 2018). Iron is usually found as part of heme or prosthetic groups of protein (Pereira et al., 2017, Valasatava et al., 2018), regulatory proteins, including several Fur species in the intestinal bacterium Salmonella enterica (Troxell et al., 2011). These proteins are essential to fundamental physiological processes: cell breathing, metabolism, and nucleic acids repair. Unbound iron can act as a catalyst to biomolecular damage. To prevent damage that may result from the production of oxyradicals, iron must be handled thoroughly in cells to keep low levels of the molecular intracellular iron. Iron's ability to carry physiologically pH electrons makes it valuable and dangerous for cells. Consequently, bacteria have developed multiple mechanisms to actively obtain iron from the medium under iron limitation and control the availability of molecular intracellular iron under iron stress or excess (Cassat and Skaar, 2013).
Many studies have focused on the process of iron and other metals bacteria acquiring and their metabolism global regulation. However, some of essential metals can be toxic for bacteria at certain levels, therefore protection mechanisms against this problem are increasingly recognized. The discharge of free intracellular iron during iron-containing proteins damaging is an underestimated aspect of iron ions homeostasis in bacteria. Therefore, this work aimed to study the effect of iron cations on the metabolism of propionic acid bacteria, including the activity of antioxidant system enzymes, adhesion ability, and their growth rate, proving the importance of iron for normal cell metabolism. The stated objective includes the following tasks to be completed:
-
•
identifying propionic acid bacteria strains for the experiment and selecting optimal conditions for their cultivation;
-
•
studying the adhesive properties of propionic acid bacteria of the Propionibacterium genus;
-
•
analyzing the effect of iron sulfate on the growth and biosynthesis of extracellular adaptation factors of propionic acid bacteria;
-
•
studying the impact of casein phosphopeptides on iron solubilization in nutrient media.
2. Methods and materials
2.1. Bacteria and cultivation conditions
In this study, the following strains of PAB were used:
-
•
P. cyclohexanicum (Kusano AC-2259 and Kusano AC-2260);
-
•
P. freudenrichii (subsp. freudenrichii AC- 2500 and subsp. shermanii AC-2503),
from the collection of microorganisms at I.I. Mechnikov Institute of Microbiology and Immunology (Kharkiv, Ukraine).
Divalent iron sulphate salt was used as an iron source. PABs were cultivated in growth factors containing serum medium (Aldén et al., 2001, Bonnet et al., 2019). A 1-day crop grown on non-fat milk was utilized as inoculum. Iron sulphate was added at a 0.3–0.6 mg/ml concentration in the growing medium. Propionic acid bacteria were cultivated with ferrous sulfate for 1 day at 28 °C. Crop growth kinetics were derived using the generally accepted method (Kovárová-Kovar and Egli, 1998).
2.2. Analytical procedures
The iron bonding process was monitored for the number of chelated Fe2+ (% of iron remaining as divalent from the total initial dose). The iron content was controlled using the reference methodology (Carter, 1976). The iron content was determined through spectrophotometry, and it was used following industry guidelines (Pfeiffer and Looker, 2017).
Products of extracellular metabolism were determined at the end of the exponential growth phase. The catalase activity was also determined by the colorimetric method (Hadwan, 2018). The peroxidase and superoxide dismutase (SOD) activities were observed by spectrophotometry using the reactant o-dianisidine (Zhuang et al., 2017) and adrenaline autooxidation (Sirota, 2017), respectively. Strain adhesion was assessed by the microbial adhesion index (MIA) and then studied on formalized erythrocytes using the advanced Briles technique (Lenchenko et al., 2020); exopolysaccharide concentration was evaluated using an Antron reagent; vitamin B12 content was determined spectrophotometrically (Tsiminis et al., 2016). The casein phosphopeptides (CPPs) solution was derived from pepsin and trypsin treated sodium caseinate. The phosphopeptides molecular weight distribution in solution was evaluated by medium pressure exclusion chromatography on a TOSOH TSK-GEL Amide-80 column. The content of iron in chelated form was determined by a mass spectrometer.
2.3. Statistical data analysis
The mean and standard deviation (SD) were calculated for the statistical analysis of the resulting data. The variance analysis was used to estimate the difference between the average values of analyzed criteria using Microsoft Excel and Statistica 10 (de Smith, 2021) software. The differences between the results obtained are reliable at the significance level of P ≤ 0.05 as per the Student's criterium.
3. Results
3.1. PAB adhesive properties
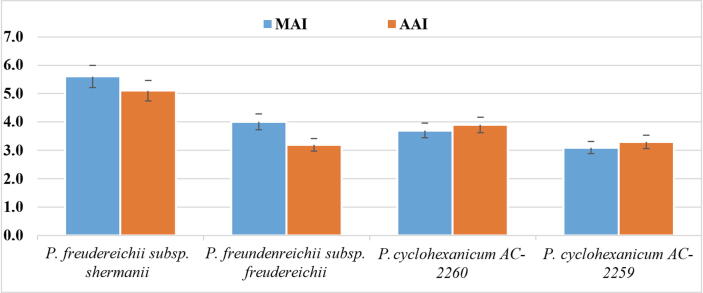
PABs differ in their ability to adhere to red blood cells. Some of the strains adhere as separate cells or their aggregates that almost completely cover the surface of erythrocytes. The adhesion properties of the cultures are considered in Fig. 1.
Fig. 1.
Adhesion properties of cultures were studied according to MAI (Microbial Adhesion Index) and AAI (Average Adhesion Index).
According to the methodology, bacteria were considered as:
-
•
non-adhesive (IAM < 1.80);
-
•
low-adhesive (IAM = 1.81–2.60);
-
•
moderately adhesive (IAM = 2.61–4.10);
-
•
very adhesive (IAM > 4.10).
Of all the cultures studied, P. fredenreichii subsp. shermanii AC-2503 has high adhesion, which is confirmed by the value of AAI = 5.1 and the MIA = 5.60 and the erythrocyte participation rate (87%). As for the other strains of the microorganisms studied, the strain P. freudenrichii subsp. freudenrichii had the lowest erythrocyte participation rate (81%), the other two had similar indices at the level of 84%.
3.2. Effect of iron sulphate on PAB growth and metabolism
This study shows that ferrous sulfate increases the specific bacteria growth rate is low: P. freudenrichii subsp. freudenrichii AC-2500 (0.3 mg/ml) and other strains (0.4 mg/ml), that sows the importance of iron. Ferrous sulfate content increasing leads to growth rate slowing. It follows that excessive iron content inhibits bacterial metabolism.
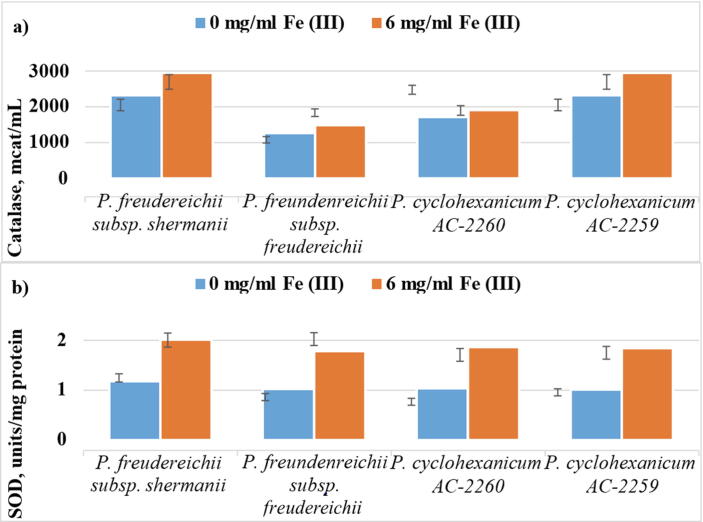
Given that the iron ions affect the synthesis and activity of iron-containing enzymes, the effect of ferrous sulfate on them was investigated. The data in Fig. 2 demonstrate an increase in the activity of enzymes with an increase in the number of iron ions for all strains. Ferrous sulfate level increasing up to 0.50–0.60 mg/ml leads to a 1.3-fold increase of catalase activity and 2-fold increase of SOD activity (on average). Regarding peroxidase, its activity has remained virtually unchanged in all experimental samples.
Fig. 2.
Effect of Fe2+ ions on the activity of catalase (a) and superoxide dismutase (b) in different strains of propionic acid bacteria.
When studying the PAB morphology, cell aggregates (adhesion) were observed when the dose of iron sulfate was increased to 0.60 mg/ml. Likely, the cells were able to aggregate due to changes in charge of the outer cell surface.
3.3. Effect of phosphopeptides on iron solubilization
During experimental studies, it was noted that at iron concentrations of 0.5 mg/ml and higher, the color of the concentrate changes, forming a precipitate, which indicates the formation of insoluble Fe (III) ions. That was the prerequisite for studying the effect of casein phosphopeptides on iron solubilization (chelation) in a nutrient medium.
The findings suggest that CPPs form nano-sized chelate complexes with iron. These compounds could bind to the cell surface, effectively transport iron ions through the cell wall, and protect the mineral from interaction with other components of the cell's internal environment.
Technological parameters of CPPs isolation were revised to obtain a hydrolyzate with the maximum compound concentration capable of forming chelates with iron. Accordingly, the technological regime for the isolation of CPPs has been changed. Synthetic chelates of minerals are considered to degrade during storage and lose their efficacy, making them inferior to organic salts. Therefore, the preservation of chelated divalent iron with CPPs during long-term storage has been studied. The data show that the content of chelated iron in concentrated solutions containing CPPs almost did not change during storage. In contrast, a significant decrease in the content of soluble Fe (II) ions was observed in controls.
One of the current fields of modern microbiology is the study of adhesive processes for microorganisms. Adhesion is an intercellular interaction that occurs through the tight attachment of cells to a substrate. Considering that microorganisms' microbiota properties depend on adhesion ability and information about PAB adhesive properties is not enough, the adhesive properties of PAB strains have been studied.
4. Discussion
The results show that PAB has relatively strong adhesion properties, especially P. fredenreichii subsp. shermanii AC-2503. It is important to emphasize that among the many functions of self-regulators, the factors that ensure the adaptation of microorganisms to adverse physical and chemical conditions have been insufficiently studied. The metal concentration can affect the interaction between microorganisms and metals (Tarekegn et al., 2020). Moreover, excess metal concentration inhibits metabolism, including defense mechanisms that offset the adverse effects of the metal.
In a biotechnology study, PAB was found to synthesize a significant number of iron-containing proteins, including enzymes (Piwowarek et al., 2018). The increase of catalase and SOD activity significantly exceeded the ability of PAB to protect themselves from oxidative stress. Higher antimutagenic activity of propionic acid bacteria was observed with increasing FeSO4 concentration, indicating induction of antimutagenesis (Słoczyńska et al., 2014).
One of the protection mechanisms against toxic metal concentrations in microorganisms is to form low-toxic compounds (Gadd, 2009). The increased biosynthesis of exocompounds (polysaccharides) upon iron addition is an example of nonenzymatic defense mechanisms, where EPS prevents excessive iron penetration into cells through the bacterial surface coating. The cell agglomerates forming can be explained by the medium's protective response to the metal excess. Also, according to the literature, the presence of di- and trivalent cations reduce electrostatic repulsion, promoting adhesion due to charged layers' shrinkage on the surface (Deng et al., 2018). Results suggest that exometabolite synthesis supports the adaptation of PAB to iron ions (Inami et al., 2017). The revealed trends allow understanding of the PAB metabolism and create a theoretical basis for developing biologically active supplements.
The effect of CPPs on iron chelation in nutritional media has also been studied. It is known that the capacity of CPPs to bond metal depends on the degree of phosphorylation. Technological parameters of CPPs extraction were revised to obtain a solution of low-molecular-weight compounds (peptides and amino acids) capable of forming chelates with iron (Arunachalam and Raja, 2010).
In general, the data show that CPPs are promising chelators for new forms of bio-available iron. Optimum doses of iron sulphate (III) and an aqueous solution of CPPs providing the maximum amount of iron solubilized have been determined.
The subsequent studies will focus on the effect of other metal ions on the metabolism of bacteria, mainly lactic acid bacteria, which are critical biotechnological objects of the industry similar to propionic acid bacteria.
5. Conclusions
Optimal iron ions Fe2+ doses to ensure active PAB growth were determined. Of all the cultures studied P. fredenreichii subsp. shermanii AC-2503 has high adhesion: AAI = 5.1; MAI = 5.60; erythrocyte involvement rate = 87%. As for the other strains of the microorganisms studied, the strain P. freudenrichii subsp. freudenrichii had the lowest erythrocyte involvement rate (81%), the other two had similar indices at the level of 84%.
The synthesis of extracellular metabolites that provide PAB protection from metal can be stimulated by iron ions adding to the nutrient medium. It was shown that particular iron ion concentrations increased the specific growth rate of PAB (P. freudenrichii subsp. freudenrichii AC-2500 (0.3 mg/ml) and other strains (0.4 mg/ml). A further increase in the iron ions concentration slows down the bacterial growth, and excess content inhibits metabolism, including defense mechanisms that offset the negative effects of the metal.
Results show that increased iron concentration facilitated enzyme activity in all strains studied. An increase of iron ions level increasing up to 0.50–0.60 mg/ml leads to a 1.3-fold increase of catalase activity and a 2-fold increase of SOD activity (on average). Peroxidase activity has remained virtually unchanged in all experimental samples.
The results achieved can enhance the culture of propionic acid bacteria to increase their productivity. Under industrial conditions, it will also be possible to implement this data into the SBA production technology. According to the results described in the article, subsequent studies will be focused on the effect of other metal ions on the metabolism of bacteria, especially lactic acid bacteria, which, like propionic acid bacteria, are essential biotechnological industrial objects.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aldén L., Demoling F., Bååth E. Rapid method of determining factors limiting bacterial growth in soil. Appl. Environ. Microbiol. 2001;67(4):1830–1838. doi: 10.1128/AEM.67.4.1830-1838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunachalam K.D., Raja R. Isolation and characterization of C.P.P. (casein phosphopeptides) from fermented milk. Afr. J. Food Sci. 2010;4:167–175. [Google Scholar]
- Bonnet M., Lagier J.C., Raoult D., Khelaifia S. Bacterial culture through selective and non-selective conditions: the evolution of culture media in clinical microbiology. New Microbes New Infect. 2019;34 doi: 10.1016/j.nmni.2019.100622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bücher C., Burtscher J., Domig K.J. Propionic acid bacteria in the food industry: An update on essential traits and detection methods. Compr. Rev. Food Sci. Food Saf. 2021;20(5):4299–4323. doi: 10.1111/1541-4337.12804. [DOI] [PubMed] [Google Scholar]
- Carter P. The ICSH Reference Method for Serum Iron Assay: Recommendation for a Viable Automated Alternative. J. Qin. Chem. Clin. Biochem. 1976;14:151–153. doi: 10.1515/cclm.1976.14.1-12.151. [DOI] [PubMed] [Google Scholar]
- Cassat J.E., Skaar E.P. Iron in infection and immunity. Cell Host Microbe. 2013;13(5):509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Assis D.A., Machado C., Matte C., Ayub M.A.Z. High cell density culture of dairy Propionibacterium sp. and Acidipropionibacterium sp.: A review for food industry applications. Food Bioprocess Technol. 2022 doi: 10.1007/s11947-021-02748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smith M.J. The Winchelsea Press; 2021. Statistical analysis handbook. [Google Scholar]
- Deng C., Li X., Xue X., Pashley R.M. The effects of low levels of trivalent ions on a standard strain of Escherichia coli (ATCC 11775) in aqueous solutions. Microbiol. Open. 2018;7(3) doi: 10.1002/mbo3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H., Kang J., Zhang D. Microbial production of vitamin B12: a review and future perspectives. Microb. Cell Factories. 2017;16(1):15. doi: 10.1186/s12934-017-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadd G.M. Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiol. 2009;156(3):609–643. doi: 10.1099/mic.0.037143-0. [DOI] [PubMed] [Google Scholar]
- Gaucher F., Bonnassie S., Rabah H., Marchand P., Blanc P., Jeantet R., Jan G. Review: Adaptation of beneficial propionibacteria, lactobacilli, and bifidobacteria improves tolerance toward technological and digestive stresses. Front. Microbiol. 2019;10:841. doi: 10.3389/fmicb.2019.00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Garcia R., McCubbin T., Navone L., Stowers C., Nielsen L., Marcellin E. Microbial Propionic Acid Production. Fermentation. 2017;3(2):21. doi: 10.3390/fermentation3020021. [DOI] [Google Scholar]
- Hadwan M.H. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem. 2018;19:7. doi: 10.1186/s12858-018-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inami K., Mine Y., Tatsuzaki J., Mori C., Mochizuki M. Isolation and characterization of antimutagenic components of Glycyrrhiza aspera against N-methyl-N-nitrosourea. Genes Environ. 2017;39:5. doi: 10.1186/s41021-016-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovárová-Kovar K., Egli T. Growth kinetics of suspended microbial cells: from single-substrate- controlled growth to mixed-substrate kinetics. MMBR. 1998;62(3):646–666. doi: 10.1128/mmbr.62.3.646-666.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenchenko E., Blumenkrants D., Sachivkina N., Shadrova N., Ibragimova A. Morphological and adhesive properties of Klebsiella pneumoniae biofilms. Vet. World. 2020;13(1):197–200. doi: 10.14202/vetworld.2020.197-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira J.C., Giese E.C., Moretti M.M.de S., Gomes A.C.dos S., Perrone O.M., Boscolo M., Martins D.A.B. Effect of metal ions, chemical agents and organic compounds on lignocellulolytic enzymes activities. Enzyme Inhibit. Activ. 2017;29:139–164. 10.5772/65934. [Google Scholar]
- Pfeiffer C.M., Looker A.C. Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. Am. J. Clin. Nutr. 2017;106(S6):1606–1614. doi: 10.3945/ajcn.117.155887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwowarek K., Lipińska E., Hać-Szymańczuk E., Bzducha-Wróbel A., Synowiec A. Research on the ability of propionic acid and vitamin B12 biosynthesis by Propionibacterium freudenreichii strain T82. Antonie van Leeuwenhoek. 2018;111(6):921–932. doi: 10.1007/s10482-017-0991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota T.V. Standardization and regulation of the rate of the superoxide-generating reaction of adrenaline autoxidation used for evaluation of pro/antioxidant properties of various materials. Biochem. Moscow Suppl. Ser. B. 2017;11:128–133. doi: 10.1134/S1990750817020068. [DOI] [Google Scholar]
- Słoczyńska K., Powroźnik B., Pękala E., Waszkielewicz A.M. Antimutagenic compounds and their possible mechanisms of action. J. Appl. Genet. 2014;55(2):273–285. doi: 10.1007/s13353-014-0198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarekegn M.M., Salilih F.Z., Ishetu F.I. Microbes used as a tool for bioremediation of heavy metal from the environment. Cogent Food Agric. 2020;6(1):1783174. doi: 10.1080/23311932.2020.1783174. [DOI] [Google Scholar]
- Troxell B., Fink R.C., Porwollik S., McClelland M., Hassan H.M. The Fur regulon in anaerobically grown Salmonella enterica sv. Typhimurium: identification of new Fur targets. BMC Microbiol. 2011;11(1):236. doi: 10.1186/1471-2180-11-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiminis G., Schartner E.P., Brooks J.L., Hutchinson M.R. Measuring and tracking vitamin B12: A review of current methods with a focus on optical spectroscopy. Appl. Spectrosc. Rev. 2016;52(5):439–455. doi: 10.1080/05704928.2016.1229325. [DOI] [Google Scholar]
- Valasatava Y., Rosato A., Furnham N., Thornton J.M., Andreini C. To what extent do structural changes in catalytic metal sites affect enzyme function? J. Inorg. Biochem. 2018;179:40–53. doi: 10.1016/j.jinorgbio.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Zhang R., Xia M., Bai X., Mou J., Zheng Y., Wang M. Effect of aspartic acid and glutamate on metabolism and acid stress resistance of Acetobacter pasteurianus. Microb. Cell Factories. 2017;16(1):109. doi: 10.1186/s12934-017-0717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahed O., Massoud R., Khosravi-Darani K., Mortazavian A.M., Mohammadi A. Propionic acid bio-fortification of yogurts by adjunct culture of Propionibacterium freudenreichii: Propionic acid biosynthesis in yogurt. Appl. Food Biotechnol. 2021;9(1):31–40. doi: 10.22037/afb.v9i1.36451. [DOI] [Google Scholar]
- Zhuang Q.Q., Lin Z.H., Jiang Y.C., Deng H.H., He S.B., Su L.T., Shi X.Q., Chen W. Peroxidase-like activity of nanocrystalline cobalt selenide and its application for uric acid detection. Int. J. Nanomed. 2017;12:3295–3302. doi: 10.2147/IJN.S128556. [DOI] [PMC free article] [PubMed] [Google Scholar]