Abstract
Gingival mesenchymal stem cells (GMSCs) have significant regenerative potential. Their potential applications range from the treatment of inflammatory diseases, wound healing, and oral disorders. Preconditioning these stem cells can optimize their biological properties. Hypoxia preconditioning of MSCs improves stem cell properties like proliferation, survival, and differentiation potential. This research explored the possible impact of hypoxia on the pluripotent stem cell properties that GMSCs possess. We evaluated the morphology, stemness, neurotrophic factors, and stemness-related genes. We compared the protein levels of secreted neurotrophic factors between normoxic and hypoxic GMSC-conditioned media (GMSC-CM). Results revealed that hypoxic cultured GMSC’s had augmented expression of neurotrophic factors BDNF, GDNF, VEGF, and IGF1 and stemness-related gene NANOG. Hypoxic GMSCs showed decreased expression of the OCT4 gene. In hypoxic GMSC-CM, the neurotrophic factors secretions were significantly higher than normoxic GMSC-CM. Our data demonstrate that culturing of GMSCs in hypoxia enhances the secretion of neurotrophic factors that can lead to neuronal lineage differentiation.
Keywords: Gingival mesenchymal stem cell, Hypoxia, Neurotrophic factors, Normoxia
1. Introduction
Cell-based therapy refers to modifying a patient’s cells (or donor cells) to fight disease and alleviate medical conditions. Therapeutics using bioengineered cells have shown significant progress in recent years such as the successful treatment of (B-lymphocyte) cell or B cell lymphoma using CAR-T (Chimeric-Antigen-Receptor-T cell) treatment. (Woodsworth and Holt, 2017). Clinical trials on stem cell therapies and their impact on diseases like diabetes and cardiomyopathy have opened new avenues in regenerative modalities. Adult stem cells are a common stem cell subtype with multipotent characteristics, capable of forming tissue-restricted or lineage-specific cell types. The oldest and most accepted stem-cell treatment at present is transplanting hematopoietic stem cells (HSC), also considered the standard therapy in malignancies which are blood-related, acquired or innate hematopoietic diseases or ailments (Henig and Zuckerman, 2014, Watt and Driskell, 2010). The second most commonly used stem cell type in a clinical setting is MSCs or the mesenchymal stem cells, which arise from the bone marrow, blood, muscular, fatty tissues, teeth, etc. and have multipotent potential. Mesenchymal stem cells, due to their ability to regenerate on their own, easy isolation and expansion, relatively easy availability from numerous sources and most importantly, their potential to proliferate into different blood cell types, more commonly known as multilineage differentiation potential, find numerous clinical applications in the field of regenerative medicine. (Hmadcha et al., 2020, Parekkadan and Milwid, 2010).
Gingiva acts as a primary immune defense shield of the oral cavity (Xu et al., 2013, Zhang et al., 2009). Zhang et al., in their study found that GMSCs or the mesenchymal cells found in the human gingiva possess the ability to modulate immunity and form cells of differential lineages (Zhang et al., 2009) such as the adipocytic, chondrocytic, or osteocytic lineages. However, GMSCs also have the potential to differentiate into ectodermal as well as endodermal origins which include nervous cells of several types (Diniz et al., 2016, Pittenger et al., 1999, Zhang et al., 2009). Xu et al. in their study, discovered that a major part of the gingival mesenchymal stem cells (GMSCs) arise from the neural crest cells of the cranium while the remaining portion is mesodermal. Neural-crest-derived GMSCs were found to have augmented differentiating potential towards chondrocytes and neural cells. The abundance and easy accessibility of GMSCs make them the treatment modality of choice in tissue engineering as well as regenerative medicine (Tomar et al., 2010, Angelopoulos et al., 2018). However, they have a reduced survival rate after transplantation, which is considered one of the greatest limitations using MSCs. In addition, there is an added complication of differentiating GMSCs from gingival fibroblast, which as demonstrated by Diar-Bakirly et al was not possible based solely on independent surface markers including the potential MSC marker CD146 (Diar-Bakirly and El-Bialy, 2021).
The poor survivability of GMSCs is due to several hosts, environmental reasons including in vitro and physiological conditions such as active inflammation, massive necrosis, protein denaturation, the action of proteases, hypoxic conditions, and lack of nutrients (Vu et al., 2016). Numerous studies have reported optimization strategies to improve their MSC properties for efficient regenerative therapies (Kim et al., 2015, Haque et al., 2015). BMSCs or the bone marrow mesenchymal stem cells from marrow incubated in hypoxic conditions displayed enhanced proliferation as well as migration capabilities. The hypoxic environment also promoted osteogenic differentiation via the upregulation of growth factors (Hung et al., 2012) Hypoxia preconditioning is a widely adopted strategy as the concentration of O2 employed in in vitro conditions is high whereas the oxygen concentration in an ischemic environment is very low. Preconditioning of MSCs in low oxygen (O2) concentration will enhance the genomic stability, chemokine receptor expression, dynamics of growth, and the proliferative capacity (Haque et al., 2013, Ejtehadifar et al., 2015). Earlier investigations culturing GMSCs have described a three-dimensional sphere-like culture technique so as to enhance the traits of the stem cells (Gugliandolo et al., 2019, Zhang et al., 2012a).
The study investigates the influence of hypoxia in GMSCs. The impact of low O2 concentration (0.5% O2) on GMSC properties such as morphology, stem cell marker expression, expression of trophic factors, stemness-associated genes, and secretion of trophic factors in conditioned media was looked into, in our study. All the changes were compared with GMSCs cultured in normoxic conditions (20% O2).
2. Methodology
2.1. Gathering samples
Gingivectomy performed for cosmetic reasons in healthy patients served as a source for the gingival samples. Following informed consent, sterile containers were used to store the samples containing PBS (phosphate buffered saline) (Sigma, St. Louis, Missouri, USA) & immediately transported to a laboratory for further processing.
2.2. Tissue-based preparation of single-cell and culture
PBS antibiotic antimycotic solution rinsed tissue was cut into small pieces and digested using 0.2% dispase II and 0.4% collagenase I. This was followed by a 20-min incubation period at 37° Celsius. Once this was done, enzymatic action was neutralized using fetal bovine serum (FBS) (Gibco, Rockville, MD, USA). A 70-µm strainer in sterile condition was used to pass the tissue through, following centrifugation was carried out at 1800 rpm for 5 min. Collected pellets were plated in five percent Carbon-dioxide at a temperature of 37° Celsius with DMEM (Dulbecco’s Modified Eagle Medium) (Invitrogen, Carlsbad, CA, USA) in addition to ten percent FBS (Foetal bovine serum) (and an antibiotic–antimycotic solution). Each week 2 times the culture medium was changed. Conditions of cell growth, morphology, and health were visualized periodically with the help of a microscope (inverted phase-contrast). Following confluence of 70–80% 0.25 percent Trypsin-Ethylene-diamine-tetraacetic-acid (EDTA) (Invitrogen, Carlsbad, CA, USA) solution was used for isolating and transferring to a larger polystyrene 25-cm2 culture flask. Confluent GMSCs were isolated using 0.25 percent Trypsin-Ethylene-diamine-tetraacetic-acid (EDTA) solution and passed on in a sustained fashion to allow development which in turn could be used for forthcoming investigations. Experiments were done for cells in the 2nd to 4th passages.
2.3. Treatment groups
GMSCs from passage 2 were placed in a 6 plate well with the growth medium (2.5 × 105 cells per well: density). Incubation was performed with twenty percent O2 level in the normoxic cells. The hypoxia group cells were incubated at 0.5–1% oxygen level in the incubator for 7 days.
2.4. Surface marker assessment
From each sample pellet, the PBS resuspended cell was placed in varying tubes and subjected to anti human CD73 –APC, anti human CD90-APC, anti human-CD34-PE, anti human-CD45-FITCanti-human-CD105-APC, & Anti human-HLA-DR-APC antibodies incubated at a temperature of 4-degree Celsius for a half hour. Cells washed with Phosphate Buffered Saline washed were pelleted, labelled, and suspended again in the sheath fluid, and subjected to flow cytometric analysis (Attune NxT Flow Cytometer, Thermo Fisher Scientific, Waltham, MA, USA). Each sample acquired at least 10,000 events. The difference between isotype (control) and the sample positive staining degree was estimated in %. Individual protein median fluorescence intensities were also noted.
2.5. Reverse Transcriptase- quantitative polymerase chain reaction (RT-qPCR)
Employing a Gene JET RNA purification kit (Thermo Scientific, Vilnius, Lithuania), ribonucleic acid was drawn out from the cells. Using a cDNA synthesis kit (High Capacity, Applied Biosystems, Carlsbad, CA, USA) 2 μg of RNA was reverse transcribed. Individual gene (20 μg) reaction volume utilized 100 ng cDNA. SYBR-Green-PCR-master-mix on a Real-Time PCR setup was used to quantitatively assess the genes (Quant Studio 5, Applied Biosystems, Foster City, CA, USA). Target genes HIF1A, BDNF, GDNF, VEGFA, IGF1, NANOG, and OCT4 expressions were normalized to reference gene GAPDH with the ΔΔCt method. Table 1 summarizes the primers employed in the study.
Table 1.
List of primers and sequences used for quantitative polymerase chain reactions.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| HIF1A | 5′-TAT GAG CCA GAA GAA CTT TTA GGC-3′ | 5′-CAC CTC TTT TGG CAA GCA TCC TG-3′ |
| BDNF | 5′-CAT CCG AGG ACA AGG TGG CTT G-3′ | 5′-GCC GAA CTT TCT GGT CCT CAT C-3′ |
| GDNF | 5′-CGC CGA AGA CCG CTC CCT CG-3′ | 5′-ATC CAT GAC ATC ATC GAA CTG ATC-3′ |
| VEGFA | 5′-TTG CCT TGC TGC TCT ACC TCC A-3′ | 5′-GAT GGC AGT AGC TGC GCT GAT A-3′ |
| IGF1A | 5′-CTC TTC AGT TCG TGT GTG GAG AC-3′ | 5′-CAG CCT CCT TAG ATC ACA GCT C-3′ |
| NANOG | 5′-CTC CAA CAT CCT GAA CCT CAG C-3′ | 5′-CGT CAC ACC ATT GCT ATT CTT CG-3′ |
| OCT4 | 5′-CCT GAA GCA GAA GAG GAT CAC C-3′ | 5′-AAA GCG GCA GAT GGT CGT TTG G-3′ |
| GAPDH | 5′-GTC TCC TCT GAC TTC AAC AGC G-3′ | 5′-ACC ACC CTG TTG CTG TAG CCA A-3′ |
2.6. Western blot analysis
RIPA buffer (Sigma Aldrich, St. Louis, MO, USA) was used to lyse the cells, following which centrifugation was carried out for 15 min at 12,000 rpm. Protein assay determined the protein content from the collected supernatant. Lysate was subjected to a sample buffer SDS-PAGE and was heated for 8 min at 100 °C. In individual wells, 20 μg protein was placed with ten percent on Mini-PROTEAN Tetra Cell with SDS-PAGE gel. HIF-1α, BDNF, GDNF, VEGF, IGF, and GAPDH served as the major antibodies (Abcam, Cambridge, UK) while HRP-conjugated secondary served as protein-spotting tools through Western blot.
2.7. Quantitative analysis of neurotrophic factors using ELISA
KRIBIOLISA human ELISA kits were used to analyze soluble proteins like neurotrophic factors BDNF, GDNF, IGF, and VEGF. Cells were obtained from the conditioned media after incubating it with a complete medium. Multiskan FC spectrophotometer was employed to read absorbance at 450 nm.
2.8. Statistical analysis
Triplicates led to the generation of three values from which a summation of data was obtained in the form of mean ± standard deviation was presented for all the 5 assessed samples. An individual comparison of both the study groups was made. The unpaired t-test (two-tailed) was performed to assess each cytokine using version 8 GraphPad Prism software (San Diego, California, USA). A p value of 0 < 0.05 and 0 < 0.01 was considered significant and highly significant respectively.
3. Results
3.1. Gmscs show MSC-like phenotype and cell-surface marker expression
GMSCs were isolated from six patients and cultured. Phenotypic characterization of GMSCs showed MSC-like morphology in the passage 2 population (Fig. 1A). Characterization of GMSCs for the cell surface markers expression was performed with flow cytometry. Cells exhibited positive expression (>95%) for principal mesenchymal markers CD105, CD73 and CD90, (Fig. 1C–E) & -ve expression (<3%) for hematopoietic markers HLA-DR, CD34 and CD45, and (Fig. 1F–H).
Fig. 1.
Isolation and characterization of GMSCs. (A) Photomicrograph of passage 2 with GMSCs. Scale bar = 100 μm. (B-H) Characterization of GMSCs for MSC-specific positive markers CD73, CD90, CD105 and negative markers CD34, CD45, HLA-DR. (GMSCs: Gingival mesenchymal stem cells).
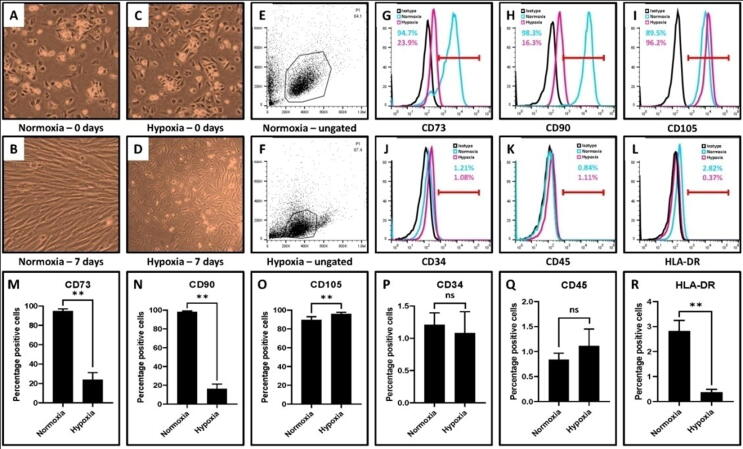
3.2. Gmscs showed different morphological features. Hypoxic GMSCs showed decreased marker expression for CD73, CD90, and HLA-DR, but increased marker expression for CD105
GMSCs were incubated at normoxic (∼20% oxygen) and hypoxic (0.5% – 1% oxygen) conditions for seven days. Changes in the morphology and MSC-specific immunophenotype were characterized in both populations. Hypoxic GMSCs showed a classical fibroblast-like morphology. In normoxic conditions, GMSCs showed elongated and flattened morphology (Fig. 2A–F). Noticeable changes were observed in cell surface markers CD90 and CD73 expression after 7 days of culture among both the groups. GMSCs incubated in a hypoxic environment showed reduced expression of positive markers CD73 (23.9%), CD90 (16.3%) markers. However, there was no difference in CD 105 marker expression between the two populations. HLA-DR, CD34 and CD45, markers expression was –ve in both populations (Fig. 2G–R).
Fig. 2.
Comparative characterization of GMSCs incubated at normoxia (∼20% oxygen) and hypoxia (0.5% – 1% oxygen) for MSC-specific markers. (A,B) Photomicrograph of GMSCs after normoxic incubation at day 0 and day 7. (C,D) Photomicrograph of GMSCs after hypoxic incubation at day 0 and day 7. Scale bar = 100 μm. (E-R) Comparative characterization of GMSCs incubated at normoxia and hypoxia for MSC-specific positive markers CD73, CD90, CD105 and negative markers CD34, CD45, HLA-DR. (ns: insignificant, **p < .01).
3.3. Hypoxia led to the upregulation of the HIF1A gene and trophic factors
GMSCs incubated at two different O2 concentrations were examined for HIF-1α gene expression & trophic factors BDNF, GDNF, VEGF, and IGF1. GMSCs collected after 7 days of culture were subjected to RT-PCR analysis for the target genes. Hypoxia increased the expression of the HIF-1α gene more than sevenfold (p < .01). The expression of neurotrophic factors - BDNF and GDNF increased considerably under hypoxic conditions compared to normoxic conditions (twofold and two and half fold respectively; p < .01, Fig. 3). The expression of VEGF (twelvefold; p > .05) and IGF-1 (sixfold; p < .01) genes were upregulated in GMSCs cultured under hypoxic conditions.
Fig. 3.
RT-qPCR analysis for gene expression in GMSCs incubated at normoxia and hypoxia. (A) Comparative gene expression (fold change) for gene expressing hypoxia-inducible factor (HIF-1α). (B-E) Comparative gene expression (fold change) for genes expressing neurotrophic factors BDNF, GDNF, VEGF, and IGF. (ns: not significant, **p < .01).
3.4. Protein expression of HIF-1α along with neurotrophic factors
Western blot analysis revealed an upregulation in HIF-1α (Hypoxia-inducible factor-1-alpha) protein expression in the hypoxic GMSCs (Fig. 4A). Consistently, the expression of neurotrophic factors BDNF and GDNF as well as the angiogenic factors VEGF and IGF1 were upregulated in the hypoxic GMSCs (Fig. 4A). Protein quantification was done using Enzyme-Linked-Immuno-Sorbent-Assay (ELISA). The expression of HIF-1α protein and other neurotrophic factors were significantly increased in hypoxic GMSCs (p < .01), displayed in Fig. 4B–F.
Fig. 4.
Western blot analysis for protein expression in GMSCs incubated at normoxia and hypoxia. (A, B) Comparative protein expression (optical density) for hypoxia-inducible factor (HIF-1α). (A, C-F) Comparative protein expression (optical density) for neurotrophic factors BDNF, GDNF, VEGF, and IGF. (**p < .01).
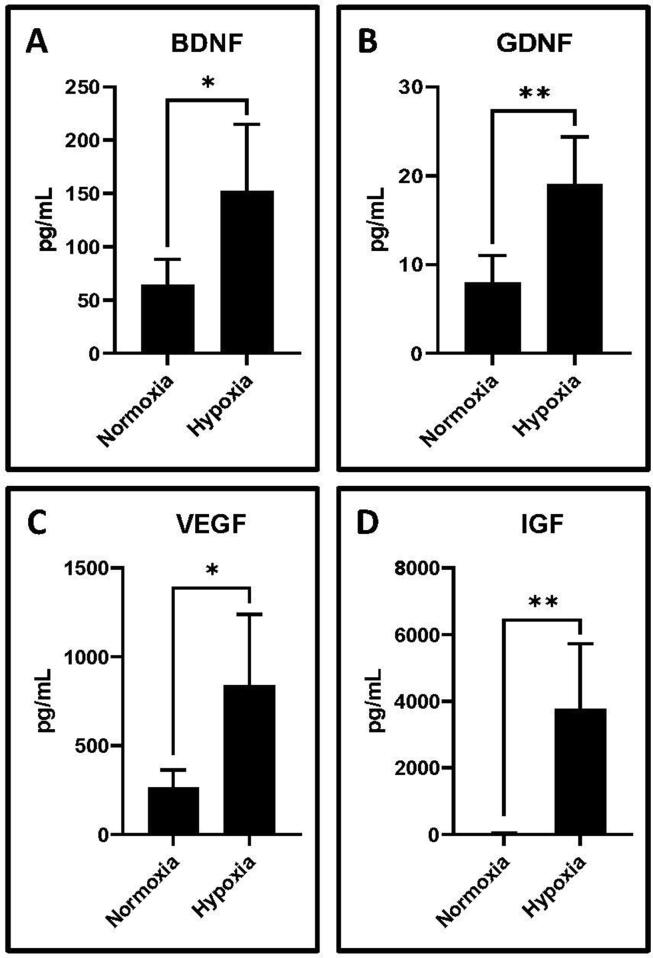
3.5. Secreted neurotrophic factors were higher in hypoxic cultured GMSC conditioned medium
ELISA was performed on GMSC-conditioned media obtained from hypoxic as well as normoxic conditions to assess neurotrophic & angiogenic factor expression in the GMSC secretome. Hypoxic GMSC-CM showed a significant increase of neurotrophic/angiogenic factors BDNF, GDNF, VEGF, and IGF compared to normoxic GMSC-CM (p < .05) (Fig. 5).
Fig. 5.
Bead-based flow cytometry analysis of secretome in GMSC-conditioned medium for secreted proteins by GMSCs incubated at normoxia and hypoxia. Comparative protein levels (pg/mL) for neurotrophic factors (A) BDNF, (B) GDNF, (C) VEGF, and (D) IGF. (*p < .05, **p < .01).
3.6. Differential expression of stemness-related gene NANOG and OCT4 in hypoxic GMSCs
Reverse Transcriptase Polymerase Chain Reaction helped evaluate pluripotency marker levels OCT4 and NANOG and. NANOG expression was significantly upregulated by 1.5 fold in response to hypoxia (p < .01). OCT4 gene expression was significantly downregulated (p < .05) compared to normoxic GMSCs (Fig. 6).
Fig. 6.
RT-qPCR analysis for gene expression in GMSCs incubated at normoxia and hypoxia. (A, B) Comparative gene expression (fold change) for genes expressing stemness-related transcription factors NANOG and OCT4. (*p < .05, **p < .01).
4. Discussion
MSCs can proliferate into cells of multiple origins & possess self-renewal properties. They are multipotent and can differentiate into mesodermic tissue or trans-differentiate into ectodermic (neural or glial) cells (Gugliandolo et al., 2018). Multipotent MSCs originally derived from bone marrow have high therapeutic potential required for stem-cell treatment. However, BMSCs and their clinical application is limited due to difficulty in obtaining an adequate amount of cells that can be used therapeutically use (Zhang et al., 2009). Oral tissues such as the gingiva have abundant GMSCs. Harvesting these cells is a minimally invasive procedure (Gugliandolo et al., 2019) These are similar to BMSCs and stem cells derived from the adipose or the fatty tissue (ADSCs) in that they possess compelling immunomodulatory effects and anti-inflammatory functions. In vivo studies show GMSCs as an ideal source of pluripotent stem cells that can be used in therapeutic applications such as wound healing & inflammatory disorders. (Zhang et al., 2009, Zhang et al., 2012b).
Clinical studies (both animal and human) have demonstrated that transplanting MSCs is an effective treatment modality in case of tissue injuries (Nakagami et al., 2005, Nagaya et al., 2005, Jaussaud et al., 2013). However, their greatest drawback is their low survival rate after grafting into injured sites which have reduced O2 supply (0.4–2.3%) (Rosová et al., 2008) (Leroux et al., 2010). Preconditioning of MSCs has proved to enhance their biological attributes and increased the efficacy of these therapies. Hypoxia is a significant environmental factor that can affect a cell’s metabolism, proliferation, differentiation, and apoptosis. A number of studies in the literature have stated that the preconditioning of MSCs in hypoxic conditions improves its biological properties (Rosová et al., 2008, Gugliandolo et al., 2019). Leroux et al. investigated the effect hypoxia has on preconditioned MSCs (1% O2) in vascular and skeletal muscle regeneration. They found that hypoxia preconditioning improved the functional capabilities of MSC and enhanced the recovery of ischemic tissue (Leroux et al., 2010) Previous research has shown that the culturing of DPSCs (Dental pulp stem cells) under hypoxic conditions (1–5%) enhanced proliferation, angiogenesis, migration, and differentiation. The expression of genes for immunomodulation, markers for pluripotency detection as well as trophic factors increased in hypoxic conditions (Sakdee et al., 2009, Aranha et al., 2010, Ahmed et al., 2016). Hypoxic preconditioning has proved to be a useful technique which improves immunomodulatory activity, enhance skin and wound repair in GMSCs in mice, and promote regenerative characteristics (Jiang et al., 2015). However, few studies have assessed the impact of a hypoxic environment on GMSCs’ differentiation potential.
The current study was proposed with the aim of assessing and comparing the impact of hypoxia on morphology, stem cell marker expression, mRNA & protein expression of trophic factors as well as stemness associated genes in GMSCs. Flow cytometry analysis of hypoxic and normoxic GMSCs was done for immunophenotype characterization. Our results revealed that mesenchymal stem-cell marker CD 105 expression slightly increased in hypoxic (96.2%) condition than normoxic (89.5%) condition. Ahmed et al. examined DPSCs under hypoxic conditions and discovered that the expression of CD105 was the same in normoxic (20% O2) and as well as in hypoxic (3% O2 and 5% O2) groups (Ahmed et al., 2016). The association of CD105 expression in the process of differentiation is not yet clearly explained. We found that the expression of CD73 and CD90 was significantly reduced in hypoxic conditions. Our result of decreased expression CD90 in hypoxic conditions concurs with previous studies where CD90 expression was considerably reduced in hypoxic BMSCs (Adesida et al., 2012, Li et al., 2017). Even then, quite a few studies have argued that there is no detectable difference in the expression CD70 and CD93 markers in MSCs between normoxic and hypoxic conditions (Antebi et al., 2018, Wagegg et al., 2012).
In our study, we analyzed mRNA and protein expression of neurotrophic and angiogenic trophic factors along with the HIF-1α gene under hypoxic conditions.RT-PCR and blot analysis suggested that the HIF-1α expression was significantly increased in hypoxic conditions. Under normoxic conditions (O2 > 5%), translated HIF-1α proteins are denatured in the presence of oxygen-activated prolyl hydroxylase domain proteins. In a hypoxic environment, HIF 1α stabilizes & enters the nucleus to get dimerized with HIF-1β and activates target genes of downstream pathways (Semenza, 2007, Wagegg et al., 2012). HIF-1α protein regulates genes which a play a key role in cellular processes including angiogenesis, anaerobic metabolism, programmed cell death, & inflammation (Wagegg et al., 2012, Ziello et al., 2007). In the current study, GMSC-culturing hypoxia considerably upregulated neuro/angiogenic trophic factor BDNF, GDNF, VEGF, and IGF expression. Protein expression data also proved a greater expression of these in hypoxic-GMSCs as opposed to normoxic cells. We checked trophic factor expression in the GMSC secretome using GMSC-CM harvested from GMSCs cultured in hypoxia as well as normoxia. We found that the level of these factors overlaps with mRNA and protein expression. Neurotrophic factors BDNF and GDNF primarily regulate the development, survival as well as the function of neurons and neural precursor cell differentiation (Lim et al., 2011, Pierce and Bari, 2001, Wagenaar et al., 2018). Lim et al. found that overexpression of BDNF in umbilical cord blood-derived MSCs (UCB-MSCs) induced neural differentiation through the increased phosphorylation of extracellular signal-regulated kinases and β-catenin. Transplantation of BDNF-overexpressed MSCs enhanced the secretion of neurotrophic factors in a model of stroke (Jeong et al., 2014). VEGF and IGF1 are potent angiogenic factors that contribute to the differentiation of MSCs (Ge et al., 2017). Recently conducted investigations have shown that VEGF expression significantly increased in MSCs under hypoxia (Aranha et al., 2010, Dai et al., 2007). Aranha et al. evaluated the impact of a hypoxic environment on DPSCs and observed that hypoxia significantly increased the expression of HIF-1α & VEGF and led to an angiogenic response in DPSCs (Aranha et al., 2010). Overexpression of IGF in BMSCs enhanced the proliferation, increased Nestin expression, and reduced the apoptosis thus promoting differentiation of Mesenchymal Stem Cells into neural precursor cells (NPCs) (Huat et al., 2014, Youssef et al., 2017). In UC-MSCs overexpression of IGF1 stimulated neural differentiation. The derived NPCs have higher proliferation with positive expression of NPC markers PAX6, Nestin, and astrocyte differentiation ability (Zhao et al., 2016). Liang et al. assessed hypoxia's influence on mesenchymal stromal cells derived from the limbus stroma (L-MSC). They prepared a conditioned media (L-MSC-CM) and co-cultured it with cortical neurons under normoxic and hypoxic conditions. Hypoxic L-MSC-CM showed enhanced neurotrophic function and neuroprotective potential. Growth factor expression (VEGF, BDNG & IGF1) was also upregulated under hypoxic L-MSC-CM (Liang et al., 2014). A transcriptional study of hypoxia-preconditioned GMSCs by Gugliandolo et al. revealed a significantly higher expression of genes related to neuronal & cortical differentiation. Upon differentiation, GMCs preconditioned with hypoxia showed greater expression of neurogenic markers PAX6, GAP43, and Nestin, suggesting that hypoxia preconditioning might have a role in enhancing biological properties & neuronal differentiation potential of GMSCs (Gugliandolo et al., 2019).
One of the most crucial traits of MSCs is their pluripotent potential. Transcription factors namely SOX2, OCT4 and NANOG maintain this nature in embryonic stem cells (Pierantozzi et al., 2011, Liu et al., 2009). OCT4 & NANOG regulate the network of differentiation-associated genes by hindering target gene expression. During this process, pluripotent markers are downregulated in embryonic stem cells, activating the differentiation-associated genes (Boyer et al., 2005). In the current study, NANOG upregulation and OCT4 downregulation was seen in the GMSCs cultured in hypoxic conditions. Our results did not conform to those of previous studies. Earlier studies reported that hypoxia enhances the stem-cell related gene expression such as (Klf4, OCT4, NANOG and SOX2) (Kwon et al., 2017, Yamamoto et al., 2013). Tsai et al. verified the escalated expression of OCT4 & NANOG in the hypoxic cultured MSCs. In order to evaluate the function of these genes in the maintenance of stem cell properties in hypoxic Mesenchymal Stem Cells, an shRNA-based knockdown was performed. Knockdown of NANOG and OCT4 considerably reduced their ability to differentiate and proliferate (Tsai et al., 2012). Culturing of DPSCs in hypoxic conditions showed no alterations in the mRNA level of OCT4 & NANOG. Mesenchymal stem cell markers in different MSCs may be controlled by several factors including the origin of stem cells, oxygen concentration used, as well as the duration of exposure to the hypoxic environment which leads to differential expression.
5. Conclusion
This research examined the influence of hypoxia on GMSCs by assessing the expression of stem-cell markers, neurotrophic & angiogenic factors. Increased expression and secretion of neurotrophic factors in hypoxic cultured GMSCs suggests that GMSC might possess therapeutic applications in the management and treatment of neuronal diseases.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The publication was supported by the Taif University Researchers Supporting Program (Project number: TURSP-2020/151), Taif University, Saudi Arabia.
Institutional ethics committee approval
The current study was conducted based on the protocol approved via the Scientific Research Committee, College of Dentistry, Jazan University, Kingdom of Saudi Arabia (CODJU-19243)
Informed consent statement
All subjects that were included in the study gave their informed consent after a detailed and comprehensive explanation.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adesida A.B., Mulet-Sierra A., Jomha N.M. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res. Ther. 2012;3:9. doi: 10.1186/scrt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N.-E.-M.-B., Murakami M., Kaneko S., Nakashima M. The effects of hypoxia on the stemness properties of human dental pulp stem cells (DPSCs) Sci. Rep. 2016;6:35476. doi: 10.1038/srep35476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelopoulos I., Brizuela C., Khoury M. Gingival Mesenchymal Stem Cells Outperform Haploidentical Dental Pulp-derived Mesenchymal Stem Cells in Proliferation Rate, Migration Ability, and Angiogenic Potential. Cell Transplant. 2018;27(6):967–978. doi: 10.1177/0963689718759649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antebi B., Rodriguez L.A., Walker K.P., Asher A.M., Kamucheka R.M., Alvarado L., Mohammadipoor A., Cancio L.C. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res. Ther. 2018;9(1) doi: 10.1186/s13287-018-1007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranha A.M.F., Zhang Z., Neiva K.G., Costa C.A.S., Hebling J., Nör J.E. Hypoxia Enhances the Angiogenic Potential of Human Dental Pulp Cells. J. Endod. 2010;36(10):1633–1637. doi: 10.1016/j.joen.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Boyer L.A., Lee T.I., Cole M.F., Johnstone S.E., Levine S.S., Zucker J.P., Guenther M.G., Kumar R.M., Murray H.L., Jenner R.G., Gifford D.K., Melton D.A., Jaenisch R., Young R.A. Core Transcriptional Regulatory Circuitry in Human Embryonic Stem Cells. Cell. 2005;122(6):947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Xu M., Wang Y., Pasha Z., Li T., Ashraf M. HIF-1α induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J. Mol. Cell. Cardiol. 2007;42(6):1036–1044. doi: 10.1016/j.yjmcc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diar-Bakirly S., El-Bialy T. Human gingival fibroblasts: Isolation, characterization, and evaluation of CD146 expression. Saudi J. Biol. Sci. 2021;28(4):2518–2526. doi: 10.1016/j.sjbs.2021.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz I.M.A., Chen C., Ansari S., Zadeh H.H., Moshaverinia M., Chee D., Marques M.M., Shi S., Moshaverinia A. Gingival Mesenchymal Stem Cell (GMSC) Delivery System Based on RGD-Coupled Alginate Hydrogel with Antimicrobial Properties: A Novel Treatment Modality for Peri-Implantitis. J. Prosthodont. 2016;25(2):105–115. doi: 10.1111/jopr.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejtehadifar M., Shamsasenjan K., Movassaghpour A., Akbarzadehlaleh P., Dehdilani N., Abbasi P., Molaeipour Z., Saleh M. The Effect of Hypoxia on Mesenchymal Stem Cell Biology. Adv. Pharm. Bull. 2015;5:141–149. doi: 10.15171/apb.2015.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge, Q., Zhang, H., Hou, J., Wan, L., Cheng, W., Wang, X., Dong, D., Chen, C., Xia, J., Guo, J., Chen, X., Wu, X., 2017. VEGF secreted by mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Mol. Med. Rep. https://doi.org/10.3892/mmr.2017.8059 [DOI] [PMC free article] [PubMed]
- Gugliandolo A., Diomede F., Cardelli P., Bramanti A., Scionti D., Bramanti P., Trubiani O., Mazzon E. Transcriptomic analysis of gingival mesenchymal stem cells cultured on 3D bioprinted scaffold: A promising strategy for neuroregeneration. J. Biomed. Mater. Res. Part A. 2018;106(1):126–137. doi: 10.1002/jbm.a.36213. [DOI] [PubMed] [Google Scholar]
- Gugliandolo A., Diomede F., Scionti D., Bramanti P., Trubiani O., Mazzon E. The Role of Hypoxia on the Neuronal Differentiation of Gingival Mesenchymal Stem Cells: A Transcriptional Study. Cell Transplant. 2019;28(5):538–552. doi: 10.1177/0963689718814470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque N., Kasim N.H.A., Rahman M.T. Optimization of Pre-transplantation Conditions to Enhance the Efficacy of Mesenchymal Stem Cells. Int. J. Biol. Sci. 2015;11(3):324–334. doi: 10.7150/ijbs.10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque N., Rahman M.T., Abu Kasim N.H., Alabsi A.M. Hypoxic Culture Conditions as a Solution for Mesenchymal Stem Cell Based Regenerative Therapy. Sci. World J. 2013;2013:1–12. doi: 10.1155/2013/632972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henig I., Zuckerman T. Hematopoietic Stem Cell Transplantation—50 Years of Evolution and Future Perspectives. Rambam Maimonides Med. J. 2014;5(4):e0028. doi: 10.5041/RMMJ.10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmadcha A., Martin-Montalvo A., Gauthier B.R., Soria B., Capilla-Gonzalez V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huat T.J., Khan A.A., Pati S., Mustafa Z., Abdullah J.M., Jaafar H. IGF-1 enhances cell proliferation and survival during early differentiation of mesenchymal stem cells to neural progenitor-like cells. BMC Neurosci. 2014;15:91. doi: 10.1186/1471-2202-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung S.-P., Ho J.H., Shih Y.-R., Lo T., Lee O.K. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. J. Orthop. Res. 2012;30(2):260–266. doi: 10.1002/jor.21517. [DOI] [PubMed] [Google Scholar]
- Jaussaud J., Biais M., Calderon J., Chevaleyre J., Duchez P., Ivanovic Z., Couffinhal T., Barandon L. Hypoxia-preconditioned mesenchymal stromal cells improve cardiac function in a swine model of chronic myocardial ischaemia. Eur. J. Cardio-Thoracic Surg. 2013;43:1050–1057. doi: 10.1093/ejcts/ezs549. [DOI] [PubMed] [Google Scholar]
- Jeong C.H., Kim S.M., Lim J.Y., Ryu C.H., Jun J.A., Jeun S.-S. Mesenchymal Stem Cells Expressing Brain-Derived Neurotrophic Factor Enhance Endogenous Neurogenesis in an Ischemic Stroke Model. Biomed Res. Int. 2014;2014:1–10. doi: 10.1155/2014/129145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C.M., Liu J., Zhao J.Y., Xiao L., An S., Gou Y.C., Quan H.X., Cheng Q., Zhang Y.L., He W., Wang Y.T., Yu W.J., Huang Y.F., Yi Y.T., Chen Y., Wang J. Effects of Hypoxia on the Immunomodulatory Properties of Human Gingiva-Derived Mesenchymal Stem Cells. J. Dent. Res. 2015;94(1):69–77. doi: 10.1177/0022034514557671. [DOI] [PubMed] [Google Scholar]
- Kim K.-W., Moon S.-J., Park M.-J., Kim B.-M., Kim E.-K., Lee S.-H., Lee E.-J., Chung B.-H., Yang C.-W., Cho M.-L. Optimization of adipose tissue-derived mesenchymal stem cells by rapamycin in a murine model of acute graft-versus-host disease. Stem Cell Res. Ther. 2015;6:202. doi: 10.1186/s13287-015-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S.Y., Chun S.Y., Ha Y.-S., Kim D.H., Kim J., Song P.H., Kim H.T., Yoo E.S., Kim B.S., Kwon T.G. Hypoxia Enhances Cell Properties of Human Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2017;14(5):595–604. doi: 10.1007/s13770-017-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux L., Descamps B., Tojais N.F., Séguy B., Oses P., Moreau C., Daret D., Ivanovic Z., Boiron J.-M., Lamazière J.-M., Dufourcq P., Couffinhal T., Duplàa C. Hypoxia Preconditioned Mesenchymal Stem Cells Improve Vascular and Skeletal Muscle Fiber Regeneration After Ischemia Through a Wnt4-dependent Pathway. Mol. Ther. 2010;18(8):1545–1552. doi: 10.1038/mt.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Li C., Zhu M., Zhang Y., Du J., Xu Y., Liu B., Gao F., Liu H., Cai J., Yang Y. Hypoxia-Induced Mesenchymal Stromal Cells Exhibit an Enhanced Therapeutic Effect on Radiation-Induced Lung Injury in Mice due to an Increased Proliferation Potential and Enhanced Antioxidant Ability. Cell. Physiol. Biochem. 2017;44:1295–1310. doi: 10.1159/000485490. [DOI] [PubMed] [Google Scholar]
- Liang C.-M., Weng S.-J., Tsai T.-H., Li I.-H., Lu P.-H., Ma K.-H., Tai M.-C., Chen J.-T., Cheng C.-Y., Huang Y.-S. Neurotrophic and neuroprotective potential of human limbus-derived mesenchymal stromal cells. Cytotherapy. 2014;16(10):1371–1383. doi: 10.1016/j.jcyt.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Lim J.Y., Park S.I., Kim S.M., Jun J.A., Oh J.H., Ryu C.H., Jeong C.H., Park S.H., Park S.A., Oh W., Chang J.W., Jeun S.-S. Neural Differentiation of Brain-Derived Neurotrophic Factor-Expressing Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells in Culture via TrkB-Mediated ERK and β-Catenin Phosphorylation and following Transplantation into the Developing Brain. Cell Transplant. 2011;20(11-12):1855–1866. doi: 10.3727/096368910X557236. [DOI] [PubMed] [Google Scholar]
- Liu T.M., Wu Y.N., Guo X.M., Hui J.H.P., Lee E.H., Lim B. Effects of Ectopic Nanog and Oct4 Overexpression on Mesenchymal Stem Cells. Stem Cells Dev. 2009;18(7):1013–1022. doi: 10.1089/scd.2008.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya N., Kangawa K., Itoh T., Iwase T., Murakami S., Miyahara Y., Fujii T., Uematsu M., Ohgushi H., Yamagishi M., Tokudome T., Mori H., Miyatake K., Kitamura S. Transplantation of Mesenchymal Stem Cells Improves Cardiac Function in a Rat Model of Dilated Cardiomyopathy. Circulation. 2005;112(8):1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- Nakagami H., Maeda K., Morishita R., Iguchi S., Nishikawa T., Takami Y., Kikuchi Y., Saito Y., Tamai K., Ogihara T., Kaneda Y. Novel Autologous Cell Therapy in Ischemic Limb Disease Through Growth Factor Secretion by Cultured Adipose Tissue-Derived Stromal Cells. Arterioscler. Thromb. Vasc. Biol. 2005;25(12):2542–2547. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- Parekkadan B., Milwid J.M. Mesenchymal Stem Cells as Therapeutics. Annu. Rev. Biomed. Eng. 2010;12(1):87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierantozzi E., Gava B., Manini I., Roviello F., Marotta G., Chiavarelli M., Sorrentino V. Pluripotency Regulators in Human Mesenchymal Stem Cells: Expression of NANOG But Not of OCT-4 and SOX-2. Stem Cells Dev. 2011;20(5):915–923. doi: 10.1089/scd.2010.0353. [DOI] [PubMed] [Google Scholar]
- Pierce R.C., Bari A.A. The Role of Neurotrophic Factors in Psychostimulant-induced Behavioral and Neuronal Plasticity. Rev. Neurosci. 2001;12 doi: 10.1515/REVNEURO.2001.12.2.95. [DOI] [PubMed] [Google Scholar]
- Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science (80-. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Rosová I., Dao M., Capoccia B., Link D., Nolta J.A. Hypoxic Preconditioning Results in Increased Motility and Improved Therapeutic Potential of Human Mesenchymal Stem Cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakdee J.B., White R.R., Pagonis T.C., Hauschka P.V. Hypoxia-amplified Proliferation of Human Dental Pulp Cells. J. Endod. 2009;35(6):818–823. doi: 10.1016/j.joen.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Semenza G.L. Hypoxia-Inducible Factor 1 (HIF-1) Pathway. Sci. STKE. 2007;2007(407) doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- Tomar G.B., Srivastava R.K., Gupta N., Barhanpurkar A.P., Pote S.T., Jhaveri H.M., Mishra G.C., Wani M.R. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem. Biophys. Res. Commun. 2010;393(3):377–383. doi: 10.1016/j.bbrc.2010.01.126. [DOI] [PubMed] [Google Scholar]
- Tsai C.-C., Su P.-F., Huang Y.-F., Yew T.-L., Hung S.-C. Oct4 and Nanog Directly Regulate Dnmt1 to Maintain Self-Renewal and Undifferentiated State in Mesenchymal Stem Cells. Mol. Cell. 2012;47(2):169–182. doi: 10.1016/j.molcel.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Vu, M.Q., Der Sarkissian, S., Borie, M., Bessette, P.-O., Noiseux, N., 2016. Optimization of Mesenchymal Stem Cells to Increase Their Therapeutic Potential. pp. 275–288. https://doi.org/10.1007/978-1-4939-3584-0_16 [DOI] [PubMed]
- Wagegg, M., Gaber, T., Lohanatha, F.L., Hahne, M., Strehl, C., Fangradt, M., Tran, C.L., Schönbeck, K., Hoff, P., Ode, A., Perka, C., Duda, G.N., Buttgereit, F., 2012. Hypoxia Promotes Osteogenesis but Suppresses Adipogenesis of Human Mesenchymal Stromal Cells in a Hypoxia-Inducible Factor-1 Dependent Manner. PLoS One 7, e46483. https://doi.org/10.1371/journal.pone.0046483 [DOI] [PMC free article] [PubMed]
- Wagenaar N., de Theije C.G.M., de Vries L.S., Groenendaal F., Benders M.J.N.L., Nijboer C.H.A. Promoting neuroregeneration after perinatal arterial ischemic stroke: neurotrophic factors and mesenchymal stem cells. Pediatr. Res. 2018;83(1-2):372–384. doi: 10.1038/pr.2017.243. [DOI] [PubMed] [Google Scholar]
- Watt F.M., Driskell R.R. The therapeutic potential of stem cells. Philos. Trans. R. Soc. B Biol. Sci. 2010;365(1537):155–163. doi: 10.1098/rstb.2009.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodsworth D.J., Holt R.A. Cell-Based Therapeutics: Making a Faustian Pact with Biology. Trends Mol. Med. 2017;23(2):104–115. doi: 10.1016/j.molmed.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Xu X., Chen C., Akiyama K., Chai Y., Le A.D., Wang Z., Shi S. Gingivae Contain Neural-crest- and Mesoderm-derived Mesenchymal Stem Cells. J. Dent. Res. 2013;92(9):825–832. doi: 10.1177/0022034513497961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Fujita M., Tanaka Y., Kojima I., Kanatani Y., Ishihara M., Tachibana S. Low Oxygen Tension Enhances Proliferation and Maintains Stemness of Adipose Tissue-Derived Stromal Cells. Biores. Open Access. 2013;2(3):199–205. doi: 10.1089/biores.2013.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef A., Aboalola D., Han V.K.M. The Roles of Insulin-Like Growth Factors in Mesenchymal Stem Cell Niche. Stem Cells Int. 2017;2017:1–12. doi: 10.1155/2017/9453108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Nguyen A.L., Shi S., Hill C., Wilder-Smith P., Krasieva T.B., Le A.D. Three-Dimensional Spheroid Culture of Human Gingiva-Derived Mesenchymal Stem Cells Enhances Mitigation of Chemotherapy-Induced Oral Mucositis. Stem Cells Dev. 2012;21(6):937–947. doi: 10.1089/scd.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Shi S., Liu Y.i., Uyanne J., Shi Y., Shi S., Le A.D. Mesenchymal Stem Cells Derived from Human Gingiva Are Capable of Immunomodulatory Functions and Ameliorate Inflammation-Related Tissue Destruction in Experimental Colitis. J. Immunol. 2009;183(12):7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.Z., Nguyen A.L., Yu W.H., Le A.D. Human Oral Mucosa and Gingiva. J. Dent. Res. 2012;91(11):1011–1018. doi: 10.1177/0022034512461016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Feng Y., Chen X., Yuan J., Liu X., Chen Y., Zhao Y., Liu P., Li Y. Effects of IGF-1 on neural differentiation of human umbilical cord derived mesenchymal stem cells. Life Sci. 2016;151:93–101. doi: 10.1016/j.lfs.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Ziello J.E., Jovin I.S., Huang Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J. Biol. Med. 2007;80:51–60. [PMC free article] [PubMed] [Google Scholar]