Abstract
Lung injuries are attributed due to exposure to Drugs or chemicals. One of the important challenging situations for the clinicians is to manage treatments of different diseases with acute lung injury (ALI). The objective of this study was to investigate the possible protective mechanisms and action of a novel Phosphodiesterase-4 inhibitor “Apremilast” (AP) in lipopolysaccharide (LPS)-induced lung injury. Blood sample from each animals were collected in a vacuum blood collection tube. The rat lungs were isolated for oxidative stress assessment, western blot analysis and their mRNA expressions using RT-PCR. Exposure of LPS in rats causes significant increase in oxidative stress, activates the pro-inflammatory cytokines release like tissue necrotic factor-alpha (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6), modulated gene expression, protein expression and histopathological changes which were reversed by administration of AP. Finding of the research enlighten the protective role of AP against LPS-induced ALI.
Keywords: Acute lung injury, Lipopolysaccharides, Apremilast, Oxidative stress, mRNA expressions
1. Introduction
Damage to lungs parenchyma and alveolar septa results in merging of two or more alveolar cells which decrease gaseous exchange that is considered as pulmonary or alveolar toxicity, which may be short periods or everlasting (Johnson and Matthay, 2010). Reversible damage refers as acute lung injury (ALI)/toxicity (Satkirin et al., 2007; Khalsa et al., 2007). Consequently acute inflammation/parenchymal lung disease causes disruption of the endothelial and epithelial barrier in the lung, which is characterized by dyspnoea to a rapid terminal failure of the respiration. Irreversible or long lasting or permanent damage to the lungs is called as chronic or late pulmonary toxicity.
The membrane of the alveolar capillary is comprised of microvascular endothelium, interstitium and alveolar epithelium. ALI considered as a foundation of acute respiratory distress syndrome (ARDS) manifested by severe form of hypoxemia, swelling and decreased neutrophils (Lee et al., 2001; Lee and Downey 2001). ARDS is generated by high grade sepsis, strain, shock, hyperacidosis, inspiration of harmful gas, etc. (Avecillas et al., 2006). The pathological mechanisms of ALI presents apoptotic derangements and unregulated release of granulocytes, the maladies associated with cytokines and oxidative free radicals/anti-oxidant mechanisms (Rubenfeld et al., 2005). Clinical presentation of ALI imposes as problem that cause high mortality rates of 30–50 % (Ware and Matthay, 2000), and effective measures are still lacking to manage it. Therefore, it becomes necessary to explore the mechanism of ALI development and to search for new efficacious drugs options for its treatment.
Lipopolysaccharides (LPS) are metabolic end products of the Gram-negative bacterial as a main component of outer cell wall (membrane) which are able to induce infections, tissue injuries and/or inflammation, as well as biochemical changes in the form of inflammation. It is biologically active having potential to stimulates an innate immune response and agonizes the Toll-like receptor 4 (TLR4) mediated action (Park and Lee, 2013). Activated TLR4 leads to further activation of the nuclear factor-kappa β (NF-kβ) and mitogen-activated protein kinases (MAPK) signaling pathway (Park and Lee, 2013; Jesse et al., 1999; Chow et al., 1999).
In NF-kB signaling pathways, IKK activate NF-kB by phosphorylating the IKKα or IKKβ in an IKKγ-dependent manner which is triggered by release and interaction of inflammatory mediators (cytokines), endo/exo toxins, and proteins serving as receptors of the antigens (Li et al., 1999, Chen and Greene, 2004, Liang et al., 2014, Lai et al., 2017, Liu et al., 2019, Cheng et al., 2021). Studies reported in knock-out mice that, in the developing liver (embryonic stage) high rate of embryonic mortality in IKKβ-deficient mice is mediated by extensive liver apoptosis because of defective TNF or IL signaling to NF-κB (Liu, 2019 #476; Lai, 2017 #455). IKK's role in conventional NF-κB signaling is still unclear; however, more recent research has discovered that IKK modulates gene expression through modifying histone and p65 phosphorylation status (Chen and Greene, 2004).
It was reported in the literature that exposure of LPS to mammalian cell/tissue can causes release of inflammation triggering cytokines which results in further activation of inflammatory cascades including cytokines; tumor necrosis factor-α (TNF-α), interneukine-1β (IL-1β), ROS, iNOS and cyclooxygenase −2 (CO-2) (Kharitonov and Sjöbring 2007). Recently, increasing evidences suggested that activation of numerous inflammatory responses by LPS results in prolonged obstructive respiratory diseases/COPD, diabetes, neurodegenerative diseases and osteoporosis etc. (Lee et al., 2011, Wang et al., 2007, Campbell, 2004).
A well-known intracellular messenger cyclic adenosine monophosphate (cAMP) is abundantly present in inflammatory cells and is responsible for regulating inflammatory responses. Among the super family of Phosphodiesterase (PDE) enzymes, the four-gene PDE4 family is claimed to be responsible for the degradation of cAMP in the inflammatory cells, vascular endothelial cell linings, and cells of the outermost layer of the skin. Currently, it is believed that Phosphodiesterase type-4 (PDE-4) enzymes is playing a central role in all kinds of cAMP mediated cell signaling. (Page and Spina, 2011, Houslay et al., 2005). PDE-4 inhibitors are emerged as a new class of drugs having broad spectrum anti-inflammatory influences (in both in-vitro and in-vivo) against asthma, pulmonary granulocytosis, diseases of bones and joints (osteoporosis and arthritis), inflammatory diseases of large intestine, sclerosis, and other conditions (Houslay et al., 2005, Keshavarzian et al., 2007). Apremilast is an orally available selective inhibitor of the PDE-4 enzyme and inhibits spontaneous production of TNF-a. (Conti and Beavo, 2007, Gobejishvili et al., 2008). It is a small-molecular novel compound available orally taken by mouth and approved by FDA for psoriatic arthritis (March 2014) and plaque psoriasis (September 2014) (Otezla SmPC, 2020). Apremilast inhibits PDE-4 which inturn elevates intracellular levels of cAMP and thus prevents generations of several pro-inflammatory mediators such as TNF-a, IL-2, IFN-γ, leukotrienes and nitric oxide synthase (Page and Spina, 2011, Keshavarzian et al., 2007, Conti and Beavo, 2007, SmPC, 2020). The contemporary research protocol was an attempt to determine the protective role of apremilast and involvement of protein kinase B (PKB), also known as Akt, is the collective name of a set of three serine/threonine-specific protein kinases and extracellular-signal-regulated kinase (ERK) pathways against LPS-induced Lung grievance through modulation of oxidative stress and expressions of mediators of inflammation.
2. Methods
2.1. Chemicals and reagents
The drug Apremilast procured from Beging Mesochem Ltd., (Beiging, China) and LPS from Sigma Aldrich (St Louis, USA). ELISA kits from EMD Millipore (Massachusetts, USA). The primer for the mRNA gene expression, PCR Master Mix, High-capacity cDNA reverse transcription kits and SYBR® Green were of Applied Biosystems (Paisley, UK). Reagent TRIzol® was of Life Technologies (Grand Island, USA). RIPA lysis buffer were purchased from Thermos Scientific (Rockford, USA). Reagent Bradford for protein quantification, Sigma Aldrich (, USA). Primary and secondary antibody for western blot analysis Santa Cruz (USA). Immobilon®-FL PVDF transfer membrane and Chemiluminescent HRP Substrate for Western blot detection kits were delivered by Merck Millipore Ltd (Oakville, Canada) and Millipore Corporation, (Billerica, USA) respectively. For histopathological staining Hematoxylin and Eosin were of Sigma Aldrich (St Louis, USA). All other chemicals of analytical purity were purchased from commercial suppliers and used in experimental protocol.
2.2. Animals
Albino Wistar rats (male) of 8–10 weeks (200 ± 20 g) were procured from Experimental animal care center, College of Pharmacy, King Saud University, Riyadh, KSA and were maintained at standard laboratory condition of light and darkness cycle (12/12 h), relative humidity (45–55%), temperature (23 ± 2° C), standard pallet diet and water ad-libitum. All procedure were carried out as per the approved protocol and according to the standard guideline of institutional ethical committee, King Saud University and was in compliance with ARRIVE guidelines.
2.3. Experimental scheme
Thirty (30) healthy rats were carefully chosen and allocated to five (5) groups (n = 6): Group 1 was Normal control (NC) group and received 50 µL of 1% carboxymethyl cellulose (CMC) only, intranasally (i.n.) daily, under ketamine anesthesia for seven days. Second group served as toxic group and was administered LPS (20 µg/rats i.n.) with normal saline (50 µL) to induce lung injury on day 7. 3rd 4th groups were treatment group, exposed to LPS same as in group 2 plus apremilast (10 & 20 mg/kg/day, p.o.) by oral gavage respectively for 7 days. Dose of apremilast was selection on the basis of previous studies conducted (Imam et al., 2019, Imam et al., 2018). Group 5 served as standard group and receives dexamethasone 5 mg/kg/day, i.p., for seven days.
At the end of experimental procedures blood sample were collected 24 h after last dose from retro-orbital plexuses and later using ketamine and sacrificed by decapitation method. The rats lung were incised and cleaned blood stains with ice cold phosphate buffer and then divided in to several pieces for oxidative stress marker analysis, mRNA expression, Protein expression and right lobe for histopathological examination under light microscope. These tissue samples were kept at −70 °C until analysis except histopathological sample which were stored at room temperature. The blood sample collected from rats were centrifuge at 3000g for 10 min to separate serum for biochemical analysis.
3. Biochemical analysis
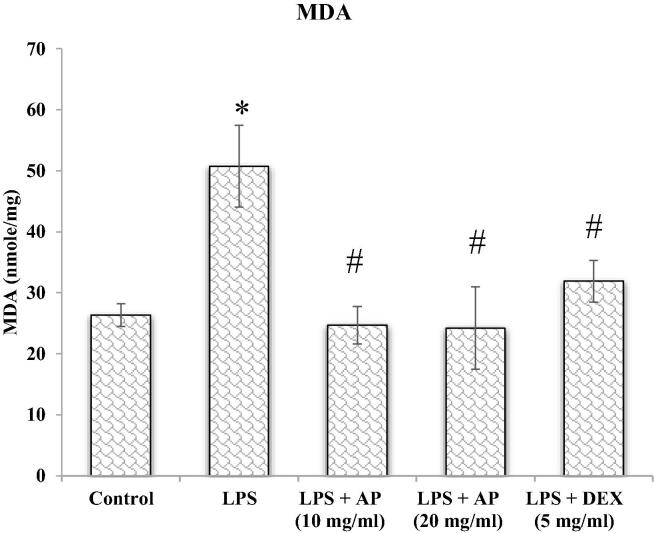
3.1. Malondialdehyde (MDA)
Tissue MDA levels concludes lipid peroxidation, which finally advises about the tissue/cellular stress, due to formation of reactive free radical facilitated response. Increased MDA levels in tissue are referred to as higher lipid peroxidation and increased tissue/cellular damage (Imam et al., 2016). MDA were assayed as per the modified methods of (Impellizzeri et al., 2011). In a reaction mixture 2 ml of sample or blank were taken followed by addition of l ml of acetic acid and thiobarbituric acid (TBA) each, respectively. The reaction mix was then heated (for 1 h at 90 °C) followed by cooling under running water, later centrifuged for 10 min at 8000g. The supernatant was separated and absorbance recorded at 650 nm against a blank by a UV spectrophotometer. The results of the assay were presented as Nano-moles of MDA per mg of protein.
3.2. Tissue glutathione (GSH) intensities
The levels of GSH was measured by using modified method of Jolly and Colleague’s (Jollow et al., 1974). Took 2 ml homogenates in a test tube, then added 2.5 ml of 0.02 M Ethylenediamine tetraacetic acid (EDTA) followed by 4 ml of distilled water and 1 ml of 50% Trichloroacetic acid (TCA) solution. The resulting mixture was shaken intermittently for 15 min followed by centrifugation at 3000g for 10 min. Fresh 2 ml of supernatant were taken in another tube and mixed by shaking with 4 ml of 0.4 M tris buffer (pH-8.9) and 0.1 ml DTNB solution. The reading was measured by measuring optical density using UV-spectrophotometer (Shiamszu, UV-1601, Japan) at 412 nm within 5 min after of adding DTNB solution. The results are presented micromoles of GSH per mg of protein.
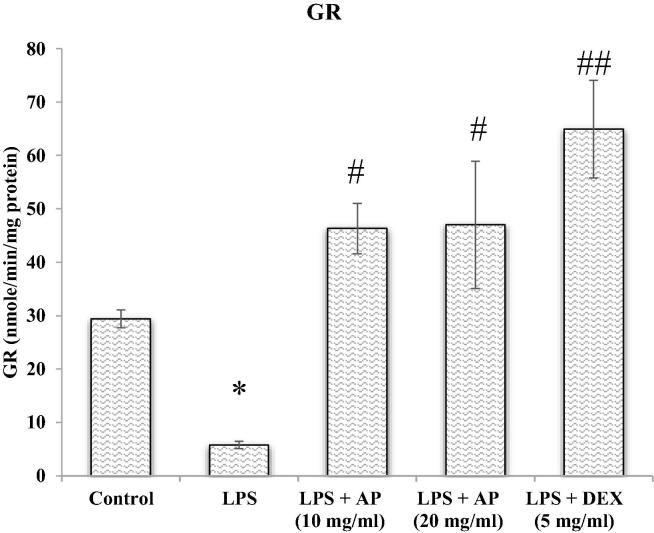
3.3. Glutathione reductase (GR) potential
Modified Calberg and Mannervik's method was followed using a microplate reader to determine the glutathione reductase activity (Carlberg and Mannervik 1985). For the conversion from oxidized form of glutathione (GSSG) to reduced form, the GR enzymes use NADPH as an electron donor (GSH). The amount of NADPH oxidized per minute was recorded spectrophotometer at 25 °C and 340 nm represents an enzymatic activity. The GR activity was represented by nmol of NADPH oxidized/min/mg of protein using a molar extinct coefficient of 6.22 × 103 per mole per cm.
3.4. Elisa assay for interleukins (IL-1β, IL-6) and TNF-α (Kit, 2019)
The levels of serum TNF-α and (IL-1β, IL-6) were quantified using ELISA kits and a 96-well strip plate pre-coated with an incarcerated antibody. The kits were specific for the detection and accurate quantifications of inflammatory markers (IL-1β, IL-6 and TNF-α) in serum. All the kit reagent were first brought to room temperature and sample were prepared freshly as per the manufacture instructions.
Added Matrix-C and Assay buffer–A to the wells containing standard dilution and samples respectively. Post incubation of 2 h plate content were rejected and washed, then added detection antibody, the plate was vacuum-packed and incubated in a shaking incubator at normal room temperature for 1 h. Waste discarded, plate washed and added solution Avidin-HRP-A to each well. The plate vacuum sealed again and incubated on shaker for 30 min. Waste rejected, plate washed and added with substrate (solution F) to each well and incubated in dark for 10 min. Wells turned blue with an intensity relative to concentration. After addition of stop solution to each wells color of the solution changed to yellow. Measure the absorbance at 450 nm within 30 min.
3.5. RNA extraction and cDNA synthesis
Ice cold reagents and chilled plate form were used for all the extraction procedures. Reagent Trizol was used to isolate entire cellular RNA from pulmonary homogenates (Invitrogen, California, USA) according to standard procedures instructed by the manufacturer and quantified by recording the absorbance at wavelength of 260 nm. The quality and purity of the RNA was measured by the 260/280 ratio (>2.0) were used for the extraction. For cDNA synthesis, 1.25 µg of total (extracted) RNA of each sample added to a blend containing reverse transcription buffer (2.0 µL of 10X), dNTP mix. {0.8 µL (100 mM) of 25X}, random primers (reverse transcriptase) {2.0 µL of 10X}, reverse transcriptase (multi-scribe) (1.0 µL), and the water free from nuclease (3.2 µL). Finally obtained reaction mixture set aside for 10 mins at room temperature, later on heated for 2 Hrs (37 °C) followed by 85 °C for 5 min and then cooled at 4 °C (Imam et al., 2015).
3.6. Quantitative mRNA expressions via RT-PCR
Quantification of particular gene mRNA expressions was done by RT-PCR using synthesized cDNA from above methods though PCR amplification done by optical 96-well reaction plate (ABI Prism 7500 System, Applied Bio-system, USA). The whole reaction mix was roughly 25 µL that comprised of (0.1 µL of 10 µM) of forward primer and reverse primer each, Master Mix (SYBR green) 12.5 µL, Water (Nuclease free) 11.5 µL and cDNA (1.25 µL). The primers (forward and reverse) were purchased from Integrated DNA technologies, (USA) and were selected from PubMed and other data base which are listed in Table 1. The alteration in mRNA expressions were recorded as fold of change in the expressions of target mRNA between controls and treatment groups of animals and were correlated by the level of B-actin. Data of the individual gene expressions determined by comparative gene expression (i.e. ΔΔ CT) method (user bulletin number 2, Applied Bio-system). Precisely data are expressed as the fold of variation in mRNA expressions normalized to an endogenous reference gene (B-actin) and comparative to calibrator (Imam et al., 2019).
Table 1.
Rat primers sequence used for RT-PCR reactions.
| Gene | Forward primer | Reverse Primer |
|---|---|---|
| TNF-α | ATTGACAGACGGCGAAGA | ATTTGGAGGAGCGAACTAAG |
| GST | GCTTTACTGTGCAAGGGAGACA | GGAAGGAGGATTCAAGTCAGGA |
| Akt 1 | TGGACTACCTGCACTCGGAGAA | GTGCCGCAAAAGGTCTTCATGG |
| ERK | GCTGACCCTGAGCACGACCA | CTGGTTCATCTGTCGGATCA |
| p53 | ACAGCGTGGTGGTACCGTAT | GGAGCTGTTGCACATGTACT |
| β-actin | CCAGATCATGTTTGAGACCTTCAA | GTGGTACGACCAGAGGCATACA |
3.7. Western blot analysis
Lung tissue sample was homogenized with lysis buffer having protease enzyme inhibitor cocktail for extraction of protein and bicinchoninic acid protein assay method was used for the quantitative analysis of protein sample for running western blot analysis as described previously (Lowry et al., 1951, Barakat et al., 2001, Al-Harbi et al., 2020). Concisely, 25–50 µg samples from each group were loaded in the well of 10% SDS-polyacrylamide gel electrophoresis (PAGE) and separated by electrophoresis followed by transfer of blot from the gel to PVDF/nitrocellulose membrane (Bio-Rad, USA) by electrophoresis. After transfer membrane was kept immersed in blocking solution at 4 °C with rocking followed by incubation overnight with primary antibodies against NF-kB-p65, and IkB-a (Santa Cruz, USA). The membrane having blot then washed with TBST 3–5 time followed by incubation with secondary peroxidase-conjugated antibodies at room temperature with rocking. Immobilon Western Chemiluminescent HRP substrate were used for the visualization of bands using enhanced Chemiluminescence method and the band was quantified relative to housekeeping protein standard (B-actin) bands using the ImageJ® image processing program (National Institute of Health, Bethesda, USA).
3.8. Histopathological evaluation
Lung tissue were excised and fixed in 10% formal saline (formalin) solution, decalcified with ethylene diamine tetra-acetic acid using 5% formic acid as base, fixed in paraffin and sectioned (3–4 μm). Staining of the cut section were done with 1% hematoxylin for 3 min, rinsed with distilled water then treated with 1% eosin in 90% alcohol for 1 min. Finally slides were dried for histology microscopically. Necrotic Alveolar cells, angiogenesis, and phagocytosis were assessed.
3.9. Statistical analysis
All data expressed as mean ± Standard error of Mean (n = 6). One-way ANOVA followed by the Newman-Keuls Multiple comparison test were used for statistical analysis. p < 0.05 stated as significant statistically, *p < 0.05 designated to show comparison between treatment and normal control groups; #p < 0.05 denotes comparison between treatment to LPS group. Graph Pad PRISM (version 6.0; GraphPad software, La Jolla, CA, USA) used for statistical analysis.
4. Results
Lipopolysaccharides (component of Gram-negative bacteria cell wall) can be able to induce infections, tissue injuries as well as biochemical changes in the form of inflammatory response. It was reported in the literature that exposure of LPS to mammalian cell/tissue can causes pro-inflammatory cytokines release which may results in subsequent activation of other inflammatory cascade which includes tumor necrosis factor-α (TNF-α) interlukin-1β (IL-1β), ROS, iNOS, and cyclooxygenase-2 (CO-2) (Tsukamoto et al., 2018). Recently, increasing evidences suggested that activation of numerous inflammatory responses by LPS results in chronic obstructive pulmonary diseases (COPD), diabetes, neurodegenerative diseases, and osteoporosis (Gomes et al., 2017). Therefore, this study was designed to demonstrate the possible protective role of AP against LPS-induced acute lung injury.
4.1. Effect of AP and LPS on oxidative stress in lung tissue (MDA, GSH &GR)
Reactive oxygen free radicals are byproducts of ordinary cellular metabolic recations. Oxidative free radicals are produced in response of various conditions including NSAIDs drugs, ischemia reperfusion injury, chronic infection and inflammatory disorders such as acute lung injury (de Lima et al., 2013). Thus, we thought that AP may play a protective role in LPS-induced lung injury by inhibiting ROS formation. Therefore, we measured the possible protective role of AP on oxidative stress parameters investigated as an index reflecting oxidative damage which were summarized in Fig. 1, Fig. 2, Fig. 3. The evidence of oxidative stress induced by LPS was indicated by inclined MDA levels with declined GSH levels and GR enzyme activity relative to control group. AP treatment significantly (p < 0.05) diminished MDA levels with improved GSH levels and GR activity relative to LPS administered group. Inclined GSH levels and GR activity was found to be superior in DEX treated group than AP, but decrease in MDA was found to be superior in AP treated groups. Above result showed that AP and DEX both have antioxidant properties and may protect lung from LPS-induced acute lung injury. Therefore, AP and DEX both decreases oxidative stress mediated damage to the lung tissue and may protect lung from LPS-mediated lung injury. LPS also stimulated pro-inflammatory cytokines release from the airways of the pulmonary epithelial cells and alveolar macrophages, therefore employed as a model for ALI (Fig. 1, Fig. 2, Fig. 3).
Fig. 1.
MDA.
Fig. 2.
GSH.
Fig. 3.
GR.
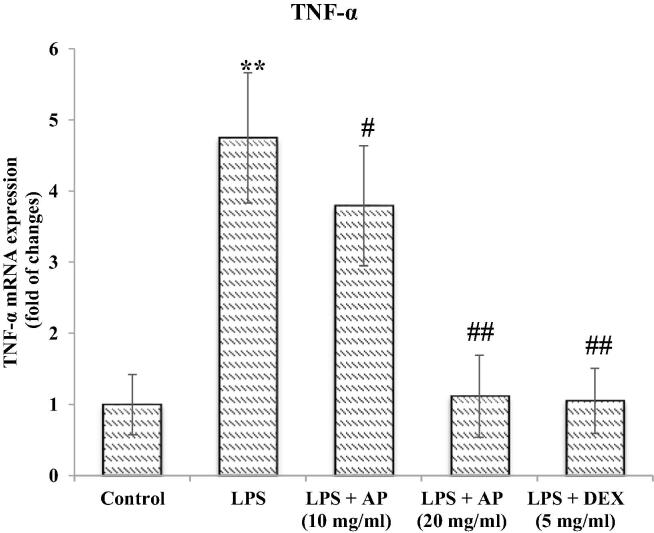
4.2. Effect of AP and LPS on changes in inflammatory markers
Cellular responses either in the cytosol or nucleoplasm due to oxidative stress may also leads to activation of inflammatory cascades which may contribute to pharmacological intervention. A sign of airway inflammation were noted, therefore, we evaluated response of AP in attenuation of pro-inflammatory markers in serum. First the serum were separated from whole blood obtained from each animals and process the sample to confirm that LPS-induces acute lung inflammation presented in Fig. 4, Fig. 5, Fig. 6. Administration of LPS resulted in an activation of release of IL-1β, IL-6 and TNF-α contributing to infiltration of neutrophils, lymphocyte, macrophages and eosinophils as detected in the histopathological examination. The animal groups treated with AP and DEX showed attenuation in IL-1β, IL-6 and TNF-α pro-inflammatory markers significantly. TNF-α were more improved with DEX as compared to both dose of AP whereas IL-1β was less improved than AP. As mentioned above our results showing that AP exhibited suppressive role in the release of inflammatory cytokines, demonstrating that AP improved LPS-induced lung inflammation by inhibiting pro-inflammatory mediator’s release. Of note, our histopathological results showed that, AP treatment significantly reversed LPS-induced infiltration of neutrophils, lymphocyte, macrophages and eosinophils in the lungs in a dose dependent manner whereas, IL-1β and TNF-α. IL-1β specially confirmed inflammation triggering mediator in many acute and chronic illnesses. The high level of IL-1β in serum accompanied by native surge of the pro-inflammatory IL-6 and TNF-α and an enthusiastic acute tissue immune response with sign of tissue grievance. Additionally, an increased level of pro-inflammatory markers indicating lung inflammation which were attenuated by AP treatment (Fig. 4, Fig. 5, Fig. 6).
Fig. 4.
TNF-α.
Fig. 5.
IL-6.
Fig. 6.
IL-1β.
4.3. Effects of AP and LPS on changes in mRNA expression of TNF-α, GST, AKT 1, & ERK
Administration of LPS induces damaged to the lung tissue leads to alteration in oxidative stress parameters and causes release of inflammatory mediators as mentioned above which involves TNF-a/NF-kB signalling pathways. There must be some correlations between signalling involved in theses cascade at molecular level or gene level with the above inflammatory changes. Therefore, we attempted to investigate mRNA gene expression through RT-PCR to explore how AP attenuated acute lung injury induced by LPS. In this study. In the present study we found that administration of LPS results an increased in TNF-α and GST expression as compared to control. Treatment with AP attenuated TNF-α and GST mRNA expression induced by LPS. Akt and ERK pathway act synergistically, upon stimulation promote mTOR signalling and evidenced that the Akt1 and ERK signalling converge on TSC2 AKt phosphorylated residues on TSC-2 distinct from those phosphorylated by ERK (Winter et al., 2011). Thus they reported that, activation of ERK and/or Akt may perhaps active mTORC1 through both TSC-2 dependent and independent mechanism. In our study mRNA expression of ERK was upregulated and Akt1 was downregulated in a dose dependent manner. In the DEX treated groups ERK was upregulated and Akt1 was downregulated. We did not measured downstream effector for both genes. Therefore, there is need of further investigation to confirm our findings. Treatment with apremilast downregulated ERK and TNF-a mRNA expression whereas upregulated AKT1 mRNA expression significantly in a dose depended manner. Dexamethasone taken as a standard also showed similar effect (Fig. 7, Fig. 8, Fig. 9, Fig. 10).
Fig. 7.
TNF-α.
Fig. 8.
GST.
Fig. 9.
AKT1.
Fig. 10.
ERK.
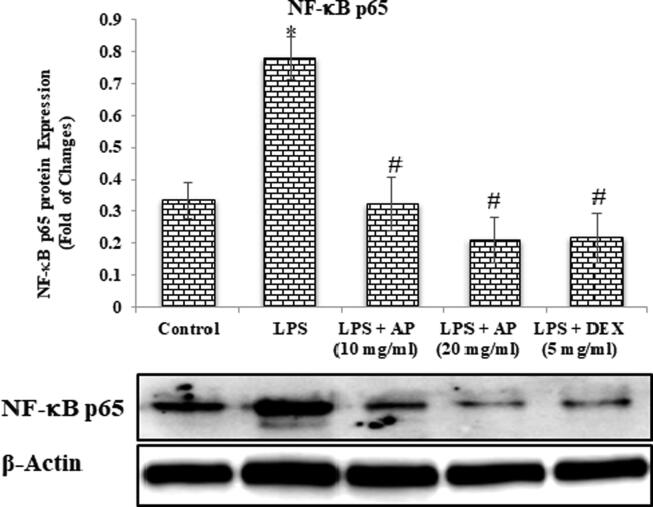
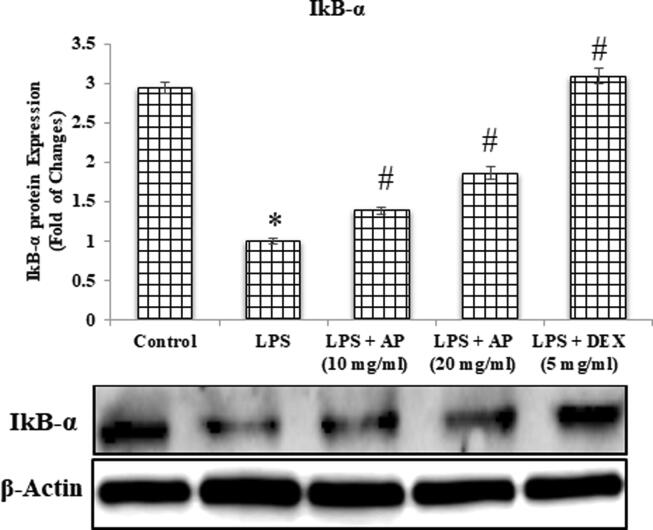
4.4. Effects of AP and LPS on changes in protein expression of NF-κB p65 and IkB-α
LPS administration causes lung tissue damage, which leads to changes in protein expression which found to be involved in inflammatory changes through the TNF-a/NF-kB signaling pathways, as previously stated. Therefore, we attempted to investigate protein expression through western analysis to explore how AP attenuated LPS-induced acute lung injury. In the present study we found that administration of LPS results an increased in NF-kB p65 protein expression whereas decrease in IkB-α protein expression as compared to control. Treatment with AP attenuated LPS-induced activation of NF-kB p65 in a dose dependent manner and improved IkB-α levels. DEX also showed similar pattern in both NF-kB p65 and IkB-α protein expression (Fig. 11, Fig. 12).
Fig. 11.
NF-κB p65.
Fig. 12.
IκB-α.
4.5. Histopathology
Administration of LPS induces damaged to the lung tissue evident by structural as well histological changes in the lung tissue. In order to see if AP had any effects on airway inflammation against LPS-induced lung injury. Therefore, in order to confirm, we did H&E staining for histopathological investigations. In our study, we find that administration of LPS showed acute inflammatory changes, including Neutrophils, lymphocytes, and macrophages with plasma cells, thickened septa by inflammation, alveolus, alveolar septae (Thickened, inflammation, fibrosis), and pulmonary vessels having inflammatory cells as compared to control (Fig. 13B-D). Lymphoid follicles with the germinal center, histiocytes, and macrophages were also seen as compared to control. Due to severe inflammation mainly due to macrophages, the normal lung histology can no longer be appreciated. These changes were normalized with pretreatment with AP as well as with DEX. Alveolar septae were normal, no sign of inflammation, no sign of fibrosis was seen in AP as well as DEX treated group. Bronchiole and pulmonary vessels were also seen normal in groups pre-treated with AP as well as DEX (Fig. 13E,F and G). It was found that the inflammatory response was significantly reduced after AP administration. Therefore, according to the above findings, it is said that AP protect against LPS-induced acute lung injury (Fig. 13).
Fig. 13.
LPS= Lipopolysaccharides: AP= Apremilast: DEX= Dexamethasone: IkB-α = Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha.
5. Discussion
LPS (a component of bacterial cell wall) induced Acute lung injury (ALI) reported to cause mortalities in patients due to respiratory failure. This toxin may cause either direct or indirect injury which either causes unstable reduction and oxidation states that lead to DNA damage or protein and lipid oxidative damages (Park et al., 2018). Scientific research findings also suggest that LPS can trigger intense inflammatory signals and cause pulmonary injuries which is evidenced by leukocyte infiltrations. The severity of pneumonia is accelerated via sacculated neutrophils, hyper oxidative states and pro-inflammatory cascade of reactions such as abnormally high levels of inflammatory cytokines TNF-α, interleukins especially IL-6 ad IL-1β which exaggerate inflammation, that may cause alveolar damage further (Boje, 2004, Takeuchi et al., 2006). The present research was designed to find some potential agents which can alleviate the above-mentioned pathways and can prevent lung injuries induced by triggering factors.
Scientific reports have already confirmed that reactive oxygen molecules associated injuries lead to numerous pathological states such as hyperglycemia, cardiac injury, hepatic and nephritic injury which finally play crucially in lung failure. The reactive oxidative free moieties such as superoxide ((O2—), hydroxyl ions (OH—) and Hydrogen peroxide (H2O2) transduce signals which disrupts intercellular processes and cause tissue injuries (Valavanidis et al., 2015; Valavanidis et al., 2013). The protective mechanism involved in such situations believed to be the antioxidant enzyme signaling against hyper oxidative stress (Chang et al., 2018). Reactive free oxygen species are deleterious to all organisms in every condition if left unchecked. When antioxidant defense is not enough and oxidative radicals exceeds then a cell is said to be in “oxidative stress”. The oxidative states developed in environmental stresses can be harmful to the cells as oxidative free radicals can cause lipid peroxidation, oxidize the proteins, and can damage the nucleic material, retards enzymatic activities, and can induce apoptotic pathways, all these cellular changes can lead to cellular death ultimately (Srivastava and Dubey, 2011, Meriga et al., 2004).
Peroxidation of lipids usually goes on constantly in every individual, although lipid per oxidation is harmful in normal conditions too, but the homeostatic biological mechanisms maintain homeostasis. Severe states of oxidative stress and insufficient antioxidant defense cause lipid peroxidation and aldehydic products such as free fatty acids and MDA are generated and these chemicals are generally referred as thibarbituric acid reacting substances (Nakai et al., 2000). Reactive oxygen free radicals affects gene signaling and disturbs almost all normal physiologic behavior of the cells including gene expressions, protein transcription, and cell death (Brandes and Kreuzer 2005). Since plasma membrane primarily constituted of phospholipids, unchecked lipid peroxidation causes cellular injuries and ultimately the cell death via direct damage to the nucleic material or deceptive protein synthesis. Researchers have already reported that depleted GSH levels and increased oxidative free radicals generation are the primary cause of unwanted and unchecked lipid peroxidation (Konukoğlu et al., 1998, Inoue, 2011). Reduced GSH considered primary signaling event in cell death pathways. Hence depleted GSH levels indicates oxidative stress and considered as marker of weakened antioxidant defense or hyper oxidative stress states.
GSH is present abundantly in mammalian cells and is potent antioxidant defense maintains tightly the reduction and oxidation status of the cell (Aquilano et al., 2014). The peptide link between glutamate and cysteine, C-terminal glycine and cysteinyl moiety protects GSH from hydrolytic reactions of Intracellular peptidases and cleavage of GSH by cysteine moiety liberate reactive thiol group (SH group), which are responsible for biological functions of GSH (oxidation–reduction and nucleophilic reaction) (Haddad and Harb 2005). GSH oxidizes itself to GSSG and reduces oxidative radicals (Wu et al., 2004) and act as antioxidant defense (Zidenberg-Cherr et al., 1991). Following the above-mentioned scientific claims, we estimated MDA, reduced GSH and GR activity in pulmonary cells as a marker of oxidative stress. Results of our experimental research are also in agreement to earlier findings, the animals exposed to LPS showed statistically significant increment in MDA levels whereas the levels of GSH and GR activity was observed significantly low. While the pulmonary tissue analysis of AP treated animals restored these altered levels MDA, GSH and GR. Hence it can be inferred that the elevated levels of MDA were due to over production of reactive free radicals and the reduced levels of GSH and GR could have been due to free radical neutralization as GSH activated by GR. Results of our experimental protocol highlights antioxidant properties of AP that prevent LPS-induced acute lung injury.
Free radical initiated cellular and intranuclear events induce inflammatory cascade of reactions which requires pharmacological interventions. Elevated levels of TNF-α interleukins (IL-2, IL6, and IL8) and few other cytokines are common to heterogeneous Lung injuries. (Soromou et al., 2012). LPS found to stimulate the production and release of pro inflammatory cytokines from bronchial epithelial cells and pulmonary macrophages and serve a model of ALI. Cytokines importantly TNF-α and IL-6 contribute significantly in initial reactions inflammation and pathogenicity of ALI (Soromou et al., 2012, Giebelen et al., 2007). Importantly elevated TNF-α, IL-6 and IL-1β considered as primary mediators of inflammation which finally triggers diverse inflammatory responses. (Soromou et al., 2012, Giebelen et al., 2007, Jin et al., 2013). LPS have been reported to increase (TNF-α, IL-6 and IL-1β) the primary pro-inflammatory cellular chemokine in lung tissues. Scientist have already reported that pharmacological entities which inhibit mRNA expressions for TNF-α protected from ALI. In experimental models (Wolthuis et al., 2009). Findings of our experimental protocol also confirmed the earlier works and administration of AP significantly reduced the elevated levels of TNF-α, IL-6 and IL-1β in lung tissues and which is believed to play confirmatory roles in lung protection which were elevated on LPS exposure.
For further clarification of protective mechanism of action of AP the mRNA expressions of TNF-α, GST, AKt1 and ERK in lung tissues were assessed using RT-PCR analytic technique. Therefore, AP was further examined whether it could attenuate these biomarkers in lung tissues which were induced by LPS.
Glutathione S-Transfereases are the phase-II biotransformation isozymes of eukaryotic and prokaryotic cells which catalyzes the conjugation reaction of xenobiotics substrates with Reduced GSH for detoxification purposes. The mRNA expression of GST and pro inflammatory marker TNF-α, AKT-I elevated significantly although ERK were significantly down regulated after LPS exposures (Csiszar et al., 2008, Yu et al., 2013). Treatment with AP significantly attenuated the altered levels of mRNA expressions as compared to LPS administered rats. The findings clearly implicate that AP can protect from LPS induced ALI in mice by modulating the mRNA expressions of these biomarkers. Since the discovery of ERKs in mammalian cells, research on MAPK signaling accelerated very rapidly (Conese and Assael, 2001; Sun et al., 2017; Huang et al., 2017). Hence now it is supposed that down regulation of these pro inflammatory biological molecules and oxidative radicals are the important target in the management of ALI.
MAPKs are abundantly found in mammals and diverse series of signals such as mechanical stress, inflammation, growth factors etc can stimulate them. MAPKs are elements of important enzymatic cascade of reactions that play key role in diverse extracellular stimuli and believed to regulate cellular processes. MAPK signals are transduced via three different forms of kinases. Mitogen activated protein cause phosphorylation of MAPKKK, phosphorylated MAPKKK cause phosphorylation of MAPKK and then finally MAPKK phosphorylates to MAPK which translocates into nucleus (Cargnello and Roux, 2011, Plotnikov et al., 2019).
Till date almost eight subfamilies of MAPKs have been reported and of these subfamilies composes numerous pathways (Janus /kinase pathway, ERK 1/2 pathway and p38 pathway) (Fecher et al., 2008). The intracellular responses of different MAPK signals is different ERK pathway mainly involved in cell survival and growth and sometimes assist in cellular differentiations in special situations (Sun et al., 2017; Conese and Assael, 2001, Huang et al., 2017).
Scientists claimed on the basis of their research findings that ERK and AKT signaling pathways synergize mTORC1 signaling and TSC2 (Yin et al., 2008, Kerkelä et al., 2011), researchers have also reported that AKT and ERK triggors mTORC1 pathways via TSC2 dependent and independent mechanisms. (Zhang et al., 2003; Brown et al., 2016, Roberts and Der, 2007, Kerkelä et al., 2011). Findings of our experimental protocol pinpointed that administration of AP the expressions of ERK mRNA was unregulated while mRNA expressions for Akt1 down regulated, although the findings further need to be attested knowing the effects on both genes. Treatment with AP down regulated the ERK signaling and TNF-a mRNA expressions whereas elevated the AKT1 mRNA expressions in dose dependent manners. The results were found as dexamethasone treated animals which was designated as standard treatment.
The ERK1/2 and MAPK Pathway; ERK1/2 are the first cloned and characterized human MAPKs. ERK1/2 are important players in cell growth, differentiation, and survival signaling, oftenly stimulated by mitogenic factors. As ERK 1/2 are the critical regulators of cellular growth and survival, the ERK1/2 MAPKs attracted researcher’s interest to develop ERK inhibitors in cancer chemotherapy. (Roberts and Der, 2007). Scientists have reported that ERK1/2 sometimes are also stimulated by cell proliferating signals such as brain-derived neurotrophic factor (BDNF) and platelet-derived growth factor (PDGF), proinflammatory cytokines IL-1β (Ruhul Amin et al., 2003), pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs). Hence, undoubtedly, ERK1/2 and MAPK pathway usually operate in cancerous states and several other pathologies.
Phosphoprylation and intern activation of ERK1/2 occurs at Thr185 and Tyr187 and MAPKK1 and MAPKK2, respectively catalyze this reaction. Phosphorylated ERK1/2 translate into the nucleus and phosphorylate and regulate transcription of various other factors that ultimately alter gene expressions.
Cytokine expressions primarily controlled at transcriptional levels. NF-κB binds with DNA and brings about mRNA expressions and transcribed numerous inflammatory cytokines, and its activation confirmed in ALI (Schwartz et al., 1996, Le Tulzo et al., 1997, Moine et al., 1997). NF-κB remains segregated in cytoplasm in the recessive state associating with IκB. When stimulated, IκB phosphorylates and separates from NF-κB, the free NF-κB enters inside the nucleus and binds to DNA and brings about the desired genetic changes. Scientific evidences suggest NF-κB over expression in ALI and controlled by IκB expressions which ultimately dependent on xanthine oxidase resulted oxidants (Schwartz et al., 1996, Le Tulzo et al., 1997, Moine et al., 1997).
Furthermore, inflammation is considered a key pathological process which give rise to plenty of diseases, controlled by NF-κB signaling pathway (Hoesel and Schmid, 2013; Valavanidis et al., 2013). Further to asses and confirm the mechanics of AP on ALI in LPS associated lung injury in mice, we analyzed the intracellular traces of NF-κB, p65, IkB-α. Further to note, NF-κB is a well-known and established transcriptional factor which checks the expressions of TNF-α and Interleukins (IL-1B and IL-6) (Imam et al., 2015). Western blot analysis in present research protocol carried out to confirm the NF-κB p65 activation. Findings conclude that administration of LPS to experimental animals significantly elevated the intracellular levels of NF-κB p65. Treatment with AP-(10 & 20 mg/kg) resulted significant suppression in the expressions of NF-κB p65 when results compared with LPS exposed animals (Fig. 6a). Findings clearly point out that AP possess anti-inflammatory actions and attenuate the expressions inflammatory cytokines by checking NF-κB pathways. The focus of our research protocol was to examine inhibitory effect of AP on NF-κB activation via quantification of p-IκB-α. The experimental outcomes exhibited that prophylactic administration of AP significantly declined LPS-mediated IκBα phosphorylation and intranuclear admission of NF-κB p65 in rat’s lung tissues. Results inferred that the inflammatory cascade of reaction play pivotal role rather than oxidative stress in LPS related pulmonary toxicities in rodents, AP being anti-inflammatory in nature resist pulmonary injuries via NF-κB pathway mediated down regulation of TNF-α.
Histological examinations; Histological examination reports of alveolar tissue confirms lung degeneration, broken parenchyma and protoplasmic vacuoles in LPS group. hypochromatic cells clusters, pyknotic nuclei and infiltrate of immune cells were observed on everywhere in lung tissues. Earlier reports documented almost same histological remarks in LPS related lung injuries. (Inoue, 2011, Soromou et al., 2012, Valavanidis et al., 2013). The morphological and structural changes induced by LPS exposure in lung tissue were promisingly reversed by AP treatment. Lung parenchymal structural changes found restored in the animal treated with AP.
Therefore, the overall outcomes of this study clearly implicates that LPS induced ALI in experimental animals via up regulation of NF-κB levels in experimental animals. Over expression of NF-κB was confirmed by estimating the intracellular TNF-α, IL-6, and IL-1β levels. Results demonstrated that AP administration prevented LPS-stimulated activation of NF-κB p65 and intranuclear translocation of NF-κB p65 in rodent’s lung tissues.
6. Conclusion
In conclusion, inference can be made that the inflammatory reactions play critical roles rather than oxidative free radicals stimulated oxidative stress in LPS leaded acute pulmonary injuries in rodents, and NF-κB p65 influences AKT and ERK signaling system. Therefore, the anti-inflammatory drug entity “AP” prevent these injuries via scrutinizing AKT and ERK signaling pathway.
7. Declarations
The present study was conducted after the protocol was approved by Institutional ethical committee, King Saud University, Kingdom of Saudi Arabia.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no (Project No. RGP-VPP-305), Riyadh Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Harbi N.O., Imam F., Alharbi M.M., Khan M.R., Qamar W., Afzal M., Algahtani M., Alobaid S., Alfardan A.S., Alshammari A., Albekairi T.H., Alharbi K.S. Role of rivaroxaban in sunitinib-induced renal injuries via inhibition of oxidative stress-induced apoptosis and inflammation through the tissue nacrosis factor-α induced nuclear factor-κappa B signaling pathway in rats. J. Thromb. Thrombol. 2020;50(2):361–370. doi: 10.1007/s11239-020-02123-6. [DOI] [PubMed] [Google Scholar]
- Aquilano K., Baldelli S., Ciriolo M.R. Glutathione: new roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014;5:196. doi: 10.3389/fphar.2014.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avecillas J.F., Freire A.X., Arroliga A.C. Clinical epidemiology of acute lung injury and acute respiratory distress syndrome: incidence, diagnosis, and outcomes. Clin. Chest Med. 2006;27(4):549–557. doi: 10.1016/j.ccm.2006.06.001. abstract vii. [DOI] [PubMed] [Google Scholar]
- Barakat A., Szick-Miranda K., Chang I.F., Guyot R., Blanc G., Cooke R., Delseny M., Bailey-Serres J. The organization of cytoplasmic ribosomal protein genes in the Arabidopsis genome. Plant Physiol. 2001;127(2):398–415. [PMC free article] [PubMed] [Google Scholar]
- Boje K.M. Nitric oxide neurotoxicity in neurodegenerative diseases. Front. Biosci. 2004;9:763–776. doi: 10.2741/1268. [DOI] [PubMed] [Google Scholar]
- Brandes R.P., Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc. Res. 2005;65(1):16–27. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Brown M., Strudwick N., Suwara M., Sutcliffe L.K., Mihai A.D., Ali A.A., Watson J.N., Schröder M. An initial phase of JNK activation inhibits cell death early in the endoplasmic reticulum stress response. J. Cell Sci. 2016;129(12):2317–2328. doi: 10.1242/jcs.179127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. Inflammation, neurodegenerative diseases, and environmental exposures. Ann. N. Y. Acad. Sci. 2004;1035:117–132. doi: 10.1196/annals.1332.008. [DOI] [PubMed] [Google Scholar]
- Cargnello M., Roux P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- Chang H.Y., Chen Y.C., Lin J.G., Lin I.H., Huang H.F., Yeh C.C., Chen J.J., Huang G.J. Asatone Prevents Acute Lung Injury by Reducing Expressions of NF-[Formula: see text]B, MAPK and Inflammatory Cytokines. Am. J. Chin. Med. 2018;46(3):651–671. doi: 10.1142/S0192415X18500349. [DOI] [PubMed] [Google Scholar]
- Chen L.F., Greene W.C. Shaping the nuclear action of NF-kappaB. Nat. Rev. Mol. Cell Biol. 2004;5(5):392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- Cheng X., Cao Z., Luo J., Hu R., Cao H., Guo X., Xing C., Yang F., Zhuang Y., Hu G. Baicalin ameliorates APEC-induced intestinal injury in chicks by inhibiting the PI3K/AKT-mediated NF-kappaB signaling pathway. Poult. Sci. 2021;101(1) doi: 10.1016/j.psj.2021.101572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J.C., Young D.W., Golenbock D.T., Christ W.J., Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999;274(16):10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Conese M., Assael B.M. Bacterial infections and inflammation in the lungs of cystic fibrosis patients. Pediatr. Infect. Dis. J. 2001;20(2):207–213. doi: 10.1097/00006454-200102000-00018. [DOI] [PubMed] [Google Scholar]
- Conti M., Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu. Rev. Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- Csiszar A., Wang M., Lakatta E.G., Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J. Appl. Physiol. (1985) 2008;105(4):1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima F.M., Albertini R., Dantas Y., Maia-Filho A.L., Santana Cde L., Castro-Faria-Neto H.C., França C., Villaverde A.B., Aimbire F. Low-level laser therapy restores the oxidative stress balance in acute lung injury induced by gut ischemia and reperfusion. Photochem. Photobiol. 2013;89(1):179–188. doi: 10.1111/j.1751-1097.2012.01214.x. [DOI] [PubMed] [Google Scholar]
- Fecher L.A., Amaravadi R.K., Flaherty K.T. The MAPK pathway in melanoma. Curr. Opin. Oncol. 2008;20(2):183–189. doi: 10.1097/CCO.0b013e3282f5271c. [DOI] [PubMed] [Google Scholar]
- Giebelen I.A., van Westerloo D.J., LaRosa G.J., de Vos A.F., van der Poll T. Local stimulation of alpha7 cholinergic receptors inhibits LPS-induced TNF-alpha release in the mouse lung. Shock. 2007;28(6):700–703. doi: 10.1097/shk.0b013e318054dd89. [DOI] [PubMed] [Google Scholar]
- Gobejishvili L., Barve S., Joshi-Barve S., McClain C. Enhanced PDE4B expression augments LPS-inducible TNF expression in ethanol-primed monocytes: relevance to alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295(4):G718–G724. doi: 10.1152/ajpgi.90232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes J.M.G., Costa J.A., Alfenas R.C.G. Metabolic endotoxemia and diabetes mellitus: A systematic review. Metabolism. 2017;68:133–144. doi: 10.1016/j.metabol.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Haddad J.J., Harb H.L. L-gamma-Glutamyl-L-cysteinyl-glycine (glutathione; GSH) and GSH-related enzymes in the regulation of pro- and anti-inflammatory cytokines: a signaling transcriptional scenario for redox(y) immunologic sensor(s)? Mol. Immunol. 2005;42(9):987–1014. doi: 10.1016/j.molimm.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Houslay M.D., Schafer P., Zhang K.Y. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov. Today. 2005;10(22):1503–1519. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- Huang H.T., Sun Z.G., Liu H.W., Ma J.T., Hu M. ERK/MAPK and PI3K/AKT signal channels simultaneously activated in nerve cell and axon after facial nerve injury. Saudi J. Biol. Sci. 2017;24(8):1853–1858. doi: 10.1016/j.sjbs.2017.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam F., Al-Harbi N.O., Al-Harbi M.M., Ansari M.A., Al-Asmari A.F., Ansari M.N., Al-Anazi W.A., Bahashwan S., Almutairi M.M., Alshammari M., Khan M.R., Alsaad A.M., Alotaibi M.R. Apremilast prevent doxorubicin-induced apoptosis and inflammation in heart through inhibition of oxidative stress mediated activation of NF-κB signaling pathways. Pharmacol. Rep. 2018;70(5):993–1000. doi: 10.1016/j.pharep.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Imam F., Al-Harbi N.O., Al-Harbi M.M., Ansari M.A., Zoheir K.M., Iqbal M., Anwer M.K., Al Hoshani A.R., Attia S.M., Ahmad S.F. Diosmin downregulates the expression of T cell receptors, pro-inflammatory cytokines and NF-κB activation against LPS-induced acute lung injury in mice. Pharmacol. Res. 2015;102:1–11. doi: 10.1016/j.phrs.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Imam F., Al-Harbi N.O., Al-Harbi M.M., Qamar W., Aljerian K., Belali O.M., Alsanea S., Alanazi A.Z., Alhazzani K. Apremilast ameliorates carfilzomib-induced pulmonary inflammation and vascular injuries. Int. Immunopharmacol. 2019;66:260–266. doi: 10.1016/j.intimp.2018.11.023. [DOI] [PubMed] [Google Scholar]
- Impellizzeri D., Esposito E., Mazzon E., Paterniti I., Di Paola R., Bramanti P., Cuzzocrea S. Effect of apocynin, a NADPH oxidase inhibitor, on acute lung inflammation. Biochem. Pharmacol. 2011;81(5):636–648. doi: 10.1016/j.bcp.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Inoue M. In: The liver: Biology and pathobiology. Arias I.M., Fausto N., Jokoby W.B., Schachter D.A., Shafritz D.A., editors. Raven Press; New York: 2011. Protective mechanisms against reactive oxygen species. [Google Scholar]
- Jin J.O., Han X., Yu Q. Interleukin-6 induces the generation of IL-10-producing Tr1 cells and suppresses autoimmune tissue inflammation. J. Autoimmun. 2013;40:28–44. doi: 10.1016/j.jaut.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E.R., Matthay M.A. Acute lung injury: epidemiology, pathogenesis, and treatment. J. Aerosol Med. Pulm. Drug Deliv. 2010;23(4):243–252. doi: 10.1089/jamp.2009.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollow D.J., Mitchell J.R., Zampaglione N., Gillette J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Kerkelä R., Ilves M., Pikkarainen S., Tokola H., Ronkainen V.P., Majalahti T., Leppäluoto J., Vuolteenaho O., Ruskoaho H. Key roles of endothelin-1 and p38 MAPK in the regulation of atrial stretch response. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300(1):R140–R149. doi: 10.1152/ajpregu.00853.2009. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A., Mutlu E., Guzman J.P., Forsyth C., Banan A. Phosphodiesterase 4 inhibitors and inflammatory bowel disease: emerging therapies in inflammatory bowel disease. Expert Opin. Invest. Drugs. 2007;16(9):1489–1506. doi: 10.1517/13543784.16.9.1489. [DOI] [PubMed] [Google Scholar]
- Khalsa S.K., Roberts C.C., Underhill M.S. Acute Pulmonary Toxicity from Thalidomide in a Patient with Multiple Myeloma. Radiol. Case Rep. 2007;2(2):41–43. doi: 10.2484/rcr.v2i2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonov S.A., Sjöbring U. Lipopolysaccharide challenge of humans as a model for chronic obstructive lung disease exacerbations. Contrib. Microbiol. 2007;14:83–100. doi: 10.1159/000107056. [DOI] [PubMed] [Google Scholar]
- Kit E. Enzyme-linked immunosorbent assay principle. EMD Millipore. 2019 [Google Scholar]
- Konukoğlu D., Serin O., Demiriz Kemerli G., Serin E., Hayirhoğlu A., Oner B. A study on the carotid artery intima-media thickness and its association with lipid peroxidation. Clin. Chim. Acta. 1998;277(1):91–98. doi: 10.1016/s0009-8981(98)00117-x. [DOI] [PubMed] [Google Scholar]
- Lai J.L., Liu Y.H., Liu C., Qi M.P., Liu R.N., Zhu X.F., Zhou Q.G., Chen Y.Y., Guo A.Z., Hu C.M. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-kB and MAPK Signaling Pathways. Inflammation. 2017;40(1):1–12. doi: 10.1007/s10753-016-0447-7. [DOI] [PubMed] [Google Scholar]
- Le Tulzo Y., Shenkar R., Kaneko D., Moine P., Fantuzzi G., Dinarello C.A., Abraham E. Hemorrhage increases cytokine expression in lung mononuclear cells in mice: involvement of catecholamines in nuclear factor-kappaB regulation and cytokine expression. J. Clin. Invest. 1997;99(7):1516–1524. doi: 10.1172/JCI119314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.L., Downey G.P. Neutrophil activation and acute lung injury. Curr. Opin. Crit. Care. 2001;7(1):1–7. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Lee Y.G., Lee J., Byeon S.E., Yoo D.S., Kim M.H., Lee S.Y., Cho J.Y. Functional role of Akt in macrophage-mediated innate immunity. Front. Biosci. (Landmark Ed.) 2011;16:517–530. doi: 10.2741/3702. [DOI] [PubMed] [Google Scholar]
- Li Z.W., Chu W., Hu Y., Delhase M., Deerinck T., Ellisman M., Johnson R., Karin M. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J. Exp. Med. 1999;189(11):1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D., Li F., Fu Y., Cao Y., Song X., Wang T., Wang W., Guo M., Zhou E., Li D., Yang Z., Zhang N. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-kappaB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation. 2014;37(1):214–222. doi: 10.1007/s10753-013-9732-x. [DOI] [PubMed] [Google Scholar]
- Liu C., Tang X., Zhang W., Li G., Chen Y., Guo A., Hu C. 6-Bromoindirubin-3'-Oxime Suppresses LPS-Induced Inflammation via Inhibition of the TLR4/NF-kappaB and TLR4/MAPK Signaling Pathways. Inflammation. 2019;42(6):2192–2204. doi: 10.1007/s10753-019-01083-1. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- Meriga B., Reddy B.K., Rao K.R., Reddy L.A., Kishor P.B. Aluminium-induced production of oxygen radicals, lipid peroxidation and DNA damage in seedlings of rice (Oryza sativa) J. Plant Physiol. 2004;161(1):63–68. doi: 10.1078/0176-1617-01156. [DOI] [PubMed] [Google Scholar]
- Moine P., Shenkar R., Kaneko D., Le Tulzo Y., Abraham E. Systemic blood loss affects NF-kappa B regulatory mechanisms in the lungs. Am. J. Physiol. 1997;273(1 Pt 1):L185–L192. doi: 10.1152/ajplung.1997.273.1.L185. [DOI] [PubMed] [Google Scholar]
- Nakai A., Oya A., Kobe H., Asakura H., Yokota A., Koshino T., Araki T. Changes in maternal lipid peroxidation levels and antioxidant enzymatic activities before and after delivery. J. Nippon Med. Sch. 2000;67(6):434–439. doi: 10.1272/jnms.67.434. [DOI] [PubMed] [Google Scholar]
- Page C.P., Spina D. Phosphodiesterase inhibitors in the treatment of inflammatory diseases. Handb. Exp. Pharmacol. 2011;204:391–414. doi: 10.1007/978-3-642-17969-3_17. [DOI] [PubMed] [Google Scholar]
- Park B.S., Lee J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013;45(12) doi: 10.1038/emm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Chen Y., Zheng M., Ryu J., Cho G.J., Surh Y.J., Sato D., Hamada H., Ryter S.W., Kim U.H., Joe Y., Chung H.T. Pterostilbene 4'-β-Glucoside Attenuates LPS-Induced Acute Lung Injury via Induction of Heme Oxygenase-1. Oxid. Med. Cell Longev. 2018;2018:2747018. doi: 10.1155/2018/2747018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov A., Chuderland D., Karamansha Y., Livnah O., Seger R. Nuclear ERK Translocation is Mediated by Protein Kinase CK2 and Accelerated by Autophosphorylation. Cell. Physiol. Biochem. 2019;53(2):366–387. doi: 10.33594/000000144. [DOI] [PubMed] [Google Scholar]
- Roberts P.J., Der C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- Rubenfeld G.D., Caldwell E., Peabody E., Weaver J., Martin D.P., Neff M., Stern E.J., Hudson L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- Ruhul Amin A.R., Senga T., Oo M.L., Thant A.A., Hamaguchi M. Secretion of matrix metalloproteinase-9 by the proinflammatory cytokine, IL-1beta: a role for the dual signalling pathways, Akt and Erk. Genes Cells. 2003;8(6):515–523. doi: 10.1046/j.1365-2443.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- Satkirin Khalsa K., Roberts Catherine C., Underhill Michael S. Acute Pulmonary Toxicity from Thalidomide in a Patient with Multiple Myeloma. Radiol Case Rep. 2007;2(2):41–43. doi: 10.2484/rcr.v2i2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.D., Moore E.E., Moore F.A., Shenkar R., Moine P., Haenel J.B., Abraham E. Nuclear factor-kappa B is activated in alveolar macrophages from patients with acute respiratory distress syndrome. Crit. Care Med. 1996;24(8):1285–1292. doi: 10.1097/00003246-199608000-00004. [DOI] [PubMed] [Google Scholar]
- SmPC, O., 2020. Film-Coated Tablets - Summary of Product Characteristics (SmPC) (emc). e.m.c.
- Soromou L.W., Chu X., Jiang L., Wei M., Huo M., Chen N., Guan S., Yang X., Chen C., Feng H., Deng X. In vitro and in vivo protection provided by pinocembrin against lipopolysaccharide-induced inflammatory responses. Int. Immunopharmacol. 2012;14(1):66–74. doi: 10.1016/j.intimp.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Dubey R.S. Manganese-excess induces oxidative stress, lowers the pool of antioxidants and elevates activities of key antioxidative enzymes in rice seedlings. Plant Growth Regul. 2011;64:16. [Google Scholar]
- Takeuchi H., Jin S., Wang J., Zhang G., Kawanokuchi J., Kuno R., Sonobe Y., Mizuno T., Suzumura A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J. Biol. Chem. 2006;281(30):21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H., Takeuchi S., Kubota K., Kobayashi Y., Kozakai S., Ukai I., Shichiku A., Okubo M., Numasaki M., Kanemitsu Y., Matsumoto Y., Nochi T., Watanabe K., Aso H., Tomioka Y. Lipopolysaccharide (LPS)-binding protein stimulates CD14-dependent Toll-like receptor 4 internalization and LPS-induced TBK1-IKK∊-IRF3 axis activation. J. Biol. Chem. 2018;293(26):10186–10201. doi: 10.1074/jbc.M117.796631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanidis A., Vlachogianni T., Fiotakis K., Loridas S. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health. 2013;10(9):3886–3907. doi: 10.3390/ijerph10093886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.J., Lin S.Z., Kuo J.S., Huang H.Y., Tzeng S.F., Liao C.H., Chen D.C., Chen W.F. Urocortin modulates inflammatory response and neurotoxicity induced by microglial activation. J. Immunol. 2007;179(9):6204–6214. doi: 10.4049/jimmunol.179.9.6204. [DOI] [PubMed] [Google Scholar]
- Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Winter J.N., Jefferson L.S., Kimball S.R. ERK and Akt signaling pathways function through parallel mechanisms to promote mTORC1 signaling. Am. J. Physiol. Cell Physiol. 2011;300(5):C1172–C1180. doi: 10.1152/ajpcell.00504.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolthuis E.K., Vlaar A.P., Choi G., Roelofs J.J., Haitsma J.J., van der Poll T., Juffermans N.P., Zweers M.M., Schultz M.J. Recombinant human soluble tumor necrosis factor-alpha receptor fusion protein partly attenuates ventilator-induced lung injury. Shock. 2009;31(3):262–266. doi: 10.1097/SHK.0b013e31817d42dd. [DOI] [PubMed] [Google Scholar]
- Wu G., Fang Y.Z., Yang S., Lupton J.R., Turner N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004;134(3):489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Yin H., Zhang J., Lin H., Wang R., Qiao Y., Wang B., Liu F. p38 mitogen-activated protein kinase inhibition decreases TNFalpha secretion and protects against left ventricular remodeling in rats with myocardial ischemia. Inflammation. 2008;31(2):65–73. doi: 10.1007/s10753-007-9050-2. [DOI] [PubMed] [Google Scholar]
- Yu P.J., Li J.R., Zhu Z.G., Kong H.Y., Jin H., Zhang J.Y., Tian Y.X., Li Z.H., Wu X.Y., Zhang J.J., Wu S.G. Praeruptorin D and E attenuate lipopolysaccharide/hydrochloric acid induced acute lung injury in mice. Eur. J. Pharmacol. 2013;710(1–3):39–48. doi: 10.1016/j.ejphar.2013.03.050. [DOI] [PubMed] [Google Scholar]
- Zidenberg-Cherr S., Olin K.L., Villanueva J., Tang A., Phinney S.D., Halsted C.H., Keen C.L. Ethanol-induced changes in hepatic free radical defense mechanisms and fatty-acid composition in the miniature pig. Hepatology. 1991;13(6):1185–1192. [PubMed] [Google Scholar]