Abstract
This study aimed to evaluate the efficacy of cefquinome in treatment and controlling of Escherichia coli experimentally infected broiler chickens, in addition of detection of its residues using High performance liquid chromatography (HPLC). In this study, 150 one-day old Cobb broiler chicks were used. On the 14th day chicks experimentally infected and divided into 3 equal groups (50 each); control group (G1) non-infected, non-treated, (G2) infected with E. coli O78 non treated, (G3) infected with E. coli O78, cefquinome treated. Cefquinome was administrated 5th day post infection, intramuscularly by a dose of (2 mg/ kg b w.t) for 3 consecutive days. Experimental E. coli infection in broilers induced weakness, loss of appetite, depression, cough and watery diarrhea in addition to a recorded mortality (30%) with reduction in growth performance, erythrogram, total proteins, albumin, antioxidants and haemagglutination inhibition (HI) titers. In addition, a significant increase in feed conversion rate (FCR), leukocytic count, liver enzymes, kidney functions, total globulins, malondialdehyde, nitric oxide and lysozyme activity. Treatment with cefquinome led to decreased mortality rate, improvement in clinical signs, growth performance and modulated most of these altered parameters. Cefquinome's residues was not detected in breast muscles 3rd day and liver and kidneys 7th days post treatment. Therefore, it's recommended that cefquinome is a good choice for controlling of colibacillosis in broilers and its withdrawal time 3 days in breast muscles and 7 days in liver and kidney post treatment.
Keywords: Colibacillosis, Cefquinome, Immunity, Residues, HPLC
1. Introduction
Chicken meat provides an animal protein of high biological value, where it contain all the essential amino acids required for growth with unsaturated fatty acids and low cholesterol value (Abou Hussein, 2007). Avian pathogenic Escherichia coli is the causative agent of colibacillosis (Gross, 1991). E. coli, which belongs to family Enterobacteriaceae, is a Gram-negative, rod shaped, non-spore forming bacilli and facultative anaerobe, which causes high mortalities and economic loses in poultry industry (Abd El-Tawab et al., 2015). Some factors like poor ventilation, overcrowding, dehydration and extremes of temperature affecting the occurrence of colibacillosis (Kaul et al., 1992). Lutful (2010) reported that broilers affected with E. coli showed weakness, loss of appetite, depression, cough, sneezing and watery diarrhea, which were more prominent clinical symptoms with morbidity and mortalities.
Gross pathological lesions of colibacillosis infection cause a gross pathological lesions as air saculitis, peritonitis, osteomyelitis, enteritis, pericarditis, hemorrhage and congestion in liver, kidneys, spleen and other parenchymatous organs (Abd El-Tawab et al., 2015)
Cephalosporins were widely used in veterinary medicine because of their broad-spectrum activity and safety, as they were used for treatment respiratory, urinary and genital infections (Greene and Watson, 2001). Cefquinome is one of the fourth generation cephalosporins, mainly used in veterinary medicine (Papich, 2014, Smiet et al., 2012).
Cefquinome has a broad-spectrum in vivo and in vitro activity against Gram positive and Gram negative bacterial species with great resistance against β-lactamase (Limbert et al., 1991). Like other β-lactam antimicrobials, they inhibit bacterial cell wall formation by interfering with penicillin binding proteins (PBPs), it composed of a β-lactam ring and a six-membered dihydrothiazine ring which is essential for their antibacterial activity (Prescott, 2013).
A quaternary ammonium side chain attached to the C-3 position in cefquinome, make it differ from the third generation, it can easily penetrate the biological membranes and porins of the bacterial cell wall due to its zwitter ionic property thus enhancing its spectrum activity and bioavailability compared with the second and third generation cephalosporins (Thomas et al., 2006). Cefquinome is resistant to inactivation by β-lactamases due to addition of a methoxyimino-aminothiazolyl moiety into the acyl side chain which improve its antimicrobial potency and extensive antibacterial spectrum (Marshall and Blair, 1999, Neu, 1982). Fourth generation has free radical scavenging potential and good stability against enzymatic hydrolysis (Soejima et al., 2000). Cephalosporins protect against hypochlorous acid (HOCl) oxidative damage. This defense is a consequence of a direct drug scavenging capacity towards HOCl (Lapenna et al., 1995).
Small metabolites of medicinal products or chemical substances that may accumulate within the tissues or edible parts of treated animals are called drug residues (EC-European Commission, 2012).
The aim of this study was to evaluate the efficacy of cefquinome in treatment of E. coli infection in broiler chickens, with focus on its effects on growth performance, hematobiochemical, oxidative status and immunological profile. In addition, determination of its residues using high performance liquid chromatography (HPLC) and its withdrawal times in different broiler tissues (breast muscles, liver and kidney).
2. Material and methods
2.1. Reagents for mobile phase in HPLC:
Tri-floro acetic acid (TFA) was spectrophotometric grade ≥ 99.9% (ALDRICH), Acetonitrile (ACN), de-ionized water (D.W)
2.2. Reagents for tissue sample extraction
Ammonium acetate buffer 0.05 M; pH5, Isooctane, Methanol.
2.3. Instrument and chromatographic conditions
The HPLC system (Agilent Technologies, 1200 series Japan) consists of a quaternary pump and a degasser to pump the mobile phase, an auto-sampler and a column oven. The detection by using a multi-wave detector (MWD) set at 267 nm. The column temperature was kept at 40 °C. The reverse-phase chromatography was performed with an analytical Agilent C18 column (250 mm by 4.6 mm; internal diameter, 5 μm: Agilent Technologies) The HPLC 2D Chemstation software (Hewlett-Packard, Les Ulis, France).
2.4. Drug and treatment
Cefquinome sulphate (50 ml cobactan) ® 2.5%, each 1 ml contain 25 mg cefquinome. It was obtained from Pharma Sweed-Egypt Company and was intramuscularly injected with a dose (2 mg/kg body weight) once daily for 3 successive days (El-Sayed et al., 2015).
2.5. Chicks
A total of 150, 1-day old Cobb broiler chicks were reared in litter system under hygienic measures. Chicks were fed commercial ready-made ration obtained from Feed Mix Company. All experimental chickens were used according to the Committee of Animal Welfare and Research Ethics (protocol ZU-IACUC/2/F/166/2021) in Zagazig University, Egypt.
2.6. Vaccines and vaccination
All chicks were vaccinated with Newcastle disease virus (NDV) (Hitchner B1 on 7th day and LaSota on 18th day of age).
- ND vaccines: Hitchner B1 and LaSota live virus vaccines were obtained from Intervet Boxmeer Company. Vial containing 1 × 106 EID50 Newcastle disease virus/dose, was dissolved in physiological saline (30 ml per 1000 doses) as eye drops.
2.7. Experimental infection:
Escherichia coli O78 was obtained from Animal Health Research Institute, EL-Dokki, Cairo. At 14th day of age, each bird in the challenged groups was orally inoculated with 1 ml of saline containing 1 × 108 colony forming unit (CFU) of E. coli/ ml (El-Boushy et al., 2006).
2.8. Experimental design
On day 14th of age, chicks were experimentally infected with E. coli O78 and divided into 3 groups (50 chicks each) randomly. Group (G1) non-infected, non-treated (control), (G2) infected with E. coli and non treated, (G3) infected and treated with cefquinome. Treatment was started after 5 days of experimental infection for 3 consecutive days (19–21 days of age) intramuscularly. Chicks were observed daily throughout the whole experimental period. Body weights were recorded to calculate the weight gain. Clinical signs, feed intake, feed conversion rate (FCR) and mortality rate were recorded as well.
2.9. Sampling
2.9.1. Blood samples
Two blood samples from the wing vein of 3 birds of each group at 7 and 14 days post treatment (28th day and 35th day of age respectively). The 1st sample was taken with anticoagulant for measuring erythrogram and leukogam (total erythrocytic, leukocytic and differential leucocytic count) (Natt and Herrick, 1952), Packed cell Volume (PCV) (Cohen, 1967) and hemoglobin content (Wintrobe, 1967). The 2nd blood sample collected without anticoagulant for serum separation for measuring aspartate aminotransferase and alanine aminotransferase (AST-ALT) (Tietz, 1976), Alkaline phosphatase (ALP) (Belfield and Goldberg, 1971), serum total protein (Hennry, 1974), serum uric acid (Weissman et al., 1974), creatinine (Hennry, 1974), super oxide dismutase (SOD) (Nishikimi et al., 1972), glutathione peroxidase (GPX) (Paglia and Valentine, 1967) and malondialdehyde (MDA) (Draper and Hadley, 1990). Serum protein fractions were performed using cellulose acetate electrophoresis test (Davis, 1964), Hemagglutination inhibition (HI) test for estimation titer of ND (Villagas, 1991). Nitric oxide (Green et al., 1982) and lysozyme activity (Schultz, 1987) measured at 1st and 3rd day post treatment.
2.9.2. Tissue samples
Three birds was slaughtered at 3rd, 5th, 7th days post last dose of cefquinome treatment, 1 g tissues was taken from liver, kidney and breast muscles and stored at −20 °C until assayed for detection of cefquinome residues using HPLC (Abd Elhafeez and Fadel, 2021).
2.10. Sample extraction
One gram of tissue sample was added in 15 ml poly-propylene centrifugal tube. 4 ml of 0.05 M ammonium acetate buffer (TAC5) pH 5 and 1 ml isooctane, were added, and mixed for few seconds, then centrifuged at 2400 × g/ 10 min. The isooctane was discarded and the supernatant was transferred to previously activated solid phase cartridges with 1 ml MeOH, 1 ml D.W and 1 ml TAC5; then cartridges was washed using 1 ml TAC5, 1 ml D.W, then 1 ml 10% ACN. Air was passed via the cartridge for 5 min. elution by 1 ml elution solution 20% ACN; at a flow rate of 3 ml/min, the elute was evaporated at 50 °C under nitrogen stream till complete dryness, then re-constituted in 500 μl D.W, filtration with 0.45 um Acro-discs before injection into HPLC system (Elazab et al., 2016).
2.11. Calibration curve
The cefquinome calibration standard was prepared at concentrations of 25, 50, 100, 250, 500, 1000, 2500 ppb in blank.
2.12. Statistical analysis
The obtained data were statistically analyzed by a one-way analysis of variance (ANOVA) method followed by Tukey's HSD multiple comparison test. Differences were considered significant at p < 0.05.
3. Results
The E. coli infected non treated group (G2) appeared symptoms of E. coli infection represented by loss of appetite, depression, ruffled feathers and diarrhea with mortality rate 30%. (Fig. 1).
Fig. 1.
Chickens of infected non treated group showed signs of E. coli infection (A) depression with ruffled feathers, (B) diarrhea.
Growth performance parameters were badly affected in the E. coli infected group, as there were a significant decrease in body weight, weight gain and feed consumption with a significant increase in FCR when compared with control group. Cefquinome treatment improve the body weight, weight gain and FCR with reduction in mortalities 6% (Table1).
Table 1.
The effect of cefquinome on growth performance and feed consumption on the 7th and 14th day post treatment of E. coli experimentally infected broiler chickens. (Mean ± S.E) (n = 3).
| Group | 14 day of age |
3rd day post treatment |
7th day post treatment |
14th day post treatment |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Body weight(g) | Body weight(g) | Body weight(g) | Weight gain(g) | feed consumption(gm) | FCR | Body weight(g) | Weight gain(g) | feed consumption(gm) | FCR | |
| G1 | 663.1 | 1101.86 a±1.21 | 1527.27 a±5.7 | 425.42 a±4.68 | 603.33 a±4.62 | 1.42c±0.02 | 2624.26 a±5.77 | 1096.99 a±7.01 | 1606.76 a±3.41 | 1.47c±0.01 |
| G2 | 665.5 | 903.86c±1.8 | 1103.99c±2.02 | 200.13c±2.19 | 369.5c±1.97 | 1.85a±0.01 | 1712.87c±1.2 | 608.87c±0.83 | 1174.3c±2.05 | 1.93 a±0.02 |
| G3 | 662.31 | 991.35b±1.38 | 1375.59b±2.79 | 384.23b±4.01 | 572.65b±2.56 | 1.49b±0.02 | 2404.02b±1.71 | 1028.43b±4.44 | 1555.38b±2.91 | 1.51b±0.2 |
E. coli infection cause a significant reduction in erythrogram and lymphocytes beside a significant increase in total leukocytic count, neutrophils compared with control group (G1). Cefquinome treated group showed a significant increase in erythrogram (RBCS, Hb, and PCV) and lymphocytes with a significant decrease in total leukocytic count and neutrophile compared with infected non treated group (Table 2).
Table 2.
The effect of cefquinome on erythrogram and leukogram of E. coli experimentally infected broiler chickens. (Mean ± S.E) (n = 3).
| Group | Erythrogram |
Leukogram |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 days post-treatment |
14 days post-treatment |
7 days post-treatment |
14 days post-treatment |
|||||||||
| Hb(g/dl) | RBCs(1012/l) | PCV(%) | Hb(g/dl) | RBCs(1012/l) | PCV(%) | t.WBCs(109/l) | Neutrophils(109/l) | Lymphocytes(109/l) | t.WBCs(109/l) | Neutrophils(109/l) | Lymphocytes(109/l) | |
| G1 | 10.49 a±0.28 | 4.29 a±0.01 | 31.06 a±0.22 | 10.35 a±0.05 | 4.43 a±0.02 | 30.74 a±0.23 | 10.2c±0.05 | 3.13c±0.07 | 5.44 a±0.07 | 10.57b±0.24 | 3.2b±0.05 | 5.38 a±0.07 |
| G2 | 7.12c±0.01 | 2.9c±0.02 | 26.35c±0.25 | 7.52b±0.1 | 2.65b±0.02 | 24.73b±0.11 | 14.74 a±0.07 | 6.73 a±0.07 | 4.23b±0.06 | 14.05 a±0.02 | 6.46 a±0.13 | 3.89b±0.1 |
| G3 | 9.1b±0.01 | 4.1b±0.05 | 30.14b±0.2 | 10.12 a±0.01 | 4.38 a±0.01 | 30.63 a±0.25 | 11.45b±0.08 | 3.97b±0.1 | 5.27 a±0.05 | 10.87b±0.03 | 3.37b±0.07 | 5.4 a±0.05 |
Infection with E. coli cause oxidative stress by significant reduction in SOD and GPX with increase in MDA levels compared to control. Meanwhile, oxidative status improved in treated group (Table 3).
Table 3.
The effect of cefquinome on serum antioxidants of E. coli experimentally infected broiler chickens. (Mean ± S.E) (n = 3).
| Group | Antioxidants |
|||||
|---|---|---|---|---|---|---|
| 7th day post-treatment |
14th day post-treatment |
|||||
| MDA(nmol/ml) | SOD(U/ml) | GPX(U/ml) | MDA(nmol/ml) | SOD(U/ml) | GPX(U/ml) | |
| G1 | 4.1c±0.03 | 12.51 a±0.22 | 3.87 a±0.01 | 4.21b±0.03 | 12.87 a±0.17 | 3.82 a±0.01 |
| G2 | 5.94 a±0.02 | 8.22c±0.05 | 2.32c±0.01 | 6.15 a±0.05 | 8.7b±0.07 | 2.48b±0.01 |
| G3 | 4.77b±0.01 | 11.85b±0.02 | 3.35b±0.01 | 4.23b±0.01 | 12.73 a±0.06 | 3.8 a±0.04 |
On comparison with control group, nitric oxide and lysozymes activity were significantly increased (P < 0.05), meanwhile HI titers against Newcastle was significantly decrease in infected non treated broilers, while E. coli infected cefquinome treated group showed significant decrease in nitric oxide, lysozymes activity and increase in HI titers compared G2 (Table 4).
Table 4.
The effect of cefquinome on Nitric oxide, lysozyme activity and HI titer against Newcastle of E. coli experimentally infected broiler chickens. (Mean ± S.E) (n = 3).
| Group | Nitric Oxide- Lysozyme |
H.I antibody titer |
||||
|---|---|---|---|---|---|---|
| 1 days post-treatment |
3 days post-treatment |
7 days post-treatment | 14 days post-treatment | |||
| N.O(umol/l) | Lysozymes (Ug/ml) | N.O(umol/l) | Lysozymes(Ug/ml) | |||
| G1 | 19.85c±0.16 | 11.45c±0.12 | 19.19b±0.13 | 12.84b±0.12 | 4.45 a±0.13 | 5.15 a±0.08 |
| G2 | 27.44 a±0.17 | 17.67 a±0.09 | 28.13 a±0.11 | 20.57 a±0.23 | 2.92c±0.03 | 2.96b±0.01 |
| G3 | 22.15b±0.17 | 15.45b±0.15 | 19.48b±0.31 | 13.19b±0.15 | 3.41b±0.05 | 5.12 a±0.01 |
Infection with E. coli cause significant reduction in total protein, albumin level and A/G ratio, while total globulins and γ globulin was significantly increased (P < 0.05) with non-significant change in α and β globulin compared to the control. Cefquinome treatment of the infected broilers significantly improve these parameters toward the control levels (Table 5).
Table 5.
The effect of cefquinome on serum total protein, albumin, globulin and A/G ratio of E. coli experimentally infected broiler chickens. (Mean ± S.E) (n = 3).
| Group | Serum total protein, Albumin and Globulin |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7th day post-treatment |
14th day post-treatment |
|||||||||||||
| Serum total protein(g/dl) | Albumin(g/dl) | Globulin(g/dl) | ˠ | α | β | A/G ratio (g/dl) | Serum total protein(g/dl) | Albumin(g/dl) | Globulin(g/dl) | ˠ | α | β | A/G ratio (g/dl) | |
| G1 | 5.93 a±0.19 | 2.85 a±0.03 | 3.08b±0.16 | 1.24c±0.01 | 0.93 a±0.01 | 0.9 a±0.15 | 0.93 a±0.04 | 5.49 a±0.22 | 2.68 a±0.05 | 2.81b±0.19 | 1.23b±0.01 | 0.81 a±0.01 | 0.76 a±0.19 | 0.96 a±0.05 |
| G2 | 4.76c±0.07 | 1.14c±0.04 | 3.62 a±0.03 | 1.81 a±0.02 | 0.92 a±0.02 | 0.89 a±0.03 | 0.31c±0.01 | 4.71b±0.01 | 1.30b±0.05 | 3.41 a±0.06 | 1.84 a±0.01 | 0.79 a±0.01 | 0.77 a±0.06 | 0.38b±0.02 |
| G3 | 5.37b±0.04 | 2.11b±0.06 | 3.26b±0.03 | 1.42b±0.01 | 0.95 a±0.02 | 0.89 a±0.05 | 0.65b±0.02 | 5.38 a±0.01 | 2.56 a±0.1 | 2.83b±0.1 | 1.24b±0.01 | 0.82 a±0.02 | 0.76 a±0.08 | 0.91 a±0.07 |
Liver enzymes and kidney function were significantly (P < 0.05) increased in E. coli infected group, cefquinome treatment improve these functions (Table 6).
Table 6.
The effect of cefquinome on liver and kidney function tests of E. coli experimentally infected broiler chickens. (Mean ± S.E) (n = 3).
| Group | liver enzymes (U/L) |
kidney function (mg/dl) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7 days post-treatment |
14 days post-treatment |
7 days post-treatment |
14 days post-treatment |
|||||||
| ALT | AST | ALP | ALT | AST | ALP | Creatinine | U.Acid | Creatinine | U.Acid | |
| G1 | 48.07c±0.34 | 46.32c±0.49 | 235.02c±0.4 | 48.8b±0.53 | 45.69b±0.33 | 234.25b±0.33 | 0.99c±0.01 | 5.39b±0.11 | 1.01b±0.02 | 5.25b±0.01 |
| G2 | 64.52 a±0.2 | 61.39 a±0.15 | 303.63 a±0.79 | 62.35 a±0.2 | 59.77 a±0.08 | 314.08 a±1.62 | 1.92 a±0.01 | 7.95 a±0.02 | 2.15 a±0.09 | 7.86 a±0.02 |
| G3 | 56.57b±0.23 | 48b±0.49 | 244.61b±0.22 | 49.29b±0.12 | 46.07b±0.14 | 237.17b±0.48 | 1.37b±0.02 | 5.52b±0.01 | 1.06b±0.01 | 5.3b±0.01 |
3.1. Standard curve concentration
Cefquinome calibration curve was prepared at concentrations of 25, 50, 100, 250, 500, 1000, 2500 ppb respectively. Linearity existed within the range of 25 ppb and 2500 ppb with a correlation coefficient (r2) of 0.99921. The retention time (R.T.) of cefquinome was 13.745 min (Table 7), (Fig. 2, Fig. 3).
Table 7.
Area under the curve corresponding to standard cefquinome concentrations.
| Retention time | Level | Amount(ppb) | Area | Resp. Factor |
|---|---|---|---|---|
| 13.745 min | 1 | 25.000 | 5.601 | 4.464 |
| 2 | 50.000 | 10.919 | 4.579 | |
| 3 | 100.00 | 21.533 | 4.644 | |
| 4 | 250.000 | 56.581 | 4.418 | |
| 5 | 500.000 | 112.270 | 4.454 | |
| 6 | 1000.000 | 220.060 | 4.544 | |
| 7 | 2500.000 | 552.120 | 4.528 |
Fig. 2.
Calibration curve of cefquinome.
Fig. 3.
HPLC chromatograms of cefquinome standard at concentration (A) 25 ppb, (B) 1000 ppb, (C). 2500 ppb.
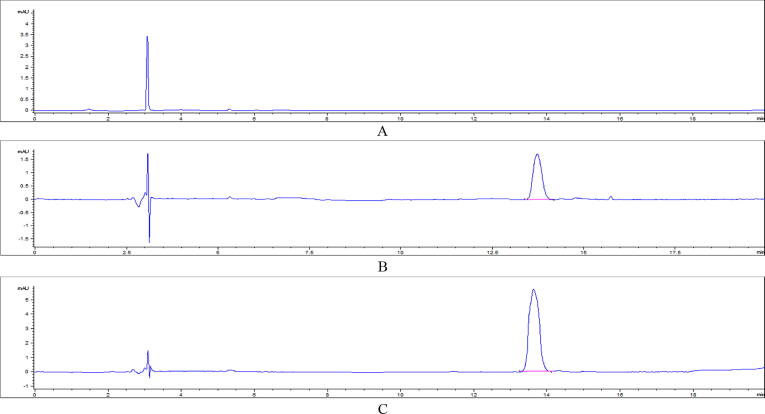
Cefquinome's residues were highly concentrated in kidney followed by liver and not detected in breast muscles at 3rd day post treatment. On 7th day post treatment, it completely not detected in liver and kidney tissues of broiler (Table 8), (Fig. 4).
Table 8.
Concentrations of cefquinome (ppb) after repeated intramuscular administration in breast muscles, liver, and kidney of E. coli experimentally infected, cefquinome treated broiler chickens (group 3). (Mean ± S.E) (n = 3).
| Organs | Residue level (ppb) |
||
|---|---|---|---|
| 3rd day post treatment | 5th day post treatment | 7th day post treatment | |
| Brest muscles | ND | ND | ND |
| Liver | 102.4 ± 0.473 | 35.4 ± 0.252 | ND |
| Kidney | 353.8 ± 0.635 | 55.74 ± 0.399 | ND |
ppb: part per billion, ND: not detected.
Fig. 4.
HPLC chromatograms of cefquinome residual level in E. coli experimentally infected, treated broiler chicken tissues in 3rd day post I/M cefquinome treatment. (A) cefquinome residues in breast muscle, (B). cefquinome residues in liver ,(C). cefquinome residues in kidney.
4. Discussion
The current study applied for evaluation efficacy of cefquinome in treatment E. coli infected broilers by its effects on growth performance, hematobiochemical, oxidative status and immunological parameters with detection of its residues in different tissues of boilers.
The clinical signs as loss of appetite, depression, respiratory symptoms including sneezing, gasping, mild conjunctivitis and diarrhea of colibacillosis with high mortalities in G2 because of excretion of endotoxins by E. coli (Roushdy, 2007, Mabrouk, 2016).
Medication of broilers infected with E. coli using cefquinome improve the general health status, growth performance with disappearance of clinical signs (El-Gendy et al., 2009, Nasr El Deen et al., 2015).
In this study, decreased body weight, weight gain and increased feed conversion rate throughout the experimental period were clearly appeared in infected broilers, which might be attributed to intestinal damage and poor digestion caused by microorganisms; these results were agreed with (Mabrouk, 2016, Fadl et al., 2020). Cefquinome treated group showed decreased feed conversion compared with non-treated, this previous data is supported by (Radwan and Radi, 2010).
Alexander (1985) reported that cefquinome cause increase nutrients absorption leading to improving in weight gain.
Reduction in erythrogram parameters caused by E. coli infection could be attributed to bacterial endotoxins, which cause intravascular destruction of erythrocytic cells and consequently lead to hemolysis with breakdown of hemoglobin (Karaivanov, 1984). E. coli produces cell damaging protein toxin that causes changes in cell membrane permeability and formation of surface lesions causing RBCs destruction (Dagmar et al., 2002). In addition, (Tserenpuntsag et al., 2005) stated that E. coli lipopolysaccharide has direct effect as it inhibits bone marrow cells and its nephrotoxicity decrease erythropoietin in blood. Arhoumah (2018) reported that cefquinome has bactericidal activity and improve erythrogram.
Leukocytosis was detected in chickens suffering from colibacillosis could be due to tissue destruction (Coles, 1967), this results agreed with (Nasr El Deen et al., 2015) in broilers and (Tharwat et al., 2013) in turkey.
Arhoumah, 2018, Shawky, 2006 reported that cefquinome administration decrease the elevated TLC in infected broilers.
Malondialdehyde (MDA) reactive aldehydes, because of lipid peroxidation indicating oxidative stress (Mandal et al., 2015).
Imbalance between oxidants production and organism's respective defense systems defined as oxidative stress (Berridge et al., 1996).
In this study, E. coli infection induced an elevation in MDA level in infected group related to excessive production of free radicals causing stress and cellular toxicity, this results agreed with (Fadl et al., 2020) who found high levels of MDA in E. coli infected broilers.
Accumulation of reactive oxygen species (ROS) and oxidant/antioxidant imbalance will lead to decrease levels of SOD and GPX in infected chicks (Kilany et al., 2018).
Treatment with cefquinome improve the antioxidant levels as according to (Soejima et al., 2000). Cephalosporins reduce hepatic oxidative damage, as they are thioether having free radical scavenging properties by preventing the free radical-mediated oxidation of sulfhydryl group. Its antioxidant defense activity related to protection against HOCl-driven oxidative injury (Lapenna et al., 1995).
Lysozyme are protein found in the body fluids, tissue and cells of living organisms having digestive and defense functions by lysis to the bacterial cell wall (Maraghi et al., 2012). During infection, lysozyme activity increased because of lysozyme gene transcription increased (Lowry et al., 2005, Paulsen et al., 2003) and phagocytosis-induced degranulation (Dey and Bishayi, 2015).
Treatment of E. coli infected broiler chickens with cefquinome cause reduction in lysozyme activity; in particular, neutralization of the medium by its acidification properties, which significantly reduces the lysozyme exocytosis, also inhibition of NADPH oxidase needed for activation of lysosomes (Bassler et al., 1982).
Nitric oxide produced by the enzyme nitric oxide synthase from L-arginine, it's a short highly reactive free radical (Thippeswamy et al., 2006). Herein, E.coli infection remarkably elevate serum nitric oxide, explained by increase of nuclear factor kappa B (NF-κB) leading over release of inflammatory mediators like cytokines and inducible NO synthase (iNOs) causing more NO formation (Macdonald et al., 2003). Tumor necrosis factor alpha (TNF-α) elevation led to increasing NO causing tissue damage (Zhao et al., 2002).
Treated group showed decrease NO level due to fourth generation of cephalosporin able to suppress TNF-α induced by E.coli endotoxin consequently led to decrease NO (Coleman, 2001).
The reduction in HI titer of ND in infected group was returned to changes in the acid-base balance due to digestive disorders, enteritis and diarrhea (Sil et al., 2002) and down regulatory effect and stress of infection post vaccination (Abd El Tawab et al., 2015, Abd El-Ghany and Ismail, 2014, Hassanin et al., 2014, Hegazy et al., 2010), reported the same results as higher HI titers were detected in healthy vaccinated chickens than other E. coli infected vaccinated.
Cefquinome treated group showed significant increase in HI titer compared with infected one, due to improvement the levels of TNF-α and IL-10 elevated by E.coli infection (Arhoumah, 2018).
E. coli infection cause a significant decreases in total serum protein, attributed to kidney diseases, causing protein loss and congestive heart failure (Blood et al., 1994), albumin level were significantly decreased due to decrease in feed intake, anorexia and hepatic damage (Deshmukh, 2006), these resulted were agreed with that of (EL-Nemr, 2011, Godbole, 2017, Manafi et al., 2016, Zaki et al., 2012). Also, E. coli infection cause significant increase in globulins, as a result of liver cirrhosis, hepatitis, and Kupffer cell proliferation (Sharma et al., 2015), this result reinforced with (Fadl et al., 2020). ˠ-globulins elevation in infected group associated with immune system activation (Gharieb and Youssef, 2014) this results agreed with (Hashem et al., 2020).
Cefquinome treatment of E. coli infected broiler chickens improved proteinogram compared with infected non treated group (Elkomy et al., 2019).
Infected broiler chickens showed increase in liver enzymes, this might be due to the hepatic degenerative changes, causing escape of liver enzymes with high abnormal levels into serum due to alteration of cellular permeability (Joan and Pannel, 1981).
Unger et al. (1989) attributed the increase of liver enzymes in E.coli infection to perfusion of certain microvascular segments and decrease perfusion of others because of redistribution of hepatic microvascular blood flow within the liver lobule. These results were supported by that obtained by (El-Kadeem, 2009, Shawky, 2006).
Degenerative changes in renal tubules and impairment of their function, decreases the excretion of uric acid and creatinine leading to increase its levels in serum in E. coli infected group (Kaneko, 1980). Our results were in accordance with that obtained by (Abdalla and Adayel, 2006, El-Sayed, 2007). Treatment of E. coli infected broiler with cefquinome showed reduction in liver enzymes which agreed with (Elbaz et al., 2020), kidney functions as reported by (Nasr El Deen et al., 2015).
Many countries have monitoring scheme to avoid antibiotic residues in food of animal origin to ensure food safety (Albayoumi, 2015).
In the present investigation, repeated intramuscular administration of cefquinome revealed that kidneys retains the highest drug concentrations followed by the liver in the infected treated broilers while the lowest drug concentrations found in muscles. These findings suggested that cefquinome excreted mainly via the kidney (Limbert et al., 1991, San Martin et al., 1998). These results were agreed with that reported by (El-Sayed et al., 2015, Gaber, 2005) in chickens and (Abd Elhafeez and Fadel, 2021) in rabbits.
5. Conclusion
The repeated intramuscular administrations of cefquinome (2 mg/kg b.wt.) once daily for three consecutive days provides an effective treatment against E. coli infection in broiler chickens. The recommended withdrawal time is 3 days in muscles and 7 days for liver and kidneys in E. coli infected cefquinome treated broilers to be safe for human consumption.
6. Ethics statement
The protocol was conducted according to the Committee of Animal Welfare and Research Ethics (protocol ZU-IACUC/2/F/166/2021) in Zagazig University, Egypt.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to thank Taif University Research Supporting Project number (TURSP-2020/76), Taif University, Taif, Saudi Arabia for supporting this research.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Ghany W.A., Ismail M. Tackling experimental colisepticaemia in broiler chickens using phytobiotic essential oils and antibiotic alone or in combination. Iran. J. Vet. Res. 2014;15:110–115. [Google Scholar]
- Abd El-Tawab A.A., El-komy A.A., El-Ekhnawy K.I., Talaie A.T. Effect of fosfomycin on E. Coli O78 isolated from broiler chickens in-vitro and in-vivo. Benha Vet. Med. J. 2015;28:294–300. [Google Scholar]
- Abd El Tawab A.A., El-Hofy F.I., El-Eknawy K.I., El-Shora H.E. Effect of Synbiotic on immune response of experimentally infected broiler chickens with E. coli and salmonella. Benha Vet. Med. J. 2015;28:188–194. [Google Scholar]
- Abd Elhafeez M.S., Fadel M.A. Investigation of kinetic and tissue residues of cefquinome after its intramuscular administration in experimentally infected rabbits with Klebsiella pneumonia. Egypt. J. Animal Health. 2021;1:23–33. [Google Scholar]
- Abdalla O., Adayel S. Sci. Vet. Med. Zag Hurghada; 2006. Concurrent use of kanamycin and spiramycin for controlling chronic respiratory disease in broiler chicks; pp. 556–563. [Google Scholar]
- Abou Hussein, R., 2007. Detection of food mediated pathogens in some meat and chicken products by using recent techniques. Ph. D. Thesis (Meat Hygiene), Faculty of Veterinary Medicine, Banha
- Albayoumi M.A. Islamic University Gaza, Faculty of Science; 2015. Detection of Antibiotic Residues in Broiler Chickens in Gaza Strip, Biological Sciences Microbiology. [Google Scholar]
- Alexander F. fourth ed. Longman; London and New York: 1985. An introduction of vet. pharm. [Google Scholar]
- Arhoumah A. Mansoura University, Faculty Veterinary; 2018. Clinicopathological studies on feverish rats treatedwith antibiotic and anti-Inflammatory. [Google Scholar]
- Bassler M., Blaschke H., Just M., Daschner F. Effect of ceftriaxone on Pseudomonas aeruginosa and Staphylococcus aureus in broth, serum, and in combination with human polymorphonuclear leukocytes. Chemotherapy. 1982;28:390–396. doi: 10.1159/000238127. [DOI] [PubMed] [Google Scholar]
- Belfield A., Goldberg D. Revised assay for serum phenyl phosphatase activity using 4-amino-antipyrine. Enzyme. 1971;12:561–573. doi: 10.1159/000459586. [DOI] [PubMed] [Google Scholar]
- Berridge M., Tan A., McCoy K., Wang R. The biochemical and celular basis of cell proliferation assays. Biochemica. 1996:15–20. [Google Scholar]
- Blood D., Radostits O., Henderson J. The English Language Book Society and Bailliere Tindall; 1994. Veterinary Medicine, a textbook of disease of cattle, sheep, goats, pigs and horses. [Google Scholar]
- Cohen R.R. Anticoagulation, Centrifligation Time, and Sample Replicate Number in the Microhematocrit Method for Avian Blood. Poult. Sci. 1967;46:214–218. doi: 10.3382/ps.0460214. [DOI] [PubMed] [Google Scholar]
- Coleman J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001;1:1397–1406. doi: 10.1016/s1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- Coles E.H. fourth ed. Sounders Company; 1967. Veterinary clinical pathology. [Google Scholar]
- Dagmar J., Muhsin O., Ntondo B. Production and characterization E coli enterohemolysin and its structure effects of erythrocyte membranes. Cell Biol. Int. 2002;26:75–86. doi: 10.1006/cbir.2001.0831. [DOI] [PubMed] [Google Scholar]
- Davis B. Method and application tohuman serum protein. Ann. NY Acad. Sci. 1964;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Deshmukh V. MAFSU; Nagpur, College of Veterinary and Animal Sciences, Udgir: 2006. Studies on Efficacy of Levofloxacin In Experimental Colibacillosis in Broilers. [Google Scholar]
- Dey S., Bishayi B. Killing of Staphylococcus aureus in murine macrophages by chloroquine used alone and in combination with ciprofloxacin or azithromycin. J. Inflamm. Res. 2015;8:29. doi: 10.2147/JIR.S76045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper H.H., Hadley M. Malondialdehyde determination as index of lipid Peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- EC-European Commission, 2012. Commission staff working document on the implementation of national residue monitoring plans in the member states in 2009.
- El-Boushy, M., Sanaa, S., Abeer, H., 2006. Immunological, hematological and biochemical studies on pefloxacin in broilers infected with E. coli, Proceedings of the 8th Sciences Veterinary Medicine Zagazig Conference, pp. 55–59
- El-Gendy A., Tohamy M., Radi M., A., Pharmacokinetic profile and some pharmacodynamic aspects of cefquinome in chickens. J. Vet. Med. Res. 2009;19:33–37. [Google Scholar]
- El-Kadeem A.E.-S.M. Pharmacology. Zagazig University, Faculty of Veterinary Medicine; 2009. Efficacy of danofloxacin and ceftiofur sodium on e. coli infection in chickens. [Google Scholar]
- EL-Nemr A.E.-s.A. Faculty of Veterinary Medicine; Cairo University: 2011. Efficacy of florfenicol on E. coli infection in chicken. [Google Scholar]
- El-Sayed A.A. Pharmacology. Zagazig university, Faculty of Veterinary Medicine; 2007. Efficacy of spiramycin on colibacillosis in chickens. [Google Scholar]
- El-Sayed M., El-Komy A., Mobarez E., El-Mahdy A. Pharmacokinetics and Tissue residues of cefquinome in normal and salmonella entretidis infected chickens. World J. Pharm. Pharmaceut. Sci. 2015;10:1974–1987. [Google Scholar]
- Elazab S.T., Gabr M.G., Amer M.S., El-nabtity S.M., Hsu W.H. Depletion of cefquinome from rabbit tissues. Int. J. Innov. Appl. Stud. 2016;18:743. [Google Scholar]
- Elbaz H., Hamed M., Abdelhamid F., Abdalla O. Effect of cefepime on hematological, immunological and oxidant/antioxidant parameters in rats experimentally infected with E. coli ATCC 25922. Mansoura Vet. Med. J. 2020;21:36–45. [Google Scholar]
- Elkomy A., Aboubakr M., Elsayed Emam M.K. Studies on the effects of cephradine and colibacellosis on immunological status of broiler chicken vaccinated with newcastle virus vaccine. Int. J. Pharm. Toxicol. 2019;7:17–21. [Google Scholar]
- Fadl S.E., El-Gammal G.A., Sakr O.A., Salah A.A., Atia A.A., Prince A.M., Hegazy A.M. Impact of dietary Mannan-oligosaccharide and β-Glucan supplementation on growth, histopathology, E-coli colonization and hepatic transcripts of TNF-α and NF-ϰB of broiler challenged with E. coli O 78. BMC Vet. Res. 2020;16:1–14. doi: 10.1186/s12917-020-02423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaber M. Parmacokinetics of cefquinome and tissue concentrations in broilers. Cairo Univ; 2005. p. 43. [Google Scholar]
- Gharieb M.M., Youssef F.M. Effect of echinacea purpurea and garlic on growth performance, immune response, biochemical and hematological parameters in broiler chicks. Assiut Vet. Med. 2014;60:218–228. [Google Scholar]
- Godbole P. MAFSU; Nagpur: 2017. Evaluation of Prophylactic and Therapeutic Efficacy of Curcumin Against Escherichia coli-Induced Infection in Broiler Chicks. [Google Scholar]
- Green L.C., Wanger D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tanninbaum S.R. Analysis of nitrites and nitrates in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Greene G., Watson A. Infectious Diseases of the dog and cat. WB Saunders Philadelphia; 2001. Antibacterial chemotherapy. [Google Scholar]
- Gross W. In: Diseases of poultry. ninth ed. Calnek B.W., Barnes H.J., Beard C.W., McDougald L.R., Saif Y.M., editors. Iowa State University Press; Ames, IA: 1991. Colibacillosis. [Google Scholar]
- Hashem M.A., Neamat-Allah A.N., Hammza H.E., Abou-Elnaga H.M. Impact of dietary supplementation with Echinacea purpurea on growth performance, immunological, biochemical, and pathological finding in broiler chickens infected by pathogenic E. coli. Tropical Animal Health Prod. 2020;52:1599–1607. doi: 10.1007/s11250-019-02162-z. [DOI] [PubMed] [Google Scholar]
- Hassanin O., Abdallah F., Awad A. Effects of florfenicol on the immune responses and the interferon-inducible genes in broiler chickens under the impact of E. coli infection. Vet. Res. Commun. 2014;38:51–58. doi: 10.1007/s11259-013-9585-7. [DOI] [PubMed] [Google Scholar]
- Hegazy A., Abd-ElSamie L., ELSayed E. The immunosuppressive effect of E. coli in chickens vaccinated with Infectious Bronchitis (IB) or Infectious Bursal Disease (IBD) vaccines. J. Am. Sci. 2010;6:762–767. [Google Scholar]
- Hennry R.J. 2 ed. Harper and Row Publ; New York, USA: 1974. Clinical Chemistry Principles and Techniques. [Google Scholar]
- Joan F.Z., Pannel P.R. third ed. Liayed; Luke, London: 1981. Clinical Chemistry in Diagnosis and Treatment. [Google Scholar]
- Kaneko J.J. fourth ed. Academic press Inc.; New York, London: 1980. Clinical biochemistry of domestic animals. [Google Scholar]
- Karaivanov L. Somatic antigens of Pasteurella multocida strains. Vet. Med. Nauki. 1984;21:12–16. [PubMed] [Google Scholar]
- Kaul L., Kaul P., Shah N. An outbreak of colibacillosis in broiler chicks at an organized poultry farm under semi-arid zone of North Gujarat. Indian Vet. J. 1992;69:373–374. [Google Scholar]
- Kilany O., Youssef F., Mabrouk M., Fares I. Clinicopathological studies on the effect of some antibacterial medicinal plants in broilers. Clin. Pathol. Forecast. 2018;1:1003. [Google Scholar]
- Lapenna D., Cellini L., De Gioia S., Mezzetti A., Ciofani G., Festi D., Cuccurullo F. Cephalosporins are scavengers of hypochlorous acid. Biochem. Pharmacol. 1995;49:1249–1254. doi: 10.1016/0006-2952(95)00044-z. [DOI] [PubMed] [Google Scholar]
- Limbert M., Isert D., Klesel N., Markus A., Seeger K., Seibert G., Schrinner E. Antibacterial activities in vitro and in vivo and pharmacokinetics of cefquinome (HR 111V), a new broad-spectrum cephalosporin. Antimicrob. Agents Chemother. 1991;35:14–19. doi: 10.1128/aac.35.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry V., Farnell M., Ferro P., Swaggerty C., Bahl A., Kogut M. Purified β-glucan as an abiotic feed additive up-regulates the innate immune response in immature chickens against Salmonella enterica serovar Enteritidis. Int. J. Food Microbiol. 2005;98:309–318. doi: 10.1016/j.ijfoodmicro.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Lutful K. Avian Colibacillosis and Salmonellosis: A Closer Look at Epidemiology, Pathogenesis, Diagnosis, Control and Public Health Concerns. Int. J. Environ. Res. Public Health. 2010;7:89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabrouk, M., 2016. Concurrent use of ciprofloxacin and metronidazole for controlling of some bacterial infections in broiler chickens. Ph. D. Thesis, Faculty. of Vet. Med. (Pharmacology) Zagazig University.
- Macdonald J., Galley H.F., Webster N.R. Oxidative stress and gene expression in sepsis. Br. J. Anaesth. 2003;90:221–232. doi: 10.1093/bja/aeg034. [DOI] [PubMed] [Google Scholar]
- Manafi M., Hedayati M., Khalaji S., Kamely M. Assessment of a natural, non-antibiotic blend on performance, blood biochemistry, intestinal microflora, and morphology of broilers challenged with Escherichia coli. Revista Brasileira de Zootecnia. 2016;45:745–754. [Google Scholar]
- Mandal A., Patra A., Mandal S., Roy S., Mahapatra S.D., Mahapatra T.D., Paul T., Das K., Mondal K.C., Nandi D.K. Therapeutic potential of different commercially available synbiotic on acetaminophen-induced uremic rats. Clin. Exp. Nephrol. 2015;19:168–177. doi: 10.1007/s10157-014-0971-4. [DOI] [PubMed] [Google Scholar]
- Maraghi S., Molyneux D., Wallbanks K. Lysozyme activity in the plasma of rodents infected with their homologous trypanosomes. Iran. J. Parasitol. 2012;7:86. [PMC free article] [PubMed] [Google Scholar]
- Marshall W.F., Blair J.E. The cephalosporins: Symposium on Antimicrobial Agents. Mayo Clinic Proc. Elsevier. 1999:187–195. doi: 10.4065/74.2.187. [DOI] [PubMed] [Google Scholar]
- Nasr El Deen N.A., Isamail S.A., Hassan W.M., Kaser A.N. Antibacterial Activity of Ceftiofur Sodium and Garlic in Escherichia coli Infected Chickens. Zagazig Vet. J. 2015;43:81–97. [Google Scholar]
- Natt M.P., Herrick C.A. A new blood diluent for counting the erythrocytes and leucocytes of the chicken. Poult. Sci. 1952;31:735–738. [Google Scholar]
- Neu H. Clinical uses of cephalosporins. Lancet. 1982;320:252–255. doi: 10.1016/s0140-6736(82)90333-6. [DOI] [PubMed] [Google Scholar]
- Nishikimi M., Rao A., Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- Papich M.G. Pharmacokinetic–pharmacodynamic (PK–PD) modeling and the rational selection of dosage regimes for the prudent use of antimicrobial drugs. Vet. Microbiol. 2014;171:480–486. doi: 10.1016/j.vetmic.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Paulsen S.M., Lunde H., Engstad R.E., Robertsen B. In vivo effects of β-glucan and LPS on regulation of lysozyme activity and mRNA expression in Atlantic salmon (Salmo salar L.) Fish Shellfish Immunol. 2003;14:39–54. doi: 10.1006/fsim.2002.0416. [DOI] [PubMed] [Google Scholar]
- Prescott J.F. Beta-lactam antibiotics: cephalosporins. Antimicrobial Therapy Vet. Med. 2013:139–157. [Google Scholar]
- Radwan I., Radi A.M. Antimicrobial activity of some cephalosporins with special reference to their effects on body weight and immune response to Newcastle disease vaccine in fayoumy chicks. J. Vet. Med. Res. 2010;20:236–242. [Google Scholar]
- Roushdy M. Zagazig University; 2007. . Some pharmacological studies on pefloxacin in chickens. [Google Scholar]
- San Martin B., Bataglia J., Hernandez P., Quiroz A., Canon H., A, r., Absorption and excretion of cefquinome in coho salmon (Oncorhynchus kisutch) in freshwater at 10 degrees C. Zentralblatt Veterinarmedizin. 1998;45:615–623. doi: 10.1111/j.1439-0442.1998.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Schultz L. The C.V. Mosby cost Louis; 1987. Methods in clinical chemistry. [Google Scholar]
- Sharma V., Jakhar K., Nehra V., Kumar S. Biochemical studies in experimentally Escherichia coli infected broiler chicken supplemented with neem (Azadirachta indica) leaf extract. Vet. World. 2015;8:1340–1345. doi: 10.14202/vetworld.2015.1340-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawky N.A. Zagazig university; 2006. Antibacteial Efficacy Of Cefoperazone And Its Combination With Sulbactam In Chickens. [Google Scholar]
- Sil G., Das P., Islam M., Rahman M. Management and disease problems of cockrels in some farms of Mymensingh, Bangladesh. Int. J. Poult. Sci. 2002;1:102–105. [Google Scholar]
- Smiet E., Haritova A., Heil B., Fink-Gremmels J., Wijnberg I. Comparing the pharmacokinetics of a fourth generation cephalosporin in three different age groups of New Forest ponies. Equine Vet. J. 2012;44:52–56. doi: 10.1111/j.2042-3306.2011.00501.x. [DOI] [PubMed] [Google Scholar]
- Soejima A., Ishizuka S., Miyake N., Fukuoka K., Suzuki M., Kamiya Y., Nagasawa T. Simultaneous inhibition of renal phospholipase A2 and glutathione synthesis by manoalide and DL-buthionine sulfoximine induces acute tubular dysfunction in rats. Nephron Exp. Nephrol. 2000;8:84–90. doi: 10.1159/000020653. [DOI] [PubMed] [Google Scholar]
- Tharwat I.A., El Nabarawy E.A., Aly Salah B., Hassan A.A. Effect Of Apramycin On Pathological, Hematological And Biochemical Changes In Turkey Infected With Coli-Bacillosis. Zagazig Vet. J. 2013;41:124–136. [Google Scholar]
- Thippeswamy T., McKay J., Quinn J., Morris R. Nitric oxide, a biological double-faced janus-Is this good or bad? Histol. Histopathol. 2006;21:445–458. doi: 10.14670/HH-21.445. [DOI] [PubMed] [Google Scholar]
- Thomas E., Thomas V., Wilhelm C. Antibacterial activity of cefquinome against equine bacterial pathogens. Vet. Microbiol. 2006;115:140–147. doi: 10.1016/j.vetmic.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Tietz N.W. WB Saunders Co; Philadelphia PA: 1976. Fundamentals of clinical chemistry; pp. 47–51. [Google Scholar]
- Tserenpuntsag B., Chang H.-G., Smith P.F., Morse D.L. Hemolytic uremic syndrome risk and Escherichia coli O157: H7. Emerg. Infect. Dis. 2005;11:1955. doi: 10.3201/eid1112.050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger L., Cryer H., Garrison R. Differential response of the microvasculature in the liver during bacteremia. Circulatory Shock. 1989;29:335–344. [PubMed] [Google Scholar]
- Villagas P. Newcastle Disease Virus Titration. Avian Virology (AM-508) Lab. Manual. 1991 [Google Scholar]
- Weissman M., Pileggi V., Henry R., Cannon D., Winkelman J. Harper and Row Publishers; Hagerstown, MD: 1974. Clinical chemistry: principles and techniques; pp. 437–440. [Google Scholar]
- Wintrobe M.M. Lea and Febiger; Philadelphia: 1967. Clinical hematology. [Google Scholar]
- Zaki M.S., Fawzy O., Osfor M. Effect of E-coli 0H157 on Baladi Broiler Chicken and some Biochemical studies. Life Sci. J.-Acta Zhengzhou Univ. Overseas Ed. 2012;9:91–94. [Google Scholar]
- Zhao Y.L., Cen X.B., Ito M., Yokoyama K., Takagi K., Kitaichi K., Nadai M., Ohta M., Takagi K., Hasegawa T. Shiga-like toxin II derived from Escherichia coli O157: H7 modifies renal handling of levofloxacin in rats. Antimicrob. Agents Chemother. 2002;46:1522–1528. doi: 10.1128/AAC.46.5.1522-1528.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading
- Tamhane A., Dunlop D. Prentice Hall; Upper Saddle River, USA: 2000. Statistics and data analysis: from elementary to intermediate. [Google Scholar]