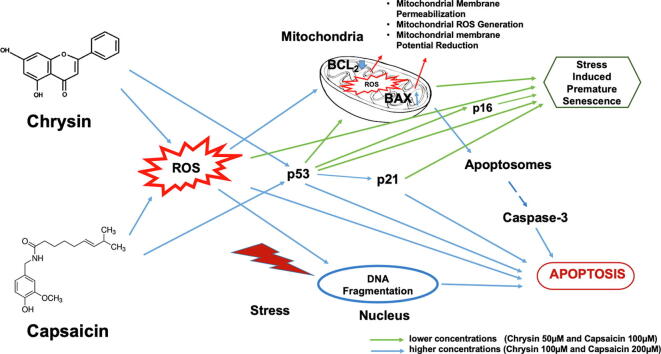
Graphical abstract
Keywords: Apoptosis, Chrysin, Capsaicin, Oxidative stress, Senescence, Mitochondria
Abstract
Current studies are focusing on the anti-cancerous properties of natural bioactive compounds, primarily those included in the human diet. These compounds have the potential to alter the redox balance that can hinder cancer cell's growth. In cancer cells, an abnormal rate of ROS production is balanced with higher antioxidant activities, which if not maintained, results in cancer cells being prone to cell death due to oxidative stress. Here, we have analyzed the effects of Chrysin and Capsaicin on the HeLa cells viability and cellular redox signaling. Both these compounds stimulate cellular and mitochondrial ROS overproduction that perturbs the cellular redox state and results in mitochondrial membrane potential loss. Apart from this, these compounds induce cell cycle arrest and induce premature senescence, along with the overexpression of p21, p53, and p16 protein at lower concentration treatment of Chrysin or Capsaicin. Moreover, at higher concentration treatment with these compounds, pro-apoptotic activity was observed with the high level of Bax and cleaved caspase-3 along with suppression of the Bcl-2 protein levels. In-Silico analysis with STITCH v5 also confirms the direct interaction of Chrysin and Capsaicin with target protein p53. This suggests that Chrysin and Capsaicin trigger an increase in mitochondrial ROS, and p53 interaction leading to premature senescence and apoptosis in concentration dependent manner and have therapeutic potential for cancer treatment.
1. Introduction
Oxidative stress is described as a disparity between the levels of Reactive oxygen species (ROS) production, which are called oxidants, and their removal by the protective mechanism of antioxidants. ROS acts both as a tumor promoter and suppressor depending on the cellular system involved and the level of oxidative stress generated (Reuter et al., 2011, Valko et al., 2006). Several compounds are reported that elevate the levels of ROS are used in the treatment of cancer (Nasri et al., 2014). These compounds control cancer growth by decreasing the level of proteins involved in cell proliferation, survival, and growth. They instigate cells towards the apoptotic pathway by increasing oxidative insult. Thus, the level of ROS decides cell fate and whether it will promote growth or will follow the apoptotic pathway for cell death (Mustafa et al., 2021, Mustafa and Mobashir, 2020). Extensive screening efforts have produced plentiful plant-based nutraceuticals with remarkable therapeutic abilities (Kumar & Pandey, 2013). Very limited information is available on the mechanism of activation of these compounds.
Here, we have examined the effect of Chrysin and Capsaicin on the growth of Human Cervical Adenocarcinoma HeLa cells. Chrysin (5,7-dihydroxy-2-phenyl-4H-chromen-4-one) belongs to the group of naturally occurring flavonoids, which possesses effective anti-inflammatory, properties (Bansal et al., 2009). It is naturally extracted from passionflower, honey, and bee propolis. Capsaicin ((E)-N-[(4-hydroxy-3-methoxyphenyl) methyl]-8-methylnon-6-enamide) an alkylamide is mainly extracted from Capsicum. It has analgesic properties and peripheral nerve pain can be controlled by using Capsaicin topically (Anand & Bley, 2011). Here we aim to investigate the anti-cancerous properties of Chrysin and Capsaicin with the insight of modulation in signaling pathways on the Human Adenocarcinoma HeLa cell line.
2. Materials and methods
2.1. Chemical reagents
Chrysin, Capsaicin, DMEM medium, antibiotics, DMSO, and other chemicals were obtained from USA based Sigma Aldrich, Antibodies were bought through Santa Cruz, USA. FBS were purchased from Invitrogen, USA. All the other chemicals were research quality.
2.2. Cell maintenance and dosage
HeLa cells were cultured in DMEM medium consisting of 10% FBS, 50 µg/ml fungizone and pen-stripe. Cells were cultured at 37 °C, supplied with of 5% CO2. Cells were grown for 24 h before treatment and treated when they reached 60–70% confluency and treated with DMSO or Chrysin or Capsaicin for 24 h or otherwise mentioned.
2.3. Cell viability assay
For MTT Assay, cells were seeded in 96 well plates and treated with various concentrations of Chrysin (0–300 μM) or Capsaicin (0–300 μM) or DMSO as control for 24, 48, and 72 h. After the respective treatment 10 µl/ml, of MTT (stock 5 mg/ml) were kept in dark for 2.5 h at 37 °C. Formazan crystals were dissolved in DMSO. The IC50 value was calculated with nonlinear regression analysis. For all the experiments absorbance was taken with UV–visible spectramax, for MTT assay at 570 nm.
2.4. Haematoxylin-Eosin (HE) staining for morphology analysis
Briefly, cells were grown over coverslip, and after the required treatment are processed for HE staining, as described (Saha et al., 2013). Hematoxylin and eosin stains used for the demonstration of nucleus and the cytoplasmic inclusions. For all the experiments images were taken by a Carl Zeiss microscope, enabled with Axiovision software.
2.5. Cellular ROS measurement and mitochondrial membrane potential detection
Cells were seeded in six well plates and after the indicated doses treatment for 24 h. Added 10 µM H2DCFDA in dark for 45 mins at 37 °C (Ali et al., 2021). Change in Mitochondrial membrane potential was detected using 10 μM Rhodamine 123 solution in media in dark condition for 30 mins at 37 °C. Cells were rinsed thrice with PBS and single-cell suspension in PBS was obtained by trypsinization (Eruslanov & Kusmartsev, 2010). For all the experiments, Cells were analyzed immediately flow cytometrically using BD FACS Verse flow cytometer, were examined with BD FACS Suite software. For Facs experiment we have acquired 10,000 cells per experimental sample. At excitation 488 nm, and emission at 525 nm.
2.6. Mitochondrial ROS measurement
Change in Mitochondrial ROS was detected using MitoSOX™ Red reagent. For analysis HeLa cells after appropriate treatment were exposed with 2.5 μM MitoSOX™ Red in media for 30 min at 37 °C. Cells were washed and analyzed at excitation 510 nm and emission at 580 nm (Eruslanov & Kusmartsev, 2010).
2.7. Immuno-cytochemical analysis
Briefly, cells were grown over coverslip and after the required treatment, they were fixed and permeabilized. After blocking, primary antibodies were added for 2 h at 37 °C, washed and incubated with fluorescence-tagged secondary antibodies. Washed and the nucleus was stained using DAPI, washed again, and mounted (Magdaleno et al., 2021). Images were taken and min. 200 cells were analyzed.
2.8. Cell cycle evaluation
Hela cells after treatment were fixed in 70% chilled ethanol at least 30 min at −20 °C. Fixed cells were rehydrated in PBS and then permeabilized with 0.1% Triton X-100, incubated with RNase A and propidium iodide for 30 mins, washed and then analyzed (Agarwal et al., 2016).
2.9. Cell size and Auto-fluorescence analysis
For analysis HeLa cells after appropriate treatment were suspended in PBS and analyzed by flow cytometrically, variations in Autofluorescence (AF), Cell Size (FSC-A), and Granularity (SSC-A) were analyzed at the excitation 450 nm and emission at 490 nm, (Agarwal et al., 2016).
2.10. β-Galactosidase assay
Cells were cultured over coverslips and after appropriate treatment, they were fixed and then stained with X-gal solution for 16–20 h at 37 °C in a humid environment and then rinsed with PBS. Images were taken by a phase-contrast microscope (Agarwal et al., 2016).
2.11. Protein quantification flow cytometrically
For analysis HeLa cells after appropriate treatment, were collected in PBS and fixed in 70% ethanol. After permeabilization cells were probed with specific antibodies for 2 h at 4 °C and then with fluorescence-tagged secondary antibodies Alexa flour 488 or with Alexa flour 546 for 1 h. Cells were analyzed immediately, at excitation 488 nm and emission at 525 nm, and excitation 546 nm and emission at 625 nm (Forment & Jackson, 2015).
2.12. Compound target protein interaction
To validate the compounds and the target protein's interaction STITCH v5 was used (Szklarczyk et al., 2016).
2.13. Statistical analysis
One-way Anova analysis (ANOVA) using GraphPad prism was done for all the statistical analysis. p-value < 0.05 was considered statistically significant.
3. Results
3.1. Chrysin and Capsaicin decreases the growth and alters the morphology of HeLa cells
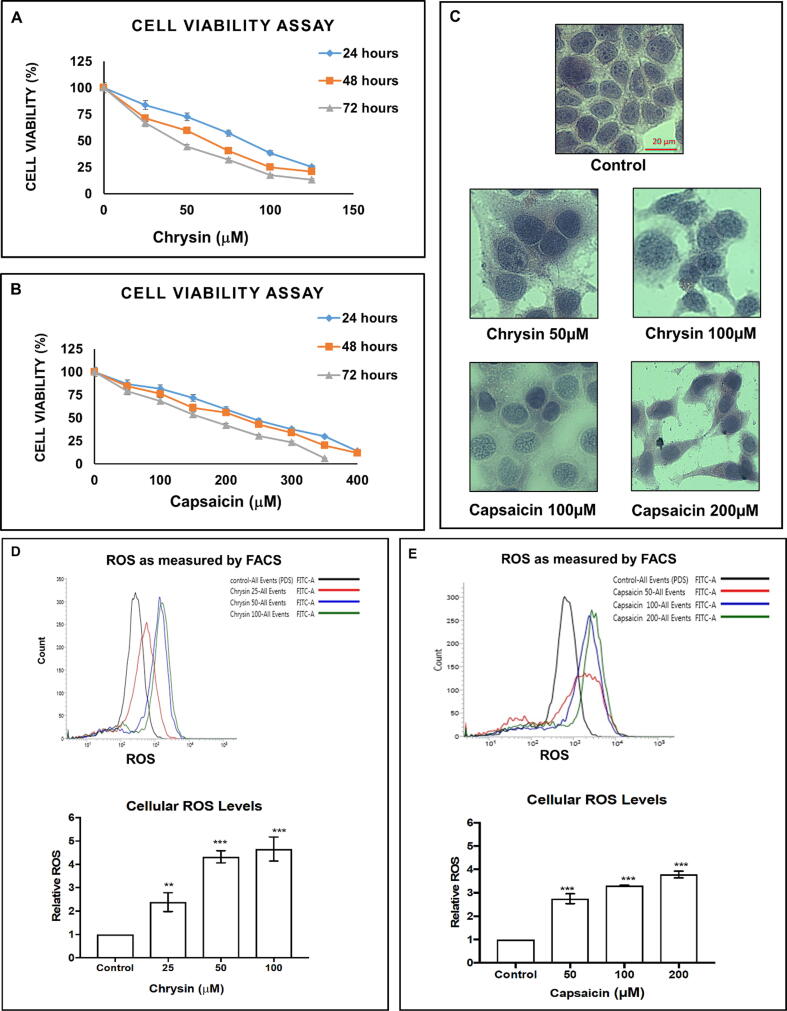
To access the impact of Chrysin and Capsaicin on the cell viability, HeLa cells were treated with various concentration of Chrysin and Capsaicin for 24, 48, and 72 h. The IC50 for Chrysin were 84 µM for 24 h, 67 µM for 48 h, and 56 µM for 72 h treatment (Fig. 1A) respectively. Capsaicin IC50 values were 241 µM for 24 h, 218 µM for 48 h, and 177 µM for 72 h treatment (Fig. 1B) respectively, confirming dose and time-dependent cytotoxic activity. Morphology of cells changes in response to external stress as an internal protection mechanism for the impediment of death. Hematoxylin-eosin staining was done and at lower doses (Chrysin 50 µM and Capsaicin 100 µM) showed (Fig. 1C) rounding up of cell contours, condensed and vacuolated nuclei, morphological features of senescence, and at the higher doses (Chrysin 100 µM and Capsaicin 200 µM) shrinkage of cell size, along with the unique feature of apoptosis were visible indicating the multiplicity of the insulting actions respectively.
Fig. 1.
Chrysin and Capsaicin retard growth and increases oxidative stress. MTT assay for cell viability analysis shows time and dose-dependent decrease in cell viability post Chrysin and Capsaicin treatment. (A) IC50 was at 84 µM for 24 h, 67 µM for 48 h, and 56 µM for 72 h of Chrysin treatment, (B) IC50 for Capsaicin 241 µM for 24 h, 218 µM for 48 h and 177 µM for 72 h of treatment. (C) Morphological analysis showing rounding up of cell contours, condensed nuclei at lower doses (Chrysin 50 µM and Capsaicin 100 µM) and shrinkage of cell size at the higher doses (Chrysin 100 µM and Capsaicin 200 µM) treatment for 24 h. ROS as level as measured after Chrysin and Capsaicin treatment. (D) Chrysin treatment (E) Capsaicin treatment compared with the Control. N = 3 ± SE (*P < 0.1; **P < 0.05 and ***P < 0.01).
3.2. Chrysin and Capsaicin increases oxidative stress in HeLa cells
Analysis of ROS generation with H2DCFDA that oxidized by ROS to green fluorescent 2′,7′-dichlorofluorescein, revealed that the intracellular ROS level is significantly amplified in a concentration-dependent manner after Chrysin and Capsaicin treatment. Chrysin displayed a 2.38, 4.32, and 4.65-fold increase in intracellular ROS at 25 µM, 50 µM, and 100 µM concentrations respectively for 24 h of treatment (Fig. 1D). Capsaicin showed a 2.74, 3.30, and 3.78-fold increase in intracellular ROS at 50 µM, 100 µM, and 200 µM concentrations respectively for 24 h of treatment (Fig. 1E).
3.3. Mitochondrial superoxide production leads to the alteration in mitochondrial membrane potential (ΔΨm) in HeLa cells
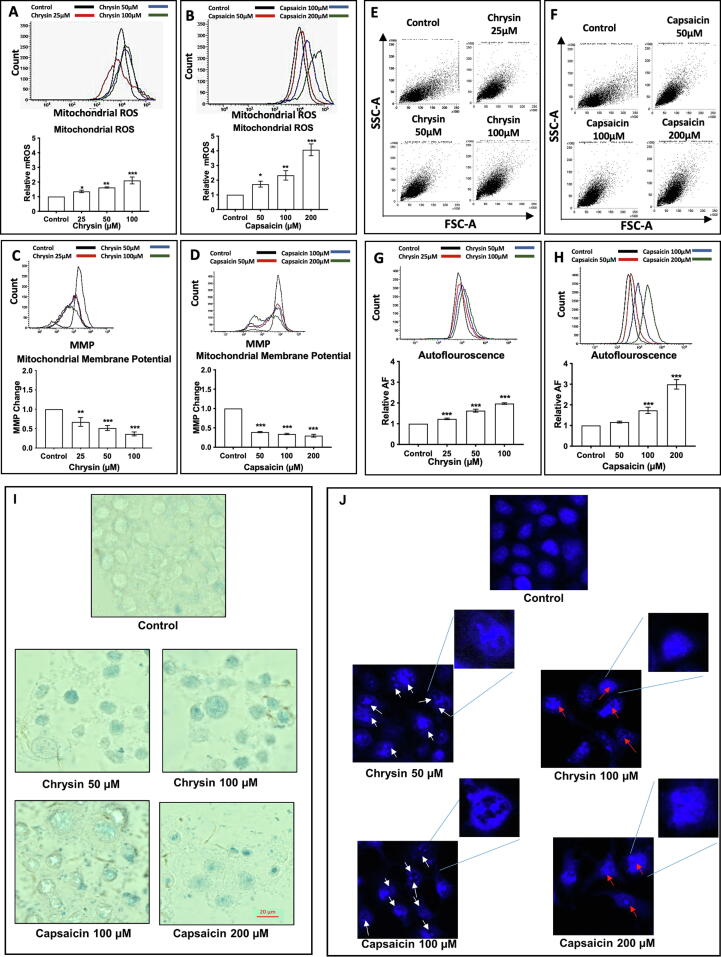
Since, the generation of ROS is inseparably linked to mitochondria as it is the major site of production/target of ROS within the cell (Chernyak et al., 2006). Chrysin showed a 1.36, 1.62- and 2.10-fold increase in mitochondrial ROS at 25, 50, and 100 µM concentration treatment for 24 hr (Fig. 2A) respectively. Capsaicin also showed a 1.73, 2.33- and 4.07-fold increase in mitochondrial ROS at 50, 100, and 200 µM concentrations respectively after 24 h (Fig. 2B). ΔΨm is an important parameter of the mitochondrial task and is often employed as an indicator of mitochondrial functioning and cell viability (Foster et al., 2009). In the case of Chrysin and Capsaicin treated cell ΔΨm decreased drastically in a concentration-dependent manner. Chrysin showed 33%, 48% and 64% reduction in ΔΨm at 25, 50 and 100 µM concentration treatment for 24 h respectively (Fig. 2C). Capsaicin also showed 61%, 66%, and 70% reduction in ΔΨm at 50, 100, and 200 µM concentrations respectively after 24 h (Fig. 2D).
Fig. 2.
Chrysin and Capsaicin stimulates Mitochondrial ROS and induces premature senescence in HeLa cells. Change in Mitochondrial ROS production (A) Chrysin treatment (B) Capsaicin treatment compared with the Control. Mitochondrial Membrane Potential was measured after (C) Chrysin treatment (D) Capsaicin treatment. Increase in cellular size (FSC-A) and granularity (SSC-A) post (E) Chrysin and, (F) Capsaicin treatment demonstrating senescence induction. Cellular autofluorescence (AF) and histogram profile (G) Chrysin and, (H) Capsaicin treatment showing enhanced AF relative to the Control indicates senescence. (I) Senescence-associated β-galactosidase (SA–β-Gal) staining in cells treated with Chrysin and Capsaicin. (J) Senescence-associated heterochromatic foci (SAHF) detected by DAPI staining in cells treated with Chrysin 50 µM and Capsaicin 100 µM and nuclear fragmentation at Chrysin 100 µM and Capsaicin 200 µM concentration treatment.
3.4. Low concentration treatment leads to premature senescence
An increase in Forward scatter FSC tells the size of the cells and side scatter tells about the intracellular structures, such as granules and nucleus. Indicates that most of the cells treated with Chrysin (Fig. 2E) and Capsaicin (Fig. 2F) are senescent. FACS analysis showed that most of the treated cells have higher autofluorescence (AF), Chrysin (Fig. 2G) showed 1.23, 1.63, and 1.97-fold increase in AF at 25, 50, and 100 µM concentration, and Capsaicin (Fig. 2H) showed 1.17, 1.73 and 2.99-fold increase in AF at 50, 100 and 200 µM concentration respectively after 24 h with a small number of cells showing reduced AF, indicating apoptotic induction. Senescence-associated β-galactosidase (SA–β-Gal) staining for aging pigment lipofuscin and autofluorescence were used as readouts. Positive β-galactosidase, intense blue staining was observed in cells treated with Chrysin and Capsaicin. Cells treated with Chrysin 50 and 100 µM and Capsaicin 100 and 200 µM for 24 h showed intense SA–β-Gal staining with flattened and enlarged cell morphology (Fig. 2I). (Hwang et al., 2009).
3.5. Senescence-Associated heterochromatin formation (SAHF) in the nucleus over Chrysin and Capsaicin exposure
A distinct change in the nuclear morphology is observed in HeLa cells under Chrysin, and Capsaicin treatment. Immunofluorescence staining with DAPI shows the development of big black spots inside the nucleus which depicts the gradual process of chromosome condensation, nuclear remodeling at low dose treatment, and finally nuclear fragmentation under higher doses Chrysin, and Capsaicin treatment. (Cremer, 2010). Prominent chromosomal condensation at low dose treatment with Chrysin 50 µM and Capsaicin 100 µM (Fig. 2J) as per the senescence cell morphology.
3.6. Chrysin and Capsaicin differentially blocked cell cycle in HeLa cell
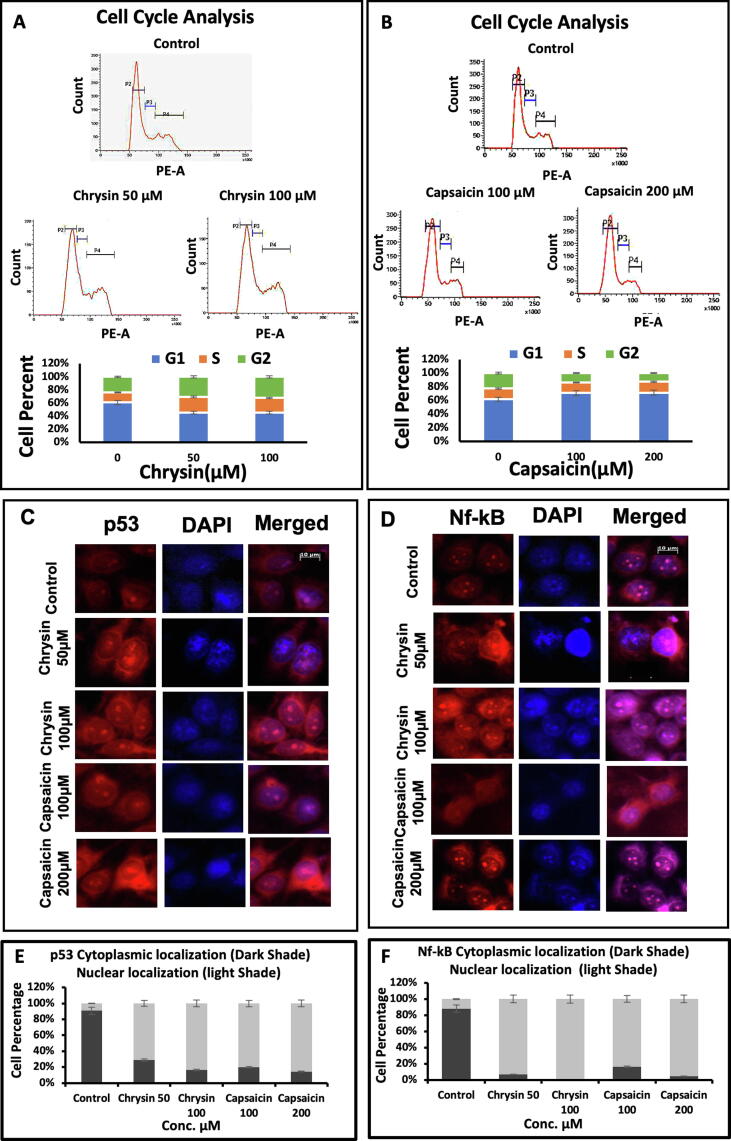
The effect of Chrysin on cell cycle progression was analyzed. The G1 population of 59.32% in control changed to 44.86% at 50 µM and 44.79% at 100 µM. G2 phase population from 22.99% in control to 30% at 50 µM and 31.56% at 100 µM. The S-phase population of 14.66% in control to 23.77% at 50 µM and S-phase 21.16% at 100 µM Chrysin treatment implying the cell cycle blockage in G2/M phase (Fig. 3A). Whereas the case of capsaicin showed an increase in the percentage of G1 phase from 61.39% in control to 67.23% at 100 µM and 72.41% at 200 µM. S-phase 15.76% at control to 14.25% at 100 µM and 16.35% at 200 µM. The G2 population of 22.56% in control changed to 13.25% at 100 µM and 12.85% at 200 µM Capsaicin treatment implying the cell cycle blockage in G1/S phase (Fig. 3B). Altogether the above results significantly elucidate inhibition of cell cycle progression and cell death in HeLa cells upon Chrysin and Capsaicin treatment.
Fig. 3.
Cell Cycle analysis and Immuno-cytochemical detection of activated apoptotic signaling upon nuclear translocation of P53 and NF-κB. (A) Cell cycle analysis shows an arrest in the G2/M phase with the increase in the G2 population in cells with treated Chrysin 100 µM concentration. (B) Cell cycle analysis shows G1/S phase arrest with the increase in the G1 population in cells treated with Capsaicin 200 µM concentration. Immuno-cytostaining showing (C) nuclear translocation of p53 and (D) NF-κB in both Chrysin and Capsaicin treated cells. (E) Histogram profile showing NF-κB Cytoplasmic and nuclear localization as denoted by dark shades and light shades respectively. (F) Histogram profile showing p53 Cytoplasmic and nuclear localization as denoted by dark shades and light shades respectively. The level of proteins was measured in 200 + cells. N = 3 ± SE (*P < 0.1; **P < 0.05 and ***P < 0.01).
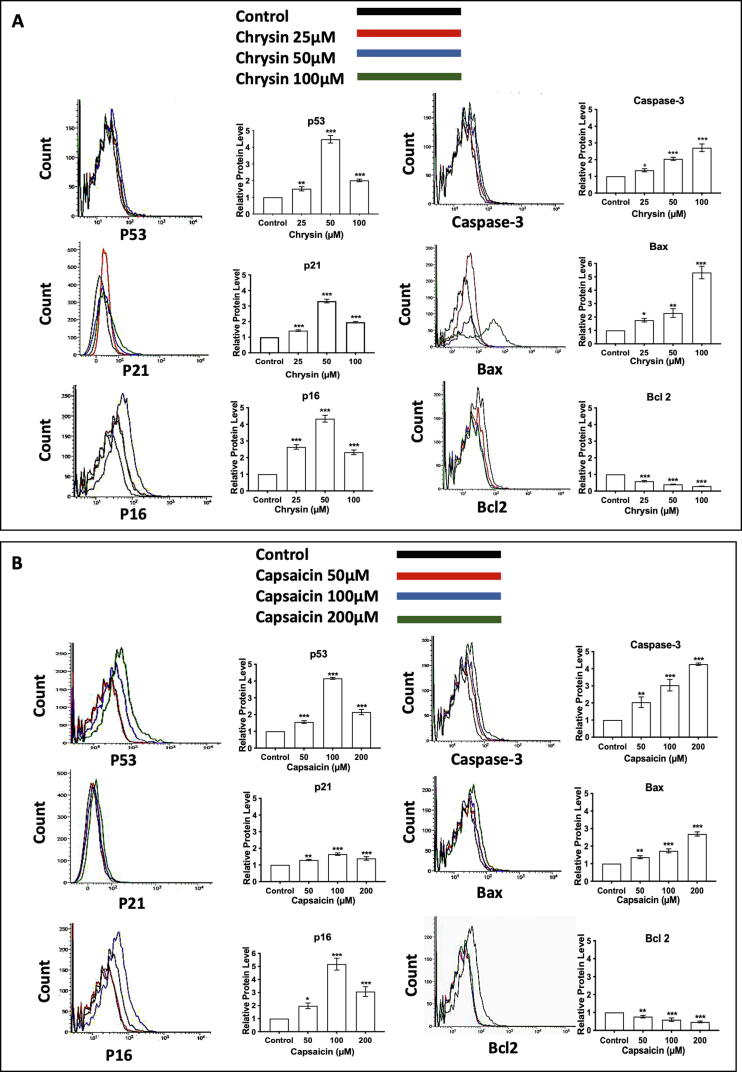
3.7. Up-regulation of p53, p16, and p21 confirms premature cellular senescence
Protein p53 response to cell stress, principally cell cycle insults. It is shown that premature senescence stimulated by oxidative stress is regulated by p53 which intern activates cyclin-dependent kinase inhibitor, p21, and initiates senescence. p16 initiate and maintain cellular senescence playing an important role (Mirzayans et al., 2012, Vurusaner et al., 2012). We observed a maximum increase of p53 (4.48-fold), p16 (4.34-fold), and p21 (3.31-fold) in cells treated with 50 µM Chrysin (Fig. 4A) and an increase of p53 (4.15-fold), p16 (5.17-fold) and p21 (1.65-fold) in cells treated with 100 µM Capsaicin (Fig. 4B). It is also reported that there is a nuclear accumulation of p53 in cells undergoing senescence. We also observed a similar change in localization of P53 which was cytoplasmic in untreated control cells. Nuclear localization of P53 (Fig. 3C) along with the elevated level of p53, p16, and P21 is driving cells towards senescence when cells were treated with Chrysin and Capsaicin independently. However, the expression of p53 and p21 (Fig. 4A) increase on treatment with higher doses of Chrysin (100 µM), p53 (2.02-fold) and p21 (1.96-fold), Capsaicin (200 µM) with p53 (2.15-fold) and p21 (1.4-fold) (Fig. 4B) thus leads to the confirmation of apoptosis induction at higher treatment concentrations.
Fig. 4.
Protein expression levels confirm dose-dependent Premature senescence and Apoptosis. High level of p21, p53, p16 confirms senescence induction by (A) Chrysin at 50 µM, (B) by Capsaicin at 100 µM and later their level decrease due to apoptosis induction at the high dose of Chrysin 100 µM and Capsaicin 200 µM. Elevated expression of apoptotic markers Caspase-3, Bax, and a decrease in the level of Bcl2 indicates apoptosis induction. N = 3 ± SE (*P < 0.1; **P < 0.05 and ***P < 0.01).
3.8. NF-κB nuclear translocation approves apoptosis induction in HeLa cells
Oxidative stress has been found to activate NF-κB in HeLa cells. NF-κB is involved in the transcription of the genes involved in several aspects of tumorigenesis such as invasion, cell proliferation, migration, and angiogenesis. ROS has been reported to both activate and repress NF-κB. NF-κB is the most important transcription factor that responds to inflammatory molecular signals (Oeckinghaus & Ghosh, 2009). Cytoplasmic localization of NF-κB was observed for untreated cells but nuclear localization (Fig. 3D) predominated for cells treated with Chrysin and Capsaicin.
3.9. Cleaved Caspase-3, Bax, and Bcl-2 expression confirm apoptosis at a higher treatment concentration
The Bcl-2 family of proteins regulators apoptosis by regulating mitochondrial permeability (Favaloro et al., 2012). Here we observed a maximum increase of Bax, (5.32-fold) and a decrease in Bcl-2 (71%) in cells treated with 100 µM Chrysin (Fig. 4A). In the case of Capsaicin, an increase in Bax (2.69-fold) and a decrease in Bcl-2 (53%) (Fig. 4B) is observed in cells treated with 200 µM Capsaicin. The activation of caspase-3 protein is a central feature of apoptosis. Caspase-3 is an important facilitator of apoptosis in the process of programmed cell death (Chang & Yang, 2000). Here we have observed an increase in the expression of caspase-3 maximum increase of (2.71-fold) in cells treated with 100 µM Chrysin (Fig. 4A) confirming apoptotic cell death.
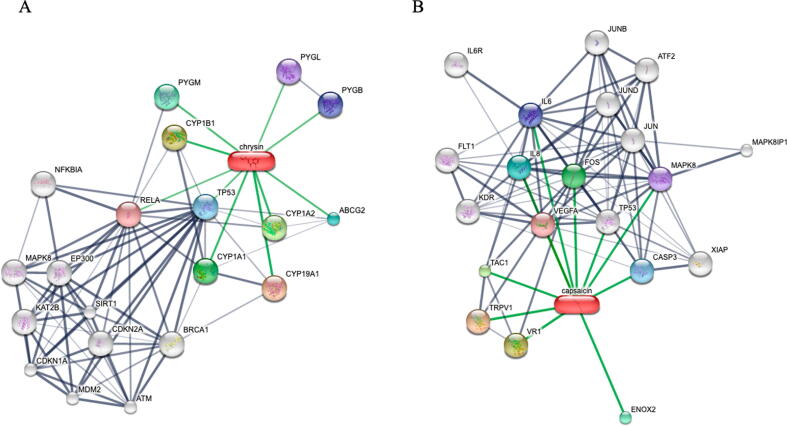
3.10. Putative networks validated the drugs and target proteins interaction
To further validate the drug targeting protein interaction, visualization of compounds and interacting proteins was performed by using the STITCHv5 software. It shows that both Chrysin (Fig. 5A) and Capsaicin (Fig. 5B) directly interacts with protein like p53 and caspase 3 to regulate cancer cell. Further confirming biochemical regulation.
Fig. 5.
Visualization of Drug-Protein interaction further confirms target engagement. The drug targeting protein interaction, visualization of compounds and interacting proteins was performed by using the STITCH v5 software shows that both Chrysin (Fig. 5A) and Capsaicin (Fig. 5B) directly interact with proteins like p53 and caspase 3 to regulate cancer cell.
4. Discussion
ROS act as signaling molecules and are appreciated for their functions that modulate cellular activity. ROS can initiate both senescence and apoptotic cell death depending on its level (Childs et al., 2014). Senescence is a condition in which a cell undergoes a persistent growth arrest and stops dividing. It can be stimulated by low dose stresses like DNA damage, exposure to UVB, oxidative stress, etc (Liao et al., 2021). Whereas, apoptosis is the phenomenon of programmed cell death, an extreme response to cellular stress and damage (Vicencio et al., 2008). Despite the presence of the enhanced antioxidant mechanism in proportion to the cellular function, cancer cells have far higher levels of ROS compared with healthy cells. The occurrence of elevated ROS levels in the cancer cell makes them more sensitive and hence provides a remarkable therapeutic frame (Pal et al., 2015).
In the present study, Chrysin and Capsaicin both show time and dose-dependent cytotoxic effects on the viability of HeLa cells. Low concentration of Chrysin 50 µM or Capsaicin 100 µM showed rounding up of cell contours, condensed and vacuolated nuclei, large and flat shaped morphological features of senescence, highly granular cytoplasmic vacuoles, and elevated expression of lysosomal β-galactosidase activity (SA-βgal), senescence-associated heterochromatin factors (SAHF) and overexpression of p53, p16, p21 proteins, the most common features of senescence (Childs et al., 2014). Whereas at the higher concentration treatment with Chrysin 100 µM or Capsaicin 200 µM, shrinkage of cell size, apoptotic bodies were observed with DAPI staining along with the unique feature of apoptosis were visible indicating the multiplicity of the insulting actions.
Transcription factor p53 has a principal function in the cell cycle progression, checkpoint activation, and is assumed to favor senescence and apoptosis. As a multitasking agent, it is critical to suppress tumor formation and mediate appropriate cellular outcomes to DNA damage damaging cancer treatments (Akhter et al., 2021, Rasul et al., 2013). Under normal conditions p53 protein, activity remains low, but when the cell is under stress condition p53 becomes functional and activates CDK inhibitor p21, which blocks cell cycle progression (Vicencio et al., 2008). Chrysin and Capsaicin considerably upregulates the level of p53 in HeLa cells with the simultaneous rise of its downstream target p21 and block the cell cycle. Chrysin incites cell-cycle arrest in G2/M phase, whereas Capsaicin-induced cell-cycle arrest in the G1/S phase, a critical task of p53 in the senescent cell. Up-regulation of tumor suppressor p16 in cells treated with Chrysin and Capsaicin at lower concentrations results in senescence, a significant event in cancer prevention. Besides p53, cells have an evolved mechanism for activation and inactivation of p16 as it plays a significant role in checking tumorigenesis. Studies indicate that p16 and p53 are interrelated and research has established p16 and p53 facilitated senescence as a substitute mechanism in controlling tumor growth (Rayess et al., 2012).
The redox-sensitive NF-κB dimers exist in the cytoplasm in a stable complex with inhibitor molecules. Upon extracellular stimulus, free NF-κB migrates to the cell nuclei and stimulates activates its target genes and contributes to cell death (Perkins & Gilmore, 2006). The main signaling pathways of NF-κB controlled ROS-mediated cellular mortality is via the stimulation of pro-apoptotic protein bax (Khandelwal et al., 2011). Chrysin and Capsaicin treatment leads to the nuclear translocation NF-κB and upregulation of bax and cleaved caspase-3 at higher dose treatment with the suppression in the bcl-2 protein level.
Remarkably, during initial onset of apoptosis, the surge of caspase-activating proteins is predominantly controlled by the bcl-2 protein family, that are the principal regulator of apoptosis. Bax expression increases, it forms homodimers and activates Caspase-3, the executor of apoptosis, and promotes apoptosis (Mo et al., 2016, Yang et al., 2020). This infers that at lower concentration treatment leads to senescence and at higher concentration treatment leads to apoptotic induction in a ROS-dependent manner. Thus, the present finding infers the therapeutic potential of Chrysin and Capsaicin in a dose-dependent manner.
5. Conclusion
Our study provides new findings exploring the redox-altering potential of Chrysin and Capsaicin. It provides evidence that Chrysin and Capsaicin induce ROS-dependent premature senescence, along with concomitant induction of apoptosis in HeLa cells. These findings suggest the therapeutic potential of Chrysin and Capsaicin. However, further studies in animal models are required to ascertain their efficacy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The author(s) received financial support from UGC (University Grant Commission), UPOE-II JNU funding from the Government of India for the research.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Jogendra S. Pawar, Email: jogendrapawar@gmail.com.
Saad Mustafa, Email: saadmustafa24@gmail.com.
Ilora Ghosh, Email: ighosh@mail.jnu.ac.in.
References
- Agarwal N.R., Maurya N., Pawar J.S., Ghosh I. A combined approach against tumorigenesis using glucose deprivation and mitochondrial complex 1 inhibition by rotenone. Cell Biol. Int. 2016;40(7):821–831. doi: 10.1002/cbin.10619. [DOI] [PubMed] [Google Scholar]
- Akhter N., Dar S.A., Haque S., Wahid M., Jawed A., Akhtar S., Alharbi R.A., Sindi A.A.A., Alruwetei A., Zubair Choudhry H.M., Ahmad A. Crosstalk of Cyclin-dependent kinase inhibitor 1A (CDKN1A) gene polymorphism with p53 and CCND1 polymorphism in breast cancer. Euro. Rev. Med. Pharmacol. Sci. 2021;25(12):4258–4273. doi: 10.26355/eurrev_202106_26131. [DOI] [PubMed] [Google Scholar]
- Ali R., Tabrez S., Akand S.K., Rahman F., Husein A., Arish M., Alqahtani A.S., Ahmed M.Z., Husain M., Rub A. Sesamol induces apoptosis-like cell death in leishmania donovani. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.749420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P., Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8 patch. Brit. J. Anaesth. 2011;107(4):490–502. doi: 10.1093/bja/aer260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal T., Jaggi M., Khar R.K., Talegaonkar S. Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. J. Pharm. Pharmaceut. Sci. 2009;12(1):46–78. doi: 10.18433/J3RC77. [DOI] [PubMed] [Google Scholar]
- Chang H.Y., Yang X. Proteases for cell suicide: functions and regulation of caspases. Microbiol. Mol. Biol. Rev. 2000;64(4):821–846. doi: 10.1128/mmbr.64.4.821-846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyak B.V., Izyumov D.S., Lyamzaev K.G., Pashkovskaya A.A., Pletjushkina O.Y., Antonenko Y.N., Sakharov D.V., Wirtz K.W.A., Skulachev V.P. Production of reactive oxygen species in mitochondria of HeLa cells under oxidative stress. Biochim. Biophys. Acta – Bioenerg. 2006;1757(5–6):525–534. doi: 10.1016/j.bbabio.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Childs B.G., Baker D.J., Kirkland J.L., Campisi J., Deursen J.M. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 2014;15(11):1139–1153. doi: 10.15252/embr.201439245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer T. Chromosome territories–a functional nuclear landscape. Curr. Opin. Cell Biol. 2010;19(5):424–436. doi: 10.1016/j.gde.2009.07.005.Nuclear. [DOI] [PubMed] [Google Scholar]
- Eruslanov E., Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Meth. Mol. Biol. (Clifton N.J.) 2010;594:57–72. doi: 10.1007/978-1-60761-411-1_4. [DOI] [PubMed] [Google Scholar]
- Favaloro B., Allocati N., Graziano V., Di Ilio C., De Laurenzi V. Role of apoptosis in disease. Aging. 2012;4(5):330–349. doi: 10.18632/aging.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forment J.V., Jackson S.P. A flow cytometry-based method to simplify the analysis and quantification of protein association to chromatin in mammalian cells. Nat. Prot. 2015;10(9):1297–1307. doi: 10.1038/nprot.2015.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D.B., Van Eyk J.E., Marbán E., O’Rourke B. Redox signaling and protein phosphorylation in mitochondria: progress and prospects. J. Bioenerg. Biomembr. 2009;41(2):159–168. doi: 10.1007/s10863-009-9217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, E.S., Yoon, G., Kang, H.T., 2009. A comparative analysis of the cell biology of senescence and aging. 66(15). [DOI] [PMC free article] [PubMed]
- Khandelwal N., Simpson J., Taylor G., Rafique S., Whitehouse A., Hiscox J., Stark L.A. Nucleolar NF-κB/RelA mediates apoptosis by causing cytoplasmic relocalization of nucleophosmin. Cell Death Differ. 2011;18(12):1889–1903. doi: 10.1038/cdd.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: an overview. Sci. World J. 2013;2013:1–16. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z., Yeo H.L., Wong S.W., Zhao Y. Cellular senescence: mechanisms and therapeutic potential. Biomedicines. 2021;9(12):1769. doi: 10.3390/biomedicines9121769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdaleno C., House T., Pawar J.S., Carvalho S., Rajasekaran N., Varadaraj A. Fibronectin assembly regulates lumen formation in breast acini. J. Cell. Biochem. 2021;122(5):524–537. doi: 10.1002/jcb.29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzayans R., Andrais B., Scott A., Murray D. New insights into p53 signaling and cancer cell response to DNA damage: Implications for cancer therapy. J. Biomed. Biotechnol. 2012;2012:1–16. doi: 10.1155/2012/170325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Z.-T., Li W.-n., Zhai Y.-R., Gong Q.-H. Icariin attenuates OGD/R-induced autophagy via Bcl-2-dependent cross talk between apoptosis and autophagy in PC12 cells. Evid.-Based Complement. Altern. Med. 2016;2016:1–6. doi: 10.1155/2016/4343084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa S., Mobashir M. LC–MS and docking profiling reveals potential difference between the pure and crude fucoidan metabolites. Int. J. Biol. Macromol. 2020;143:11–29. doi: 10.1016/j.ijbiomac.2019.11.232. [DOI] [PubMed] [Google Scholar]
- Mustafa S., Pawar J.S., Ghosh I. Fucoidan induces ROS-dependent epigenetic modulation in cervical cancer HeLa cell. Int. J. Biol. Macromol. 2021;181:180–192. doi: 10.1016/j.ijbiomac.2021.03.110. [DOI] [PubMed] [Google Scholar]
- Nasri H., Baradaran A., Shirzad H., Kopaei M.R. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prevent. Med. 2014;5(12):1487–1499. [PMC free article] [PubMed] [Google Scholar]
- Oeckinghaus, A., Ghosh, S., 2009. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harbor Perspect. Biol. 1(4), a000034–a000034. <https://doi.org/10.1101/cshperspect.a000034>. [DOI] [PMC free article] [PubMed]
- Pal R., Ramdzan Z.M., Kaur S., Duquette P.M., Marcotte R., Leduy L., Davoudi S., Lamarche-vane N., Iulianella A., Nepveu A. CUX2 functions in the repair of oxidative DNA damage CUX2 functions as an accessory factor in the repair of oxidative DNA damage. J. Biol. Chem. 2015;290(37):22520–22531. doi: 10.1074/jbc.M115.651042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins N.D., Gilmore T.D. Good cop, bad cop: The different faces of NF-κB. Cell Death Differ. 2006;13(5):759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- Rasul A., Khan M., Ali M., Li J., Li X. Targeting apoptosis pathways in cancer with alantolactone and isoalantolactone. Sci. World J. 2013;2013:1–9. doi: 10.1155/2013/248532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayess H., Wang M.B., Srivatsan E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer. 2012;130(8):1715–1725. doi: 10.1002/ijc.27316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked? Free Rad. Biol. Med. 2011;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha P., Chowdhury A.R., Dutta S., Chatterjee S., Ghosh I., Datta K., Anto R. Autophagic vacuolation induced by excess ROS generation in HABP1/p32/gC1qR overexpressing fibroblasts and its reversal by polymeric hyaluronan. PLoS ONE. 2013;8(10):e78131. doi: 10.1371/journal.pone.0078131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D., Santos A., Von Mering C., Jensen L.J., Bork P., Kuhn M. STITCH 5: augmenting protein-chemical interaction networks with tissue and affinity data. Nucl. Acids Res. 2016;44(D1):D380–D384. doi: 10.1093/nar/gkv1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M., Rhodes C.J., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Vicencio J.M., Galluzzi L., Tajeddine N., Ortiz C., Criollo A., Tasdemir E., Morselli E., Ben Younes A., Maiuri M.C., Lavandero S., Kroemer G. Senescence, apoptosis or autophagy? When a damaged cell must decide its path – a mini-review. Gerontology. 2008;54(2):92–99. doi: 10.1159/000129697. [DOI] [PubMed] [Google Scholar]
- Vurusaner B., Poli G., Basaga H. Tumor suppressor genes and ROS: Complex networks of interactions. Free Rad. Biol. Med. 2012;52(1):7–18. doi: 10.1016/j.freeradbiomed.2011.09.035. [DOI] [PubMed] [Google Scholar]
- Yang J., Zhan X.-Z., Malola J., Li Z.-Y., Pawar J.S., Zhang H.-T., Zha Z.-G. The multiple roles of Thy-1 in cell differentiation and regeneration. Differentiation. 2020;113:38–48. doi: 10.1016/j.diff.2020.03.003. [DOI] [PubMed] [Google Scholar]