Abstract
Potato cyst nematodes caused by Globodera rostochiensis, are quarantine-restricted pests causing significant yield losses to potato growers. The phytohormone ethylene play significant roles in various plant-pathogen interactions, however, the molecular knowledge of how ethylene influences potato–nematode interaction is still lacking. Precise detection of potato-induced genes is essential for recognizing plant-induced systemic resistance (ISR). Candidate genes or PR- proteins with putative functions in modulating the response to potato cyst nematode stress were selected and functionally characterized. Using real-time polymerase chain reaction (RT-PCR), we measured the quantified expression of four pathogenesis-related (PR) genes, PR2, PR3, peroxidase, and polyphenol oxidase. The activation of these genes is intermediate during the ISR signaling in the root tissues. Using different ethylene concentrations could detect and induce defense genes in infected potato roots compared to the control treatment. The observed differences in the gene expression of treated infected plants are because of different concentrations of ethylene treatment and pathogenicity. Besides, the overexpressed or suppressed of defense- related genes during developmental stages and pathogen infection. We concluded that ethylene treatments positively affected potato defensive genes expression levels against cyst nematode infection. The results emphasize the necessity of studying molecular signaling pathways controlling biotic stress responses. Understanding such mechanisms will be critical for the development of broad-spectrum and stress-tolerant crops in the future.
Keywords: Potato-nematode interactions, Induced systemic resistance, Ethylene signaling pathways, Pathogenesis-related proteins
1. Introduction
Cyst nematodes (Heteroderidae; Globodera rostochiens) is causing a loss of approximately US $125 billion annually worldwide, as major root parasitic nematodes (Chitwood, 2003, Fouda et al., 2020, Kumar et al., 2021, Ochola et al., 2021). Crops, such as wheat, soybean, potato, and rice, have been destroyed because of cyst nematode species. Nematodes are obligate and sedentary endoparasites that interact with their host plants (Cotton et al., 2014). They feed on their host roots where parasitic cells within or around the central cylinder and plant tissues are damaged due to nematode invasion and feeding (Holbein et al., 2016).
Significant variations in host gene expression are induced owing to the interaction between plants and pathogens. Ethylene is a crucial plant hormone with broad range of biological functions, including plant growth and development, promoting root initiation, inhibiting root elongation, stimulating fruit ripening, promoting seed germination, inducing leaf abscission, and activating the production of other phytohormones (Kang et al., 2010, Kour et al., 2019). Ethylene plays a vital role as an inducer for plant roots infected with Globodera rostochiensis and has an indirect impact on pre-injected plants so that it might result from altered plant defense and significantly elevated ethylene (ET) concentrations. Although ET plays an essential role in the cyst nematode attraction, its repression in the syncytia signals a rather negative assignment for nematode’s evolution last stages (Ali et al., 2013a, Ali et al., 2013b, Mazarei et al., 2003).
The Jasmonate (JA), ethylene (ET) and salicylic (SA)-dependent induced systemic resistance (ISR) pathway and systemic-acquired resistance (SAR) pathway act independently and enhance protection against the pathogen. SAR is an induced defense mechanism that provides long-lasting protection against a wide range of microbes. SAR also needs salicylic acid (SA) as a signal molecule and is linked with pathogenesis-related proteins accumulation, which are involved in resistance. Moreover, SAR provokes a rapid local reaction, where the pathogen is confined to a small area of the infection site. SAR is a defense mechanism plants employ against pathogen attack; this appears as resistance and protection of distal tissues (Sanz-Alférez et al., 2008, Abdelsalam et al., 2021). The beginning of this enhanced resistance is associated with a local and systemic improvement in plant hormone SA levels (Malamy et al., 1990, Métraux et al., 1990, Liu et al., 2022). Posteriorly, SAR genes, including those that encode PR (pathogen-related) proteins, are upregulated (Slavokhotova et al., 2021, Yu et al., 2022a). Systemic resistance induced in plants by pathogens can be occurred as a result of insect herbivory or due to root colonization by certain mutualistic microorganisms in rhizosphere. It enhances the plant's defense systems by the conformation of defensive compounds; therefore, ISR is critical in plant immune system. Both SAR and ISR allow differential defense against various pathogens (Ton et al., 2002, Yu et al., 2022b). The pathogen can induce resistance by activating the ET pathway (Siddiqui and Shaukat, 2005, Contreras-Cornejo et al., 2011).
Phytohormone crosstalk is critical for plants' defense response to insects and pathogens in which SA, ET, and jasmonic acid (JA) are all essential. However, pathogens have developed strategies to control the signalling network to intensify virulence on host plants. Evidence has shown that components from the pathways of ET-, JA-, and SA-dependent defense can influence each other’s signaling. For example, SA and its functional analogs such as INA and BTH inhibit JA-dependent defense gene expression, possibly by inhibiting JA biosynthesis and action (Pieterse et al., 2001, Ab Rahman et al., 2018, Elnahal et al., 2022). ET, SA, and JA are necessary signals for induced plant resistance against insects that are triggered when insects are fed, including two levels of defense. The first is a direct defense, including production of secondary chemicals like feeding deterrents or toxins. While, the second level refer to the indirect defense, producing a volatiles mixture to entice enemies to their hosts (Pieterse et al., 2001, Meisrimler et al., 2021).
Real-time PCR (RT-PCR) is an accurate and rapid assay for recognizing and quantifying G. rostochiensis from various nematode populations (Toyota et al., 2008, Yan et al., 2012). The objectives of this study are the quantitative detection of induced systemic resistance genes of potato roots upon ethylene treatment and G. arostochiensis infection during plant–nematode interactions. The expression levels of potato defensive genes, such as pathogenesis-related (PR) genes, were investigated upon ET treatments either before infection with cyst nematodes, as a plant hormone, or after infection, as elicitor signal molecules, compared with the control. The current study aims at understanding the molecular signalling pathways controlling biotic stress responses of potato against cyst nematodes upon ET treatments.
2. Materials and methods
2.1. Induction of potato root genes
Healthy potato tubers “spunta” were cultivated in 25-cm plastic pots (1:3 mixture of an autoclaved sand and loam). Potato plants were cultivated in a greenhouse treated after three weeks of planting, where the plants had two true expanded leaves. Four ethylene concentrations, 0.1, 0.5, 1.0, and 5.0 mM, were used for treatment (Fujimoto et al., 2011). All plant leaves were sprayed equally with four concentrations using an atomizer. At 1, 24, 48, and 72 h after ethylene treatment, the roots of treated potato plants were collected to examine the defensin genes before nematode infection. After, pathogenicity assay was done for G. rostochiensis, which was isolated and identified (Elkobrosy et al., 2018, Elkobrosy et al., 2020); 60 cysts of G. rostochiensis for each pot were inoculated on potato roots. The cyst nematode was inserted into the soil near the roots. The roots of treated infected plants have been collected at intervals after inoculation; 12, 24, 48 h, and 7, 14, 21, 28, and 35 dpi (day post-infection) treatment to compare with the treated ones (Fujimoto et al., 2011).
2.2. RNA extraction and RT-PCR assay
According to the manufacturer’s protocol, total RNA extraction from the treated infected potato roots and control ones is accomplished using an RNA isolation kit (TRIzol-Invitrogen, USA). First, reverse transcriptase (RT) first strand reactions were applied using mRNAs templates, reverse transcriptase enzyme and the dNTPs. The components were then mixed with the primer DNA in the reverse transcriptase buffer for 1 h at 42 °C. Finally, the obtained cDNA was used as a template for the next amplification by PCR. The components of RT-PCR assay performed in a total volume of 20 µl for each reaction mixture comprise 1 µl of 2 mg/ml total RNA, 5 µl of 10 pmol oligo dT primer (Clontech INC, USA), 2 µl of 10 × reaction buffer (Enzynomics, Korea), 1 µl of 2 mM dNTPs (SibEnzyme, Russia), 0.5 µl of 0.5 U M-MLV reverse transcriptase enzyme (Enzynomics, Korea), 0.5 µl of 40 U RNAase inhibitor, and 10 µl sterile distilled water. The RT-PCR assay was performed using a Thermocycler Gene Amp 9700 (Applied Biosystems (ABI), USA), programmed at 42 °C for 1 h and at 95 °C for 5 min to inactivate the reaction. The final step was at 4 °C for 10 min.
2.3. Quantitative detection using real-time PCR (qRT-PCR)
The prepared samples were quantified using a NanoDrop 2000 spectrophotometer (Thermo, USA) at 260 and 280 nm using 1 μl of each RNA sample extracted from treated infected potato roots to determine the quality and quantity of RNA. The RNA samples were quantified in a single-step assay after the normalization of certain concentrations using 2 × SYBER GreenqRT-PCR Master Mix kit (Applied Biotechnology, Egypt). Four primers were used in the qRT-PCR technique: β-1,3-glucanase (PR2), chitinase (PR3), peroxidase (PR9), and polyphenol oxidase (PPO) to detect induced genes of potato roots treated with ethylene and infected with cyst nematode. The sequences of these primers are shown in Table 1. β-actin was used as a reference or housekeeping gene for normalizing RNA levels of the target defense genes. Amplification reactions were performed using a Thermo Rotor-Gene Q 560-system (Qiagen, Germany), and 1.5 μl (0.1 mM) of each primer (forward and reverse) (Metabion International AG, Germany), 1 μl (10 ng) template RNA, 8.5-μl distilled sterile water, and 12.5-μl qPCR SYBER Green Master Mix (2×) (Applied Biotechnology, Egypt) were added to make a final volume of 25 µl. Amplification reactions were performed in a 36-well Thermo Rotor-Gene Q qRT-PCR system. The manufacturer’s recommended universal thermal protocol used includes 3 min activation of thermo-start at 95 °C followed by 15 s at 95 °C for initial template denaturation, 45 cycles of 60 °C for 30 s each, and combined annealing/extension phase of 72 °C for 30 s. Biological repetition of qRT-PCR samples was performed twice.
Table 1.
The pathogenesis-related (PRs) protein primers used in RT-PCR.
| Primer | Type | Primer sequence 5′ → 3′ |
|---|---|---|
| β-Actin | Forward | GTG GGC CGC TCT AGG CAC CAA |
| Reverse | CTC TTT GAT GTC ACG CAC GAT TTC | |
| β-1,3-glucanases (PR2) | Forward | TCC GGG GTA TGT TAT GGA AGA |
| Reverse | GGC CAT CCA CTC TCA GAC ACA | |
| Chitinase (PR3) | Forward | CGG TGG TAC TCC TCC TGG ACC C |
| Reverse | CGG CGC CAC GGT CGG CGT CTG A | |
| Peroxidase (PR9) | Forward | GCT TTG TCA GGG GTT GTG AT |
| Reverse | TGC ATC TCT AGC AAC CAA CG | |
| Polyphenol oxidase (PPO) | Forward | CAT GCT CTT GAT GA GGC GTA |
| Reverse | CCA TCT ATG GAA CGG GAA GA |
2.4. Data analysis
The amplification curves of each reaction were created using sequence detection software. Also, an automatic setting of the baseline calculated the threshold cycle number (Ct). A disassociation curve was created after each reaction to distinguish treatment amplicons. Delta–delta threshold cycle (ΔΔCq) expression values were calculated for RNA samples of each treatment to determine gene expressions using β-actin (reference gene) and other defense genes.
Δ Cq = Cq – reference gene.
ΔΔ Cq = Δ Cq – control.
ΔΔ Cq expression = 2 (−ΔΔ Cq).
The equations show the mathematical model of the relative expression ratio for the RT-PCR (Schmittgen and Livak, 2008). Additionally, it expressed the ratio of the targeted gene in samples vs. control compared with the housekeeping gene included in each experiment with RT-PCR.
3. Results
3.1. Influence of Globodera rostochiensis on potato plants morphologically
The field symptoms of ethylene treatment and nematode infection include leaf wilting and discoloration, growth retardation, and reduced root system, which was abnormally branched, direct damage to the roots in a case of high population densities, dwarfing, and early senescence of plants because of nutrient deficiencies and stress (Fig. 1, Fig. 2).
Fig. 1.
Representing; control roots (a), potato roots infected with G. rostochiensis and treated with 0.1-mM ethylene (b), potato roots infected with G. rostochiensis and treated with 0.5-mM ethylene (c), potato roots infected with G. rostochiensis and treated with 1.0-mM ethylene (d), and potato roots infected with G. rostochiensis and treated with 5.0-mM ethylene (e).
Fig. 2.
Representing; control shoots (a), shoots of potato infected with G. rostochiensis and treated with 0.1-mM ethylene (b), shoots of potato infected with G. rostochiensis and treated with 0.5-mM ethylene (c), shoots of potato infected with G. rostochiensis and treated with 1.0-mM ethylene (d), and shoots of potato infected with G. rostochiensis and treated with 5.0-mM ethylene (e).
3.2. Quantitative detection using qRT- PCR
The mRNAs of healthy potato roots and those infected with G. rostochiensis and treated with different ethylene concentrations were extracted. RT-PCR was used to detect the precise amounts of mRNA for some genes of potato roots infected with G. rostochiensis and treated with four ethylene concentrations (0.1, 0.5, 1.0, and 5.0 mM). These pathogen-related genes are PR2, PR3, PR9, and PPO, while β-actin gene was used as a reference gene.
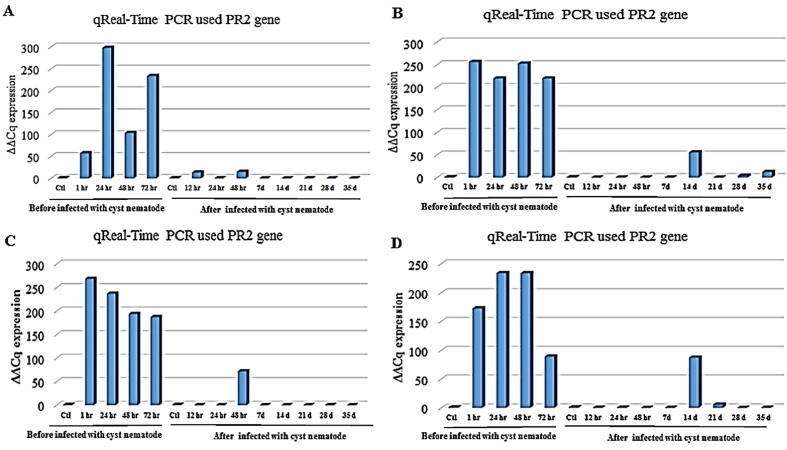
The uppermost expression of β-1,3-glucanase gene signified after 24 h of root treatment with 0.1 mM ethylene before infection with G. rostochiensis compared with the controls (Fig. 3A). Also, gene expression was highly upregulated in response to 0.1 mM ethylene root treatment after 72, 48, and 1 h before infection with G. rostochiensis compared with the controls. The elevated expression was after 1 and 48 h of root treatment with 0.5 mM ethylene; also, after 24 and 72 h of 67 root treatments with 0.5 mM ethylene compared with the control before infection with cyst nematodes. Additionally, results revealed that after 14 d after infection with G. rostochiensis, gene expression was high for infected treated roots with 0.5% ethylene (Fig. 3B). Furthermore, it showed a high expression (see Fig. 3C) after 1, 24, 48, and 72 h of root treatment with 1 mM ethylene compared with the control before infection with cyst nematodes. There is only one uppermost expression of β-1,3-glucanase gene signified after 48 h of infection with G. rostochiensis for infected treated roots with 1 mM ethylene. Fig. 3D; showed that the gene expression of β-1,3-glucanase elevated at 24, 48, 1, and 72 h of root treatment with 5 mM ethylene compared with the controls before infection with cyst nematodes. Additionally, after 14 d of infection with G. rostochiensis, the gene expression was high for infected treated roots with 5 mM ethylene and relatively after 21 d of infection compared with the control.
Fig. 3.
Histogram of quantitative estimation for PR2 gene expression in roots of potato “Spunta” cultivar treated with (A) 0.1 mM, (B) 0.5 mM, (C) 1.0 mM, and (D) 5 mM of ethylene before infection with G. rostochiensis; healthy plants as controls at 1, 24, 48, and 72 h after infection with G. rostochiensis; healthy plants as control at 12, 24, 48 h, and 7, 14, 21, 28, and 35 d.
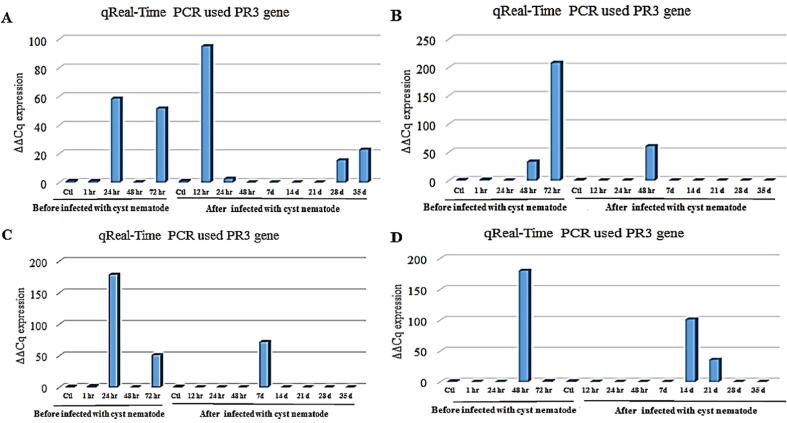
In contrast, according to Fig. 4A, quantitative detection of chitinase gene expression revealed that the elevated expression was after 12 h of infection with G. rostochiensis for the infected treated roots with 0.1 mM ethylene compared with the control. The highest level of chitinase gene expression was after 72 h of root treatment with 0.5 mM ethylene before infection with G. rostochiensis compared with the control (Fig. 4B). The quantitative estimation of chitinase gene expression showed that the uppermost expression was after 24 h of root treatment with 1 mM ethylene, which was before infection with G. rostochiensis compared with the control (Fig. 4C). Also, after 7 d of infection with G. rostochiensis, the gene expression for infected treated roots with the same concentration (1 mM) ethylene was compared with the control. However, Fig. 4D; showed that all treatments recorded a low expression, except for three, where the elevated expression was after 48 h of root treatment with 5 mM ethylene before infection with G. rostochiensis, subsequently after 14 d of infection with G. rostochiensis for treated roots with 5 mM.
Fig. 4.
Histogram of the quantitative estimation for PR3gene expression in potato roots “Spunta” cultivar treated with (A) 0.1 mM, (B) 0.5 mM, (C) 1.0 mM, and (D) 5 mM of ethylene before infection with G. rostochiensis; healthy plants as control at 1, 24, 48, and 72 h after infection with G. rostochiensis; healthy plants as control at 12, 24, and 48 h and 7, 14, 21, 28, and 35 d.
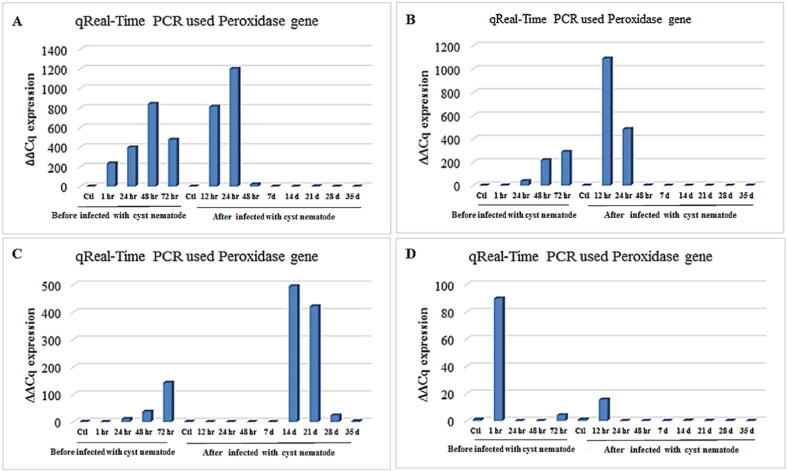
The quantitative detection of peroxidase gene expression showed high expression after 24 and 12 h of infection with G. rostochiensis for treated roots infected with 0.1 mM ethylene compared with the control (Fig. 5A). Additionally, results showed that all root treatments with 0.1-mM ethylene signified and high expressed before the 68 infections of cyst nematodes, beginning with 1 h of root treatment until 72 h after treatment. According to Fig. 5B; the high level of gene expression was after 12 and 24 h of infection with G. rostochiensis for infected roots treated with 0.5-mM ethylene compared with the control, whereas the gene expression of root treatment with 0.5-mM ethylene was relatively high after 72 h before cyst nematode infection. Fig. 5C; shows the quantitative estimation for peroxidase gene expression in potato roots treated with 1-mM ethylene after 14 and 21 d of infection with G. rostochiensis. Also, the gene expression of root treatment with 1-mM ethylene was relatively raised after 72 h before cyst nematode infection compared with the controls. In contrast, Fig. 5D; revealed that it estimated only high expression after 1 h of root treatment with 5-mM ethylene before cyst nematode infection. Also, the gene expression of roots infected with G. rostochiensis and treated with 5-mM ethylene was low, except for one treatment, which was relatively elevated after 12 h of infection compared with the control.
Fig. 5.
Histogram of quantitative estimation for peroxidase gene expression in roots of potato “Spunta” cultivar treated with (A) 0.1 mM, (B) 0.5 mM, (C) 1.0 mM, and (D) 5 mM of ethylene before infection with G. rostochiensis; healthy plants as control at 1, 24, 48, and 72 h after infection with G. rostochiensis; healthy plants as control at 12, 24, and 48 h and 7, 14, 21, 28, and 35 d.
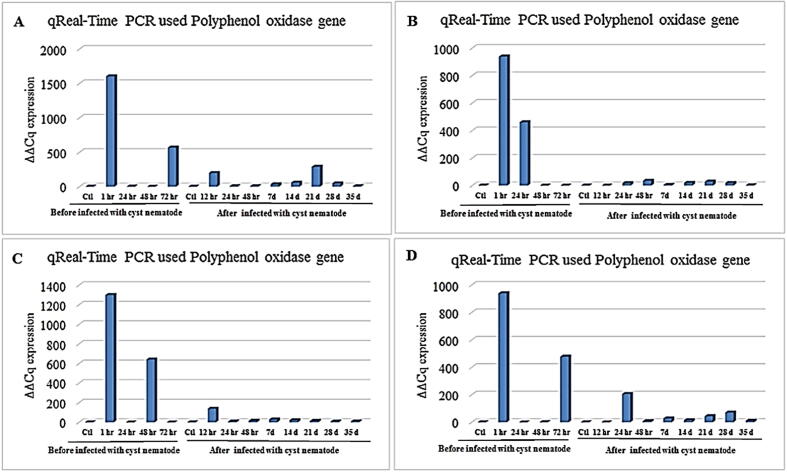
The gene expression of polyphenol oxidase was high in only one treatment after 1 h of root treatment with 0.1-mM ethylene before cyst nematode infection compared with the controls (Fig. 6A). Also, there was a raise after 72 h of root treatment with the same concentration (0.1 mM) ethylene before infection. The results of Fig. 6B indicated that the uppermost gene expression of polyphenol oxidase was after 1 h of root treatment with 0.5-mM ethylene before cyst nematode infection compared with the control. Also, after 24 h of root treatment with 0.5-mM ethylene, the gene expression of polyphenol oxidase was elevated before the infection. The high expression of polyphenol oxidase was shown in Fig. 6C after 1 and 48 h of root treatment with 1-mM ethylene compared with the controls before infection with the cyst nematodes. There is only one relatively raised expression of polyphenol oxidase gene after 12 h of infection with G. rostochiensis for treated roots infected with 1-mM ethylene. Results in Fig. 6D; showed that the quantitative estimation of polyphenol oxidase gene expression was high after 1 and 72 h of root treatment with 5-mM ethylene compared with the control before infection with the cyst nematodes. Additionally, the polyphenol oxidase gene expression was raised after 24 h and 28 d of infection with G. rostochiensis for treated roots infected with 5-mM ethylene compared with the controls and elevated after 21 d of infection with G. rostochiensis for treated roots infected with the same concentration (5 mM) ethylene.
Fig. 6.
Histogram of the quantitative estimation for polyphenol oxidase gene expression in roots of potato “Spunta” cultivar treated with (A) 0.1 mM, (B) 0.5 mM, (C) 1.0 mM, and (D) 5 mM of ethylene before infection with G. rostochiensis; healthy plants as control at 1, 24, 48, and 72 h after infection with G. rostochiensis; healthy plants as control at 12, 24, and 48 h and 7, 14, 21, 28, and 35 d.
4. Discussion
The gene expression levels were high upon ethylene treatment in potato roots (the control tissue is most scarce) by qRT- PCR. Similar studies confirmed that most cyst nematodes were more attracted to the ethylene-treated plants' root exudates than untreated control plants. Furthermore, the attractiveness of the treated plants was much higher than that of the untreated ones according to the triggers of Heterodera schachtii, which induced an elevated expression of ethylene-related genes in the arabidopsis roots (Abdelsalam et al., 2019a, Kammerhofer et al., 2015).
The qRT-PCR experiments for potato roots treated with different ethylene concentrations were accomplished, and they confirmed their roles in regulating gene expression. For example, the high concentration of ethylene treatments (5 mM) revealed low gene expression levels for the roots infected with cyst nematodes. Similar results confirmed that using several hormones on arabidopsis roots showed high concentrations of certain hormones, which might alter the gene expressions, indicating that root tissue contains the transcripts of shoot-specific genes (Abdelsalam et al., 2019b, Wieczorek et al., 2006, Zhao et al., 2020).
The gene expression levels were high upon ethylene treatment in potato roots (the control tissue is most scarce) by qRT-PCR. Similar studies confirmed that most cyst nematodes were higher attracted to ethylene-treated plants' root exudates than those of untreated control. Furthermore, these treated plants exhibited a significantly higher attractiveness than untreated ones, as seen in the triggers of Heterodera schachtii, which induced an elevated expression of ethylene-related genes in the arabidopsis roots (Abdelsalam et al., 2020, Kammerhofer et al., 2015, Zhao et al., 2021).
Plants commonly activate the ethylene production and signalling pathway in response to pathogen attack, resulting in the development of ethylene-dependent defensive signalling (Broekaert et al., 2006). Studies showed that ET-insensitive mutants to the exogenous ET application on tomato and Medicago truncatula were found to be more susceptible to M. javanica, M. hapla, and M. incognita (Hu et al., 2017, Čepulytė et al., 2018, Costa et al., 2020). While, ET-insensitive Arabidopsis mutants were more attractive to M. hapla juveniles, whereas ET-overproducing plants were less attractive (Fudali et al., 2013). Similarly, the external ET application in rice decreased M. graminicola infection (Nahar et al., 2011, Sikder et al., 2021). In a research by Wubben et al. (2001), five ethylene insensitive mutants of A. thaliana – etr1-1, ein2-1, ein3-1, eir1-1, and axr2 were shown to be less vulnerable to the nematode H. schachtii than wild type plants (Gutierrez et al., 2009). Consequently, the diverse roles of ethylene in plant–nematode interactions appear to be substantially influenced by host plant, nematode species and infection stage (Piya et al., 2019).
Exogenous application of ethylene was also found to be efficient in improving the baseline defensive responses of sugar beet against cyst nematode and significant reduction of plant infection (Ghaemi et al., 2020). Likewise, ethylene influences cyst nematode attraction to the root in Arabidopsis (Kammerhofer et al., 2015). The overexpression of the ET transcription factor RAP2.6 improved plant resistance toward H. schachtii, which was associated with the activation of JA-dependent defensive genes and the increase of callose deposition in the syncytium (Ali et al., 2013a, Ali et al., 2013b). As well as ET has also been shown to have a function in syncytium formation (Wubben et al., 2001).
Moreover, the expression of genes encoding for the enzymes 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) and oxidase (ACO), involved in ET-signalling and ET-biosynthesis, is usually thought to have a role in plant defense against endoparasitic sedentary nematodes (Li et al., 2015, Leonetti et al., 2017). Three ET-responsive genes such as one APETALA2/ethylene-responsive transcription factor (AP2/ERF) gene were down-regulated in the infected roots at 10 dpi (Ghaemi et al., 2020). Besides, another two ET-defensive genes were up-regulated, EIL1 and ERF1, which have been implicated in ISR signalling and reduction of disease vulnerability (Nakano et al., 2006, Spence et al., 2014). Transcriptomic analysis identified genes putatively implicated in host resistance, for example genes involved in phenylpropanoid pathway and genes encoding F-box proteins, chitinase, CYSTM domain-containing proteins, CASP-like protein and galactono-1,4-lactone dehydrogenase (Ghaemi et al., 2020). The synthesis of chitinase enzymes leads to the lysis of chitin-rich cell walls of a variety of pathogens and pests, such as pathogenic fungi, insects, and plant parasitic nematodes (Sharp, 2013, Subbanna et al., 2018).
Cross-talk with other phytohormones such as JA (Nahar et al., 2011), cytokinin or SA (Piya et al., 2019) and AUX (Strader et al., 2010) might affect the outcome of ET–nematode interactions. For example, Plants that have been treated with ET Activated many genes in the JA signalling pathway, which result in increased defensin synthesis and activation of several PR-proteins (Nahar et al., 2011, Sikder et al., 2021). Also, the ET-responsive genes EIL1 and EIN3 have been demonstrated to engage synergistically with JA to regulate defense responses against Botrytis cinerea, but they interact adversely with SA to regulate host defensive response against Pseudomonas syringae (Chen et al., 2009, Zhu et al., 2011, Piya et al., 2019). This study offers new molecular insights into plant-nematode interactions, which can be exploited to develop new management techniques for the potato cyst nematodes.
5. Conclusion
Unraveling plant-nematode interactions is essential for understanding their harmful effects on plants. The use of molecular techniques can provide a better and more comprehensive knowledge of various effects and microbial interactions. The current study focused on enhancing potato resistance against cyst nematodes, G. rostochiensis using different ethylene concentrations. Additionally, we are tracking the quantification of the expressed genes due to plant-nematode interactions, elucidating the molecular mechanisms associated with disease resistance. Interestingly, high levels of PR proteins, which are involved in the induced systemic resistance of plants, were found, confirming the ethylene role in the development of potato resistance against cyst nematode. This may not only lead to a better understanding of the role of phytohormones such as ethylene in potato-cyst nematode interactions but also improve the potential to find sustainable agriculture solutions and novel agrochemicals. This study extend knowledge about plant-nematode interactions and can be used for breeding programs targeting cyst nematode resistance in potato plants. Finally, our findings will pave the way for the implementation of novel management approaches to maximize stress tolerance of agro-ecosystems, which will produce value for the world's growing population, for both food production and consumption.
CRediT authorship contribution statement
Dina H. Elkobrosy: Data curation, Formal analysis, Funding acquisition, Methodology, Resources, Writing – original draft. Dalia G. Aseel: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. Elsayed E. Hafez: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. Mohamed A. El-Saedy: Data curation, Formal analysis, Writing – review & editing. Asma A. Al-Huqail: Writing – original draft, Writing – review & editing, Formal analysis, Data curation. Hayssam M. Ali: Writing – original draft, Writing – review & editing. Jebril Jebril: Writing – original draft, Writing – review & editing. Saad Shama: Data curation, Formal analysis, Investigation, Methodology. Nader R. Abdelsalam: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Ahmed S.M. Elnahal: Software, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
Authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP-2021/186), King Saud University, Riyadh, Saudi Arabia.
Funding
This research was funded by the Researchers Supporting Project number (RSP-2021/186) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Dalia G. Aseel, Email: daliagamil52@yahoo.com.
Elsayed E. Hafez, Email: elsayedhafez@gmail.com.
Mohamed A. El-Saedy, Email: anwer.elsaedy@yahoo.com.
Asma A. Al-Huqail, Email: aalhuqail@ksu.edu.sa.
Hayssam M. Ali, Email: hayhassan@ksu.edu.sa.
Jebril Jebril, Email: jebril@ksu.edu.
Saad Shama, Email: shamasaad@alexu.edu.eg.
Nader R. Abdelsalam, Email: nader.wheat@alexu.edu.eg.
Ahmed S.M. Elnahal, Email: ahmedelnahal2015@gmail.com.
References
- Ab Rahman S.F. Syed, Singh E., Pieterse C.M.J., Schenk P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018;267:102–111. doi: 10.1016/j.plantsci.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Abdelsalam N.R., Ali H.M., Salem M.Z.M., El-Wakil H.E. Quantitative and qualitative genetic studies of some Acacia species grown in Egypt. Plants. 2020;9(2):243. doi: 10.3390/plants9020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelsalam N.R., Balbaa M.G., Osman H.T., Ghareeb R.Y., Desoky E.-S., Elshehawi A.M., Aljuaid B.S., Elnahal A.S.M. Inheritance of resistance against northern leaf blight of maize using conventional breeding methods. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelsalam N.R., Botros W.A., Khaled A.E., Ghonema M.A., Hussein S.G., Ali H.M., Elshikh M.S. Comparison of uridine diphosphate-glycosyltransferase UGT76G1 genes from some varieties of Stevia rebaudiana Bertoni. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-44989-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelsalam N.R., Salem M.Z.M., Ali H.M., Mackled M.I., EL-Hefny M., Elshikh M.S., Hatamleh A.A. Morphological, biochemical, molecular, and oil toxicity properties of Taxodium trees from different locations. Ind. Crops Prod. 2019;139:111515. doi: 10.1016/j.indcrop.2019.111515. [DOI] [Google Scholar]
- Ali M.A., Abbas A., Kreil D.P., Bohlmann H. Overexpression of the transcription factor RAP2. 6 leads to enhanced callose deposition in syncytia and enhanced resistance against the beet cyst nematode Heterodera schachtiiin Arabidopsis roots. BMC Plant Biol. 2013;13(1):1–17. doi: 10.1186/1471-2229-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.A., Abbas A., Kreil D.P., Bohlmann H. Overexpression of the transcription factor RAP2.6 leads to enhanced callose deposition in syncytia and enhanced resistance against the beet cyst nematode Heterodera schachtiiin Arabidopsis roots. BMC Plant Biol. 2013;13:47. doi: 10.1186/1471-2229-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekaert W.F., Delauré S.L., De Bolle M.F.C., Cammue B.P.A. The role of ethylene in host-pathogen interactions. Annu. Rev. Phytopathol. 2006;44(1):393–416. doi: 10.1146/annurev.phyto.44.070505.143440. [DOI] [PubMed] [Google Scholar]
- Čepulytė R., Danquah W.B., Bruening G., Williamson V.M. Potent attractant for root-knot nematodes in exudates from seedling root tips of two host species. Sci. Rep. 2018;8(1):1–10. doi: 10.1038/s41598-018-29165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H., Xue, L., Chintamanani, S., Germain, H., Lin, H., Cui, H., Cai, R., Zuo, J., Tang, X., Li, X., Guo, H., 2009. Ethylene insensitive3 and ethylene insensitive3-like1 repress salicylic acid induction deficient2 expression to negatively regulate plant innate immunity in Arabidopsis. Plant Cell 21(8), 2527–2540. [DOI] [PMC free article] [PubMed]
- Chitwood D.J. Research on plant-parasitic nematode biology conducted by the United States Department of Agriculture-Agricultural Research Service. Pest Manage. Sci. 2003;59(6–7):748–753. doi: 10.1002/ps.684. [DOI] [PubMed] [Google Scholar]
- Contreras-Cornejo H.A., Macías-Rodríguez L., Beltrán-Peña E., Herrera-Estrella A., López-Bucio J. Trichoderma-induced plant immunity likely involves both hormonal-and camalexin-dependent mechanisms in Arabidopsis thaliana and confers resistance against necrotrophic fungi Botrytis cinerea. Plant Signal. Behav. 2011;6:1554–1563. doi: 10.4161/psb.6.10.17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, S.R., Chin, S., Mathesius, U., 2020. Infection of Medicago truncatula by the root-knot nematode Meloidogyne javanica does not require early nodulation genes. Front. Plant Sci. 1050. [DOI] [PMC free article] [PubMed]
- Cotton J.A., Lilley C.J., Jones L.M., Kikuchi T., Reid A.J., Thorpe P., Tsai I.J., Beasley H., Blok V., Cock P.J., Eves-van den Akker S., Holroyd N., Hunt M., Mantelin S., Naghra H., Pain A., Palomares-Rius J.E., Zarowiecki M., Berriman M., Jones J.T., Urwin P.E. The genome and life-stage specific transcriptomes of Globodera pallidaelucidate key aspects of plant parasitism by a cyst nematode. Genome Biol. 2014;15(3):R43. doi: 10.1186/gb-2014-15-3-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkobrosy D.H., Aseel D.G., Abdelsalam N.R., El-Saedy M.A., Shama S., Hafez E.E. Molecular and physiological detection of cyst nematodes on potato during plant nematode interactions. Alex. Sci. Exc. J. 2018;39(3):478–481. [Google Scholar]
- Elkobrosy D.H., Aseel D.G., Abdelsalam N.R., El-Saedy M.A., Shama S., Hafez E.E. The effect of cyst nematode (globodera rostochiensis) isolate ddh1 on gene expression in systemic leaves of potato plant. J. Microbiol. Biotechnol. Food Sci. 2020;10:93–97. [Google Scholar]
- Elnahal A.S., El-Saadony M.T., Saad A.M., Desoky E.S.M., El-Tahan A.M., Rady M.M., AbuQamar S.F., El-Tarabily K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: a review. Euro. J. Plant Pathol. 2022:1–34. [Google Scholar]
- Fouda M.M.G., Abdelsalam N.R., Gohar I.M.A., Hanfy A.E.M., Othman S.I., Zaitoun A.F., Allam A.A., Morsy O.M., El-Naggar M. Utilization of High throughput microcrystalline cellulose decorated silver nanoparticles as an eco-nematicide on root-knot nematodes. Colloids Surf. B: Biointerf. 2020;188:110805. doi: 10.1016/j.colsurfb.2020.110805. [DOI] [PubMed] [Google Scholar]
- Fudali S.L., Wang C., Williamson V.M. Ethylene signaling pathway modulates attractiveness of host roots to the root-knot nematode Meloidogyne hapla. Mol. Plant-Microbe Inter. 2013;26(1):75–86. doi: 10.1094/MPMI-05-12-0107-R. [DOI] [PubMed] [Google Scholar]
- Fujimoto T., Tomitaka Y., Abe H., Tsuda S., Futai K., Mizukubo T. Expression profile of jasmonic acid-induced genes and the induced resistance against the root-knot nematode (Meloidogyne incognita) in tomato plants (Solanum lycopersicum) after foliar treatment with methyl jasmonate. J. Plant Physiol. 2011;168(10):1084–1097. doi: 10.1016/j.jplph.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Ghaemi R., Pourjam E., Safaie N., Verstraeten B., Mahmoudi S.B., Mehrabi R., De Meyer T., Kyndt T. Molecular insights into the compatible and incompatible interactions between sugar beet and the beet cyst nematode. BMC Plant Biol. 2020;20(1):1–16. doi: 10.1186/s12870-020-02706-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez O.A., Wubben M.J., Howard M., Roberts B., Hanlon E., Wilkinson J.R. The role of phytohormones ethylene and auxin in plant-nematode interactions. Russ. J. Plant Physiol. 2009;56(1):1–5. [Google Scholar]
- Holbein J., Grundler F.M.W., Siddique S. Plant basal resistance to nematodes: an update. J. Exp. Botany. 2016;67(7):2049–2061. doi: 10.1093/jxb/erw005. [DOI] [PubMed] [Google Scholar]
- Hu Y., You J., Li C., Williamson V.M., Wang C. Ethylene response pathway modulates attractiveness of plant roots to soybean cyst nematode Heterodera glycines. Sci. Rep. 2017;7(1):1–13. doi: 10.1038/srep41282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerhofer N., Radakovic Z., Regis J.M.A., Dobrev P., Vankova R., Grundler F.M.W., Siddique S., Hofmann J., Wieczorek K. Role of stress-related hormones in plant defence during early infection of the cyst nematode Heterodera schachtii in Arabidopsis. New Phytol. 2015;207(3):778–789. doi: 10.1111/nph.13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B.G., Kim W.T., Yun H.S., Chang S.C. Use of plant growth-promoting rhizobacteria to control stress responses of plant roots. Plant Biotechnol. Rep. 2010;4(3):179–183. [Google Scholar]
- Kour D., Rana K.L., Yadav N., Yadav A.N., Kumar A., Meena V.S., Singh B., Chauhan V.S., Dhaliwal H.S., Saxena A.K. Plant Growth Promoting Rhizobacteria for Agricultural Sustainability. Springer; Singapore: 2019. Rhizospheric microbiomes: biodiversity, mechanisms of plant growth promotion, and biotechnological applications for sustainable agriculture; pp. 19–65. [Google Scholar]
- Kumar A., Harloff H.-J., Melzer S., Leineweber J., Defant B., Jung C. A rhomboid-like protease gene from an interspecies translocation confers resistance to cyst nematodes. New Phytol. n/a. 2021;231(2):801–813. doi: 10.1111/nph.17394. [DOI] [PubMed] [Google Scholar]
- Leonetti P., Zonno M.C., Molinari S., Altomare C. Induction of SA-signaling pathway and ethylene biosynthesis in Trichoderma harzianum-treated tomato plants after infection of the root-knot nematode Meloidogyne incognita. Plant Cell Rep. 2017;36(4):621–631. doi: 10.1007/s00299-017-2109-0. [DOI] [PubMed] [Google Scholar]
- Li R., Rashotte A.M., Singh N.K., Weaver D.B., Lawrence K.S., Locy R.D. Integrated signaling networks in plant responses to sedentary endoparasitic nematodes: a perspective. Plant Cell Rep. 2015;34(1):5–22. doi: 10.1007/s00299-014-1676-6. [DOI] [PubMed] [Google Scholar]
- Liu Z., Sun Z., Zeng C., Dong X., Li M., Liu Z., Yan M. The elemental defense effect of cadmium on Alternaria brassicicola in Brassica juncea. BMC Plant Biol. 2022;22(1):1–14. doi: 10.1186/s12870-021-03398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy J., Carr J.P., Klessig D.F., Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990;250(4983):1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Mazarei M., Lennon K.A., Puthoff D.P., Rodermel S.R., Baum T.J. Expression of an Arabidopsis phosphoglycerate mutase homologue is localized to apical meristems, regulated by hormones, and induced by sedentary plant-parasitic nematodes. Plant Mol. Biol. 2003;53(4):513–530. doi: 10.1023/B:PLAN.0000019062.80459.80. [DOI] [PubMed] [Google Scholar]
- Meisrimler C.-N., Allan C., Eccersall S., Morris R.J. Interior design: how plant pathogens optimize their living conditions. New Phytol. 2021;229(5):2514–2524. doi: 10.1111/nph.17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux J.P., Signer H., Ryals J., Ward E., Wyss-Benz M., Gaudin J., Raschdorf K., Schmid E., Blum W., Inverardi B. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science. 1990;250(4983):1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- Nahar K., Kyndt T., De Vleesschauwer D., Höfte M., Gheysen G. The jasmonate pathway is a key player in systemically induced defense against root knot nematodes in rice. Plant Physiol. 2011;157(1):305–316. doi: 10.1104/pp.111.177576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T.K., Suzuki K., Fujimura T., Shinshi H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006;140:411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochola J., Coyne D., Cortada L., Haukeland S., Ng'ang'a M., Hassanali A., Opperman C., Torto B. Cyst nematode bio-communication with plants: implications for novel management approaches. Pest Manage. Sci. 2021;77(3):1150–1159. doi: 10.1002/ps.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse C.M., Ton J., Van Loon L. Cross-talk between plant signalling pathways: boost or burden. AgBiotechNet. 2001;3:1–8. [Google Scholar]
- Piya S., Binder B.M., Hewezi T. Canonical and noncanonical ethylene signaling pathways that regulate Arabidopsis susceptibility to the cyst nematode Heterodera schachtii. New Phytol. 2019;221(2):946–959. doi: 10.1111/nph.15400. [DOI] [PubMed] [Google Scholar]
- Sanz-Alférez S., Mateos B., Alvarado R., Sánchez M. SAR induction in tomato plants is not effective against root-knot nematode infection. Euro. J. Plant Pathol. 2008;120(4):417–425. [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C T method. Nat. Proto. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sharp R.G. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy. 2013;3:757–793. [Google Scholar]
- Siddiqui I.A., Shaukat S.S. Pseudomonas aerugmosa-mediated induction of systemic resistance in tomato against root-knot nematode. Plant Pathol. J. 2005;4:21–25. [Google Scholar]
- Sikder M.M., Vestergård M., Kyndt T., Kudjordjie E.N., Nicolaisen M. Phytohormones selectively affect plant parasitic nematodes associated with Arabidopsis roots. New Phytol. 2021;232(3):1272–1285. doi: 10.1111/nph.17549. [DOI] [PubMed] [Google Scholar]
- Slavokhotova A., Korostyleva T., Shelenkov A., Pukhalskiy V., Korottseva I., Slezina M., Istomina E., Odintsova T. Transcriptomic analysis of genes involved in plant defense response to the cucumber green mottle mosaic virus infection. Life. 2021;11(10):1064. doi: 10.3390/life11101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence C., Alff E., Johnson C., Ramos C., Donofrio N., Sundaresan V., Bais H. Natural rice rhizospheric microbes suppress rice blast infections. BMC Plant Biol. 2014;14(1):1–17. doi: 10.1186/1471-2229-14-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader L.C., Chen G.L., Bartel B. Ethylene directs auxin to control root cell expansion. Plant J. 2010;64(5):874–884. doi: 10.1111/j.1365-313X.2010.04373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbanna A.R.N.S., Rajasekhara H., Stanley J., Mishra K.K., Pattanayak A. Pesticidal prospectives of chitinolytic bacteria in agricultural pest management. Soil Biol. Biochem. 2018;116:52–66. [Google Scholar]
- Ton J., Van Pelt J.A., Van Loon L.C., Pieterse C.M.J. Differential effectiveness of salicylate-dependent and jasmonate/ethylene-dependent induced resistance in arabidopsis. Mol. Plant-Microbe Interact.®. 2002;15:27–34. doi: 10.1094/MPMI.2002.15.1.27. [DOI] [PubMed] [Google Scholar]
- Toyota K., Shirakashi T., Sato E., Wada S., Min Y.Y. Development of a real-time PCR method for the potato-cyst nematode Globodera rostochiensis and the root-knot nematode Meloidogyne incognita. Soil Sci. Plant Nutr. 2008;54:72–76. [Google Scholar]
- Wieczorek K., Golecki B., Gerdes L., Heinen P., Szakasits D., Durachko D.M., Cosgrove D.J., Kreil D.P., Puzio P.S., Bohlmann H., Grundler F.M.W. Expansins are involved in the formation of nematode-induced syncytia in roots of Arabidopsis thaliana. Plant J. 2006;48:98–112. doi: 10.1111/j.1365-313X.2006.02856.x. [DOI] [PubMed] [Google Scholar]
- Wubben M.J., Su H., Rodermel S.R., Baum T.J. Susceptibility to the sugar beet cyst nematode is modulated by ethylene signal transduction in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2001;14(10):1206–1212. doi: 10.1094/MPMI.2001.14.10.1206. [DOI] [PubMed] [Google Scholar]
- Yan G., Smiley R.W., Okubara P.A. Detection and quantification of Pratylenchus thornei in DNA extracted from soil using real-time PCR. Phytopathology. 2012;102:14–22. doi: 10.1094/PHYTO-03-11-0093. [DOI] [PubMed] [Google Scholar]
- Yu, H.L., Kim, J.Y., Lim, S.H., Kang, H.W., 2022. The defense response of pepper (Capsicum annuum L.) induced by exopolysaccharide from Schizophyllum commune. Physiol. Mole. Plant Pathol. p. 101810.
- Yu Y., Gui Y., Li Z., Jiang C., Guo J., Niu D. Induced systemic resistance for improving plant immunity by beneficial microbes. Plants. 2022;11(3):386. doi: 10.3390/plants11030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Abdelsalam N.R., Xu Y., Chen M.-S., Feng Y., Kong L., Bai G. Identification of two novel Hessian fly resistance genes H35 and H36 in a hard winter wheat line SD06165. Theor. Appl. Gene. 2020;133:2343–2353. doi: 10.1007/s00122-020-03602-3. [DOI] [PubMed] [Google Scholar]
- Zhao L., Liu S., Abdelsalam N.R., Carver B.F., Bai G. Characterization of wheat curl mite resistance gene Cmc4 in OK05312. Theor. Appl. Gene. 2021;134:993–1005. doi: 10.1007/s00122-020-03737-3. [DOI] [PubMed] [Google Scholar]
- Zhu Z., An F., Feng Y., Li P., Xue L., Mu A., Jiang Z., Kim J.M., To T.K., Li W., Zhang X. Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc. Natl. Acad. Sci. 2011;108(30):12539–12544. doi: 10.1073/pnas.1103959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
Further reading
- Dong X. Genetic dissection of systemic acquired resistance. Curr. Opin. Plant Biol. 2001;4(4):309–314. doi: 10.1016/s1369-5266(00)00178-3. [DOI] [PubMed] [Google Scholar]
- Métrauxs J.-P. Systemic acquired resistance and salicylic acid: current state of knowledge. Euro. J. Plant Pathol. 2001;107:13–18. [Google Scholar]