Abstract
5-Fluorouracil (5-FU) is a drug of choice for colorectal-cancer. But oral therapeutic efficacy of 5-FU is restricted due to their very little bioavailability because of poor membrane permeability and GIT-absorption. We have developed a multiple nanoemulsion (w/o/w i.e. 5-FU-MNE) in which 5-FU incorporated to improve their oral-absorption. Globule-size of opt-5-FU-MNE was 51.64 ± 2.61 nm with PDI and ZP 0.101 ± 0.001 and −5.59 ± 0.94, respectively. In vitro 5-FU-release and ex vivo permeation studies exhibited 99.71% release and 83.64% of 5-FU from opt-nanoformulation. Cytotoxic in vitro studies-exhibited that 5-FU in opt-5-FU-MNE was 5-times more potent than 5-FU-S on human-colon-cancer-cell-lines (HT-29). The enhanced Cmax with AUC0-8h with opt-5-FU-MNE was shown extremely significant (p < 0.001) in wistar rat’s plasma in the comparison of oral and i.v. treated group of 5-FU-S by PK-observations. Furthermore, opt-5-FU-MNE was showed much more significant (p < 0.001) results as compared to 5-FU-S (free) on cell lines for human colon cancer (HT-29).
Keywords: 5-Fluorouracil, Multiple-nanoemulsion (w/o/w), Enhancement of oral bioavailability, UHPLC-MS/MS bioanalytical-method, Pharmacokinetics, Chemoprevention of colorectal cancer, Cytotoxic activity
1. Introduction
Colorectal-cancer (CRC) is a very important reason of morbidity and mortality all over the world (WHO, 2002). CRC is 9% happened of total types of cancer occurrence (World Cancer Research Fund and American Institute for Cancer Research, 2007, Boyle and Langman, 2000). CRC is 3rd most frequent cancer globally and 4th most regular reason of death. In 2002, CRC have seen over on 1 million fresh peoples that approximately influenced uniformly on both gender (men and women), this year for which international evaluates have been presented the data (World Health Organization, 2002, Boyle and Ferlay, 2005, Parkin et al., 2005, Ferlay et al., 2004). 5-FU is a drug of choice in the treatment of all kinds of cancers (Youssof et al., 2019). 5-FU is a drug that showed a wide range of effect against solid-tumors e.g. pancreas, liver, breast, GI-tract, brain, and ovary. 5-FU can be utilized single or taken as with additional chemotherapy treatments. 5-FU inhibits thymidylate synthase as a result it produces interference in the nucleoside metabolism of DNA and RNA which is the main cause of 5-FU cytotoxicity of cancerous cells (Youssof et al., 2019, Arias, 2008). Therefore, 5-FU is a most famous cytotoxic medicine in the classification of anticancer drugs i.e. antimetabolite which is suggested for the treatment of different kinds of cancers, and solid tumors like ovarian, breast and colorectal tumor (Alanazi et al., 2015; Rossella et al., 2005; Dong et al., 2013). 5-FU is an III class of BCS category drug that showed good water solubility and poor permeability i.e. hydrophilic drug having 12.20 mg ml−1. 5-FU exhibited a problem with non-optimum pharmacokinetic profile via oral delivery. Thus, it is given through IV administration in which maintenance of 5-FU concentration in the blood is still little because of its fast metabolism (Presant et al., 2002, Longley et al., 2003). Now a days, a novel drug delivery system i.e. nanoemulsions are taken as a transporter/carrier vehicle for the encapsulation of drug. Nanoemulsions are the novel carriers that showed stability in terms of thermodynamically, containing mixture of oils, surfactant/co-surfactants, and water (Ahmad et al., 2018a & 2019).
A latest novel nanocarrier approach i.e. a double or multiple (w/o/w i.e. water/oil/water) nanoemulsions are the systems containing dispersions of minute water droplets inside bigger oil droplets followed by the whole (w/o) dispersed in an outer surface water-phase by the support of surfactants (Shakeel et al., 2014, Hanson et al., 2008, Zhao et al., 2011). This is not a easy formulation to prepare due to it contains <100.0 nm globule size of external and internal phases for double nanoemulsions (Garti, 1997, Loscertales et al., 2002, Udata et al., 2003). In last few years, a novel approach comes in the form of SNEDDS (i.e. self-nanoemulsifying-drug-delivery-systems) was examined for the delivery of lipophilic drugs to improve their solubility or dissolution property (in vitro) in addition to bioavailability (in vivo) (Udata et al., 2003, Shakeel et al., 2013). It is not an easy task to prepare hydrophilic drugs for oral delivery e.g. hydrophilic anticancer drugs or proteins to encapsulate in the SNEDDS. SNEDDS should contain aqueous phase as an external phase for the dilution in the GI fluids without any precipitation of drug or phase separation. We can formulate the w/o nanoemulsions for Hydrophilic drugs to entrapped in the internal phase i.e. water. If we will give w/o nanoemulsions orally that produces precipitation of drug or phase separation due to oil taken as an external phase. Thus, it is a very huge challenge to give hydrophilic drugs via oral route as in SNEDDS form. The nanoemulsions (w/o/w, double) have given us solution for this problem as compare to oral nanoemulsions or SNEDDS or microemulsions. We can entrap these hydrophilic drugs inside water-in-oil nanoemulsions after that it can disperse finally into the external water phase by the use of surfactants. On the basis of reported literature, it is not an easy-task to formulate nanoemulsions (w/o/w) that it contains <100.0 nm globule sizes of external and internal phases via spontaneous emulsification methods (Hanson et al., 2008, Zhao et al., 2011, Garti, 1997). It was successfully formulated and characterized before by the use of surfactant as single-component block copolypeptides via high technique of energy emulsification (Hanson et al., 2008). This is a first-time design to deliver a drug in the form double nanoemulsion via oral route. In addition to, the main difficulty is the use of only one surfactant in which the maximum ratio of hydrophilic to lipophilic remains products that supports stabilization of oil droplets into the water. Even so many nanoemulsions were examined as a nano-vehicles of drug-delivery for oral (Thomas et al., 2012, Shanmugam et al., 2011), dermal and transdermal (Ahmad et al., 2020) of various lipophilic active constituents. For delivery of hydrophilic drugs in the form of NEs/double-NEs as vehicles, we didn’t find any previous reported method. In some cases, multiple or double-w/o/w-emulsions has been reported for the delivery of drugs via dermally like DNAzymes and acyclovir (Schwarz et al., 2012, Schmidts et al., 2011, Schmidts et al., 2012). 5-FU has characteristic hydrophilicity that showed a wide range of effect against solid tumors e.g. pancreas, lungs, colon, neck, head, liver, breast, GI-tract, brain, and ovary (Shah et al., 2011, Li et al., 2008, Yassin et al., 2010). Various kinds of colloidal or dispersion carriers like niosomes (Cosco et al., 2009), ethiosomes (Thomas et al., 2011), nanogels (Zhang et al., 2012), nanoparticles (Ahmad et al., 2012, Lin et al., 2012), microemulsions (Gupta et al., 2005, Yanyu et al., 2012, Shishu et al., 2012), and microparticles (Ahmad et al., 2012, Lin et al., 2012) was already used to enhance the solubility, strong-delivery, and 5-FU-bioavailability. Based on the previously reported information, multiple (w/o/w) nanoemulsions have a good novel nano-approach as in the form of SNEDDS for 5-FU delivery via oral administration. Double SNEDDS have various advantages e.g. improvement of therapeutic effects, decrement of adverse effects, reduction of oral-dose and enhancement in PK-profile for 5-FU through oral delivery. Thus, the main purpose of presented-research study was to develop a novel double or multiple w/o/w SNEDDS of 5-FU, characterize, and also to assess the treatment of colorectal cancer. The proposed nanoformulation will be formulated first time through easiest method like low energy emulsification or spontaneous emulsification method. This 5-FU-MNE (w/o/w) SNEDDS approach for oral chemotherapy will increase a very good attraction and interest. In this way, the patients will take the drug orally at their home that can be good patient’s compliance and their quality of life avoiding uneasiness of injections. Besides this, anticancerous drugs should be exposed to a long time to the cancerous cells and maintained the drug plasma concentration. In this way, we can enhance efficacy of 5-FU and decrease their side effects as well as make easy the use of 5-FU as an anticancer drug for chronic treatment schedule as prophylactics beside recurrence and metastasis.
Based on literature survey, it was reported many methods for 5-FU plasma samples analysis. But the main drawbacks of these reported methods have simultaneously developed with other drugs at the same time that means not a single bioanalytical-method for plasma analysis of 5-FU is available individually (Remaud et al., 2005, Licea-Perez et al., 2009, Vainchtein et al., 2010, Ganti et al., 2013, Peer et al., 2012, Bobin-Dubigeon et al., 2013, Liu et al., 2010, Chen and Zhou, 2010, Büchel et al., 2013). All of these reported methods have run time>5.0 min and all of them have lack of research reports for quantification of 5-FU in the plasma upto picogram-range. Because it is very important to us for our opt-5-FU-multiple-NE needs a method to determine drug concentration upto picogram level for PK-parameters evaluations. We have developed and validated successfully of a new 5-FU- UHPLC-ESI-triple-quadrupole-MS/MS method. It was applied to examine PK-parameters for multiple w/o/w nanoemulsion. Our optimized-method contained many-applications that are maximum-sensitivity, maximum efficiency, very less retention and run time for the calculations of plasma-pharmacokinetic outcomes in a very less time.
The proposed present study is a first time proposed to formulate a novel-nanoformulation of 5-FU-multiple w/o/w SNEDDS for increased colorectal targeting of 5-FU. Our most important purpose is to improve 5-FU-bioavailability in the plasma after the oral delivery of 5-FU-nanoformulation. 5-FU-multiple w/o/w SNEDDS exhibited best effective-solubility and permeability. 5-FU-multiple-nanoemulsion (w/o/w, SNEDDS) has been formulated and characterize on the basis of various physicochemical parameters to find out their suitability for oral 5-FU-delivery. All the PK-parameters (AUC0–t, t1/2, Cmax, Kel etc.) of 5-FU-S and 5-FU-multiple-nanoemulsion (w/o/w, SNEDDS) have been calculated and it was compared successfully with the help of a novel-developed and validated UHPLC-MS/MS method in the treatment of colorectal cancer.
2. Materials and methods
2.1. Materials
We have purchased 5-FU from Chem-Impex International, Inc. Milli-Q-water was used in this study by ELGA, purification system, UK. Transcutol-HP (Gattefosse, France), IPA (Sigma Aldrich), Cremophor EL (BASF, UK), and castor oil were purchased from different sources. Acetonitrile, Methanol, ethanol i.e. LC-MS–grade with highly pure was purchased from Fluka, Sigma Aldrich.
2.2. Experimental
2.2.1. Formulation of primary nanoemulsion (w/o) and their thermodynamic stability tests
5-FU primary nanoemulsions (w/o) were formulated through oil phase titration method in which Transcutol-HP (surfactant), IPA (cosurfactant), water (aqueous phase), and castor oil (oil phase) were used. Surfactant (Transcutol-HP) and cosurfactant (IPA) were used to prepare various ratio of Smix i.e. 1:0, 1:2, 1:1, 2:1, 3:1, and 4:1. Various ratios of Smix and water were mixed together from 9:1 to 1:9. Specific ratios of Smix and water were titrated to plot the pseudo-ternary phase diagrams followed by added slowly castor oil as an oil phase. Based on pseudo-ternary phase diagram, zones of primary nanoemulsion w/o (1°NE) were determined and also examined visually via transparent, clear, and easily flowable formulations (Ahmad et al., 2019). Based on pseudo-ternary phase diagram, many formulations were chosen and also performed the tests for thermodynamic stability e.g. centrifugation, heating & cooling cycles, and freeze–thaw cycles was used by Ahmad et al. (2019) & (2018a).
2.2.2. Characterization of NE (1°, w/o)
5-FU-NE (1°, w/o) have chosen when those have passed thermodynamic stability tests for the characterization. All the important characterization parameters have been performed e.g. PDI, droplet-size, and ZP of the opt–NEs. All the complete procedures are mentioned in the 5-FU-MNE (w/o/w) characterization.
2.2.3. Development of 5-FU-multiple-nanoemulsions (w/o/w, SNEDDS)
5-FU-NE (1°, w/o) were chosen as a result obtained like lowest PDI, smallest-globule-size, smallest viscosity with optimum ZP-values, and RI. 5-FU1-NE (1°, w/o, 5-FU1) were taken as oil phase for next step formulation of multiple-nanoemulsions i.e. w/o/w 5-FU-MNEs (SNEDDS). 5-FU-MNEs (w/o/w, SNEDDS) were prepared via aqueous-phase-titration method by the use of 5-FU1-NE (1°, w/o, 5-FU1) as an oil phase, Cremophor-EL as a surfactant, Transcutol-HP as a cosurfactant and Milli-Q-water as an aqueous phase. Various ratios of Smix (Cremophor-EL as a surfactant and Transcutol-HP as a cosurfactant) i.e. 1:0, 1:2, 1:1, 2:1, 3:1, and 4:1 were mixed together. 5-FU1-NE (1°, w/o, 5-FU1 as an oil phase) and definite Smix weight ratios from 9:1 to 1:9 were mixed together and developed pseudo-ternary phase diagrams via titrating mixture of 5-FU1-NE with the help of water-phase added slowly. Zones of MNEs (w/o/w) were determined via region in the pseudo-diagram. Pseudo-diagrams were used for the selection of various preparations and then finally for thermodynamic stability tests (Schmidts et al., 2011, Schmidts et al., 2012, Shah et al., 2011).
2.3. Self-nanoemulsification efficiency (SNEDDS) of 5-FU-multiple-nanoemulsions (w/o/w, 5-FU-MNEs)
5-FU-MNEs were showed stable on different stress conditions of thermodynamic-tests and it was selected to SNEDDS-test. Standard USP dissolution apparatus was used for the efficiency of opt-SNEDDS (Arias, 2008, Li et al., 2008, Yassin et al., 2010). Each 5-FU-MNE (w/o/w, 1 gm) was mixed with Milli-Q-water (500.0 ml) at a temperature 37.0 ± 0.50 °C. A dissolution paddle (made up of standard stainless steel) agitated gentle at 50.0 rpm the self-nanoemulsification efficiency of developed-formulations was determined by our open-eyes (Li et al., 2008, Yassin et al., 2010).
2.4. 5-FU-multiple-nanoemulsions (w/o/w, 5-FU-MNEs) characterization
Malvern Zetasizer (Malvern Instruments, Malvern, UK) based on dynamic laser light scattering was used to determine ZP, PDI, and globule size for 5-FU-MNEs (w/o/w). All the samples were diluted 20-times in Milli-Q-water and sonicated it for one minute to reduce the scattering property and it was examined at 25.0 ± 1.0 °C. 5-FU1-NE (1°, w/o, 5-FU1) were examined by TEM (transmission electron microscopy) to determine the globule-size and surface morphological structures (Morgagni 268D; FEI Company, Hillsboro, OR) with the help of bright field imaging combinations to enhanced their magnification. We have diluted the 5-FU-MNEs (w/o/w) samples 100 times in Milli-Q-water and placed on the copper grid followed by Ahmad et al., method (Schmidts et al., 2011, Schmidts et al., 2012, Shah et al., 2011).
5-FU-MNEs (w/o/w) viscosity was examined without dilution with help of Brookfield viscometer (Brookfield Engineering Laboratories, Inc, Middleboro, MA) by the spindle (CPE40 at 25.0 ± 0.50 °C). 5-FU-MNEs (w/o/w) RI was examined without dilution with help of Abbes type refractometer (Precision Standard Testing Equipment Corporation, Germany). %T of 5-FU-MNEs (w/o/w) was estimated through UV–VIS spectrophotometer (Shimadzu, Japan) at 550.0 nm (Gupta et al., 2005). Each 5-FU-MNEs (w/o/w) was taken 1.0 ml in which 5-FU (25.0 mg) present and further diluted with methanol and made up volume upto 10.0 ml in a volumetric flask. The flask was shaken vigorously. 5-FU-amount was examined through a developed-UHPLC-MS/MS-method.
2.5. DSC (Differential scanning calorimetry)
5-FU, IPA, Transcutol HP, castor oil, opt-5-FU-NE (°1), Cremophor EL, and opt-5-FU-MNE (w/o/w) were analysed by DSC 214 Polyma (NETZSCH‑Wittelsbacherstraße 42, 95,100 Selb, Germany) to identify the whole-solubilization as well as encapsulation of the 5-FU in the nanoemulsion (w/o) & (w/o/w). Briefly, reference is taken as empty pan whereas for sample analysis, we have taken sample (10 mg) inside pan. 20–400 °C temperature range was chosen to determine thermal analysis (DSC) with 10.0°K/min followed by 60 ml/min nitrogen flow (Ahmad et al. 2019). The data was determined by the software of DSC 214 Polyma (Netzsch Proteus 70, Germany)
2.6. 5–FU release studied (in vitro)
Selected 5-FU-MNE1–5-FU-MNE12 (w/o/w SNEDDS) were chosen to calculate the 5–FU release studies (in vitro) with the help of USP dissolution apparatus at 37.0 ± 0.50 °C and 50.0 rpm rotating speed in Milli-Q-water (900.0 ml). 5-FU-MNE1–5-FU-MNE12 (1.0-gram, w/o/w) was taken in separate hard gelatin capsule (000 sizes) in which also taken 25.0 mg 5-FU. 3.0 ml of sample from every 5-FU-MNE was taken at 1.0, 3.0, 5.0, 10.0, 15.0, 30.0, 45.0- and 60.0-minutes time intervals from already prepared-dissolution-medium and kept it in fresh Milli-Q-water (Alanazi et al., 2015). The amount of 5-FU was determined by every sample with the help of LC-MS-method. We have not used any 5-FU oral dosage form because any 5-FU-brand is not marketed for comparative in vitro 5-FU-release profile of MNE (w/o/w). Furthermore, our opt-oral dosage 5-FU-MNE (w/o/w) dissolution conditions can’t be same as any brand of 5-FU injection or cream.
2.7. Ex vivo rat intestinal membrane permeability
Rat intestine was used to examine 5-FU-permeation (Ruan et al., 2006, Avadi et al., 2011). In a brief, we have kept all the rats overnight fasted and then sacrificed through cervical dislocation with the help of xylazine (5.0 mg kg−1) and ketamine HCl (50.0 mg kg−1) intraperitoneally for anaesthesia. Rat ileum tissue was removed out and smoothly rinsed by Hank’s balanced salt solution (HBSS). We adopted the same method reported by Ruan et al., 2006, Avadi et al., 2011. 5-FU-S, 5-FU-MNE1, 5-FU-MNE5, and 5-FU-MNE9 was selected to use for the intestinal mucosal cells-permeation study with the help of 0.7850 cm2 franz-diffusion-cells and receiving chamber containing 12.0 ml capability. DHC-6 T Logan-transdermal-diffusion-cell sampling system was used to study the ex vivo permeation. Receptor compartment was filled with PBS (Phosphate buffer saline, 12.0 ml, 6.80-pH) whereas magnetic bar was used for stirring followed by maintained temperature (37 ± 1 °C). The instrument (DHC-6 T) was first cleaned properly and then it was stabilized for ten-minutes. 5-FU-S (10.0 mg/g), 5-FU-MNE1, 5-FU-MNE5, and 5-FU-MNE9 (5-FU:10 mg/g) were putted in each donor compartment when instrument was stabilized. All the withdrawn samples were examined at different intervals (10.0, 20.0, 30.0, 40.0, 50.0, 60.0, 90.0, and 120.0 min). All the samples were filtered (0.250 µm syringe filter) and putted in separate HPLC-vials for analysis by LC-MS method.
2.8. Stability studies on 5-FU-MNE1
We have performed the stability study of opt-5-FU-MNE1 on the 2-temperatures i.e. 4 °C (refrigerator) and 25 °C (room temperature). PDI, globule-size, viscosity, % Transmittance, RI, and 5-FU-content were estimated in different intervals (0, 30, 60, and 90 days). The mentioned-physicochemical-parameters were examined as per method mentioned in 5-FU-multiple-nanoemulsions (w/o/w, 5-FU-MNEs) Characterization.
2.9. A bioanalytical UHPLC–MS/MS method-development
LC–MS-based method was developed on Pinnacle-DB-C18 column (50X30 mm; 1.9 µm) with the help of binary solvent manager and a highly sensitive and peak-resolution tunable mass detector (ESI-triple-quadrupole, Shimadzu-LCMS-8050, Japan). Mobile phase was optimized methanol: ammonium formate i.e. 5 mM (75:25) with a 0.150 ml/min flow rate and 10 µl injected. Guidelines of US-FDA were used to validate method whereas weigh factor i.e. 1/x2 was calculated to determine the concentration vs. detector-response (US FDA, 2001, Ahmad et al., 2018b).
2.10. In vivo study
For the animal-study, we have got an ethical approval from IRB committee of Imam Abdulrahman Bin Faisal University to conduct the animal-study with ethical approval number IRB-UGS-2019–05-379. Wistar rats were taken that contain an age (8–10 weeks) and weight (200–250 g) which was putted in the dark-light cycle for 12.0 h. The humidity and temperature were maintained 60 ± 4% and 25 ± 3 °C, respectively.
2.11. PK (Pharmacokinetic) study
To perform PK, total numbers of rats were 36 = 12X3 and divided into three groups as G-1 (i.v. 5-FU-S), G-2 (oral 5-FU-S), and G-3 (oral, 5-FU-MNE-SNEDDS, w/o/w). The blood was collected at 0.50, 0.75, 1.00, 1.50, 2.00, 4.00, 6.00, and 8.00 h time intervals. The plasma was separated by the 4000-rpm centrifugation process (upto 10.0 min) and it was stored at –40.0 °C. LC-MS-method was used to bioanalyse the plasma extracted samples whereas the details are mentioned in this manuscript.
2.11.1. Cell lines based on human colon cancer (HT-29) for the cytotoxicity activity (in vitro)
2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)–2H-tetrazolium based WST-1 evaluation was performed to examine the cytotoxic effects of 5-FU-S, opt-5-FU-MNE (w/o/w, SNEDDS), and MNE (w/o/w, SNEDDS) without 5-FU. In this activity, tetrazolium salt was used to colored-water-soluble formazan-compounds via succinate-tetrazolium reductase that was found in viable-cells. The formazan is water soluble and their quantity is equivalent to number of viable cells. RPMI-1640 i.e. Roswell-Park Memorial-Institute-1640 medium was used for culture of cells (HT-29) followed by the addition of ABM (1.0%, GIBCO), fetal bovine serum (10.0%, FBS), penicillin (100.0 IU ml−1), L-glutamine (2.0 mmol L-1), and streptomycin (100 mg ml−1) in the CO2 atmosphere (5.0%) and maintained the temperature 37.0 ± 0.50 °C. 0.40X104 cells per well was used to grow cells ninety six well plates and then incubated with the help of incubator in the CO2 atmosphere (5.0%) and maintained the temperature 37.0 ± 0.50 °C. We were always changed the medium from 5-FU-S, 5-FU-MNE (SNEDDS, w/o/w), and MNE (SNEDDS, w/o/w) without 5-FU. WST-1 reagent (10.0 μl) was mixed after seventy-two-hours in every well and plates followed by incubation at 37.0 ± 0.5 °C for four hours. Formazan quantity was analysed with the help of ELISA-reader at 450.0 nm.
2.12. Statistical calculations
Student's t-test was applied for parameters of 5-FU-MNE characterization, permeation, PK, and cytotoxicity (in vitro) etc. Significance levels were calculated in terms of p-values that were significant-statistically at all levels of p < 0.05
3. Results
3.1. Formulation of primary nanoemulsion (w/o) and their thermodynamic stability studies
For 5-FU-NE (1°, w/o), pseudo-diagrams were prepared to examine the region of NE and optimum concentration ratio of co-surfactant, surfactant, and oil in which oil phase (Castor oil), surfactant (Transcutol HP), and co-surfactant (IPA) at various 1°Smix ratios (1:1, 1:2, and 2:1) (Fig. 1A to C). Like, surfactant quantity was greater than the co-surfactant quantity i.e. 1°Smix (2:1) or 1°Smix (1:1) area was greater than the NE-area that denotes 1° Smix solubilized lower to oil phase. Smix (44% w/w) incorporation was solubilized highest by concentration of water (18 %w/w) that means Transcutol-HP dominated for the size of nanoformulation (Fig. 1C). On the other hand, more increase of the concentration of surfactant in 1°Smix ratios (2:1 & 3:1) (Fig. 1A and 1C), a significant reduction was seen in the NE-region. Transcutol-HP may be produced liquid crystalline phase whereas the quantity of IPA was not mollified. Here our findings showed that Gibbs free energy of NE-preparation is dependent on the degree of surfactants and co-surfactants submissively reduces interfacial-tension of water or oil interface and also alter in dispersion entropy (Thomas et. al., 2012; Shanmugam et. al., 2011). We have selected only those formulations in which phase diagrams showed quantity could contain optimum amount of water-phase through using smallest possible Smix. Zones of highest NEs area were showed through Smix ratio (3:1) based on phase diagrams results. Based on phase diagrams results, NEs with various formulae were specifically chosen to establish the tests of various thermodynamic stability. 5-FU (25.0 mg) was solubilized in aqueous-phase and then required quantity of Smix was added in recommended ratio via addition of oil drop by drop until the clear and transparent NE was prepared. The prepared-NEs were ready to use for various tests (i.e. thermodynamic-stability). Those NEs were selected which didn’t exhibited no phase separation, cracking, coalescence, no phase inversion, and turbidity at all stress conditions. It was further chosen for characterization and their composition of such NEs shown in Table 1.
Fig. 1.
Pseudo-ternary phase diagrams of w/o nanoemulsion region of Castor Oil (oil phase), Transcutol-HP (surfactant), Isopropyl Alcohol, IPA (co-surfactant) with different Smix ratios: Smix 2:1 [A], Smix 1:1 [B], and Smix 3:1 [C], and for multiple-NE (w/o/w) region of the 1°-NE with Smix 1:2 (oil phase), Cremophor-EL (surfactant), Transcutol HP (co-surfactant) with different Smix ratios: Smix 2:1 [D], Smix 1:1 [E], and Smix 1:2 [F]. Note: Primary emulsion was composed of 18.0% aqueous phase, 38.0% Smix, and 44.0% castor oil (oil phase).
Table 1.
Preparation of 1° nanoemulsion (w/o) and their characterization (n = 3).
| Code | % Composition of Formulations (w/w) |
Smix Ratio | Globule Size ± SD | PDI ± SD | ZP in mV ± SD | RI ± SD | Viscosity ± SD (cps) | ||
|---|---|---|---|---|---|---|---|---|---|
| Water Phase | Smix | Oil Phase | |||||||
| 5-FU1 | 18 | 38 | 44 | 3:1 | 70.34 ± 2.71 | 0.213 ± 0.003 | −25.74 ± 1.63 | 1.481 ± 0.012 | 49.86 ± 3.74 |
| 5-FU2 | 15 | 40 | 45 | 3:1 | 85.37 ± 2.91 | 0.273 ± 0.005 | −24.84 ± 1.72 | 1.483 ± 0.013 | 66.94 ± 7.16 |
| 5-FU3 | 10 | 40 | 50 | 3:1 | 104.61 ± 3.01 | 0.291 ± 0.006 | −24.91 ± 1.78 | 1.484 ± 0.006 | 82.01 ± 6.33 |
| 5-FU4 | 5 | 35 | 60 | 3:1 | 120.17 ± 5.33 | 0.316 ± 0.005 | −26.42 ± 1.83 | 1.484 ± 0.009 | 101.72 ± 7.41 |
| 5-FU5 | 5 | 25 | 70 | 3:1 | 129.63 ± 6.31 | 0.356 ± 0.009 | −26.14 ± 1.96 | 1.488 ± 0.010 | 112.34 ± 7.98 |
| 5-FU6 | 20 | 50 | 30 | 2:1 | 81.36 ± 3.19 | 0.274 ± 0.010 | −25.39 ± 1.66 | 1.482 ± 0.004 | 62.07 ± 5.09 |
| 5-FU7 | 15 | 40 | 45 | 2:1 | 93.67 ± 3.61 | 0.281 ± 0.008 | −25.32 ± 1.69 | 1.481 ± 0.007 | 76.47 ± 6.02 |
| 5-FU8 | 10 | 40 | 50 | 2:1 | 114.31 ± 4.31 | 0.324 ± 0.007 | −25.84 ± 1.78 | 1.485 ± 0.010 | 95.64 ± 6.67 |
| 5-FU9 | 5 | 35 | 60 | 2:1 | 131.64 ± 6.31 | 0.371 ± 0.009 | −24.63 ± 1.91 | 1.483 ± 0.006 | 112.07 ± 8.36 |
| 5-FU10 | 5 | 25 | 70 | 2:1 | 141.06 ± 7.09 | 0.376 ± 0.010 | −24.90 ± 1.61 | 1.488 ± 0.007 | 123.43 ± 8.39 |
| 5-FU11 | 20 | 50 | 30 | 1:1 | 89.26 ± 4.65 | 0.319 ± 0.008 | −26.07 ± 1.80 | 1.482 ± 0.011 | 73.42 ± 6.16 |
| 5-FU12 | 15 | 40 | 45 | 1:1 | 100.28 ± 4.91 | 0.329 ± 0.010 | −25.38 ± 1.36 | 1.486 ± 0.005 | 88.03 ± 5.17 |
| 5-FU13 | 10 | 40 | 50 | 1:1 | 126.34 ± 6.11 | 0.348 ± 0.011 | −25.26 ± 1.33 | 1.482 ± 0.006 | 109.67 ± 9.18 |
| 5-FU14 | 5 | 35 | 60 | 1:1 | 139.64 ± 6.87 | 0.378 ± 0.009 | −25.31 ± 1.34 | 1.484 ± 0.008 | 123.81 ± 10.11 |
| 5-FU15 | 5 | 25 | 70 | 1:1 | 145.09 ± 8.01 | 0.392 ± 0.011 | −25.31 ± 1.92 | 1.487 ± 0.010 | 139.14 ± 10.34 |
PDI: Polydispersity Index; ZP: zeta potential; mV: milli volt; RI: Reference Index; cps: cycles per second
3.2. 5-FU-NE (1°, w/o) characterization
The data for selected (w/o)-1°-5-FU-NE i.e. 5-FU1–5-FU15 were showed in Table 1 in which the size range from 70.34 ± 2.71 to 145.09 ± 8.01 nm. 5-FU15 showed the highest globule-size that could be because of presence of maximum oil concentrations and smaller surfactants concentration (Table 1) conducting the preparation of inflexible interfacial tension. A cosurfactant insufficient quantity was not able to generate flexible into rigid-film for the 2°nanosizing. The globule-size of chosen-NEs analysis exhibited enhanced in globule size with enhancement of oil concentration. 5-FU1 globule-size was showed the smallest (70.34 ± 2.71 nm) may be due to smaller oil concentrations, optimum-surfactants concentration and maximum Smix ratio. Mostly, it was observed that globule-size of all NEs exhibited reduced in their globule size due to enhancing the surfactants concentration and their Smix ratio. PDI was showed <0.392 for all NEs that representing the narrow distribution. 5-FU1-NE showed lowest PDI value i.e. 0.213 ± 0.003 recommending the same globule-sizes of NEs. It was already published that if ZP values is in-between 25 and 30 mV illustrated a stable-nanoformulation (Ahmad et al., 2019, Ahmad et al., 2020). ZP values of nanoemulsions (5-FU1–5-FU15) were found in the range of − 24.63 ± 1.91 to − 26.42 ± 1.83 mV. NE of 5-FU1 exhibited the ZP-value i.e. −25.74 ± 1.63 mV (Table 1). 5-FU1–5-FU15 NEs didn’t not find any alteration in ZP-values (p ≤ 0.050) significantly. Mentioned ZP-values showed the stability potential of all NEs as stated previously (Ahmad et al., 2019, Ahmad et al., 2020). All NEs showed –ve-value of ZP may be due to fatty-acids and esters present in oil i.e. castor-oil as an oil phase for preparation of 5-FU-NE (1°, w/o). RI of prepared-NEs (5-FU1–5-FU15) was found in the range of 1.481 ± 0.012 to 1.488 ± 0.010 at 25.0 °C (Table 1). All the observed values basically 5-FU1 was very near to RI of castor oil as used as oil phases i.e. 1.481 ± 0.012. On this basis, we can characterize w/o form of 5-FU-1°-NE. 5-FU1-NE showed the 1.481 value for RI. We have selected 15-nanoemulsion i.e. 5-FU1–5-FU15 showed the viscosity ranged from 49.86 ± 3.74 to 139.14 ± 10.34 cps. Transcutol-HP, castor oil, and IPA have been participated and correlated their ratio in stabilization of screened-NEs (5-FU1–5-FU15) on the basis of viscosity findings. 5-FU1 NE showed smallest viscosity-value i.e. 49.86 ± 3.74 cps as compared to rest 5-FU2 to 5-FU15 NEs. Here, it showed lowest viscosity of 5-FU1 that can be due to amount of surfactant and smallest oil concentration based on rheological properties.
3.3. Development of 5-FU-multiple-nanoemulsions (w/o/w, SNEDDS)
5-FU1-NE (1°, w/o) was chosen as an oil phase on the basis of optimized parameters i.e. smallest globule-size (70.34 ± 2.71), smallest PDI (0.213 ± 0.003), smallest viscosity (49.86 ± 3.74 cps) and optimum ZP values i.e. −25.74 ± 1.63 mV), and RI (1.481 ± 0.012) for the preparation of 5-FU-multiple-nanoemulsions (w/o/w, 5-FU-MNEs, SNEDDS) (Fig. 2, Table 1). 5-FU-MNEs (w/o/w) were formulated through aqueous phase titration method using 5-FU1-NE (1°, w/o) as an oil phase, Cremophor-EL as a surfactant, Transcutol-HP as a cosurfactant, and Milli-Q-water (ultrapure) as a water-phase. Pseudo-ternary phase diagrams were prepared for 5-FU-MNEs (w/o/w) with various 2° Smix which were shown in Fig. 1D to 1F. We have determined enhance the surfactant concentration which parallely increased the NE-area. The zone of 5-FU-MNEs (w/o/w, SNEDDS) was increased significantly due to the same ratio of surfactant and cosurfactant concentrations (2°Smix; 1:1) when it compared to 1:0 and 1:2 (Smix ratio) (Fig. 1F). Highest quantity of 5-FU1-NE (1°, w/o) was solubilized 33.0% (w/w) with smaller 2° Smix concentration (i.e. 22.0% w/w). We have observed that enhanced the surfactant concentration with regard to cosurfactant (2:1 Smix ratio), 5-FU-MNEs (w/o/w, SNEDDS) zones were reduced their area when it compared to 1:1 ratio (Fig. 1D). Highest quantity of 5-FU1-NE (1°, w/o) was solubilized through 2:1 ratio was 28.0% (w/w) entrapping inside 2° Smix concentration (i.e. 41.0% w/w). But 5-FU-MNEs (w/o/w, SNEDDS) zones were reduced when surfactant concentration was added more quantity with regard to cosurfactant (3:1, Smix). Thus, highest quantity of 5-FU1-NE (1°, w/o) was solubilized by the use of 22.0% (w/w) entrapping inside 2° Smix concentration (i.e. 52.0% w/w). As 5-FU-MNEs (w/o/w, SNEDDS) zones were reduced again through enhancing surfactant concentration with regard to cosurfactant. Finally, it was concluded it is not require going for 5:1 or 4:1 Smix ratio to prepare more phase diagrams (Arias, 2008, Longley et al., 2003). Based on phase diagrams, we have selected twelve 5-FU-MNEs (w/o/w, MNE1-MNE12, SNEDDS) to perform the tests for thermodynamic stability of NEs.
Fig. 2.
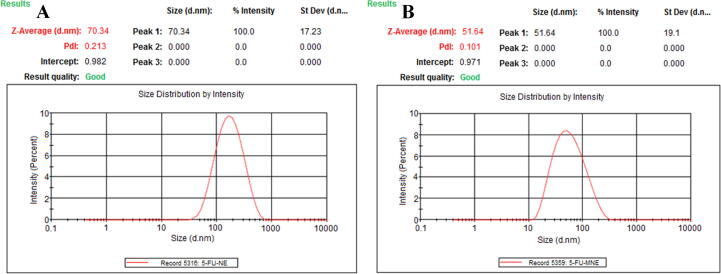
Dynamic light scattering techniques (A): globule size of 5-FU-primary-NE (w/o) and (B): globule size of opt-5-FU-MNEs (w/o/w, SNEDDS).
3.4. Efficiency of 5-FU-MNEs (w/o/w, MNE1-MNE12, SNEDDS) for Self-nanoemulsification
We have selected thermodynamically stable 5-FU-MNEs (w/o/w, SNEDDS) to perform the nanoemulsification efficiency. 5-FU-MNEs (w/o/w, SNEDDS) have diluted many-times because to examine the possibility of phase separation or precipitation of a 5-FU that could be produced at a specific % of water-, surfactant-, and -oil. Current research examination, for dispersion medium, milli-Q-water was chosen. Based on previous study nonionic-surfactants was dispersed in the H2O or intestinal fluids or simulated-gastric and found it no significant variations in opt-SNEDDS (Arias, 2008, Zhang et al., 2012). 5-FU-MNEs (w/o/w, SNEDDS) were passed the selfnanoemulsification efficiency test in Grades (A & B) after that they were chosen to more characterization (Table 2).
Table 2.
Preparation of multiple nanoemulsion (w/o/w) and their characterization (n = 3). All of them passed test for SNEDDS-test.
| Code | % Composition of Formulations (w/w) | Smix Ratio | Globule Size ± SD | PDI ± SD | ZP in mV ± SD | RI ± SD | Viscosity ± SD (cps) | Transmittance (%) ± SD | Drug Content ± SD | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1° 5-FU1-NE (Oil Phase) | Smix | Water Phase | |||||||||
| MNE1 | 10 | 25 | 65 | 1:1 | 51.64 ± 2.61 | 0.101 ± 0.001 | −5.59 ± 0.94 | 1.331 ± 0.003 | 7.97 ± 0.021 | 99.16 ± 0.41 | 99.63 ± 0.23 |
| MNE2 | 15 | 30 | 55 | 1:1 | 79.47 ± 4.03 | 0.169 ± 0.009 | −3.34 ± 0.16 | 1.339 ± 0.008 | 27.64 ± 3.02 | 96.13 ± 1.05 | 97.61 ± 1.47 |
| MNE3 | 20 | 35 | 45 | 1:1 | 103.14 ± 6.58 | 0.316 ± 0.034 | −2.34 ± 0.09 | 1.335 ± 0.013 | 49.14 ± 4.26 | 92.67 ± 2.67 | 95.47 ± 1.51 |
| MNE4 | 25 | 40 | 35 | 1:1 | 129.47 ± 8.73 | 0.334 ± 0.049 | −4.34 ± 1.13 | 1.349 ± 0.011 | 72.01 ± 6.89 | 89.92 ± 2.97 | 93.11 ± 1.16 |
| MNE5 | 10 | 25 | 65 | 1:2 | 48.14 ± 3.05 | 0.112 ± 0.002 | −0.16 ± 0.01 | 1.332 ± 0.006 | 8.94 ± 0.038 | 98.01 ± 0.38 | 99.02 ± 0.19 |
| MNE6 | 15 | 30 | 55 | 1:2 | 83.14 ± 5.01 | 0.159 ± 0.010 | −4.04 ± 0.13 | 1.337 ± 0.012 | 30.21 ± 4.31 | 95.84 ± 3.21 | 96.49 ± 2.34 |
| MNE7 | 20 | 35 | 45 | 1:2 | 119.65 ± 8.19 | 0.364 ± 0.040 | −3.12 ± 0.18 | 1.346 ± 0.016 | 54.92 ± 3.61 | 91.06 ± 3.52 | 93.02 ± 2.36 |
| MNE8 | 25 | 40 | 35 | 1:2 | 145.93 ± 7.18 | 0.398 ± 0.051 | −1.46 ± 0.09 | 1.346 ± 0.003 | 69.45 ± 2.15 | 87.45 ± 2.84 | 91.64 ± 1.45 |
| MNE9 | 10 | 25 | 65 | 2:1 | 53.64 ± 2.83 | 0.126 ± 0.002 | −0.13 ± 0.53 | 1.349 ± 0.008 | 9.39 ± 0.043 | 97.62 ± 1.06 | 98.45 ± 0.58 |
| MNE10 | 15 | 30 | 55 | 2:1 | 92.41 ± 6.33 | 0.161 ± 0.019 | −5.32 ± 0.78 | 1.346 ± 0.026 | 32.81 ± 5.01 | 94.02 ± 4.18 | 95.64 ± 3.45 |
| MNE11 | 20 | 35 | 45 | 2:1 | 131.54 ± 9.57 | 0.456 ± 0.061 | −2.81 ± 0.53 | 1.348 ± 0.019 | 58.21 ± 4.43 | 89.24 ± 3.82 | 91.41 ± 2.98 |
| MNE12 | 25 | 40 | 35 | 2:1 | 153.23 ± 9.75 | 0.482 ± 0.064 | −1.32 ± 0.23 | 1.349 ± 0.004 | 72.95 ± 2.02 | 85.10 ± 2.11 | 88.79 ± 3.64 |
PDI: Polydispersity Index; ZP: zeta potential; mV: milli volt; RI: Reference Index; cps: cycles per second
3.5. 5-FU-MNEs (w/o/w, SNEDDS) characterization
TEM was taken to determine the shape and surface morphology of 5-FU-MNEs (w/o/w, SNEDDS) globules. TEM images of MNE1 (w/o/w) was selected and studied for distribution of globule-size and their morphology of surface. All globules were observed contains <100.0 nm sizes which is very important characterization of 5-FU-MNEs (w/o/w) (Ahmad et al., 2019). Fig. 2, Fig. 3 showed all the globules <100.0 nm size and their globules-size distribution (Table 2) determined through Malvern Zeta sizer. All globules were showed distributed uniformly with nano-range.
Fig. 3.
TEM image of globule-size for opt-5-FU-MNEs (w/o/w, SNEDDS).
Table 2 exhibited PDI, globule-size, ZP of all 5-FU-MNEs (w/o/w) which showed globule size range from 51.64 ± 2.61 to 153.23 ± 9.75 nm with a PDI smaller than 0.482 of 5-FU-MNEs (w/o/w; MNE1– MNE12) that denoting uniform and narrow distribution of globule-size. MNE12 showed the maximum globule-size and also contains maximum % of oil phase (w/o 5-FU-1°-NE) concentration. If the oil phase (10.0–25.0%) concentration increased with 2°Smix ratio which were also increased in globule-size parallely like globule-size of MNE1 to MNE4: 51.64 ± 2.61 to 129.47 ± 8.73 nm at Smix,2° 1:1; 48.14 ± 3.05 to 145.93 ± 7.18 nm at Smix,2° 1:2; 53.64 ± 2.83 to 153.23 ± 9.75 nm at Smix,2° 2:1. Based on these findings, globule-size of all 5-FU-MNEs (w/o/w) is directly proportional to the oil phase concentration. If oil phase concentration was kept 10.0% or 15% as constant which were showed decreased in globule-sizes with increasing of aqueous phase concentration. Hence, MNE1 showed smallest surfactant concentration i.e. 25% (2° Smix, 1:1), oil phase (10.0%), and aqueous phase (65%) to produce smallest size of globule. On the other hand, we have also observed that > 10.0% of the oil phase showed the higher globule size (79.47 ± 4.03 nm) with increase in 2° Smix ratio. 2° Smix ratio (1:1) was used for the formulation of MNE (w/o/w, SNEDDS) produced smallest globule-size i.e. 51.64 ± 2.61 nm and smallest 0.101 ± 0.001 PDI of MNE1 whereas MNE12 was showed the maximum globule-size (153.23 ± 9.75) as compared to rest of MNEs (MNE1 to MNE11) (Table 2).
MNEs (MNE1 to MNE12) were not diluted to determine the RIs showed the range i.e. 1.331–1.349 (Table 2). All these RI-values were found to be very near to RI of water phase i.e. (RI:1.33) that is clear indication of w/o/w nature of MNEs (SNEDDS) formulated. MNE1 (w/o/w, SNEDDS) showed smallest RI i.e. 1.331 ± 0.003 as compared to other MNEs (w/o/w, MNE2 to MNE12, SNEDDS). All the MNEs (w/o/w, MNE1 to MNE12, SNEDDS) were not diluted to examine the viscosity that all were showed 7.97 to 72.95 cps (Table 2). MNE1 was showed the smallest viscosity i.e. 7.97 cps as compared to other MNEs (w/o/w, MNE2 to MNE12, SNEDDS). We observed that increased in oil concentration for the preparation of MNEs that showed the parallely increase in the viscosity. The results of viscosity were showed same pattern as globule-size evaluations. MNEs (w/o/w, MNE1 to MNE12, SNEDDS) were showed the results that all of them are free flowing based on the low viscosity value. %T of MNEs (w/o/w, MNE1 to MNE12, SNEDDS) exhibited the range from 85.10 ± 2.11 to 99.16 ± 0.41% (Table 2). MNE1 (w/o/w, SNEDDS) showed highest %T i.e. 99.16 ± 0.41% as compared to other MNEs (w/o/w, MNE2 to MNE12, SNEDDS). We observed that decreased slightly in %T in the MNEs that showed the inversely increase in the viscosity and globule-sizes. MNE1 was showed the highest % Drug content i.e. 99.63 ± 0.23% as compared to other MNEs (w/o/w, MNE2 to MNE12, SNEDDS). We again observed that decreased slightly in %Drug content in the MNEs that showed the inversely increase in the viscosity and globule-sizes. Thus, it might be because of highest-concentration of oil phase. MNE12 (w/o/w, SNEDDS) showed smallest % drug content i.e. 88.79 ± 3.64% as compared to other MNEs (w/o/w, MNE1 to MNE11, SNEDDS).
3.6. DSC study
5-FU showed melting point i.e. 282.60 °C of excellent endothermic peak on DSC examination (Fig. 4) which is same as reported before and mentioned in the COA a range 282.0–283.0 °C and also indicates the 5-FU crystalline nature. DSC of Castor oil (only one line), and Transcutol HP showed different peaks like 46.1 °C, 168.0 °C, 172.8 °C, 212.9 °C, and IPA (121.8 °C and 127.5 °C). Opt-5-FU1-NE (1°, w/o) has given one split peak for Transcutol HP and IPA in the thermogram of DSC. In the DSC thermogram of opt-5-FU1-NE (1°, w/o) was not found any peak of 5-FU. Based on DSC of opt-5-FU1-NE (1°, w/o), we finally-determined that 5-FU entirely encapsulated within the core of opt-5-FU1-NE (1°, w/o). Cremophor EL showed a prominent endothermic peak on 65.6 °C which showed a crystalline nature of Cremophor EL. At last, 5-FU-MNE (w/o/w, SNEDDS) exhibited a tiny peak and one split peak. To conclude, we didn’t find any peak of 5-FU and diminished-peaks of opt-5-FU1-NE (1°, w/o) i.e. a clear indication of prepared opt-5-FU-MNE (w/o/w, SNEDDS).
Fig. 4.
DSC-thermograms showed endothermic peaks of castor oil, 5-FU, Transcutol HP, IPA, 5-FU-NE, Cremophor EL, and opt-5-FU-MNEs (w/o/w, SNEDDS).
3.7. 5-FU release (in vitro) evaluations
5-FU-release (in vitro) was examined and compare from twelve 5-FU-MNEs (w/o/w, MNE1 to MNE12, SNEDDS) in which initial loaded quantity of 5-FU (25.0 mg) were taken. 5-FU-release (in vitro) from 5-FU-MNEs (w/o/w, MNE1, MNE5, and MNE9 SNEDDS) were showed highly significant (p<0.05) as compared to other 5-FU-MNEs (w/o/w, SNEDDS) (Fig. 5A). Percentage of 5-FU-release was found to be 99.71%, 92.33%, and 88.02% from MNE1, MNE5, and MNE9, respectively. 5-FU-MNE1 (w/o/w, SNEDDS) was showed 99.71% highest 5-FU-release. 5-FU-MNE1 (w/o/w, SNEDDS) was also showed>86.0% 5-FU-release in the initial fifteen minutes assessment itself as compared to other 5-FU-MNEs (w/o/w, SNEDDS) (Fig. 5A). 5-FU was highest released from 5-FU-MNE1 (w/o/w, SNEDDS) may be because of smallest PDI, minimum-globule-size, smallest viscosity that gives a maximum surface area of 5-FU-MNE1 was given offered fast 5-FU-release. But rest of 5-FU-MNEs (w/o/w, SNEDDS) contained optimized quantity of oil phase but still they showed 5-FU-release was significantly smaller i.e. p<0.05 as compared to 5-FU-MNE1 (w/o/w, SNEDDS) may be because of maximum value of PDI, bigger globule-size, and maximum-viscosity (Table 2). Hence, 5-FU was found more surface area for dissolution in case of 5-FU-MNE1 (w/o/w, SNEDDS) as compared to other MNEs (w/o/w, MNE2 to MNE12, SNEDDS). As a result, opt-5-FU-MNE1 (w/o/w, SNEDDS) showed maximum 5-FU-release (99.71%), smallest-globule-size (51.64 nm), smallest PDI value (0.101), smallest viscosity (7.97 cps), %Transmittance (99.16 ± 0.41), %Drug Content (99.63 ± 0.23) stability of opt-5-FU-MNE1 (w/o/w, SNEDDS) and 5-FU. Therefore, we optimized 25.0% surfactant concentration is excellent for rest of PK, cytotoxicity (in vitro) studies.
Fig. 5.
[A] %age-cumulative-release showed 5-FU from various 5-FU-NEs and [B] %age-intestinal permeation study of 5-FU from opt-5-FU-MNEs (w/o/w, SNEDDS).
3.8. Ex vivo intestinal membrane permeation examination
MNE1, MNE5, MNE9 have showed significantly enhanced intestinal membrane permeability as compare to 5-FU-S after the conversion of MNEs (w/o/w, SNEDDS) (Fig. 5B). We have observed increased the membrane permeability for 5-FU as reduced the % of oil phase at the entire levels of 2° Smix ratios. But MNE1, MNE5, and MNE9 showed very small variations in intestinal membrane permeability due to their same % of oil content and much closed to globule sizes. 5-FU permeability from MNE1 (w/o/w, SNEDDS) i.e. 83.64% (2°Smix, 1:1) was higher as compared to other MNEs (MNE5 i.e. 77.19% & MNE9 i.e. 70.54%) followed by free 5-FU-S (19.89%). Based on the ex vivo intestinal membrane permeability of 5-FU, 2° Smix (1:1) resulted an optimized % ratio of the oil phase to 2° Smix, MNE1 (w/o/w, SNEDDS) for increment of 5-FU oral absorption of (Fig. 5B, Table 2).
3.9. 5-FU-MNE1 (w/o/w, SNEDDS) stability studies
Any drug formulation can’t be ideal until that should passed all the parameters related with stability like chemical, physical, and microbiological stability the totally intended shelf life period (Ahmad et al., 2019). Thus, opt-5-FU-MNE1 (w/o/w, SNEDDS) was characterized by all physicochemical parameters like globule-size, PDI, RI, viscosity, %Transmittance, and 5-FU-content. Therefore, we selected two temperatures (4.0 °C & 25.0 °C) to determine these parameters. All results related with above mentioned parameters have not shown any alteration at refrigerator temperature (Table 3) which were very minute at refrigerator temperature. At room temperature, it was observed very small alteration of these parameters. On the basis of these findings, opt-5-FU-MNE1 (w/o/w, SNEDDS) is satisfactorily stable at both temperatures.
Table 3.
Determination of physicochemical parameters for stability studies on MNE1.
| Time (months) | Temperature (°C) | Globule Size ± SD | PDI ± SD | RI ± SD | Viscosity ± SD (cps) | Transmittance (%) ± SD | % Drug Content ± SD |
|---|---|---|---|---|---|---|---|
| 0 | 4.0 ± 1.0 | 51.64 ± 2.61 | 0.101 ± 0.001 | 1.331 ± 0.003 | 7.97 ± 0.021 | 99.16 ± 0.41 | 99.63 ± 0.23 |
| 1 | 4.0 ± 1.0 | 51.99 ± 2.73 | 0.109 ± 0.002 | 1.335 ± 0.005 | 8.21 ± 0.023 | 98.97 ± 0.46 | 99.20 ± 0.29 |
| 2 | 4.0 ± 1.0 | 52.21 ± 2.91 | 0.114 ± 0.004 | 1.338 ± 0.007 | 8.26 ± 0.026 | 98.42 ± 0.52 | 99.00 ± 0.31 |
| 3 | 4.0 ± 1.0 | 53.89 ± 2.99 | 0.119 ± 0.008 | 1.342 ± 0.008 | 8.30 ± 0.030 | 98.06 ± 0.61 | 98.69 ± 0.38 |
| 0 | 25.0 ± 1.0 | 51.64 ± 2.61 | 0.101 ± 0.001 | 1.331 ± 0.003 | 7.97 ± 0.021 | 99.16 ± 0.41 | 99.63 ± 0.23 |
| 1 | 25.0 ± 1.0 | 53.91 ± 3.01 | 0.110 ± 0.005 | 1.339 ± 0.007 | 8.88 ± 0.032 | 98.03 ± 0.34 | 98.81 ± 0.33 |
| 2 | 25.0 ± 1.0 | 58.76 ± 3.68 | 0.119 ± 0.007 | 1.343 ± 0.005 | 9.37 ± 0.048 | 97.18 ± 0.29 | 98.20 ± 0.38 |
| 3 | 25.0 ± 1.0 | 61.05 ± 3.84 | 0.123 ± 0.004 | 1.346 ± 0.011 | 10.53 ± 0.056 | 95.56 ± 0.33 | 97.72 ± 0.31 |
3.10. UHPLC-ESI-triple-quadrupole-MS/MS based 5-FU-developed method- and validation
5-FU parent and daughter ions scan were displayed in Fig. 6A & B whereas their chromatograms are shown in Fig. 7. For 5-FU, plasma recovery (>78.28 ± 2.31%) with r2 > 0.9963 value of linear regression was determined at 1.0–1000.0 ng/ml concentration ranges.
Fig. 6.
Mass spectrum of [A] 5-FU-MS parent ion scan [M−H]– at m/z 129.06 and [B] 5-FU-MSMS daughter ion scan at m/z 42.05.
Fig. 7.
[A] Chromatogram of extracted blank plasma, [B] extracted lower limit of quantification (LLOQ), and [C] one of standard of 5-FU.
Fig. 7 showed chromatograms that indicated for selectivity of method for extracted-plasma of blank as well as 5-FU. The % CV for 5-FU was as; 2.07–3.03 (intra-batch) and 1.74–4.08 (inter-batch) while % accuracy was as; 97.06–99.32 (intra-batch) and 96.08–98.97 (inter–batch) mentioned in Table 4. Table 5 showed the data for Robustness and Ruggedness which were found to be acceptance limit in all parameters. Table 6 was also showed the data for stability which were found to be acceptance limit in all parameters as mentioned validation parameters in US-FDA, 2001 and used by Ahmad et. al., 2018b.
Table 4.
Precision and Accuracy Data for 5-Fluorouracil (5-FU).
| Intra–batch Inter–batch |
% Recovery | |||||||
|---|---|---|---|---|---|---|---|---|
| QC ID | Theoretical Content (ng mL−1) | Mean concentration observed (ng mL−1) | Accuracya (%) | CVb (%) | Mean concentration observed (ng mL−1) | Accuracya (%) | CVb (%) | |
| LOQQC | 1.02 | 0.99 ± 0.03 | 97.06 | 3.03 | 0.98 ± 0.04 | 96.08 | 4.08 | 78.28 ± 2.31 |
| LQC | 2.92 | 2.90 ± 0.06 | 99.32 | 2.07 | 2.87 ± 0.05 | 98.97 | 1.74 | 79.13 ± 2.02 |
| MQC | 415.00 | 410.03 ± 11.06 | 98.80 | 2.70 | 408.24 ± 10.25 | 98.37 | 2.51 | 81.87 ± 3.27 |
| HQC | 815.00 | 795.62 ± 21.03 | 97.62 | 2.64 | 791.54 ± 20.14 | 97.12 | 2.54 | 83.10 ± 4.11 |
Values (Mean ± SD) are derived from 6 replicates: aAccuracy (%) = Mean value of [(mean observed concentration)/(theoretical concentration)] × 100; bPrecision (%): Coefficient of variance (percentage) = standard deviation divided by mean concentration found × 100; Theoretical contents; LOQQC: 1.010 ng mL−1, LQC: 2.92 ng mL−1; MQC: 415.0 ngmL−1; and HQC: 815.0 ngmL−1.
Table 5.
Robustness of the Method for 5-Fluorouracil (5-FU).
| (A) Robustness | |||
|---|---|---|---|
| Conditions | LQC (2.92 ng mL−1) | MQC(415.0 ng mL−1) | HQC (815.0 ng mL−1) |
| Mobile Phase [Methanol: 5 mM Ammonium Formate (75:25 : v/v)] | |||
| Negative level (74.7:25.3, n = 3) | 2.87 ± 0.04 (1.39%) | 400.11 ± 9.32 (2.33%) | 795.67 ± 20.16 (2.53%) |
| Zero level (75:25, n = 3) | 2.89 ± 0.06 (2.08%) | 407.31 ± 10.16 (2.49%) | 803.41 ± 21.32 (2.65%) |
| Positive level (75.3:24.7, n = 3) | 2.79 ± 0.09 (3.23%) | 403.21 ± 12.43 (3.08%) | 793.49 ± 19.05 (2.40%) |
| Flow Rate (0.15 ml /min) | |||
| Negative level (0.14, n = 3) | 2.81 ± 0.10 (3.56%) | 398.51 ± 9.64 (2.42%) | 786.18 ± 22.58 (2.87%) |
| Zero level (0.15, n = 3) | 2.86 ± 0.06 (2.10%) | 400.30 ± 10.13 (2.53%) | 806.33 ± 19.24 (2.39%) |
| Positive level (0.16, n = 3) | 2.82 ± 0.05 (1.77%) | 395.09 ± 11.09 (2.81%) | 791.04 ± 20.36 (2.57%) |
| pH of Mobile Phase(Default pH = 6.8) | |||
| Negative level (6.6, n = 3) | 2.82 ± 0.08 (2.84%) | 391.36 ± 10.08 (2.58%) | 789.64 ± 21.08 (2.67%) |
| Zero level (6.8, n = 3) | 2.86 ± 0.07 (2.45%) | 406.47 ± 9.31 (2.29%) | 807.09 ± 20.13 (2.49%) |
| Positive level (7.0, n = 3) | 2.79 ± 0.09 (3.23%) | 399.94 ± 11.21 (2.80%) | 790.11 ± 19.31 (2.44%) |
|
(B) Ruggedness | ||||
|---|---|---|---|---|
| QC ID | Theoretical content (ng mL−1) | Mean concentration observed (ng mL−1) | Accuracy a (%) | CV (%) b |
| LOQQC | 1.02 | 0.99±0.03 | 98.12 | 3.03 |
| LQC | 2.92 | 2.90±0.08 | 99.32 | 2.76 |
| MQC | 415.0 | 406.31±10.12 | 97.91 | 2.49 |
| HQC | 815.0 | 801.44±16.32 | 98.34 | 2.04 |
Values (Mean ± SD) are derived from 6 replicates: aAccuracy (%) = Mean value of [(mean observed concentration)/(theoretical concentration)] × 100; bPrecision (%): Coefficient of variance (percentage) = standard deviation divided by mean concentration found × 100; Theoretical contents; LOQQC: 1.010 ng mL−1, LQC: 2.92 ng mL−1; MQC: 415.0 ngmL−1; and HQC: 815.0 ngmL−1.
Table 6.
Ex vivo stability data for 5-Fluorouracil (5-FU).
| Conditions | LQC (2.92 ngmL−1) | HQC (815.0 ngmL−1) |
|---|---|---|
| Long term stability; recovery (ng) after storage (–40 °C) | ||
| Previous day | 2.90 ± 0.07 | 804.94 ± 20.31 |
| 30th Day | 2.86 ± 0.06 (98.62%) | 791.36 ± 19.46 (98.31%) |
| Freeze–thaw stress; recovery (ng) after freeze–thaw cycles (–40 °C to 25 °C) | ||
| Pre-Cycle | 2.89 ± 0.08 | 803.57 ± 21.08 |
| First Cycle | 2.87 ± 0.06 (99.31%) | 796.21 ± 20.11 (99.08%) |
| Second Cycle | 2.83 ± 0.05 (97.92%) | 791.25 ± 21.01 (98.47%) |
| Third Cycle | 2.79 ± 0.07 (96.54%) | 785.05 ± 19.11 (97.70) |
| Heating-cooling stress; recovery (ng) after Heating-cooling cycles (50 °C to 4 °C) | ||
| Pre-Cycle | 2.89 ± 0.07 | 804.94 ± 20.16 |
| First Cycle | 2.84 ± 0.06 (97.57%) | 799.04 ± 19.41 (99.27%) |
| Second Cycle | 2.79 ± 0.05 (94.83%) | 791.25 ± 20.34 (98.30%) |
| Third Cycle | 2.74 ± 0.06 (93.37%) | 784.16 ± 21.19 (97.42%) |
| Bench top stability; recovery (ng) at room temperature (25 °C) | ||
| 0 hr | 2.90 ± 0.07 | 803.18 ± 19.83 |
| 24 hr | 2.84 ± 0.08 (97.93%) | 785.61 ± 22.07 (97.81%) |
| Post processing stability; recovery (ng) after storage in auto sampler (4 °C) | ||
| 0 hr | 2.88 ± 0.07 | 801.54 ± 20.93 |
| 24 hr | 2.82 ± 0.06 (98.85%) | 786.14 ± 21.15 (98.08%) |
Values (Mean ± SD) are derived from six replicates. Figures in parenthesis represent analyte concentration (%) relative to time zero. Theoretical contents; LOQQC: 1.010 ng mL−1, LQC: 2.92 ng mL−1; MQC: 415.0 ngmL−1; and HQC: 815.0 ngmL−1.
3.11. PK parameters calculations
For PK parameters determination, we used the independent-non-compartmental model as a one-dose of 5-FU-S (oral) 5-FU-S (IV), and opt-5-FU-MNE1 (w/o/w, SNEDDS; Oral) in Fig. 8 signified plasma-drug-concentration Vs time profile in rats. Trapezoidal method was used to examine plasma 5-FU-concentration in terms of Tmax, Cmax, and AUC in rats. In the Fig. 8 showed highest value of Cmax i.e.767.36 ± 24.93 ng/ml for i.v. 5-FU-S and their 5-FU showed fast clearance due to 5-FU-decay at plasma level. Opt-5-FU-MNE1 (w/o/w, SNEDDS) was enhanced significantly (p < 0.001) in terms of Cmax, Tmax as well as AUC0–t parameters as compared to oral 5-FU-S (Table 7). 5-FU-MNE SNEDDS is also significantly enhanced the bioavailability, Cmax, Tmax as well as AUC0–t when it also compared to 5-FU-S (i.v.) (p < 0.01) (Table 7). A 5-FU released in a sustained form (dissolution studies) for Tmax while increased absorption supports the enhanced to Cmax together with AUC0–t. Opt-5-FU-MNE1 (w/o/w, SNEDDS) showed increased absorption could be due to 1) Opt-5-FU-MNE1 (w/o/w, SNEDDS)-encapsulation i.e. GI-shielding 2) also shield from P-gp as well as CYP-450 metabolism 3) enterocytic endocytosis and based on mentioned additive effects resulted an enhanced oral bioavailability (Ahmad et al., 2017).
Fig. 8.
Pharmacokinetic was studied in wistar rats after 5 mg/kg single dose of 5-FU-S (intravenous), 5-FU-S (Oral; 10.0 mg/kg), and 5-FU-MNE (w/o/w, SNEDDS; Oral; 10.0 mg/kg). Significantly high AUC was achieved with opt-5-FU-MNE (w/o/w, SNEDDS; Oral; 10.0 mg/kg) (p < 0.01, mean ± SD, n = 6).
Table 7.
Pharmacokinetic parameters of 5-Fluorouracil (5-FU) after single i.v. dose of 5-FU-S (5 mg/kg body weight), 5-FU-S (Oral, 20 mg/kg body weight), 5-FU-MNE (Oral, 20 mg/kg body weight) (mean ± SD; n = 6).
| Parameters | Cmax (ng/mL) | Tmax (h) | t1/2 | AUC0−t (ng h/mL) | AUC0−∞ (ng h/mL) | Keli (h−1) |
|---|---|---|---|---|---|---|
| 5-FU-S (i.v.) | 767.36 ± 34.93 | 0.50 | 1.58 | 1036.51 ± 67.92 | 1077.34 ± 69.32 | 0.43742 |
| 5-FU-S (Oral) | 93.84 ± 11.46 | 0.50 | 2.41 | 199.41 ± 10.82 | 231.04 ± 11.32 | 0.28709 |
| 5-FU-MNE (Oral) | 411.67 ± 22.64** | 1.00 | 6.68** | 2021.54 ± 113.71*** | 3530.22 ± 167.43*** | 0.10370 |
* p < 0.1, ** p < 0.01; *** p < 0.001
3.12. Cell lines based on human colon cancer (HT-29) for the cytotoxicity activity (in vitro)
We examined the therapeutic efficacy (in vitro) of opt-5-FU-MNE1 (w/o/w, SNEDDS) on colon cancer cell lines (HT-29). We have prepared various molar concentrations of 5-FU for opt-5-FU-MNE1 (w/o/w, SNEDDS) and 5-FU-S to expose to perform the cytotoxicity (%, in vitro) study (Table 8). Opt-MNE1 (w/o/w, SNEDDS) without 5-FU was also used to treated in the same molar concentration as a control. Percentage Cell survival was estimated through addition of 35.0, 75.0, 150.0, 300.0, 600.0 and 1200.0 μM concentration of 5-FU-S and opt-5-FU-MNE1 (w/o/w, SNEDDS) (Figure 9). The highest cytotoxicity of 5-FU-S (i.e. free aqueous-solution) was found to be 57.72 ± 9.08% at 1200.0 μM concentration (Table 8). Though, 5-FU used same concentration in opt-5-FU-MNE1 (w/o/w, SNEDDS) exhibited 95.62 ± 1.64% cytotoxicity that have been found highly significant than 5-FU-S (p <0.050). The smallest concentration (35.50 μM) of 5-FU in Opt-MNE1 given results 72.08 ± 5.81% cytotoxicity as compared to 46.36 ± 6.31% cytotoxicity of 5-FU-S (Table 8). Overall, 5-FU in the form of opt-5-FU-MNE1 (w/o/w, SNEDDS) showed highly potent and efficacious than in 5-FU-S (free form) on colon cancer cell lines which signified the ability of opt-5-FU-MNE1 (w/o/w, SNEDDS) for the chemoprevention of colon cancer. The viability of cells for the colon cancer cells treated with free form of 5-FU-S showed 42.28% at 1200.0 μM concentration (Figure 9).
Table 8.
IC50 and % Cytotoxicity of 5-FU-S, opt-5-FU-MNE, and control on colon cancer cell lines (n = 3).
| Cell Death (% ±SD) |
IC50 | |||||
|---|---|---|---|---|---|---|
| Concentration (µM) | 5-FU-S | 5-FU-MNE | Control (MNE) | 5-FU-S | 5-FU-MNE | Control (MNE without 5-FU) |
| 0.00 | 0.00 | 0.00 | 0.00 | |||
| 35.50 | 46.36 ± 6.31 | 72.08 ± 5.81 | 0.18 ± 0.01 | |||
| 75.00 | 48.72 ± 8.93 | 74.68 ± 6.46 | 1.75 ± 0.08 | |||
| 150.00 | 52.06 ± 3.21 | 78.57 ± 4.59 | 4.57 ± 1.03 | 100.0 | 19.9 | – |
| 300.00 | 53.74 ± 2.12 | 79.83 ± 5.64 | 8.45 ± 1.98 | |||
| 600.00 | 54.69 ± 5.64 | 91.62 ± 1.89 | 12.54 ± 2.14 | |||
| 1200.00 | 57.72 ± 9.08 | 95.62 ± 1.64 | 14.54 ± 2.39 | |||
The concentration at which 50% cell death (IC50)
Fig. 9.
%age-Cell viability (n = 3, SD, mean) of 5-FU in control, 5-FU aqueous-free-solution, and optimized-5-FU-NE nanoformulation on human colon (HT-29) cancer cell lines. Opt-5-FU-MNE (w/o/w, SNEDDS) (p < 0.01) were highly significant than 5-FU-S (without formulation).
4. Discussion
A UHPLC-MS/MS based 5-FU-bioanalytical method is also developed in current research which gives many useful applications efficient, sensitive (upto pg), <3.0 min run time with sharp RT, economic as well as very competent for the calculations of PK parameters in rats with well-defined findings. 5-FU was delivered orally to increase the intestinal membrane permeability and their bioavailability with the help of self-nanoemulsifying system. 5-FU is a hydrophilic drug i.e. very hard task to deliver orally but we optimized-SNEDDS (w/o) in which oil as an external phase produces phase separation or rapid precipitation of 5-FU i.e. encapsulated inside water phase can take place after contact to GI-fluids (Shakeel et al., 2014, Sigward et al., 2013). To solve this difficulty, we were taken on a multiple-self-nanoemulsifying-system i.e. w/o/w in which 1°-w/o-5-FU-NE of water 5-FU-solution was dispersed in the oil droplets. After that 1°-w/o-5-FU-NE has been easily dispersed in the outer-water phase for example GI-fluids via a 2°surfactant. Current research, Labrasol and Cremophor EL is used as surfactants containing maximum-HLB-value i.e. 14. Both surfactants were reduced interfacial energy which is essential for the preparation of NE in which they are not much influenced through pH and alteration of ionic strength in the GI-tract (Sigward et al., 2013). Though, Cremophor EL was shown to play a role in hypersensitivity reactions together with neurotoxicity, nephrotoxicity, and cardiotoxicity linked with i.v. delivery of paclitaxel (Gelderblom et al., 2001, Kiss et al., 2013, Pangeni et al., 2016). Transcutol HP was also employed in w/o part as a surfactant and w/o/w part as co-surfactant for the preparation of NE which also decreases interfacial tension and development of mechanical barriers to coalescence. Thus, it was observed that surfactant and co-surfactant adsorbed at the interface to decrease the interfacial energy and offering a mechanical barrier to coalescence (Pangeni et al., 2016). It is very important to optimize the Smix ratio for the oral delivery of NE to enhance the entropy of dispersion and area of interfacial with decrement of interfacial-tension followed by free energy of the system for the thermodynamically stable system. It is also very important to play a role for oil-phase-to-Smix-ratio at the time of development of NE in which surfactants were also reduces interfacial-tension and decrement of globule-size (Gelderblom et al., 2001, Kiss et al., 2013, Pangeni et al., 2016). In this way, it reduces the globule-size from 153.23 to 51.64 nm with enhance in more surfactant come to closer at the site of adsorption & the construction of a extra closely-packed-surfactant film on the position of interface between to oil & water resulted a strongest stabilization. On the opposite side, the globule-size was enhanced through enhancing co-surfactant concentration. Globule-size was increased may be due to presence of co-surfactant which spreading the interfacial film (Shakeel et al., 2014, Pangeni et al., 2016).
Cremophor EL is also worked as an enhancer via freeing from tight junction and enhancing cell membrane fluidity (Udata et al., 2003, Buyukozturk et al., 2010, Lu et al., 2012). Thus, a surfactant reduced the membrane integrity which is very helpful to support for the permeation of 5-FU in the membrane in another way we can say a hydrophilic very tiny molecule has gone through a paracellular pathway. Cremophor EL was published earlier that bind to hydrophobic-part of P-gp which gives the confirmation alter and directing to decreased drug efflux (Shakeel et al., 2014, Pangeni et al., 2016). Oral absorption was enhanced due to the great protection from P-gp-mediated 5-FU efflux.
Opt-5-FU-MNE1 (w/o/w, SNEDDS) exhibited a significantly inhibited the growth of same cell lines. Opt-5-FU-MNE1 (w/o/w, SNEDDS) treated cells showed the cell viability (4.38%) at 1200.0 μM which is highly significant than 5-FU-S (p < 0.05). Opt-5-FU-MNE1 (w/o/w, SNEDDS) showed highest cell viability (27.92%) as compared to 5-FU-S (freely available i.e. 53.64%) at 35.50 μM. We have observed that opt-5-FU-MNE1 (w/o/w, SNEDDS) showed very low viability of cells at all levels of concentrations which are a clear indication of superiority of opt-5-FU-MNE1 (w/o/w, SNEDDS) as compared to 5-FU-S. In an opposite way, opt-MNE1 (without 5-FU, w/o/w, SNEDDS) didn’t inhibit more cell growth to the exposure of HT-29 cells (Figure 9). It means a clear indication of opt-MNE1 (without 5-FU, w/o/w, SNEDDS) inhibit negligibly cell growth to the exposure of HT-29 cells. The cell death (50.0% i.e. IC50) was also calculated from concentration dependent cell viability curves for opt-5-FU-MNE1 (w/o/w, SNEDDS) and 5-FU-S (Figure 9). 100.0 μM is a concentration for IC50 value of 5-FU-S. On the other hand, opt-5-FU-MNE1 (w/o/w, SNEDDS) showed very low IC50 value of 5-FU as compare to 5-FU-S. On basis of IC50 values findings, the 5-FU of opt-5-FU-MNE1 (w/o/w, SNEDDS) was calculated 5.0 times more potent and effective than 5-FU-S therefore, we reduced the dose of 5-FU and to avoid the adverse effects of 5-FU at the time delivery of opt-5-FU-MNE1 (w/o/w, SNEDDS) orally (i.e. 10 mg/kg/body-weight). Finally, it was concluded that opt-5-FU-MNE1 (w/o/w, SNEDDS) will be successfully used orally for chemoprevention of colon cancer after the clinical studies.
5. Conclusion
We selected w/o 1°NE as oil phase, maximum 5-FU-release and their highest permeation, smallest PDI with smallest globule-size & viscosity, and optimized concentration of surfactant and cosurfactant was prepared and optimized-5-FU-MNE1 (w/o/w, SNEDDS) in which it contains (w/o 1°NE : 10.0% w/w); 12.5% w/w Cremophor-EL as a surfactant, 12.5% w/w Transcutol-HP as a co-surfactant, and 65.0% w/w Milli-Q-water. Opt-5-FU-MNE1 (w/o/w, SNEDDS) was calculated 5.0 times more potent and effective than 5-FU-S based on in vitro cytotoxicity studies. Based on these findings, opt-5-FU-MNE1 (w/o/w, SNEDDS) is a better vhicle for oral delivery of hydrophilic anticancer 5-FU as a drug for the prevention of colorectal cancer. A UPLC-MS/MS method was developed, validated and used successfully for PK-studies of opt-5-FU-MNE1. An orally administered nanoemulsion containing 5-FU showed 4.5 times enhanced in bioavailability as compared to 5-FU-S and enhanced pharmacokinetic effect. Furthermore, 5-FU was enhanced successfully in the GIT-absorption by oral delivery of multiple-nanoemulsion via PK-study on wistar-rats. Hopefully, our opt-5-FU-MNE1 (w/o/w, SNEDDS) will be applied for an oral delivery of 5-FU. It will be a great achievement of patient compliance via as better substitute of conventional IV-infusion and also a great application of treatment of cancer-patients with prophylactically-use in future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are very grateful thanks to Animal House, IRMC, Imam Abdulrahman Bin Faisal University, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad M.Z., Akhter S., Anwar M., Ahmad F.J. Assam Bora rice starch based biocompatible mucoadhesive microsphere for targeted delivery of 5-fluorouracil in colorectal cancer. Mol. Pharm. 2012;9(11):2986–2994. doi: 10.1021/mp300289y. [DOI] [PubMed] [Google Scholar]
- Ahmad N., Ahmad R., Alam M.A., Ahmad F.J. Enhancement of oral bioavailability of doxorubicin through surface modified biodegradable polymeric nanoparticles. Chem. Cent. J. 2018;12(1):65. doi: 10.1186/s13065-018-0434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Niyaz, Ahmad Rizwan, Al-Qudaihi Ali, Alaseel Salman Edrees, Fita Ibrahim Zuhair, Khalid Mohammed Saifuddin, Pottoo Faheem Hyder. Preparation of a novel curcumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation. RSC Advances. 2019;9(35):20192–20206. doi: 10.1039/C9RA03102B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N., Ahmad R., Buheazaha T.M., AlHomoud H.S., Al-Nasif H.A., Sarafroz M. A comparative ex vivo permeation evaluation of a novel 5-Fluorocuracil nanoemulsion-gel by topically applied in the different excised rat, goat, and cow skin. Saudi J. Biol. Sci. 2020;27(4):1024–1040. doi: 10.1016/j.sjbs.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N., Ahmad R., Naqvi A.A., Alam M.A., Rub R.A., Ahmad F.J. Enhancement of Quercetin Oral Bioavailability by Self-Nanoemulsifying Drug Delivery System and their Quantification Through Ultra High-Performance Liquid Chromatography and Mass Spectrometry in Cerebral Ischemia. Drug Res (Stuttg). 2017;67(10):564–575. doi: 10.1055/s-0043-109564. [DOI] [PubMed] [Google Scholar]
- Ahmad Niyaz, Alam Md Aftab, Ahmad Farhan Jalees, Sarafroz Md, Ansari Khalid, Sharma Sonali, Amir Mohd. Ultrasonication techniques used for the preparation of novel Eugenol-Nanoemulsion in the treatment of wounds healings and anti-inflammatory. Journal of Drug Delivery Science and Technology. 2018;46:461–473. [Google Scholar]
- Alanazi F.K., Haq N., Radwan A.A., Alsarra I.A., Shakeel F. Formulation and evaluation of cholesterol-rich nanoemulsion (LDE) for drug delivery potential of cholesteryl-maleoyl-5-fluorouracil. Pharm. Dev. Technol. 2015;20(3):266–270. doi: 10.3109/10837450.2013.860551. [DOI] [PubMed] [Google Scholar]
- Arias J.L. Novel strategies to improve the anticancer action of 5-fluorouracil by using drug delivery systems. Molecules. 2008;13(10):2340–2369. doi: 10.3390/molecules13102340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avadi M.R., Sadeghi A.M.M., Mohamadpour Dounighi Naser, Dinarvand R., Atyabi F., Rafiee-Tehrani M. Ex vivo evaluation of insulin nanoparticles using chitosan and arabic gum. ISRN Pharm. 2011;2011:1–6. doi: 10.5402/2011/860109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobin-Dubigeon C., Amiand M., Percheron C., Audeval C., Rochard S., Leynia P., Bard J.M. A new, validated wipe-sampling procedure coupled to LC-MS analysis for the simultaneous determination of 5-fluorouracil, doxorubicin and cyclophosphamide in surface contamination. J. Anal. Toxicol. 2013;37(7):433–439. doi: 10.1093/jat/bkt045. [DOI] [PubMed] [Google Scholar]
- Boyle P., Ferlay J. Mortality and survival in breast and colorectal cancer. Nat. Clin. Pract. Oncol. 2005;2(9):424–425. doi: 10.1038/ncponc0288. [DOI] [PubMed] [Google Scholar]
- Boyle P., Langman J.S. ABC of colorectal cancer: Epidemiology. BMJ. 2000;321(7264):805–808. doi: 10.1136/bmj.321.7264.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel Barbara, Rhyn Peter, Schürch Stefan, Bühr Claudia, Amstutz Ursula, R. Largiadèr Carlo. LC-MS/MS method for simultaneous analysis of uracil, 5,6-dihydrouracil, 5-fluorouracil and 5-fluoro-5,6-dihydrouracil in human plasma for therapeutic drug monitoring and toxicity prediction in cancer patients. Biomed. Chromatogr. 2013;27(1):7–16. doi: 10.1002/bmc.2741. [DOI] [PubMed] [Google Scholar]
- Buyukozturk F., Benneyan J.C., Carrier R.L. Impact of emulsion-based drug delivery systems on intestinal permeability and drug release kinetics. J. Control Release. 2010;142(1):22–30. doi: 10.1016/j.jconrel.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Chen J., Zhou M. Determination of eniluracil and 5-fluorouracil in human plasma by LC-MS/MS. Bioanalysis. 2010;2(12):2011–2017. doi: 10.4155/bio.10.152. [DOI] [PubMed] [Google Scholar]
- Cosco D., Paolino D., Muzzalupo R., Celia C., Citraro R., Caponio D., Picci N., Fresta M. Novel PEG-coated niosomes based on bola-surfactant as drug carriers for 5-fluorouracil. Biomed. Microdevices. 2009;11(5):1115–1125. doi: 10.1007/s10544-009-9328-2. [DOI] [PubMed] [Google Scholar]
- Dong Zhikui, Zheng Wenyi, Xu Zaiyang, Yin Zongning. Improved stability and tumor targeting of 5-fluorouracil by conjugation with hyaluronan. J. Appl. Polym. Sci. 2013;130(2):927–932. doi: 10.1002/app.39247. [DOI] [Google Scholar]
- Ferlay J., Bray F., Pisani P., Parkin D.M. International Agency for Research on Cancer; Lyon: 2004. GLOBOCAN 2002: Cancer incidence, mortality and prevalence worldwide. [Google Scholar]
- Ganti V., Walker E.A., Nagar S. Pharmacokinetic application of a bio-analytical LC-MS method developed for 5-fluorouracil and methotrexate in mouse plasma, brain and urine. Biomed Chromatogr. 2013;27(8):994–1002. doi: 10.1002/bmc.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garti N. Double emulsions: scope, limitations and new achievements. Colloids Surf A. 1997;123:233–246. doi: 10.1016/S0927-7757(96)03809-5. [DOI] [Google Scholar]
- Gelderblom H., Verweij J., Nooter K., Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer. 2001;37(13):1590–1598. doi: 10.1016/S0959-8049(01)00171-X. [DOI] [PubMed] [Google Scholar]
- Gupta Reeta R., Jain Swantrant K., Varshney Manoj. AOT water-in-oil microemulsions as a penetration enhancer in transdermal drug delivery of 5-fluorouracil. Colloids and Surfaces B: Biointerfaces. 2005;41(1):25–32. doi: 10.1016/j.colsurfb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Hanson J.A., Chang C.B., Graves S.M., Li Z., Mason T.G., Deming T.J. Nanoscale double emulsions stabilized by single-component block copolypeptides. Nature. 2008;455(7209):85–88. doi: 10.1038/nature07197. [DOI] [PubMed] [Google Scholar]
- Kiss Lóránd, Walter Fruzsina R., Bocsik Alexandra, Veszelka Szilvia, Ózsvári Béla, Puskás László G., Szabó-Révész Piroska, Deli Mária A. Kinetic analysis of the toxicity of pharmaceutical excipients Cremophor EL and RH40 on endothelial and epithelial cells. J. Pharm. Sci. 2013;102(4):1173–1181. doi: 10.1002/jps.23458. [DOI] [PubMed] [Google Scholar]
- Li S., Wang A., Jiang W., Guan Z. Pharmacokinetic characteristics and anticancer effects of 5-fluorouracil loaded nanoparticles. BMC Cancer. 2008;8:103. doi: 10.1186/1471-2407-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licea-Perez H., Wang S., Bowen C. Development of a sensitive and selective LC-MS/MS method for the determination of alpha-fluoro-beta-alanine, 5-fluorouracil and capecitabine in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009;877(11–12):1040–1046. doi: 10.1016/j.jchromb.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Lin Y., Li Y., Ooi C.P. 5-Fluorouracil encapsulated HA/PLGA composite microspheres for cancer therapy. J. Mater. Sci. Mater. Med. 2012;23:2453–2460. doi: 10.1007/s10856-012-4723-2. [DOI] [PubMed] [Google Scholar]
- Liu K., Zhong D., Zou H., Chen X. Determination of tegafur, 5-fluorouracil, gimeracil and oxonic acid in human plasma using liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2010;52(4):550–556. doi: 10.1016/j.jpba.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Longley D.B., Harkin D.P., Johnston P.G. Reviews and comments from the nature publishing group. Nat. Rev. Cancer. 2003;3:330. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- Loscertales I.G., Barrero A., Guerrero I., Cortijo R., Marquez M., Ganan-Calvo A.N. Micro/nano encapsulation via electrifies coaxial liquid jets. Science. 2002;295:1695–1698. doi: 10.1126/science.1067595. [DOI] [PubMed] [Google Scholar]
- Lu Y., Qi J., Wu W. Absorption, disposition and pharmacokinetics of nanoemulsions. Curr Drug Metab. 2012;13(4):396–417. doi: 10.2174/138920012800166544. [DOI] [PubMed] [Google Scholar]
- Pangeni R., Choi S.W., Jeon O.C., Byun Y., Park J.W. Multiple nanoemulsion system for an oral combinational delivery of oxaliplatin and 5-fluorouracil: preparation and in vivo evaluation. Int. J. Nanomedicine. 2016;11:6379–6399. doi: 10.2147/IJN.S121114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Peer C.J., McManus T.J., Hurwitz H.I., Petros W.P. Development and utilization of a combined LC-UV and LC-MS/MS method for the simultaneous analysis of tegafur and 5-fluorouracil in human plasma to support a phase I clinical study of oral UFT®/leucovorin. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012;898:32–37. doi: 10.1016/j.jchromb.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Presant C.A., Jacobson J., Wolf W., et al. Does leucovorin alter the intratumoral pharmacokinetics of 5-fluorouracil? A Southwest oncology group study. Invest. New Drugs. 2002;20:369–376. doi: 10.1023/a:1020651311866. [DOI] [PubMed] [Google Scholar]
- Remaud G., Boisdron-Celle M., Morel A., Gamelin A. Sensitive MS/MS-liquid chromatography assay for simultaneous determination of tegafur, 5-fluorouracil and 5-fluorodihydrouracil in plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;824(1–2):153–160. doi: 10.1016/j.jchromb.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Ruan L.P., Chen S., Yu B.Y., Zhu D.N., Cordell G.A., Qiu S.X. Prediction of human absorption of natural compounds by the non-everted rat intestinal sac model. Eur. J. Med. Chem. 2006;41(5):605–610. doi: 10.1016/j.ejmech.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Schmidts T., Dobler D., von den Hoff S., Schlupp P., Garn H., Runkel F. Protective effect of drug delivery systems against the enzymatic degradation of dermally applied DNAzyme. Int. J. Pharm. 2011;410(1–2):75–82. doi: 10.1016/j.ijpharm.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Schmidts T., Marquardt K., Schlupp P., Dobler D., Heinz F., Mäder U., Garn H., Renz H., Zeitvogel J., Werfel T., Runkel F. Development of drug delivery systems for the dermal application of therapeutic DNAzymes. Int. J. Pharm. 2012;431(1–2):61–69. doi: 10.1016/j.ijpharm.2012.04.034. [DOI] [PubMed] [Google Scholar]
- Schwarz J.C., Klang V., Karall S., Mahrhauser D., Resch G.P., Valenta C. Optimisation of multiple W/O/W nanoemulsions for dermal delivery of aciclovir. Int. J. Pharm. 2012;435(1):69–75. doi: 10.1016/j.ijpharm.2011.11.038. [DOI] [PubMed] [Google Scholar]
- Shah N., Shah T., Amin A. Polysaccharides: a targeting strategy for colonic drug delivery. Expert Opin. Drug Deliv. 2011;8(6):779–796. doi: 10.1517/17425247.2011.574121. [DOI] [PubMed] [Google Scholar]
- Shakeel F., Haq N., Al-Dhfyan A., Alanazi F.K., Alsarra I.A. Double w/o/w nanoemulsion of 5-fluorouracil for self-nanoemulsifying drug delivery system. J. Mol. Liq. 2014;200:183–190. doi: 10.2147/IJN.S121114. [DOI] [Google Scholar]
- Shakeel F., Haq N., Elbadry M., Alanazi F.K., Alsarra I.A. Ultra fine super selfnanoemulsifying drug delivery system (SNEDDS) enhanced solubility and dissolution of indomethacin. J. Mol. Liq. 2013;180:89–94. doi: 10.1016/j.molliq.2013.01.008. [DOI] [Google Scholar]
- Shanmugam S., Baskaran R., Balakrishnan P., Thapa P., Yong C.S., Yoo B.K. Solid selfnanoemulsifying drug delivery system (S-SNEDDS) containing phosphatidycholine for enhanced bioavailability of highly lipophilic bioactive carotenoid lutein. Eur. J. Pharm. Biopharm. 2011;79:250–257. doi: 10.1016/j.ejpb.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Shishu, Kamalpreet, Maheshwari Manjul. Development and evaluation of novel microemulsion based oral formulations of 5-fluorouracil using non-everted rat intestine sac model. Drug Dev. Ind. Pharm. 2012;38(3):294–300. doi: 10.3109/03639045.2011.602407. [DOI] [PubMed] [Google Scholar]
- Sigward E., Mignet N., Rat P., Dutot M., Muhamed S., Guigner J.M., Scherman D., Brossard D., Crauste-Manciet S. Formulation and cytotoxicity evaluation of new self-emulsifying multiple W/O/W nanoemulsions. Int. J. Nanomedicine. 2013;8:611–625. doi: 10.2147/IJN.S35661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A.M., Kapanen A.I., Hare J.I., Ramsay E., Edwards K., Karlsson G., Bally M.B. Development of a liposomal nanoparticle formulation of 5-fluorouracil for parenteral administration: formulation design, pharmacokinetics and efficacy. J. Control Release. 2011;150(2):212–219. doi: 10.1016/j.jconrel.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Thomas N., Holm R., Müllertz A., Rades T. In vitro and in vivo performance of novel super saturated self-nanoemulsifying drug delivery systems (super-SNEDDS) J. Control. Release. 2012;160(1):25–32. doi: 10.1016/j.jconrel.2012.02.027. [DOI] [PubMed] [Google Scholar]
- Udata C., Patel J., Pal D., Hejchman E., Cushman M., Mitra A.K. Enhanced transport of a novel anti-HIV agent – cosalane and its congeners across human intestinal epithelial (Caco-2) cell monolayers. Int. J. Pharm. 2003;250(1):157–168. doi: 10.1016/s0378-5173(02)00523-9. [DOI] [PubMed] [Google Scholar]
- US FDA. Guidance for Industry Bioanalytical Method Validation; 2001. Available from:http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf. [Last accessed on 2018 May 24].
- Vainchtein L.D., Rosing H., Schellens J.H., Beijnen J.H. A new, validated HPLC-MS/MS method for the simultaneous determination of the anti-cancer agent capecitabine and its metabolites: 5'-deoxy-5-fluorocytidine, 5'-deoxy-5-fluorouridine, 5-fluorouracil and 5-fluorodihydrouracil, in human plasma. Biomed. Chromatogr. 2010;24(4):374–386. doi: 10.1002/bmc.1302. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund and American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research; 2007.
- World Health Organization. Cancer Incidence in Five Continents. Lyon: The World Health Organization and The International Agency for Research on Cancer; 2002.
- Yanyu Xiao, Fang Liu, Qineng Ping, Hao Cai. The influence of the structure and the composition of water/AOT-Tween 85/IPM microemulsion system on transdermal delivery of 5-fluorouracil. Drug Dev. Ind. Pharm. 2012;38(12):1521–1529. doi: 10.3109/03639045.2012.654795. [DOI] [PubMed] [Google Scholar]
- Yassin A.E., Anwer M.K., Mowafy H.A., El-Bagory I.M., Bayomi M.A., Alsarra I.A. Optimization of 5-flurouracil solid-lipid nanoparticles: a preliminary study to treat colon cancer. Int. J. Med. Sci. 2010;7(6):398–408. doi: 10.7150/ijms.7.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssof A.M.E., Alanazi F.K., Salem-Bekhit M.M., Shakeel F., Haq N. Bacterial Ghosts Carrying 5-Fluorouracil: A Novel Biological Carrier for Targeting Colorectal Cancer. AAPS PharmSciTech. 2019;20(2):20–48. doi: 10.1208/s12249-018-1249-z. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wo Y., Zhang Y., Wang D., He R., Chen H., Cui D. In vitro study of ethosome penetration in human skin and hypertrophic scar tissue. Nanomedicine. 2012;8(6):1026–1033. doi: 10.1016/j.nano.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhang J., Wang Q., Li J., Han B. Water-in-oil-in-water double nanoemulsion induced by CO2. Phys Chem Chem Phys. 2011;13(2):684–689. doi: 10.1039/C0CP00869A. [DOI] [PubMed] [Google Scholar]