Graphical abstract
The graphical abstract is summarized in
This graphical abstract reveals the time scale and the experimental approach of the current study. The rats were let to acclimatize for one week and following that they were assigned into 3 groups: control, and two experimental groups (groups 2 & 3). The rats in group 2 were sacrificed at day 14 whilst groups 1 and 3 were sacrificed on day 28. Follwing sacrifice the liver tissue was removed for histopathology, biochemical and genetic assays.
Keywords: Silver Nanoparticles (Ag NPs), Hydrogen peroxide, Hepatic Bdnf, Hepatotoxicity, Masson's trichrome stains, Ultraviolet–Visible Spectroscopy, Transmission Electron Microscopy, Liver enzymes
Abbreviations: Ag NPs, Silver Nanoparticles; ALT, Alanine Transaminase; AST, Aspartate Transaminase; Bdnf, Brain-derived neurotrophic factor; H&E, Hematoxylin & Eosin stain; H2o2, Hydrogen Peroxide; HCC, Hepatocellular Carcinoma; NaBH4, Sodium Borohydride; Nanoparticles, (NPs); TRI, Masson's trichrome stains; ppm, Parts Per Million; PAS, Periodic acid-Schiff; PVP, Polyvinyl Pyrrolidone; RNA, Ribonucleic acid; ROS, Reactive Oxygen Species; SGOT, Serum Glutamate oxaloacetate Transaminase; SGPT, Serum Glutamate pyruvate Transaminase; TEM, Transmission Electron Microscopy; UV–Vis, Ultraviolet–Visible Spectroscopy
Abstract
Products containing Silver nanoparticles (Ag NPs) are becoming vastly used in our daily life. The widespread increased introduction of Ag NPs in many aspects of life has raised researchers' concerns regarding their safety and toxicity for biological and environmental life in the past few years. The current study aimed to explore the subsequent effects of Ag NPs withdrawal, following short-term oral administration. Eighteen rats were assigned randomly into three groups (control group "1" and AG NPs treated groups "2" and "3"; 6 animals each). The control group received normal food and tap water while groups 2 & 3 received 0.5 ml of a solution containing 25 ppm Ag NPs for 14 days. Group 2 rats were sacrificed on day 14 whereas group 3 was left for another 14 days of particle cessation followed by euthanasia on day 28. Functional assessment was done by liver enzyme assays, hydrogen peroxide activity, hepatic Bdnf expression, and P53 immunoreactivity. Hepatic tissue structural assessment was done via hematoxylin and eosin, periodic acid-Schiff as well as Masson's trichrome stains. The results revealed a significant elevation of Hydrogen peroxide in group 2 only compared to the control group. Hepatic Bdnf and liver enzymes were both insignificantly affected. Structural abnormalities and enhanced apoptosis in hepatic tissue were found 14 days after ceasing the nanoparticles. In conclusion: Structural and functional insults following Ag NPs oral administration continues after particle withdrawal, and interestingly they do not necessitate apparent reflection on liver enzyme assays.
1. Introduction
Nanotechnology breakthroughs and the invention of nanoparticles (NPs), notably silver nanoparticles (Ag NPs), have made significant contributions to health, drug research, food processing, and agriculture (Yildirimer et al., 2011). Nanoparticles are microscopic particles with a diameter of <100 nm. Nanoparticles are more dangerous than particles of a different size. Ag NPs of various sizes can be consumed directly through food, water, cosmetics, and pharmaceuticals (Sardari et al., 2012). In the field of medicine, Ag NPs are used due to their antibacterial, antiviral, and antifungal properties. It is also used as a disinfectant in swimming pools and drinking water (El Mahdy et al., 2014). Furthermore, it was observed in the literature that oral administration of Ag NPs prepared solution as a treatment raised the risk of argyria (Griffith et al., 2015). Argyria is a benign health condition related to the ingestion of Ag which leads to blue-grayish skin discoloration through the preferential deposition of Ag in the “basal lamina” of different internal soft tissues as the spleen, blood vessels, liver, gastrointestinal tract, and kidney (Griffith et al., 2015;Flores-López et al., 2019).
The hazardous effect of Ag NPs on humans has recently gotten a lot of attention, notably in airborne occupational exposures during nanomaterial manufacturing. Ag NPs' tiny size enables their transfer from natural barriers such as the lungs, gastrointestinal system, or skin, resulting in acute and chronic harmful consequences (Bakand and Hayes, 2016). Therefore, the widespread increased introduction of Ag NPs in many aspects of life has raised researchers' concerns regarding their safety and toxicity for biological and environmental life in the past few years. Consequently, this necessitates a thorough investigation of their dramatically variable interactions at the molecular levels (Akter et al., 2018; Gupta and Xie, 2018). Ag NPs toxicity was proven by the existing data on possible human adverse health consequences. AgNPs release silver ions in the presence of water, which cause considerable change in oxidative and immunity responses, as well as a multitude of harmful effects on cells and genes(Akter et al., 2018, Chernousova and Epple, 2013; Cho et al., 2013; Deckers et al., 2008). The form, size, concentration, dose, route of entry into the body, and duration of exposure of Ag NPs, as well as the presence of the aggregated state, are all aspects that can influence Ag NPs toxicity (Ajdary et al., 2018, Mao et al., 2018). Therefore, characterization is essential for determining the properties of nanoparticles under investigation (Zhang et al., 2016).
The consumption of various-sized particles through the gastrointestinal tract may have various toxicological implications. However, research on the toxicological effects of nanoparticles on the gastrointestinal tract is lacking. The spleen and liver have previously been identified as key target organs after intravenous injection of several nanomaterials. This is because these organs are part of the reticuloendothelial system (RES), which is responsible for removing foreign substances from the circulatory system (De Jong et al., 2013). Therefore, a more thorough examination of Ag NP's ability to infiltrate the liver is required. However, a rat liver cell line research proved that 25 ppm of Ag NPs was the most toxic concentration (Akter et al., 2018).
Previous researches suggest that the cytotoxicity effect of Ag NPs is due to the initiation of oxidative stress (Flores-López et al., 2019; Kim et al., 2009, Manke et al., 2013; Rahman et al., 2009). Reactive oxygen species (ROS) are normally produced in cellular reactions. However, when their production rate exceeds the natural cellular antioxidant defense mechanisms, they can lead to an increase in the process of lipid peroxidation, mitochondrial damage, and apoptosis (Flores-López et al., 2019). The enhanced ROS activity in response to Ag NPs leads to production of hydrogen peroxide (H2o2) (Patlolla et al., 2015). Therefore, this can provoke the intrinsic apoptotic pathway in the mitochondria via the production of H2o2 (Lorenzo et al., 2009, Manke et al., 2013). Brain-derived neurotrophic factor (BDNF) is a neurotrophic factor to help neurons differentiate, develop, and survive (Bus et al., 2011). The function of BDNF in regulating apoptosis in the central nervous system has been studied (Patlolla et al., 2015). BDNF has been detected in numerous tissues such as adipose tissue, skeletal muscle, kidneys, liver and prostate (Lorenzo et al., 2009). Other peripheral tissues were investigated for BDNF and its receptors after tissue damage (Shu et al., 2019). Due to the extensive variance in the morphological and physicochemical characteristics of Ag NPs, which affect their interactions with the body's biological systems, there are significant differences in the findings of conducted investigations (Akter et al., 2018).
Although researches are confirming the toxic effects of Ag NPs in vivo, only a few studies have looked into the persistent hazardous effects of Ag NPs following their withdrawal (Recordati, 2016). Therefore, in the current study, we are focusing on the subacute effects of Ag NPs subacute oral administration and the persistence of harmful effects following the stoppage of Ag NPs oral administration. The oral method was chosen to mimic Ag NP consumption in everyday life such as in the water and food industry and due to the sacristy of researches that used the oral route. The administration of a small dose was conducted to examine the effect of continued daily exposure and how much harm it would cause when it was withdrawn.
2. Methods
2.1. Animals
Healthy adult male Albino Wistar rats (n = 18; 3 groups, 6 rats each; age: 8 ± 1 weeks; 220–225 g) were purchased (Source: The Ophthalmic Research Institute in Giza, Egypt). Female rats were not included in this investigation. This is attributed to the erratic nature of female data resulting from hormonal changes during the female reproductive cycle. Rats were housed in plastic cages in a temperature and humidity-controlled setting (25 2 °C, 55 percent humidity, and 12 h light/dark cycles). The animals were fed a conventional chow diet (Al-Gomhoureya Co.) that had a carbohydrate content of 48.8%, a protein content of 21%, and a fat content of 3%. Rats were acclimatized for a week prior to the beginning of the experiment. This experimental study was conducted in the animal house of the college of Medicine, Ismailia, Egypt (2020). All experimental procedures were performed according to the Declaration of Helsinki and were approved by the Research Ethics Committee of the Faculty of Medicine, Suez Canal University research number (4234–2020).
2.2. Materials
The nanoparticles were prepared in the Center of Excellence, Faculty of Medicine, Suez Canal University by reduction technique as per standard protocol (Sigma Aldrich Co., Saint Louis, MO 63103, USA). 30 ml of 0.002 M of sodium borohydride (NaBH4) was added to an Erlenmeyer flask. The solution was freshly prepared. The solution was prepared by a chemical reduction technique using sodium borohydride (NaBH4). Polyvinyl pyrrolidone (PVP) was used to prevent agglomeration of Ag NPs as per previously published protocols (El Mahdy et al., 2015; Wang et al., 2005; Zhang and Liu, 2020). Particles were prepared under optimal physiochemical circumstances including temperature and speed of stirring, according to the standard protocol. A magnetic stir bar was used with the placement of an ice bath on the stir plate. Stirring on ice was to decrease the rate of decomposition during the preparation. 0.07gm of PVP was weighed and added to borohydrate solution. 2 ml of 0.001 silver nitrate (AgNO3) was dropped into the stirring NaBH4 solution (1 drop/ sec). After adding all the AgNO3 stirring was stopped. The resultant solution has concentration of 200 ppm of Ag NP was diluted with distilledwater until a concentration of 25 ppm was obtained (1 ml of Ag NP solution to 7 ml of distilled water).
Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), and Hydrogen peroxide (H2O2) kits were obtained (Biodiagnostics Co.; Cairo, Egypt).
2.3. Characterization of silver nanoparticles
2.3.1. Transmission electron microscopic (TEM) analysis
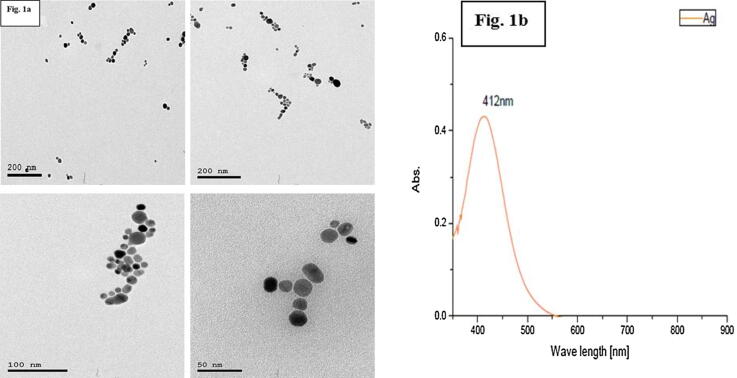
Characterization of Ag NPs was done via TEM in a specialized laboratory, Egypt. to evaluate the diameter and morphological properties of Ag NPs. TEM analysis revealed a sphere-shaped Ag NPs particles that showed a limited size distribution range from 12.81 to 23.83 nm (Average: 16.5 nm) as shown in Fig. 1a.
Fig. 1.
a TEM characterization, showing spherical Ag NPs. Fig. 1b: UV Vis reveals no stable aggregates and homogeneity in the size distribution of Ag NPs.
2.3.2. Ultraviolet–Visible (UV–Vis) Spectroscopy measurements analysis
The (UV–Vis) analysis was utilized to evaluate the diameter and morphological properties of Ag NPs. The peak absorbance wavelength was at 412 nm and the particles were aggregated typically in the wavelength range of 600–800. In the absorbance wavelength 400–500, there were absence of stable particle aggregation (Cho et al., 2018). The limited distribution of the wavelength demonstrates the homogeneity of nanoparticle size; Fig. 1b.
2.4. Experiment design
At the beginning of the experiment, 18 rats were assigned randomly into three groups (n = 6/group). The groups were labeled as control group (group 1): 0.5 ml saline via oral gavage, Ag NPs groups (Group 2 & group 3): Ag NPs solution (0.5 ml of a solution containing 25 ppm Ag NPs (equal to a dose of 5 µg/kg body weight of silver). Oral gavage was given on a single daily basis. To avoid unnecessary harm or stress to the rats, oral gavage was performed using metal needles for feeding (Gauge: 16G; length: 3 in., curved) by a qualified expert (Yousof et al., 2020). On day 14, Ag NPs were stopped for both the Ag Nps groups (groups 2 & 3). To assess the direct hepatotoxic effect of Ag NPs, six animals (group 2) were anesthetized by intraperitoneal injection of thiopental Na (40 mg/kg) (Nikolić et al., 2017) and euthanized by cervical dislocation. Regarding group 3; rats were left under observation for another 14 days on the usual chow diet and free access to water to assess the presence of persistent hepatic damage following the cessation of the Ag NPs. On day 28 the same sacrificing procedures were done with groups 1 and 3. Blood samples were collected in each sacrifice day via a cardiac puncture for further analysis of liver transaminases; alanine transaminase (ALT) & aspartate transaminase (AST). Part of rat liver (10–20 g) was frozen for further homogenization to assess hydrogen peroxide (H2O2) level as an oxidative stress marker. The rest of rat livers were fixed into 10% formalin-fixed, paraffin-embedded blocks for Bdnf gene expression, histopathology, and immunohistochemistry.
2.5. Assessment of liver enzymes
The blood samples were let for clotting (30 min) and then centrifuged at (3000 rpm/15 min). The collected sera were then stored (−20 °C) for further liver enzymes assessment. To determine the level of liver enzymes UV/VIS Spectrophotometer (Unico S2100 Series, USA) were used. ALT and AST were assessed via colorimetric kinetic technique (Murray, 1984a, Murray, 1984b).
2.6. Assessment of hepatic hydrogen peroxide (H2O2)
The liver tissues were homogenized as per the manufacturer's guidelines·H2O2 was determined by colorimetric according to (Aebi, 1984) method via UV/VIS Spectrophotometer (Unico S2100 Series, USA).
2.7. Bdnf gene expression by Real-Time PCR
The RNeasy FFPE Kit (Germany, cat. no. 73504) was used for total RNA extraction from formalin-fixed, paraffin-embedded liver tissue based on the manufacturer's guidelines (Kokkat et al., 2013). The determination of the content and purity of RNA and cDNA was examined through a Nanodrop spectrophotometer (Wilmington, DE, USA). A high-capacity cDNA Reverse Transcriptase Package (USA, cat. no. Archive) and a thermocycler (BIOMETRA®, LA, USA) were used for reverse transcription.
The expression of the Bdnf and B-actin genes were determined using an Applied Biosystems Step One TM Real-Time PCR apparatus. Primer assays specific for genes (willowfort, UK) were engineered and examined utilizing web Bioinformatics tools; Details are provided in the supplemental file in Table Is & Table IIs. Normalization of every target gene was done (endogenous β-actin). The 2−ΔΔCT method was utilized for the relative expression of genes (Livak and Schmittgen, 2001).
2.8. Histopathological & immunohistochemical assessments
Neutral formalin (10%) was used for the fixation of specimens for 24 h at room temperature (23–28 °C) followed by preparation of paraffin sections (5-μm-thick). The obtained sections were stained using Hematoxylin & Eosin stain (H & E) (Clark and Biological Stain Commission, 1981) for a general architecture of the liver, Masson's trichrome stain for collagen fibers (Masson and Masson, 1929), periodic acid-Schiff reaction (PAS) (McMANUS, 1946) for the glycogen content in hepatocyte and immunohistochemical stain for the demonstration of apoptosis (P53, WWW.ABclonal.com . Catalog No.: A5761) (Anzola et al., 2004) in rat hepatocyte. Qualitative and quantitative assessments were done for histopathological and immune-histochemical changes in the liver. Quantitative measurements were done using computer software image J. Quantification of the severity of tissue damage (scoring): Regarding the liver, the severity of changes was quantitated from none (–) to severe (+++) based on the central vein congestion & dilatation; disruption of hepatic arrangement and degenerated & vacuolation of the hepatocyte.
2.9. Data analysis
IBM SPSS statistical software version 23 was applied to examine the data. Mean ± standard deviations were used to express the data, and the means were compared using ANOVA. The post-hoc LSD test was used to identify the group differences if the ANOVA was significant. Graphical abstract was presented using Mind the graph and Microsoft PowerPoint.
3. Results
3.1. Liver enzymes & H2O2 Assays
The results of our study revealed no significant difference between groups regarding the liver transaminases (p value greater than 0.05); reflecting no increase in liver enzymes in both experimental groups (groups 2 & 3). Assessment of hydrogen peroxide showed a statistically significant elevation in group two in comparison to the control group. Meanwhile, there was no statistically significant elevation of H2O2 level in group 3 in comparison to the control. Comparison between groups 2 and 3 revealed no significant differences; Table 1.
Table 1.
Assessment of Liver Transaminases (U/L) and H2O2 (Mm/L) (n = 18, 6/group):
| ALT (Mean ± SD) | AST (Mean ± SD) | H2O2 | |
|---|---|---|---|
| Group 1 | 12.6 ± 2.07 | 13 ± 1.7 | 0.04 ± 0.02 |
| Group 2 | 13.1 ± 1.4 | 14 ± 2.5 | 0.36 ± 0.02* |
| Group 3 | 12.1 ± 1.1 | 12 ± 3.2 | 0.13 ± 0.09 |
* Comparison to control reveals statistical significance (p < 0.05).
The table reveals no increase in liver enzymes in both experimental groups. Assessment of hydrogen peroxide revealed a statistically significant elevation in group 2 compared to control.
3.2. Bdnf gene Expression
Hepatic Bdnf gene expression was calculated as fold change (the control group was considered as one-fold change). Group two showed an insignificant increase in Bdnf gene expression (mean ± SD: 13.05 ± 16.81; P-value: ≥ 0.05). Group 3 in which Ag NPs were stopped for 2 weeks, showed a further but insignificant increase in the Bdnf expression (mean ± SD: 25.24 ± 39.37; P-value: ≥ 0.05).
3.3. Histopathological & immunohistochemistry Results
A qualitative assessment of the histopathological changes in the liver using H & E stain was done. It revealed ballooning of the hepatocyte, vacuolated eosinophilic cytoplasm, and nuclear pyknosis, Fig. 2a, b, c. PAS study showed decreased PAS reaction in the cytoplasm of hepatocyte in groups 2 & 3 compared to control group, Fig. 3a, b, c. Masson Trichrome study revealed, no abnormal deposition of collagen around the central vein, Fig. 4a, b, c. P53 immunostaining pointed to moderate & intense immunoreactivity for P53 in the form of brownish color in groups 2 & 3 when contrasted to the control group, Fig. 5a, b, c.
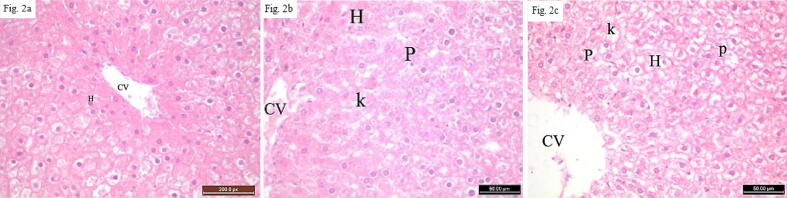
Fig. 2.
Photomicrograph sections in the hepatic tissue of rats using H& E stain.Fig. 2a: A liver tissue section from a control rat showing normal central veins and hepatocyte (H) which are arranged into hepatic cords radiating from the central vein (CV). Fig. 2b: Liver tissue from a rat treated with nanosilver (group 2) showing congested & dilatated central vein (CV) and vacuolated ballooned hepatocytes (H) with karyolytic (K) and pyknotic (P) nuclei. Fig. 2c: Hepatic tissue from a rat in group 3 showing congested & dilatated central vein (CV) and vacuolated ballooned hepatocytes (H) with karyolytic (K) and pyknotic (P) nuclei. [H&E X400].
Fig. 3.
Photomicrograph sections in the hepatic tissue of rats using PAS stain.Fig. 3a: liver tissue section from a control rat showing positive PAS reaction in the cell cytoplasm. Normal accumulations of reddish stained glycogen particles at one pole of the hepatocyte are shown. Fig. 3b: liver from rat administered nanosilver (group 2) revealing decreased PAS reaction in the cytoplasm of hepatocyte more than the control group. Fig. 3c: hepatic tissue section from a rat in group 3 displaying decreased PAS reaction in the cytoplasm of hepatocyte more than group 2. [PAS X400].
Fig. 4.
Photomicrograph sections in the hepatic tissue of rats using MTS stainFig. 4a: liver tissue section from a control rat with minimal greenish collagens around the central vein. Fig. 4b: a section from hepatic tissue from a rat given nanosilver (group 2) with no change in the greenish collagenous fibers surrounding the central vein. Fig. 4c: a section of liver from rat from group 3 displaying no change in the greenish collagenous fibers encircling the central vein [Masson's trichrome stain X 400].
Fig. 5.
Photomicrograph sections in the hepatic tissue of rats using P53 stain 5a: liver tissue section from a control rat displaying mild brownish reaction. Fig. 5b: liver tissue section from a rat given with nanosilver (group 2) revealing moderate immunoreactivity of P53 in the form of brownish color. Fig. 5c: a section of hepatic tissue from a rat from group 3 with intense immunoreactivity of P53 in the form of brownish color [P53 immunostainning × 400].
Scoring of the morphological features as assessed by histopathological examination of the liver are presented in Table 2. Quantitative measurements included the optical density of collagen fibers, PAS positive material of the hepatocyte collagen content, and P53 immunoreactivity in the hepatocyte of the liver; Table 3, Table 4.
Table 2.
Scoring of the morphological features as assessed by histopathological examination of liver:
| Group | central vein congestion & dilatation | disruption of hepatic arrangement | degenerated & vacuolation of hepatocyte |
|---|---|---|---|
| Group I (control) | (-) | (-) | (-) |
| Group II | (+) to (++) | (+) to (++) | (+) to (++) |
| Group III | (++) | (++) to (+++) | (++) to (+++) |
(−), no damage; (+), minimal (<5%); (++), moderate (5–20%); (+++), widespread (more than 20%).
Table 3.
The Optical Density of PAS Reaction in the Different Experimental Groups (n = 18, 6/group):
| Group | Optical density (Mean ± SD) |
|---|---|
| Group 1 | 101.61 ± 4.36 |
| Group 2 | 80.95 ± 10.78* |
| Group 3 | 72.13 ± 16.15* |
* Comparison to control reveals statistical significance (p < 0.05).
Table 4.
The optical density of P53 labeled cells in the hepatocyte in the experimental groups (n = 18, 6/group):
| Group | Optical density (Mean ± S.D.) |
|---|---|
| Group 1 | 64.62 ± 10.87 |
| Group 2 | 94.68 ± 7.31* |
| Group 3 | 114.017 ± 7.21* |
* Comparison to control reveals statistical significance (p < 0.05).
Quantitative measurements of the optical density of collagen fibers, PAS-positive material of the hepatocyte collagen content reveals a statistically significant diminishment in the optical density in the experimental groups contrasted to control groups.
Quantitative measurements of optical density of P53 labeled cells in the hepatocytes show a statistically significant increase in optical density in the experimental groups compared to the control.
4. Discussion
The goal of this research was to assess the potential effect of daily low Ag NPs dose administration on liver structure and function for a short-term period and the persistence of this harm after removal of the nanoparticles. Subacute exposure to Ag NPs can cause persistent hepatotoxicity, according to the current study. This hepatotoxicity impedes the liver's typical quick regenerating capacity and may even postpone it once the nanoparticles are removed.
4.1. The impact of nanoparticle characteristics on their toxicity
The morphological features of nanoparticles in general and particularly Ag NPs have a great effect on the research outcomes. This is due to the variability in the chemical reactions that they induce inside the biological media of the body, as well as the liability of small-sized particles to induce more toxicity due to their ability to find a portal to the cell (Ferdous and Nemmar, 2020; Yildirimer et al., 2011). Previous studies have provided different oral doses of Ag NPs for a variable time, documenting that the buildup of Ag NPs in different body tissues is dose-dependent (Kim et al., 2010, Kim et al., 2008). The concentration of 25 ppm (used in the current study) was reported to be the most toxic to the cell line. It is still undetermined whether the shape itself could have a role in the toxicological process or it could be a multifactorial issue (Akter et al., 2018). Another important aspect that contributes to the cytotoxic activity of AgNPs is their large surface area, which releases Ag + ions. Because of their larger surface area to volume ratio, smaller AgNPs have a faster rate of silver ion (Ag + ) dissolution in the surrounding microenvironment, resulting in improved bioavailability, enhanced distribution, and toxicity of Ag when compared to bigger NPs (Ferdous and Nemmar, 2020).
4.2. Increased H2O2 level in the liver
In this experiment, we detected a statistically marked increase in H2O2 levels in the group of animal sacrificed on day 14. This indicates an enhancement of redox activity that overcomes the cellular antioxidant capacity to scavenge the increased ROS. The increase in H2O2 persisted after stopping the nanoparticles in the group sacrificed on day 28. This may indicate the continued inability of cellular antioxidant activity to cope with the increased redox state. Overall, this refers to the persistent damage that has been caused by Ag NPs. An experiment by Patlolla et al. has reported that short-term administration of high doses of silver nanoparticles (50 and 100 mg/kg) activated ROS, induced liver injury and elevated liver enzymes (Patlolla et al., 2015). Recent research reported that Ag NPs enhanced apoptosis of HePG-2 cells in human hepatocellular carcinoma in in a dosages-dependent way. They demonstrated this by activating the caspase-3 pathway by generating reactive oxygen species (ROS) (Zhu et al., 2016). A prior study found that excessive levels of H2O2 or a failure to eliminate it caused cell necrosis and death (Rosa et al., 2006). The findings of these studies, taken together, support the findings of the current investigation in terms of the mechanistic role of ROS in the production of hepatic cell injury and the augmentation of cell death following the administration of Ag NPs.
4.3. The immune-histopathological features of subscute toxicity by Ag NPs
P53 is a tumor suppressor gene. When P53 is moderately stimulated within physiological limits, it exerts a beneficial function. The physiological apoptosis gets rid of the damaged or aged cells without releasing pro-inflammatory cytokines and eliciting an immune response. In contrast, hypo-activation or hyper-activation can lead to liver pathologies that can ultimately lead to HCC (Krstic et al., 2018; Link and Iwakuma, 2017). This is due to the improper removal of cells with mutated genes. In the same consensus, excessive or sustained apoptosis can lead to acute or chronic sustained inflammations and hepatic diseases (Cao et al., 2016; Guicciardi et al., 2013). The findings of the present study showed a toxic effect of Ag NPs on the liver at the structural level. Immuno-histochemical assessment of the apoptotic gene P53 revealed increased expression in the experimental group administered Ag NPs for 14 days. The present study has a significant finding which is the aggravated increase in P53-labeled hepatocytes in the group in which Ag NPs has stopped for fourteen days. The previous finding could reflect the persistent effect of Ag NPs after their withdrawal. A previous study could support our findings, albeit the different protocols and the type of silver nanoparticles. In that study, the researchers administrated a single dose (20% (w/v) aqueous solution − 7.9 ± 0.95 nm in size) of citrated Ag NPs into rabbits. They reported irreversible damage in liver cells that lasted for at least a month after a single dosage of citrated Ag NPs cytotoxicity (Kim et al., 2019). The main concern in this argument is the liability of this pathologic apoptosis to induce fibrosis or hepatic cancer (Guicciardi and Gores, 2005) which is an issue that should be thoroughly investigated due to the widespread introduction of Ag NPs in many aspects of daily life.
In our work, the histological alterations in the liver caused by Ag NPs were assessed qualitatively and quantitatively, proving their toxicity. A healthy liver's regeneration capacity is slow. When the liver is injured, on the other hand, this capability is increased to compensate for the cell loss (Krstic et al., 2018). Based on what has been published in earlier studies, there are two types of regeneration models of the liver tissue (Tao et al., 2017). The first model occurs in a unique pattern and follows partial hepatectomy (removal of 2/3 of liver tissue), and it takes about a week for the hepatocyte to regenerate and compensate for the loss. The other model is what occurs in liver insult via toxins or viruses. The oval stem cells operate and develop into hepatocytes and biliary cells to heal the substantial damage to the liver cells in this later model. Others have found disagreements over whether regenerated liver cells come from mature hepatocytes or a specific cell type (Gilgenkrantz and l’Hortet, A.C. de, 2018, Moreno-Marínet al., 2017). The high regenerative capacity of the liver can start as early as 24 to 72 h after drug-induced liver damage and can be seen along with the signs of liver injury on microscopic examination (Michalopoulos, 2007; Steup et al., 1993). As a result, we expected the disease to recover in two weeks, with the regenerative features overcoming the indicators of liver injury. This, however, was not the case. Many causes attribute to this unexpected outcome. To begin with, even when the nanoparticles are removed, a portion of them can precipitate in the liver and so cannot be entirely eliminated. It is uncertain if this damage can be reversed by the liver's long-term regenerative power or if it will result in chronic liver damage that has yet to be determined. Another explanation could be the ability of some noxious materials to induce long-lasting damage even in a single given dose. Ag NPs could be one of these material categories that induce persistent damage over short-term use (Kim et al., 2019). Ag NPs have also been shown to suppress the growth of pathogens like Herpes, HIV, and Hepatitis B, according to studies. The presence of the Ag + ion and its capacity to aggressively attach to the negatively charged proteins of DNA and RNA, preventing cell replication, is thought to be the cause of lower cellular proliferation caused by Ag NPs applications. This, probably, could indicate that a similar reaction could occur in somatic liver cells (Nayek et al., 2021).
4.4. The role of hepatic Bdnf gene in Ag NPs toxicity
BDNF regulates various aspects of brain activity and is crucial for maintaining central energy balance. It is found in organs in the periphery that are incorporated in fat and glucose metabolism, like the liver, although its significance in these tissues is uncertain. The findings suggest that through decreasing Peroxisome proliferator-activated receptor alpha and fibroblast growth factor 21, hepatic BDNF may aid the establishment of hepatic illnesses in response to a high fat diet (Teillon et al., 2010). A previous study found that the serum BDNF level is significantly lowered in patients with hepatitis B virus-induced liver cirrhosis (Shu et al., 2019). Another study has correlated the Ag NPs to the suppression of BDNF- stimulated cell survival in neuroblastoma cells treated with Ag NPs (Park et al., 2017). In the current study, although BDNF expression was lower in liver tissue after Ag NPs cessation, this difference was not statistically significant. BDNF and its receptor were only found in hepatic carcinoma tumor tissue and cell lines in a previous investigation, though not in nontumorous hepatocytes or normal cell lines. Therefore, elevated BDNF expression may promote tumor growth (Roesler et al., 2011; Yang et al., 2005). This may point to that although there were structural damage and apoptosis after Ag NPs withdrawal, the cells did not pass to a tumorigenic transformation, may be due to the short time of nanoparticle exposure.
4.5. Liver enzymes in Ag NPs subacute toxicity
Liver enzymes, ALT & AST, showed insignificant changes between the control and experimental groups. Although we did not expect that due to apparent cellular affection at the histological level, other studies concerned with the liver insult by diseases have shown similar results. Previous studies investigated the increase in the pro-apoptotic gene P53 in non-alcoholic liver steatosis. It has related the elevated transferases to the stage of the steatosis and indicated the insignificant correlation between liver transferases elevation and the expression of P53 (Panasiuk et al., 2006). Researchers found that when 3 groups of adult male Wister rats were given an intraperitoneal injection of Ag NPs at concentrations of 5, 10, and 100 ppm for 7 days, serum Glutamic Oxaloacetic Transaminase (SGOT) and Serum Glutamic-pyruvic Transaminase (SGPT) were insignificantly elevated at different time points compared to the control group. Nevertheless, they have reported partial damage to the liver tissue on histopathological examination (Monir Doudi, Mahbubeh Setorki, 2014). Another study that examined the effect of Ag NPs on the liver at varying doses for 28 days, documented no considerable effect of silver nanoparticles on liver transferases, although it also reported up-regulating activity of liver caspase-3 (Mahsa et al., 2016). In contrast, a prior study on silver nanoparticle toxicity found that the experimental groups had higher levels of liver enzymes than the control group (Heydrnejad et al., 2015). Other researchers documented a dose-dependent (concentrations: 5, 10, 20, and 40 ppm of Ag NPs) elevation of liver enzymes although they did not detect histopathologic changes in the liver (Shahin et al, 2014). Therefore, the structural heterogeneity of silver nanoparticles, the route of delivery, and the duration of treatment could all be factors in the disparities among experiments (Ajdary et al., 2018, Mao et al., 2018).
5. Conclusion
In general, the current study found that subacute Ag NP exposure can have long-term hepatotoxic consequences. This hepatotoxicity impedes the liver's typical quick regenerating capacity and may even postpone it when the nanoparticles are eliminated. However, long-term follow-up studies for various periods of time after terminating Ag NPs treatment are still needed to confirm these findings. Moreover, the introduction of variable doses to validate the effect of time in relation to the dosage on liver toxicity and regenerative capacity should be considered. The role of BDNF in subacute hepatic toxicity by Ag NPs particles and its interaction with the P53 needs to be elucidated. As a consequence, these findings raise concerns about the uncontrolled and unmonitored massive use of Ag NPs in recent decades. Normal levels of liver enzymes, if not paired with evident symptoms indicating hepatic disease, might be deceiving and postpone medical attention, which can lead to future health dangers as a result of the cumulative effect of using these nanoparticles.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
Great thanks should be provided to Dr. Ayman Salah Salem, specialist of clinical pathology, Ministry of Health, Egypt; for his rapid and keen response on analyzing tissue homogenate specimens for H2o2 assay. We would like to convey our profound gratitude to the Oncology Diagnostic Unit laboratory at our institution for providing the molecular biology tools used in this work.
Funding
The research is completely funded by the authors.
Author contribution
ShY: generating the idea of the research, methodology formulation and follow up of laboratory experiments, searching the methods, editing of the manuscript and discussing the results. HE: editing & performing methodology of histopathologic and immune-histochemistry assessment, and performing statistical analysis and discussing & editing of their results. MH: Molecular Biology analysis,shared in methodology and shared in result analysis and presentation. KEl-S: generating the idea of the research and methodology formulation and follow up of laboratory experiments, partial share in editing of the manuscript and discussing the results. ShA editing the paper, editing the methodology and conceptualization. All authors shared in revising the manuscript, performing the statistical analysis and results presentation. The first and the last authors provided similar contributions in authorship.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2022.02.054.
Contributor Information
Shimaa Mohammad Yousof, Email: drshimaay@gmail.com.
Horeya Erfan, Email: Horeya.arafat@med.suez.edu.eg.
Marwa Mohamed Hosny, Email: marwahosny@med.suez.edu.eg.
Shaimaa A. Shehata, Email: shaimaa_shehata@med.suez.edu.eg.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Aebi, H., 1984. [13] Catalase in vitro, in: Methods in Enzymology, Oxygen Radicals in Biological Systems. Academic Press, pp. 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
- Ajdary M., Moosavi M.A., Rahmati M., Falahati M., Mahboubi M., Mandegary A., Jangjoo S., Mohammadinejad R., Varma R.S. Health concerns of various nanoparticles: a review of their in vitro and in vivo toxicity. Nanomaterials. 2018;8 doi: 10.3390/nano8090634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter M., Sikder MdT., Rahman MdM., Ullah A.K.M.A., Hossain K.F.B., Banik S., Hosokawa T., Saito T., Kurasaki M. A systematic review on silver nanoparticles-induced cytotoxicity: physicochemical properties and perspectives. J. Adv. Res. 2018;9:1–16. doi: 10.1016/j.jare.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzola M., Saiz A., Cuevas N., Lopez-Martinez M., Martinez de Pancorbo M.A., Burgos J.J. High levels of p53 protein expression do not correlate with p53 mutations in hepatocellular carcinoma. J. Viral Hepat. 2004;11:502–510. doi: 10.1111/j.1365-2893.2004.00541.x. [DOI] [PubMed] [Google Scholar]
- Bakand S., Hayes A. Toxicological considerations, toxicity assessment, and risk management of inhaled nanoparticles. Int. J. Mol. Sci. 2016;17:E929. doi: 10.3390/ijms17060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bus B., Molendijk M., Penninx B., et al. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology. 2011;36:228–239. doi: 10.1016/j.psyneuen.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Cao L., Quan X.-B., Zeng W.-J., Yang X.-O., Wang M.-J. Mechanism of hepatocyte apoptosis. J. Cell Death. 2016;9:19–29. doi: 10.4137/JCD.S39824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernousova S., Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013;52:1636–1653. doi: 10.1002/anie.201205923. [DOI] [PubMed] [Google Scholar]
- Cho J.-G., Kim K.-T., Ryu T.-K., Lee J., Kim J.-E., Kim J., Lee B.-C., Jo E.-H., Yoon J., Eom I., Choi K., Kim P. Stepwise embryonic toxicity of silver nanoparticles on oryzias latipes. BioMed. Res. Int. 2013;2013 doi: 10.1155/2013/494671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.-M., Mizuta Y., Akagi J., Toyoda T., Sone M., Ogawa K. Size-dependent acute toxicity of silver nanoparticles in mice. J. Toxicol. Pathol. 2018;31:73–80. doi: 10.1293/tox.2017-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, G., Biological Stain Commission, 1981. Staining procedures. Williams & Wilkins, Baltimore, Md. [etc.
- De Jong W.H., Van Der Ven L.T.M., Sleijffers A., Park M.V.D.Z., Jansen E.H.J.M., Van Loveren H., Vandebriel R.J. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials. 2013;34:8333–8343. doi: 10.1016/j.biomaterials.2013.06.048. [DOI] [PubMed] [Google Scholar]
- El Mahdy M.M., Eldin T.A.S., Aly H.S., Mohammed F.F., Shaalan M.I. Evaluation of hepatotoxic and genotoxic potential of silver nanoparticles in albino rats. Exp. Toxicol. Pathol. 2015;67:21–29. doi: 10.1016/j.etp.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Ferdous Z., Nemmar A. Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci. 2020;21:2375. doi: 10.3390/ijms21072375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-López L.Z., Espinoza-Gómez H., Somanathan R. Silver nanoparticles: electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2019;39:16–26. doi: 10.1002/jat.3654. [DOI] [PubMed] [Google Scholar]
- Gilgenkrantz, H., l’Hortet, A.C. de, 2018. Understanding Liver Regeneration: From Mechanisms to Regenerative Medicine. Am. J. Pathol. 188, 1316–1327. https://doi.org/10.1016/j.ajpath.2018.03.008 [DOI] [PubMed]
- Griffith R.D., Simmons B.J., Bray F.N., Falto-Aizpurua L.A., Abyaneh M.-A.Y., Nouri K. 1064 nm Q-switched Nd:YAG laser for the treatment of Argyria: a systematic review. J. Eur. Acad. Dermatol. Venereol. 2015;29:2100–2103. doi: 10.1371/journal.pcbi.1004393. [DOI] [PubMed] [Google Scholar]
- Guicciardi M.E., Gores G.J. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guicciardi M.E., Malhi H., Mott J.L., Gores G.J. Apoptosis and necrosis in the liver. Compr. Physiol. 2013;3 doi: 10.1002/cphy.c120020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Xie H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J Environ Pathol Toxicol Oncol. 2018;37(3):209–230. doi: 10.1615/JEnvironPatholToxicolOncol.2018026009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydrnejad M.S., Samani R.J., Aghaeivanda S. Toxic effects of silver nanoparticles on liver and some hematological parameters in male and female mice (Mus musculus) Biol. Trace Elem. Res. 2015;165:153–158. doi: 10.1007/s12011-015-0247-1. [DOI] [PubMed] [Google Scholar]
- Kim S., Choi J.E., Choi J., Chung K.-H., Park K., Yi J., Ryu D.-Y. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol. In Vitro. 2009;23:1076–1084. doi: 10.1016/j.tiv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Kim Y.J., Rahman M.M., Lee S.M., Kim J.M., Park K., Kang J.-H., Seo Y.R. Assessment of in vivo genotoxicity of citrated-coated silver nanoparticles via transcriptomic analysis of rabbit liver tissue [WWW Document] Int. J. Nanomed. 2019 doi: 10.2147/IJN.S174515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.S., Kim J.S., Cho H.S., Rha D.S., Kim J.M., Park J.D., Choi B.S., Lim R., Chang H.K., Chung Y.H., Kwon I.H., Jeong J., Han B.S., Yu I.J. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in sprague-dawley rats. Inhal. Toxicol. 2008;20:575–583. doi: 10.1080/08958370701874663. [DOI] [PubMed] [Google Scholar]
- Kim, Y.S., Song, M.Y., Park, J.D., Song, K.S., Ryu, H.R., Chung, Y.H., Chang, H.K., Lee, J.H., Oh, K.H., Kelman, B.J., Hwang, I.K., Yu, I.J., 2010. Subchronic oral toxicity of silver nanoparticles. Part. Fibre Toxicol. 7, 20. https://doi.org/10.1186/1743-8977-7-20 [DOI] [PMC free article] [PubMed]
- Kokkat T.J., Patel M.S., McGarvey D., LiVolsi V.A., Baloch Z.W. Archived formalin-fixed paraffin-embedded (FFPE) blocks: a valuable underexploited resource for extraction of DNA, RNA, and protein. Biopreserv. Biobank. 2013;11:101–106. doi: 10.1089/bio.2012.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krstic J., Galhuber M., Schulz T.J., Schupp M., Prokesch A. p53 as a Dichotomous Regulator of Liver Disease: The Dose Makes the Medicine. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19030921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara Yildirimer, Nguyen T.K. Thanh, Marilena Loizidou, Alexander M. Seifalian, Toxicology and clinical potential of nanoparticles, Nano Today, Volume 6, Issue 6, 2011, Pages 585-607, ISSN 1748-0132, https://doi.org/10.1016/j.nantod.2011.10.001. (https://www.sciencedirect.com/science/article/pii/S1748013211001137) [DOI] [PMC free article] [PubMed]
- Link T., Iwakuma T. Roles of p53 in extrinsic factor-induced liver carcinogenesis. Hepatoma Res. 2017;3:95–104. doi: 10.20517/2394-5079.2017.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K., Schmittgen, T., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods 25, 402–8. [DOI] [PubMed]
- Lorenzo, P., Barbara, P., Thomas, L., Florenzo, I., Leonardo, R., Floriana, G., Rosanna, M., Maura, B., Daniela, S., Francesco, V., Elisabetta, M.M., Antonio, F., 2009. Protective effect of augmenter of liver regeneration on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Free Radic. Res. 43, 865–875. 10.1080/10715760903100125 [DOI] [PubMed]
- Magdy Mohamed El Mahdy, Taher Ahmed Salah Eldin, Halima Sayed Aly, Faten Fathy Mohammed, Mohamed Ibrahim Shaalan, 2014. Evaluation of hepatotoxic and genotoxic potential of silvernanoparticles in albino rats 67, 21–29. [DOI] [PubMed]
- Mahsa P., Zahra G.M., Massoud S., Mohamad Javad A., Zohreh A. The effect of silver nanoparticles on the biochemical parameters of liver function in serum, and the expression of caspase-3 in the liver tissues of male rats. Avicenna J. Med. Biochem. 2016;4:7–35557. doi: 10.17795/ajmb-35557. [DOI] [Google Scholar]
- Manke, A., Wang, L., Rojanasakul, Y., 2013. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity [WWW Document]. BioMed Res. Int. https://doi.org/10.1155/2013/942916 [DOI] [PMC free article] [PubMed]
- Mao B.-H., Chen Z.-Y., Wang Y.-J., Yan S.-J. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Sci. Rep. 2018;8:2445. doi: 10.1038/s41598-018-20728-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson, P., Masson, P., 1929. Some histological methods: Trichrome staining and their preliminary technique. undefined.
- McMANUS J.F.A. Histological demonstration of mucin after periodic acid. Nature. 1946;158:202. doi: 10.1038/158202a0. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G.K. Liver regeneration. J. Cell. Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monir Doudi, Mahbubeh Setorki, 2014. Acute Effect of Nanosilver to Function and Tissue Liver of Rat after Intraperitioneal Injection [WWW Document]. URL https://scialert.net/fulltext/?doi=jbs.2014.213.219&org=11 (accessed 7.11.19)
- Moreno-Marín, N., Barrasa, E., Morales-Hernández, A., Paniagua, B., Blanco-Fernández, G., Merino, J.M., Fernández-Salguero, P.M., 2017. Dioxin Receptor Adjusts Liver Regeneration After Acute Toxic Injury and Protects Against Liver Carcinogenesis. Sci. Rep. 7, 10420. https://doi.org/10.1038/s41598-017-10984-w [DOI] [PMC free article] [PubMed]
- Murray R. Mosby Co.; St Louis, Toronto, Princeton: 1984. Alanine aminotransferase.In: Kaplan A and Peace AL (ed) Clinical Chemistry. The C.V. [Google Scholar]
- Murray R. In: Clinical Chemistry. Kaplan A., Peace A.L., editors. The C.V. Mosby Co.; St Louis, Toronto, Princeton: 1984. Aspartate aminotransferase. [Google Scholar]
- Nayek S., De Silva I.W., Aguilar R., Lund A.K., Verbeck G.F. Toxicological alterations induced by subacute exposure of silver nanoparticles in Wistar rats. J. Appl. Toxicol. JAT. 2021;41:972–986. doi: 10.1002/jat.4086. [DOI] [PubMed] [Google Scholar]
- Nikolić, T., Petronijević, M., Sopta, J., Velimirović, M., Stojković, T., Jevtić Dožudić, G., Aksić, M., Radonjić, N.V., Petronijević, N., 2017. Haloperidol affects bones while clozapine alters metabolic parameters - sex specific effects in rats perinatally treated with phencyclidine. BMC Pharmacol. Toxicol. 18, 65. https://doi.org/10.1186/s40360-017-0171-4 [DOI] [PMC free article] [PubMed]
- Panasiuk A., Dzieciol J., Panasiuk B., Prokopowicz D. Expression of p53, Bax and Bcl-2 proteins in hepatocytes in non-alcoholic fatty liver disease. World J. Gastroenterol. WJG. 2006;12:6198–6202. doi: 10.3748/wjg.v12.i38.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.H., Gurunathan S., Choi Y.-J., Han J.W., Song H., Kim J.-H. Silver nanoparticles suppresses brain-derived neurotrophic factor-induced cell survival in the human neuroblastoma cell line SH-SY5Y. JIEC. 2017;47:62–73. [Google Scholar]
- Patlolla A.K., Hackett D., Tchounwou P.B. Silver nanoparticle induced oxidative stress-dependent toxicity in sprague-dawley rats. Mol. Cell. Biochem. 2015;399:257–268. doi: 10.1007/s11010-014-2252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.F., Wang J., Patterson T.A., Saini U.T., Robinson B.L., Newport G.D., Murdock R.C., Schlager J.J., Hussain S.M., Ali S.F. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol. Lett. 2009;187:15–21. doi: 10.1016/j.toxlet.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Recordati, 2016. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: nano-specific and size-dependent effects. [DOI] [PMC free article] [PubMed]
- Roesler R., de Farias C.B., Abujamra A.L., Brunetto A.L., Schwartsmann G. BDNF/TrkB signaling as an anti-tumor target. Expert Rev. Anticancer Ther. 2011;11:1473–1475. doi: 10.1586/era.11.150. [DOI] [PubMed] [Google Scholar]
- Laura Conde de la Rosa, Marieke H. Schoemaker, Titia E. Vrenken, Manon Buist-Homan, Rick Havinga, Peter L.M. Jansen, Han Moshage, Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: Involvement of JNK and ERK MAP kinases, Journal of Hepatology, Volume 44, Issue 5, 2006, Pages 918–929, ISSN 0168–8278, doi: 10.1016/j.jhep.2005.07.034. [DOI] [PubMed]
- Sardari Roshan. Toxicological effects of silver nanoparticles in rats. African Journal of Microbiology Research. 2012;6 doi: 10.5897/AJMR11.1070. [DOI] [Google Scholar]
- Shahin Gavanji, Sana Sayedipour,Mohsen Doostmohamadi, Behrouz Larki, 2014. (PDF) The Effect of different Concentrations of Silver Nanoparticles on Enzyme Activity and Liver Tissue of Adult Male Wistar Rats in-vivo Condition [WWW Document]. URL https://www.researchgate.net/publication/273221752_The_Effect_of_different_Concentrations_of_Silver_Nanoparticles_on_Enzyme_Activity_and_Liver_Tissue_of_Adult_Male_Wistar_Rats_in-vivo_Condition (accessed 7.8.19)
- Shu H.-C., Hu J., Jiang X.-B., Deng H.-Q., Zhang K.-H. BDNF gene polymorphism and serum level correlate with liver function in patients with hepatitis B-induced cirrhosis. Int. J. Clin. Exp. Pathol. 2019;12:2368–2380. [PMC free article] [PubMed] [Google Scholar]
- Simon-Deckers, A., Gouget, B., Mayne-L’Hermite, M., Herlin-Boime, N., Reynaud, C., Carrière, M., 2008. In vitro investigation of oxide nanoparticle and carbon nanotube toxicity and intracellular accumulation in A549 human pneumocytes. Toxicology, This issue includes: Proceedings of the Annual Congress of The British Toxicology Society 253, 137–146. https://doi.org/10.1016/j.tox.2008.09.007 [DOI] [PubMed]
- Steup D.R., Hall P., McMillan D.A., Sipes I.G. Time course of hepatic injury and recovery following coadministration of carbon tetrachloride and trichloroethylene in Fischer-344 rats. Toxicol. Pathol. 1993;21:327–334. doi: 10.1177/019262339302100309. [DOI] [PubMed] [Google Scholar]
- Tao Y., Wang M., Chen E., Tang H. Liver regeneration: analysis of the main relevant signaling molecules [WWW Document] Mediat. Inflamm. 2017 doi: 10.1155/2017/4256352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teillon S., Calderon G.A., Rios M. Diminished diet-induced hyperglycemia and dyslipidemia and enhanced expression of PPARalpha and FGF21 in mice with hepatic ablation of brain-derived neurotropic factor. J. Endocrinol. 2010;205:37–47. doi: 10.1677/joe-09-0405. [DOI] [PubMed] [Google Scholar]
- Wang H., Qiao X., Chen J., Wang X., Ding S. Mechanisms of PVP in the preparation of silver nanoparticles. Mater. Chem. Phys. 2005;94:449–453. doi: 10.1016/j.matchemphys.2005.05.005. [DOI] [Google Scholar]
- Yang Z.F., Ho D.W., Lam C.T., Luk J.M., Lum C.T., Yu W.C., Poon R.T., Fan S.T. Identification of brain-derived neurotrophic factor as a novel functional protein in hepatocellular carcinoma. Cancer Res. 2005;65:219–225. [PubMed] [Google Scholar]
- Yousof S.M., Awad Y.M., Mostafa E.M.A., Hosny M.M., Anwar M.M., Eldesouki R.E., Badawy A.E. The potential neuroprotective role of Amphora coffeaeformis algae against monosodium glutamate-induced neurotoxicity in adult albino rats. Food Funct. 2021 Jan 21;12(2):706–716. doi: 10.1039/d0fo01957g. Epub 2020 Dec 18 PMID: 33337454. [DOI] [PubMed] [Google Scholar]
- Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-F., Liu Z.-G., Shen W., Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian-Kang Zhu, Abiotic Stress Signaling and Responses in Plants, Cell, Volume 167, Issue 2, 2016, Pages 313–324, ISSN 0092–8674, doi: 10.1016/j.cell.2016.08.029. (https://www.sciencedirect.com/science/article/pii/S0092867416310807). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.