Abstract
Formalin is used for different purposes due to its preservation capability. But continuous exposure to formalin may result various health related issues leading to cancer and death. A new alcohol-based fixative, EMA (ethanol, methanol and acetic acid = 3:1:1) could be a safer option in this regard. To compare the health hazards of formalin and EMA, a total 15 adult male mice were randomly distributed into three groups- exposure groups (formalin and EMA) and control group. The mice were subjected to natural inhalation exposure of the fixatives followed by behavioral depression test (forced swimming test), histopathology and serum biochemical tests. Our results showed that the hazardous effects of formalin were remarkably higher than that of EMA. Formalin exposed group showed severe depression (P < 0.001) in the forced swimming test compared to EMA and control groups. Histopathologically, diffuse lymphocytic infiltrations around the lung alveoli and bronchioles and severe inflammation with accumulation of reactive cells in the cerebral cortex were detected in the formalin exposed group, whereas little or no inflammation with fibrinous exudates in the bronchioles was reported in the EMA group and no inflammatory cells were detected in the cerebral tissues. The serum biochemical analysis of the inflammatory mediators (Interleukin-6 and C-reactive protein) revealed that both significantly (P < 0.001) increased in the formalin exposed group compared to EMA and control groups. These results confer that EMA could be a safer option to reduce health hazards of formalin in the workplace environment.
Keywords: Formalin, EMA, Histopathology, Lung, Cerebrum, Interleukin-6, C-reactive protein
1. Introduction
Humans are constantly exposed to chemicals from the environment, some of which are harmful to their health (Aguwa et al., 2018). The aqueous preparation of formaldehyde (37–40%) is commonly known as formalin. Because of advantageous fixative qualities, formalin is routinely utilized in tissue preservation techniques. Its widespread avail in medical management, however, may expose related persons to unappreciated risks.
The ubiquitous use of formalin in a plethora of sectors like production of building materials, textile industry, product sterilization, cosmetics, plastics, paper, and plywood, it’s role as a fixative to preserve biological specimens in various academic sectors and laboratories like forensic, biological, and pathological; formaldehyde has become a habitual adulterant of earth’s environment (Bakar et al., 2015, Checkoway et al., 2015, Ciftci et al., 2015). Cigarette smoke, combusting byproducts, combustible oil and fossil fuel, fumes from the timber or oil from the earth, automobile exhaust, paint vapors, chipboard, hardboard, and toxic emissions from dye used as painting of buildings and wooded objects are all contributors of formaldehyde to the nature (Zararsiz et al., 2007, Cheney and Collins, 1995, Restani and Galli, 1991, World Health Organization, 2010, Bernstein et al., 1984, Flyvholm and Menne, 1992, Kilburn, 1994).
In studies, it was reported that the initial exposure to formaldehyde, the major constituent of formalin, has been associated with certain health hazards such as respiratory tract and skin irritation. Not only that, chronic exposure to formaldehyde has also been linked to an increased risk of nasopharyngeal cancer, cancer of nasal sinus, and myeloid leukemia. In fact, fundamental scientific experiments and investigations using numerous animal models have demonstrated formaldehyde's mutagenesis potential, highlighting the necessity for exposure safety precautions (Biosafe, US, 2019). If certain downstream biochemical and molecular investigations are taken into account, 40% formaldehyde is necessarily not the ideal fixative (Panzacchi et al., 2016). As a matter of fact, the merits of formalin in tissue preservation approach are offset due to a slew of disadvantages; the most significant of which are diminished immunohistochemical reactivity and fast degradation of DNA or RNA (Cox et al., 2006, Moelans et al., 2011). The proportion of epitope modification is correlated with the availability of molecular target. Formalin as a fixative, works on the target protein through crosslinking mechanism, which ultimately reduces the number of epitopes available to bind by antibodies. (Bogen et al., 2009, Hayat, 2000, O’Leary et al., 2009, Otali et al., 2009, Paavilainen et al., 2010).
The high level of exposure to formaldehyde in different settings may cause irritations to the eyes and upper respiratory tracts. The acute toxicity and the prevalence of contact dermatitis are undeniable (Pabst, 1987). In contrast to formalin, ethanol and methanol combination as a fixative provides comparatively a safer option. This combination of fixative works via coagulation mechanism and targets the hydrogen bonds of protein to break down and coagulate it. While the combination of this two alcohol have proven their potency as tissue fixative through their usefulness in cytology; when used alone, both can act as causal factor for shrinkage and fragility of tissue (Adrian et al., 2000). An alcohol-based fixative is a safer alternate of formalin which keeps nucleic acid intact during preservation as well facilitate the microscopic and immunohistochemical investigations (Haque et al., 2020, Rahman et al., 2021).
Chronic formalin inhalation might have undesirable effects on kidneys, skin, brain, and appetite. As a result, some precautions are suggested for medical students, academicians and technicians which include short time exposure, ensuring sufficient ventilation, using embalming chemicals with minimum toxicity, restrict formalin use as preservative in food industry, and finally taking personal precaution during procedures (Egwurugwu et al., 2018). Inhaled formaldehyde has a deleterious effect on mice's spatial learning and memory. This is similar to those in occupationally exposed populations losing their attention and recall (Lu et al., 2008, Inci et al., 2013). Furthermore, alcoholic fixation has advantages over formalin fixation in terms of penetration, preservation, and a safer working environment. Furthermore, the EMA's preservation capacity has already been investigated in one of our prior experiments (Haque et al., 2020, Rahman et al., 2021). However, its health hazards due to exposure need to be explored compared to the gold standard fixative, formalin. Therefore, our current work was framed to explore the health hazards of formalin and EMA in mice.
2. Materials and methods
2.1. Ethical approval
As per the standardized instruction, all experimental animals were reared humanely. The current research was conducted as per approval and guidance of the Animal Welfare and Experimental Ethics Committee, Bangladesh Agricultural University, Mymensingh, Bangladesh (Protocol Number: AWEEC/BAU/2021–06).
2.2. Experimental animals
A total of fifteen (N = 15) Swiss albino mice (Mus musculus) were randomized into control and exposed groups (formalin and EMA); each group having five (n = 5) male mice. The experimental mice were purchased from the Animal Resource Center, International Centre for Diarrheal Disease Research, Bangladesh, Mohakhali, Dhaka, at the age of 30 days, with an average body weight of 25–28 g. Before introducing the mice to the experimental setup, they were reared for 15 days with an aim to be accustomed with the circumstances as well to attain reproducing ability (at 45–48 postnatal days, Swiss albino mice of either sex obtain their offspring producing capability). After that, when the mice were aged day 60, the experiment began. During the whole experimental tenure, compartmentalized rectangular metallic cages (9 × 11 × 7 cube inches) wrapped with wire mesh were used to house the mice. The mice were thoroughly examined for any developmental abnormalities, detectable genital defects or diseases that might have some influence on the study or the results thereby; however, found neither one.
The mice were cared at Animal Care Room, Department of Pharmacology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh, in proper hygienic conditions, with experimental and normal feeding (standard pelleted feed for mice from ICDDR, B) ad libitum. Special attention was given to the consistency of the day to day routine practices from the start of the experiment till the end. To be more specific, special concentration was given to the ventilation system of the rearing house which maintained at standard level. At natural day and light condition, the room temperature was kept at 28 ± 2 °C with a relative humidification of 70–80%. Before starting the experiment, it was mandatory to check the reproductive ability of the experimental mice. For that purpose, both male and female mice were kept together under close observation for a normal cycle while they were fed the normal mice pellet and water ad libitum.
2.3. Inhalation exposure of the fixatives in mice
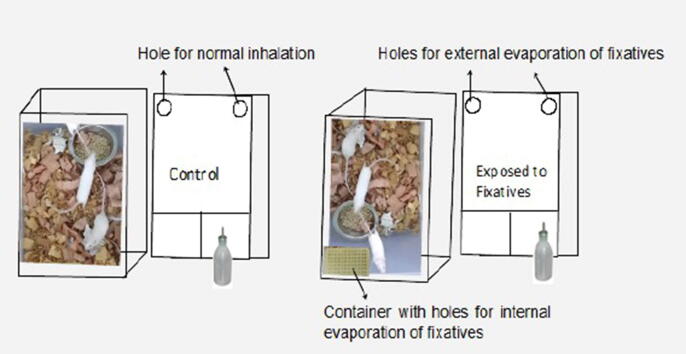
The inhalation process of the fixatives were facilitated via exposing the mice to natural vaporization of formalin and alcoholic fixatives, EMA (ethanol: methanol: acetic acid = 3:1:1). The specific containers having openings on the roof surface were used to prevent direct dermal contact with the fixative solution but a continuous flow of evaporated fixatives was maintained. The top surface of each mouse cage was covered with aluminium foil with two circular holes (about 1.5 cm in diameter) close to the corners for external evaporation of the fixatives. For chronic exposure, each container was filled with 10 ml of formalin (10%) and EMA solution which was placed in the mouse cage @ 8 h a day, 5 times a week, for a period of 4 weeks (Fig. 1).
Fig. 1.
A natural inhalation exposure of the fixatives in mice.
2.4. Behavioral depression study in mice (forced swimming test)
The exposed mice were subjected to forced swimming test for behavioral depression study. Here, the degree of passive stress-coping strategy or depression like behavior, immobility is taken into consideration (behavioral despair). For this purpose, the mice were introduced to successive double trials where they were imposed to swim in the three separate acrylic glasses beakers (one for each group) filled with more than half of it with fresh water (temperature between 23 and 25 °C) so that the mice can easily swim into it without touching the bottom of the beaker or grabbing the upper surface of it. The first trial was performed for habituation of mice which was lasted for 15 min. After 24 h, a second trial with duration of 5 min (300 sec) was conducted. In the second trial, the time lapse the mice spent with no movements except keeping its head above water was recorded.
2.5. Sample collection
Chloroform solution was used to anesthetize the mice. Followed by collection of blood samples (2–3 ml) directly from the heart via 2 ml insulin syringe and collected blood was kept into the tubes with coagulating agent. The serum samples were obtained by centrifugation at the maximum speed (15,000 rpm) for 15 min and stored at −20 °C for future analysis. Finally, cervical subluxation method was used to ethically sacrifice the mice from both the control and treatment groups. For histo-pathological analysis, immediately after sacrifice, desired tissue samples (lungs and brain) were collected and processed.
2.6. Analysis of inflammatory response
The systemic inflammatory mediators were detected by markers like interleukin-6 (IL-6) and C-reactive protein (CRP). Commercially available enzyme-linked Immunosorbent assay (R&D Systems, Minneapolis, Minn) was used to detect serum concentration of IL-6. On the other hand, in a BN II analyzer (Dade Behring, Newark, Del) using a high-sensitivity latex-enhanced Immunonephelometric assay, the serum concentration of CRP was measured.
2.7. Histopathological analysis of tissues
The lung and cerebral tissues were collected for histological processing and staining by Hematoxylin and Eosin (H & E). In brief, 70% alcohol was used to rinse as well to remove the fixative solution from the tissues. The processing schedule was dehydration using ascending grade of alcohols (70% alcohol, 80% alcohol, 90% alcohol, two changes of 100% alcohol, 2 h in each) and clearing through two changes of xylene (1 h each). After that, the tissue samples were treated through three changes of graded paraffin wax for 45 min each. Following embedding, codding of each block was performed. A sliding microtome (MIC 509, Euromex, Japan) was used and the paraffin blocks were sectioned at thickness of 6 μm. Lukewarm water at 37 °C was used to stretch the sections (KF-WS-100 Tissue Flotation Water Bath). The glass slides were coated with adhesive on both sides and the tissue sections were mounted on the slides followed by dried in a slide warmer at 40 °C, overnight. With following protocol, the sections were stained: deparaffinization using xylene-I, II & III (3 min each); rehydration of tissues via descending graded alcohols-100% alcohol, 95% alcohol, 80% alcohol & 70% alcohol (3 min each); deionized water with gentle shaking (10 min); Harris Hematoxylin (10 min); deionized water with gentle shaking (10 min); Eosin-Y (2 min), dehydration through ascending grade of alcohol-70% alcohol, 80% alcohol, 95% alcohol and 2 changes of 100% alcohol (3 min each); 50% xylene + 50% alcohol and finally clearing of alcohol using xylene-I, II & III (3 min each). At the next step, DPX, a mounting medium is used to put the cover slip over the tissue and finally examination under light microscope was done.
2.8. Imaging
Nikon photo microscope was used to take the images at 10X magnification directly from the slides.
2.9. Statistical analysis
During the course of experiment, data were collected and subjected to the statistical analysis. On way of ANOVA was performed within a completely randomized design (CRD) using Graph Pad Prism (version 9.0). Significant means were separated using multiple comparisons at 5% level of significance.
3. Results
3.1. Behavioral depression study (forced swimming test)
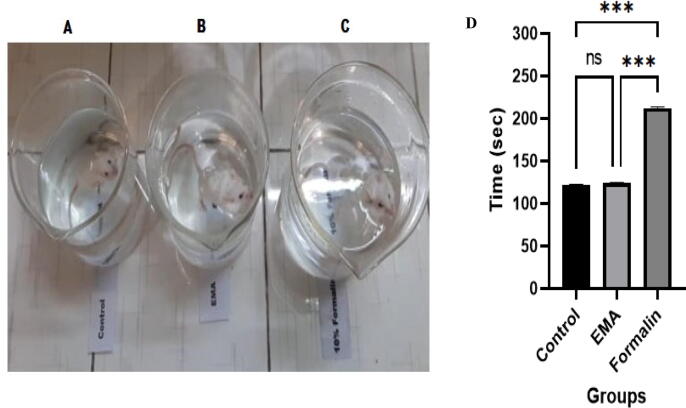
This study aimed to evaluate the level of depression due to exposure of fixatives in mice. To test the depression level, forced swimming tests were performed within the given period of time. The experimental animals were forced to swim in the glass beakers for 300 sec and the time they spent without any locomotive functions (floating) was recorded. Our data showed severe depression in the formalin exposed group which was significantly (P < 0.001) higher in comparison to EMA and control groups (Fig. 2). However, the result was non-significant (P > 0.05) between EMA and control groups.
Fig. 2.
Forced swimming test in mice (A = control, B = EMA and C = 10% formalin exposed groups. Bar diagrams (D) illustrating the immobility (floating) period (sec) in the control, EMA and formalin exposed mice during forced swimming test of 300 sec (mean ± SEM). Significant at the level of 5% (P < 0.05).
3.2. Histopathology
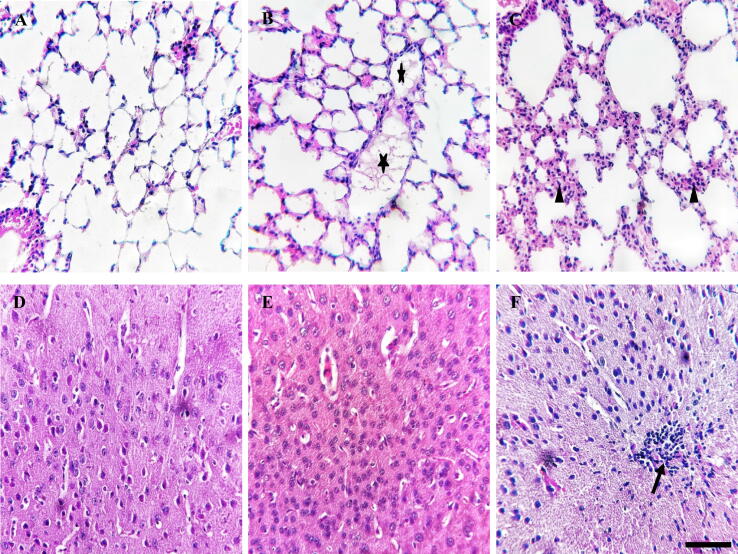
The lung and cerebral tissues of the exposed (EMA and formalin) and control groups were processed for histopathological study using H & E staining. In the formalin exposed group, we observed diffuse lymphocytic infiltrations around the lung alveoli and bronchioles. In the cerebrum, mild inflammation with accumulation of reactive cells in the cortical region was detected. However, little or no inflammation with fibrinous exudates in the bronchioles was reported in the EMA exposed group. But no reactive cells were found in the cerebral tissues (Fig. 3). These results indicated the histo-toxic reactions of formalin at cellular level other than EMA.
Fig. 3.
Histopathological analysis of lung (A–C) and cerebral tissues (D–F) in mice (A, D = control, B, E = EMA and C, F = formalin exposed groups). Arrow heads and arrows indicate reactive cells and asterisks indicate exudates (A–F). Scale bar in all images (A–F) = 250 μm.
3.3. Serum biochemical analysis
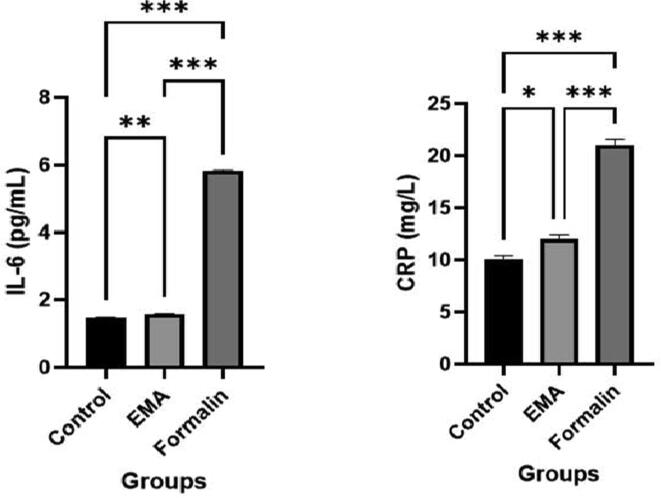
The plasma cytokine interleukin-6 (IL-6) acts as a mediator of inflammation which in case of acute-phase response functions like a primary stimulus. CRP is a well-known pro-inflammatory marker. The serum biochemical analysis of the inflammatory mediators (IL-6 and CRP) revealed that both significantly (P < 0.001) increased beyond normal range in the formalin exposed group compared to EMA and control groups (Fig. 4). Both IL-6 and CRP also increased (P = 0.003 and P = 0.036, respectively) in the EMA and control groups within the normal range. These results suggested stronger inflammation in the organs of formalin exposed group compared to EMA and control groups.
Fig. 4.
Bar diagrams illustrating the serum cytokines, interleukin-6 (mean ± SEM) and CRP in the control, EMA and formalin exposed groups. Significant at the level of 5% (P < 0.05).
4. Discussion
Formaldehyde is a colorless, extremely combustible gas that has been linked to human cancer (Cogliano et al., 2005). The noxious aspect of formaldehyde has been the subject of numerous research in past decades (Liu et al., 2006, Lu et al., 2008, Lu et al., 2005), highlighting the need for an alternative. Formalin could be replaced with alcohol-based fixatives such as EMA. The validity of improving EMA has been established in the current study using various methods of hazards analysis.
The forced swimming test is one of the most reliable methods for assessing behavioral depression in mice (Petit-Demouliere et al., 2005). Our findings revealed that the formalin-exposed group suffered from severe depression, which was significantly (P < 0.001) higher than EMA and control groups. However, statistical analysis showed non-significant difference (P > 0.05) between EMA and control groups. In their experiment, Aguwa et al., 2018 discovered similar results. The results of their neurobehavioral investigations revealed that the muscular strength of experimental groups were significantly lower than control group. They concluded that inhaling formalin vapor had induced neurodegeneration of cerebellum in adult male Wistar rats based on their findings. The proposed explanation for these effects could be oxidative stress-induced neuron damage in the brain (Janero, 1990).
In histomorphological research, we discovered that formalin breathed mice had multiple conspicuous evidences in the lungs and cerebrum, whereas such lesions were not identified in EMA inhaled mice, indicating that EMA is a better preservative than formalin. Due to the formalin exposure, scattered lymphocytic infiltrations surrounding the lung alveoli and bronchioles were detected in the lungs, but the EMA group had minimal or no inflammation with fibrinous exudates in the bronchioles. Mild inflammation was discovered in the subcortical region, as well as an accumulation of reactive cells. In the case of EMA, however, there were no reactive cells in the brain regions.
These findings revealed that formalin, unlike EMA and control, had histotoxic effects at the cellular level. Gladfelter discovered a normal histological framework of the cerebella in rats in the control group (Gladfelter 1973), but cell death and a faint nuclear outline of the purkinje cell in the experimental group (Gladfelter 1973). In the formalin-exposed groups, they also discovered evidence of nuclear damage (pyknosis and Karyorhexis). The granule and basket cells in the molecular layer were similarly atrophied and scattered more sparsely. These findings point to cerebellar pathology, which could elicit motor dysfunction in upcoming days.
Martin and Fischer (1995) published a review article focusing on morphohistological outcomes on different animal models. And he found that animals unveiled to formalin have specific lesions such as inflammation induced changes within the lungs. The lungs of the rats and guinea pigs that were exposed for an hour and a half exhibited significant congestion. A uniform red-staining exudate filled parts of the alveoli. There had been some desquamation of the alveolar lining cells. Many polynuclear leukocytes were discovered in the capillaries, lung connective tissue, and free in the alveoli. There were a lot of eosinophils. There were some mononeuclear leukocytes, but they were in small quantities. The bronchi were filled with serum containing a few leukocytes and bronchial epithelium that was desquamated and deteriorated. The findings are very similar to those we found in formalin-exposed animals, but no such lesions were found in the alcohol-based fixatives, EMA.
Inflammation is a complex process involving a large number of chemicals. Researchers in their studies mainly focused on two functionally connected biomarkers: Interlukin-6 and C-reactive protein in acute phase. Liver is the activation site of CRP is activated by IL-6 (Baumeister et al., 2016, Baumeister et al., 2014). These molecules are easily detectable in serum and are produced in significant quantities during infections. Interlukin-6 and C-reactive protein are widely acknowledged as inflammatory biomarkers in the behavioral literature, and both are frequently employed to measure the existence and degree of low-grade inflammation (Fagundes and Way, 2014). From the histopathological analysis we could see the infiltration of the inflammatory cells remarkably higher in the formalin inhaled group compared to EMA and control. So it is quite obvious that the IL-6 and CRP were significantly higher in case of the formalin inhaled group than that of EMA group and control. However, both IL-6 and CRP also increased moderately in the EMA group compared to the control but still within the normal range. These results are in agreement with Miller et al. (2011).
5. Conclusions
From the results of hazards analysis, we can conclude that alcoholic fixative, EMA could be an effective option for safer workplace environment. Considering the serious health concerns of formalin, its usage has been limited throughout the world by concerned authorities for protecting people. However, the most rational way of prevention would be the avoidance of imprudent usage of formalin and its replacement with a comparatively safer and cost effective option, such as EMA.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We greatly acknowledge the professional supports from Professor Dr. Md. Mahmudul Alam, Department of Surgery and Obstetrics, Faculty of Veterinary Science, Bangladesh Agricultural University (BAU), Mymensingh-2202.
Funding
This study was supported by Bangladesh Agricultural University (BAU), Mymensingh, Bangladesh; University Grants Commission (UGC), Bangladesh; Ministry of Science and Technology (MoST) and Ministry of Education (MoE), Government of the People’s Republic of Bangladesh.
Author contributions
Rubayat Rezoana: Conception or design, Data acquisition, Analysis, or Interpretation, Drafting the manuscript. Latifa Akter: Conception or design, Data acquisition, Analysis, or Interpretation. Rafiqul Islam: Conception or design, Critically revising the manuscript. Sonali Bhakta: conception or design, Drafting the manuscript. Ummay Ayman: Data acquisition, Analysis, or Interpretation, Drafting the manuscript. Mohammad Rabiul Karim: critically revising the manuscript. Ziaul Haque: Conception or design, Data acquisition, Analysis, or Interpretation, Drafting the manuscript, Critically revising the manuscript. All authors have provided their final opinion and concerned about accuracy and originality of the work.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Ummay Ayman, Email: ayman@bau.edu.bd.
Ziaul Haque, Email: zhaqueah80@bau.edu.bd.
References
- Adrian W.R., Jenny W.M., Clyde B.R. Evaluation of Ethanol-based Fixatives as a Substitute for Formalin in Diagnostic Clinical Laboratories. J. Histotechnol. 2000;23:299–308. doi: 10.1179/0147888007948. [DOI] [Google Scholar]
- Aguwa U.S., Ovie F.O., Keme E.T., Olu S.I. Effect of Formalin Inhalation on the Cerebellum of Adult Male Wistar Rat. Int. Invent. Sci. J. 2018;2(2):80–84. http://iisj.in/index.php/iisj/article/view/16 [Google Scholar]
- Bakar E., Ulucam E., Cerkezkayabekir A. Protective effects of proanthocyanidin and vitamin E against toxic effects of formaldehyde in kidney tissue. Biotech. Histochem. 2015;90(1):69–78. doi: 10.3109/10520295.2014.954620. [DOI] [PubMed] [Google Scholar]
- Baumeister D., Russell A., Pariante C.M., Mondelli V. Inflammatory biomarker profiles of mental disorders and their relation to clinical, social and lifestyle factors. Soc. Psychiatry Psychiatr. Epidemiol. 2014;49:841–849. doi: 10.1007/s00127-014-0887-z. [DOI] [PubMed] [Google Scholar]
- Baumeister D., Akhtar R., Ciufolini S., Pariante C.M., Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol. Psychiatry. 2016;21:642–649. doi: 10.1038/mp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein R.S., Styner L.T., Elliot L.J., Kimbrough R., Falk H., Blade L. Inhalation exposure to formaldehyde: An overview of its toxicity, epidemiology, monitoring and control. Am. Ind. Hyg. Assoc. J. 1984;45:778–785. doi: 10.1080/15298668491400601. [DOI] [PubMed] [Google Scholar]
- Biosafe, US, 2019. Formalin Exposure: A Review of Known Health Hazards and the Role of Innovation in Improving Safety. Biosafe.us. 2019. 1.801.253.1600.
- Bogen S.A., Vani K., Sompuram S.R. Molecular mechanisms of antigen retrieval: antigen retrieval reverses steric interference caused by formalin-induced cross-links. Biotech. Histochem. 2009;84:207–215. doi: 10.1136/jclinpath-2011-200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkoway H., Dell L.D., Bofffetta P., Gallagher A.E., Crawford L., Lees P.S., Mundt K.A. Formaldehyde exposure and mortality risks from acute myeloid leukemia and other lymphohematopoietic malignancies in the US National Cancer Institute cohort study of workers in formaldehyde industries. J. Occup. Environ. Med. 2015;57(7):785–794. doi: 10.1097/JOM.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney J.E., Collins C.H. Formaldehyde disinfection in laboratories: Limitations and hazards. Br. J. Biomed. Sci. 1995;523:195–201. https://pubmed.ncbi.nlm.nih.gov/8527997/ [PubMed] [Google Scholar]
- Ciftci G., Aksoy A., Cenesiz S., Sogut M.U., Yarim G.F., Nisbet C., Guvene D., Ertekin A. Therapeutic role curcumin in oxidative DNA damage caused by formadenldehyde. Microsc. Res. Tech. 2015;78(5):391–395. doi: 10.1002/jemt.22485. [DOI] [PubMed] [Google Scholar]
- Cogliano V.J., Grosse Y., Baan R.A., Straif K., Secretan M.B., El Ghissassi F. Meeting report: summary of IARC monographs on formaldehyde, 2-butoxyethanol, and 1-tert-butoxy-2- propanol. Environ. Health. Perspect. 2005;113:1205–1208. doi: 10.1289/ehp.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M.L., Schray C.L., Luster C.N., Stewart Z.S., Korytko P.J., Khan K.N.M., et al. Assessment of fixatives, fixation, and tissue processing on morphology and RNA integrity. Exp. Mol. Pathol. 2006;80:183–191. doi: 10.1016/j.yexmp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Egwurugwu J.N., Ohamaeme M.C., Ekweogu C.N., Ngwu E.E., Ugwuezumba P.C., Ogunnaya F.U., Azudialu B.C., Izunwanne D.I., Nwamkpa P., Elendu M.U., Eberendu I.G. Effects of Formalin Inhalation on Physical Characteristics and Renal Profile of Albino Wistar Rats. Asian J. Med. Health. 2018;12(4):1–11. doi: 10.9734/AJMAH/2018/44412. [DOI] [Google Scholar]
- Fagundes C.P., Way B. Early-life stress and adult inflammation. Curr. Directions Psychol. Sci. 2014;23:277–283. doi: 10.1177/0963721414535603. [DOI] [Google Scholar]
- Flyvholm M.A., Menne T. Allergic contact dermatitis from formaldehyde: A rare study focusing on sources of formaldehyde exposure. Contact Dermatitis. 1992;27(1):27–36. doi: 10.1111/j.1600-0536.1992.tb05194.x. [DOI] [PubMed] [Google Scholar]
- Gladfelter W.G. A comparative analysis of the locomotory systems of medusoid Cnidaria. Helgolender wiss. Meeresunters. 1973;25:228–272. [Google Scholar]
- Haque Z., Rahman M.A., Khan M.Z.I. Alcohol-Based Fixatives can Better Preserve Tissue Morphology than Formalin. Int. J. Morphol. 2020;38:1371–1375. doi: 10.4067/S0717-95022020000501371. [DOI] [Google Scholar]
- Hayat M.A. Springer Science & Business Media; 2000. Microscopy, immunohistochemistry, and antigen retrieval methods: for light and electron microscopy.https://link.springer.com/book/10.1007/b112626 [Google Scholar]
- Inci M., Zararsız İ., Davarc M., Görür S. Toxic effects of formaldehyde on the urinary system. Turk. J. Urol. 2013;39(1):48–52. doi: 10.5152/tud.2013.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janero D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indexes of lipid-peroxidation and peroxidative tissue-injury. Free Radic. Biol. Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131. [DOI] [PubMed] [Google Scholar]
- Kilburn K.H. Neurobehavioral impairment and seizures from formaldehyde. Arch. Environ. Health. 1994;49(1):37–44. doi: 10.1080/00039896.1994.9934412. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li C.M., Lu Z., Ding S., Yang X., Mo J. Studies on formation and repair of fromaldehyde-damaged DNA by detection of DNA-protein crosslink and DNA breaks. Frontiers Biosci. 2006;11:991–997. doi: 10.2741/1856. [DOI] [PubMed] [Google Scholar]
- Lu Z., Li C.M., Qiao Y., Liu Y., Yan Y., Yang X. Type II Vanilloid receptor message system: one of the possible mechanism for the rise in asthma cases. Frontiers Biosci. 2005;10:2527–2533. doi: 10.2741/1717. [DOI] [PubMed] [Google Scholar]
- Lu Z., Li C.M., Qiao Y., Yan Y., Yang X. Effect of inhaled formaldehyde on learning and memory of mice. Indoor Air. 2008;18:77–83. doi: 10.1111/j.1600-0668.2008.00524.x. [DOI] [PubMed] [Google Scholar]
- Martin, H., Fischer, M.D., 1995. The Toxic Effects Of Formaldehyde And Formalin. San Francisco. (From the l∼athological Laboratory of Rush Medical College, Chicago.).
- Miller G.E., Chen E., Parker K.J. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol. Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelans C.B., Oostenrijk D., Moons M.J., et al. Formaldehyde substitute fixatives: effects on nucleic acid preservation. J. Clin. Pathol. 2011;64:960–967. doi: 10.1136/jclinpath-2011-200152. [DOI] [PubMed] [Google Scholar]
- O’Leary T.J., Fowler C.B., Evers D.L., et al. Protein fixation and antigen retrieval: chemical studies. Biotech. Histochem. 2009;84:217–221. doi: 10.3109/10520290903039086. [DOI] [PubMed] [Google Scholar]
- Otali D., Stockard C.R., Oelschlager D.K., et al. Combined effects of formalin fixation and tissue processing on immunorecognition. Biotech. Histochem. 2009;84:223–247. doi: 10.3109/10520290903039094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavilainen L., Edvinsson Å., Asplund A., et al. The impact of tissue fixatives on morphology and antibody-based protein profiling in tissues and cells. J. Histochem. Cytochem. 2010;58:237–246. doi: 10.1369/jhc.2009.954321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst R. Exposure to formaldehyde in anatomy: An occupational health hazard. Anatomical Rec. 1987;219:109–112. doi: 10.1002/ar.1092190202. [DOI] [PubMed] [Google Scholar]
- Panzacchi S., Gnudi F., Mandrioli D., Montella R., Strollo V., Merrick B.A., Belpoggi F., Tibaldi E. Effects of short and long-term alcohol-based fixation on Sprague-Dawley rat tissue morphology, protein and nucleic acid preservation. Acta Histochem. 2016;121(6):750–760. doi: 10.1016/j.acthis.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Demouliere B., Chenu F., Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharma. 2005;177(3):245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Rahman A.M., Sultana N., Ayman U., Bhakta S., Afrose M., Afrin M., Haque Z. Alcoholic fixation over formalin fixation: A new, safer option for morphologic and molecular analysis of tissues. Saudi J. Biol. Sci. 2021:1–8. doi: 10.1016/j.sjbs.2021.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restani P., Galli C.L. Oral toxicity of formaldehyde and its derivatives. Crit. Rev. Toxicol. 1991;21(5):315–328. doi: 10.3109/10408449109019569. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO), 2010. WHO Guidelines for Indoor Air Quality. Selected Pollutants. WHO Regional Office for Europe, Copenhagen. [PubMed]
- Zararsiz I., Sarsilmaz M., Tas U., Kus I., Meydan S., Ozan E. Protective effect of melatonin against formaldehyde-induced kidney damage in rats. Toxicol. Ind. Health. 2007;23(10):573–579. doi: 10.1177/0748233708089022. [DOI] [PubMed] [Google Scholar]