Abstract
Multispecies cropping systems contribute to sustainable agriculture with multiple ecosystem services. Effects of intercropping of various crops with faba beans on growth and yield parameters and disease severity of root rot, damping off and broomrape were investigated. This study was implemented in the laboratory, greenhouse and field to investigate the effect of the intercropping systems (fenugreek + faba bean, lupine + faba bean, garlic + faba bean and sole faba bean). The intercropping systems were combined with the application of arbuscular mycorrhiza fungi (AMF) and yeast as bio-control agents, compared to chemical application of herbicides (Glyphosate) and fungicides (Rizolex-T50), to control rot root diseases and broomrape weeds, Orobanche spp., of faba bean plants in vivo and under the naturally infested field. In vitro, yeast and Rizolex-T50 significantly inhibited mycelial growth of root pathogenic fungi. Intercropping with garlic and/or application of Rizolex-T, significantly decreased the incidence and disease index of root rot and damping-off diseases, meanwhile increased percentage of survival plants. In vivo, intercropping with fenugreek and/or application of Glyphosate, significantly reduced the number/weight of spikes/plot of broomrapes. Intercropping with fenugreek combined with AMF application promoted crop growth and significantly increased yield components. The AMF enhanced seed yield/ha when applied to the intercropping of faba bean + fenugreek and faba bean + garlic, showing the highest seed yield/ha with 3.722 and 3.568 ton/ha, respectively. Intercropping of faba bean with garlic integrated with AMF revealed the highest values of LER, 2.45, and net return, 2341 US$/ha. Our results suggested that using faba bean–garlic intercrop along with AMF inoculation can reduce root rot disease, damping off and broomrapes, as well as enhance the profitability of Egyptian farmer and sustainable production.
Keywords: Orobanche crenate, AMF, Yeast extract, Rizolex-T, Glyphosate
1. Introduction
The economic significance of growing faba bean (Vicia faba L.) in the world and Egypt owing to its high nutritional value (Lizarazo et al, 2015). Generally, frequent cultivation lead to development, persistence and high build-up of soil pathogens and parasitic weeds causing serious yield losses. On the other hand, intercropping system, also known as polyculture or mixed cropping, is an important farming practice that is commonly emphasized worldwide to avoid the inoculum buildup of soil borne plant pathogens (Panth et al. 2020). Intercropping practices, with varied crop cultivation, use comparatively low inputs and enhance quality of the agro-ecosystem as well as help to manage diseases, weeds and pests (Sharma et al., 2021, Maitra et al., 2021), by antagonistic secondary metabolites released by one plant root, which can effectively suppress the pathogen of another plant (Hao et al. 2010). Intercropping ensures many benefits like enhancing yield components, environmental safety, sustainability production and better ecosystem services. In intercropping systems, two crop species or more are grown simultaneously as they coexist and interact among themselves and the agro-ecosystems (Maitra et al. 2021).
Intercropping garlic, onion or caraway with faba bean significantly reduced both pre and post-emergence damping-off and rot root, compared to sole faba bean cultivation (Abdel-Monaim and K., A., M., Abo-Elyousr, , 2012, Mousa and El-Sayed, 2016). Likewise, intercropping fenugreek, lupine, Egyptian clover or flax with faba bean reduced the O. crenata infestation of faba bean and achieved high yield, LER and net return (Bakheit and A.Y., Allam, A.H., Galal, , 2002, Safina, 2017). The inhibition of O. crenata seeds germination when fenugreek was intercropped with legume plants is due to allelopathy and isolation the main inhibiting metabolite (Abbes et al., 2019). However, percentages of Orobanche infestation varied among different intercropping species (Abu-Shall and Ragheb, 2014).
Using Arbuscular Mycorrhiza Fungi (AMF) and yeast as bio-control agents for promoting plant growth and inducing plant resistance against certain soil-borne pathogenic fungi has recently received a lot of interest (Abouzeid and K.A., El-Tarabily, , 2010, Abdel-Razik and N. M.A., Sallam, A. M.I., Eraky, and M.H.A., Hassan, , 2012, Imara and A., N., A. Reyad, and S., E., El-Abeid, , 2018, Abd El-Hai and Ali, 2019). AMF exudates may be reduced seed germination of the Orobanche and Phelipanche species (Fernández-aparicio et al., 2010, Hassan and M., and R., A., Abakeer, , 2013, Fernãndez-aparicio et al., 2010). Soil treatment with Trichoderma spp. alone or in combination with aerial spray of glyphosate (50 ppm) was effective in reducing infection of Orobanches and increasing yields of peas, faba bean and tomatoes (Samejima and Y., Sugimoto, , 2018, El-Dabaa and H., Abd-El-Khair, , 2020).
Currently, Chemical fungicides are the most often used method for fungal disease management. However, chemical compounds leave toxic residues in plants that are harmful to animals and humans (Li et al. 2019). Furthermore, chemical fungicides pollute the environment owing to their sluggish biodegradation rates. As a result, the use of biocontrol agents instead of chemical fungicides has gained traction as an alternative disease management method that is both ecologically benign and decreases the harmful effects of fungicides (Khunnamwong et al. 2019).
Therefore, the present research was conducted to clarify the efficacy of intercropping systems combined with AMF or yeast extract as bio-control agents, in comparison to herbicides and fungicides application, for decreasing root rot diseases and O. crenata weed, as well as enhance growth and yield parameters of faba bean under field conditions.
2. Materials and methods
2.1. Laboratory experiment
2.1.1. In vitro isolation of root rot pathogens and yeast extract preparation
R. solani and F. solani was isolated from naturally infected roots of faba bean (Vicia faba L.) collected from Sers El-Layian Agricultural Research Station, ARC in Egypt. Infected roots tissues were cut into small pieces and surface sterilized using sodium hypochlorite 5% (NaOCl) for two minutes and washed three times with dH2O before being dried on double layers of sterile filter papers. The samples were firstly plated on water agar media. Three pieces were put in each plate then the plates were incubated at 25–27 °C for four days. The isolates were purified using single spore or hyphal tip techniques (Choi et al., 1999, Torbati et al., 2016). The causal organisms were initially identified microscopically according to the morphological features of mycelial, sclerotia and asexual spores using the description of (Barnett and Hunter, 1972, Barnett and Hunter, 1998) and confirmed the identification at the Department of Mycology, Plant Pathology Institute, Agricultural Research Center, Giza, Egypt. On the other hand, yeast extract was obtained from commercial bread yeast was employed as a source for Saccharomyces cerevisiae and prepared according to (Wehr and Frank, 2004, Gül et al., 2005).
2.2. In vitro screening of yeast extract and Rhizolex-T50 for antagonism to R. Solani and F. Solani
Approximately 20 μL of yeast extract (OD660 = 0.8–1.26 × 107 cells/mL) was added in the middle of agar plates having 15 ml of YPD agar medium. Each of R. solani and F. solani was cultured with 5 × 5 mm2 agar discs of pathogens, mycelium, which were placed on the middle of plates. Three replicates were prepared for each pathogen and the plates was sealed with parafilm. The control treatment represents the yeast-free agar plates with only the inoculation of the pathogenic fungus. The cultures were incubated at 25–28 °C for 6 days (Chen et al. 2018). On the other hand, the Rhizolex-T50 fungicide was used at 1000 ppm of the active ingredients. About 15 ml of sterilized PDA medium mixed with the fungicide was poured in Petri dishes. After solidification, dishes were inoculated using 5 × 5 mm2 discs of 7 days old PDA cultures of both fungi. Three replicates were used for each particular concentration of each fungicide. Three replicates of PDA dishes received no fungicides served as a control. The inoculated plates were incubated at 25-28˚C. The mycelial growth diameter was calculated 5–7 dpi (days post inoculation) comparing to the control where the dishes completely covered with the fungal growth. Reduction percentage of the mycelial growth was calculated based on the previous formula described by (Sundar et al. 1995).
2.3. Greenhouse experiment
Study the effect of intercropping, mycorrhiza fungus, yeast extract and Rizolex-T50 on faba bean root rot disease caused by R. solani and F. solani was carried out under greenhouse conditions in Sers El-Layian Agricultural Research Station, ARC, Egypt. Pots (60 cm diameter) and soil were sterilized with 5% formalin solution. After formalin evaporation, soil infestation was performed by inoculation with R. solani and F. solani at 2% of the soil weight (Papvizas and Davey, 1962). Inoculums were prepared by growing each of them in 500 ml conical flasks containing 200 ml of autoclaved potato dextrose broth (PDB) medium and incubated at 26 ± 1 °C for 10–15 days. The fungal growth was blended to homogenize the inoculum before infestation at the rate of 100 ml homogenized culture per pot as mentioned by (Abdel-Monaim et al. 2011).
Ten seeds of faba bean cv. Sakha 1 were treated with mycorrhiza fungus, yeast extract and Rizolex-T50 (50 %WSC) before intercropped with fenugreek, lupin and garlic separately in three pots, then replicated three for each treatment. The percentages of pre-, post-emergence damping-off, root-rotted plants and survived plants calculated after 15, 30 and 90 days of sowing, respectively, according the following formula:
2.4. Field experiment
This work was carried out at Experimental Farm of Sers El-Layian Agric. Res. Stat., (30° 25′ 60 N ; 30° 58′ 0E), Egypt during 2019/2020 and 2020/2021 seasons. Experimental soil was clay texture and naturally infested with root rot diseases and Orobanche crenate. The experimental design was split-plot with three replicates. Sub-plot area was 10.5 m2 (3 X 3.5 m). The main plots were assigned to intercropping treatments (fenugreek + faba bean, lupine + faba bean, garlic + faba bean and sole faba bean), while the sub-plots were assigned to bio-control agent (Mycorrhiza Fungi and yeast extract) and chemical control agent (Rizolex-T50 as fungicide and Glyphosate (Roundup 48%) as herbicide) compared to control (without any control agents). The herbicide Glyphosate was applied at recommenced dose (180 cm3 /hectare). Sowing date of faba bean (Vicia faba L.), fenugreek (Trigonella foenum-graecum L.) and lupin (Lupinus termis L.) were on November 20th and 21th in 2019/20 and 2020/21, respectively, while garlic (Allium sativum L.) was sown 30 days earlier than faba bean in both seasons, under sole and intercropping system. Faba bean seeds cv. Sakha 1 was planted on both sides of ridge (60 cm) at 25 cm and one plants/hill. Fenugreek seeds cv. Giza 30 was broadcast on top of the ridges at rate of 4 g/m2, while lupine seeds cv. Giza1 and garlic cloves cv. Balady were planted in one row on the top of ridge at 20 and 10 cm apart between hills, respectively. Cultural management of all crops was practiced as recommended by the Egyptian Ministry of Agriculture. Data was recorded as follows:
-
a)
Soil-borne pathogens
The percentage of infection was recorded as pre-, post-emergence damping-off, root rot and the survived faba bean plants.
-
b)
Orobanche parasitic weed
The number and weight (g) of emerged O. crenata spikes was recorded /plot.
-
c)
Faba bean and the three intercrops
At harvest, data on faba bean plant height (cm), number of branches/plant, number of pods/plant and seeds weight/plant (g) were collected for ten randomly plants taken from the internal ridges. Yield (ton/ha) of faba bean and intercrops was estimated from all plants/plot and converted to ton/hectare.
-
d)
Land equivalent ratio
Land Equivalent Ratio (LER) according to Willey (1979) was calculated as follows:
Where: Yaa and Ybb = pure stand yield of a (faba bean) and b (intercrops). Yab and Yba = intercropping yield of a and b
-
e)
Economic evaluation
Total and net return of intercropping were compared with sole faba bean. Price of main products and cost were presented by Bulletin of Statistical Cost Production and Net Return (2019 and 2020).
2.5. Statistical analysis
Statistical analysis was performed using analysis of variance using MSTATC statistical package (Freed, 1991). Treatments mean were compared by L.S.D. test at 0.05 level of probability (Gomez and Gomez 1984).
3. Results
3.1. Laboratory experiment
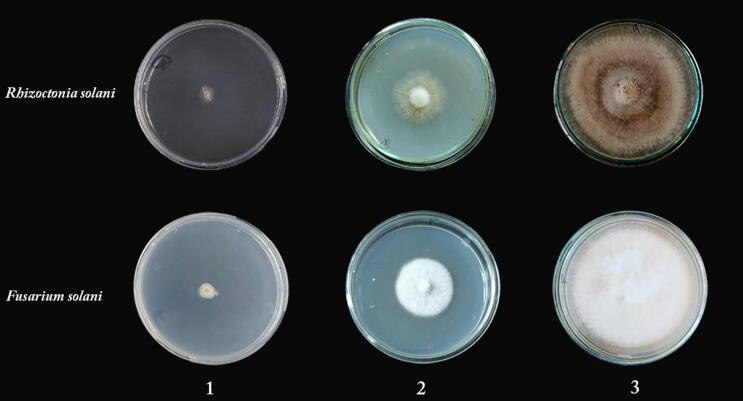
Both of yeast extract and the fungicide Rizolex-T50 were evaluated to inhibit the mycelial growth of R. solani and F. solani and the results were taken after 7 dpi (days post inoculation), indicating a significant inhibition against both pathogenic fungi as compared to the control, as shown in (Fig. 1). The fungicide Rizolex-T50 showed better inhibition than yeast extract against both pathogens, R. solani and F. solani. Since Rizolex-T50 showed a nearly complete reduction of mycelial growth for both pathogens, while yeast suspension recorded 44, 40% inhibition towards R. solani and F. solani.
Fig. 1.
Effect of yeast extract and Rizolex-T50 on mycelial growth of R. Solani and F. solani. (1) Rizolex-T50, (2) Yeast extract and (3) Control (fungus without any treatment).
3.2. Greenhouse experiment
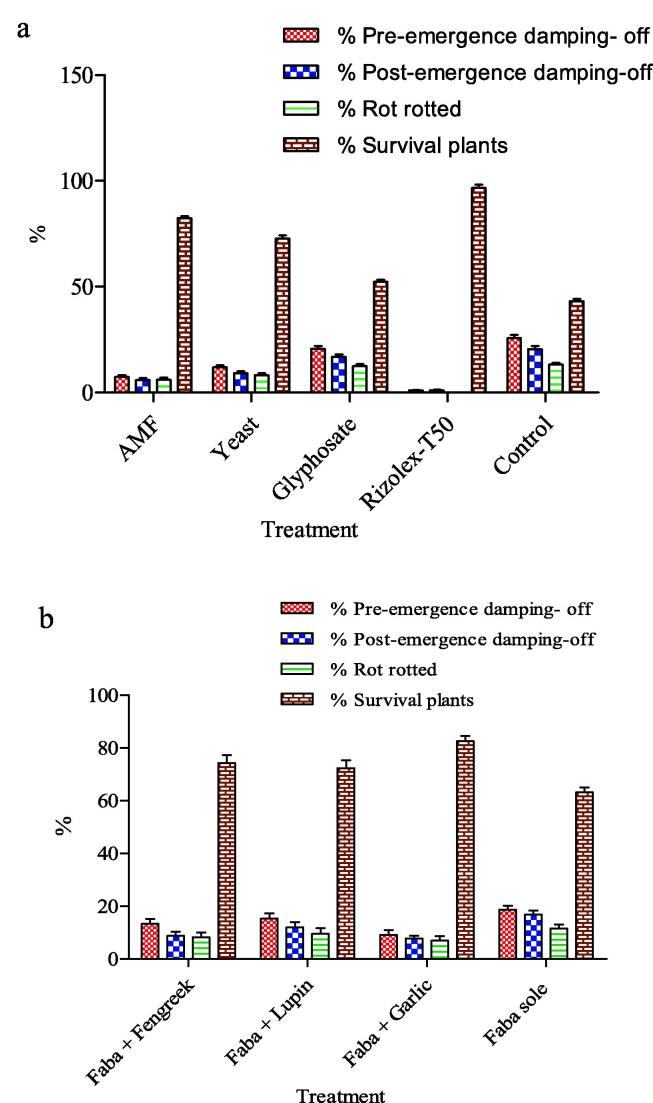
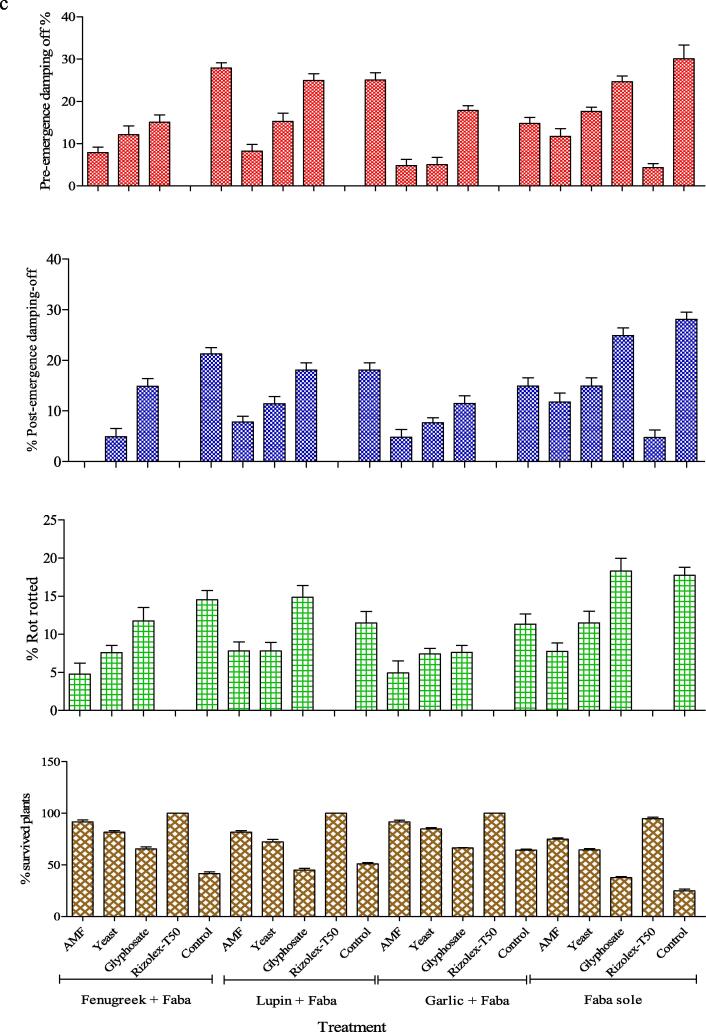
Fig. 2 indicated that intercropping systems, bio-agents and chemical controls and their interaction significantly decreased pre, post-emergence damping-off and root rot diseases, consequently increase plants survival %, as compared to sole faba bean. The most effective intercropping system was garlic, followed by fenugreek. In addition, Rizolex-T50 ranked in the first position, while mycorrhiza treatment ranked in the second position, and yeast was the third in reducing faba bean damping-off and root rot diseases. Generally, the lowest values of pre, post-emergence and root rot diseases occurred under the application of Rizolex-T50 in all intercropping systems, which significantly decreased the occurrence of root rot and damping-off diseases, followed by mycorrhiza treatment and yeast under any intercropping system.
Fig. 2.
Effect of (a) bio and chemical controls, (b) the intercropping system, and (c) their interactions on damping off, rot root and survived plants percentage of faba bean in greenhouse.
4. Field experiment
4.1. Disease assessment
Intercropping three intercrops with faba bean significantly reduce damping-off and root rot disease, as well as significantly increased plant survival % compared with sole faba bean (Table 1). Also, Rizolex-T50 was the most effective treatment in decrease damping-off and root rot diseases, followed by mycorrhiza then yeast compared to without treatment, irrespective of the intercropping system. Concerning interactions between two factors, the results indicated that no infections were recorded under treatment with Rizolex-T50 coupled with garlic intercropping system in both seasons (Table 1). Likewise, intercropping fenugreek with applied Rizolex-T50 and /or intercropping garlic coupled with mycorrhiza had the lowest pre and post-emergence damping-off and root rot disease, compared to the other treatments, while the highest values of survival plants were recorded with these treatments.
Table 1.
Effect of the intercropping system, bio and chemical control agents and their interactions on damping off, rot root and survived plants percentage of faba bean in field during both seasons.
| Treatment | Trait |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-emergence damping off % |
Post-emergence damping off % |
Rot rotted% |
% survived plants |
||||||
| 2019/20 | 2020/21 | 2019/20 | 2020/21 | 2019/20 | 2020/21 | 2019/20 | 2020/21 | ||
| Faba + Fenu. | AMF | 10.00 | 8.33 | 2.00 | 1.66 | 3.00 | 2.33 | 85.00 | 87.66 |
| Yeast | 11.66 | 9.33 | 6.33 | 5.33 | 5.00 | 3.33 | 77.00 | 82.00 | |
| Glyphosate | 15.00 | 14.66 | 11.00 | 6.66 | 5.66 | 4.33 | 68.33 | 74.33 | |
| Rizolex-T50 | 0.00 | 0.33 | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 | 99.66 | |
| Control | 17.33 | 18.00 | 12.33 | 9.00 | 7.33 | 6.00 | 63.00 | 67.00 | |
| Mean | 10.80 | 10.13 | 6.33 | 4.53 | 4.20 | 3.20 | 78.66 | 82.13 | |
| Faba + Lupin | AMF | 13.33 | 9.66 | 4.33 | 3.66 | 4.33 | 3.00 | 78.00 | 83.66 |
| Yeast | 14.00 | 11.00 | 7.66 | 7.00 | 6.33 | 4.66 | 72.00 | 77.33 | |
| Glyphosate | 22.66 | 18.66 | 12.00 | 10.66 | 7.00 | 6.00 | 58.33 | 64.66 | |
| Rizolex-T50 | 0.33 | 0.33 | 0.00 | 0.00 | 0.00 | 0.00 | 99.66 | 99.66 | |
| Control | 24.33 | 18.66 | 14.66 | 13.00 | 9.00 | 7.66 | 52.00 | 60.66 | |
| Mean | 14.93 | 11.66 | 7.73 | 6.86 | 5.33 | 4.26 | 72.00 | 77.20 | |
| Faba + Garlic | AMF | 7.00 | 7.00 | 1.66 | 1.33 | 1.66 | 1.33 | 89.66 | 90.33 |
| Yeast | 9.00 | 8.00 | 3.33 | 3.33 | 2.33 | 2.00 | 85.33 | 86.66 | |
| Glyphosate | 12.00 | 13.33 | 8.00 | 5.33 | 4.66 | 4.33 | 75.00 | 77.00 | |
| Rizolex-T50 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 | 100.00 | |
| Control | 13.33 | 14.33 | 10.00 | 6.66 | 6.66 | 4.66 | 70.00 | 74.66 | |
| Mean | 8.26 | 8.46 | 4.60 | 3.33 | 3.06 | 2.46 | 84.06 | 85.73 | |
| Faba sole | AMF | 17.33 | 11.00 | 8.00 | 7.00 | 6.00 | 4.00 | 68.66 | 78.00 |
| Yeast | 19.66 | 13.33 | 11.00 | 7.66 | 8.00 | 5.66 | 61.66 | 73.33 | |
| Glyphosate | 26.00 | 20.33 | 13.33 | 12.33 | 11.33 | 7.33 | 49.33 | 60.00 | |
| Rizolex-T50 | 0.00 | 0.33 | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 | 99.66 | |
| Control | 28.66 | 24.66 | 21.66 | 20.66 | 15.00 | 9.66 | 34.66 | 45.00 | |
| Mean | 18.33 | 13.93 | 10.80 | 9.53 | 8.06 | 5.33 | 62.86 | 71.20 | |
| AMF | 11.91 | 9.00 | 4.00 | 3.41 | 3.75 | 2.66 | 80.33 | 84.91 | |
| Yeast | 13.58 | 10.41 | 7.08 | 5.83 | 5.41 | 3.91 | 74.00 | 79.83 | |
| Glyphosate | 18.91 | 16.75 | 11.08 | 8.75 | 7.16 | 5.50 | 62.83 | 69.00 | |
| Rizolex-T50 | 0.08 | 0.25 | 0.00 | 0.00 | 0.00 | 0.00 | 99.91 | 99.75 | |
| Control | 20.91 | 18.83 | 14.66 | 12.33 | 9.50 | 7.00 | 54.91 | 61.33 | |
| LSD 0.05 A | 1.75 | 0.53 | 1.39 | 1.11 | 0.44 | 0.61 | 1.81 | 0.80 | |
| LSD 0.05B | 1.02 | 0.99 | 1.02 | 0.88 | 0.76 | 0.54 | 1.54 | 1.40 | |
| LSD at 0.05 A × B | 2.04 | 1.98 | 2.03 | 1.76 | 1.51 | 1.07 | 3.09 | 2.80 | |
4.2. Broomrape parasitic weed
Results in Table 2 indicated that the intercropping fenugreek, lupin and garlic exhibited highly significant decreasing in the number of spikes/plot by 55.50, 41.42 and 48.83% and dry weight of spikes/plot by 48.48, 35.98 and 43.57%, respectively, compared to sole faba bean as average of both seasons. That is indicated that intercropping treatments would help to decrease O. crenate infestation in faba bean fields. Glyphosate as chemical control agent was superior in reduced the previous mentioned characters followed by inoculation faba bean seeds with AMF then yeast as bio-control. Regarding the interactions effect of intercropping and control agents on O. crenate infection, highly significant reduction in number of spikes/plot and spikes weight/plot were obtained, in both season. Spray glyphosate coupled with intercropping fenugreek or garlic was the best effective treatment for reduced O. crenate infection in faba bean fields, which reduced number and weight of broomrape by 82.16 and 77.67% for fenugreek and 83.33 and 78.07 for garlic over sole faba bean with control, orderly, as average of both seasons.
Table 2.
Effect of intercropping system, bio and chemical control and their interactions on Orobanche weed and growth characters of faba bean during both seasons.
| Treatment | Trait |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Orobanche/plot |
Plant height (cm) |
No. of Branches /plant |
Orobanche/plot |
Plant height (cm) |
No. of Branches/plant |
||||
| No.of spikes |
Weight(g) |
No.of spikes |
weight(g) |
||||||
| 2019/2020 season | 2020/2021 season | ||||||||
| Faba + Fenu. | AMF | 33.33 | 53.33 | 106.93 | 4.10 | 29.00 | 43.25 | 123.83 | 3.37 |
| Yeast | 34.00 | 59.98 | 103.17 | 4.13 | 31.00 | 48.76 | 123.83 | 3.30 | |
| Glyphosate | 28.67 | 47.40 | 97.83 | 3.70 | 6.00 | 35.90 | 113.83 | 3.20 | |
| Rizolex-T50 | 61.67 | 80.62 | 98.50 | 3.87 | 48.00 | 68.84 | 115.40 | 3.50 | |
| Control | 75.67 | 105.12 | 89.50 | 2.90 | 73.00 | 93.12 | 86.15 | 2.97 | |
| Mean | 46.67 | 69.29 | 99.19 | 3.74 | 37.40 | 57.97 | 112.61 | 3.27 | |
| Faba + Lupin | AMF | 52.33 | 87.93 | 111.23 | 3.33 | 40.00 | 69.31 | 128.33 | 3.27 |
| Yeast | 52.00 | 70.43 | 100.67 | 4.00 | 78.00 | 62.56 | 133.17 | 3.00 | |
| Glyphosate | 33.00 | 41.80 | 99.33 | 2.70 | 28.00 | 36.59 | 116.67 | 2.80 | |
| Rizolex-T50 | 70.67 | 104.68 | 101.00 | 3.03 | 61.00 | 95.84 | 124.83 | 3.17 | |
| Control | 88.33 | 117.10 | 93.87 | 2.10 | 80.00 | 104.49 | 90.25 | 2.47 | |
| Mean | 59.27 | 84.39 | 101.22 | 3.03 | 51.40 | 73.75 | 118.65 | 2.94 | |
| Faba + Garlic | AMF | 44.33 | 60.64 | 105.67 | 4.33 | 36.00 | 47.69 | 122.00 | 4.00 |
| Yeast | 52.00 | 64.98 | 101.37 | 4.00 | 42.00 | 59.98 | 115.17 | 3.70 | |
| Glyphosate | 32.00 | 40.90 | 96.33 | 3.33 | 25.00 | 36.09 | 109.67 | 3.47 | |
| Rizolex-T50 | 60.67 | 92.94 | 100.00 | 4.03 | 52.00 | 89.16 | 116.00 | 3.57 | |
| Control | 73.33 | 108.20 | 86.13 | 3.03 | 66.00 | 96.33 | 85.67 | 3.00 | |
| Mean | 52.47 | 73.53 | 97.90 | 3.75 | 44.20 | 65.85 | 109.70 | 3.55 | |
| Faba sole | AMF | 77.00 | 121.71 | 103.17 | 4.40 | 67.00 | 104.57 | 131.17 | 4.23 |
| Yeast | 102.33 | 115.41 | 100.67 | 3.93 | 83.00 | 108.50 | 116.67 | 3.93 | |
| Glyphosate | 46.67 | 69.18 | 95.00 | 3.77 | 31.00 | 59.24 | 97.50 | 3.70 | |
| Rizolex-T50 | 100.67 | 155.45 | 98.67 | 4.20 | 95.00 | 150.00 | 115.33 | 3.63 | |
| Control | 225.00 | 175.20 | 83.20 | 1.87 | 117.00 | 175.80 | 79.52 | 3.23 | |
| Mean | 110.33 | 127.39 | 96.34 | 3.63 | 78.60 | 119.62 | 108.04 | 3.75 | |
| Mycorrhizal | 51.75 | 80.90 | 106.75 | 4.04 | 43.00 | 66.20 | 126.33 | 3.72 | |
| Yeast | 60.08 | 77.70 | 101.72 | 4.02 | 51.00 | 69.95 | 122.21 | 3.48 | |
| Glyphosate | 35.08 | 49.82 | 97.13 | 3.38 | 22.50 | 41.95 | 109.42 | 3.29 | |
| Rizolex | 73.42 | 108.42 | 99.54 | 3.78 | 64.00 | 100.96 | 117.89 | 3.47 | |
| Without | 115.58 | 126.40 | 88.18 | 2.48 | 84.00 | 117.62 | 85.40 | 2.92 | |
| LSD 0.05 A | 3.72 | 2.76 | N.S | 0.06 | 6.20 | 6.99 | N.S | 0.53 | |
| LSD 0.05B | 5.34 | 4.50 | 2.79 | 0.17 | 5.08 | 5.05 | 8.11 | 0.40 | |
| LSD 0.05 A × B | 10.69 | 9.00 | N.S | 0.33 | 10.15 | 10.11 | N.S | N.S | |
4.3. Faba bean growth characters
Results in Table 2 confirm that the intercropping systems had insignificant effect on plant height. Conversely, growing faba bean separately significantly increased number of branches/plant over the intercropping. Meanwhile, inoculating faba bean seeds with AMF significantly increased plant height and number of branches/plant, followed by yeast.
Significant increases in number of branches/plant by the interaction effect only in first season (Table 2). However, the highest plant height was obtained by intercropping faba bean with lupine and application of AMF. Whereas, the maximum branches/plant (4.40) was achieved by inoculation of sole faba bean seeds with AMF, followed by intercropping garlic wit faba bean along with AMF application (4.33). Contrary, control produces the lowest values of plant height and number of branches/plant.
4.4. Faba bean yield characters
Results in (Table 3) showed that intercropping fenugreek significantly increased pods number/plant, seed weight/plant and seed yield/ha, followed by garlic, without significant differences in most traits except 100-seed weight and seed yield/fad in both seasons. Intercropping fenugreek, lupin and garlic with faba bean increased seed yield/fad comparison to sole faba bean by 37.21, 13.94 and 30.71% as average of both seasons. Concerning bio and chemical control agents, the maximum values of yield and yield components for faba bean were achieved with AMF, followed by Rizolex-T50 compared to other treatments (Table 3). Application AMF, Yeast, glyphosate and Rizolex-T50 significantly increased faba bean seed yield/ha by 35.98, 14.20, 16.31 and 30.38 %, respectively, compared to without, as average of both seasons.
Table 3.
Effect of intercropping system, bio and chemical control and their interactions on yield and its components characters of faba bean during both seasons.
| Treatment | Trait |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pods /plant (No.) |
Seed wt. /plant (g) |
100-seed wt. (g) |
Seed yield (t/ha) |
Pods /plant (No.) |
Seed wt. /plant (g) |
100-seed wt. (g) |
Seed yield (t/ha) |
||
| 2019/2020 season | 2020/2021 season | ||||||||
| Faba + Fenu. | AMF | 19.10 | 23.10 | 86.10 | 3.470 | 24.30 | 37.35 | 95.00 | 3.975 |
| Yeast | 16.00 | 19.00 | 76.99 | 2.951 | 19.57 | 17.26 | 90.00 | 3.315 | |
| Glyphosate | 16.53 | 19.53 | 82.72 | 3.206 | 16.20 | 21.61 | 91.33 | 3.094 | |
| Rizolex-T50 | 17.60 | 20.60 | 85.81 | 3.375 | 17.33 | 29.01 | 94.00 | 3.953 | |
| Control | 12.10 | 12.52 | 75.14 | 2.723 | 15.43 | 16.52 | 89.98 | 2.863 | |
| Mean | 16.27 | 18.95 | 81.35 | 3.146 | 18.57 | 24.35 | 92.06 | 3.440 | |
| Faba + Lupin | AMF | 13.47 | 16.47 | 86.84 | 2.996 | 14.47 | 18.80 | 90.33 | 3.251 |
| Yeast | 11.13 | 14.13 | 76.77 | 2.530 | 13.67 | 16.88 | 90.33 | 2.697 | |
| Glyphosate | 12.67 | 15.67 | 81.98 | 2.692 | 12.13 | 17.27 | 90.67 | 2.835 | |
| Rizolex-T50 | 12.93 | 15.93 | 85.56 | 2.697 | 13.93 | 16.25 | 91.33 | 3.065 | |
| Control | 9.77 | 10.00 | 72.00 | 2.206 | 11.63 | 14.33 | 90.13 | 2.373 | |
| Mean | 11.99 | 14.44 | 80.63 | 2.625 | 13.17 | 16.71 | 90.56 | 2.844 | |
| Faba + Garlic | AMF | 18.00 | 23.00 | 84.41 | 3.382 | 22.10 | 27.92 | 94.33 | 3.753 |
| Yeast | 13.20 | 16.20 | 78.14 | 2.939 | 19.53 | 25.12 | 90.33 | 3.208 | |
| Glyphosate | 16.57 | 19.57 | 80.26 | 3.082 | 19.00 | 23.16 | 93.33 | 2.999 | |
| Rizolex-T50 | 16.33 | 17.33 | 86.37 | 3.134 | 20.60 | 28.74 | 93.00 | 3.451 | |
| Control | 10.13 | 11.98 | 75.65 | 2.673 | 16.93 | 19.59 | 91.00 | 2.744 | |
| Mean | 14.85 | 17.62 | 80.97 | 3.043 | 19.63 | 24.91 | 92.40 | 3.231 | |
| Faba sole | AMF | 14.73 | 18.73 | 79.65 | 2.649 | 20.73 | 20.90 | 84.67 | 2.875 |
| Yeast | 11.17 | 15.17 | 72.78 | 1.966 | 18.43 | 18.98 | 79.00 | 2.530 | |
| Glyphosate | 13.43 | 16.43 | 75.82 | 2.185 | 16.17 | 15.77 | 82.00 | 2.404 | |
| Rizolex-T50 | 15.53 | 17.53 | 80.95 | 2.573 | 20.53 | 20.14 | 87.33 | 3.035 | |
| Control | 8.63 | 9.18 | 70.55 | 1.666 | 13.03 | 13.74 | 87.20 | 2.123 | |
| Mean | 12.70 | 15.41 | 75.95 | 2.207 | 17.78 | 17.91 | 84.04 | 2.593 | |
| Mycorrhizal | 16.33 | 20.33 | 84.25 | 3.124 | 20.40 | 26.24 | 91.08 | 3.464 | |
| Yeast | 12.88 | 16.13 | 76.17 | 2.597 | 17.80 | 19.56 | 87.42 | 2.938 | |
| Glyphosate | 14.80 | 17.80 | 80.20 | 2.792 | 15.88 | 19.45 | 89.33 | 2.833 | |
| Rizolex | 15.60 | 17.85 | 84.67 | 2.945 | 18.10 | 23.53 | 91.42 | 3.376 | |
| Without | 10.16 | 10.92 | 73.33 | 2.318 | 14.26 | 16.04 | 89.58 | 2.526 | |
| LSD 0.05 A | 1.16 | 0.89 | 0.52 | 0.109 | 0.89 | 0.95 | 1.66 | 0.202 | |
| LSD 0.05B | 0.92 | 1.09 | 1.26 | 0.095 | 0.87 | 1.06 | 1.48 | 0.146 | |
| LSD 0.05 A × B | N.S | 2.18 | N.S | N.S | 1.75 | 2.11 | 2.95 | N.S | |
Significant difference in yield and its components of faba bean were noticed due to interaction between studied factors, except number of pods/plant (Table 3). Intercropping fenugreek with faba bean, which inoculation with AMF enhanced the most faba bean yield components over the other treatments. In contrast, the lowest values of yield components were produced by control. Similarly, the highest seed yield 3.470 and 3.975 ton/ha was achieved with faba bean inoculation by AMF and intercropped with fenugreek in 1st and 2nd season, followed by 3.382 ton/ha by intercropping garlic plus AMF in 1st season and 3.953 ton/ha by intercropping fenugreek and application Rizolex-T50 in 2nd season.
4.5. Yields of three intercrops
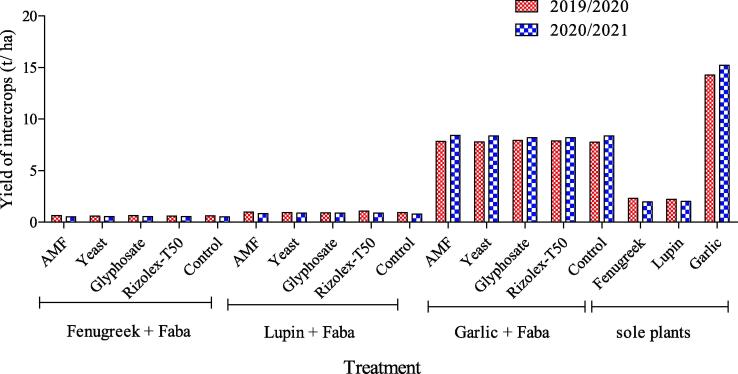
Yield of fenugreek, lupin and garlic in both seasons illustrated in Fig. 3. The garlic had the highest yield in sole and intercropping systems, while fenugreek had the lowest yield.
Fig. 3.
Yields of fenugreek, lupin and garlic under intercropping and sole culture in two seasons.
4.6. Competitive relationship and economic evaluation
4.6.1. Land equivalent ratio (LER)
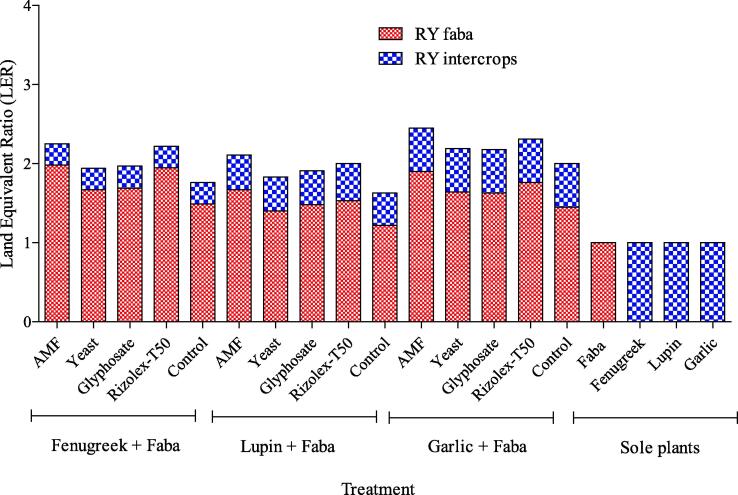
Fig. 4 illustrated that intercropping three intercrops with faba bean increase LER than one compared to control. The highest values of RYfaba 1.98 was obtained by intercropping fenugreek with faba bean, which inculcated with AMF in as average of both seasons. While the mean relative yield values of intercrops varied and were 0.27 for fenugreek, 0.44 for lupin and 0.55 for garlic. Therefore, the highest values of LER 2.45 were produce by intercropping garlic with faba bean coupled with AMF, followed by 2.30 with garlic and Rizolex-T50. While the lowest values of LER 1.64 was obtained by intercropping lupin and faba bean without applied any control agents, however it’s was more advantage than sole faba bean (1.0). The LER of 2.45 indicates that a sole faba bean would require 145% more land to achieve the same yield as intercropping.
Fig. 4.
Interaction effect of intercropping systems and bio and chemical control agents LER as average in both seasons.
4.7. Economic evaluation
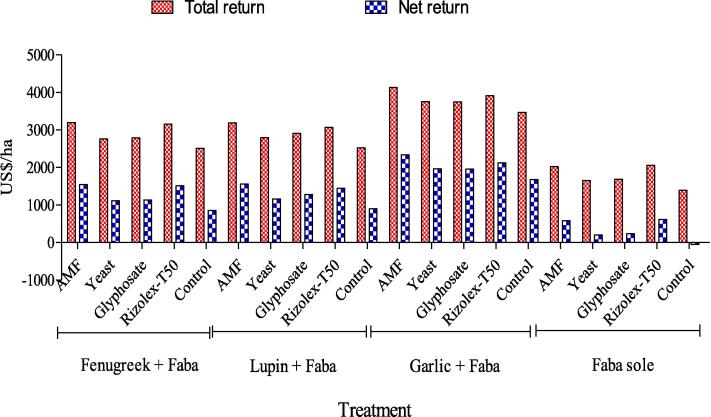
Results in Fig. 5 indicate that sowing faba bean separate with or without bio or chemical control agents had lowest total and net return than intercropping faba bean. The highest total return 4130 US$/ha and net return 2342 US$/ha were obtained by intercropping garlic with faba bean coupled with AMF as average of two seasons. Thereby, intercropping faba bean more financially rewarding to Egyptian farmer’s than sole faba bean.
Fig. 5.
Interaction effect of intercropping systems and bio and chemical control agents on total and net income as average of both seasons.
5. Discussion
Under the intercropping system, the mechanisms acting on the suppression of soil-borne pathogens and weeds may be due to reducing the host density, altering the soil environment and allelopathy. Several studies confirm the role of allelopathic substances in suppression soil-borne diseases and parasitic weed (Hao et al., 2010, Mousa and El-Sayed, 2016, Abbes and I., Trabelsi, M., Kharrat, M, Amri, , 2019). Exudates of faba bean decreased significantly, which reduced the amount of nutrients needed for pathogen growth, limited the proliferation of pathogens and contributed to the alleviation of Fusarium wilt (Lv et al 2020). The allelochemicals released by fenugreek roots significantly reduced seed germination and numbers of underground Orobanche attachments with faba bean especially in the resistant cultivar (Abbes et al., 2019). Also, garlic is an allelopathic crop that reduces the obstacles to sole culture (Cheng et al., 2016).
Mycorrhiza and yeast as biocontrol agent against soil-borne pathogens has been also investigated by (Abouzeid and K.A., El-Tarabily, , 2010, Abdel-Razik and N. M.A., Sallam, A. M.I., Eraky, and M.H.A., Hassan, , 2012), where mycorrhiza increases the solubility of phosphates in soils and enhance nitrogen fixing ability of legumes. The antagonistic potential effects of AMF controlled the incidence of root rot disease in field (Nawaz-Noor and S., and K., R., Sreeramulu, , 2017, Abd-El-Khair and K., H., E., Haggag, H., I., Seif El-Nasr, , 2018, Imara and A., N., A. Reyad, and S., E., El-Abeid, , 2018). Yeast produced the hydrolytic enzymes that degrade the cell wall of the pathogenic fungi (Jadhav and Sayyed, 2016). The inhibited effect of yeast on mycelial growth of pathogenic fungi may be explained based on the production of soluble antifungal metabolites and proteinaceous toxins (Abd El-Hai and Ali 2019). Also, yeasts may efficiently attack harmful fungus strains and reduce or inhibit growth of destructive microbes as reviwed in (Liu et al., 2013, Horváth et al., 2020). Various mechanisms, such as nutrition competition or antifungal chemical production, have been hypothesized to explain their antagonistic action (Heydari and Pessarakli, 2010, Guerrero et al., 2014, Elnahal et al., 2022). They can create siderophores, cell wall disintegrating enzymes, and other unidentified bioactive compounds (Qin et al., 2004, Mayo et al., 2015, Ghosh et al., 2017, Zhimo et al., 2017, Di Canito et al., 2021). Rizolex-T50 was the most effective treatment may be due to the nearly entirely inhibition of mycelia growth of fungal pathogens. This finding was in harmony with (Mahmoud et al 2018). Potential mechanisms by which Rizolex-T50 suppressed plant fungal diseases were previously addressed since it has significantly enhanced the production of toxic free-radicals and stimulated some defense mechanisms such as total phenolic compounds, flavonoids, ascorbic acid, antioxidant enzymes, and other metabolites, while reduced hydrogen peroxide, MDA and glutathione (Ali et al., 2009, Abdel-Monaim et al., 2011, Mohamed and Akladious, 2017). Also, it should be noted that Rizolex-T50 may also have a direct antibacterial action and are therefore implicated in cell wall crosslinking, gene expression stimulation, phytoalexin synthesis, and systemic resistance induction. (Begum et al., 2010, El-Mohamedy and Abd Alla, 2013, El-Mohamedy et al., 2015).
Also, faba bean treated by AMF can also provide good protection against parasitic weeds. The AMF symbiosis decreased strigolactone (SL), which substance stimulates parasitic germination, production by the host plant can be utilization as a biocontrol approach against parasites weed (Fernández-aparicio et al., 2010, López-Ráez et al., 2011). O. crenata infestation was successfully suppressed with AMF soil treatment. Plants infected with AMF showed fewer Orobanche attachments and formed tubercles at a slower rate (Habimana et al. 2014). However, glyphosate (Round up) superiority bio control agents in reduced the number and weight of O. crenata/plot. Glyphosate efficiency is attributed to the herbicide translocate unmetabolised to the underground Orobanche via the haustorium inflicting its suppressive action in the parasite (Gressel, 2009). The weeds can be controlled by using resistant or tolerant varieties, microbiological approach, cultural practices, chemical controls and integrated management (Samejima and Sugimoto, 2018).
The positive effect of intercropping on growth and yield of faba bean could be attributed to reduce negative effect of soil diseases and Orobanche weed, which increased plants survival percentage and improved yield components (Abbes et al., 2019). Further, biological N fixing and chemicals secreted by legume roots that is stimulate the growth of faba bean plants (Bakheit and A.Y., Allam, A.H., Galal, , 2002, Ahmed and N., G. Abdallah, R. M., Abd- El raoufn, , 2015). Similarly, inoculation faba bean seeds with mycorrhiza or yeast improve their growth and yield by increasing the nutrient uptake and producing growth promoting compounds as well as plant protection to several diseases and parasitic weeds (.Hisamuddin et al., 2015, Nawaz-Noor and S., and K., R., Sreeramulu, , 2017, Abd-El-Khair and K., H., E., Haggag, H., I., Seif El-Nasr, , 2018, Elnahal et al., 2022). AMF improving crop yield by destroying the O. crenata at its early developmental stages (El-Dabaa and Abd-El-Khair 2020). AMF root exudates had a harmful effect on the germination of O. cumana stimulated by germination stimulants and AMF spore exudates might provide a similar effect (Louarn et al. 2016). Exudation and Production of strigolactone were significantly decreased by AMF symbiosis in tomato (Lopez-Raez et al., 2011), as well as Mycorrhizal root exudates inhibited O. cumana germination (Louarn et al., 2012). Root exudates from AMF-colonized pea plants had minimal germination inducing activities toward O. crenata, O. foetida, O. minor and P. aegyptiaca (Fernández-aparicio et al., 2010, Fernández-Aparicio et al., 2013, Habimana et al., 2014). On the other hand, AMF-treated plants showed substantial increases in nutritional content, phenolics, and chlorophyll content when compared to untreated control plants. The activities of POX, PAL, and PPO were elevated in the roots of the host plant (Komeil and Badry 2021) and the nutrient uptake was enhanced by the successfully colonized roots, besides the Orobanche germination was reduced or delayed after mycorrhizal colonization (Hassan and Abakeer 2013).
Undoubtedly, integrating agronomic, chemical and biological control agents can benefit growers not only by reducing rot root disease and O. ceranata, but also by increasing productivity of faba bean (Abu-Shall and E. I. M., Ragheb, , 2014, El-Dabaa, 2019), LER, total and net return compared control (Ahmed and N., G. Abdallah, R. M., Abd- El raoufn, , 2015, Safina, 2017). The improved resistance of faba bean to aggressive broomrape might be attributable to the plant's specific defense mechanisms induced by AMF treatment (Komeil and Badry 2021). AMF can play an important role in natural and agricultural environments by influencing plant nutrition, soil biological activity, and changing nutrient availability by plants (Ingraffia et al., 2019, Elnahal et al., 2022).
Intercropping is a technique for increasing soil fertility and crop production. There is growing interest in intercropping due to the need to reduce nitrogen costs and soil erosion. Intercropping is already utilised in parts of Africa as a low-cost method of managing broomrapes (Oswald et al., 2002, Habimana et al., 2014). Intercropping with fenugreek, or berseem clover has been shown to minimize O. crenata infection on faba bean and pea owing to allelopathic interactions, for example, trigoxazonane was found in fenugreek root exudates, perhaps responsible for the prevention of O. crenata seed germination (Fernández-aparicio et al., 2010, Zeid and Komeil, 2019, Fernãndez-aparicio et al., 2010). These results agree with another study about intercropping between flax and faba bean might create unfavorable under-grown circumstances for O. crenata growth, boosting faba bean competitiveness and production during the growth and development phases (Safina 2017). Also, Fernández-Aparicio et al., (2013) suggested that the mechanism for reducing O. crenata infection is the prevention of seed germination by allelochemicals generated by cereal roots. Therefore, intercropping benefits include better resource utilization, yield stability, weed reduction, increased soil fertility conservation, and higher economic returns (Kabura et al., 2008; Mohamed, 2013, Meresa and Gebremedhin, 2020).
6. Conclusion
A number of diseases and parasitic weeds that infect faba beans can reduce yield and quality. The use of chemical control, including herbicides and fungicides, for controlling weeds and soil-borne pathogens is costly, produces environmental hazards and affects beneficial microorganisms in the soil. Therefore, consumer demand for alternative approaches is increasing, such as biocontrol agents and agronomic approaches that reduce plant pathogen populations. Bioactive compounds generated by yeasts may be potential growth-inhibiting agents against fungal growth, as well as yeasts may potentially contribute to alternate techniques to combating plant pathogenic fungi. In addition, we highlight the significant effects of the intercropping system on microbiological properties in the rhizosphere, which may contribute to enhancement of yield components. Besides, we demonstrated that faba bean–garlic intercrop along with AMF inoculation could be recommended due to the potential role of driving biological interactions among neighboring plants, as well as reduction of root rot disease and broomrapes. Accordingly, this study introduces a high potential ecofriendly approach for use in sustainable faba bean production and disease management.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Authors would like to thank Taif University Researchers Supporting Project number (TURSP-2020/138), Taif University, Taif, Saudi Arabia
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbes Z., Trabelsi I., Kharrat M., Amri M. Intercropping with fenugreek (Trigonella foenum-graecum) enhanced seed yield and reduced Orobanche foetida infestation in faba bean (Vicia faba) Biological Agric., & Hortic. 2019;35 (4):238–247 [Google Scholar]
- El-Hai K.M.A., Ali A.A. Formulation of Trichoderma, Saccharomyces and Rhizobium metabolites against damping-off and root rot pathogens in peanut plant. Asian J. Biol. Sci. 2019;12(2):114–121. [Google Scholar]
- Abd-El-Khair H., Haggag K.H.E., Seif El-Nasr H.I. Field application of Trichoderma harzianum and Bacillus subtilis combined with Rhizobium for controlling Fusarium root rot in faba bean in organic farming. Middle East J Appl Sci. 2018;8(3):865–873. [Google Scholar]
- Abdel-Monaim M.F., Abo-Elyousr K.A.M. Effect of preceding and intercropping crops on suppression of lentil damping-off and root rot disease in New Valley, Egypt. Crop Prot. 2012;32:41–46. [Google Scholar]
- Abdel-Monaim M.F., Ismail M.E., Morsy K.M. Induction of systemic resistance of benzothiadiazole and humic acid in soybean plants against Fusarium wilt disease. Mycobiology. 2011;39(4):290–298. doi: 10.5941/MYCO.2011.39.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Razik S.A., Sallam N.M.A., Eraky A.M.I., Hassan M.H.A. Integrated controls of root rots and wilt disease of faba bean by soil amendment with suppressive compost in combination with seed coating with an antagonistic yeast. Archives of Phytopathology and Plant Protection. 2012;45(14):1692–1704. [Google Scholar]
- Abouzeid M.A., El-Tarabily K.A. Fusarium spp. suppress germination and parasitic establishment of bean and hemp Orobanches. Phytopathol. Mediterr. 2010;9:51–64. [Google Scholar]
- Abu-Shall A.M.H., Ragheb E.I.M. Management of Orobanche Crenata using trap crops and Phytomyza Orobanchia kalt. in broad bean (Vicia Faba) field in Egypt. Egyptian J. of Biological Pest Control. 2014;24(1):217–223. [Google Scholar]
- Ahmed N.R., Abdallah N.G., Abd-El raoufn R.M. Intercropping fenugreek (Trigonela foenum graecum L) on the faba bean (Vicia faba) to reduce the incidence of (Orobanche crenata) World Rural Observations. 2015;7(1):88–99. [Google Scholar]
- Ali, A.A., Ghoneem, K.M., El-Metwally, M.A. and KM, A.E.H., 2009. Induce systemic resistance in lupine against root rot diseases. Pakistan journal of biological sciences: PJBS, 12(3), pp.213-221. [DOI] [PubMed]
- Bakheit B.R., Allam A.Y., Galal A.H. Intercropping faba bean with some legume crops for control of O. crenata. Acta Agronomica Hungarica. 2002;50:1–6. [Google Scholar]
- Barnett, H.L. and Hunter, B.B., 1972. Illustrated genera of imperfect fungi. Illustrated genera of imperfect fungi., (3rd ed).
- Barnett H.L., Hunter B.B. 4th ed. The American Phytopathological Society; 1998. Illustrated genera of imperfect fungi. [Google Scholar]
- Begum M.M., Sariah M., Puteh A.B., Zainal Abidin M.A., Rahman M.A., Siddiqui Y. Field performance of bio-primed seeds to suppress Colletotrichum truncatum causing damping-off and seedling stand of soybean. Biological Control. 2010;53(1):18–23. [Google Scholar]
- Bulletin of Statistical Cost Production, Net Return, 2019 and 2020. Winter Field Crops and Vegetables and Fruit. Agriculture Statistics and Economic Sector, Ministry of Egyptian Agriculture and Land Reclamation“, Part (1), February, Egypt.
- Chen P.-H., Chen R.-Y., Chou J.-Y. Screening and evaluation of yeast antagonists for biological control of Botrytis cinerea on strawberry fruits. Mycobiology. 2018;46(1):33–46. doi: 10.1080/12298093.2018.1454013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F., Cheng Z., Meng H. Transcriptomic insights into the allelopathic effects of the garlic allelochemical diallyl disulfide on tomato roots. Scientific Reports. 2016;6:1–13. doi: 10.1038/srep38902. article No.38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, Y.W., Hyde, K.D., and Ho, W.H., 1999. Single spore isolation of fungi. Fungal diversity.
- Di Canito A., Mateo-Vargas M.A., Mazzieri M., Cantoral J., Foschino R., Cordero-Bueso G., Vigentini I. The role of yeasts as biocontrol agents for pathogenic fungi on postharvest grapes: A review. Foods. 2021;10(7):1650. doi: 10.3390/foods10071650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Dabaa M.A.T., Abd-El-Khair H. Applications of plant growth promoting bacteria and Trichoderma spp. For controlling Orobanche crenata in faba bean. Bulletin of the National Research Centre. 2020;44(4):1–10. [Google Scholar]
- El-Dabaa, M.A.T., H. Abd-El-Khair, W.M.A. El-Nagdi, 2019. Field application of Clethodim herbicide combined with Trichoderma spp. for controlling weeds, root knot nematodes and Rhizoctonia root rot disease in two faba bean cultivars. J Plant Prot Res., 59(2):255–264 https://doi.org/10.24425/jppr.2019. 129287
- El-Mohamedy R.S., Shafeek M.R., Fatma A.R. Management of root rot diseases and improvement growth and yield of green bean plants using plant resistance inducers and biological seed treatments. Journal of Agricultural Technology. 2015;11(5):1219–1234. [Google Scholar]
- El-Mohamedy R.S.R., Abd Alla M.A. Bio-priming seed treatment for biological control of soil borne fungi causing root rot of green bean (Phaseolus vulgaris L.) J. Agric. Technol. 2013;9(3):589–599. [Google Scholar]
- Elnahal A.S.M., El-Saadony M.T., Saad A.M., Desoky E.S.M., El-Tahan A.M., Rady M.M., AbuQamar S.F., El-Tarabily K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022 [Google Scholar]
- Fernández-aparicio M., García-garrido J.M., Ocampo J.A., Rubiales D. Colonisation of field pea roots by arbuscular mycorrhizal fungi reduces Orobanche and Phelipanche species seed germination. Weed Research. 2010;50(3):262–268. [Google Scholar]
- Fernández-Aparicio M., Cimmino A., Evidente A., Rubiales D. Inhibition of Orobanche crenata seed germination and radicle growth by allelochemicals identified in cereals. Journal of agricultural and food chemistry. 2013;61(41):9797–9803. doi: 10.1021/jf403738p. [DOI] [PubMed] [Google Scholar]
- Fernández‐Aparicio, m., Rispail, N., Prats, E., Morandi, D., Garcia‐Garrido, J.M., DUMAS‐GAUDOT, E., Duc, G. and Rubiales, D., 2010. Parasitic plant infection is partially controlled through symbiotic pathways. Weed research, 50(1), pp.76-82.
- Freed R.D. Michigan State Unive; East Lansing, Michigan: 1991. MSTATC Microcomputer Statistical Program. [Google Scholar]
- Ghosh S.K., Banerjee S., Sengupta C. Siderophore production by antagonistic fungi. J Biopest. 2017;10(2):105–112. [Google Scholar]
- Gomez, K.A., and A.A., Gomez, 1984. Statistical Procedure for Agricultural Research. 2nd Ed. John Wiley Sons. NewYork. USA.
- Gressel J. Crops with target-site herbicide resistance for Orobanche and Striga control. Pest Manag. Sci. 2009;65:560–565. doi: 10.1002/ps.1738. [DOI] [PubMed] [Google Scholar]
- Guerrero V., Guigon C., Berlanga D., Ojeda D. Complete control of Penicillium expansum on apple fruit using a combination of antagonistic yeast Candida oleophila. Chilean J Agric Res. 2014;74(4):427–431. [Google Scholar]
- Gül H., Özçelik S., Sağdıç O., Certel M. Sourdough bread production with lactobacilli and S. cerevisiae isolated from sourdoughs. Process Biochemistry. 2005;40(2):691–697. [Google Scholar]
- Habimana S., Nduwumuremyi A., Chinama R.J.D. Managementof orobanche in field crops: A review. Journal of soil science and plant nutrition. 2014;14(1):43–62. [Google Scholar]
- Hao W.-y., Ren L.-X., Ran W., Shen Q.-R. Allelopathic effects of root exudates from water- melon and rice plants on Fusarium oxysporum f. sp. niveum. Plant Soil. 2010;336(1-2):485–497. [Google Scholar]
- Hassan M., Abakeer R.A. Effects of arbuscular mycorrhizal fungi (AMF) and bacterial strains on Orobanche crenata Forsk, on faba bean. Universal Journal of Applied Science. 2013;1(1):27–32. doi: 10.13189/ujas.2013.010105. http://www.hrpub.org [DOI] [Google Scholar]
- Heydari A., Pessarakli M. A review on biological control of fungal plant pathogens using microbial antagonists. J Biol Sci. 2010;10(4):273–290. [Google Scholar]
- Hisamuddin A., Akhtar A., Sharf R. Vesicular arbuscular mycorrhizal (VAM) fungi: A tool for sustainable agriculture. American Journal of Plant Nutrition and Fertilization Technology. 2015;5(2):40–49. [Google Scholar]
- Horváth E., Sipiczki M., Csoma H., Miklós I. Assaying the effect of yeasts on growth of fungi associated with disease. BMC microbiology. 2020;20(1):1–10. doi: 10.1186/s12866-020-01942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imara, D., A., N., A. Reyad, and S., E., El-Abeid, 2018. Combined effect of vascular arbuscular mycorrhizal fungi and yeast on controlling spearmint root rot and wilt diseases and on some plant parameters. Middle East Journal of Applied Sciences, 8 (2): 705-718.
- Ingraffia, R., Amato, G., Frenda, A.S. and Giambalvo, D., 2019. Impacts of arbuscular mycorrhizal fungi on nutrient uptake, N2 fixation, N transfer, and growth in a wheat/faba bean intercropping system. PloS one, 14(3), p. e0213672. [DOI] [PMC free article] [PubMed]
- Jadhav H.P., R.Z., Sayyed, 2016. Hydrolytic enzymes of rhizospheric microbes in crop protection. MOJ Cell Sci Rep., 3(5):135-136. DOI: 10.15406/mojcsr.2016.03.00070
- Khunnamwong P., Lertwattanasakul N., Jindamorakot S., Suwannarach N., Matsui K., Limtong S. Evaluation of antagonistic activity and mechanisms of endophytic yeasts against pathogenic fungi causing economic crop diseases. Folia microbiologica. 2020;65(3):573–590. doi: 10.1007/s12223-019-00764-6. [DOI] [PubMed] [Google Scholar]
- Komeil D.A., Badry H.H. Arbuscular Mycorrhizal Fungi Improved Host-plant Resistance Against Crenate Broomrape in Faba Bean. Asian Journal of Plant Sciences. 2021;20(3):477–487. [Google Scholar]
- Li Yankai. Ahmed A.A. Aioub, Bo Lv, Zhaonong Hu, Wenjun Wu. Antifungal activity of pregnane glycosides isolated from Periploca sepium root barks against various phytopathogenic fungi. Industrial Crops and Products. 2019 doi: 10.1016/j.indcrop.2019.02.009. [DOI] [Google Scholar]
- Liu J., Sui Y., Wisniewski M., Droby S., Liu Y. Review: Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. International Journal of Food Microbiology. 2013;167(2):153–160. doi: 10.1016/j.ijfoodmicro.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Lizarazo C.I., Lampi A.-M., Liu J., Sontag-Strohm T., Piironen V., Stoddard F.L. Nutritive quality and protein production from grain legumes in a boreal climate. J. Sci. Food Agric. 2015;95(10):2053–2064. doi: 10.1002/jsfa.6920. [DOI] [PubMed] [Google Scholar]
- López-Ráez J.A., Charnikhova T., Fernández I., Bouwmeester H., Pozo M.J. Arbuscular mycorrhizal symbiosis decreases strigolactone production in tomato. J. Plant Physiol. 2011;168(3):294–297. doi: 10.1016/j.jplph.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Louarn J., Boniface M.-C., Pouilly N., Velasco L., PérezVich B., Vincourt P., Muños S. Sunflower resistance to broomrape (Orobanche cumana) is controlled by specific QTLs for different parasitism stages. Front. Plant Sci. 2016;(14:p.). doi: 10.3389/fpls.2016.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louarn J., Carbonne F., Delavault P., Becard G., Rochange S. Reduced germination of Orobanche cumana seeds in the presence of arbuscular mycorrhizal fungi or their exudates. PloS one. 2012;7(11) doi: 10.1371/journal.pone.0049273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J., Dong Y., Dong K., Zhao Q., Yang Z., Chen L. Intercropping with wheat suppressed Fusarium wilt in faba bean and modulated the composition of root exudates. Plant Soil. 2020;448(1-2):153–164. doi: 10.1007/s11104-019-04413-2. [DOI] [Google Scholar]
- Mahmoud, N., A., N., A., Khalifa, M., S., Abbas, H.M., Sobhy, and N. M., Abou- Zeid, 2018. Efficacy of antagonistic fungal and bacterial bioagents against faba bean damping-off disease. Zagazig J. Agric. Res., 45, (3):917-929.
- Maitra S., Hossain A., Brestic M., Skalicky M., Ondrisik P., Gitari H., Brahmachari K., Shankar T., Bhadra P., Palai J.B., Jena J., Bhattacharya U., Duvvada S.K., Lalichetti S., Sairam M. Intercropping—A Low Input Agricultural Strategy for Food and Environmental Security. Agronomy. 2021;11(2):343. doi: 10.3390/agronomy11020343. [DOI] [Google Scholar]
- Mayo S., Gutiérrez S., Malmierca M.G., Lorenzana A., Campelo M.P., Hermosa R., Casquero P.A. Influence of Rhizoctonia solani and Trichoderma spp. in growth of bean (Phaseolus vulgaris L.) and in the induction of plant defense-related genes. Front. Plant Sci. 2015;6(685) doi: 10.3389/fpls.2015.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meresa B.K., Gebremedhin H.M. Faba Bean Gall (Olpidium viciae K.) as a Priority Biosecurity Threat for Producing Faba Bean in Ethiopia: Current Status and Future Perspectives. International Journal of Agronomy. 2020;2020:1–12. [Google Scholar]
- Mohamed G. Effect of intercropping of pea with some medicinal plants on microbial community of soil, damping-off and downy mildew diseases, under Beheira governorate conditions. Journal of Plant Protection and Pathology. 2013;4(7):625–641. [Google Scholar]
- Mohamed H.I., Akladious S.A. Changes in antioxidants potential, secondary metabolites and plant hormones induced by different fungicides treatment in cotton plants. Pesticide biochemistry and physiology. 2017;142:117–122. doi: 10.1016/j.pestbp.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Mousa A.M., El-Sayed S.A. Effect of intercropping and phosphorus fertilizer treatments on incidence of Rhizoctonia root-rot disease of faba bean. Int. J. Curr. Microbiol. App. Sci. 2016;5(4):850–863. [Google Scholar]
- Nawaz-Noor A.S., Sreeramulu K.R. Comparative efficiency of biocontrol agents in the management of root borne diseases of coleus (Coleus forskohlii (Willd.) Briq.) Int. J. Curr. Microbiol. App. Sci. 2017;6(4):2315–2327. [Google Scholar]
- Oswald A., Ransom J.K., Kroschel J., Sauerborn J. Intercropping controls Striga in maize based farming systems. Crop Protection. 2002;21(5):367–374. [Google Scholar]
- Panth M., Hassler S.C., Baysal-Gurel F. Methods for management of soilborne diseases in crop production. Agriculture. 2020;10(1):16. [Google Scholar]
- Papvizas G.C., Davey C.B. Isolation and Pathogenicity of Rhizoctonia saprophytically existing in soil. Phytopath. 1962;52:834–840. [Google Scholar]
- Qin G., Tian S., Xu Y. Biocontrol of postharvest diseases on sweet cherries by four antagonistic yeasts in different storage conditions. Postharv Biol Technol. 2004;31(1):51–58. [Google Scholar]
- Safina S. Effect of Ridge Width and Cropping System on Productivity and Land Use Efficiency in Faba Bean-Flax Intercrops. Egyptian Journal of Agronomy. 2017;0(0):357–381. [Google Scholar]
- Samejima H., Sugimoto Y. Recent research progress in combating root parasitic weeds. Biotechnol Biotechnol Equip. 2018;32(2):221–240. [Google Scholar]
- Sharma G., Shrestha S., Kunwar S., Tseng T.-M. Crop diversification for improved weed management: A review. Agriculture. 2021;11(5):461. [Google Scholar]
- Sundar A.R., Das N.D., Krishnaveni D. In-vitro Antagonism of Trichoderma spp. against two Fungal Pathogens of Castor. Indian J. Plant Protec. 1995;23(2):152–155. [Google Scholar]
- Torbati M., Arzanlou M., Bakhshi M. Morphological and molecular identification of ascomycetous coprophilous fungi occurring on feces of some bird species. Current Research in Environmental & Applied Mycology. 2016;6(3):210–217. [Google Scholar]
- Wehr H.M., Frank J.F. Ignatius Press; 2004. Standard methods for the examination of dairy products. [Google Scholar]
- Willey, R.,W., 1979. Intercropping its importance and research needs. Part 1. Competition and yield advantage. (c.f Field Crops Abst., 32: 1-10).
- Zeid M.M., Komeil D.A. Same-Hill Intercropping of Different Plant Species with Faba Bean for Control of Orobanche Crenata. Alexandria Science Exchange Journal. 2019;40(APRIL- JUNE):228–238. [Google Scholar]
- Zhimo V.Y., Dilip D., Sten J., Ravat V.K., Bhutia D.D., Panja B., Saha J. Antagonistic yeasts for biocontrol of the banana postharvest anthracnose pathogen Colletotrichum musae. J Phytopathol. 2017;165(1):35–43. [Google Scholar]
Further Reading
- Abdel-Kader, M, M, N, S, El-Mougy, S, M, Lashin, 2011. Essential oils and Trichoderma harzianum as an integrated control measure against faba bean root rot pathogens. Journal of Plant Protection Research, 51 (3): 306-313. DOI: 10.2478/v10045-011-0050-8
- AOAC, 2000. Official Methods of Analysis of AOAC Int. 17th Ed. by Horwitz, W. Suite (ed.) Vol. (2), Chapter, (41): 66-68.
- Chapman H.D., Pratt P.E. Division Agric. Sci., California Univ., USA; 1961. Methods of Analysis for Soil, Plant and Water. [Google Scholar]
- Parker C., Riches C.R. CAB International; Wallingford: 1993. Parasitic weeds of the world: Biology and Control. [Google Scholar]