Abstract
The major aspects of using plant-derived medications are significantly safer and secure than synthetic ones. The n-hexane seed extract of ayurvedic medicinal plants Myristica fragrans was also utilized as food ingredients have analyzed for phytochemical existence by Gas Chromatography-mass spectrometry (GC–MS). Twenty-three phytoconstituents were identified with elemicin (24.44%) as the major constituent. Lipid peroxidase, catalase and DPPH assays were performed using the isolated elemicin and the results revealed significant antioxidant activity. The antibacterial study revealed that elemicin showed MIC of 31.25 μg/mL against Escherichia coli, Pseudomonas aeruginosa, and Salmonella typhi, and 62.5 μg/mL against Klebsiella pneumonia and Staphylococcus aureus. Elemicin exhibited better antifungal activity against Candida tropicalis and Aspergillus flavus than Aspergillus niger, Penicillium chrysogenum and Trichophyton rubrum. The study implies that the elemicin isolated from Myristica fragrans possess promising bioactive properties and can be crucially utilized in the development of therapeutic agents and food preservatives.
Keywords: Myristica fragrans, GC–MS analysis, Elemicin, Antimicrobial activity, Antioxidant activity
1. Introduction
In recent times, the analysis of natural products for the revelation of dynamic compounds with antimicrobial, antioxidant, and anticancer properties from plant sources that can be applied to the food commerce has picked up intrigue (Kazemi, 2015). Food contamination and food poisoning are as yet anxiety for both the food industry and consumers regardless of the utilization of various preservation and conservation methods. Both antioxidant and antimicrobial properties are significant in expanding the life span of the usability of the specific food material (Singh et al., 2005). Essential oils are professed to have bioactive properties that can be significantly applicable in food industries (Piaru et al., 2012). It is also utilized traditionally as therapeutic agents for relaxing muscles, soothe digestive and nervous disorders, prevent liver diseases, and reduce inflammation.
Myristica fragrans is a tree which belongs to the family Myristicaceae that originated in Indonesia. The fruit part of the tree has 78% flesh, 4% mace, 5% shell and 13% seed. The kernel of this seed is ground to produce the nutmeg spice. The essential oil extracted from seed was utilized for the study since oil is found in large amounts in the seeds than any other part of the plant. In general, seeds contain more than 60 percent oil (Kuete, 2017). The essential oil extracted from M. fragrans is commonly used as a flavoring agent in food industry and fragrance incorporating agent in cosmetics. The amount of essential oil extracted by steam distillation ranges between 4% and 16% and comprises 80–90% of monoterpene hydrocarbons. It has high hepatoprotective, anticancer, antibacterial, antifungal and antitumor activities. The medicinal property of M. fragrans is due to the presence of major bioactive compounds like myristicin, eugenol, elemicin, and safrole (Kuete, 2017, Asgarpanah, 2012).
In order to utilize the medicinal potential of this ayurvedic plant, individual constituents were assessed for their biological properties. Myristicin is a colorless and volatile phenyl propane derivative that is insoluble in water. It is mainly used as an insect and pest repellent exhibiting neurotoxic and anti-cholinergic effect. Medically, it prevents liver damage and tumor formation. Eugenol is a phenolic compound and used as an over-the-counter anesthetic for toothache, antifungal activity and anti-inflammatory properties (Niu et al., 2012, Azir Uddin et al., 2017). It is an active ingredient which is mostly extracted from clove. Safrole is a colorless organic compound belonging to a class of benzodiaoxoles that is found in Sassafras root. It has genotoxic property and its use as a flavoring agent is banned by FDA in 1960. Since all these compounds have already been explored, elemicin was chosen as the compound of interest for this study.
Apart from M. fragrans, elemicin compound is also present in Petroselinum sativum, Melaleuca bracteata, P. crispum and Canarium commune. It can be converted into a trimethoxy amphetamine derivative that is a hallucinogen and hence was shown to possess psychotomimetic property. The application of elemicin in medicinal field is very limited and the research data is still in its infancy. The bioavailability of elemicin is low if it is taken in the form of essential oil. There are few reports that discusses the toxic nature of this compound (De Vincenzi et al., 2004). Hence, there is a need to explore the medicinal value of elemicin and to determine the minimum safe concentration to avoid health risk.
Thus, an attempt was initiated to identify the bioactive compounds in the essential oil of M. fragrans utilizing GC–MS, and isolate elemicin to assess its antimicrobial and antioxidant properties.
2. Materials and methods
2.1. Materials
The seeds of Myristica fragrans were procured from the National Institute of Siddha and Hospital, Tambaram, Chennai, India. The seeds were air-dried at 50˚C and pulverized to a coarse powder using electric bladder
Myristica fragrans:
2.2. Morphology
The spice tree is a large evergreen plant that thrives well under tropical climates. A fully-grown tree reaches about 50–60 feet in height and is the source of nutmeg and mace, two valuable spices. The nutmeg fruit, in fact, is a drupe, about the size of an apricot, which when ripen splits up to reveal single centrally situated oval shaped hard kernel known as “nutmeg spice.” The seed is closely enveloped by crimson-red colored lacy or thread like arils known as “mace.” Both spices have a similar warm, sweet aromatic flavor. Nutmeg is the seed of the tree, roughly egg-shaped and about 20 to 30 mm (0.8 to 1.2 in) long and 15 to 18 mm (0.6 to 0.7 in) wide, and weighing between 5 and 10 g (0.2 and 0.4 oz) dried, while mace is the dried “lacy” reddish covering or aril of the seed. The first harvest of nutmeg trees takes place 7–9 years after planting, and the trees reach full production after 20 years. Nutmeg is usually used in powdered form
2.3. Preliminary phytochemical analysis of essential oil
The seeds were air-dried at 50˚C and pulverized to a coarse powder using an electric blender. 100 g of powdered sample was extracted with 70% hexane in a shaker for 3 h followed by evaporation and filtration of crude extract using Whatman No.1 filter paper. The essential oil (EO) was obtained by hydro distillation using Clevenger's apparatus and was preserved at room temperature. Phytochemical analysis for detection of different chemical classes of phytoconstituents was carried out according to standard procedures (Ameh and Eze, 2010, Jeff-Agboola and Awe, 2016).
2.4. GC–MS profiling
GC–MS analysis of the n-hexane extract of M. fragrans was executed using GC–MS 5975C Agilent mass selective detector in the electron impact mode (70 eV). Injector and MS transfer line temperatures were set at 220 °C and 290 °C, respectively. The components were determined based on a comparison of their relative time of retention and mass spectra with those of the standards and NIST11 mass spectral library data.
2.5. Isolation of elemicin from essential oil
The EO was dissolved in chloroform and agitated with dilute NaOH solution. It is then washed with distilled water and rested using a separating funnel. The basic phase was further profiled in a column chromatography using hexane followed by ethyl ether as the mobile phase. The ether eluted fractions were compounded and were subjected to vacuum evaporator wherein the temperature was maintained at 155 °C. The solvent in the compounded fraction was removed by a rotary evaporator and dried overnight. It was again subjected to column chromatography using isooctane/chloroform/methanol (70:29:1) as the mobile phase. The eluents were characterized by UV spectrophotometer for the presence of elemicin.
2.6. Antioxidant studies
2.6.1. Lipid peroxidase assay
Lipid peroxidase assay was conducted using a 1 mL sample of isolated elemicin diluted with 9 mL of hexane. Out of the six test tubes, one was held blank and 1 mL of the above sample was taken at various concentrations (100, 200, 300, 400, 500 μg/mL) in the remaining test tubes. Then 200 μl SDS, 1.5 mL acetic acid, and 1.5 mL 0.8 percent TBA were added and vigorously shaken. After which two glass beads were added and was made up to 10 mL using distilled water. The mixture was boiled at 95 °C and 1 mL of water, n-butanol / pyridine (5 mL) was included and centrifuged for 10 min at 4000 rpm (Datta and Patil, 2020, Gyawali et al., 2020). The absorbance at 532 nm was taken and the inhibition percentage was determined using the formula,
Where, Ac is the control absorbance and Ab is the test absorbance. Experiments conducted in triplicates.
2.6.2. Catalase assay
The catalase assay was performed as per standard protocol (Höferl et al., 2014). The sample for catalase assay was prepared by mixing 200 µl of isolated elemicin along with Tris-NaOH (5 mL), EDTA, Triton, polyvinylpyrrolidone solutions. The mixture was centrifuged for 10 min at 22,000 rpm at 4 °C and the supernatant was obtained. Further, 1 mL of the reaction mixture comprising potassium phosphate buffer (50 mM), enzyme extract (250 µl), and hydrogen peroxide (60 mM) was added to the supernatant. Using the spectrophotometer, the absorbance was taken at 240 nm for 3 min. To calculate the H2O2 degradation, the following equation was used.
where, ΔA is difference in absorption, ε is extinction coefficient = 43.6 M−1cm−1. Experiments conducted in triplicates.
2.6.3. DPPH- free radical scavenging assay
The radical scavenging activity of isolated elemicin was estimated by DPPH assay according to the standard protocol (Gayathri and Sathish, 2016). In every test tube, each sample (1 mL) and hexane (9 mL) were dissolved. Each of six test tubes was held as blank and 1 mL of this sample was taken at various concentrations (100, 200, 300, 400, 500 μg/mL) in the rest of the test tubes. To the above samples, a 0.1 mm DPPH solution in methanol has been added and the absorbance was measured at 570 nm after 30 min. The scavenging percentage was calculated using the below-mentioned formula,
Where Abc = Absorbance of Control; Abt = Absorbance of Test. Results expressed as Mean ± SD (standard deviation). All experiments are performed in triplicates
2.7. Antimicrobial activity
Both antibacterial and antifungal activities were estimated through determining the scale of the inhibition zone on the cultivated layer.
2.7.1. Antibacterial activity
The elemicin isolated from M. fragrans were evaluated for in-vitro antibacterial activity by microdilution method (Elshikh et al., 2016, Veiga et al., 2019). Five strains of bacteria including E. coli (MTCC433), K. pneumoniae (MTCC109), P. aeruginosa (MTCC1035), S. typhi (MTCC3231) and Staph. aureus (MTCC1144) was chosen for the current study. All the bacterial strains were acquired from the Microbial Type Culture Collection (MTCC). 80 μl of Mueller Hinton agar (MHA) was taken in each well of the microtiter plate. The inoculum (10 μl) of each species was added to each well along with the Resazurin dye (10 μl). The isolated extract (500 μg/mL) was added in the first well and was serially diluted in subsequent wells. Plates were incubated at 37 °C for 24 h and the minimum concentration at which the dye changes its color was noted to determine the MIC. Amoxicillin (10 μl) and DMSO (10 μl) were taken as a positive and negative control respectively. The minimum concentration of elemicin with clear zone of inhibition was considered as MIC. The zone of inhibition was measured as the diameter of the clear zone and results were recorded. Experiments were conducted in in triplicate
2.7.2. Antifungal activity
In-vitro antifungal activity was performed for the isolated elemicin from M. fragrans by microdilution method (Elshikh et al., 2016, Veiga et al., 2019). Fungal strains such as A. flavus (MTCC1884), A. niger (MTCC404), C. tropicalis (MTCC1000), P. chrysogenum (MTCC1348) and T. rubrum (MTCC8477) were chosen for the current study. All the fungal strains were acquired from the Microbial Type Culture Collection (MTCC). 80 μl of potato dextrose agar was taken in each well of the microtiter plate. The inoculum (10 μl) of each species was added to each well along with the Resazurin dye (10 μl). The isolated extract (500 μg/mL) was added in the first well and was serially diluted in subsequent wells. Plates were incubated at 37 °C for 24 h and the minimum concentration at which the dye changes its color was noted to determine the MIC. Amphotericin (10 μl) and DMSO (10 μl) were taken as a positive and negative control respectively.
3. Results
3.1. Preliminary phytochemical analysis
The phytochemical analysis of the EO from M. fragrans contain the majority of the fundamental phytoconstituents including tannin, saponin, alkaloid, protein, steroid, anthraquinone, terpenoids, and cardiac glycosides. The results on the n-hexane extract of M. fragrans are presented in Table 1 and the primary essential oil showed strong presence of terpenoids than the other compounds.
Table 1.
Phytochemical analysis of the n-hexane extract of M. fragrans. (+) represents presence and (-) represents absence.
| Seed Extract | Tannin | Saponin | Flavonoids | Alkaloids | Steroid | Anthraquinone | Terpenoids | Cardiac glycosides |
|---|---|---|---|---|---|---|---|---|
| Myristica fragrans | + | + | – | + | + | + | ++ | + |
3.2. GC–MS analysis
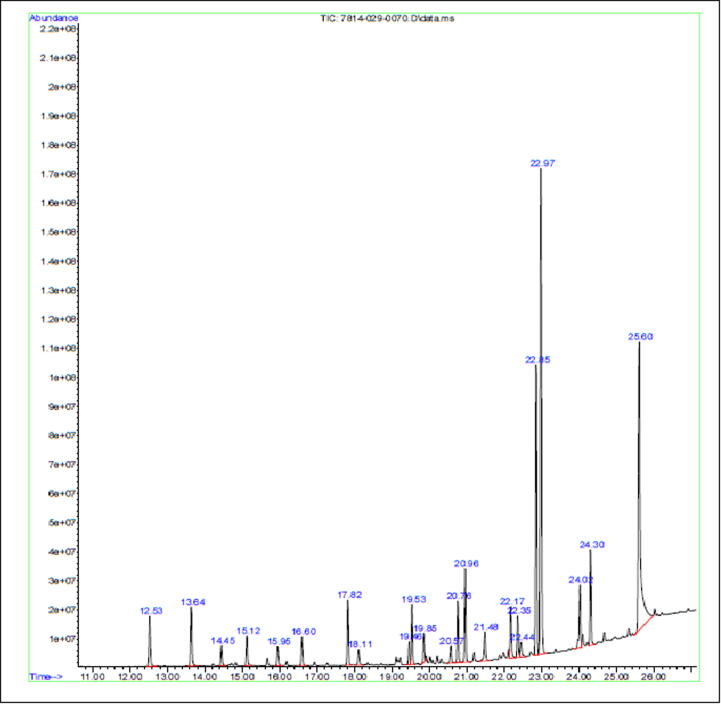
The GC–MS analysis (Fig. 1) detected the presence of twenty-three volatile components in the essential oil of M. fragrans and the interpreted results are presented in Table 2. Among the 23 phytochemical compounds present in the essential oil of M. fragrans, benzene, 1, 2, 3-trimethoxy-5-(2-propenyl)- accumulated the largest amount (24.44%) followed by tetradecanoic acid (22.25%). (See Fig. 2, Fig. 3, Fig. 4, Fig. 5).
Fig 1.
Myristica fragrans.Taxonomy: (1) Kingdom: Plantae; (2) Order: Magnoliales; (3) Family: Myristicaceae; (4) Genus: Myristica; (5) Species: M.fragrans.
Table 2.
Interpretation of the GC–MS analysis of n-hexane extract of M. fragrans.
| S.No | Compound name | RT | Area% |
|---|---|---|---|
| 1 | Bicyclo[3.1.0[hex-2-ene, 4-methyl-1-(1-methylethyl)- | 12.526 | 2.48 |
| 2 | Bicyclo[3.1.0[hex- 4-methylene-1-(1-methylethyl)- | 13.644 | 3.03 |
| 3 | 1,3-cyclohexadiene, 1-mwthyl-4-(1-methylethyl)- | 14.443 | 0.95 |
| 4 | Gamma-terpinene | 15.125 | 1.43 |
| 5 | Cyclohexanol, 1-methyl-4-(1-methyl ethyl)- | 15.953 | 0.93 |
| 6 | Cyclohexanol, 1-methyl-4-(1-methylethenyl)-cis- | 16.592 | 1.27 |
| 7 | 3-Cyclohexe, 1-ol-4-methyl-1-(1-methyl ethyl)-(R)- | 17.827 | 2.92 |
| 8 | Alpha-terpineol | 18.102 | 0.76 |
| 9 | Anethole | 19.453 | 0.95 |
| 10 | 1,3-benzodioxole, 5-(1-propenyl)-Safrole | 19.526 | 2.40 |
| 11 | Thymol | 19.845 | 1.06 |
| 12 | Alfa-Copaene | 20.571 | 0.88 |
| 13 | Eugenol | 20.760 | 2.68 |
| 14 | Methyl eugenol | 20.949 | 3.82 |
| 15 | Caryophyllene | 21.472 | 1.51 |
| 16 | Trans-Isoeugenol | 22.169 | 2.61 |
| 17 | Benzene 1,2-dimethoxy-4-(1-propenyl)- | 22.358 | 10.84 |
| 18 | Naphthalene, 1,2,3,5,6a-hexahydro-4–7-dimethyl-1-(1-methyl ethyl) -(1S-cis)- | 22.445 | 0.91 |
| 19 | 1,3-Benzodioxole,4-methoxy-6-(2-propenyl)- | 22.852 | 13.81 |
| 20 | Benzene 1,2,3-trimethoxy-5-(2-propenyl)- (or) Elemicin | 22.968 | 24.44 |
| 21 | Phenol, 2,6-dimethoxy-4-(2-propenyl)- | 24.013 | 2.97 |
| 22 | Isoelemicin | 24.304 | 4.09 |
| 23 | Tetradecanoic acid | 25.596 | 22.25 |
Fig. 2.
GC–MS analysis of n-hexane extract of Myristica fragrans. The compound at RT 22.968 shows the highest peak among the other compounds.
Fig. 3.
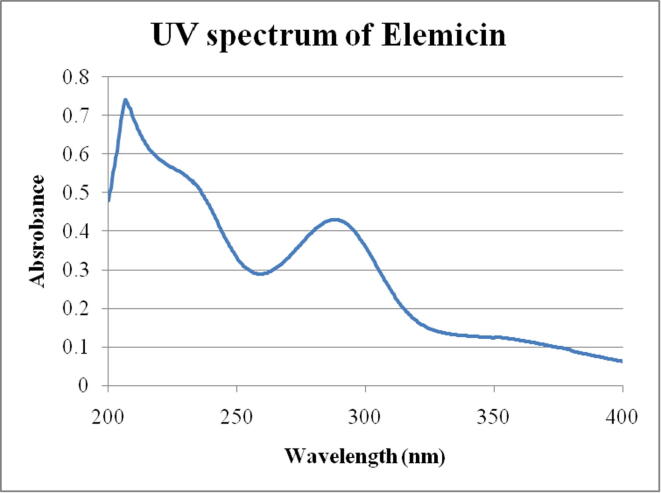
UV absorbance spectrum of elemicin showing characteristic peaks at 216 nm and 282 nm.
Fig. 4.
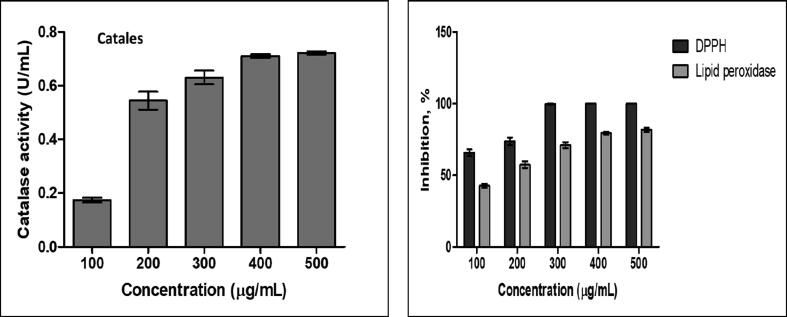
Graphical representation of in vitro antioxidant activity of elemicin. Incremental effect was observed in all the studies. Results expressed as Mean ± SD. Experiments performed in triplicates.
Fig. 5.
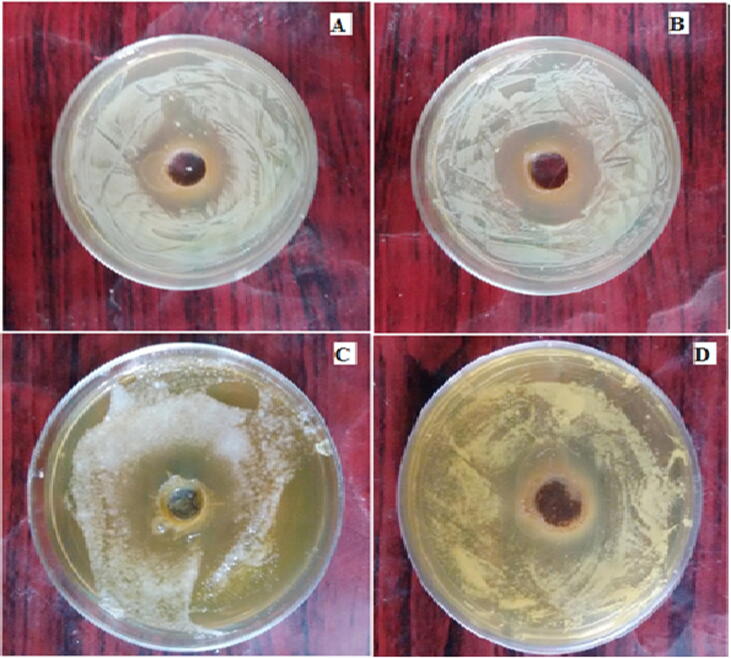
Antibacterial activity of the isolated elemicin against tested bacteria (A) E coli (B) P. aeroginosa (c) S. aureus (D) S. typhi.
The common name of benzene, 1, 2, 3-trimethoxy-5-(2-propenyl)- is elemicin. Several studies were conducted to determine the presence of various bioactive compounds in M. fragrans. The major components of its volatile extract that were identified include myristicin, eugenol, isoeugenol, elemicin, safrole, 4-terpineol and camphene (Asgarpanah, 2012, Adiani et al., 2015). Since the quantity of elemicin in the extract was more than the other compounds, attempts were made to isolate elemicin from the essential oil and study its antioxidant and antimicrobial property.
3.3. Isolation of elemicin
The choice of solvent is important in various stages of the isolation process. The dissolution in chloroform followed by agitation with dilute NaOH removed the possible acidic compound in the EO. The water wash separated the undesired acidic compound from the basic bioactive compound. The selective elution technique performed using hexane as the first mobile phase separated al non-oxygenated hydrocarbons from the oil. The ether elution isolated the desired oxygenated aromatic compounds. Totally, six eluents were collected from second round of chromatography and subjected to UV absorption spectrophotometer (Fig. 3). On comparing, the UV spectrum of the sixth eluents exhibited two major peaks at 216 nm and 282 nm and a shoulder between 220 and 240 nm characteristic to that of elemicin.
3.4. Antioxidant activity
The isolated elemicin was assessed for its antioxidant property using three methods including lipid peroxidase, catalase and DPPH assay. The isolate exhibited incremental antioxidant potential in both lipid peroxidase and catalase assay. In DPPH assay, 100% radical scavenging activity was observed at 300 µg/mL concentration. This indicates the possibility of good free radical scavenging activity with an improved the effect of ROS (reactive oxygen species) on the biological system (Ginting et al., 2018, Tan et al., 2013). The standard deviation of catalase activity, DPPH and peroxidase inhibition activity shown in Table 3 and Fig. 4 and all experiments data performed and triplicates and mentioned the respective mean and standard deviation values.
Table 3.
Minimum inhibitory concentration (MIC, µg/mL) of the isolated elemicin against tested bacteria and fungi.
| S. No | Bacterial species | Elemicin MIC concentration (µg/mL) | Fungal species | Elemicin MIC concentration (µg/mL) |
|---|---|---|---|---|
| 1 | E. coli | 31.25 | C. tropicalis | 62.5 |
| 2 | P. aeroginosa | 31.25 | A. niger | 125 |
| 3 | S. aureus | 62.5 | A. flavus | 62.5 |
| 4 | S. typhi | 31.25 | T. rubrum | 125 |
| 5 | K. pneumonia | 62.5 | P. chrysogenum | 125 |
| 7 | DMSO | – | DMSO | – |
3.5. Antibacterial activity & antifungal activity
The isolated elemicin demonstrated substantial inhibition against bacteria including E. coli, K. pneumoniae, P. aeruginosa, S. typhi and S.aureus. The isolate exhibited MIC value of 31.25 μg/mL against E. coli, P. aeruginosa and S. typhi. A MIC value of 62.5 μg/mL was assessed against K. pneumonia and S. aureus. (Table 3 and Fig. 5)
The essential oil of M. fragrans showed a significant zone of inhibition against fungi with a MIC of 125 µg/mL for Aspergillus niger, Trichophyton rubrum, Penicillium chrysogenum and 62.5 µg/mL for Candida tropicalis, Aspergillus flavus. The essential oil of Myristica fragrans showed substantial antifungal activity against Aspergillus niger (Fig. 5).
4. Discussions
The phytochemical analysis on the n-hexane extract of Myristica fragrans in the current study coincides with the results of earlier studies by Jinous and Nastaran (2012). However, there were only few studies that discussed the UV spectrum of elemicin (Victor, 1977). Turning to the antioxidant activity, Kapoor et al., (2013) extracted oleoresins from M. fragrans using Clevenger apparatus.
The major component of the oleoresin was elemicin that possessed strong DPPH radical scavenging activity (Kapoor et al., 2013). Adiani et al., (2013) identified the presence of elemicin as the major antioxidant constituent in the nutmeg essential oil (17. Furthermore, Kuete et al., (2017) discussed the antioxidant activity of nutmeg essential oil in various studies (Kuete, 2017). The current results coincide with the earlier studies and elemicin was known to be a major contributing compound. On discussing the antibacterial activity, the acetone extract of the aerial part of M. fragrans containing 17.68% elemicin inhibited the growth of Staph. aureus, K. pneumonia but was ineffective against P. aeruginosa (Singh et al., 2005). The ethanolic leaf extract of M. fragrans demonstrated MIC value of 50 mg/mL against E. coli; K. pneumonia and Staph. aureus, and 100 mg/mL against P. aeruginosa (Ibrahim et al., 2011).
The EO from M. fragrans containing elemicin (8.81%) as one of the major constituents showed better antibacterial activity against including E. coli, K. pneumoniae, and Staph. aureus than the EO from T. ammi (Soni et al., 2016). The choice of plant parts, solvent and method used for extraction played a major role. All these studies depict the fact that the presence of elemicin improved the antibacterial property of the M. fragrans extract and the same has been observed in the present study.
On considering the antifungal activity, Das et al., (2021) investigated the role of elemicin as a food preservative which depicted better antifungal activity than other bioactive compounds (Das et al., 2021). The oil from D. carota with high amounts of elemicin, demonstrated strong antifungal activity at concentrations between 0.16 μl/mL and 0.64 μl/mL. In another study, the EO from fresh leaves of P. carpunya containing 7.2% elemicin showed synergistic effect against Candida sp. These deductions from the earlier studies are a clear evident of elemicin’s antifungal potential.
5. Conclusion
Essential oils of plants should receive much more consideration as natural and safe remedies compared to synthetic ones as their ability as a traditional medicine has been proven non-scientifically. The current research was designed in such a way to authenticate the already existing medicinal potential. The present study demonstrates that the n-hexane extract of M. fragrans contained the majority of the fundamental phytoconstituents. The essential oil was analyzed using GC–MS that depicted the large presence elemicin. Elemicin was isolated using selective elution technique and was found to possess good antioxidant activity. The isolate also demonstrated promising antimicrobial activity against 5 bacterial and 5 fungal species. This implies that the essential oil of M. fragrans could be a prospective future source of natural antioxidants and antimicrobials catering to food and pharmaceutical industries.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors express thankful and financial support by the Researchers Supporting Project Number (RSP-2021/293) King Saud University, Riyadh, Saudi Arabia. The authors (Yuvaraj D.) would like to thank Vel Tech High Tech Dr. Rangarajan Dr. Sakunthala Engineering College for providing all the required facilities for performing the work.
Funding
The authors express thankful and financial support by the Researchers Supporting Project Number (RSP-2021/293) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Yuvaraj Dinakarkumar, Email: yuvarajdinakarkumar@gmail.com.
Selvaraj Arokiyaraj, Email: arokiyaraj16@gmail.com.
References
- Kazemi M. Chemical composition and antimicrobial, antioxidant activities and anti-inflammatory potential of Achillea millefolium L., Anethum graveolens L., and Carum copticum L. essential oils. J. Herb. Med. 2015;5(4) [Google Scholar]
- Singh G., Marimuthu P., De Heluani C.S., Catalan C. Antimicrobial and antioxidant potentials of essential oil and acetone extract of Myristica fragrans Houtt. (aril part) J. Food Sci. 2005;70(2) [Google Scholar]
- Piaru S.P., Mahmud R., Abdul Majid A.M.S., Ismail S., Man C.N. Chemical composition, antioxidant and cytotoxicity activities of the essential oils of Myristica fragrans and Morinda citrifolia. J. Sci. Food Agric. 2012;92(3) doi: 10.1002/jsfa.4613. [DOI] [PubMed] [Google Scholar]
- Kuete V. Myristica fragrans: A Review. In: Medicinal Spices and Vegetables from Africa [Internet]. Elsevier; 2017. p. 497–512. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128092866000236.
- Asgarpanah Jinous. Phytochemistry and pharmacologic properties of Myristica fragrans Hoyutt.: A review. AFRICAN. J. Biotechnol. 2012;11(65) [Google Scholar]
- Niu G., Pollock H.S., Lawrance A., Siegel J.P., Berenbaum M.R. Effects of a naturally occurring and a synthetic synergist on toxicity of three insecticides and a phytochemical to navel orangeworm (Lepidoptera: Pyralidae) J. Econ. Entomol. 2012;105(2) doi: 10.1603/ec10194. [DOI] [PubMed] [Google Scholar]
- Azir Uddin M., Shahinuzzaman M., Sohel Rana M., Yaakob Z. Study of chemical composition and medicinal properties of volatile oil from clove buds (Eugenia caryophyllus) Int. J. Pharm. Sci. Res. 2017;8(2) [Google Scholar]
- De Vincenzi M., De Vincenzi A., Silano M. Constituents of aromatic plants: Elemicin. Fitoterapia. 2004;75:6. doi: 10.1016/j.fitote.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Ameh G., Eze C. Phytochemical Constituents of Some Nigerian Plants. Bio-Research. 2010;8(1) [Google Scholar]
- Jeff-Agboola Y.A., Awe L.B. Antifungal and phytochemical screening of some Nigerian medicinal plant extracts against toxigenic Aspergillus flavus. Cogent Food Agric. 2016;2(1) [Google Scholar]
- Datta S., Patil S. Evaluation of traditional herb extract salvia officinalis in treatment of Alzheimer’s disease. Pharmacogn J. 2020;12(1) [Google Scholar]
- Gyawali R., Gupta R.K., Shrestha S., Joshi R., Paudel P.N. Formulation and Evaluation of Polyherbal Cream Containing Cinnamomum zeylanicum Blume, Glycyrrhiza glabra L and Azadirachta indica A Juss. Extracts to Topical Use. J. Inst. Sci. Technol. 2020;25(2) [Google Scholar]
- Höferl M., Stoilova I., Schmidt E., Wanner J., Jirovetz L., Trifonova D., et al. Chemical composition and antioxidant properties of juniper berry (Juniperus communis L.) essential oil. action of the essential oil on the antioxidant protection of saccharomyces cerevisiae model organism. Antioxidants. 2014;3(1) doi: 10.3390/antiox3010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayathri P.K., Sathish Kumar K. Antioxidant activity of essential oil extracted from enicostemma littorale. J. Chem. Pharm. Sci. 2016;9(1):256–258. [Google Scholar]
- Elshikh M., Ahmed S., Funston S., Dunlop P., McGaw M., Marchant R., et al. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016 Jun 1;38(6):1015–1019. doi: 10.1007/s10529-016-2079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga A, Toledo M da GT, Rossa LS, Mengarda M, Stofella NCF, Oliveira LJ, et al. Colorimetric microdilution assay: Validation of a standard method for determination of MIC, IC50%, and IC90% of antimicrobial compounds. J Microbiol Methods. 2019;162:50–61. [DOI] [PubMed]
- Adiani V., Gupta S., Chatterjee S., Variyar P.S., Sharma A. Activity guided characterization of antioxidant components from essential oil of Nutmeg (Myristica fragrans) J. Food Sci. Technol. 2015;52(1) [Google Scholar]
- Victor T. High performance liquid chromatography of some aromatic compounds of nutmeg and carrots. The Universtiy of Alabama. 1977 [Google Scholar]
- Ginting B, Maira R, . M, Helwati H, Desiyana LS, Mujahid R. Isolation of essensial oil of nutmeg (Myristica fragrans houtt) and antioxidant activity test with DPPH. J Nat. 2018;18(1).
- Tan K.P., Khoo H.E., Azrina A. Comparison of antioxidant components and antioxidant capacity in different parts of nutmeg (Myristica fragrans) Int. Food Res. J. 2013;20(3) [Google Scholar]
- I.P.S, Kapoor, Bandana Singh , Gurdip Singh , Carola S. De Heluani MP De, Catalan L& CAN. Chemical Composition and Antioxidant Activity of Essential Oil and Oleoresins of Nutmeg (Myristica fragrans Houtt.) Fruits. Int. J. Food Prop. 2013;16:1059–70.
- Singh G, Marimuthu P, Heluani CS de, Catalan C. Antimicrobial and Antioxidant Potentials of Essential Oil and Acetone Extract of Myristica fragrans Houtt. (Aril Part). J Food Sci [Internet]. 2005 Mar; 70(2):M141–8. Available from: http://doi.wiley.com/10.1111/j.1365-2621.2005.tb07105.x.
- Ibrahim T., Opawale B., Oyinloye J. Antibacterial activity of herbal extracts against multi drug resistant strains of bacteria from clinical origin. Life Sci. Leafl. 2011;1(15) [Google Scholar]
- Soni R., Sharma G., Jasuja N.D. Essential Oil Yield Pattern and Antibacterial and Insecticidal Activities of Trachyspermum ammi and Myristica fragrans. Scientifica (Cairo) 2016;2016 doi: 10.1155/2016/1428194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Singh V.K., Dwivedy A.K., Chaudhari A.K., Dubey N.K. Exploration of some potential bioactive essential oil components as green food preservative. LWT. 2021;137 [Google Scholar]