Abstract
An inflammation response occurs when the body reacts to exogenous and endo enous noxious stimuli, and it helps the body respond to infection and repair tissues, adapt to stress, and remove dead or damaged cells. Anti-inflammatory drugs such as non-steroidal anti-inflammatory drugs are traditionally used to treat inflammation; however, these drugs often cause negative side effects. For this reason, developing and establishing effective alternative medicines for treating many chronic diseases with underlying inflammation is critically dependent on the identification of new organic molecules and bioactive substances. Aromatic and volatile compounds found in essential oils isolated from Pimenta dioica (allspice), Cuminum cyminum (cumin), and Citrus sinensis (sweet orange) are a source of bioactive compounds. Allspice essential oil reduces ear inflammation more than 65% and the anti-inflammatory activity of allspice essential oil is enhanced when combined with sweet orange peel and cumin essential oils, resulting in the reduction of edema inflammation by more than 85%, similar to indomethacin. As an alternative to anti-inflammatory treatment, essential oil mix is pharmacologically safe as it is neither toxic nor mutagenic.
Keywords: Anti-inflammatory activity, Essential oil, Pimenta dioica (allspice), Cuminum cyminum (cumin), Citrus sinensis (sweet orange), Ear edema, Mutagenic, NSAIDs
1. Introduction
Inflammation is a protective response triggered by exogenous noxious stimuli such as irritants, allergens, toxic compounds, virulence factors and microorganisms, and as well by endogenous noxious conditions as signals released by damage tissue or malfunctioning, stressed or dead cells.
The main purpose of inflammatory response is host defense against infection, for tissue-repair and adaptation to stress, to remove dead or damaged host cells, among others (Medzhitov, 2008, Wei et al., 2015).
Non-steroidal anti-inflammatory drugs (NSAIDs) are often used for inflammatory treatment, however those drugs are associated to adverse effects as nausea, vomiting, renal and liver failure (Ferrari et al., 2016, Gias et al., 2020), as well as gastrointestinal lesions, mainly manifested as gastric ulcers (Flores-Fernandez et al., 2019). Therefore, identification of new organic molecules, and bioactive compounds is essential to develop and stablish effective alternative medicines for treatment of many chronic diseases with an underlying inflammatory. Despite the therapeutic effects desirables, its potential toxic and mutagenic effects should not be underestimated, despite its natural origin; the evaluation of any chemical compound or mixture intended to be used in humans for treatment of any disease, must be carried out in accordance with international institutions such as the FDA (Ruiz-Pérez et al., 2016a).
Medicinal plants, natural products and herbal diet in traditional, complementary and alternative medicine has been practiced since ancient times, as they are inexpensive and readily available (Flores-Fernandez et al., 2019, Padilla-Camberos et al., 2021).
Essential oils are a complex mixture of volatile and aromatic compounds isolated from whole plant or any part such as branches, leaves, bark, fruits, seeds or roots. Essential oils isolated from plants such as Citrus sinensis, Cuminum cyminum and Pimenta dioica have shown different biological activities.
Sweet orange, Citrus sinensis, that is one of the most abundant fruit crops worldwide with huge commercial applications in food, pharmaceutical preparations, perfumery, and cosmetics (Farahmandfar et al., 2020, Njoroge et al., 2005), has a great source of essential oils present in its peel (flavedo). Among the biological activities reported for this C. sinensis peel is antioxidant, bactericidal, insecticidal, antimycotic an cytotoxic activity of essential oils (Farahmandfar et al., 2020, Nair S et al., 2018, Oyedeji et al., 2020).
C. cyminum commonly known as cumin is used as a condiment and ingredient in many cuisines worldwide, as well is employed in small quantities in perfumes due to its aroma. Cumin has shown biological activities as insecticide, analgesic, antioxidant, anticancer, hypotensive, antidiabetic, antibacterial, acaricidal, among other. Those activities have been related to its components such as alkaloids, coumarin, flavonoids, glycosides, saponins, tannins and steroids (Al-snafi, 2017, Nirmala et al., 2020).
On the other hand, the P. dioica named as allspice is a tropical tree widely exploited in America, used as well as a spice and condiment to flavor food, perfume essence, and in traditional medicine to treat colds, stomach pain, muscle and joint pain, indigestion, menstrual cramps, and dyspepsia (Mérida-Reyes et al., 2020, Zhang et al., 2012). Allspice has shown antioxidant, hypotensive, antimicrobial and antiproliferative activity, as well as analgesic, antipyretic, antiulcer and cytoprotective activity in vivo models (Al-Rehaily et al., 2002, Lorenzo-Leal et al., 2019b, Zhang et al., 2012).
Anti-inflammatory activity by sweet orange juice and an ethanol extract from C. sinensis by-products is reported (Coelho et al., 2013, Li et al., 2017), but there is just a study of a peel extract alleviating the inflammatory response by ultraviolet B (Yoshizaki et al., 2014). The anti-inflammatory effect of essential oil from C. sinensis has not been reported yet, while this activity of essential oils isolated from Cumin has been reported in vitro using the macrophage cell line RAW 264.7 stimulated by lipopolysaccharide (Wei et al., 2015), and in vivo models induced by carrageenan (Shivakumar et al., 2010) and formalin (Sayyah et al., 2002) as well. Nevertheless, anti-inflammatory activity from allspice is controversial as there is a report where the P. dioica essential oil did not reduce the pro-inflammatory interleukin (IL)-6 or tumor necrosis factor alpha (TNF-α) production, nor enhanced the anti-inflammatory IL-10 production (Lorenzo-Leal et al., 2019b), but there is other study where the anti-inflammatory cytokine IL-10 increased using a ground extract instead of essential oil (Mueller et al., 2010). In addition, an allspice aqueous extract showed an anti-inflammatory effect on carrageenan-induced paw edema (Al-Rehaily et al., 2002).
Therefore, in this study, the in vivo anti-inflammatory activity of essential oils isolated from C. sinensis (sweet orange), C. cyminum (cumin) and P. dioica (allspice) was evaluated, as well as the synergistic or additive effect of a mix of these three essential oils. Moreover, in vivo acute toxicity and in vitro mutagenic potential were assessed.
2. Materials and methods
2.1. Essential oils purification from plant material
Seeds from Cuminum cyminum (cumin) and berries from and Pimenta dioica (allspice), were purchased from commercial suppliers in Jalisco, Mexico and kept at room temperature until their use.
Essential oil extraction from Cuminum cyminum and Pimenta dioica was performed for the hydrodistillation method according to Owolabi et al. (2013). 250 g of plant material for each species was placed in 5 L flasks and then 2 L distilled water was added to cover the seeds and berries, then samples were submitted to hydrodistillation at 96–97 °C using a Clevenger-type apparatus at normal atmospheric pressure for ∼ 2 h, until no more essential oil was obtained. Finally, residual water was removed with anhydrous sodium sulphate. The drying agent was filtered off and the essential oils were stored in amber vials at 4 °C for future analysis.
Sweet orange essential oil from Citrus sinensis was proportionated by Corporation International Frutech.
2.2. Chemical characterization
2.2.1. Gas chromatography and coupled to mass spectrometry (GC–MS) analysis
Essential oil chemical composition was determined according to Owolabi et al. (2014). GC–MS analysis was carried out with a gas chromatograph model 7890B coupled to a mass selective detector model 5977A (Agilent Technologies, Santa Clara, California, USA). Separation was carried out in a HP-5 capillary column (60 m × 0.25 mm ID × 0.25 μm film thickness). Analytical chromatographic conditions were injector and transfer line temperature 220 °C and 260 °C, respectively. Initial oven temperature was 60 °C for 5 min, and then it was increased to 250 °C at a rate of 3 °C/min for 15 min. Helium was used as carrier gas with at 0.8 mL/min flow rate, and the sample injection volume was 1 μL using a 1:60 split ratio. Mass spectrometer operated in scan mode at 70-eV ionization voltage, and the acquisition mass range was 30 to 300 m/z at 1.0 scan/s.
2.2.2. Identification of essential oil compounds
The identification of the volatile constituents of the essential oils was performed by (1) comparison of the mass spectra of each of component with the Wiley library spectra 275 L and NIST library; (2) comparison of the retention index (RI) obtained by GC–MS and RI reported in the literature (Soran et al., 2009) at same phases; (3) injection of pure compounds at 95% minimum purity under the same analytical conditions described for GC–MS but using gas nitrogen as carrier at 2 mL/min. The relative proportions of the oil constituents were percentages obtained by flame ionization detector peak-area normalization.
2.3. Animals
Eight-week-old female Balb-c mice (21.4–23.7 g body weight) were obtained from the Faculty of Veterinary Medicine of the University of Guadalajara, Mexico. Animals were housed in polypropylene plastic cages at 23.0 ± 2.0 °C at 44–55% RH and light and dark cycles of 12 h, with rodent food and water ad libitum. Animal were handled according to the guidelines and regulations promulgated by the Federal Government of Mexico NOM-062-ZOO-1999 (Padilla-Camberos et al., 2016) and by the “Guide for the Care and Use of Laboratory Animals” of the National Institute of Health, and under approval code 209–001-A granted by the Internal Committee for the Care and Use of Laboratory Animals (CICUAL) of the Institution (CIATEJ).
2.4. Anti-inflammatory activity
2.4.1. Ear edema induction
Animals were randomly divided into ten groups containing six animals each. Cutaneous inflammation was induced by topical application of an irritant agent prepared by croton oil dissolved at 5% v/v in acetone (Santos et al., 2015). 20 μl of the irritant agent was applied to the right ear inner surface, and 20 μl of acetone to the left ear as control of the irritant agent.
2.4.2. Essential oil application
This experiment consisted of five animal groups. Immediately after cutaneous inflammation, four groups were treated topically on the right ear by 50 mg/kg body weight of essential oil of C. sinensis, C. cyminum and P. dioica, and 10 mg/kg body weight of indomethacin (positive control). As a negative control, the fifth group did not receive any treatment.
2.4.3. Natural essential oil mix application
After ear edema induction, the remaining five groups of animals were used to test the anti-inflammatory activity of the essential oil mix containing equal parts of sweet orange peel, cumin and allspice essential oils. Three groups of animals were administered topically by 100, 500, and 1,000 mg/kg body weight of the essential oil mix. 10 mg/kg body weight of indomethacin was applied for positive control group and the negative control group was not treated.
2.4.4. Ear edema quantification
After 4 h of the ear edema induction and the treatment application, animals were euthanized by cervical dislocation, then ear biopsies of 6 mm diameter were obtained and weighed. The extent of the edema of the ear was determined by the weight differences between the inflamed (right) and the non-inflamed (left) ear.
The anti-inflammatory effect expressed as percentage inhibition of edema was calculated according to the following formula:
2.5. Pharmacological safety evaluation
2.5.1. Acute oral toxicity
The oral acute toxicity of the essential oils mix was conducted using the raise and lower procedure according to the OECD 425 method as described by Flores-Fernandez et al. (2017). A group containing five mice were evaluated in limit test by intragastric gavage at a single dose of 2,000 mg/kg body weight. All physical and behavioral changes such as aggressiveness, piloerection, tremors, convulsions, salivation, diarrhea, and lethargy, were observed during two weeks to detect signs of delayed toxicity. Before dosing, the animals were fasted for 8 hrs.
2.5.2. Mutagenic potential of essential oil mix
The Ames test (Maron and Ames, 1983) was conducted to assess the mutagenic potential of essential oil mix using Salmonella enterica serovar Typhimurium (S. typhimurium) TA100 strain was employed to identify up to 90% of mutagens (Toukourou et al., 2020). Mutagenicity test was realized by Ames test using TA 100 tester strain which detect base pair substitutions in genetic material (Maron and Ames, 1983).
A preculture was inoculated in nutrient broth and grown at 37 °C, 100 rpm, overnight to achieve a density of 1x109 cells/ml. Then, 50 μl of essential oils mix was added to 50 μl of bacterial culture, followed by short incubation at 37 °C, 100 rpm for 20 min. Subsequently, 2 mL top agar (0.5% agar, 0.5% NaCl, 0.5 mM Histidine/D-Biotin) was added, and the content was homogenized and poured onto minimal glucose agar plates (1.5 % agar, 50x VB salts, 10% glucose) and incubated at 37 °C for 72 h. As negative control 50 μl of pH 7.4 PBS was added as test substance, while for positive control 50 μg/plate of methyl methanesulfonate was used. The counting of revertant colonies was carried out with a “counter pen” (Control company, USA). Tests were performed at 1.5, 2.5 and 5 mg of essential oils mix per plate in triplicate.
2.6. Data analysis
All values were expressed as mean ± SEM. Anti-inflammatory effect was evaluated using one-way analysis of variance (ANOVA) with a Tukey post-hoc test to determine significance between groups using GraphPad Prism 8.0.1 software (GraphPad Software, San Diego, CA, USA). P < 0.05 values were considered as statistically significant.
3. Results
3.1. Anti-inflammatory activity of essential oils
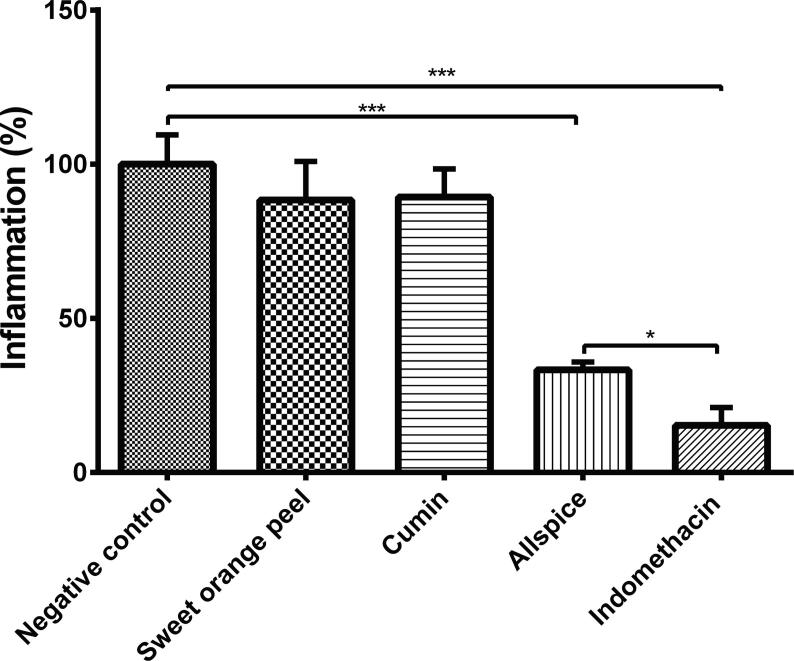
Topical application of 50 mg/kg body weight of allspice essential oil reduced ear inflammation by 66.67% (total inflammation of 33.33%) compared to the negative control (Fig. 1), but this reduction was not as much as the indomethacin-treated positive control, which significantly reduced inflammation by 84.67% (15.33% of total inflammation; Fig. 1). Essential oil of allspice showed an 18% increase in inflammation compared to Indomethacin (p < 0.05; Fig. 1). Sweet orange peel and cumin essential oils had low anti-inflammatory activity (11.60% and 10.67% reduction; total inflammation of 88.4% and 89.33%, respectively), which was not statistically significant in comparison to the negative control (Fig. 1).
Fig. 1.
Anti-inflammatory effect of topical application of essential oils from sweet orange peel, cumin, and allspice on ear edema induced by croton oil in Balb-c mice. Essential oils were tested at 50 mg/kg body weight, indomethacin was used as a positive control at 10 mg/kg body weight, while negative control did not receive any treatment. Values are represented as mean ± SE. ∗ p < 0.05 compared to positive control and ∗∗∗p < 0.001 compared to negative control.
3.2. Anti-inflammatory activity of essential oils mix
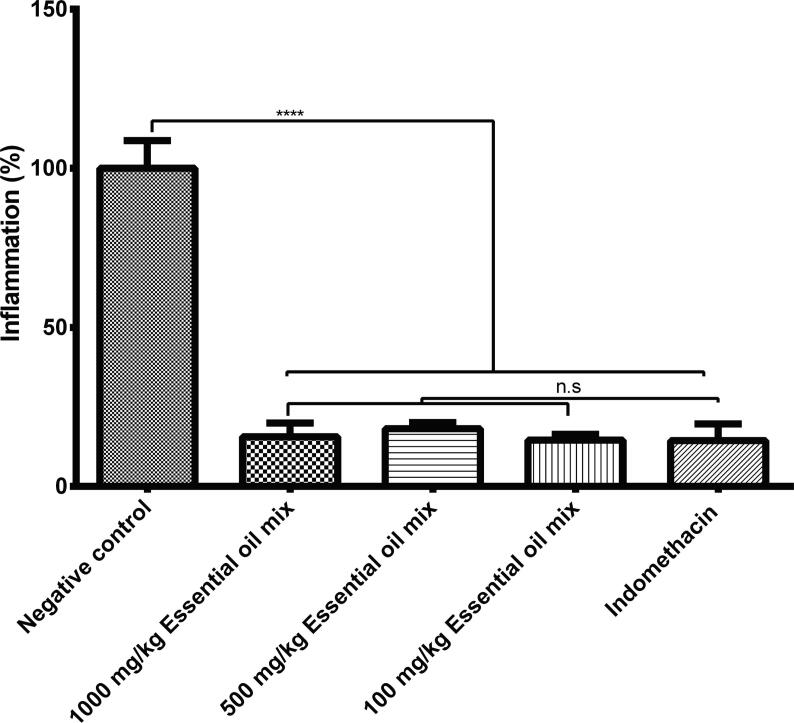
A synergistic or additive effect was observed when the three essential oils isolated from orange peel, cumin, and allspice were mixed and topically applied (Fig. 2). The essential oil mix at 100, 500, and 1000 mg/kg body weight doses reduced ear inflammation by 85.46, 81.83, and 84.34% regarding the negative control group, eliciting a total inflammation of 14.54, 18.17 and 15.66%, respectively (Fig. 2). The reduction of inflammation elicited by the essential oil mix was totally comparable to the inflammation reduction by the positive control treated with 10 mg/kg body weight of indomethacin, since this NASID reduced inflammation by 85.59% (total inflammation of 14.41%; Fig. 2).
Fig. 2.
Anti-inflammatory effect of topical application of essential oils mix of sweet orange peel, cumin, and allspice on ear edema induced by croton oil in Balb-c mice. Essential oils mix was tested at 100, 500, and 1,000 mg/kg body weight; indomethacin was used as a positive control at 10 mg/kg body weight, while no treatment was applied to the negative control. Values are represented as mean ± SE. ∗∗∗∗p < 0.001 compared to negative control. A statistically significant difference was not found = n.s.
3.3. Pharmacological safety evaluation of essential oils mix
3.3.1. Acute toxicity
A maximum fixed dose of 2000 mg/kg body weight of essential oil mix administered to mice did not cause any negative effects, clinical signs on behavior or appearance, including lethargy and inactivity, throughout the study compared to the control group. All animals survived, and both groups had similar dietary intake and changes in body weight (data not shown).
3.3.2. Mutagenic evaluation
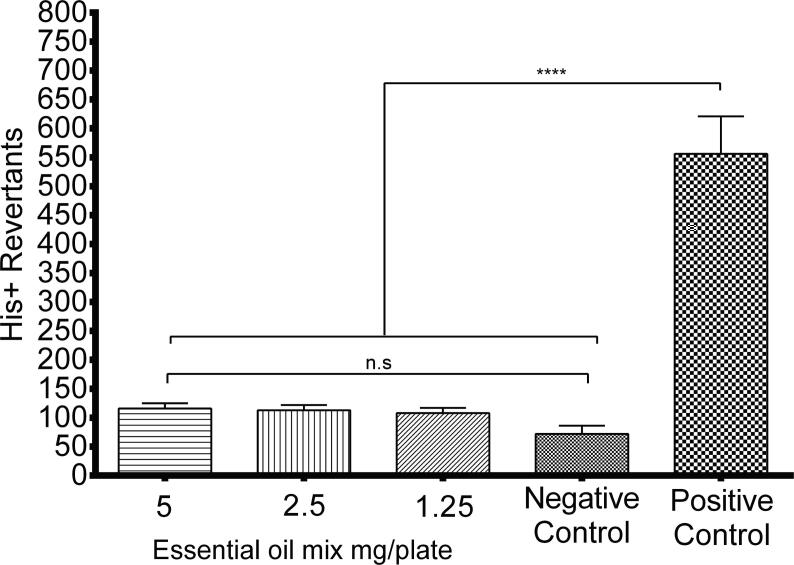
Essential oil mix of sweet orange peel, cumin, and allspice tested at 1.25, 2.5 and 5 mg per plate did not induce base-pair substitution mutations on Salmonella enterica serovar Typhimurium TA100 strain compared to the negative and control positive control of 50 μg/plate methyl methanesulfonate (Fig. 3).
Fig. 3.
Mutagenic evaluation of an essential oil mix of sweet orange peel, cumin, and allspice. The number of revertant colonies of S. typhimurium TA100 strain treated with different concentrations of essential oil mix (1.25, 2.5, and 5 mg/plate) in the absence of metabolic activation system (−S9). For the positive and negative control, 50 μg methyl methanesulfonate and 50 μl of pH 7.4 PBS were used. Values are expressed as mean ± SE of three independent experiments. Significantly different ∗∗∗∗p < 0.001 compared to the negative control.
3.4. Chemical composition of sweet orange peel, cumin and allspice essential oils
Chemical composition analysis by GC–MS identified 31 compounds in sweet orange peel essential oil, primarily limonene (82.57%) followed by β-Myrcene (4.73%; Table 1), while for cumin essential oil 30 chemical compounds were detected, being cuminaldehyde (30.85%) the most predominant, followed by γ-terpinene (15.42%), 2-caren-10-al (8.81%), β-pinene (8.76%) and cuminic alcohol (8.49%; Table 1). The most abundant compounds in allspice essential oil were eugenol (63.6%) and eucalyptol (1,8-Cineole; 3.95%; Table 1).
Table 1.
Chemical composition of essential oils isolated from sweet orange peel, cumin seeds, and allspice berries.
| Compound | RIa | Content (%) |
Identification | ||
|---|---|---|---|---|---|
| Citrus sinensis Sweet orange peel | Cuminum cyminum Cumin | Pimenta dioica Allspice | |||
| α-Thujene | 928 | 0.40 | 0.56 | 1.11 | MS, RI |
| α-Pinene | 932 | 1.52 | 1.13 | 0.31 | MS, RI, STD |
| β-Pinene | 968 | n.d. | 8.76 | 0.12 | MS, RI, STD |
| Sabinene | 973 | 1.10 | 0.99 | 0.32 | MS, RI |
| β-Myrcene | 992 | 4.73 | 1.35 | 3.77 | MS, RI |
| α-Phellandrene | 1007 | 0.72 | 0.45 | 0.86 | MS, RI |
| α-Terpinene | 1016 | n.d. | 0.47 | 0.80 | MS, RI, STD |
| o-Cymene | 1020 | 1.21 | 6.81 | 0.86 | MS, RI, STD |
| p-Cymene | 1025 | n.d. | 5.41 | 1.01 | MS, RI, STD |
| Limonene | 1029 | 82.57 | 1.24 | 1.53 | MS, RI, STD |
| Eucalyptol (1,8-Cineole) | 1037 | n.d. | 0.23 | 3.95 | MS, RI, STD |
| β-Ocimene | 1046 | 0.21 | 0.34 | 0.06 | MS, RI |
| γ-Terpinene | 1059 | 0.23 | 15.42 | 1.45 | MS, RI, STD |
| trans-Sabinene hydrate | 1085 | n.d. | 0.29 | 0.66 | MS, RI |
| Linalool | 1098 | 2.11 | 0.42 | 3.48 | MS, RI, STD |
| Nonanal | 1102 | 0.11 | n.d. | n.d. | MS, RI |
| p-Menth-2,8-dien-1-ol | 1125 | 0.20 | 0.14 | 1.33 | MS, RI |
| trans-Limonene oxide | 1137 | 0.01 | n.d. | n.d. | MS, RI |
| β-Terpineol | 1150 | 0.08 | 0.78 | 1.32 | MS, RI, STD |
| Borneol | 1158 | n.d. | n.d. | 1.04 | MS, RI |
| Citronellal | 1160 | 0.09 | n.d. | n.d. | MS, RI |
| Terpinen-4-ol | 1173 | 0.38 | 0.53 | 1.10 | MS, RI, STD |
| α-Terpineol | 1191 | 0.40 | 1.70 | 2.31 | MS, RI, STD |
| Decanal | 1207 | 0.70 | n.d. | n.d. | MS, RI |
| Nerol | 1233 | 0.10 | n.d. | n.d. | MS, RI |
| Cuminaldehyde | 1237 | n.d. | 30.85 | n.d. | MS, RI, STD |
| Neral | 1242 | 0.22 | n.d. | n.d. | MS, RI |
| Geraniol | 1263 | 0.07 | n.d. | n.d. | MS, RI |
| Phellandral | 1268 | n.d. | 0.39 | n.d. | MS, RI |
| Geranial | 1275 | 0.32 | n.d. | 0.13 | MS, RI |
| Perillaldehyde | 1280 | 0.05 | n.d. | n.d. | MS, RI |
| Cuminic alcohol | 1285 | n.d. | 8.49 | n.d. | MS, RI |
| 2-Caren-10-al | 1291 | n.d. | 8.81 | 0.40 | MS, RI |
| Perilla alcohol | 1298 | n.d. | 0.80 | n.d. | MS, RI |
| p-Mentha-1,4-dien-7-ol | 1323 | n.d. | 0.70 | n.d. | MS, RI |
| Eugenol | 1356 | n.d. | 0.41 | 63.60 | MS, RI, STD |
| α-Copaene | 1375 | 0.12 | n.d. | 0.97 | MS, RI |
| β-Cubebene | 1391 | 0.07 | n.d. | 0.58 | MS, RI |
| Dodecanal | 1413 | 0.18 | n.d. | n.d. | MS, RI |
| β-Caryophyllene | 1418 | n.d. | 0.87 | 2.49 | MS, RI, STD |
| α-Humulene | 1453 | n.d. | n.d. | 0.52 | MS, RI |
| α-Curcumene | 1479 | n.d. | 0.03 | n.d. | MS, RI, STD |
| Valencene | 1494 | 0.16 | n.d. | n.d. | MS, RI |
| α-Farnesene | 1505 | 0.03 | n.d. | n.d. | MS, RI |
| γ-Cadinene | 1516 | n.d. | n.d. | 0.29 | MS, RI, STD |
| β-Sinensal | 1698 | 0.11 | n.d. | n.d. | MS, RI |
| Total | 98.20 | 98.37 | 96.37 | ||
Major components are shown in bold.
STD: These chemical components were identified by authentic compound injection (Standard).
MS: Mass spectrum.
n.d.: not detected.
Retention index determined on HP-5 column.
4. Discussion
Current drugs used to decrease inflammation are costly and may cause adverse effects. New bioactive compounds and potential therapeutic agents in the treatment of inflammatory disorders can be identified by studying medicinal plants.
The ear edema induced by application of croton oil is a reliable animal model to evaluate the efficacy of anti-inflammatory drugs because the irritant elicits an inflammatory response in the animal, which is evident by the weight increase of the ears.
In this study, the anti-inflammatory effect of sweet orange peel and cumin essential oil using the ear edema mice model is the first time reported. In regard to allspice essential oil, a report showed that this spice did not have anti-inflammatory properties since the pro-inflammatory IL-6 and TNF-α production was not reduced and the anti-inflammatory cytokine IL-10 was not increased (Lorenzo-Leal et al., 2019b). However, an allspice ground extract increased IL-10 levels (Mueller et al., 2010). Even though discrepancies may be explained by differences in sample preparation, in the present study an inflammatory reduction of 84.67% was reported by using an essential oil extracted from allspice (P. dioica). This anti-inflammatory effect is in agreement with an allspice aqueous extract tested in the carrageenan-induced paw edema model (Al-Rehaily et al., 2002).
Although the allspice essential oil showed significant anti-inflammatory activity, the NASID drug, indomethacin used as a positive control, was still more efficacious. Therefore, a combination of essential oils, containing equal parts of essentials oils isolated from sweet orange peel, cumin, and allspice was tested in the ear edema mice model, which showed surprising synergistic or additive anti-inflammatory effects as the inflammatory reduction was similar to indomethacin. This effect is supported by analyzing in detail the lowest dosage (100 mg/kg of body weight) of the essential oils mix, since that dose contained 33.33 mg/kg of each essential oil, which was 16.67 mg/kg less than in the experiment where the anti-inflammatory activity of individual essential oils was assessed separately. It is well known that most biological processes are not linear, but assuming that 33.33 mg/kg of each essential oil could reduce inflammation by 59.28% (this value is the sum of the inflammatory reduction expected of 44.44, 7.73 and 7.11% for the essential oils of allspice, sweet orange peel and cumin for a dose of 33.3 mg/kg), whereas 85.46% was obtained for the essential oil mix.
Once the essential oil mix showed an anti-inflammatory activity similar to a NASID drug, the essential oil mix safety remained in question, as bioactive compounds and medicinal plants have shown potential to cause harmful or detrimental effects, potential toxic and mutagenic effects (Anywar et al., 2021). The acute oral toxicity test demonstrated that the use of essential oil mix is safe because no differences in body weight and food intake were noted in the present study and all animals exhibited notable tolerance without any sign of mortality, toxicity, or affectation at the limit test dose used, showing the lethal dose which causes the death of 50% (LD50) is greater than 2000 mg/kg body weight. The mutagenic evaluation by AMES test used for risk–benefit evaluation of new products to be used in humans (Ruiz-Pérez et al., 2016b) did not show evidence of base-pair substitution mutation evoked by the essential oil mix as the frequency of revertant colonies was less than the 2-fold of the negative control and not significantly different to the negative control.
The composition of essential oils varies from one plant to another, however, there are common compounds among them such as terpenes, monoterpenes, sesquiterpenes, and propenylphenols which have also been shown to function as antimicrobial, antioxidant, antiproliferative, and anti-inflammatory (Miguel, 2010). Previous studies have reported the chemical composition of sweet orange peel, cumin, and allspice essential oils (Hajlaoui et al., 2010, Ismail et al., 2020, Lorenzo-Leal et al., 2019a, Qiao et al., 2008, Rihawy et al., 2014), and the majority of compounds found in this study coincide in this study, showing some differences on the ratios and the absence of some minority compounds. These differences may be due to several factors, such as where the species were cropped, harvested or which part of the plant was used, and how the oil was obtained. However, the main components remained the same.
The anti-inflammatory properties of the oil can be attributed in part to main components such as eugenol, limonene, and γ-terpinene. Biological activities for some of these compounds have been reported. Eugenol inhibits the production of proinflammatory cytokines in acute lung injury (Huang et al., 2015). Furthermore, eugenol has been nanoemulsified demonstrating superior results versus existing inflammation-reducing gels (Esmaeili et al., 2016). IL-10 has been shown to increase with limonene compound (Bach and Bach, 2021), while TNF-α levels are reduced (Kummer et al., 2013). Gama-terpinene compound alleviates inflammation by attenuating edema, proteins extravasation, cytokines production and cell migration to inflamed tissue (Ramalho et al., 2015).
Nonetheless, minor components have also been shown to contribute to the biological activity such as β-Myrcene, eucalyptol (1,8-Cineole) and β-pinene. Myrcene reduced the expression of COX-2, a regulator of inflammation in a kidney inflammation model, downregulating proinflammatory cytokines IL-1β, IL-6, and TNF-α and anti-inflammatory markers IL-4 and IL-10 (Islam et al., 2020). Additionally, 1.8-cineole (eucalyptol) has shown anti-inflammatory activity in paw mice and was tested in a clinical trial of an airway disease, asthma, to use it as a new rationale for its use as a mucolytic agent in upper and lower respiratory tracts (Juergens et al., 2003, Martins et al., 2017). Pinene is another compound that significantly reduced the production of IL-6 and TNF-α on inflammatory responses induced by lipopolysaccharide (Kim et al., 2015).
5. Conclusions
Topical application of allspice essential oil reduces ear inflammation by more than 65%, and the anti-inflammatory effect is enhanced by combining it with sweet orange peel and cumin essential oils, reducing edema inflammation by more than 85%, showing similar therapeutic effects to indomethacin. Essential oil mix of C. sinensis, C. cyminum, and P. dioica is pharmacologically safe because it is neither toxic nor mutagenic.
Funding
This research received no external funding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Rehaily A.J., Al-Said M.S., Al-Yahya M.A., Mossa J.S., Rafatullah S. Ethnopharmacological Studies on Allspice (Pimenta dioica) in Laboratory Animals. Pharm. Biol. 2002;40(3):200–205. doi: 10.1076/phbi.40.3.200.5829. [DOI] [Google Scholar]
- Al-snafi A.E. The pharmacological activities of Cuminum cyminum -A review The pharmacological activities of Cuminum cyminum - A review Prof Dr Ali Esmail Al-Snafi. IOSR J. Pharm. 2017;6:46–65. [Google Scholar]
- Anywar G., Kakudidi E., Byamukama R., Mukonzo J., Schubert A., Oryem-Origa H., Jassoy C. A Review of the Toxicity and Phytochemistry of Medicinal Plant Species Used by Herbalists in Treating People Living With HIV/AIDS in Uganda. Front. Pharmacol. 2021;12:1–10. doi: 10.3389/fphar.2021.615147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach H., Bach H. Antimicrobial and anti-inflammatory activities of commercial aromatizing fragrances. Futur. Sci. OA. 2021;7:14–18. doi: 10.2144/fsoa-2020-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho R.C.L.A., Hermsdorff H.H.M., Bressan J. Anti-inflammatory Properties of Orange Juice: Possible Favorable Molecular and Metabolic Effects. Plant Foods Hum. Nutr. 2013;68(1):1–10. doi: 10.1007/s11130-013-0343-3. [DOI] [PubMed] [Google Scholar]
- Esmaeili F., Rajabnejhad S., Partoazar A.R., Mehr S.E., Faridi-Majidi R., Sahebgharani M., Syedmoradi L., Rajabnejhad M.R., Amani A. Anti-inflammatory effects of eugenol nanoemulsion as a topical delivery system. Pharm. Dev. Technol. 2016;21(7):887–893. doi: 10.3109/10837450.2015.1078353. [DOI] [PubMed] [Google Scholar]
- Farahmandfar R., Tirgarian B., Dehghan B., Nemati A. Changes in chemical composition and biological activity of essential oil from Thomson navel orange (Citrus sinensis L. Osbeck) peel under freezing, convective, vacuum, and microwave drying methods. Food Sci. Nutr. 2020;8(1):124–138. doi: 10.1002/fsn3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F.C., Lemos Lima R.d.C., Schimith Ferraz Filha Z., Barros C.H., de Paula Michel Araújo M.C., Antunes Saúde-Guimarães D. Effects of Pimenta pseudocaryophyllus extracts on gout: Anti-inflammatory activity and anti-hyperuricemic effect through xantine oxidase and uricosuric action. J. Ethnopharmacol. 2016;180:37–42. doi: 10.1016/j.jep.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Flores-Fernandez José Miguel, Barragán-Álvarez Carla Patricia, Díaz-Martínez Nestor Emmanuel, Villanueva-Rodríguez Socorro, Padilla-Camberos Eduardo. In vitro and in vivo postprandial glycemic activity of Citrus limetta peel flour. Pharmacogn. Mag. 2017;13(52):613. doi: 10.4103/pm.pm_158_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Fernandez J.M., Padilla-Camberos E., Fernandez-Flores O., Diaz-Martinez N., Barragan-Alvarez C., Ramirez-Rodriguez P. Gastroprotective activity and pharmacological safety evaluation of Eupatorium aschenbornianum. Exp. Ther. Med. 2019;4467–4472 doi: 10.3892/etm.2019.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gias Z.T., Afsana F., Debnath P., Alam M.S., Ena T.N., Hossain M.H., Jain P., Reza H.M. A mechanistic approach to HPLC analysis, antinociceptive, anti-inflammatory and postoperative analgesic activities of panch phoron in mice. BMC Complement. Med. Ther. 2020;20:102. doi: 10.1186/s12906-020-02891-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajlaoui H., Mighri H., Noumi E., Snoussi M., Trabelsi N., Ksouri R., Bakhrouf A. Chemical composition and biological activities of Tunisian Cuminum cyminum L. essential oil: A high effectiveness against Vibrio spp. strains. Food Chem. Toxicol. 2010;48(8–9):2186–2192. doi: 10.1016/j.fct.2010.05.044. [DOI] [PubMed] [Google Scholar]
- Huang X., Liu Y., Lu Y., Ma C. Anti-inflammatory effects of eugenol on lipopolysaccharide-induced inflammatory reaction in acute lung injury via regulating inflammation and redox status. Int. Immunopharmacol. 2015;26(1):265–271. doi: 10.1016/j.intimp.2015.03.026. [DOI] [PubMed] [Google Scholar]
- Islam A.U.S., Hellman B., Nyberg F., Amir N., Jayaraj R.L., Petroainu G., Adem A. Myrcene attenuates renal inflammation and oxidative stress in the adrenalectomized rat model. Molecules. 2020;25:1–15. doi: 10.3390/molecules25194492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail M.M., Samir R., Saber F.R., Ahmed S.R., Farag M.A. Pimenta oil as a potential treatment for Acinetobacter baumannii wound infection: In vitro and in vivo bioassays in relation to its chemical composition. Antibiotics. 2020;9:1–16. doi: 10.3390/antibiotics9100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juergens U.R., Dethlefsen U., Steinkamp G., Gillissen A., Repges R., Vetter H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: A double-blind placebo-controlled trial. Respir. Med. 2003;97(3):250–256. doi: 10.1053/rmed.2003.1432. [DOI] [PubMed] [Google Scholar]
- Kim D.-S., Lee H.-J., Jeon Y.-D., Han Y.-H., Kee J.-Y., Kim H.-J., Shin H.-J., Kang JongWook, Lee B.S., Kim S.-H., Kim S.-J., Park S.-H., Choi B.-M., Park S.-J., Um J.-Y., Hong S.-H. Alpha-Pinene Exhibits Anti-Inflammatory Activity Through the Suppression of MAPKs and the NF-κB Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2015;43(04):731–742. doi: 10.1142/S0192415X15500457. [DOI] [PubMed] [Google Scholar]
- Kummer R., Fachini-Queiroz F.C., Estevão-Silva C.F., Grespan R., Silva E.L., Bersani-Amado C.A., Cuman R.K.N. Evaluation of anti-inflammatory activity of Citrus latifolia Tanaka essential oil and limonene in experimental mouse models. Evidence-based Complement. Altern. Med. 2013;2013:1–8. doi: 10.1155/2013/859083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.-C., Hsu H.-J., Wang Y.-S., Cassidy J., Sheen S., Liu S.-C. Effects of heat treatment on the antioxidative and anti-inflammatory properties of orange by-products. Food and Function. 2017;8(7):2548–2557. doi: 10.1039/c7fo00188f. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Leal A.C., Palou E., López-Malo A. Evaluation of the efficiency of allspice, thyme and rosemary essential oils on two foodborne pathogens in in-vitro and on alfalfa seeds, and their effect on sensory characteristics of the sprouts. Int. J. Food Microbiol. 2019;295:19–24. doi: 10.1016/j.ijfoodmicro.2019.02.008. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Leal A.C., Palou E., López-Malo A., Bach H. Antimicrobial, Cytotoxic, and Anti-Inflammatory Activities of Pimenta dioica and Rosmarinus officinalis Essential Oils. Biomed Res. Int. 2019;2019:1–8. doi: 10.1155/2019/1639726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron D.M., Ames B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. Mutagen. Relat. Subj. 1983;113(3-4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Martins A.O.B.P.B., Rodrigues L.B., Cesário F.R.A.S., de Oliveira M.R.C., Tintino C.D.M., Castro F.F.e., Alcântara I.S., Fernandes M.N.M., de Albuquerque T.R., da Silva M.S.A., de Sousa Araújo A.A., Júniur L.J.Q., da Costa J.G.M., de Menezes I.R.A., Wanderley A.G. Anti-edematogenic and anti-inflammatory activity of the essential oil from Croton rhamnifolioides leaves and its major constituent 1,8-cineole (eucalyptol) Biomed. Pharmacother. 2017;96:384–395. doi: 10.1016/j.biopha.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Mérida-Reyes M.S., Muñoz-Wug M.A., Oliva-Hernández B.E., Gaitán-Fernández I.C., Simas D.L.R., Ribeiro da Silva A.J., Pérez-Sabino J.F. Composition and Antibacterial Activity of the Essential Oil from Pimenta dioica (L.) Merr. from Guatemala. Medicines. 2020;7:59. doi: 10.3390/medicines7100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules. 2010;15:9252–9287. doi: 10.3390/molecules15129252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M., Hobiger S., Jungbauer A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010;122(4):987–996. doi: 10.1016/j.foodchem.2010.03.041. [DOI] [Google Scholar]
- Nair S A., Sr R.K., Nair A.S., Baby S. Citrus peels prevent cancer. Phytomedicine. 2018;50:231–237. doi: 10.1016/j.phymed.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Nirmala M.J., Durai L., Rao K.A., Nagarajan R. Ultrasonic nanoemulsification of Cuminum cyminum essential oil and its applications in medicine. Int. J. Nanomedicine. 2020;15:795–807. doi: 10.2147/IJN.S230893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njoroge S.M., Koaze H., Karanja P.N., Sawamura M. Essential oil constituents of three varieties of Kenyan sweet oranges (Citrus sinensis) Flavour Fragr. J. 2005;20(1):80–85. doi: 10.1002/ffj.1377. [DOI] [Google Scholar]
- Owolabi M.S., Olowu R.A., Lajide L., Oladimeji M.O., Padilla-Camberos E., Flores-Fernández J.M. Inhibition of potato tuber sprouting during storage by the controlled release of essential oil using a wick application method. Ind. Crops Prod. 2013;45:83–87. doi: 10.1016/j.indcrop.2012.11.043. [DOI] [Google Scholar]
- Owolabi M.S., Padilla-Camberos E., Ogundajo A.L., Ogunwande I.A., Flamini G., Yusuff O.K., Allen K., Flores-Fernandez K.I., Flores-Fernandez J.M. Insecticidal activity and chemical composition of the Morinda lucida essential oil against pulse beetle Callosobruchus maculatus. Sci. World J. 2014;2014:1–7. doi: 10.1155/2014/784613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyedeji A.O., Okunowo W.O., Osuntoki A.A., Olabode T.B., Ayo-folorunso F. Insecticidal and biochemical activity of essential oil from Citrus sinensis peel and constituents on Callosobrunchus maculatus and Sitophilus zeamais. Pestic. Biochem. Physiol. 2020;168:104643. doi: 10.1016/j.pestbp.2020.104643. [DOI] [PubMed] [Google Scholar]
- Padilla-Camberos E., Flores-Fernández J.M., Canales-Aguirre A.A., Barragán-Álvarez C.P., Gutiérrez-Mercado Y., Lugo-Cervantes E. Wound healing and antioxidant capacity of Musa paradisiaca Linn. peel extracts. J. Pharm. Pharmacogn. Res. 2016;4:165–173. [Google Scholar]
- Padilla-Camberos E., Sanchez-Hernandez I.M., Torres-Gonzalez O.R., Ramirez-Rodriguez P., Diaz E., Wille H., Flores-Fernandez J.M. Biosynthesis of silver nanoparticles using Stenocereus queretaroensis fruit peel extract: Study of antimicrobial activity. Materials (Basel). 2021;14(16):4543. doi: 10.3390/ma14164543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y., Bi J.X., Zhang Y., Zhang Y., Fan G., Xiao L.Y., Si Y.P. Characterization of Aroma Active Compounds in Fruit Juice and Peel Oil of Jinchen Sweet Orange Fruit (Citrus sinesis (L.) Osbeck) by GC-MS and GC-O. Molecules. 2008;13:1333–1344. doi: 10.3390/molecules13061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho T., Pacheco de Oliveira M., Lima A., Bezerra-Santos C., Piuvezam M. Gamma-Terpinene Modulates Acute Inflammatory Response in Mice. Planta Med. 2015;81(14):1248–1254. doi: 10.1055/s-0035-1546169. [DOI] [PubMed] [Google Scholar]
- Rihawy M.S., Bakraji E.H., Odeh A. PIXE and GC-MS investigation for the determination of the chemical composition of Syrian Cuminum cyminum L. Appl. Radiat. Isot. 2014;86:118–125. doi: 10.1016/j.apradiso.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Ruiz-Pérez N.J., González-Ávila M., Sánchez-Navarrete J., Toscano-Garibay J.D., Moreno-Eutimio M.A., Sandoval-Hernández T., Arriaga-Alba M. Antimycotic Activity and Genotoxic Evaluation of Citrus sinensis and Citrus latifolia Essential Oils. Sci. Rep. 2016;6:1–9. doi: 10.1038/srep25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos I.J.M., Leite G.O., Costa J.G.M., Alves R.R.N., Campos A.R., Menezes I.R.A., Freita F.R.V., Nunes M.J.H., Almeida W.O. Topical Anti-Inflammatory Activity of Oil from Tropidurus hispidus (Spix, 1825) Evidence-based Complement. Altern. Med. 2015;2015:1–7. doi: 10.1155/2015/140247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayyah M., Peirovi A., Kamalinejad M. Anti-nociceptive effect of the fruit essential oil of Cuminum cyminum L. in rat. Iran. Biomed. J. 2002;6:141–145. [Google Scholar]
- Shivakumar S.I., Shahapurkar A.A., Kalmath K.V., Shivakumar B. Antiinflammatory activity of fruits of Cuminum cyminum Linn S. Der Pharm. Lett. 2010;2:22–24. [Google Scholar]
- Soran M.L., Cobzac S.C., Varodi C., Lung I., Surducan E., Surducan V. The extraction and chromatographic determination of the essentials oils from Ocimum basilicum L. by different techniques. J. Phys. Conf. Ser. 2009;182:012016. doi: 10.1088/1742-6596/182/1/012016. [DOI] [Google Scholar]
- Toukourou H., Uwambayinema F., Yakoub Y., Mertens B., Laleye A., Lison D., Quetin-Leclercq J., Gbaguidi F. In Vitro and in Vivo Toxicity Studies on Cymbopogon giganteus Chiov. Leaves Essential Oil from Benin. J. Toxicol. 2020;2020:1–12. doi: 10.1155/2020/8261058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, J., Zhang, X., Bi, Y., Miao, R., Zhang, Z., Su, H., 2015. Anti-Inflammatory Effects of Cumin Essential Oil by Blocking JNK, ERK, and NF- B Signaling Pathways in LPS-Stimulated RAW 264.7 Cells. Evidence-based Complement. Altern. Med. 2015. https://doi.org/10.1155/2015/474509. [DOI] [PMC free article] [PubMed]
- Yoshizaki N., Fujii T., Masaki H., Okubo T., Shimada K., Hashizume R. Orange peel extract, containing high levels of polymethoxyflavonoid, suppressed UVB-induced COX-2 expression and PGE2 production in HaCaT cells through PPAR-γ activation. Exp. Dermatol. 2014;23:18–22. doi: 10.1111/exd.12394. [DOI] [PubMed] [Google Scholar]
- Zhang L.L., Lokeshwar B. Medicinal Properties of the Jamaican Pepper Plant Pimenta dioica and Allspice. Curr. Drug Targets. 2012;13:1900–1906. doi: 10.2174/138945012804545641. [DOI] [PMC free article] [PubMed] [Google Scholar]