Abstract
Background
Infectious complications are a major cause of morbidity and mortality after kidney transplantation.
Methods
In this transplant cohort study at the German Center of Infectious Diseases (DZIF), we evaluated all infections occurring during the first year after renal transplantation. We assessed microbial etiology, incidence rates, and temporal occurrence of these infections.
Results
Of 804 renal transplant recipients (65.2% male, 51 ± 14 years), 439 (54.6%) had 972 infections within the first year after transplantation. Almost half of these infections (47.8%) occurred within the first 3 months. Bacteria were responsible for 66.4% (645/972) of all infections, followed by viral (28.9% [281/972]) and fungal (4.7% [46/972]) pathogens. The urinary tract was the most common site of infection (42.4%). Enterococcus was the most frequently isolated bacterium (20.9%), followed by E. coli (17.6%) and Klebsiella (12.5%). E. coli was the leading pathogen in recipients <50 years of age, whereas Enterococcus predominated in older recipients. Resistant bacteria were responsible for at least 1 infection in 9.5% (76/804) of all recipients. Viral infections occurred in 201 recipients (25.0%). Of these, herpes viruses predominated (140/281 [49.8%]), and cytomegalovirus had the highest incidence rate (12.3%). In the 46 fungal infections, Candida albicans (40.8%) was the most commonly isolated. Other fungal opportunistic pathogens, including Aspergillus fumigatus and Pneumocystis, were rare.
Conclusions
Renal allograft recipients in Germany experience a high burden of infectious complications in the first year after transplantation. Bacteria were the predominating pathogen, followed by opportunistic infections such as cytomegalovirus. Microbial etiology varied between age groups, and resistant bacteria were identified in 10% of recipients.
Keywords: infection, renal transplantation, cohort study, German, DZIF
Solid organ transplant recipients require long-term immunosuppression, which puts them at risk for life-threatening infections. Infectious diseases are a major cause of morbidity and mortality after kidney transplantation, especially in the early post-transplant period [1–3]. Infectious complications also reduce recipients’ quality of life and increase health care costs [2, 4, 5]. Although infections play a fundamental role in recipient prognosis and allograft survival [6, 7], many questions regarding the prevention and early diagnosis of infections remain unanswered, and more research is needed in this area. Post-transplant data are often restricted by a lack of both stringent patient follow-up and standardized definitions of infections. Very few prospective cohort studies of transplant recipients have investigated all infectious complications [8–12], and the occurrence and timeline of all post-transplantation infections have not been studied in a German transplant cohort.
To prevent infection and reduce infection-related morbidity and mortality, we need information on multiple factors. For example, immunosuppressive strategies can reduce the incidence of rejection but increase the recipients’ susceptibility to infection [13–15]. Furthermore, the availability of efficient prophylaxis may have modified frequencies and temporal infection patterns [1, 9, 12]. Knowing the timing and frequency of infections in the era of extended donor/recipient criteria, modern immunosuppression, and routine use of prophylaxis is crucial for implementing prevention strategies.
The transplant cohort of the German Center of Infectious Diseases (DZIF) is a unique database that evaluates infections after renal transplantation [16]. In this cohort, clinical data are being collected, together with biosamples from transplant recipients (predominantly renal allograft recipients). In this study, we assessed the microbial etiology, incidence rates, and temporal occurrence of all infections occurring in this cohort.
METHODS
Study Population and Design
The DZIF transplant cohort is a multicenter prospective cohort study at the German Center for Infection Research [16]. It includes allograft recipients from 5 of the largest German transplant centers (University Hospital Hannover, University Hospital and Renal Center Heidelberg, TU Munich, LMU Munich, and University Hospital Tuebingen). In participating transplant centers, all patients receiving organs were informed about the study and invited to participate.
In the present study, we analyzed data from all adult renal and simultaneous pancreas–kidney allograft recipients aged 18 years and older who consented to participate and received a transplant between April 2011 and November 2019. Ethics approval was obtained from all participating centers (Hannover Medical School Nr 6534, Medical Faculty of the University of Heidelberg Nr S-585/2013, Medical Faculty of the TU Munich Nr 5926/13, LMU Munich Nr 380-15, University Hospital Tuebingen Nr 327/2014BO1), and all participants provided written informed consent.
Study visits were performed immediately before transplantation and at months 3, 6, 9, and 12 after transplantation. We also performed study visits when infections were detected. At each study visit, clinical and laboratory data were collected and entered into a central web-based database. The DZIF transplant cohort monitors data and conducts quality audits regularly, and the study was approved by the DZIF Scientific Steering Committee.
Patient Consent
Ethics approval was obtained from all participating centers (Hannover Medical School Nr 6534, Medical Faculty of the University of Heidelberg Nr S-585/2013, Medical Faculty of the TU Munich Nr 5926/13, LMU Munich Nr 380-15, University Hospital Tuebingen Nr 327/2014BO1), and all participants provided written informed consent.
Outcomes
The primary outcome was the occurrence of clinically relevant infection within the first 12 months after transplantation. Infections were categorized as clinically relevant if the patient complained about specific clinical symptoms (eg, fever, malaise, pain) or if the patient was hospitalized and had antibiotic, antiviral, or antifungal treatment in therapeutic doses. Secondary outcomes were the type of pathogen, timing of infection, and site of infection.
Infectious Events
All infections were identified by trained transplant physicians using paper-based or electronic hospital records and referral documentation. Infections were defined according to Kidney Disease: Improving Global Outcomes (KDIGO) 2009 guidelines, AST 2019 infection guidelines, and European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group definitions [17–20].
A urinary tract infection was only included in case of clinical symptoms in addition to a positive urine culture [18]. Antimicrobial resistance was also recorded [21]. Herpes virus and polyomavirus infections were detected by real-time polymerase chain reaction. BK virus above the threshold of 104 copies per mL plasma was defined as clinically significant [17]. Fungal infections were reported if they met EORTC/MSG criteria with proven and probable invasive infection [19, 20]. We included all proven and probable fungal infections and fungemias. Pneumocystis jirovecii infection was diagnosed by sputum culture and bronchial lavage.
Donor-Derived Infection
Immunosuppression
Almost all patients received a standard triple-drug combination (comprising a calcineurin inhibitor, mycophenolate, and steroids) together with an interleukin 2 receptor antagonist (basiliximab) to induce immunosuppression.
Prophylaxis and Surveillance Strategy
Prophylaxis and surveillance strategy was suggested to be performed according to KDIGO 2009 guidelines [17]. Standard protocol included antiviral prophylaxis with valganciclovir for all cytomegalovirus (CMV) immunoglobulin G (IgG)–positive recipients and for all recipients of organs from CMV IgG–positive donors for at least 3 months. In case of the high-risk constellation D+/R–, 6 months of prophylaxis was suggested. If the recipient was CMV IgG positive, 3-month valganciclovir prophylaxis was recommended. Pneumocystis jirovecii infection was prevented with trimethoprim-sulfamethoxazole for 6 months. Anti-Candida prophylaxis with oral nystatin was provided within the first 1–3 months if >20 mg of methylprednisolone was administered. Urinary tract infection prophylaxis was suggested with trimethoprim-sulfamethoxazole for 6 months after transplantation. Screening for BKV was was recommended to be performed monthly for the first 6 months and every 3 months thereafter as well as in case of worsening of allograft function. No routine EBV screening was performed. Urinary tract infection surveillance was routinely performed with urine culture at each visit, in addition to recording clinical symptoms.
Recipients who received organs from bacteremic or fungemic donors had targeted antimicrobial therapy for a period of 7–14 days.
Statistical Analysis
It was assumed that all 5 centers together cover at least 20% of all solid organ transplants in Germany and provide a high number of infectious events, allowing in-depth analysis of infectious complications. The time of observation was defined as the time between transplantation and transplant failure, loss to follow-up, death, or 12 months after the transplant—whichever occurred first. Cumulative infection rates were calculated as the percentage of infected patients during the first year post-transplantation. According to respective data distribution, continuous variables were expressed as means and SDs or as medians and interquartile ranges (IQRs). Categorical variables were presented as numbers and percentages. Continuous variables were compared using the Mann-Whitney U test or Student t test, as appropriate. Crosstabs combined with the chi-square test or Fisher exact test was used to compare categorical variables. Statistical significance was defined as a P value <.05. We performed logistic regression to evaluate independent risk factors for the occurrence of multiple infections. Significant variables in univariate analysis were introduced in a multivariate model. Cox regression was used to analyze the effect of preceding infections depending on pathogen class (bacterial/viral fungal). Any preceding infection within the first year was considered a time-dependent covariate. Highly prevalent pathogens in patients suffering multiple infections were detected by logistic regression, thereby comparing first-year cumulative incidence rates of patients suffering >2 infections/year with those of patients suffering 1 or 2 infection(s)/year. All statistical analyses were performed using IBM SPSS Statistics, version 28.0 for Mac OS X (SPSS, Inc. Chicago, IL, USA).
RESULTS
Renal Allograft Recipients and Incidence of Infections
All adult renal transplant recipients consenting to participate in the DZIF transplant cohort and receiving a transplant in 1 of the 5 recruiting centers between April 2011 and November 2019 (n = 804) were enrolled for our analysis; 65.2% were male, and the mean age was 51 ± 14 years (Tables 1 and 2). Forty-two (5%) patients had a simultaneous pancreas–kidney transplantation. Follow-up was terminated early in 39 patients (15 patients died, 15 had transplant failure, and 9 were lost to follow-up). Infections were recorded as the direct cause of death in 6/15 patients (40%; pneumonia due to Pseudomonas in 1 patient, invasive aspergillosis in 2 patients, invasive mucormycosis in 1 patient, bornavirus encephalitis in 1 patient, and an outpatient infection of unknown origin in 1 patient).
Table 1.
Characteristics of Recipients, Donors, and Antimicrobial Prophylaxis
| Total | Data Complet-eness | I <50 Years | II 50–65 Years | III >65 Years | Male | Female | |
|---|---|---|---|---|---|---|---|
| Total No. of patients | 804 | 315 | 351 | 137 | 515 | 275 | |
| Demographics | |||||||
| Age at tx, mean ± SD, range, y | 51 ± 14 18–79 |
99.0 | 37 ± 8 18–49 |
57 ± 5 50–65 |
69 ± 3 66–79 |
52 ± 14 18–79 |
50 ± 14 18–74 |
| Male gender | 515 (65.2) | 98.3 | 64.2a | 62.1 | 75.0a | 100.0 | 0.00 |
| Clinical data | |||||||
| Cause of ESRD | 99.0 | ||||||
| Glomerulonephritis | 247 (31.0) | 32.1 | 31.9 | 26.5 | 34.5 | 25.5 | |
| APKD | 122 (15.3) | 10.9 | 21.6 | 9.6 | 12.4 | 20.1 | |
| Diabetes mellitus | 84 (10.6) | 10.3 | 9.8 | 13.2 | 11.0 | 9.5 | |
| Nephrosclerosis | 43 (5.4) | 2.2 | 6.3 | 10.3 | 6.3 | 4.0 | |
| Interstinal nephritis | 25 (3.1) | 3.5 | 2.9 | 5.1 | 3.1 | 3.3 | |
| Vasculitis and collagenoses | 24 (3.0) | 2.6 | 3.2 | 3.7 | 2.0 | 5.1 | |
| Urologically caused diseases | 24 (3.0) | 4.8 | 0.9 | 2.9 | 2.5 | 2.9 | |
| Other hereditary diseases | 38 (4.8) | 9.0 | 2.6 | 0.7 | 5.5 | 3.6 | |
| Other | 192 (24.1) | 24.7 | 21.0 | 30.9 | 22.7 | 25.9 | |
| Body mass index, mean ± SD, kg/m2 | 26 ± 5 | 97.8 | 25 ± 5 | 26 ± 5 | 27 ± 5 | 25 ± 3 | 25 ± 5 |
| Donor characteristics | |||||||
| Deceased donor | 522 (65.7) | 98.9 | 47.3b | 73.3b | 88.8b | 64.2 | 68.0 |
| Age group | 95.8 | ||||||
| <35 y | 78 (10.1) | 13.7 | 10.0 | 2.3 | 8.9 | 12.4 | |
| ≥35–<60 y | 363 (47.1) | 61.7 | 48.7 | 9.9 | 47.9 | 45.1 | |
| ≥60 y | 329 (42.7) | 24.7 | 41.3 | 87.8 | 43.2 | 42.5 | |
| Male sex | 330 (42.8) | 95.9 | 41.0 | 44.4 | 42.7 | 39.9 | 47.7 |
| CMV serologies | |||||||
| R+ | 407 (54.8) | 92.4 | 55.1 | 49.0 | 58.6 | 51.8g | 60.4g |
| D+ | 445 (58.1) | 95.3 | 58.7 | 54.7 | 59.1 | 58.0 | 58.3 |
| D+/R− | 157 (22.0) | 88.9 | 21.3 | 20.2 | 21.7 | 37.0 | 21.6 |
| Immunized | |||||||
| AB0 incompatibility | 45 (5.9) | 95.4 | 6.6 | 5.7 | 2.9 | 5.6 | 5.1 |
| Prior transplant | 126 (15.7) | 100.0 | 19.0 | 14.2 | 10.9 | 15.1 | 16.7 |
| Pancreas–kidney transplantation | 42 (5.2) | 99.1 | 8.2c | 4.6c | 1.5c | 6.0 | 5.4 |
| Antimicrobial prophylaxis | |||||||
| Antivirald | 568 (74.9) | 94.3 | 76.6 | 73.6 | 74.6 | 73.5 | 78.3 |
| Anti-Candidae | 521 (70.2) | 93.0 | 70.3 | 72.0 | 65.3 | 72.0 | 67.7 |
| Postoperative variables | |||||||
| In-patient stay, median (IQR), d | 18 (14–25) | 98.1 | 16 (13–24) | 18 (14–25) | 21 (15–31) | 19 (14–26) | 17 (14–24) |
| Delayed graft function | 126 (15.9) | 98.4 | 13.3f | 16.0 | 21.8f | 15.4 | 16.8 |
Data presented as No. (%) or as % (columns 3–8) unless otherwise indicated. Missing data were excluded. Delayed graft function = need for hemodialysis within the first 7 days post-transplantation.
Abbreviations: APKD, autosomal polycystic kidney disease; CMV, cytomegalovirus; D+, donor negative; ESRD, end-stage renal disease; IQR, interquartile range; R+, recipient positive; R–D+, recipient negative, donor positive; tx, transplantation.
Statistically significant result, tested with chi-square test: P = .025, χ2 = 5.03.
Statistically significant result, tested with chi-square test: I vs III: P < .001, χ2 = 67.26; I vs II: P < .001, χ2 = 46.78.
Statistically significant result, tested with chi-square test: I vs II: P = .014, χ2 = 6.04; I vs III: P = .003, χ2 = 9.01.
Mostly valganciclovir/ganciclovir.
Mostly oral nystatin.
Statistically significant result, tested with chi-square test: I vs III: P = .023, χ2 = 5.15.
Male vs female: p = .026, χ2 = 4.95.
Table 2.
Characteristics of Infected Patients
| Bacterial Infection | Viral Infection | Fungal Infection | |
|---|---|---|---|
| Total No. of patients | 332 | 201 | 39 |
| Demographics | |||
| Age at tx, mean ± SD, range, y | 53 ± 14, 18–79 | 51 ± 14, 20–79 | 58 ± 12, 27–76 |
| Male gender | 63.0 | 65.7 | 66.7 |
| Clinical data | |||
| Cause of ESRD | |||
| Glomerulonephritis | 27.4 | 33.3 | 17.9 |
| APKD | 16.9 | 14.9 | 5.1 |
| Diabetes mellitus | 12.0 | 8.5 | 25.6 |
| Nephrosclerosis | 5.7 | 4.0 | 15.4 |
| Interstinal nephritis | 3.0 | 5.0 | 5.1 |
| Vasculitis and collagenoses | 2.1 | 4.5 | 2.6 |
| Urologically caused diseases | 3.0 | 2.0 | 0.0 |
| Other hereditary diseases | 4.5 | 7.0 | 2.6 |
| Other | 25.3 | 20.9 | 25.6 |
| Body mass index, mean ± SD, kg/m2 | 26 ± 4 | 26 ± 4 | 26 ± 5 |
| Donor characteristics | |||
| Deceased donor | 68.7 | 71.1 | 87.2 |
| Age group | |||
| <35 y | 8.5 | 4.1 | 10.5 |
| ≥35–<60 y | 42.9 | 30.6 | 31.6 |
| ≥60 y | 48.6 | 26.5 | 57.9 |
| Male sex | 45.6 | 41.8 | 36.8 |
| CMV serologies | |||
| R+ | 55.4 | 49.2 | 45.9 |
| D+ | 60.5 | 64.4 | 63.2 |
| R+/D− | 23.7 | 31.9 | 30.6 |
| Immunized | |||
| AB0 incompatibility | 5.4 | 5.5 | 2.6 |
| Prior transplant | 16.6 | 16.4 | 12.8 |
| Pancreas–kidney transplantation | 6.0 | 4.5 | 17.9 |
| Antimicrobial prophylaxis | |||
| Antivirala | 76.5 | 78.4 | 74.4 |
| Anti-Candidab | 71.9 | 79.0 | 84.6 |
| Postoperative variables | |||
| In-patient stay, median (IQR), d | 21, 15–30 | 18, 14–25 | 33, 16–40 |
| Delayed graft function | 20.1 | 18.0 | 44.7 |
Data presented as % unless otherwise indicated. Missing data were excluded. Delayed graft function = need for hemodialysis within the first 7 days post-transplantation.
Abbreviations: APKD, autosomal polycystic kidney disease; CMV, cytomegalovirus; D+, donor negative; ESRD, end-stage renal disease; IQR, interquartile range; R+, recipient positive; R–D+, recipient negative, donor positive; tx, transplantation.
Mostly valganciclovir/ganciclovir
Mostly oral nystatin.
Standard prophylaxis included 6-month anti-Pneumocystis prophylaxis with trimethoprim-sulfamethoxazole for all recipients. Anti-Candida prophylaxis (mostly oral nystatin) was given to 70.2% of recipients for 1–3 months. Antiviral prophylaxis (valganciclovir/ganciclovir) was provided to 74.9% of all recipients for at least 3 months.
Burden and Timeline of Infections
In total, 972 infections were recorded in 439 patients within the first year after renal transplantation, resulting in a cumulative incidence rate of 54.9% (439/804) or 2.2 (range, 1–11) infections per infected patient (Figure 1). Infection rates were highest in the first month after transplantation at 21.1% (205/972; 8.3 episodes per 1000 transplant-days); 47.8% (465/972) of infections occurred within the first 3 months (6.4 episodes per 1000 transplant-days), 25.3% (246/972) between months 3 and 6, 15.3% (149/972) between months 6 and 9, and 11.5% (112/972) between months 9 and 12.
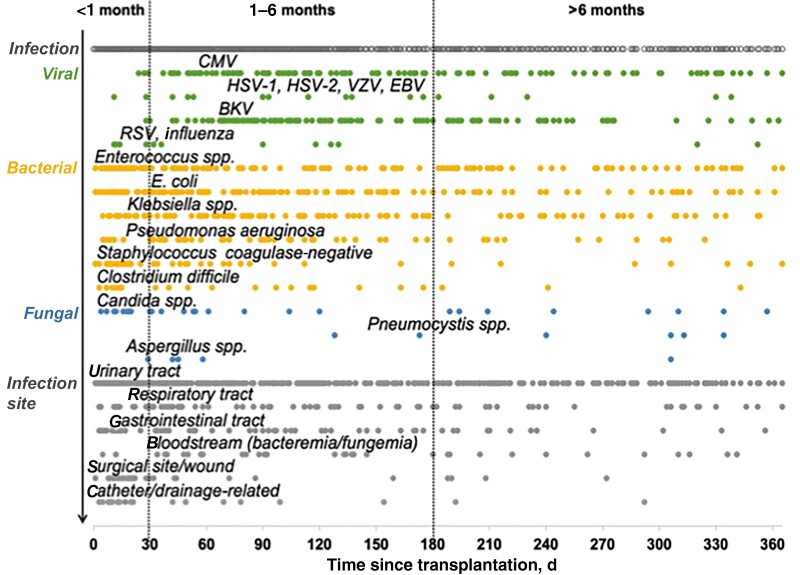
Figure 1.
Infections detected in the first year after renal transplantation. Abbreviations: BKV, BK virus; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HSV, herpes simplex virus; RSV, respiratory syncytial virus; VZV, varicella zoster virus.
Nosocomial infections (mostly bacterial urinary tract) predominated in the first months, followed by opportunistic viral infections such as CMV and BK virus (BKV) between months 1 and 5 (Figure 1). There were 2 peaks of fungal infection. Candida spp. and Aspergillus fumigatus were the leading pathogens in the first 2 months, while Pneumocystis jirovecii occurred after month 4. There was no difference in the incidence of infections between male and female recipients (male 54.2%, female 58.2%; P = .28). However, age affected the incidence of infection, with recipients aged >65 years having an increased risk of bacterial and fungal infection (Tables 1 and 2; Supplementary Figure 2).
Bacterial Infections
Bacteria accounted for 66.4% (645/972) of all infections (Figure 1, Table 3). A bacterial infection was reported in 41.3% (332/804) of all recipients (male 36.3%, female 44.7%; P = .28), and the incidence increased with recipients’ age (<50 years: 35.2%; 50–65 years: 43.9%; >65 years: 48.9%) (Supplementary Figures 1 and 2). Elderly recipients tended to experience bacterial infections later than younger recipients did (median [IQR], 39 [17–85] days vs 28 [11–131] days; P = .064). Regarding location of infection, bacterial infections were most common in the urinary tract (62.3% [402/645]), followed by the respiratory tract (8.2% [53/645]) and gastrointestinal tract (7.0% [45/645]). Bacteremia was noticed in 3.5% (34/972) of all infections, concerning 31 patients (3.9%), and E. coli was the predominant pathogen (35.7%); 54.9% (354/645) of infections occurred within the first 3 months. Enterococcus spp. predominated within the first month, and gram-negative bacteria (especially E. coli, Klebsiella spp.) predominated within months 3 and 6.
Table 3.
Characteristics of Bacterial Infections
| Bacterial Pathogen | No. of Bacterial Infections (Total = 645) | % of Bacterial Pathogen Detections (Total = 771) | First-Year Cumulative Incidence Rate, % (No. of Patients) (Total = 804) | Median Time to First Infection (IQR), d |
|---|---|---|---|---|
| All | 645 | 771 | 54.6 (439) | 34 (12–96) |
| Enterococcus spp. | 161 | 20.9 | 14.8 (119) | 31 (13–116) |
| E. coli | 136 | 17.6 | 12.9 (104) | 72 (24–153) |
| Unknown pathogen | 137 | 17.7 | 13.2 (106) | 74 (12–163) |
| Klebsiella spp. | 96 | 12.5 | 7.8 (63) | 72 (39–152) |
| Pseudomonas aeruginosa | 56 | 7.3 | 5.0 (40) | 89 (38–155) |
| Staphylococcus coagulase neg. | 52 | 6.7 | 6.0 (48) | 20 (13–64) |
| Enterobacter | 23 | 3.0 | 2.7 (22) | 102 (62–214) |
| Clostridium difficile | 22 | 2.9 | 2.7 (22) | 75 (14–135) |
| Other bacteria | 19 | 2.5 | 2.2 (18) | 46 (21–113) |
| Other gram-positive bacteria | 19 | 2.5 | 2.4 (19) | 58 (18–152) |
| Streptococcus/Streptococcus spp. | 11 | 1.4 | 1.4 (11) | 181 (44–311) |
| Other Enterobacteria | 16 | 2.1 | 1.9 (15) | 75 (42–182) |
| Other nonenteric gram-neg. bac. | 8 | 1.0 | 1.0 (8) | 99 (48–140) |
| Staphylococcus aureus | 8 | 1.0 | 0.9 (7) | 71 (32–119) |
| Other mycobacteria | 2 | 0.3 | 0.2 (2) | – |
| Stenostrophomonas | 1 | 0.1 | 0.1 (1) | – |
| Pneumococcus | 1 | 0.1 | 0.1 (1) | – |
| Legionella | 1 | 0.1 | 0.1 (1) | – |
| Nocardia | 1 | 0.2 | 0.1 (1) | – |
| Other anaerobic bacteria | 1 | 0.2 | 0.1 (1) | – |
Abbreviations: bac., bacteria; IQR, interquartile range.
Enterococcus was isolated most often (20.9% [161/771]), followed by E. coli (17.6% [136/771]) and Klebsiella (12.5% [96/771]). E. coli was the predominant pathogen among recipients aged <50 years, whereas Enterococcus species were the most frequent among recipients aged 50–65 and >65 years (Supplementary Figure 3). The percentage of Pseudomonas aeruginosa infections increased with age, and the rate of infection was almost 4 times higher in recipients >65 years old than in recipients <50 years old (14.5% vs 3.7%). Pseudomonas infection was also more common in males than in females (6.6% vs 2.2%; P = .007), whereas E. coli was more frequently detected in females (E. coli 17.4% vs 10.9%; P = .010).
Resistant bacteria were highly prevalent, involved in 19.1% (123/645) of all bacterial infections and affecting 9.5% (76/804) of our cohort. This rate was nearly 3 times higher in recipients aged >65 years than in recipients aged <50 years (16.1% [22/137] vs 5.4% [17/315]; P < .001]). Of the bacteria we isolated from recipients, 16.0% (123/771) were resistant strains. Vancomycin-resistant Enterococcus was the most predominant resistant bacteria (44.7% [55/123]), followed by multiresistant gram-negative bacteria (26.0% [32/123]). Opportunistic bacteria, such as Legionella spp., Nocardia spp., and Mycobacteria spp., were rare (0.6% [4/645]).
Viral Infections
Viral pathogens were responsible for 28.9% (281/972) of all infections (Figure 1, Table 4) and were detected in 25.0% (201/804) of recipients (25.6% male, 25.1% female; P = .87) (Supplementary Figures 1 and 2). Recipients aged >65 years were least affected by viral infections, and recipients aged 50–65 years were most affected (18.2% [25/137] vs 28.8% [101/351]; P = .002). This was particularly true for CMV infection, which had an incidence of 14.5% (51/351) in middle-aged recipients and 6.6% (9/137) in recipients >65 years old (P = .016). Of all viral infections, CMV had the highest incidence rate (12.3% [99/804]), being 3 times higher in the CMV high-risk group (D+/R−: 27.4% [43/157]) than in groups with a lower CMV risk (9.0% [50/558]; P < .001 [D−/R− = 0.6%, D+/R+ = 11.6%, D−/R+ = 12.9%]). In addition, the median (IQR) time to first CMV infection was significantly shorter in the high-risk group than in the lower-risk groups (106 [73–154] days vs 171 [82–251] days; P = .022), despite prophylaxis; 12.1% of all CMV-infected recipients were suffering from CMV disease. Herpesviridae other than CMV were more frequent in male recipients (12.8% vs 3.2%). In all, herpes viruses were predominant (140/281 [49.8%]). All recipients with herpes simplex virus (HSV) infection were male, and most (75% [6/9]) did not receive antiviral prophylaxis. The incidence of herpes infections also increased with age (<50 years: 5.0%; 50–65 years: 11.2%; <65 years: 16.2%). Polyomaviridae were the second most common viral pathogens (42.0% [118/281]), and of these, BKV was predominant (99.2% [117/118]). BKV nephropathy was confirmed in 1.5% (12/804) of all recipients. Notably, the temporal pattern of BKV and CMV infections was similar. Respiratory infections included influenza A and B (n = 8), respiratory syncytial virus (n = 6), and rhinovirus (n = 1) and were more common in female recipients than in male recipients (8.6% vs 3.6%). Gastrointestinal viral pathogens included hepatitis E (n = 2) and norovirus (n = 6).
Table 4.
Characteristics of Viral Infections
| Viral Infection | No. of Viral Infections (Total = 281) | % of Viral Pathogen Detections (Total = 281) | First-Year Cumulative Incidence Rate, % (No. of Patients) (Total = 804) | Median Time to First Infection (IQR), d |
|---|---|---|---|---|
| All | 281 | 281 | 25.0 (201) | 111 (71–183) |
| CMV | 113 | 40.2 | 12.3 (99) | 133 (74–219) |
| BKV | 117 | 41.6 | 11.4 (92) | 113 (84–182) |
| HSV-1 | 9 | 3.2 | 1.1 (9) | 90 (42–114) |
| HSV-2 | 6a | 2.1 | 0.7 (6) | 45 (31–79) |
| VZV | 6 | 2.1 | 0.7 (6) | 172 (112–202) |
| Influenza A | 6 | 2.1 | 0.7 (6) | 79 (17–129) |
| RSV | 6 | 2.1 | 0.7 (6) | 34 (29–77) |
| Norovirus | 6 | 2.1 | 0.7 (6) | 65 (36–113) |
| EBV | 5 | 1.8 | 0.6 (5) | 137 (134–330) |
| Hepatitis E | 2 | 0.7 | 0.2 (2) | – |
| Influenza B | 2 | 0.7 | 0.2 (2) | – |
| HHV-6 | 1 | 0.4 | 0.1 (1) | – |
| JCV | 1 | 0.4 | 0.1 (1) | – |
| Rhinovirus | 1 | 0.4 | 0.1 (1) | – |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV-6, human herpes virus 6; HSV, herpes simplex virus; IQR, interquartile range; JCV, JC virus; RSV, respiratory syncytial virus; VZV, varicella zoster virus.
All 6 patients had HSV-2 and HSV-1.
Fungal Infections
There were 46 documented fungal infections (5.0% male, 4.7% female; P = .84) (Figure 1, Table 5). Candida albicans was the most common fungal agent and accounted for 40.8% (20/46) of all fungal isolates.
Table 5.
Characteristics of Fungal Infections
| Fungal Pathogen | No. of Fungal Infections (Total = 46) | % of Fungal Pathogen Detections (Total = 49) | First-Year Cumulative Incidence Rate, % (No. of Patients) (Total = 804) | Median Time to First Infection (IQR), d |
|---|---|---|---|---|
| All | 46 | 49 | 39 (4.9) | 58 (20–159) |
| Candida albicans | 20 | 40.8 | 18 (2.2) | 54 (23–116) |
| Candida non-albicans spp. | 12 | 24.5 | 12 (1.5) | 25 (13–255) |
| Pneumocystis jirovecii | 7 | 14.3 | 7 (0.9) | 240 (151–310) |
| Aspergillus fumigatus | 5 | 10.2 | 5 (0.6) | 45 (42–58) |
| Other | 3 | 6.1 | 3 (0.4) | 181 (117–237) |
| Cryptococcus neoformans | 1 | 2.0 | 1 (0.1) | – |
| Zygomycetes | 1 | 2.0 | 1 (0.1) | – |
Abbreviation: IQR, interquartile range.
We documented 7 Pneumocystis jirovecii (14.3%) and 5 Aspergillus fumigatus infections (10.2%). Aspergillus fumigatus was only detected in males, particularly between months 1 and 2. Other opportunistic pathogens like Cryptococcus neoformans (n = 1) or Zygomycetes (n = 1) were rare.
Fungal infections were detected in 39/804 (4.9%) patients, and incidence rates increased with age (>65 years: 11.7%; vs <50 years: 1.9%; P < .001) (Supplementary Figures 1 and 2). The incidence of fungal infections was also high in pancreas–kidney allograft recipients (16.7% [9/42]). Of note, fungal infections were detected predominantly early in the youngest age group and predominantly late in the middle-aged group (median [IQR], 23 [13–35] days vs 128 [61–184] days; P = .052). The latest fungal infections were due to Pneumocystis jirovecii (median [IQR], 240 [151–310]), and the earliest were due to Candida non-albicans species (median [IQR], 25 [13–255]). Recipients infected with Candida albicans had a long postoperative stay (median [IQR], 45 [16–55] days), whereas recipients infected with non-albicans spp. (n = 12) were notably younger than those infected with other fungal pathogens (60 ± 9 vs 50 ± 14 years).
Almost 90% (34/39) of recipients with a fungal infection also had at least 1 bacterial infection during the first year after transplantation. In 80% (27/34) of cases, bacterial infection preceded fungal infection. All preceding bacterial infections were treated with broad-spectrum antibiotics (mostly carbapenems or glycopeptides). Only 4/39 (10.3%) patients with a fungal infection experienced no other infection within the first year. Overall, the mean number (range) of all infections per recipient was 4.2 ± 2.3 (1–11); 48.7% (19/39) were affected by resistant bacteria. The most common site of infection was the respiratory tract, mostly due to Aspergillus fumigatus and Pneumocystis spp.
Multiple Infections
More than 2 infections per year were detected in 17.0% (137/804) of recipients. The prevalence increased with age (25.5% in recipients aged >65 years vs 10.5% in recipients aged <55 years; P < .001). Univariate analysis revealed that recipient age, deceased donor, donor age, delayed graft function, and the number of postoperative inpatient-days were significantly associated with the occurrence of multiple infections (Table 6). Multivariate analysis confirmed that recipient age and number of postoperative inpatient-days were independent risk factors for multiple infections.
Table 6.
Risk Factors for Multiple Infections
| Univariate Analyses | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Covariates | OR | 95% CI | P | OR | 95% CI | P |
| Age at tx, y | 1.03 | [1.02–1.05] | <.001 | 1.02 | [1.00–1.04] | .038 |
| Male sex | 1.32 | [0.86–2.02] | .21 | … | … | |
| Cadaveric donation | 1.67 | [1.05–2.66] | .029 | 1.18 | [0.68–2.06] | .547 |
| Antiviral prophylaxis | 1.40 | [0.85–2.32] | .186 | … | … | |
| Prior tx | 1.21 | [0.69–2.13] | .504 | … | … | |
| Pancreas–kidney tx | 1.27 | [0.54–3.00] | .580 | … | … | |
| Delayed graft functiona | 1.92 | [1.18–3.15] | .009 | 1.20 | [0.67–2.14] | .547 |
| Donor ageb | 1.10 | [1.02–1.18] | .010 | 1.04 | [0.96–1.12] | .383 |
| No. of inpatient-dc | 1.32 | [1.17–1.49] | <.001 | 1.25 | [1.10–1.43] | <.001 |
Recipients with >2 infections/year (group 1, n = 137) were compared with recipients with 1–2 infection(s)/year (group 2, n = 302). Results are based on logistic regression analyses. Inclusion criteria for multivariate analysis: P < .05 in univariate analysis.
Abbreviations: OR, odds ratio; tx, transplantation.
Need for hemodialysis within the first 7 days post-transplantation.
In 5-year intervals.
Post-transplantation, in 7-day intervals.
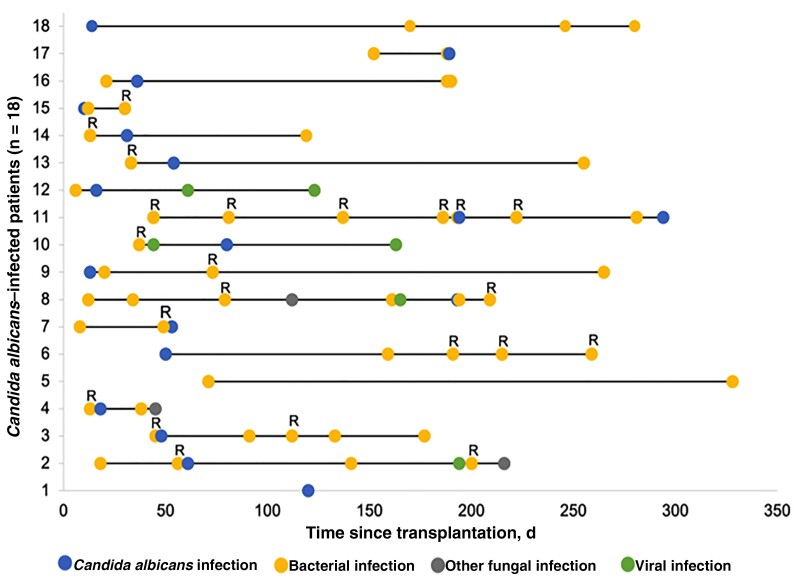
Table 7 shows pathogens that were significantly more prevalent in recipients with >2 infections per year than in recipients with 1–2 infections. Recipients infected with Candida albicans were frequently affected by multiple infections (mean [range], 5.0 [1–10] infections/patient), but also with resistant strains (66.7% [12/18]) (Figure 2).
Table 7.
Highly Prevalent Pathogens in Patients Suffering Multiple Infections
| OR | 95% CI | P | Incidence Rate Group 1 | Incidence Rate Group 2 | |
|---|---|---|---|---|---|
| Candida non-albicans spp. | 23.70 | [3.00–187.09] | .003 | 7.3 (10/137) | 0.7 (2/302) |
| Candida albicans | 19.86 | [4.50–87.57] | <.001 | 11.7 (16/137) | 0.7 (2/302) |
| EBV | 9.05 | [1.02–81.77] | .035 | 2.9 (4/137) | 0.3 (1/302) |
| Pneumocystis jirovecii | 5.68 | [1.09–29.66] | .033 | 3.6 (5/137) | 0.7 (2/302) |
| Klebsiella spp. | 5.44 | [5.44–9.59] | <.001 | 29.9 (41/137) | 7.3 (22/302) |
| Pseudomonas aerguinosa | 4.27 | [2.71–8.40] | <.001 | 18.2 (25/137) | 5.0 (15/302) |
| Enterococcus spp. | 4.60 | [2.94–7.21] | <.001 | 48.9 (67/137) | 17.2 (52/302) |
| Staph. coag. negative | 2.98 | [1.62–5.48] | <.001 | 19.0 (26/137) | 7.3 (22/302) |
| Enterobacter | 2.80 | [1.18–6.66] | .015 | 8.8 (12/137) | 3.3 (10/302) |
| E. coli | 2.64 | [1.67–4.16] | <.001 | 36.5 (50/137) | 17.8 (54/302) |
| CMV | 1.80 | [1.13–2.86] | .013 | 29.9 (41/137) | 19.2 (58/302) |
First-year pathogen incidence rates in patients suffering >2 infections/year (n = 137) were compared with those in patients suffering 1 or 2 infections/year (n = 302). Not significant: BK virus (P = .065), Streptococcus spp. (P = .090), Staphylococcus aureus (P = .140), herpes simplex virus–1 (P = .277), influenza A (P = .277), norovirus (P = .277), respiratory syncytial virus (P = .277), herpes simplex virus–2 (P = .297), Clostridium difficile (P = .314), varicella zoster virus (P = 0.393), Aspergillus fumigatus (P = .497).
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; OR, odds ratio; Staph. coag. negative, Staphylococcus coagulase negative.
Figure 2.
Patients with Candida albicans infections were frequently affected by multiple infections and resistant bacteria. Shown is a timeline of all infections during first year in renal allograft recipients with Candida albicans infection (n = 18). Abbreviation: R, bacterial resistance.
Bacterial infection increased the risk of fungal infection (hazard ratio [HR], 6.45; 95% CI, 3.23–12.90; P < .001), and fungal infection increased the risk for bacterial infection (HR, 3.50; 95% CI, 1.44–8.49; P = .006). The risk of viral infection was not affected by bacterial infection (HR, 1.13; 95% CI, 0.84–1.52; P = .43) or fungal infection (HR, 0.87; 95% CI, 0.44–2.62; P = .89).
DISCUSSION
Effective management of post-transplant infection relies on prevention, early diagnosis, and specific therapy [15, 22]. For this, detailed information on the incidence, microbial etiology, and timeline of infections is crucial. This is the first comprehensive study of a representative German renal transplant cohort comprising >800 patients evaluating all clinically relevant infections detected during the first year after transplantation.
Almost 55% of our cohort experienced at least 1 clinically relevant infection. Bacterial infections predominated (66%), followed by viral (29%) and fungal (<5%) pathogens. BKV and CMV were the most frequently identified opportunistic pathogens.
Three infection periods have been reported after solid organ transplantation: Nosocomial infections predominate in the first month, followed by opportunistic infections between months 1 and 6, and community-acquired infections after month 6 [2, 14]. We confirmed these results; Enterococcus spp. and Candida spp. infections mainly occurred during the first 30 days, and infections with opportunistic pathogens, like CMV and BKV, were highly prevalent in the intermediate period. Infections with Pneumocystis jirovecii were rare and occurred mostly in the late period, presumably because routine trimethoprim-sulfamethoxazole prophylaxis was administered for 6 months after transplantation. Only a few community-acquired respiratory infections occurred during the late period—most were detected in the first month after transplantation. This might be because pathogens are typically not evaluated in patients with mild symptoms who do not require hospitalization.
Long-term immunosuppression and antimicrobial therapy increased the incidence of resistant pathogens in transplant patients [23–25]. We identified resistant strains in 9.5% of all recipients and in 50% of patients with fungal infections. This might reflect the high prevalence of multiple infections requiring antimicrobial therapies.
An association between bacterial and fungal infections and resistant strains appears likely. Elderly recipients with prolonged postoperative hospitalization seem to be particularly vulnerable. Early and intensified surveillance is needed. This might also be appropriate for pancreas–kidney allograft recipients, appearing to be susceptible to early Candida infections, or elderly males, who are prone to Pseudomonas aeruginosa. All these predominantly nosocomial pathogens, including the most frequently isolated agent, Enterococcus, were associated with the occurrence of multiple infections. There could be a direct effect due to pathogen-specific mechanisms or an indirect effect as a consequence of frequent hospitalizations and unsuccessful therapies. The interaction of both effects seems likely. The current bacterial spectrum, with Enterococcus and E. coli predominating, does not seem to be sufficiently covered by trimethoprim-sulfamethoxazole prophylaxis. Considering the high prevalence of bacterial infections, additional agents are required. Several new immunosuppressive regimens with varying immunosuppressive potential have been developed in the last years for transplant recipients [26, 27]. Opportunistic infections have been successfully reduced in these patients through prophylaxis and surveillance [2]. An important effect of these strategies is reducing the prevalence of Pneumocystis jirovecii and CMV infections, both of which peak at 4 months after transplantation. Patients receiving a CMV IgG–seropositive organs receive a 3- or 6-month valganciclovir prophylaxis [17, 28]. This is important because more than half of viral infections were herpes viruses, with CMV having the highest incidence rate. A prolonged CMV prophylaxis and/or an intensified preemptive strategy once valganciclovir is stopped could also be beneficial. Indeed, CMV infections remain an important risk factor for graft loss and patient mortality [29–31]. Another way to reduce CMV infection would be to test CMV-specific cell-mediated immunity in susceptible patients. This would allow CMV prophylaxis to be discontinued in individual patients [32]. BKV nephropathy is rare but can result in graft loss [33, 34]. In the DZIF cohort study, Polyomaviridae were the second most common viral pathogens, in agreement with findings from the Swiss Transplant Cohort [8]. Therapeutic interventions are limited, so strict surveillance, as suggested in the KDIGO guidelines, is needed [17].
Most clinical studies have focused on specific infections and have provided limited information on the global burden of infectious complications after renal transplantation [35–40]. This cohort study has collected data of optimal quality based on standardized definitions and quality audits [16], and these data guarantee a comprehensive view of all clinically relevant infections in the first year after renal transplantation. However, the generalizability of our results to other settings might vary depending on the geographic setting and distinct microbial prophylaxis strategy.
In conclusion, renal allograft recipients in Germany experienced a high burden of infectious events during the first year after transplantation. Current prophylactic agents appear efficient in the prevention of opportunistic infections, but thereby neglect common nosocomial infections (eg, Enterococcus spp.). The prevalence of nosocomial infections was high during the first months, and the prevalence of viral opportunistic infections such as CMV and BKV increased thereafter. Invasive fungal diseases and infections by opportunistic bacteria were rare. Many bacterial pathogens were multidrug resistant, supporting the need for specific microbiologic diagnoses and critical use of antibiotics. Age not only affects the frequency but also the temporal pattern and microbial etiology of infections. The occurrence of multiple infections was common and seems to correlate with immunodeficiency. Further research is needed to understand the effects and mechanisms of the frequently involved pathogens, first detected in the present study. Targeting these would be a very effective way to prevent further infections. In all, our observations may help to guide future surveillance strategies. Therefore, further prospective multicenter studies are required to verify and update our results and to pursue novel approaches for prophylactic strategies.
Supplementary Material
Acknowledgments
We thank the study coordinators at all participating facilities of the Transplant Cohort of the German Center for Infection Research (DZIF Transplant Cohort) Consortium for their excellent support.
Financial support . This work was supported by the German Ministry of Education and Research via the German Center for Infection Research (DZIF; TTU 07.701).
Potential conflicts of interest . All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions . C.S. designed and conducted the study, recruited patients, analyzed data, and wrote the manuscript; I.S. collected and analyzed data and wrote the manuscript; K.G. and D.S. conducted the study and collected data; A.M., A.D.P., and S.N. recruited patients and collected data; R.B. analyzed data; L.R., U.H., and C.M. supervised the performance of the study; P.S., K.H., M.Z., and S.M. discussed the manuscript; T.G. supervised the study conception and performance of the study and wrote the manuscript. All authors were involved in the review of the study data and manuscript preparation.
Contributor Information
Claudia Sommerer, Nephrology, University Hospital Heidelberg, Heidelberg, Germany; German Centre for Infection Research (DZIF), Germany.
Iris Schröter, Nephrology, University Hospital Heidelberg, Heidelberg, Germany; German Centre for Infection Research (DZIF), Germany.
Katrin Gruneberg, Nephrology, University Hospital Heidelberg, Heidelberg, Germany; German Centre for Infection Research (DZIF), Germany.
Daniela Schindler, German Centre for Infection Research (DZIF), Germany; Department of Nephrology, Klinikum rechts der Isar of the Technical University Munich, Munich, Germany.
Rouven Behnisch, Institute of Medical Biometry, University Hospital Heidelberg, Heidelberg, Germany.
Christian Morath, Nephrology, University Hospital Heidelberg, Heidelberg, Germany; German Centre for Infection Research (DZIF), Germany.
Lutz Renders, German Centre for Infection Research (DZIF), Germany; Department of Nephrology, Klinikum rechts der Isar of the Technical University Munich, Munich, Germany.
Uwe Heemann, German Centre for Infection Research (DZIF), Germany; Department of Nephrology, Klinikum rechts der Isar of the Technical University Munich, Munich, Germany.
Paul Schnitzler, German Centre for Infection Research (DZIF), Germany; Department of Infectious Diseases, University Hospital Heidelberg, Heidelberg, Germany.
Anette Melk, German Centre for Infection Research (DZIF), Germany; Department of Pediatric Kidney, Liver and Metabolic Diseases, Hannover Medical School, Hannover, Germany.
Andrea Della Penna, Department of General, Visceral and Transplant Surgery, University Hospital Tuebingen, Tuebingen, Germany.
Silvio Nadalin, German Centre for Infection Research (DZIF), Germany; Department of General, Visceral and Transplant Surgery, University Hospital Tuebingen, Tuebingen, Germany.
Klaus Heeg, German Centre for Infection Research (DZIF), Germany; Department of Infectious Diseases, University Hospital Heidelberg, Heidelberg, Germany.
Stefan Meuer, German Centre for Infection Research (DZIF), Germany; Department of Immunology, University Hospital Heidelberg, Heidelberg, Germany.
Martin Zeier, Nephrology, University Hospital Heidelberg, Heidelberg, Germany; German Centre for Infection Research (DZIF), Germany.
Thomas Giese, German Centre for Infection Research (DZIF), Germany; Department of Immunology, University Hospital Heidelberg, Heidelberg, Germany.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med 2007; 357:2601–14. [DOI] [PubMed] [Google Scholar]
- 2. Fishman J. Infection in organ transplantation. Am J Transplant 2017; 17:856–79. [DOI] [PubMed] [Google Scholar]
- 3. Fiorentino M, Pesce F, Schena A, Simone S, Castellano G, Gesualdo L. Updates on urinary tract infections in kidney transplantation. J Nephrol 2019; 32:751–61. [DOI] [PubMed] [Google Scholar]
- 4. Martins BCC, Mesquita KHC, Costa I, et al. Hospital cost of complications after kidney transplant. Transplant Proc 2020; 52:1294–8. [DOI] [PubMed] [Google Scholar]
- 5. Bodro M, Linares L, Chiang D, Moreno A, Cervera C. Managing recurrent urinary tract infections in kidney transplant patients. Expert Rev Anti Infect Ther 2018; 16:723–32. [DOI] [PubMed] [Google Scholar]
- 6. Martin-Gandul C, Mueller NJ, Pascual M, Manuel O. The impact of infection on chronic allograft dysfunction and allograft survival after solid organ transplantation. Am J Transplant 2015; 15:3024–40. [DOI] [PubMed] [Google Scholar]
- 7. Vogelzang JL, van Stralen KJ, Noordzij M, et al. Mortality from infections and malignancies in patients treated with renal replacement therapy: data from the ERA-EDTA registry. Nephrol Dial Transplant 2015; 30:1028–37. [DOI] [PubMed] [Google Scholar]
- 8. van Delden C, Stampf S, Hirsch HH, et al. Burden and timeline of infectious diseases in the first year after solid organ transplantation in the Swiss Transplant Cohort Study. Clin Infect Dis 2020; 71:e159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Juan R S, Aguado JM, Lumbreras C, et al. Incidence, clinical characteristics and risk factors of late infection in solid organ transplant recipients: data from the RESITRA study group. Am J Transplant 2007; 7:964–71. [DOI] [PubMed] [Google Scholar]
- 10. Koller MT, van Delden C, Müller NJ, et al. Design and methodology of the Swiss Transplant Cohort Study (STCS): a comprehensive prospective nationwide long-term follow-up cohort. Eur J Epidemiol 2013; 28:347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sousa SR, Galante NZ, Barbosa DA, Pestana JO. Incidence of infectious complications and their risk factors in the first year after renal transplantation. J Bras Nefrol 2010; 32:75–82. [PubMed] [Google Scholar]
- 12. Garrido RS, Aguado JM, Díaz-Pedroche C, et al. A review of critical periods for opportunistic infection in the new transplantation era. Transplantation 2006; 82:1457–62. [DOI] [PubMed] [Google Scholar]
- 13. Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med 2004; 351:2715–29. [DOI] [PubMed] [Google Scholar]
- 14. Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med 1998; 338:1741–51. [DOI] [PubMed] [Google Scholar]
- 15. Fishman JA. From the classic concepts to modern practice. Clin Microbiol Infect 2014; 20(Suppl 7):4–9. [DOI] [PubMed] [Google Scholar]
- 16. Karch A, Schindler D, Kühn-Steven A, et al. The transplant cohort of the German Center for Infection Research (DZIF Tx-Cohort): study design and baseline characteristics. Eur J Epidemiol 2021; 36:233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients . Am J Transplant 2009; 9(Suppl 3): S1––155.. [DOI] [PubMed] [Google Scholar]
- 18. Goldman JD, Julian K. Urinary tract infections in solid organ transplant recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019; 33:e13507. [DOI] [PubMed] [Google Scholar]
- 19. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2020; 71:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–81. [DOI] [PubMed] [Google Scholar]
- 22. Marty FM, Rubin RH. The prevention of infection post-transplant: the role of prophylaxis, preemptive and empiric therapy. Transpl Int 2006; 19:2–11. [DOI] [PubMed] [Google Scholar]
- 23. Bodro M, Sabé N, Tubau F, et al. Risk factors and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in solid-organ transplant recipients. Transplantation 2013; 96:843–9. [DOI] [PubMed] [Google Scholar]
- 24. Patel G, Rana MM, Huprikar S. Multidrug-resistant bacteria in organ transplantation: an emerging threat with limited therapeutic options. Curr Infect Dis Rep 2013; 15:504–13. [DOI] [PubMed] [Google Scholar]
- 25. Ziakas PD, Pliakos EE, Zervou FN, Knoll BM, Rice LB, Mylonakis E. MRSA and VRE colonization in solid organ transplantation: a meta-analysis of published studies. Am J Transplant 2014; 14:1887–94. [DOI] [PubMed] [Google Scholar]
- 26. Kälble F, Schaier M, Schäfer S, et al. An update on chemical pharmacotherapy options for the prevention of kidney transplant rejection with a focus on costimulation blockade. Expert Opin Pharmacother 2017; 18:799–807. [DOI] [PubMed] [Google Scholar]
- 27. Sommerer C, Suwelack B, Dragun D, et al. An open-label, randomized trial indicates that everolimus with tacrolimus or cyclosporine is comparable to standard immunosuppression in de novo kidney transplant patients. Kidney Int 2019; 96:231–44. [DOI] [PubMed] [Google Scholar]
- 28. Kotton CN, Kumar D, Caliendo AM, et al. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2018; 102:900–31. [DOI] [PubMed] [Google Scholar]
- 29. Leeaphorn N, Garg N, Thamcharoen N, Khankin EV, Cardarelli F, Pavlakis M. Cytomegalovirus mismatch still negatively affects patient and graft survival in the era of routine prophylactic and preemptive therapy: a paired kidney analysis. Am J Transplant 2019; 19:573–84. [DOI] [PubMed] [Google Scholar]
- 30. Jehn U, Schütte-Nütgen K, Bautz J, et al. Cytomegalovirus viremia after living and deceased donation in kidney transplantation. J Clin Med 2020; 9:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stern M, Hirsch H, Cusini A, et al. Cytomegalovirus serology and replication remain associated with solid organ graft rejection and graft loss in the era of prophylactic treatment. Transplantation 2014; 98:1013–8. [DOI] [PubMed] [Google Scholar]
- 32. Kumar D, Chin-Hong P, Kayler L, et al. A prospective multicenter observational study of cell-mediated immunity as a predictor for cytomegalovirus infection in kidney transplant recipients. Am J Transplant 2019; 19:2505–16. [DOI] [PubMed] [Google Scholar]
- 33. Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med 2002; 347:488–96. [DOI] [PubMed] [Google Scholar]
- 34. Hirsch HH, Randhawa PS. BK polyomavirus in solid organ transplantation—guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019; 33:e13528. [DOI] [PubMed] [Google Scholar]
- 35. Moreno A, Cervera C, Gavaldá J, et al. Bloodstream infections among transplant recipients: results of a nationwide surveillance in Spain. Am J Transplant 2007; 7:2579–86. [DOI] [PubMed] [Google Scholar]
- 36. Vidal E, Torre-Cisneros J, Blanes M, et al. Bacterial urinary tract infection after solid organ transplantation in the RESITRA cohort. Transpl Infect Dis 2012; 14:595–603. [DOI] [PubMed] [Google Scholar]
- 37. Alevizakos M, Nasioudis D, Mylonakis E. Urinary tract infections caused by ESBL-producing Enterobacteriaceae in renal transplant recipients: a systematic review and meta-analysis. Transpl Infect Dis 2017; 19. [DOI] [PubMed] [Google Scholar]
- 38. Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 2010; 50:1101–11. [DOI] [PubMed] [Google Scholar]
- 39. Brakemeier S, Dürr M, Bachmann F, Schmidt D, Gaedeke J, Budde K. Risk evaluation and outcome of Pneumocystis jirovecii pneumonia in kidney transplant patients. Transplant Proc 2016; 48:2924–30. [DOI] [PubMed] [Google Scholar]
- 40. Illesy L, Szabo-Pap M, Toth F, et al. Bacterial infections after kidney transplantation: a single-center experience. Transplant Proc 2016; 48:2540–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.