Abstract
Solid waste disposal is a growing concern among Pacific Island nations. With severe limitations in land area, in combination with the lack of reuse or recycling options, many near-shore marine ecosystems across Oceania are highly impacted by locally derived marine debris, including plastics, microplastics and associated chemical contaminants. In order to catalyze improved solid waste management and plastic use policies, the potential ecological and public health risks must be clearly identified and communicated. Using an ecological risk assessment framework, potential risks to marine ecosystems and human health are explored by quantifying microplastics and organic contaminants in 4 study sites located in Tutuila, American Samoa. Results of sampled near-shore marine waters, marine sediments and molluscs indicate that microplastics are unevenly distributed in the marine environment, with the highest concentrations detected in marine molluscs (e.g. average of 15 and 17 particles per organism, the majority of which were microfibers identified as polyethylene terephthalate). These invertebrates also have the highest environmental concentrations of organic contaminants, including phthalates, pesticides and PCBs. However, based on estimated rates of invertebrate consumption, the risk of adverse impacts to human health are likely to be low. Regardless, future studies are recommended to better understand the environmental partitioning of microplastics in dynamic near-shore marine environments, as well as the specific pathways and consequences of the physical and chemical impacts of microplastics on marine species populations and overall marine ecosystem health.
Keywords: Risk assessment, Microplastics, Pesticides, PCBs, Molluscs, American Samoa, Pacific Islands
Risk assessment; Microplastics; Pesticides; PCBs; Molluscs; American Samoa; Pacific ocean.
1. Introduction
Over the past decade, an increasing number of studies have documented the presence of marine debris and plastic trash in the world's oceans (Eriksen et al., 2014; Woodall et al., 2014; Jambeck et al., 2015; Borrelle et al., 2020). Micrometer sized plastic particles, including microplastics from the breakdown of plastic debris into smaller pieces and microfibers from synthetic clothing, fishing gear, car tires and other sources, are increasingly documented on beaches, oceans, and in deep-sea sediments (Woodall et al., 2014; Carr 2017; See et al., 2020). Microplastics, and especially microfibers, are of growing global concern as they are easily ingested by a wide variety of marine and freshwater organisms, including fish, invertebrates and microorganisms (Galloway et al., 2017). Ingestion of plastic particles has been shown to have both physical and chemical impacts to organism digestive tracts (Sharma and Chatterjee 2017), as well as in some cases induce immuno-toxicological responses, inhibit growth, alter gene expression and cause cell death (Lithner et al., 2011; Egbeocha et al., 2018; Du et al., 2020).
Both plastic debris and micro-sized plastics are comprised of polymers (i.e., polyethylene, polyvinyl chloride, polystyrene, polyurethane, polycarbonate) and other additives such as plasticizers, colorants, flame retardants, resins and anti-oxidants during production. Additionally, plastics have been shown to “sorb” or accumulate additional hydrophobic organic contaminants from the environment, such as organochlorine pesticides, polychlorinated biphenyls (PCBs), poly aromatic hydrocarbons (PAHs), and poly brominated diethers (PBDEs) (Engler 2012; Rochman et al., 2013). As a result, microplastics are sometimes considered a vector for organic pollutant transport into marine organisms via microplastics consumption (Cole et al., 2011; Koelmans et al., 2013; Rocha-Santos and Duarte 2015), which then may pose a threat to human health when organisms are consumed as seafood (Smith et al., 2018). However, the physical, chemical and biological impacts of microplastics on human health are not well-understood (Smith et al., 2018), although some plastic additives, such as phthalates, are known endocrine disrupters that can impact human growth, development and reproductive systems (Wang and Qian 2021). For these reasons, documenting in-situ correlations between microplastic and organic contaminant concentrations in both the environment and marine fauna, is important for refining ecological and human health risk assessments.
Across Oceania, solid waste management is one of the most pressing environmental problems for Pacific island nations, given the severe lack of land area, high costs of shipping, and little to no capacity for recycling or repurposing of plastics, chemicals and other wastes (Richards and Haynes, 2014; Mohee et al., 2015). On the main island of Tutuila, American Samoa, marine pollution and marine debris are of increasing concern for territorial regulatory agencies, including the American Samoa Environmental Protection Agency (ASEPA) and the Department of Marine and Wildlife Resources (DMWR). As a consequence, the Marine Debris Program of the American Samoa Environmental Protection Agency has prioritized microplastics monitoring, research and risk assessment within their 2016–2025 strategic objectives. Previous studies (Polidoro et al., 2017), have identified organic contaminants of concern (pesticides, PAHs, PCBs, etc.), marine plastic debris, and microplastics in several near-shore coastal areas on Tutuila. Even though locally caught seafood forms a staple of American Samoan diets, no studies have been conducted to date on the presence and distribution of microplastics and potentially associated organic contaminants in marine species in American Samoa. Similarly, no studies have been conducted on the risks that microplastics and these other contaminants may pose on marine ecosystems and human health.
To preliminarily assess risk, we applied a screening-level framework developed by the U.S. EPA, 1998 that allows for rapid identification and prioritization of contaminants that may cause adverse impacts to ecological or human communities. This screening-level risk assessment methodology characterizes risk by calculating a simple hazard quotient for a given combination of an environmental exposure, or contaminant dose, divided by an available, relevant toxicological threshold. In this approach, a number of risk assessment scenarios can be rapidly evaluated by dividing the relevant measured environmental concentration or calculated ingested dose (for human risk assessment), by the selected relevant toxicological threshold of adverse impact (U.S. EPA, 1998). To facilitate this screening process, an Action Level can be calculated for each contaminant detected, based on known toxicological thresholds of adverse impact (for example measured in micrograms of contaminant ingested per kg of human body weight), in order to directly compare concentrations measured in the environment to contaminant levels estimated to cause adverse human health impacts (U.S. EPA, 2000).
Our overall objectives were: 1) to document the presence, concentration and environmental behavior of microplastics and potentially associated organic contaminants in intertidal marine waters, marine sediments and molluscs in three study sites in American Samoa, and 2) to apply a risk assessment framework to estimate any potential adverse impacts to human health based on microplastic and organic contaminant concentrations in locally consumed molluscs. Results represent the first human health risk assessment, based on the presence of microplastics and organic contaminants in seafood, conducted in American Samoa. Identification of the type, concentration and potential health risk associated with microplastics and organic contaminants in American Samoa will help local government agencies and citizen groups to prioritize mitigation of polluted areas, to improve chemical management, and to develop seafood consumption advisories where necessary. At a larger scale, the risk assessment approach presented can be applied across the globe, and especially in data poor regions, to help identify and prioritize contaminants of concern, and to assess if selected human populations are at elevated risk of adverse health outcomes.
2. Methods
2.1. Site characterization
The largest island of Tutuila (140 km2) is the center of American Samoa government and business, and supports a population of more than 56,000 residents. The climate is hot and humid throughout the year, with annual rainfall ranging from 3 to 5 m per year. The most pressing environmental concerns include extensive coastal alterations, fishing pressure, loss of wetlands, soil erosion, coastal sedimentation, solid and hazardous waste disposal, and pollution (Craig et al., 2005). Four coastal areas on the island of Tutuila, American Samoa (e.g. inner Nu'uuli Lagoon, Lions Park, Pago Harbor, and Lauli'i Beach) were sampled (Figure 1.), based on prior observations of high solid waste or marine debris content (Polidoro et al., 2017). Nu'uuli lagoon is the largest brackish marine lagoon on Tutuila, and receives freshwater input from at least two small rivers. The area is dominated by mangroves in the inner lagoon area, and the main airport on its outer edge near Lions Park. Sediments are mostly mud and silt, but large pieces of household trash and commercial debris can be found throughout the near-shore and intertidal zone. Traditionally, Nu'uuli lagoon has been an important fishing, clamming and recreational area, but significant increases in pollution and marine debris has essentially prohibited these uses. Pago Harbor serves as the main site for shipping and industry on Tutuila, and at its inner most edge, receives freshwater input from Vaipito stream. Lauli'i Beach is located on the outer, eastern tip of Pago Harbor, and also receives some freshwater input from Lauli'i stream. Sediments here contain more sand, with small patches of coral reef. However, shorelines are also littered with concrete and other types of plastic and marine debris.
Figure 1.
Location of study sites 1) Pago Harbor, 2) inner Nu'uuli Lagoon and Lions Park, and 3) Lauli'i.
2.2. Sampling design
At each of the sites, a 40m to 50m square sampling area was established (Figure 1) adjacent to the coastline, within the intertidal zone, and in the vicinity of coastal streams that have been observed to be a source of marine debris. Between September 2017 and July 2018, the inner Nu'uuli Lagoon, Pago Harbor and Lauli'i sites were sampled in the same area once a month for 8 months (e.g. in September, October, November, February, March, May, June and July). Each sampling event consisted of collecting two 1-L replicates of seawater in approximately 0.5–0.75m depth around the center of the sampling area in autoclaved 1 L borosilicate glass containers, collecting 6–10 sediment samples of approximately 200g (wet weight) each with a stainless-steel hand shovel at low tide from the top 10 cm of the intertidal zone in an E-W and S–N transect, and opportunistic hand collection of at least 20 bivalves and/or gastropods within the sampling area. During each sampling event, water chemistry (pH, temperature, dissolved oxygen, salinity) was recorded using a multiparameter water meter (HI98914, Hanna Instruments) in the same area where water samples were taken. However, because the invertebrate community in the initial inner Nu'uuli site was observed to be mostly gastropods (Neritina canalis), compared to the outer part of the lagoon near Lions Park where mostly rock oysters (Isognomon spp) were observed, Lions Park was added as a 4th site in the last 2 months of sampling, where 4 1-L water samples, 12 sediment samples and 153 bivalve samples were collected over 2 months. In sum, over the course of the project, 52 1L seawater samples, 185 sediment samples, 465 gastropods (comprised of Astraea rhodostoma, Neritina canalis, Nerita plicata, and Baltillaria spp) and 116 rock oysters (Isognomon spp), were collected across the 4 sites. Seawater samples were processed onto both 47mm glass fiber filters (Whatman, pore size 0.7 μm) and C18 solid phase extraction disks (Empore 3M) within 24 h of collection. All filters, sediments, and molluscs were frozen immediately after collection, and subsequently transported frozen to Arizona State University for extraction and analyses.
2.3. Microplastic analyses
2.3.1. Seawater and sediments
Each 1-liter seawater sample was initially filtered through a 47mm glass fiber filter (Whatman, pore size 0.7 μm). For marine sediments, a 50 g subsample was collected from each thawed and homogenized sediment sample, and then 250 mL of a 2M NaCl solution was added to each sample and agitated/stirred for 6 min. Sediment samples were allowed to settle overnight, and the liquid fraction removed by glass pipette. This process was repeated 4 times for each sample. In controlled trials, approximately 2 g of 4 polymer-type microplastics (LDPE, HDPE, PVC, and PET) ranging in size from 0.3mm to 2mm were added to 50-gram composited sediment samples collected from Nu'uuli lagoon. The combined liquid extracts from each sample were then filtered through a 47mm glass fiber filter (Whatman, pore size 0.7 μm) and treated with 30% H2O2 to remove any organic material if needed. In these spiked trials, approximately 85% or more of all microplastics were recovered, which is similar to iterative density separation techniques reported elsewhere (e.g. Thomas et al., 2020; Avio et al., 2015).
2.3.2. Marine molluscs
Half of all molluscs collected during each sampling event, or approximately 290 samples, were analyzed for microplastics. Each mollusc was weighed and measured before being shelled. After shelling, the whole tissue was weighed and placed in 30% H2O2 and catalyzed with low heat (∼50 °C) for 4 h, and left to digest for 96 h. The resulting digestate for each sample was then filtered through a 47mm glass fiber filter (Whatman, pore size 0.7 μm). This method has been reported to have at least an 85% recovery rate for approximately 200–3000 μm sized-particles (Tsangaris et al., 2021).
2.3.3. Quality control
To reduce the possibility of environmental contamination, when samples were not being actively processed, all samples and glass fiber filters remained covered with aluminum foil. During active extraction and analyses of microplastics, blank glass fiber filters were left out on the laboratory bench as well as subject to the same extraction and analyses procedures in order to account for any introduced microplastics during active laboratory analyses (Dehaut et al., 2019). All glass fiber filter blanks were visually analyzed for microplastics, and a subset was analyzed by micro-Raman. Based on the subset analyzed by micro-Raman, an average of less than 1 microfiber per sample could have been introduced.
2.3.4. Microplastic identification
All glass fiber filters were visually examined under an Olympus BX10 light microscope to visually count and map particles that looked to be either microplastic fragments or microplastic fibers based on shape, color and/or transparency. After microscopy, a random subsample of 30 glass fiber filters, or approximately 10% of the 291 marine invertebrate filtered samples, and 5 laboratory blanks to control for potentially introduced microplastics during analyses, were verified for microplastic presence and polymer type using micro-Raman (custom-built in a 180° geometry with an Andor 750 spectrometer). Based on this subsampling method, the number of microplastics reported by visual observation were estimated to be between 1.5 to 5 times higher than the number confirmed by micro-Raman, with an average overestimation factor of 3, which is lower than other studies that have reported up to 70% error rates in microscopy (Lusher et al., 2017). However, given the wide range of microplastic variation among samples, the relatively small number of microplastics detected per sample, and the larger objectives of this study, the number of microplastic particles reported here have not been corrected by the overestimation factor.
2.4. Organic contaminant analyses
2.4.1. Seawater and sediments
After pre-filtration with glass fiber filters, all seawater samples were filtered through 47mm C-18 filter disks (Empore, pore size 12μm), which were then eluted with 5mL acetone, 5mL acetonitrile and 8 mL of hexane to pull of any captured organic contaminants, and then passed through a Na2SO4 column to remove excess water. Each sediment sample was thawed, homogenized and sieved to < 5mm. A subsample of approximately 5 g was spiked with 60μg of p-terphenyl as recovery surrogate, and then homogenized in a porcelain mortar and pestle with 20g of Na2SO4 to remove water. Homogenized samples were then spun on a rotor for 48–72 h in 60ml of 1:1 hexane:acetone. Solvents were decanted and filtered with a glass fiber filter, and then passed through a column of silica gel to remove polar compounds, and Na2SO4 to remove water. Samples that were exceptionally high in organic sulfur (yellow coloration) were also passed through a column of Florisil (You and Lydy 2004).
2.4.2. Marine molluscs
Half of all molluscs sampled, or approximately 300 samples, were used for organic contaminant analyses. Each mollusc was weighed and measured before being shelled. After shelling, the whole tissue was weighed and spiked with 15μg of p-terphenyl as recovery surrogate, and then homogenized (e.g. mixed) in 1:4 parts Na2SO4 to remove excess water. All homogenized invertebrate samples were then spun on a rotor for 48 h in 60ml of 1:1 hexane:acetone. Extracts were passed through several cleanup columns to remove larger molecules (e.g. Biobeads SX-3, BioRad) and polar compounds (e.g. Silica Gel), and occasionally Florisil if highly colored.
2.4.3. Contaminant identification and quality control
All samples were concentrated of a final volume of 0.5 ml using Nitrogen gas and then analyzed for organic contaminants using a using a Varian 3800 gas chromatograph in tandem with a Saturn 2200 electron ionization mass spectrometer. Minimum detection limits (MDL) were estimated by doubling the lowest standard concentration that showed a peak, with a signal-to-noise ratio greater than 3. To estimate method recoveries for bivalves and sediments, selected samples were homogenized and spiked with known concentrations of PCBs, pesticides, phthalates, and PAHs. Method recoveries ranged from 40% to 90% for PCBs, 25%–70% for pesticides, from 30% to 80% for phthalates, and from 20% to 90% for PAHs. Method recoveries for sea water are very similar, and are reported in Polidoro et al. (2017). All results presented are uncorrected for method recoveries.
2.5. Risk assessment
In order to estimate risk for human health, a screening-level hazard quotient approach was used (US EPA 1998), where risk = dose of contaminant (in mg consumed per day, per kg body weight) divided by a relevant toxicological threshold for adverse health impacts for that contaminant (in mg of contaminant per kg body weight). To calculate contaminant dose, average body weights of 100 kg were used for adults and 50 kg for children (Rodriguez-Martinez et al., 2020), with estimated shelled seafood consumption rates of 200 g/day for adults and 100 g/day for children. For toxicological thresholds, the EPA oral reference doses (Oral RfD) for detected contaminants were used (Table 1). However, it is important to note that oral reference doses and/or other toxicological thresholds were not available for all contaminants detected. This is especially true for microplastics, which currently do not have any toxicological thresholds for adverse impacts related to number of particles consumed due to their extreme variation in chemical composition, size, shape, degradation rates, etc. Until more research is available on physical toxicological thresholds for microplastics ingestion, thresholds based on the chemical composition of microplastics are assumed to be the best surrogate (e.g. toxicological thresholds for plasticizers including phthalates, and other chemical additives).
Table 1.
Calculated Action Levels (mg/kg or ppm) based on estimated seafood consumption rates. Potential exceedances based on maximum contaminant concentrations detected are highlighted.
| Chemical | Oral RfD (mg/kg/day) | Adult Weight (kg) | Average Daily Serving (kg/day) | Child Weight (kg) | Average Daily Serving (kg/day) | Molluscs Action Level (mg/kg or ppm) |
|---|---|---|---|---|---|---|
| Dibutyl Phthalate | 0.10000 | 100 | 0.200 | 50 | 0.100 | 50.000 |
| Diethyl Phthalate | 0.80000 | 100 | 0.200 | 50 | 0.100 | 400.000 |
| Di-ethylhexyl Phthalate (DEHP) | 0.02000 | 100 | 0.200 | 50 | 0.100 | 10.000 |
| Aldrin | 0.00003 | 100 | 0.200 | 50 | 0.100 | 0.015 |
| Alpha-Lindane | 0.00018 | 100 | 0.200 | 50 | 0.100 | 0.090 |
| Benthiocarb | 0.01000 | 100 | 0.200 | 50 | 0.100 | 5.000 |
| Carbaryl | 0.10000 | 100 | 0.200 | 50 | 0.100 | 50.000 |
| Chlordane/Nonachlor | 0.00050 | 100 | 0.200 | 50 | 0.100 | 0.250 |
| DDT | 0.00050 | 100 | 0.200 | 50 | 0.100 | 0.250 |
| Endosulfan | 0.00600 | 100 | 0.200 | 50 | 0.100 | 3.000 |
| Gamma-Lindane | 0.00030 | 100 | 0.200 | 50 | 0.100 | 0.150 |
| Heptachlor | 0.00050 | 100 | 0.200 | 50 | 0.100 | 0.250 |
| Hexachlorobenzene | 0.00080 | 100 | 0.200 | 50 | 0.100 | 0.400 |
| Methoxychlor | 0.00500 | 100 | 0.200 | 50 | 0.100 | 2.500 |
| Mirex | 0.00020 | 100 | 0.200 | 50 | 0.100 | 0.100 |
| PCBs | 0.00002 | 100 | 0.200 | 50 | 0.100 | 0.010 |
∗Values in bold show all Action Level exceedances for gastropods collected at Lauli'i Beach. Gastropods collected from inner Nu'uuli exceeded for summed PCBs, Chlordane/Nonachlor, and Mirex, and from Pago Harbor exceeded for Chlordane/Nonachlor and DEHP. Rock oysters at Lions Park exceeded for Chlordane/Nonachlor and summed PCBs.
In order to directly compare consumption rates and different risk scenarios with contaminant results reported in parts per million (ppm), an Action Level for each detected contaminant with an available oral RfD was calculated based on the formula: Action Level (ppm or mg/kg) = (Oral RfD (mg/kg) x body weight (kg))÷average daily serving size (kg). Calculation of an Action Level for different body weight and consumption rate scenarios allows for direct comparisons of detected contaminant concentrations (in ppm) with Action Levels (ppm). Where maximum or average detected contaminant concentrations in molluscs exceed calculated Action Levels, the risk of adverse health impacts is considered to be elevated.
3. Key results
3.1. Microplastics
Microplastic concentrations detected in nearshore marine waters were very low, and ranged from 0 to approximately 10 microfibers per liter across all sites, most of which were not able to be identified to polymer type. Microplastics in marine sediments were not well-detected (e.g. only few fibers detected in just a few samples), and therefore, given our visual observation rate error and background blank subtraction, sediment microplastic concentrations are not reported here. However, the sediment extraction method employed in this study may not work well for higher density microplastics comprised of PVC or PET, which have been shown to resistant to removal from saturated NaCl solutions (Thomas et al., 2020). In addition, some marine sediments collected from Nu'uuli lagoon and Pago Harbor were relatively high in organic material with fine silty-clay textures, which has been shown to significantly reduce recovery rates (Cashman et al., 2020).
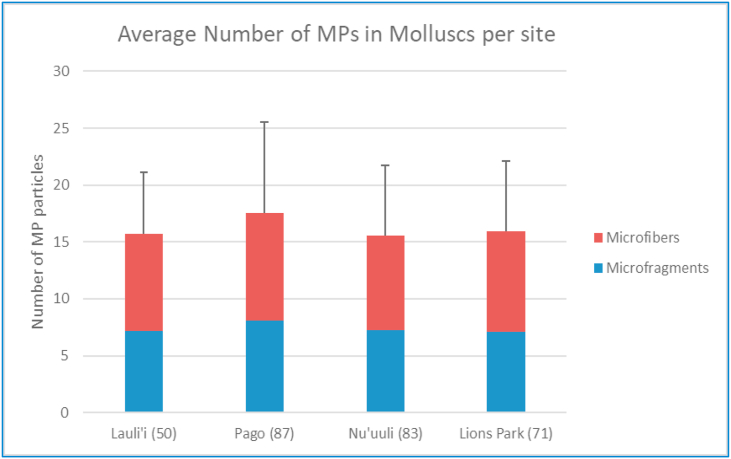
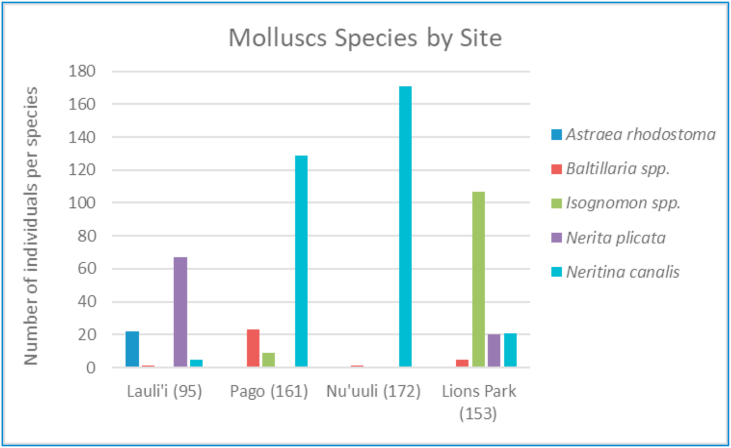
Microplastics in marine molluscs averaged between 15 and 17 particles per organism (minimum of 0 and maximum of 69) (Figure 2), of which about 55% of microplastics were in the shape of microfibers. Based on the subsampled 10% of molluscs analyzed by micro-Raman, approximately 75% of the identifiable plastic was polyethylene terephthalate (PET). Other polymers identified included polycarbonate (PC), polyamide (PA) and polyvinyl chloride (PVC). There were no observed differences in the number of microplastics observed in molluscs among different sites, even though mollusc species composition varied by site (Figure 3).
Figure 2.
Average number of microplastics (MP) in molluscs across sampled sites. Error bars represent standard deviation, number in parenthesis after site indicates number of total samples collected and analyzed over the study period.
Figure 3.
Species names of marine molluscs collected in each sampling site. Number in parenthesis after site indicates number of total samples collected and analyzed over the study period.
3.2. Organic contaminants
A wide range of phthalates, pesticides, polychlorinated biphenyls (PCBs), and polycyclic aromatic hydrocarbons (PAHs), were detected in seawater, sediments, and molluscs (Figure 4). Based on equivalent concentration units, the average concentration of contaminants clearly increased from marine waters (less than 0.35 μg per Liter or ppb) < sediments (less than 0.05 μg per gram or ppm) < molluscs (less than 25 μg per gram or ppm). Across sampled sites, the widest variety of organic contaminants were detected in molluscs collected from Lauli'i. Although there were no observed differences among sites in terms of the average organic contaminant concentrations in marine waters, sediments and molluscs, molluscs from collected from Lauli'i had the highest maximum concentrations detected for PCBs and most pesticides.
Figure 4.
a–c: Average summed organic contaminant concentrations in marine waters, sediments, and molluscs per site. Error bars represent standard deviation, number in parenthesis after site indicates number of total samples collected and analyzed over the study period.
3.3. Risk assessment
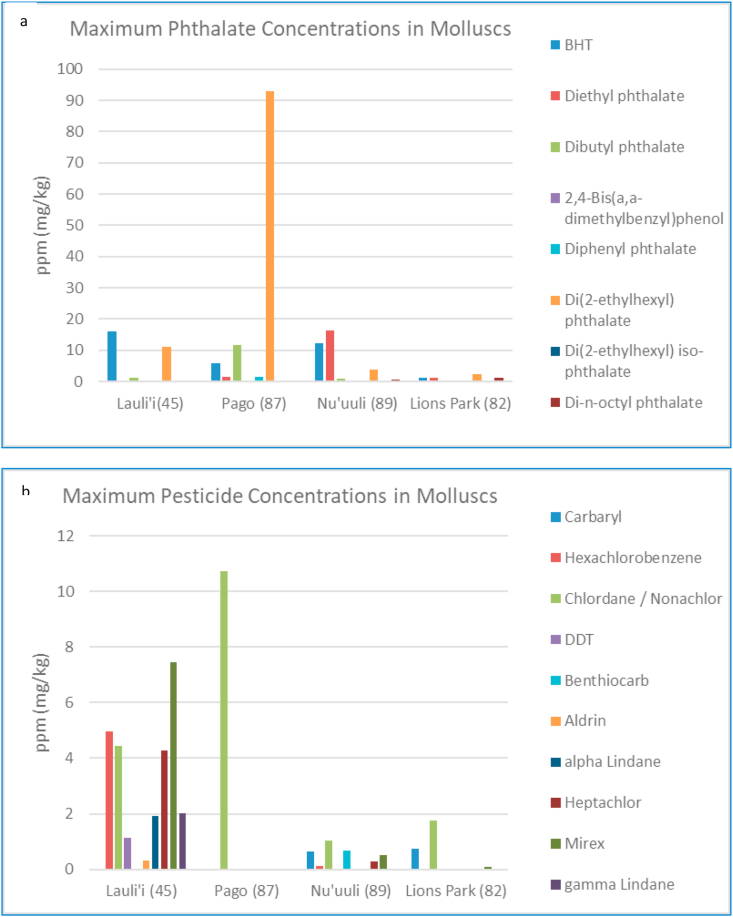
Based on the maximum concentrations detected of individual contaminants in molluscs (e.g. phthalates and pesticides in Figure 5), several detected pesticides, summed PCBs and di-ethylhexyl phthalate (DEHP) exceeded the calculated Adult and Child Action Levels (Table 1). Specifically, maximum summed PCB concentrations detected in molluscs from Lauli'i, inner Nu'uuli Lagoon and Lions Park exceeded Action Levels, while only maximum DEHP concentrations detected in molluscs from Pago Harbor exceeded calculated Action Levels. Eight of the detected pesticides in gastropods collected from Lauli'i Beach exceeded calculated Action Levels. Most importantly, Chlordane (e.g. including cis-Nonachlor and trans-Nonachlor which are bioaccumulating components of Chlordane), was detected in exceedances of Action Levels at all 4 sites, including in rock oysters from Lions Park, and as such, should be prioritized for further studies as a potential contaminant of concern.
Figure 5.
a–b. Maximum concentrations of individual phthalates and pesticides detected in molluscs across all study sites. Number in parenthesis after site indicates number of total samples collected and analyzed over the study period.
It is important to note that it is very unlikely that the American Samoan population is consuming these molluscs at the rates indicated, given informal observations on the decline in molluscs traditionally collected for consumption. Although Action Levels become more “protective” or lower with higher consumption rates and lower body weights, it is unlikely that the gastropods collected from Lauli'i, inner Nu'uuli Lagoon, and Pago Harbor pose a risk to human health, as they are probably being consumed at lower rates than used in the Action Level calculations. However, of higher concern are the levels of Chlordane and PCBs detected in rock oysters from Lions Park, which may be more regularly consumed. In terms of environmental and/or ecological risk, all of the marine sites sampled show varying levels of PCBs and pesticides, with the highest concentrations of these persistent contaminants detected at Lauli'i Beach.
4. Discussion
Overall, very few microplastics were found in near-shore marine waters and intertidal sediments, compared to intertidal molluscs. Although the concentrations of detected microplastics in marine waters were within the range of other studies (Burns and Boxall 2018; Bucci et al., 2020), microplastics were not well-detected in marine sediments. It is likely that microplastics in the intertidal areas sampled in American Samoa are not being uniformly deposited in this dynamic marine environment, but rather are patchily distributed and/or being carried out to offshore areas. Other studies have also shown that microplastic abundance in the intertidal zone is negatively associated with the strength of hydrological processes, including flow velocity and submergence time (Wu et al., 2020). Regardless, comparison of microplastic particles in sea water and marine sediments across different studies is increasingly problematic, given the extreme variation in extraction methods, temporal and spatial sampling regimes, net or filtration pore sizes, and the general lack of reporting of recovery rates (Cutroneo et al., 2020; Phoung et al., 2021). For example, studies of microplastics in seawater in the United Kingdom with 5μm sized filters found an average of only 1.5 to 6.7 particles per liter (Li et al., 2018), while other studies with much larger sample sizes (e.g. more water filtered) over larger spatial scales and with larger 300 μm pore-sized nets found between 8 to 9200 particles/m3 (or 0.008 to 9.2 particles per liter) (Desforges et al., 2014). Given the low numbers of microplastics detected in marine sediments in our study, potential issues with the selected extraction methods cannot be ruled out (Cashman et al., 2020; Phuong et al., 2021). In a systematic review of 70 studies of microplastics extracted from marine sediments, Phoung et al. (2021) found that only 22 reported method recovery rates. Of the reviewed studies that used extraction methods similar to ours (e.g. wet sediment, NaCl floatation with or without H2O2 treatment, and filtration through 0.7μm glass fiber filter), only two studies reported recovery rates (e.g. Fries et al., 2013; Karlsson et al., 2017), both of which reported recovery rates very similar to ours (e.g. greater than 80%). However, several field studies that have used similar methods of extraction found much higher numbers of microplastics in marine sediments compared to our study, including averages of 730–2300 particles per kg of sediment in tidal sediments in the United Kingdom (Blumenroder et al., 2017), and from 672 to 2175 particles per kg of sediment in marine lagoons near Venice, Italy (Vianello et al., 2013). Although increased efforts are needed to include microplastic recovery rates in all field and laboratory studies, optimal recovery of most polymers found in the environment appear to be based on pre-sieving sediment through a 5mm mesh, floatation with NaCI or ZnCl2 (rather than NaCl which may only be best only for polyethylene and polystyrene), followed by 30% H2O2 digestion either before or after filtration (Phoung et al., 2021). Lastly, confirmation of polymers, regardless of size, needs to be confirmed through an appropriate method (e.g. FTIR, Raman, LDIR, or GCMS-pyrolysis).
Regardless, microplastics are most certainly present in the near-shore marine environment in American Samoa as the filter feeding bivalves collected in our study contained very fine microfragments and microfibers likely from the water column, or in the case of gastropods, grazed from microplastic particles potentially trapped in algae or other plant material attached to hard substrate. Interestingly, other studies have found similar results in terms of the number of microplastics and microfibers in different species of intertidal molluscs. For example, a study of a variety of bivalves and gastropods in Hong Kong tidal flats also found a mean of approximately 0–18 particles per organism, dominated by microfibers, with a higher abundance found in gastropods (Xu et al., 2020). In another study of both bivalves and gastropods in the Persian Gulf, the mean number of microplastic particles was 0–21 particles, also dominated by microfibers (Naji et al., 2018).
Across the different sites sampled in American Samoa, there were no significant differences in concentration of microplastics and organic contaminants. This indicates that microplastics and the detected contaminants are likely ubiquitous and diffuse across the study sites, and may be from different non-point sources of pollution, such as agriculture, industrial runoff, buried legacy waste, etc. However, on a wet weight basis, it is clear that molluscs are bioaccumulating more microplastics and organic contaminants compared to in-situ waters and intertidal sediments. What is not clear, is if the microplastics themselves are a primary vector of organic contaminant transport and bioaccumulation in marine molluscs, or if contaminants are accumulating in molluscs from the ambient environment. To address this data gap, chemicals could be extracted directly from the microplastics isolated from molluscs using for example, Pyrolysis-GCMS (Primpke et al., 2020). However, this can be limited and/or extremely difficult due to small sample sizes and the microscopic sizes of some microfibers. Under controlled settings, complementary feeding studies where the chemical composition of microplastics is determined before ingestion and after egestion, could also increase understanding of potential transfer or release of contaminants from microplastics into organism tissue. As such, it is increasingly important that laboratory feeding studies are environmentally relevant, and that more rigorous guidelines and protocols are established for environmental field studies to help unify microplastic sampling, extraction and identification methods.
The maximum (but not the average) detected concentration of some organic contaminants (Chlordane, PCBs, and DEHP) quantified in bivalves and gastropods exceeded calculated Action Levels. These maximum concentration exceedances can indicate potential elevated risk of adverse health impacts for populations that regularly consume moderate to high amounts of these molluscs, and/or have body weights that are lower than those used here, especially as reported concentrations of organic contaminants were not corrected for method recoveries. However, marine molluscs are not as widely consumed as locally-caught fishes or other protein sources available in American Samoa, and the consumption rates used to calculate Action Levels are likely higher than actual consumption rates of the current population. Similarly, it is important to note that oral reference doses, and other safety standards, are calculated based on assumed chronic consumption by an adult of average height and weight on a regular basis over a long-term period of time, and not set on the premise of one-time consumption.
Regardless, based on the known toxicological impacts of the contaminants with Action Level exceedances (US EPA 2020; US EPA 2003; US EPA 1991) certain populations of American Samoans may be at elevated risk for cancer from chronic exposure to DEHP, PCBs, or Chlordane. Although the sources of DEHP are likely from existing plastic pollution, PCBs and Chlordane have been essentially banned for use in the United States since the late 1970s. These contaminants may be legacy pollutants from agriculture, industrial or military activities prior to the 1980s (Polidoro et al., 2017). Further studies are needed to determine the source, transport pathways, ecological impacts and subsequent mitigation strategies for these and the other contaminants detected in American Samoan near-shore marine environments. Given the enormity of this task and the limited amount of data available on specific toxicological thresholds for most marine species and ecosystems, the impacts of microplastics and organic contaminants on marine populations and ecosystems could potentially be examined more efficiently within a trait-based risk assessment framework that ranks species’ relative vulnerabilities to contaminants (Polidoro et al., 2020).
Lastly, more research is needed to determine if physical thresholds for microplastic ingestion or environmental presence can be systematically linked to adverse ecological or negative health outcomes. Given the extreme variation in the composition, shape and size of microplastics in the environment, this will likely be very difficult to standardize, especially across different types of organisms. For example, the 48-hour acute toxicity (EC50) of polyethylene microfragments (37.24 ± 11.76 μm) on the common test organism Daphnia magna was found to be 80 times higher than that of polyethylene microbeads (37.05 ± 3.96 μm), potentially due to the irregular shape and high specific surface area of fragments vs. beads (Na et al., 2021). Similarly, smaller polystyrene microbeads (7.3 μm) have been found to significantly reduce algal feeding in the marine copepod Centropages typicus compared with larger polystyrene microbeads (20.6 μm) (Cole et al., 2013). One option towards harmonization of physical (e.g. size, shape, polymer type) toxicological thresholds for microplastics could be through the development of standards for microplastic particulates similar to those for total suspended solids and/or total dissolved solids in current drinking and surface water regulation.
5. Conclusions
In conclusion, ecological and human health risk assessment frameworks can help to prioritize contaminants, species, geographical areas and selected populations for contaminant mitigation and improved management actions. As molluscs are an important source of protein across the globe, this study provides a framework for scientific or regulatory agencies working in similar, data-poor regions, to conduct screening-level risk assessments using in-situ, baseline studies at the local or regional scale. Additionally, this project relied on extensive participatory training, education, and capacity building opportunities for local researchers, community fishers, community college students, and the general public in American Samoa, which will not only strengthen local career opportunities and skillsets, but will also increase community awareness and action to reduce microplastic, solid waste and other pollutants in near-shore coastal ecosystems.
Although the amounts of microplastics detected in marine molluscs in American Samoa were somewhat comparable with other studies, the amounts of microplastics detected in marine waters were very low, and basically negligible in marine sediments. These results show the critical importance of continued method development for optimizing extraction of different sizes, shapes, and types of microplastics from widely variable environmental media. Additionally, field collection of environmental samples must consider that microplastics are not evenly distributed across the land or seascape. Rather, microplastics are highly likely to be patchily distributed, with higher concentrations in some areas compared to others, due to varying oceanographic, organismal and polymer conditions that control input, transport, deposition, uptake, degradation and accumulation of microplastics.
Further studies are also needed to address both the chemical and physical impacts of microplastic ingestion on human and marine species health, for use within risk assessment frameworks. However, given that the physical impacts of microplastic ingestion on organism health is highly dependent not only on the amounts of microplastics ingested, but also their shape, size, chemical composition, and egestion or excretion rate, it seems unlikely that an impacts threshold for physical ingestion of microplastics can be feasibly developed for a wide range of organisms (including humans). At present, characterization of the chemical constituents of microplastics (including polymer additives and sorbed or associated environmental contaminants) can at the very minimum, provide a measure of the potential chemical impacts of plastics, based on oral or other exposures for different organisms (including humans). These chemical exposures, or doses, can then be compared to available data on health or ecosystem impacts, based on the oral reference doses or other relevant toxicological thresholds.
Declarations
Author contribution statement
Beth Polidoro: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Tiffany Lewis: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Cassandra Clement: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by National Ocean Service (NA17_NOS9990026).
Data availability statement
Data associated with this study has been deposited at NOAA Marine Debris Clearinghouse under the Project ID NA17NOS9990026.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors dedicate this publication to the late, honorable Ameko Pato for his vision and leadership of environmental protection, and commitment to training the next generation of environmental leaders in American Samoa. We also thank Fa'amao Asalele, Jewel Tuiasosopo, and Mia Comeros-Raynal from the American Samoa Environmental Protection Agency, Alice Lawrence from the American Samoa Department of Marine and Wildlife Resources, and Rose Pato and Meagan Curtis from American Samoa Community College (ASCC). This project would not have been possible without the support and effort of many students, which include Alphina Liusamoa, Feterika Tavila, Fuamai Togo, Raijele Toanivere, Ruby Tapuat, Senerivi Gago, Warren Sevaaestasi from ASCC, and Fiona Bellows, Cecilia Fernandez, Sonia Lopez, Daisy Rodrigues, Baye Summers, and Jasmine Palacios from Arizona State University.
References
- Avio C.G., Gorbi S., Regoli F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: first observations in commercial species from Adriatic Sea. Mar. Environ. Res. 2015;111:18–26. doi: 10.1016/j.marenvres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Blumenroder J., Sechet P., Kakkonen J.E., Hartl M.G.J. Microplastic contamination of intertidal sediments of Scapa Flow, Orkney: a first assessment. Mar. Pollut. Bull. 2017;124(1):112–120. doi: 10.1016/j.marpolbul.2017.07.009. [DOI] [PubMed] [Google Scholar]
- Borrelle S., Ringma J., Lavender Law K., Monnahan C.C., Lebreton L., McGivern A., Murphy E., Jambeck J., Leonard G.H., Hilleary M.A., Eriksen M., Possingham H., Gerber L., Polidoro B., Tahir A., Bernard M., De Frond H., Mallos N., Savelli H., Barnes M., Rochman C. Predicted growth in global plastic waste exceeds efforts to mitigate plastic pollution. Science. 2020;369(6510):1515–1518. doi: 10.1126/science.aba3656. [DOI] [PubMed] [Google Scholar]
- Bucci K., Tulio M., Rochman C.M. Ecological Applications; 2020. What Is Known and Unknown about the Effects of Plastic Pollution_meta Analysis and Systematic Review. [DOI] [PubMed] [Google Scholar]
- Burns E.E., Boxall A.B.A. Microplastics in the aquatic environment: evidence for or against adverse impacts and major knowledge gaps. Environ. Toxicol. Chem. 2018;37(11):2776–2796. doi: 10.1002/etc.4268. [DOI] [PubMed] [Google Scholar]
- Carr S.A. Sources and dispersive modes of micro-fibers in the environment. Integrated Environ. Assess. Manag. 2017;13(3):466–469. doi: 10.1002/ieam.1916. [DOI] [PubMed] [Google Scholar]
- Cashman M.A., Ho K.T., Boving T.B., Russo S., Robinson S., Burgess R.M. Comparison of microplastic isolation and extraction procedures from marine sediments. Mar. Pollut. Bull. 2020;159:111507. doi: 10.1016/j.marpolbul.2020.111507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M., Lindeque P., Halsband C., Galloway T.S. Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 2011;62(12):2588–2597. doi: 10.1016/j.marpolbul.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Cole M., Lindeque P., Fileman E., Halsband C., Goodhead R., Moger J., Galloway T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013;47(12):6646–6655. doi: 10.1021/es400663f. [DOI] [PubMed] [Google Scholar]
- Craig P., Di Donato G., Fenner D., Hawkins C. In: The State of Coral Reef Ecosystems of the United States and Pacific Freely Associated States, NOAA Technical Memorandum NOS NCCOS 11. NOAA/NCCOS Center for Coastal Monitoring and Assessment’s Biogeography Team. Waddell J., editor. Silver Spring; Maryland USA: 2005. The state of coral reef ecosystems of American Samoa; p. 522. [Google Scholar]
- Cutroneo L., Reboa A., Besio G., Borgogno F., Canesi L., Canuto S., Dara M., Enrile F., Forioso I., Greco G., Lenoble V. Microplastics in seawater: sampling strategies, laboratory methodologies, and identification techniques applied to port environment. Environ. Sci. Pollut. Control Ser. 2020;27(9):8938–8952. doi: 10.1007/s11356-020-07783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaut A., Hermabessiere L., Duflos G. Current frontiers and recommendations for the study of microplastics in seafood. Trac. Trends Anal. Chem. 2019;116:346–359. [Google Scholar]
- Desforges J.P.W., Galbraith M., Dangerfield N., Ross P.S. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014;79:94–99. doi: 10.1016/j.marpolbul.2013.12.035. [DOI] [PubMed] [Google Scholar]
- Du J., Xu S., Zhou Q., Li H., Fu L., Tang J., Wang Y., Peng X., Xu Y., Du X. Environmental Science and Pollution Research; 2020. A Review of Microplastics in the Aquatic Environmental: Distribution, Transport, Ecotoxicology, and Toxicological Mechanisms; pp. 1–12. [DOI] [PubMed] [Google Scholar]
- Egbeocha C.O., Malek S., Emenike C.U., Milow P. Feasting on microplastics: ingestion by and effects on marine organisms. Aquat. Biol. 2018;27:93–106. [Google Scholar]
- Engler R.E. The complex interaction between marine debris and toxic chemicals in the ocean. Environ. Sci. Technol. 2012;46(22):12302–12315. doi: 10.1021/es3027105. [DOI] [PubMed] [Google Scholar]
- Eriksen M., Lebreton L.C., Carson H.S., Thiel M., Moore C.J., Borerro J.C., Galgani F., Ryan P.G., Reisser J. Plastic pollution in the world's oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0111913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E., Dekiff J.H., Willmeyer J., Nuelle M.T., Ebert M., Remy D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Processes Impact. 2013;15(10):1949–1956. doi: 10.1039/c3em00214d. [DOI] [PubMed] [Google Scholar]
- Galloway T.S., Cole M., Lewis C. Interactions of microplastic debris throughout the marine ecosystem. Nature Ecol. Evol. 2017;1(5):1–8. doi: 10.1038/s41559-017-0116. [DOI] [PubMed] [Google Scholar]
- Jambeck J.R., Geyer R., Wilcox C., Siegler T.R., Perryman M., Andrady A., Narayan R., Law K.L. Plastic waste inputs from land into the ocean. Science. 2015;347(6223):768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- Karlsson T.M., Vethaak A.D., Almroth B.C., Ariese F., van Velzen M., Hassellöv M., Leslie H.A. Screening for microplastics in sediment, water, marine invertebrates and fish: method development and microplastic accumulation. Mar. Pollut. Bull. 2017;122(1–2):403–408. doi: 10.1016/j.marpolbul.2017.06.081. [DOI] [PubMed] [Google Scholar]
- Koelmans A.A., Besseling E., Wegner A., Foekema E.M. Plastic as a carrier of POPs to aquatic organisms: a model analysis. Environ. Sci. Technol. 2013;47(14):7812–7820. doi: 10.1021/es401169n. [DOI] [PubMed] [Google Scholar]
- Li J., Green C., Reynolds A., Shi H., Rotchell J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018;241:35–44. doi: 10.1016/j.envpol.2018.05.038. [DOI] [PubMed] [Google Scholar]
- Lithner D., Larsson Å., Dave G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011;409(18):3309–3324. doi: 10.1016/j.scitotenv.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Lusher A.L., Welden N.A., Sobral P., Cole M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods. 2017;9(9):1346–1360. [Google Scholar]
- Mohee R., Mauthoor S., Bundhoo Z.M., Somaroo G., Soobhany N., Gunasee S. Current status of solid waste management in small island developing states: a review. Waste Manag. 2015;43:539–549. doi: 10.1016/j.wasman.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Na J., Song J., Achar J.C., Jung J. Synergistic effect of microplastic fragments and benzophenone-3 additives on lethal and sublethal Daphnia magna toxicity. J. Hazard Mater. 2021;402:123845. doi: 10.1016/j.jhazmat.2020.123845. [DOI] [PubMed] [Google Scholar]
- Naji A., Nuri M., Vethaak A.D. Microplastics contamination in molluscs from the northern part of the Persian Gulf. Environ. Pollut. 2018;235:113–120. doi: 10.1016/j.envpol.2017.12.046. [DOI] [PubMed] [Google Scholar]
- Phuong N.N., Fauvelle V., Grenz C., Ourgaud M., Schmidt N., Strady E., Sempéré R. Science of The Total Environment; 2021. Highlights from a Review of Microplastics in marine Sediments; p. 146225. [Google Scholar]
- Polidoro B.A., Comeros-Raynal M.T., Cahill T., Clement C. Land-based sources of marine pollution: pesticides, PAHs and phthalates in coastal stream water, and heavy metals in coastal stream sediments in American Samoa. Mar. Pollut. Bull. 2017;116(1-2):501–507. doi: 10.1016/j.marpolbul.2016.12.058. [DOI] [PubMed] [Google Scholar]
- Polidoro B., Matson C.W., Ottinger M.A., Renegar D.A., Romero I.C., Schlenk D., Wise J.P., González J.B., Bruns P., Carpenter K.E., Cobián Rojas D., Collier T.K., Duda T.F., González-Díaz P., Di Giulio R., Grubbs R.D., Haney J.C., Incardona J.P., Horta-Puga G., Linardich C., Moore J.A., Pech D., Perera Valderrama S., Ralp G.M., Strongin K., Ringwood A.H., Würsig B. Vol. 763. Science of The Total Environment; 2020. p. 142986. (A Multi-Taxonomic Framework for Assessing Relative Petrochemical Vulnerability of Marine Biodiversity in the Gulf of Mexico). [DOI] [PubMed] [Google Scholar]
- Primpke S., Fischer M., Lorenz C., Gerdts G., Scholz-Böttcher B.M. Comparison of pyrolysis gas chromatography/mass spectrometry and hyperspectral FTIR imaging spectroscopy for the analysis of microplastics. Anal. Bioanal. Chem. 2020;412(30):8283–8298. doi: 10.1007/s00216-020-02979-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards E., Haynes D. Municipal Solid Waste Management in Asia and the Pacific Islands. Springer; Singapore: 2014. Solid waste management in Pacific Island countries and territories; pp. 255–279. [Google Scholar]
- Rocha-Santos T., Duarte A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. Trends Anal. Chem. 2015;65:47–53. [Google Scholar]
- Rochman C.M., Hoh E., Hentschel B.T., Kaye S. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: implications for plastic marine debris. Environ. Sci. Technol. 2013;47(3):1646–1654. doi: 10.1021/es303700s. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Martinez A., Zhou B., Sophiea M.K., Bentham J., Paciorek C.J., Iurilli M.L., Carrillo-Larco R.M., Bennett J.E., Di Cesare M., Taddei C., Bixby H. Height and body-mass index trajectories of school-aged children and adolescents from 1985 to 2019 in 200 countries and territories: a pooled analysis of 2181 population-based studies with 65 million participants. Lancet. 2020;396(10261):1511–1524. doi: 10.1016/S0140-6736(20)31859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See M., Gilchrist C., Cooper N., Ratcliffe D., Siddle R. Microplastics in the marine environment: a literature review and northeast England case study. Water Environ. J. 2020 [Google Scholar]
- Smith M., Love D.C., Rochman C.M., Neff R.A. Microplastics in seafood and the implications for human health. Cur. Environ. Health Rep. 2018;5(3):375–386. doi: 10.1007/s40572-018-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Chatterjee S. Microplastic pollution, a threat to marine ecosystem and human health: a short review. Environ. Sci. Pollut. Control Ser. 2017;24(27):21530–21547. doi: 10.1007/s11356-017-9910-8. [DOI] [PubMed] [Google Scholar]
- Thomas D., Schütze B., Heinze W.M., Steinmetz Z. Sample preparation techniques for the analysis of microplastics in soil—a review. Sustainability. 2020;12(21):9074. [Google Scholar]
- Tsangaris C., Panti C., Compa M., Pedà C., Digka N., Baini M., D'Alessandro M., Alomar C., Patsiou D., Giani D., Romeo T., Deudero S., Fossi M.C. Interlaboratory comparison of microplastic extraction methods from marine biota tissues: a harmonization exercise of the Plastic Busters MPAs project. Mar. Pollut. Bull. 2021;164:111992. doi: 10.1016/j.marpolbul.2021.111992. [DOI] [PubMed] [Google Scholar]
- U.S. EPA . 1991. Di(2-ethylhexyl)phthalate (DEHP) (CASRN 117-81-7): Reference Dose for Chronic Oral Exposure. (RfD) [Google Scholar]
- U.S. EPA . Vol. 1998. US Environmental Protection Agency; Washington, DC: 1998. Guidelines for Ecological Risk Assessment. EPA/630/R-95/002F. [Google Scholar]
- U.S. EPA . third ed. US Environmental Protection Agency, Office of Water; Washington, DC: 2000. Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories: Vol 1. Fish Sampling and Analysis. EPA-823-B-00-007. [Google Scholar]
- U.S. EPA . US Environmental Protection Agency; Cincinnati, OH: 2003. IRIS Toxicological Review of Mirex (2003 External Review Draft) NCEA-C-1343. [Google Scholar]
- U.S. EPA . National Center for Environmental Assessment; Cincinnati, OH: 2020. Integrated Risk Information System (IRIS) Online. [Google Scholar]
- Vianello A., Boldrin A., Guerriero P., Moschino V., Rella R., Sturaro A., Da Ros L. Microplastic particles in sediments of Lagoon of Venice, Italy: first observations on occurrence, spatial patterns and identification. Estuar. Coast Shelf Sci. 2013;130:54–61. [Google Scholar]
- Wang Y., Qian H. Healthcare. Vol. 9. Multidisciplinary Digital Publishing Institute; 2021. Phthalates and their impacts on human health; p. 603. No. 5. [Google Scholar]
- Woodall L.C., Sanchez-Vidal A., Canals M., Paterson G.L., Coppock R., Sleight V., Calafat A., Rogers A.D., Narayanaswamy B.E., Thompson R.C. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014;1(4):140317. doi: 10.1098/rsos.140317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Pennings S.C., Tong C., Xu Y. Variation in microplastics composition at small spatial and temporal scales in a tidal flat of the Yangtze Estuary, China. Sci. Total Environ. 2020;699:134252. doi: 10.1016/j.scitotenv.2019.134252. [DOI] [PubMed] [Google Scholar]
- Xu X., Wong C.-Y., Tam N.F.Y., Lo H.-S., Cheung S.G. Microplastics in invertebrates on soft shores in Hong Kong: influence of habitat, taxa and feeding mode. Sci. Total Environ. 2020;715:136999. doi: 10.1016/j.scitotenv.2020.136999. [DOI] [PubMed] [Google Scholar]
- You J., Lydy M.J. Evaluation of desulfuration methods for pyrethroid, organophosphate, and organochlorine pesticides in sediment with high sulfur content. Arch. Environ. Contam. Toxicol. 2004;47(2):148–153. doi: 10.1007/s00244-003-3166-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has been deposited at NOAA Marine Debris Clearinghouse under the Project ID NA17NOS9990026.