Abstract
Breast cancer is the leading cause of cancer-related deaths among women. Among breast cancer types, triple negative breast cancer (TNBC) is the most aggressive, and is resistant to hormonal and chemotherapeutic treatments. As such, alternative approaches that may provide some benefit in fighting this debilitating pathology are critically needed; hence the utilization of herbal medicine. Origanum syriacum L., one of the most regularly consumed plants in the Mediterranean region, exhibits antiproliferative effect on several cancer cell lines. However, whether this herb modulates the malignant phenotype of TNBC remains poorly investigated. Here, we show that in MDA-MB-231, a TNBC cell line, Origanum syriacum L. aqueous extract (OSE) inhibited cellular viability, induced autophagy determined by the accumulation of lipidized LC3 II, and triggered apoptosis. We also show that OSE significantly promoted homotypic cell-cell adhesion while it decreased cellular migration, adhesion to fibronectin, and invasion of MDA-MB-231 cells. This was supported by decreased activity of focal adhesion kinase (FAK), reduced α2 integrin expression, and downregulation of secreted PgE2, MMP2 and MMP-9, in OSE-treated cells. Finally, we also show that OSE significantly inhibited angiogenesis and downregulated the level of nitric oxide (NO) production. Our findings demonstrate the ability of OSE to attenuate the malignant phenotype of the MDA-MB-231 cells, thus presenting Origanum syriacum L. as a promising potential source for therapeutic compounds for TNBC.
Keywords: Origanum syriacum L., ethnopharmaclogy, Za’atar, phytotherapy, breast cancer
Introduction
Breast cancer remains the most frequent cancer among females, affecting 2.1 million women each year (1, 2). It is the leading cause of cancer-related mortalities accounting for approximately 15% of all cancer deaths among women (1–3). In addition, breast cancer rates are increasing in almost every region worldwide (1, 2).
Literature reported several classifications of breast cancer. Early classification depended on histological features (4). However, recent “microarray revolution” showed that breast cancer types differ in their mRNA expression profiles (5, 6). Consequently, breast cancer was categorized into molecular subtypes (4), with the triple-negative breast cancer (TNBC) being among the most aggressive types (7). Immunoistochemical analysis reveals that TNBC is characterized by the lack of expression of estrogen receptor (ER) and progesterone receptor (PR), in addition to an absence of human epidermal growth factor receptor 2 (HER2) protein overexpression (8). These three receptors are the most commonly targeted biomarkers, and their absence renders most conventional hormonal and chemotherapeutic treatments relatively ineffective (9). This is reflected by the poor prognosis and low 5-year survival rate of TNBC patients (10). In addition, most TNBC cells evade apoptosis and acquire chemoresistance as well as avoid DNA repair mechanisms (11). It is well known that chemotherapy is often associated with many undesirable and severe side effects (12). Taken together, these observations mandate that alternative approaches, such as herbal medicine, be sought to develop novel treatments that could be more efficacious or exerts less undesired effects. Indeed, recent studies focus on herbal medicine as a treatment of several diseases, including aggressive breast cancer (13–22). In addition, a large percentage of women appear to be highly interested in using herbal remedies to prevent or treat breast cancer (23, 24).
Origanum syriacum L. is an aromatic perennial herb belonging to the mint family, Lamiaceae (25). It is native to the Mediterranean region, especially Lebanon, where it is used in culinary and other preparations (26). Its various medical effects were long described in Avicenna’s “The Canon of Medicine” (980 – 1037 AD) (26). These effects are likely due to the richness of Origanum syriacum L. in bioactive compounds such as terpinene, carvacrol, p-cymene, thymoquinone, thymol, and β-caryophyllene (25, 26). These compounds were reported to have anti-inflammatory (27), antimicrobial (28), anti-glycemic (29), and anticancer (30) effects. In addition, many of these bioactive compounds exhibit antioxidant effects (31) known to inhibit the malignant phenotype of human breast cancer cells (32, 33). Indeed, Origanum syriacum L. showed strong anti-proliferative activities against THP-1 human leukemia cells (34), LoVo, SW620, HCT 116, and Vero colon cancer cells (35, 36), and MCF-7 breast cancer cells (37). Furthermore, the anticancer effect of other Origanum species such as Origanum acutidens, Origanum vulgare, and Origanum majorana on MDA-MB-231, a highly aggressive and metastatic breast cancer cells, was also reported (38–40). However, the anticancer effects of Origanum syriacum L. on MDA-MB-231, which represents an excellent representative model of TNBC, are still poorly investigated.
In this study, we evaluated the effect of Origanum syriacum L. aqueous extract on the malignant phenotype of a battery of breast cancer cell lines, but we focus on MDA-MB-231 cells as a classic representative of TNBC. Our results showed that OSE potently inhibited the proliferation, migration, invasion and adhesion of MDA-MB-231. In addition, OSE induced autophagy and apoptosis, and showed an anti-angiogenic effect. These results highlight the promising therapeutic potential of Origanum syriacum L. against invasive TNBC.
Materials and Methods
Cell Culture and Reagents
Cell lines were purchased from American Type Culture Collection (ATCC number: HTB-26™)(Manassas, VA, USA). Cells were cultured in DMEM media supplemented with 10% FBS, 1% penicillin/streptomycin and 2mM L-Glutamine. Cells were maintained in a humidified incubator at 37°C with 5% CO2 atmosphere. LC3I/II antibody, HRP-conjugated Goat Anti-Mouse antibody, HRP-conjugated Goat Anti-Rabbit antibody were purchased from Abcam (Cambridge, UK). Anti-GAPDH antibody, anti-caspase-3, were obtained from Cell Signaling Technology (Leiden, The Netherlands). Methylthiazolyldiphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (Schnelldorf, Germany). PgE2 and NO colorimetric ELISA quantification kits were purchased from Cayman Chemical (Ann Arbor, Michigan, USA). MMP-2 and MMP-9 ELISA quantification kits were obtained from R&D Systems (Minneapolis, MN, USA).
Preparation of Origanum syriacum Aqueous Extract (OSE)
Origanum syriacum L. leaves were obtained from local farm (South of Lebanon) in the Spring season. The plant identity was confirmed by a plant taxonomist at Lebanese International University, Beirut, Lebanon, and the nomenclature is checked with http://www.theplantlist.org. Dried plant and its leaves were donated to the herbarium located at Qatar University. The Origanum syriacum aqueous extract was prepared as previously described (36). Briefly, leaves were rinsed to remove dust particles and then air-dried in a dark room. Then, leaves were ground into a fine powder using a porcelain mortar and pestle. The powder was suspended in distilled water (1:3 weight to volume ratio) and stirred overnight with continuous shaking. The mixture was then was vacuum-filtered and dried using a rotary evaporator. The extract was then lyophilized and kept at -20°C for further use. For cell treatment, OSE was diluted in culture media and sterilized using 0.2 µm filter.
MTT Assay
Cells were seeded in 96-well plate at a concentration of 104 cells/well. The following day, cells were treated with increasing concentrations of OSE (0.8, 1.2 and 1.6 mg/ml) for 24, 48, and 72 hours. MTT solution (20 µL, 5 mg/mL) was added to each well, and cells were incubated for an hour in a 5% CO2 incubator. Then the medium was removed and 200 µL of 100% DMSO was added into each well. The plates were then shaken for 15 min before reading the absorbance at 570 nm using TECAN M200 Pro (Tecan Group Ltd., Austria) microplate reader. Absorbance is directly proportional to cell viability.
Wound Healing Assay
Cells were grown in 12-well plates until 90–95% confluent. The monolayer was then scratched using a yellow (20–200 µL) tip. Wells were washed three times with phosphate buffered saline (PBS) to remove cellular debris, then medium with or without OSE (1.2 mg/mL) was added into the wells. Wound healing was monitored, and photomicrographs were taken using Olympus IX53 inverted microscope (OLYMPUS Co., Tokyo, Japan). The width of the scratch was measured using Olympus cell Sens software (OLYMPUS Co, Tokyo, Japan).
Transwell Migration Assay
Cells (5 x 104/well) were seeded in the upper chamber of an insert (BD Biosciences, USA) in serum-free media, with or without OSE (1.2 mg/mL). This concentration was chosen since the time needed for this experiment must be short enough so as not to induce cell toxicity. As such, using the lower concentration of 0.8 mg/ml, even if effective, would complicate the results as it would need more than 24 hours, by which time, decreased viability may interfere with the results. The lower chamber was loaded with complete medium (containing 10% FBS), with this FBS acting as a chemoattractant. Cells were allowed to migrate for 6 hours; then they were washed with PBS and fixed with formaldehyde. After washing, migrated cells were stained with 1% crystal violet for 10 minutes, then were washed twice with PBS. Cells from at least five different random fields were counted under an inverted microscope (Objective x10). The number of migrated cells is presented as means ± SEM.
Matrigel Invasion Assay
Transwell inserts were coated with matrigel and allowed to dry overnight. Cells in serum-free media were seeded onto the rehydrated upper transwell chamber in the absence or presence of OSE (1.2 mg/mL). The lower chamber was loaded with complete medium (serum acted as a chemotactic attractant). Following incubation for 24 hours, the medium was aspired and cells on the upper side of the insert membrane were removed with a cotton swab. Invading cells on the lower side of the membrane were fixed with methanol and stained with DAPI. The membrane was then cut with a blade and mounted on an anti-fade agent. Cells from at least five different random fields were counted.
Cell Adhesion to Fibronectin
Wells of 96-well plates were coated with fibronectin and incubated at 37°C overnight. The following day, wells were blocked with 3% Bovine Serum Albumin (BSA) for 3 hours at room temperature. Cells (5× 103/well) were then seeded and incubated for 60 minutes in the humidified incubator (37°C with 5% CO2). Nonadherent cells were removed by washing with PBS (3x) and adherent cells were stained with crystal violet and observed under the microscope. At least five random fields were counted and the experiment repeated three times.
Quantification of Secreted of MMP-2, MMP-9 and PgE2
MDA-MB-231 cells (2× 105/well) grown to a sub-confluent level in 6-well plate, then cultured for 24 hours in the presence or absence of OSE. The conditioned medium from OSE (0.8 and 1.2 mg/mL) treated cells and untreated cells was collected and the levels of secreted MMP-2, MMP-9 (Invitrogen, Camarillo, CA, USA) or PgE2 (Cayman Chemical, MI, USA) were determined using colorimetric ELISA kitsas per the manufacturer’s instruction. The optical density of each sample was measured using an AMP Platos R 496 microplate reader (AMP Diagnostics, Poland). The proteins present in the conditioned media were concentrated using the Amicon Ultra-0.5 protein purification and concentration column (Millipore) and protein concentration was assayed using the BCA protein assay kit (Thermo Scientific). Levels of the PgE2 and MMPs were normalized to the total protein level in each sample.
Quantification of Nitrate/Nitrite Production
The amount of Nitrate/Nitrite production was determined with a colorimetric ELISA kit (Cayman Chemical, Ann Arbor, Michigan, USA), which is based on the Griess reaction, according to the manufacturer’s instructions. Briefly, this kit utilizes the conversion of nitrate to nitrite, followed by the conversion of this nitrite to a deep purple azo product that can be measured photometrically. The value of nitrate/nitrite presented is the total value measured in the well treated without or with OSE (0.8 and 1.2 mg/mL) for 24h, minus the value determined from the media alone in the absence of any growing cells. Assays were performed in triplicates and three independent experiments were performed.
Chorioallontoic Membrane (CAM) Assay
Fifteen fertilized eggs were incubated while rotating at 37°C with 50% humidity for 6 days (E6). Then, a small window was cut in the eggshell above the CAM. The window was loaded with or without OSE, sealed with parafilm, and incubated at 37°C with 50% humidity. Images were taken at 0 hours and after 24 hours after treatment. Vessels area and number of junctions were quantified using AngioTool software.
Western Blotting
Cells were washed with PBS and then lysed using lysis buffer (2% SDS, 60 mM Tris, pH 6.8) as we previously described (41). Following protein quantification, equal amounts of protein (20–30 µg) were resolved onto 5–11% SDS-PAGE. Proteins were then transferred onto polyvinylidene difluoride (PVDF) membrane (Biorad). The membrane was then blocked (5% fat-free milk in TBS-T, 1 hour at room temperature) and incubated overnight with the relevant primary antibody at 4°C. The membrane was then washed with TBS-T (3x 10 minutes) and incubated with the appropriate HRP-conjugated secondary antibody (1 hour, room temperature). The membrane was washed again with TBS-T (3x 10 minutes) and immunoreactive bands were detected by ECL chemiluminescent substrate (ECL clarity, Biorad) and quantified using Chemidoc MP Imaging system (Biorad).
Caspase 3/7 Activity Assay
As we previously reported (42), cells (5,000/well) were seeded in triplicates in 96-well plates and treated without or with OSE. Caspase 3/7 activity was measured using a Caspase 3/7 Glo® kit (Promega Corporation, USA) as per the manual’s instructions. Luminescence was measured, and data plotted as mean ± SEM for three independent experiments.
Statistical Analysis
Results were expressed as means ± S.E.M. of the number of experiments. A Student’s t-test for paired or unpaired values was performed and a p value of <0.05 was considered statistically significant.
Results
OSE Inhibits the Proliferation of a Battery of Breast Cancer Cells
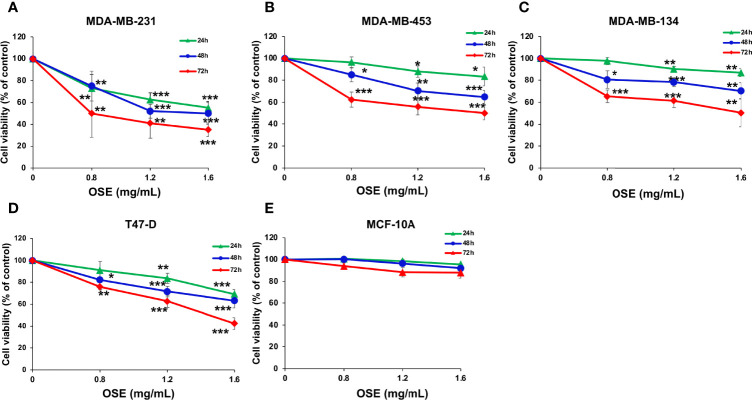
We first examined the effect of Origanum syriacum aqueous extract (OSE) on cellular viability of MDA-MB-231 cells. Cells were exposed to increasing concentrations of OSE for 24, 48 and 72 hours and cell viability was examined as described in materials and methods. We found that OSE significantly reduced the viability of these cells in concentration- and time-dependent manner ( Figure 1A ). The IC50 is 1.69, 1.47 or 0.8 mg/mL at 24, 48 or 72 hours, respectively. Similar results were obtained in other breast cancer cell lines including T-47D (ER+, PR+/-, HER2-), MDA-MB-453 (ER-, PR-, HER2+), MDAMB-134 (ER+, PR-, HER2-) ( Figure 1 ). Importantly, OSE did not significantly affect viability of the normal human mammary epithelial cell line, MCF-10A, which was used as a control cell line ( Figure 1 )
Figure 1.
OSE Inhibits proliferation of a battery of breast cancer cell lines (MDA-MB 231 (A), MDAMB-453 (B), MDAMB-134 (C) T47-D (D), without significantly inducing cytotoxicity in normal human mammary epithelial cells (MCF10A) (E). Cells were treated with increasing concentrations of OSE (0.8, 1.2 and 1.6 mg/ml) for 24, 48, and 72 h Cell viability was assessed by the overall metabolic activity measured by MTT. Values are calculated as % of the corresponding vehicle control value and represented as mean ± standard error of the mean (SEM) of three independent experiments, each run in triplicate. (*p < 0.05, **p < 0.01 and ***p < 0.005).
OSE Induces Autophagy and Apoptosis in MDA-MB-231 Cells
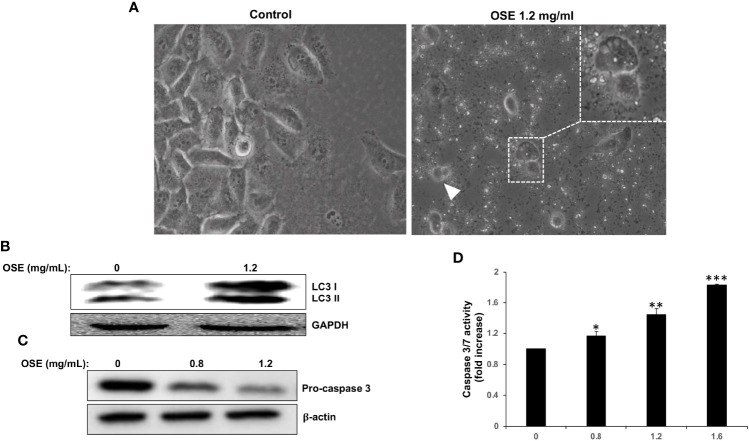
Light microscopy observation of MDA-MB-231 cells treated with OSE revealed cytoplasmic vacuolation ( Figure 2A , thin arrow) in subpopulation of treated cells, suggestive of autophagy in those cells. In addition, some treated cells appeared smaller and rounded, characteristic of dying cells ( Figure 2 , arrow head). In order to determine whether the cytoplasmic vacuolation resulted from activation of autophagy, we examined by Western blotting the accumulation of the lipidized LC3 II a marker of autophagosome formation. As shown in Figure 2B , OSE induced the accumulation of LC3 II, hence arguing in favor of autophagy induction in treated cells. Next, we looked whether apoptosis is also activated in response to OSE. We found that OSE induces a concentration-dependent cleavage of the effector procaspase 3 in MDA-MB-231 cells ( Figure 2C ). In addition, OSE, in a concentration and time-dependent manner, significantly increased the activity of Caspase 3/7, a major apoptosis-executing enzyme ( Figure 2D ). Altogether, our date suggests that OSE inhibits breast cancer through induction of autophagy and activation of apoptosis.
Figure 2.
OSE induces autophagy and apoptosis in MDA-MB-231. (A) Cells were treated with OSE (1.2 mg/ml) for 48 hrs. Micrographs were captured at magnifications of 200X. (B) Induction of autophagy by OSE. Cells were treated with OSE (1.2 mg/ml) for 48 hrs, and cytoplasmic (LC3 I) and lipidized LC3 II accumulation was detected by Western blotting. (C) Activation of apoptosis by OSE. Cells were treated with OSE (0.8 and 1.2 mg/ml) for 48 hrs, and cleavage of procaspase-3 was examined by Western blotting. (D) Cells were treated without (0) or with OSE (0.8, 1.2 or 1.6 mg/ml) for 24 hours, and then Caspase 3/7 activity measured. (* denotes p<0.05, ** denotes p<0.01, and *** denotes p<0.001).
OSE Attenuates the Migration and Downregulates Activated Focal Adhesion Kinase in MDA-MB-231 Cells
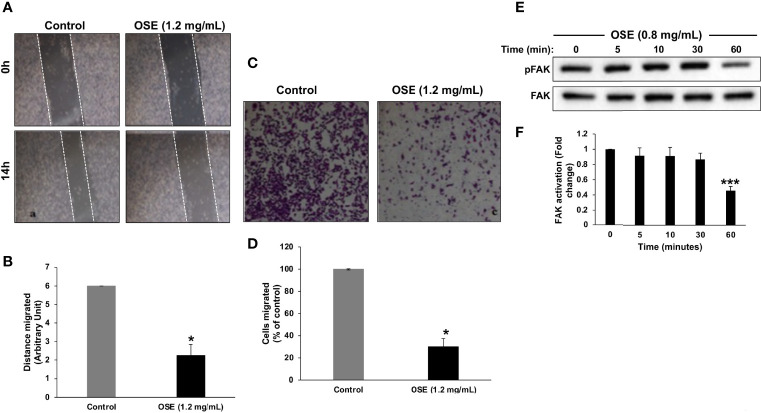
Next, we examined the effect of OSE on the migratory potential of cells. Results of wound healing assay showed that OSE significantly attenuated the migration potential of MDA-MB-231 cells ( Figures 3A, B ). OSE-mediated inhibition of migration was further confirmed by transwell migration assay. As shown in Figures 3C, D , the number of OSE-treated cells was dramatically reduced by ~60% compared to control cells. It is important to mention here that the timepoint chosen for this assay (i.e. 6 hours post-treatment) was so that we can be sure that any decrease in migration is not due to decrease in viability. Indeed, at this timepoint, there was no decrease in viability and no increase in caspase activity (data not shown).
Figure 3.
OSE inhibits migration and downregulates activated FAK in MDA-MB-231 cells. (A, B) Cells were treated with OSE (1.2 mg/ml) and cell migration was assessed by wound healing assay. (C, D) Inhibition of cell migration in transwell migration assay. Images of crystal violet-stained migratory cells were taken 6 h post-treatment. Values are represented as mean ± SEM of distance migrated (n = 3 replicates) (*p < 0.05). (E) Downregulation of pFAK by OSE. Cells were treated with OSE (0.8 mg/ml) for 5, 10, 30 and 60 mins, and the phosphorylation level of FAK was determined by Western blotting. (F) Quantitation of three independent replicates plotted as mean of fold change (***p < 0.001).
It is well documented that the activation of focal adhesion kinase (FAK) is associated with cell adhesion and migration of various cancer cell lines. Thus, we sought to determine the effect of OSE on FAK activity. Western blotting analysis revealed that a low concentration of OSE (0.8 mg/mL) significantly downregulated the level of activated FAK (pFAK) as early as 1 hour post-treatment ( Figures 3E, F ). Our data demonstrate that OSE inhibits the migration potential of MDA-MB-231 cells most likely through inactivation of FAK.
OSE Inhibits Adhesion to Fibronectin and Downregulates Integrin- α2 in MDA-MB-231 Cells
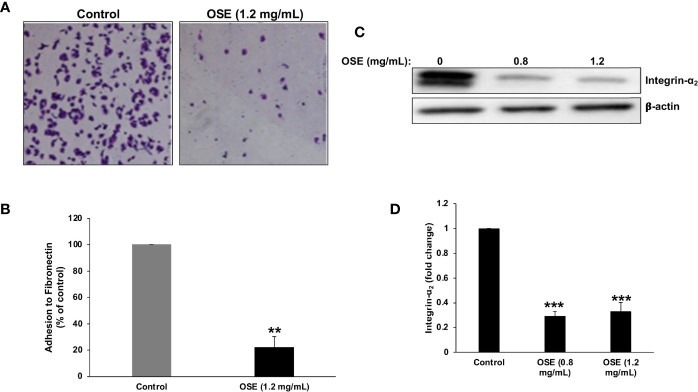
Cellular adhesion to the ECM is a crucial step for migration and invasion of cancer cells. So, we examined the effect of OSE on the ability of MDA-MB-231 to adhere to fibronectin, a key component in the extra cellular matrix (ECM). As it is shown in Figures 4A, B , OSE significantly inhibited the adhesion of MDA-MB-231cells to fibronectin by ~78%. This is supported by our finding that untreated cells exhibit significantly reduced adhesion to plates coated with RGD, a peptide known to mediate cell adhesion to fibronectin (data not shown).
Figure 4.
OSE inhibits adhesion of MDA-MB-231 cells to fibronectin and downregulates integrin α2. (A, B) OSE impairs the ability of MDA-MB-231 cells to adhere to fibronectin. Cells were treated with OSE (1.2 mg/ml) and allowed to adhere to fibronectin for 1 hr. Representative photomicrographs show the effect of OSE on MDA-MB-231 adhesion. Values are represented as mean ± SEM of relative fold inhibition of control cells (**p < 0.005). (C, D) Cells were treated with OSE (0.8 mg/ml) for 24h, and the level of integrin α 2 was detected by Western blotting. Values represent mean fold change of three independent replicates. One-way ANOVA was performed (***p < 0.001).
Integrins, including integrin α2, are major mediators of cell adhesion of invasive breast cancer to fibronectin. Having shown that OSE inhibits the adhesion of MDA-MB-231 cells to fibronectin, we sought to determine whether this inhibition involves disruption of the integrin-fibronectin interaction. Toward this, we measured, by western blotting, the effect of OSE on the protein level of integrin α2. As shown in Figures 4C, D , OSE significantly reduced the level of integrin α2 in OSE-treated cells and therefore strongly suggest that the anti-adhesive effect of OSE is potentially mediated by downregulation of integrin α2 in MDA-MB-231 cells.
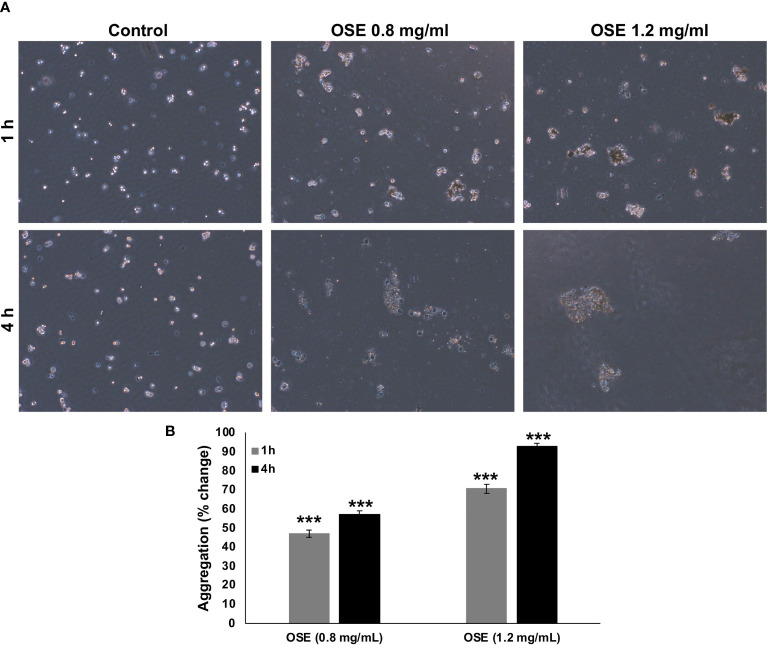
OSE Induces Aggregation of MDA-MB-231 Cells
Epithelial-mesenchymal transition (EMT) has been implicated in conferring metastatic properties to cancer cells. Hallmarks of EMT includes loss of cell connection between neighboring cells. To examine whether OSE cell-cell homotypic adhesion, aggregation assay was carried out. OSE treatment was able to promote aggregation in a time- and concentration- dependent manner of MDA-MB-231 cells ( Figure 5 ).
Figure 5.
OSE promotes MDA-MB-231 cell-cell aggregation. (A, B) Cells were incubated with or without OSE treatment (0.8 and 1.2 mg/ml) for 1 or 4h and subjected to aggregation assay. Percentage aggregation was calculated according to the following formula: % aggregation = (1-Nt/Nc) x 100, where Nt and Nc represent the number of single cells in OSE-treated or control groups respectively. Values are represented as mean ± standard error of the mean (SEM) of three independent experiments (***p < 0.001).
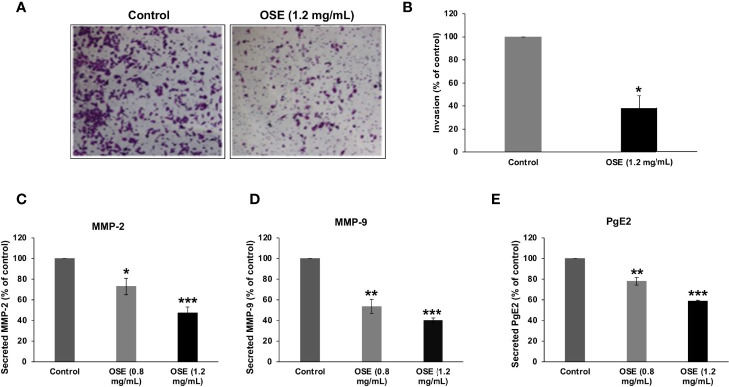
OSE Inhibits Invasion of MDA-MB-231 Cells and Downregulates the Level of Secreted MMP-2, -9 and PgE2
Next, we evaluated the effect of OSE on the invasive capacity of MDA-MB-231 cells in Matrigel-coated Boyden chambers. Our results show that OSE inhibited cell invasion, evident by the significant decrease by 60% in number of OSE-treated cells that has passed through the Matrigel coated membrane ( Figures 6A, B ).
Figure 6.
OSE inhibits invasion and downregulates MMP-2, MMP-9 and PgE2 in MDA-MB-231 cells. (A, B) Cells were treated with OSE (1.2 mg/ml) and cell invasion was evaluated using invasion assay as described in materials and methods. Representative photomicrographs show the effect of OSE on invading cells. (C–E) Cells were grown in with (0.8 and 1.2 mg/mL) or without OSE. Levels of secreted MMP-2 (C), MMP-9 (D), and PgE2 (E) were evaluated by ELISA as described in material and methods. Values are represented as mean ± standard error of the mean (SEM) of three independent experiments (*p < 0.05, **p < 0.005, ***p < 0.001).
Degradation of the ECM by matrix metalloproteinases (MMP) is an important event in cancer invasion and metastasis. Thus, we sought to evaluate the effect of OSE on MMP-2 and MMP-9 activity. We found that OSE decreased, in concentration-dependent manner, the levels of secreted MMP-2 ( Figure 6C ) and MMP-9 ( Figure 6D ) in MDA-MB-231 cells.
Prostaglandin E2 (PgE2), highly expressed in breast tumors, was reported to regulate cell growth, migration and invasion of several tumors. We found that OSE was also able to significantly reduce the level of PgE2 in MDA-MB-231 cells ( Figure 6E ). Altogether, our findings strongly suggest that downregulation of MMP-2, MMP-9 and PgE2 secretion account, although may be not solely, to the impaired invasive ability of OSE-treated MDA-MB-231 cells.
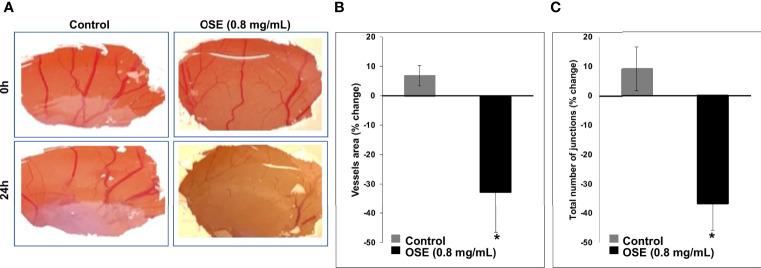
OSE Inhibits In Ovo Angiogenesis
Angiogenesis is crucial for tumor development and metastasis. Thus, we sought to determine whether OSE could affect the formation of new blood vessels using chick chorioallantoic membrane (CAM) angiogenesis assay, a powerful tool for this objective. Figure 7A shows that OSE (0.8 mg/mL) significantly decreased not only the vessels area but also the number of junctions in OSE-treated compared to control embryos. Indeed, the vessel area dropped from 6.85 ± 3.49% in control to -32.68 ± 13.75% in OSE-treated embryo ( Figure 7B ). The number of junctions were also reduced from 9.12 ± 7.45% in control to -36.68 ± 9.14% in OSE-treated embryo ( Figure 7C ). These results demonstrated that one possible mechanism through which OSE might exert its anti-breast cancer activity is through inhibition of angiogenesis.
Figure 7.
OSE inhibits angiogenesis in ovo. OSE (0.8 mg/ml) was added on the CAM of E6 embryo. Photographs (A) taken 24h after treatment show the change in vessels area (B) and the total number of junctions (C). Values are represented as mean ± SEM (*p < 0.05).
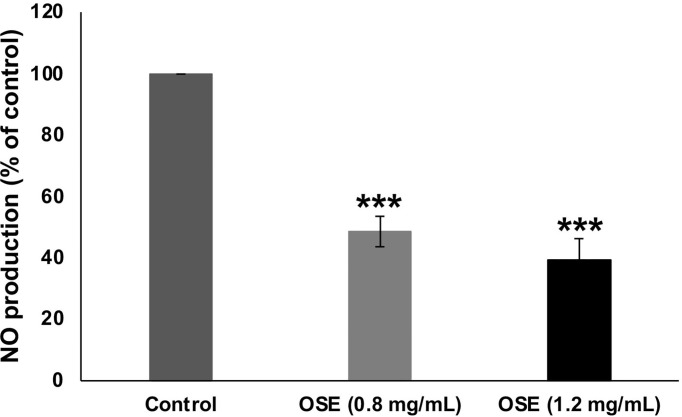
OSE Reduces Nitric Oxide Production in OSE-Treated MDA-MB-231 Cells
Nitric oxide (NO) signaling was shown to promote breast tumor progression and metastasis by altering the expression of genes implicated in cellular migration, invasion and angiogenesis. Having shown that OSE inhibited migration, invasion and angiogenesis in MDA-MB-231 cells, we decided then to examine the effect of OSE on the NO production in those breast cancer cells. As shown in Figure 8 , OSE significantly decreased NO production in a concentration-dependent manner in MDA-MB-231 cells. Based on this finding, we can hypothesize that OSE could also exert its anti-metastatic effect on breast cancer cells though modulation of NO production.
Figure 8.
OSE reduces NO in MDA-MB-231 cells. MDA-MB-231 cells were treated with OSE (0.8 and 1.2 mg/ml) for 24h and Nitrate/Nitrite production was quantified as described in Materials and Methods. Values are represented as mean ± SEM (***p < 0.001).
Discussion
Breast cancer is the most common incident cancer (43), with the TNBC accounting for 10% to 15% of all breast cancer types (44). TNBC is irresponsive to hormonal therapies and acquires resistance to chemotherapeutic agents, making TNBC treatment a real challenge. A potential candidate for TBNC is herbal medicine, especially that many prescribed anticancer drugs are of plant origin (45). In this study we assessed the effect of Origanum syriacum on the malignant phenotype of MDA-MB-231, a TNBC cell line. Our results showed that OSE inhibited the proliferative, migratory, invasive and adhesive capacities of MDA-MB-231 cells. In addition, it efficiently inhibited angiogenesis in ovo. Hence, OSE targets several key contributors to breast cancer malignancy, validating not only its potential medicinal use against TNBC but also making it a potential source for identification of new phytochemicals with potent anticancer activity.
The significance of this study arises from the relevance of the used model and the availability of the used plant. Knowing that TNBC has higher proliferative, migratory and invasive characteristics; it was of obvious importance and more clinical relevance to use the MDA-MB-231 as our model. Another important point is the availability of Origanum syriacum in a region with high incidence of breast cancer. Indeed, this plant native is to some Arab countries, where the average onset of breast cancer in Arab women is 10 years younger than in American and European women (46). As such, this study opens a new horizon to accessible cost-effective breast cancer therapy in a high incident region.
The major hallmark of cancer is uncontrolled cell proliferation, which is markedly exacerbated in TNBC. Our results showed that OSE reduced proliferation of MDA-MB-231 cells. Other plant species belonging to the Origanum genus were also reported to have cytotoxic effect on MDA-MB-231 cells. These include Origanum acutidens, Origanum vulgare, and Origanum majorana (38–40). Apoptosis is a crucial process that aims at getting rid of abnormal cells (47). Malignant cells, including TNBC cells, can evade apoptosis and acquire chemoresistance (48). Thus, inducing apoptosis is an efficient approach in cancer treatment. When tumor cells escape apoptosis, autophagy appears as an alternative mechanism for cell death (49). Autophagy attenuates malignancy by promoting cancer cell death (50). Our results show that the antiproliferative effect of OSE was due to the induction of both, autophagy and apoptosis. This was concomitant with the downregulation of procaspase-3 and accumulation of lipidized LC3I/IIproteins. Although autophagy may be an escape or resistance mechanism for some cancers, it is not likely to be the case in these OSE-treated MDA-MB-231 cells. This is due to the notion that the other hallmarks of malignancy tested here (viability, migration, adhesion, aggregation, angiogenesis) are suppressed by OSE, likely indicating that these cells are executing a death pathway, rather than acquiring resistance.
Our results are in accordance with a several studies showing that the cytotoxic effect of anticancer herbs is mediated by autophagy and apoptosis (19). For instance, carnosol, a herbal compound, elicited its cytotoxic effects on MDA-MB-231 cells by inducing autophagy and apoptosis (51). Two Origanum species, Origanum acutidens and Origanum majorana, showed antiproliferative effects in MDA-MB-231 by inducing cell apoptosis (39, 42). In fact, Origanum majorana induced autophagy and apoptosis of colorectal cancer cells (HT-29 and Caco-2) (52, 53). This effect was mediated by p38 activation (53). Whether this same activation takes place to induce apoptosis in MDA-MB-231 cells is still to be determined. On the other hand, Rhus coriaria and tetrandrine, a compound extracted from Stephania tetrandra, induced autophagy in MDA-MB-231 (49, 54). This autophagy was achieved via inhibiting the PI3K/AKT/mTOR pathway by tetrandrine (49) and by inhibiting NFκB, STAT3 and nitric oxide pathways by Rhus coriaria (54). Whether Origanum syriacum promotes MDA-MB-231 autophagy by inhibiting these signaling pathways is yet to be investigated.
Patients with metastatic TNBC have a poor prognosis (55). This is related to the strong invasive and migratory abilities of TNBC cells (56). These events require ECM degradation by proteases, including MMPs. In fact, MMP upregulation results in reinforced migration, whereas their downregulation weakens migration (57). Interestingly, the expression of MMP-2 and MMP-9 is upregulated in breast cancer, and their expression level is correlated with lymph node metastasis and tumor staging (58). In our study, OSE attenuated the migration and invasion of MDA-MB-231 cells. This was accompanied by inhibition of FAK and downregulation of MMP-2 and MMP-9. These events likely underlie the compromised migratory and invasive capabilities. In addition, OSE inhibited cell adhesion. Thus, by hindering cell adhesion, migration, and invasion, OSE may potently inhibit cancer metastasis. In accordance, we have previously shown that another species of Origanum, namely Origanum majorana, inhibits migration and invasion of the MDA-MB-231 cells and decreases their adhesion to endothelial cells (59). These effects were accompanied by suppressing the activities and expression of MMP-2 and MMP-9 (59). Other plants such as Rhus coriaria also attenuated the migration, invasion, and blocked adhesion to fibronectin of MDA-MB-231 cells likely by downregulating MMP-9 (54).
PgE2 production, upregulated in breast cancer, is a marker for its progression (60). In fact, PgE2 is involved in almost all cancer processes including tumor development, migration, invasion, angiogenesis, and immunosuppression (61–63). Our results showed that OSE reduced secretion of PgE2. This downregulation may contribute to disseminating the previously described inhibitory effects of OSE on cell proliferation, migration, and invasion. Our results are in line with the Rhus coriaria-induced downregulation of PgE2, which likely underlies the anti-migratory, anti- invasive and anti-adhesive effects of the plant (54). It is worth mentioning that reducing PgE2 secretion by inhibiting COX-2 resulted in greatly compromised migration and invasion of MDA-MB-231 cells (64). As such, inhibitors of COX-2/PgE2 may be present in Origanum syriacum and may be used to suppress tumorigenicity of breast cancer.
The association of TNBC with epithelial–mesenchymal transition (EMT) is well established (65). During EMT, cancer cells gain migratory advantage via the disruption of cell-to-cell adhesion (66). Indeed, cells with reduced cell-cell adhesive capacity tend to be more migratory, invasive and malignant. Interestingly, OSE reversed the EMT phenotype by promoting cell aggregation as well as attenuating cell migration and adhesion to ECM, particularly the RGD domain of fibronectin. Knowing that drug resistance is associated with the expression of an EMT phenotype (67), it is not unreasonable to speculate that OSE potently reverses chemoresistance.
Angiogenesis promotes tumor vascularization and is a marker of tumor development and metastasis (68, 69). Invasive human breast cancers express several angiogenic factors, with vascular endothelial growth factor (VEGF) being the predominating factor (70). Patients with TNBC upregulated levels of VEGF and poor prognosis (71). OSE inhibited angiogenesis evident by decreased vessel length and junction number. This inhibition is probably mediated by the downregulation of the major angiogenic factor, VEGF. We have previously shown similar inhibitory effect with Origanum majorana (59). Furthermore, Rhus coriaria inhibited angiogenesis by reducing the production of VEGF in MDA-MB-231 (54). This anti-angiogenic potential of OSE is further cemented by our observation that it also inhibits NO, a gasotransmitter with pro-angiogenic capacity. It is important to mention here that we opted to use the 0.8 mg/ml rather than the 1.2 mg/ml due to the potential toxicity of the extract on the very sensitive developing embryo. Indeed, at a 0.8 mg/ml, it is obvious that a dramatic decrease in vascularization was noted. Thus, it would only be expected that a higher dose would so severely hamper vasculogenesis and angiogenesis that the sensitive embryo may die, rendering any finding at such a high dose rather obsolete. Together, this anti-angiogenic ability of Origanum syriacum further supports its potential as an anticancer drug.
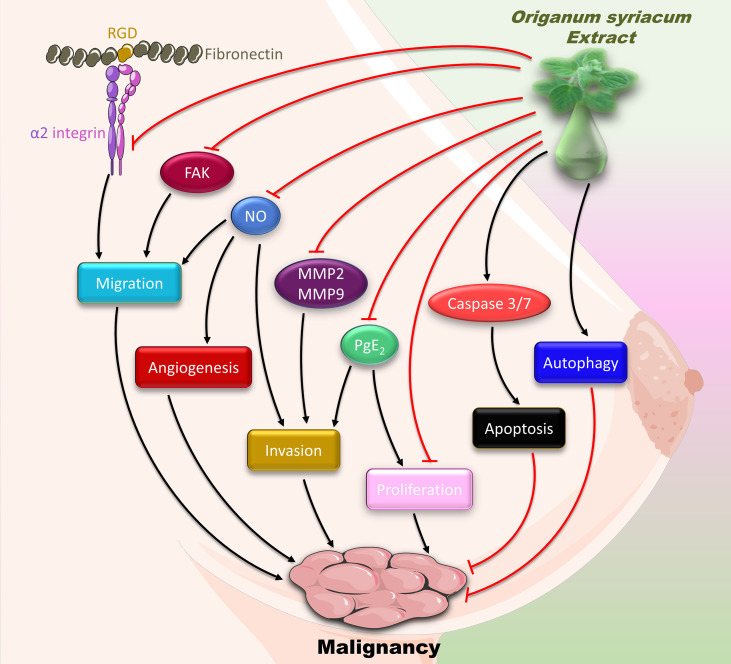
In conclusion, our results show that OSE inhibits the major hallmarks of cancer, namely proliferation, migration, invasion, and angiogenesis ( Figure 9 ). These effects attenuate the malignant phenotype of MDA-MB-231cells, and thus may alleviate TNBC. However, further research is warranted to test OSE in an in vivo model and to dissect its molecular mechanism, especially that the protective effect of some natural compounds is controversial (72). Given the association between inflammation and cancer, assessing the anti-inflammatory effect of OSE would be interesting, especially that increased cytokine levels are linked to poor prognosis of TNBC. In addition, isolating and characterizing the bioactive molecule causing these OSE-induced anticancer and anti-inflammatory effects would be worth investigating. This is especially relevant since HPLC studies on O. syriacum have already been performed, and bioactives have been isolated long time ago (73). Some of these bioactives include apigenin, naringenin, rosmarinic acid, and caffeic acid (73). Interestingly, most of these bioactives possess potential anti-cancer effects. For instance, apigenin has been shown to suppress malignancy of MDAMB-231-derived tumors (74). Similarly, naringenin inhibits proliferation of MCF-7 and MDAMB-231 breast cancer cells (75). Rosmarinic acid inhibits proliferation and promotes apoptosis in MDAMB-231 and MDA-MB-468 cells, both being TNBC cells (76). Knowing that hormonal therapy is not sufficiently effective on TNBC and that it may impart resistance to chemotherapy, the search for alternative therapeutic approaches including herbal medicine becomes of utmost importance. It is critically important here to mention that oftentimes, the total extract or the whole herb provide more benefit than a single or a few bioactives. This may partly be due to inherent synergistic effects between the various bioactives, which could be lost when one or a few bioactives are separately consumed. Equally importantly, the plant is a typical part of the Mediterranean breakfast, and is consumed as a whole, not as individual ingredients per se. To the best of our knowledge, there is no clinical evidence to support a specific dose for consuming this plant. Nonetheless, this plant is well-known to be safe for human consumption (77), and is indeed one of the most consumed herbs in Lebanon and the Mediterranean region. Indeed, a study showed that the consumption of this plant’s essential oils had no adverse effects on animals (78) making it an attractive target for further studies that may eventually establish this herb as an important resource for potential drugs. However, the lack of in vivo animal studies is a limitation of this study, especially given the toxic effects of high doses of OSE in the developing embryo. Altogether, our study provides evidence for the in vitro anti-malignant effect of O. syriacum and opens new horizons to the potential of this plant as a source of bioactives that possess anti-cancer effects.
Figure 9.
Summary of the anti-malignant mechanisms of OSE in MDA-MB-231 cells.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
Conceptualization, AE; methodology, AA, MF, JM, RA, AB, EB, and SB; validation, FK, AE, SB, and EB; formal analysis, MF; investigation, AA, MF, JM, RA, and RI; resources, AB, EB, SN, and AE; data curation, AA, MF, JM, and RA.; writing— AA, MF, JM, RA, RI, SB, EB, AS, and SN; writing—review and editing, KM and AE; supervision, EB, AE, FK, RI, and AB; project administration, AE; funding acquisition, KM, AE, EB, AB, FK. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funds from Qatar National Research Fund (UREP 16-145-3-030 to A.A and A.H.E), Qatar University fund (QUST-CAS-FALL-13/14-9 to A.A. and A.H. E.), the American University of Beirut (MPP #320133 (AE) and URB (EB)), University of Petra (AB, EB, and AE), and UAE University, UAE (funds#G3458 and G3908 to KM).
Conflict of Interest
Author AA was employed by Kurome Therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank UAE University for offsetting the article processing charges of this publication.
References
- 1. WHO . Cancer. Geneva, Switzerland. (2020). [Google Scholar]
- 2. World Health, O . WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All. Geneva: World Health Organization; (2020). [Google Scholar]
- 3. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int J Cancer (2019) 144(8):1941–53. doi: 10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- 4. Nounou MI, ElAmrawy F, Ahmed N, Abdelraouf K, Goda S, Syed-Sha-Qhattal H. Breast Cancer: Conventional Diagnosis and Treatment Modalities and Recent Patents and Technologies. Breast Cancer (Auckl) (2015) 9(Suppl 2):17–34. doi: 10.4137/BCBCR.S29420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inic Z, Zegarac M, Inic M, Markovic I, Kozomara Z, Djurisic I, et al. Difference Between Luminal A and Luminal B Subtypes According to Ki-67, Tumor Size, and Progesterone Receptor Negativity Providing Prognostic Information. Clin Med Insights Oncol (2014) 8:107–11. doi: 10.4137/CMO.S18006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sonnenblick A, Fumagalli D, Sotiriou C, Piccart M. Is the Differentiation Into Molecular Subtypes of Breast Cancer Important for Staging, Local and Systemic Therapy, and Follow Up? Cancer Treat Rev (2014) 40(9):1089–95. doi: 10.1016/j.ctrv.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 7. Geyer FC, Pareja F, Weigelt B, Rakha E, Ellis IO, Schnitt SJ, et al. The Spectrum of Triple-Negative Breast Disease: High- and Low-Grade Lesions. Am J Pathol (2017) 187(10):2139–51. doi: 10.1016/j.ajpath.2017.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foulkes WD, Smith IE, Reis-Filho JS. Triple-Negative Breast Cancer. N Engl J Med (2010) 363(20):1938–48. doi: 10.1056/NEJMra1001389 [DOI] [PubMed] [Google Scholar]
- 9. Goncalves H, Jr., Guerra MR, Duarte Cintra JR, Fayer VA, Brum IV, Bustamante Teixeira MT. Survival Study of Triple-Negative and Non-Triple-Negative Breast Cancer in a Brazilian Cohort. Clin Med Insights Oncol (2018) 12:1179554918790563. doi: 10.1177/1179554918790563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin Cancer Res (2007) 13(15):4429–34. doi: 10.1158/1078-0432.CCR-06-3045 [DOI] [PubMed] [Google Scholar]
- 11. Nedeljkovic M, Damjanovic A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells (2019) 8(9). doi: 10.3390/cells8090957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nurgali K, Jagoe RT, Abalo R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front Pharmacol (2018) 9:245. doi: 10.3389/fphar.2018.00245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kong Q, Sun F, Chen X. Impact of Fixed-Dose Combination of Germacrone, Curdione, and Furanodiene on Breast Cancer Cell Proliferation. Cell J (2013) 15(2):160–5. [PMC free article] [PubMed] [Google Scholar]
- 14. Al-Menhali A, Al-Rumaihi A, Al-Mohammed H, Al-Mazrooey H, Al-Shamlan M, AlJassim M, et al. Thymus Vulgaris (Thyme) Inhibits Proliferation, Adhesion, Migration, and Invasion of Human Colorectal Cancer Cells. J Med Food (2015) 18(1):54–9. doi: 10.1089/jmf.2013.3121 [DOI] [PubMed] [Google Scholar]
- 15. Anwar MA, Al Disi SS, Eid AH. Anti-Hypertensive Herbs and Their Mechanisms of Action: Part II. Front Pharmacol (2016) 7:50. doi: 10.3389/fphar.2016.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saleh Al-Shehabi T, Iratni R, Eid AH. Anti-Atherosclerotic Plants Which Modulate the Phenotype of Vascular Smooth Muscle Cells. Phytomedicine (2016) 23(11):1068–81. doi: 10.1016/j.phymed.2015.10.016 [DOI] [PubMed] [Google Scholar]
- 17. Alali FQ, El-Elimat T, Khalid L, Hudaib R, Al-Shehabi TS, Eid AH. Garlic for Cardiovascular Disease: Prevention or Treatment? Curr Pharm Des (2017) 23(7):1028–41. doi: 10.2174/1381612822666161010124530 [DOI] [PubMed] [Google Scholar]
- 18. Anwar MA, Samaha AA, Ballan S, Saleh AI, Iratni R, Eid AH. Salvia Fruticosa Induces Vasorelaxation In Rat Isolated Thoracic Aorta: Role of the PI3K/Akt/eNOS/NO/cGMP Signaling Pathway. Sci Rep (2017) 7(1):686. doi: 10.1038/s41598-017-00790-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Athamneh K, Hasasna HE, Samri HA, Attoub S, Arafat K, Benhalilou N, et al. Rhus Coriaria Increases Protein Ubiquitination, Proteasomal Degradation and Triggers non-Canonical Beclin-1-Independent Autophagy and Apoptotic Cell Death in Colon Cancer Cells. Sci Rep (2017) 7(1):11633. doi: 10.1038/s41598-017-11202-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anwar MA, Samaha AA, Baydoun S, Iratni R, Eid AH. Rhus Coriaria L. (Sumac) Evokes Endothelium-Dependent Vasorelaxation of Rat Aorta: Involvement of the cAMP and cGMP Pathways. Front Pharmacol (2018) 9:688. doi: 10.3389/fphar.2018.00688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maaliki D, Shaito AA, Pintus G, El-Yazbi A, Eid AH. Flavonoids in Hypertension: A Brief Review of the Underlying Mechanisms. Curr Opin Pharmacol (2019) 45:57–65. doi: 10.1016/j.coph.2019.04.014 [DOI] [PubMed] [Google Scholar]
- 22. Laskar YB, Lourembam RM, Mazumder PB. Herbal Remedies for Breast Cancer Prevention and Treatment. In: Medicinal Plants-Use in Prevention and Treatment of Diseases. London, United Kingdom: IntechOpen; (2020). [Google Scholar]
- 23. Morris KT, Johnson N, Homer L, Walts D. A Comparison of Complementary Therapy Use Between Breast Cancer Patients and Patients With Other Primary Tumor Sites. Am J Surg (2000) 179(5):407–11. doi: 10.1016/s0002-9610(00)00358-5 [DOI] [PubMed] [Google Scholar]
- 24. Cassidy A. Are Herbal Remedies and Dietary Supplements Safe and Effective for Breast Cancer Patients? Breast Cancer Res (2003) 5(6):300–2. doi: 10.1186/bcr724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lukas B, Schmiderer C, Franz C, Novak J. Composition of Essential Oil Compounds From Different Syrian Populations of Origanum Syriacum L.(Lamiaceae). J Agric Food Chem (2009) 57(4):1362–5. doi: 10.1021/jf802963h [DOI] [PubMed] [Google Scholar]
- 26. Farhat M, Tóth J, Héthelyi B, Szarka S, Czigle S. Analysis of the Essential Oil Compounds of Origanum Syriacum L. Eur Pharm J (2012) 59(2):6–14. doi: 10.2478/v10219-012-0020-x [DOI] [Google Scholar]
- 27. de Santana MF, Guimaraes AG, Chaves DO, Silva JC, Bonjardim LR, de Lucca Junior W, et al. The Anti-Hyperalgesic and Anti-Inflammatory Profiles of P-Cymene: Evidence for the Involvement of Opioid System and Cytokines. Pharm Biol (2015) 53(11):1583–90. doi: 10.3109/13880209.2014.993040 [DOI] [PubMed] [Google Scholar]
- 28. Marchese A, Arciola CR, Barbieri R, Silva AS, Nabavi SF, Tsetegho Sokeng AJ, et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on P-Cymene. Mater (Basel) (2017) 10(8). doi: 10.3390/ma10080947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sangi SM, Sulaiman MI, El-Wahab MF, Ahmedani EI, Ali SS. Antihyperglycemic Effect of Thymoquinone and Oleuropein, on Streptozotocin-Induced Diabetes Mellitus in Experimental Animals. Pharmacogn Mag (2015) 11(Suppl 2):S251–257. doi: 10.4103/0973-1296.166017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Legault J, Pichette A. Potentiating Effect of Beta-Caryophyllene on Anticancer Activity of Alpha-Humulene, Isocaryophyllene and Paclitaxel. J Pharm Pharmacol (2007) 59(12):1643–7. doi: 10.1211/jpp.59.12.0005 [DOI] [PubMed] [Google Scholar]
- 31. Viuda-Martos M, El Gendy A.E.-N.G., Sendra E, Fernandez-Lopez J, Abd El Razik K, Omer EA, et al. Chemical Composition and Antioxidant and Anti-Listeria Activities of Essential Oils Obtained From Some Egyptian Plants. J Agric Food Chem (2010) 58(16):9063–70. doi: 10.1021/jf101620c [DOI] [PubMed] [Google Scholar]
- 32. Dastpeyman M, Motamed N, Azadmanesh K, Mostafavi E, Kia V, Jahanian-Najafabadi A, et al. Inhibition of Silibinin on Migration and Adhesion Capacity of Human Highly Metastatic Breast Cancer Cell Line, MDA-MB-231, by Evaluation of β1-Integrin and Downstream Molecules, Cdc42, Raf-1 and D4GDI. Med Oncol (2012) 29(4):2512–8. doi: 10.1007/s12032-011-0113-8 [DOI] [PubMed] [Google Scholar]
- 33. Loganathan R, Selvaduray K, Nesaretnam K, Radhakrishnan A. Tocotrienols Promote Apoptosis in Human Breast Cancer Cells by Inducing Poly (ADP-Ribose) Polymerase Cleavage and Inhibiting Nuclear Factor Kappa-B Activity. Cell Proliferation (2013) 46(2):203–13. doi: 10.1111/cpr.12014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ayesh BM, Abed AA, Doa’a MF. In Vitro Inhibition of Human Leukemia THP-1 Cells by Origanum Syriacum L. And Thymus Vulgaris L. Extracts. BMC Res Notes (2014) 7(1):612. doi: 10.1186/1756-0500-7-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Öztürk Ş., Duran N. Antiproliferative Effects of Origanum Syriacum L. And Myrtus Communis L. @ on Human Colon Cancer Cell Line. In: International Conference on Advanced Materials and Systems (ICAMS). The National Research & Development Institute for Textiles and Leather-INCDTP. p. 255–60. [Google Scholar]
- 36. Aldisi SS, Jaganjac M, Eid AH, Goktepe I. Evaluation of Apoptotic, Antiproliferative, and Antimigratory Activity of Origanum Syriacum Against Metastatic Colon Cancer Cells. J Herbs Spices Medicinal Plants (2019) 25(3):202–17. doi: 10.1080/10496475.2019.1587674 [DOI] [Google Scholar]
- 37. Al-Kalaldeh JZ, Abu-Dahab R, Afifi FU. Volatile Oil Composition and Antiproliferative Activity of Laurus Nobilis, Origanum Syriacum, Origanum Vulgare, and Salvia Triloba Against Human Breast Adenocarcinoma Cells. Nutr Res (2010) 30(4):271–8. doi: 10.1016/j.nutres.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 38. Grbovic F, Stankovic MS, Curcic M, Dordevic N, Seklic D, Topuzovic M, et al. In Vitro Cytotoxic Activity of Origanum Vulgare L. @ on HCT-116 and MDA-MB-231 Cell Lines. Plants (Basel) (2013) 2(3):371–8. doi: 10.3390/plants2030371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tuncer E, Unver-Saraydin S, Tepe B, Karadayi S, Ozer H, Sen M, et al. Antitumor Effects of Origanum Acutidens Extracts on Human Breast Cancer. J BUON (2013) 18(1):77–85. [PubMed] [Google Scholar]
- 40. Makrane H, El Messaoudi M, Melhaoui A, El Mzibri M, Benbacer L, Aziz M. Cytotoxicity of the Aqueous Extract and Organic Fractions From Origanum Majorana on Human Breast Cell Line MDA-MB-231 and Human Colon Cell Line HT-29. Adv Pharmacol Sci (2018) 2018:3297193. doi: 10.1155/2018/3297193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eid AH, Chotani MA, Mitra S, Miller TJ, Flavahan NA. Cyclic AMP Acts Through Rap1 and JNK Signaling to Increase Expression of Cutaneous Smooth Muscle Alpha2c-Adrenoceptors. Am J Physiol Heart Circ Physiol (2008) 295(1):H266–272. doi: 10.1152/ajpheart.00084.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Al Dhaheri Y, Eid A, AbuQamar S, Attoub S, Khasawneh M, Aiche G, et al. Mitotic Arrest and Apoptosis in Breast Cancer Cells Induced by Origanum Majorana Extract: Upregulation of TNF-Alpha and Downregulation of Survivin and Mutant P53. PloS One (2013) 8(2):e56649. doi: 10.1371/journal.pone.0056649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. WHO . Cancer (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/cancer (Accessed 3 February 2022).
- 44. Society AC. Triple-Negative Breast Cancer [Online] (2022). Available at: https://www.cancer.org/cancer/breast-cancer/about/types-of-breast-cancer/triple-negative.html#:~:text=Triple%2Dnegative%20breast%20cancer%20(TNBC,15%25%20of%20all%20breast%20cancers (Accessed March 1, 2022).
- 45. Levitsky DO, Dembitsky VM. Anti-Breast Cancer Agents Derived From Plants. Natural Products Bioprospecting (2015) 5(1):1–16. doi: 10.1007/s13659-014-0048-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chouchane L, Boussen H, Sastry KS. Breast Cancer in Arab Populations: Molecular Characteristics and Disease Management Implications. Lancet Oncol (2013) 14(10):e417–424. doi: 10.1016/S1470-2045(13)70165-7 [DOI] [PubMed] [Google Scholar]
- 47. Elmore S. Apoptosis: A Review of Programmed Cell Death. Toxicol Pathol (2007) 35(4):495–516. doi: 10.1080/01926230701320337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liberio MS, Joanitti GA, Fontes W, Castro MS. Anticancer Peptides and Proteins: A Panoramic View. Protein Pept Lett (2013) 20(4):380–91. doi: 10.2174/092986613805290435 [DOI] [PubMed] [Google Scholar]
- 49. Guo Y, Pei X. Tetrandrine-Induced Autophagy in MDA-MB-231 Triple-Negative Breast Cancer Cell Through the Inhibition of PI3K/AKT/mTOR Signaling. Evid Based Complement Alternat Med (2019) 2019:7517431. doi: 10.1155/2019/7517431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yun CW, Lee SH. The Roles of Autophagy in Cancer. Int J Mol Sci (2018) 19(11). doi: 10.3390/ijms19113466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Al Dhaheri Y, Attoub S, Ramadan G, Arafat K, Bajbouj K, Karuvantevida N, et al. Carnosol Induces ROS-Mediated Beclin1-Independent Autophagy and Apoptosis in Triple Negative Breast Cancer. PloS One (2014) 9(10):e109630. doi: 10.1371/journal.pone.0109630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Benhalilou N, Alsamri H, Alneyadi A, Athamneh K, Alrashedi A, Altamimi N, et al. Origanum Majorana Ethanolic Extract Promotes Colorectal Cancer Cell Death by Triggering Abortive Autophagy and Activation of the Extrinsic Apoptotic Pathway. Front Oncol (2019) 9:795. doi: 10.3389/fonc.2019.00795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Athamneh K, Alneyadi A, Alsamri H, Alrashedi A, Palakott A, El-Tarabily KA, et al. Origanum Majorana Essential Oil Triggers P38 MAPK-Mediated Protective Autophagy, Apoptosis, and Caspase-Dependent Cleavage of P70S6K in Colorectal Cancer Cells. Biomolecules (2020) 10(3). doi: 10.3390/biom10030412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. El Hasasna H, Saleh A, Al Samri H, Athamneh K, Attoub S, Arafat K, et al. Rhus Coriaria Suppresses Angiogenesis, Metastasis and Tumor Growth of Breast Cancer Through Inhibition of STAT3, NFkappaB and Nitric Oxide Pathways. Sci Rep (2016) 6:21144. doi: 10.1038/srep21144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kassam F, Enright K, Dent R, Dranitsaris G, Myers J, Flynn C, et al. Survival Outcomes for Patients With Metastatic Triple-Negative Breast Cancer: Implications for Clinical Practice and Trial Design. Clin Breast Cancer (2009) 9(1):29–33. doi: 10.3816/CBC.2009.n.005 [DOI] [PubMed] [Google Scholar]
- 56. Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, et al. The IL-6/JAK/Stat3 Feed-Forward Loop Drives Tumorigenesis and Metastasis. Neoplasia (2013) 15(7):848–62. doi: 10.1593/neo.13706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nabeshima K, Inoue T, Shimao Y, Sameshima T. Matrix Metalloproteinases in Tumor Invasion: Role for Cell Migration. Pathol Int (2002) 52(4):255–64. doi: 10.1046/j.1440-1827.2002.01343.x [DOI] [PubMed] [Google Scholar]
- 58. Li H, Qiu Z, Li F, Wang C. The Relationship Between MMP-2 and MMP-9 Expression Levels With Breast Cancer Incidence and Prognosis. Oncol Lett (2017) 14(5):5865–70. doi: 10.3892/ol.2017.6924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Al Dhaheri Y, Attoub S, Arafat K, AbuQamar S, Viallet J, Saleh A, et al. Anti-Metastatic and Anti-Tumor Growth Effects of Origanum Majorana on Highly Metastatic Human Breast Cancer Cells: Inhibition of Nfκb Signaling and Reduction of Nitric Oxide Production. PloS One (2013) 8(7):e68808. doi: 10.1371/journal.pone.0068808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rolland PH, Martin PM, Jacquemier J, Rolland AM, Toga M. Prostaglandin in Human Breast Cancer: Evidence Suggesting That an Elevated Prostaglandin Production is a Marker of High Metastatic Potential for Neoplastic Cells. J Natl Cancer Institute (1980) 64(5):1061–70. [PubMed] [Google Scholar]
- 61. Timoshenko AV, Xu G, Chakrabarti S, Lala PK, Chakraborty C. Role of Prostaglandin E2 Receptors in Migration of Murine and Human Breast Cancer Cells. Exp Cell Res (2003) 289(2):265–74. doi: 10.1016/S0014-4827(03)00269-6 [DOI] [PubMed] [Google Scholar]
- 62. Wang D, Dubois RN. Prostaglandins and Cancer. Gut (2006) 55(1):115–22. doi: 10.1136/gut.2004.047100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zheng H, Li Y, Wang Y, Zhao H, Zhang J, Chai H, et al. Downregulation of COX-2 and CYP 4A Signaling by Isoliquiritigenin Inhibits Human Breast Cancer Metastasis Through Preventing Anoikis Resistance, Migration and Invasion. Toxicol Appl Pharmacol (2014) 280(1):10–20. doi: 10.1016/j.taap.2014.07.018 [DOI] [PubMed] [Google Scholar]
- 64. Kim MJ, Kim HS, Lee SH, Yang Y, Lee MS, Lim JS. NDRG2 Controls COX-2/PGE(2)-Mediated Breast Cancer Cell Migration and Invasion. Mol Cells (2014) 37(10):759–65. doi: 10.14348/molcells.2014.0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jang MH, Kim HJ, Kim EJ, Chung YR, Park SY. Expression of Epithelial-Mesenchymal Transition–Related Markers in Triple-Negative Breast Cancer: ZEB1 as a Potential Biomarker for Poor Clinical Outcome. Hum Pathol (2015) 46(9):1267–74. doi: 10.1016/j.humpath.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 66. Roche J. The Epithelial-To-Mesenchymal Transition in Cancer. Cancers (2018) 10(2):52. doi: 10.3390/cancers10020052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Du B, Shim JS. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules (2016) 21(7). doi: 10.3390/molecules21070965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Brem SS, Gullino PM, Medina D. Angiogenesis: A Marker for Neoplastic Transformation of Mammary Papillary Hyperplasia. Science (1977) 195(4281):880–2. doi: 10.1126/science.402692 [DOI] [PubMed] [Google Scholar]
- 69. Brem SS, Jensen HM, Gullino PM. Angiogenesis as a Marker of Preneoplastic Lesions of the Human Breast. Cancer (1978) 41(1):239–44. doi: [DOI] [PubMed] [Google Scholar]
- 70. Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, et al. Expression of the Angiogenic Factors Vascular Endothelial Cell Growth Factor, Acidic and Basic Fibroblast Growth Factor, Tumor Growth Factor β-1, Platelet-Derived Endothelial Cell Growth Factor, Placenta Growth Factor, and Pleiotrophin in Human Primary Breast Cancer and its Relation to Angiogenesis. Cancer Res (1997) 57(5):963–9. [PubMed] [Google Scholar]
- 71. Linderholm B, Hellborg H, Johansson U, Elmberger G, Skoog L, Lehtiö J, et al. Significantly Higher Levels of Vascular Endothelial Growth Factor (VEGF) and Shorter Survival Times for Patients With Primary Operable Triple-Negative Breast Cancer. Ann Oncol (2009) 20(10):1639–46. doi: 10.1093/annonc/mdp062 [DOI] [PubMed] [Google Scholar]
- 72. Shaito A, Posadino AM, Younes N, Hasan H, Halabi S, Alhababi D, et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int J Mol Sci (2020) 21(6). doi: 10.3390/ijms21062084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dorman HJ, Bachmayer O, Kosar M, Hiltunen R. Antioxidant Properties of Aqueous Extracts From Selected Lamiaceae Species Grown in Turkey. J Agric Food Chem (2004) 52(4):762–70. doi: 10.1021/jf034908v [DOI] [PubMed] [Google Scholar]
- 74. Lee HH, Jung J, Moon A, Kang H, Cho H. Antitumor and Anti-Invasive Effect of Apigenin on Human Breast Carcinoma Through Suppression of IL-6 Expression. Int J Mol Sci (2019) 20(13). doi: 10.3390/ijms20133143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kanno S, Tomizawa A, Hiura T, Osanai Y, Shouji A, Ujibe M, et al. Inhibitory Effects of Naringenin on Tumor Growth in Human Cancer Cell Lines and Sarcoma S-180-Implanted Mice. Biol Pharm Bull (2005) 28(3):527–30. doi: 10.1248/bpb.28.527 [DOI] [PubMed] [Google Scholar]
- 76. Messeha SS, Zarmouh NO, Asiri A, Soliman KFA. Rosmarinic Acid-Induced Apoptosis and Cell Cycle Arrest in Triple-Negative Breast Cancer Cells. Eur J Pharmacol (2020) 885:173419. doi: 10.1016/j.ejphar.2020.173419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Benelli G, Pavela R, Petrelli R, Cappellacci L, Bartolucci F, Canale A, et al. Origanum Syriacum Subsp. Syriacum: From an Ingredient of Lebanese ‘Manoushe’ to a Source of Effective and Eco-Friendly Botanical Insecticides. Ind Crops Products (2019) 134:26–32. doi: 10.1016/j.indcrop.2019.03.055 [DOI] [Google Scholar]
- 78. Lakis Z, Mihele D, Nicorescu I, Vulturescu V, Udeanu DI. The Antimicrobial Activity of Thymus Vulgaris and Origanum Syriacum Essential Oils on Staphylococcus Aureus, Streptococcus Pneumoniae and Candida Albicans. Farmacia (2012) 60(6):857–65. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.