Abstract
Background and objective
Poor oral health increases systemic inflammation, which has complex pathophysiologic links with atrial fibrillation (AF). The aim of this comprehensive systematic review was to investigate the association between oral health and AF in terms of new-onset AF and AF recurrence.
Methods
After PROSPERO protocol was registered, PubMed, Scopus, and Cochrane Database of Systematic Reviews were standardly searched from database inception to February 2021. The included studies were assessed for quality and risk of bias using the modified Newcastle-Ottawa scale. The indicators of poorer oral health were the presence of periodontitis, lower frequency of dental scaling, lower frequency of toothbrushing, and lower number of missing teeth.
Results
We initially identified 424 studies; however, only 5 studies met the inclusion criteria. The included studies comprised 3 nationwide population-based retrospective cohort studies, 1 large prospective cohort study, and 1 case-control study that reported the association between oral health and AF. These studies demonstrated that poor oral health was associated with new-onset AF, and may promote AF recurrence and progression. Moreover, AF patients with poorer oral health may have a higher risk of arrhythmias and major adverse cardiovascular events during long-term follow-up.
Conclusion
Improved oral health potentially reduces new-onset AF. Periodontitis prevention, regular dental visits for professional dental scaling, and frequent tooth brushing, are oral health care interventions that contribute to AF protection. Therefore, promoting oral health should be integrated as a part of AF primary prevention.
Keywords: Atrial fibrillation, Cardiovascular disease, Oral health, Oral hygiene, Periodontitis, Tooth loss
Highlights
-
•
The association between oral health and AF as well as the possible mechanisms were reviewed.
-
•
Periodontitis, which can cause systemic inflammation, tends to play major role in AF risk.
-
•
Oral disease prevention and treatment are associated with the decreased occurrence of new-onset AF.
-
•
Frequent toothbrushing, regular dental scaling and dental check-up could be ones of the oral hygiene protocols to prevent periodontitis.
-
•
Clinical implications and future perspective are elaborated and summarized.
Atrial fibrillation; Cardiovascular disease; Oral health; Oral hygiene; Periodontitis; Tooth loss.
1. Introduction
Atrial fibrillation (AF) is the most common significant cardiac arrhythmia in clinical practice [1, 2]. AF has a prevalence of approximately 2–4% of the general population, and rises with increased age [2]. AF independently increases the risk of ischemic stroke and heart failure, contributing to serious morbidity and mortality. Thus, AF consumes a large amount of health resources worldwide and is one of the most important public health concerns [3]. Several animal and human studies support that systemic inflammation plays a major role in the pathogenesis of atrial remodeling, which is established as the initial substrate for AF development [4]. This is supported by evidence showing that the atria of AF patients are infiltrated with inflammatory cells [5]. Several inflammatory biomarkers influence signaling pathways of AF pathogenesis, for example, C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) [6]. These inflammatory markers are also associated with AF occurrence, recurrence, perpetuation, burden, and thromboembolic complications [4]. Therefore, any intervention that reduces systemic inflammation process may potentially benefit AF prevention.
In addition to general health, poor oral health is a major health burden in many countries, and it shares common risk factors with several noncommunicable diseases. Dental caries and periodontitis, oral infection, and inflammatory diseases, are the major oral health concerns commonly caused by poor oral hygiene [7]. Extensive dental caries and severe periodontitis can lead to tooth loss, which is associated with mortality [8]. In addition to oral diseases, the proxy indicators for poor oral health include the number of missing teeth and poor oral hygiene, such as a lower frequency of dental scaling and tooth brushing [9, 10, 11]. The evidence reveals that poor oral hygiene enhances inflammatory biomarkers levels, and is associated with various cardiovascular diseases, including coronary artery diseases and AF [6].
Previous animal studies have supported a causal relationship between systemic inflammation produced by poor oral health, especially periodontitis and AF [12, 13]. Multiple clinical studies have also found an association between oral health and AF [9, 10, 11, 14, 15]. However, the consensus and recommendation guidelines of oral health care for AF prevention have never been determined. Therefore, the aim of this study was to conduct a systematic review to investigate the association between oral health and AF based on new-onset AF and AF recurrence in human studies.
2. Materials and methods
2.1. Literature review and search strategy
The protocol for this systematic review was registered with PROSPERO (International Prospective Register of Systematic Reviews): No. CRD2020219729. The literature search was independently conducted by the two investigators (P.L. and N.L.) using two strategies. First, an electronic search was performed using PubMed, Scopus, and Cochrane Database of Systematic Reviews databases from their inception to February 2021. The search was restricted to human studies published in English. To identify the association between oral health and AF, the search protocols included the following electronic Medical Subject Headings search terms and keywords terms: “oral disease” OR “oral health” OR "oral hygiene" OR “dental care” OR “dental caries” OR “dental health” OR “dental hygiene” OR “dental service” OR “periodontal disease” OR “periodontitis” OR “tooth loss” combined with the term "atrial fibrillation" OR “AF” OR “AFib”. In addition, a manual search for obtainable relevant studies using references from the included articles was performed. This study was conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines and statement [16]. The raw data is publicly available through the Open Science Framework (URL: https://osf.io/rb2fp/?view_only=fe98591ca44549d0af7cfae970c8e755).
2.2. Selection criteria
The selected studies eligible for reviewing were based on the PICOS (population, intervention (exposure), comparison group, outcome, and study design) criteria (Table 1). All cohort and case-control studies that reported an association between oral health and AF were eligible for inclusion. The oral health indicators were having periodontal disease, the number of missing teeth, professional dental cleaning, frequency of tooth brushings, and dental service utilization. The outcomes of interest were the occurrence of new-onset AF and AF recurrence. The inclusion criteria were not limited by study size. Systematic and narrative reviews were excluded. The retrieved articles were individually reviewed for their eligibility criteria by P.L. and N.L. Discrepancies were discussed and resolved by complementary consensus. The remaining discrepancies were resolved by a senior third-person.
Table 1.
PICOS criteria for study inclusion.
| Parameter | Criteria |
|---|---|
| Population | Adult and older individuals |
| Intervention (Exposure) | Exposed to any proxy indicators of oral health and oral hygiene status e.g., periodontitis, dental scaling, dental cleaning, tooth brushing, tooth loss |
| Comparison | Not exposed to any proxy indicators of oral health and oral hygiene status, or general population |
| Outcome | Occurrence of new-onset AF, AF recurrence |
| Study design | Original data from observational studies. Excluded studies were narrative and systematic reviews. |
AF, atrial fibrillation.
2.3. Data abstraction
A structured data collecting form was created, which was used to record the information extracted from each study. The extracted information included the title, year of the study, year of the publication, name of the first author, country of the conducted study, study design, demographic and characteristics the study population, duration of the follow-up period, oral health status, and AF outcomes (e.g., incidence of new-onset AF, incidence of AF recurrence, arrhythmic events, and major adverse cardiovascular events (MACE)). Quantitative estimates comprising hazard ratio (HR), relative risk (RR), odds ratio (OR), and 95% confidence intervals (95% CI) of the association between oral health and AF were recorded. The variables included in the multivariable analysis models were also extracted from the published reports.
2.4. Quality assessment
Quality assessment of the studies was performed independently by two reviewers (P.L., N.L.) using the modified Newcastle-Ottawa quality assessment scale [17]. The selection criterion of the reviewers were that they included both experienced dentist and cardiologist. The 3 quality assessment categories were: 1) selection, 4 items; 2) comparability, 1 item; 3) outcome, 3 items. The ascertainment of the exposure was used for case-control studies, while the ascertainment of the outcome was used for cohort studies. Each item received a dichotomous score of 0 (not performed on inadequate) or 1 (adequate), except for a single item in the comparability category that employed scores of 0–2. The summation score of all items ranged from 0–7.
3. Results
3.1. Literature identified
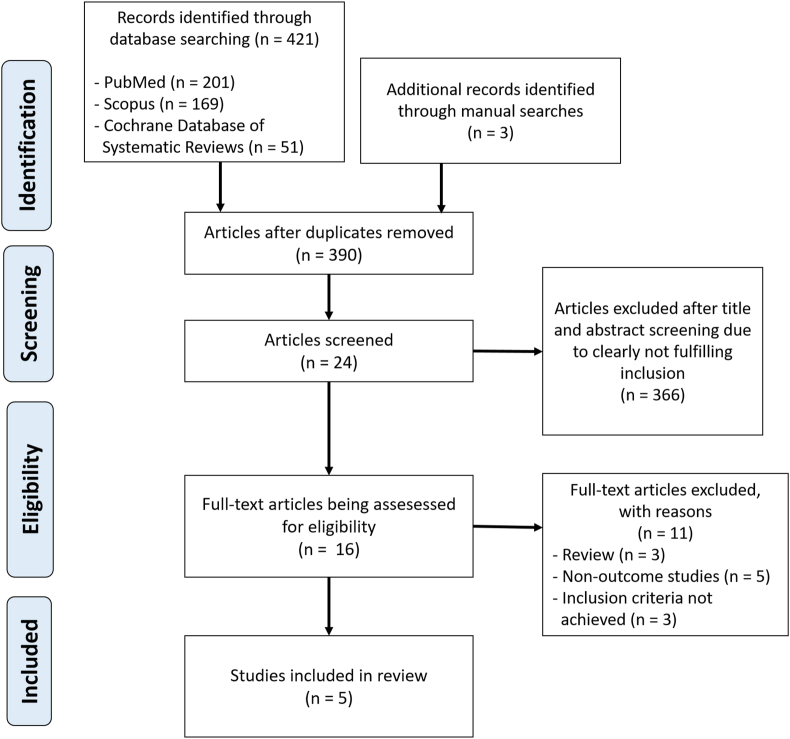
We identified 424 potentially eligible articles (421 articles from the electronic searching strategy and 3 articles from the additional manual searches). We excluded 34 duplicated articles, and the other 366 articles because they were case reports, correspondences, review articles, in vitro studies, pediatric populations, animal studies, or not relevant. Thus, 24 articles remained for abstract review and screening. Eight studies were excluded because they did not meet the inclusion criteria. Sixteen articles remained for the full-length review and 11 were excluded because they did not report the outcome of interest. Ultimately, 5 observational studies related to oral health and AF risk were identified. Oral health indicators in the studies were the presence of periodontal disease, frequency of dental scaling or professional dental cleaning, frequency of tooth brushings, number of missing teeth, and dental service use [9, 10, 11, 14, 15]. The literature retrieval, review, and selection process are demonstrated in Figure 1.
Figure 1.
The literature retrieval, review, and selection process.
3.2. Quality assessment
The items and results of bias risk assessment by the modified Newcastle-Ottawa scale are shown in Table 2. The quality assessment scores of each study using the modified Newcastle-Ottawa scale ranged from 3 to 7 points. Among all included studies, one case-control study had relatively poor-quality score because the information regarding follow-up duration of the outcome was absent and the allocation of the exposure started after the outcome [14]. Moreover, the outcome of interest was not only specific to AF, but also included MACE and other arrhythmic event.
Table 2.
A modified Newcastle-Ottawa scale for quality assessment of included studies.
| Study | Selection (0–1) |
Comparabilitya (0–2) |
Outcome (0–1) |
Total (7) | ||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of exposed cohort | Selection of non-exposed cohort | Ascertainment of exposure | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Adequate of follow-up | |||
| Chen SJ 2013 | 1 | 1 | 1 | 2 | 1 | 1 | 7 | |
| Chen DY, et al. (2016) | 0 | 1 | 0 | 2 | 0 | 1 | 4 | |
| Im SI, et al. (2018) | 1 | 1 | 0 | 1 | 0 | 0 | 3 | |
| Chang Y, et al. (2020) | 1 | 1 | 1 | 0 | 1 | 1 | 5 | |
| Sen S, et al. (2021) | 1 | 1 | 1 | 2 | 1 | 1 | 7 | |
Comparability were assessed as followed: 1 point when the exposed subjects were matched with non-exposed subjects, 2 points if the study also adjusted for age, sex, and comorbidities.
3.3. Study and study population characteristics
The studies in the final analysis comprised 5 observational studies; 3 nationwide population-based retrospective cohort studies, 1 large prospective cohort study, and 1 case-control study. One study population was from the United States of America, while the other 4 study populations were from Asian countries; Taiwan (n = 2) and Korea (n = 2). The PICOS of the included studies are presented in Table 3. The four cohort studies consisted of patients without a past history of AF, whereas one case-control study evaluated patients with known non-valvular AF. The primary outcomes were the occurrence of new-onset AF and AF recurrence. The sample size of the study populations ranged from 227 to 787,490, whereas the follow-up duration ranged from 18 months to 17 years.
Table 3.
Characteristic of the studies included in the systematic review.
| Author | Chen et al. [11] | Chen et al. [10] | Im et al. [14] | Chang et al. [9] | Sen et al. [15] |
|---|---|---|---|---|---|
| Year | 2013 | 2016 | 2018 | 2020 | 2021 |
| Country | Taiwan | Taiwan | Korea | Korea | United States of America |
| Study design | A nationwide, population-based, retrospective cohort (NHIRD) | A nationwide, population-based, retrospective cohort (NHIRD) | Case-control | A nationwide, population-based, retrospective cohort (NHIS-HEALS) | A large prospective cohort study (ARIC) |
| Follow-up duration | 4.6 ± 1 years | 4,075,682/3,405,292 person-years of follow-up | 18 months | 10.5 years | 17 years |
| Subjects |
16,955 adults who were age | 787,490 subjects without previous history of AF/AFL | 227 patients with non-valvular AF | 161,286 adults who were age | 5,958 subjects without previous history of AF |
| 60 or more without past history of cardiac arrhythmias | Exp: 393,745 | Exp: 47 | 40-79 without past history of AF | M: 45.5% | |
| Exp: 3,391 | Control: 393,745 | Control: 180 | M: 61% | Age: 59.5 ± 5.6 years | |
| Control: 13,564 | M: 49% | M: 68% | Age: 52 ± 9 years | DM: 6.7% | |
| M: 55% | Age: 42 ± 17 years | Age: 60 ± 11 years | DM: 9% | HT: 34.5$ | |
| Age: 68 ± 6 | DM: 5% | DM: 17% | HT: 39% | HF: 0.6% | |
| DM: 22% | HT: 9% | HT: 56% | CKD: 8% | CAD: 3.3% | |
| HT: 44% | HF: 0.5% | HF: 11% | |||
| HF: 5% | CAD: 3% | Stroke/TIA: 3% | |||
| CAD: 21% | CKD: 1% | ||||
| Stroke/TIA: 13% | |||||
| CKD: 8% |
|||||
| Intervention/Exposure (Oral health indicators) | |||||
| 1) Periodontal disease | N/A | Presence of periodontitis Control: No periodontitis (1:1 age and sex matched) Note: Diagnosis of periodontitis was defined if one of the following criteria was present; 1) At least 1 dental visit recorded with periodontitis and received antibiotic therapy or periodontal treatment 2) Having more than 2 dental visits with periodontitis diagnosis in one year |
Presence of periodontitis Control: No periodontitis Note: Periodontitis was evaluated using WHO CPI score and diagnosis of periodontitis was confirmed when CPI score ≥3. |
Presence of periodontitis Control: No periodontitis Note: Diagnosis of periodontitis was defined if one of the ICD-10 codes was present; 1) Acute periodontitis, 2) Chronic periodontitis, 3) Periodontosis, 4) Other periodontal disease, and 5) Unspecified periodontal disease. |
Presence of periodontitis (mild, moderate, severe) Control: Periodontal health Note: Periodontitis severity was diagnosed using the periodontal profile class (PPC) |
| 2) Tooth loss | Number of missing teeth: 1–7, 8–14, 15–21, ≥22 Control: no missing teeth |
||||
| 3) Dental scaling/professional dental cleaning | Frequency of dental scaling at least 1 once a year for 3 consecutive years Control: No dental scaling (1:4 matched age, sex, and significant underlying disease, including HT, DM, CHF, CAD, CKD, ischemic stroke/TIA, and asthma/COPD) |
Covariate of periodontitis: Frequency of dental scaling (none, 1–2, >2 times per year) (patients with periodontitis received more frequent dental scaling than the others without periodontitis) | Professional dental cleaning (Yes) Control: no professional dental cleaning |
||
| 4) Toothbrushing | Frequency of tooth brushing: 2, ≥3 (times/day) Control: 0–1 times/day |
||||
| 5) Dental service utilization | Dental visit for any reasons in the last year Control: no dental visit in the last year |
Dental care utilization: regular users (those who sought routine dental care) Control: episodic users (those who sought dental care only when in discomfort, something needed to be fixed, never, or did not receive regular dental care). |
|||
| Methods for oral health assessment |
Review patients' record |
- Oral examination by trained and calibrated dentists to assess periodontitis - Review patients' record to assess dental scaling frequency |
- Oral examination by dentist to assess periodontitis (diagnosed when >2 times of claims), and number of missing teeth - Self-reported questionnaire to assess dental symptoms, dental visit for any reasons, oral hygiene behavior |
- Oral examination by a single examiner to assess periodontitis - Self-reported questionnaire to assess dental care utilization |
|
| Outcome | Primary: Occurrence of new-onset AF | Primary: Time from the inclusion to the first AF/AFL diagnosis during outpatient or inpatient visit | Primary: MACE Secondary: arrhythmic events, including AT, AF, VT, PAC, PVC. |
Primary: Occurrence of new-onset AF and heart failure (HF) | Primary: Occurrence of new-onset AF |
| AF diagnosis |
To assure AF diagnostic accuracy, the occurrence of AF was only defined in subjects with AF diagnosis at discharge or repeatedly confirmed more than twice in outpatient department, by documented ICD9-CM code: 427.31 |
Incident AF/AFL was defined in patients with at least one outpatient or inpatient diagnosis of AF/AFL, by documented ICD9-CM code: 427.31–2 |
Paroxysmal AF was defined when previous ECG showed sinus rhythm. Persistent AF was defined when perpetuating 7 days or more. Chronic AF was defined as an ongoing long-term period. During follow-up, diagnosis of AF recurrence or onset of AF progression was based on the first time that all ≥3 consecutive ECGs at interval of ≥1 week indicated AF, or based on the clinical judgement of the physicians if ECGs were not obtained thrice during the defined period. |
To assure AF and HF diagnostic accuracy, the occurrence of AF and HF were defined in subjects with documented diagnostic ICD10 code: I48 at least 2 claims per year. |
Incident AF was defined by any of the followings; 1) Documented standard 12-lead ECG during follow-up (tracings with automatically-reported AF were reviewed by a cardiologist) 2) Hospital discharge diagnostic codes (defined by ICD9-CM codes 427.31 or 427.32) 3) Death certificates (defined by ICD9-CM codes 427.31 or 427.32) |
| Potential covariate/confounder adjustment |
- Age, Sex - Comorbidities at baseline (heart failure, hypertension, diabetes mellitus, vascular disease, hyperlipidemia ischemic heart disease, valvular heart disease, chronic obstructive pulmonary disease, sleep apnea, renal disease, hypothyroidism, hyperthyroidism) - Average number of outpatient visits |
- Age, sex, socioeconomic status - Regular exercise - Alcohol consumption - Anthropometric measurements (body mass index, systolic- and diastolic blood pressure) - Comorbidities (Hypertension, diabetes, dyslipidemia, current smoking, renal disease, cancer history) - Laboratory findings (total cholesterol, fasting blood sugar, aspartate aminotransferase, gamma-glutamyl transferase, proteinuria) |
- Age, sex, education level, race - Comorbidities (hypertension, diabetes, LDL, obesity, smoking, alcohol use, CAD, CHF) |
||
| Results |
Significantly lower occurrence of new-onset AF in subjects who received dental scaling more than 1 time per year (HR = 0.340, 95% CI = 0.248–0.489; p < 0.001). Dental scaling was independently associated with a reduced risk of new-onset AF (HR = 0.671, 95% CI = 0.524–0.859; p = 0.002). |
Significantly higher occurrence of new-onset AF in subjects with periodontitis (HR = 1.31, 95% CI = 1.25–1.36). |
Significantly higher MACE and arrhythmic events were found in subjects with periodontitis (adjusted OR = 17.8, 95% CI = 3.46–91.3; p < 0.001). Periodontitis was an independent risk factor for MACE and arrhythmic events (p < 0.001). |
Significantly lower occurrence of new-onset AF (HR = 0.90, 95% CI = 0.83–0.98) and HF (HR = 0.88, 95% CI = 0.82–0.94) in subjects with tooth brushings at least 3 times/day. |
Significantly higher occurrence of new-onset AF in subjects with severe periodontitis (adjusted HR = 1.31, 95% CI = 1.06–1.62). Significantly lower occurrence of new-onset AF in regular dental care utilization users (adjusted HR = 0.88, 95% CI = 0.78–0.99). |
| Main findings and comments | Oral health promotion by dental scaling was associated with lower occurrence of new-onset AF. | Patients with periodontitis had an increased risk of new-onset AF/AFL. Dental scaling at least 2 times/year in this study was associated with increased AF/AFL risk because it indicated presence of periodontitis. |
Periodontitis in patients with known valvular AF was associated with higher incidence of arrhythmic events and MACE | Lower risk of incident AF was associated with higher frequency of tooth brushings (≥3 times/day) Lower risk of incident HF was associated with higher frequency of tooth brushings (≥2 times/day), professional dental cleaning, and higher number of missing teeth (≥22 teeth). |
Patients with severe periodontitis and episodic dental care users had higher risk of incident AF than those with periodontal health and mild/moderate periodontitis, and regular denture care users. The association between severe periodontitis and ischemic stroke mediated by AF. |
AF, atrial fibrillation; AFL, atrial flutter; ARIC, Atherosclerosis Risk in Communities; AT, atrial tachycardia; CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CPI, community periodontal index; DM, diabetes mellitus; ECG, electrocardiogram; Exp, exposed; HF, heart failure; HT, hypertension; HR, hazard ratio; ICD, International Classification of Diseases, M, male; MACE, major adverse cardiovascular events; NHIRD, National Health Insurance Research Database; NHIS-HEALS: National Health Insurance System-National Health Screening Cohort; PAC, premature atrial complex; PVC, premature ventricular complex; TIA, transient ischemic attack; VT, ventricular tachycardia; WHO, World Health Organization.
Four cohort studies comprised adult and older individuals aged (mean ± SD) 42 ± 17 to 68 ± 6 years without previous AF history. The exposure, i.e. the oral health indicators, varied between studies, including the presence of periodontitis [9, 10, 14, 15], number of missing teeth [9], frequency of dental scaling or professional dental cleaning [9, 10, 11], frequency of tooth brushings [9], and dental service utilization [9, 15]. The primary outcome in the four cohort studies was the occurrence of new-onset AF. In the case-control study by Im et al (2018) [14], the study population was adult and older individuals aged 60 ± 11 years with known non-valvular AF. The exposed group in the study comprised subjects diagnosed with periodontitis based on their community periodontal index (CPI) score ≥3. The outcome variables were MACE and arrhythmic events, i.e., atrial tachycardia (AT), AF, ventricular tachycardia (VT), premature ventricular complex (PVC), and premature atrial complex (PAC). During the follow-up period, the onset of AF recurrence or onset of AF progression was also examined.
3.4. AF assessment methods
AF diagnosis was defined using either the documented ICD codes for AF (ICD-9CM 427.31, ICD-9CM 427.32, and ICD10 I48) [[9], [10], [11],15], or reviewing standard 12-lead ECGs [14, 15]. Most of the included studies reviewed only ICD codes [9, 10, 11], while Im et al. (2018) reviewed the consecutive ECGs [14], and Sen et al. (2021) reviewed both [15]. New-onset AF was defined in subjects with at least one documented ICD code for AF during follow-up [10, 15], or at least twice to assure AF diagnostic accuracy [9, 11]. The onset of AF recurrence or onset of AF progression was defined based on the first time that ≥3 consecutive ECGs at an interval of ≥1 week indicated AF. If consecutive ECGs were not obtained three times during the period, the physicians’ clinical judgement was used to define the onset of AF progression [14].
3.5. Oral health assessment methods
Oral health indicators in the included studies were categorized into oral diseases (periodontitis and tooth loss) and oral hygiene behaviors (dental scaling, tooth brushing, and dental visits/dental service utilization). To determine the periodontal status and number of missing teeth, an intraoral examination was conducted by one [10, 15] or more dentists [9, 10, 14]. Periodontitis was confirmed when the periodontitis was recorded in at least one dental visit and received antibiotic therapy or periodontal treatment simultaneously [10], or when the periodontitis was recorded in more than two dental visits [9, 10]. Periodontitis was evaluated through different criteria; ICD code, WHO CPI criteria, and periodontal profile class (PPC). The PPC allowed categorizing periodontitis into different severity levels. The number of tooth loss was intraorally examined by dentists regardless of the causes such as periodontal disease or dental caries [9].
To assess oral hygiene behavior, frequency of dental scaling was collected from the patients’ record [10, 11] and self-reported questionnaire [9]. Moreover, the frequency of tooth brushing and dental visit/dental care utilization was obtained from a self-reported questionnaire [9, 14, 15].
3.6. Association between oral health variables and AF
Oral health status was identified across the included studies. A distinction was made between each oral health status and associated AF outcomes.
-
1)
Periodontitis and AF
Except the study by Chen et al. (2013), four out of five studies reported an association between periodontitis and AF. Chen et al. (2016) found that having periodontitis was significantly associated with an increased the risk of new-onset AF/AFL [10], while Chang et al. (2020) found an association only in a univariate analysis [9]. The case-control study by Im et al. (2018) consistently reported a significant association between periodontitis and developing major adverse cardiac events and arrhythmic events (adjusted OR (95% CI) = 17.8 (3.5–91.3) and 9.2 (1.2–68.0), respectively) [14]. Sen et al. (2021) revealed a dose-response relationship between an increased severity level of periodontitis and AF risk in a univariate analysis crude HR (95% CI): control (periodontal health) = 1.00, mild = 1.05 (0.86–1.29), moderate = 1.40 (1.16–1.69), and severe periodontitis = 1.54 (1.26–1.87) [15]. However, significant association was found only in severe periodontitis (adjusted HR (95% CI) = 1.31 (1.06–1.62)). A mediation analysis was also conducted suggesting that AF mediated the association between periodontitis and cardioembolic stroke.
-
2)
Dental scaling, dental service utilization, and AF
Chen et al. (2013), Chen et al. (2016), and Chang et al. (2020) revealed an association between professional dental cleaning or dental scaling and lower AF risk [9, 10, 11]. Chen et al. (2013) found that a dental scaling frequency of at least once a year for 3 consecutive years was associated with reduced AF risk [11], whereas Chang et al. (2020) reported an association between AF risk and professional dental scaling at least once during the year of data collection [9]. Consistent with these findings, Chen et al. (2016) reported that patients who received dental scaling 1–2 times a year had lower AF risk (adjusted HR (95% CI) = 0.39 (0.38–0.41)).
Sen et al. (2021) reported that the regular dental service users had a lower risk of AF incidence compared with the episodic users [15]. In contrast, Chang et al. (2020) found that having a dental visit for any reason during the past year was not associated with AF risk [9].
-
3)
Tooth brushing and AF
Chang et al. (2020) determined the association between the frequency of tooth brushing and AF risk [9]. The univariate analysis revealed a dose-response relationship between an increased toothbrushing frequency and a decreased AF risk (crude HR (95% CI): none to once a day (control) = 1.00, two times/day = 0.79 (0.73–0.85), at least three times/day = 0.61 (0.57–0.67), p for trend <0.001). A multivariable analysis reported a significant association only between toothbrushing at least three times a day and a decreased AF risk (adjusted HR (95% CI) = 0.89 (0.82–0.97), p for trend = 0.004). A significant dose-response relationship between an increased number of tooth loss and heart failure was found in the univariate and multivariable analyses.
-
4)
Association between the number of missing teeth and AF
Chang et al. (2020) determined the association between the number of missing teeth and AF risk was in the nationwide, population-based, retrospective cohort study [9]. The univariate analysis revealed a dose-response relationship between an increased number of missing teeth and an increased risk of AF and heart failure (crude HR (95% CI) for AF: no missing tooth (control) = 1.00, 1–7 teeth = 1.22 (1.14–1.30), 8–14 teeth = 2.05 (1.72–2.44), 15–21 teeth = 1.65 (1.17–2.32), and ≥22 teeth = 2.98 (2.32–3.84), p for trend <0.001). However, the multivariable analysis reported the association only between an increased number of tooth loss and heart failure. In this study, whether tooth loss was a consequence of periodontitis or extensive dental caries was not determined [9].
4. Discussion
The present comprehensive systematic aimed to investigate the causal association between oral health and AF in human studies. Five studies identifying the association between oral health and AF in adults and older individuals were included. All studies supported that better oral health status was associated with a lower occurrence of new-onset AF. In particular, the absence of periodontitis, dental scaling at least once a year, frequent tooth brushing, and regular dental service utilization independently resulted in lower AF incidence. Periodontitis may increase MACE and aggravate arrhythmic events, including AF recurrence and progression [14]. Moreover, a mediation analysis revealed that AF may link periodontitis to cardioembolic stroke. One study suggested the association between AF and a higher number of missing teeth, however, the multivariate analysis found no association after adjusting for confounding factors [9].
Previous studies revealed the effects of poor oral health on atherosclerotic cardiovascular disease [18]. Evidence supports the association between poor oral health and several cardiovascular risk and disorders, including hypertension, diabetes mellitus, dyslipidemia, abdominal aortic aneurysm, and coronary artery disease [19, 20, 21, 22]. AF, a cardiovascular disease risk equivalent [23], is also linked to oral inflammation [24]. Our findings support that poor oral health is associated with an increased risk of a new-onset AF and may promote its progression.
In contrast to our findings, Holm-Pedersen et al. (2005) reported that having periodontitis was not associated with cardiac arrhythmias in community-dwelling older persons aged 80 and above [25]. Their study found an association between cardiac arrhythmias and active dental caries. However, this study did not define a specific type of arrhythmias in the prespecified outcomes, but only stated that AF was responsible for 58% of the arrhythmic events. Therefore, the study was not included in the present systematic review.
4.1. Possible underlying mechanisms
The current evidence supports that inflammatory factors and AF are linked [4, 5, 6]. Most inflammatory states, including metabolic syndrome, increase lifetime risk of new-onset AF. For example, obesity, one of the root causes of multiple noncommunicable diseases and closely linked to metabolic syndrome, is responsible for 50%-enhanced risk of new-onset AF [26]. The underlying pathogenesis facilitating AF incidence and perpetuation in obese patients can be mainly explained by three mechanisms. First, most patients with obesity are eventually accompanied by diabetes mellitus, hypertension, obstructive sleep apnea and ischemic heart disease, which can themselves increase risk of new-onset-AF. Second, direct adipocyte infiltration of the atria in obese patients is reported [27]. Third, fatty tissue can release adipocytokines, such as activin A and matrix metalloproteinases (MMPs), which are inflammatory mediators and pro-fibrotic molecules [28]. In brief, lipotoxicity results in inflammation and fibrosis, contributing to AF initiation. Theoretically, conditions that increase systemic inflammatory biomarkers can lead to AF.
Periodontitis and poor oral hygiene are interrelated. Poor oral hygiene can lead to biofilm formation that is attached to the supragingival or subgingival tooth surface. The biofilm comprises bacterial cell layers surrounded by extracellular polymeric substances that can develop into dental calculus when it is calcified [29]. Although the biofilm can be removed by oral hygiene care, such as tooth brushing and interdental cleaning, dental calculus has to be removed by professional dental cleaning or dental scaling. Biofilm formation can cause gingivitis or inflammation of the gingival tissue, which can progress to periodontitis when the inflammation extends along the root surface of the tooth and penetrates into the bone and supporting tissue [30]. Periodontitis progression can lead to tooth loss due to periodontal attachment and supporting bone loss.
Oral biofilms, i.e., microbial communities, develop from early colonizers that are typically facultative saccharolytic anaerobes, and late colonizers that are often proteolytic obligate anaerobes when oxygen is progressively depleted. The bacterial species associated with the clinical parameters of periodontal disease comprise a high proportion of the three gram-negative anaerobic, proteolytic, and asaccharolytic bacteria; Porphyromonas gingivalis (Pg), Tannerella forsythia (Tf), and Treponema denticola (Td) [29, 31]. The potential roles of oral microorganisms and periodontal pathogens that link poor oral hygiene, periodontitis, and AF can be described by two major host-response mechanisms; a systemic inflammatory response, and an autoimmune response to periodontal pathogens.
The subgingival biofilm in a periodontal pocket enables translocation of periodontal pathogens into the blood circulation, and induces peripheral and systemic inflammatory responses by elevating serum inflammatory biomarkers and cytokines, such as CRP, TNF-α, and IL-6 [6]. CRP plays an important role in endothelial dysfunction by down-regulating endothelial nitric oxide (NO) synthase transcription in endothelial cells and destabilizing endothelial NO-synthase messenger RNA. Inhibiting NO production promotes endothelial cell apoptosis [32]. TNF-α causes inflammation-related AF by increasing pulmonary vein arrhythmogenicity and inducing abnormal calcium homeostasis [33].
Clinical evidence consistently demonstrates that patients with AF have increased CRP, TNF-α, and plasma IL-6 levels, compared with patients without AF [34]. Increased CRP results in AF development and recurrence after successful electrical cardioversion, and the associated prothrombotic state [35]. In addition, AF patients with a higher IL-6 level tend to have a higher risk of thromboembolic events and cardiovascular disease death [36]. Meanwhile, human studies reported that periodontal intervention and oral hygiene improvement significantly decreased inflammatory biomarkers, such as CRP, TNF-α, and IL-6 [37]. Therefore, oral hygiene improvement may decrease the inflammatory responses and reduce AF risk [11].
Another host-response mechanism is the autoimmune response to heat-shock proteins (HSPs) produced by micro-organisms, especially gram-negative bacteria. In humans, HSPs function in cellular stress protection by maintaining proteostasis and preventing cardiomyocyte damage [38]. Oral microorganisms, particularly periodontal pathogens, and human HSP60 express structural homology, sharing similar amino acid sequences, which causes an immunologic cross-reaction between the two molecules, so called “molecular mimicry” [38]. The antibodies generated against bacterial HSP60 also become autoantibodies recognizing human autologous HSPs, leading to an autoimmune response [38]. The HSP 60/65 homolog of Pg, called GroEL, induces a humoral and cellular immune response in humans, which is cross-reacting with the endogenous HSPs expressed by the host [39]. In addition to the autoimmune response resulting from the presence of common epitopes in host proteins and microbial HSPs, the cytotoxicity of certain bacterial HSPs may contribute to tissue destruction [39]. Changes in cardiac HSP60 expression have been found in patients with AF [40], and auto-reactivity to HSPs has been found in patients with periodontitis [41].
A systematic review and meta-analysis by Roca-Millan et al. (2018) reported a beneficial effect of periodontal treatment on inflammatory biomarkers [18]. They concluded that C-reactive protein (CRP) and leukocyte values were significantly lower after periodontal treatment. Increased systemic inflammatory biomarkers, especially CRP, TNF-α, and IL-6, might be the main pathophysiologic link between poor oral health and cardiovascular disease [42, 43, 44] and these biomarkers are also associated with AF [36, 45, 46]. Thus, periodontal treatment along with oral hygiene improvement might decrease the systemic inflammatory response, leading to AF protective effects.
4.2. Limitations
There are some limitations in this systematic review. Due to limited number of the included studies in the present systematic review, the association between AF and some oral health variables, such as number of missing teeth, frequency of toothbrushing, and dental service utilization, may provide indefinite clinical implications. A meta-analysis could not be performed due to variations in the oral health variables and assessment methods, and thus, we could not provide a pooled estimate for the association between oral health status and AF. Moreover, the AF diagnosis in most of the included studies was not a comprehensive examination. Three studies defined AF from ICD9-CM or ICD10 codes without further confirmation by reviewing standard 12-lead ECGs [9, 10, 11]. However, the accuracy of AF diagnosis using the ICD code is considered acceptable based on a positive predictive value of 89%, sensitivity of 80–85%, and specificity of 97–99% reported in a previous study [47].
Another limitation was that the criteria and accuracy of the periodontitis diagnosis in some studies were unclear. A standard diagnosis of periodontitis includes a direct intraoral examination to record clinical attachment loss, periodontal pocket depth, and bleeding on probing, as well as intraoral radiographic examination to identify the amount of bone loss and severity of periodontitis [48]. However, this information was lacking, especially the intraoral radiographic examination [10]. One study lacked the intra- or inter-examiner reliability of the periodontitis diagnosis [15]. The severity level of periodontitis was identified in only one study [15]; therefore, determining the dose-response relationship between increased periodontitis severity levels and AF risk was limited. A calibration tool for periodontitis diagnosis is necessary for further studies to reduce bias of the results.
Only limited number of studies had used oral hygiene behaviors as proxy indicators, and hence, a recommendation cannot be made. The protective effect of frequent toothbrushing and dental scaling on periodontitis has been reported [49]. An increased dental scaling frequency was associated with decreased AF risk. However, Chen et al. (2016) reported that dental scaling more than 3 times a year increased AF risk. This was because the patients who received dental scaling more than twice a year had already developed periodontitis [10]. Moreover, self-report questionnaires of oral hygiene behaviors may introduce a recall bias. Therefore, toothbrushing and dental scaling frequency should be used as a proxy, but not a true diagnostic indicator of periodontitis due to the limited number of the included studies.
Lastly, some included studies lacked information concerning potential risk factors and confounders that might affect oral hygiene behavior, dental service utilization, and therefore, the new onset of AF. The variables include socioeconomic status (educational level, marital status) [9, 11], obesity, health behaviors such as alcohol consumption and tobacco use [10], as well as life style factors [11]. The heterogeneity of demographic, economic and educational status may influence the results, and therefore, the large number of samples was included in the evaluated studies.
4.3. Clinical implications and future perspectives
The current 2019 AHA/ACC/HRS [50] and 2020 ESC [51] AF guidelines emphasize the primary preventive approach to reduce lifetime risk of new-onset AF. Increasing evidence supports that managing cardiovascular risk factors and concomitant diseases reduces the AF incidence, and improves sinus rhythm maintenance. The interventions to prevent and control AF mentioned in most of the guidelines are modifying unhealthy lifestyles, blood pressure control, comorbidities management, weight reduction, and avoiding alcohol [50, 51, 52]. The underlying pathophysiologic mechanism between these interventions and the protective effect on AF is because they reduce systemic inflammation [53]. Therefore, any intervention that reduces inflammation can theoretically prevent AF.
By reducing systemic inflammation, oral disease prevention and oral health promotion may decrease the risk and progression of AF. Our findings suggest that preventing and treating periodontitis potentially reduces the risk of developing AF. To prevent periodontitis occurrence and progression, several protocols for oral hygiene care are recommended. The principles are to reduce biofilm and calculus formation, and thus, reducing local and systemic inflammation. Our findings suggest frequent tooth brushing (at least 2 times/day) and regular dental visits to receive professional dental scaling (1–2 times/year) should be more frequent in patients with severe periodontitis [9, 10, 11]. Due to a limited number of studies, however, the associations between the increased AF risk and more frequent professional dental scaling (more than 2 times/day) remain inconclusive. Additionally, oral health promotion strategies should be implemented, e.g., improving oral health literacy and providing universal health coverage to reduce the financial hardship and increase accessibility to oral health care services. Therefore, oral disease prevention and oral health promotion should be an integral part of the interventions for preventing new-onset AF, which might further reduce the risk and severity of ischemic stroke and heart failure.
Some topics are recommended for future studies. The role of the CHA2DS2-VASc score, the parameter for predicting thromboembolic risk in patients with AF, was not included in the present study. Thus, the associations between poor oral health, AF, CHA2DS2-VASc score, and stroke should be further investigated. Because periodontitis is a chronic inflammatory condition, a greater CHA2DS2-VASc score in periodontitis patients without AF may lead to a higher risk of ischemic stroke. Similarly, a CHA2DS2-VASc score in periodontitis patients with AF may not be similar to the general non-valvular AF population. Moreover, the association between AF occurrence and other oral health indicators, such as dental caries and tooth loss should be investigated, and the underlying mechanisms clarified. In addition to tooth brushing, preventing periodontitis can be implemented by several mechanical cleaning and chemical rinsing methods [49]. Thus, the association between AF and oral hygiene care with other intraoral mechanical and chemical cleaning including interdental cleaning and mouthwash should be further investigated. Lastly, other periodontal indicators, e.g., pocket depth and clinical attachment loss, should be examined on dental radiographs to determine the severity of periodontitis and identify a dose-response relationship between the increased severity of periodontitis and AF.
5. Conclusions
Better oral health potentially reduces the occurrence of new-onset AF. Periodontitis prevention, including regular professional dental visits and frequent tooth brushing, are oral health care interventions that can protect against AF. Therefore, oral health promotion should be considered as a part of the interventions for AF primary prevention.
Declarations
Author contribution statement
Pattranee Leelapatana and Nareudee Limpuangthip: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Benjamin E.J., Muntner P., Alonso A., et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y., Barnes M.E., Gersh B.J., et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Copley D.J., Hill K.M. Atrial fibrillation: a review of treatments and current guidelines. AACN Adv. Crit. Care. 2016;27(1):120–128. doi: 10.4037/aacnacc2016281. [DOI] [PubMed] [Google Scholar]
- 4.Korantzopoulos P., Letsas K.P., Tse G., et al. Inflammation and atrial fibrillation: a comprehensive review. J. Arrhythm. 2018;34(4):394–401. doi: 10.1002/joa3.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harada M., Van Wagoner D.R., Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ. J. 2015;79(3):495–502. doi: 10.1253/circj.CJ-15-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaizot A., Vergnes J.N., Nuwwareh S., et al. Periodontal diseases and cardiovascular events: meta-analysis of observational studies. Int. Dent. J. 2009;59(4):197–209. [PubMed] [Google Scholar]
- 7.Petersen P.E., Estupinan-Day S., Ndiaye C. WHO's action for continuous improvement in oral health. Bull. World Health Organ. 2005;83(9):642. [PMC free article] [PubMed] [Google Scholar]
- 8.Polzer I., Schwahn C., Volzke H., et al. The association of tooth loss with all-cause and circulatory mortality. Is there a benefit of replaced teeth? A systematic review and meta-analysis. Clin. Oral Invest. 2012;16(2):333–351. doi: 10.1007/s00784-011-0625-9. [DOI] [PubMed] [Google Scholar]
- 9.Chang Y., Woo H.G., Park J., et al. Improved oral hygiene care is associated with decreased risk of occurrence for atrial fibrillation and heart failure: a nationwide population-based cohort study. Euro. J. Preven. Cardiol. 2020;27(17):1835–1845. doi: 10.1177/2047487319886018. [DOI] [PubMed] [Google Scholar]
- 10.Chen D.-Y., Lin C.-H., Chen Y.-M., et al. Risk of atrial fibrillation or flutter associated with periodontitis: a nationwide, population-based, cohort study. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0165601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S.J., Liu C.J., Chao T.F., et al. Dental scaling and atrial fibrillation: a nationwide cohort study. Int. J. Cardiol. 2013;168(3):2300–2303. doi: 10.1016/j.ijcard.2013.01.192. [DOI] [PubMed] [Google Scholar]
- 12.Yu G., Yu Y., Li Y.N., et al. Effect of periodontitis on susceptibility to atrial fibrillation in an animal model. J. Electrocardiol. 2010;43(4):359–366. doi: 10.1016/j.jelectrocard.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Aoyama N., Suzuki J.I., Kobayashi N., et al. Detrimental effects of specific Periodontopathic bacterial infection on tachyarrhythmia compared to Bradyarrhythmia. BMC Cardiovasc. Disord. 2017;17(1):267. doi: 10.1186/s12872-017-0703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Im S.I., Heo J., Kim B.J., et al. Impact of periodontitis as representative of chronic inflammation on long-term clinical outcomes in patients with atrial fibrillation. Open Heart. 2018;5(1) doi: 10.1136/openhrt-2017-000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sen S., Redd K., Trivedi T., et al. Periodontal disease, atrial fibrillation and stroke. Am. Heart J. 2021;235:36–43. doi: 10.1016/j.ahj.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 18.Roca-Millan E., Gonzalez-Navarro B., Sabater-Recolons M.M., et al. Periodontal treatment on patients with cardiovascular disease: systematic review and meta-analysis. Med. Oral Patol. Oral Cir. Bucal. 2018;23(6):e681–e690. doi: 10.4317/medoral.22725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding F., Wu D., Han X., et al. Oral hygiene and periodontal conditions in the Chinese patients with aortic aneurysm. BMC Oral Health. 2018;18(1):136. doi: 10.1186/s12903-018-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietropaoli D., Del Pinto R., Ferri C., et al. Poor oral health and blood pressure control among US hypertensive adults. Hypertension. 2018;72(6):1365–1373. doi: 10.1161/HYPERTENSIONAHA.118.11528. [DOI] [PubMed] [Google Scholar]
- 21.Lamster I.B., Lalla E., Borgnakke W.S., et al. The relationship between oral health and diabetes mellitus. J. Am. Dent. Assoc. 2008;139(Suppl):19S–24S. doi: 10.14219/jada.archive.2008.0363. [DOI] [PubMed] [Google Scholar]
- 22.Ashraf J., Hussain Bokhari S.A., Manzoor S., et al. Poor oral health and coronary artery disease: a case-control study. J. Periodontol. 2012;83(11):1382–1387. doi: 10.1902/jop.2012.110563. [DOI] [PubMed] [Google Scholar]
- 23.Barrios V., Escobar C. Atrial fibrillation, an equivalent of cardiovascular disease risk. Eur. Heart J. 2020;41(48):4599. doi: 10.1093/eurheartj/ehaa771. [DOI] [PubMed] [Google Scholar]
- 24.Aarabi G., Schnabel R.B., Heydecke G., et al. Potential impact of oral inflammations on cardiac functions and atrial fibrillation. Biomolecules. 2018;8(3) doi: 10.3390/biom8030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holm-Pedersen P., Avlund K., Morse D.E., et al. Dental caries, periodontal disease, and cardiac arrhythmias in community-dwelling older persons aged 80 and older: is there a link? J. Am. Geriatr. Soc. 2005;53(3):430–437. doi: 10.1111/j.1532-5415.2005.53160.x. [DOI] [PubMed] [Google Scholar]
- 26.Pouwels S., Topal B., Knook M.T., et al. Interaction of obesity and atrial fibrillation: an overview of pathophysiology and clinical management. Expert Rev. Cardiovasc. Ther. 2019;17(3):209–223. doi: 10.1080/14779072.2019.1581064. [DOI] [PubMed] [Google Scholar]
- 27.Mahajan R., Lau D.H., Brooks A.G., et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J. Am. Coll. Cardiol. 2015;66(1):1–11. doi: 10.1016/j.jacc.2015.04.058. [DOI] [PubMed] [Google Scholar]
- 28.Goudis C.A., Korantzopoulos P., Ntalas I.V., et al. Obesity and atrial fibrillation: a comprehensive review of the pathophysiological mechanisms and links. J. Cardiol. 2015;66(5):361–369. doi: 10.1016/j.jjcc.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Velsko I.M., Fellows Yates J.A., Aron F., et al. Microbial differences between dental plaque and historic dental calculus are related to oral biofilm maturation stage. Microbiome. 2019;7(1):102. doi: 10.1186/s40168-019-0717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchesan J.T., Morelli T., Moss K., et al. Interdental cleaning is associated with decreased oral disease prevalence. J. Dent. Res. 2018;97(7):773–778. doi: 10.1177/0022034518759915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holt S.C., Ebersole J.L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the "red complex", a prototype polybacterial pathogenic consortium in periodontitis. Periodontol. 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 32.Verma S., Wang C.H., Li S.H., et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106(8):913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 33.Lee S.H., Chen Y.C., Chen Y.J., et al. Tumor necrosis factor-alpha alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci. 2007;80(19):1806–1815. doi: 10.1016/j.lfs.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 34.Conway D.S., Buggins P., Hughes E., et al. Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation. J. Am. Coll. Cardiol. 2004;43(11):2075–2082. doi: 10.1016/j.jacc.2003.11.062. [DOI] [PubMed] [Google Scholar]
- 35.Liu T., Li G., Li L., et al. Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. J. Am. Coll. Cardiol. 2007;49(15):1642–1648. doi: 10.1016/j.jacc.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 36.Zhou P., Waresi M., Zhao Y., et al. Increased serum interleukin-6 level as a predictive biomarker for atrial fibrillation: a systematic review and meta-analysis. Rev. Port. Cardiol. 2020;39(12):723–728. doi: 10.1016/j.repc.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Sun W.L., Chen L.L., Zhang S.Z., et al. Changes of adiponectin and inflammatory cytokines after periodontal intervention in type 2 diabetes patients with periodontitis. Arch. Oral Biol. 2010;55(12):970–974. doi: 10.1016/j.archoralbio.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Rizzo M., Macario A.J., de Macario E.C., et al. Heat shock protein-60 and risk for cardiovascular disease. Curr. Pharmaceut. Des. 2011;17(33):3662–3668. doi: 10.2174/138161211798220981. [DOI] [PubMed] [Google Scholar]
- 39.Goulhen F., Grenier D., Mayrand D. Oral microbial heat-shock proteins and their potential contributions to infections. Crit. Rev. Oral Biol. Med. 2003;14(6):399–412. doi: 10.1177/154411130301400603. [DOI] [PubMed] [Google Scholar]
- 40.Schafler A.E., Kirmanoglou K., Balbach J., et al. The expression of heat shock protein 60 in myocardium of patients with chronic atrial fibrillation. Basic Res. Cardiol. 2002;97(3):258–261. doi: 10.1007/s003950200019. [DOI] [PubMed] [Google Scholar]
- 41.Ando T., Kato T., Ishihara K., et al. Heat shock proteins in the human periodontal disease process. Microbiol. Immunol. 1995;39(5):321–327. doi: 10.1111/j.1348-0421.1995.tb02208.x. [DOI] [PubMed] [Google Scholar]
- 42.Demmer R.T., Trinquart L., Zuk A., et al. The influence of anti-infective periodontal treatment on C-reactive protein: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0077441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koppolu P., Durvasula S., Palaparthy R., et al. Estimate of CRP and TNF-alpha level before and after periodontal therapy in cardiovascular disease patients. Pan Afr. Med. J. 2013;15:92. doi: 10.11604/pamj.2013.15.92.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nibali L., Fedele S., D'Aiuto F., et al. Interleukin-6 in oral diseases: a review. Oral Dis. 2012;18(3):236–243. doi: 10.1111/j.1601-0825.2011.01867.x. [DOI] [PubMed] [Google Scholar]
- 45.Weymann A., Popov A.F., Sabashnikov A., et al. Baseline and postoperative levels of C-reactive protein and interleukins as inflammatory predictors of atrial fibrillation following cardiac surgery: a systematic review and meta-analysis. Kardiol. Pol. 2018;76(2):440–451. doi: 10.5603/KP.a2017.0242. [DOI] [PubMed] [Google Scholar]
- 46.Yo C.H., Lee S.H., Chang S.S., et al. Value of high-sensitivity C-reactive protein assays in predicting atrial fibrillation recurrence: a systematic review and meta-analysis. BMJ Open. 2014;4(2) doi: 10.1136/bmjopen-2013-004418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen P.N., Johnson K., Floyd J., et al. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol. Drug Saf. 2012;21(Suppl 1):141–147. doi: 10.1002/pds.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tonetti M.S., Greenwell H., Kornman K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018;89(Suppl 1):S159–S172. doi: 10.1002/JPER.18-0006. [DOI] [PubMed] [Google Scholar]
- 49.Loe H. Oral hygiene in the prevention of caries and periodontal disease. Int. Dent. J. 2000;50(3):129–139. doi: 10.1111/j.1875-595x.2000.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 50.January C.T., Wann L.S., Calkins H., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. 2019;140(2):e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 51.Hindricks G., Potpara T., Dagres N., et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2020;2020 doi: 10.1093/eurheartj/ehab648. [DOI] [PubMed] [Google Scholar]
- 52.Andrade J.G., Aguilar M., Atzema C., et al. The 2020 Canadian cardiovascular society/Canadian heart rhythm society comprehensive guidelines for the management of atrial fibrillation. Can. J. Cardiol. 2020;36(12):1847–1948. doi: 10.1016/j.cjca.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Chung M.K., Eckhardt L.L., Chen L.Y., et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American heart association. Circulation. 2020;141(16):e750–e772. doi: 10.1161/CIR.0000000000000748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.