Abstract
The potentially toxic freshwater cyanobacterium Cylindrospermopsis raciborskii has become increasingly prevalent in tropical and temperate water bodies worldwide. This paper investigates the effects of different nitrogen sources (NO3−, NH4+, and omission of a fixed form of nitrogen) on the growth rates, morphologies, and cylindrospermopsin (CYL) concentrations (expressed as a percentage of the freeze-dried weight) of seven C. raciborskii isolates obtained from a range of water bodies in northern Australia and grown in batch culture. In general, growth rates were lowest in the absence of a fixed-nitrogen source and highest with NH4+ as the nitrogen source. Conversely, the highest concentrations of CYL were recorded in cultures grown in the absence of a fixed-nitrogen source and the lowest were found in cultures supplied with NH4+. Cultures supplied with NO3− were intermediate with respect to both CYL concentration and growth rate. Different nitrogen sources resulted in significant differences in the morphology of C. raciborskii trichomes. Most notable were the loss of heterocysts and the tapering of end cells in cultures supplied with NH4+ and the statistically significant increase in vegetative cell length (nitrogen depleted < NO3− < NH4+). The morphological changes induced by different nitrogen sources were consistent for all isolates, despite measurable differences in vegetative-cell and heterocyst dimensions among isolates. Such induced morphological variation has implications for Cylindrospermopsis taxonomy, given that distinctions between species are based on minor and overlapping differences in cell lengths and widths. The close phylogenetic association among all seven isolates was confirmed by the high level (>99.8%) of similarity of their 16S rRNA gene sequences. Another genetic technique, analysis of the HIP1 octameric-palindrome repeated sequence, showed greater heterogeneity among the isolates and appears to be a useful method for distinguishing among isolates of C. raciborskii.
The freshwater cyanobacterium Cylindrospermopsis raciborskii (order Nostocales), first named by Seenayya and Subba Raju (33), was initially assigned to the genus Anabaenopsis as A. raciborskii Woloszynska (41) but was subsequently recommended for exclusion from that genus because of its quite different pattern of heterocyst development, which more closely resembles that of the genus Cylindrospermum. On this basis, the genus Cylindrospermopsis was proposed (33). This was later supported by Horecká and Komárek (17), who distinguished Cylindrospermopsis from Cylindrospermum by the presence in the former of gas vacuoles, attenuated and pointed ends of trichomes, and spores (akinetes) positioned near one or both ends of the trichomes, with one to three vegetative cells between the terminal heterocysts and akinetes. Using numerical taxonomic methods based on a wide range of morphological features, Horecká and Komárek (17) also confirmed the close relationship between the two genera and a considerably more distant relationship between the genera Cylindrospermopsis and Anabaenopsis. In his description of natural populations of these two genera from western Slovakia, Hindák (16) confirmed that while in Cylindrospermopsis heterocysts develop primarily from terminal cells, in Anabaenopsis they are formed in pairs in an intercalary position.
Eight Cylindrospermopsis species have now been described: Cylindrospermopsis africana, C. cuspis, C. philippinensis, C. raciborskii (19), C. allantoidispora, C. catemaco, C. tavernae (20, 21), and C. curvispora (37). These species, most of which have been described from natural populations, are distinguished by minor and overlapping differences in vegetative-cell and heterocyst dimensions and by akinete shape, although akinetes have not been found in all species (e.g., C. curvispora).
C. raciborskii, the most frequently reported species in this genus, is of interest from a water quality perspective due to its ability to produce a potent hepatotoxic alkaloid, cylindrospermopsin (CYL) (13, 14, 28). This toxin, which has been implicated in outbreaks of human sickness (4, 5, 15) and in cattle mortality (30), can accumulate in the tissues of aquatic organisms (29). The ability of C. raciborskii to produce paralytic shellfish-poisoning toxins, similar to those found in dinoflagellates and the cyanobacterium Anabaena circinalis, has also been demonstrated (22). Given the potential for serious health concerns, there is a clear need to investigate the limits of morphological variation within C. raciborskii so that natural populations of this cyanobacterium can be identified correctly for the purposes of ecological monitoring and toxicological studies.
In this paper we describe investigations into the effects of different nitrogen sources on the growth, morphology (including those characteristics which distinguish the different species of the genus Cylindrospermopsis), and gravimetric CYL concentrations of seven isolates of C. raciborskii taken from a range of water bodies in northern Australia and grown in pure culture. The isolates have been characterized genetically by two techniques. Firstly, 16S rRNA gene (rDNA) sequence analysis was performed. This has been shown to be useful in determining differences among cyanobacterial genera (39) and species (25). Second, genomic polymorphism analysis, employing cyanobacterium specific highly iterative palindrome (HIP1) repeats (11), was carried out. This technique has been applied to other cyanobacteria and has been shown to be useful as a typing technique at the strain level for many genera of cyanobacteria (35). This is the first report of the application of this technique to the genus Cylindrospermopsis.
MATERIALS AND METHODS
Isolation and culturing of C. raciborskii.
Seven isolates of C. raciborskii (five with straight trichomes and two with coiled trichomes), all with vegetative and heterocyst cell dimensions within the reported range for that species (1, 19), were brought into pure culture as previously described (31). These isolates originated from a range of water bodies in northern Australia. Cultures were grown in ASM-1 medium (6) (pH 7.6) modified by the exclusion of the primary nitrogen source (NaNO3). The sources of isolates, types of source water body, trichome morphologies, and relevant publications are given in Table 1.
TABLE 1.
Sources and trichome morphologies of C. raciborskii isolates used in this study
| Isolate | Source | Impoundment type | Date isolated | Trichome morphology | Reference |
|---|---|---|---|---|---|
| CR1 | Solomon Dam (18°45′S, 146°35′E) | Potable supply | February 1996 | Straight | 31 |
| CR2 | Solomon Dam (18°45′S, 146°35′E) | Potable supply | February 1996 | Coiled | 31 |
| CR3 | Townsville (19°16′S, 146°49′E) | Aquaculture pond | August 1997 | Straight | 29 |
| CR4 | Goonyella Dam (21°48′S, 147°58′E) | Potable supply | April 1998 | Straight | NAa |
| CR5 | McKinlay (21°16′S, 141°18′E) | Farm dam | August 1997 | Straight | 30 |
| CR6 | Townsville (19°16′S, 146°49′E) | Aquaculture pond | August 1997 | Coiled | 29 |
| CR7 | Lake Julius (20°08′S, 139°44′E) | Potable supply | November 1995 | Straight | NA |
NA, not available.
Experimental culturing conditions.
To investigate the influence of the nitrogen source on the growth rates, morphologies, and gravimetric CYL concentrations of the seven isolates, the primary nitrogen source of the ASM-1 media (2 mM NaNO3) was either included (NO3−), omitted, or replaced by NH4Cl (NH4+) to give equivalent final concentrations of nitrogen. Urea was previously shown to be unsuitable for the growth of this cyanobacterium (31). Media for the three nitrogen treatments were also modified by the addition of a buffer (0.02 M HEPES, pH 7.6).
Triplicate 150-ml cultures in sterile 250-ml Erlenmeyer flasks, initiated by the aseptic transfer of 1 ml of stock culture (containing ca. 100,000 cells ml−1), were placed in a controlled-environment cabinet at 25°C with a light intensity of 50 μmol m−2 s−1 (12 h of light/12 h of darkness) provided by cool white fluorescent tubes.
Growth, CYL concentration determinations, and morphological analyses.
Maximum growth rates of cultures, in divisions per day, were determined from growth curves based on periodic (24- to 48-h) estimation of cell concentrations as indicated by previously calibrated optical density (750 nm) measurements (31). Cyanobacterial biomass at the end of the exponential growth phase (determined individually for each of the isolates and nitrogen treatments) was measured as freeze-dried weight following the filtration (0.45-μm-pore-size Whatman GF/C membranes) of pooled triplicate cultures. CYL concentrations in the harvested cultures were analyzed by mass spectrometry coupled with high-performance liquid chromatography (9) and expressed per unit of freeze-dried weight.
For each of the isolates and nitrogen treatments, representative subsamples of pooled culture (ca. 20 ml) were preserved in Lugol's iodine solution for microscopic (Olympus CH-2) measurement of morphological features including vegetative-cell length (VCL), vegetative-cell width (VCW), heterocyst length (HL), and heterocyst width (HW). For each of the isolates and nitrogen treatments, measurements were made on at least 30 heterocysts and vegetative cells. End cells were excluded from the analysis due to the greater variability in end-cell shape (34).
Statistical analysis of the data (appropriately transformed to satisfy the requirements for a parametric test) was by two-way analysis of variance using SPSS version 6.1 (SPSS, Inc., Chicago, Ill.). The experimental design allowed evaluation of differences between isolates, the effect of the nitrogen source, and the interaction between these two variables.
DNA extraction.
Total genomic DNA was extracted from lyophilized samples of the seven isolates grown under stock culture conditions (as described above) by using a modification of a technique for purification of DNA from Gram-negative bacteria (24). Briefly, lyophilized samples were suspended in 500 μl of a solution containing 50 mM Tris-HCl (pH 8.0), 5 mM EDTA (pH 8.0), and 50 mM NaCl. Lysozyme was added to a final concentration of 1 mg ml−1, and the solution was incubated at 55°C for 30 min. After addition of 10 μl of a proteinase K solution (10 mg ml−1) and 20 μl of 10% sodium dodecyl sulfate, the mixture was incubated at 55°C for 10 min or until the solution cleared (indicating complete cell lysis). The solution was then chilled on ice and extracted twice with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1, vol/vol/vol). The supernatant was then added to an equal volume of 4 M ammonium acetate, and total genomic DNA was precipitated by addition of 2 volumes of isopropanol and centrifugation (12,000 × g) for 10 min at room temperature. The integrity and concentration of the extracted genomic DNA were determined spectrophotometrically at 260 and 280 nm.
16S rDNA amplification.
PCR amplification of the 16S rRNA gene was performed using primers 27F1(UFP) and 1494Rc(URP) together with PCR reagents as previously described (25). Thermal cycling was performed at 94°C for 4 min followed by 30 cycles of 94°C for 20, 50°C for 30, and 72°C for 2 min. The amplification reaction products were purified by using the Wizard PCR purification system (Promega, Madison, Wis.) to remove reaction components including unincorporated primers, enzyme, and nucleotides. Approximately 100 ng of PCR product and 10 pmol of previously described 16S rRNA gene sequencing primers (25) were used to determine the primary structure of the Cylindrospermopsis 16S rDNA. Automated DNA sequencing was performed with the PRISM cycle sequencing system and an ABI 373 sequencer (Applied Biosystems Inc., Foster City, Calif.). Oligonucleotide primers were synthesized on an Oligo 1000 DNA synthesis system (Beckman, Fullerton, Calif.) and purified by reverse-phase chromatography.
16S rDNA phylogenetic analysis.
DNA sequences were aligned by using the 6CG Pileup program (Genetics Computer Group, Madison, Wis.) and the multiple sequence alignment tool from Clustal X (36). Manual confirmation of the sequence alignment was performed and checked against both primary- and secondary-structure considerations of the 16S rRNA molecule. The aligned sequences were applied to genetic distance and maximum-parsimony methods for phylogenetic inference. Ambiguous characters, where a deletion, insertion, or unidentified state was recorded for any isolate, were not subjected to further analysis. For all multiple sequence alignments and phylogenetic inference programs, the input order of each of the taxa was randomized. Genetic distances (D) were calculated (18) with the formula D = −3/4 ln(1 − 4/3d), where d is the sequence dissimilarity. Phylogenetic inference protocols DNADIST, NEIGHBOR, DNAPARS, CONSENSE, and SEQBOOT were supplied in the PHYLIP package (version 3.57c) (10). All sequence manipulation and phylogeny programs were made available by the Australian National Genomic Information Service (Sydney, Australia).
Cyanobacterial repeated-sequence PCR.
HIP1 PCR amplifications were performed with primers HipCA and HipTG (35). Twenty-microliter reaction volumes contained 2 μl of a 2 mM deoxynucleoside triphosphate solution, 2 μl of 25 M MgCl2, 2 μl (100 ng) of DNA preparation, 1 μl (10 pmol) of each primer solution, 11.8 μl of H2O, and 0.2 μl (1 U) of Taq polymerase. Reactions were cycled using a temperature profile consisting of 95°C for 5 min; 30 cycles of 95°C for 10 s, 40°C for 20 s, and 72°C for 60 s; and 1 cycle of 72°C for 5 min. Reactions were also performed as described above but with only the HipTG primer employed to initiate strand extension. PCR products were separated by 1.5% agarose gel electrophoresis in Tris-borate-EDTA buffer according to standard protocols (32). The quantities of PCR products loaded onto the gel differed slightly (between 3 and 6 μl) for the seven isolates so as to result in approximately equivalent intensities of banding patterns. Gel electrophoresis was performed in triplicate to confirm the resultant profiles. Gels were corrected for brightness and contrast and photographed. The photographic image was then used to construct a binary matrix based on the visual presence or absence of DNA bands on the electrophoresis gel. Phylogenetic analysis of this binary data matrix was achieved by using the DNAPARS, CONSENSE, and SEQBOOT programs of the phylogenetic inference package (PHYLIP, version 3.57c) (10).
RESULTS
Effect of nitrogen source on growth rate and CYL concentration of C. raciborskii.
Five of the seven isolates, CR1, CR2, CR3, CR4, and CR5, produced detectable concentrations of CYL. Isolates CR6 and CR7 did not produce detectable concentrations of this toxin under any of the growth conditions investigated (Table 2). There was no correlation between the ability of the isolates to produce CYL and whether the isolates had coiled or straight trichomes, since both coiled and straight morphotypes were found among those isolates producing high concentrations (0.21 to 0.46% of freeze-dried weight) and those that did not contain detectable concentrations (less than ca. 0.00002%) of CYL. The highest concentrations of this toxin were found in cultures grown in the absence of a fixed nitrogen source, for which growth rates were lower (with the exception of CR3) (Table 2). The lowest concentrations of CYL occurred in cultures supplied with NH4+, for which growth rates were highest. Cultures supplied with NO3− were, in general, intermediate with respect to both CYL concentration and growth rate (Table 2) with the exception of isolate CR3, for which this nitrogen source resulted in the highest CYL concentration.
TABLE 2.
Effects of different nitrogen sources on growth rates and CYL concentrations (measured at the end of the period of exponential growth) of seven C. raciborskii isolates in pure cultures
| Isolate | Value obtained with N source:
|
|||||
|---|---|---|---|---|---|---|
| No nitrogen addition
|
NO3−
|
NH4+
|
||||
| GRa | CYLb | GRa | CYLb | GRa | CYLb | |
| CR1 | 0.63 ± 0.05 (4) | 0.46 | 0.65 ± 0.05 (4) | 0.35 | 0.83 ± 0.16 (3) | 0.23 |
| CR2 | 0.75 ± 0.16 (4) | 0.30 | 0.93 ± 0.01 (3) | 0.27 | 1.05 ± 0.01 (3) | 0.21 |
| CR3 | 0.65 ± 0.09 (4) | 0.38 | 0.69 ± 0.12 (3) | 0.42 | 0.51 ± 0.03 (3) | 0.34 |
| CR4 | 0.77 ± 0.13 (4) | 2.9 × 10−2 | 0.64 ± 0.3 (3) | 2.8 × 10−2 | 1.30 ± 0.18 (3) | 2.1 × 10−2 |
| CR5 | 0.87 ± 0.31 (3) | 1.2 × 10−4 | 1.41 ± 0.27 (3) | 7.0 × 10−5 | 1.44 ± 0.31 (3) | NDc |
| CR6 | 0.89 ± 0.15 (3) | ND | 1.20 ± 0.14 (3) | ND | 1.49 ± 0.29 (3) | ND |
| CR7 | 0.86 ± 0.06 (4) | ND | 0.71 ± 0.10 (4) | ND | 1.10 ± 0.31 (3) | ND |
Growth rate in divisions day−1 ± 1 of standard deviation. Values in parentheses are numbers of replicates.
Percentage of freeze-dried weight.
ND, not detected.
Morphological variation among isolates of C. raciborskii.
The characteristic gross morphology of the inoculum (i.e., straight or coiled trichomes [Table 1]) was maintained through successive generations in culture. There was no evidence of straightening of coiled trichomes or of coiling of straight trichomes. The numerical taxonomic analysis of morphological characteristics detected a number of statistically significant differences in VCL, VCW, VCL:VCW ratio, HL, HW, and HL:HW ratio among the seven isolates (P <0.00) (Table 3), indicating that there were measurable morphological differences among isolates taken from different water bodies. Nitrogen source had no effect on VCL (P = 0.3) or HL:HW ratio (P = 0.2) but had a significant effect on all other morphological variables (Table 3). VCW increased as follows: no added N < NO3− < NH4+ (Fig. 1). In general there was a strong consistency in the responses of all isolates to different nitrogen sources. The most striking morphological change induced by different nitrogen sources was the complete loss of heterocysts in cultures that occurred with NH4+. The terminal cells of trichomes grown in this treatment also became more tapered in appearance.
TABLE 3.
Results of a two-way analysis of variance comparing some morphological characteristics of seven C. raciborskii isolates grown with different N sources
| Variable | Total sample size | Statistical results when evaluating:
|
|||||
|---|---|---|---|---|---|---|---|
| Differences between isolates
|
Effect of N source
|
Isolate differences × N source effectsa
|
|||||
| F | P | F | P | F | P | ||
| VCLb | 779 | 33.79 | 0.00 | 1.21 | ≥0.30 | 2.54 | 0.00 |
| VCWb | 779 | 170.87 | 0.00 | 1,098.4 | 0.00 | 18.88 | 0.00 |
| VCL:VCWb | 779 | 70.82 | 0.00 | 182.57 | 0.00 | 5.30 | 0.00 |
| HL | 521 | 15.30 | 0.00 | 54.49 | 0.00 | 2.0 | ≥0.06 |
| HW | 521 | 35.83 | 0.00 | 80.74 | 0.00 | 4.19 | 0.00 |
| HL:HWb | 521 | 31.44 | 0.00 | 1.68 | ≥0.20 | 0.22 | ≥0.97 |
Interaction of isolate differences and nitrogen source effects.
Data required log10 transformation to improve normality.
FIG. 1.
VCL (a), VCW (b), VCL:VCW ratio (c), HL (d), HW (e), and HL:HW ratio (f) for seven isolates of C. raciborskii (CR1 to CR7 as described in Table 1) grown in the presence of NO3− (▨) or NH4+ (▧) or without the addition of a combined nitrogen source (□). The coiled forms, CR2 and CR6, are labeled (C) in the upper panels. In panels d to f, only two bars are shown because cultures grown in the presence of NH4+ did not produce heterocysts.
None of the differences in vegetative-cell and heterocyst dimensions could be related either to the ability of the isolates to produce CYL (Table 2) or to gross morphology (i.e., straight or coiled trichomes) (Fig. 1).
Genetic comparison.
Analysis of 16S rRNA gene nucleotide sequences confirmed a strong (>99.8%) genetic similarity among all seven isolates of C. raciborskii (Fig. 2). It was not possible to define Australian Cylindrospermopsis isolates by specific sequence signatures. Therefore, the design of specific 16S rDNA-directed PCRs for the delineation of strains used here with regard to toxicity was not undertaken. Other cyanobacteria which are known to produce CYL, including Aphanizomenon ovalisporum ILC-146 (93.8% similar), Umezakia natans TAC101 (87.5% similar), and Anabaena bergii AWQC283A (92% similar), showed divergent 16S rDNA sequences in comparison to those of C. raciborskii. These other species were more distant from the C. raciborskii strains than Anabaena cylindrica and therefore were not used in the phylogenetic reconstruction.
FIG. 2.
Phylogenetic affiliations between seven isolates of C. raciborskii (CR1 to CR7) (Table 1) and other cyanobacteria, derived from complete 16S rRNA gene sequences. The phenogram was reconstructed from a pairwise distance matrix (18) by the neighbor-joining method (27). The scale represents two substitutions per 100 nucleotide positions. Bootstrap values (1,000 resampling cycles) indicate the statistical significance of each node. GenBank accession numbers for the 16S rDNA sequences of strains M. aeruginosa PCC7941, N. muscorum PCC7120, and Chlorogloeopsis spp. strain PCC7518 are U40340, X59559, and X68780, respectively. Other sequence data were obtained from the Ribosomal Database Project under the accession codes cyls. 7417 (Cylindrospermum spp. strain PCC7417), chrc. 7203 (C. thermalis PCC7203), and glb.violac (Gloeobacter spp. strain PCC7421). Strains in boldface type were characterized during the present study.
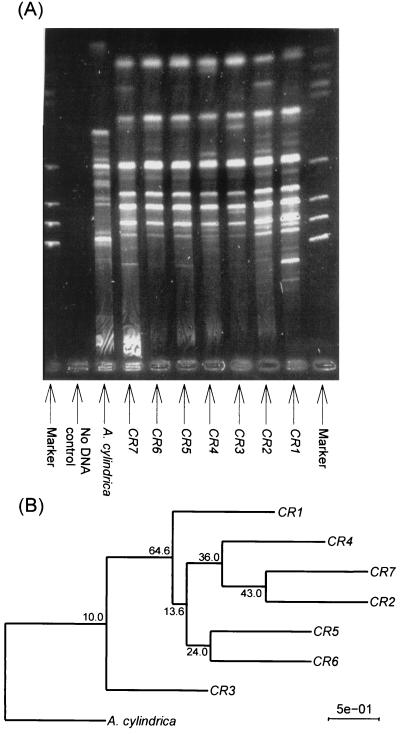
The technique using short tandem-repeat sequences, HIP-PCR, was more sensitive in detecting genetic heterogeneity among isolates. Clear differences in banding patterns were observed among some of the isolates when both HipAC and HipTG primers were used in the one PCR (Fig. 3A). These genomic profiles were employed for the construction of a binary matrix which was subsequently used as the basis for the construction of a phylogenetic tree with Anabaena cylindrica serving as the outgroup. CR1 and CR3 (both of which possess straight trichomes and produce CYL) were distinct from the other isolates (Fig. 3B). This distinction was validated statistically by (i) the lack of significant bootstrap values separating CR1 and CR3 (10%) and (ii) consistent clustering of the remaining strains distinct from the CR3 lineage in 64.6% of resampled trees (Fig. 3B). The remainder of the isolates clumped together in two closely related groups, the first consisting of three isolates (CR2, CR7, and CR4) and the second containing two (CR5 and CR6). Both of these closely related groups contained representatives of straight and coiled isolates as well as toxic and nontoxic members. Only three fragments with no polymorphism were revealed for each of the seven strains when only the HipGT primer was used in a PCR (data not shown).
FIG. 3.
(A) Electrophoretic comparison of the PCR products formed in reactions primed with HipCA and HipTG primers for the seven isolates of C. raciborskii (CR1 to CR7), Anabaena cylindrica, and a no-DNA control. (B) Phenogram constructed from analysis of electrophoresis gels resulting from the HIP-PCR in panel A. A binary matrix was tabulated based on the presence or absence of bands and consisted of 25 characters across the eight operational taxonomic units. Tree reconstruction procedures are described in the text. All bootstrap values (1,000 resampling events) are shown.
DISCUSSION
Five of the seven isolates produced detectable concentrations of CYL. This proportion (ca. 70%) is in fairly close agreement with the results of mouse toxicity tests of isolates of C. raciborskii taken from water bodies in southern Australia (2) and suggests that the CYL toxin is widespread in many Australian water bodies. Only CR1, CR2, and CR3 were found to produce CYL at concentrations of >0.1% of the dry weight. The nitrogen source was found to have a significant effect on CYL concentration. While the growth rate was significantly reduced in the absence of a fixed-nitrogen source, growth under these conditions produced the greatest gravimetric toxin concentration. Similar findings have been reported for some other cyanobacterial species, including Aphanizomenon flos-aquae and Anabaena flos-aquae, both of which have been shown to produce higher concentrations of the propane alkaloid anatoxin-a under nitrogen-depleted conditions (26).
Different nitrogen sources were found to induce statistically significant changes to the morphologies of all seven isolates. The loss of heterocysts, as occurred in cultures supplied with NH4+, considerably increases the difficulty in identifying members of the genus Cylindrospermopsis, considering that the terminal nature of heterocysts is the primary diagnostic feature of the genus. Furthermore, the provision of NH4+ as the primary nitrogen source led to a 33 to 61% increase in VCW and effected a 23 to 45% reduction in the VCL:VCW ratio. Considering that species in the genus Cylindrospermopsis are distinguished by minor differences in vegetative-cell and heterocyst dimensions, there are clear implications for the intrageneric taxonomy of Cylindrospermopsis strains. Similar observations have been reported for other genera of cyanobacteria in which the primary taxonomic characteristics vary under different culture conditions (7, 8, 38). The morphological variants induced by the different nitrogen supplies as reported here are in strong correspondence with the seasonal variants described by Singh (34). This observation is further supported by the findings of Komárková et al. (21) showing that populations of C. raciborskii lacking heterocysts were predominant in a tropical reservoir during periods of higher NH4+ concentration. Clearly, cell length and width measurements are insufficient to distinguish between strains or species of the genus Cylindrospermopsis, an observation in agreement with the suggestion that many morphological features of microorganisms in general may not be under tight genetic control (12).
All isolates, despite exhibiting statistically significant differences in many morphological characteristics, were found to be extremely similar in terms of their 16S rRNA gene nucleotide sequences. While Anabaena cylindrica grouped closely to C. raciborskii, Aphanizomenon ovalisporum and U. natans, two species also known to produce CYL, were considerably more genetically distant. With our increasing recognition of the ubiquity of some cyanobacterial toxins, such as microcystin and saxitoxin, throughout a range of distantly related cyanobacterial groups, it is not surprising that the CYL toxin is also produced by other genera of cyanobacteria. Given the data presented here, it would seem that the genus Cylindrospermopsis is a genetically well-defined population exhibiting considerable morphological and toxicological plasticity, unlike some other nostocalean cyanobacteria (3, 23).
The HIP-PCR analysis was found here to be useful for distinguishing among isolates of C. raciborskii at the strain level. Interestingly, this technique detected a significant difference between isolate CR3 and all other isolates. This isolate, which was obtained from an aquaculture pond (Table 1), responded quite differently than the other isolates to different nitrogen sources, most notably with respect to growth rate responses.
Among the isolates which could not be distinguished by this technique were representatives with both straight and coiled trichomes and those which were toxic or nontoxic. The HIP-PCR analysis supports the proposal that morphologies, including heterocyst differentiation, trichome coiling, and CYL production, are inducible or repressible characters and are not necessarily linked to the phylogeny of Cylindrospermopsis. This confirmed the earlier finding (31) that neither of these characteristics is a valid taxonomic criterion, even though preliminary DNA profiling based on heptamer repeats indicates some linkage between trichome coiling and genotype (40). Identification and characterization of the genetic basis for CYL biosynthesis will assist in the detection of toxigenic strains and provide evidence for the evolution of CYL production in C. raciborskii. This may be achievable given the state of proteomics and the effect of altered diazotrophy on CYL production.
ACKNOWLEDGMENTS
Financial assistance for this study was provided by Mount Isa Mines Ltd., the Australian Research Council, and the Queensland Department of Natural Resources.
We thank G. K. Eaglesham (Queensland Scientific Services) for performing CYL analyses, M. Pratchet (James Cook University) for assistance with statistical analysis, B. P. Burns (University of New South Wales) for performing HIP-PCRs, and M. Watanabe (National Science Museum, Tokyo, Japan) for supplying Umezakia natans TAC101.
REFERENCES
- 1.Baker P D. Identification of common noxious cyanobacteria. Part I. Nostocales. Research report no. 29. Melbourne, Australia: Urban Water Research Association of Australia; 1991. [Google Scholar]
- 2.Baker P D, Humpage A R. Toxicity associated with commonly occurring cyanobacteria in surface waters of the Murray Darling Basin. Aust J Mar Freshw Res. 1994;45:773–786. [Google Scholar]
- 3.Beltran E C, Neilan B A. Geographical segregation of the neurotoxin-producing cyanobacterium Anabaena circinalis. Appl Environ Microbiol. 2000;66:4468–4474. doi: 10.1128/aem.66.10.4468-4474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourke A T C, Hawes R B, Neilson A, Stallman N D. An outbreak of hepato-enteritis (the Palm Island mystery disease) possibly caused by algal intoxication. Toxicon. 1983;3:45–48. [Google Scholar]
- 5.Byth S. Palm Island mystery disease. Med J Aust. 1980;2:40–42. doi: 10.5694/j.1326-5377.1980.tb131814.x. [DOI] [PubMed] [Google Scholar]
- 6.Carmichael W W, Gorham P R. An improved method for obtaining axenic clones of planktonic blue-green algae. J Phycol. 1974;10:238–240. [Google Scholar]
- 7.Chang T P. Morphological remarks on Pseudoanabaena mucicola (Huber-Pestalozzi et Naumann) (Bourrelly) Chang. Algol Stud. 1988;50–53:59–70. [Google Scholar]
- 8.Doers M P, Parker D L. Properties of Microcystis aeruginosa and M. flos-aquae (cyanobacteria) in culture: taxonomic implications. J Phycol. 1988;24:502–508. [Google Scholar]
- 9.Eaglesham G K, Norris R L, Shaw G R, Smith M J, Chiswell R K, Davis B C, Neville G R, Seawright A A, Moore M R. Use of HPLC-MS/MS to monitor cylindrospermopsin, a blue-green algal toxin, for public health purposes. Environ Toxicol. 1999;14:151–155. [Google Scholar]
- 10.Felsenstein J. PHYLIP. Phylogeny inference package. Cladistics. 1989;5:164–166. [Google Scholar]
- 11.Gupta A, Morby A P, Turner J S, Whitton B A, Robinson N J. Deletion within the metallothionein locus of cadmium tolerant Synechococcus PCC6301 involving a highly iterated palindrome (Hip1) Mol Microbiol. 1993;5:825–834. doi: 10.1111/j.1365-2958.1993.tb01110.x. [DOI] [PubMed] [Google Scholar]
- 12.Harold F M. To shape a cell: an inquiry into the causes of morphogenesis of microorganisms. Microbiol Rev. 1990;54:381–431. doi: 10.1128/mr.54.4.381-431.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkins P R, Runnegar M T C, Jackson A R B, Falconer I R. Severe hepatotoxicity caused by the tropical cyanobacterium (blue-green alga) Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju isolated from a domestic water supply reservoir. Appl Environ Microbiol. 1985;50:1292–1295. doi: 10.1128/aem.50.5.1292-1295.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawkins P R, Chandrasena N R, Jones G J, Humpage A R, Falconer I R. Isolation and toxicity of Cylindrospermopsis raciborskii from an ornamental lake. Toxicon. 1997;35:341–346. doi: 10.1016/s0041-0101(96)00185-7. [DOI] [PubMed] [Google Scholar]
- 15.Hayman J. Beyond the Barcoo—probable human tropical cyanobacterium poisoning in outback Australia. Med J Aust. 1992;157:794–796. doi: 10.5694/j.1326-5377.1992.tb141290.x. [DOI] [PubMed] [Google Scholar]
- 16.Hindák F. Planktonic species of two related genera, Cylindrospermopsis and Anabaenopsis, from western Slovakia. Arch Hydrobiol. 1988;80:283–302. [Google Scholar]
- 17.Horecká M, Komárek J. Taxonomic position of three planktonic blue green algae from the genera Aphanizomenon and Cylindrospermopsis. Preslia (Prague) 1979;51:289–312. [Google Scholar]
- 18.Jukes T H, Cantor C R. Evolution of protein molecules. In: Munro H N, editor. Mammalian protein metabolism. Vol. 3. New York, N.Y: Academic Press Inc.; 1969. pp. 21–132. [Google Scholar]
- 19.Komárek J, Kling H. Variation in six planktonic cyanophyte genera in Lake Victoria (East Africa) Arch Hydrobiol. 1991;88:21–46. [Google Scholar]
- 20.Komárková J. The tropical planktonic genus Cylindrospermopsis (Cyanophytes, Cyanobacteria) In: Azevedo T, editor. Anais dos IV Congresso Latino-Americano de Ficologia, II Reunião Ibero Americana de Ficologia e VII Reunião Brasileira de Ficologia. I. 1998. pp. 327–340. São Paulo, Brazil. [Google Scholar]
- 21.Komárková J, Laudares-Silva R, Senna P A C. Extreme morphology of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) in the Lagoa do Peri, a freshwater coastal lagoon, Santa Catarina, Brazil. Algol Stud. 1999;94:207–222. [Google Scholar]
- 22.Lagos N, Onodera H, Zagatto P A, Andrinolo D, Azevedo S M, Oshima Y. The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii, isolated from Brazil. Toxicon. 1999;37:1359–1373. doi: 10.1016/s0041-0101(99)00080-x. [DOI] [PubMed] [Google Scholar]
- 23.Moffitt, M. C., S. E. Blackburn, and B. A. Neilan. Ribosomal RNA sequences reflect the ecophysiology and define the toxic cyanobacteria of the genus Nodularia. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 24.Neilan B A. Identification and phylogenetic analysis of toxigenic cyanobacteria by multiplex randomly amplified polymorphic DNA PCR. Appl Environ Microbiol. 1995;61:2286–2291. doi: 10.1128/aem.61.6.2286-2291.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neilan B A, Jacobs D, Del Dot T, Blackall L L, Hawkins P R, Cox P T, Goodman A E. rRNA sequences and evolutionary relationships among toxic and nontoxic cyanobacteria of the genus Microcystis. Int J Syst Bacteriol. 1997;47:693–697. doi: 10.1099/00207713-47-3-693. [DOI] [PubMed] [Google Scholar]
- 26.Rapala J, Sivonen K, Luukkainen R, Niemelä S. Anatoxin-a concentration in Anabaena and Aphanizomenon under different environmental conditions and comparison of growth by toxic and non-toxic Anabaena isolates: a laboratory study. J Appl Phycol. 1993;5:581–591. [Google Scholar]
- 27.Saitou N, Nei M. The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 28.Saker M L, Griffiths D J. The effect of temperature on growth and cylindrospermopsin content of seven isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from water bodies in northern Australia. Phycologia. 2000;39:349–354. [Google Scholar]
- 29.Saker M L, Eaglesham G K. The accumulation of cylindrospermopsin from the cyanobacterium Cylindrospermopsis raciborskii in tissues of the Redclaw crayfish Cherax quadricarinatus. Toxicon. 1999;37:1065–1077. doi: 10.1016/s0041-0101(98)00240-2. [DOI] [PubMed] [Google Scholar]
- 30.Saker M L, Thomas A D, Norton J H. Cattle mortality attributed to the toxic cyanobacterium Cylindrospermopsis raciborskii in an outback region of north Queensland. Environ Toxicol. 1999;14:179–183. [Google Scholar]
- 31.Saker M L, Neilan B A, Griffiths D J. Two morphological forms of Cylindrospermopsis raciborskii (Cyanobacteria) isolated from Solomon Dam, Palm Island, Queensland. J Phycol. 1999;35:599–606. [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 33.Seenayya G, Subba Raju N. On the ecology and systematic position of the alga known as Anabaenopsis raciborskii (Wolosz.) Elenk. and a critical evaluation of the forms described under the genus Anabaenopsis. In: Desikachary T V, editor. First International Symposium on Taxonomy and Biology of Blue-Green Algae. Madras, India: Madras University; 1972. pp. 52–57. [Google Scholar]
- 34.Singh R N. Seasonal variants of Anabaenopsis raciborskii Wolosz. Hydrobiologia. 1962;20:87–91. [Google Scholar]
- 35.Smith J K, Parry J D, Day J G, Smith R J. A PCR technique based on the Hip1 interspersed repetitive sequence distinguishes cyanobacterial species and strains. Microbiology. 1998;144:2791–2801. doi: 10.1099/00221287-144-10-2791. [DOI] [PubMed] [Google Scholar]
- 36.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe M. Studies on planktonic blue-green algae. 5. A new species of Cylindrospermopsis (Nostocaceae) from Japan. Bull Natl Sci Mus Ser B. 1995;21:45–48. [Google Scholar]
- 38.Wilmotte A. Growth and morphological variability of six strains of Phormidium cf. ectocarpi Gomont (Cyanophyceae) cultivated under different temperatures and light intensities. Algol Stud. 1988;50–53:35–46. [Google Scholar]
- 39.Wilmotte A, Golubic S. Morphological and genetic criteria in the taxonomy of cyanophyta/cyanobacteria. Algol Stud. 1991;64:1–24. [Google Scholar]
- 40.Wilson K M, Schembri M A, Baker P D, Saint C P. Molecular characterization of the toxic cyanobacterium Cylindrospermopsis raciborskii and design of a species-specific PCR. Appl Environ Microbiol. 2000;66:332–338. doi: 10.1128/aem.66.1.332-338.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woloszynska J. Das phytoplankton einiger Javanian seen mit Berücksichtigung des sawa-Planktons. Bull Int Acad Sci Cracoviae Ser B. 1912;6:649–709. [Google Scholar]